Abstract
Sex chromosome trisomies (SCT), including Klinefelter syndrome/XXY, Trisomy X, and XYY syndrome, occur in 1 of every 500 births. The past decades of research have resulted in a broadening of known associated medical comorbidities as well as advances in psychological research. This review summarizes what is known about early neurodevelopmental, behavioral, and medical manifestations in young children with SCT. We focus on recent research and unanswered questions related to the risk for neurodevelopmental disorders that commonly present in the first years of life and discuss the medical and endocrine manifestations of SCT at this young age. The increasing rate of prenatal SCT diagnoses provides the opportunity to address gaps in the existing literature in a new birth cohort, leading to development of the eXtraordinarY Babies Study. This study aims to better describe and compare the natural history of SCT conditions, identify predictors of positive and negative outcomes in SCT, evaluate developmental and autism screening measures commonly used in primary care practices for the SCT population, and build a rich data set linked to a bank of biological samples for future study. Results from this study and ongoing international research efforts will inform evidence-based care and improve health and neurodevelopmental outcomes.
Keywords: XXY, XYY, Trisomy X, neurodevelopment, testosterone
1 |. INTRODUCTION
Sex chromosome trisomy (SCT) is common, and the rate of prenatal SCT diagnosis is rapidly increasing. SCTs occur in 1 of every 500 births and are the most common chromosomal abnormalities, including XXY/Klinefelter syndrome (1/600 males), XXX/Trisomy X (1/1000 females), and XYY syndrome (1/1000 males) (Coffee et al., 2009; Hamerton, Canning, Ray, & Smith, 1975; Nielsen, 1990). Historically, less than 10% of individuals with SCT were diagnosed before adolescence, however the rate of prenatal diagnosis is increasing exponentially as testing of cell free-fetal DNA (cfDNA) in maternal blood evolves to become standard screening in prenatal care (Abramsky & Chapple, 1997; Bojesen, Juul, & Gravholt, 2003). In May 2016, the American College of Obstetrics and Gynecology expanded its support for prenatal cfDNA screening from high-risk pregnancies to all pregnancies, potentially increasing rates of prenatal SCT diagnosis by 10-fold (Bianchi & Wilkins-Haug, 2014; Lo, Cori, Norton, & Caughey, 2014; ACOG Practice Bulletin No. 163: Screening for Fetal Aneuploidy 2016). In addition to the rapidly evolving status of prenatal screening, universal newborn screening for a variety of new genetic conditions is being considered in the near future. Genetic testing technologies being used in some newborn screening pilot programs will incidentally diagnose SCT, and in some of these cases SCT may likely be identified more frequently than the rare genetic conditions the tests are designed to ascertain (Coffee et al., 2009; Esposito et al., 2018; Inaba et al., 2013; Park et al., 2013; Vorsanova et al., 2001). Finally, with emerging evidence of earlier age of diagnosis and potential early treatments that may affect outcomes in SCT (Davis, Reynolds, Dabelea, Zeitler, & Tartaglia, 2019; Samango-Sprouse et al., 2013; Wigby et al., 2016), there is advocacy for SCT to be considered for newborn screening studies (Nieschlag et al., 2016). With these changes in the landscape and a rapidly growing population of infants with a prenatal diagnosis, updated research investigating early determinants contributing to phenotypic variability and increased morbidities is overdue and needed to inform care (Herlihy, Gillam, Halliday, & McLachlan, 2011).
The goal of this review is to summarize what is known about early neurodevelopmental, behavioral, and medical manifestations in young children with SCT in order to guide early care and highlight research needs. In what follows, we briefly summarize older literature from the 1970’s to the 1990’s that laid the foundation for what is known about SCT, and then highlight advances in the field over the last 20 years. We then introduce the eXtraordinarY Babies Study, a prospective study of infants with a prenatal diagnosis of SCT, and highlight background neurodevelopmental and endocrine questions this study will address, as well as the implications study results may have on the topic of newborn screening for SCT.
2 |. EARLY LONGITUDINAL AND CROSS-SECTIONAL STUDIES
Our core knowledge of the natural history of SCT in infancy and childhood is largely based from birth cohort studies conducted from the 1970’s to 1990’s. These studies included children with SCT identified through newborn screening research protocols who were then followed prospectively at seven sites across the United States, Canada, and Europe into young adulthood (Robinson, Bender, & Linden, 1990). At the time of these studies, clinical chromosomal testing was largely reserved for patients with congenital malformations, marked dysmorphisms, or more severe developmental disabilities, and thus clinical descriptions of SCT patients in the medical literature were biased toward severely involved cases. Thus, these birth cohort studies represent the first studies in the SCT population without significant ascertainment bias (Abramsky & Chapple, 1997; Bishop et al., 2010). Although there were some differences in protocols between study sites, results described SCT growth patterns, development, clinical features, and clinical labs summarized in Table 1.
TABLE 1.
Summary of neurodevelopmental, medical, and gonadal function in SCT described in the 1970’s birth cohorts
| XXY (Klinefelter syndrome) N = 95 | XYY (Jacobs syndrome) N = 59 | XXX (triple X) N = 46 | ||
|---|---|---|---|---|
| Neurodevelopmental | Early developmental delays 1–7 | 70% Usually mild, speech delay>motor |
80% Usually mild, speech del>motor |
55% Usually mild, speech del>motor |
| Learning disabilities 4,5,8–11 | 64–85% (esp. reading) | ∼55% | 75–100% (esp. reading) | |
| Mean cognitive (IQ) 12–18 | 10–15 points lower than normal; Verbal < nonverbal |
5–10 points lower than normal; verbal usually = nonverbal | 10–15 points lower than normal; verbal < nonverbal | |
| Behavior/Social-emotional, 13,15,17–22 | Shy, social difficulties; immature, attentional problems | Hyperactivity; negative mood; impulsivity 2 | Shy, anxiety, social difficulties; sensory integration problems | |
| Motor skills 6,7,13 | Motor delays, coordination and motor planning problems | Increased rate of balance and coordination problems 1, 12 | Motor delays, coordination problems, low strength | |
| Physical/medical | Average birth size 1,2,3,23 | Slightly smaller | Normal | Smaller |
| Congenital anomalies 1,2,3,13 | Modest increase | Rare | Rare | |
| Dysmorphisms 1,2,3,13 | Minimal: Hypertelorism, epicanthal folds, clinodactyly, small head circumference | No dysmorphisms reported normal head circumference | Minimal: Hypertelorism, epicanthal folds, clinodactyly, small head circumference | |
| Growth and body habitus 1,13–15,22,23 | Tall stature; long legs increased growth velocity starting at 5yo; excess weight gain | Tall stature, long legs thinner than XXY delayed growth spurt 1 | Tall stature; increased growth velocity starting at 7yo; abdominal pain (25%) | |
| Muscle tone 1–3,6,7 | Hypotonia | Hypotonia | Hypotonia | |
| Gonadal function | Prepubertal gonadal function 1–3,13,17,22,24,25,26 | Cryptorchidism in 10–20%; small testes <0.5ml in 65% at 6m; slow penile growth; T under assay detection limit; bone age delayed (−2 SD) | Testes and penile size in the normal range; Testo concentrations normal | Nothing reported in infancy, high FSH in mid-childhood; bone age delayed (−1 SD) |
| Puberty timing, tempo, and course 13,15,17,20,22,24 | Testes enlarge to max of 10ml, high LH & FSH after Tanner 3, Testo plateaus in late puberty | Early onset of testicular enlargement, normal testosterone levels | Thelarche and menarche late-normal, but precocious puberty also reported | |
| Adult function/fertility 14,18,19,21,22 | Infertility, 90% with low Testo | Assumed normal fertility | ∼10% secondary amenorrhea; pregnancies in 9/37 |
(1) Stewart et al., 1982; (2) Robinson et al., 1982; (3) Ratcliffe et al., 1982; (4) Pennington, Puck, & Robinson, 1980; (5) Ratcliffe, 1982a; (6) Salbenblatt, Meyers, Bender, Linden, & Robinson, 1989; (7) Salbenblatt, Meyers, Bender, Linden, & Robinson, 1987; (8) Rovet, Netley, Keenan, Bailey, & Stewart, 1996; (9) Pennington, Bender, Puck, Salbenblatt, & Robinson, 1982; (10) Bender, Puck, Salbenblatt, & Robinson, 1986; (11) Bender, Linden, & Robinson, 1993; (12) Bender, Linden, & Harmon, 2001, (13) Robinson, Bender, & Linden, 1990; (14) Ratcliffe, Masera, Pan, & McKie, 1994; (15) Stewart, Bailey, Netley, & Park, 1990; (16) Rovet, Netley, Bailey, Keenan, & Stewart, 1995; (17) Ratcliffe, Murray, & Teague, 1986; (18) Bancroft, Axworthy, & Ratcliffe, 1982; (19) Bender, Harmon, Linden, & Robinson, 1995; (20) Robinson, Bender, Linden, & Salbenblatt, 1990; (21) Linden, Bender, Harmon, Mrazek, & Robinson, 1988, (22) Ratcliffe, Butler, & Jones, 1990; (23) Ratcliffe, 1985; (24) Ratcliffe, 1982b.
3 |. RESEARCH ADVANCES SINCE THE NEWBORN SCREENING STUDIES
Since these initial newborn screening trials in the 1970’s, most SCT research has been cross-sectional in design with participants primarily recruited through advocacy groups or clinical settings. These cohorts include both children identified with SCT in the prenatal period and those diagnosed during childhood due to clinical findings such as developmental delay, signs of pubertal/gonadal failure, or other medical concerns leading to genetic testing. Thus, there is ascertainment bias in all of these studies that limits generalizability of results to the entire SCT population. However, acknowledging this bias, many important discoveries in the SCT field have occurred over the past 40 years which we describe in more detail in sections below. For example, there has been a broadening of known associated medical comorbidities like insulin resistance, decreased bone health, and cardio-metabolic disorders, including epidemiologic studies in Europe revealing increased morbidity and mortality in all SCT conditions (Bojesen & Gravholt, 2011; Bojesen, Juul, Birkebaek, & Gravholt, 2004; Gravholt, Jensen, Host, & Bojesen, 2011; Pasquali et al., 2013; Stagi et al., 2016; Stagi et al., 2017; Stochholm, Juul, & Gravholt, 2010a; Stochholm, Juul, & Gravholt, 2010b; Swerdlow, Higgins et al., 2005). We have also learned that infants with XXY show lower levels of serum testosterone levels during the mini puberty of infancy at 2–4 months of age (Aksglaede, Davis, Ross, & Juul, in press; Lahlou, Fennoy, Carel, & Roger, 2004; Ross et al., 2005), and both retrospective, nonrandomized cohort (Samango-Sprouse et al., 2013) and prospective, blinded trials (Davis et al., 2017; Davis et al., 2019; Ross et al., 2017) of different androgen treatments in infants and young children with XXY suggest that there may be improvements in health outcomes and some psychological domains. There have also been significant advances in fertility research in XXY such that now sperm can be retrieved through surgical microdissection in ~50% of men with XXY, however there is little current understanding of factors predicting success in sperm retrieval (Madureira et al., 2014; Plotton et al., 2015;Rohayem et al., 2015; Takeda et al., 2017; Ragab et al., 2018; Deebel et al., 2020). Additionally, while hormonal impacts have traditionally been considered more important in XXY compared with the other SCT conditions, there have been reports of increased rates of primary ovarian insufficiency and decreased ovarian volumes in Trisomy X (Ayed et al., 2014; Davis et al., in press; Goswami et al., 2003; Jiao et al., 2012; Stagi et al., 2016; Stagi, Di Tommaso, Manoni, et al., 2016; Villanueva & Rebar, 1983), and sub-fertility and impaired testicular function in XYY which also are deserving of additional study (Davis et al., in press; Hofherr, Wiktor, Kipp, Dawson, & Van Dyke, 2011; Kim, Khadilkar, Ko, & Sabanegh, 2013).
Elevated rates of important and treatable neurodevelopmental problems such as autism spectrum disorder (ASD) and attention deficit hyperactivity disorder (ADHD) have been identified by various researchers utilizing diagnostic criteria that were not available during the initial prospective studies (Bruining, Swaab, Kas, & van Engeland, 2009; Lee et al., 2011; Ross et al., 2012; Tartaglia et al., 2017; Tartaglia, Ayari, Hutaff-Lee, & Boada, 2012), and theoretical models have been proposed linking deficits in social cognition and executive functioning to behavioral and social outcomes (van Rijn, 2019). The important role of background family history and early environmental experiences in their contribution to phenotypic variability has also been introduced (Samango-Sprouse et al., 2014; van Rijn, Barneveld, Descheemaeker, Giltay, & Swaab, 2016). Psychosocial research extending into adulthood has also reported the effects of SCT diagnoses on quality of life (Close, Fennoy, Smaldone, & Reame, 2015; Skakkebæk, Wallentin, & Gravholt, 2015; Turriff, Levy, & Biesecker, 2011; Turriff, Macnamara, Levy, & Biesecker, 2016).
Preliminary genetic studies have suggested specific polymorphisms or expression patterns that may be associated with phenotypic features including height in all SCTs (Ottesen et al., 2010), penile length and pubertal development in XXY (Wikstrom, Hoei-Hansen, Dunkel, & Rajpert-De Meyts, 2007; Zinn et al., 2005), language abilities in XXY (Vawter, Harvey, & DeLisi, 2007), and autism symptoms in XYY (Ross, Tartaglia, Merry, Dalva, & Zinn, 2015). Further, larger genomic and transcriptomic studies are being initiated that have shown effects of altered gene dosage of both sex chromosome and autosomal genes in SCT (Raznahan et al., 2018; Skakkebæk et al., 2018), raising the complexity of approaches needed to better understand the genes that contribute to the neurodevelopmental and medical phenotypes across SCT conditions.
While these cross-sectional studies add to the understanding of SCT, natural history of these new findings in unbiased cohorts are lacking. SCT researchers reporting on cross-sectional samples often try to partially address ascertainment bias by comparing participants with a prenatal versus postnatal diagnosis. Because young children with a postnatal diagnosis are typically identified because of a developmental disorder and thus biased toward a more severe phenotype, description of the prenatally identified subset of a research sample is more likely to represent the broad phenotypic variation that can occur in SCT (Bishop et al., 2010; Geerts, Steyaert, & Fryns, 2003; Wigby et al., 2016). However, this approach often leaves lower sample sizes and remains biased by the involvement of both prenatally and postnatally diagnosed participants recruited through clinical samples or support groups. There have been no updated longitudinal studies of prenatally diagnosed children with SCT. This is a major limitation and challenge in prenatal and postnatal genetic counseling, where ascertainment bias of published literature has to be explained and the phenotypic variability has to be emphasized while there is limited evidence-based information to share about predictors of the broad neurodevelopmental and medical outcomes. Given the rise in prenatal diagnoses from cfDNA screening, there is the opportunity to study a new cohort of infants from birth with consideration of current research gaps and the marked need for updated information to guide genetic counseling and clinical care for this understudied and rapidlygrowing population of children.
4 |. THE EXTRAORDINARY BABIES STUDY
These research gaps and opportunities led to the development of the eXtraordinarY Babies Study, a prospective natural history study of infants prenatally diagnosed with SCT designed to examine trajectories of neurodevelopment and physical health from birth through the first few years of life as well as psychosocial factors such as quality of life and parental experiences. This project is funded by NICHD as a project of the American College of Medical Genetics and Genomics (ACMG) Newborn Screening Translational Research Network (NBSTRN) (ClinicalTrials.gov NCT03396562), and is being conducted at two sites including University of Colorado/Children’s Hospital Colorado and Nemours-Dupont Hospital for Children. The study is currently enrolling infants between 2 and 12 months of age with a prenatal diagnosis of SCT, and aims to better describe and compare the natural history of SCT conditions, identify predictors of positive and negative outcomes in SCT, evaluate developmental and autism screening measures commonly used in primary care practices for the SCT population, and to build a rich data set linked to a bank of biological samples for future study. Participants are seen for study visits at 2, 6, and 12 months of age, and then annually. Each study visit includes collection of a comprehensive battery of historical, developmental, psychological, and physical examination data, as well as collection of biological samples as shown in Table 2.
TABLE 2.
Data collected at study visits for the eXtraordinarY Babies Study
| Demographic/family information | Race/ethnicity Family history (medical, learning, psychological, education levels, work) Household and family factors (siblings, birth order, household members, location, language exposure) Socioeconomic status Parental height/weight measurement Brief parental cognitive, language, executive function skills Parenting stress/attachment/quality of life |
| Development/behavior assessments | Cognition Language skills Motor skills Social development and play Adaptive functioning Eyetracking: Early social cognition Pre-academic skills (3+ years) Behavioral questionnaires Temperament scales Therapy/intervention/daycare history Developmental/autism screening measures |
| Medical data | Prenatal, birth, medical, and surgical history Growth parameters Physical examination Body composition (PeaPOD, BodPOD) Laboratory studies (hormonal profiles, etc.) |
| Biological samples for biorepository | Blood processed for DNA, RNA, serum, plasma Urine Stool |
The primary analysis plan includes predictors of phenotypic outcomes at 3 years of age, although interim findings and longitudinal trajectories will also be analyzed, and renewal funding to expand and follow this cohort through the school age years into adolescence and adulthood is planned. Over 160 infants with SCT have been enrolled to date, with target enrollment of 200 infants during this funding period (2017–2022). A de-identified data set will be contributed to the NIH/NICHD Newborn Screening Translational Research Network Longitudinal Pediatric Data Repository (NBSTRN LPDR) per NIH data sharing guidelines to facilitate secondary analyses and combination with other data sets. Results will be disseminated through presentations at scientific and family meetings, peer-reviewed publications, webinars, electronic newsletters, and social media postings as the cohort reaches key timepoints in development. While detailed genetic and metabolomic studies are not funded under the current protocol, the biorepository will allow these investigations and collaborative translational projects in future studies. This combination of developmental, hormonal, physical, and quality of life data collected in a prospective fashion will allow for investigation of many interesting and important research questions introduced below that will allow us to advance care, improve counseling, inform discussions of newborn screening, and move toward research-based intervention studies for infants and children with SCT.
5 |. NEURODEVELOPMENT IN EARLY CHILDHOOD IN SCT
The list of neurodevelopmental and psychological risks associated with SCT that can manifest from infancy into adulthood is long (Tartaglia et al., 2015; Urbanus, van Rijn, & Swaab, 2020). These risks include cognitive, language, and learning disabilities, attention and executive functioning difficulties, and internalizing and externalizing behavioral and psychological disorders, although the marked variability in the presence and severity of these features is a consistent research finding. Here we focus on recent research and unanswered questions related to the neurodevelopmental disorders that commonly present in the first 3–5 years of life that can be addressed during the early years of the eXtraordinarY Babies Study, specifically language impairment, ASD, and motor skills deficits.
5.1 |. Language impairment and early social cognition
Children with SCT are at increased risk for developmental delays, and over 75% have been reported to receive early speech therapy to support acquisition of developmental milestones (Bender et al., 1993; Robinson, Bender, Linden, & Salbenblatt, 1990; Thompson et al., 2020). Early language profiles reported include increased risk for mild delays in language milestones, with more challenges reported in expressive language compared to receptive skills (Simpson et al., 2003; Walzer, Graham Jr., Bashir, & Silbert, 1982). Expressive language disorder and Receptive-Expressive language disorder are common diagnoses assigned to young children with SCT to support the need for speech therapy. However, these diagnoses are fairly generic in describing that children have difficulties with language expression and/or comprehension, without further analyses of the specific language components that are affected. Some early studies identified domains of word retrieval, syntactic production, and narrative formation in the speech-language profile of young children with SCT (Walzer et al., 1982; Walzer, Bashir, & Silbert, 1990). A more recent study of language in all SCT conditions by Bishop et al. (2018) showed that in a “low bias” group age 5–16 there was indeed a higher rate of overall language difficulties, however around one third had no evidence of language problems. Further, in those with language difficulties, the profile of language skills in domains of core language, verbal production/memory, and literacy skills were highly variable and not different compared with the comparison group of children with language concerns without a genetic etiology (Bishop et al., 2018).
Other important studies have reported deficits in phonological processes, oromotor skills, articulation, and motor planning of speech in SCT (verbal apraxia or dyspraxia) (Bender et al., 1983; Samango-Sprouse & Rogol, 2002; St John et al., 2019; Walzer et al., 1990). These types of speech disorders are approached by speech pathologists with different therapy techniques compared with those used for more generalized receptive-expressive language delays, and thus further characterization of these patterns in SCA is important to differentiate whether alternative therapy techniques need to be incorporated into a speech-language therapy program. Prospective study of the trajectory and profile of speech and language development during these early years of speech-language acquisition will allow for analysis of the natural history of speech-language components at multiple levels to guide further study into intervention points and approaches. Targeting early language development in SCT is also important since early language deficits are known to precede literacy and academic problems, which occur in 50–75% of SCT (Bender et al., 1983; Pennington et al., 1980; Peterson, Pennington, Shriberg, & Boada, 2009; St John et al., 2019), and also contribute to social-emotional and behavior domains.
Social skills difficulties are commonly reported in SCT, and older literature has supported that language deficits are the main contributor to social deficits (Harkulich, Marchner, & Brown, 1979; Ratcliffe, 1982a). Further correlations between verbal/language skills and social skills or autism traits have been identified in more recent studies as well (Cordeiro, Tartaglia, Roeltgen, & Ross, 2012; van Rijn, Bierman, Bruining, & Swaab, 2012). However, more recent research led by Dr. van Rijn from the Leiden University in the Netherlands has shown that social difficulties in SCT result from more than just language delays, but that deficits exist in core aspects of social cognition. Through eye tracking studies, functional MRI, and other psychological experiments, older children and adults with an extra X chromosome have demonstrated higher risk for deficits in the domain of social attention, defined as the automatic and spontaneous visual orientation towards meaningful aspects of social interaction. This deficit is associated with subsequent difficulties interpreting social scenarios, reading facial expressions, and understanding tone of voice, and also correlates with self-report of social skills in adult XXY patients (Chawarska, Macari, & Shic, 2012; van Rijn, 2015; van Rijn et al., 2012; van Rijn, Barendse, van Goozen, & Swaab, 2014; van Rijn, de Sonneville, & Swaab, 2018; van Rijn, Stockmann, van Buggenhout, van Ravenswaaij-Arts, & Swaab, 2014; van Rijn, Swaab, Aleman, & Kahn, 2006).
The developmental origins of social cognitive deficits and emotion regulation problems in infants and young children with SCT is a topic of current study (TRIXY study, PI: Sophie van Rijn, Grant #016.165.397, NWO Netherlands Organization for Scientific Research). In typically developing children, eyetracking studies show that by 3-months of age there is a social preference towards voices and faces, and a strong tendency to focus on the face during social interaction (Haith, Bergman, & Moore, 1977; Salva, Farroni, Regolin, Vallortigara, & Johnson, 2011; Simion, Regolin, & Bulf, 2008). By 12 months, the majority of infants are skilled in coordinating attention between social partners to share awareness of an object or event (Carpenter, Nagell, & Tomasello, 1998). In young children with autism, deficits in social attention have shown a correlation with language deficits (Bradshaw et al., 2019; Stagg, Linnell, & Heaton, 2014). Perhaps early language deficits in SCT are rooted from deficits in social attention, contrary to previous theories that social deficits in SCT stem from language problems? If some infants with SCT are unable to select and encode relevant aspects of social interactions such as facial expressions or eye gaze and are unable to attend to the critical conversations in their social world, then perhaps these are the same infants with more significant deficits in subsequent language development? (Birmingham & Kingstone, 2009; Frank, Vul, & Saxe, 2012). This prospective study of infants will incorporate direct language and social skills assessments with eye tracking technology to investigate the developmental course and relationship between social attention and language in the three SCT conditions.
5.1.1 |. Autism Spectrum Disorder
In the clinical setting, this combination of deficits in communication and social interactions suggests a possible diagnosis of ASD, and indeed some children with SCT are diagnosed with ASD. In 2017, Tartaglia et al., reported results from research-recruited samples at two sites that included ASD evaluation as part of larger studies of health and development in SCA. Results showed that 5–10% of boys with XXY and up to 38% of boys with XYY in these samples met criteria for ASD (Tartaglia et al., 2017). Although ascertainment bias must be acknowledged, these rates are 6 to more than 30 times higher than the risk of ASD in typical XY males in the United States (Christensen, Baio et al., 2016). The significantly higher rate of ASD in XYY compared to XXY is important to further study and explain. ASD rates in females with Trisomy X have been less studied, with some research suggesting few features of ASD while others show that ~10% of girls with Trisomy X screen positive for ASD (Bishop et al., 2010; van Rijn, Stockmann, et al., 2014; Wigby et al., 2016).
Given the prevalence of ASD in SCT documented thus far, a longitudinal study to compare early developmental profiles between young children with SCT who do and do not develop ASD could identify predictors. Do early deficits in social attention and language indeed predict ASD? If not, what factors do? Are there differences between ASD predictors in XXY compared with XYY? Identifying early markers or risks factors for ASD can provide an earlier point of intervention and prepare families for the possibility of later challenges. Prospective study also provides an opportunity to compare early ASD trajectories in the different SCT conditions to idiopathic ASD. A large body of literature exists describing developmental trajectories of infants at high risk for idiopathic ASD due to the presence of ASD in an older sibling. For example, in these high risk samples early skills such as decreased used of communicative gestures, decreased attention to social stimuli, and failure to orient to the speaker in response to hearing their name have been identified as “red flags” in infants and toddlers who go on to develop a clinical diagnosis (Chawarska et al., 2014; Messinger et al., 2013; Miller et al., 2015; Newschaffer et al., 2012; Ozonoff et al., 2010). It is unclear is whether the same early markers of ASD that have been identified in infant-sibling ASD studies are also present in the subset of infants and toddlers with SCT who later develop social deficits and/or ASD, and these questions will be able to be explored with the comprehensive early social skills profiling and ASD assessments included as part of the eXtraordinarY Babies Study. In addition to earlier diagnosis, this comparison is also important in helping identify if traditional ASD intervention targets could be effective in the different SCT conditions.
5.1.2 |. Motor deficits
Increased risk for motor delays and later deficits in motor coordination, endurance, and strength is present for all three SCT conditions (Salbenblatt et al., 1987; Salbenblatt et al., 1989). The lower frequency of motor deficits in XYY (50%) compared to XXY (75%) suggests that androgen insufficiency, genes on the X chromosome, or another neuromuscular or metabolic problem may affect motor development and function. Martin, Cordeiro, Richardson, Davis, and Tartaglia (2019) reported an association between visual-motor skills and adaptive functioning abilities (a measure of general functioning in day-to-day life) in a cross-sectional sample of males with XXY (Martin et al., 2019). The eXtraordinarY Babies Study will allow for more investigation of the variability in early motor development, and will help answer questions on whether early motor skills may predict later self-care skills, physical activities, and overall health.
5.1.3 |. Neurodevelopmental disorders beyond early childhood in SCT—Learning disabilities, executive dysfunction/ADHD, and emotional disorders
In addition to neurodevelopmental disorders presenting in early childhood, important features of SCT emerge beyond early childhood that likely have roots in early development. These include increased risk for cognitive problems and learning disabilities, including dyslexia and disorders of written expression in all three SCT conditions (Bender et al., 1986; Bender et al., 2001; Pennington et al., 1980; Pennington et al., 1982; Ratcliffe, 1982b). Attentional problems are also common, and studies of cohorts ascertained through advocacy organizations and clinical samples report ADHD rates of 20–40% in XXY and XXX, and up to 75% in XYY compared to 5–10% in the general population (Bruining et al., 2009; Geerts et al., 2003; Lee et al., 2011; Tartaglia et al., 2012). Children with SCT also have increased risk for difficulties with executive function, including initiation, planning, organization, working memory, and cognitive flexibility (Boada, Janusz, Hutaff-Lee, & Tartaglia, 2009; Fales et al., 2003; Lee et al., 2011; van Rijn, Bierman, et al., 2012). This combination of learning and executive disabilities subsequently hinders academic outcomes and overall adaptive functioning. Further, behavioral and emotional disorders including anxiety, depression, and mood disorders are commonly reported from clinical settings (Bruining et al., 2009; Otter, Schrander-Stumpel, Didden, & Curfs, 2012; Ratcliffe & Field, 1982), also with evidence of features beginning to emerge in the first years of life in some children (Urbanus, Swaab, Tartaglia, Cordeiro, & Van Rijn, 2020). Together with medical features, all of these areas of difficulty can lead to poorer overall outcomes and quality of life (Close, Fennoy, et al., 2015; Close, Sadler, & Grey, 2015). While all of these additional features associated with SCT later in childhood or adulthood are beyond the age of the current eXtraordinarY Babies Study cohort, plans to continue to closely evaluate this cohort into school age, through adolescence, and into adulthood across all psychological domains will allow us to explore the developmental origins and early risk factors for these challenges across academic, psychological, and social-emotional domains and to evaluate potential treatment approaches.
6 |. MEDICAL AND ENDOCRINE MANIFESTATIONS OF SCT
6.1 |. Overview of testicular and ovarian function and cardiometabolic health in SCT
The atypical sex chromosomes in SCT also have important effects on development and function of the gonads. The testes of boys with XXY/Klinefelter syndrome often fail to produce normal amounts of testosterone, and testosterone replacement therapy is considered standard of care in adolescence for normal pubertal development and health (Davis et al., 2016; Rogol & Tartaglia, 2010). Sperm production is also impaired in XXY, leading to impaired fertility (Deebel et al., 2020). Girls with trisomy X typically have normal puberty, but are at increased risk for premature ovarian failure, however, no studies exploring longitudinal gonadal function in young children with trisomy X have been completed (Tartaglia, Howell, Sutherland, Wilson, & Wilson, 2010; Villanueva & Rebar, 1983). Males with XYY have traditionally been reported to have normal male hormone production with a slight increase in fertility problems, however recent research supports that there may indeed be some impairment in testicular functioning in adolescents with XYY as further described by Davis, Soares, et al. (in press) in this article collection (Davis, Kowal, et al., in press; Ismail, el-Beheiry, Hashishe, & el-Bahaei, 1993). Gonadal function for all SCTs during infancy and early childhood is of interest not just for need for hormonal or reproductive treatments, but also because hypogonadism and hormonal differences may also affect aspects of neurodevelopment, energy metabolism, and body composition (Bojesen, Host, & Gravholt, 2010).
Important progress has also been made in the identification of medical risks in SCT, primarily through cohort studies and population-based studies in adult populations. As with the neurodevelopmental and psychological features described above, the developmental origins and trajectories of these medical features are of great interest for development of preventative measures and treatment recommendations. Type 2 diabetes and cardiovascular diseases yield a standardized mortality ratio of 5.8 in men with XXY, 2.2 in men with XYY, and 2.5 in women with trisomy X compared with the general population (Stochholm et al., 2010a; Stochholm et al., 2010b; Swerdlow, Higgins et al., 2005). Metabolic syndrome, a constellation of signs including large waist circumference, dyslipidemia, elevated fasting blood glucose, and high blood pressure, is present in around 50% of men with XXY (Bojesen et al., 2010; Gravholt et al., 2011). In addition to reports of the high prevalence of cardiometabolic disorders in adults with SCT (Boisen, Owen, Rasmussen, & Sergeant, 1981; Bojesen et al., 2006; Bojesen & Gravholt, 2011; Stochholm et al., 2010a; Swerdlow, Higgins et al., 2005), research groups have also begun to report cardiometabolic biomarkers in children and adolescents with XXY (Aksglaede, Molgaard, Skakkebaek, & Juul, 2008; Bardsley, Falkner, Kowal, & Ross, 2011; Davis et al., 2016; Davis et al., in press). These studies describe increased risk for a higher body fat percentage and frequency of cardiometabolic risk factors such as increased waist circumference and dyslipidemia in adolescents and school-age children with XXY. Further, one study also identified that nearly 20% of prepubertal children with XXY had low inhibin B, a hormone reflecting testicular function in prepubertal boys, and that inhibin B was negatively associated with features of metabolic syndrome (Davis, Lahlou, et al., 2016). This is consistent with what is known in adults with XXY—hypogonadism strongly correlates with, and may be causative of, cardiometabolic dysfunction. Given the presence of this relationship in pre-pubertal boys with XXY, longitudinal studies in younger children are needed to discern what comes first so we can later test plausible interventions. Studies of cardiometabolic features in younger cohorts with XYY and trisomy X and the relationship to hormonal profile are lacking, although beginning to be explored. See Davis et al., in this article collection for new investigations in XYY (Davis, Kowal, et al., in press).
6.2 |. Testicular function in infants with XXY
Primary testicular failure resulting in hypogonadism during adolescence is one of the hallmarks of XXY syndrome, and is nearly universal (Davis, Rogol, & Ross, 2015). Despite this, there has been limited investigation of testicular function in infants and children with XXY. All infants have an activation of their hypothalamic-pituitary-gonadal axis in the first months of life called the “mini-puberty” period. The purpose of this brief mini-puberty remains quite speculative. Research demonstrates many tissues are sexually dimorphic, and exposure to testosterone or estrogen during sensitive time points is required for masculinization or feminization of these organs. Mouse models that manipulate exposure to sex steroids in the neonatal period suggest that early testosterone exposure may have lasting effects on cognition and metabolism mediated through epigenetic mechanisms induced by testosterone or estrogen (Ghahramani et al., 2014; Swift-Gallant, Coome, Ramzan, & Monks, 2016). Blocking the testosterone surge in male mice leads to higher leptin levels and greater fat to muscle ratio for life, and testosterone given to neonatal female rodents results in masculinized pattern of gene expression (de Mello et al., 2012; Dkhil et al., 2015). Neonatal mice exposed to testosterone have sexually dimorphic differences in brain DNA methylation as adults (Nugent et al., 2015).
In human studies, six studies with less than 100 total subjects have evaluated the testosterone surge in the first few months of life (called the mini-puberty of infancy) in XXY with mixed results (Davis et al., 2015). See Aksglaede et al. (in press) in this article collection for review of mini-puberty in XXY (Aksglaede et al., in press). In summary, the largest study showed that testosterone levels fell below the median in 83% of XXY infants (Lahlou et al., 2004). Since these publications showing lower testosterone during mini-puberty in XXY, there has been increasing interest in supplementing testosterone in XXY infants and the potential effects on health and neurodevelopment.
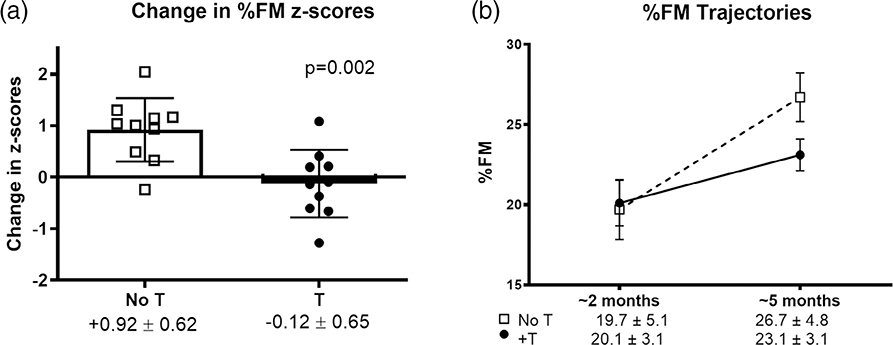
A recent pilot study enrolled 20 prenatally diagnosed infants with XXY at 1–3 months of age who were randomized to a short 3-month course of intramuscular T or no treatment. Body composition was assessed by PeaPod (air displacement plethysmography) at baseline and at the end of 3 months, and results showed increased accumulation of body fat in the first months of life in untreated infants compared with the treated infants. During a 12-week time period, body fat percentage z-scores increased by 0.92 ± 0.6 SD in the untreated group, compared with T-treated infants whose body fat percentage z-scores did not change (−0.12 ± 0.65 SD, p = .002) (Figure 1). Further, the T-treated infants had similar body fat percentage at 5 months of age to a control group of 316 male infants, while the untreated group had higher body fat percentage (p = .037) as well as longer penile length and no serious adverse events from treatment. It has previously been published that boys with XXY as young as 4 years of age have high percent body fat (mean + 0.9 SDS), (Aksglaede et al., 2008) and together these results support that the natural history of body fat accumulation likely originates in infancy in XXY. The eXtraordinarY Babies Study will also explore whether altered body composition is associated with developmental delays across developmental domains. Lower lean muscle mass, for example, may result in lower stamina for interaction with the infant’s environment and slower acquisition of motor developmental milestones. Further, some research has linked early motor skills with language outcomes in typical children (Iverson, 2010), and thus this association can also be explored in the SCT population in the context of muscle mass as well. Knowledge of the trajectories of body composition and gonadal function throughout infancy and early childhood for all three SCT conditions will help determine if body fat accumulation will inversely correlate with motor outcomes. Future studies on this cohort can then examine whether poor testicular or ovarian function in early infancy will predict later body composition.
FIGURE 1.
(a) Change in %FM z scores was significantly greater in untreated (open squares) than in testosterone-treated (closed circles) boys with XXY. Bars and error bars represent mean and SD, respectively, and symbols represent individual participants. (b) Absolute %FM was similar at baseline but higher in the untreated boys after 3 months, although this difference did not reach statistical significance (p = .061). Error bars represent SEM. FM, fat mass; SD, standard deviation; T, testosterone treatment; SEM, standard error of the mean. Reprinted with permission (Davis et al., 2019)
Studies have also explored the relationship of early androgen treatment and neurodevelopmental features of XXY with the consideration that androgen treatment may mimic the normal testosterone surge seen during the mini-puberty period and act more directly to impact brain development and/or function. Retrospective description of a clinical cohort followed in a developmental clinic reported improved cognitive and psychosocial outcomes in the subset who had received testosterone injections from outside endocrinologists during the first years of life (Samango-Sprouse et al., 2013; Samango-Sprouse et al., 2015), although lack of randomization or blinding, unknown baseline endogenous androgen levels, and varied timing of treatment beyond the typical mini-puberty period complicate interpretation of results. These results serve as important background, however, for prospective, randomized, double-blind trials that would satisfy scientific criteria for changes in care practices if results were replicated in other centers. The role of androgen treatment in psychological and motor development beyond the mini-puberty period is also an area of interest in XXY, and a double-blind placebo-controlled study of the androgen oxandrolone in 80 prepubertal boys with XXY for 24 months showed improvement in one of five primary endpoints of motor function (visual-motor function), while secondary analyses demonstrated positive effects of androgen on aspects of psychosocial function (anxiety, depression, social problems), without significant effects on cognitive function, hyperactivity, or aggressive behaviors (Ross et al., 2017). Interestingly, another study recently reported a correlation of social anxiety symptoms with salivary testosterone levels in male children and adults with XXY, however, there was not an association with other measures of social cognition (van Rijn, 2018). More investigation is needed to determine the role of androgens in consideration of both the early impact on long-term neurodevelopmental differences, as well as on shorter-term changes in neural systems or functioning that could affect psychological symptoms. The eXtraordinarY Babies Study will allow us to track and compare the endogenous hormone profiles in large SCT cohorts across the first year of life, as well as to compare neurodevelopmental and physical outcomes in those with XXY who have received exogenous hormone treatments at different timepoints to those who did not receive treatment.
6.3 |. Ovarian function in trisomy X
The limited available data on early hormonal function in trisomy X comes from 12 girls in the newborn screening studies that showed prolonged elevation of FSH until 5–6 years of age similar to a pattern seen in gonadal dysgenesis (Stewart et al., 1979; Stewart et al., 1982). A recent Italian cross-sectional study of 15 girls age 7–11 showed elevated LH and FSH, lower estradiol, and decreased ovarian volumes compared to controls (Stagi, di Tommaso, Scalini, et al., 2016). Adult women withtrisomy X are frequently reported to have premature ovarian insufficiency (POI), however, actual prevalence of POI in trisomy X is unknown (Ayed et al., 2014; Jiao et al., 2012; Tartaglia et al., 2010). The eXtraordinarY Kids Clinic at Children’s Hospital Colorado has recently begun testing AMH (a marker of ovarian reserve) in adolescents with trisomy X, and recently conducted a pilot case-control study in 15 girls 5 to 24 years of age with trisomy X compared with 26 controls of similar age. Results showed that females with trisomy X had significantly lower serum AMH compared to controls (0.7 ng/ml [IQR 0.2–1.7] vs. 2.7 [IQR 1.3–4.8], p < .001). Additionally, girls with trisomy X were much more likely to have an AMH below the 2.5th percentile for age with 67% of them meeting these criteria (OR 11, 95% CI 2.3–42) (Davis, Soares, et al., in press). These results suggest that markers of decreased ovarian reserve begin to present in childhood and adolescence in trisomy X. A better understanding of the natural history of low AMH concentrations and the prevalence of subsequent POI in this patient population may be important for considerations of fertility preservation in adolescents or young adults with trisomy X with decreasing ovarian reserve. Further studies are also important as ovarian function includes production of female sex hormones across the lifespan that can affect bone and cardiometabolic health, as well as myriad aspects of psychological functioning. There are no available data on ovarian function in trisomy X infants, and exploring hormonal profiles prospectively from the newborn period as part of the eXtraordinarY Babies Study will define the prevalence and timing of onset of ovarian dysfunction and perhaps identify opportunities for early hormonal interventions.
6.4 |. Hormonal and genetic research considerations
The role of sex hormones on neurodevelopment and behavioral functioning is a critical area of research and preliminary study results described above are intriguing and deserving of additional investigations. However, it is important to consider that hormonal treatments are unlikely to normalize neurodevelopmental and brain function in XXY, as there are hundreds of genes that have shown differential expression both on the extra sex chromosome as well as the autosomes in all SCT conditions that affect neurodevelopment and plasticity, neuroanatomy, and intracellular signaling pathways (Liu et al., 2019; Raznahan et al., 2018; Skakkebæk et al., 2018; Xenophontos et al., 2019; Zitzmann et al., 2015). Overt hypogonadism is not commonly associated with XYY or trisomy X as it is with XXY, and the profile of neurodevelopmental, cognitive, and psychological risks across the three SCT conditions share more overall similarities than differences. Further, neurodevelopmental involvement increases as the number of sex chromosomes increase in the tetrasomy and pentasomy conditions (Linden, Bender, & Robinson, 1995; Tartaglia, Ayari, Howell, D’Epagnier, & Zeitler, 2011), further supporting the primary role of undiscovered genetic factors in neurodevelopmental phenotypic variability. Thus, while complex, efforts to identify changes in biological systems and cellular pathways caused by the excess gene dosage are likely to yield important pathophysiologic information and potential therapeutic targets that will apply across all sex chromosome aneuploidy conditions. The biobank developed by the eXtraordinarY Babies Study that combines rich longitudinal phenotypic data with biological samples can be used to further explore these questions of the interplay between genetic and hormonal factors in infancy and early childhood. Expanded studies on metabolomics, transcriptomics, and other cell model approaches may then point to pathways that could be targeted by medications or other therapeutics.
6.5 |. Congenital malformations and other health problems
All SCT conditions have been associated with increased risk for other congenital malformations and medical diagnoses in cross-sectional studies and/or case reports. Increased risk for congenital cardiac and renal malformations, allergies, autoimmunity, eosinophilic esophagitis, dental problems, velopharyngeal insufficiency, elbow abnormalities, hypotonia, pes planus, tremor, white matter MRI abnormalities, and seizures have been described across all SCT conditions (Boisen & Rasmussen, 1978; Campbell & Price, 1981; Giedd et al., 2007; Harris et al., 2016; Lepage et al., 2014; Liu et al., 2016; Pasquali et al., 2013; Rock & McLellan, 1990; Steinness & Nielsen, 1970; Stochholm et al., 2010a; Stochholm et al., 2010b; Varrela & Alvesalo, 1988; Wigby et al., 2016; Zeger et al., 2008). XXY has been further associated with increased rates of hernias, venous thrombosis, and certain malignancies such as germ cell tumors (Bojesen et al., 2004; Campbell & Price, 1981; Williams et al., 2018). While detailed description of risks for these comorbidities are beyond the scope of this review, larger studies and population-based cohort studies are needed to better understand the true prevalence of these other conditions so that screening recommendations can be developed, as well as to explore any differences in presentation or considerations for treatment in SCT. In the short term, the eXtraordinarY Babies Study will allow for better description of congenital malformations, feeding differences, and growth patterns in the first few years of life, again with correlation to neurodevelopmental and hormonal profiles.
7 |. CONSIDERATIONS OF NEWBORN SCREENING FOR SCT
Many investigators involved with the original newborn screening studies advocated that routine neonatal screening would satisfy cost-benefit analysis given the low cost of testing and the benefit of parents, physicians, and teachers anticipating behavior, learning, and psychosocial problems so modifications could be made (Dickens, 1982; Stewart et al., 1982). Recent studies identifying neurodevelopmental disorders such as ASD that have evidence-based early treatments also support that newborn screening may provide opportunity for interventions to improve long-term outcomes. Importantly, the recent research showing potential benefits of hormonal therapy in infants and young children with XXY in both neurodevelopmental and physical health outcomes further supports that newborn screening may be beneficial as it could offer a disease-modifying intervention during an early critical period. However, others argue that SCT conditions do not meet the criteria for newborn screening due to the broad phenotypic variability and milder phenotype without severe or immediate medical needs. It is argued that developmental screening practices and routine medical care should identify the subset of those with SCT with delays severe enough to require early intervention therapies, that these delays should prompt genetic testing leading to a diagnosis, and that we do not currently have evidence-based interventions specifically for SCT. While routine developmental screening and medical care should theoretically identify these children, Visootsak, Ayari, Howell, Lazarus, and Tartaglia (2013) published a study that included 89 males with XXY where parents reported a mean gap of 4.8 years between when they first raised developmental or behavioral concerns to providers and when genetic testing was performed leading to XXY diagnosis (Visootsak et al., 2013). For these cases, it is clear that relying on screening was not effective, and similar experiences of families going through a “diagnostic odyssey” before obtaining an SCT diagnosis have been reported (Bourke, Snow, Herlihy, Amor, & Metcalfe, 2014; Close, Sadler, & Grey, 20120156). As with other genetic conditions, ethical issues and concerns remain about whether knowledge of an SCT from birth may also affect the parent-child relationship, decrease parental expectations, and also negatively affect self-identity due to the known predisposition to risks and conditions associated with SCT. In the previous newborn screening studies where families were told of the diagnosis, parents reported increased anxiety related to the knowledge of their child’s condition (Valentine, 1979). On the other hand, many would also argue that knowledge of these risks and potential areas of challenge from infancy also allows a more proactive and compassionate approach to raising a child, and that knowledge of the diagnoses allows for more timely interventions of developmental, academic, mental health, and medical care if needed.
In March 2016, over 100 clinicians and researchers of many disciplines with expertise in SCT gathered at the 2nd International Meeting for Klinefelter Syndrome in Muenster, Germany. A summary of the lively professional discussions were published, and there was strong support for newborn screening to allow anticipatory guidance and early intervention, and others noted that realistically it may not be a matter of whether SCT should be screened for, but when it will be identified as part of more universal newborn genetic testing studies (Nieschlag et al., 2016). It was acknowledged that our understanding of the natural history and interventions to improve SCT outcomes are lacking, and pilot newborn screening studies were recommended to explore these topics (Lanfranco, Kamichke, Zitzmann, & Nieschlag, 2004; Nieschlag et al., 2016). The eXtraordinary Babies Study will further evaluate parental experiences in a national, diverse cohort of families with a prenatal SCT diagnosis that will help inform this discussion about the implications of newborn screening. Specifically, measures of parenting stress, maternal and paternal attachment, and parental quality of life will be followed prospectively, and qualitative experiences will also be captured. Results of these measures will also be examined in the context of other variables such as the developmental and medical course of the child, the need for therapies/interventions, or other household factors that may impact parenting experiences such as number of other children and socioeconomic status. The population of parents with a prenatal diagnosis differs somewhat from those that would learn of the diagnosis through newborn screening, as they elected to continue the pregnancy following prenatal diagnosis. However, their experiences, in combination with developmental outcomes of the study participants, will provide important information to guide discussions related to newborn screening.
8 |. CONCLUSIONS AND FUTURE DIRECTIONS
The eXtraordinarY Babies Study aims to improve care for all individuals with SCT by building upon previous research and answering new questions about the interplay of health, hormones, and neurodevelopment using a rigorous, prospective design. Results will be considered along with the findings of other important studies in SCT being conducted internationally to inform genetic counseling, guide considerations for newborn screening, improve care recommendations, and to identify targets for intervention trials. With the myriad of ongoing efforts of dedicated translational research teams internationally in collaboration with advocacy groups, funding agencies, research participants and their families, there is great promise that discoveries from current research efforts will lead to broader understanding of the origin and pathophysiology of known risks, new treatment options, evidence-based care recommendations, and improved quality of life for individuals with all types of sex chromosome disorders.
ACKNOWLEDGMENTS
Supported by NIH/NICHD R01HD091251, NICHD K23HD092588, NIH/NCATS Colorado CTSA Grant Number UL1 TR002535. Contents are the authors’ sole responsibility and do not necessarily represent official NIH views. This work was also supported by a grant from the Dutch Organization for Scientific Research (NWO funding # 016.165.397) to Sophie van Rijn. Additional acknowledgements to members of the eXtraordinarY Kids Research teams at each site including Richard Boada, Sophia Deklotz, Stevenson Yip, Caroline Harrison, Stephanie Takamatsu, Lisa Cordeiro, Sydney Martin, Jacqueline Frazier, Lindsey Cohen, Laura Pyle, and Andrea Osypuk.
Funding information
Eunice Kennedy Shriver National Institute of Child Health and Human Development, Grant/Award Numbers: K23HD092588, R01HD091251; National Center for Advancing Translational Sciences, Grant/Award Number: UL1 TR002535; Nederlandse Organisatie voor Wetenschappelijk Onderzoek, Grant/Award Number: 016.165.397
REFERENCES
- Abramsky L, & Chapple J (1997). 47,XXY (Klinefelter syndrome) and 47,XYY: Estimated rates of and indication for postnatal diagnosis with implications for prenatal Counselling. Prenatal Diagnosis, 17(4), 363–368. [DOI] [PubMed] [Google Scholar]
- Aksglaede L, Davis S, Ross J, & Juul A (in press). Minipuberty in Klinefelter syndrome current status and future directions. American Journal of Medical Genetics. Part C, Seminars in Medical Genetics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksglaede L, Molgaard C, Skakkebaek NE, & Juul A (2008). Normal bone mineral content but unfavourable muscle/fat ratio in Klinefelter syndrome. Archives of Disease in Childhood, 93(1), 30–34. [DOI] [PubMed] [Google Scholar]
- American College of Obstetrics and Gynecology. (2016). Practice bulletin no. 163: Screening for fetal aneuploidy. Obstetrics and Gynecology, 127(5), e123–e137. [DOI] [PubMed] [Google Scholar]
- Ayed W, Amouri A, Hammami W, Kilani O, Turki Z, Harzallah F, … Slama CB (2014). Cytogenetic abnormalities in Tunisian women with premature ovarian failure. Comptes Rendus Biologies, 337(12), 691–694. [DOI] [PubMed] [Google Scholar]
- Bancroft J, Axworthy D, & Ratcliffe S (1982). The personality and psycho-sexual development of boys with 47,XXY chromosome constitution. Journal of Child Psychology and Psychiatry, 23(2), 169–180. [DOI] [PubMed] [Google Scholar]
- Bardsley MZ, Falkner B, Kowal K, & Ross JL (2011). Insulin resistance and metabolic syndrome in prepubertal boys with Klinefelter syndrome. Acta Paediatrica, 100(6), 866–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender B, Fry E, Pennington B, Puck M, Salbenblatt J, & Robinson A (1983). Speech and language development in 41 children with sex chromosome anomalies. Pediatrics, 71(2), 262–267. [PubMed] [Google Scholar]
- Bender B, Harmon RJ, Linden MG, & Robinson A (1995). Psychosocial adaptation in 39 adolescents with sex chromosome abnormalities. Pediatrics, 96, 302–308. [PubMed] [Google Scholar]
- Bender BG, Linden MG, & Harmon RJ (2001). Neuropsychological and functional cognitive skills of 35 unselected adults with sex chromosome abnormalities. American Journal of Medical Genetics, 102(4), 309–313. [DOI] [PubMed] [Google Scholar]
- Bender BG, Linden MG, & Robinson A (1993). Neuropsychological impairment in 42 adolescents with sex chromosome abnormalities. American Journal of Medical Genetics, 48(3), 169–173. [DOI] [PubMed] [Google Scholar]
- Bender BG, Puck MH, Salbenblatt JA, & Robinson A (1986). Dyslexia in 47,XXY boys identified at birth. Behavior Genetics, 16(3), 343–354. [DOI] [PubMed] [Google Scholar]
- Bianchi DW, & Wilkins-Haug L (2014). Integration of noninvasive DNA testing for aneuploidy into prenatal care: What has happened since the rubber met the road? Clinical Chemistry, 60(1), 78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmingham E, & Kingstone A (2009). Human social attention. Progress in Brain Research, 176, 309–320. 10.1016/S0079-6123(09)17618-5. [DOI] [PubMed] [Google Scholar]
- Bishop DV, Jacobs PA, Lachlan K, Wellesley D, Barnicoat A, Boyd PA, … Scerif G (2010). Autism, language and communication in children with sex chromosome trisomies. Archives of Disease in Childhood, 96(10), 954–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop DVM, Brookman-Byrne A, Gratton N, Gray E, Holt G, Morgan L, … Thompson PA (2018). Language phenotypes in children with sex chromosome trisomies. Wellcome Open Research, 3, 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boada R, Janusz J, Hutaff-Lee C, & Tartaglia N (2009). The cognitive phenotype in Klinefelter syndrome: A review of the literature including genetic and hormonal factors. Developmental Disabilities Research Reviews, 15(4), 284–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisen E, Owen DR, Rasmussen L, & Sergeant J (1981). Cardiac functioning and blood pressure of 47,XYY and 47,XXY men in a doubleblind, double-matched population survey. American Journal of Human Genetics, 33(1), 77–84. [PMC free article] [PubMed] [Google Scholar]
- Boisen E, & Rasmussen L (1978). Tremor in XYY and XXY men. Acta Neurologica Scandinavica, 58(1), 66–73. [DOI] [PubMed] [Google Scholar]
- Bojesen A, & Gravholt CH (2011). Morbidity and mortality in Klinefelter syndrome (47,XXY). Acta Paediatrica, 100(6), 807–813. [DOI] [PubMed] [Google Scholar]
- Bojesen A, Host C, & Gravholt CH (2010). Klinefelter’s syndrome, type 2 diabetes and the metabolic syndrome: The impact of body composition. Molecular Human Reproduction, 16(6), 396–401. [DOI] [PubMed] [Google Scholar]
- Bojesen A, Juul S, Birkebaek N, & Gravholt CH (2004). Increased mortality in Klinefelter syndrome. The Journal of Clinical Endocrinology and Metabolism, 89(8), 3830–3834. [DOI] [PubMed] [Google Scholar]
- Bojesen A, Juul S, & Gravholt CH (2003). Prenatal and postnatal prevalence of Klinefelter syndrome: A national registry study. The Journal of Clinical Endocrinology and Metabolism, 88(2), 622–626. [DOI] [PubMed] [Google Scholar]
- Bojesen A, Kristensen K, Birkebaek NH, Fedder J, Mosekilde L, Bennett P, … Gravholt CH (2006). The metabolic syndrome is frequent in Klinefelter’s syndrome and is associated with abdominal obesity and hypogonadism. Diabetes Care, 29(7), 1591–1598. [DOI] [PubMed] [Google Scholar]
- Bourke E, Snow P, Herlihy A, Amor D, & Metcalfe S (2014). A qualitative exploration of mothers’ and fathers’ experiences of having a child with Klinefelter syndrome and the process of reaching this diagnosis. European Journal of Human Genetics, 22(1), 18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw J, Shic F, Holden AN, Horowitz EJ, Barrett AC, German TC, & Vernon TW (2019). The use of eye tracking as a biomarker of treatment outcome in a pilot randomized clinical trial for young children with autism. Autism Research, 12(5), 779–793. [DOI] [PubMed] [Google Scholar]
- Bruining H, Swaab H, Kas M, & van Engeland H (2009). Psychiatric characteristics in a self-selected sample of boys with Klinefelter syndrome. Pediatrics, 123(5), e865–e870. [DOI] [PubMed] [Google Scholar]
- Campbell WA, & Price WH (1981). Venous thromboembolic disease in Klinefelter’s syndrome. Clinical Genetics, 19(4), 275–280. [DOI] [PubMed] [Google Scholar]
- Carpenter M, Nagell K, & Tomasello M (1998). Social cognition, joint attention, and communicative competence from 9 to 15 months of age. Monographs of the Society for Research in Child Development, 63(4), 1–143. [PubMed] [Google Scholar]
- Chawarska K, Macari S, & Shic F (2012). Context modulates attention to social scenes in toddlers with autism. Journal of Child Psychology and Psychiatry, 53(8), 903–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawarska K, Shic F, Macari S, Campbell DJ, Brian J, Landa R, … Bryson S (2014). 18-month predictors of later outcomes in younger siblings of children with autism spectrum disorder: A baby siblings research consortium study. Journal of the American Academy of Child and Adolescent Psychiatry, 53(12), 1317–1327.e1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen DL, Baio J, Van Naarden Braun K, Bilder D, Charles J, Constantino JN, … C. f. D. C. a. P. (CDC). (2016). Prevalence and characteristics of autism Spectrum disorder among children aged 8 years- -autism and developmental disabilities monitoring network, 11 sites, United States, 2012. MMWR Surveillance Summaries, 65 (3), 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close S, Fennoy I, Smaldone A, & Reame N (2015). Phenotype and adverse quality of life in boys with Klinefelter syndrome. The Journal of Pediatrics, 167(3), 650–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close S, Sadler L, & Grey M (2015). In the dark: Challenges of caring for sons with Klinefelter syndrome. Journal of Pediatric Nursing, 31(1), 11–20. [DOI] [PubMed] [Google Scholar]
- Coffee B, Keith K, Albizua I, Malone T, Mowrey J, Sherman SL, & Warren ST (2009). Incidence of fragile X syndrome by newborn screening for methylated FMR1 DNA. American Journal of Human Genetics, 85(4), 503–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordeiro L, Tartaglia N, Roeltgen D, & Ross J (2012). Social deficits in male children and adolescents with sex chromosome aneuploidy: A comparison of XXY, XYY, and XXYY syndromes. Research in Developmental Disabilities, 33(4), 1254–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S, Deklotz S, Nadeau K, Kelsey M, Zeitler P, & Tartaglia N (in press). High prevalence of cardiometabolic risk features in adolescents with 47,XXY / Klinefelter syndrome. American Journal of Medical Genetics. Part C, Seminars in Medical Genetics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S, Howell S, Wilson R, Tanda T, Ross J, Zeitler P, & Tartaglia N (2016). Advances in the interdisciplinary care of children with Klinefelter syndrome. Advances in Pediatrics, 63(1), 15–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S, Kowal K, Tahsin A, Bloy L, Roberts T, & Ross J (in press). Testicular function in boys with 47,XYY and relationship to phenotype. American Journal of Medical Genetics. Part C, Seminars in Medical Genetics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S, Lahlou N, Bardsley M, Temple MC, Kowal K, Pyle L, … Ross J (2016). Gonadal function is associated with cardiometabolic health in pre-pubertal boys with Klinefelter syndrome. Andrology, 4(6), 1169–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S, Soares S, Howell S, Cree-Green M, Buyers E, Johnson J, & Tartaglia N (in press). Diminished ovarian reserve in girls and adolescents with trisomy X syndrome. Reproductive Sciences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SM, Cox-Martin MG, Bardsley MZ, Kowal K, Zeitler PS, & Ross JL (2017). Effects of oxandrolone on cardiometabolic health in boys with Klinefelter syndrome: A randomized controlled trial. The Journal of Clinical Endocrinology and Metabolism, 102(1), 176–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SM, Reynolds RM, Dabelea DM, Zeitler PS, & Tartaglia NR (2019). Testosterone treatment in infants with 47,XXY: Effects on body composition. Journal of the Endocrine Society, 3(12), 2276–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SM, Rogol AD, & Ross JL (2015). Testis development and fertility potential in boys with Klinefelter syndrome. Endocrinology and Metabolism Clinics of North America, 44(4), 843–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Mello WG, de Morais SR, Dornelles RC, Kagohara Elias LL, Antunes-Rodrigues J, & Bedran de Castro JC (2012). Effects of neonatal castration and androgenization on sexual dimorphism in bone, leptin and corticosterone secretion. Bone, 50(4), 893–900. [DOI] [PubMed] [Google Scholar]
- Deebel NA, Galdon G, Zarandi NP, Stogner-Underwood K, Howards S, Lovato J, … Sadri-Ardekani H (2020). Age-related presence of spermatogonia in patients with Klinefelter syndrome: A systematic review and meta-analysis. Human Reproduction Update, 26(1), 58–72. [DOI] [PubMed] [Google Scholar]
- Dickens BM (1982). Ethical and legal issues in medical management of sex chromosome-abnormal adolescents. Birth Defects Original Article Series, 18(4), 227–246. [PubMed] [Google Scholar]
- Dkhil MA, Al-Quraishy S, Abdel-Baki AA, Ghanjati F, Arauzo-Bravo MJ, Delic D, & Wunderlich F (2015). Epigenetic modifications of gene promoter DNA in the liver of adult female mice masculinized by testosterone. The Journal of Steroid Biochemistry and Molecular Biology, 145, 121–130. [DOI] [PubMed] [Google Scholar]
- Esposito G, Tremolaterra MR, Savarese M, Spiniello M, Patrizio MP, Lombardo B, … Carsana A (2018). Unraveling unusual X-chromosome patterns during fragile-X syndrome genetic testing. Clinica Chimica Acta, 476, 167–172. [DOI] [PubMed] [Google Scholar]
- Fales CL, Knowlton BJ, Holyoak KJ, Geschwind DH, Swerdloff RS, & Gonzalo IG (2003). Working memory and relational reasoning in Klinefelter syndrome. Journal of the International Neuropsychological Society, 9(6), 839–846. [DOI] [PubMed] [Google Scholar]
- Frank MC, Vul E, & Saxe R (2012). Measuring the development of social attention using free-viewing. Infancy, 17(4), 355–375. [DOI] [PubMed] [Google Scholar]
- Geerts M, Steyaert J, & Fryns JP (2003). The XYY syndrome: A follow-up study on 38 boys. Genetic Counseling, 14(3), 267–279. [PubMed] [Google Scholar]
- Ghahramani NM, Ngun TC, Chen PY, Tian Y, Krishnan S, Muir S, … Vilain E (2014). The effects of perinatal testosterone exposure on the DNA methylome of the mouse brain are late-emerging. Biology of Sex Differences, 5, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Clasen LS, Wallace GL, Lenroot RK, Lerch JP, Wells EM, … Samango-Sprouse CA (2007). XXY (Klinefelter syndrome): A pediatric quantitative brain magnetic resonance imaging case-control study. Pediatrics, 119(1), e232–e240. [DOI] [PubMed] [Google Scholar]
- Goswami R, Goswami D, Kabra M, Gupta N, Dubey S, & Dadhwal V (2003). Prevalence of the triple X syndrome in phenotypically normal women with premature ovarian failure and its association with autoimmune thyroid disorders. Fertility and Sterility, 80(4), 1052–1054. [DOI] [PubMed] [Google Scholar]
- Gravholt CH, Jensen AS, Host C, & Bojesen A (2011). Body composition, metabolic syndrome and type 2 diabetes in Klinefelter syndrome. Acta Paediatrica, 100(6), 871–877. [DOI] [PubMed] [Google Scholar]
- Haith MM, Bergman T, & Moore MJ (1977). Eye contact and face scanning in early infancy. Science, 198(4319), 853. [DOI] [PubMed] [Google Scholar]
- Hamerton JL, Canning N, Ray M, & Smith S (1975). A cytogenetic survey of 14,069 newborn infants. I. Incidence of chromosome abnormalities. Clinical Genetics, 8(4), 223–243. [DOI] [PubMed] [Google Scholar]
- Harkulich JF, Marchner TJ, & Brown EB (1979). Neurological, neuropsychological, and behavioral correlates of Klinefelter’s syndrome. The Journal of Nervous and Mental Disease, 167(6), 359–363. [DOI] [PubMed] [Google Scholar]
- Harris VM, Sharma R, Cavett J, Kurien BT, Liu K, Koelsch KA, … Scofield RH (2016). Klinefelter’s syndrome (47,XXY) is in excess among men with Sjögren’s syndrome. Clinical Immunology, 168, 25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlihy AS, Gillam L, Halliday JL, & McLachlan RI (2011). Postnatal screening for Klinefelter syndrome: Is there a rationale? Acta Paediatrica, 100(6), 923–933. [DOI] [PubMed] [Google Scholar]
- Hofherr SE, Wiktor AE, Kipp BR, Dawson DB, & Van Dyke DL (2011). Clinical diagnostic testing for the cytogenetic and molecular causes of male infertility: The Mayo Clinic experience. Journal of Assisted Reproduction and Genetics, 28(11), 1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba Y, Herlihy AS, Schwartz CE, Skinner C, Bui QM, Cobb J, … Godler DE (2013). Fragile X-related element 2 methylation analysis may provide a suitable option for inclusion of fragile X syndrome and/- or sex chromosome aneuploidy into newborn screening: A technical validation study. Genetics in Medicine, 15(4), 290–298. [DOI] [PubMed] [Google Scholar]
- Ismail SR, el-Beheiry AH, Hashishe MM, & el-Bahaei ME (1993). Cytogenetic study in idiopathic infertile males. The Journal of the Egyptian Public Health Association, 68(1–2), 179–204. [PubMed] [Google Scholar]
- Iverson JM (2010). Developing language in a developing body: The relationship between motor development and language development. Journal of Child Language, 37(2), 229–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao X, Qin C, Li J, Qin Y, Gao X, Zhang B, … Chen ZJ (2012). Cytogenetic analysis of 531 Chinese women with premature ovarian failure. Human Reproduction, 27(7), 2201–2207. [DOI] [PubMed] [Google Scholar]
- Kim IW, Khadilkar AC, Ko EY, & Sabanegh ES (2013). 47,XYY syndrome and male infertility. Revista de Urología, 15(4), 188–196. [PMC free article] [PubMed] [Google Scholar]
- Lahlou N, Fennoy I, Carel JC, & Roger M (2004). Inhibin B and anti-Mullerian hormone, but not testosterone levels, are normal in infants with nonmosaic Klinefelter syndrome. The Journal of Clinical Endocrinology and Metabolism, 89(4), 1864–1868. [DOI] [PubMed] [Google Scholar]
- Lanfranco P, Kamichke A, Zitzmann M, & Nieschlag E (2004). Klinefelter’s syndrome. Lancet, 364(July 17), 273–283. [DOI] [PubMed] [Google Scholar]
- Lee NR, Wallace GL, Clasen LS, Lenroot RK, Blumenthal JD, White SL, … Giedd JN (2011). Executive function in young males with Klinefelter (XXY) syndrome with and without comorbid attentiondeficit/hyperactivity disorder. Journal of the International Neuropsychological Society, 17(3), 522–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepage JF, Hong DS, Raman M, Marzelli M, Roeltgen DP, Lai S, … Reiss AL (2014). Brain morphology in children with 47, XYY syndrome: A voxel- and surface-based morphometric study. Genes, Brain, and Behavior, 13(2), 127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden MG, Bender BG, Harmon RJ, Mrazek DA, & Robinson A (1988). 47,XXX: What is the prognosis? Pediatrics, 82(4), 619–630. [PubMed] [Google Scholar]
- Linden MG, Bender BG, & Robinson A (1995). Sex chromosome tetrasomy and pentasomy. Pediatrics, 96(4 Pt 1), 672–682. [PubMed] [Google Scholar]
- Liu K, Kurien BT, Zimmerman SL, Kaufman KM, Taft DH, Kottyan LC, … Scofield RH (2016). X chromosome dose and sex bias in autoimmune diseases: Increased prevalence of 47,XXX in systemic lupus erythematosus and Sjögren’s syndrome. Arthritis & Rhematology, 68(5), 1290–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Tang D, Zheng F, Xu Y, Guo H, Zhou J, … Dai Y (2019). Single-cell sequencing reveals the relationship between phenotypes and genotypes of Klinefelter syndrome. Cytogenetic and Genome Research, 159(2), 55–65. [DOI] [PubMed] [Google Scholar]
- Lo JO, Cori DF, Norton ME, & Caughey AB (2014). Noninvasive prenatal testing. Obstetrical & Gynecological Survey, 69(2), 89–99. [DOI] [PubMed] [Google Scholar]
- Madureira C, Cunha M, Sousa M, Neto AP, Pinho MJ, Viana P, … Barros A (2014). Treatment by testicular sperm extraction and intracytoplasmic sperm injection of 65 azoospermic patients with nonmosaic Klinefelter syndrome with birth of 17 healthy children. Andrology, 2(4), 623–631. [DOI] [PubMed] [Google Scholar]
- Martin S, Cordeiro L, Richardson P, Davis S, & Tartaglia N (2019). The association of motor skills and adaptive functioning in XXY/Klinefelter and XXYY syndromes. Physical & Occupational Therapy in Pediatrics, 39 (4), 446–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messinger D, Young GS, Ozonoff S, Dobkins K, Carter A, Zwaigenbaum L, … Sigman M (2013). Beyond autism: A baby siblings research consortium study of high-risk children at three years of age. Journal of the American Academy of Child and Adolescent Psychiatry, 52 (3), 300–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M, Iosif AM, Young GS, Hill M, Phelps Hanzel E, Hutman T, … Ozonoff S (2015). School-age outcomes of infants at risk for autism spectrum disorder. Autism Research, 9(6), 632–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newschaffer CJ, Croen LA, Fallin MD, Hertz-Picciotto I, Nguyen DV, Lee NL, … Shedd-Wise KM (2012). Infant siblings and the investigation of autism risk factors. Journal of Neurodevelopmental Disorders, 4(1), 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J (1990). Sex chromosome abnormalities found among 34,910 newborn children: Results from a 13-year incidence study in Arhus, Denmark. Birth Defects Original Article Series, 26(4), 209–223. [PubMed] [Google Scholar]
- Nieschlag E, Ferlin A, Gravholt CH, Gromoll J, Kohler B, Lejeune H, … Wistuba J (2016). The Klinefelter syndrome: Current management and research challenges. Andrology, 4(3), 545–549. [DOI] [PubMed] [Google Scholar]
- Nugent BM, Wright CL, Shetty AC, Hodes GE, Lenz KM, Mahurkar A, … McCarthy MM (2015). Brain feminization requires active repression of masculinization via DNA methylation. Nature Neuroscience, 18(5), 690–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otter M, Schrander-Stumpel CT, Didden R, & Curfs LM (2012). The psychiatric phenotype in triple X syndrome: New hypotheses illustrated in two cases. Developmental Neurorehabilitation, 15(3), 233–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottesen AM, Aksglaede L, Garn I, Tartaglia N, Tassone F, Gravholt CH, … Juul A (2010). Increased number of sex chromosomes affects height in a nonlinear fashion: A study of 305 patients with sex chromosome aneuploidy. American Journal of Medical Genetics. Part A, 152A(5), 1206–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Iosif AM, Baguio F, Cook IC, Hill MM, Hutman T, … Young GS (2010). A prospective study of the emergence of early behavioral signs of autism. Journal of the American Academy of Child and Adolescent Psychiatry, 49(3), 256–266. [PMC free article] [PubMed] [Google Scholar]
- Park SJ, Jung EH, Ryu RS, Kang HW, Chung HD, & Kang HY (2013). The clinical application of array CGH for the detection of chromosomal defects in 20,126 unselected newborns. Molecular Cytogenetics, 6(1), 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquali D, Arcopinto M, Renzullo A, Rotondi M, Accardo G, Salzano A, … Cittadini A (2013). Cardiovascular abnormalities in Klinefelter syndrome. International Journal of Cardiology, 168(2), 754–759. [DOI] [PubMed] [Google Scholar]
- Pennington B, Puck M, & Robinson A (1980). Language and cognitive development in 47,XXX females followed since birth. Behavior Genetics, 10(1), 31–41. [DOI] [PubMed] [Google Scholar]
- Pennington BF, Bender B, Puck M, Salbenblatt J, & Robinson A (1982). Learning disabilities in children with sex chromosome anomalies. Child Development, 53(5), 1182–1192. [PubMed] [Google Scholar]
- Peterson RL, Pennington BF, Shriberg LD, & Boada R (2009). What influences literacy outcome in children with speech sound disorder? Journal of Speech, Language, and Hearing Research, 52(5), 1175–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotton ISG, d’Estaing B, Cuzin A, Brosse M, Benchaib J, Lornage R, … Lejeune and F group. (2015). Preliminary results of a prospective study of testicular sperm extraction in Young versus adult patients with nonmosaic 47,XXY Klinefelter syndrome. The Journal of Clinical Endocrinology and Metabolism, 100(3), 961–967. [DOI] [PubMed] [Google Scholar]
- Ragab MW, Cremers JF, Zitzmann M, Nieschlag E, Kliesch S, & Rohayem J (2018). A history of undescended testes in young men with Klinefelter syndrome does not reduce the chances for successful microsurgical testicular sperm extraction. Andrology, 6(4), 525–531. [DOI] [PubMed] [Google Scholar]
- Ratcliffe SG (1982a). Speech and learning disorders in children with sex chromosome abnormalities. Developmental Medicine and Child Neurology, 24(1), 80–84. [DOI] [PubMed] [Google Scholar]
- Ratcliffe SG (1982b). The sexual development of boys with the chromosome constitution 47,XXY (Klinefelter’s syndrome). Clinics in Endocrinology and Metabolism, 11(3), 703–716. [DOI] [PubMed] [Google Scholar]
- Ratcliffe SG (1985). Longitudinal growth studies on children with sex chromosome abnormalities. Progress in Clinical and Biological Research, 200, 301–309. [PubMed] [Google Scholar]
- Ratcliffe SG, Butler GE, & Jones M (1990). Edinburgh study of growth and development of children with sex chromosome abnormalities. IV. Birth Defects Original Article Series, 26(4), 1–44. [PubMed] [Google Scholar]
- Ratcliffe SG, & Corker CS (1975). Proccedings: Testicular activity in infants with sex chromosome abnormalities. Archives of Disease in Childhood, 50(5), 404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe SG, & Field MA (1982). Emotional disorder in XYY children: Four case reports. Journal of Child Psychology and Psychiatry, 23(4), 401–406. [DOI] [PubMed] [Google Scholar]
- Ratcliffe SG, Masera N, Pan H, & McKie M (1994). Head circumference and IQ of children with sex chromosome abnormalities. Developmental Medicine and Child Neurology, 36(6), 533–544. [DOI] [PubMed] [Google Scholar]
- Ratcliffe SG, Murray L, & Teague P (1986). Edinburgh study of growth and development of children with sex chromosome abnormalities. III. Birth Defects Original Article Series, 22(3), 73. [PubMed] [Google Scholar]
- Ratcliffe SG, Tierney I, Nshaho J, Smith L, Springbett A, & Callan S (1982). The Edinburgh study of growth and development of children with sex chromosome abnormalities. Birth Defects Original Article Series, 18(4), 41–60. [PubMed] [Google Scholar]
- Raznahan A, Parikshak NN, Chandran V, Blumenthal JD, Clasen LS, Alexander-Bloch AF, … Geschwind DH (2018). Sex-chromosome dosage effects on gene expression in humans. Proceedings of the National Academy of Sciences of the United States of America, 115(28), 7398–7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A, Bender B, Borelli J, Puck M, Salbenblatt J, & Webber ML (1982). Sex chromosomal abnormalities (SCA): A prospective and longitudinal study of newborns identified in an unbiased manner. Birth Defects Original Article Series, 18(4), 7–39. [PubMed] [Google Scholar]
- Robinson A, Bender BG, & Linden MG (1990). Summary of clinical findings in children and young adults with sex chromosome anomalies. Birth Defects Original Article Series, 26(4), 225–228. [PubMed] [Google Scholar]
- Robinson A, Bender BG, Linden MG, & Salbenblatt JA (1990). Sex chromosome aneuploidy: The Denver prospective study. Birth Defects Original Article Series, 26(4), 59. [PubMed] [Google Scholar]
- Rock WP, & McLellan NJ (1990). Severe hypodontia in association with Klinefelter (47 XXY) syndrome. A case report. British Journal of Orthodontics, 17(4), 321–323. [DOI] [PubMed] [Google Scholar]
- Rogol AD, & Tartaglia N (2010). Considerations for androgen therapy in children and adolescents with Klinefelter syndrome (47, XXY). Pediatric Endocrinology Reviews, 8(Suppl 1), 145–150. [PubMed] [Google Scholar]
- Rohayem J, Fricke R, Czeloth K, Mallidis C, Wistuba J, Krallmann C, … Kliesch S (2015). Age and markers of Leydig cell function, but not of Sertoli cell function predict the success of sperm retrieval in adolescents and adults with Klinefelter’s syndrome. Andrology, 3(5), 868–875. [DOI] [PubMed] [Google Scholar]
- Ross JL, Kushner H, Kowal K, Bardsley M, Davis S, Reiss AL, … Roeltgen D (2017). Androgen treatment effects on motor function, cognition, and behavior in boys with Klinefelter syndrome. The Journal of Pediatrics, 185, 193–199.e194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross JL, Roeltgen DP, Kushner H, Zinn AR, Reiss A, Bardsley MZ, … Tartaglia N (2012). Behavioral and social phenotypes in boys with 47,XYY syndrome or 47,XXY Klinefelter syndrome. Pediatrics, 129(4), 769–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross JL, Samango-Sprouse C, Lahlou N, Kowal K, Elder FF, & Zinn A (2005). Early androgen deficiency in infants and young boys with 47,XXY Klinefelter syndrome. Hormone Research, 64(1), 39–45. [DOI] [PubMed] [Google Scholar]
- Ross JL, Tartaglia N, Merry DE, Dalva M, & Zinn AR (2015). Behavioral phenotypes in males with XYY and possible role of increased NLGN4Y expression in autism features. Genes, Brain, and Behavior, 14(2), 137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovet J, Netley C, Bailey J, Keenan M, & Stewart D (1995). Intelligence and achievement in children with extra X aneuploidy: A longitudinal perspective. American Journal of Medical Genetics, 60(5), 356–363. [DOI] [PubMed] [Google Scholar]
- Rovet J, Netley C, Keenan M, Bailey J, & Stewart D (1996). The psychoeducational profile of boys with Klinefelter syndrome. Journal of Learning Disabilities, 29(2), 180–196. [DOI] [PubMed] [Google Scholar]
- Salbenblatt J, Meyers DC, Bender B, Linden MG, & Robinson A (1987). Gross and fine motor development in 47,XXY and 47,XYY males. Pediatrics, 80(2), 240–244. [PubMed] [Google Scholar]
- Salbenblatt JA, Meyers DC, Bender BG, Linden MG, & Robinson A (1989). Gross and fine motor development in 45,X and 47,XXX girls. Pediatrics, 84(4), 678–682. [PubMed] [Google Scholar]
- Salva OR, Farroni T, Regolin L, Vallortigara G, & Johnson MH (2011). The evolution of social orienting: Evidence from chicks (Gallus gallus) and human newborns. PLoS ONE, 6(4), e18802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samango-Sprouse C, & Rogol A (2002). XXY the hidden disability and a prototype for an infantile presentation of developmental dyspraxia (IDD). Infants and Young Children, 15(1), 11–18. [Google Scholar]
- Samango-Sprouse C, Stapleton EJ, Lawson P, Mitchell F, Sadeghin T, Powell S, & Gropman AL (2015). Positive effects of early androgen therapy on the behavioral phenotype of boys with 47,XXY. American Journal of Medical Genetics. Part C, Seminars in Medical Genetics, 169(2), 150–157. [DOI] [PubMed] [Google Scholar]
- Samango-Sprouse CA, Sadeghin T, Mitchell FL, Dixon T, Stapleton E, Kingery M, & Gropman AL (2013). Positive effects of short course androgen therapy on the neurodevelopmental outcome in boys with 47,XXY syndrome at 36 and 72 months of age. American Journal of Medical Genetics. Part A, 161A(3), 501–508. [DOI] [PubMed] [Google Scholar]
- Samango-Sprouse CA, Stapleton EJ, Mitchell FL, Sadeghin T, Donahue TP, & Gropman AL (2014). Expanding the phenotypic profile of boys with 47, XXY: The impact of familial learning disabilities. American Journal of Medical Genetics. Part A, 164A(6), 1464–1469. [DOI] [PubMed] [Google Scholar]
- Simion F, Regolin L, & Bulf H (2008). A predisposition for biological motion in the newborn baby. Proceedings of the National Academy of Sciences, 105(2), 809–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson JL, de la Cruz F, Swerdloff RS, Samango-Sprouse C, Skakkebaek NE, Graham JM Jr., … Paulsen CA (2003). Klinefelter syndrome: Expanding the phenotype and identifying new research directions. Genetics in Medicine, 5(6), 460–468. [DOI] [PubMed] [Google Scholar]
- Skakkebæk A, Nielsen MM, Trolle C, Vang S, Hornshøj H, Hedegaard J, … Gravholt CH (2018). DNA hypermethylation and differential gene expression associated with Klinefelter syndrome. Scientific Reports, 8(1), 13740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skakkebæk A, Wallentin M, & Gravholt CH (2015). Neuropsychology and socioeconomic aspects of Klinefelter syndrome: New developments. Current Opinion in Endocrinology, Diabetes, and Obesity, 22(3), 209–216. [DOI] [PubMed] [Google Scholar]
- St John M, Ponchard C, van Reyk O, Mei C, Pigdon L, Amor DJ, & Morgan AT (2019). Speech and language in children with Klinefelter syndrome. Journal of Communication Disorders, 78, 84–96. [DOI] [PubMed] [Google Scholar]
- Stagg SD, Linnell KJ, & Heaton P (2014). Investigating eye movement patterns, language, and social ability in children with autism spectrum disorder. Development and Psychopathology, 26(2), 529–537. [DOI] [PubMed] [Google Scholar]
- Stagi S, Di Tommaso M, Manoni C, Scalini P, Chiarelli F, Verrotti A, … de Martino M (2016). Bone mineral status in children and adolescents with Klinefelter syndrome. International Journal of Endocrinology, 2016, 3032759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagi S, di Tommaso M, Scalini P, Lapi E, Losi S, Bencini E, … de Martino M (2016). Triple X syndrome and puberty: Focus on the hypothalamus-hypophysis-gonad axis. Fertility and Sterility, 105(6), 1547–1553. [DOI] [PubMed] [Google Scholar]
- Stagi S, Di Tommaso M, Scalini P, Sandini E, Masoni F, Chiarelli F, … de Martino M (2017). Cross-sectional study shows that impaired bone mineral status and metabolism are found in nonmosaic triple X syndrome. Acta Paediatrica, 106(4), 619–626. [DOI] [PubMed] [Google Scholar]
- Steinness E, & Nielsen J (1970). The electrocardiogram in males with the 47, XYY karyotype. The Lancet, 295(7661), 1402–1403. [DOI] [PubMed] [Google Scholar]
- Stewart DA, Bailey JD, Netley CT, & Park E (1990). Growth, development, and behavioral outcome from mid-adolescence to adulthood in subjects with chromosome aneuploidy: The Toronto study. Birth Defects Original Article Series, 26(4), 131–188. [PubMed] [Google Scholar]
- Stewart DA, Bailey JD, Netley CT, Rovet J, Park E, Cripps M, & Curtis JA (1982). Growth and development of children with X and Y chromosome aneuploidy from infancy to pubertal age: The Toronto study. Birth Defects Original Article Series, 18(4), 99–154. [PubMed] [Google Scholar]
- Stewart DA, Netley CT, Bailey JD, Haka-Ikse K, Platt J, Holland W, & Cripps M (1979). Growth and development of children with X and Y chromosome aneuploidy: A prospective study. Birth Defects Original Article Series, 15(1), 75–114. [PubMed] [Google Scholar]
- Stochholm K, Juul S, & Gravholt CH (2010a). Diagnosis and mortality in 47,XYY persons: A registry study. Orphanet Journal of Rare Diseases, 5, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stochholm K, Juul S, & Gravholt CH (2010b). Mortality and incidence in women with 47,XXX and variants. American Journal of Medical Genetics. Part A, 152A(2), 367–372. [DOI] [PubMed] [Google Scholar]
- Swerdlow AJ, Higgins CD, Schoemaker MJ, Wright AF, Jacobs PA, & G. United Kingdom Clinical Cytogenetics. (2005). Mortality in patients with Klinefelter syndrome in Britain: A cohort study. The Journal of Clinical Endocrinology and Metabolism, 90(12), 6516–6522. [DOI] [PubMed] [Google Scholar]
- Swift-Gallant A, Coome LA, Ramzan F, & Monks DA (2016). Nonneural androgen receptors affect sexual differentiation of brain and behavior. Endocrinology, 157(2), 788–798. [DOI] [PubMed] [Google Scholar]
- Takeda T, Iwatsuki S, Hamakawa T, Mizuno K, Kamiya H, Umemoto Y, … Yasui T (2017). Chromosomal anomalies and sperm retrieval outcomes of patients with non-obstructive azoospermia: A case series. Andrology, 5(3), 473–476. [DOI] [PubMed] [Google Scholar]
- Tartaglia N, Ayari N, Howell S, D’Epagnier C, & Zeitler P (2011). 48,XXYY, 48,XXXY and 49,XXXXY syndromes: Not just variants of Klinefelter syndrome. Acta Paediatrica, 100(6), 851–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia N, Howell S, Wilson R, Janusz J, Boada R, Martin S, … Zeitler P (2015). The eXtraordinarY kids clinic: An interdisciplinary model of care for children and adolescents with sex chromosome aneuploidy. Journal of Multidisciplinary Healthcare, 8, 323–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia NR, Ayari N, Hutaff-Lee C, & Boada R (2012). Attentiondeficit hyperactivity disorder symptoms in children and adolescents with sex chromosome aneuploidy: XXY, XXX, XYY, and XXYY. Journal of Developmental and Behavioral Pediatrics, 33(4), 309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia NR, Howell S, Sutherland A, Wilson R, & Wilson L (2010). A review of trisomy X (47,XXX). Orphanet Journal of Rare Diseases, 5, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia NR, Wilson R, Miller JS, Rafalko J, Cordeiro L, Davis S, … Ross J (2017). Autism Spectrum disorder in males with sex chromosome aneuploidy: XXY/Klinefelter syndrome, XYY, and XXYY. Journal of Developmental and Behavioral Pediatrics, 38(3), 197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson T, Howell S, Davis S, Wilson R, Boada R, Janusz J, & Tartaglia N (2020). A current survey of early childhood intervention services in sex chromosome aneuploidies. American Journal of Medical Genetics. Part C, Seminars in Medical Genetics, 182, 1–14. 10.1002/ajmg.c.31785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turriff A, Levy HP, & Biesecker B (2011). Prevalence and psychosocial correlates of depressive symptoms among adolescents and adults with Klinefelter syndrome. Genetics in Medicine, 13(11), 966–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turriff A, Macnamara E, Levy HP, & Biesecker B (2016). The impact of living with Klinefelter syndrome: A qualitative exploration of adolescents and adults. Journal of Genetic Counseling, 26(4), 728–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanus E, Swaab H, Tartaglia N, Cordeiro L, & Van Rijn S (2020). The behavioral profile of children aged 1–5years with sex chromosome trisomy (47,XXX, 47,XXY, 47,XYY). American Journal of Medical Genetics. Part C, Seminars in Medical Genetics, 182 10.1002/ajmg.c.31788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanus E, van Rijn S, & Swaab H (2020). A review of neurocognitive functioning of children with sex chromosome trisomies: Identifying targets for early intervention. Clinical Genetics, 97(1), 156–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine G (1979). The growth and developmental of 6 XYY children In Robinson A, Lubs H, & Bergsma D (Eds.), Sex chromosomal aneuploidy: Prospective studies on children (pp. 175–190). New York: Alan R Liss. [Google Scholar]
- van Rijn S (2015). Social attention in 47,XXY (Klinefelter syndrome): Visual scanning of facial expressions using Eyetracking. Journal of the International Neuropsychological Society, 21(5), 364–372. [DOI] [PubMed] [Google Scholar]
- van Rijn S (2018). Salivary testosterone in relation to social cognition and social anxiety in children and adolescents with 47,XXY (Klinefelter syndrome). PLoS ONE, 13(7), e0200882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijn S (2019). A review of neurocognitive functioning and risk for psychopathology in sex chromosome trisomy (47,XXY, 47,XXX, 47, XYY). Current Opinion in Psychiatry, 32(2), 79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijn S, Barendse M, van Goozen S, & Swaab H (2014). Social attention, affective arousal and empathy in men with Klinefelter syndrome (47,XXY): Evidence from eyetracking and skin conductance. PLoS ONE, 9(1), e84721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijn S, Barneveld P, Descheemaeker MJ, Giltay J, & Swaab H (2016). The effect of early life stress on the cognitive phenotype of children with an extra X chromosome (47,XXY/47,XXX). Child Neuropsychology, 24(1), 1–10. [DOI] [PubMed] [Google Scholar]
- van Rijn S, Bierman M, Bruining H, & Swaab H (2012). Vulnerability for autism traits in boys and men with an extra X chromosome (47,XXY): The mediating role of cognitive flexibility. Journal of Psychiatric Research, 46(10), 1300–1306. [DOI] [PubMed] [Google Scholar]
- van Rijn S, de Sonneville L, & Swaab H (2018). The nature of social cognitive deficits in children and adults with Klinefelter syndrome (47,XXY). Genes, Brain, and Behavior, 17(6), e12465. [DOI] [PubMed] [Google Scholar]
- van Rijn S, Stockmann L, van Buggenhout G, van Ravenswaaij-Arts C, & Swaab H (2014). Social cognition and underlying cognitive mechanisms in children with an extra X chromosome: A comparison with autism spectrum disorder. Genes, Brain, and Behavior, 13(5), 459–467. [DOI] [PubMed] [Google Scholar]
- van Rijn S, Swaab H, Aleman A, & Kahn R (2006). X chromosomal effects on social cognitive processing and emotion regulation: A study with Klinefelter men (47,XXY). Schizophrenia Research, 84(2–3), 194–203. [DOI] [PubMed] [Google Scholar]
- van Rijn S, Swaab H, Baas D, de Haan E, Kahn RS, & Aleman A (2012). Neural systems for social cognition in Klinefelter syndrome (47,XXY): Evidence from fMRI. Social Cognitive and Affective Neuroscience, 7(6), 689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varrela J, & Alvesalo L (1988). Taurodontism in 47,XXY males: An effect of the extra X chromosome on root development. Journal of Dental Research, 67(2), 501–502. [DOI] [PubMed] [Google Scholar]
- Vawter MP, Harvey PD, & DeLisi LE (2007). Dysregulation of X-linked gene expression in Klinefelter’s syndrome and association with verbal cognition. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics, 144B(6), 728–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva AL, & Rebar RW (1983). Triple-X syndrome and premature ovarian failure. Obstetrics and Gynecology, 62(3 Suppl), 70s–73s. [PubMed] [Google Scholar]
- Visootsak J, Ayari N, Howell S, Lazarus J, & Tartaglia N (2013). Timing of diagnosis of 47,XXY and 48,XXYY: A survey of parent experiences. American Journal of Medical Genetics. Part A, 161A(2), 268–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorsanova SG, Yurov YB, Ulas VY, Demidova IA, Sharonin VO, Kolotii AD, … Soloviev IV (2001). Cytogenetic and molecular-cytogenetic studies of Rett syndrome (RTT): A retrospective analysis of a Russian cohort of RTT patients (the investigation of 57 girls and three boys). Brain and Development, 23(Suppl 1), S196–S201. [DOI] [PubMed] [Google Scholar]
- Walzer S, Bashir A, & Silbert A (1990). Cognitive and behavioral factors in the learning disabilities of XXY and XYY boys. Birth Defects Original Article Series, 26(4), 45–58. [PubMed] [Google Scholar]
- Walzer S, Graham JM Jr., Bashir AS, & Silbert AR (1982). Preliminary observations on language and learning in XXY boys. Birth Defects Original Article Series, 18(4), 185–192. [PubMed] [Google Scholar]
- Webber ML, Puck MH, Maresh MM, Goad WB, & Robinson A (1982). Short communication: Skeletal maturation of children with sex chromosome abnormalities. Pediatric Research, 16(5), 343–346. [DOI] [PubMed] [Google Scholar]
- Wigby K, D’Epagnier C, Howell S, Reicks A, Wilson R, Cordeiro L, & Tartaglia N (2016). Expanding the phenotype of triple X syndrome: A comparison of prenatal versus postnatal diagnosis. American Journal of Medical Genetics. Part A, 170(11), 2870–2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikstrom AM, Hoei-Hansen CE, Dunkel L, & Rajpert-De Meyts E (2007). Immunoexpression of androgen receptor and nine markers of maturation in the testes of adolescent boys with Klinefelter syndrome: Evidence for degeneration of germ cells at the onset of meiosis. The Journal of Clinical Endocrinology and Metabolism, 92(2), 714–719. [DOI] [PubMed] [Google Scholar]
- Williams LA, Pankratz N, Lane J, Krailo M, Roesler M, Richardson M, … Poynter JN (2018). Klinefelter syndrome in males with germ cell tumors: A report from the Children’s oncology group. Cancer, 124(19), 3900–3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xenophontos A, Seidlitz J, Liu S, Clasen LS, Blumenthal JD, Giedd JN, … Raznahan A (2020). Altered Sex Chromosome Dosage Induces Coordinated Shifts in Cortical Anatomy and Anatomical Covariance. Cerebral Cortex, 30, (4), 2215–2228. 10.1093/cercor/bhz235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeger MP, Zinn AR, Lahlou N, Ramos P, Kowal K, Samango-Sprouse C, & Ross JL (2008). Effect of ascertainment and genetic features on the phenotype of Klinefelter syndrome. The Journal of Pediatrics, 152(5), 716–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinn AR, Ramos P, Elder FF, Kowal K, Samango-Sprouse C, & Ross JL (2005). Androgen receptor CAGn repeat length influences phenotype of 47,XXY (Klinefelter) syndrome. The Journal of Clinical Endocrinology and Metabolism, 90(9), 5041–5046. [DOI] [PubMed] [Google Scholar]
- Zitzmann M, Bongers R, Werler S, Bogdanova N, Wistuba J, Kliesch S, … Tüttelmann F (2015). Gene expression patterns in relation to the clinical phenotype in Klinefelter syndrome. The Journal of Clinical Endocrinology and Metabolism, 100(3), E518–E523. [DOI] [PubMed] [Google Scholar]