Abstract
The development of thyroid hormone (TH) analogues was prompted by the attempt to exploit the effects of TH on lipid metabolism, avoiding cardiac thyrotoxicosis. Analysis of the relative distribution of the α and β subtypes of nuclear TH receptors (TRα and TRβ) showed that TRα and TRβ are responsible for cardiac and metabolic responses, respectively. Therefore, analogues with TRβ selectivity were developed, and four different compounds have been used in clinical trials: GC-1 (sobetirome), KB-2115 (eprotirome), MB07344/VK2809, and MGL-3196 (resmetirom). Each of these compounds was able to reduce low-density lipoprotein cholesterol, but a phase 3 trial with eprotirome was interrupted because of a significant increase in liver enzymes and the contemporary report of cartilage side effects in animals. As a consequence, the other projects were terminated as well. However, in recent years, TRβ agonists have raised new interest for the treatment of nonalcoholic fatty liver disease (NAFLD). After obtaining excellent results in experimental models, clinical trials have been started with MGL-3196 and VK2809, and the initial reports are encouraging. Sobetirome turned out to be effective also in experimental models of demyelinating disease. Aside TRβ agonists, TH analogues include some TH metabolites that are biologically active on their own, and their synthetic analogues. 3,5,3′-triiodothyroacetic acid has already found clinical use in the treatment of some cases of TH resistance due to TRβ mutations, and interesting results have recently been reported in patients with the Allan–Herndon–Dudley syndrome, a rare disease caused by mutations in the TH transporter MCT8. 3,5-diiodothyronine (T2) has been used with success in rat models of dyslipidemia and NAFLD, but the outcome of a clinical trial with a synthetic T2 analogue was disappointing. 3-iodothyronamine (T1AM) is the last entry in the group of active TH metabolites. Promising results have been obtained in animal models of neurological injury induced by β-amyloid or by convulsive agents, but no clinical data are available so far.
Keywords: TH analogues; sobetirome; eprotirome; resmetirom; triac; 3,5-diiodothyronine; 3-iodothyronamine
Introduction
The term thyroid hormone (TH) analogue is used with regard to compounds that have a similar molecular structure as TH and can therefore interact with at least some of its molecular targets.
It should be kept in mind that TH signaling is particularly complex. Canonical TH signaling is based on the interaction with nuclear TH receptors (TRs), leading to either activation or repression of the transcription of a large number of genes. Two gene subtypes exist, TRα and TRβ, and different isoforms, which are designated by numerical subscripts, can be produced by alternative splicing (1). TRα1 is the major TR isoform in the heart, and it is also expressed in other tissues, including skeletal muscle, brain, and bone. TRα2 lacks part of the TH binding domain, so it is unable to bind TH and is thought to mediate constitutive repression of transcription. TRβ1 is widely expressed in most tissues, while TRβ2 expression is more circumscribed and it is particularly relevant in the brain, pituitary, retina, and inner ear. TRβ3 has been identified in rat adipose tissue, but it does not appear to be present in humans.
With regard to the topic of this review, the main functional consequence of TR subtype distribution is that the metabolic effects of TH are largely mediated by TRβ1, while cardiac TH actions depend on TRα1.
In addition to canonical signaling, TH may activate a number of noncanonical signaling pathways (2). The latter involves TRs that do not bind DNA directly, extranuclear TRs that activate the phosphatidylinositol 3-kinase pathway, and membrane receptors belonging to the integrin family (3).
In addition, some TH metabolites have been suggested to be potentially active, although their physiological role is still unclear. By definition, these compounds should be regarded as TH analogues, although they are endogenous molecules. They have also been used as templates to develop novel classes of synthetic analogues. Active TH metabolites include 3,5-diiodothyronine (T2), 3-iodothyronamine (T1AM), and several thyroacetic acids (3,5,3′,5′-tetraiodothyroacetic acid or Tetrac; 3,5,3′-triiodothyroacetic acid or Triac; 3-iodothroacetic acid or TA1). They have been the object of several recent reviews (4–8), and their properties are briefly recalled in the subsequent paragraphs only in as much as this is relevant to the object of the present succinct review.
TH analogues have been developed for clinical and therapeutic purposes, namely, to exploit some aspects of TH signaling to produce beneficial effects in the disease. Therefore, in the following paragraphs, these analogues are discussed with the perspective of their potential therapeutic use. Special emphasis is placed on clinical investigations, although some results obtained in experimental models of disease are mentioned when appropriate.
TH Analogues with Selective TRβ Activity in Dyslipidemia
The development of selective TRβ agonists was prompted by the aim of treating dyslipidemia, particularly hypercholesterolemia, avoiding cardiac thyrotoxicosis. The ligand binding pocket is more flexible in TRβ than in TRα and one of the amino acids involved in triiodothyronine (T3) binding is different, since TRα serine 277 is replaced by asparagine 311 in TRβ. These differences have been exploited to synthesize selective TRβ agonists (9,10).
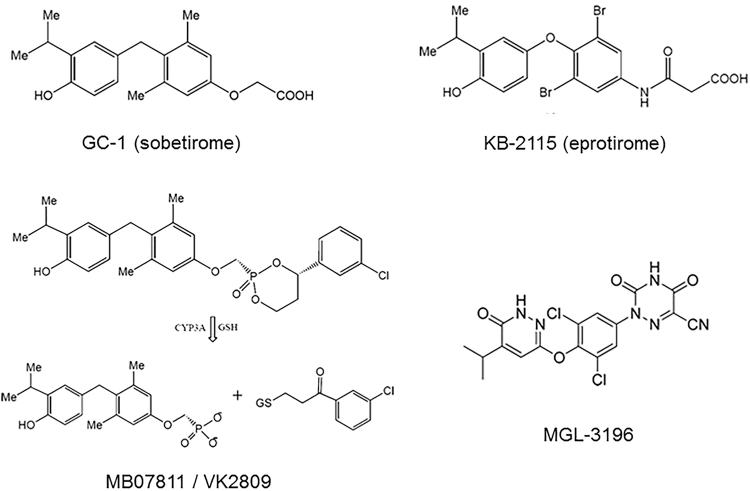
The analogues that have reached the clinical field are shown in Figure 1. In GC-1, now called sobetirome, the iodine atoms and the oxygen linking the two aromatic rings are replaced by alkyl groups, and the amino acid side chain is replaced by an oxoacetic chain. The ratio of TRβ to TRα affinity is 10-fold higher for GC-1 than for T3, and an additional useful property is represented by selective liver uptake.
FIG. 1.
Chemical structure of the synthetic TRβ analogues that have been used in clinical trials. In the left lower panel, please note that MB07811, now known as VK2809, is a prodrug; in the liver, it is converted into the active principle, formerly known as MB07344, by the hydroxylase CYP3A in a reaction requiring glutathione (GSH). See text for further details.
In KB-2115, now referred to as eprotirome, iodine is replaced by bromine (in the tyrosyl ring) or isopropyl (in the phenolic ring) and an amido-acetic side chain is present, yielding nearly 20-fold TRβ selectivity.
A different strategy was followed by researchers at Metabasis. Their compound, known as MB07811 and now renamed VK2809, is a prodrug, selectively taken up by the liver, where it is converted into the active principle (MB07344), which differs from GC-1 only for the presence of a phosphoryl group in the side chain.
Another compound that has been used in human is MGL-3196, also called resmetirom, whose more complex chemical structure (a substituted pyridazinone ring replaces the phenolic ring and a heterocyclic cyanoazauracil group is included in the side chain) allows nearly 30-fold TRβ selectivity.
All these compounds have been used in animal models of hypercholesterolemia, where they reduced total and low-density lipoprotein (LDL) cholesterol without significant changes in heart rate (11). Based on these preclinical findings, clinical trials were started. In 2008, both sobetirome (12) and eprotirome (13) were reported to produce a significant reduction in total and LDL cholesterol after 2–3 weeks of treatment in small groups of patients affected by hypercholesterolemia. Similar results were obtained with MGL-3196, which reduced serum triglycerides and LDL cholesterol after 2 weeks of treatment in 48 hypercholesterolemic patients (14). In patients treated with statins, the addition of eprotirome caused further reduction in serum LDL cholesterol and triglycerides (15).
No significant side effects were reported in any of these investigations, and therefore, a phase 3 trial was undertaken to compare eprotirome (at the daily doses of 50 and 100 μg) versus placebo in 236 patients with familial hypercholesterolemia. After 6 weeks, total cholesterol, LDL cholesterol, and triglycerides were significantly reduced in both treatment groups, without any change in HDL cholesterol (16). However, the trial was interrupted (it was originally planned to last 52–76 weeks) because parallel experimental investigations found that eprotirome caused cartilage damage in dogs. Notably, during the study period, a significant increase in liver enzymes was detected, which determined treatment interruption in four patients.
Resulting in a partial domino effect, termination of the eprotirome project caused parallel projects to be terminated as well. In particular, the sobetirome project was interrupted and Metabasis announced that a phase 1 investigation with MB07344 was aborted after observing increased liver enzymes in some patients. Additional reasons probably contributed to these decisions. While selective TRβ agonists were devised and tested, excellent clinical results were obtained with statins in the primary and secondary preventions of major cardiac events. At the same time, investigations performed with other experimental drugs showed that LDL cholesterol reduction per se was not necessarily associated with reduced cardiovascular risk. In aggregate, selective TRβ stimulation was no longer regarded as a promising strategy to treat hypercholesterolemia.
TH Analogues with Selective TRβ Activity in Liver Disease
Despite the disappointing results obtained with TRβ agonists in hypercholesterolemia, in recent years new potential uses have been proposed for these agents. The most attractive field is probably represented by nonalcoholic fatty liver disease (NAFLD).
This is a highly prevalent condition, since it is estimated to affect 17–46% of the adult population in the United States and Europe (17). Its clinical presentation is quite variable. Most NAFLD patients show a simple increase in liver enzymes with a histological pattern of increased hepatocyte triglyceride (steatosis). Symptoms may be minimal or absent and the clinical picture may be stable over time. However, a significant fraction of NAFLD patients (up to 40% in some series) develop histological evidence of lobular inflammation and hepatocyte ballooning, defining a variant of the disease known as nonalcoholic steatohepatitis (NASH). NASH is a serious condition, since it is associated with a high risk (about 10–20%) of evolution into cirrhosis, that is, derangement in liver architecture leading to hepatic insufficiency. Patients with NASH and/or cirrhosis are also prone to develop hepatocellular carcinoma (HCC).
NAFLD is frequently associated with insulin resistance, hypercholesterolemia, or other components of the so-called metabolic syndrome (17) and patients are usually treated for these underlying conditions, but no specific treatment to prevent liver damage has been approved so far. In principle, TH should be beneficial in NAFLD, due to reduced fatty acid synthesis, increased triglyceride and fatty acid breakdown, and enhanced hepatocyte regeneration (18,19). The liver effects of TH are mediated by TRβ, so selective TRβ agonists have been tested in experimental models, and several compounds (including sobetirome, eprotirome, and MB07811) were able to reduce liver steatosis (18–20). Sobetirome was also reported to prevent the development of HCC induced by activation of the catenin pathway (21).
The study of TRβ agonists in NAFLD has just entered the clinical field, and the results of two clinical trials have recently been reported. In 116 patients with biopsy-confirmed NASH treated with MGL-3196 (resmetirom) (80 mg daily) for 12 weeks, liver fat, as assessed by magnetic resonance imaging, was significantly reduced: −7.0% versus −2.7% with placebo, if expressed as percentage of absolute liver mass; −32.9% versus −10.4% if expressed as percentage of baseline liver fat (22). The effect was retained after 36 weeks, and in a subgroup of patients, biopsy revealed a reduction of histological markers of inflammation.
Similar results were reported with VK2809 (alias MB07811) in 45 patients with NAFLD treated for 12 weeks at the dosages of 10 mg every other day or 10 mg daily (23). In this trial, liver fat content, assessed by magnetic resonance imaging, decreased by 8.9–10.6% versus 0.9% with placebo (−56.5–59.7% vs. −8.9% if expressed as percentage of baseline liver fat). While these results have raised a great interest, and discussions have already started about the comparison of these two drugs, it should be stressed that the final results of the latter trial have not been published yet.
TH Analogues with Selective TRβ Activity in Central Nervous System Disease
In recent years, theoretical arguments have been developed, suggesting the potential usefulness of TH analogues in demyelinating disease, since TH favors both oligodendrocyte differentiation and myelin sheet synthesis (24). Although TRα1 is widely expressed in the central nervous system, efforts have been focused on TRβ agonists because of the necessity to avoid cardiac side effects. To increase bioavailability, an ethanolamine ester of sobetirome has been synthesized, which acts as a prodrug, since it crosses the blood/brain barrier and is converted into sobetirome within the central nervous system (25). This compound has been recently tested in different experimental models of demyelination, with good results: biochemical evidence of remyelination was confirmed by morphological findings obtained by nuclear magnetic imaging, and it was associated with improved functional recovery (26).
Clinical tests may be imminent for a specific disease known as X-linked adrenoleukodystrophy (ALD). This is a rare congenital disease due to mutations in the ALD protein, a transporter for very-long-chain fatty acids (i.e., with ≥22 carbon atoms), physiologically located in the peroxisomal membrane and encoded by the ABCD1 gene. The consequence is the accumulation of very-long-chain fatty acids and their derivatives, which are eventually incorporated in cellular membranes, whose structure and function are deranged. Affected males develop adrenal insufficiency in childhood and progressive myelopathy occurs in adulthood. Demyelinating lesions in cerebral white matter also appear, since the age of 3 years (27).
ALD patients may benefit from hematopoietic stem cell transplantation, but this treatment is effective only if performed in the early stages of the disease, and it carries a significant risk of mortality (5–10%). No specific pharmacological therapy is available for ALD. Notably, TH induces the expression of ABCD2, coding for an additional peroxisomal transporter, and in a transgenic mouse model of ALD, sobetirome administration reduced the brain and adrenal content of very-long-chain fatty acids (28). Based on these observations, a clinical trial with sobetirome in X-linked ALD has been posted in the NIH database (NCT01787578). The trial is presently labeled as withdrawn, and the alleged reason is the need for revisions to the original protocol.
Triac in Syndromes of Reduced Sensitivity to TH
Reduced sensitivity to TH is diagnosed when symptoms of hypothyroidism occur despite normal or increased serum TH. This finding may be the consequence of mutations in TRs, transporters, or metabolizing enzymes (29). The expression “resistance to TH” is usually reserved for syndromes caused by TR mutations. Most cases are associated with TRβ mutations. Since TRβ is involved in the inhibition of thyrotropin (TSH) secretion by T3 and thyroxine (T4), circulating levels of T4 and T3 are usually high, TSH is normal or high, and goiter may be present. The clinical picture is quite variable, and includes signs and symptoms of both hypothyroidism, in TRβ-dependent organs, and thyrotoxicosis, in TRβ-dependent organs. The most common findings are delayed growth, delayed bone maturation, cognitive impairment, and tachycardia (1,29).
Treating TH resistance is not easy. Exogenous T4 or T3 administration may improve some symptoms, but it may worsen others, and symptomatic therapy is often prescribed, for example, beta-blockers to reduce heart rate. The ideal treatment would be represented by a T3 analogue able to activate the mutated receptor.
In some patients, this can occur with Triac (Fig. 2). The lack of the amine group does not prevent Triac from activating TRs, and it has similar affinity as T3 for the wild-type receptor. Apparently, the different shape and charge of the Triac molecule allow several classes of mutated TRβ to be activated as well. In these patients, chronic Triac administration at dosages on the order of 25–35 μg per kg of body weight daily represents the best therapeutic regimen (30). In general, Triac-sensitive cases of TH resistance are caused by mutations in the carboxy-terminal region of the T3 binding domain, while mutations located close to the hinge region do not respond to Triac. No positive response to Triac has been reported in TRα mutations, so far.
FIG. 2.
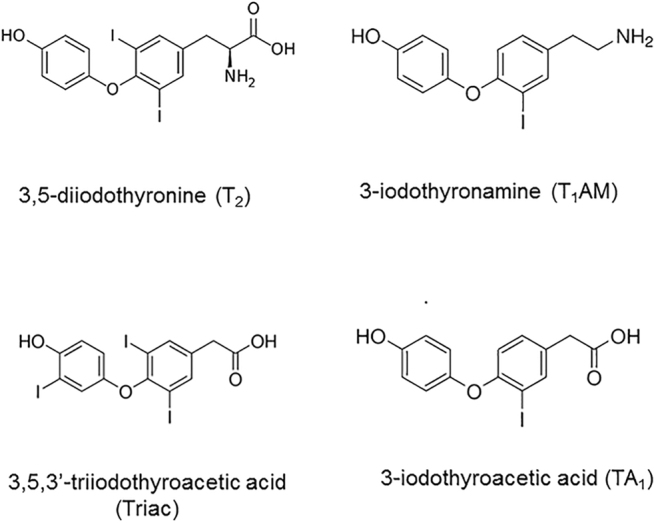
Chemical structure of some active thyroid hormone metabolites that have been used in patients or in animal models of human disease. See text for further details.
A different cause of reduced sensitivity to TH is represented by mutations in MCT8, a T3/T4 transporter that is the major pathway mediating T4 and T3 uptake in the central nervous system (31). The clinical syndrome associated with MCT8 mutations is known as the Allan–Herndon–Dudley syndrome. The MCT8 gene is located on the X chromosome, and therefore, virtually all patients are male. Diagnosis is suspected in the presence of high T3 with low to normal T4 and low to normal TSH, associated with congenital brain hypothyroidism. The phenotype is variable, depending on the location and type of the mutation. Symptoms usually include cognitive impairment associated with congenital hypotonia and weakness, which may progress to spasticity. Paroxysmal dyskinesias and seizures may also occur. Peripheral hyperthyroidism often causes tachycardia, muscle wasting, and progressive body weight reduction (31–33).
Since it has been observed that cellular Triac uptake does not depend on MCT8, a clinical trial has been undertaken with this endogenous TH analogue in 50 patients, with a median age of 7 years. The results obtained after 12 months of treatment at the dosage of 350–4200 μg daily have recently been published (33). The treatment was highly effective in reducing serum T3, and Triac dosage was actually titrated on T3 reduction. Signs of peripheral hypothyroidism, notably tachycardia and body weight reduction, were significantly attenuated. On the contrary, the effects on the neurological symptoms were limited, and improvement in the indices of motor function was limited to patients younger than 5 years. It seems therefore that once the neurological phenotype is fully developed, it is hardly reversible.
On this basis, another phase 2 trial is underway (NCT02396459), in which Triac treatment will be started as early as possible in postnatal life.
Synthetic TH analogues might also be useful. Notably, in a small clinical study diiodothyropropionic acid (DITPA) administration (2.1–2.4 mg/kg per day) was able to normalize T3 in four children affected by MCT8 deficiency, aged 8–25 months (34). Heart rate decreased in three patients and weight gain occurred in two. Although DITPA was introduced over 25 years ago as a relatively weak and poorly selective TR agonist, its mode of interaction with TRs has not been specifically investigated so far.
Potential Uses of T2 and T1AM
While Triac is basically a thyromimetic, other active TH metabolites (Fig. 2) can interact with different molecular targets. T2 has direct mitochondrial actions, allegedly on respiratory chain complex IV (subunit Va), and it stimulates mitochondrial respiration and fatty acid oxidation (3,4,8). These metabolic responses are further supported by genomic effects, whose molecular basis is unclear, since they appear to be different from those elicited by T3. In any case, in rats treated with high-fat diet, exogenous T2 decreased serum triglycerides and LDL cholesterol, as well as liver fat and biochemical indices of liver injury, suggesting potential therapeutic value for either dyslipidemia or NAFLD (4,35). However, it is still controversial whether these positive effects may be achieved without inducing tachycardia or cardiac hypertrophy, since cardiac thyrotoxicosis has been reported in mice (36).
A single pilot investigation was performed in two human volunteers taking T2 (300 μg/kg) for 3 weeks (37). A slight but significant decrease in body weight was reported (−4%), without any change in serum T3, T4, TSH, and cardiac function. More recently, a synthetic T2 analogue (TRC150094) was used in 40 patients with metabolic syndrome, but the results were rather disappointing, since no significant effect on insulin sensitivity and plasma lipid profile was observed (38).
The last entry into the group of active TH metabolites is represented by T1AM. It does not interact with canonical or noncanonical TH targets, and it was discovered as a high affinity agonist of TAAR1, a membrane G protein-coupled receptor (GPCR) that is expressed in the brain and many other tissues (39,40). T1AM is a biogenic amine and shares several properties of this class of compounds, including the ability to interact with multiple targets, namely, other GPCRs (e.g., α2A adrenergic receptor), membrane ionic channels (e.g., TRPM8), and monoamine transporters (6,7).
In experimental animals, the administration of exogenous T1AM induced many different functional effects. Neurological and metabolic effects are particularly interesting, since they appear to be elicited at relative low doses, and might have physiological relevance (40,41). The former includes modulation of feeding behavior and sleep/wake cycle, reduction of pain threshold, prolearning, and antiamnestic responses. The major metabolic effects consist in the stimulation of triglyceride and fatty acid catabolism, but anti-insulin effects on glucose metabolism are also elicited at slightly higher doses.
Although the administration of exogenous T1AM has not been tested in humans, some experimental results obtained in murine models of disease have raised interest about potential therapeutic applications. T1AM reduced serum cholesterol in spontaneously obese mice (42), as well as liver triglycerides in a mouse model of polycystic ovary syndrome (43).
Neuroprotective effects appear even more promising. Intracerebral T1AM rescued long-term potentiation and behavioral evidence of cognitive dysfunction in an in vivo model of β-amyloid toxicity, namely, transgenic mice overexpressing human-mutated amyloid precursor protein (44). In addition, the intracerebral injection of T1AM metabolite 3-iodothyroacetic acid protected from the convulsive effect of pentylenetetrazole and from kainate toxicity (45), while exogenous T1AM reduced apoptosis and functional injury in a mouse model of spinal cord clamp (46).
Because of the multitude of T1AM effects, an active research line is focused on the development of synthetic derivatives with more favorable biodistribution and/or receptor selectivity (47).
In conclusion, the development TH analogues was initially prompted by the attempt to exploit the effects of TH on lipid metabolism, while avoiding unwanted cardiac effects. TRβ agonists have provided good results in a few small clinical trials performed in hypercholesterolemic patients, but these projects have been terminated after the report of potential side effects. In recent years, TRβ agonists have raised new interest for the treatment of NAFLD, and a couple of clinical trials have provided encouraging initial results. Triac has already found clinical use in the treatment of selected cases of TH resistance due to TRβ mutations, and interesting results have recently been reported in the Allan–Herndon–Dudley syndrome.
Other TH analogues are under consideration for neurological diseases, although human results are not yet available. In particular, sobetirome derivatives have been successful in animal models of multiple sclerosis, and T1AM has been beneficial in an animal model of β-amyloid toxicity.
Overall, research on TH analogues is experiencing a shift in its focus, but it still appears to be an active and promising field.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This work was supported by a grant from Pisa University (PRA 2018 to R.Z.).
References
- 1. Ortiga-Carvalho TM, Sidhaye AR, Wondisford FE. 2014. Thyroid hormone receptors and resistance to thyroid hormone disorders. Nat Rev Endocrinol 10:582–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Flamant F, Cheng SY, Hollenberg AN, Moeller LC, Samarut J, Wondisford FE, Yen PM, Refetoff S. 2017. Thyroid hormone signaling pathways: time for a more precise nomenclature. Endocrinology 158:2052–2057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Davis PJ, Goglia F, Leonard JL. 2016. Nongenomic actions of thyrid hormone. Nat Rev Endocrinol 17:111–121 [DOI] [PubMed] [Google Scholar]
- 4. Senese R, de Lange P, Petito G, Moreno M, Goglia F, Lanni A. 2018. 3,5-diiodothyronine: a novel thyroid hormone metabolite and a potent modulator of energy metabolism. Front Endocrinol (Lausanne) 9:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Groeneweg S, Peeters RP, Visser TJ, Visser WE. 2017. Triiodothyroacetic acid in health and disease. J Endocrinol 234:R99–R121 [DOI] [PubMed] [Google Scholar]
- 6. Hoefig CS, Zucchi R, Köhrle J. 2016. Thyronamines and derivatives: physiological relevance, pharmacological actions, and future research directions. Thyroid 26:1656–1673 [DOI] [PubMed] [Google Scholar]
- 7. Köhrle J, Biebermann H. 2019. 3-iodohyronamine—a thyroid hormone metabolite with distinct target profiles and modes of action. Endocrine Rev 40:602–630 [DOI] [PubMed] [Google Scholar]
- 8. Zucchi R, Rutigliano G, Saponaro F. 2019. Novel thyroid hormones. Endocrine 66:95–104 [DOI] [PubMed] [Google Scholar]
- 9. Joharapurak AA, Dhote VV, Jain MR. 2012. Selective thyromimetics using receptor and tissue selectivity approaches: prospects for dyslipidemia. J Med Chem 55:5649–5675 [DOI] [PubMed] [Google Scholar]
- 10. Mondal S, Mugesh G. 2017. Novel thyroid hormone analogues, enzyme inhibitors and mimetics, and their action. Mol Cell Endocrinol 458:91–104 [DOI] [PubMed] [Google Scholar]
- 11. Tancevski I, Rudling M, Eller P. 2011. Thyromimetics: a journey from bench to bed-side. Pharmacol Ther 131:33–39 [DOI] [PubMed] [Google Scholar]
- 12. Lin VW, Klepp HM, Hanley RM. 2008. Sobetirome is a TRβ- and liver-selective thyromimetic that can affect substantial LDL-C lowering without significant changes in heart rate or the thyroid axis in euthyroid men [abstract]. San Francisco: the Endocrine Society Annual Meeting ENDO 08, OR36-33
- 13. Berkenstam A, Kristensen J, Mellström K, Carlsson B, MalmJ, Rehnmark S, Garg N, Andersson CM, Rudling M, Sjöberg F, Angelin B, Baxter JD. 2008. The thyroid mimetic compound KB2115 lowers plasma LDL cholesterol and stimulates bile acid synthesis without cardiac effects in humans. Proc Natl Acad Sci U S A 105:663–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Taub R, Chiang E, Chabot-Blanchet M, Kelly MJ, Reeves RA, Guertin MC, Tardif JC. 2013. Lipid lowering in healthy volunteers treated with multiple doses of MGL-3196, a liver-targeted thyroid hormone receptor-β agonist. Atherosclerosis 230:373–380 [DOI] [PubMed] [Google Scholar]
- 15. Ladenson PW, Kristensen JD, Ridgway EC, Olsson AG, Carlsson B, Klein I, Baxter JD, Angelin B. 2010. Use of the thyroid hormone analogue eprotirome in statin-treated dyslipidemia. N Engl J Med 362:906–916 [DOI] [PubMed] [Google Scholar]
- 16. Sjouke B, Langslet G, Ceska R, Nicholls SJ, Nissen SE, Ohlander M, Ladenson PW, Olsson AG, Hovingh GK, Kastelein JJ. 2014. Eprotirome in patients with familial hypercholesterolaemia (the AKKA trial): a randomized, double-blind, placebo-controlled phase 3 study. Lancet Diabetes Endocrinol 2:355–463 [DOI] [PubMed] [Google Scholar]
- 17. European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO) 2016. ESAL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 64:1388–1402 [DOI] [PubMed] [Google Scholar]
- 18. Coppola M, Glinni D, Moreno M, Cioffi F, Silvestri E, Goglia F. 2014. Thyroid hormone analogues and derivatives: actions in fatty liver. World J Hepatol 6:114–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sinha RA, Singh BK, Yen PM. 2018. Direct effects of thyroid hormones on hepatic lipid metabolism. Nat Rev Endocrinol 14:259–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Martagon AJ, Lin JZ, Cimini SL, Webb P, Phillips KJ. 2015. The amelioration of hepatic steatosis by thyroid hormone receptor agonists is insufficient to restore insulin sensitivity in ob/ob mice. PLoS One 10:e0122987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Puliga E, Min Q, Tao J, Zhang R, Pradhan-Sundd T, Poddar M, Singh S, Columbano A, Yu J, Momga SP. 2017. Thyroid hormone receptor-β agonist GC-1 inhibits met-β-catenin-driven hepatocellular cancer. Am J Pathol 187:2473–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Harrison SA, Bashir MR, Guy CD, Zhou L, Moylan CA, Frias JP, Alkhouri N, Bansal MB, Baum S, Neuschwander-Tetri BA, Taub R, Moussa SE. 2019. Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: a multicenter, randomized, double-blind, placebo-controlled phase 2 trial. Lancet 394:2012–2024 [DOI] [PubMed] [Google Scholar]
- 23. Loomba R, Neutel J, Bernard D, Severance R, Mohseni R, Dao M, Saini S, Margaritescu C, Homer K, Tran B, Mancini M, Masamune H, Lian B. 2018. VK2809, a novel liver-directed thyroid receptor beta agonist, significantly reduces liver fat in patients with nonalcoholic fatty liver disease: a phase 2 randomized placebo-controlled trial [abstract]. Hepatology 68:1447A [Google Scholar]
- 24. Zhang M, Ma Z, Qin H, Yao Z. 2016. Thyroid hormone potentially benefits multiple sclerosis via facilitating remyelination. Mol Neurobiol 53:4406–4416 [DOI] [PubMed] [Google Scholar]
- 25. Placzek AT, Ferrara SJ, Hartley MD, Sanford-Crane HS, Meining M, Scanlan TS. 2016. Sobetirome prodrug esters with enhanced blood-brain barrier permeability Bioorg Med Chem 24:5842–5854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hartley MD, Banerji T, Tagge IJ, Kirkemo LL, Chaudhary P, Calkins E, Galipeau D, Shokat MD, DeBell MJ, Van Leuven S, Miller H. 2019. Myelin repair stimulated by CNS-selective thyroid hormone action. JCI Insights 4:e126329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kemp S, Huffnagel IC, Linthorst GE, Wanders RJ, Engelen M. 2016. Adrenoleukodystrophy—neuroendocrine pathogenesis and redefinition of natural history. Nat Rev Endocrinol 12:606–615 [DOI] [PubMed] [Google Scholar]
- 28. Hartley MD, Kirkemo LL, Banerji T, Scanlan TS. 2017. A thyroid hormone-based strategy for correcting the biochemical abnormality in X-linked adrenoleukodystrophy. Endocrinology 158:1328–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Refetoff S, Dumitrescu AM. 2007. Syndromes of reduced sensitivity to thyroid hormone: genetic defects in hormone receptors, cell transporters and deiodination. Best Pract Clin Endocrinol Metabolism 21:277–305 [DOI] [PubMed] [Google Scholar]
- 30. Groeneweg S, Peeters RP, Visser TJ, Visser WE. 2017. Therapeutic applications of thyroid hormone analogues in resistance to thyroid hormone (RTH) syndromes. Mol Cell Endocrinol 458:82–90 [DOI] [PubMed] [Google Scholar]
- 31. Friesema ECH, Visser WE, Visser TJ. 2010. Genetics and phenomics of thyroid hormone transport by MCT8. Mol Cell Endocrinol 322:107–113 [DOI] [PubMed] [Google Scholar]
- 32. Schwartz CE, Stevenson RE. 2007. The MCT8 thyroid hormone transporter and Allan-Herndon-Dudley Syndrome. Best Pract Clin Endocrinol Metab 21:307–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Groeneweg S, Peeters RP, Moran C, Stoupa A, Aurial F, Tonduti D, Dica A, Paone L, Rozenkova K, Malikova J, van der Walt A, de Coo IFM, McGowan A, Lyons G, Aarsen FK, Barca D, van Beynum IM, van der Knoop MM, Jansen J, Manshande M, Lunsing RJ, Nowak S, den Uil CA, Zillikens MC, Visser FE, Vrijmoeth P, de Wit MCY, Wolf NI, Zandstra A, Ambegaonkar G, Singh Y, de Rijke YB, Medici M, Bertini ES, Depoorter S, Lebl J, Cappa M, De Meirleir L, Krude H, Craiu D, Zibordi F, Oliver Petit I, Polak M, Chatterjee K, Visser TJ, Visser WE. 2019. Effectiveness and safety of the tri-iodothyronine analogue Triac in children and adults with MCT8 deficiency: an international, single arm, open-label, phase 2 trial. Lancet Diabetes Endocrinol 7:695–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Verge CF, Konrad D, Cohen M, Di Cosmo C, Dumitrescu AM, Marcinkowski T, Hameed S, Hamilton J, Weiss RE, Refetoff S. 2012. Diiodothyropionic acid (DITPA) in the treatment of MCT8 deficiency. J Clin Endocrinol Metab 97:4515–4523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Goglia F. 2015. The effects of 3,5-diiodothyronine on energy balance. Front Physiol 5:528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jonas W, Lietzow J, Wohlgemuth F, Hoefig CS, Wiedmer P, Schweizer U, Köhrle J, Schurmann A. 2015. 3,5-Diiodo-L-thyronine (3,5-T2) exerts thyromimetic effects on hypothalamus-pituitary-thyroid axis, body composition, and energy metabolism in male diet-induced obese mice. Endocrinology 156:389–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Antonelli A, Fallahi P, Ferrari SM, Di Domenicantonio A, Moreno M, Lanni A, Goglia F. 2011. 3,5-diiodo-L-thyronine increases resting metabolic rate and reduces body weight without undesirable side effects. J Biol Regul Homeost Agents 25:655–660 [PubMed] [Google Scholar]
- 38. Van der Val F, Hassing C, Visser M, Thakkar P, Mohanan A, Pathak K, Dutt C, Chauthaiwale V, Ackermans M, Nederveen A, Serlie M, Nieuwdorp M, Stroes E. 2014. The effect of a diodothyronine mimetic on insulin sensitivity in male cardiometabolic patients: a double-blind randomized controlled trial. PLoS One 9:e86890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zucchi R, Chiellini S, Scanlan TS, Grandy DK. 2006. Trace amine-associated receptors and their ligands. Br J Pharmacol 149:967–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rutigliano G, Accorroni A, Zucchi R. 2018. The case for TAAR1 as a modulator of central nervous system function. Front Pharmacol 8:987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zucchi R, Accorroni A, Chiellini G. 2014. Update on 3-iodothyronamine and its neurological and metabolic actions. Front Physiol 5:402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Assadi-Porter FM, Reiland H, Sabatini M, Lorenzini L, Carnicelli V, Rogowski M, Selen Alpergin ES, Tonelli M, Ghelardoni S, Saba A, Zucchi R, Chiellini G. 2018. Metabolic reprogramming by 3-iodothyronamine (T1AM): a new perspective to reverse obesity through regulation of sirtuin 4 and 6 expression. Int J Mol Sci 19:e1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Selen Alpergin ES, Bolandnazar Z, Sabatini M, Rogowski M, Chiellini G, Zucchi R, Assadi-Porter FM. 2017. Metabolic profiling reveals reprogramming of lipid metabolic pathways in treatment of polycystic ovary syndrome with 3-iodothyronamine. Physiol Rep 5:e13097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Accorroni A, Rutigliano G, Sabatini M, Frascarelli S, Borsò M, Novelli E, Bandini L, Ghelardoni L, Saba A, Zucchi R, Origlia N. 2020. Exogenous 3-iodothyronamine rescues the entorhinal cortex from β-amyloid toxicity. Thyroid 30:147–160 [DOI] [PubMed] [Google Scholar]
- 45. Laurino A, Landucci E, Resta F, De Siena F, Pellegrini-Giampietro DE, Masi A, Mannaioni G, Raimondi L. 2018. Anticonvulsant and neuroprotective effects of the thyroid hormone metabolite 3-Iodothyroacetic Acid. Thyroid 28:1387–1397 [DOI] [PubMed] [Google Scholar]
- 46. Lv J, Liao J, Tan W, Yang L, Shi X, Zhang H, Chen L, Wang S, Li Q. 2018. 3-Iodothyronamine acting through an anti-apoptotic mechanism is neuroprotective against spinal cord injury in rats. Ann Clin Lab Sci 48:736–742 [PubMed] [Google Scholar]
- 47. Chiellini G, Bellusci L, Sabatini M, Zucchi R. 2017. Thyronamines and analogues—the route from rediscovery to translational research on thyronergic amines. Mol Cell Endocrinol 458:149–155 [DOI] [PubMed] [Google Scholar]