Abstract
Background:
Numerous triggers have been implicated in adult female acne including endogenous (hormonal dysfunction and genetic predisposition) and exogenous causes (drugs, cosmetics, sunscreens, stress, and smoking).
Aims:
To evaluate the role of various trigger factors in adult female acne and to analyze the androgenic hormone pattern including anti-Mullerian hormone (AMH) in these patients.
Materials and Methods:
Patients having acne of age ≥25 years were analyzed using a pre devised proforma to elicit trigger factors while the severity was graded using the Global Acne Grading System (GAGS). A detailed hormonal assessment was undertaken that assessed total testosterone (TT), sex hormone-binding globulin (SHBG), free androgen index (FAI), AMH, 17-hydroxyprogesterone (17-OHP), dehydroepiandrosterone sulfate (DHEAS), follicle-stimulating hormone (FSH), luteinizing hormone (LH), thyroid-stimulating hormone (TSH), and prolactin.
Results:
Out of the 165 cases seen and sub-analyzed for triggers, premenstrual flare, diet, cosmetics, and stress were the most commonly implicated causes. Among cosmetics, fairness creams and foundations were implicated. The hormonal analysis revealed deranged values of all hormones with the most common being 17-OHP and AMH. Almost 42.8% patients with DHEAS derangement and 58.75% females with raised 17-OHP suffered from moderate to severe stress.
Limitations:
A prospective cohort correlation study of the implicated triggers is needed to confirm the association with adult female acne.
Conclusions:
Adult female acne may be triggered by diet, stress, and cosmetics and there is a distinct hormonal milieu that accounts for hyperandrogenemia. We noted high levels of adrenal androgens which have been known to be associated with stress and sleep deprivation. Our study shows the value of counseling adult female acne patients about various acne triggers.
Keywords: Adult female acne, hyperandrogenemia, trigger factors, stress, diet, cosmetics
Introduction
Adult acne, predominantly seen in females, is defined as the presence of acne beyond the age of 25 years.[1] It has two subtypes: “persistent acne” and “late-onset acne.” The “persistent acne” which presents in adolescence and continues into adulthood is the most frequent subtype.[2,3,4] The “late-onset acne” appears for the first time after 25 years of age.[5,6]
The most common endogenous causes of adult acne are hormonal dysfunction[1] and a genetic predisposition. The various external triggers implicated include drugs, cosmetics, exposure to comedogenic substances, stress, and smoking.[7,8] Premenstrual flare is also seen in many acne patients.[2]
As androgen excess consequent to ovarian or adrenal pathology is one of the most common endocrine dysfunction in adult female acne,[9] a hormonal evaluation is indispensable to specify the androgen source.[10]
The aims of our study were to evaluate various trigger factors implicated in adult female acne in Indian patients and to analyze the androgenic hormone pattern in them. As polycystic ovary syndrome (PCOS) is a common cause of androgenic excess in acne we also studied the levels of anti-Mullerian hormone (AMH) which has been known to correlate well with ovarian reserve, polycystic ovarian morphology (PCOM), and PCOS.[11,12]
Materials and Methods
This prospective study examined nonpregnant females with acne aged ≥25 years, who were not on hormonal therapy for last 3 months attending the OPD in a tertiary referral hospital in north India from November 2017 to March 2019. The study was conducted after due ethics approval by the institutional ethics committee (IEC). A detailed history and examination were undertaken according to a preapproved proforma. The Global Acne Grading System (GAGS) was used to assess the acne. The patients were subdivided into persistent and late-onset acne.
The patient reported trigger factors associated with acne flare were labelled as “subjective triggers” while objective evaluation of triggers was arrived by specific questions pertaining to diet, cosmetics, stress, and sleep pattern [Table 1]. Dietary history was assessed by the analysis of the glycemic index of foods[13] and a note was made of the intake of milk and dairy products, processed foods, chocolates, and “oily” foods. Ingredients of cosmetics were assessed for comedogenicity and includeddecyl oleate, isopropyl palmitate, isopropyl myristate, isopropyl isostearate, myristyl myristate, butyl stearate, isocetyl stearate, and cocoa butter.[14] Sunscreen ingredients were assessed for implicated comedogenic ingredients such as homosalate, red petrolatum, zinc oxide, N, N-dimethylamino benzoate, dioxybenzone, oxybenzone, and dihydroxyacetone.[15] Stress during the past 1 month was assessed using perceived stress scale (PSS). The adequacy of sleep cycle was evaluated using Pittsburgh sleep quality index (PSQI). An acne flare was defined as a history of increase in the number, type, and extent of acne lesions as reported by the patients. We also elicited history and cross-verified relevant drug intake including androgens, anticonvulsants (carbamazepine, phenytoin, gabapentin), antidepressants (lithium), antipsychotics, antitubercular drugs (isoniazid, pyrazinamide), antivirals (ganciclovir), and use of over-the-counter medications such as vitamin B12 and various vitamin supplements commonly implicated to cause or exacerbate acne.
Table 1.
Proforma
| History of presenting acne complaint | |
|---|---|
| History of acne trigger factors (subjective) | None |
| What causes or exacerbates acne according to you? | Cosmetics |
| Drugs* | |
| Prescription/nonprescription (including vitamin supplements) | |
| Hormonal/nonhormonal | |
| Oily food (patient perception) | |
| Premenstrual flare Travel | |
| Personal history for objective assessment of acne trigger | |
| Dietconsumption (days/week) | What type of milk do you consume and how frequently? (skimmed/partially skimmed/whole) |
| What are the various dairy products (cheese/yogurt) you consume and how frequently? | |
| What are the various starchy food and processed foods you consume (pasta/bread/rice) and how frequently? | |
| What are the various oily foods you consume and how frequently? | |
| How frequently do you eat fruits, vegetables, fish, and dietary fibers? | |
| Assessment of glycemic index of foods† | |
| Cosmetics (frequency and duration) | Which sunscreen do you use and how frequently? |
| Which fairness cream do you use and how frequently? | |
| Which foundation do you use and how frequently? | |
| What is the frequencyof getting Facial? | |
| Assessment of cosmetics for comedogenic ingredients‡ | |
| Stress¦ | None |
| Mild | |
| Moderate | |
| Severe | |
| Sleep-wake cycle‖ | Adequate |
| Inadequate | |
| Smoking | Smoker/nonsmoker |
*Drug history included androgens, anticonvulsants, antidepressants, antipsychotics, antitubercular drugs, and antivirals. Drugs causing acneiform eruptions such as corticosteroids were excluded (only acne vulgaris assessed). †based on glycemic index chart[13], ‡Based on data derived from previous studies[14][15], §Perceived stress scale, Mild—low stress: Moderate—moderate stress; Severe—high perceived stress. ‖Pittsburgh sleep quality index (PSQI) score >5—inadequate sleep (poor sleepers)
On days 3–5 of the menstrual cycle, a morning sample after overnight fasting was taken to evaluate hormones including total testosterone (TT), sex hormone-binding globulin (SHBG), free androgen index (FAI), AMH, 17-hydroxyprogesterone (17-OHP), dehydroepiandrosterone sulfate (DHEAS), follicle-stimulating hormone (FSH), luteinizing hormone (LH), thyroid-stimulating hormone (TSH), and prolactin, using the random access fully automated immunoassay system (DXI-600, Beckman Coulter, USA). While DHEAS and 17-OHP were representative of adrenal androgens, AMH was used to evaluate PCOS. The free androgen index (FAI) was calculated as a surrogate marker of hyperandrogenemia. Free testosterone was not tested as it requires highly sensitive tools and is not standardized across laboratories or studies.
Additionally, a transabdominal ultrasound was performed on the same dayof blood sampling using a curvilinear transabdominal probe (3 MHz, Medison—SonoAceX1) in the transverse and sagittal plane. PCOS was diagnosed on the basis of Rotterdam's criteria and biochemical hyperandrogenism was defined by serum TT levels more than or equal to 1.89 nmol/Lusing the Rotterdam's criteria.
Statistical methods
The data were entered in MS EXCEL spreadsheet and analysis was done using Statistical Package for Social Sciences (SPSS) version 21.0.
Categorical variables were presented in number and percentage (%) and continuous variables were presented as mean ± SD and median. The normality of data was tested by the Kolmogorov-Smirnov test. If the normality was rejected then the nonparametric test was used.
Quantitative variables were compared using the independent t-test/Mann-Whitney test (when the data sets were not normally distributed) between the two groups. Qualitative variables were correlated using Chi-Square test/Fisher's Exact test. A P value of <0.05 was considered statistically significant.
Results
Of the 165 adult female acne patients seen, 56% of females had late-onset acne and 43% presented as persistent acne. As per the inclusion criterion for hormonal testing, only 120 were further studied. Most of the patients with acne (70%) were in the age range of 25–30 years while only 5.83% were aged above 40 years. Working women, students, and housewives constituted 36.67%, 15.83%, and 47.50%, respectively in the study. PCOS was diagnosed as per Rotterdam's criteria in 31 (25.83%) patients. The most common lesion at presentation was papule (66.67%) followed by mixed lesions including pustules, nodules, and comedones. The patient acne severity was graded as mild (79.17%) to moderate (20.83%) with a mean GAGS of 15.57 ± 4.04. The subjective evaluation revealed that 30% of females had a distinct premenstrual flare [Table 2]. The objective assessment implicated the role of diet in 23.33% of patients with the common foods being “oily”and dairy products [Figure 1]. Among cosmetics; fairness creams, foundations, and facials were implicated. The mean duration was found to be the highest of “fairness creams” while the maximum frequency per month was highest for sunscreens [Table 3]. Females with persistent acne had a significantly higher frequency of foundation usage with a mean of 14.88 days per month when compared to only 5.08 days per month in the late-onset acne patients [Figure 2]. On the basis of PSS, moderate to severe stress was reported by 65.83% females while inadequate sleep was reported by 35% of patients. The majority (97.61%) of our patients with inadequate sleep suffered from stress as well. 77.27% of working women and 52.63% of students in the study suffered from moderate to severe stress in addition to acne.
Table 2.
Frequency table of subjective acne trigger factors
| Aggravation factor | Number of women | Percentage |
|---|---|---|
| Nil | 77 | 64.17 |
| Cosmetics | 2 | 1.67 |
| Drugs | 1 | 0.83 |
| Oily food | 3 | 2.50 |
| Premenstrual flare | 36 | 30 |
| Travel | 1 | 0.83 |
| Total | 120 | 100 |
Figure 1.
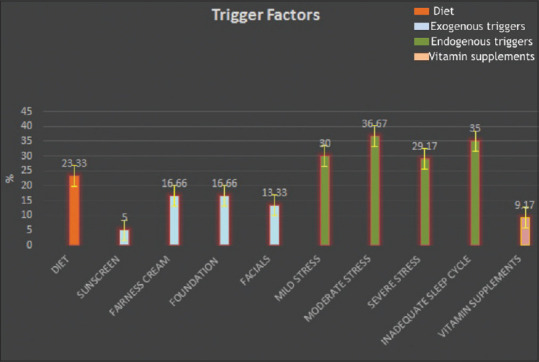
An analysis of trigger factors in adult female acne based on objective evaluation (X-axis—trigger factors, Y-axis—the percentage of affected women)
Table 3.
Cosmetics usage in total acne population
| Cosmetics usage | Sample size | Mean±SD | Median | Min-max | Interquartile range |
|---|---|---|---|---|---|
| Sunscreenuse duration (month) | 6 | 10.33±12.68 | 6 | 2-36 | 4-8 |
| Sunscreenuse frequency (days) | 6 | 25.83±3.54 | 25 | 21-30 | 24-30 |
| Fairness cream use duration (month) | 20 | 29.7±34.57 | 12 | 20-120 | 3-54 |
| Fairness cream use frequency (days) | 20 | 24.95±7.67 | 29 | 4-30 | 25-30 |
| Foundationuse duration (month) | 20 | 21.5±25.82 | 12 | 2-120 | 6-24 |
| Foundationuse frequency (days) | 20 | 9±10.63 | 4 | 1-30 | 2-15 |
| Facial duration (month) | 16 | 7.56±5.37 | 6 | 2-24 | 5-10 |
| Facial frequency (days) | 16 | 1.25±0.45 | 1 | 1-2 | 1-1.500 |
Frequency: Days/month, duration: number of months. Frequency of sunscreen usage was highest while duration of sunscreen usage was highest
Figure 2.
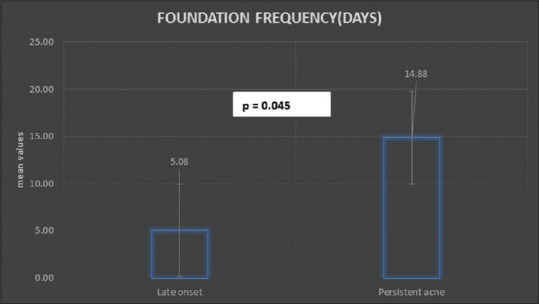
Persistent acne was associated with increased frequency of foundation usage
The hormonal analysis revealed that the mean values of all the hormones assessed in the study cohort were within the normal range as depicted in Table 4. Individual analysis of hormones revealed that elevated 17-OHPand AMH were noted in 66.67% and 60.83% patients, respectively [Figure 3]. Out of the 80 females with deranged 17 OHP,12 (10%) had a value >2 ng/mL and only 2 (1.66%) had a value of more than 4 ng/mL. DHEAS was raised in 17.5% patients. Interestingly, 42.8% patients with DHEAS derangement and 58.75% females with raised 17-OHP suffered from moderate to severe stress as well. Apart from this, only 26 (21.67%) and 14 (11.67%) patients had deranged value of serum prolactin and TSH.
Table 4.
Hormonal analysis of total acne population
| Hormones | Sample size | Mean±SD | Median | Min-max | Interquartile range | Normal value |
|---|---|---|---|---|---|---|
| 17OHP (ng/mL) | 120 | 1.56±0.68 | 1.5 | 0.33-5.74 | 1.231-1.727 | 0.2-1.3 |
| DHEAS (µg/mL) | 120 | 1.94±0.88 | 1.82 | 0.46-5.4 | 1.305-2.400 | 0.48-2.75 |
| TT (nmol/L) | 120 | 1.07±0.78 | 0.84 | 0.16-6.06 | 0.640-1.295 | <1.89 |
| SHBG (nmol/L) | 120 | 53.61±34.39 | 44.35 | 7-230 | 31.700-66.350 | 11.7-137.2 |
| FAI | 120 | 2.97±3.31 | 1.9 | 0.27-26.46 | 1.126-3.628 | <5 |
| AMH (ng/mL) | 120 | 4.13±3.26 | 3.44 | 0.01-17.2 | 1.715-5.710 | 1.62-5.1 |
| LH (mIU/mL) | 120 | 5.73±3.45 | 5.01 | 0.22-24.21 | 3.765-6.705 | 0.8-15.5 |
| FSH (mIU/mL) | 120 | 6.36±2.23 | 6.1 | 0.86-14.3 | 5.150-7.385 | 1.3-23.4 |
| LH/FSH ratio | 120 | 0.97±0.64 | 0.8 | 0.23-4.03 | 0.604-1.066 | <2 |
| Prolactin (ng/mL) | 120 | 16±15.07 | 13.05 | 2.8-152.8 | 10.350-17.300 | 3-18.6 |
| TSH (mIU/mL) | 120 | 2.9±1.8 | 2.6 | 0.33-9.98 | 1.515-3.785 | 0.5-5 |
Data were expressed as mean±standard deviation (SD). 17-OHP: 17-hydroxyprogesterone; DHEAS: Dehydroepiandrosterone sulfate; TT: total testosterone; SHBG: sex hormone-binding globulin; FAI: free androgen index; AMH: anti-Mullerian hormone; LH: luteinizing hormone; FSH: follicle-stimulating hormone; TSH: thyroid-stimulating hormone
Figure 3.
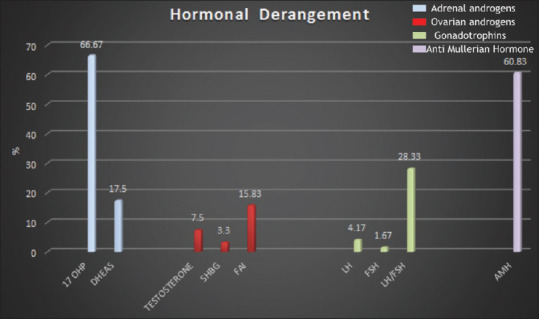
A depiction of the percentage of patients with deranged hormones (X-axis—hormonal parameters, Y-axis—the percentage of women with hormonal derangement). 17-OHP: 17-hydroxyprogesterone; DHEAS: dehydroepiandrosterone sulfate; SHBG: sex hormone-binding globulin; FAI: free androgen index; LH: luteinizing hormone; FSH: follicle-stimulating hormone; AMH: anti-Mullerian hormone
Discussion
The mean age of adult females with acne in our study was 29.73 ± 5.04 years which is akin to a previous study where the mean age of adult acne females was found to be 28.8 ± 3.64 years.[16] Most of our patients had mild to moderate acne which is consistent with previous studies.[1,17,18] Family history was noted in 10% of our study sample.
The premenstrual flare was the most common self-reported trigger factor (30%) which is less than the published literature wherein it ranges from 40%[19] to 84.8%.[1] This factor is significant as it is the most convincing proof of elevated androgens and is a clinical manifestation of a relatively lower mid-cycle peak of estrogen in women with acne. We found that 23.33% of adult females had a history of regular intake of oily foods. Landro et al. reported that 50% of adult acne patients consumed milk and dairy products regularly and further highlighted that the rate of affected women consuming vegetables, fruits, and fish was significantly less than the normal population.[20] George et al. also observed that oily foods and dairy products precipitated acne in 37.3% and 1.8% adult acne patients, respectively.[21] Diet has been propounded to cause acne via deranged nutrient signaling-induced hyperkeratosis and hyperseborrhea.[22,23] This with over-activation of mTORC1 by a high glycemic dietcan cause acne in conjunction with increased levels of androgens.[22]
Various cosmetics implicated in acne included sunscreens, fairness cream, foundation, and facials. It has been historically believed that low-grade persistent acne in females could occur majorly due to cosmetics use which lead to the use of the term “acne cosmetica” coined by Kligman.[24] A hospital-based study noted that 40% of adult acne patients experienced an aggravation of lesions after cosmetic use[21] which was supported by another study, which also concurred that a statistically significant difference in acne severity between “make-up” users and nonusers was seen.[18] Significantly, females with persistent acne were found to have a higher use of foundation. This is an important and overlooked cause and may play a role in persistent acne. This is in consonance with the Chlebus study where a significant correlation was seen between acne and the use of “full cover foundation.”[8] Similarly, the Dumont-Wallon study also found cosmetic use as an important factor in adult acne pathogenesis.[6] While a variety of cosmetic ingredients are known to be comedogenic and it is believed that many cosmetic companies now replace these ingredients with non-comedogenic alternatives,[25] this might not be the case in our country. Though sunscreens were a minor cause, they have been well-established as a cause of “acne cosmetica”and should be considered in our population. Here it must be noted that it is the vehicle that is the culprit and this is compounded by the enhanced comedogenicity with concomitant UV radiation[15,26] which is one of the possible causes of the reported summer flare in acne.[27]
Chronic stress activating the hypothalamic-pituitary-adrenal (HPA) axis has been purported to exacerbate acne mediated by the enhanced secretion of adrenal androgens and neuropeptides such as corticotrophin-releasing hormone (CRH). Recent studies have also demonstrated that CRH promotes lipid synthesis in the sebaceous gland and that the CRH system is abundantly expressed in acne-involved skin.[28] In our analysis, stress was an important trigger factor which is in consonance with previous studies where almost 50% of cases noted this association.[5,8] Goulden et al. also noted that 71% of females reported acne flare during stressful times but only 12% of females accepted to be under chronic stress.[1] An elegant study has demonstrated that patients with acne experience worsening of the disease during examinations and changes in acne severity correlate highly with increasing stress.[29] More than half of the students in our study also complained of being under moderate to severe stress. One significant aspect was the inadequate sleep cycle which can be closely related to stress as 97.61% patients with lack of adequate sleep suffered from stress as well. A well-established relation between stress and lack of sleep has been established[29] and it has been surmised that “work” stress of the modern “work environment’’may impact on acne in the adult female patient.[30] This was also evident in our study where a large proportion of working women with acne reported being under stress. Here it must be noted that while there is a link between stress and acne possibly due to the changing lifestyle of the modern women,[31,32,33] ideally a prospective study with a correlation with stressful events and acne flares is needed to arrive at a firm conclusion of its relationship with acne.
Biochemical hyperandrogenemia can be studied by analyzing the deranged values of TT, FAI, 17-OHP, and DHEAS as these can assess both the ovarian and adrenal sources of androgens. The elevated values of DHEAS (17.5%) and FAI (15.83%) are consistent with a previous study by Lucky et al.[34] who observed that DHEAS was raised in 18.5% acne patients and SHBG was low in 33.8% in acne females, the latter accounting for the increased FAI. Darley et al. also noted a higher prevalence of raised testosterone and low SHBG in 46% of adult females with persistent or late-onset acne.[35] Cibula et al. also found that testosterone and DHEAS were raised in 24% and 30% patients, respectively.[36]
The high level of 17-OHP in our work was above the reference range in 66.67% adult females though none of them had a value higher than 8 ng/mL which is considered diagnostic of non classic congenital adrenal hyperplasia (NCCAH). A study by Aziz et al. concurred that a value of more than 4 ng/mL has a sensitivity of 90% in diagnosing NCCAH.[37] In our work, only 10% and 1.66% patients had a value >2 ng/mL and >4 ng/mL, respectively. The high value of 17-OHP in our work is comparable to other studies that have analyzed this hormone in acne[38,39] which concurred that 17 OHP can be elevated in 31% patients of acne alone and in 40% patients of acne with hirsutism.[34]
Our hormonal milieu is significant for two reasons. Apart from the accepted role of androgens in stimulating the sebaceous gland, our work supports the propositus that chronic stress leads to enhanced secretion of adrenal androgens, resulting in sebaceous hyperplasia and the subsequent induction of acne.[40] This is more so as the adrenal gland is intricately linked to stress,[41] which has been, in turn, found to be a trigger for acne[42,43] and this possibly accounts for the raised adrenal hormones. Significantly, 42.8% of patients with elevated DHEAS and 58.75% with raised 17-OHP had a documented history of moderate to severe stress.
AMH has been extensively studied to diagnose PCOS and PCOM and has been found to correlate with various aspects of PCOS,[44,45,46,47,48,49] but has been scarcely studied in adult acne. We found AMH levels to be significantly higher and this is accounted for by the patients with PCOSor PCOM which is a common underlying cause of adult female acne.
Thus, our work shows that adult female acne may be triggered by diet, stress, and cosmetics, and there is a distinct hormonal milieu that accounts for hyperandrogenemia and the increased adrenal androgen levels. We found that lack of sleep and stress were both seen in a large proportion of patients which with a high level of adrenal hormones, suggestive of a tenuous link between stress and acne. We have also reconfirmed the raised levels of AMH in adult female acne and this hormone should be studied further in adult acne females.
Further analysis of the other trigger factors and the interrelationship between the various hormonal parameters, PCOS, PCOM, and AMH in adult female acne is needed and is being analyzed in our centre to shed light on the role of various androgens in acne.
The clinical applicability of our study is that it can help counsel adult female acne patients about the possible trigger factors and their link with adult acne. Also, we reiterate the role of androgens and as a corollary the need for appropriate and timely anti-androgen therapy in adult female acne.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Goulden V, Clark S, Cunliffe W. Post-adolescent acne: A review of clinical features. Br J Dermatol. 1997;136:66–70. [PubMed] [Google Scholar]
- 2.Goulden V, Stables GI, Cunliffe WJ. Prevalence of facial acne in adults. J Am Acad Dermatol. 1999;41:577–80. [PubMed] [Google Scholar]
- 3.Shaw JC, White LE. Persistent acne in adult women. Arch Dermatol. 2001;137:1252–53. [PubMed] [Google Scholar]
- 4.Schmitt JV, Masuda PY, Miot HA. Acne in women: Clinical patterns in different age-groups. An Bras Dermatol. 2009;84:349–54. doi: 10.1590/s0365-05962009000400005. [DOI] [PubMed] [Google Scholar]
- 5.Poli F, Dreno B, Verschoore M. An epidemiological study of acne in female adults: Results of a survey conducted in France. J Eur Acad Dermatol Venerol. 2001;15:541–5. doi: 10.1046/j.1468-3083.2001.00357.x. [DOI] [PubMed] [Google Scholar]
- 6.Dumont-Wallon G, Dreno B. Specificity of acne in women older than 25 years. Presse Med. 2008;37:585–91. doi: 10.1016/j.lpm.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 7.Williams C, Layton AM. Persistent acne in women. Implications for the patient and for therapy. Am J Clin Dermatol. 2006;7:281–90. doi: 10.2165/00128071-200607050-00002. [DOI] [PubMed] [Google Scholar]
- 8.Chlebus E, Chlebus M. Factors affecting the course and severity of adult acne. Observational cohort study. J Dermatolog Treat. 2017;28:737–44. doi: 10.1080/09546634.2017.1329500. [DOI] [PubMed] [Google Scholar]
- 9.Harper JC. Evaluating hyperandrogenism: A challenge in acne management. J Drugs Dermatol. 2008;7:527–30. [PubMed] [Google Scholar]
- 10.Uysal G, Sahin Y, Unluhizarci K, Ferahbas A, Uludag SZ, Aygen E, et al. Is acne a sign of androgen excess disorder or not? Eur J Obstet Gynecol Reprodu Biol. 2017;211:21–5. doi: 10.1016/j.ejogrb.2017.01.054. [DOI] [PubMed] [Google Scholar]
- 11.Durlinger AL, Gruijters MJ, Kramer P, Karels B, Ingraham HA, Nachtigal MW, et al. Anti-mullerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology. 2002;143:1076–84. doi: 10.1210/endo.143.3.8691. [DOI] [PubMed] [Google Scholar]
- 12.Rajpert-De Meyts E, Jorgensen N, Graem N, Muller J, Cate RL, Skakkebaek NE. Expression of anti-Mullerian hormone during normal and pathological gonadal development: Association with differentiation of sertoli and granulosa cells. J Clin Endocrinol Metab. 1999;84:3836–44. doi: 10.1210/jcem.84.10.6047. [DOI] [PubMed] [Google Scholar]
- 13.Krishnaswamy K, Sesikeran B, Laxmaiah A, Vajreswari A, Ramalaxmi BA, Dube AK, et al. Dietary Guidelines for Indians–A Manual. Hyderabad: National Institute of Nutrition, ICMR; 2011. [Google Scholar]
- 14.Nguyen SH, Dang TP, Maibach HI. Comedogenicity in rabbit: Some cosmetic ingredients/vehicles. Cutan Ocul Toxicol. 2007;26:287–92. doi: 10.1080/15569520701555383. [DOI] [PubMed] [Google Scholar]
- 15.Mills OH, Jr, Kligman AM. Comedogenicity of sunscreens: Experimental observations in rabbits. JAMA Dermatol. 1982;118:417–9. [PubMed] [Google Scholar]
- 16.Shrestha S. Correlation of hormonal profile and lipid levels with female adult acne in a Tertiary care center of Nepal. J Nepal Health Res Counc. 2018;16:222–7. [PubMed] [Google Scholar]
- 17.Sardana K, Singh C, Narang I, Bansal S, Garg VK. The role of antiMullerian hormone in the hormonal workup of women with persistent acne. J Cosmet Dermatol. 2016;15:343–9. doi: 10.1111/jocd.12235. [DOI] [PubMed] [Google Scholar]
- 18.Kaminsky A, Florez-White M, Bagatin E, Arias MI. Large prospective study on adult acne in Latin America and the Iberian peninsula: Risk factors, demographics, and clinical characteristics. Int J Dermatol. 2019;58:1277–82. doi: 10.1111/ijd.14441. [DOI] [PubMed] [Google Scholar]
- 19.Stoll S, Shalita AR, Webster GF, Kaplan R, Danesh S, Penstein A. The effect of the menstrual cycle on acne. J Am Acad Dermatol. 2001;45:957–60. doi: 10.1067/mjd.2001.117382. [DOI] [PubMed] [Google Scholar]
- 20.Di Landro A, Cazzaniga S, Cusano F, Bonci A, Carla C, Musumeci ML, et al. Adult female acne and associated risk factors: Results of a multicenter case-control study in Italy. J Am Acad Dermatol. 2016;75:1134–41.e1. doi: 10.1016/j.jaad.2016.06.060. [DOI] [PubMed] [Google Scholar]
- 21.George RM, Sridharan R. Factors aggravating or precipitating acne in Indian adults: A hospital-based study of 110 cases. Indian J Dermatol. 2018;63:328–31. doi: 10.4103/ijd.IJD_565_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romańska-Gocka K, Woźniak M, Kaczmarek-Skamira E, Zegarska B. The possible role of diet in the pathogenesis of adult female acne. Postepy Dermatol Alergol. 2016;33:416–20. doi: 10.5114/ada.2016.63880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melnik BC, Zouboulis CC. Potential role of FoxO1 and mTORC1 in the pathogenesis of Western diet-induced acne. Exp Dermatol. 2013;22:311–5. doi: 10.1111/exd.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kligman AM, Mills OH., Jr Acne cosmetica. Arch Dermatol. 1972;106:843–50. [PubMed] [Google Scholar]
- 25.Nelson FP, Rumsfield J. Cosmetics content and function. Int J Dermatol. 1988;27:665–72. doi: 10.1111/j.1365-4362.1988.tb01258.x. [DOI] [PubMed] [Google Scholar]
- 26.Mills OH, Porte M, Kligman AM. Enhancement of comedogenic substances by ultraviolet radiation. Br J Dermatol. 1978;98:145–50. doi: 10.1111/j.1365-2133.1978.tb01615.x. [DOI] [PubMed] [Google Scholar]
- 27.Narang I, Sardana K, Bajpai R, Garg VK. Seasonal aggravation of acne in summers and the effect of temperature and humidity in a study in a tropical setting. J Cosmet Dermatol. 2019;18:1098–1104. doi: 10.1111/jocd.12777. [DOI] [PubMed] [Google Scholar]
- 28.Ganceviciene R, Graziene V, Fimmel S, Zouboulis C, Ganceviciene R, Graziene V, et al. Involvement of the corticotropin-releasing hormone system in the pathogenesis of acne vulgaris. Br J Dermatol. 2009;160:345–52. doi: 10.1111/j.1365-2133.2008.08959.x. [DOI] [PubMed] [Google Scholar]
- 29.Chiu A, Chon SY, Kimball AB. The response of skin disease to stress: Changes in the severity of acne vulgaris as affected by examination stress. JAMA Dermatol. 2003;139:897–900. doi: 10.1001/archderm.139.7.897. [DOI] [PubMed] [Google Scholar]
- 30.Albuquerque RG, Rocha MA, Bagatin E, Tufik S, Andersen ML. Could adult female acne be associated with modern life? Arch Dermatol Res. 2014;306:683–8. doi: 10.1007/s00403-014-1482-6. [DOI] [PubMed] [Google Scholar]
- 31.Bondade S, Hosthota A, Basavaraju V. Stressful life events and psychiatric comorbidity in acne—a case control study. Asia Pac Psychiatry. 2019;11:e12340. doi: 10.1111/appy.12340. [DOI] [PubMed] [Google Scholar]
- 32.Dreno B, Bagatin E, Blume-Peytavi U, Rocha M, Gollnick H. Female type of adult acne: Physiological and psychological considerations and management. J Dtsch Dermatol Ges. 2018;16:1185–94. doi: 10.1111/ddg.13664. [DOI] [PubMed] [Google Scholar]
- 33.Chatzikonstantinou F, Miskedaki A, Antoniou C, Chatzikonstantinou M, Chrousos G, Darviri C. A novel cognitive stress management technique for acne vulgaris: A short report of a pilot experimental study. Int J Dermatol. 2019;58:218–20. doi: 10.1111/ijd.14227. [DOI] [PubMed] [Google Scholar]
- 34.Lucky AW, McGuire J, Rosenfield RL, Lucky PA, Rich BH. Plasma androgens in women with acne vulgaris. J Invest Dermatol. 1983;81:70–4. doi: 10.1111/1523-1747.ep12539043. [DOI] [PubMed] [Google Scholar]
- 35.Darley CR, Moore JW, Besser GM, Munro DD, Edwards CR, Rees LH, et al. Androgen status in women with late onset or persistent acne vulgaris. Clin Exp Dermatol. 1984;9:28–35. doi: 10.1111/j.1365-2230.1984.tb00751.x. [DOI] [PubMed] [Google Scholar]
- 36.Cibula D, Hill M, Vohradnikova O, Kuzel D, Fanta M, Zivny J. The role of androgens in determining acne severity in adult women. Br J Dermatol. 2000;143:399–404. doi: 10.1046/j.1365-2133.2000.03669.x. [DOI] [PubMed] [Google Scholar]
- 37.Azziz R, Hincapie LA, Knochenhauer ES, Dewailly D, Fox L, Boots LR. Screening for 21-hydroxylase-deficient nonclassic adrenal hyperplasia among hyperandrogenic women: A prospective study. Fertil Steril. 1999;72:915–25. doi: 10.1016/s0015-0282(99)00383-0. [DOI] [PubMed] [Google Scholar]
- 38.Borgia F, Cannavo S, Guarneri F, Cannavo SP, Vaccaro M, Guarneri B. Correlation between endocrinological parameters and acne severity in adult women. Acta Derm Venereol. 2004;84:201–4. doi: 10.1080/00015550410023248. [DOI] [PubMed] [Google Scholar]
- 39.Cinar N, Cetinozman F, Aksoy DY, Elcin G, Yildiz BO. Comparison of adrenocortical steroidogenesis in women with post-adolescent severe acne and polycystic ovary syndrome. J Eur Acad Dermatol Venereol. 2015;29:875–80. doi: 10.1111/jdv.12696. [DOI] [PubMed] [Google Scholar]
- 40.Kligman AM. Postadolescent acne in women. Cutis. 1991;48:75–7. [PubMed] [Google Scholar]
- 41.Berger I, Werdermann M, Bornstein SR, Steenblock C. The adrenal gland in stress – Adaptation on a cellular level. J Steroid Biochem Mol Biol. 2019;190:198–206. doi: 10.1016/j.jsbmb.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 42.Lucky AW, Rosenfield RL, McGuire J, Rudy S, Helke J. Adrenal androgen hyperresponsiveness to adrenocorticotropin in women with acne and/or hirsutism: Adrenal enzyme defects and exaggerated adrenarche. J Clin Endocrinol Metab. 1986;62:840–8. doi: 10.1210/jcem-62-5-840. [DOI] [PubMed] [Google Scholar]
- 43.Schulpis K, Georgala S, Papakonstantinou ED, Michas T. Psychological and sympatho-adrenal status in patients with cystic acne. J Eur Acad Dermatol Venereol. 1999;13:24–7. [PubMed] [Google Scholar]
- 44.Laven JS, Mulders AG, Visser JA, Themmen AP, De Jong FH, Fauser BC. Anti-Müllerian hormone serum concentrations in normoovulatory and anovulatory women of reproductive age. J Clin Endocrinol Metab. 2004;89:318–23. doi: 10.1210/jc.2003-030932. [DOI] [PubMed] [Google Scholar]
- 45.Sahmay S, Atakul N, Aydogan B, Aydın Y, Imamoglu M, Seyisoglu H. Elevated serum levels of anti-müllerian hormone can be introduced as a new diagnostic marker for polycystic ovary syndrome. Acta Obstet Gynecol Scand. 2013;92:1369–74. doi: 10.1111/aogs.12247. [DOI] [PubMed] [Google Scholar]
- 46.Woo HY, Kim KH, Rhee EJ, Park H, Lee MK. Differences of the association of anti-Mullerian hormone with clinical or biochemical characteristics between women with and without polycystic ovary syndrome. Endocr J. 2012;59:781–90. doi: 10.1507/endocrj.ej12-0055. [DOI] [PubMed] [Google Scholar]
- 47.Li L, Chen X, Mo Y, Chen Y, Wenig M, Yang D. Elevated serum anti-Mullerian hormone in adolescent and young adult Chinese patients with polycystic ovary syndrome. Wien Klin Wochenschr. 2010;122:519–24. doi: 10.1007/s00508-010-1426-x. [DOI] [PubMed] [Google Scholar]
- 48.Iliodromiti S, Kelsey TW, Anderson RA, Nelson SM. Can anti-Mullerian hormone predict the diagnosis of polycystic ovary syndrome. A systematic review and meta-analysis of extracted data? J Clin Endocrinol Metab. 2013;98:3332–40. doi: 10.1210/jc.2013-1393. [DOI] [PubMed] [Google Scholar]
- 49.Saxena U, Ramani M, Singh P. Role of AMH as diagnostic tool for polycystic ovarian syndrome. J Obstet Gynaecol India. 2018;68:117–22. doi: 10.1007/s13224-017-1066-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


