Abstract
Background
The COVID-19 pandemic response is affecting maternal and neonatal health services all over the world. We aimed to assess the number of institutional births, their outcomes (institutional stillbirth and neonatal mortality rate), and quality of intrapartum care before and during the national COVID-19 lockdown in Nepal.
Methods
In this prospective observational study, we collected participant-level data for pregnant women enrolled in the SUSTAIN and REFINE studies between Jan 1 and May 30, 2020, from nine hospitals in Nepal. This period included 12·5 weeks before the national lockdown and 9·5 weeks during the lockdown. Women were eligible for inclusion if they had a gestational age of 22 weeks or more, a fetal heart sound at time of admission, and consented to inclusion. Women who had multiple births and their babies were excluded. We collected information on demographic and obstetric characteristics via extraction from case notes and health worker performance via direct observation by independent clinical researchers. We used regression analyses to assess changes in the number of institutional births, quality of care, and mortality before lockdown versus during lockdown.
Findings
Of 22 907 eligible women, 21 763 women were enrolled and 20 354 gave birth, and health worker performance was recorded for 10 543 births. From the beginning to the end of the study period, the mean weekly number of births decreased from 1261·1 births (SE 66·1) before lockdown to 651·4 births (49·9) during lockdown—a reduction of 52·4%. The institutional stillbirth rate increased from 14 per 1000 total births before lockdown to 21 per 1000 total births during lockdown (p=0·0002), and institutional neonatal mortality increased from 13 per 1000 livebirths to 40 per 1000 livebirths (p=0·0022). In terms of quality of care, intrapartum fetal heart rate monitoring decreased by 13·4% (−15·4 to −11·3; p<0·0001), and breastfeeding within 1 h of birth decreased by 3·5% (−4·6 to −2·6; p=0·0032). The immediate newborn care practice of placing the baby skin-to-skin with their mother increased by 13·2% (12·1 to 14·5; p<0·0001), and health workers' hand hygiene practices during childbirth increased by 12·9% (11·8 to 13·9) during lockdown (p<0·0001).
Interpretation
Institutional childbirth reduced by more than half during lockdown, with increases in institutional stillbirth rate and neonatal mortality, and decreases in quality of care. Some behaviours improved, notably hand hygiene and keeping the baby skin-to-skin with their mother. An urgent need exists to protect access to high quality intrapartum care and prevent excess deaths for the most vulnerable health system users during this pandemic period.
Funding
Grand Challenges Canada.
Introduction
The scale of the COVID-19 outbreak has brought in an unprecedented change in the global and national landscape on daily wellbeing.1 As a result, many countries are responding to restrict the spread of disease through national or local lockdowns. As health institutions show strain in responding to the pandemic,2 concern is increasing that COVID-19 will disrupt health-service delivery, including for maternal and newborn health services, particularly in resource-limited countries.3
In 2019, almost 80 million women gave birth at health institutions globally, which is three times the number of institutional births in 2000.4 Because more women and their babies have been able to access effective and respectful care before, during, and after pregnancy, maternal and neonatal mortality and stillbirth rates have all decreased substantially in the past 20 years (by 44% for maternal mortality, 41% for neonatal mortality, and 25% for stillbirth until 2019).5, 6, 7
Modelling studies have estimated the potential impact of the COVID-19 pandemic on mortality due to reduced access to maternal and neonatal health services. Estimates by the Guttmacher Institute suggest that even a moderate decrease of 10% in coverage of pregnancy-related and neonatal health care could result in an additional 28 000 maternal deaths and 168 000 neonatal deaths globally.8 An analysis of 118 countries using the Lives Saved Tool suggested reductions in coverage of around 15% for 6 months could result in 253 500 additional child deaths and 12 190 additional maternal deaths, while reductions of around 45% for 6 months would result in 1 157 000 additional child deaths and 56 700 additional maternal deaths.9
Research in context.
Evidence before this study
During the Ebola virus disease outbreak in west Africa, the response to the outbreak reduced coverage of essential health services. A modelling exercise using the Lives Saved Tool estimated an excess of 56 700 maternal and 1 157 000 child deaths assuming up to 45% coverage reductions in 118 countries for 6 months during the COVID-19 pandemic outbreak. We searched MEDLINE and Google Scholar on July 5, 2020, for articles in English published in the past 5 years on changes in use of health facilities for childbirth, quality of intrapartum care, and mortality during the COVID-19 pandemic response using the terms “COVID-19” AND “utilization of health services” AND “quality of intrapartum care” AND “mortality”. We found no published primary data regarding changes in coverage or quality of care, or stillbirth or neonatal mortality.
Added value of this study
To our knowledge, this study is one of the first and largest documentations of service reduction during COVID-19. We provide empirical evidence from nine hospitals in Nepal. Compared with before the COVID-19 lockdown, the number of institutional births was reduced by approximately half, with increased inequality by ethnicity. We also found a significantly increased risk of preterm birth, institutional stillbirth, and neonatal mortality during lockdown. Improvement was seen in the hand hygiene practices of health workers during childbirth and in neonatal skin-to-skin contact during the lockdown. During lockdown, significant reductions were seen in intrapartum fetal heart rate surveillance and breastfeeding within 1 h of birth.
Implications of all the available evidence
The COVID-19 outbreak and response has reduced coverage of health facility births and widened inequalities in Nepal, with significantly increased institutional stillbirth and neonatal mortality rates. These outcomes are concerning in a fragile health system and raise questions on policies regarding strict lockdowns in low-income and middle-income countries during the COVID-19 outbreak.
Although prioritised as an essential core health service,10 some reports indicate that maternal and newborn health services are being reduced due to COVID-19 restrictions in low-income and middle-income countries, and that quality of care might be deteriorating, risking deaths and reversals of hard-won gains over the past two decades.11 Reports published after the 2014–16 Ebola virus disease outbreaks in west Africa showed decreases in maternity services, including facility births, as a result of restrictions on travel and fear of using services.12 The full scope of the impact that COVID-19 is having on service delivery access and health outcomes, including on mortality, is difficult to appreciate while the pandemic is ongoing. Although routine data systems could be used to show changes in service delivery or mortality, the COVID-19 pandemic might have added extra strains to data collection in low-income and middle-income countries.13 To date, we could not identify published reports of primary data showing reduced coverage of intrapartum care, or effects on birth outcomes (appendix 3 p 3).
Nepal is an example of a country that has had substantial gains in maternal and neonatal survival over the past two decades,14 yet these gains are at risk due to COVID-19. As of 2019, maternal mortality has decreased by 76%, stillbirth rate has decreased by 58%, and newborn mortality has decreased by 62% since 2000.14 The number of institutional births increased by four times between 2001 and 2016 as a result of social mobilisation and financial incentives.13 In Nepal, the first case of COVID-19 was detected on Jan 23, 2020, with additional cases detected throughout March, 2020.15 As a result of these additional cases being detected, the high-level committee for COVID-19 management for Nepal intensified their preparations for hospitals to deal with the COVID-19 cases through supportive management and isolation.15 A countrywide lockdown was announced on March 21, 2020, with directives to frontline health-care providers to prepare for cases.15 The lockdown consisted of multiple restrictions including on all forms of travel except for emergency services, and grocery stores and food services with authorisation from security personnel from local law enforcement throughout the country. The lockdown was implemented abruptly, within a few hours of the announcement. Speculation is widespread that the COVID-19 pandemic response through the national lockdown has had an indirect impact on women and babies due to travel restrictions and fear of going to hospitals due to COVID-19 and possible poor care.11 Health workers were provided with directives to use personal protective equipment and improve infection preventive practices in all hospitals to prevent transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
We aimed to assess the indirect impact of the COVID-19 lockdown on use of public health facilities for childbirth, quality of intrapartum care, institutional stillbirth rate, and neonatal mortality rate before and during lockdown.
Methods
Study design and participants
This is a prospective, observational study nested within two quality improvement studies, REFINE (ISRCTN16741720) and SUSTAIN (ISRCTN18148368), being done in nine health institutions in Nepal to implement a safer birth bundle package for 24 months (from January, 2019, to December, 2020; appendix 3 p 3).16 We report data over a period of 5 months including 12·5 weeks before lockdown implementation (Jan 1–March 20, 2020) and 9·5 weeks during lockdown (March 21–May 30, 2020).
The nine hospitals were distributed across all seven provinces of the country (appendix 3 p 11). The annual number of births in these nine hospitals covered 11·2% of the national number of births for 2019.16 The hospitals in the study provided referral obstetric services through Comprehensive and Emergency Obstetrics and Neonatal Care services. All vaginal births took place in delivery units and caesarean births took place in operating theatres. At these nine hospitals, during the study period, no cases of COVID-19 were reported before lockdown and 1401 cases were reported during lockdown, but no cases were reported among pregnant women. There was no closure of any of the nine hospitals in the study as a result of reporting COVID-19 cases during the study period.
Participants who consented and were enrolled in the REFINE and SUSTAIN studies were considered for this study. Women at 22 weeks of gestation or more admitted in the labour room and whose fetal heart sound was heard at the time of admission were eligible for inclusion. For use of the participant-level data for this study, additional approval was sought from the ethical review board of Nepal Health Research Council (registration number 439/2020). For this study, we excluded women who had multiple births and their babies.
Participants provided informed written consent at the time of admission to the hospital. The SUSTAIN and REFINE studies were granted ethical approval by the ethical review board of Nepal Health Research Council.
Data sources and management
We extracted participant-level data from the existing data collection systems for the REFINE and SUSTAIN studies. For these studies, a validated clinical observation checklist was used to observe the labour and delivery event for all vaginal births possible, and women's obstetric and neonatal information was collected from patient case notes. A data collection system was set up at each hospital and observations were done by independent clinical researchers using a tablet-based application. All the data entered in the tablet-based application were reviewed on a weekly basis by an independent database manager. For this study, data were extracted by OB into SPSS software (version 17.0) for cleaning of extracted data of all births and observed data from all vaginal births.
Definitions and measurements
Institutional stillbirth rate was defined as the number of babies born in the institution with no signs of life, with a gestational age of 22 weeks or more, per 1000 births. Institutional neonatal mortality rate was defined as the number of neonates who died before discharge per 1000 livebirths. The health worker's performance during intrapartum care was measured on the basis of WHO's 2016 Standards for improving quality of maternal and newborn care in health facilities quality of care statement and process of care.17 The nine components of these standards are (1) health worker's handwashing practice during childbirth, defined as health-care staff who cleaned their hands correctly as per WHO's five moments for hand hygiene; (2) health worker's use of gloves and gown to reduce infection transmission during childbirth; (3) preparation of equipment to be used during childbirth; (4) health worker greeting the mother at the time of admission; (5) women having a companion during labour; (6) intrapartum fetal heart rate monitoring at 30 min intervals; (7) neonate's cord clamped 1 min after birth; (8) neonatal skin-to-skin contact with mother after birth; and (9) breastfeeding within 1 h of birth.
For sociodemographic characteristics, ethnicity was recorded on the basis of the caste system in Nepal (ie, relatively disadvantaged ethnic groups [Janajati, Madeshi, Muslim, Dalit] and relatively advantaged ethnic groups [Brahmin and Chhetri-Hill, and Brahmin-Tarai]).18 We report women's age as mean (SD) and categorised as 18 years or younger, 19–24 years, 25–29 years, 30–34 years, and 35 years or older. Parity was defined as no previous births, at least one previous birth, or two or more previous births. Obstetric characteristic measurements included were complication at the time of admission, induced labour, and mode of birth, including spontaneous vaginal birth, assisted vaginal birth, and caesarean birth. For neonatal characteristics, we captured preterm birth (defined as <37 weeks of gestation on the basis of first day of mother's last menstrual period), low birthweight (≤2500 g), and sex of the baby (boy, girl, or ambiguous).
Data analysis
We compared demographic, obstetric, and neonatal characteristics before and during lockdown using Pearson's χ2 test. We analysed the coverage of health worker's performance before and during lockdown using Pearson's χ2 test.
To measure the weekly change in the number of births, we used a segmented time series model. We checked for autocorrelation using the autocorrelation factor for the outcome variable and found no significant autocorrelation.19
We used a generalised linear model with Poisson distribution and log-link function to calculate the risk of preterm birth, institutional stillbirth, and institutional neonatal mortality before and during lockdown. We adjusted for ethnicity, maternal age, and obstetric characteristics to calculate the risk of preterm birth, institutional stillbirth, and institutional neonatal mortality. We assessed the between-hospital heterogeneity on preterm birth, institutional stillbirth, and institutional neonatal mortality. We compared the weekly trend in the number of institutional births between January and May, 2019, and between January and May, 2020, to assess the difference between the two different time periods. To assess trends in outcome variables and health worker performance before and during the COVID-19 lockdown, we used locally weighted scatterplot smoothing regression analysis.
We imputed missing values for gestational age using the Classification and Regression Tree method in the mice package in R. We did all data analyses using R (version 3.6.2).
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the manuscript. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
During the study period, 24 167 women were admitted into the hospitals for delivery and 22 907 were eligible for enrolment, of whom 21 763 enrolled and 20 354 gave birth in the hospitals during the study period, with 13 189 (64·8%) before the COVID-19 lockdown and 7165 (35·2%) during the COVID-19 lockdown (appendix 3 p 12). 10 453 vaginal births were observed over the study period, with 8228 (78·7%) before lockdown and 2225 (21·3%) during lockdown.
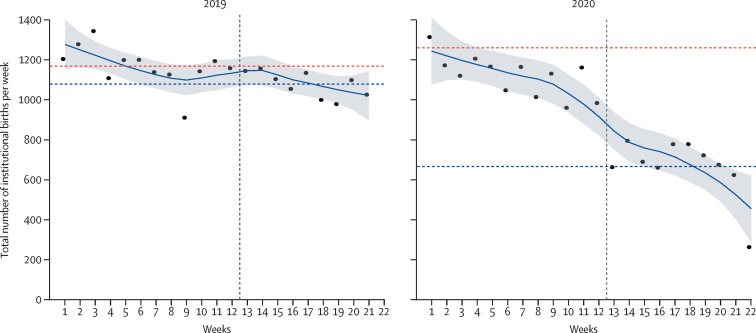
In comparison with the weekly number of institutional births between January and May, 2019, a substantial decrease was seen between January and May, 2020, especially after week 12·5 when the COVID-19 lockdown was announced (figure 1 ). In 2020, we observed a decreasing trend in the weekly number of institutional births since the start of the study period, before lockdown started, which continued to decrease sharply after the lockdown was announced. The average weekly reduction in institutional births during lockdown was 7·4%, with a total decrease of 52·4% by the end of lockdown (figure 1; appendix 3 p 4). Before lockdown, the mean weekly number of institutional births was 1261·1 births (SE 66·1). The average number of weekly institutional births decreased by 197·7 (SE 92·3) to 651·4 (49·9) during lockdown (appendix 3 p 5).
Figure 1.
Number of weekly institutional births for the first 22 weeks of 2019 and of 2020 in Nepal, indicative of implementation of national lockdown in 2020
Datapoints are mean weekly number of births, smoothed line is the locally weighted scatterplot smoothing curve with shaded grey area showing the 95% CI. The vertical dashed line indicates week 12·5 when lockdown was announced in 2020. The horizontal red dashed line is the mean weekly number of births before week 12·5 and the blue dashed line is the mean weekly number of births during the remaining 9·5 weeks.
We observed an increase in the use of childbirth services by women of the relatively advantageous ethnic group Brahmain and Chhetri-Hill during lockdown (2429 [33·9%] of 7165 births) compared with before lockdown (4055 [30·7%] of 13 189 births; p<0·0001; appendix 3 p 6). Decrease in attendance was seen among the more disadvantaged ethnic group Madhesi during lockdown (1228 [17·1%]) compared with before lockdown (2840 [21·5%]; p=0·0015). The mean age of women giving birth across study sites before lockdown was 24·1 years (SD 4·4), which increased to 24·3 years (4·5) during lockdown. The proportion of women who had a complication during admission increased from 6·7% (n=884) before lockdown to 8·7% (n=587) during lockdown (p=0·0126). The proportion of women whose labour was induced increased from 17·1% (n=2258) before lockdown to 32·1% (n=2282) during lockdown (p<0·0001). The proportion of women who had caesarean section increased from 24·5% (n=3234) before lockdown to 26·2% (n=1879) during lockdown (p=0·0075). The proportion of babies born preterm (before 37 weeks) increased (16·7% [n=2125] before lockdown vs 20·0% [n=1342] during lockdown; p=0·0016) and the proportion that were of low birthweight did not increase (11·1% [n=1429] vs 10·5% [773]; p=0·37; appendix 3 p 6).
Across the study period we recorded 3467 preterm births, 332 institutional stillbirths (179 before lockdown, 153 during lockdown), and 43 institutional neonatal deaths (18 before lockdown, 25 during lockdown; appendix 3 p 6). The risk ratio of preterm birth for during lockdown versus before lockdown was 1·30 (95% CI 1·20–1·40), after adjusting for ethnicity, maternal age, and complication during admission (table 1 ). The institutional stillbirth rate increased from 14 per 1000 total births before lockdown to 21 per 1000 total births during lockdown. The adjusted risk ratio of institutional stillbirth rate during the lockdown versus before lockdown was 1·46 (95% CI 1·13–1·89). The institutional neonatal mortality rate increased from 13 deaths per 1000 livebirths before lockdown to 40 deaths per 1000 livebirths during the lockdown. The adjusted risk ratio of neonatal mortality during the lockdown versus before lockdown was 3·15 (95% CI 1·47–6·74). We found little between-hospital heterogeneity (appendix 3 p 10).
Table 1.
Change in preterm birth rate, institutional stillbirth rate, and institutional neonatal mortality before and during the COVID-19 lockdown using generalised linear model with Poisson distribution
|
Preterm birth rate |
Institutional stillbirth, rate per 1000 total births |
Institutional neonatal mortality rate, per 1000 livebirths |
|||||
|---|---|---|---|---|---|---|---|
| Estimate (95% CI) | p value | Estimate (95% CI) | p value | Estimate (95% CI) | p value | ||
| Unadjusted effect, exp(β) | |||||||
| Baseline risk (risk before lockdown) | 0·167 (0·160–0·174) | <0·0001 | 14 (12–16) | <0·0001 | 13 (8–20) | <0·0001 | |
| Lockdown risk (risk during lockdown) | 0·187 (0·178–0·204) | <0·0001 | 21 (18–25) | <0·0001 | 40 (23–57) | <0·0001 | |
| Risk ratio during lockdown vs before lockdown | 1·198 (1·113–1·295) | <0·0001 | 1·57 (1·27–1·95) | 0·0002 | 2·58 (1·41–4·72) | 0·0022 | |
| Adjusted effect, β | |||||||
| Baseline risk (risk before lockdown) | 0·14 (0·11–0·17) | <0·0001 | 3 (2–7) | <0·0001 | 0·9 (0·1–8) | <0·0001 | |
| Risk ratio during lockdown vs before lockdown | 1·30 (1·20–1·40) | <0·0001 | 1·46 (1·13–1·89) | 0·0042 | 3·15 (1·47–6·74) | 0·0037 | |
| Adjusting factors | |||||||
| Preterm birth | NA | NA | 5·54 (4·24–7·24) | <0·0001 | 6·74 (3·05–14·91) | <0·0001 | |
| Complication during admission | 2·61 (2·36–2·88) | <0·0001 | 3·17 (2·38–4·21) | <0·0001 | 3·25 (1·42–7·43) | 0·0054 | |
| Ethnicity | |||||||
| Dalit* | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | |
| Janajati* | 0·88 (0·77–1·01) | 0·062 | 0·55 (0·38–0·81) | 0·0025 | 0·78 (0·31–1·96) | 0·594 | |
| Madhesi* | 1·52 (1·33–1·73) | <0·0001 | 0·80 (0·54–1·19) | 0·263 | 0·194 (0·04–0·94) | 0·042 | |
| Muslim* | 1·57 (1·30–1·89) | <0·0001 | 0·74 (0·41–1·35) | 0·330 | 0·37 (0·05–3·01) | 0·350 | |
| Brahmin and Chhetri-Hill† | 0·99 (0·87–1·12) | 0·852 | 0·46 (0·31–0·67) | <0·0001 | 0·26 (0·08–0·82) | 0·022 | |
| Brahmin-Tarai† | 1·15 (0·82–1·61) | 0·426 | 0·15 (0·02–1·11) | 0·063 | 0·00 (NA) | .. | |
| Maternal age, years | 1·00 (0·99–1·01) | 0·472 | 1·04 (1·02–1·07) | 0·0015 | 1·00 (0·92–1·08) | 0·914 | |
For unadjusted effect, estimate is exp(β), the risk of birth outcome; for adjusted effect, estimates are β, with 95% CI in parentheses. NA=not applicable.
Relatively disadvantaged ethnic groups.
Relatively advantaged ethnic groups.
Trend analyses of the stillbirth rate show a sharp increase immediately at the start of lockdown from 8 stillbirths per 1000 total births in week 1 of lockdown (week 12 of the study period) to 33 per 1000 total births in week 4 of lockdown and then some variability between 18 per 1000 births in week 6 of lockdown to 20 per 1000 births on week 9·5 (appendix 3 p 14).
Compared with before lockdown, during lockdown health workers' hand hygiene practices during childbirth increased by 12·9% (95% CI 11·8 to 13·9; table 2 ), health workers greeting the mother decreased by 2·2% (−3·1 to −1·3), the use of gloves and gown for childbirth decreased by 2·4% (−3·1 to −1·9), companionship during labour decreased by 6·0% (−6·9 to −5·1), intrapartum fetal heart rate monitoring decreased by 13·4% (−15·4 to −11·3), placing the baby skin-to-skin with the mother increased by 13·2% (12·1 to 14·5), and breastfeeding within 1 h of birth decreased by 3·5% (−4·6 to −2·6). Changes among the other standards were not significant (table 2).
Table 2.
Health worker performance for labour and childbirth before lockdown and during the COVID-19 lockdown
| Before lockdown (n=8228) | During lockdown (n=2225) | Change in proportion (95% CI) | p value | |
|---|---|---|---|---|
| Health workers wash hands during childbirth (n=10 450) | 2350 (28·6%) | 921 (41·4%) | 12·9% (11·8 to 13·9) | <0·0001 |
| Health workers use gloves and gown during childbirth (n=10 450) | 7818 (95·0%) | 2058 (92·6%) | −2·4% (−3·1 to −1·9) | 0·0007 |
| Preparation of equipment to be used during childbirth (n=10 450) | 6646 (80·6%) | 1814 (81·6%) | 0·8% (0·0 to 1·6) | 0·197 |
| Health worker greets the mother (n=10 450) | 2748 (33·4%) | 693 (31·2%) | −2·2% (−3·1 to −1·3) | 0·026 |
| Companionship during labour (n=10 157) | 7133 (89·4%) | 1816 (83·4%) | −6·0% (−6·9 to −5·1) | 0·0014 |
| Intrapartum fetal heart rate monitoring at 30 min interval (n=9705) | 4394 (56·8%) | 851 (43·4%) | −13·4% (−15·4 to −11·3) | <0·0001 |
| Baby keeps skin-to-skin contact with the mother's chest after birth (9705) | 1005 (13·0%) | 515 (26·2%) | 13·2% (12·1 to 14·5) | <0·0001 |
| Breastfeeding within 1 h of birth (n=10 453) | 4056 (49·3%) | 1020 (45·8%) | −3·5% (−4·6 to −2·6) | 0·0032 |
| Cord clamping 1 min after birth (n=9819) | 5291 (67·7%) | 1315 (65·8%) | −1·9% (−2·9 to −0·9) | 0·060 |
Data are n (%) or change in proportion with 95% CI in parentheses.
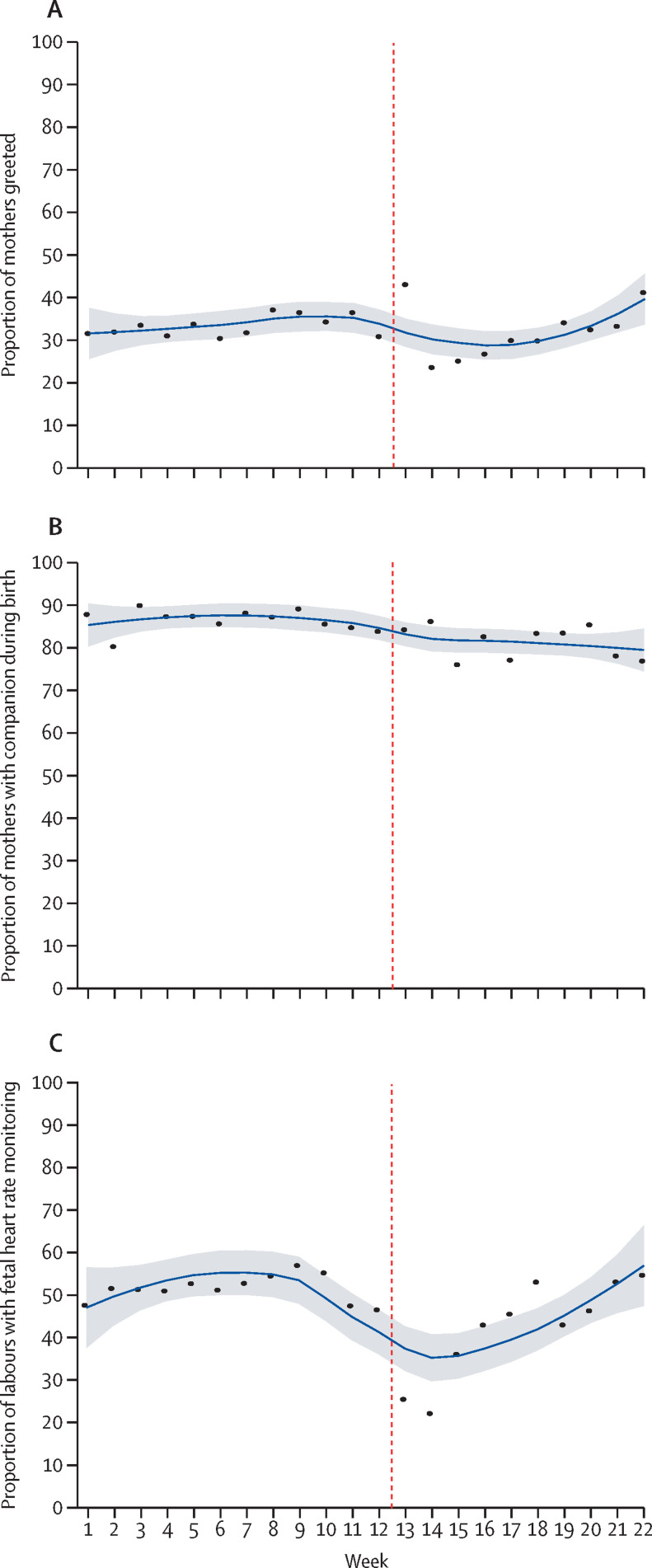
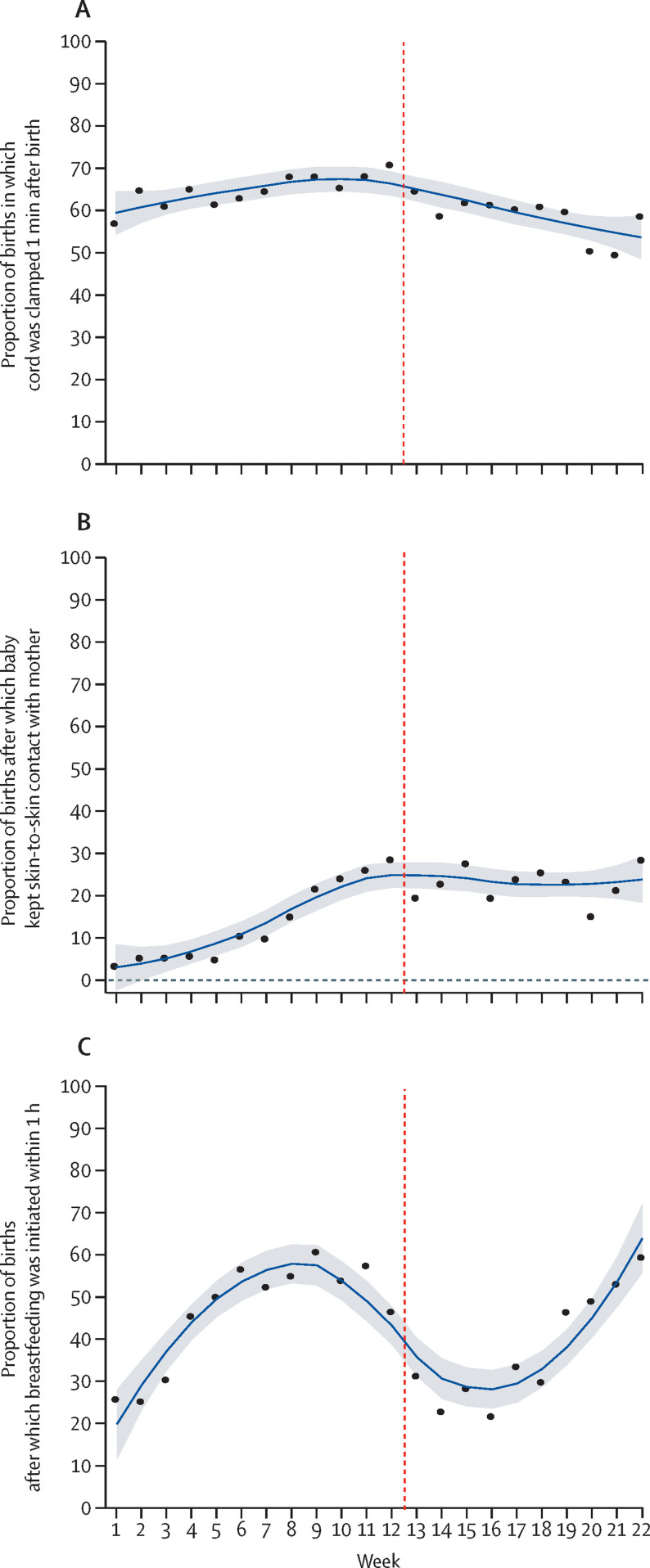
Analysis of trends in health worker practices on infection prevention during childbirth showed that the use of gloves and gown and preparation of equipment for use during childbirth decreased between week 2 and week 4 of lockdown (appendix 3 pp 16, 18). We saw a general increase in handwashing practices 3 weeks before lockdown, which continued to increase during lockdown (appendix 3 p 17). Our analysis of intrapartum care showed that occurrence of health workers greeting mothers decreased 2 weeks before the start of lockdown until week 3 of lockdown (ie, week 14 of the study period), and then gradually increased thereafter (figure 2 ). Companionship during labour decreased gradually 2 weeks before the start of lockdown (study week 10) and continued to decrease slightly during the lockdown period (figure 2). The analysis of trends in health workers' performance on intrapartum fetal heart rate monitoring shows a decrease from 3 weeks before the lockdown, which continued until week 2 of lockdown. From week 3 of lockdown, intrapartum fetal heart rate monitoring increased until the end of the study period (figure 2). Analysis of the immediate newborn care practice of cord clamping 1 min after birth increased slightly from the beginning of the study period to the beginning of lockdown, after which point a decrease was seen until the end of the study period (figure 3 ). Analysis of immediate newborn care practice of babies receiving skin-to-skin contact showed an increase from 3% from week 1 of the study period to 30% at the end of the study period (figure 3). Immediately after the start of lockdown, a decrease was seen in the skin-to-skin contact care to 20% and with fluctuation across the rest of the study period (figure 3). Breastfeeding within 1 h of birth started to decrease 4 weeks before lockdown and continued to decrease after the start of lockdown. After week 4 of lockdown, the practice of breastfeeding within 1 h of birth gradually increased until the end of the study period (figure 3; appendix 3 p 8).
Figure 2.
Change in health worker intrapartum care performance before and after implementation of lockdown in Nepal
(A) Health worker greets mother. (B) Companion during labour. (C) Fetal heart rate monitoring every 30 min. Vertical dashed line indicates week 12·5, when lockdown began. Datapoints are coverage at each timepoint across institutions and the smoothed line is the locally weighted scatterplot smoothing curve with shaded area showing the 95% CI.
Figure 3.
Immediate newborn care practice performance before and after implementation of lockdown in Nepal
(A) Cord clamping 1 min after birth. (B) Neonate keeps skin-to-skin contact with mother after birth. (C) Neonate breastfed within 1 h of birth. Vertical dashed line indicates week 12·5, when lockdown began. Datapoints are coverage at each timepoint across institutions and the smoothed line is the locally weighted scatterplot smoothing curve with shaded area showing the 95% CI.
Discussion
During the COVID-19 lockdown period in Nepal, institutional births in study hospitals reduced by approximately half compared with the beginning of the study period. The adjusted risk ratio for preterm birth was 1·30, for institutional stillbirth was 1·46, and for institutional neonatal mortality was 3·15 during the lockdown period compared with before lockdown. A decrease was seen in the coverage of intrapartum fetal heart rate monitoring during labour and in breastfeeding within 1 h of birth, but some improvements were seen in some immediate newborn care practices, such as skin-to-skin contact, and hand hygiene practice. Decreasing rates of companionship during labour might be due to hospital-level restrictions on visitors and patient support.
The decrease in use of health facilities started in the weeks before lockdown, possibly indicating a heightened fear of disease transmission, which might have stopped women from seeking care at health facilities. During lockdown in Nepal, the rate of decrease in the use of health facilities was heightened because the national lockdown halted public transport and restricted movement of people, similar to in other countries.11 Similar reductions in institutional births of 33% were reported during the Ebola virus disease outbreak in Liberia.20 In Sierra Leone, fear of Ebola virus disease and mistrust in health-care workers were reported as factors that stopped women from accessing care from health facilities.21 We found a relative decrease in the use of services compared with before lockdown among relatively disadvantaged ethnic groups highlighting already known disparities in Nepal.22 Our findings indicate the need to continue communication with policy makers and programme coordinators to address such inequalities and coverage gaps so that additional deaths can be averted.9
The increased proportion of admitted women having complications during admission, including preterm birth, during the COVID-19 lockdown period, might suggest that women at high risk of complications are disproportionately attending health facilities or that the number of complicated cases has increased due to delays and other challenges of the lockdown. Using the three delays of care model,23 the lockdown travel restrictions might have resulted in delays in patients reaching health facilities or delays in providing quality of care for those at the facility due to a shortage of skilled health-care workers, resulting in adverse outcomes among mothers and neonates. The increase in preterm birth could also be associated with the distress of COVID-19-related social restrictions, considering psychosocial stress during pregnancy.24
The decrease in coverage of preparation of equipment for childbirth and immediate newborn care 2 weeks before lockdown and until week 4 of lockdown could indicate the scarcity of protective equipment for infection prevention. This hypothesis also corroborates the reports of shortages in protection equipment in health facilities in Nepal.25 3 weeks before lockdown, intrapartum fetal heart rate monitoring reduced substantially, which might have been because health workers restricted their contact with women due to a scarcity of protective equipment. The inadequate use of gloves and equipment for managing childbirth in these health facilities indicates the poor readiness of the health facilities for maternal and neonatal care. The improvements observed in hand hygiene practices during childbirth reflect hospital COVID-19 interventions to prevent transmission of SARS-CoV-2 among health-care providers.16, 26
We also observed that companionship during labour began to decrease 3 weeks before the lockdown and continued to drop during the lockdown. Hospital protocols restricted visitors and companions to women during labour to reduce the risk of nosocomial transmission of SARS-CoV-2.27 However, WHO recommends companionship during labour and childbirth for an improved childbirth experience during this pandemic.28
Improvements in immediate neonatal care practice, such as placing neonates skin-to-skin with mothers, shows the promotion of immediate newborn practices by health workers during the pandemic. This finding is contradictory to the speculations that implementation of immediate newborn care practices might decrease due to a lack of pandemic preparedness. However, the reduction in breastfeeding within 1 h of birth 3 weeks before lockdown might reflect health workers' initial misunderstanding of breastfeeding protocols during the COVID-19 pandemic,29 despite global recommendations of breastfeeding.30 The improvement in the health workers' practice of intrapartum fetal heart rate monitoring and breastfeeding within 1 h of birth after a sudden decrease before and during the initial phase of lockdown, followed by a later increase, can be attributed to the ongoing quality improvement initiatives of the SUSTAIN and REFINE studies in these hospitals.16
Our study has several limitations. We did not explore the prevalence or the direct impact of COVID-19 on health outcomes. None of the women admitted to the hospital were tested for COVID-19, so we do not know the prevalence of COVID-19 among the study population. Other limitations include that observations of health worker practice were only done for vaginal births, not caesarean births, which introduces the potential for selection bias. Additionally, some observer reporting bias might have been present, especially because the workload in labour and delivery rooms is generally high, which could be a challenging environment for the observation of health worker performance by an independent researcher. Finally, here we report service use for nine hospitals, and so our findings should not be interpreted as a population-level reduction in institutional births. Our study also has several strengths including a large sample size before and during the COVID-19 lockdown period, with data systems in multiple hospitals established through existing studies. Prospective data collection using trained researchers to collect information using observation checklists strengthened our confidence in these findings. This study focused on understanding the indirect impact of COVID-19 on disruption in health service delivery for facility-based maternal and neonatal health. To our knowledge, this study provides one of the first and largest documentations of service reduction during COVID-19 and underlines the urgent need to protect care for women and their babies.
In summary, during the COVID-19 pandemic, women and their babies (both in utero and neonates) are susceptible and at risk due to gaps in care that can result in adverse birth outcomes including mortality. The decrease in the number of institutional births and increase in adverse outcomes are especially concerning because of Nepal's fragile health system and raise questions on policies regarding strict lockdowns in low-income and middle-income countries.13 Pandemic lockdowns threaten lives and jeopardise progress that has been made in the past two decades in Nepal, potentially derailing on-track efforts to achieve the Sustainable Development Goals by 2030, especially for maternal and neonatal survival, and efforts to build stronger health systems after the pandemic.
Acknowledgments
Acknowledgments
This study was funded by Grand Challenges Canada, which is funded by the Government of Canada. We thank Honey Malla, Srijana Sharma, and Ankit Acharya for their support in research implementation. We thank the technical advisors to the SUSTAIN project, particularly Anjani Kumar Jha, Yasho Vardhan Pradhan, Sushil Nath Pyakurel, Kiran Bajracharya, Sheela Verma, Amit Bhandari, Anjana K C Thapa, Krishna Prasad Bista, Jhalak Sharma Paudel, and Leela Paudel. We thank our colleagues from Laerdal Medical and the Laerdal Global Health team for their support in the implementation of SUSTAIN. We also thank all the data site coordinators, data collectors, and mothers who participated in the study.
Contributors
AKC, RG, and JEL conceptualised the study. PP, PB, MPS, KS, and RG supervised the main study implementation. OB did the data cleaning and mining. AKC, MMå, MMo, and AKS did the data analysis. AKC, MVK, and RG prepared the first draft of the manuscript, with revisions by JEL. All authors reviewed and approved the final version of the manuscript.
Declaration of interests
We declare no competing interests.
Supplementary Materials
References
- 1.Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McMahon SA, Ho LS, Brown H, Miller L, Ansumana R, Kennedy CE. Healthcare providers on the frontlines: a qualitative investigation of the social and emotional impact of delivering health services during Sierra Leone's Ebola epidemic. Health Policy Plan. 2016;31:1232–1239. doi: 10.1093/heapol/czw055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Menendez C, Gonzalez R, Donnay F, Leke RGF. Avoiding indirect effects of COVID-19 on maternal and child health. Lancet Glob Health. 2020;8:e863–e864. doi: 10.1016/S2214-109X(20)30239-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boerma T, Requejo J, Victora CG, et al. Countdown to 2030: tracking progress towards universal coverage for reproductive, maternal, newborn, and child health. Lancet. 2018;391:1538–1548. doi: 10.1016/S0140-6736(18)30104-1. [DOI] [PubMed] [Google Scholar]
- 5.WHO. UNICEF. United Nations Population Fund. World Bank Group. United Nations Population Division . World Health Organization; Geneva: 2015. Trends in maternal mortality: 1990 to 2015.https://www.who.int/reproductivehealth/publications/monitoring/maternal-mortality-2015/en/ [Google Scholar]
- 6.Blencowe H, Cousens S, Jassir FB, et al. National, regional, and worldwide estimates of stillbirth rates in 2015, with trends from 2000: a systematic analysis. Lancet Glob Health. 2016;4:e98–e108. doi: 10.1016/S2214-109X(15)00275-2. [DOI] [PubMed] [Google Scholar]
- 7.UNICEF. WHO. World Bank. UN . United Nations International Children's Emergency Fund; New York, NY: 2019. Level and trend of child mortality 2019.https://www.unicef.org/reports/levels-and-trends-child-mortality-report-2019 [Google Scholar]
- 8.Riley T, Sully E, Ahmed Z, Biddlecom A. Estimates of the potential impact of the COVID-19 pandemic on sexual and reproductive health in low- and middle-income countries. Int Perspect Sex Reprod Health. 2020;46:73–76. doi: 10.1363/46e9020. [DOI] [PubMed] [Google Scholar]
- 9.Roberton T, Carter ED, Chou VB, et al. Early estimates of the indirect effects of the COVID-19 pandemic on maternal and child mortality in low-income and middle-income countries: a modelling study. Lancet Glob Health. 2020;8:e901–e908. doi: 10.1016/S2214-109X(20)30229-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO . World Health Organization; Geneva: 2020. COVID-19 guidance on maintaining essential health services and systems.https://www.who.int/westernpacific/emergencies/covid-19/technical-guidance/maintaining-essential-services-systems [Google Scholar]
- 11.Graham WJ, Afolabi B, Benova L, et al. Protecting hard-won gains for mothers and newborns in low-income and middle-income countries in the face of COVID-19: call for a service safety net. BMJ Glob Health. 2020;5 doi: 10.1136/bmjgh-2020-002754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delamou A, Ayadi AME, Sidibe S, et al. Effect of Ebola virus disease on maternal and child health services in Guinea: a retrospective observational cohort study. Lancet Glob Health. 2017;5:e448–e457. doi: 10.1016/S2214-109X(17)30078-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kc NP, Kc A, Sharma N, et al. Community participation and mobilization in community-based maternal, newborn and child health programmes in Nepal. J Nepal Health Res Counc. 2011;9:101–106. [PubMed] [Google Scholar]
- 14.Kc A, Jha AK, Shrestha MP, et al. Trends for neonatal deaths in Nepal (2001-2016) to project progress towards the SDG target in 2030, and risk factor analyses to focus action. Matern Child Health J. 2020;24(suppl 1):5–14. doi: 10.1007/s10995-019-02826-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ministry of Health and Population . Government of Nepal; Kathmandu: 2020. Coronavirus disease (COVID-19) outbreak updates & resource materials. Situation report 42.https://heoc.mohp.gov.np/update-on-novel-corona-virus-covid-19/ [Google Scholar]
- 16.Gurung R, Jha AK, Pyakurel S, et al. Scaling Up Safer Birth Bundle Through Quality Improvement in Nepal (SUSTAIN)—a stepped wedge cluster randomized controlled trial in public hospitals. Implement Sci. 2019;14:65. doi: 10.1186/s13012-019-0917-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO . World Health Organization; Geneva: 2016. Standards for improving quality of maternal and newborn care in health facilities.https://www.who.int/maternal_child_adolescent/documents/improving-maternal-newborn-care-quality/en/ [Google Scholar]
- 18.Subedi M. Caste system: theories and practices in Nepal. Himal J Sociol Anthropol. 2011;4:134–159. [Google Scholar]
- 19.Newey WK, West KDA. Simple, positive semi-definite, heteroskedasticity and autocorrelation consistent covariance matrix. Econometrica. 1987;55:703–708. [Google Scholar]
- 20.Iyengar P, Kerber K, Howe CJ, Dahn B. Services for mothers and newborns during the Ebola outbreak in Liberia: the need for improvement in emergencies. PLoS Curr. 2015;7:7. doi: 10.1371/currents.outbreaks.4ba318308719ac86fbef91f8e56cb66f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones S, Sam B, Bull F, et al. ‘Even when you are afraid, you stay’: provision of maternity care during the Ebola virus epidemic: a qualitative study. Midwifery. 2017;52:19–26. doi: 10.1016/j.midw.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 22.Målqvist M, Pun A, Raaijmakers H, Kc A. Persistent inequity in maternal health care utilization in Nepal despite impressive overall gains. Glob Health Action. 2017;10 doi: 10.1080/16549716.2017.1356083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thaddeus S, Maine D. Too far to walk: maternal mortality in context. Soc Sci Med. 1994;38:1091–1110. doi: 10.1016/0277-9536(94)90226-7. [DOI] [PubMed] [Google Scholar]
- 24.Shapiro GD, Fraser WD, Frasch MG, Séguin JR. Psychosocial stress in pregnancy and preterm birth: associations and mechanisms. J Perinat Med. 2013;41:631–645. doi: 10.1515/jpm-2012-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Himalayan News Service . The Himalayan Times; March 26, 2020. Protective gear shortage imperils health workers: coronavirus pandemic.https://thehimalayantimes.com/nepal/protective-gear-shortage-imperils-health-workers [Google Scholar]
- 26.Ministry of Health and Population . Government of Nepal; Kathmandu: 2020. Interim guidance for RMNCH services in COVID-19 pandemic.https://heoc.mohp.gov.np/update-on-novel-corona-virus-covid-19/ [Google Scholar]
- 27.WHO . World Health Organization; Geneva: 2018. WHO recommendations: intrapartum care for a positive childbirth experience.www.who.int/publications/i/item/9789241550215 [PubMed] [Google Scholar]
- 28.Arora KS, Mauch JT, Gibson KS. Labor and delivery visitor policies during the COVID-19 pandemic: balancing risks and benefits. JAMA. 2020;323:2468–2469. doi: 10.1001/jama.2020.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tomori C, Gribble K, Palmquist AEL, Ververs MT, Gross MS. When separation is not the answer: breastfeeding mothers and infants affected by COVID-19. Matern Child Nutr. 2020;2020 doi: 10.1111/mcn.13033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.WHO . World Health Organization; Geneva: May 7, 2020. Q&A: breastfeeding and COVID-19 for health care workers.www.who.int/news-room/q-a-detail/q-a-on-covid-19-and-breastfeeding [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.