Abstract
Engineered male and female biomimetic reproductive tissues are being developed as autonomous in vitro units or as integrated multi-organ in vitro systems to support germ cell and embryo function, and to display characteristic endocrine phenotypic patterns, such as the 28-day human ovulatory cycle. In this Review, we summarize how engineered reproductive tissues facilitate research in reproductive biology, and overview strategies for making engineered reproductive tissues that might eventually allow the restoration of reproductive capacity in patients.
Individuals can face reproductive or endocrine failure because of genetic predisposition, age, iatrogenic effects of treatment or disease. Over a century of progress that began with advancements in reproductive tissue and reproductive organ transplantation, followed by technological developments at the interface of reproductive biology, materials science, bioengineering and advanced manufacturing, has resulted in engineered reproductive tissues that can restore and support normal organ function1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20 (Table 1). Modern-engineered reproductive tissues and culture systems are enabling the increasingly physiological in vitro modelling of homeostasis, development, disease, pregnancy and aging. Engineered reproductive tissues are used for the efficient screening of new pharmacologic agents (for both therapeutic efficacy and toxicity), or are transplanted to restore damaged or diseased reproductive tissue. In this Review, we highlight recent progress in the development of engineering strategies employed in reproductive science and medicine, with a focus on biomaterials and microfluidic approaches that permit the generation of functional constructs at the tissue and organ levels for use in research and in clinical applications. Approaches at the cellular, molecular and genetic levels, such as the use of induced pluripotent stem cells21,22, the controlled delivery of drugs and other bioactive agents23,24,25,26,27, and the use of microfluidic devices in assisted reproductive technology28,29, have been discussed elsewhere.
Table 1.
Major technological advancements in reproductive science and medicine
| Year | Advancement | References |
|---|---|---|
| 1895 | First ovarian graft transplantation | 1 |
| 1931 | First human uterus transplantation | 2 |
| 1936 | First modern penile implant | 3 |
| 1941 | First testis prosthesis | 4 |
| 1978 | First human birth from IVF First human testis transplantation |
5 6 |
| 1984 | Human birth from frozen embryo | 7 |
| 1986 | Successful cryopreservation of human oocytes | 8 |
| 1992 | IVF by intracytoplasmic sperm injection | 9 |
| 2004 | Human birth from ovarian tissue transplantation Human whole ovary transplantation |
10 11 |
| 2006 | Cryopreservation of whole human ovary Oncofertility field established Live animal birth from follicles grown in vitro within a biomaterial |
12 13 14 |
| 2014 | Engineered vagina transplant | 15 |
| 2015 | Human metaphase-II oocyte in vitro Human birth from uterus transplantation |
16 17 |
| 2017 | EVATAR Biobag Functional 3D-printed ovarian bioprosthetic |
18 19 20 |
Table 1 displays current trends in engineering reproductive tissues evolved from scientific and technological developments aimed to improve reproductive-health outcomes.
The female mammalian reproductive system consists of the ovaries, fallopian tubes (also called oviducts in non-primate species), uterus, cervix and vagina (Fig. 1). Sexual intercourse, fertilization, implantation and maintenance of a pregnancy to term all depend on the dynamic interactive physiology of the reproductive tract organs, which function in response to hormonal instructions produced by the ovaries, the pituitary gland and the hypothalamus throughout the ovulatory cycle30 (Fig. 2a). The main components of the mammalian reproductive tract in males include the testes, epididymis, seminal vesicle, vas deferens, prostate gland and penis (Fig. 3). In contrast to the female ovulatory cycle, the reproductive endocrine cycle of the male is characterized by a daily rise and fall of testosterone, largely produced by the Leydig cells of the testes in response to pituitary and hypothalamic signals30 (Fig. 2b).
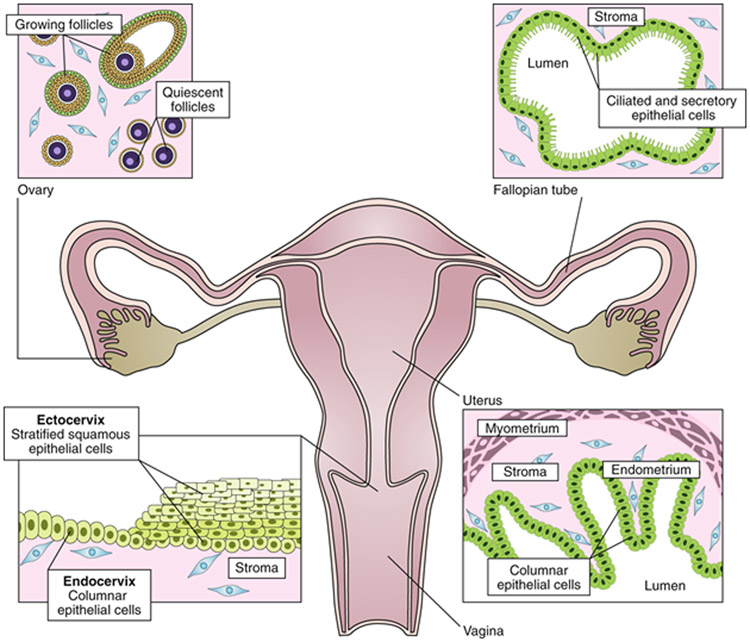
Fig. 1: The female reproductive tract.
The female reproductive tract consists of the ovaries, fallopian tubes, uterus, cervix and vagina. The ovaries contain follicles—that is, cellular aggregates in which an oocyte is surrounded by hormone-producing granulosa cells. Each month, a cohort of quiescent follicles are activated and begin growth, ultimately resulting in the ovulation of a single oocyte. The oocyte travels through the fallopian tube where the ciliated epithelial cells generate a current that sperm swim against for fertilization. The next organ in the tract is the uterus, where embryo implantation occurs. The muscular layer of the uterus (myometrium) generates contractions. The cervix forms the boundary between the uterus and the vagina. The endocervix resembles the uterus histologically, while the ectocervix is more similar to the vagina. Developing technology for the female reproductive tract requires the understanding of the native tissue and of endocrine interactions.
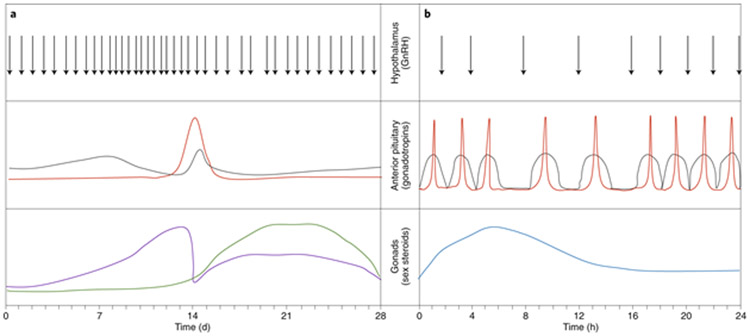
Fig. 2: Endocrinology of the hypothalamic–pituitary–gonadal axis.
a, The female hypothalamic–pituitary–gonadal axis cycles every 28 d in humans. Pulses of gonadotropin-releasing hormone (GnRH) from the hypothalamus drive the secretion of the gonadotropins FSH (grey) and luteinizing hormone (red) from the anterior pituitary. The gonadotropins instruct the female gonad (that is, the ovary) to produce estradiol (purple) and progesterone (green). b, The male hypothalamic–pituitary–gonadal axis cycles every 24 h in humans. GnRH pulses from the hypothalamus drive the secretion of FSH (grey) and luteinizing hormone (red) from the anterior pituitary. The gonadotropins stimulate production of testosterone (blue) by the Leydig cells of the male gonad (that is, the testis). Engineered reproductive tissue constructs aim to mimic the hormonal changes ex vivo or to restore the complex physiological interactions in vivo.
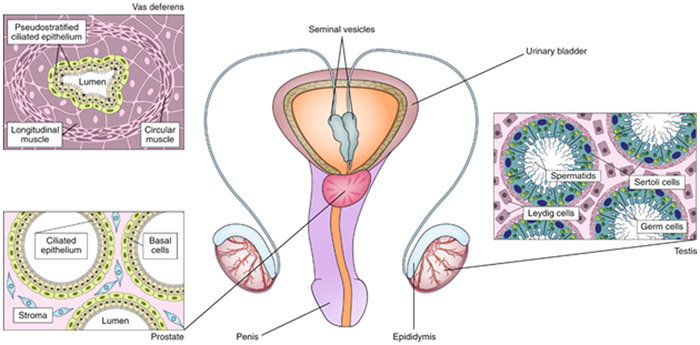
Fig. 3: The male reproductive tract.
The male reproductive tract consists of the testes, the epididymis, the vas deferens, the seminal vesicles, the prostate and the penis. The testis is composed of seminiferous tubules, which are lined with a layer of germ cells that give rise to spermatids. Within the seminiferous tubules, Sertoli cells support the developing gametes. Leydig cells, located between the tubules, secrete testosterone. The developing sperm travels out of the testis to the epididymis, then to the vas deferens, and is expelled through the penis. The prostate and seminal vesicles secrete fluid that makes up the semen. Engineering reproductive tissue involves the recapitulation of the complex cellular tissue and endocrine relationships that exist in the male reproductive tract towards developing in vitro models or making biomimetic organs for transplantation.
To date, knowledge regarding these tissues comes from studies using animal models and two-dimensional (2D) cell culture; however, these methods are far from ideal. First, although there are many similarities in mammalian reproductive biology across species, there are also a number of differences in terms of both reproductive physiology and pathophysiology. For example, regarding physiology, there are interspecies differences in receptor expression31, metabolism32, ovulation rate and cycle length33, ranging from four days in the mouse to 28 days in the human female. With respect to pathophysiology, there are a number of conditions, such as endometriosis or cervical cancer, for which there is no equivalent animal model. Second, in 2D culture, cells from reproductive tissues often lose some of their native properties, precluding the generalization of the results of in vitro experiments to the in vivo situation. For example, the epithelium of the female reproductive tract, like most epithelia, de-differentiates when cultured in vitro, becoming basal-like, and the cell–cell interactions necessary for ovarian follicle function are lost in 2D culture30. Similarly, Sertoli cells, the somatic epithelial cells that contribute to germ cell expansion and differentiation in the testis, also lose key expression markers and secretory profiles when cultured in 2D, a challenge that has been difficult to overcome, even with the use of genetically engineered Sertoli cells or other feeder cell types34. Third, both male and female reproductive organs and tissues produce and maintain hormone cycles that are critical for regulating numerous physiological processes within and beyond the reproductive tract. In the female, the uterus is dependent on the rise and fall of oestrogen and progesterone produced by the ovary in order to drive endometrial proliferation and subsequent shedding. Cycling ovarian hormones also influence the function of the fallopian tube; for example, progesterone decreases the beat frequency of fallopian tube cilia35. In the male, testosterone is required for the normal maintenance of epithelial cells in the prostate36,37,38. Testosterone induces fluid production in the seminal vesicles and is required for normal spermatogenesis39. Organs outside of the reproductive tract, such as the heart and bone, also respond to steroid hormones and, in turn, the reproductive tissues and organs respond to hormones produced by distant organs such as the thyroid, pituitary and pancreas. For example, the testis–bone–pancreas axis, which involves signalling through osteocalcin and insulin-like 3, remains poorly understood40,41,42. Therefore, in vitro models that lack these hormonal factors do not fully capture the complexity of in vivo endocrine loops. Furthermore, owing to the complexity of the endocrine loops that occur throughout the female ovulatory cycle, biomedical research has historically focused on the ‘simpler’ male cycle43. Taken together, current approaches that rely on 2D in vitro monoculture and in vivo animal models to study the human reproductive tract have often fallen short of elucidating mechanistic answers and human-relevant findings. Developing advanced engineered solutions for reproductive science and medicine that recapitulate both the tissue and organ environments as well as endocrine factors of the reproductive tract makes it possible to reintroduce variables that have thus far been missing. These solutions will facilitate in vitro drug screening and toxicity testing, tissue transplantation and ex vivo foetal development, and serve to fill knowledge gaps by providing effective models for the study of sex differences in health and disease.
Engineered models of reproductive tissues and organs
To understand the mechanisms of development, disease and normal function in reproductive systems, approaches leveraging biomaterials, tissue engineering and microfluidics have led to the creation of in vitro models of individual reproductive tissues and integrated tissue systems. Table 2 outlines the recent bioengineered systems recapitulating aspects of the male or female reproductive biology that are discussed in this Review.
Table 2.
Overview of recent literature in engineered reproductive tissues
| Tissue | Engineered system |
References | |
|---|---|---|---|
| Female | Ovary | Hydrogels | 14,16,46,47,48,49,50,51,52,53,133,142,143,144,145,146,147,148,149,150,151 |
| Decellularized or recellularized | 63,64,65,66,67,68,81,88 | ||
| 3D printing | 20 | ||
| Microfluidic | 18 | ||
| Fallopian tube | Hydrogels | 61 | |
| 3D co-culture | 116,117 | ||
| Microfluidic | 18,118 | ||
| Uterus | Hydrogels | 62,135 | |
| Decellularized or recellularized | 69,70,71,72,73,74 | ||
| 3D printing | 98 | ||
| Scaffold-free | 110 | ||
| Microfluidic | 18,122 | ||
| Cervix | Decellularized or recellularized | 168 | |
| Scaffold-free | 102,103 | ||
| Microfluidic | 18 | ||
| Vagina | Decellularized or recellularized | 15,169,170,171,172,173 | |
| Male | Testis | Hydrogels | 58,59,60,165 |
| Decellularized or recellularized | 75,76,77,78,83,84,85,86,89 | ||
| Scaffold-free | 104,105,106,107,108,109,111,112 | ||
| 3D printing | 95 | ||
| Microfluidic | 119,120,121 | ||
| Peripheral | Placenta | Decellularized or recellularized | 79,80 |
| 3D bioprinting | 96,97 | ||
| Microfluidic | 123,124,125,126,127,128,129,130,131 |
Hydrogel encapsulation
Perhaps the most straightforward of recent developments is the encapsulation of reproductive cells and tissues within hydrogels for 3D in vitro culture (Fig. 4a). Hydrogels have a high degree of structural similarity to the native extracellular matrix (ECM) and have been widely used to encapsulate cells and tissues for tissue-engineering applications44,45,46. Hydrogels are composed of more than 90% water, permit efficient diffusion of nutrients and waste, and provide physical support and critical physical cues to encapsulated cells and tissues. The functional unit of the ovary, the ovarian follicle, is an example of a reproductive tract component whose function is highly dependent on physical cues from its microenvironment to maintain its cellular architecture. An ovarian follicle consists of a central oocyte surrounded by layers of hormone-producing somatic cells. Without this 3D architecture, the oocyte–somatic cell connections are lost, and the follicle dissociates and dies. Follicles have been encapsulated for in vitro 3D culture in natural hydrogels (such as collagen47, alginate14,16,48,49,50, fibrin51 and hyaluronic acid52) and synthetic hydrogels (such as poly(ethylene glycol)53), and combinations thereof54,55. This work established that when the 3D architecture is maintained in culture, follicles can survive and function autonomously, supporting hormone production, oocyte maturation and ovulation (independently of the hypothalamic–pituitary–ovarian axis)56,57. The hydrogel encapsulation technique has also been used to generate in vitro models of the testis using Matrigel58, agarose59 or solubilized decellularized ECM60. In particular, a model was constructed using a three-layer gradient system composed of a layer of murine testicular-cell-laden Matrigel surrounded by two layers of cell-free Matrigel58. The testicular cells migrated within the Matrigel, forming organized testicular organoids with proliferating germ cells, a functional blood–testis barrier and a physiological response to retinoic acid, tumour necrosis factor, and retinoic acid inhibitors, thus creating a more physiological in vitro model than traditional 2D culture methods. Matrigel encapsulation has also been employed for the generation of human fallopian tube organoids61, and human umbilical vein endothelial cells, human endometrial stromal cells and trophoblast spheroids have been embedded within photocrosslinked gelatin methacrylate hydrogels to study decidualization and placentation, which are key phenomena of uterine physiology and early pregnancy62. The development of hydrogel encapsulation techniques and their application to reproductive tissue culture have enabled the creation of more physiologically relevant in vitro models that replicate the in vivo architecture necessary for normal function.
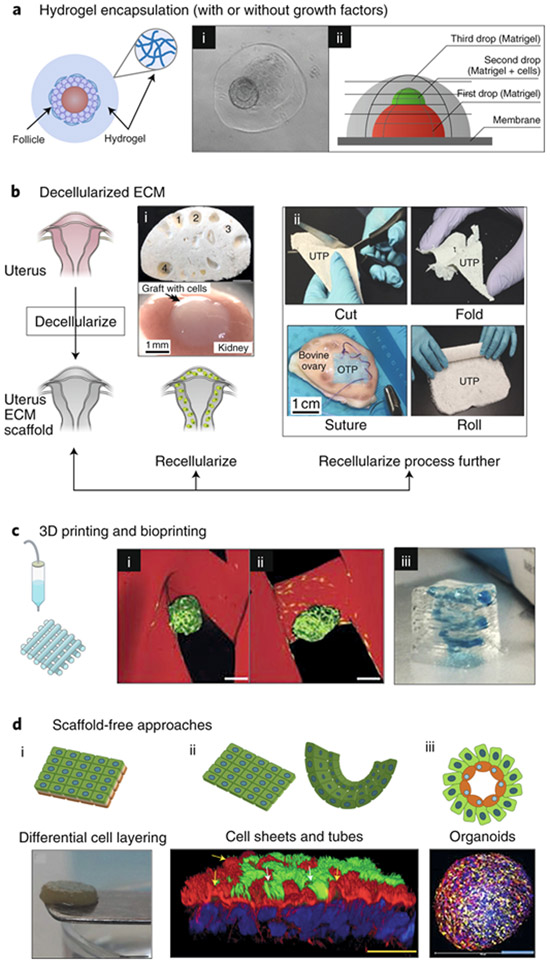
Fig. 4: Strategies for the use of engineered biomaterials in reproductive science and medicine.
a, Hydrogel encapsulation enables the in vitro culture of ovarian follicles and testicular cells in a manner that mimics their in vivo architecture. (i) 3D structure of the ovarian follicle (arrow) was maintained within collagen–alginate beads55. (ii) A three-layer gradient system consisting of testis-cell-containing Matrigel placed between two layers of cell-free Matrigel186. The gradient of cells promotes cellular reorganization into functional testicular organoids. b, Decellularization techniques for ovarian, uterine and testis tissues, for both basic research and transplantation. Following decellularization, tissue scaffolds can be (i) directly recellularized or (ii) further processed into other biomaterials. (i) Transplanted decellularized bovine ovary scaffolds (top) seeded with murine ovarian cells (bottom) to restore puberty in ovariectomized mice64. The numbers indicate visible pores where large follicles resided and that are maintained following decellularization. (ii) Decellularized composite materials from a variety of organ sources. UTP, bovine uterus ECM; OTP, bovine ovary ECM; CTP, bovine collagen88. The composite materials are ideal surgical materials as they can be cut, folded, sutured and rolled, and can support cell adhesion and survival. c, 3D printing and bioprinting provide precise control in the construction of engineered scaffolds for reproductive tissues. (i) 3D-printed bioprosthetic ovaries restore both fertility and endocrine function after transplantation into ovariectomized mice20. A tortuous pore network (i) better maintains the ovarian-follicle (green) architecture within the 3D-printed gelatin scaffold (red) compared to a grid-like 90° pore network (ii). Scale bars, 100 μm. (iii) Bioprinted model of the placenta by using human trophoblast cells to study pre-eclampsia96. The 3D-printed spiral (blue) resembles the maternal spiral arteries, which trophoblasts invade during normal placentation. d, Scaffold-free approaches, including self-assembling organoids, cell sheets and differential cell layering, have been used to create 3D models of reproductive tissues. (i) Gross appearance of a disk-shaped, scaffold-free 3D cervical stromal equivalent, onto which primary human cervical cells were seeded so as to generate a 3D-human ectocervix model102. (ii) Bovine oviduct epithelial cells self-assembled to form floating vesicles with outward facing actively beating cilia (green; indicated by white arrows). Actin filaments appear in red; nuclei appear in blue118; yellow arrows indicate primary cilia and actin-rich secretory protrustions. (iii) By using the hanging-drop method, testicular organoids composed of all testicular cell subtypes were created. Spermatogonial stem cells appear in blue, Sertoli cells in yellow and Leydig cells in red108.
Decellularized ECM scaffolds
In decellularization—commonly used in regenerative medicine—physical, enzymatic or chemical treatments are used to remove cellular material while the biochemical and structural features of the ECM (and thus its signalling roles) are preserved. Unlike cell surface markers, the biochemical components of the ECM are largely conserved between individuals and between species, thereby reducing the risk of a serious immune response when decellularized ECMs are transplanted63. Methods for the decellularization of tissue have been applied to generate engineered ovarian64,65,66,67,68, uterine69,70,71,72,73,74, testis75,76,77,78 and placenta79,80 tissue (Fig. 4b). Non-tissue-specific, ‘universal’ decellularized ECMs, such as the human amniotic membrane, have also been used for the culture of murine ovarian follicles81.
Recellularized scaffolds have been transplanted to improve or restore tissue function in preclinical70 and clinical studies82, and have also been used to study the molecular mechanisms of reproductive physiology. For example, a human decellularized endometrial scaffold was repopulated with primary epithelial and stromal cells in order to establish long-term cultures that respond to a 28-day ovulatory cycle72. In conventional 2D culture, endometrial cells tend to lose polarity and have altered gene expression and function with respect to the in vivo condition. Within the bioactive decellularized scaffold, however, the endometrial cells proliferate, survive throughout an extended culture period and retain their characteristic morphology and hormone responsiveness. In another example, human testis organoids were formed within a human testis decellularized scaffold83,84,85. These organoids possessed spermatogonia (undifferentiated male germ cells) and secreted testosterone and inhibin B, two major markers of somatic cell function; however, it is unclear whether the tissue-specific decellularized scaffold provided a benefit (when compared to other ECMs) in terms of testicular tissue formation. For example, porcine spermatogonial stem cells have been successfully cultured on ECM scaffolds from various organ sources, suggesting that laminin, rather than testis-specific factors, is the most important environmental protein for undifferentiated germ cell expansion86. Human Sertoli cells have also been cultured on decellularized porcine testis ECM, suggesting that it might be feasible to use decellularized pig testis, or other sources of testis ECM, as a scaffold for future human testicular engineering57,77.
A disadvantage of decellularized tissue scaffolds is that their microarchitecture is ‘locked in’, which often makes it challenging to repopulate the scaffold with cells87. However, with respect to conventional or injection-based scaffold seeding, the seeding of a human decellularized ovarian scaffold was improved by using a rotational seeding method (a spinner flask)68. To mitigate this issue, decellularized ECM from various organ sources, including bovine ovary and uterus, has been milled into a powder and suspended in a biocompatible polymer matrix (made of poly(lactic-co-glycolic acid)), creating ‘tissue papers’ that can be cut, folded and sutured88. The ovary tissue paper is able to support murine ovarian follicle adhesion and viability and to function in vitro and maintain the viability and function of non-human primate and human ovarian tissue for up to eight weeks88. Another strategy involves digesting decellularized tissue by using an acidic pepsin solution into tissue-specific hydrogels, which can then be used for cell encapsulation60 or freeze-dried and chemically crosslinked prior to being seeded with a cell suspension89. The Leydig cells in porcine testicular organoids survived better in decellularized testis-derived hydrogels than in collagen gels, indicating the preservation of growth factors necessary for the maintenance of the testicular niche60. Decellularized ECM-derived materials are likely to take on a bigger role in reproductive tissue engineering, both as biomaterials in their own right and as templates for the design of increasingly instructive and biomimetic synthetic materials. In fact, analyses of the composition of decellularized ECM, as exemplified by proteomic analysis of decellularized porcine90 and human91 ovarian tissue, will enable the generation of synthetic matrices with bioinspired composition.
3D-printed scaffolds and bioprinting
With 3D printing, materials can be fabricated with precise control of bulk geometry and of their internal pore architecture, thus allowing for sophisticated biomimicry and personalization (Fig. 4c). 3D printing of biological scaffolds refers to the printing of a cell-free scaffold that may be subsequently seeded with cells, whereas 3D bioprinting describes the process of depositing cell-laden ‘bioinks’ in precise 3D locations. 3D-printing technologies, materials and cell selection have been amply discussed92,93. One example of 3D printing of reproductive tissue is the bioprosthetic ovary20. Murine follicles seeded into 3D-printed gelatin scaffolds with a tortuous (rather than grid-like) network of pores maintained the 3D architecture, survival and function (specifically, hormone production) of the follicles. A similar meshwork of interconnected pores—in this case, fabricated by electrospinning of polycaprolactone (PCL)—was also able to maintain the 3D architecture of porcine follicles94. For the testis, 3D-printed alginate scaffolds have been explored for organoid generation; however, a biomimetic morphology similar to the native testis was not observed95. Another example is an in vitro model of the placenta96,97—a transient organ that exchanges nutrients, waste and gas between the mother and the developing fetus and that secretes hormones that support pregnancy—used to elucidate the mechanisms of pre-eclampsia, a disease of poor placental development. By using extrusion-based 3D bioprinting, human trophoblast-laden gelatin methacrylate hydrogel bioinks can be printed alongside the cell-free bioinks to study trophoblast migration, an important step in placental development. Also, human mesenchymal stem cells derived from human endometrial biopsies have been bioprinted on top of conventional PCL meshes used in the treatment of pelvic organ prolapse. In comparison to cell-free meshes, the addition of the bioprinted endometrial stem cells resulted in improved tissue integration and in the maintenance of an anti-inflammatory macrophage phenotype98. The development of 3D printing and bioprinting for making personalized scaffolds with customized cell-specific niches will aid the development of clinical solutions for patients and the study of in vivo physiology.
Scaffold-free approaches
The traditional tissue-engineering paradigm involves cells, signals and a scaffold. Yet because scaffold materials can affect cellular behaviour, scaffold-free methods have emerged as an alternative (Fig. 4d). By relying on the self-assembly of cells, scaffold-free approaches generate 3D multicellular aggregates that secrete their own matrices99,100,101. Several physiological models of cervical epithelium (such as primary human fibroblasts secreting a primarily collagen matrix to support epithelial differentiation102 as well as cancerous and normal cervical models that use cell line cultures of human dermis from neonatal foreskin103) have used scaffold-free techniques. These models typically exhibit properties of epithelial differentiation, yet the overall epithelial thickness is reduced and the morphology of the epithelial cells resemble a neoplastic state, perhaps owing to sex mismatch between the cell source (male neonatal foreskin) and the target tissue (female cervix). These foreskin-derived models have been used to study cervical infection, hormone regulation, epithelial–stromal interactions, neoplasia and cancer, yet they do not adequately represent cervical biology and ignore any effects of sex on disease progression.
In vitro models of human testicular organoids that perform testis-specific functions have also been created via scaffold-free approaches104. However, the morphology of these organoids does not resemble testicular tissue. In vitro testis tissues have also been created by using fish, murine and non-human-primate (marmoset) cells in suspension-based (non-adherent) 3D culture105,106,107. Suspension culture models better mimic testicular architecture as they allow for the expansion of germ cells and the incorporation of somatic cells. However, non-human-primate experiments have not demonstrated spermatogenesis progression, only spermatogonial expansion, possibly suggesting that additional factors (in particular, more physiological microenvironments) are needed. Beyond their use as a scaffold, soluble human testis ECM has been used as a media additive for culturing human testicular organoids as a way to mimic the cues of the in vivo testis microenvironment without providing a structural scaffold on which to grow de novo tissues108. Created via hanging drop culture, these organoids contain all major testis cell types, increased in size over three weeks of culture and showed an upregulation of post-meiotic germ cell gene transcription over the culture period. This organoid system has been used to study the persistence of the Zika virus within the different cell types of the testis109. Furthermore, cell-adhesion-resistant microwell arrays have been used to induce self-assembly of organoids with reproducible and controllable diameters to generate models of endometrial110 and testicular111,112 tissues with in-vivo-like 3D organization.
Microfluidic, co-culture and multi-tissue culture systems
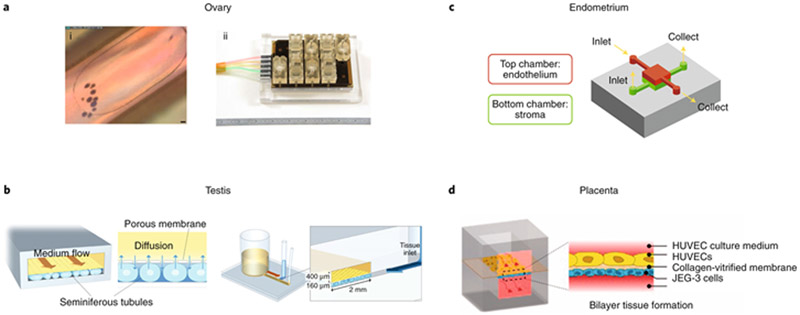
No reproductive tissue exists in isolation in vivo; this is especially true of the reproductive tract tissues, which are highly endocrine-active. These tissues, most notably the ovary and testis, secrete factors that influence the growth, differentiation and function of other tissues in the tract and in other organ systems. In order to understand the crosstalk between reproductive tissues, co-culture techniques, including microfluidic platforms (Fig. 5), have been used. Microfluidic culture systems allow for easy co-culture and for the addition or subtraction of media factors, such as pituitary or sex hormones, in order to replicate dynamic hormone cycles (such as the female ovulatory cycle) that are required for downstream tissue function (Fig. 5a). Microfluidic culture systems for the recapitulation of the physiology of tissue systems, often called ‘organs-on-chips’, also provide the benefits of prompt oxygen and nutrient delivery as well as waste removal and permit further mechanical input from fluid flow (including shear force, bulk flow and peristalsis-like contraction113,114,115).
Fig. 5: Microfluidic culture models of components of both the male and female reproductive tracts.
a, The ovary, specifically the follicular microenvironment, can be recreated via microfluidic culture conditions (i; scale bar, 50 μm) within a microphysiological system (ii). The cultures were supplemented with the pituitary hormones FSH and hCG to recreate the estradiol and progesterone profiles of the human 28-d ovulatory cycle18. b, A simple two-channel microfluidic device enabled the long-term functional maintenance of ex vivo testis tissue119. c, A compartmentalized model of the human endometrium that includes both the endothelium and stroma exhibited a physiological response via prolactin production122. d, A two-channel microfluidic system modelled the placental barrier and displayed enhanced mass-transfer dynamics123. HUVEC, human umbilical vein endothelial cell.
To better understand the microenvironment of the fallopian tube and oviduct during fertilization and embryo development, 3D co-culture methods that maintain epithelial polarity and differentiation are employed. Human fallopian tube epithelium has been cultured on a Transwell at the liquid–air interface, with hormonal cues provided by microfluidically connected murine ovarian follicles hormonally directed to mimic the human reproductive cycle116. In both cases, the researchers detected beating cilia and secreted factors in the culture medium, mimicking in vivo oviduct fluid. Fallopian tube epithelium that was co-cultured with hormone-secreting ovarian follicles showed cyclic differences in secreted factors and a thicker epithelium. Interestingly, the addition of fallopian tube epithelial cells to ovarian follicle cultures seemed to enhance ovarian function, as evidenced by the increased levels of progesterone secreted by the corpus luteum following ovulation, thus illustrating the importance of crosstalk between reproductive organs in reproductive processes. Co-cultures of bovine oviductal epithelium and embryos have revealed that crosstalk may involve signalling mediated by bone morphogenetic proteins (BMPs), a subfamily of growth factors in the transforming growth factor-β superfamily117. The introduction of bovine sperm and oocytes in an oviduct-on-a-chip system capable of supporting fertilization, developed to study the oviduct microenvironment within a microfluidic culture system, showed the support of oocyte penetration as well as the prevention of polyspermy and parthenogenic activation, which are common occurrences in current in vitro fertilization (IVF) systems118. These advances have increased the understanding of the microenvironment of the fallopian tube during fertilization and of early embryo development, and may enable the creation of more physiologically accurate conditions for IVF and embryo culture.
Microfluidic technology has also been applied to the male’s reproductive biology, for instance in the development of a microfluidic system for the culture of testis fragments from mice119 (Fig. 5b). This system involves a testis culture chamber separated from dynamic media flow by a microporous membrane, to mimic the microcirculation–tissue relationship in the in vivo microenvironment of the testis. Whereas traditional interphase culture methods allow for tissue maintenance for extended time periods (up to 139 days), the use of microfluidic culture techniques enables functional maintenance for up to 180 days, with testosterone production in response to stimulation by the luteinizing hormone and production of sperm that resulted in the live birth of healthy mice after intracytoplasmic sperm injection119,120,121. This system used hydrostatic pressure to create continuous microfluidic flow for the duration of the culture, forgoing the need for pumps and power sources. Although in its current form the throughput of the system is limited, its development may improve the study of testis function and should stimulate the incorporation of male endocrine function into other in vitro systems.
The male endocrine cycle occurs on a much shorter time scale (one day) than that of the female’s, which is characterized by continually changing levels of estradiol and progesterone across a 28-day cycle. The incorporation of microfluidic technology into the culture of integrated female reproductive tissues has led to systems that more accurately replicate the in vivo microenvironment and recreate the complex female endocrine cycle. The co-culture of human endometrial stromal cells and endothelial cells within a microfluidic environment has enabled the study of the crosstalk between the two uterine cell types122 (Fig. 3c). In this system, estradiol and progesterone were supplemented according to an idealized ovulatory cycle while only the endothelial cells were directly exposed to the shear stress of the flow of media, thus mimicking perivascular blood flow. The cultures were maintained for 28 days, during which decidualization was observed in the stromal cell population. Also, the endothelial cells responded positively to shear stress exposure, as evidenced by cytoskeletal alignment and the formation of tight junctions. In a dynamic culture system (named EVATAR) consisting of a series of fluidically connected wells containing microphysiological cultures of ovary, fallopian tube, endometrium, cervix and liver tissues18, ovulation occurred after supplementing a base ‘universal media’ with varying levels of the pituitary hormones follicle-stimulating hormone (FSH) and human chorionic gonadotropin (hCG). This also led to the production, by the ovary, of follicular and luteal-phase estradiol-and-progesterone profiles according to an idealized 28-day human ovulatory cycle. Via the microfluidic dissemination of media, the cyclical ovarian hormone profile also informed tissue function in the system’s fallopian tube, endometrium, cervix and liver tissues. Such an integrated tissue culture system provided an alternative way to study the endocrine loops of the reproductive tract in vitro.
Beyond the modelling of ‘normal’ reproductive biology, microfluidic systems can be used to model non-normal states and disease states. For example, during pregnancy, the endocrine milieu is altered when compared to the normal ovulatory cycle throughout gestation, owing to the continued maintenance of the progesterone-producing corpus luteum and the development of the placenta. There are a number of microfluidic models of the placenta (Fig. 3d)—the major endocrine organ responsible for maintaining pregnancy. These models typically consist of a membrane-separated co-culture of endothelial cells and placental trophoblasts as a simple recreation of the maternal–foetal interface123,124,125. Such placenta-on-a-chip models have been used to study the transport of caffeine126, anti-depressants127,128 nanoparticles129 and the Zika virus130 across the placental barrier. Also, a microfluidic invasion assay allowed the study of the migration of primary human trophoblasts, a critical component to placentation that, if irregular or inhibited, can lead to gestational disorders such as pre-eclampsia131.
The integration of fluidic forces with in vitro placental culture was a step forward in the developing of higher-quality placental models. In particular, compared to traditional static culture, these microfluidic models incorporate a shear stress component that is critical for the normal function of the placenta in vivo. In the EVATAR system, the initial endocrine state of pregnancy was recreated by maintaining levels of hCG in the media throughout the luteal phase (Fig. 3e). This resulted in both the maintenance of the corpus luteum of the ovulated follicle and the subsequent sustained production of progesterone. And in a microfluidic model of ovarian cancer in the peritoneal cavity during metastasis132, ovarian cancer spheroids were co-cultured in channels coated with human peritoneal mesothelial cells and exposed to shear stress, which has previously been shown to induce functional responses in ovarian cancer. Such microfluidic models, created on systems that capture complex tissue–tissue interactions, will produce cutting edge disease models that address the shortcomings of current in vitro and animal models of reproductive disease for disorders such as endometriosis, polycystic ovary syndrome and hypogonadism.
Applications of engineered tissues, organs and organ systems
Engineered tissues, organs and organ system models have been used for a range of applications, in particular as in vitro models for toxicology and drug-discovery studies, as transplantated tissues to replace or restore damaged or diseased organs, and as devices to support ex vivo foetal development.
In vitro toxicology testing and drug discovery
Beyond the general use of engineered in vitro models for research purposes, the models can also be used in toxicology studies. For instance, encapsulated follicle cultures have been used to predict reproductive toxicity in vitro, as exemplified by the use of murine ovarian follicles encapsulated in a fibrin–alginate composite system for the high-throughput toxicity testing of doxorubicin133. Other studies have also shown that doxorubicin has a dose-dependent toxicity on alginate-encapsulated murine ovarian follicles134, and human testis organoids respond in a dose-dependent fashion to four commonly used antimitotic chemotherapeutic drugs108. These testis organoid cultures showed IC50 values that were significantly higher than those seen in 2D cultures, which might reduce the number of false positive results. The testis organoid model was also amenable to cryopreservation via slow freezing and vitrification, which will likely be necessary in the banking of organoids for use in large-scale high-throughput toxicity testing108.
Beyond toxicology, in vitro tissue models can also be used to understand the mechanisms of action of drugs, or to screen for potential therapeutic agents. For example, 3D human endometrial tissue models have been used to understand the effects of the two commonly used fertility drugs levonorgestrel and mifepristone135. This model system could also be used both to study drug mechanisms and to discover new agents for fertility control. Engineered in vitro reproductive tissue models are also likely to take on a greater role in toxicology testing and drug discovery, especially in the testing of drug safety in pregnant women. Additionally, induced pluripotent stem cell (iPSC) technologies, which are becoming more accessible, will likely be incorporated into engineered reproductive tissue models for personalized medicine21,136.
Ovarian tissue transplantation
Ovarian tissue engineering is a promising strategy to treat both idiopathic and iatrogenic female infertility resulting from exposure to gonadotoxic chemotherapy and radiation therapy137. Despite numerous attempts to protect fertility in patients with cancer, the cryopreservation of ovarian tissue with subsequent transplantation is the only fertility preservation option available to pre-pubertal patients and to patients who cannot delay cancer treatment138,139,140. Although there have been more than 130 live births reported following the transplantation of cryopreserved ovarian tissue141, the technique is contraindicated for patients with cancers that have a moderate-to-high likelihood of ovarian metastasis because of the risk of reintroducing malignant cells leading to disease recurrence. The various biomaterial-engineering approaches described earlier may lead to new fertility restoration options that can avoid the need for the transplantation of intact tissue and that may thus mitigate this risk.
Engineered ovarian tissue has been transplanted in mice in many forms: as encapsulated follicles, as recellularized ovarian ECM scaffolds and as 3D-printed ‘bioprostheses’. Transplanted encapsulated follicles grow and mature in vivo within Matrigel, collagen, fibrin, alginate (a natural hydrogel derived from algae) and poly(ethylene glycol) hydrogels142,143,144,145,146. Primordial follicles are even able to mature into antral follicles and produce steroid hormones when seeded into macroporous alginate scaffolds with affinity-bound BMP-4 (ref. 147). Furthermore, artificial follicles—multi-layered constructs composed of an inner core of granulosa cell and bone-marrow-derived mesenchymal-stem-cell-laden alginate surrounded by a sheath of theca-cell-laden alginate—restore estradiol secretion for at least 90 days in ovariectomized mice and improve estrogen-deficiency-induced uterine atrophy without causing endometrial hyperplasia, a precursor to cancer148. To test the preclinical safety of this strategy for restoring fertility in survivors of childhood cancer, donor follicles were isolated from a mouse with breast cancer, encapsulated in fibrin matrices (with or without vascular endothelial growth factor (VEGF)) and transplanted into ovariectomized mice143. All mice receiving transplants resumed cycling, but live birth was only achieved in mice who received VEGF-containing fibrin matrices. This study showed the feasibility of reducing the risk of recurrent metastatic breast cancer by isolating and transplanting isolated follicles, rather than intact ovaries, from tumour-laden donor mice. Another method to reduce the risk of tumour recurrence when using cryopreserved ovarian tissue for auto-transplantation is to encapsulate the tissue in a biomaterial that can act as a barrier to impede the migration of any residual cancer cells out of the transplanted tissue and into the body. Because the biomaterial forms a barrier between the transplanted ovarian tissue and the rest of the body, this technique cannot be used to restore natural fertility; yet it can be used to restore physiologic endocrine function149.
Alginate is a promising candidate biomaterial for use as a tissue barrier because it cannot be degraded by mammalian cells. As a hydrogel, alginate would enable the passive diffusion of nutrients to the transplanted tissue and the egress of hormones away from the graft while preventing the migration of tumour cells away from the transplant (and immune cells towards it). TheraCyte, an FDA-approved poly(tetrafluroethylene) membrane, has also been used to shield transplanted ovarian tissue from immune responses150. Transplanted follicles encapsulated in both alginate and TheraCyte restored endocrine function with a decrease in FSH levels in ovariectomized mice. Although these barrier devices prevent the release of mature oocytes, they are capable of supporting in vivo follicle maturation and are compatible with IVF. Another immune isolation strategy involves the use of poly-L-ornithine and alginate to reduce the risk of immune reactions against foreign granulosa and theca cells (the hormone-producing cells of the ovary). Multilayer hydrogel beads (two alginate layers, containing either granulosa or theca cells, separated by a poly-L-ornithine layer to provide additional immune isolation) were created to administer hormone-replacement therapy for post-menopausal women and cancer patients after exposure to gonadotoxic treatments151. These engineered constructs delivered stable levels of hormones for over 90 days that were sufficient for the maintenance of the mineral density of normal bone and of a healthy body composition in a murine model of menopause. Although similar biomaterials have been used in humans for the transplantation of pancreatic islets, further studies are needed to test the safety and effectiveness of such biomaterial barriers in humans152,153,154.
Recellularized ovarian ECM scaffolds have been transplanted into pre-pubertal ovariectomized mice, which mimic the physiology of young cancer survivors with premature ovarian failure. Following transplantation, the mice initiated puberty, with increasing levels of oestrogen and inhibin A20. First pregnancies and live births following IVF have been reported for women who had received minimally invasive transplantation of previously cryopreserved ovarian tissue with a commercially available decellularized ECM scaffold prepared from human cadaver skin82 (trade name, Alloderm). In addition to live births, ovarian function was continuous for up to two years after transplantation, which indicates that ECM from non-tissue-specific sources may provide sufficient support for a functional transplant. The 3D-printed ovarian bioprosthetic also restored fertility and endocrine function in ovariectomized mice. Following transplantation, the follicle-seeded 3D-printed scaffolds became highly vascularized, resulting in live births through natural mating and in supported maternal lactation, which indicates that the 3D-printed bioprosthetic restored both physiological fertility (via ovulation through the porous scaffold) and endocrine function20. Recellularization of decellularized ovarian tissue and 3D-printed scaffolds may therefore be useful strategies to restore endocrine function after gonadotoxic therapy, thereby preventing sequelae of premature ovarian failure, such as osteopenia and cardiovascular disease. Although more research and development is needed before these approaches can become standard practice in humans, the recent animal work and the small pilot studies in humans82 suggest that there will be new possibilities for restoring ovarian function in patients.
Testis tissue transplantation
As with pre-pubertal females, there is a need for improved fertility preservation options for pre-pubertal males. Before the onset of puberty, the testis does not produce haploid, fertility-competent sperm for cryopreservation30. Therefore, cryopreservation of immature testicular tissue, although still an investigational technique, is the only available option for fertility preservation in this patient population155. In 1978, the first transplantation of an intact human testis carried out in identical twins (one of whom was born without testes) resulted in a live birth6. This study indicated that testicular transplantation could be a viable technique for fertility restoration. Unfortunately, existing protocols cannot cryopreserve intact testes; instead, only testicular tissue fragments are cryopreserved. In theory, fragments of cryopreserved immature testicular tissue can be subsequently thawed and transplanted to restore fertility; yet this procedure has not yet been performed in humans. Preclinical studies have achieved live offspring in autografted and xenografted testis fragments from rodents, pigs and non-human primates156,157,158. However, xenotransplantation of human testicular tissue or cells into rodent models often fails to maintain spermatogonia, and few studies in higher-mammal testicular transplant have observed the development of fully mature spermatocytes156,159,160,161,162. The principal challenges of human immature testicular tissue transplantation (ITT) are hypoxia and reperfusion injury, the maintenance of early spermatogonial populations and the poor or late neovascularization of the testicular graft163. Also, many of the same fundamental concerns of ovarian tissue transplantation also apply to ITT, including the reintroduction of malignant cells. A study of the in vitro production of haploid germ cells within cultured human immature testicular tissue suggested that in vitro maturation prior to transplantation may improve ITT164. The development of successful protocols for ITT, which will likely involve biomaterial scaffolds for the provision of an appropriate niche for testicular cells, immunoisolation and ex vivo tissue maturation within microfluidic systems, will be critical for this patient population.
To date, the use of engineered biomaterials to improve transplant outcomes has been relatively unexplored for testis transplantation (compared to ovarian transplantation). Two studies have encapsulated testicular cells within Matrigel to promote vascularization158,165. As in previous reports wherein testicular cell pellets were transferred without a matrix166, the Matrigel-encapsulated testicular cells self-assembled into de novo seminiferous tubules; however, few germ cells were observed, suggesting that the technique cannot restore fertility. One study used hydrogels (alginate or fibrin) loaded with VEGF nanoparticles to encapsulate testicular tissue for transplantation as a mechanism to further promote vascularization23. After five days, grafts containing the VEGF nanoparticles displayed increased vascularity compared to the naked hydrogel and to unencapsulated grafts; however, this benefit disappeared 21 days post-transplantation.
As with ovarian bioprotheses, decellularized ECMs and scaffold fabrication technologies such as 3D-printing could be used for the design of a testis bioprosthesis. Such engineered biomaterials may mitigate the challenges associated with ITT by promoting angiogenesis and preserving spermatogonia. Similarly, as in vitro grown testicular tissues become more common, testicular organoids might provide an alternative to native tissue for transplantation. Additionally, biomaterials may improve spermatogonial stem cell transplantation into recipient seminiferous tubules, a promising fertility-preserving technique that has seen success in several mammalian research species157,167.
Uterine tissue transplantation
With advances in assisted reproductive technologies such as IVF, many women who struggle with infertility are able to get pregnant. However, in the case of absolute uterine factor infertility (owing to a missing uterus or to a non-functional uterus), gestational surrogacy has been the only option. The use of tissue-engineered uterine constructs for researching and treating absolute uterine factor infertility and other reproductive syndromes that affect uterine function has been explored. For example, decellularized uterine ECM was transplanted into murine uteri with artificially induced defects69. Uterine epithelial cells migrated into the decellularized ECM, forming an intact epithelial layer within a week. Stromal cell and myometrial cell migration and regeneration followed thereafter, indicating that the use of a decellularized matrix is a viable strategy for the repair and regeneration of uterine tissue near defect sites. In a rat model, recellularized uterine ECM scaffolds were used to repair defects in native uterine tissue in vivo and to support a healthy pregnancy70. Collagen scaffolds, loaded with human umbilical cord mesenchymal stem cells, have similarly been used to restore endometrial tissue structure and fertility in a murine uterine defect model168. Furthermore, the first whole reproductive organ of a large animal to undergo decellularization was a porcine uterus. The decellularized organ was then recellularized with primary human endometrial cells, providing proof-of-concept evidence that porcine scaffolds can support human endometrial regeneration and that recellularized ECM scaffolds may be a promising solution for uterine factor infertility71.
Cervicovaginal tissue transplantation
Although rare, women without a functional cervix or vagina at birth (known as cervical or vaginal aplasia), which can be caused by Mayer-Rokitansky-Küster-Hauser syndrome and other various disorders, suffer from infertility. In a clinical study of 53 patients with this syndrome that used decellularized dermal ECM (a commonly used and commercially available ‘universal’ decellularized ECM) to reconstruct the vagina provided near-normal sexual function to all patients and improved their body image perception169. In another clinical study, decellularized porcine small-intestinal submucosa (another commonly used and commercially available ‘universal’ decellularized ECM) was used to reconstruct the cervix and vagina in women with missing or malformed anatomy170,171. All patients resumed menstruation, and the engineered cervix and vagina remained patent. To accelerate tissue regeneration further, the scaffolds were seeded with human bone marrow mesenchymal stem cells (induced to possess a vaginal epithelial phenotype)172 or with autologous cells from a vulvar biopsy125 prior to transplantation. In the latter case, isolated epithelial and muscle cells expanded in culture, seeded onto the scaffolds and matured in an incubator prior to transplantation. For up to eight years following surgical transplantation, yearly biopsies revealed that the vaginal implants had normal structure and function. Decellularized ECM scaffolds have also been used to reconstruct human cervicovaginal tissue in clinical trials15, yet further work is necessary to understand the factors that accelerate tissue regeneration and support long-term function. For example, a case report described the use of a tilapia skin scaffold for vaginal reconstruction with the formation of a stratified squamous epithelium when assessed by histology at 180 days post-transplanation173.
Ex vivo foetal development
An artificial uterus that is able to carry out uterine and placental functions ex vivo19, known as the ‘biobag’, supported foetal lambs for at least four weeks without organ failure. The biobag consisted of a pumpless arteriovenous circuit within a closed fluid environment with continuous fluid exchange, with blood flow driven by the foetal heart and accessed through umbilical vasculature. Another pumpless, perfusion-driven microfluidic device was integrated into a lung assist device to improve oxygenation status in preterm neonates174. Although the factors causing premature birth (such as cervical incompetency) are not completely understood, biobags and microfluidic lung assist devices could provide better outcomes in cases of premature birth.
Outlook
Reproductive tissues can be engineered to serve as in vitro models for research and to replace or regenerate damaged tissues in order to restore reproductive function (fertility and endocrine function). Additionally, the use of biomaterials and advanced culture systems to create or model functional reproductive tissues ex vivo has resulted in significant advances in engineering reproductive phenomena at the cellular and molecular levels. However, one aspect of new biomaterials that is often overlooked is the potential for adverse effects on reproductive health, such as gamete development and quality. Biomaterials often contain plastics or leachates that may appear to be biocompatible with non-reproductive tissues but are later found to exhibit reproductive toxicity175. It is thus necessary to ensure that tissue constructs, whether for reproductive or non-reproductive tissues, are assessed for their true biocompatibility, especially with the reproductive system. Moreover, with nearly 2% of Americans born through assisted reproductive technology since 1976 (ref. 176), medical intervention to aid men and women with infertility is a reality. In future, the support of endocrine function in human beings living and working in space may be needed, and animal reproductive capacity may require the same interventions that are under consideration for human applications.
Even as new technologies develop, there should be close consideration of the ethics associated with interventions that challenge traditional notions of reproductive abilities177,178,179. New concerns will arise as gametes and niches in which gametes develop are created, and the existential questions associated with self and personhood will be debated. Because what will actually be developmentally possible in a laboratory setting and in the human context cannot be predicted, reproductive health ethics, law and religious beliefs need to be considered (as happened in the area of oncofertility178). Long-lasting transplantable reproductive tissues will likely require a combination of the strategies described in this Review: customized biomaterials, native matrix cues and sophisticated fabrication technologies capable of creating personalized bioprostheses. They will also require the involvement of medical ethicists and legal experts to ensure that future interventions are crafted in a manner consistent with human values and needs. Moreover, the created solutions should eliminate societal disparities180,181,182 and ensure that the science is also informed by the people in whose interest the advances are being made177,183,184,185.
The development of fundamental new knowledge from the engineered approaches described here will hopefully enable the endocrine and fertility needs of existing patients and the people of tomorrow. After all, reproductive health is central to human persistence as a species.
Acknowledgements
We thank Stacey C. Tobin for editorial assistance on the manuscript. The work in the Woodruff laboratory has been supported by the National Institutes of Health (NIH) through a variety of Institutes and Offices, including the National Center for Advancing Translational Sciences (NCATS), the National Institute of Environmental Health Sciences (NIEHS), the Eunice Kennedy Shriver National Institute of Child Health and Development (NICHD), the Office of Women's Health Research (ORWH) and the NIH Common Fund. We are currently funded by the National Institute for Environmental Health Sciences/National Center for Advancing Translational Sciences (grant nos. UH3TR001207 and 4UH3ES029073-03), the Bill & Melinda Gates Foundation (grant no. OPP1161206), the Thomas J. Watkin’s Memorial Professorship, the National Institute of Aging (grant no. F30AG058387, awarded to E.S.G.) and the National Institute for Child Health and Development (grant no. F31HD089693, awarded to M.E.E.). All other grants mentioned are awarded to T.K.W.
References
- 1.Morris RT The ovarian graft. New York Med. J 62, 436 (1895). [Google Scholar]
- 2.Favre-Inhofer A, Rafii A, Carbonnel M, Revaux A & Ayoubi JM Uterine transplantation: review in human research. J. Gynecol. Obstet. Hum. Reprod 47, 213–221 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez KM & Pastuszak AW A history of penile implants. Transl. Androl. Urol 6, S851–S857 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Girsdansky J & Newman HF Use of a vitallium testicular implant. Am. J. Surg 53, 514 (1941) [Google Scholar]
- 5.Steptoe PC & Edwards RG Birth after the reimplantation of a human embryo. Lancet 312, 366 (1978) [DOI] [PubMed] [Google Scholar]
- 6.Silber SJ Transplantation of a human testis for anorchia. Fertil. Steril 30, 181–187 (1978). [PubMed] [Google Scholar]
- 7.Zeilmaker GH, Alberda AT, van Gent I, Rijkmans CMPM & Drogendijk AC Two pregnancies following transfer of intact frozen-thawed embryos. Fertil. Steril 42, 293–296 (1984) [DOI] [PubMed] [Google Scholar]
- 8.Chen C Pregnancy after human oocyte cryopreservation. Lancet 1, 884–886 (1986). [DOI] [PubMed] [Google Scholar]
- 9.Palermo G, Joris H, Devroey P & Van Steirteghem AC Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet 340, 17–18 (1992). [DOI] [PubMed] [Google Scholar]
- 10.Donnez J et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet 364, 1405–1410 (2004). [DOI] [PubMed] [Google Scholar]
- 11.Hilders CG, Baranski AG, Peters L, Ramkhelawan A & Trimbos JB Successful human ovarian autotransplantation to the upper arm. Cancer 101, 2771–2778 (2004). [DOI] [PubMed] [Google Scholar]
- 12.Jadoul P et al. Laparoscopic ovariectomy for whole human ovary cryopreservation: technical aspects. Fertil. Steril 87, 971–975 (2007). [DOI] [PubMed] [Google Scholar]
- 13.Woodruff TK The emergence of a new interdiscipline: oncofertility. Cancer Treat. Res 138, 3–11 (2007) [DOI] [PubMed] [Google Scholar]
- 14.Xu M, Kreeger PK, Shea LD & Woodruff TK Tissue-engineered follicles produce live, fertile offspring. Tissue Eng. 12, 2739–2746 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raya-Rivera AM et al. Tissue-engineered autologous vaginal organs in patients: a pilot cohort study. Lancet 384, 329–336 (2014 [DOI] [PubMed] [Google Scholar]
- 16.Xiao S et al. In vitro follicle growth supports human oocyte meiotic maturation. Sci. Rep 5, 17323 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brännström M et al. Livebirth after uterus transplantation. Lancet 385, 607–616 (2015). [DOI] [PubMed] [Google Scholar]
- 18.Xiao S et al. A microfluidic culture model of the human reproductive tract and 28-day menstrual cycle. Nat. Commun 8, 14584 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Partridge EA et al. An extra-uterine system to physiologically support the extreme premature lamb. Nat. Commun 8, 15112 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laronda MM et al. A bioprosthetic ovary created using 3D printed microporous scaffolds restores ovarian function in sterilized mice. Nat. Commun 8, 15261 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Botman O & Wyns C Induced pluripotent stem cell potential in medicine, specifically focused on reproductive medicine. Front. Surg 1, 5 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amini Mahabadi J et al. Derivation of male germ cells from induced pluripotent stem cells by inducers: a review. Cytotherapy 20, 279–290 (2018) [DOI] [PubMed] [Google Scholar]
- 23.Poels J et al. Transplantation of testicular tissue in alginate hydrogel loaded with VEGF nanoparticles improves spermatogonial recovery. J. Control. Release 234, 79–89 (2016) [DOI] [PubMed] [Google Scholar]
- 24.Shikanov A et al. Fibrin encapsulation and vascular endothelial growth factor delivery promotes ovarian graft survival in mice. Tissue Eng. Part A 17, 3095–3104 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ensign LM, Cone R & Hanes J Nanoparticle-based drug delivery to the vagina: a review. J. Control. Release 190, 500–514 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanaka A et al. Effect of sustained release of basic fibroblast growth factor using biodegradable gelatin hydrogels on frozen-thawed human ovarian tissue in a xenograft model. J. Obstet. Gynaecol. Res 44, 1947–1955 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Prakapenka AV, Bimonte-Nelson HA & Sirianni RW Engineering poly(lactic-co-glycolic acid) (PLGA) micro- and nano-carriers for controlled delivery of 17β-estradiol. Ann. Biomed. Eng 45, 1697–1709 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kashaninejad N, Shiddiky MJA & Nguyen N-T Advances in microfluidics-based assisted reproductive technology: from sperm sorter to reproductive system-on-a-chip. Adv. Biosyst 2, 1700197 (2018). [Google Scholar]
- 29.Weng L et al. On-chip oocyte denudation from cumulus-oocyte complexes for assisted reproductive therapy. Lab Chip 18, 3892–3902 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strauss JF & Barbieri RL Yen and Jaffe’s Reproductive Endocrinology (Elsevier, 2014) [Google Scholar]
- 31.Saunders PTK et al. Differential expression of oestrogen receptor alpha and beta proteins in the testes and male reproductive system of human and non-human primates. Mol. Hum. Reprod 7, 227–236 (2001). [DOI] [PubMed] [Google Scholar]
- 32.Toutain P-L, Ferran A & Bousquet-Mélou A in Comparative and Veterinary Pharmacology (eds Cunningham F et al. ) 19–48 (Springer, 2010). [Google Scholar]
- 33.Sato J, Nasu M & Tsuchitani M Comparative histopathology of the estrous or menstrual cycle in laboratory animals. J. Toxicol. Pathol 29, 155–162 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kanatsu-Shinohara M et al. Reconstitution of mouse spermatogonial stem cell niches in culture. Cell Stem Cell 11, 567–578 (2012). [DOI] [PubMed] [Google Scholar]
- 35.Bylander A et al. Rapid effects of progesterone on ciliary beat frequency in the mouse fallopian tube. Reprod. Biol. Endocrinol 8, 48 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cunha GR et al. The endocrinology and developmental biology of the prostate. Endocr. Rev 8, 338–362 (1987). [DOI] [PubMed] [Google Scholar]
- 37.Roberts RO et al. Androgen receptor gene polymorphisms and increased risk of urologic measures of benign prostatic hyperplasia. Am. J. Epidemiol 159, 269–276 (2004). [DOI] [PubMed] [Google Scholar]
- 38.Goldenberg SL, Koupparis A & Robinson ME Differing levels of testosterone and the prostate: a physiological interplay. Nat. Rev. Urol 8, 365–377 (2011). [DOI] [PubMed] [Google Scholar]
- 39.O’Shaughnessy PJ Hormonal control of germ cell development and spermatogenesis. Semin. Cell Dev. Biol 29, 55–65 (2014). [DOI] [PubMed] [Google Scholar]
- 40.Herbst KL & Bhasin S Testosterone action on skeletal muscle. Curr. Opin. Clin. Nutr. Metab. Care 7, 271–277 (2004). [DOI] [PubMed] [Google Scholar]
- 41.Karsenty G & Oury F Regulation of male fertility by the bone-derived hormone osteocalcin. Mol. Cell. Endocrinol 382, 521–526 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oury F et al. Osteocalcin regulates murine and human fertility through a pancreas-bone-testis axis. J. Clin. Invest 123, 2421–2433 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim AM, Tingen CM & Woodruff TK Sex bias in trials and treatment must end. Nature 465, 688–689 (2010). [DOI] [PubMed] [Google Scholar]
- 44.Nicodemus GD & Bryant SJ Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Eng. Part B Rev 14, 149–165 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khetan S & Burdick J Cellular encapsulation in 3D hydrogels for tissue engineering. J. Vis. Exp 32, 1590 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.West ER, Shea LD & Woodruff TK Engineering the follicle microenvironment. Semin. Reprod. Med 25, 287–299 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Joo S et al. The effect of collagen hydrogel on 3D culture of ovarian follicles. Biomed. Mater 11, 065009 (2016). [DOI] [PubMed] [Google Scholar]
- 48.Skory RM, Xu Y, Shea LD & Woodruff TK Engineering the ovarian cycle using in vitro follicle culture. Hum. Reprod 30, 1386–1395 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amorim CA, Van Langendonckt A, David A, Dolmans M-M & Donnez J Survival of human pre-antral follicles after cryopreservation of ovarian tissue, follicular isolation and in vitro culture in a calcium alginate matrix. Hum. Reprod 24, 92–99 (2008). [DOI] [PubMed] [Google Scholar]
- 50.Pangas SA, Saudye H, Shea LD & Woodruff TK Novel approach for the three-dimensional culture of granulosa cell–oocyte complexes. Tissue Eng. 9, 1013–1021 (2003). [DOI] [PubMed] [Google Scholar]
- 51.Xu J et al. Fibrin promotes development and function of macaque primary follicles during encapsulated three-dimensional culture. Hum. Reprod 28, 2187–2200 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Desai N, Abdelhafez F, Calabro A & Falcone T Three dimensional culture of fresh and vitrified mouse pre-antral follicles in a hyaluronan-based hydrogel: a preliminary investigation of a novel biomaterial for in vitro follicle maturation. Reprod. Biol. Endocrinol 10, 29 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shikanov A, Smith RM, Xu M, Woodruff TK & Shea LD Hydrogel network design using multifunctional macromers to coordinate tissue maturation in ovarian follicle culture. Biomaterials 32, 2524–2531 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shikanov A, Xu M, Woodruff TK & Shea LD Interpenetrating fibrin–alginate matrices for in vitro ovarian follicle development. Biomaterials 30, 5476–5485 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Choi JK, Agarwal P, Huang H, Zhao S & He X The crucial role of mechanical heterogeneity in regulating follicle development and ovulation with engineered ovarian microtissue. Biomaterials 35, 5122–5128 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lebbe M & Woodruff TK Involvement of androgens in ovarian health and disease. Mol. Hum. Reprod 19, 828–837 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.West-Farrell ER et al. The mouse follicle microenvironment regulates antrum formation and steroid production: alterations in gene expression profiles. Biol. Reprod 80, 432–439 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alves-Lopes JP, Söder O & Stukenborg JB Testicular organoid generation by a novel in vitro three-layer gradient system. Biomaterials 130, 76–89 (2017). [DOI] [PubMed] [Google Scholar]
- 59.Gholami K, Pourmand G, Koruji M, Ashouri S & Abbasi M Organ culture of seminiferous tubules using a modified soft agar culture system. Stem Cell Res. Ther 9, 249 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vermeulen M et al. Generation of organized porcine testicular organoids in solubilized hydrogels from decellularized extracellular matrix. Int. J. Mol. Sci 20, 5476 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kessler M et al. The Notch and Wnt pathways regulate stemness and differentiation in human fallopian tube organoids. Nat. Commun. 6, 8989 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zambuto SG, Clancy KBH & Harley BAC A gelatin hydrogel to study endometrial angiogenesis and trophoblast invasion. Interface Focus 9, 20190016 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Crapo PM, Gilbert TW & Badylak SF An overview of tissue and whole organ decellularization processes. Biomaterials 32, 3233–3243 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Laronda MM et al. Initiation of puberty in mice following decellularized ovary transplant. Biomaterials 50, 20–29 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Eivazkhani F et al. Evaluating two ovarian decellularization methods in three species. Mater. Sci. Eng C 102, 670–682 (2019). [DOI] [PubMed] [Google Scholar]
- 66.Hassanpour A, Talaei-Khozani T, Kargar-Abarghouei E, Razban V & Vojdani Z Decellularized human ovarian scaffold based on a sodium lauryl ester sulfate (SLES)-treated protocol, as a natural three-dimensional scaffold for construction of bioengineered ovaries. Stem Cell Res. Ther 9, 252 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Alshaikh AB et al. Decellularization of the mouse ovary: comparison of different scaffold generation protocols for future ovarian bioengineering. J. Ovarian Res 12, 58 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mirzaeian L et al. Optimizing the cell seeding protocol to human decellularized ovarian scaffold: Application of dynamic system for bio-engineering. Cell J. 22, 227–235 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hiraoka T et al. STAT3 accelerates uterine epithelial regeneration in a mouse model of decellularized uterine matrix transplantation. JCI Insight 1, e87591 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hellström M et al. Bioengineered uterine tissue supports pregnancy in a rat model. Fertil. Steril 106, 487–496 (2016). [DOI] [PubMed] [Google Scholar]
- 71.Campo H et al. De- and recellularization of the pig uterus: a bioengineering pilot study. Biol. Reprod 96, 34–45 (2017). [DOI] [PubMed] [Google Scholar]
- 72.Olalekan SA, Burdette JE, Getsios S, Woodruff TK & Kim JJ Development of a novel human recellularized endometrium that responds to a 28-day hormone treatment. Biol. Reprod 96, 971–981 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Miyazaki K & Maruyama T Partial regeneration and reconstruction of the rat uterus through recellularization of a decellularized uterine matrix. Biomaterials 35, 8791–8800 (2014). [DOI] [PubMed] [Google Scholar]
- 74.Daryabari SS et al. Development of an efficient perfusion-based protocol for whole-organ decellularization of the ovine uterus as a human-sized model and in vivo application of the bioscaffolds. J. Assist. Reprod. Genet. 36, 1211–1223 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baert Y & Goossens E in Methods in Molecular Biology (Ed. Turksen K) 121–127 (Humana Press, 2017). [Google Scholar]
- 76.Vermeulen M, del Vento F, de Michele F, Poels J & Wyns C Development of a cytocompatible scaffold from pig immature testicular tissue allowing human Sertoli cell attachment, proliferation and functionality. Int. J. Mol. Sci 19, 227 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kargar-Abarghouei E, Vojdani Z, Hassanpour A, Alaee S & Talaei-Khozani T Characterization, recellularization, and transplantation of rat decellularized testis scaffold with bone marrow-derived mesenchymal stem cells. Stem Cell Res. Ther 9, 324 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Akbarzadeh A et al. Decellularised whole ovine testis as a potential bio-scaffold for tissue engineering. Reprod. Fertil. Dev 31, 1665–1673 (2019). [DOI] [PubMed] [Google Scholar]
- 79.Barreto RSN, Romagnolli P, Fratini P, Mess AM & Miglino MA Mouse placental scaffolds: a three-dimensional environment model for recellularization. J. Tissue Eng 10, 1–11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Favaron PO et al. Establishment of 3-dimensional scaffolds from hemochorial placentas. Placenta 81, 32–41 (2019). [DOI] [PubMed] [Google Scholar]
- 81.Motamed M et al. Tissue engineered human amniotic membrane application in mouse ovarian follicular culture. Ann. Biomed. Eng 45, 1664–1675 (2017). [DOI] [PubMed] [Google Scholar]
- 82.Oktay K, Bedoschi G, Pacheco F, Turan V & Emirdar V First pregnancies, live birth, and in vitro fertilization outcomes after transplantation of frozen-banked ovarian tissue with a human extracellular matrix scaffold using robot-assisted minimally invasive surgery. Am. J. Obstet. Gynecol 214, 94.e1–94.e9 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Baert Y et al. Derivation and characterization of a cytocompatible scaffold from human testis. Hum. Reprod 30, 256–267 (2015). [DOI] [PubMed] [Google Scholar]
- 84.Baert Y et al. Primary human testicular cells self-organize into organoids with testicular properties. Stem Cell Rep. 8, 30–38 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Baert Y, Rombaut C & Goossens E in Methods in Molecular Biology (Ed. Turksen K) 283–290 (Humana, 2017). [DOI] [PubMed] [Google Scholar]
- 86.Park MH et al. Effects of extracellular matrix protein-derived signaling on the maintenance of the undifferentiated state of spermatogonial stem cells from porcine neonatal testis. Asian-Australasian J. Anim. Sci 29, 1398–1406 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Scarrit ME, Pashos NC & Bunnell BA A review of cellularization strategies for tissue engineering of whole organs. Front. Bioeng. Biotechnol 3, 43 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jakus AE et al. “Tissue papers” from organ-specific decellularized extracellular matrices. Adv. Funct. Mater 27, 1700992 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rezaei Topraggaleh T, Rezazadeh Valojerdi M, Montazeri L & Baharvand H A testis-derived macroporous 3D scaffold as a platform for the generation of mouse testicular organoids. Biomater. Sci 7, 1422–1436 (2019). [DOI] [PubMed] [Google Scholar]
- 90.Henning NF, LeDuc RD, Even KA & Laronda MM Proteomic analyses of decellularized porcine ovaries identified new matrisome proteins and spatial differences across and within ovarian compartments. Sci. Rep 9, 20001 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ouni E, Vertommen D, Chiti MC, Dolmans MM & Amorim CA A draft map of the human ovarian proteome for tissue engineering and clinical applications. Mol. Cell. Proteomics 18, S159–S173 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Murphy SV & Atala A 3D bioprinting of tissues and organs. Nat. Biotechnol 32, 773–785 (2014). [DOI] [PubMed] [Google Scholar]
- 93.Billiet T, Vandenhaute M, Schelfhout J, Van Vlierberghe S & Dubruel P A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering. Biomaterials 33, 6020–6041 (2012). [DOI] [PubMed] [Google Scholar]
- 94.Raffel N et al. Novel approach for the assessment of ovarian follicles infiltration in polymeric electrospun patterned scaffolds. PLoS ONE 14, e0215985 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Baert Y, Dvorakova-Hortova K, Margaryan H & Goossens E Mouse in vitro spermatogenesis on alginate-based 3D bioprinted scaffolds. Biofabrication 11, 035011 (2019). [DOI] [PubMed] [Google Scholar]
- 96.Kuo C-Y et al. Development of a 3D printed, bioengineered placenta model to evaluate the role of trophoblast migration in preeclampsia. ACS Biomater. Sci. Eng 2, 1817–1826 (2016). [DOI] [PubMed] [Google Scholar]
- 97.Kuo CY et al. Trophoblast–endothelium signaling involves angiogenesis and apoptosis in a dynamic bioprinted placenta model. Biotechnol. Bioeng 116, 181–192 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Paul K et al. 3D bioprinted endometrial stem cells on melt electrospun poly ε-caprolactone mesh for pelvic floor application promote anti-inflammatory responses in mice. Acta Biomater. 97, 162–176 (2019). [DOI] [PubMed] [Google Scholar]
- 99.Ovsianikov A, Khademhosseini A & Mironov V The synergy of scaffold-based and scaffold-free tissue engineering strategies. Trends Biotechnol. 36, 348–357 (2018). [DOI] [PubMed] [Google Scholar]
- 100.Huch M & Koo B-K Modeling mouse and human development using organoid cultures. Development 142, 3113–3125 (2015). [DOI] [PubMed] [Google Scholar]
- 101.Cui X, Hartanto Y & Zhang H Advances in multicellular spheroids formation. J. Roy. Soc. Interface 14, 20160877 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.De Gregorio V et al. An engineered cell-instructive stroma for the fabrication of a novel full thickness human cervix equivalent in vitro. Adv. Healthc. Mater 6, 1601199 (2017). [DOI] [PubMed] [Google Scholar]
- 103.Karolina Zuk A, Wen X, Dilworth S, Li D & Ghali L Modeling and validating three dimensional human normal cervix and cervical cancer tissues in vitro. J. Biomed. Res 31, 240–247 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.von Kopylow K et al. Dynamics, ultrastructure and gene expression of human in vitro organized testis cells from testicular sperm extraction biopsies. Mol. Hum. Reprod 24, 123–134 (2018). [DOI] [PubMed] [Google Scholar]
- 105.Higaki S et al. In vitro differentiation of fertile sperm from cryopreserved spermatogonia of the endangered endemic cyprinid honmoroko (Gnathopogon caerulescens). Sci. Rep 7, 42852 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Azizi H, Skutella T & Shahverdi A Generation of mouse spermatogonial stem-cell-colonies in a non-adherent culture. Cell J. 19, 238–249 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lin ZYC et al. Sphere-formation culture of testicular germ cells in the common marmoset, a small New World monkey. Primates 57, 129–135 (2016). [DOI] [PubMed] [Google Scholar]
- 108.Pendergraft SS, Sadri-Ardekani H, Atala A & Bishop CE Three-dimensional testicular organoid: a novel tool for the study of human spermatogenesis and gonadotoxicity in vitro. Biol. Reprod 96, 720–732 (2017). [DOI] [PubMed] [Google Scholar]
- 109.Strange DP et al. Human testicular organoid system as a novel tool to study Zika virus pathogenesis. Emerg. Microbes Infect 7, 82 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Murphy AR, Wiwatpanit T, Lu Z, Davaadelger B & Kim JJ Generation of multicellular human primary endometrial organoids. J. Vis. Exp 152, e60384 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sakib S, Yu Y, Voigt A, Ungrin M & Dobrinski I Generation of porcine testicular organoids with testis specific architecture using microwell culture. J. Vis. Exp 152, e60387 (2019). [DOI] [PubMed] [Google Scholar]
- 112.Sakib S et al. Formation of organotypic testicular organoids in microwell culture†. Biol. Reprod 100, 1648–1660 (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang B, Korolj A, Lai BFL & Radisic M Advances in organ-on-a-chip engineering. Nat. Rev. Mater 3, 257–278 (2018). [Google Scholar]
- 114.Bhatia SN & Ingber DE Microfluidic organs-on-chips. Nat. Biotechnol 32, 760–772 (2014). [DOI] [PubMed] [Google Scholar]
- 115.Kim MS, Bae CY, Wee G, Han YM & Park JK A microfluidic in vitro cultivation system for mechanical stimulation of bovine embryos. Electrophoresis 30, 3276–3282 (2009). [DOI] [PubMed] [Google Scholar]
- 116.Zhu J et al. Human fallopian tube epithelium co-culture with murine ovarian follicles reveals crosstalk in the reproductive cycle. Mol. Hum. Reprod 22, 756–767 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.García EV et al. Bovine embryo-oviduct interaction in vitro reveals an early cross talk mediated by BMP signaling. Reproduction 153, 631–643 (2017). [DOI] [PubMed] [Google Scholar]
- 118.Ferraz MAMM et al. Improved bovine embryo production in an oviduct-on-a-chip system: prevention of poly-spermic fertilization and parthenogenic activation. Lab Chip 17, 905–916 (2017). [DOI] [PubMed] [Google Scholar]
- 119.Komeya M et al. Long-term ex vivo maintenance of testis tissues producing fertile sperm in a microfluidic device. Sci. Rep 6, 21472 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.De Michele F et al. Preserved seminiferous tubule integrity with spermatogonial survival and induction of Sertoli and Leydig cell maturation after long-term organotypic culture of prepubertal human testicular tissue. Hum. Reprod 32, 32–45 (2017). [DOI] [PubMed] [Google Scholar]
- 121.Yamanaka H et al. A monolayer microfluidic device supporting mouse spermatogenesis with improved visibility. Biochem. Biophys. Res. Commun 500, 885–891 (2018). [DOI] [PubMed] [Google Scholar]
- 122.Gnecco JS et al. Compartmentalized culture of perivascular stroma and endothelial cells in a microfluidic model of the human endometrium. Ann. Biomed. Eng 45, 1758–1769 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lee JS et al. Placenta-on-a-chip: a novel platform to study the biology of the human placenta. J. Matern. Neonatal Med. 29, 1046–1054 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Blundell C et al. A microphysiological model of the human placental barrier. Lab Chip 16, 3065–3073 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nishiguchi A et al. In vitro placenta barrier model using primary human trophoblasts, underlying connective tissue and vascular endothelium. Biomaterials 192, 140–148 (2019). [DOI] [PubMed] [Google Scholar]
- 126.Pemathilaka RL, Caplin JD, Aykar SS, Montazami R & Hashemi NN Placenta-on-a-chip: in vitro study of caffeine transport across placental barrier using liquid chromatography mass spectrometry. Glob. Challenges 3, 1800112 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Arumugasaamy N, Hurley-Novatny A, Lembong J, Kim PCW & Fisher JP Assessing SSRIs’ effects on fetal cardiomyocytes utilizing placenta-fetus model. Acta Biomater. 99, 258–268 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Arumugasaamy N, Gudelsky A, Hurley-Novatny A, Kim PCW & Fisher JP Model placental barrier phenotypic response to fluoxetine and sertraline: a comparative study. Adv. Healthc. Mater 8, e1900476 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yin F et al. A 3D human placenta-on-a-chip model to probe nanoparticle exposure at the placental barrier. Toxicol. Vitr 54, 105–113 (2019). [DOI] [PubMed] [Google Scholar]
- 130.Arumugasaamy N et al. Biomimetic placenta-fetus model demonstrating maternal–fetal transmission and fetal neural toxicity of Zika virus. Ann. Biomed. Eng 46, 1963–1974 (2018). [DOI] [PubMed] [Google Scholar]
- 131.Abbas Y et al. A microfluidics assay to study invasion of human placental trophoblast cells. J. R. Soc. Interface 14, 20170131 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li S-S et al. Modeling ovarian cancer multicellular spheroid behavior in a dynamic 3D peritoneal microdevice. J. Vis. Exp 120, e55337 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhou H et al. Hydrogel based 3-dimensional (3D) system for toxicity and high-throughput (HTP) analysis for cultured murine ovarian follicles. PLoS ONE 10, e0140205 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Xiao S et al. Doxorubicin has dose-dependent toxicity on mouse ovarian follicle development, hormone secretion, and oocyte maturation. Toxicol. Sci 157, 320–329 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Meng C-X, Andersson KL, Bentin-Ley U, Gemzell-Danielsson K & Lalitkumar PGL Effect of levonorgestrel and mifepristone on endometrial receptivity markers in a three-dimensional human endometrial cell culture model. Fertil. Steril 91, 256–264 (2009). [DOI] [PubMed] [Google Scholar]
- 136.Laronda MM, Burdette JE, Kim J & Woodruff TK Recreating the female reproductive tract in vitro using iPSC technology in a linked microfluidics environment. Stem Cell Res. Ther 4, S13 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cooper AR et al. The time is now for a new approach to primary ovarian insufficiency. Fertil. Steril 95, 1890–1897 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.De Vos M, Smitz J & Woodruff TK Fertility preservation in women with cancer. Lancet 384, 1302–1310 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jeruss JS & Woodruff TK Preservation of fertility in patients with cancer. N. Engl. J. Med 360, 902–911 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Donnez J & Dolmans M-M Fertility preservation in women. N. Engl. J. Med 377, 1657–1665 (2017). [DOI] [PubMed] [Google Scholar]
- 141.Donnez J, Dolmans M-M, Diaz C & Pellicer A Ovarian cortex transplantation: time to move on from experimental studies to open clinical application. Fertil. Steril 104, 1097–1098 (2015). [DOI] [PubMed] [Google Scholar]
- 142.Amorim CA & Shikanov A The artificial ovary: current status and future perspectives. Future Oncol. 12, 2323–2332 (2016). [DOI] [PubMed] [Google Scholar]
- 143.Kniazeva E et al. Primordial follicle transplantation within designer biomaterial grafts produce live births in a mouse infertility model. Sci. Rep 5, 17709 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rios PD et al. Retrievable hydrogels for ovarian follicle transplantation and oocyte collection. Biotechnol. Bioeng 115, 2075–2086 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Vanacker J et al. Transplantation of an alginate-matrigel matrix containing isolated ovarian cells: first step in developing a biodegradable scaffold to transplant isolated preantral follicles and ovarian cells. Biomaterials 33, 6079–6085 (2012). [DOI] [PubMed] [Google Scholar]
- 146.Day JR et al. Immunoisolating poly(ethylene glycol) based capsules support ovarian tissue survival to restore endocrine function. J. Biomed. Mater. Res. Part A 106, 1381–1389 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Felder S et al. Reconstruction of the ovary microenvironment utilizing macroporous scaffold with affinity-bound growth factors. Biomaterials 205, 11–22 (2019). [DOI] [PubMed] [Google Scholar]
- 148.Sittadjody S et al. Encapsulation of mesenchymal stem cells in 3D ovarian cell constructs promotes stable and long-term hormone secretion with improved physiological outcomes in a syngeneic rat model. Ann. Biomed. Eng 48, 1058–1070 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.David A, Day J & Shikanov A Immunoisolation to prevent tissue graft rejection: current knowledge and future use. Exp. Biol. Med 241, 955–961 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.David A et al. Restoring ovarian endocrine function with encapsulated ovarian allograft in immune competent mice. Ann. Biomed. Eng 45, 1685–1696 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sittadjody S et al. In vivo transplantation of 3D encapsulated ovarian constructs in rats corrects abnormalities of ovarian failure. Nat. Commun 8, 1858 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yakhnenko I, Wong WK, Katkov II & Itkin-Ansari P Cryopreservation of human insulin expressing cells macro-encapsulated in a durable therapeutic immunoisolating device theracyte. Cryo-Lett. 33, 518–531 (2012). [PubMed] [Google Scholar]
- 153.Veiseh O et al. Size- and shape-dependent foreign body immune response to materials implanted in rodents and non-human primates. Nat. Mater 14, 643–651 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Calafiore R et al. Microencapsulated pancreatic islet allografts into nonimmunosuppressed patients with type 1 diabetes: first two cases. Diabetes Care 29, 137–138 (2006). [DOI] [PubMed] [Google Scholar]
- 155.Edmonds ME, Orwig KE & Brannigan RE in Textbook of Oncofertility Research and Practice: A Multidisciplinary Approach (eds Woodruff TK et al. ) 385–394 (Springer, 2019). [Google Scholar]
- 156.Kaneko H et al. Generation of live piglets for the first time using sperm retrieved from immature testicular tissue cryopreserved and grafted into nude mice. PLoS ONE 8, e70989 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hermann BP et al. Spermatogonial stem cell transplantation into rhesus testes regenerates spermatogenesis producing functional sperm. Cell Stem Cell 11, 715–726 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Fayomi AP et al. Autologous grafting of cryopreserved prepubertal rhesus testis produces sperm and offspring. Science 363, 1314–1319 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Liu Z et al. Generation of macaques with sperm derived from juvenile monkey testicular xenografts. Cell Res. 26, 139–142 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ohta H & Wakayama T Generation of normal progeny by intracytoplasmic sperm injection following grafting of testicular tissue from cloned mice that died postnatally. Biol. Reprod 73, 390–395 (2005). [DOI] [PubMed] [Google Scholar]
- 161.Poels J, Van Langendonckt A, Many M-C, Wese F-X & Wyns C Vitrification preserves proliferation capacity in human spermatogonia. Hum. Reprod 28, 578–589 (2013). [DOI] [PubMed] [Google Scholar]
- 162.Medrano JV et al. Germ cell transplantation into mouse testes procedure. Fertil. Steril 102, e11–e12 (2014). [DOI] [PubMed] [Google Scholar]
- 163.Poels J et al. In search of better spermatogonial preservation by supplementation of cryopreserved human immature testicular tissue xenografts with N-acetylcysteine and testosterone. Front. Surg 1, 47 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.de Michele F et al. Haploid germ cells generated in organotypic culture of testicular tissue from prepubertal boys. Front. Physiol 9, 1413 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lee KH et al. Vitrified canine testicular cells allow the formation of spermatogonial stem cells and seminiferous tubules following their xenotransplantation into nude mice. Sci. Rep 6, 21919 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Honaramooz A, Megee SO, Rathi R & Dobrinski I Building a testis: formation of functional testis tissue after transplantation of isolated porcine (Sus scrofa) Testis Cells. Biol. Reprod 76, 43–47 (2007). [DOI] [PubMed] [Google Scholar]
- 167.Brinster RL Germline stem cell transplantation and transgenesis. Science 296, 2174–2176 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Xin L et al. A collagen scaffold loaded with human umbilical cord-derived mesenchymal stem cells facilitates endometrial regeneration and restores fertility. Acta Biomater. 92, 160–171 (2019). [DOI] [PubMed] [Google Scholar]
- 169.Zhu L, Zhou H, Sun Z, Lou W & Lang J Anatomic and sexual outcomes after vaginoplasty using tissue-engineered biomaterial graft in patients with Mayer-Rokitansky-Küster-Hauser syndrome: a new minimally invasive and effective surgery. J. Sex. Med 10, 1652–1658 (2013). [DOI] [PubMed] [Google Scholar]
- 170.Ding J-X, Chen X-J, Zhang X.-y, Zhang Y & Hua K-Q Acellular porcine small intestinal submucosa graft for cervicovaginal reconstruction in eight patients with malformation of the uterine cervix. Hum. Reprod 29, 677–682 (2014). [DOI] [PubMed] [Google Scholar]
- 171.Ding J-X, Zhang X, Chen L & Hua K-Q Vaginoplasty using acellular porcine small intestinal submucosa graft in two patients with Meyer-von-Rokitansky-Küster-Hauser syndrome: a prospective new technique for vaginal reconstruction. Gynecol. Obstet. Invest 75, 93–96 (2013). [DOI] [PubMed] [Google Scholar]
- 172.Li Y et al. Bone marrow mesenchymal stem cells could acquire the phenotypes of epithelial cells and accelerate vaginal reconstruction combined with small intestinal submucosa. Cell Biol. Int 39, 1225–1233 (2015). [DOI] [PubMed] [Google Scholar]
- 173.Pinto Medeiros Dias MT et al. Tilapia fish skin as a new biologic graft for neovaginoplasty in Mayer-Rokitansky-Kuster-Hauser syndrome: a video case report. Fertil. Steril 112, 174–176 (2019) [DOI] [PubMed] [Google Scholar]
- 174.Dabaghi M et al. An artificial placenta type microfluidic blood oxygenator with double-sided gas transfer microchannels and its integration as a neonatal lung assist device. Biomicrofluidics 12, 044101 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hunt PA et al. Bisphenol a exposure causes meiotic aneuploidy in the female mouse. Curr. Biol 13, 546–553 (2003). [DOI] [PubMed] [Google Scholar]
- 176.ART Success Rates. Centers for Disease Control and Prevention https://www.cdc.gov/art/artdata/index.html (2019).
- 177.Campo-Engelstein L, Chen D, Baratz AB, Johnson EK & Finlayson C The ethics of fertility preservation for pediatric patients with differences (disorders) of sex development. J. Endocr. Soc 1, 638–645 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Woodruff TK, Zoloth L, Campo-Engelstein L & Rodriguez S Oncofertility: ethical, legal, social, and medical perspectives. Preface. Cancer Treat. Res 156, 5–7 (2010). [PubMed] [Google Scholar]
- 179.Segers S, Mertes H, de Wert G, Dondorp W & Pennings G Balancing ethical pros and cons of stem cell derived gametes. Ann. Biomed. Eng 45, 1620–1632 (2017). [DOI] [PubMed] [Google Scholar]
- 180.Basco D, Campo-Engelstein L & Rodriguez S Insuring against infertility: expanding state infertility mandates to include fertility preservation technology for cancer patients. J. Law Med. Ethics 38, 832–839 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Fleetwood A & Campo-Engelstein L The impact of infertility: why ART should be a higher priority for women in the global South. Canc. Treat 156, 237–248 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Campo-Engelstein L Consistency in insurance coverage for iatrogenic conditions resulting from cancer treatment including fertility preservation. J. Clin. Oncol 28, 1284–1286 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Chatzinikolaou N The ethics of assisted reproduction. J. Reprod. Immunol 85, 3–8 (2010). [DOI] [PubMed] [Google Scholar]
- 184.Brezina PR & Zhao Y The ethical, legal, and social issues impacted by modern assisted reproductive technologies. Obstet. Gynecol. Int 2012, 686253 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Campo-Engelstein L Offering testicular tissue cryopreservation to boys: the increasing importance of biological fatherhood. Am. J. Bioeth 13, 39–40 (2013). [DOI] [PubMed] [Google Scholar]
- 186.Alves-Lopes JP, Söder O & Stukenborg JB Use of a three-layer gradient system of cells for rat testicular organoid generation. Nat. Protoc 13, 248–259 (2018). [DOI] [PubMed] [Google Scholar]