Abstract
Background/Objectives:
The Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy Phase 2 (CALERIE) study showed that individuals who are non-obese were able to undergo significant calorie restriction (CR), yet the time course changes in adherence, weight, and appetite are unknown. This analysis aimed to investigate the time course changes in adherence, body weight, and appetite during the CALERIE study.
Subjects/Methods:
Overall, 143 participants (body mass index: 21.9–28.0 kg/m2) were randomized to a CR group that aimed to achieve 25% CR for 2 years. Throughout the intervention, body weight was measured, and appetite was assessed through visual analogue scales. Algorithms were utilized with body weight measurements to calculate adherence percentile score. Participants targeted an adherence percentile score of 50, though being between 80 (lowest acceptable adherence) and 10 (highest acceptable adherence) was adequate. Polynomial regression analyses were used to assess time course changes.
Results:
Polynomials indicated that adherence percentile score increased above 50 after approximately week 20, although adherence remained acceptable (adherence percentile score less than 80) (R2 = 0.89; P < 0.001). Weight loss occurred until approximately week 60 and then plateaued (R2 ≥ 0.92; P < 0.001). Hunger and thirst increased (R2 ≥ 0.30; P < 0.001), but the total increase in scale scores were less than 10 mm throughout the intervention.
Conclusions:
In individuals who are non-obese, adherence to 25% CR declines after 20 weeks, but two years of CR that stimulates a meaningful reduction in weight, promotes aging-related benefits and negligibly affects appetite is viable.
Introduction
Calorie restriction (CR) is the only known intervention that attenuates the rate of biological aging and improves lifespan in animals (1). In humans, individuals who self-impose CR exhibit numerous biological adaptions linked to lower cardiometabolic disease risk and reduced age-related comorbidities (1).
Over two years, the Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy Phase 2 (CALERIE) study showed that prolonged CR causes 10% weight loss during the first year and essentially maintained weight loss over the second year (2). However, these observations were derived from clinical assessment visits conducted once every 6 months, meaning the precise time course changes in body weight during CALERIE are unknown. It is likewise unknown how adherence shifted during the intervention. Doubly labelled water measurements were used to assess adherence during CR over the two years (2), but these data were again collected once every 6 months and thus fail to capture fluctuations that may arise between measurement points. Elucidating these fluctuations with more frequent measurements would identify periods where individuals require additional resources to augment adherence and maximize the benefits of prolonged CR.
Generally, in response to energy deficits, appetite increases (3–5), although changes are not always seen (6). Anton et al.(7) showed CR did not alter subjective appetite ratings in individuals who are non-obese, yet this study was performed over 6 months, meaning the prolonged influence of CR on appetite ratings is unstudied in individuals who are exclusively non-obese. We recently reported that hunger changes in CALERIE were negligible (8); however, further parameters of appetite such as fullness, prospective food consumption (PFC), general satisfaction, satisfaction with foods and thirst have not been examined.
Accordingly, the aim of the current analysis was to investigate the time course changes in adherence, body weight and appetite perceptions in participants who are non-obese and undergoing prolonged CR.
Methods
2.1. Ethics and trial registration
CALERIE (clinicaltrials.gov registration: NCT00427193) was performed according to the principles in the Declaration of Helsinki, and received approval from the institutional review boards at Pennington Biomedical Research Center, Washington University, Tufts University, and Duke University (9). Participants provided written informed consent before partaking in the study, and a data and safety monitoring board provided oversight of the study.
This study was a 2-year randomized controlled trial conducted from January 2007 to March 2012. Participants were randomized to a CR group or an ad libitum control group in a 2:1 ratio in favor of CR (Supplementary Figure 1). A permuted block randomization technique was employed to achieve this desired ratio and treatment assignment was completed using a telephone-based voice-response system (2).
2.2. Participants
Study recruitment, the screening process and exclusion criteria are detailed elsewhere (10). Briefly, healthy participants with a body mass index (BMI) of 22.0 to 28.0 kg/m2 and an age of 20–50 years (male) and 20–47 years (female) were considered. Participants received a telephone interview and completed three screening visits to determine eligibility.
2.3. Intervention
Details of the intervention have been reported elsewhere (11). In short, participants in the CR group targeted an immediate and sustained 25% reduction in energy intake from energy requirements determined at baseline, while the control group were instructed to maintain habitual energy intake levels (12). Participants in the CR group attended individual and group sessions that provided nutritional and behavioral strategies focused on optimizing compliance (11). Conversely, the control group did not receive any intervention and counselling sessions (11).
2.4. Computer tracking system
The computer tracking system (CTS) was employed to track participants in the CR group to individually tailor the intervention and provide support based on the participant’s needs (11,13). Information stored by the CTS was used as a barometer of compliance by documenting session attendance, self-monitoring, adherence, and body weight (11). Furthermore, toolbox algorithms adapted from previous regimens (14) guided counsellors in providing suitable and specific strategies that assisted participants in reaching their energy intake and weight loss goals.
All data derived from the CTS were input at dated individual and group counselling sessions throughout the intervention. During the first 26 weeks, participants attended weekly sessions, alternating between individuals and group sessions. Both individual and group sessions continued to occur twice monthly until month 12 and then monthly unless compliance to the intervention was compromised and more sessions were required (11). At counselling sessions, participants’ body weight was measured using calibrated scales (Scale Tronix 5200; Welch Allyn; NY) and recorded into the CTS. The CTS simultaneously utilized weight loss algorithms and body weight measures to supply adherence percentile scores, which served as an estimate of CR adherence (15). Described elsewhere (15), these algorithms were created using inputs based on body weight, weight loss data from a previous CR study (16), measurements of total daily energy expenditure at baseline and BMI (15). They also take account of the curvilinear changes in weight typically seen during weight loss regimens (15) A greater adherence percentile score is indicative of a lower percent CR achieved, while a lower adherence percentile score denotes a higher percent CR attained (15). Participants aimed to reach the 50th adherence percentile score, although to account for inter-individual variability, being between the 80th percentile (lowest acceptable weight loss) and the 10th percentile (highest acceptable weight loss) was considered acceptable, meaning that participants were considered adherent at less than 25% CR (11). These percentile limits were illustrated, providing a zone within which weight loss and compliance to CR was satisfactory (Supplementary Figure 2) (11).
The CTS was used to obtain participants’ subjective appetite and thirst perceptions at each counselling session during the two-year intervention period (11). This was achieved by participants completing a single set of 100-mm visual analogue scales (VAS) that obtained participants appetite ratings during the previous week. Specifically, measures of hunger, fullness, satisfaction, PFC, satisfaction with type of food (STF), satisfaction with amount of food (SAF) and thirst over the previous week were measured (17). Though we did not enforce any standardization stipulations prior to ratings, retrospective VAS are uniformly in agreement with daily appetite ratings (18).
2.5. Anthropometry
At baseline, month 6, month 12, month 18 and month 24, participants completed assessment visits where body weight was obtained. Calibrated scales standardized across study sites (Scale Tronix 5200; Welch Allyn; NY; USA) were used to measure participants body weight. Body weight measurements occurred in the morning after an overnight fast (≥ 8 h), with participants only wearing a pre-weighed hospital gown.
2.6. Energy intake and percent CR
Energy intake was calculated using the intake balance method, which estimated energy intake through doubly labelled water-determined energy expenditure and the change in body composition (12). Energy intake values were then used to calculate percent CR at months 6, 12, 18 and 24 in the CR group.
Statistical analysis
The present study is an exploratory analysis of secondary data; therefore, the sample size obtained in the CR group was used (9). Adherence percentile scores, weight loss and appetite rating values were averaged to yield a biweekly mean value. Data collected up to week 96 were considered in all analyses, since very few subjects attended sessions beyond this point. Repeated measures analysis of variance assessed time-course changes in outcome measures at 12-week intervals irrespective of normality (19). If data were aspherical, a Greenhouse-Geisser correction was applied for epsilon < 0.75, whereas the Huynh Feldt correction was utilized for lower levels of asphericity (> 0.75). Polynomial regression analyses were used to fit trend lines for the intervention (2 years). Week was represented in the model through linear (week), quadratic (week squared) and cubic (week cubed) terms. The term that best represented the data was determined using the lowest Akaike Information Criteria (AIC) (20), although polynomial regressions models greater than the third-order were not applied to avoid over-parameterization. Each model fit examined the main effect of the week parameter (week, week squared, week cubed) and comprised of the average line of best fit and 95% confidence limits. Unless otherwise stated, data were presented as mean (±SEM), while descriptive data were displayed as mean (±SD). Data were analyzed through SAS version 9.3 (SAS Institute, Cary, NC, USA). Statistical significance was set at P < 0.05 for all analyses.
Results
3.1. Participant characteristics
Overall, 145 participants were randomized to the CR group, although two dropped out prior to the start of the intervention, meaning 143 participants began the trial and were analyzed. Most participants in the CR group were female (69.2%), and the mean (±SD) age and BMI of the CR group were 38.0 (7.3) y and 25.2 (1.8 kg/m2), respectively (Supplementary Table 1). These and additional baseline characteristics of the CR group are detailed in Supplementary Table 1.
3.2. Weight loss and percent CR at assessment visits
Data from clinic visits showed weight loss from baseline to month 6, 12, 18 and 24 was 9.9% (0.3%), 11.5% (0.4%), 11.4% (0.4%) and 10.4% (0.4%), respectively. The CR group achieved an average CR of 11.7% (0.7%) over the two years. Mean (±SEM) percent CR attained at month 6, month 12, month 18 and month 24 was 19.5% (0.8%), 10.8% (0.7%), 8.7% (0.8%) and 8.0% (0.9%), respectively.
3.3. Computer tracking system data
Table 1 shows changes in CTS-derived outcome measures at 12-week intervals. There was a main effect of time for adherence percentile score, weight loss and percent weight loss (P < 0.001). Furthermore, subjective perceptions of hunger and thirst changed during the protocol (P ≤ 0.022), though no changes in ratings of fullness, satisfaction, PFC, SAF and STF were observed (P ≥ 0.225).
Table 1.
Computer tracking system data collected at 3-month intervals in the calorie restriction group (n = 143) of the CALERIE trial.
| Week 12 | Week 24 | Week 36 | Week 48 | Week 60 | Week 72 | Week 84 | Week 96 | P value* | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | ||
| Adherence percentile score | 43.9 | 2.4 | 49.3 | 2.4 | 57.6 | 2.2 | 65.5 | 2.1 | 66.8 | 3.1 | 71.9 | 3.0 | 69.9 | 3.0 | 68.9 | 2.9 | 0.002 |
| Weight loss (kg) | 5.6 | 0.2 | 7.8 | 0.2 | 8.9 | 0.2 | 9.4 | 0.3 | 9.9 | 0.5 | 9.2 | 0.4 | 9.7 | 0.4 | 9.6 | 0.4 | <0.001 |
| Percent weight loss | 7.6 | 0.2 | 10.6 | 0.2 | 12.0 | 0.3 | 12.7 | 0.3 | 13.0 | 0.5 | 12.4 | 0.5 | 13.0 | 0.5 | 13.1 | 0.5 | <0.001 |
| Hunger (mm) | 35 | 2 | 36 | 2 | 37 | 2 | 40 | 2 | 40 | 3 | 40 | 3 | 38 | 3 | 39 | 3 | 0.022 |
| Satisfaction (mm) | 71 | 2 | 70 | 2 | 69 | 2 | 68 | 2 | 71 | 3 | 69 | 3 | 72 | 3 | 71 | 3 | 0.225 |
| Fullness (mm) | 69 | 2 | 68 | 2 | 66 | 2 | 65 | 2 | 64 | 3 | 66 | 3 | 70 | 3 | 67 | 3 | 0.512 |
| PFC (mm) | 55 | 2 | 54 | 2 | 54 | 2 | 54 | 2 | 53 | 3 | 57 | 3 | 56 | 3 | 60 | 3 | 0.809 |
| SAF (mm) | 71 | 2 | 70 | 2 | 69 | 2 | 69 | 2 | 70 | 3 | 69 | 3 | 72 | 3 | 72 | 3 | 0.643 |
| STF (mm) | 73 | 2 | 73 | 1 | 71 | 2 | 70 | 2 | 73 | 2 | 72 | 2 | 74 | 2 | 73 | 2 | 0.916 |
| Thirst (mm) | 36 | 2 | 41 | 2 | 39 | 2 | 39 | 2 | 41 | 3 | 39 | 4 | 41 | 3 | 45 | 4 | 0.012 |
Abbreviations: PFC, prospective food consumption; SAF, satisfaction with amount of food; STF, satisfaction with type of food.
Repeated measures analysis of variance.
Values are mean (±SEM).
Figure 1A, 1B and 1C display the mean (±SEM) adherence percentile score, weight loss from baseline and percent weight loss from baseline, respectively, while Table 2 documents the results of the modelled trajectories for these endpoints. The trajectory of the adherence percentile score was best fit with a third-order polynomial (R2 = 0.89; P < 0.001). This showed that adherence percentile score was increasing after approximately week 20 of the intervention, indicating adherence to the prescribed CR was decreasing (Figure 2A). Adherence percentile score increased until approximately week 60, with a steady plateau thereafter (Figure 2A). Weight loss over the two years was best represented with a second-order polynomial (R2 = 0.93; P < 0.001), illustrating that body weight declined rapidly within the first 40 weeks and then more steadily until it began to plateau after approximately week 60 (Figure 2B). A second-order polynomial regression was similarly the best fit for percent weight loss (R2 = 0.92; P < 0.001), with the line of best fit showing the greatest decline during the first 26 weeks before plateauing after approximately week 60 (Figure 2C).
Figure 1.
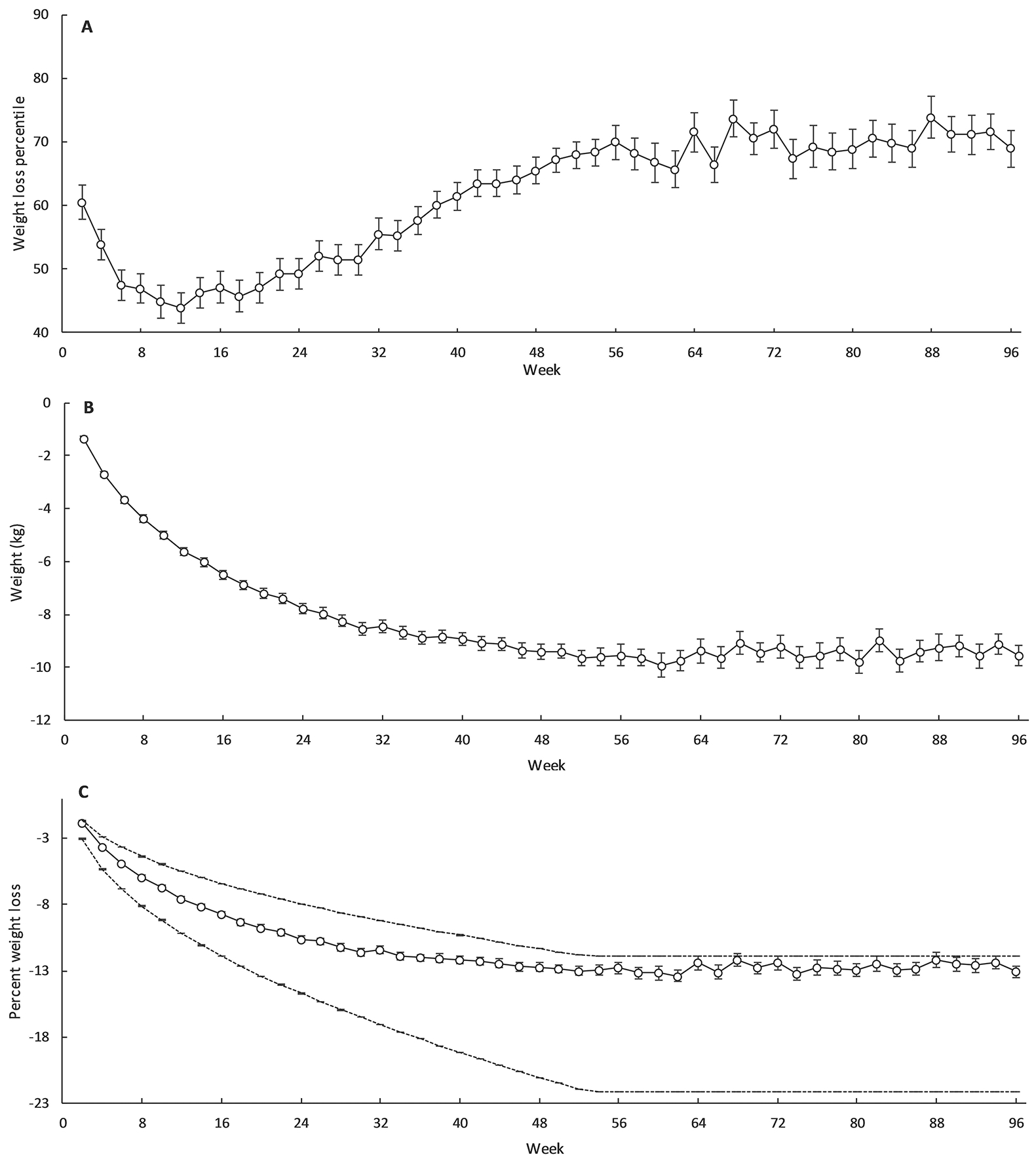
Adherence percentile score (A), weight loss (B) and percentage weight loss along with the accepted upper (80th percentile) and lower (10th percentile) boundaries of percent weight loss (C) and in the calorie restriction group during CALERIE. Values are mean ± SEM. Bold line indicates mean values, dashed lines indicate 10th and 80th percentile.
Table 2.
Parameter estimates of weight loss, percentage weight loss, adherence percentile score and visual analogue scale ratings during CALERIE in the calorie restriction group (n = 143) from polynomial regression analyses.
| Intercept | Week | Week squared | Week cubed | ||
|---|---|---|---|---|---|
| Adherence percentile score | Estimate | 50.483 | −0.356 | 0.020 | −0.0002 |
| SEM | 2.052 | 0.180 | 0.004 | <0.001 | |
| P | <0.001 | 0.054 | <0.001 | <0.001 | |
| Weight loss | Estimate | −2.899 | −0.216 | 0.001 | - |
| SEM | 0.251 | 0.012 | <0.001 | ||
| P | <0.001 | <0.001 | <0.001 | ||
| Percentage weight loss | Estimate | −3.926 | −0.294 | 0.002 | - |
| SEM | 0.346 | 0.016 | <0.001 | ||
| P | <0.001 | <0.001 | <0.001 | ||
| Hunger | Estimate | 35.275 | 0.056 | - | - |
| SEM | 0.715 | 0.013 | |||
| P | <0.001 | <0.001 | |||
| Fullness | Estimate | 66.374 | 0.007 | - | - |
| SEM | 0.630 | 0.011 | |||
| P | <0.001 | 0.494 | |||
| Satisfaction | Estimate | 69.115 | −0.004 | - | - |
| SEM | 0.542 | 0.010 | |||
| P | <0.001 | 0.704 | |||
| PFC | Estimate | 54.433 | 0.019 | - | - |
| SEM | 0.579 | 0.010 | |||
| P | <0.001 | 0.067 | |||
| STF | Estimate | 71.651 | 0.002 | - | - |
| SEM | 0.749 | 0.013 | |||
| P | <0.001 | 0.888 | |||
| SAF | Estimate | 70.308 | −0.010 | - | - |
| SEM | 0.576 | 0.010 | |||
| P | <0.001 | 0.334 | |||
| Thirst | Estimate | 35.814 | 0.058 | - | - |
| SEM | 0.652 | 0.012 | |||
| P | <0.001 | <0.001 |
Abbreviations: PFC, prospective food consumption; SAF, satisfaction with amount of food; STF, satisfaction with type of food.
Values are estimates (±SEM).
Figure 2.
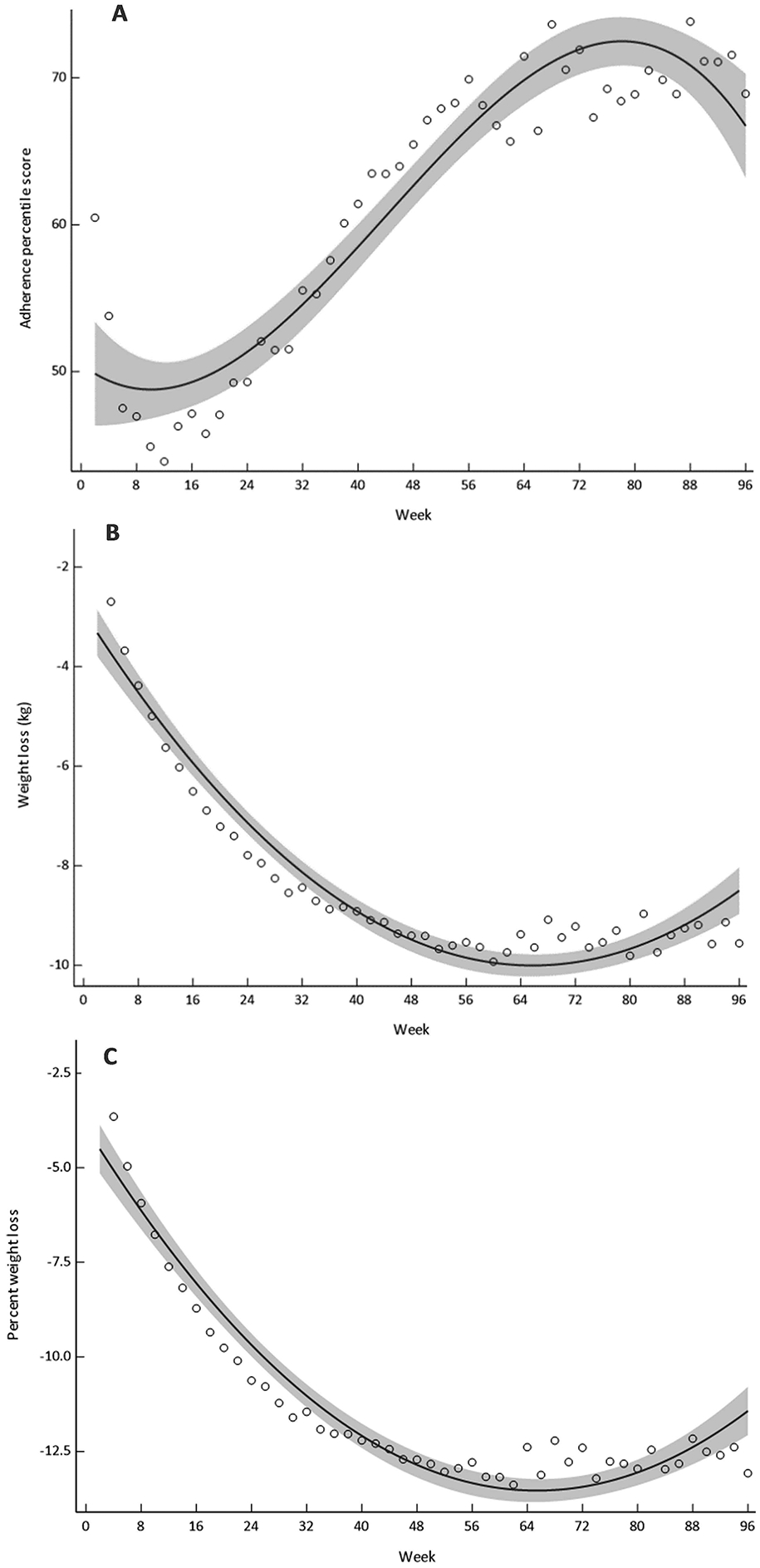
Modelled regression curves for adherence percentile score (A), weight loss (B) and percentage weight loss (C) during CALERIE. Bold line indicates best fit, shaded area indicates 95% confidence limits.
Mean (±SEM) VAS ratings during the intervention are shown in Figures 3A, 3B, 2C, 3D, 3E, 3F and 3G; modelled trajectories for VAS ratings are documented in Table 2. Hunger values increased over the CR intervention, with the best-fit regression showing a linear increase of 0.056 mm every 2 weeks (R2 = 0.30; P < 0.001; Figure 4A). Likewise, the best-fit regression for ratings of thirst was linear, indicating that thirst perceptions increased by 0.058 mm every 2 weeks during the intervention (R2 = 0.35; P < 0.001; Figure 4G). There were, on the contrary, no changes in subjective ratings of fullness, satisfaction, PFC, STF and SAF during the intervention (R2 ≤ 0.07; P ≥ 0.07; Figure 4).
Figure 3.
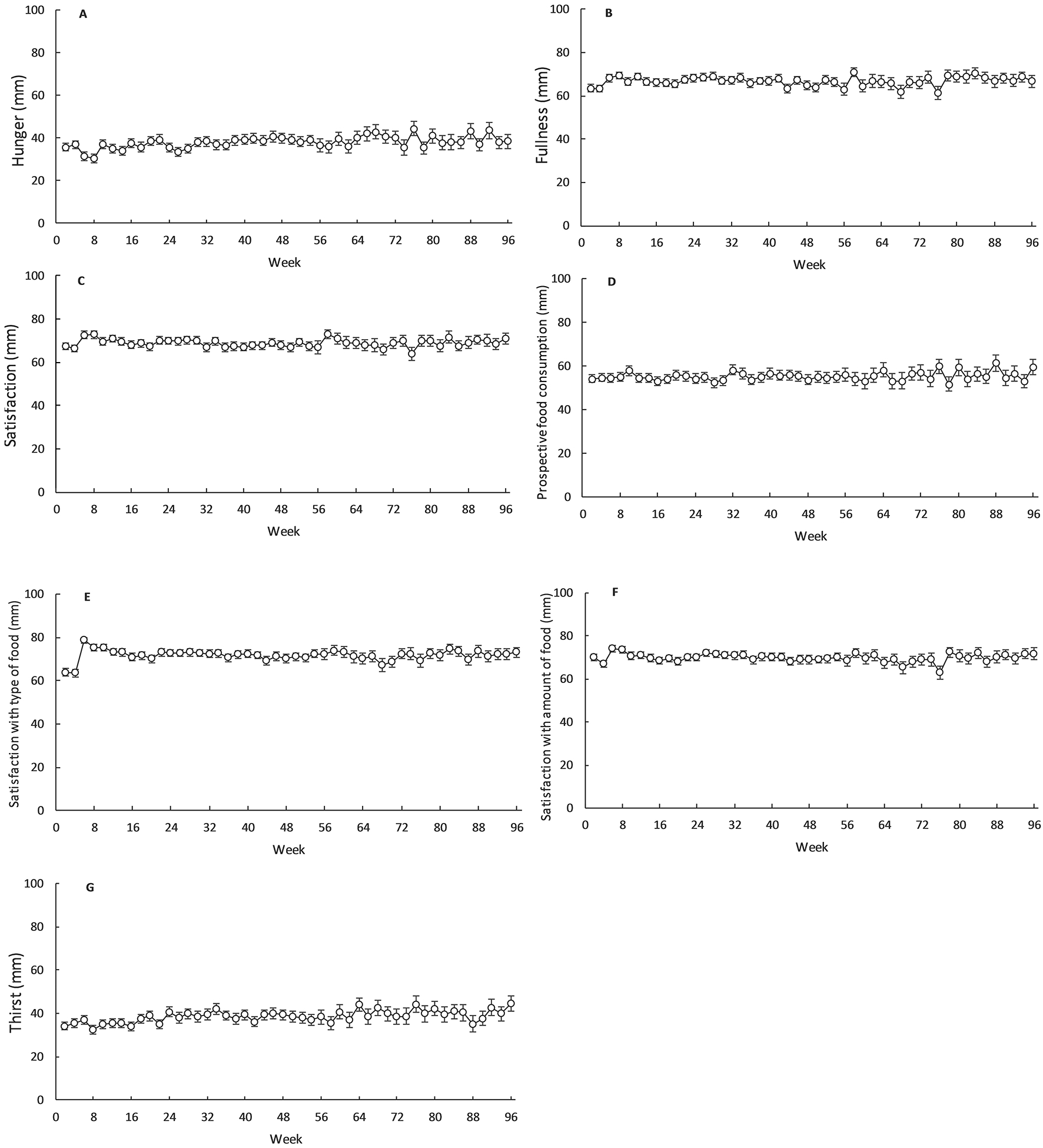
Hunger (A), fullness (B), satisfaction (C), prospective food consumption (D), satisfaction with type of food (E), satisfaction with amount of food (F) and thirst (G) in the calorie restriction group during CALERIE. Values are mean ± SEM.
Figure 4.
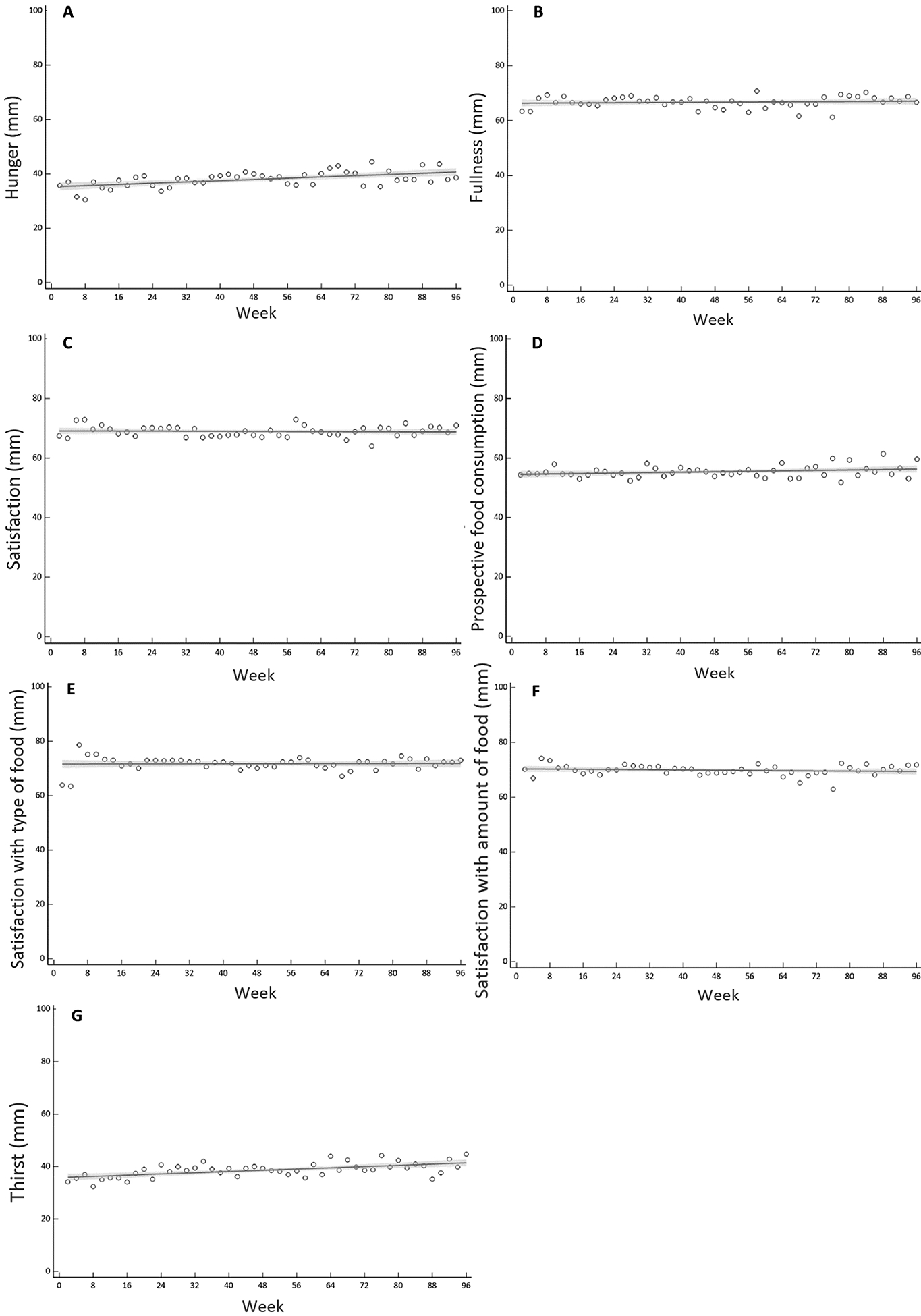
Modelled regression curves for hunger (A), fullness (B), satisfaction (C), prospective food consumption (D), satisfaction with type of food (E), satisfaction with amount of food (F) and thirst (G) during CALERIE. Bold line indicates best fit, shaded area indicates 95% confidence limits.
Discussion
In the present study, we observed that adherence to a two-year CR intervention decreased within 20 weeks and then continued to fall to week 60. However, an acceptable degree of CR was still evident relative to baseline, and we showed that body weight declined within the first 60 weeks of the intervention before plateauing. Furthermore, hunger and thirst VAS ratings increased in a linear fashion, but there were no changes in other appetite parameters.
The CALERIE study induced a reduction in oxidative damage (21), improved cardiometabolic disease risk factors (22) and did not alter quality of life (23) in the CR group. Our novel findings showed a rise in adherence percentile score during CALERIE from the intercept (50th percentile) at approximately week 20, with a continual increase until roughly week 60, indicating that percent CR continued to fall until this point. These results imply that compliance to 25% CR is challenging and are consonant with our energy intake measures at clinic visits, which showed percent CR decreased to 10.8% by the end of year 1 and then further lowered to 8.0% CR at the end of the intervention (2). Given greater CR has been associated with greater benefits to longevity (24), further innovative strategies may be needed to bolster percent CR and age-related improvements, particularly after 20 weeks. However, participants were experiencing CR relative to baseline and were generally within the trials acceptable limits (adherence percentile score between 80 and 10), which were implemented to account for diverse adherence between participants (9,11). The plateau in adherence percentile score at approximately week 60 suggests that participants reached a percent CR that is more sustainable. Indeed, at present, for individuals in obesogenic environments who are non-obese and looking to adopt CR as a means of improving longevity, average CR at approximately 8–10% represents an achievable and safe degree of energy restriction over two years.
Our regression models suggest that weight loss peaked at approximately 12% at week 60 and then plateaued. This extends our clinic measures which showed that the CR group displayed 8.3 kg of weight loss during the first 52 weeks prior to plateauing to 7.6 kg weight loss at the end of the trial (2). Though weight loss in the first year would have been greater had compliance (percent CR) not declined, participants were notably within acceptable boundaries of weight loss (between the 80th and 10th percentile), and our data shows that individuals who are non-obese are able to lose and maintain clinically significant weight loss beyond one year. The maintenance of weight in year 2, in particular after week 60, suggests that the CR group reached a new equilibrium where energy intake met energy expenditure and weight was relatively stable (25). That is, despite the reduction in CR adherence, energy intake after week 60 was: 1) still lower than baseline, 2) relatively stable, and 3) commensurate with lower energy expenditure values reported previously (2).
The weight loss achieved in CALERIE was impressive, particularly due to the inclusion of normal weight participants. In individuals who are overweight or obese, a meta-analysis showed body weight decreased by 5% after 26 weeks in response to diet interventions, with weight loss maintained at 4.6% and 4.4% at weeks 52 and 104, respectively (26). Some also see significant weight regain at year 2 of weight loss trials (27,28), and this has been attributed to body weight set points that initiate biological processes to defend body weight once it falls below a particular threshold (29). The weight changes seen in our group of individuals without obesity are an ostensible contradiction to this set point hypothesis. More in line with alternative models of weight control that underscore the importance of environment as well as inherent biology (30), our findings potentially imply that conserved mechanisms of weight control can be overcome by rigorous lifestyle interventions (29). Such interventions could have included the evidence-based sessions supplied throughout the trial that aimed to promote goal setting, enlist social support, improve calorie estimations, limit portion size, increase self-monitoring and prevent relapse (11). It is, however, noteworthy that our model proposed an upswing at weight at the end of the two-year CR period. Although it is difficult to predict the weight loss changes if CALERIE had been extended, these data could imply that marked weight regain may have occurred if the trial had been lengthened.
The stability of appetite perceptions is crucial in attenuating weight regain and maintaining dietary regimens within obesogenic environments (31). Our data indicates that hunger and thirst increased in the CR group, yet the mean increase was only 0.056 mm and 0.058 mm every 2 weeks, respectively, which equates to an increase of ~3 mm over the course of the intervention. Researchers have suggested that a change of 10% (10 mm) in VAS scores is meaningful and reasonable in the context of appetite (17,32); thus, it is likely that the rise in hunger and thirst seen in the CR group has little clinical relevance. We additionally saw no change in fullness, satisfaction, PFC, STF and SAF, affirming that the CR group experienced trivial changes in appetite and satisfaction with food. This supports Anton et al. (7) who documented that appetite VAS ratings were not significantly altered during 6 months of CR in individuals who were non-obese. These also corroborate our findings from clinic visits that show that hunger changes, examined with the food craving-state questionnaire and eating inventory, were negligible in the CR group compared to the ad libitum group (8). It is particularly striking that appetite perceptions were not meaningfully altered in the first 12 months when weight loss was greatest. Indeed, 12 months of weight loss has been shown to decrease concentrations of tonic appetite suppressant leptin (33) and increase the orexigenic hormone ghrelin (34), potentially leading to a rise in appetite and weight regain in individuals with obesity (5). While our findings may indicate that appetite perceptions are differentially altered during CR because of obesity status, it is equally possible that the implementation of strategies that assisted the CR group manage hunger, satiety and food cravings are implicated in our findings (11). This included recommending diets rich in protein and fiber, which have been effective in ameliorating elevations in appetite (35,36) and should be considered in controlling appetite during prolonged energy deficits. Further, it is viable that rises in appetite were blunted by the general decline in adherence and therefore additional work is needed to determine how changes in appetite are related to fixed changes in energy intake during prolonged CR regimens.
The CALERIE trial was an extensive study with high quality outcome measures, but this work is a secondary analysis and limitations are present. Though our goal was to examine appetite in the preceding week using validated measures, a potential limitation of our study was that we did not standardize the timing of appetite measurements. We must also acknowledge that our weight loss algorithms were derived from only one year of CR, which means our weight loss projections in the second year are more hypothetical (2). In addition, the current analysis did not contain data from the ad libitum control group, as ratings were principally used to monitor the CR group, and the control group were not provided any interventional sessions during the trial, rendering consistent appetite measurements difficult. Although it is possible the rigorous nature of the CR intervention lessened the need for a control group, appetite has been shown to increase in CR and control groups during short-term CR studies (7). This suggests our analyses would have benefited from the control group and additional randomized controlled trials are needed. Finally, given our data was averaged biweekly, our time course analyses did not incorporate a true baseline value.
In conclusion, in humans who are non-obese, a well-assisted CR intervention induces significant weight loss for just over a year and maintains weight loss without triggering meaningful changes in appetite. Adherence to a 25% CR diet is attainable for approximately 20 weeks, with continued reductions until 8–10% CR. While these findings indicate that 25% CR is challenging in obesogenic environments, a tailored CR protocol can stimulate a reduction in body weight that does not increase appetite and leads to health benefits related to longevity. Longer follow-ups are now required to determine if these changes are maintained beyond two years of CR.
Supplementary Material
Acknowledgements
We are indebted to the study participants. The efforts of the CALERIE data coordinating center (James Rochon, Kim Huffman and William Krauss) are also acknowledged and greatly appreciated. CALERIE 2 data, including the data reported in this manuscript, can be accessed at https://calerie.duke.edu/.
Funding
The research was supported by the National Institute on Aging and the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (grants U01AG022132, U01AG020478, U01AG020487, and U01AG020480); National Obesity Research Center (grant P30 DK072476), sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases; and the National Institute of General Medical Sciences of the National Institutes of Health, which funds the Louisiana Clinical and Translational Science Center (grant 1 U54 GM104940).
The CALERIE data repository is supported by the CALERIE Research Network and contains an array of clinical, physiologic, and molecular data. More information about repository contents and how to request data and samples can be found at the following website: https://calerie.duke.edu/
Footnotes
Conflicts of interest
The authors declare no conflict of interest.
References
- 1.Redman LM, Ravussin E. Caloric restriction in humans: Impact on physiological, psychological, and behavioral outcomes. Antioxid Redox Signal. 2011;14:275–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ravussin E, Redman LM, Rochon J, Das SK, Fontana L, Kraus WE, et al. A 2-year randomized controlled trial of human caloric restriction: Feasibility and effects on predictors of health span and longevity. Journals Gerontol - Ser A Biol Sci Med Sci. 2015;70(9):1097–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doucet E, St-Pierre S, Alméras N, Tremblay A. Relation between appetite ratings before and after a standard meal and estimates of daily energy intake in obese and reduced obese individuals. Appetite. 2003;40:137–43. [DOI] [PubMed] [Google Scholar]
- 4.McGuire MT, Wing RR, Klem ML, Lang W, Hill JO. What predicts weight regain in a group of successful weight losers? J Consult Clin Psychol. 1999;67:177–85. [DOI] [PubMed] [Google Scholar]
- 5.Sumithran P, Prendergast LA, Delbridge E, Purcell K, Shulkes A, Kriketos A, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365(17):1597–604. [DOI] [PubMed] [Google Scholar]
- 6.Wadden TA, Vogt RA, Andersen RE, Bartlett SJ, Foster GD, Wilk J, et al. Exercise in the treatment of obesity: Effects of four interventions on body composition, resting energy expenditure, appetite, and mood. J Consult Clin Psychol. 1997;65:269–77. [DOI] [PubMed] [Google Scholar]
- 7.Anton SD, Han H, York E, Martin CK, Ravussin E, Williamson DA. Effect of calorie restriction on subjective ratings of appetite. J Hum Nutr Diet. 2009;22:141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dorling JL, Bhapkar M, Das SK, Racette SB, Apolzan JW, Fearnbach SN, et al. Change in self-efficacy, eating behaviors and food cravings during two years of calorie restriction in humans without obesity. Appetite. 2019;143:104397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rochon J, Bales CW, Ravussin E, Redman LM, Holloszy JO, Racette SB, et al. Design and conduct of the CALERIE study: comprehensive assessment of the long-term effects of reducing intake of energy. Journals Gerontol Ser A Biol Sci Med Sci. 2011;66(1):97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stewart TM, Bhapkar M, Das S, Galan K, Martin CK, McAdams L, et al. Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy Phase 2 (CALERIE Phase 2) screening and recruitment: Methods and results. Contemp Clin Trials. 2013;34(1):10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rickman AD, Williamson DA, Martin CK, Gilhooly CH, Stein RI, Bales CW, et al. The CALERIE Study: Design and methods of an innovative 25% caloric restriction intervention. Contemp Clin Trials. 2011;32:874–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Racette SB, Das SK, Bhapkar M, Hadley EC, Roberts SB, Ravussin E, et al. Approaches for quantifying energy intake and %calorie restriction during calorie restriction interventions in humans: the multicenter CALERIE study. AJP Endocrinol Metab. 2012;302:E441–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anton SD, LeBlanc E, Allen HR, Karabetian C, Sacks F, Bray G, et al. Use of a computerized tracking system to monitor and provide feedback on dietary goals for calorie-restricted diets: The POUNDS LOST study. J Diabetes Sci Technol. 2012;6(5):1216–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wadden TA, West D, Delahanty LM, Jakicic JM, Rejeski J, Williamson DA, et al. The look AHEAD study: A description of the lifestyle intervention and the evidence supporting it. Obesity. 2006;14:737–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pieper C, Redman L, Racette S, Roberts S, Bhapkar M, Rochon J, et al. Development of adherence metrics for caloric restriction interventions. Clin Trials. 2011;8(2):155–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Racette SB, Weiss EP, Villareal DT, Arif H, Steger-May K, Schechtman KB, et al. One year of caloric restriction in humans: Feasibility and effects on body composition and abdominal adipose tissue. Journals Gerontol - Ser A Biol Sci Med Sci. 2006;61(9):943–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Dsorders J Int Assoc Study Obes. 2000;24(1):38–48. [DOI] [PubMed] [Google Scholar]
- 18.Womble LG, Wadden TA, Chandler JM, Martin AR. Agreement between weekly vs. daily assessment of appetite. Appetite. 2003;40(2):131–5. [DOI] [PubMed] [Google Scholar]
- 19.Maxwell S, Delaney H Designing experiments and analyzing data: A model comparison perspective. 2nd ed. Routledge; 1990. 1990 p. [Google Scholar]
- 20.Akaike H Information theory and an extension of the maximum likelihood principle In: Petrov B, Csaki F, editors. Second International Symposium on Information Theory. Budapest; 1998. p. 267–81. [Google Scholar]
- 21.Redman LM, Smith SR, Burton JH, Martin CK, Il’yasova D, Ravussin E. Metabolic slowing and reduced oxidative damage with sustained caloric restriction support the rate of living and oxidative damage theories of aging. Cell Metab. 2018;27:805–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Most J, Gilmore LA, Smith SR, Han H, Ravussin E, Redman LM. Significant improvement in cardiometabolic health in healthy nonobese individuals during caloric restriction-induced weight loss and weight loss maintenance. Am J Physiol Metab. 2018;314:E396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin CK, Bhapkar M, Pittas AG, Pieper CF, Das SK, Williamson DA, et al. Effect of calorie restriction on mood, quality of life, sleep, and sexual function in healthy nonobese adults the CALERIE 2 randomized clinical trial. JAMA Intern Med. 2016;176(6):743–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weindruch R, Walford RL, Fligiel S, Guthrie D. The retardation of aging in mice by dietary restriction: Longevity, cancer, immunity and lifetime energy intake. J Nutr. 1986;116(4):641–54. [DOI] [PubMed] [Google Scholar]
- 25.Heymsfield SB, Thomas D, Nguyen AM, Peng JZ, Martin C, Shen W, et al. Voluntary weight loss: Systematic review of early phase body composition changes. Obes Rev. 2011;12:e348–61. [DOI] [PubMed] [Google Scholar]
- 26.Franz MJ, VanWormer JJ, Crain AL, Boucher JL, Histon T, Caplan W, et al. Weight-loss outcomes: A systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J Am Diet Assoc. 2007;107(10):1755–67. [DOI] [PubMed] [Google Scholar]
- 27.The Look AHEAD Research Group. Eight-year weight losses with an intensive lifestyle intervention: The look AHEAD study. Obesity. 2014;22(1):5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Müller MJ, Geisler C, Heymsfield SB, Bosy-Westphal A. Recent advances in understanding body weight homeostasis in humans. F1000Research. 2018;7:F1000 Faculty Rev-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Speakman JR. The evolution of body fatness: Trading off disease and predation risk. J Exp Biol. 2018;221:jeb167254. [DOI] [PubMed] [Google Scholar]
- 31.Giskes K, van Lenthe F, Avendano-Pabon M, Brug J. A systematic review of environmental factors and obesogenic dietary intakes among adults: Are we getting closer to understanding obesogenic environments? Obes Rev. 2011;12(5):e95–106. [DOI] [PubMed] [Google Scholar]
- 32.Blundell J, De Graaf C, Hulshof T, Jebb S, Livingstone B, Lluch A, et al. Appetite control: Methodological aspects of the evaluation of foods. Obes Rev. 2010;11(3):251–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keim NL, Stern JS, Havel PJ. Relation between circulating leptin concentrations and appetite during a prolonged, moderate energy deficit in women. Am J Clin Nutr. 1998;68:794–801. [DOI] [PubMed] [Google Scholar]
- 34.Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346(21):1623–30. [DOI] [PubMed] [Google Scholar]
- 35.Howarth NC, Saltzman E, Roberts SB. Dietary fiber and weight regulation. Nutr Rev. 2001;59:129–39. [DOI] [PubMed] [Google Scholar]
- 36.Perri M, JA C. Improving the maintenance of weight lost in behavioral treatment of obesity Wadden TA, Stunkard AJ, editors. Handbook of Obesity Treatment, New York, NY: Guilford Press; 2002. 357–379 p. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


