Abstract
Prostate cancer chemoprevention by sulforaphane (SFN), which is a metabolic by-product of glucoraphanin found in broccoli, in preclinical models is associated with induction of both apoptosis and autophagy. However, the molecular mechanism underlying SFN-mediated autophagy, which is protective against apoptotic cell death by this phytochemical, is still poorly understood. The present study demonstrates a role for lysosome-associated membrane protein 2 (LAMP2) in SFN-mediated autophagy and apoptosis. Western blotting revealed dose-dependent induction of LAMP2 protein after treatment with SFN as well as its naturally-occurring analogs in PC-3 and 22Rv1 human prostate cancer cell lines that was confirmed by microscopy (SFN). The mRNA level of LAMP2 was also increased upon treatment with SFN in both cell lines. SFN-mediated increase in the level of autophagy marker microtubule-associated protein light-chain 3B (LC3BII) was augmented by RNA interference of LAMP2 in PC-3 and 22Rv1 cells. Apoptosis induction by SFN treatment was also increased significantly by knockdown of the LAMP2 protein in PC-3 and 22Rv1 cells. Augmentation of SFN-mediated apoptosis by RNA interference of LAMP2 was accompanied by induction and activation of pro-apoptotic protein Bak. Oral administration of SFN to TRAMP mice also resulted in induction of LAMP2 protein expression. Targeted microarray in SFN-treated PC-3 cells revealed induction of many autophagy-related genes (e.g., HSP90AA1, NRF2, etc.) and their expression positively correlated with that of LAMP2 in prostate cancer TCGA. In conclusion, the present study reveals that induction of LAMP2 by SFN inhibits its ability to induce apoptotic cell death at least in human prostate cancer cells.
Keywords: Sulforaphane, LAMP2, Autophagy, Prostate Cancer, Chemoprevention
Introduction
Prostate cancer is a leading cause of cancer-related fatality in American men (1). Chemoprevention is conceptually attractive for reducing the death and suffering from prostate cancer, but a clinically acceptable intervention for this purpose is still lacking. For example, the likelihood of prostate cancer chemoprevention was not illustrated by large clinical trials of 5α-reductase inhibitors (finasteride or dutasteride) or selenium plus vitamin E combination regimen (2–4). At the same time, naturally-occurring small molecules isolated from dietary sources (e.g., broccoli and other cruciferous vegetables) or medicinal plants continue to be investigated for chemoprevention of different cancers including malignancy of the prostate (5–7). Sulforaphane (SFN), a metabolic by-product of a glucosinolate (glucoraphanin) that is abundant in broccoli sprouts, has received wide attention for chemoprevention of prostate and other cancers (8). Preclinical research to determine the mechanisms of cancer chemoprevention by SFN was sparked by epidemiological studies that suggested a possible reduction in prostate cancer risk with increased dietary intake of broccoli and other cruciferous vegetables (9–11). In one such epidemiological study, intake of cruciferous vegetables as well as broccoli was associated with decreased prostate cancer risk (11).
Both human prostate cancer cell lines and rodent models (xenograft and transgenic mouse models) have been utilized to demonstrate inhibitory effect of SFN on prostate cancer growth in vitro and in vivo (8). Survival of LNCaP and PC-3 human prostate cancer cell lines was inhibited significantly by SFN treatment in vitro in association with apoptosis induction (12–14). Interestingly, a normal human prostate epithelial cell line PrEC was insensitive to apoptosis induction by SFN (14). Mouse models of prostate cancer have been used to evaluate the in vivo anticancer activity of SFN and broccoli sprout (13,15,16). Our own group showed that oral administration of SFN [5.6 μmol SFN in 0.1 mL of phosphate-buffered saline (PBS), 3 times/week] inhibited the growth of PC-3 human prostate cancer cell line subcutaneously implanted in male athymic mice by >50% without any side effects (13). We showed further that oral administration of 6 μmol SFN 3 times/week significantly inhibited prostate carcinogenesis in TRAMP mice (15). The incidence and multiplicity of pulmonary metastasis in the TRAMP model was also decreased upon SFN administration when compared to control mice (15). Prevention of prostate cancer in TRAMP mice has also been demonstrated by dietary feeding of broccoli sprout (16).
Our laboratory was the first to demonstrate autophagy induction by SFN in PC-3 and LNCaP human prostate cancer cell lines in vitro (17). We also found that SFN-mediated autophagy represented a defense mechanism against apoptotic cell death by this phytochemical (17). Because apoptotic cell death constitutes an important mechanism in anticancer effect of SFN (8,12–14), we hypothesized that in vivo prostate cancer chemoprevention by SFN may be amenable to augmentation by autophagy inhibition. Indeed, the incidence of poorly differentiated prostate cancer was decreased significantly upon treatment of TRAMP mice with SFN plus an autophagy inhibitor (chloroquine) when compared to control mice (18).
In this study, we examined potential contribution of lysosome-associated membrane protein 2 (LAMP2) in autophagy and apoptosis induction by SFN. The rationale for investigating potential impact of LAMP2 on anticancer activity of SFN stemmed from the following observations: (a) the expression of LAMP2 was suggested to be a prognostic factor in prostate cancer (19), and (b) because of its localization to the lysosomal membrane, which is a critical cellular component in the autophagic machinery (20), the LAMP2 has been implicated in regulation of autophagy in the context of prostate and other cancers (21,22). For example, up-regulated expression of LAMP2 and autophagy induction was linked to neuroendocrine differentiation in a human prostate cancer cell line (21). LAMP2A overexpression in breast cancer cells was suggested to promote their survival (23). Moreover, LAMP2A deficiency in breast cancer cells enhanced their sensitivity to a clinically used drug doxorubicin (23). Another study demonstrated that LAMP2A was required for hepatocellular carcinoma xenograft growth by overcoming apoptosis (24). A microRNA (miR-487b-5p) was shown to regulate temozolomide resistance in lung cancer cells involving LAMP2-mediated autophagy (25). Interestingly, LAMP2A knockdown by small-hairpin RNA (shRNA) transfection prevented activation of tumor-associated macrophages as well as tumor growth (26). Collectively, these studies implicate LAMP2 in prostate and other cancer types (21–26).
Materials and Methods
Ethics statement
Mouse study to determine the chemopreventive efficacy of oral SFN administration in TRAMP mice (18) was approved by the Institutional Animal Care and Use Committee. Prostate adenocarcinoma sections from control and SFN-treated mice from this study were used for immunohistochemical analysis of LAMP2 expression.
Reagents
SFN and its naturally-occurring analogs, including thio-derivatives (iberverin, erucin, and berteroin), sulfinyl-derivatives (iberin and alyssin), and sulfonyl-derivatives (cheirolin, erysolin, and alyssin sulfone) were purchased from LKT Laboratories (St. Paul, MN), whereas 4′,6-diamidino-2-phenylindole (DAPI) was purchased from Sigma-Aldrich (St. Louis, MO). Stock solutions of SFN and its analogs were prepared in dimethyl sulfoxide (DMSO) and diluted with complete media immediately before use. An equal volume of DMSO (final concentration, 0.03%) was added to controls. Enzalutamide (ENZ) was purchased from Selleckchem (Houston, TX). LysoTracker Green, Mitotracker Red, Alexa Fluor 568 goat anti-mouse antibody, Alexa Fluor 488 goat anti-rabbit antibody, and Alexa Fluor 568 goat anti-rabbit antibody were purchased from Invitrogen-Life Technologies (Carlsbad, CA). Annexin V-FITC/propidium iodide (PI) Apoptosis Detection kit was purchased from BD Biosciences (San Diego, CA). Anti-β-Actin antibody was from Sigma-Aldrich; antibodies against LAMP2 for immunoblotting and immunocytochemistry were from Abcam (Cambridge, MA); an antibody against LAMP2 for immunohistochemistry was from LSBio (Seattle, WA); anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody was from GeneTex (Irvine, CA); antibodies against LC3B, UV radiation resistance associated (UVRAG), nuclear factor erythroid 2-related factor 2 (NRF2), and Bcl-2 were from Cell Signaling Technology (Danvers, MA); anti-LC3 antibody for immunocytochemistry was from MBL International (Woburn, MA); antibodies against Bcl-xL, Bak, heat shock protein (HSP) 90AA1, HSPA8, nuclear factor κB (NFκB) p50 subunit, and Bax were from Santa Cruz Biotechnology (Dallas, TX); anti-active Bax (clone 6A7) antibody for immunocytochemistry was from BD Pharmingen (San Diego, CA); anti-Bak (Ab-1) antibody for immunocytochemistry was from Millipore (Burlington, MA). The LAMP2-targeted shRNA was obtained from Santa Cruz Biotechnology, whereas a non-specific control shRNA was from Qiagen (Valencia, CA).
Cell lines
The PC-3 and 22Rν1 cells were obtained from the American Type Culture Collection (Manassas, VA). PC-3 cells were cultured in F-12K nutrient mixture (Kaighn’s modification) supplemented with 10% fetal bovine serum and antibiotic mixture. Growth medium for 22Rv1 cells was RPMI 1640 supplemented with 10% fetal bovine serum, 10 mmol/L HEPES, 1 mmol/L sodium pyruvate, 2.5 g/L glucose, and antibiotic mixture. These cell lines were last authenticated by us in March of 2017. PC-3 and 22Rv1 cells stably transfected with control shRNA or LAMP2-targeted shRNA were cultured in growth media in the presence of 1 μg/mL of puromycin.
Determination of cell viability
PC-3 (5×104 cells) and 22Rv1 cells (1.5×105 cells) were plated in 12-well plates in triplicate and then treated with DMSO or SFN or enzalutamide (ENZ) for specified time points. Harvested cells were mixed with trypan blue solution and viable cells were counted under an inverted microscope.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA from control and SFN-treated cells was isolated using RNeasy kit from Qiagen. The cDNA was synthesized and reverse transcribed using oligo(dT)20 primer and SuperScript III Reverse Transcriptase. The PCR was performed using SYBR™ Green PCR Master Mix (Thermo Fisher Scientific). PCR primers and the amplification conditions were as follows: LAMP2 forward: 5’-TGGTGTTGCAGCTGTTGTTG-3’; LAMP2 reverse: 5’-CGTAAGCAATCACTATAACGATAATCAA-3’; GAPDH forward: 5’-GGACCTGACCTGCCGTCTAGAA-3’; GAPDH reverse: 5’-GGTGTCGCTGTTGAAGTCAGAG-3’; 95ºC for 5 minutes followed by 40 cycles of 95ºC for 30 seconds, 60ºC for 1 minute, and 72ºC for 30 seconds.
Western blotting
Cells (7~7.5×105 cells/10-cm dish for PC-3 and 2×106 cells/10-cm dish for 22Rv1) were treated with DMSO (control) or the indicated doses of SFN or its analogs for specified time periods, and both floating and attached cells were collected. Cells were lysed as described by us previously (27). Other details of immunoblotting have been described by us previously (27). Immunoreactive bands were visualized by Chemiluminescence method. The blots were stripped and re-probed with anti-β-Actin or anti-GAPDH antibody to correct for differences in protein loading. Change in protein level was determined by densitometric scanning of the immunoreactive band and corrected for the loading control.
Immunocytochemical analysis
Cells (5×104 cells for PC-3 and 1×105 cells for 22Rv1) were plated on coverslips in 12-well plates, allowed to attach for overnight, and then exposed to DMSO (control) or SFN for specified time points. Cells were incubated with MitoTracker or Lysotracker (Lyso) to stain mitochondria or lysosomes for 15 or 30 minutes, respectively. Cells were then fixed, permeabilized, blocked with 0.5% bovine serum albumin and 0.15% glycine in PBS for 1 hour, and incubated with appropriate primary antibody (LAMP2, LC3, Bax, and Bak) overnight at 4°C. Cells were further incubated with Alexa Fluor 568-conjugated secondary antibody or Alexa Fluor 488-conjugated secondary antibody for 1 hour at room temperature. Subsequently, the cells were incubated with DAPI to stain nucleus, and then mounted. Images were captured using a fluorescence or confocal microscope.
Determination of apoptosis
Apoptosis induction was assessed by quantitation of histone-associated DNA fragment release into the cytosol using an ELISA kit from Roche Applied Sciences (Indianapolis, IN) or by flow cytometry using Annexin V/propidium iodide (PI) Apoptosis Detection kit. Quantitation of histone-associated DNA fragment release into the cytosol was performed according to the manufacturer’s instructions. For quantitation of apoptosis by flow cytometry, cells were treated with DMSO or SFN for 24 hours. Cells were harvested and washed with PBS. Cells were suspended in binding buffer and stained with Annexin V and PI for 15 minutes at room temperature in the dark. Samples were then diluted with binding buffer. Stained cells were analyzed using Coulter Epics XL or Accuri C6 Flow Cytometer.
Immunohistochemistry
Archived prostate adenocarcinoma sections from our previous study (18) were de-paraffinized, hydrated, and immersed in boiling citrate retrieval buffer solution (pH 6.0) for 30 minutes followed by treatment with 0.3% hydrogen peroxide in 100% methanol for 20 minutes at room temperature. Sections were exposed to PBS consisting of 5% normal goat serum and 0.1% Triton X-100 for 24 hours at 4°C followed by incubation with LAMP2 antibody for 72 hours in humidified chambers at 4°C. Sections were washed with PBS, incubated with horseradish peroxidase-conjugated secondary antibody for 2 hours at room temperature, incubated with 3,3’-diaminobenzidine tetrahydrochloride for 10 minutes, and then counterstained with hematoxylin. Stained sections were examined under Leica microscope at ×200 magnification. At least 6 non-overlapping and non-necrotic regions were captured from each section and analyzed using Aperio ImageScope software (positive pixel count V9 algorithm) to quantitate H-score as described previously by us (28,29).
Human autophagy RT2 Profiler™ polymerase chain reaction (PCR) array
PC-3 cells treated with DMSO or SFN (40 μmol/L) for 6 or 9 hours were harvested by trypsinization and total RNA was extracted using the RNeasy® Mini kit (Qiagen) following the instructions provided by the manufacturer. Reverse transcription was performed using 2~3 μg of total RNA and RT2 First-Strand kit (SABiosciences-Qiagen) following the supplier’s protocol. The mixtures of cDNA and RT2 SYBR® Green ROX™ qPCR Mastermix were prepared immediately before the real-time PCR. Real-time PCR was performed in two-step cycling program using an ABI 7700. The amplification conditions were as follows: 10 minutes at 95°C followed by 40 cycles of 15 seconds at 95°C and 1 minute at 60°C. The data were analyzed using the web-based software provided by the manufacturer. The threshold cycles were calculated and the genes with values above 35 were considered undetected. The cut-off was ± 1.5-fold change in gene expression and P < 0.05.
Analysis of The Human Genome Atlas (TCGA) data
The University of California Santa Cruz Xena Browser (http://xena.ucsc.edu/public-hubs/) was used to analyze prostate cancer TCGA RNA-Seq data to determine the correlation between expression of LAMP2 and that of genes associated with autophagy.
Statistical analysis
All data were analyzed using the Prism 8 (version 8.0.0.224) of the GraphPad Software (San Diego, CA). Statistical methods used in this study were analysis of variance (ANOVA) followed by Dunnett’s or Bonferroni’s multiple comparison test or unpaired t test. P < 0.05 was considered statistically significant. For gene correlation evaluation, Pearson correlation analysis was used.
Results
SFN treatment increased mRNA and proteins levels of LAMP2 in prostate cancer cells
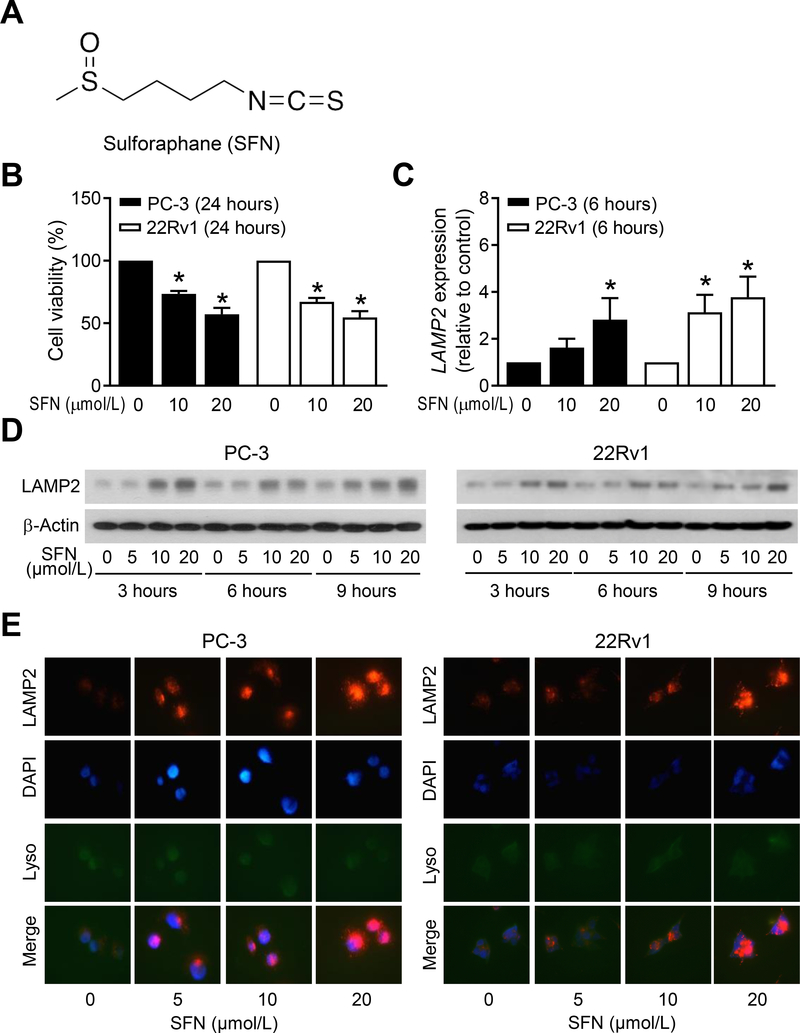
Initially, we determined the effect of SFN treatment (structure of SFN is shown in Fig. 1A) on viability of PC-3 and 22Rv1 cells by trypan blue dye exclusion assay (Fig. 1B). The viability of both cell lines was decreased dose-dependently by SFN treatment. As can be seen in Fig. 1C, exposure of PC-3 and 22Rv1 cells to SFN resulted in induction of LAMP2 mRNA expression as revealed by qRT-PCR. We next tested whether SFN-mediated induction of LAMP2 mRNA was accompanied by an increase in its protein level. Western blotting revealed induction of LAMP2 protein expression upon SFN exposure in both PC-3 and 22Rv1 cells that was evident as early as 3-hour post-treatment but most pronounced at 10 and 20 μmol/L concentrations (Fig. 1D). Consistent with western blotting data, level of LAMP2 protein was very low in DMSO-treated control PC-3 and 22Rv1 cells (Fig. 1E) but its expression increased dose-dependently upon SFN treatment in both cell lines (Fig. 1E). Collectively, these results indicated induction of mRNA and protein levels of LAMP2 in SFN-treated PC-3 and 22Rv1 cells when compared to solvent-treated control cells.
Figure 1.
SFN treatment induces LAMP2 expression in human prostate cancer cells. A, Chemical structure of SFN. B, Viability of PC-3 and 22Rv1 cells after 24-hour of treatment with DMSO or the indicated doses of SFN. Results shown are mean ± SD (n=3). Experiment was repeated with comparable results. *Statistically significant compared with DMSO-treated control by one-way ANOVA with Dunnett’s adjustment (P<0.05). C, qRT-PCR analysis for LAMP2 mRNA level in PC-3 and 22Rv1 cells treated with DMSO or the indicated doses of SFN for 6 hours. Results shown are mean ± SD (n=3). *Statistically significant compared with DMSO-treated control by one-way ANOVA with Dunnett’s adjustment (P<0.05). Experiment was repeated with comparable results. D, Immunoblotting for LAMP2 and β-Actin proteins using lysates from PC-3 and 22Rv1 cells after treatment with DMSO or the indicated doses of SFN for specified time points. Experiment was done twice and the results were generally consistent. E, Representative immunofluorescence microscopy images for LAMP2 (red), DAPI (blue), and LysoTracker (Lyso, green) in PC-3 and 22Rv1 cells treated with DMSO or the indicated doses of SFN for 9 hours. Experiment was repeated with generally comparable results.
Treatment with SFN analogs resulted in induction of LAMP2 protein
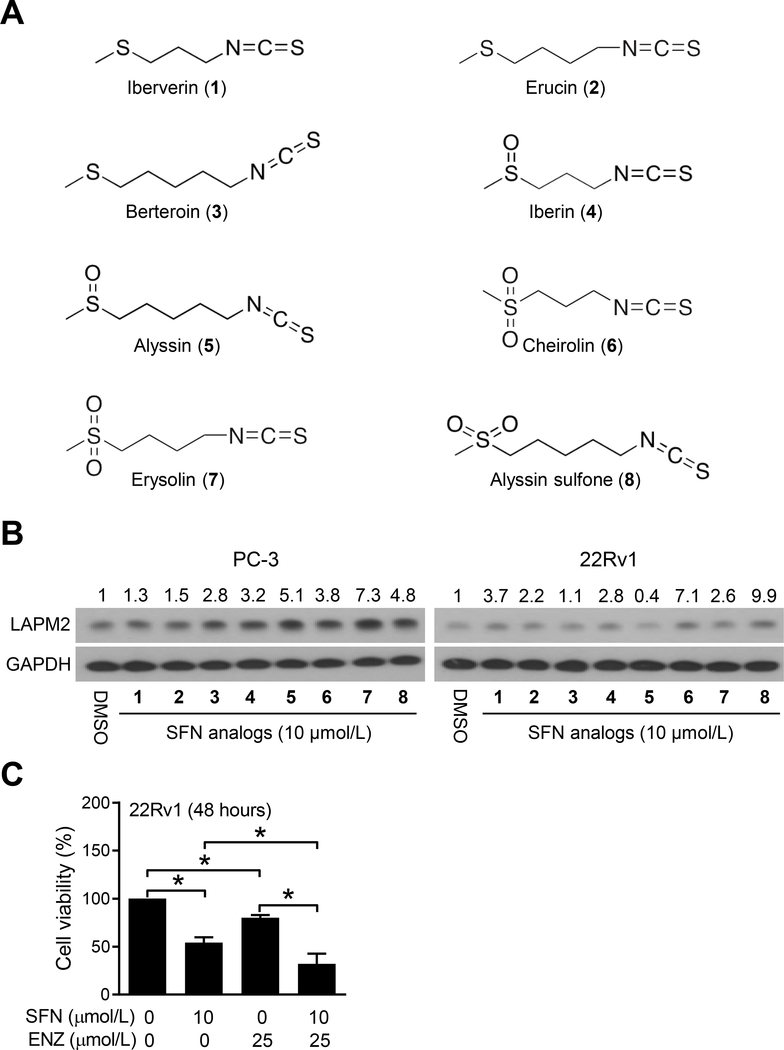
SFN belongs to the sulfur-containing family of isothiocyanates. We next raised the question of whether SFN-mediated induction of LAMP2 protein was unique to this agent. Structures of thio-derivatives (iberverin, erucin, and berteroin), sulfinyl-derivatives (iberin and alyssin), and sulfonyl-derivatives (cheirolin, erysolin, and alyssin sulfone) of SFN are shown in Fig. 2A. Treatment of PC-3 and 22Rv1 cells with each tested analogue resulted in some level of induction of LAMP2 protein expression except for Alyssin in 22Rv1 cells (Fig. 2B). Generally, the order of induction potency was sulfonyl-derivatives > sulfinyl-derivatives > thio-derivatives. These results indicated that the oxidation state of the sulfur affected LAMP2 induction potency of the SFN analogues. We focused on SFN itself for further investigation because this isothiocyanate is most well-characterized and has been tested clinically (8,30).
Figure 2.
Naturally occurring SFN analogs are inducers of LAMP2 protein in human prostate cancer cells. A, Chemical structures of naturally occurring SFN analogs. B, Immunoblotting for LAMP2 and GAPDH proteins using lysates from PC-3 and 22Rv1 cells after treatment with DMSO or 10 μmol/L of SFN analogs for 6 hours. Numbers on top of the bands are fold change in LAMP2 protein level relative to corresponding DMSO-treated controls. Experiment was repeated at least twice with consistent results. C, Viability of 22Rv1 cells after 48-hour treatment with DMSO or the indicated agents. Results shown are mean ± SD (n=3). Experiment was repeated with comparable results. *Statistically significant between the indicated groups by one-way ANOVA with Bonferroni’s multiple comparison test (P<0.05). ENZ, enzalutamide.
It is interesting to note that PC-3 and 22Rv1 cells exhibit differential response to LAMP2 induction upon treatment with Alyssin, which is a methylsulfinyl-pentyl analog of SFN. Alyssin is a potent inducer of LAMP2 protein expression in PC-3 cells but no such induction was evident in 22Rv1 cells (Fig. 2B). Without detailed investigation, it is difficult to speculate on the molecular basis for this cell line-specific discrepancy, but it could be attributable to genetic differences between PC-3 and 22Rv1 cells. For example, PC-3 cells lack androgen receptor (AR) as well as phosphatase and tensin homolog (PTEN) whereas 22Rv1 cells have wild-type PTEN but mutant AR. Additional work is necessary to determine whether LAMP2 induction potency of Alyssin is affected by AR and/or PTEN status or related to other unknown mechanism(s).
SFN increased anticancer activity of ENZ
We explored the possibility of whether anticancer effect of AR antagonist ENZ was affected by SFN. Because PC-3 cells lack AR expression, only 22Rv1 cells were used for this experiment. Viability of 22Rv1 cells was decreased significantly upon treatment with SFN as well as ENZ when used singly (Fig. 2C). Moreover, the anticancer activity of ENZ was increased significantly by co-treatment with SFN when compared to SFN alone (Fig. 2C).
SFN-mediated increase in LC3BII protein level was augmented by LAMP2 knockdown
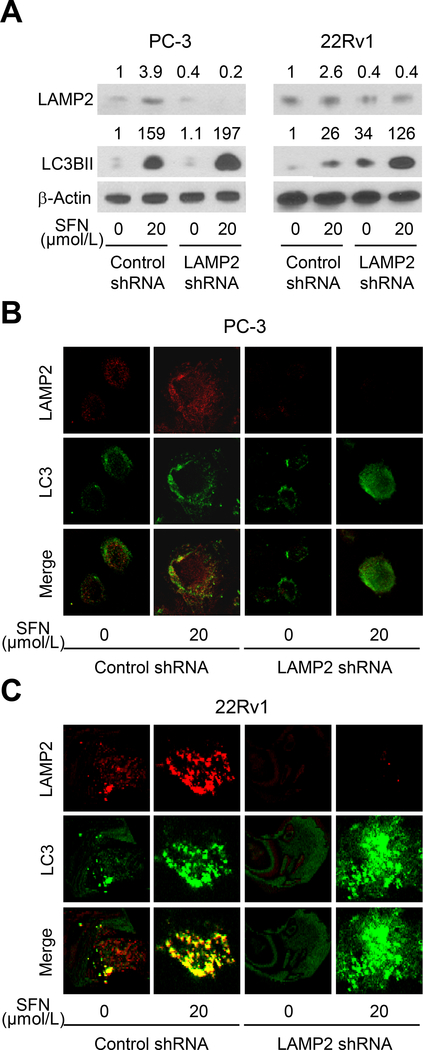
The level of LAMP2 protein was decreased by 60% upon stable transfection of PC-3 and 22Rv1 cells with a LAMP2-targeted shRNA (Fig. 3A). Consistent with the results of our prior publication (17), SFN treatment increased the protein level of autophagy biomarker LC3BII in both cell lines (Fig. 3A). Interestingly, the increase in LC3BII protein level resulting from SFN treatment was augmented by knockdown of the LAMP2 protein (Fig. 3A). We confirmed these results by confocal microscopy. Representative images for LAMP2 (red fluorescence) and LC3 (green fluorescence) in DMSO-treated control and SFN-treated PC-3 and 22Rv1 cells are shown in Fig. 3B and Fig. 3C, respectively. Merging of the red and green fluorescence leading to yellow-orange color indicated possible co-localization of these proteins in DMSO-treated control cells. The intensity of co-staining was increased further after treatment with SFN (Fig. 3B, C). Consistent with the results of western blotting (Fig. 3A), the increase in LC3BII protein level resulting from exposure of cells to SFN was boosted even further after LAMP2 protein knockdown (Fig. 3B, C). These results indicated an increase in the level of autophagy marker LC3BII by SFN treatment and LAMP2 knockdown.
Figure 3.
Knockdown of LAMP2 enhances SFN-mediated LC3BII accumulation in human prostate cancer cells. A, Immunoblotting for LAMP2, LC3BII, and β-Actin proteins using lysates from LAMP2 knockdown PC-3 and 22Rv1 cells and control shRNA transfected cells after 8-hour treatment with DMSO or 20 μmol/L of SFN. Numbers on top of the bands are fold change in protein level compared with respective DMSO-treated control shRNA transfected cells. Experiment was done at least twice, and the results were generally consistent. B-C, Representative confocal microscopic images for LAMP2 (red) and LC3 (green) in PC-3 and 22Rv1 cells stably transfected with control shRNA or LAMP2-targeted shRNA and treated with DMSO or 20 μmol/L of SFN for 6 hours. Experiment was repeated with comparable results.
The confocal microscopy data (Fig. 3B, C) suggests colocalization of LC3 and LAMP2 proteins that was diminished by knockdown of LAMP2 protein. Expression of LC3 and LAMP2A proteins has also been examined in normal human prostate and prostate adenocarcinoma (31). These authors showed intense staining for LAMP2A but less intense staining for LC3 with poor co-localization in normal prostate gland (31). The expression of these proteins was variable in prostate adenocarcinoma ranging from intense expression of LC3 and LAMP2A and intense co-localization to intense expression of LAMP2A but poor LC3 expression or intense expression of LC3 but poor expression of LAMP2A (31).
LAMP2 knockdown augmented SFN-induced apoptosis
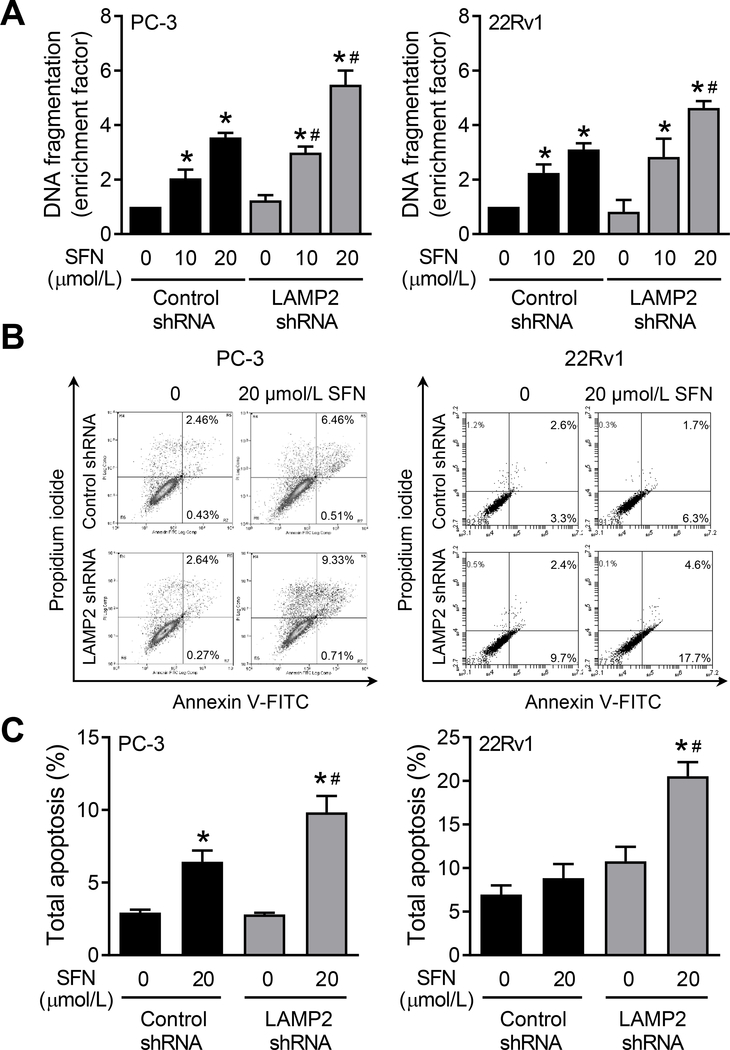
SFN treatment resulted in dose-dependent apoptosis in control shRNA transfected PC-3 and 22Rv1 cells when compared to respective DMSO-treated control cells as revealed by DNA fragmentation assay (Fig. 4A). The DNA fragmentation resulting from SFN treatment was further boosted by depletion of the LAMP2 protein by shRNA transfection (Fig. 4A). We confirmed these results by flow cytometric quantitation of apoptotic cells (Fig. 4B). The fraction of apoptotic cells was highest in the SFN-treated LAMP2 shRNA transfected cells (Fig. 4C). Moreover, the apoptotic population following SFN treatment in cells transfected with LAMP2-targeted shRNA was significantly higher than those in control shRNA transfected cells (Fig. 4C). These results indicated that induction of LAMP2 protein expression by treatment with SFN attenuated its pro-apoptotic activity.
Figure 4.
LAMP2 knockdown augments SFN-induced apoptosis in prostate cancer cells. A, Determination of histone-associated DNA fragment release into cytosol in PC-3 and 22Rv1 cells stably transfected with control shRNA or LAMP2-targeted shRNA and treated with DMSO or the indicated doses of SFN for 24 hours. Results shown are mean ± SD (n=3). Statistically significant *compared with respective control or #between control shRNA and LAMP2 shRNA groups by one-way ANOVA with Bonferroni’s multiple comparison test (P<0.05). Experiment was repeated at least twice and the results were generally consistent. B, Representative Annexin V/PI flow histograms from PC-3 and 22Rv1 cells stably transfected with control shRNA or LAMP2-targeted shRNA and treated with DMSO or 20 μmol/L of SFN for 24 hours. C, Quantitation of total apoptosis from data shown in panel B. Results shown are mean ± SD (n=3). Statistically significant *compared with respective control or #between control shRNA and LAMP2 shRNA groups by one-way ANOVA with Bonferroni’s multiple comparison test (P<0.05). Each experiment was repeated at least twice, and the results were generally consistent.
Bak activation by SFN was augmented by LAMP2 knockdown
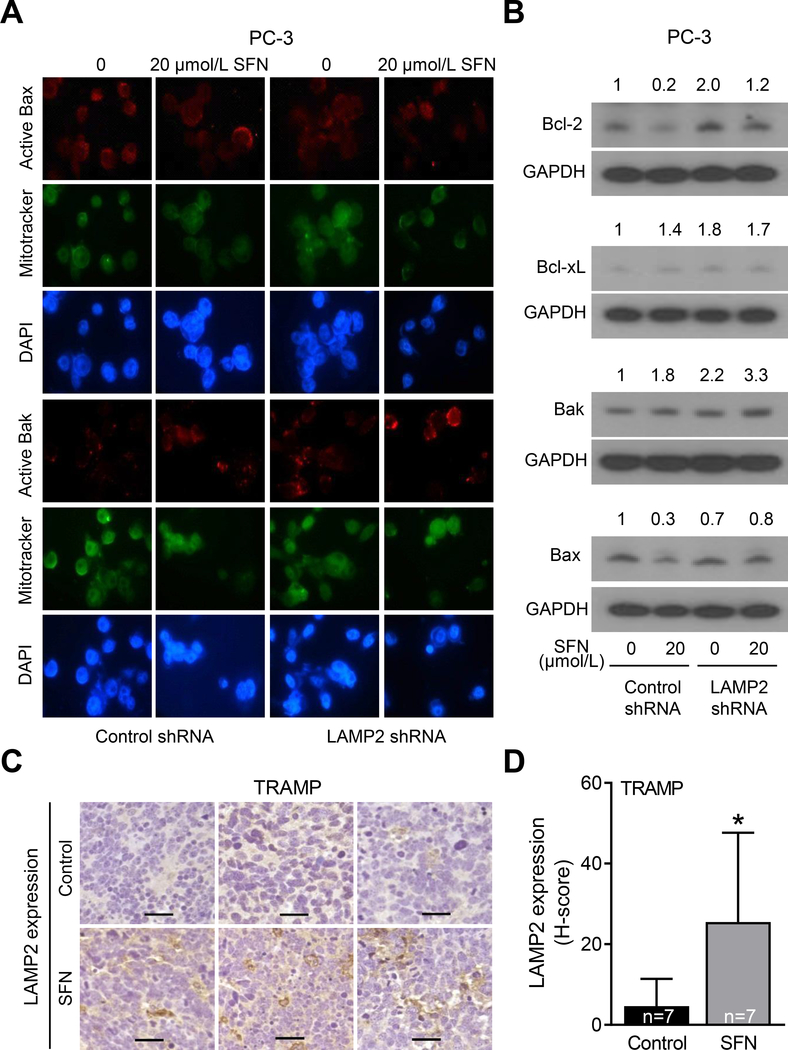
We have shown previously that Bax and Bak proteins are essential for SFN-mediated apoptosis (14). We raised the question of whether augmentation of SFN-induced apoptosis by LAMP2 deficiency was related to Bax and/or Bak proteins. Fig. 5A shows immunocytochemical staining for active Bax and active Bak in PC-3 cells. SFN treatment increased the immunostaining for active Bak, but not active Bax, in control shRNA transfected cells (Fig. 5A). The SFN-mediated increase in the staining for active Bak was increased even further after LAMP2 knockdown (Fig. 5A). The effect of SFN treatment and/or LAMP2 knockdown was also determined on levels of other apoptosis regulation proteins. Consistent with the results of our prior work (13), SFN treatment caused a decrease (about 80% decrease) in protein level of anti-apoptotic Bcl-2 protein in control shRNA transfected cells that was slightly reversed by LAMP2 knockdown (Fig. 5B). Interestingly, LAMP2 knockdown alone caused induction of Bcl-2, Bcl-xL, and Bak proteins, but suppression of Bax protein expression (Fig. 5B). Strikingly, maximum level of Bak protein was observed in the SFN plus LAMP2 knockdown group (Fig. 5B) that likely explains the maximum apoptosis seen in this group (Fig. 4).
Figure 5.
Effect of SFN treatment and LAMP2 knockdown on levels of Bcl-2 family of apoptosis regulating proteins. A, Representative immunofluorescence microscopy images for active Bax (red) and active Bak (red), MitoTracker (green), and DAPI (blue) in PC-3 cells stably transfected with control shRNA or LAMP2-targeted shRNA and treated with DMSO or 20 μmol/L SFN for 24 hours. Consistent results were observed in replicate experiments. B, Immunoblots for Bcl-2, Bcl-xL, Bak, Bax, and GAPDH proteins using lysates from PC-3 cells stably transfected with control shRNA or LAMP2-targeted shRNA and treated for 24 hours with DMSO or 20 μmol/L SFN. Numbers on top of the bands are fold changes in protein levels compared with DMSO-treated control shRNA transfected cells. Experiment was done at least twice, and the results were consistent. C, Representative immunohistochemical images (×200 magnification; scale bar= 50 μm) for LAMP2 protein expression in prostate adenocarcinoma from 3 different TRAMP mice of control and SFN-treatment groups. D, Quantification of LAMP2 protein expression from data shown in panel C. Results shown are mean ± SD (n=7). *Statistically significant compared with control by Student’s t-test (P<0.05).
SFN administration to TRAMP mice resulted in induction of LAMP2 protein expression in prostate adenocarcinoma
Fig. 5C shows immunohistochemical staining for LAMP2 protein in representative prostate adenocarcinoma sections of control and SFN-treated TRAMP mice. Consistent with the results in human prostate cancer cell lines (Fig. 1D, E), the staining for LAMP2 protein was very weak in the prostate adenocarcinoma sections of control TRAMP mice. On the other hand, SFN administration resulted in a significant increase in protein level of LAMP2 as evidenced by the quantitation of immunohistochemical staining (Fig. 5D). These results provided in vivo evidence for induction of LAMP2 protein expression in prostate cancer cells by SFN treatment.
Analysis of LAMP2 expression in prostate cancer TCGA dataset
Previous studies have suggested a prognostic value of LAMP2 expression in prostate cancer (19). Therefore, it was of interest to further examine the association of LAMP2 expression in prostate cancer using RNA-Seq data from TCGA. The expression of LAMP2 was not significantly different between normal human prostate and prostate adenocarcinoma (Supplementary Fig. S1A). Likewise, there was no correlation between Gleason score and expression of LAMP2 (Supplementary Fig. S1B). These results indicated that the expression of LAMP2 itself was not altered in human prostate adenocarcinoma at least based on prostate cancer TCGA RNA-Seq data.
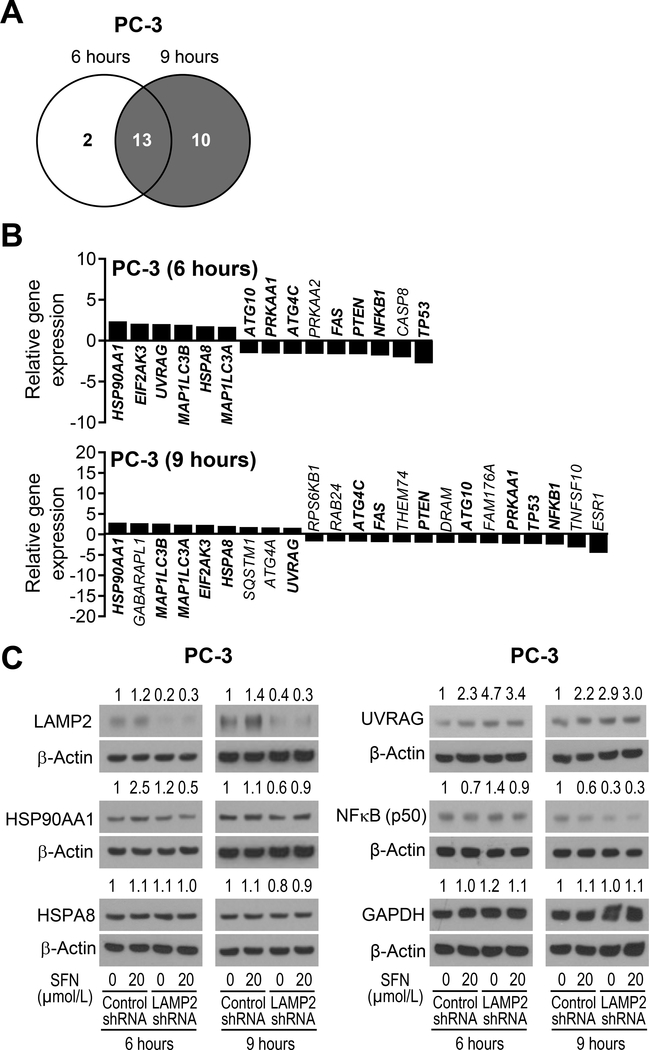
Targeted microarray for determination of the effect of SFN on expression of genes related to autophagy in PC-3 cells
Next, we performed targeted microarray for autophagy regulation genes using PC-3 cells treated with SFN for 6 or 9 hours and DMSO-treated control cells. The Venn diagram in Fig. 6A shows unique and overlapping gene expression changes following SFN treatment compared to DMSO-treated control cells. The up-regulated and down-regulated genes upon SFN treatment are specified in Fig. 6B. The SFN-mediated gene expression changes that were consistent at both 6 and 9 hour-time points (a total of 13 genes) are specified in bold in Fig. 6B. The common up-regulated genes after 6 and 9-hour treatment with SFN included HSP90AA1, MAP1LC3B, MAP1LC3A, EIF2AK3, HSPA8, and UVRAG. The common down-regulated genes in SFN-treated PC-3 cells at both time points were ATG4C, FAS, PTEN, ATG10, PRKAA1, TP53, and NFKB1. Interestingly, analysis of the prostate cancer TCGA revealed a significant positive correlation between expression of LAMP2 with that of HSP90AA1, MAP1LC3B, and HSPA8 (Supplementary Fig. S2). Transcription factor NRF2, which is a well-known target of SFN-mediated chemoprevention (32), has been shown to regulate chaperone-mediated autophagy involving LAMP2A (33). The expression of NRF2 was also positively associated with that of LAMP2 in prostate cancer TCGA (Supplementary Fig. S2). Finally, we have shown previously that Bax and Bak proteins play critical role in apoptosis induction by SFN (14). In this study, we show activation and induction of Bak by SFN treatment that was increased even further by LAMP2 knockdown (Fig. 5A, B). Analysis of the prostate cancer RNA-Seq data revealed a significant inverse association between expression of LAMP2 with that of Bak and Bax as shown in Supplementary Fig. S2.
Figure 6.
SFN treatment alters expression of genes associated with autophagy regulation in PC-3 cells. A, Venn diagram showing unique and common genes between 6-hour and 9-hour SFN-treated PC-3 cells identified from RT2 Profiler PCR Array (Human Autophagy) experiment. Cut-off value was set to 1.5-fold change in expression and P<0.05. B, Relative gene expression change in response to SFN treatment for 6 hours (upper panel) and 9 hours (lower panel) in PC-3 cells. Genes in bold are common at both 6 and 9-hour time points. C, Immunoblotting for some proteins associated with autophagy from data shown in panel B. Lysates from PC-3 cells stably transfected with control shRNA or LAMP2-targeted shRNA and treated for 6 or 9 hours with DMSO or 20 μmol/L of SFN were used for immunoblot analysis. Numbers on top of the bands are fold changes in protein levels compared with DMSO-treated control shRNA transfected cells. Experiment was done at least twice with comparable results.
Immunoblotting for multiple proteins was performed to determine whether SFN-mediated gene expression changes resulted in alteration of respective protein levels. The level of HSP90AA1 protein, which plays an important role in autophagy and it is a therapeutic target in cancer (34), was increased after SFN treatment for 6 hours in control shRNA transfected cells but this response was abolished in LAMP2 shRNA cells (Fig. 6C). The level of HSPA8 protein was not affected by SFN treatment or LAMP2 knockdown. Consistent with gene expression data, SFN treatment resulted in induction of UVRAG protein, which is another key regulator of autophagy (35), in control shRNA cells that was increased even further after LAMP2 knockdown (Fig. 6C). Consistent with published literature (36), SFN treatment downregulated NFκB p50 subunit level in control shRNA cells that was either partly reversed at 6-hour time point (Fig. 6C). Finally, SFN-mediated induction of NRF2 protein was partially attenuated after LAMP2 knockdown especially at the 6-hour time point (Supplementary Fig. S3). However, further work is necessary to determine the contribution of these proteins in autophagy and apoptosis regulation by SFN.
Because GAPDH was used as a loading control in some immunoblotting experiments (Fig. 2B, 5B), we tested the possibility of whether SFN treatment affected its expression. As shown in Fig. 6C, expression of GAPDH was not affected by SFN treatment in control shRNA or LAMP2 shRNA cells.
Discussion
SFN belongs to the isothiocyanate family of naturally-occurring phytochemicals that are abundant as their respective glucosinolate precursors in various cruciferous vegetables like cabbage, watercress, broccoli, mustard, and so forth. Even though prostate cancer chemoprevention has also been demonstrated by an aromatic class of isothiocyanate [phenethyl isothiocyanate (PEITC) from watercress], there are striking differences in underlying mechanisms for SFN and PEITC. SFN treatment inhibits the incidence of early-stage [prostatic intraepithelial neoplasia (PIN) and well-differentiated prostate cancer)] in TRAMP mice, whereas the incidence of only poorly-differentiated prostate adenocarcinoma is suppressed significantly by dietary administration of PEITC in the same mouse model (15,28). Even though PEITC administration results in autophagy induction in vivo (37), the results of in vitro studies on PEITC are strikingly different from that of SFN (17,37). Unlike SFN, the PEITC-mediated apoptotic DNA fragmentation is significantly attenuated by pharmacologic inhibition of autophagy using 3-methyladenine, and these results were confirmed by RNA interference of autophagy regulator Atg5 (17,37). Together, these results suggest that the interplay between autophagy and apoptosis is complex and may be context-dependent for isothiocyanate family of cancer chemopreventive agents.
The results of the present study indicate that SFN treatment increases mRNA and protein levels of LAMP2 in vitro in cultured human prostate cancer cells. Naturally-occurring analogs of SFN are also potent inducers of LAMP2 protein expression although some structure-activity relationship is evident with regards to their LAMP2 induction potency. The prostate cancer chemoprevention by SFN administration in TRAMP mice is also associated with in vivo induction of the LAMP2 protein expression in the prostate adenocarcinoma. The LAMP2 induction by SFN is functionally relevant as knockdown of LAMP2 augments apoptosis induction by SFN. These results indicate that prostate cancer chemoprevention by SFN can be further enhanced by a combination regimen involving LAMP2 inhibitor. Further work is necessary to explore this combination in vivo using TRAMP or other transgenic mouse models of prostate cancer.
Ultimately the autophagic degradation process takes place in lysosome where LAMP2 is localized and maintains lysosomal stability. Therefore, loss of lysosomal activity in part resulting from LAMP2 knockdown could inhibit autophagy flux leading to failure in autophagic degradation. Other autophagy inhibitors such as Bafilomycin A1 and chloroquine also exhibit the same pattern as LAMP2 knockdown.
Both Bax and Bak proteins seem essential for apoptosis induction by SFN based on in vitro results using immortalized mouse embryonic fibroblasts (MEF) form Bax and/or Bak knockout mice (14). The MEF from Bax or Bak knockout mice are resistant to apoptotic cell death by SFN, and the MEF derived from Bax and Bak double knockout mice exhibit even greater protection against SFN-induced cytochrome c release, caspase activation, and apoptosis compared with wild-type or single knockout cells (14). The results of the present study implicate Bak in potentiation of SFN-induced apoptosis by LAMP2 knockdown. It is interesting to note that LAMP2 knockdown alone results in induction of not only Bak but also anti-apoptotic proteins Bcl-2 and Bcl-xL, which may explain lack of any appreciable effect of LAMP2 knockdown alone on apoptosis.
Analysis of the LAMP2 expression in 50 prostate cancer specimens and adjacent normal tissues from same subjects as well as the same number of benign prostatic hyperplasia revealed suppression of its level in prostate cancer when compared to other two groups (19). Another group showed up-regulation of LAMP2 expression in LNCaP cells conditioned for neuroendocrine differentiation by culture in serum-free medium for 6 days (21). The neuroendocrine prostate cancer is highly aggressive and associated with poor prognosis (38,39). The LAMP2 induction after neuroendocrine differentiation in LNCaP cells was associated with increased levels of autophagy marker LC3 (21). Analysis of the TCGA did not indicate up-regulation or suppression of the LAMP2 gene expression. Further studies are needed to determine whether up-regulation of LAMP2 is unique to neuroendocrine prostate cancers.
Previous published studies have revealed SFN-mediated sensitization of prostate cancer cells to cisplatin (40). SFN was also shown to decrease nephrotoxicity from cisplatin (41). We have shown previously that SFN is a potent inhibitor of AR in prostate cancer cells (42). We therefore tested the possibility of sensitization of prostate cancer cells to AR antagonist ENZ. Indeed, we found an increase in cytotoxicity in 22Rv1 cells by SFN + ENZ combination when compared to single agent treatment. However, in vivo validation of these findings in preclinical models (xenograft or transgenic mouse models) is necessary for clinical translation of the SFN + ENZ combination regimen.
In conclusion, the present study identifies LAMP2 protein induction as an undesirable pharmacological effect of SFN with regards to its pro-apoptotic effect, and consequently chemopreventive activity at least in prostate cancer cells. We propose that prostate cancer chemoprevention by SFN may be augmented by a combination regimen using chemical inhibition of LAMP2.
Supplementary Material
Acknowledgments
This study was supported by the National Cancer Institute grant R01 CA225716 (to S.V.S.). The following UPMC Hillman Cancer Center core facilities partly supported by the National Cancer Institute grant P30 CA047904 were utilized in this study: Animal Facility, Cell and Tissue Imaging Facility, Flow Cytometry Facility, and Tissue and Research Pathology Services. The authors thank Min Wang for technical assistance.
Footnotes
Conflict of Interest: None of the authors has any conflict of interest.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med 2003;349:215–24. [DOI] [PubMed] [Google Scholar]
- 3.Andriole GL, Bostwick DG, Brawley OW, Gomella LG, Marberger M, Montorsi F, et al. Effect of dutasteride on the risk of prostate cancer. N Engl J Med 2010;362:1192–202. [DOI] [PubMed] [Google Scholar]
- 4.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2009;301:39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ju J, Picinich SC, Yang Z, Zhao Y, Suh N, Kong AN, et al. Cancer-preventive activities of tocopherols and tocotrienols. Carcinogenesis 2010;31:533–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee KW, Bode AM, Dong Z. Molecular targets of phytochemicals for cancer prevention. Nat Rev Cancer 2011;11:211–8. [DOI] [PubMed] [Google Scholar]
- 7.Zi X, Grasso AW, Kung HJ, Agarwal R. A flavonoid antioxidant, silymarin, inhibits activation of erbB1 signaling and induces cyclin-dependent kinase inhibitors, G1 arrest, and anticarcinogenic effects in human prostate carcinoma DU145 cells. Cancer Res 1998;58:1920–9. [PubMed] [Google Scholar]
- 8.Singh SV, Singh K. Cancer chemoprevention with dietary isothiocyanates mature for clinical translational research. Carcinogenesis 2012;33:1833–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen JH, Kristal AR, Stanford JL. Fruit and vegetable intakes and prostate cancer risk. J Natl Cancer Inst 2000;92:61–8. [DOI] [PubMed] [Google Scholar]
- 10.Kolonel LN, Hankin JH, Whittemore AS, Wu AH, Gallagher RP, Wilkens LR, et al. Vegetables, fruits, legumes and prostate cancer: a multiethnic case-control study. Cancer Epidemiol Biomarkers Prev 2000;9:795–804. [PubMed] [Google Scholar]
- 11.Joseph MA, Moysich KB, Freudenheim JL, Shields PG, Bowman ED, Zhang Y, et al. Cruciferous vegetables, genetic polymorphisms in glutathione S-transferases M1 and T1, and prostate cancer risk. Nutr Cancer 2004;50:206–13. [DOI] [PubMed] [Google Scholar]
- 12.Chiao JW, Chung FL, Kancherla R, Ahmed T, Mittelman A, Conaway CC. Sulforaphane and its metabolite mediate growth arrest and apoptosis in human prostate cancer cells. Int J Oncol 2002;20:631–6. [DOI] [PubMed] [Google Scholar]
- 13.Singh AV, Xiao D, Lew KL, Dhir R, Singh SV. Sulforaphane induces caspase-mediated apoptosis in cultured PC-3 human prostate cancer cells and retards growth of PC-3 xenografts in vivo. Carcinogenesis 2004;25:83–90. [DOI] [PubMed] [Google Scholar]
- 14.Choi S, Singh SV. Bax and Bak are required for apoptosis induction by sulforaphane, a cruciferous vegetable-derived cancer chemopreventive agent. Cancer Res 2005;65:2035–43. [DOI] [PubMed] [Google Scholar]
- 15.Singh SV, Warin R, Xiao D, Powolny AA, Stan SD, Arlotti JA, et al. Sulforaphane inhibits prostate carcinogenesis and pulmonary metastasis in TRAMP mice in association with increased cytotoxicity of natural killer cells. Cancer Res 2009;69:2117–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keum YS, Khor TO, Lin W, Shen G, Kwon KH, Barve A, et al. Pharmacokinetics and pharmacodynamics of broccoli sprouts on the suppression of prostate cancer in transgenic adenocarcinoma of mouse prostate (TRAMP) mice: implication of induction of Nrf2, HO-1 and apoptosis and the suppression of Akt-dependent kinase pathway. Pharm Res 2009;26:2324–31. [DOI] [PubMed] [Google Scholar]
- 17.Herman-Antosiewicz A, Johnson DE, Singh SV. Sulforaphane causes autophagy to inhibit release of cytochrome C and apoptosis in human prostate cancer cells. Cancer Res 2006;66:5828–35. [DOI] [PubMed] [Google Scholar]
- 18.Vyas AR, Hahm ER, Arlotti JA, Watkins S, Stolz DB, Desai D, et al. Chemoprevention of prostate cancer by D,L-sulforaphane is augmented by pharmacological inhibition of autophagy. Cancer Res. 2013;73:5985–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jamali L, Moradi A, Ganji M, Ayati M, Kazeminezhad B, Fazeli Attar Z, et al. Potential prognostic role for SPOP, DAXX, RARRES1, and LAMP2 as an autophagy related genes in prostate cancer. Urol J 2019;in press (doi: 10.22037/uj.v0i0.4935). [DOI] [PubMed] [Google Scholar]
- 20.Li X, He S, Ma B. Autophagy and autophagy-related proteins in cancer. Mol Cancer 2020;19:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morell C, Bort A, Vara-Ciruelos D, Ramos-Torres Á, Altamirano-Dimas M, Díaz-Laviada I et al. Up-regulated expression of LAMP2 and autophagy activity during neuroendocrine differentiation of prostate cancer LNCaP cells. PLoS One 2016;11:e0162977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alessandrini F, Pezzè L, Ciribilli Y. LAMPs: Shedding light on cancer biology. Semin Oncol 2017;44:239–53. [DOI] [PubMed] [Google Scholar]
- 23.Saha T LAMP2A overexpression in breast tumors promotes cancer cell survival via chaperone-mediated autophagy. Autophagy 2012;8:1643–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding ZB, Fu XT, Shi YH, Zhou J, Peng YF, Liu WR, et al. Lamp2a is required for tumor growth and promotes tumor recurrence of hepatocellular carcinoma. Int J Oncol 2016;49:2367–76. [DOI] [PubMed] [Google Scholar]
- 25.Bao L, Lv L, Feng J, Chen Y, Wang X, Han S, et al. miR-487b-5p Regulates Temozolomide resistance of lung cancer cells through LAMP2-medicated autophagy. DNA Cell Biol 2016;35:385–92. [DOI] [PubMed] [Google Scholar]
- 26.Wang R, Liu Y, Liu L, Chen M, Wang X, Yang J, et al. Tumor cells induce LAMP2a expression in tumor-associated macrophage for cancer progression. BioMed 2019;40:118–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiao D, Srivastava SK, Lew KL, Zeng Y, Hershberger P, Johnson CS, et al. Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits proliferation of human prostate cancer cells by causing G2/M arrest and inducing apoptosis. Carcinogenesis 2003;24:891–7. [DOI] [PubMed] [Google Scholar]
- 28.Powolny AA, Bommareddy A, Hahm ER, Normolle DP, Beumer JH, Nelson JB, et al. Chemopreventative potential of the cruciferous vegetable constituent phenethyl isothiocyanate in a mouse model of prostate cancer. J Natl Cancer Inst 2011;103:571–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hahm ER, Lee J, Kim SH, Sehrawat A, Arlotti JA, Shiva SS, et al. Metabolic alterations in mammary cancer prevention by withaferin A in a clinically relevant mouse model. J Natl Cancer Inst 2013;105:1111–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cipolla BG, Mandron E, Lefort JM, Coadou Y, Della Negra E, Corbel L, et al. Effect of sulforaphane in men with biochemical recurrence after radical prostatectomy. Cancer Prev Res (Phila) 2015;8:712–9. [DOI] [PubMed] [Google Scholar]
- 31.Kalamida D, Giatromanolaki A, Koukourakis MI. Characterization of the “Autophagic Flux” in Prostate Cancer Tissue Biopsies by LC3A/LAMP2a Immunofluorescence and Confocal Microscopy. Methods Mol Biol 2019;1880:555–60. [DOI] [PubMed] [Google Scholar]
- 32.Juge N, Mithen RF, Traka M. Molecular basis for chemoprevention by sulforaphane: a comprehensive review. Cell Mol Life Sci 2007;64:1105–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pajares M, Rojo AI, Arias E, Díaz-Carretero A, Cuervo AM, Cuadrado A. Transcription factor NFE2L2/NRF2 modulates chaperone-mediated autophagy through the regulation of LAMP2A. Autophagy 2018;14:1310–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang B, Chen Z, Yu F, Chen Q, Tian Y, Ma S, et al. Hsp90 regulates autophagy and plays a role in cancer therapy. Tumour Biol 2016;37:1–6. [DOI] [PubMed] [Google Scholar]
- 35.Song Y, Quach C, Liang C. UVRAG in autophagy, inflammation, and cancer. Autophagy 2020;16:387–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu C, Shen G, Chen C, Gélinas C, Kong AN. Suppression of NF-κB and NF-κB-regulated gene expression by sulforaphane and PEITC through IκBα, IKK pathway in human prostate cancer PC-3 cells. Oncogene 2005;24:4486–95. [DOI] [PubMed] [Google Scholar]
- 37.Bommareddy A, Hahm ER, Xiao D, Powolny AA, Fisher AL, Jiang Y, et al. Atg5 regulates phenethyl isothiocyanate-induced autophagic and apoptotic cell death in human prostate cancer cells. Cancer Res 2009;69:3704–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun Y, Niu J, Huang J. Neuroendocrine differentiation in prostate cancer. Am J Trans Res 2009;1:148–62. [PMC free article] [PubMed] [Google Scholar]
- 39.Terry S, Beltran H. The many faces of neuroendocrine differentiation in prostate cancer progression. Front Oncol 2014;4:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kallifatidis G, Labsch S, Rausch V, Mattern J, Gladkich J, Moldenhauer G, et al. Sulforaphane increases drug-mediated cytotoxicity toward cancer stem-like cells of pancreas and prostate. Mol Ther 2011;19:188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guerrero-Beltrán CE, Calderón-Oliver M, Tapia E, Medina-Campos ON, Sánchez-González DJ, Martínez-Martínez CM, et al. Sulforaphane protects against cisplatin-induced nephrotoxicity. Toxicol Lett 2010;192:278–85. [DOI] [PubMed] [Google Scholar]
- 42.Kim SH, Singh SV. D,L-Sulforaphane causes transcriptional repression of androgen receptor in human prostate cancer cells. Mol Cancer Ther. 2009. July;8(7):1946–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.