Abstract
Purpose
To describe a case of severe Streptococcus pneumoniae endophthalmitis in a patient with a cystic, avascular filtering bleb who had been implanted with a Xen® Gel Stent 21 months previously.
Observations
A 64-year-old woman with open-angle glaucoma developed severe endophthalmitis 21 months after Xen® Gel Stent implantation. On presentation, visual acuity was limited to light perception. Examination revealed a 100% hypopyon, blebitis and an exposed stent, along with orbital cellulitis. Immediate explantation of the exposed Xen® was performed, and intravitreal antibiotics were administered. S. pneumoniae was isolated from an anterior chamber paracentesis. Based on the antibiogram, the patient was treated with topical fortified piperacillin, gentamicin and vancomycin along with appropriate systemic antibiotics (intravenous imipenem and oral levofloxacin). After 3 days of antibiotics, she received a daily intravenous bolus of methylprednisolone at a dose of 1 mg/kg/day for three days. Despite these measures, the patient's condition declined, with purulent melting of the globe requiring evisceration.
Conclusionsand Importance
As for other filtering surgeries, blebitis and severe endophthalmitis can occur after Xen® Gel Stent implantation. Patients with thin conjunctiva and/or cystic blebs over the stent should be followed particularly closely.
Keywords: Glaucoma, Trabeculectomy, Xen®, Intraocular pressure, Endophthalmitis
Highlights
-
•
As for other filtering surgeries, blebitis and severe endophthalmitis can occur after Xen® Gel Stent implantation.
-
•
Erosion of the Xen® Gel Stent through the conjunctiva can lead to severe infection such as blebitis and endophthalmitis.
-
•
Patients with a cystic bleb should be followed regularly, as they may develop stent exposure and infectious complications.
1. Introduction
Glaucoma is a blinding optic neuropathy affecting over 60 million people world-wide.1,2 Intraocular pressure (IOP) lowering is the only effective therapeutic strategy to slow the progression of glaucomatous optic neuropathy.3, 4, 5 The most commonly performed glaucoma surgeries are filtering surgeries, with trabeculectomy remaining the gold standard technique. Trabeculectomy aims to create a new pathway for aqueous humor from the anterior chamber (AC) into the subconjunctival space, with development of a filtering bleb (FB). In recent years, new surgical techniques, “Minimally Invasive Glaucoma Surgeries” (MIGS), have been developed in order to limit intra- and post-operative complications of filtering surgery and to allow for faster recovery.6,7 The Xen® Gel Stent is one of these newer minimally invasive surgical techniques. It consists of the ab interno implantation of a 6 mm long collagen tube with a 45 μm lumen, connecting the AC with the subconjunctival space.8, 9, 10 Unlike trabeculectomy, this newer technique avoids conjunctival dissection. It is therefore touted as reducing the risk of postoperative bleb leakage and, thus, endophthalmitis. Since it received a CE mark in December 2015 and FDA approval in November 2016, published safety and efficacy studies have not yet addressed long-term complications.11
2. Case report
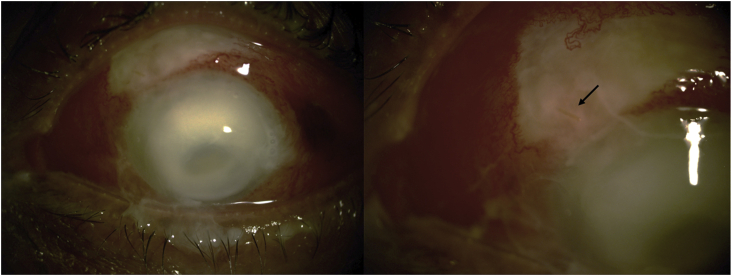
In September 2017, a 64-year-old woman with progressive open-angle glaucoma on maximum medical treatment underwent cataract surgery combined with placement of a Xen® Gel Stent (Allergan, Dublin, Ireland) according to the manufacturer's instructions. A subconjunctival injection of 0.1 ml mitomycin C (MMC) 0.2 mg/ml was administered in the superonasal quadrant prior to implantation of the stent. At the conclusion of the procedure, 0.1 ml cefuroxime (1mg) was injected into the anterior chamber. Postoperative treatment consisted of combination antibiotic-steroid drops (Tobradex®, dexamethasone 0.1 g and tobramycin 0.3 g per unit dose, Novartis Pharma, Rueil-Malmaison, France) instilled three times a day for 4 weeks and an anti-inflammatory drop (Indocollyre® 0.1% indomethacin, Bausch & Lomb, Montpellier, France) instilled three times a day for 6 weeks, and all glaucoma medications were discontinued on the day of surgery. Postoperative visits took place on day 1, day 7, day 21, 2 months and 5 months, after which the patient was referred back to her primary ophthalmologist to continue follow-up every 6 months. The postoperative follow-ups were uneventful, with good IOP control between 10 and 12 mmHg (central corneal pachymetry 490μm). The filtering bleb appeared very cystic, avascular and of moderate size, and the stent appeared to be directed toward the conjunctival surface (Fig. 2). At no time during follow-up did the bleb show any signs of overt or subclinical leakage.
Fig. 2.
Photograph of the patient two months after Xen® implantation. The appearance of the filtering bleb was very cystic, avascular and of moderate size; the tube appeared to be directed toward the conjunctival surface.
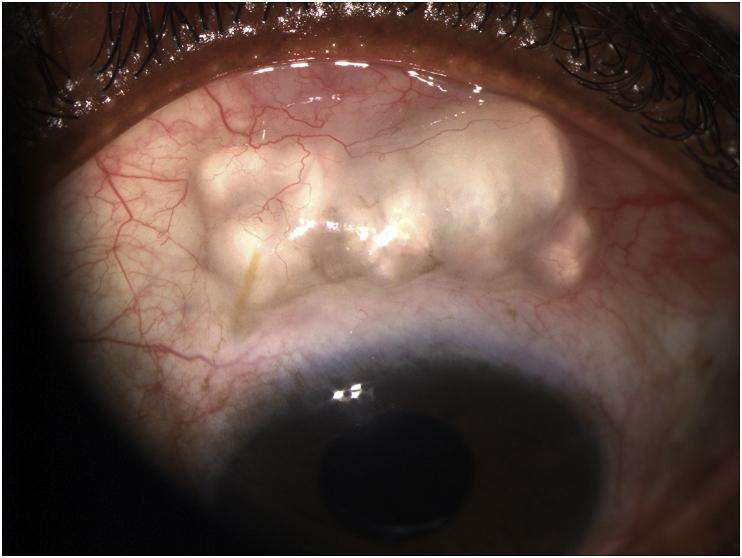
Twenty-one months later, in June 2019, the patient presented to the emergency department with periorbital edema, redness, pain and nearly complete loss of vision in her left eye. The symptoms had begun five days previously and gradually worsened. On presentation, examination revealed conjunctival hyperemia, a 100% hypopyon and an exposed stent which stained with fluorescein (Fig. 1). The diagnosis of endophthalmitis of the left eye secondary to blebitis was made, and the patient was admitted to the hospital.
Fig. 1.
Photograph of the patient on the day of admission, showing 100% hypopyon and blebitis. The black arrow shows the exposed Xen®.
Explantation of the exposed Xen® was performed along with anterior chamber paracentesis to obtain a sample of the purulent fluid. Bacteriologic, viral and fungal examinations were performed on this sample, the explanted Xen®, and a corneal scraping, revealing antibiotic-susceptible Streptococcus pneumoniae. A fungal culture and real-time PCR for herpes simplex 1 and 2 and varicella-zoster were negative.
Therapeutic management consisted of immediate intravitreal injection of 0.1 ml of vancomycin 0.1 mg/0.1 ml and 0.1 ml of ceftazidime 2 mg/0.1 ml in combination with systemic antibiotic treatment with imipenem and levofloxacin as well as topical antibiotics: hourly fortified piperacillin, gentamicin and vancomycin. After 3 days of appropriate antibiotics, the patient received a daily bolus of methylprednisolone 1 mg/kg for three days in a row. An additional intravitreal injection of 0.1 ml of vancomycin 0.1 mg/0.1 ml and ceftazidime 2 mg/0.1 ml was administered at day 3 and day 6. B-scan ultrasonography revealed vitreous condensation and choroidal thickening without retinal detachment. In the absence of improvement, vitrectomy was considered and discussed at a staff meeting. Given the poor visibility due to the corneal infiltrate, vitrectomy was deferred. Because of the severe periorbital inflammation associated with limitation of ocular motility, a brain and orbital CT scan was performed, revealing preseptal and orbital cellulitis. Despite all of our efforts, the eye deteriorated, with purulent melting of the globe requiring evisceration three months after the diagnosis of endophthalmitis.
3. Discussion
In the present case, erosion of the conjunctiva and extrusion of the Xen® Gel Stent was the most likely cause of an extremely severe ocular infection which led to the total loss of the eye. In the past two years, 8 other cases of endophthalmitis have also been reported. Of the 8 cases of post-Xen® endophthalmitis reported in the literature, 5 of them occurred after stent exposure. Ibáñez-Muñoz et al.12 reported one case of endophthalmitis occurring secondary to Xen® Gel Stent extrusion in a 12-month retrospective study of 68 patients. Similar cases have been described by Heidinger et al.,13 Karri et al.14 and Lim et al.15 They all noted that the tube was exposed at its point of exit from the subconjunctival space, enabling infection. Olgun et al. reported 2 cases of endophthalmitis without blebitis, 4 and 5 months after surgery. The blebs were of moderate size, avascular in the first case, and in the second case, 2 mm of the Xen® Gel Stent was protruding from the conjunctiva.16 Kerr et al.17 respectively reported three cases of blebitis occurring 8, 16 and 24 months after surgery, from which two of the patients developed endophthalmitis. Two of them showed an epithelial defect and leak, but the implant was not exposed, and explantation of the devices was not required. In our case, the bleb was avascular and very cystic, and the implant appeared to be directed toward the surface, thus increasing the likelihood of extrusion.
As underlined by Lim et al., flat blebs are at higher risk of leakage, due to friction of the implant against the overlying conjunctiva, which can lead to blebitis or endophthalmitis.15 Unlike the cases presented previously, our patient had a cystic bleb, and it is thus the position of the stent directed toward the inner surface of the bleb that might have allowed friction with the conjunctival bleb tissue and ultimately extrusion. Gedde et al.18 showed that tube exposure was the greatest risk factor for endophthalmitis. In a case where there is a risk of erosion, removal of the implant should be discussed. Although a successful technique of a free conjunctival autograft covered with an additional amniotic membrane graft has been described in a case of recurrent exposure of a Xen® Gel Stent,19 the risk of long-term blebitis should be carefully evaluated.
The use of mitomycin C (MMC) injected into the subconjunctival space without irrigation raises a long-term safety issue.20 Mitomycin C (MMC) is an antimetabolite used during the initial stages of a trabeculectomy to prevent excessive postoperative scarring and thus reduce the risk of failure. The use of the antimetabolites such as MMC has improved the success rate of trabeculectomy surgery but may increase the risk of developing a bleb-related infection due to leakage.21, 22, 23 In trabeculectomy, MMC is held in the subconjunctival space with sponges for 30 seconds to 3 minutes before being removed and the subconjunctival space irrigated thoroughly. With Xen® Gel Stent implantation, in the absence of conjunctival dissection, we inject and leave MMC in place in the subconjunctival space. This might be a risk factor for the development of thin, avascular blebs and, consequently, late endophthalmitis.
4. Conclusion
Erosion of the Xen® Gel Stent through the conjunctiva can lead to severe infection such as blebitis and endophthalmitis. Patients with cystic blebs and an abnormal stent orientation toward the conjunctival surface should be followed regularly, as they may develop stent exposure and severe infectious complications.
Patient consent
Written informed consent was obtained from the patient described in this case report.
Funding
No funding or grant support
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Supervised by AL. The patient was under the care of AL. The report was written by JB, AL and CB.
All the authors mentioned in the manuscript have agreed to authorship, read and approved the manuscript, and given consent for submission and subsequent publication of the manuscript.
Declaration of competing interest
The following authors have no financial disclosures: JB, CB, AL.
Acknowledgements
The authors thank Dr Kevin D. Clark for editorial assistance with the manuscript.
References
- 1.Tham Y.-C., Li X., Wong T.Y., Quigley H.A., Aung T., Cheng C.-Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081–2090. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 2.Kingman S. Glaucoma is second leading cause of blindness globally. Bull World Health Organ. 2004;82(11):887–888. doi:/S0042-96862004001100019. [PMC free article] [PubMed] [Google Scholar]
- 3.Heijl A., Leske M.C., Bengtsson B. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120(10):1268–1279. doi: 10.1001/archopht.120.10.1268. [DOI] [PubMed] [Google Scholar]
- 4.Kass M.A., Heuer D.K., Higginbotham E.J. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):701–713. doi: 10.1001/archopht.120.6.701. discussion 829-830. [DOI] [PubMed] [Google Scholar]
- 5.The Advanced Glaucoma Intervention Study (AGIS): 7 The relationship between control of intraocular pressure and visual field deterioration.The AGIS Investigators. Am J Ophthalmol. 2000;130(4):429–440. doi: 10.1016/s0002-9394(00)00538-9. [DOI] [PubMed] [Google Scholar]
- 6.Richter G.M., Coleman A.L. Minimally invasive glaucoma surgery: current status and future prospects. Clin Ophthalmol. 2016;10:189–206. doi: 10.2147/OPTH.S80490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Glaucoma Society . February 2014. AGS and FDA MIGS Workshop. [Google Scholar]
- 8.Widder R.A., Dietlein T.S., Dinslage S., Kühnrich P., Rennings C., Rössler G. The XEN45 Gel Stent as a minimally invasive procedure in glaucoma surgery: success rates, risk profile, and rates of re-surgery after 261 surgeries. Graefes Arch Clin Exp Ophthalmol. January 2018 doi: 10.1007/s00417-018-3899-7. [DOI] [PubMed] [Google Scholar]
- 9.De Gregorio A., Pedrotti E., Russo L., Morselli S. Minimally invasive combined glaucoma and cataract surgery: clinical results of the smallest ab interno gel stent. Int Ophthalmol. May 2017 doi: 10.1007/s10792-017-0571-x. [DOI] [PubMed] [Google Scholar]
- 10.Schlenker M.B., Gulamhusein H., Conrad-Hengerer I. Efficacy, safety, and risk factors for failure of standalone ab interno gelatin microstent implantation versus standalone trabeculectomy. Ophthalmology. 2017;124(11):1579–1588. doi: 10.1016/j.ophtha.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Buffault J., Baudouin C., Labbé A. XEN® Gel Stent for management of chronic open angle glaucoma: a review of the literature. J Fr Ophtalmol. 2019;42(2):e37–e46. doi: 10.1016/j.jfo.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Ibáñez-Muñoz A., Soto-Biforcos V.S., Rodríguez-Vicente L. XEN implant in primary and secondary open-angle glaucoma: a 12-month retrospective study. Eur J Ophthalmol. April 2019 doi: 10.1177/1120672119845226. 1120672119845226. [DOI] [PubMed] [Google Scholar]
- 13.Heidinger A., Schwab C., Lindner E., Riedl R., Mossböck G. A retrospective study of 199 Xen45 stent implantations from 2014 to 2016. J Glaucoma. 2019;28(1):75–79. doi: 10.1097/IJG.0000000000001122. [DOI] [PubMed] [Google Scholar]
- 14.Karri B., Gupta C., Mathews D. Endophthalmitis following XEN stent exposure. J Glaucoma. June 2018 doi: 10.1097/IJG.0000000000001012. [DOI] [PubMed] [Google Scholar]
- 15.Lim R., Lim K.S. XEN implant-related endophthalmitis. Ophthalmology. 2018;125(2):209. doi: 10.1016/j.ophtha.2017.10.019. [DOI] [PubMed] [Google Scholar]
- 16.Olgun A., Imamoğlu S., Karapapak M., Düzgün E., Kaçar H. Endophthalmitis after XEN gel stent implantation: 2 cases. J Glaucoma. 2018;27(12):e191–e194. doi: 10.1097/IJG.0000000000001076. [DOI] [PubMed] [Google Scholar]
- 17.Kerr N.M., Wang J., Sandhu A., Harasymowycz P.J., Barton K. Ab interno gel implant-associated bleb-related infection. Am J Ophthalmol. 2018;189:96–101. doi: 10.1016/j.ajo.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 18.Gedde S.J., Scott I.U., Tabandeh H. Late endophthalmitis associated with glaucoma drainage implants. Ophthalmology. 2001;108(7):1323–1327. doi: 10.1016/S0161-6420(01)00598-X. [DOI] [PubMed] [Google Scholar]
- 19.Arnould L., Theillac V., Moran S., Gatinel D., Grise-Dulac A. Recurrent exposure of XEN gel stent implant and conjunctival erosion. J Glaucoma. 2019;28(3):e37–e40. doi: 10.1097/IJG.0000000000001146. [DOI] [PubMed] [Google Scholar]
- 20.Ono T., Yuki K., Ozeki N., Shiba D., Tsubota K. Ocular surface complications after trabeculectomy: incidence, risk factors, time course and prognosis. Ophthalmologica. 2013;230(2):93–99. doi: 10.1159/000351649. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto T., Sawada A., Mayama C. The 5-year incidence of bleb-related infection and its risk factors after filtering surgeries with adjunctive mitomycin C: collaborative bleb-related infection incidence and treatment study 2. Ophthalmology. 2014;121(5):1001–1006. doi: 10.1016/j.ophtha.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 22.Rai P., Kotecha A., Kaltsos K. Changing trends in the incidence of bleb-related infection in trabeculectomy. Br J Ophthalmol. 2012;96(7):971–975. doi: 10.1136/bjophthalmol-2011-300926. [DOI] [PubMed] [Google Scholar]
- 23.Wilkins M., Indar A., Wormald R. Intraoperative Mitomycin C for glaucoma surgery. Cochrane Database Syst Rev. 2005;(4) doi: 10.1002/14651858.CD002897.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]