Abstract
Introduction
Ischemic brain injury due to stroke or other pathologies is a major contributor to disability and mortality worldwide. Upon the occurrence of stroke, neuronal cells undergo apoptosis due to the deprivation of oxygen and nutrients and failure of the blood–brain barrier (BBB). In the moments immediately following a stroke, widespread perfusion resulting from hyperpermeability is accompanied by an acute inflammatory response, which induces neovascularization and often permanent neurological injury. Vascular endothelial growth factor (VEGF) and its receptor VEGF receptor 2 (VEGFR2) have been targeted to suppress cerebral ischemia. Recently, natural products including flavonoids, such as juglanin, have been receiving increasing attention for their impressive physiological effects.
Methods
Twenty mg/kg body weight juglanin was administrated for 3 weeks before inducing middle cerebral artery occlusion (MCAO) in mice. The animal brain infarction volume, neurological deficit score, blood–brain barrier permeability, and the expression of tight junction proteins were evaluated. Endothelial permeability and tight junction protein expression were also assessed in brain microvascular endothelial cells (HMBVECs) exposed to oxygen–glucose deprivation/reperfusion (OGD/R).
Results
Juglanin significantly reduced occlusion-induced infarct volume and improved neurological score by suppressing BBB hyperpermeability. Juglanin inhibited both the mRNA and protein expression of VEGF and VEGFR2 and restored the normal expression of occludin and zonula occludens-1 (ZO-1), two important tight junction proteins, in MCAO mice. Meanwhile, the results of in vitro experiments show that the protective effects of juglanin against increased BBB permeability and reduced tight junction functionality are dependent on the VEGF/VEGFR2 signaling pathway, as evidenced by the capacity of exogenous VEGF-A to abolish the effects of juglanin.
Conclusion
Our findings indicate a potent ability of juglanin to prevent neuronal injury resulting from cerebral ischemia by modulating the VEGF/VEGFR2 signaling pathway. Further research will help elucidate the exact mechanisms behind the protective effects of juglanin.
Keywords: cerebral ischemia, stroke, juglanin, VEGF, VEGFR2, tight junction proteins, blood–brain barrier, BBB permeability
Introduction
Cerebral ischemia is associated with various pathologies, including stroke, traumatic brain injury,1 and Alzheimer’s disease,2 among others. Stroke is the third most common cause of death worldwide and the leading cause of permanent disability, with 15–30% of patients deemed permanently disabled in the first 3 months following the occurrence of a stroke.3 Ischemic stroke is by far the most common, accounting for over 85% of all stroke cases.4 In ischemic conditions, tissues are starved of vital oxygen and nutrients due to reduced or obstructed blood flow, which leads to neural cell apoptosis, oxidative stress, and a robust inflammatory response.5 The blood–brain barrier (BBB) plays a critical role in maintaining homeostasis by acting as a selectively permeable barrier between the vasculature and the central nervous system (CNS). Functional failure of the BBB is a major aspect of ischemic stroke, which causes an increase in infarct volume, edema, and hemorrhage, thereby inducing widespread neuronal damage. Recent research has shown that following acute ischemic stroke, the permeability of the BBB continually increases.6 Increased permeability of the BBB has been associated with an increase in infarct volume, interstitial fluid pressure, and vasogenic cerebral edema.7 Tight junction proteins such as occludin and zonula occludens-1 (ZO-1) serve to maintain the integrity of the BBB while selectively allowing certain substances to permeate the BBB monolayer. Therapies designed to increase the expression of tight junction proteins and thereby promote BBB integrity have been suggested as a treatment approach for ischemic brain injury.8
Angiogenesis plays a significant role in increasing infarct volume in ischemic stroke. After the tissues are starved of oxygen, the body reacts by rushing blood to the deprived regions of the brain via newly formed microvessels. Vascular endothelial growth factor (VEGF) is regarded as the main factor driving angiogenesis, and thus, a key effector in ischemic stroke.9 VEGF also acts as a major regulator of vascular permeability and has been shown to cause a significant increase in BBB permeability. In response to middle cerebral artery occlusion (MCAO) in mice, VEGF is increased in various CNS-associated cell types. This is at least in part mediated by VEGF receptor 2 (VEGFR2), which induces vascular permeability by activating the endothelial nitric oxide synthase (eNOS) pathway and reducing the expression of tight junction proteins.10 Inhibiting VEGFR2 signaling has been suggested as a method to prevent ischemia-induced neurovascular remodeling, thereby reducing infarct volume and improving outcomes.11
In recent decades, natural products have gained recognition as an important source for the successful development of new drugs. The application of natural products in the treatment of brain disorders requires the drug to cross the BBB. The development of neuroprotective natural compounds and novel delivery approaches have been extensively investigated.12,13 For example, curcumin from plants in the ginger family has been widely used in supplementary drugs, and developed curcumin-nanoparticles have demonstrated potent anti-oxidant activity in MCAO mice.14 Rutin from citrus fruit is known to benefit the vasculature, and the administration of rutin-encapsulated nanoparticles significantly reduces infarction volume in MCAO mice.15 Safranal extracted from saffron is a powerful antioxidant. Therapies employing the nanoencapsulation of safranal exhibit significant improvement in neurobehavior and antioxidant activity in MCAO mice.16 Glycyrrhizic acid from the root of the licorice plant is well known for its anti-inflammatory effect. Therapies employing glycyrrhizic acid-encapsulated nanoparticles results in enhanced neurobehavioral activity in MCAO animals.17 Contemporary research indicates that naringenin, a natural compound derived from grapefruit, exerts a neuroprotective effect in cerebral ischemic rats.18
Juglanin is a naturally occurring flavonoid glycoside derived from Polygonum aviculare and other plants, which has been shown to exert impressive anti-inflammatory and antioxidant effects. Additionally, juglanin can hinder cancer progression.19 Presently, the effects of juglanin in ischemic brain injury are incompletely understood. Cyclic diarylheptanoids of the juglanin class have been shown to reduce neuronal cell death, including juglanin A and juglanin C isolated from Juglans sinensis.20 Therefore, we hypothesized that juglanin might confer other neuroprotective effects. In the present study, we investigated the effects of juglanin in an MCAO mouse model of ischemic stroke. We also performed a series of in vitro experiments using human brain microvascular endothelial cells (HBMVECs) to elucidate the mechanism of juglanin-mediated neuroprotection. Our findings show that juglanin may have potential as a treatment to prevent BBB hyperpermeability and reduce infarct volume.
Materials and Methods
Mouse Model and Drug Administration
For our in vivo experiments, C57/BL6 mice were purchased from Jackson Laboratory. All animal experimentations in the present research were followed through in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of Health’s National Organizations (National Institutes of Health, US). Animal experimental procedures were carried out under a protocol approved by the Institutional Animal Care and Use Committee at Qingdao University (NO. 20,160,332) and were in accordance with Qingdao University guidelines for the care and use of laboratory animals. Experiments with human subjects were designed in accordance with the World Medical Association Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects. Human subject experiments were approved by the ethics committee of Qingdao University (NO. 20,160,125). The mice were divided into four groups: (1). Vehicle group; (2) juglanin group; (3) MCAO group; (4) MCAO + juglanin group. In the MCAO groups, mice were subjected to cerebral ischemia by inserting a surgical filament into the middle cerebral artery and closing with sutures for 2 h, followed by reperfusion for 24 h. In the juglanin treatment group, mice were treated with juglanin at a dose of 20 mg/kg body weight via oral gavage for 3 weeks before the MCAO experiment, as described above. Once the mice had recovered from anesthesia, successful ischemia/reperfusion injury was confirmed by determining the neurological deficit score in all mice. TTC staining was used to determine infarct volume. After TTC staining, the infarct region appears in white on the striatum, cortex, and adjacent areas of the right hemisphere. The infarct area was delimited and expressed as a percentage of the contralateral normal area on the left hemisphere.
Neurological Deficit Scoring Method
Neurological deficit was determined using a 5-point grading scale where grade 0 = no observable sign of neurological deficit; grade 1 = inability to fully extend the contralateral forepaw when grasped by the tail; grade 2 = moving in circles toward the ipsilateral side; more severe, grade 3 = falling to the side contralateral to brain damage; grade 4 = creasing to move and displaying minimal signs of consciousness.
Blood–Brain Barrier Permeability Assay
Evans blue dye was used to assess BBB permeability in mice in vivo. Directly after stereotactic injection, Evans blue dye (EBD) (2%; 4 mL/kg) was injected into the tail vein. At the end of the study, the mice in all three groups were sacrificed, and the tissues were perfused with saline. The brains of the mice were quickly harvested and then weighed and homogenized in 50% trichloroacetic acid (TCA). The concentration of EBD was indexed by measuring the fluorescence intensity of the dye with excitation at 620 nm and emission at 680 nm.
Immunostaining of Tight Junction Proteins
The tight junction proteins occludin and ZO-1 were stained with corresponding antibodies. Briefly, phosphate buffer saline (PBS) was used to perfuse the sacrificed mice from each group. Whole-brain tissues were then harvested and sliced into 8 μm-thick sections. The sections were embedded using OCT compound. After rehydration and blocking for 1 h with 10% horse serum solution (Dako China), the sections were stained with primary antibodies against occludin (#91,131, Cell Signaling Technology, 1:50) and ZO-1 (#13,663, Cell Signaling Technology, 1:100) overnight at 4°C, followed by Alexa Fluor 488-labeled anti-mouse secondary antibody (#A32731, Thermo fisher scientific, 1:1000) for 1 h at room temperature in darkness. The slides were mounted with ProLong Antifade Mountant (Thermo Fisher Scientific, USA). A Zeiss fluorescence microscope was used to visualize the images.
Cell Culture, OGD/R, and Treatment
For our in vitro experiments, bEnd.3 human brain microvascular endothelial cells (HBMVECs) were obtained from Cell Systems. The HBMVECs used in all experiments were maintained using a Complete Medium Kit (Cell Systems) containing 10% serum and CultureBoost-R™. To induce OGD, the cells were incubated with deoxygenated media in an air-tight incubator for 6 h followed by flushing with 1% O2, 5% CO2, and 94% N2. The cells were then placed into normal culture media under normoxic conditions (21% O2, 5% CO2) at 37°C for reperfusion. For juglanin treatment, the cells were incubated in the presence or absence of 2.5 and 5 µM juglanin.21,22
Endothelial Cell Permeability in vitro Assay
Endothelial layer permeability was determined using an FITC-Dextran assay kit. Briefly, HBMVECs were seeded onto collagen-coated individual hanging inserts in a 24-well receiver plate. After an endothelial monolayer had formed, the cells were exposed to hypoxic media for 6 h followed by reperfusion media for 24 h in the presence or absence of 2.5 and 5 µM juglanin. FITC-Dextran was added on top of the endothelial monolayer to allow the fluorescent molecules to pass through. Endothelial monolayer permeability was proportional to the rate at which the molecules passed through the monolayer. The fluorescence intensity of the well solution in the receiver plate was normalized to baseline values and comparisons were made at multiple time points to determine the extent of permeability.
Real-Time PCR Analysis
To determine the mRNA expression of the target genes, total RNA was extracted from bEnd.3 HBMVECs using an RNeasy Micro kit (Qiagen (Cat.74004)). The concentration and quality of the isolated RNA was determined using a NanoDrop spectrophotometer. Then, cDNA was synthesized using 1 µg total RNA with iScript™ reverse transcription Supermix for RT-qPCR (Invitrogen (Cat. 1,708,840)). The total mRNA transcripts of occludin, ZO-1, VEGF, and VEGFR2 were detected via SYBR-based real-time PCR on an ABI 7500 platform.
Western Blot Analysis
To determine the protein expression of the target genes, HBMVECs were lysed using radio-immunoprecipitation assay (RIPA) buffer containing protease inhibitor cocktail. Briefly, total cell lysates (20 μg) were loaded onto 4–20% precast polyacrylamide gel electrophoresis (PAGE) gels and the proteins were separated by size.
Then, the separated protein mixture was transferred onto polyvinylidene fluoride (PVDF) membranes and blocked with 5% skim milk. To detect the specific proteins, blots were probed with specific primary antibodies overnight at 4°C and followed by 1-hour incubation with secondary antibody at room temperature. The primary and secondary antibodies used were: occludin (#91,131, Cell Signaling Technology, 1:500), ZO-1 (#13,663, Cell Signaling Technology, 1:1000); VEGF (#2463, Cell Signaling Technology, 1:1000), VEGFR2 (#2472, Cell Signaling Technology, 1:1000), β-actin (#4970, Cell Signaling Technology, 1:2000); anti-rabbit IgG, HRP-linked antibody (#7074, Cell Signaling Technology, USA); anti-mouse IgG, HRP-linked Antibody (#7076, Cell Signaling Technology, USA).
Statistical Analysis
The experimental results contained herein are expressed as means ± standard deviation (SD). The significance of differences between the groups in this study was determined using the SPSS statistical software package (v15.0). Analysis of variance (ANOVA) followed by the Tukey’s post hoc test was used to calculate statistical significance. P < 0.05 was considered to represent statistical significance.
Results
Juglanin Reduces Infarct Volume and Improves Neurological Score by Reducing BBB Permeability
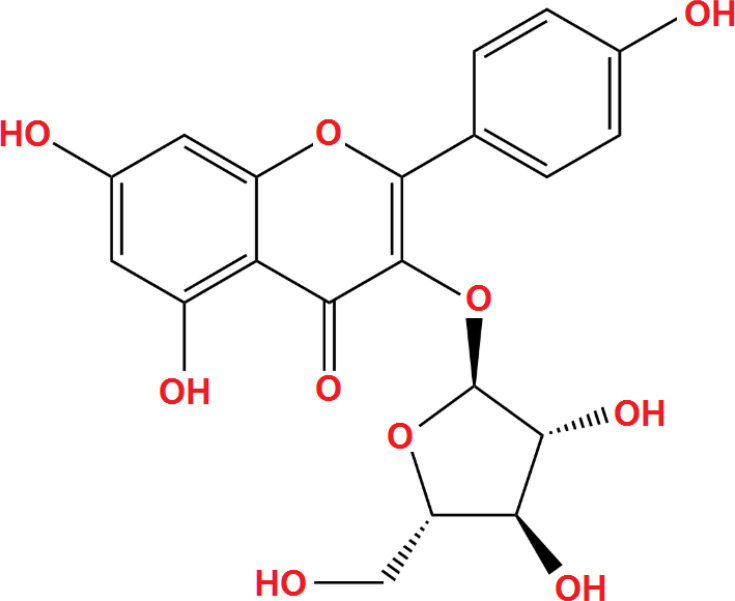
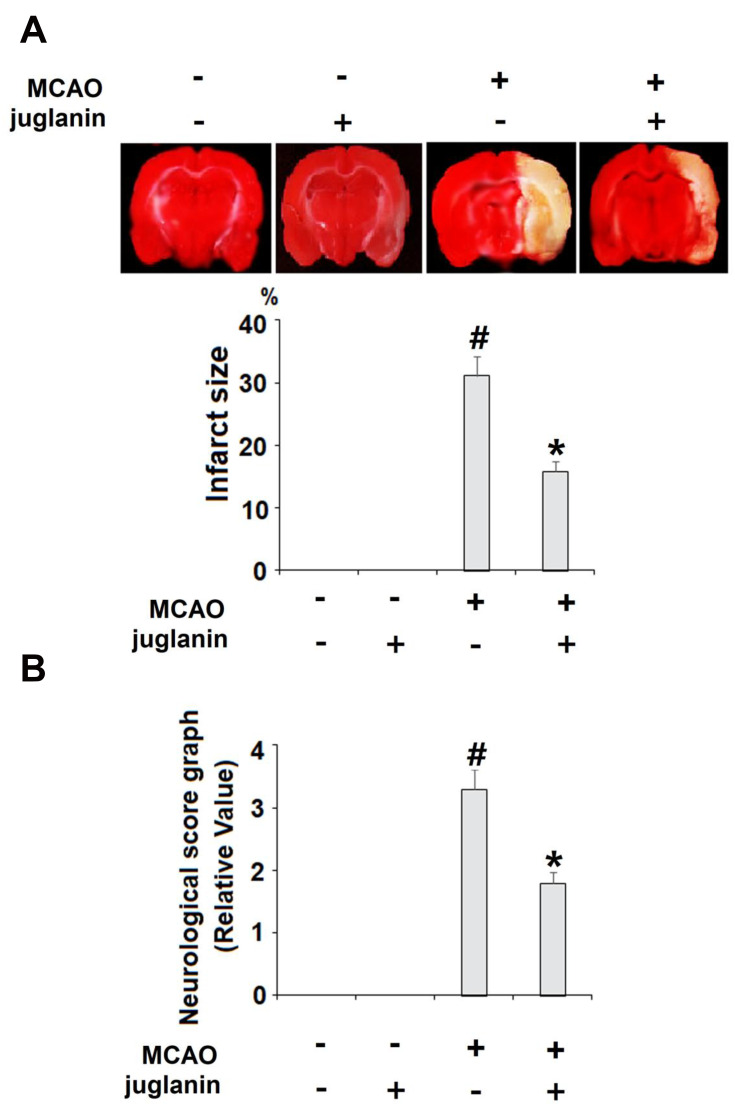
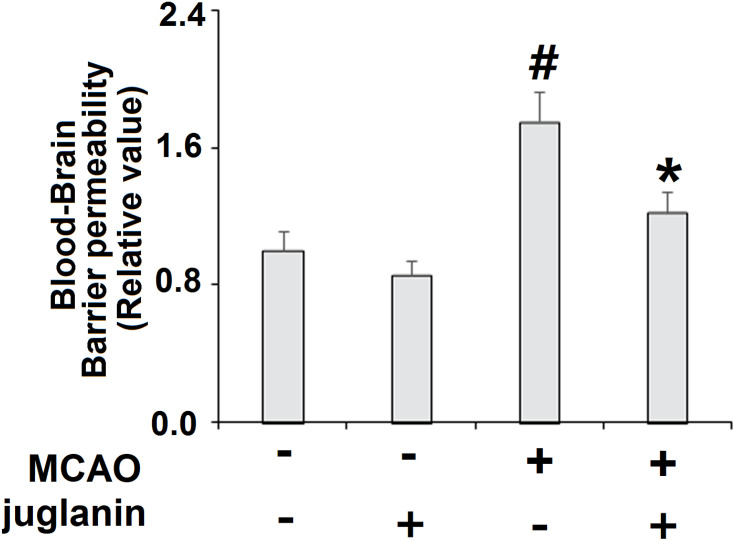
Juglanin (kaempferol-3-O-α-L-arabinofuranoside) has a molecular formula of C20H18O10 and a weight of 418.3 g/mol. The molecular structure of juglanin is shown in Figure 1. We began our study by determining the effects of juglanin in vivo by measuring brain infarct volume and neurological score in an MCAO mouse model. The mice were divided into a vehicle group, a juglanin alone group, an MCAO group, and an MCAO plus juglanin pretreatment group. All animals in the vehicle and juglanin alone groups had no obvious infarct detected, while the MCAO groups had about 30% infarct area. However, the MCAO plus juglanin group showed a significant reduction in infarct volume of about half (Figure 2A). Accordingly, animals in the vehicle and juglanin alone groups had no visible neurological deficit, and the MCAO group had an average deficit score of about 3. However, animals in the MCAO plus juglanin group had an average score below 2, indicating that the drug ameliorated the deficit in neurological score induced by MCAO (Figure 2B). Next, we explored the underlying mechanism by measuring the effect of juglanin on BBB permeability. Compared to the vehicle group, the results in Figure 3 demonstrate that juglanin reduced MCAO-induced hyperpermeability from an initial increase of 75% to an increase of only 22%. These findings imply that juglanin exerts significant neuroprotective effects against ischemic injury.
Figure 1.
Molecular structure of juglanin.
Figure 2.
Juglanin reduced brain infarction volume and improved neurological dysfunction in a middle cerebral artery occlusion (MCAO) mice model. Mice were divided into 3 experimental groups: (1) Vehicle group; (2) juglanin group: mice were treated with juglanin (20 mg/kg body weight via oral gavage) (3) MCAO group: mice were subjected to cerebral ischemia for 2 h, followed by reperfusion for 24 h; (4) MCAO + juglanin: mice were treated with juglanin (20 mg/kg body weight via oral gavage) for 3 weeks before the MCAO experiment. (A) Representative images of brain infarction and quantification of infarction volume. (B) Neurological score graph of the three experimental groups (#P<0.01 vs vehicle group; *P<0.01 vs MCAO group).
Figure 3.
Juglanin reduced blood–brain barrier (BBB) permeability in a middle cerebral artery occlusion (MCAO) mice model. Blood–brain barrier permeability was assayed (#P<0.01 vs vehicle group; *P<0.01 vs MCAO group).
Juglanin Reduces the Expression of VEGF, VEGFR2 and Increases Tight Junctions
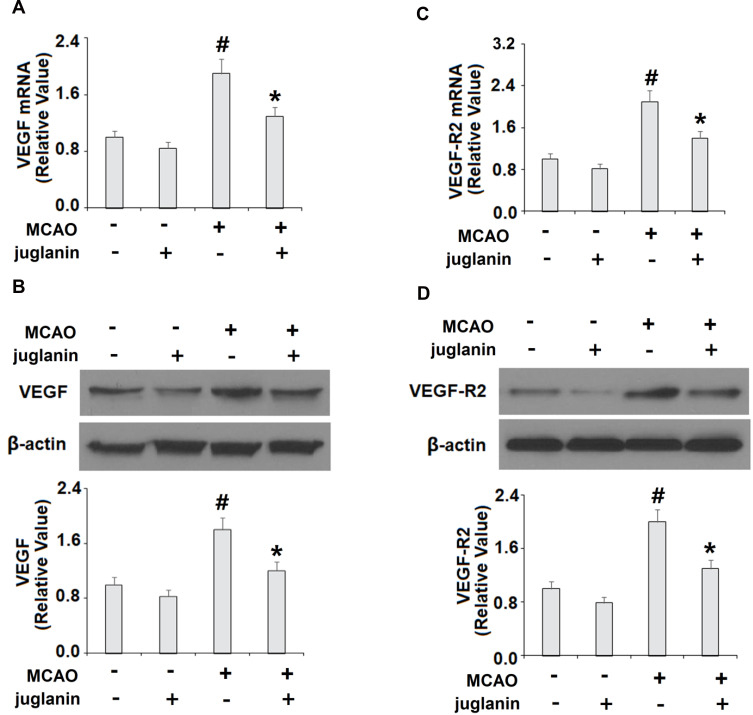
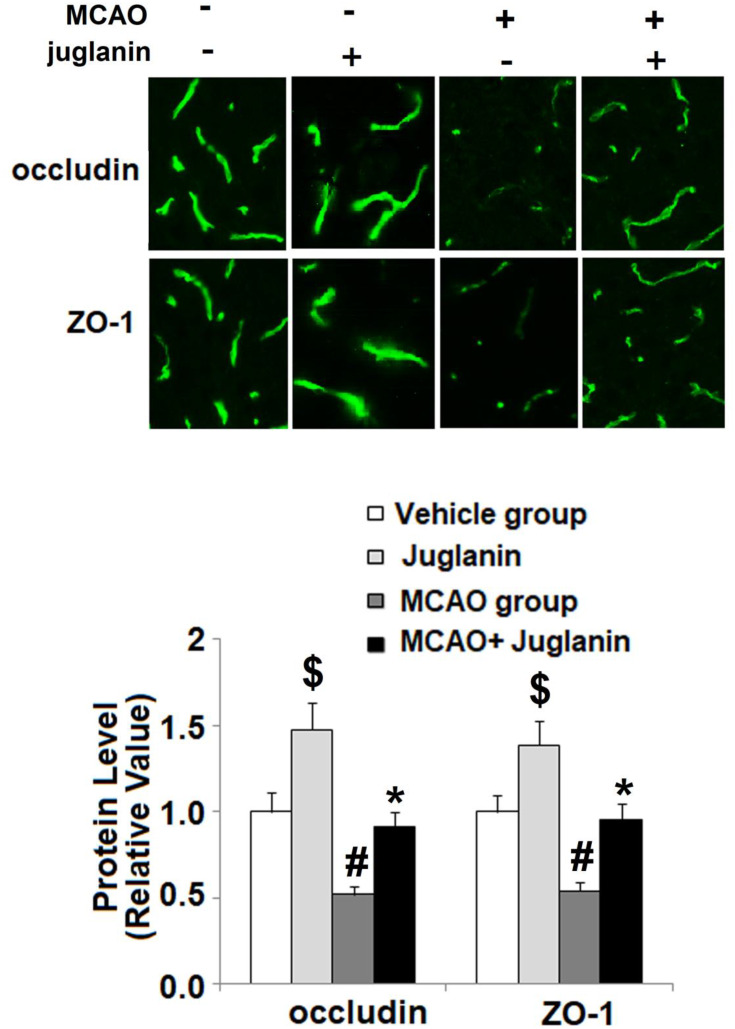
To determine the mechanism through which juglanin protected against ischemic brain injury in MCAO mice, we measured the levels of VEGF and its receptor VEGFR2 in vehicle and MCAO mice. The mRNA and protein expressions of VEGF are shown in Figure 4A and B. Compared to vehicle mice, MCAO mice had a 90% increase in VEGF mRNA and an 80% increase in protein expression, respectively, while MCAO mice pretreated with juglanin had only a 30% increase in mRNA and a 20% increase in protein. The mRNA and protein expressions of VEGFR2 are shown in Figure 4C and D. Compared to the vehicle group, MCAO mice had a 110% increase in VEGFR2 mRNA and a 100% increase in protein expression, respectively. Meanwhile, MCAO mice pretreated with juglanin had only a 40% increase in VEGFR2 mRNA and a 30% increase in protein. Thus, juglanin significantly reduced VEGF and VEGFR2 expression, thereby inhibiting MCAO-induced angiogenesis. Next, we assessed whether juglanin improves BBB permeability by modulating the expression of the tight junction proteins occludin and ZO-1. The immunostaining results for these two proteins are shown in Figure 5. Compared to vehicle mice, MCAO mice had only about half the amount of both occludin and ZO-1, while pretreatment with juglanin almost completely ameliorated the decrease in these proteins.
Figure 4.
Juglanin reduced the expression of VEGF and VEGFR2 in a middle cerebral artery occlusion (MCAO) mice model. (A) mRNA of VEGF as measured by real-time PCR. (B) Protein of VEGF as measured by Western blot. (C) mRNA of VEGFR2 as measured by real-time PCR. (D) Protein of VEGFR2 as measured by immunostaining (#P<0.01 vs vehicle group; *P<0.01 vs MCAO group).
Figure 5.
Juglanin restored the expression of occludin and ZO-1 in a middle cerebral artery occlusion (MCAO) mice model. Protein of occludin and ZO-1 as measured by immunostaining ($, #P<0.01 vs vehicle group; *P<0.01 vs MCAO group).
Juglanin Prevents OGD/R Injury in vitro
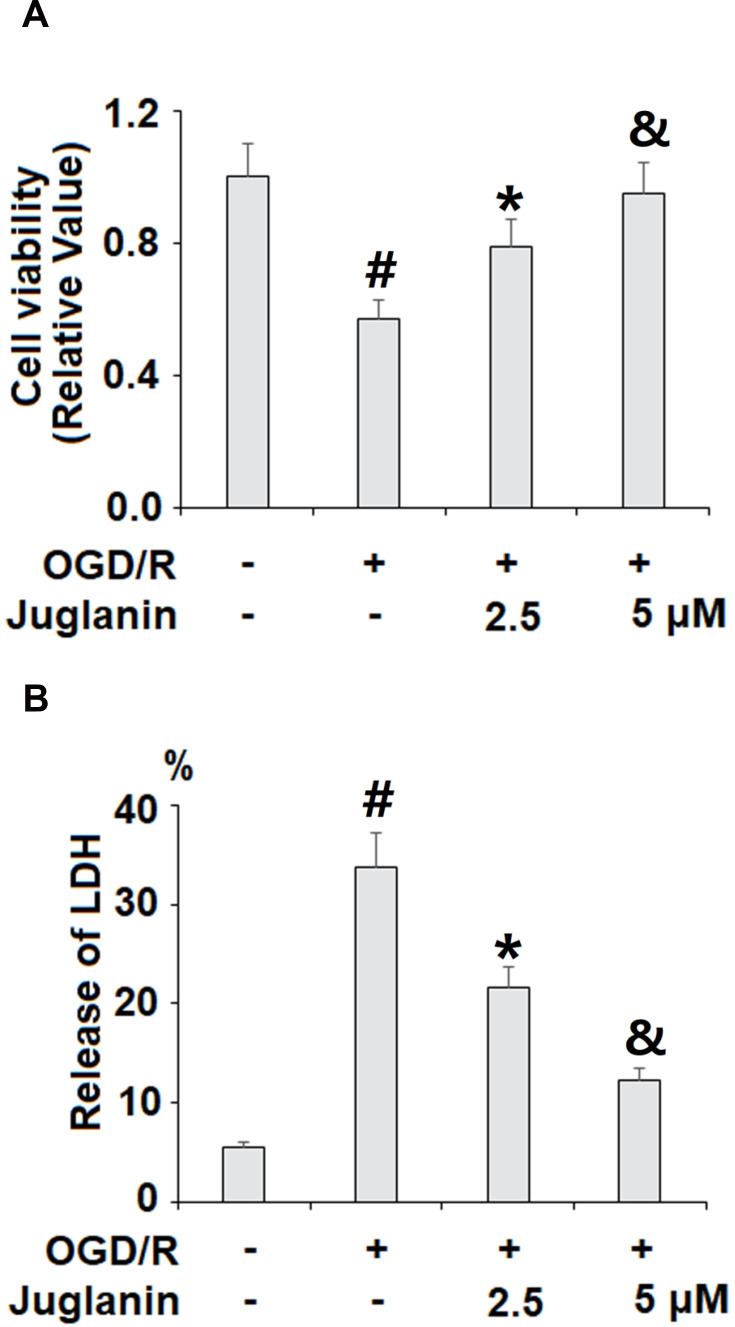
Next, we performed a series of in vitro experiments to determine the mechanisms of action of juglanin against ischemic injury using HBMVECs exposed to OGD/R. We began by determining the effect of juglanin on cell viability via MTT assay and the release of LDH. As shown in Figure 6A, OGD/R reduced cell viability by 43%, which was dose-dependently ameliorated by 2.5 and 5 µM juglanin, mitigating the reduction of cell viability to only 21% and 5%, respectively. The LDH cytotoxicity results are shown in Figure 6B. At baseline, the release of LDH into the culture media was 5.6%, which increased to 33.8% by OGD/R exposure. However, juglanin dose-dependently reduced OGD/R-induced cell death to 21.6% and 12.3%, respectively.
Figure 6.
Juglanin prevented oxygen–glucose deprivation/reperfusion (OGD/R) in human bEnd.3 brain microvascular endothelial cells (HBMVECs). Cells were exposed to hypoxic conditions for 6 h, followed by exposure to reperfusion media for (24 h) in the presence or absence of juglanin (2.5, 5 μM). (A) Cell viability was determined by 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide, Thiazolyl Blue Tetrazolium Bromide (MTT) assay. (B) Release of lactate dehydrogenase (LDH) (#, *, &P<0.01 vs previous column group).
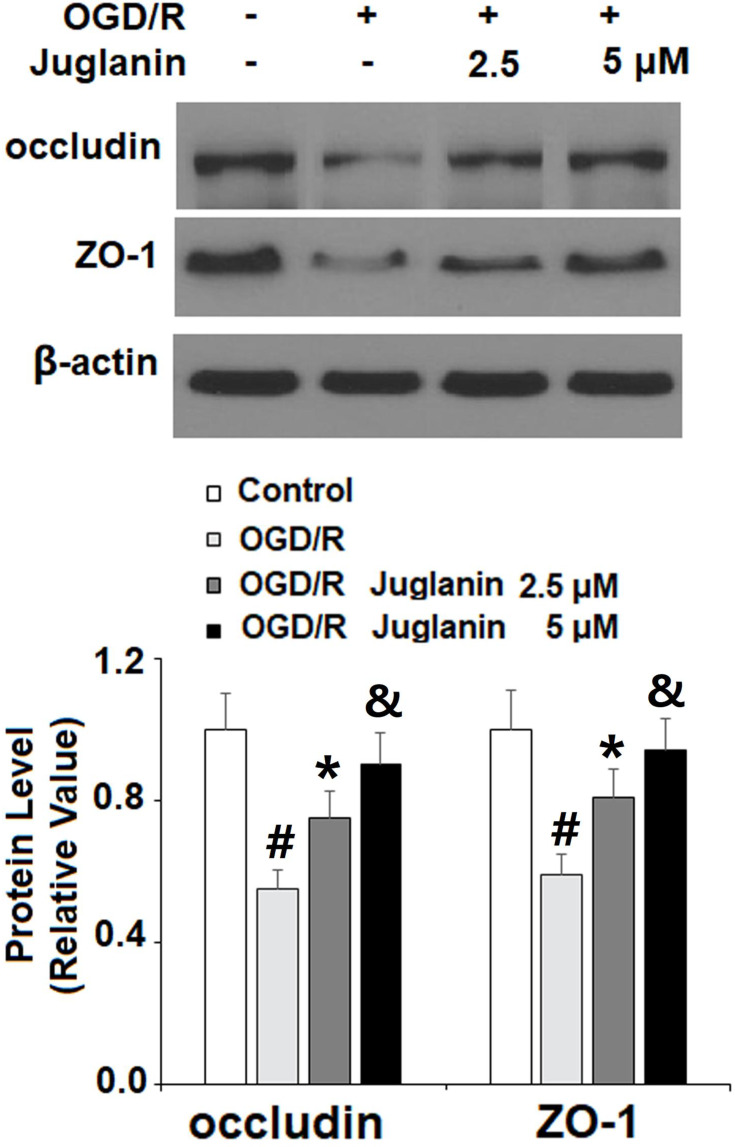
Subsequently, we determined endothelial monolayer permeability in HBMVECs. As shown in Supplementary Figure 1, time course fluorescence tracking showed that non-OGD/R-treated cells had a steady fluorescence intensity at the different measured time points, while OGD/R-exposed cells showed a gradual increase in efflux, which peaked at the 24-h time point. Therefore, 24 h was used in this study. The results of FITC-dextran permeation in Figure 7 reflect an increase from 8.3% to 78.6%, which was dose-dependently mitigated by juglanin to only 46.5% and 32.6%. Mechanistically, we found that juglanin at least in part reduced endothelial permeability by dose-dependently preventing the decrease in tight junction protein expression induced by OGD/R. As shown in Figure 8, OGD/R reduced the expression of occludin and ZO-1 by roughly 50%, which as almost completely rescued by juglanin.
Figure 7.
Juglanin prevented oxygen–glucose deprivation/reperfusion (OGD/R)-induced increased brain endothelial monolayer permeability in human bEnd.3 brain microvascular endothelial cells (HBMVECs). Cells were exposed to hypoxic conditions for 6 h, followed by exposure to reperfusion media for (24 h) in the presence or absence of juglanin (2.5, 5 μM). Brain endothelial permeability was assessed by FITC-dextran permeation (#, *, &P<0.01 vs previous column group).
Figure 8.
Juglanin prevented oxygen–glucose deprivation/reperfusion (OGD/R)-induced reduced occludin and ZO-1 in human bEnd.3 brain microvascular endothelial cells (HBMVECs). Cells were exposed to hypoxic conditions for 6 h, followed by exposure to reperfusion media for (24 h) in the presence or absence of juglanin (2.5, 5 μM). Protein of occludin and ZO-1 as measured by Western blot analysis (#, *, &P<0.01 vs previous column group).
The Effects of Juglanin are Mediated by VEGF
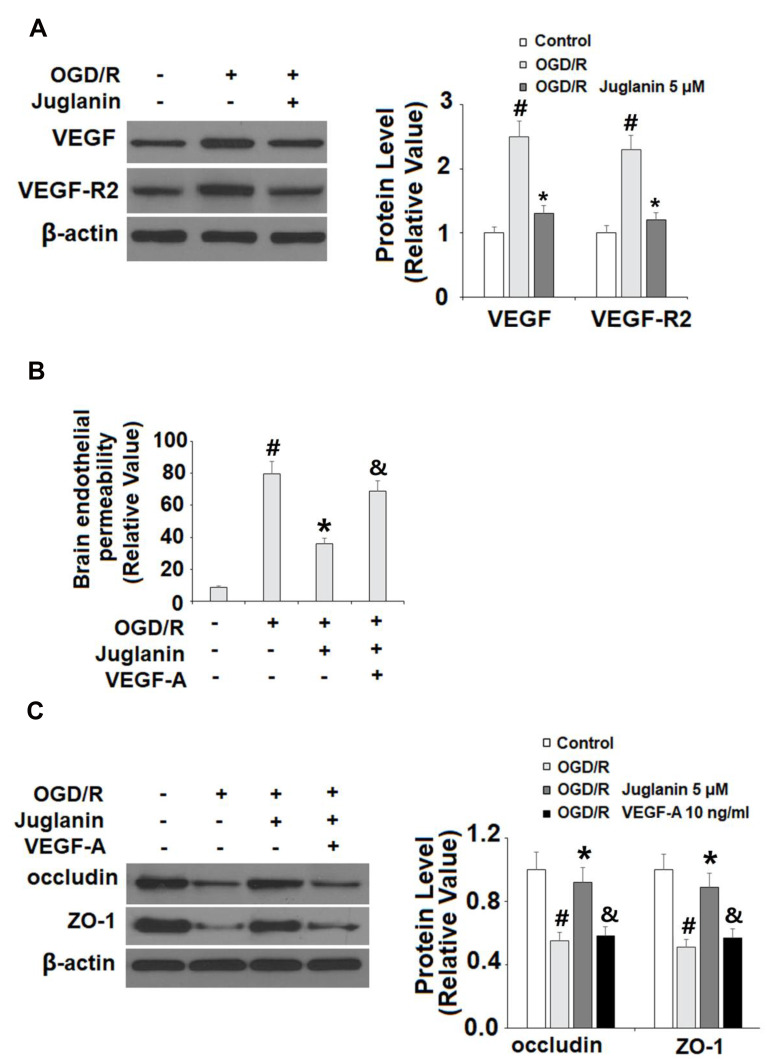
Finally, we determined whether the effects of juglanin observed in our study are mediated by its effect on VEGF expression. Compared to non-treated cells, juglanin significantly suppressed the increase in VEGF and VEGFR2 expression induced by OGD/R, reducing the 150% increase in VEGF to only 30%, and the 130% increase in VEGFR2 to only 20% (Figure 9A). Next, we employed exogenous VEGF-A, which regulates angiogenesis and BBB permeability by activating VEGFR2,23 to confirm our findings. We found that the addition of VEGF-A abolished the effects of juglanin on brain endothelial permeability (Figure 9B) as well as occludin and ZO-1 expression, thereby demonstrating the dependence of juglanin-mediated neuroprotection on VEGF/VEGFR2 signaling (Figure 9C).
Figure 9.
The effects of juglanin on brain endothelial monolayer permeability are mediated by VEGF. (A) Human bEnd.3 brain microvascular endothelial cells (HBMVECs) were exposed to OGD/R in the presence or absence of juglanin (5 μM). The expression of VEGF and VEGFR2 were measured by Western blot analysis. (B, C) Human bEnd.3 brain microvascular endothelial cells (HBMVECs) were exposed to OGD/R in the presence or absence of juglanin (5 μM) or 10 ng/mL VEGF-A. Brain endothelial permeability and the expression of occludin and ZO-1 (#, *, &P<0.01 vs previous column group).
Discussion
In the present study, we investigated the potential protective effects of juglanin against ischemic brain injury both in vivo and in vitro. Previous research has shown that juglanin could reduce lipopolysaccharide-induced brain inflammation in a mouse model of Parkinson’s disease.24 Other flavonoids have also been shown to confer protective effects against ischemic brain injury. For example, naringenin has been shown to inhibit angiogenesis in mice through two-pore channel 2 (TPC2) signaling.25 Pinocembrin, a flavonoid derived from honey as well as several plant species, has been suggested as a treatment for ischemic stroke due to its ability to reduce inflammation and oxidative stress, among other things.26 A recent report demonstrated that flavonoids including curcumin, lycopene, ginsenoside, vitexin, and baicalin confer neuroprotective effects through various mechanisms including preventing ischemia-induced apoptosis and increasing cell viability and tissue perfusion.27 Currently, there is limited information regarding the role of juglanin in ischemic brain injury and other diseases. Our results indicate that pretreatment with juglanin could prevent ischemic brain injury in mice by significantly reducing infarct volume and improving neurological score in MCAO mice, thereby suggesting a potent neuroprotective function of juglanin.
Mechanistically, we hypothesized that the neuroprotective effects of juglanin described above might involve modulation of VEGF, a critical factor in angiogenesis following ischemic stroke. VEGF is expressed by various cells in the CNS, including glial cells, astrocytes, microglial cells, and endothelial cells, and promotes BBB permeability while suppressing edema. VEGFR2, a receptor for VEGF, has been shown to induce decreased expression of tight junction proteins, thereby increasing vascular permeability.10 Currently, the effects of juglanin on VEGF/VEGFR2 activation have not been thoroughly studied. Here, we found that juglanin pretreatment significantly reduced the expression of VEGF as well as that of VEGFR2 both in vivo in an MCAO mouse model and in vitro in HBMVECs. This finding is congruent with the results of previous studies on other flavonoid compounds. Quercetin, for example, has been shown to reduce tumor angiogenesis and proliferation by targeting VEGFR2.28 Previous research suggests that the inhibitory effect of juglanin observed in our study may be due to its molecular structure. Factors including 3-galloylation, C2=C3 double bonds, total OH, B-ring catechol, and C-ring 3-OH have been shown to affect the capacity of various flavonoids to suppress VEGF/VEGFR2 signaling.29 Consistently, we found that juglanin prevented the reduction in tight junction protein transcription and expression induced by ischemia in vivo, which was confirmed in HBMVECs. Alterations in the expression levels of tight junction proteins such as occludin and ZO-1 are a major determining factor of BBB permeability and, therefore, neurovascular homeostasis. Similar to our findings, the flavonoid baicalin has been shown to reduce BBB permeability by increasing the expression of tight junction proteins including ZO-1.30 Other flavonoids have also demonstrated an ability of flavonoids to alter tight junction function, especially in the intestinal epithelium.31,32 Finally, we showed that the effects of juglanin against ischemic injury could be abolished by VEGF-A, which is known to trigger pathological neovascularization.33
Taken together, the findings of the present study demonstrate the vast potential of juglanin to ameliorate ischemic brain injury by reducing infarct volume and preserving tight junction functionality.
Highlights
Juglanin reduces infarct volume and improves neurological score;
Juglanin reduces blood–brain barrier permeability by increasing the expression of occludin and ZO-1;
The protective effects of juglanin are mediated through inhibition of VEGF/VEGFR2 signaling;
The protective effects of juglanin are evident both in vivo in MCAO mice and in vitro in HBMVECs.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Veenith TV, Carter EL, Geeraerts T, et al. Pathophysiologic mechanisms of cerebral ischemia and diffusion hypoxia in traumatic brain injury. JAMA Neurol. 2016;73(5):542–550. doi: 10.1001/jamaneurol.2016.0091 [DOI] [PubMed] [Google Scholar]
- 2.Kalaria RN. The role of cerebral ischemia in alzheimer’s disease. Neurobiol Aging. 2000;21(2):321–330. doi: 10.1016/S0197-4580(00)00125-1 [DOI] [PubMed] [Google Scholar]
- 3.Fang J, Alderman MH. Trend of stroke hospitalization, United States, 1988–1997. Stroke. 2001;32(10):2221–2226. doi: 10.1161/hs1001.096193 [DOI] [PubMed] [Google Scholar]
- 4.Khaku AS, Tadi P. Cerebrovascular disease. Stroke. 2020. [Google Scholar]
- 5.Khoshnam SE, Winlow W, Farzaneh M, et al. Pathogenic mechanisms following ischemic stroke. Neurol Sci. 2017;38(7):1167–1186. [DOI] [PubMed] [Google Scholar]
- 6.Merali Z, Huang K, Mikulis D, et al. Evolution of blood-brain-barrier permeability after acute ischemic stroke. PLoS One. 2017;12(2):e0171558. doi: 10.1371/journal.pone.0171558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sandoval KE, Witt KA. Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiol Dis. 2008;32(2):200–219. doi: 10.1016/j.nbd.2008.08.005 [DOI] [PubMed] [Google Scholar]
- 8.Abdullahi W, Tripathi D, Ronaldson PT. Blood-brain barrier dysfunction in ischemic stroke: targeting tight junctions and transporters for vascular protection. Am J Physiol Cell Physiol. 2018;315(3):C343–C356. doi: 10.1152/ajpcell.00095.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mărgăritescu OT, Pirici D, Mărgăritescu C. VEGF expression in human brain tissue after acute ischemic stroke. Rom J Morphol Embryol. 2011;52(4):1283–1292. [PubMed] [Google Scholar]
- 10.Wang Q, Deng Y, Huang L, et al. Hypertonic saline downregulates endothelial cell-derived VEGF expression and reduces blood-brain barrier permeability induced by cerebral ischaemia via the VEGFR2/eNOS pathway. Int J Mol Med. 2019;44(3):1078–1090. doi: 10.3892/ijmm.2019.4262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li W-L, Fraser JL, Yu SP, et al. The role of VEGF/VEGFR2 signaling in peripheral stimulation-induced cerebral neurovascular regeneration after ischemic stroke in mice. Exp Brain Res. 2011;214(4):503. doi: 10.1007/s00221-011-2849-y [DOI] [PubMed] [Google Scholar]
- 12.Scheff SW, Ansari MA. Natural compounds as a therapeutic intervention following traumatic brain injury: the role of phytochemicals. J Neurotrauma. 2017;34(8):1491–1510. doi: 10.1089/neu.2016.4718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang YJ, Cutler EG, Cho H. Therapeutic nanoplatforms and delivery strategies for neurological disorders. Nano Converg. 2018;5(1):35. doi: 10.1186/s40580-018-0168-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmad N, Umar S, Ashafaq M, et al. A comparative study of PNIPAM nanoparticles of curcumin, demethoxycurcumin, and bisdemethoxycurcumin and their effects on oxidative stress markers in experimental stroke. Protoplasma. 2013;250(6):1327–1338. doi: 10.1007/s00709-013-0516-9 [DOI] [PubMed] [Google Scholar]
- 15.Ahmad N, Ahmad R, Naqvi AA, et al. Rutin-encapsulated chitosan nanoparticles targeted to the brain in the treatment of cerebral ischemia. Int J Biol Macromol. 2016;91:640–655. doi: 10.1016/j.ijbiomac.2016.06.001 [DOI] [PubMed] [Google Scholar]
- 16.Ahmad N, Ahmad R, Abbas Naqvi A, et al. The effect of safranal loaded mucoadhesive nanoemulsion on oxidative stress markers in cerebral ischemia. Artif Cells Nanomed Biotechnol. 2017;45(4):775–787. doi: 10.1080/21691401.2016.1228659 [DOI] [PubMed] [Google Scholar]
- 17.Ahmad N, Al-Subaiec AM, Ahmad R, et al. Brain-targeted glycyrrhizic-acid-loaded surface decorated nanoparticles for treatment of cerebral ischaemia and its toxicity assessment. Artif Cells Nanomed Biotechnol. 2019;47(1):475–490. doi: 10.1080/21691401.2018.1561458 [DOI] [PubMed] [Google Scholar]
- 18.Ahmad N, Ahmad R, Ahmad FJ, et al. Poloxamer-chitosan-based naringenin nanoformulation used in brain targeting for the treatment of cerebral ischemia. Saudi J Biol Sci. 2020;27(1):500–517. doi: 10.1016/j.sjbs.2019.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Catanzaro E, Greco G, Potenza L, et al. Natural products to fight cancer: a focus on juglans regia. Toxins. 2018;10(11):469. doi: 10.3390/toxins10110469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jirásek P, Amslinger S, Heilmann J. Synthesis of natural and non-natural curcuminoids and their neuroprotective activity against glutamate-induced oxidative stress in HT-22 cells. J Nat Prod. 2014;77(10):2206–2217. doi: 10.1021/np500396y [DOI] [PubMed] [Google Scholar]
- 21.Chen L, Xiong YQ, Xu J, Wang JP, Meng ZL, Hong YQ. Juglanin inhibits lung cancer by regulation of apoptosis, ROS and autophagy induction. Oncotarget. 2017;8(55):93878–93898. doi: 10.18632/oncotarget.21317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hou GR, Zeng K, Lan HM, Wang Q. Juglanin ameliorates UVB-induced skin carcinogenesis via anti-inflammatory and proapoptotic effects in vivo and invitro. Int J Mol Med. 2018;42(1):41–52. doi: 10.3892/ijmm.2018.3601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shibuya M. Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: a crucial target for anti-and pro-angiogenic therapies. Genes Cancer. 2011;2(12):1097–1105. doi: 10.1177/1947601911423031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang FX, Xu RS. Juglanin ameliorates LPS-induced neuroinflammation in animal models of parkinson’s disease and cell culture via inactivating TLR4/NF-κB pathway. Biomed Pharmacother. 2018;97:1011–1019. doi: 10.1016/j.biopha.2017.08.132 [DOI] [PubMed] [Google Scholar]
- 25.Pafumi I, Festa M, Papacci F, et al. Naringenin impairs two-pore channel 2 activity and inhibits VEGF-induced angiogenesis. Sci Rep. 2017;7(1):1. doi: 10.1038/s41598-017-04974-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lan X, Wang W, Li Q, Wang J. The natural flavonoid pinocembrin: molecular targets and potential therapeutic applications. Mol Neurobiol. 2016;53(3):1794–1801. doi: 10.1007/s12035-015-9125-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Putteeraj M, Lim WL, Teoh SL, Yahaya MF. Flavonoids and its neuroprotective effects on brain ischemia and neurodegenerative diseases. Curr Drug Targets. 2018;19(14):1710–1720. doi: 10.2174/1389450119666180326125252 [DOI] [PubMed] [Google Scholar]
- 28.Kashyap D, Garg VK, Tuli HS, et al. Fisetin and quercetin: promising flavonoids with chemopreventive potential. Biomolecules. 2019;9(5):174. doi: 10.3390/biom9050174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cerezo AB, Winterbone MS, Moyle CW, et al. Molecular structure‐function relationship of dietary polyphenols for inhibiting VEGF‐induced VEGFR‐2 activity. Mol Nutr Food Res. 2015;59(11):2119–2131. doi: 10.1002/mnfr.201500407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu H, Wang Z, Xing Y, et al. Baicalin reduces the permeability of the blood–brain barrier during hypoxia in vitro by increasing the expression of tight junction proteins in brain microvascular endothelial cells. J Ethnopharmacol. 2012;141(2):714–720. doi: 10.1016/j.jep.2011.08.063 [DOI] [PubMed] [Google Scholar]
- 31.Chen XM, Kitts DD. Flavonoid composition of orange peel extract ameliorates alcohol-induced tight junction dysfunction in Caco-2 monolayer. Food Chem Toxicol. 2017;105:398–406. doi: 10.1016/j.fct.2017.04.009 [DOI] [PubMed] [Google Scholar]
- 32.Schulzke JD, Ploeger S, Amasheh M, et al. Epithelial tight junctions in intestinal inflammation. Mol Struct Funct Tight Junction. 2009;1165:294. [DOI] [PubMed] [Google Scholar]
- 33.Nagy JA, Dvorak AM, Dvorak HF. VEGF-A and the induction of pathological angiogenesis. Annu Rev Pathol Mech Dis. 2007;2:251–275. doi: 10.1146/annurev.pathol.2.010506.134925 [DOI] [PubMed] [Google Scholar]