Highlights
-
•
High demand for diagnostic testing for COVID-19 has depleted commercially available consumables for diagnostic testing.
-
•
Alternative swabs that are not approved for Nasopharyngeal sampling work well for COVID-19 diagnostics.
-
•
Alternative fluids that are readily available in hospital settings can function as alternatives to Viral Transport Media.
-
•
No meaningful difference in viral yield from different swabs and most transport mediums for the collection and detection of SARS-CoV-2, indicating swab and medium alternatives could be used if supplies run out.
Keywords: SARS-CoV-2, Swab, Diagnostic testing
Abstract
On March 11, 2020, the World Health Organization (WHO) assessed COVID-19, caused by SARS-CoV-2, as a pandemic. As of June 1, 2020, SARS-CoV-2 has had a documented effect of over 6 million cases world-wide, amounting to over 370,000 deaths (World Health Organization, 2020. Novel Coronavirus (COVID-19) Situation. http://https://covid19.who.int/). Consequently, the high demand for testing has resulted in a depletion of commercially available consumables, including the recommended swabs and viral transport media (VTM) required for nasopharyngeal sampling. Therefore, the potential use of unvalidated alternatives must be explored to address the global shortage of testing supplies. To tackle this issue, we evaluated the utility of different swabs and transport mediums for the molecular detection of SARS-CoV-2. This study compared the performance of six swabs commonly found in primary and tertiary health care settings (PurFlock Ultra, FLOQSwab, Puritan Pur-Wraps cotton tipped applicators, Puritan polyester tipped applicators, MedPro 6” cotton tipped applicators, and HOLOGIC Aptima) for their efficacy in testing for SARS-CoV-2. Separately, the molecular detection of SARS-CoV-2 was completed from different transport mediums (DMEM, PBS, 100 % ethanol, 0.9 % normal saline and VTM), which were kept up to three days at room temperature (RT). The results indicate that there is no meaningful difference in viral yield from different swabs and most transport mediums for the collection and detection of SARS-CoV-2, indicating swab and medium alternatives could be used if supplies run out.
1. Introduction
In December 2019, a cluster of acute respiratory illnesses were reported in Wuhan, Hubei Province, China, initially classified as ‘pneumonia of unknown etiology’ (Lu et al., 2020). The etiology has since been attributed to a novel positive-sense RNA virus from the Coronaviridae family, named Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), the causative agent of Coronavirus Disease 2019 (COVID-19). The clinical spectrum of SARS-CoV-2 infections can range from asymptomatic/mild, moderate, severe to critical (Lipsitch et al., 2020; Wang et al., 2020). Mild patients present minimal symptoms with no radiographic features; moderate patients exhibit fever, respiratory symptoms and radiographic features; whereas severe patients demonstrate dyspnea, reduced oxygen saturation (<93 %), or reduced PaO2/FiO2 (<300 mmHg); and critical patient meet one of the following criteria: respiratory failure, septic shock or multi-system organ failure (Wang et al., 2020). An important finding related to the global spread of SARS-CoV-2 is that people can be highly infectious and shed virus during the asymptomatic phase (Wei et al., 2020). Due to fast global spread and high morbidity and mortality rates, the World Health Organization (WHO) declared COVID-19 a pandemic. Data provided by the WHO Health Emergency Dashboard (June 1, 2020) shows more than 6 million reported cases and over 370,000 associated deaths have occurred since the beginning of the pandemic, high-lighting the highly infectious nature of SARS-CoV-2 (World Health Organization, 2020).
Most countries established clinical and epidemiological screening criteria to determine if testing a patient for SARS-CoV-2 is warranted. Screening criteria are implemented to ensure diagnostic testing is available for patients who have high pretest probability and for which the results will impact public health management. If screening criteria for testing is met, the WHO recommends collecting samples from the upper respiratory tract, including naso- (NP) or oropharyngeal (OP) swabs. If NP or OP swabs cannot be obtained, lower respiratory tract samples (endotracheal aspirate, expectorated sputum or bronchoalveolar lavage) can be submitted (Centre for Disease Control and Prevention, 2020). In terms of NP collection, the Center for Disease Control and Prevention (CDC) has stated that NP swabs must be completed using synthetic fiber swabs with plastic shafts, due to the deleterious effect that calcium alginate swabs or swabs with wooden shaft may have on virus inactivation or polymerase chain reaction (PCR). The CDC also recommends that all swabs are to be transported in 2−3 mL of viral transport media (VTM) (Centre for Disease Control and Prevention, 2020).
More recently, countries with more liberal testing paradigms, have noted better success in managing this pandemic. While unlimited testing is unrealistic, expanded testing, not limited to individuals with symptoms, those in hospitals, long-term care facilities, travelers, and health workers, have made early recognition, contact tracing, isolation and management more effective. This high demand for testing has depleted key supply stocks, including recommended testing swabs and transport medium. This study aimed to examine the efficacy of six different swabs that are commonly found in hospital settings (PurFlock Ultra, FLOQSwab, Puritan Pur-Wraps cotton tipped applicators, Puritan polyester tipped applicators, MedPro 6” cotton tipped applicators, and HOLOGIC Aptima), along with more readily available alternative transport mediums (DMEM, PBS, 100 % ethanol, 0.9 % normal saline and VTM) for their use in molecular detection of SARS-CoV-2.
2. Methods
2.1. Swab comparisons
The swabs that were tested included: PurFlock Ultra (Puritan Medical Products LLC, USA), FLOQSwab (Copan Diagnotics Inc, Italy), Puritan Pur-Wraps cotton tipped applicators (Puritan Medical Products LLC, USA), Puritan polyester tipped applicators (Puritan Medical Products LLC, USA), MedPro 6” cotton tipped applicators (A.M.G Medical Inc, Canada), and HOLOGIC Aptima (Hologic Inc, USA). PurFlock Ultra and FLOQSwabs Ultra minitips are synthetic flocked swabs; flocked swabs are those that have variable-lengthed adhesive synthetic fibres, suggested to improve patient comfort, expedite maximum liquid uptake and release through nylon capillaries, and increase test sensitivity (Copan Diagnostics, 2020). MedPro and Puritan Pur-Wraps applicators are both cotton tipped swabs, but MedPro comprises a wooden applicator shaft while the Puritan Pur-Wraps swab has a flexible aluminum shaft. HOLOGIC swabs, designed for clinical sampling of endocervical and male urethral specimens for Chlamydia, Gonorrhea, Trichomonas, herpes simplex virus, bacterial vaginosis and mycoplasma, contains a polyester swab with a polystyrene shaft.
To compare the amount of fluid each swab could retain after being dipped and rotated in DMEM, 1200 μL of medium was placed into a 2 mL cryovial and weighed on an analytical scale. Each swab, in its own cryovial, was dipped and rotated making sure all sides of the tip were coated. The swab was then taken out and remaining medium was weighed. Each swab was tested 5 separate times and the mean volume of media retained (μL) was used to help determine virus recovery efficiency (Table 1 ).
Table 1.
Posterior estimates of the mean sampled volume for each swab.
| Swab | Swab Material | Shaft Material | Median (uL) | Lower 95 % HPDI (uL) | Upper 95 % HPDI (uL) |
|---|---|---|---|---|---|
| Puritan 5.5" Cotton Swab | Cotton | Aluminum | 13.4 | 5.1 | 23 |
| Hologic Aptima Multitest | Synthetic (Polyester) |
Polystyrene | 26 | 17.5 | 37 |
| FLOQSwab | Synthetic | Polystyrene | 25 | 20 | 31 |
| PurFLock Ultra | Synthetic | Polystyrene | 115 | 104 | 126 |
| Puritan Standard Polyester Tip | Synthetic (Polyester) |
Polystyrene | 127 | 114 | 141 |
| MedPro Cotton Tipped | Cotton | Wooden | 218 | 202 | 233 |
To test the efficacy of each swab to detect SARS-CoV-2, virus was serially diluted 10-fold in DMEM for concentrations of 5.5 × 105 down to 5.5 × 10−4 PFU/ mL. 500 μL of each virus dilution was then dispensed into separate sterile reagent reservoir trays (Corning Inc, 4870). Each swab tip was submerged into the separate virus dilutions and rotated, ensuring the tip was entirely coated. The swabs were then placed into 2 mL cryovials containing 500 μL plain DMEM until the remainder of the swabs and dilutions were completed and virus inactivation initiated following the protocol outlined below. All swab testing was completed in two biological replicates. As a comparison control, samples from each virus dilution were inactivated and extracted without the use of a swab.
2.2. Transport medium comparisons
To test SARS-CoV-2 viral yield from different transport mediums over time, SARS-CoV-2 was serially diluted into plain DMEM for concentrations of 5.5 × 103 and 5.5 × 101. Subsequently, 500 μL of each virus dilution was then placed into separate sterile reagent reservoir trays (Corning Incorporated, 4870). Using a MedPro 6” cotton tipped applicator (A.M.G Medical Inc, Canada), the tip of the swab was immersed into the virus dilution aliquot and rotated to absorb as much virus dilution as possible. The swab was then placed into a 2 mL cryovial containing 500 μL of either DMEM, PBS, 0.9 % normal saline, 100 % ethanol or VTM and the shaft was broken off to allow capping of the 2 mL cryovial tube. The tips were left in the various transport mediums at RT until time of virus inactivation at 0, 24, 48, and 72 h post-inoculation. Each transport medium time point was completed in two biological replicates. The VTM used in this study was prepared following protocols from Lennette et al. Lennette et al. (1985). As a control comparison for all mediums, 500 μL of the virus dilution was placed into 2 mL cryovials and inactivated and extracted at the same time-points as the swab samples.
2.3. Virus inactivation, RNA extraction, RT-PCR
All experiments with live SARS-CoV-2 were performed in a biosafety level (BSL) 4 laboratory. To remove samples from BSL4 for further analysis, 140 μl of sample was inactivated in 560 μl Buffer AVL for 10 min, then the contents were transferred to a tube containing 560 μl 100 % ethanol for an additional 10 min. RNA was extracted from samples using QIAmp viral RNA Minikit (QIAGEN, Valencia, CA) following manufacturer’s instructions. Primers and probes used to detect SARS-CoV-2 by real time quantitative PCR (RT-qPCR) was based on the E gene (E_Sarbeco_F1: ACAGGTACGTTAATAGTTAATAGCGT, E_Sarbeco_R2: ATATTGCAGCAGTACGCACACA, E_Sarbeco_P1: FAM-ACACTAGCCATCCTTACTGCGCTTCG-BHQ), described by Corman et al. (Corman et al., 2020). RT-qPCR was performed using the Lightcycler 480 RNA Master Hydolysis Probes kit and run on a QuantStudio 5 RT-qPCR system to measure the quantification cycle (Cq).
2.4. Statistics
The data were analyzed using two models. A 4-parameter logistic curve was fit to the dose-Cq data for the different swabs. A linear model was fit to the time-Cq data for the recovery media. The swab absorption volumes are modeled as Student t-distributed, with each swab having its own mean and standard deviation, with shared degrees of freedom. In all cases, the models were fit using a Bayesian framework and sampled using R (Stan Development Team, 2020). The details of each model are provided in the Supplementary statistical analysis.
3. Results
3.1. Swab comparisons
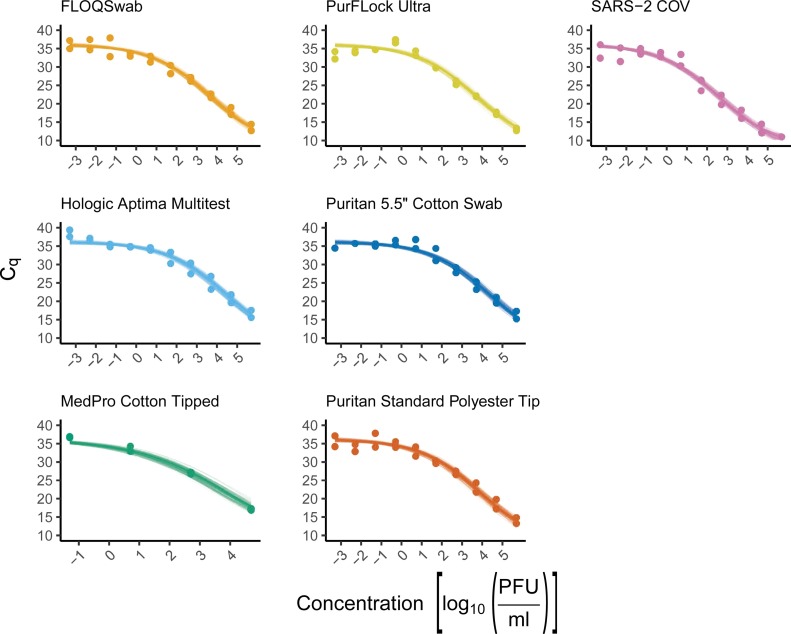
Each swab was dipped in serially diluted SARS-CoV-2 and then broken off into cryovial tubes containing 500 μL DMEM. The viral medium was then inactivated, and the RNA was extracted for analysis by RT-qPCR. Our data shows that all swab types (PurFlock Ultra, FLOQSwab, Puritan Pur-Wraps cotton tipped applicators, Puritan polyester tipped applicators, MedPro 6” cotton tipped applicators, and HOLOGIC Aptima) give similar yields of SARS-CoV-2 RNA when compared to each other at all virus dilutions (Fig. 1 ). We also determined how much volume of media each swab could retain (Table 1) and used this information to calculate the efficacy at which the swabs are able to pick up virus from the dilutions and elute into the media. Although each swab gave similar levels of RNA at each dilution (Fig. 1), the volume that each swab was able to retain and elute varied giving a range in percentage of virus recovery (Table 2 ).
Fig. 1.
Comparison analysis of six different swabs efficacy in detecting SARS-CoV-2 RNA at concentrations from 5.5 × 105 down to 5.5 × 10−4 PFU/ mL. Inactivation and RNA extraction of SARS-CoV-2 virus dilutions (pink) was used as a control. The dark line shows the expected average Cq based on the posterior median of the slope and intercept parameters. The transparent lines present 100 random draws from the posterior distribution, providing a visual estimate of the uncertainty around the mean.
Table 2.
Posterior estimates of the recovery percentage of each swab in DMEM.
| Swab | Material Type | Shaft Material | Median (%) | Lower 95 %HPDI (%) | Upper 95 % HPDI (%) |
|---|---|---|---|---|---|
| Puritan 5.5″ Cotton Swab | Cotton | Aluminum | 68 | 16.2 | 168 |
| Hologic Aptima Multitest | Synthetic (Polyester) |
Polystyrene | 31 | 10.4 | 65 |
| FLOQSwab | Synthetic | Polystyrene | 166 | 60 | 332 |
| PurFLock Ultra | Synthetic | Polystyrene | 42 | 15.9 | 81 |
| Puritan Standard Polyester Tip | Synthetic (Polyester) |
Polystyrene | 27 | 10.0 | 52 |
| MedPro Cotton Tipped | Cotton | Wooden | 22 | 5.9 | 49 |
3.2. Transport medium comparisons
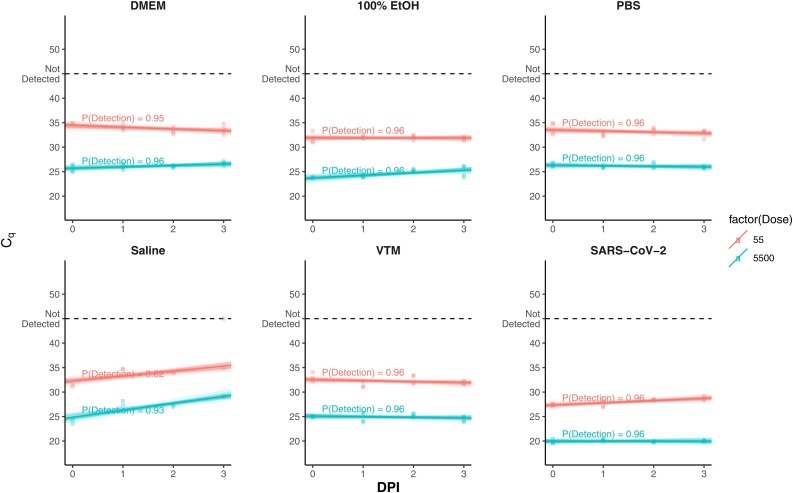
Comparison of the different transport media was done at two virus dilutions, 5.5 × 103, 5.5 × 101 PFU/mL respectively. These concentrations were chosen as they span the upper and lower cycle threshold limits of the assay. SARS-CoV-2 was diluted in DMEM, MedPro 6” cotton tipped applicators were dipped in each dilution and then broken off into 2 mL cryovials containing either DMEM, 100 % ethanol, PBS, 0.9 % normal saline or VTM and left at RT for up to 73 h (3 days). Our results indicate that DMEM, 100 % ethanol, PBS, and VTM eluted and preserved similar amounts of viral RNA for molecular diagnostics at both concentrations (Fig. 2 ). However, 0.9 % Normal Saline did show substantial loss of detectable RNA over time.
Fig. 2.
Quantification cycle for SARS-CoV-2 assessed for 5 different transport mediums (DMEM, Ethanol, PBS, 0.9 % normal saline and VTM) over 72-h at RT. X-axis shows time as day post inoculation (DPI). The dark line shows the expected average Cq based on the posterior median of the slope and intercept parameters. The transparent lines present 100 random draws from the posterior distribution, providing a visual estimate of the uncertainty around the mean.
4. Discussion
Molecular based assays such as RT-qPCR have supplanted viral culture as the reference assay and the most widely used modality for diagnostics of viral respiratory pathogens such as SARS-CoV-2. However, effective specimen collection and transport to centralized laboratories is required, and as exemplified by the current COVID-19 pandemic, recommended materials may be limited. This study addressed the issue of the shortage of swabs and transport mediums by evaluating unvalidated alternatives. We found little variation between different swabs and media for the molecular detection of SARS-CoV-2. We were unable to find other studies comparing different types of swabs and transport media for the efficacy of detecting for SARS-CoV-2 under controlled conditions.
Comparison of different swab types have been completed in the past for other respiratory viruses. NP and OP testing using nylon swabs or rayon, a material similar to cotton, has shown that nylon swabs show better viral genome yield compared to rayon swabs (Daley et al., 2006; Hernes et al., 2011). Another study assessing cotton versus flocked swabs found that both retained virus at room temperature for 15 days, allowing for successful extraction and detection; however, the flocked swab did allow for consistently higher yields (Moore et al., 2008).
For all respiratory sampling, including SARS-CoV-2, the CDC does not recommend the use of cotton or wood shafted swabs as it is difficult to elute virus and the wooden shaft can absorb elution buffer, thereby inhibiting PCR (Centre for Disease Control and Prevention, 2020, 2018). We, however, did not observe any significant decrease in SARS-CoV-2 yield with the use of wooden shafted cotton swabs compared to a synthetic fiber swab (Fig. 1). Therefore, our results suggest that the cotton and wood shafted swabs could be an appropriate specimen collection alternative if stocks of other swabs are unavailable.
For the portion of the study focusing on alternative transport media, we assessed the ability of DMEM, PBS, 0.9 % Normal Saline, and 100 % ethanol compared to VTM to be used as medium for the preservation and recovery of viral RNA to be quantified by molecular detection. Our results indicate that all media, with the exception of 0.9 % saline, preserved similar amounts of SARS-CoV-2 RNA for extraction and detection over 72 h (Fig. 2). Although our study focused on if virus yield decreased over time, it should be noted that there are slight differences in the virus recovery when comparing the different mediums to each other. From this data it seems that 100 % ethanol has the best virus recovery while still maintaining stable viral RNA levels over 72 h. This is possibly due to its denaturing effects on the virus, changing the way it interacts with the fibers of the swab allowing it to elute into the transport medium at a higher efficiency. These results indicate the 100 % ethanol may be the best recommended transport medium in the event that viral transport medium stocks are depleted. Supporting the results of our study, another study compared VTM to saline, Universal Transport Medium (UTM), liquid Amies medium and Amies gel containing charcoal, finding slight differences in yield but resulting in all media able to give amplifiable product over 7 days (Druce et al., 2012). Additionally, ethanol-based transport medium (CyMol) has been studied, finding that it preserved influenza viral RNA for at least 21 days at temperatures from 4 °C to RT (Luinstra et al., 2011).
To ensure virus-spiked fluid is acceptable to use to recapitulate actual human samples (for retention and release as well as for the presence of PCR inhibitors), we also tested the most relevant biological fluid (NP swab sample obtained from a volunteer). This was chosen since, NP samples are widely regarded as the ‘gold-standard’ in most labs/testing facilities. To this end, we have tested control NP swab samples spiked with SARS-CoV-2 and found that the addition of the NP swab sample made no difference to the RT-qPCR results when compared to similarly spiked control samples lacking the human NP swab sample (unpublished data).
It should also be noted that this study did not test the viability of the different swab collections and transport media alternatives to retain culturable virus. While 100 % ethanol precludes culture, the other recovery media may not be able to deal with potential bacterial, fungal, protease and nuclease contamination that may be associated with samples from the respiratory tract. This may be an important consideration as this outbreak moves into a different phase that may rely on determining infectious risks of patients and timing of disease onset/recovery (Bullard et al., 2020).
Despite finding similar levels of viral RNA collected using different swabs and transport media, there is variation when evaluating different respiratory clinical samples while testing for SARS-CoV-2. Comparing NP swabs, OP swabs and sputum at different points in infection, studies have found that sputum samples generally showed the highest viral loads (Lin et al., 2020; Pan et al., 2020). These conclusions were also seen from a detailed study which found that 14/15 bronchoalveolar lavage (BAL) and 75/104 sputum samples were positive for SARS-CoV-2, compared to 5/8 nasal swabs, 6/13 brush biopsies and 126/398 pharyngeal swabs (Kaul, 2020). Another study found that sputum induction was more sensitive in detection of SARS-CoV-2 RNA when compared to throat swabs in convalescent patients (Han et al., 2020). However, it should be noted that most jurisdictions are discouraging induced sputum due to the generation of aerosols and potential associated spread. Comparing NP and OP swabs for SARS-CoV-2 molecular diagnostics, it has been found that NP swabs gave significantly stronger positive tests than OP swabs from the same patient from day 0 to day +15 of symptom onset (Yang et al., 2020). Alternatively, a study evaluating 9 SARS-CoV-2 patients in Germany found that both NP and OP swabs gave similar PCR-positive results on days 1–5 post symptom onset (Woelfel et al., 2020).
It is of paramount importance to identify different swab and transport medium alternatives for the molecular detection of SARS-CoV-2 as supplies are limited during this unprecedented pandemic. Our observations suggest that, with the exception off 0.9 % saline, the collection swabs and transport medium types we tested are appropriate options for collection, transport and preservation of SARS-CoV-2 RNA for molecular detection. It should be noted that a major limitation of this study is not testing these alternatives on actual patients or human volunteers. Our artificial swabbing environment compared to swabbing epithelial tissue in patients may abrogate the benefits of flocked swabs which are optimized with brush-like textures for efficient dislodging of epithelial target cells (Copan Diagnostics, 2020). To address this, future studies could involve testing swab efficacy through an animal model or involve ethics approval and clinical trials to prove the efficacy of these swabs and transport medium in a clinical setting. Select provinces in Canada have already determined alternate collection kits, such as HOLOGIC Aptima, for NP sampling for SARS-CoV-2 if needed during shortages, therefore validation in the clinical setting is achievable (Public Health Ontario, 2020). Investigation of different swab and transport mediums from patient samples would give a more accurate representation of their efficacy to collect SARS-CoV-2 samples leading to its detection.
Funding
Funding was provided by the Canadian Institutes of Health Research (CIHR - Operating Grant FRN:143481, Canada) and the Government of Canada.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jviromet.2020.113947.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Bullard J., Dust K., Funk D., Strong J.E., Alexander D., Garnett L., Boodman C., Bello A., Hedley A., Schiffman Z., Doan K., Bastien N., Li Y., Van Caeseele P.G., Poliquin G. Predicting infectious SARS-CoV-2 from diagnostic samples. Clin. Infect. Dis. 2020:1–18. doi: 10.1093/cid/ciaa638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centre for Disease Control and Prevention . 2018. Collecting Specimens for Varicella Zoster Virus (VZV) Testing. [Google Scholar]
- Centre for Disease Control and Prevention . 2020. Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens from Persons for Coronavirus Disease 2019 (COVID-19) [WWW Document] URL https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html (Accessed 3.24.20) [Google Scholar]
- Copan Diagnostics . 2020. FLOQSwab [WWW Document] [Google Scholar]
- Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., Bleicker T., Brünink S., Schneider J., Schmidt M.L., Mulders D.G., Haagmans B.L., van der Veer B., van den Brink S., Wijsman L., Goderski G., Romette J.L., Ellis J., Zambon M., Peiris M., Goossens H., Reusken C., Koopmans M.P., Drosten C. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25:1–8. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley P., Castriciano S., Chernesky M., Smieja M. Comparison of flocked and rayon swabs for collection of respiratory epithelial cells from uninfected volunteers and symptomatic patients. J. Clin. Microbiol. 2006;44:2265–2267. doi: 10.1128/JCM.02055-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druce J., Garcia K., Tran T., Papadakis G., Birch C. Evaluation of swabs, transport media, and specimen transport conditions for optimal detection of viruses by PCR. J. Clin. Microbiol. 2012;50:1064–1065. doi: 10.1128/JCM.06551-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H., Luo Q., Mo F., Long L., Zheng W. SARS-Cov-2 RNA more readily detected in induced sputum than in throat swabs of convalescent COVID-19 patients. Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30174-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernes S.S., Quarsten H., Hagen E., Lyngroth A.L., Pripp A.H., Bjorvatn B., Bakke P.S. Swabbing for respiratory viral infections in older patients: a comparison of rayon and nylon flocked swabs. Eur. J. Clin. Microbiol. Infect. Dis. 2011;30:159–165. doi: 10.1007/s10096-010-1064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul D. Pharyngeal and nasal swabs may not have adequate sensitivity for SARS-CoV-2. New Engl. J. Med. J. Watch. 2020;323(18):1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [Google Scholar]
- Lennette D.A. Collection and preparation of specimens for virological examination. In: Lennette E.H., Balows W.J., Hausler J., Shadomy H.J., editors. Manual of Clinical Microbiology. American Society for Microbiology; Washington, D.C: 1985. pp. 687–693. [Google Scholar]
- Lin C., Xiang J., Yan M., Li H., Huang S., Shen C. medRxiv; 2020. Comparison of Throat Swabs and Sputum Specimens for Viral Nucleic Acid Detection in 52 Cases of Novel Coronavirus (SARS-Cov-2) Infected Pneumonia (COVID-19) 2020.02.21.20026187. [DOI] [PubMed] [Google Scholar]
- Lipsitch M., Swerdlow D.L., Finelli L. Defining the epidemiology of Covid-19 - studies needed. N. Engl. J. Med. 2020 doi: 10.1056/NEJMp2002125. [DOI] [PubMed] [Google Scholar]
- Lu H., Stratton C.W., Tang Y.W. Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. J. Med. Virol. 2020;92:401–402. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luinstra K., Petrich A., Castriciano S., Ackerman M., Chong S., Carruthers S., Ammons B., Mahony J.B., Smieja M. Evaluation and clinical validation of an alcohol-based transport medium for preservation and inactivation of respiratory viruses. J. Clin. Microbiol. 2011;49:2138–2142. doi: 10.1128/JCM.00327-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore C., Corden S., Sinha J., Jones R. Dry cotton or flocked respiratory swabs as a simple collection technique for the molecular detection of respiratory viruses using real-time NASBA. J. Virol. Methods. 2008;153:84–89. doi: 10.1016/j.jviromet.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Pan Y., Zhang D., Yang P., Poon L.L.M., Wang Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect. Dis. 2020;20:411–412. doi: 10.1016/S1473-3099(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Public Health Ontario . 2020. Coronavirus Disease 2019 (COVID-19) Testing [WWW Document] [Google Scholar]
- Stan Development Team . 2020. The R Interface to Stan. R Package Version 2.19.3 [WWW Document] URL http://mc-stan.org/ (Accessed 6.1.20) [Google Scholar]
- Wang Yixuan, Wang Yuyi, Chen Y., Qin Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J. Med. Virol. 2020 doi: 10.1002/jmv.25748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W.E., Li Z., Chiew C.J., Yong S.E., Toh M.P., Lee V.J. Presymptomatic transmission of SARS-CoV-2-Singapore. Morb. Mortal. Wkly. Rep. 2020;69:411–415. doi: 10.15585/mmwr.mm6914e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woelfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Mueller M.A., Niemeyer D., Vollmar P., Rothe C., Hoelscher M., Bleicker T., Bruenink S., Schneider J., Ehmann R., Zwirglmaier K., Drosten C., Wendtner C. medRxiv; 2020. Virological Assessment of Hospitalized Cases of Coronavirus Disease 2019. 2020.03.05.20030502. [DOI] [Google Scholar]
- World Health Organization . 2020. Novel Coronavirus (COVID-19) Situation [WWW Document] URL https://covid19.who.int/ (Accessed 3.24.20) [Google Scholar]
- Yang Y., Yang M., Shen C., Wang F., Yuan J., Li Jinxiu, Zhang M., Wang Z., Xing L., Wei J., Peng L., Wong G., Zheng H., Liao M., Feng K., Li Jianming, Yang Q., Zhao J., Zhang Z., Liu L., Liu Y. medRxiv; 2020. Evaluating the Accuracy of Different Respiratory Specimens in the Laboratory Diagnosis and Monitoring the Viral Shedding of 2019-nCoV Infections. 2020.02.11.20021493. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.