Abstract
Purpose
We examined inferior oblique muscles from subjects with over-elevation in adduction for characteristics that might shed light on the potential mechanisms for their abnormal eye position.
Methods
The inferior oblique muscles were obtained at the time of surgery in subjects diagnosed with either primary inferior oblique overaction or Apert syndrome. The muscles were frozen and processed for morphometric analysis of myofiber size, central nucleation, myosin heavy chain (MyHC) isoform expression, nerve density, and numbers of neuromuscular junctions per muscle section.
Results
The inferior oblique muscles from subjects with Apert Syndrome were smaller, and had a much more heterogeneous profile relative to myofiber cross-sectional area compared to controls. Increased central nucleation in the Apert syndrome muscles suggested on-going myofiber regeneration or reinnervation over time. Complex changes were seen in the MyHC isoform patterns that would predict slower and more sustained contractions than in the control muscles. Nerve fiber densities were significantly increased compared to controls for the muscles with primary inferior oblique overaction and Apert syndrome that had no prior surgery. The muscles from Apert syndrome subjects as well as those with primary inferior oblique overaction with no prior surgery had significantly elevated numbers of neuromuscular junctions relative to the whole muscle area.
Conclusions
The muscles from both sets of subjects were significantly different from control muscles in a number of properties examined. These data support the view that despite similar manifestations of eye misalignment, the potential mechanism behind the strabismus in these subjects is significantly different.
Keywords: strabismus, extraocular muscle, inferior oblique, inferior oblique overaction, Apert syndrome, neuromuscular junctions
Strabismus is a complex disorder of manifest or latent misalignment of the eyes. Eye movements are controlled by the ocular motor system in the brain, and the final common pathway ends at the six pairs of extraocular muscles (EOMs) that attach to the eyes and control all the eye movements. The etiology of strabismus when not associated with anisometropia or muscle paralysis from identified nerve or brain injury is not well understood. Part of the problem in assessing the potential cause of idiopathic childhood onset strabismus is the increasing evidence that despite a similar abnormal eye position, the underlying cause of the overt strabismus is not always the same.
Strabismus involving the inferior oblique muscles serves as a good example of this problem. The main role of the inferior oblique muscle is to elevate the eye when it is adducted, and additionally to extort the eyes. One form of strabismus is excessive elevation of the eye in adduction, which is a relatively common form of eye misalignment.1,2 There are a variety of potential etiologies in individuals with over-elevation in adduction, making correct diagnosis critical for effective surgical alignment of the eyes.3,4 In addition to what has been termed primary inferior oblique overaction,5 which is presumed to be an inferior oblique that is “too strong” and thus “overacting”3,4 or a superior oblique that is “too weak” or “underacting,” over-elevation in adduction is associated with a number of associated conditions.5–13 It should be noted that in individuals with Apert syndrome, imaging studies suggest that bony orbital features and EOM heterotopy also produce excyclorotation.14,15
If a decision is made to proceed with surgery to improve eye alignment in a patient with over-elevation in adduction, a variety of surgical approaches can be used to correct this, including muscle recession,16 various forms of muscle transpositions,17–19 temporal or nasal inferior oblique myectomy,20–23 and fixation of the inferior oblique to the orbital wall.24 Unfortunately, recurrence of the over-elevation in adduction is a common problem in these patients, and is often difficult to predict.4,8,22,25,26
A number of studies appeared in the 1970s and 1980s that examined EOMs from patients with a variety of strabismus diagnoses; including those with Duane's syndrome, Brown's syndrome, nystagmus, and nerve paralysis.27–30 These studies described a number of “unusual findings.” What is particularly interesting about these early studies is their focus on electron microscopic analysis. In the past 2 decades, we have learned a great deal about normal EOM cell biology and physiology, reviewed in McLoon et al.31 For example, we now understand that normal EOMs remodel continuously throughout life and thus contain a population of activated satellite cells.32–34 Interestingly, recent studies showed an increased number of activated satellite cells in muscles from subjects with strabismus compared to normal control EOMs.35,36 The normal skeletal muscle fiber type distinctions do not apply to the EOM,37,38 and aspects of mitochondrial and calcium biology,39,40 as well as other aspects of metabolism, and physiology differ significantly when the EOMs are compared to limb and body skeletal muscles. Thus, despite a long history of surgical treatment for this type of eye misalignment in children, we still do not understand the molecular and cellular biological underpinnings of strabismus in general, and, more specifically, over-elevation in adduction.
In this study, the inferior oblique muscles from subjects who were being treated by surgical myectomy for over-elevation in adduction were examined histologically, and various parameters were analyzed morphometrically in a masked fashion. After analysis, the data from individual muscles were grouped based on the diagnosis of primary inferior oblique overaction (IOOA) or subjects with Apert Syndrome and compared to control inferior oblique muscles.
Methods
The inferior oblique muscles were collected as surgical waste specimens during normal surgery for correction of over-elevation in adduction, and were described in detail previously.41 These studies were approved by the Institutional Review Board of the University of Texas Southwestern Medical Center, including review of human subjects’ medical records in compliance with the guidelines of the Health Insurance Portability and Accountability Act (HIPAA). This study adhered to the tenets of the Declaration of Helsinki.
All surgical waste specimens were collected from patients scheduled for myectomy of one or both inferior oblique muscles for primary or secondary over-elevation in adduction. Control muscles were collected either at the time of enucleation during Eye Bank tissue harvesting or at the time of enucleation for other diagnoses. None of the control subjects received cytotoxic drugs or radiation. Only one muscle per subject was used for the analyses performed, selected randomly if two muscle specimens had been collected. For the control muscles and for the muscles from subjects for whom it was a first surgery, all were removed from the more temporal portion of the length of the inferior oblique. Control muscles were obtained from this same location. For the subjects for whom the muscle specimens were from a second surgery, all were obtained using a nasal myectomy procedure. Studies have examined changes along the length of EOMs in a number of species,42–44 including humans.45 These studies show that the EOMs are very similar to each other in the insertional and origin regions, but quite different in the mid-region of the muscles.45 Thus, we worked under the assumption that comparisons are reasonable based on these known biochemical and structural similarities in these regions.
The following muscle samples were collected and described in detail previously41: nine muscles from subjects with primary inferior oblique overaction with no prior inferior oblique surgery (IOOA NPS) with a median age of 6.9 +/− 4.9 years, 7 samples from patients with recurrent inferior oblique overaction after previous surgery (IOOA) with a median age of 7.3 +/− 5.1 years, 6 specimens from subjects with Apert syndrome and over-elevation in adduction and no prior inferior oblique surgery (Apert NPS) with a median age of 4.4 +/− 2.1 years, 2 specimens from patients with Apert syndrome re-operated after recurrent over-elevation in adduction (Apert) with a median age of 6 years (range 4–8 years), and 9 control samples removed by an ophthalmologist at the time of enucleation or eye donation with a median age of 25.2 +/− 28 years. In the control group, there were inferior oblique muscles from five children and four adults. All variables were compared between these two age groups within the control group, and none of the parameters differed significantly based on dividing the muscles into these categories. Thus, all the control muscle data were averaged. The control subjects had no history of strabismus, and none had prior EOM surgery. Details of the severity of patient over-elevation in adduction have been described in detail previously.41
The inferior oblique muscles were embedded in tragacanth gum, frozen in 2-methylbutane chilled on liquid nitrogen, and stored frozen at −80 deg Celsius (°C) until processed. The frozen inferior oblique muscles were sectioned at 12 µm and stored at −30o. One set of sections was stained with hematoxylin and eosin, which were used to determine total myofiber number as well as the percent of centrally nucleated myofibers in the muscle samples. All morphometric analyses were performed masked relative to the subject from which it was obtained. The total myofiber number was determined by counting every muscle fiber in each cross-section. The percentage of central nucleation was determined by counting all myofibers with a centrally located nucleus as a percent of all the myofibers counted in each field. Three sections were counted for each sample and averaged to determine a mean for that patient/muscle. A minimum of 200 myofibers were counted per muscle cross-section, and 4 cross-sections were quantified spread along the length of each muscle sample. Mean myofiber cross-sectional areas were determined by manually circling a minimum of 200 myofibers per muscle section, with 3 sections analyzed per muscle specimen. All sections were examined using a Leica microscope equipped with a video camera, and this interfaced with a computer-based morphometry program (Bioquant, Nashville, TN, USA).
Representative samples of inferior oblique muscle cross-sections were immunostained for myosin heavy chain (MyHC) isoform expression as well as density of nerves and neuromuscular junctions. The sections were rinsed in PBS, followed by a 1-hour incubation with primary antibody against the following (MyHC) isoforms: pan fast (1:40, Vector Laboratories, Burlingame, CA, USA), slow twitch (1:40, MyHCI, Vector Laboratories) and the following from the Hybridoma Bank (University of Iowa, Ames, IA, USA): fast IIA (1:20; MyHCIIA, SC-71); fast IIX (1:20; MyHCIIX, 6H1); embryonic (1:20, MyHCemb, F1.652); neonatal (1:20, MyHCemb, F1.652); slow tonic 1:20, MyHCslowtonic S46), and EOM specific (1:5–1:10; MyHCeom, 4A6). The tissue sections were air-dried, and incubated in control serum for 15 minutes, followed by incubation in primary antibody for 1 hour at room temperature. The sections were rinsed in PBS and incubated using the Vectastain Elite mouse ABC peroxidase Elite kit (Vector Laboratories). For visualization, the reacted tissue sections were incubated in a diaminobenzidine solution containing heavy metals. Specificity of antibody binding was verified by immunostaining sections in the absence of primary antibody. The immunostained serial sections were examined microscopically and photographed for quantification.
For nerve fiber identification, the SMI-31 antibody was used (Covance, Princeton, NJ, USA; dilution, 1:1000). The slides were rinsed and incubated with the Vectastain horseradish peroxidase labeling kit. After rinsing in PBS, they were reacted with the heavy metal intensified diaminobenzidine procedure, dehydrated, and coverslipped for analysis. The percent positive for each MyHC isoform was determined based on total fiber number per microscope field for the orbital and global layers of all muscles, reported as a percent of all myofibers counted. A minimum of 250 myofibers were counted per section analyzed, and sections were analyzed along the length of each muscle specimen. For nerve density calculations, thresholds of staining intensity were used to determine all the nerve fiber bundles in micrometers (µm)2 and the total muscle area per field was measured. Nerve fiber density was calculated as a percent of muscle area.
An additional series of slides were immunostained for neuromuscular junctions and nerve fibers using the SMI-31 protocol as described followed by incubation with alpha-bungarotoxin conjugated to AlexaFluor 488 overnight (Invitrogen, Carlsbad, CA, USA). Sections were washed and mounted with Vectashield (Vector Laboratories). All neuromuscular junctions in entire muscle cross-sections were counted, and the area of the muscle cross-section determined, resulting in the number of neuromuscular junctions per muscle area in µm2. An average of three sections per subject were measured, and the means of each subject were used to calculate the overall mean of neuromuscular junction density.
Data were examined statistically using analysis of variance (ANOVA) followed by post hoc tests of significance. These were performed using Prism software (GraphPad, La Jolla, CA, USA), with significance at P < 0.05.
Results
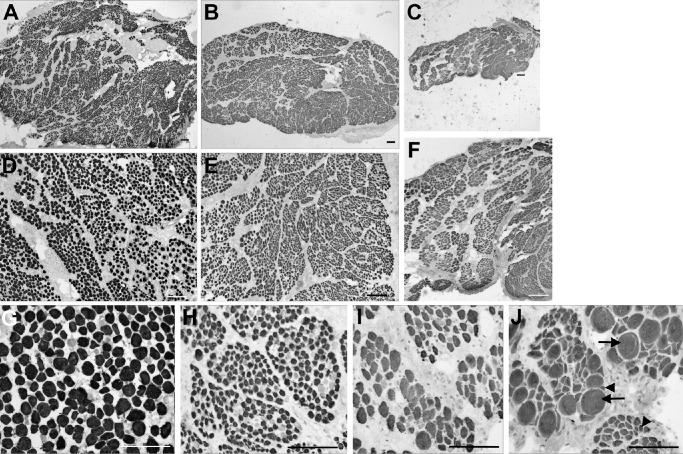
Pronounced differences were seen both qualitatively and quantitatively upon histological examination of the muscle cross-sections of subjects diagnosed with IOOA, Apert syndrome, and the control muscles (Fig. 1). As is well known, the control inferior oblique muscles had a distinct layer of smaller fibers, defining the orbital layer, and larger myofibers, defining the global layer. The myofibers were heterogeneous in size, with loose connective tissue between neighboring myofibers (Figs. 1A, 1D, 1G). The inferior oblique muscles from subjects with over-elevation in adduction showed considerable variability from subject to subject, and several distinct differences were observed (see Fig. 1). In the inferior oblique muscle from one typical subject diagnosed with IOOA, the specimen had extremely small myofibers, and the global and orbital layers could not be distinguished by myofiber size alone (Figs. 1B, 1E). In a muscle from a subject diagnosed as having Apert syndrome, the entire muscle was small, with an obvious reduction in total fiber number (Figs. 1C, 1F). At higher power in an inferior oblique from a subject with Apert syndrome, there was an extreme heterogeneity of myofiber cross-sectional areas from very small to very large and hypertrophic (Figs. 1H–J). Total myofiber numbers were determined for inferior oblique muscles from subjects diagnosed with primary IOOA and Apert syndrome compared to control muscles (Fig. 2). The inferior oblique muscles from subjects with IOOA had significantly more myofibers in the orbital layer, with the global layer similar to controls, albeit trending toward significant difference. However, both orbital and global layers in inferior oblique muscles from subjects with Apert syndrome had significantly fewer total myofiber numbers compared to controls and compared to muscles from subjects with IOOA and controls (see Fig. 2). No significant differences were seen between those specimens that were from subjects with no prior surgery or had prior surgery (data not shown).
Figure 1.
Photomicrographs of inferior oblique muscles immunostained with an antibody to pan-fast myosin heavy chain isoform, which labels the majority of myofibers in EOM. Control muscles (A, D, G), muscle from a subject diagnosed with inferior oblique overaction (B, E, H), and muscle from a subject diagnosed with Apert syndrome (C, F, I, J). Photographs D to J are from the global layer of the muscles. Control inferior oblique muscle (A, D, G), muscle from subject diagnosed with inferior oblique overaction (B, E, H), and muscle from subject with Apert syndrome (C, F, I, J). There are extremely hypertrophic myofibers (arrows) as well as severely atrophic fibers (arrowheads). The scale bar is 200 µm for each image.
Figure 2.
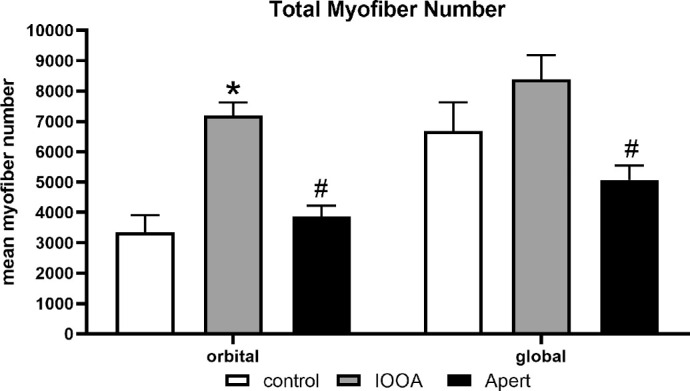
Mean total fiber number in inferior oblique muscles from control, inferior oblique muscles from subjects diagnosed with inferior oblique overaction (IOOA), and muscle from subjects diagnosed with Apert syndrome (Apert). Data represent the mean ± SEM. The asterisk indicates significant difference from control. (F = 11.3; Control orbital compared to IOOA orbital P = 0.001). The hashtag indicates significant difference from the muscles from subjects with primary inferior oblique overaction (orbital IOOA versus Apert P = 0.0025; global IOOA versus Apert P = 0.003).
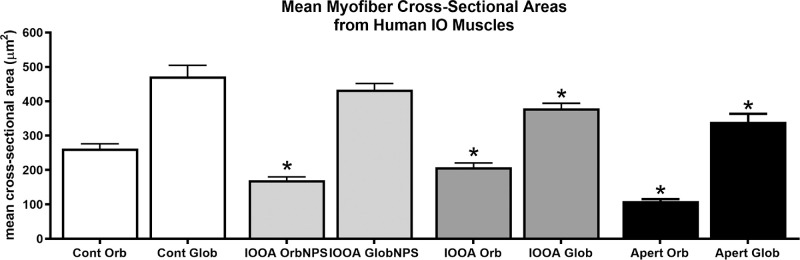
For the analysis of mean myofiber cross-sectional areas, muscles were divided into three groups: Apert syndrome, IOOA with no prior surgery (NPS), and IOOA where a prior surgery on the inferior oblique has been performed. The mean cross-sectional areas were determined for the orbital and global layers of each of these three groups of subjects and compared to control muscles (Fig. 3). The orbital layer fibers were significantly smaller in the muscles from the IOOA and Apert syndrome subjects, 21% and 82% smaller, respectively, than those of the control muscles. The global layer fibers were also significantly 32.5% smaller in the muscles from the Apert syndrome subjects than the global layer myofibers from the control muscles.
Figure 3.
Mean myofiber cross-sectional area for the muscles from subjects with primary inferior oblique overaction (IOOA) with no prior surgery (NPS) or prior surgery and Apert syndrome (Apert) compared to control muscles. Asterisks indicate significant difference from control muscle. (F = 6.884; *orbital control versus orbital IOOA NPS P = 0.0001; orbital control versus IOOA orb P = 0.007; orbital control versus orbital Apert P = 0.0001; global control versus global IOOA P = 0.009; global control versus global Apert P = 0.003.)
The unusual appearance of many of the muscle specimens from the IOOA and Apert syndrome subjects prompted an examination of centrally nucleated myofibers. Central nucleation of skeletal muscle fibers is a hallmark of degeneration and regeneration or denervation and reinnervation. The control inferior oblique muscles contained few centrally nucleated myofibers (Fig. 4), with approximately 0.92% ± 0.1% and 0.48% ± 0.06% in the orbital and global layers. In the orbital layer and global layers from the inferior oblique muscles from IOOA subjects with no prior surgery or with prior surgery, central nucleation was significantly elevated. In the orbital layer, central nucleation was 4- to 8-fold higher than the control muscle for both groups of subjects and 4-fold higher in the global layers. In the muscles from subjects with Apert syndrome, central nucleation was five-fold higher than control in the orbital layers and four-fold higher in the global layers.
Figure 4.
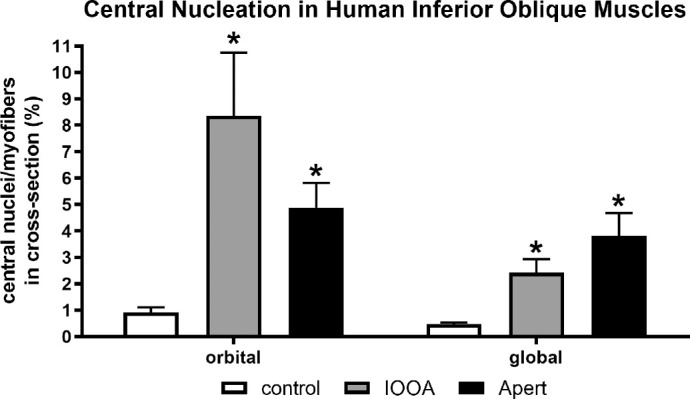
Central nucleation for the muscles from subjects with primary inferior oblique overaction (IOOA) with no prior surgery (NPS) or prior surgery and Apert syndrome (Apert) compared to control muscles. Asterisks indicate significant difference from control muscles. (F = 5.025; P = 0.0001 compared to control.)
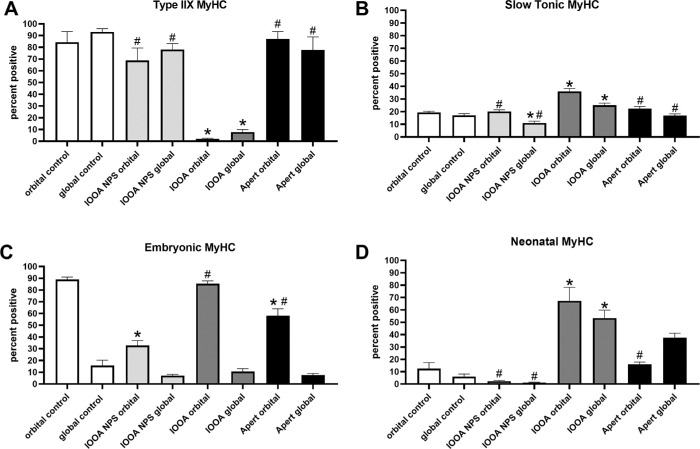
Shortening velocity is, in part, controlled by MyHC isoform expression patterns.46,47 Of the eight MyHC isoforms examined, only four showed significant changes in the examined muscles (Fig. 5, Supplementary Fig. S1). In the orbital layer of the muscles from those with IOOA and with Apert syndrome and no prior surgery, a significant decrease in myofibers positive for embryonic MyHC isoform was seen. With prior surgery, the muscles from subjects with IOOA showed a significant increase compared to those without prior surgery (Fig. 5C). For neonatal MyHC expression, there was a significant increase in expression in both sets of subjects in the muscles where a previous surgery had been performed (Fig. 5D). In all cases, there was a significant reduction in type IIX-positive myofibers (Fig. 5A), and an increase in numbers of fibers positive for the slow tonic MyHC isoform (Fig. 5B). Embryonic and neonatal MyHC isoforms would be expected to produce slower shortening velocities, which would be further slowed with reduction in the fast type IIX isoform. The increase in slow tonic MyHC expression would result in a muscle that maintained a tonic contraction over a longer period of time compared to the normal twitch contraction profiles.
Figure 5.
Myosin heavy chain (MyHC) isoform expression patterns in inferior oblique muscles from children with primary inferior oblique overaction (IOOA), Apert syndrome (Apert), and controls immunostained for (A) IIX (F = 18.23; * orbital control versus orbital IOOA P = 0.0001; global control versus global IOOA P = 0.0001; # orbital IOOA versus orbital IOOA NPS P = 0.0005; orbital IOOA versus orbital Apert P = 0.0001; global IOOA versus global IOOA NPS P = 0.0001; global IOOA versus global Apert P = 0.0001), (B) slow tonic (F = 22.87; * orbital control versus orbital IOOA NPS P = 0.02; orbital control versus orbital IOOA P = 0.0001; global control versus global IOOA NPS P = 0.02; # orbital IOOA versus orbital IOOA NPS P = 0.0001; orbital IOOA versus orbital Apert P = 0.0002; global IOOA versus global IOOA NPS P = 0.0001; global IOOA versus global Apert P = 0.03), (C) embryonic (F = 32.43; * orbital control versus orbital IOOA NPS P = 0.0001; * orbital control versus orbital Apert P = 0.0003; # orbital IOOA NPS versus orbital IOOA P = 0.0001, orbital IOOA NPS versus orbital Apert P = 0.000), and (D) neonatal (F = 29.08; * orbital control versus orbital IOOA P = 0.0001; global control versus global IOOA P = 0.0001; global control versus global Apert P = 0.0003; # orbital IOOA versus orbital IOOA NPS P = 0.0001; orbital IOOA versus orbital Apert P = 0.0006; global IOOA versus global IOOA NPS P = 0.0001). Asterisks indicate significant difference from control muscle. Hashtag indicates significant difference from the IOOA muscles.
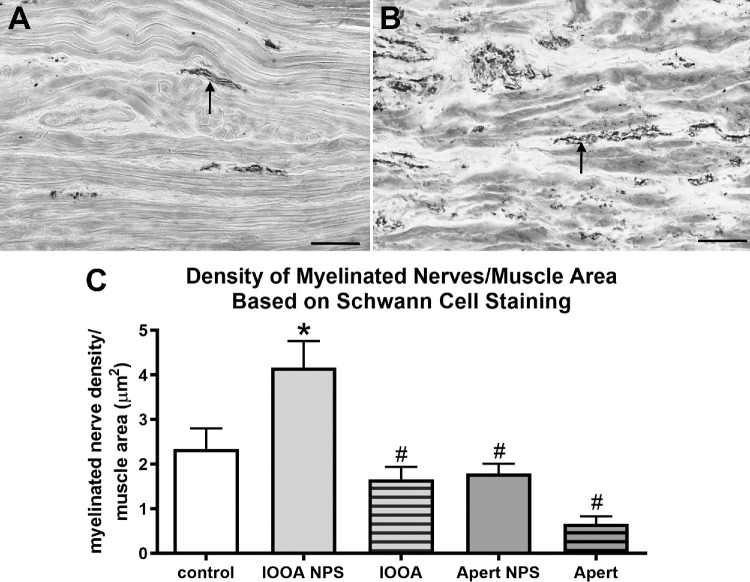
A prominent view for conditions like over-elevation in adduction is the suggestion that these muscles are “too strong.” As a result, we examined the density of myelinated nerves in the muscles using an antibody to Schwann cells (Fig. 6). In the muscles from subjects with no prior surgery and inferior oblique overaction, their overall density of myelinated nerves was increased by 78.2% over controls. Interestingly, surgery resulted in a significant drop over the “no prior surgery” muscles, resulting in no difference from control muscles, and a decrease of 66.2% over the non-operated muscles. Both non-operated and previously operated muscles from subjects with Apert syndrome had levels similar to controls and significantly decreased compared to the non-operated IOOA muscles.
Figure 6.
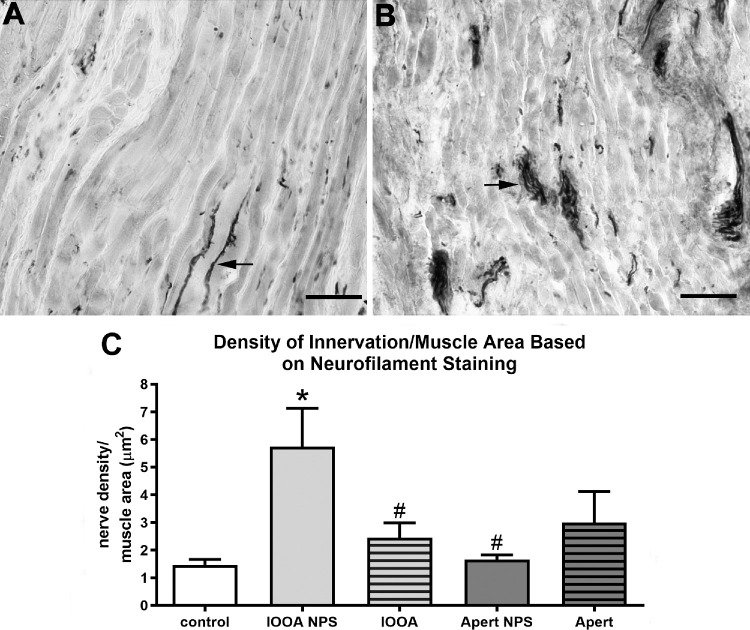
Myelinated nerves as a percent of muscle area based on Schwann cell immunostaining. (A) Inferior oblique muscle from a control subject. (B) Inferior oblique muscle from a subject with primary inferior oblique overaction (IOOA) and no prior surgery (NPS). (C) Percent of myelinated nerves per muscle area after immunostaining for Schwann cells. Bar is 100 µm. Asterisks indicate significant difference from control muscle. (F = 3.233; control versus IOOA NPS: P = 0.029). Hashtag indicates significant difference from the muscles from subjects with primary IOOA with NPS. (IOOA NPS versus IOOA P = 0.0001; IOOA NPS versus Apert NPS P = 0.015; IOOA NPS versus Apert P = 0.0001.)
Similarly, when total nerve fiber density was assessed using immunostaining for neurofilament protein, the muscles from non-operated subjects with IOOA and Apert syndrome had an almost six-fold increase in nerve density compared to controls (Fig. 7). For the muscles from subjects with IOOA who had prior surgery, the nerve levels were similar to the muscles without prior surgery and were still almost five-fold greater in the amount of nerve tissue per muscle area than the control muscles. In subjects with Apert syndrome, the effect of prior surgery was to increase the amount of nerves in the muscle by 60%.
Figure 7.
Nerves as a percent of muscle area based on neurofilament (SMI-31) immunostaining. (A) Inferior oblique muscle from a control subject. (B) Inferior oblique muscle from a subject with primary inferior oblique overaction (IOOA) and no prior surgery (NPS). (C) Percent of all nerves per muscle area after immunostaining for neurofilament protein. Bar is 100 µm. Asterisks indicate significant difference from control muscle. (F = 3.442; control versus IOOA NPS P = 0.0001.) Hashtag indicates significant difference from the muscles from subjects with primary IOOA with NPS. (IOOA NPS versus IOOA P = 0.006; IOOA NPS versus Apert NPS P = 0.004.)
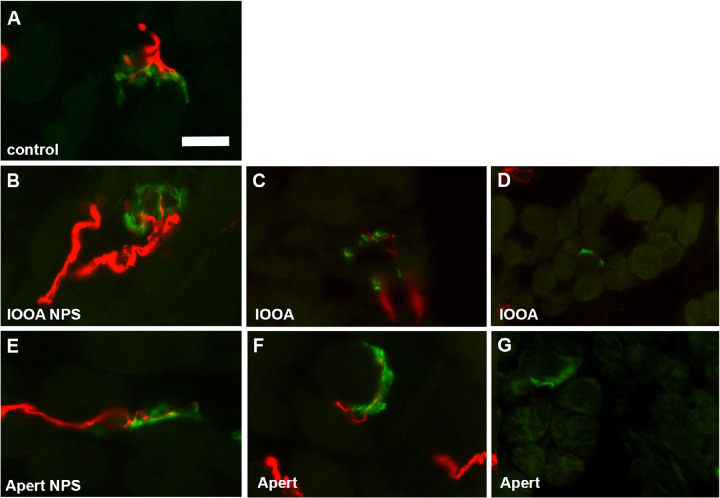
Neuromuscular junctions per whole muscle cross-sectional area were examined and quantified (Fig. 8). Most of the en plaque endings of the specimens from subjects with IOOA or Apert syndrome without prior surgery looked relatively normal. In the muscle specimens from both IOOA and subjects with Apert syndrome that had a prior surgery, many appeared to be smaller than normal, with many without an obvious nerve (Figs. 8D, 8G). However, the number of neuromuscular junctions were significantly elevated in the inferior oblique muscle specimens from subjects with Apert syndrome and no prior surgery and those with IOOA and no prior surgery (Figs. 8, 9), with over four- to six-fold more neuromuscular junctions relative to muscle area in these muscle specimens. Interestingly, prior surgery resulted in neuromuscular junction density that was not significantly different from controls, albeit still with 2- to 3-fold more neuromuscular junctions than in the control muscles.
Figure 8.
Photomicrographs of neuromuscular junctions and their innervating nerves in inferior oblique muscles immunostained with an antibody to neurofilament protein (smi-31; red) and a fluorescently tagged alpha-bungarotoxin (green) to label the neuromuscular junctions. Control muscle (A), muscles from subjects diagnosed with inferior oblique overaction (IOOA) (B, C, D) without prior surgery B (IOOA NPS), or after a previous surgery C, D (IOOA), and muscle from subjects diagnosed with Apert syndrome (Apert) (E, F, G) without prior surgery E or after a previous surgery F, G.
Figure 9.
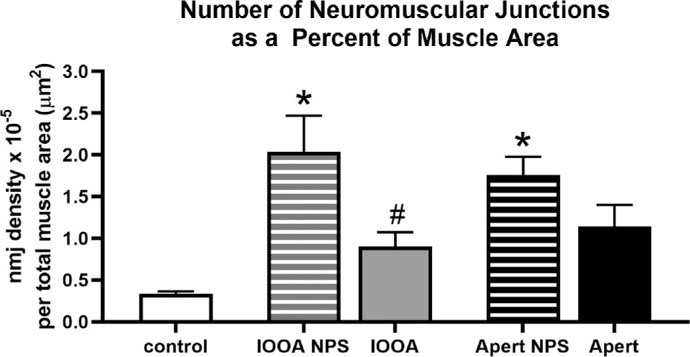
Neuromuscular junction numbers per total muscle cross-sectional area in µm2. Primary inferior oblique overaction (IOOA), Apert syndrome (Apert), no prior surgery (NPS), neuromuscular junction (nmj). Asterisks indicate significant difference from control muscle. (F = 7.071; P = 0.005 and P = 0.04, respectively). Hashtag indicates significant difference from the non-operated muscles with a similar diagnosis (F = 1.193; P = 0.05).
Discussion
Several major conclusions can be drawn from these analyses. Both sets of muscles from subjects with over-elevation in adduction showed a number of striking differences compared to the control inferior oblique muscles. The inferior oblique muscles also showed significant differences in patients with IOOA and in patients with Apert syndrome. The inferior oblique muscle specimens from previously unoperated subjects of both types were significantly more densely innervated than the muscles from the control subjects. It is important to note that these muscle specimens were both derived from the same region of the muscles. In addition, the muscles from the subjects with Apert syndrome were smaller than the muscles from the subjects with IOOA, again comparing muscles from the same anatomic location along the muscle length.
Strabismus has a prevalence of 39 to 90% of patients with craniosynostosis, including Apert syndrome.48–50 It is thought that the premature fusion of cranial sutures in Apert syndrome restricts the volume of the orbit, leading to lateralization and shallowing of the orbits. Imaging studies also demonstrate that EOM heterotopy and bony orbital alterations results in excyclorotation of the axes of the bony orbit, which, in turn, results in excyclorotation of the EOMs.14,15 These alterations result in proptosis, strabismus, and exposure keratopathy, which are commonly seen in this syndrome.51 However, on top of the bony abnormalities relative to muscle position within the orbit, muscle-specific differences were evident. Previous research identified abnormal EOM anatomy in patients with Apert syndrome and other craniosynostosis syndromes, including anomalous EOM attachments to the eye, anomalous EOM insertion, and atrophic or absent EOMs.10,52–57 A case report also noted structural anomalies in the inferior oblique of a patient with Apert syndrome, with abnormal mitochondrial and myofibrillar organization seen in light and electron microscopy.58 Although they only examined one muscle from a single subject, their results support the findings of our study that strabismus in Apert syndrome may be caused by both structural and functional differences in the EOMs themselves in addition to or in part due to the gross anatomic variations in muscle origins and insertions. In Apert syndrome, strabismus in these patients is clearly the result of a complex multifactorial process, with known abnormal genetic, orbital, and muscle anomalies. The abnormal inferior oblique muscles on a cellular level seen in this study reflects and adds to this complexity.
Our results show that subjects with Apert syndrome had smaller inferior oblique muscles, with fewer myofibers and a large heterogeneity of myofiber size compared to both controls and subjects with IOOA. The inferior oblique muscles from these subjects also showed decreased nerve density compared to subjects with IOOA. What is particularly interesting about the reduction in the overall amount of nerve density is that Apert syndrome is often associated with sixth nerve palsies and other changes in muscle number or overall muscle size.10 Increased central nucleation suggests an ongoing process of either muscle denervation and/or re-innervation. The presence of ongoing regenerative processes is supported by a prior study using electron microscopy, where interspersed with normal fibers were those that were vacuolated as well as the sporadic appearance within the muscle tissue of degenerating nerves.58
Genetic analyses showed that 99% of patients with Apert syndrome have one of two missense mutations in the gene for fibroblast growth factor receptor 2 (FGFR2).59 These mutations are thought to result in sustained FGFR2 signaling due to increased affinity of the ligand to the receptor in the absence of sustained FGF2 expression.60,61 Although the effects of these changes have been studied in bone formation, little is known about the effects on the EOMs specifically. It is important to note that an examination of fetal orbital tissues showed that the EOMs specifically express FGFR2.62 This is in contrast to studies that showed only low levels of FGFR2 expression in limb skeletal muscle.63 We hypothesize that FGFR2 is likely to be expressed in the EOMs, and the detailed expression pattern of FGFR2 is the subject of on-going studies. The hypothesized sequelae to the expression of the mutations associated with Apert syndrome would be reduced satellite cell self-renewal and an enhanced rate of differentiation.64 This, in turn, would result in the reduced muscle fiber size and overall muscle size seen in the inferior oblique muscles from subjects with Apert syndrome. Further studies examining these effects are also ongoing in our laboratory.
Not only did the inferior oblique muscles from subjects with Apert syndrome have smaller muscles, they also had a decreased amount of nerves. We predict this would manifest as reduced muscle innervation, suggesting that successful correction of the abnormal eye position might be better treated with a different pharmacologic or surgical approach compared to patients with primary IOOA. This agrees with studies that show that patients with Apert syndrome who undergo strabismus surgery yield more variable and unpredictable surgical outcomes.65 Our study suggests that in subjects with over-elevation in adduction, whether this eye position is associated with Apert syndrome or other forms of strabismus is important and should direct management.
Our study suggests that EOMs from patients with Apert syndrome are particularly abnormal, and there are a number of alterations in muscle lengths and bony orbital shape that affect muscle attachments in individuals with Apert syndrome. Taking these complex issues into account will be important for optimizing their visual outcomes. Management of patients with Apert syndrome, as reviewed by Elhusseiny et al.,66 is known to be challenging, and a variety of approaches have been recommended based on consideration of muscle tone, excyclorotation of the EOMs, and imaging of the orbit prior to surgery.66 These studies also suggest that a pharmacological approach, using molecules that alter the function of the FGF signaling pathways perhaps, may have an effect in this group of patients and deserves further study.
In the inferior oblique muscles from subjects with primary IOOA, there was a significantly greater number of nerve fibers per muscle fiber compared to controls. As these muscles are assumed to be contracting more strongly than needed, this finding is in alignment with the current assumptions in the field. It is interesting that prior surgery had a significant effect on many of the properties examined in our study. In particular, the inferior oblique muscle specimens from previously operated subjects showed decreased nerve per myofiber number and decreased neuromuscular junction numbers compared to the levels in unoperated subjects with either IOOA or Apert syndrome. The muscle specimens from individuals where this was a second surgery were nasal myectomies, and thus from a different location along the overall muscle length, compared to the myectomy at the scleral attachment end of the muscle. This may be a confounding variable, and further analysis of additional muscle specimens are needed for added assurance of these differences. However, studies have shown that these regions are similar to each other compared to the properties of the middle region of these muscles when examined for such properties as: MyHC isoform expression patterns, myofiber cross-sectional areas, and innervation pattern.42–45 These data collectively suggest that comparisons between these regions is reasonable and informative. Based on previous results, this is likely to be the result of extensive adaptation due to changes in the length/tension curve secondary to the surgery. Thus, the changes are in the direction that are predicted, based on known biochemical and functional properties, to reduce a presumably overacting muscle. We have described extensive decreases in muscle connective tissue content in previously operated muscles compared to specimens from subjects with strabismus for whom this was their first surgery.41 These decreases were hypothesized to cause reduced overall muscle tension in the months after surgery.41 In experimental studies in rabbits, we showed that strabismus surgery resulted in bilateral and rapid muscle adaptation, such as increased neonatal and slow MyHC isoform expression.67,68 Further support that adaptation of agonist/antagonist muscle pairs occurs after muscle surgery was the demonstration of functional changes in both muscle force generation and neuronal firing rates. Recession of EOMs in cats produced significant weakening in force development of the antagonist muscle.69 These results correlate with adaptation of saccadic gains measured as soon as 1 day after a surgical resection/recession surgery in the non-human primate.70 Thus, the changes seen in the previously operated inferior oblique muscles in the present study are in harmony with data from a number of other laboratories. Studies using a monkey model of strabismus demonstrated that these changes are likely driven by adaptive changes in neuronal drive of the innervating motor neurons that begin almost immediately after strabismus surgery.71 Patients typically undergoing repeat strabismus surgery only do so if their surgery was not as successful as needed – in the short term averaging 20 to 67% of subjects.72–75 It would be interesting to assess the EOMs of individuals who had better surgical outcomes compared to patients with less predictable outcomes, in order to see if there were intrinsic differences in the EOMs that may have led to a poorer response to surgery. Knowing more about the role of the EOMs in disease and in the context of systemic diseases, such as genetic disorders, in specific patient populations would lead to improved outcomes for our patients.
Supplementary Material
Acknowledgments
Supported by National Institutes of Health (NIH) RO1EY15313 and EY11375 from the National Eye Institute (LKM), Minnesota Medical Foundation, Minnesota Lions and Lionesses, and an unrestricted grant to the Department of Ophthalmology from Research to Prevent Blindness, Inc.
Disclosure: J.C. Rudell, None; D. Stager Jr, None; J. Felius, None; L.K. McLoon, None
References
- 1. Wilson ME, Parks MM.. Primary inferior oblique overaction in congenital esotropia, accommodative esotropia, and intermittent exotropia. Ophthalmology. 1989; 96: 950–955. [DOI] [PubMed] [Google Scholar]
- 2. Tollefson MM, Mohney BG, Diehl NN, Burke JP. Incidence and types of childhood hypertropia: a population-based study. Ophthalmology. 2006; 113: 1142–1145. [DOI] [PubMed] [Google Scholar]
- 3. Kushner BJ. Incomitant strabismus: does extraocular muscle form denote function? Arch Ophthalmol. 2010a; 128: 1604–1609. [DOI] [PubMed] [Google Scholar]
- 4. Parks MM. The weakening surgical procedures for eliminating overaction of the inferior oblique muscle. Am J Ophthalmol. 1972; 73: 107–122. [DOI] [PubMed] [Google Scholar]
- 5. Isenberg ST, Apt L.. Inferior oblique weakening procedures: technique and indications. In: Rosenbaum AL, Santiago AP, eds. Clinical Strabismus Management: Principles and Surgical Techniques. 1st ed. Philadelphia: W.B. Saunders; 1999: 449–458. [Google Scholar]
- 6. Madison WP, Zein WM.. Recent developments in the field of superior oblique palsies. Curr Opin Ophthalmol. 2008; 19: 379–383. [DOI] [PubMed] [Google Scholar]
- 7. Meyer E, Ludatscher RM, Zonis S. Primary and secondary overacting inferior oblique muscles: an ultrastructural study. Brit J Ophthalmol. 1984; 68: 416–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Caldeira JA. V-pattern esotropia: a review; and a study of the outcome after bilateral recession of the inferior oblique muscle: a retrospective study of 78 consecutive patients. Binocul Vis Strabismus Q. 2003; 18: 35–48. [PubMed] [Google Scholar]
- 9. Kushner BJ. Effect of ocular torsion on A and V patterns and apparent oblique muscle overaction. Arch Ophthalmol. 2010b; 128: 712–718. [DOI] [PubMed] [Google Scholar]
- 10. Carruthers JD. Strabismus in craniofacial dysostosis. Graefes Arch Clin Exp Ophthalmol. 1988; 226: 230–234. [DOI] [PubMed] [Google Scholar]
- 11. Cuttone JM, Brazis PT, Miller MT, Folk ER. Absence of the superior rectus muscle in Apert's syndrome. J Pediatr Ophthalmol Strabismus. 1979; 16: 349–354. [DOI] [PubMed] [Google Scholar]
- 12. Hussein MA, Stager DR Sr, Beauchamp GR, Jr Stager DR, Felius J. Anterior and nasal transposition of the inferior oblique muscles in patients with missing superior oblique tendons. J AAPOS. 2007; 11: 29–33. [DOI] [PubMed] [Google Scholar]
- 13. Stager DR Jr, GR Beauchamp, Wright WW, Felius J, Stager D Sr. Anterior and nasal transposition of the inferior oblique muscles. J AAPOS. 2003; 7: 167–173. [DOI] [PubMed] [Google Scholar]
- 14. Tan KP, Sargent MA, Poskitt KJ, Lyons CJ. Ocular overelevation in adduction in craniosynostosis: is it the result of excyclorotation of the extraocular muscles? J AAPOS. 2005; 9: 550–557. [DOI] [PubMed] [Google Scholar]
- 15. Dagi LR, MacKinnon S, Zurakowski D, Prabhu SP. Rectus muscle excyclorotation and V-pattern strabismus: a quantitative appraisal of clinical relevance in syndromic craniosynostosis. Br J Ophthalmol. 2017; 101: 1560–1565. [DOI] [PubMed] [Google Scholar]
- 16. Apt L, Call NB. Inferior oblique muscle recession. Am J Ophthalmol. 1978; 85: 95–100. [DOI] [PubMed] [Google Scholar]
- 17. Elliott RL, Nankin SJ.. Anterior transposition of the inferior oblique. J Pediatr Ophthalmol Strabismus. 1981; 18: 35–38. [DOI] [PubMed] [Google Scholar]
- 18. Gobin MH. Anteroposition of the inferior oblique muscle in V-esotropia. Ophthalmologica. 1964; 149: 138–141. [DOI] [PubMed] [Google Scholar]
- 19. Stager DR Sr, GR Beauchamp, Stager DR Jr. Anterior and nasal transposition of the inferior oblique muscle: a preliminary case report on a new procedure. Binocul Vis Strabismus Q. 2001; 16: 43–44. [PubMed] [Google Scholar]
- 20. Toosi SH, von Noorden GK. Effect of isolated inferior oblique muscle myectomy in the management of superior oblique palsy. Am J Ophthalmol. 1979; 88: 602–608. [DOI] [PubMed] [Google Scholar]
- 21. Chimonidou E, Chatzistefanou K, Theodossiadis G. Treatment of inferior oblique muscle overaction with myectomy or with anterior transposition. Eur J Ophthalmol. 1996; 6: 11–13. [DOI] [PubMed] [Google Scholar]
- 22. Shipman T, Burke J.. Unilateral inferior oblique myectomy and recession in the treatment of inferior oblique muscle overaction: a longitudinal study. Eye (Lond). 2003; 17: 1013–1018. [DOI] [PubMed] [Google Scholar]
- 23. Stager DR Jr, Wang X, Stager DR Sr, Beauchamp GR, Felius J. Nasal myectomy of the inferior oblique muscles for recurrent elevation in adduction. J AAPOS. 2004; 8: 462–465. [DOI] [PubMed] [Google Scholar]
- 24. Ela-Dalman N, Velez FG, Felius J, Stager DR Sr, Rosenbaum AL. Inferior oblique muscle fixation to the orbital wall: a profound weakening procedure. J AAPOS. 2007; 11: 17–22. [DOI] [PubMed] [Google Scholar]
- 25. Ziffer AJ, Isenberg SJ, Elliott RL, Apt L. The effect of anterior transposition of the inferior oblique muscle. Am J Ophthalmol. 1993; 116: 224–227. [DOI] [PubMed] [Google Scholar]
- 26. Squirrell DM, Sears KS, Burke JP. Reexploration and inferior oblique myectomy temporal to the inferior rectus to treat persistent inferior oblique overaction. J AAPOS. 2007; 11: 48–51.31. [DOI] [PubMed] [Google Scholar]
- 27. Berard-Badier M, Pelissier JF, Toga M, Mouillac N, Berard PV. Ultrastructural studies of extraocular muscles in ocular motility disorders. II. Morphological analysis of 38 biopsies. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1978; 208: 193–205. [DOI] [PubMed] [Google Scholar]
- 28. Martinez AJ, Biglan AW, Hiles DA. Structural features of extraocular muscles of children with strabismus. Arch Ophthalmol. 1980; 98: 533–539. [DOI] [PubMed] [Google Scholar]
- 29. Spencer RF, McNeer KW.. Structural alterations in overacting inferior oblique muscles. Arch Ophthalmol. 1980; 98: 128–133. [DOI] [PubMed] [Google Scholar]
- 30. Domenici-Lombardo L, Corsi M, Mencucci R, Scrivanti M, Faussone-Pelligrini MS, Salvi G. Extraocular muscles in congenital strabismus: muscle fiber and nerve ending ultrastructure according to different regions. Ophthalmologica. 1992; 205: 29–39. [DOI] [PubMed] [Google Scholar]
- 31. McLoon LK, Willoughby CL, Andrade FH. Extraocular Muscles: Structure and Function. In: Craniofacial Muscles: A New Framework for Understanding the Effector Side of Craniofacial Muscles. McLoon LK, Andrade F, (Eds.). Springer, 2012; pp. 31–88. [Google Scholar]
- 32. McLoon LK, Wirtschafter J.. Activated satellite cells in extraocular muscles of normal adult monkeys and humans. Invest Ophthalmol Vis Sci. 2003; 44: 1927–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McLoon LK, Wirtschafter JD.. Continuous myonuclear addition to single extraocular myofibers in uninjured adult rabbits. Muscle Nerve. 2002; 25: 348–358. [DOI] [PubMed] [Google Scholar]
- 34. McLoon LK, Rowe J, Wirtschafter J, McCormick KM. Continuous myofiber remodeling in uninjured extraocular myofibers: myonuclear turnover and evidence for apoptosis. Muscle Nerve. 2004; 29: 707–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Antunes-Foschini RM, Ramalho FS, Ramalho LN, Bicas HE. Increased frequency of activated satellite cells in overacting inferior oblique muscles from humans. Invest Ophthalmol Vis Sci. 2006; 47: 3360–3365. [DOI] [PubMed] [Google Scholar]
- 36. Antunes-Foschini R, Miyashita D, Bicas HE, McLoon LK. Activated satellite cells in medial rectus muscles of patients with strabismus. Invest Ophthalmol Vis Sci. 2008; 49: 215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Asmussen G, Punkt K, Bartsch B, Soukup T. Specific metabolic properties of rat oculorotatory extraocular muscles can be linked to their low force requirements. Invest Ophthalmol Vis Sci. 2008; 49: 4865–4871. [DOI] [PubMed] [Google Scholar]
- 38. McLoon LK, Park HN, Kim JH, Pedrosa-Domellöf F, Thompson LV. A continuum of myofibers in adult rabbit extraocular muscles: force, shortening velocity, and patterns of myosin heavy chain colocalization. J Appl Physiol. 2011; 111: 1178–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Andrade FH, McMullen CA, Rumbaut RE. Mitochondria are fast Ca2+ sinks in rat extraocular muscles: a novel regulatory influence on contractile function and metabolism. Invest Ophthalmol Vis Sci. 2005; 46: 4541–4517. [DOI] [PubMed] [Google Scholar]
- 40. Patel SP, Gamboa JL, McMullen CA, Rabchevsky A, Andrade FH. Lower respiratory capacity in extraocular muscle mitochondria: evidence for intrinsic differences in mitochondrial composition and function. Invest Ophthalmol Vis Sci. 2009; 50: 180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jr Stager DR, J Felius, McLoon LK. Postulating a role for connective tissue elements in inferior oblique overaction. Trans Am Ophthalmol Soc. 2013; 111: 119–132. [PMC free article] [PubMed] [Google Scholar]
- 42. Rubinstein NA, Porter JD, Hoh JFY. The development of longitudinal variation of myosin isoforms in the orbital fibers of extraocular muscles of rats. Invest Ophthalmol Vis Sci. 2004; 45: 3067–3072. [DOI] [PubMed] [Google Scholar]
- 43. Harrison AR, Anderson BC, Thompson LV, McLoon LK. Myofiber length and three-dimensional localization of NMJs in normal and botulinum toxin-treated adult extraocular muscles. Invest Ophthalmol Vis Sci. 2007; 48: 3594–3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bicer S, Reiser PJ.. Myosin isoform expression in dog rectus muscles: patterns in global and orbital layers and among single fibers. Invest Ophthalmol Vis Sci. 2009; 50: 157–167. [DOI] [PubMed] [Google Scholar]
- 45. Park KA, Lim J, Sohn S, Oh SY. Myosin heavy chain isoform expression in human extraocular muscles: Longitudinal variation and patterns of expression in global and orbital layers. Muscle Nerve. 2012; 45: 713–720. [DOI] [PubMed] [Google Scholar]
- 46. Bottinelli R, Schiaffino S, Reggiani C. Force-velocity relations and myosin heavy chain isoform compositions of skinned fibres from rat skeletal muscle. J Physiol. 1991; 437: 655–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nelson AG, Thomspon WJ.. Contractile properties and myosin phenotype of single motor units from neonatal rats. Am J Physiol. 1994; 266: C919–924. [DOI] [PubMed] [Google Scholar]
- 48. Khan SH, Nischal KK, Dean F, Hayward RD, Walker J. Visual outcomes and amblyogenic risk factors in craniosynostotic syndromes: a review of 141 cases. Br J Ophthalmol. 2003; 87: 999–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Khong JJ, Anderson P, Gray TL, Hammerton M, Selva D, David D. Ophthalmic findings in Apert's syndrome after craniofacial surgery: twenty-nine years’ experience. Ophthalmology. 2006; 113: 347–352. [DOI] [PubMed] [Google Scholar]
- 50. Tay T, Martin F, Rowe N, Johnson K, Poole M, Tan K, Kennedy I, Gianoutsos M. Prevalence and causes of visual impairment in craniosynostotic syndromes. Clin Exp Ophthalmol. 2006; 34: 434–440. [DOI] [PubMed] [Google Scholar]
- 51. Buncic JR. Oscular aspects of Apert syndrome. Clin Plast Surg. 1991; 18: 315–319. [PubMed] [Google Scholar]
- 52. Weinstock FJ, Hardesty HH.. Absence of superior recti in craniofacial dysostosis. Arch Ophthalmol. 1965; 74: 152–153. [DOI] [PubMed] [Google Scholar]
- 53. Diamond GR, Katowitz JA, Whitaker LA, Quinn GE, Schaffer DB. Variations in extraocular muscle number and structure in craniofacial dysostosis. Am J Ophthalmol. 1980; 90: 416–418. [DOI] [PubMed] [Google Scholar]
- 54. Pinchoff BS, Sandall G.. Congenital absence of the superior oblique tendon in craniofacial dysostosis. Ophthalmic Sur. 1985; 16: 375–377. [PubMed] [Google Scholar]
- 55. Pollard ZF. Bilateral superior oblique muscle palsy associated with Apert's syndrome. Am J Ophthalmol. 1988; 106: 337–340. [DOI] [PubMed] [Google Scholar]
- 56. Bustos DE, Donahue SP.. Absence of all cyclovertical extraocular muscles in a child who has Apert syndrome. J AAPOS. 2007; 11: 408–409. [DOI] [PubMed] [Google Scholar]
- 57. Coats DK, Ou R.. Anomalous medial rectus muscle insertion in a child with craniosynostosis. Binocul Vis Strabismus Q. 2001; 16: 119–120. [PubMed] [Google Scholar]
- 58. Margolis S, Pachter BR, Breinin GM. Structural alterations of extraocular muscle associated with Apert's syndrome. Br J Ophthalmol. 1977; 61: 683–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Park WJ, Theda C, Maestri NE, et al. Analysis of phenotypic features and FGFR2 mutations in Apert syndrome. Am J Hum Genet. 1995; 57: 321–328. [PMC free article] [PubMed] [Google Scholar]
- 60. Anderson J, Burns HD, Enriquez-Harris P, Wilkie A, Heath JK. Apert syndrome mutations in fibroblast growth factor receptor 2 exhibit increased affinity for FGF ligand. Hum Mol Genet. 1998; 7: 1475–1483. [DOI] [PubMed] [Google Scholar]
- 61. Wheldon LM, Khodabukus N, Patey SJ, Smith TG, Heath JK. Identification and characterization of an inhibitory fibroblast growth factor receptor-2 (FGFR2) molecule, up-regulated in an Apert Syndrome mouse model. Biochem J. 2011; 436: 71–81. [DOI] [PubMed] [Google Scholar]
- 62. Khan SH, Britto JA, Evans RD, Nischal KK. Expression of FGFR-2 and FGFR-3 in the normal human fetal orbit. Br J Ophthalmol. 2005; 89: 1643–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pawlikowski B, Vogler TO, Gadek K, Olwin BB. Regulation of skeletal muscle stem cells by fibroblast growth factors. Dev Dyn. 2017; 246: 359–367. [DOI] [PubMed] [Google Scholar]
- 64. Liu Y, Schneider MF. FGF2 activates TRPC and Ca2+ signaling leading to satellite cell activation. Front Physiol. 2014;Feb 11; 5: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Coats DK, Paysse EA, Stager DR. Surgical management of V-pattern strabismus and oblique dysfunction in craniofacial dysostosis. J AAPOS. 2000; 4: 338–342. [DOI] [PubMed] [Google Scholar]
- 66. Elhusseiny AM, Huynh EM, Dagi LR. Evaluation and management of V pattern strabismus in craniosynostosis. J Binocul Vis Ocul Motil. 2020; 70: 40–45. [DOI] [PubMed] [Google Scholar]
- 67. Christiansen SP, McLoon L.K.. The effect of resection on satellite cell activity in rabbit extraocular muscle. Invest Ophthalmol Vis Sci. 2006; 47: 605–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Christiansen SP, Antunes-Foschini RS, McLoon LK. The effects of recession versus tenotomy without recession in adult rabbits extraocular muscle. Invest Ophthalmol Vis Sci. 2010; 51: 5646–5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Christiansen SP, Soulsby ME, Seifen EE. Effect of antagonist weakening on developing tension in cat extraocular muscle. Invest Ophthalmol Vis Sci. 1995; 36: 2547–2550. [PubMed] [Google Scholar]
- 70. Pullela M, Degler BA, Coats DK, Das VE. Longitudinal evaluation of eye misalignment and eye movements following surgical correction of strabismus in monkeys. Invest Ophthalmol Vis Sci. 2016; 57: 6040–6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Pullela M, Agaoglu MN, Joshi AC, Agaoglu S, Coats DK, Das VE. Neural plasticity following surgical correction of strabismus in monkeys. Invest Ophthalmol Vis Sci. 2018; 59: 5011–5021.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Livir-Rallatos G, Gunton KB, Calhoun JH. Surgical results in large-angle exotropia. J AAPOS. 2002; 6: 77–80. [DOI] [PubMed] [Google Scholar]
- 73. Trigler L, Siatkowski RM.. Factors associated with horizontal reoperation in infantile esotropia. J AAPOS. 2002; 6: 15–20. [DOI] [PubMed] [Google Scholar]
- 74. Lee SY, Rosenbaum AL.. Surgical results of patients with A-pattern horizontal strabismus. J AAPOS. 2003; 7: 251–255. [DOI] [PubMed] [Google Scholar]
- 75. Pineles SL, Ela-Dalman N, Zvansky AG, Yu F, Rosenbaum AL. Long-term results of the surgical management of intermittent exotropia. J AAPOS. 2010; 14: 298–304. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.