Abstract
All organisms encounter abiotic stress but only certain organisms are able to cope with extreme conditions and enter into cryptobiosis (hidden life). Previously, we have shown that C. elegans dauer larvae can survive severe desiccation (anhydrobiosis), a specific form of cryptobiosis. Entry into anhydrobiosis is preceded by activation of a set of biochemical pathways by exposure to mild desiccation. This process called preconditioning induces elevation of trehalose, intrinsically disordered proteins, polyamines and some other pathways that allow the preservation of cellular functionality in the absence of water. Here, we demonstrate that another stress factor, high osmolarity, activates similar biochemical pathways. The larvae that acquired resistance to high osmotic pressure can also withstand desiccation. In addition, high osmolarity significantly increases the biosynthesis of glycerol making larva tolerant to freezing. Thus, to survive abiotic stress, C. elegans activates a combination of genetic and biochemical pathways that serve as a general survival program.
Subject terms: Biochemistry, Genetics, Physiology
Introduction
Organisms in nature encounter abiotic stress, defined as negative impact on living organisms of non-living factors (this does not include starvation), but only a few can survive conditions such as complete absence of water or oxygen, high temperature, freezing or extreme salinity. To achieve this, such organisms enter into a state known as anabiosis or cryptobiosis (hidden life), in which they reduce metabolism to an undetectable level1. Once conditions become favorable, they exit the cryptobiotic state and resume their reproductive life cycle. There are many spectacular examples where organisms enter a cryptobiotic state, among them are bacterial spores2, plant seeds3 and multicellular eukaryotes4–6. Recently, it was discovered that nematodes from the Siberian permafrost could be revived to life after dwelling up to 45,000 years in ice7. Organisms capable of entering cryptobiosis display dramatic differences to living creatures (absence of motility, metabolism and reproduction) but they are still alive, indicating they undergo a peculiar change in their cellular organization. Understanding the mechanisms by which the cryptobiotic state is induced and maintained could provide important insights on fundamental concepts of dead and alive.
Anhydrobiosis, the most common form of cryptobiosis, is observed in both prokaryotes8 and eukaryotes9–16. Here, organisms can lose up to 95% of their body water and suspend their metabolism. Previously, we demonstrated that dauer larvae of Caenorhabditis elegans is a true anhydrobiote and survives to harsh desiccation17. These larvae, which are formed in response to unfavorable environmental conditions such as scarcity of food or high population density, have a different metabolic signature than L3 larvae. This difference is manifested in reduced oxygen consumption, heat production and transition to gluconeogenic mode18.
To survive harsh desiccation dauer larvae need to be prepared (preconditioned) by exposure to mild desiccation17. Preconditioning, is a specific program that comprises extensive remodeling of transcriptome and proteome, affecting the activity of many regulatory and metabolic pathways19. Among these are elevation of a disaccharide trehalose, polyamine biosynthesis, fatty acid desaturation pathways and massive upregulation of an intrinsically disordered protein, LEA-1. Recently it was shown that similar to C. elegans dauer larvae, tardigrades upon preconditioning survive to extreme desiccation20. Preconditioning upregulates several intrinsically disordered proteins (IDP) essential for desiccation tolerance. These IDPs might protect them against severe desiccation by forming glass-like amorphous matrix21. Recent studies have demonstrated that IDPs mediate stress responses by their ability to undergo liquid–liquid phase separation (LLPS)22,23.
It remains unclear, whether these pathways are induced only by mild desiccation or other stress factors can induce them as well. Here, we show that elevated osmotic pressure induces similar pathways to preconditioning with mild desiccation and makes them resistant to harsh desiccation. Remarkably, in addition to previously described pathways, high osmotic pressure induces also upregulation of glycerol that enables dauer larvae to survive freezing. Our data indicate that dauer larvae possess a general program to survive different kinds of abiotic stress.
Results
Osmotic preconditioning enhances survival of C. elegans dauer larvae subjected to harsh desiccation
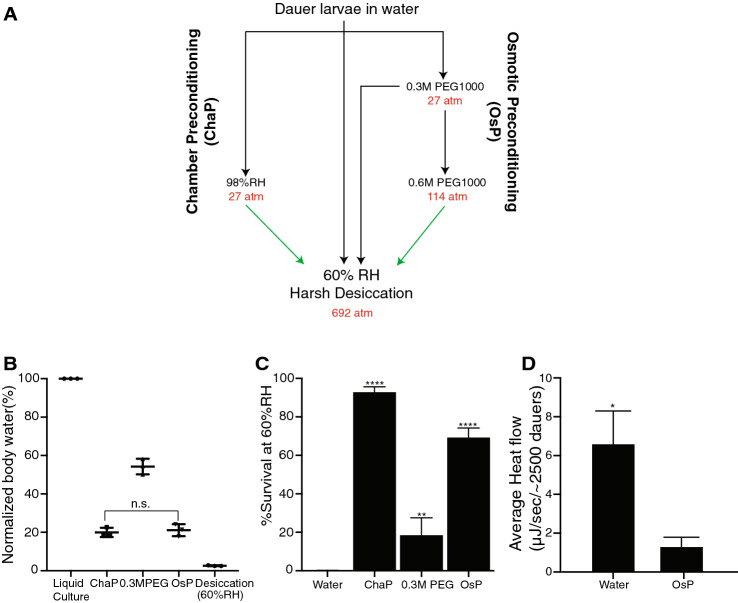
As mentioned earlier C. elegans dauer larva survives to harsh desiccation only when the larvae are preconditioned at mild desiccation (98% RH) for 4 days17. Here in, this preconditioning will be defined as chamber preconditioning (ChaP). We asked whether other stress factors like high osmolarity, can precondition the dauer larva to survive harsh desiccation. To obtain uniform dauer population of same age, we used dauer constitutive strain daf-2(e1370) that forms 100% dauer larvae at 25 °C24. As a starting point of our efforts to develop osmotic preconditioning (OsP), we decided to treat dauer larvae with osmotic pressure comparable to ChaP. Osmotic pressure of air at a given relative humidity was calculated using modified van’t Hoff’s equation and corresponds to 27 atm for 98% RH (Fig. 1A)17. Under these conditions, larvae lose about 80% of their body water (Fig. 1B). For OsP we applied the membrane impermeable polyethylene glycol (PEG1000). Using van’t Hoff’s equation and published experimental data, we determined that an osmotic pressure of 27 atm is achieved by 0.3 M PEG25,26. However, the water loss at this concentration of PEG is much lower than with ChaP (about 40%, Fig. 1B). The dauer larvae exposed to this concentration of PEG were sensitive to harsh desiccation (Fig. 1C), although the treatment induced high levels of trehalose (Supplementary Fig. S1A) which is essential for desiccation tolerance. We reasoned that in addition to elevated trehalose, in order to be effective, a preconditioning procedure should deplete more water. This was achieved by subsequent exposure to 0.6 M PEG. Thus, osmotic preconditioning (OsP) (Fig. 1A) can be achieved using a two-step treatment with PEG. It should be mentioned that in our preliminary experiments, the larvae directly exposed to 0.6 M PEG showed negligible survival and thus in the following only two-step procedure was used. The amount of water loss in dauer larvae upon OsP was indeed similar to amounts in ChaP treated dauer larvae (Fig. 1B) and furthermore the survival in response to harsh desiccation was significantly increased (Fig. 1C). Resistance to desiccation via osmotic preconditioning is not only specific to daf-2(1370) dauer larvae but also to wild type (N2) dauer larvae (Supplementary Fig. S4A, B).
Figure 1.
Osmotic preconditioning renders C. elegans dauers resistant to harsh desiccation. (A) Schematic diagram illustrating exposure of osmotic and chamber preconditioned dauers to harsh desiccation. (B) Osmotic and chamber preconditioned daf-2(e1370) dauers lose similar amounts of water. Error bars indicate standard deviation of three biological replicates. Unpaired two-tailed t test with Welch correction, n.s. p > 0.05. (C) Osmotic preconditioning enhances survival of daf-2(e1370) dauers to harsh desiccation. For water n = 2,758, ChaP n = 2,588, 0.3 M PEG1000 n = 2,488, OsP n = 4,113. Error bars indicate standard deviation of three independent experiments with two technical replicates. Statistical comparison was performed with one-way ANOVA with Dunnett’s multiple comparisons test. **p < 0.01, ****p < 0.0001. (D) Osmotic preconditioned daf-2(e1370) dauers have reduced heat flow (heat dissipation per unit time) in contrast to non-preconditioned dauers. Error bars indicate standard deviation of three biological replicates with four technical replicates performed on two different days. Statistical comparison was performed with unpaired two-tailed t test with Welch correction. *p < 0.05.
Cryptobiosis is associated with cessation of the metabolism. Measurement of the true caloric output of an organism during cryptobiosis is difficult27. Calorimetry of a dauer larvae experiencing ChaP or harsh desiccation is not possible due to inability to maintain a constant relative humidity during the measurement. In particular, calorimetric vials are not equipped to contain hygroscopic medium such as sodium hydroxide or calcium chloride, thus constant relative humidity cannot be maintained due to change in vapor equilibrium28. OsP occurs in a fluid medium and therefore provides an opportunity to estimate the metabolic rate of preconditioned dauer larvae. As a proxy of metabolic rate, heat flow was used29 to estimate the average metabolic rate of OsP-treated dauer larvae. Heat flow was significantly decreased by OsP treatment compared to non-preconditioned larvae (approximately 16% of non-preconditioned larvae, Fig. 1D). These results indicate that dauer larvae subjected to OsP reduces metabolism to low levels.
Osmotic preconditioning and chamber preconditioning induce similar biochemical pathways
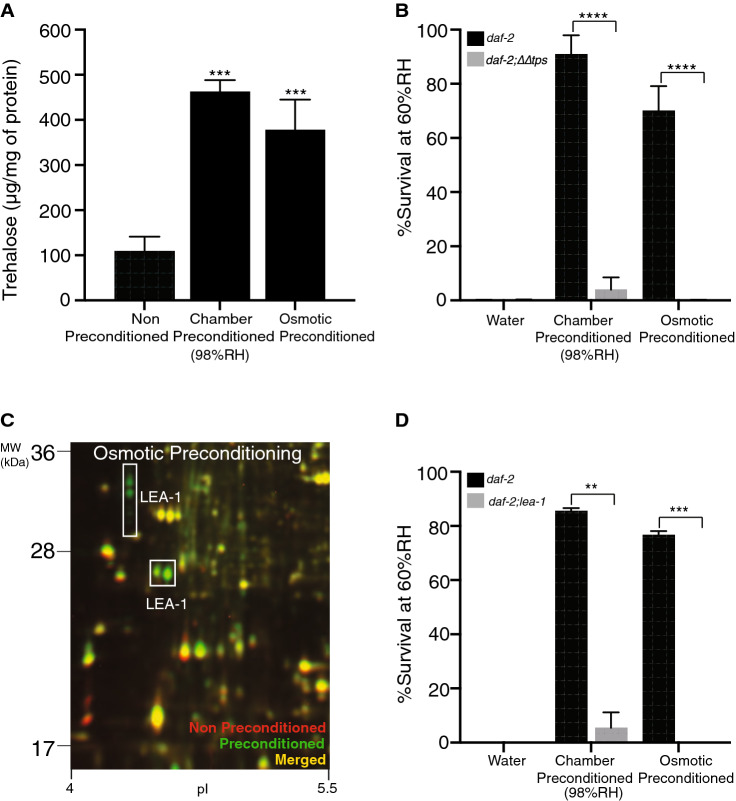
ChaP induces several biochemical pathways that are essential for the desiccation tolerance, including elevated biosynthesis of trehalose and an intrinsically disordered protein LEA-119. We therefore asked whether OsP also affects these pathways. As shown in Fig. 2A, OsP of larvae induces up to 3.4-fold enhancement of trehalose levels. Moreover, similar to ChaP, the mutants deficient in trehalose biosynthesis pathway (daf-2;ΔΔtps)17 do not survive desiccation when preconditioned by OsP (Fig. 2B).
Figure 2.
Trehalose and LEA-1 are upregulated upon osmotic preconditioning in C. elegans dauers. (A) Osmotic preconditioning induces elevation of trehalose levels in dauers. Error bars indicate standard deviation of three independent experiments with three technical replicates. Statistical comparison was performed with one-way ANOVA with Dunnett’s multiple comparisons test. ****p < 0.0001. (B) Trehalose elevation upon osmotic preconditioning is essential for desiccation tolerance in dauers. Survival rates of ChaP and OsP daf-2(e1370), daf-2;ΔΔ tps dauer larvae to harsh desiccation. For daf-2(e1370) water n = 1,912, ChaP n = 2,027, OsP n = 4,334, daf-2;ΔΔ tps water n = 1,017, ChaP n = 1,401, OsP n = 1,839. Error bars indicate standard deviation of two independent experiments with two technical replicates. Statistical comparison was performed with unpaired t-test using Holm–Sidak method. ****p < 0.0001. (C) Comparison of proteomic changes in daf-2(e1370) dauers upon osmotic preconditioning. Overlay of false-colored 2D-DIGE images comparing dauer proteomes before (red) and after (green) osmotic preconditioning. Representative portion of the proteome is shown. (D) LEA-1 upregulation upon osmotic preconditioning is essential for desiccation tolerance. Survival rates of ChaP and OsP daf-2(e1370), daf-2; lea-1(tag1676) dauer larvae to harsh desiccation. For daf-2(e1370) water n = 1,192, ChaP n = 1,581, OsP n = 5,347, daf-2; lea-1(tag1676) water n = 521, ChaP n = 1,060, OsP n = 1,448. Error bars indicate standard deviation of two independent experiments with two technical replicates. Statistical comparison was performed with unpaired t test using Holm–Sidak method. **p < 0.01, ***p < 0.001. All the experiments were performed in the daf-2(e1370) background unless otherwise stated.
Several isoforms of LEA-1 were strongly expressed upon OsP treatment (Fig. 2C).
Previously, we reported that lea-1(RNAi) renders dauer larvae very sensitive to desiccation19. Similarly, a mutant bearing a complete deletion of lea-1 displayed very poor survival upon desiccation following both ChaP and OsP (Fig. 2D). These results demonstrate that similar to ChaP, OsP induces elevation of trehalose biosynthesis and LEA-1 in dauer larvae and that these are essential for survival under harsh desiccation. Thus, similar pathways seem to mediate the effect of different preconditioning protocols on survival following desiccation.
In addition to trehalose elevation, osmotic preconditioning induces glycerol biosynthesis
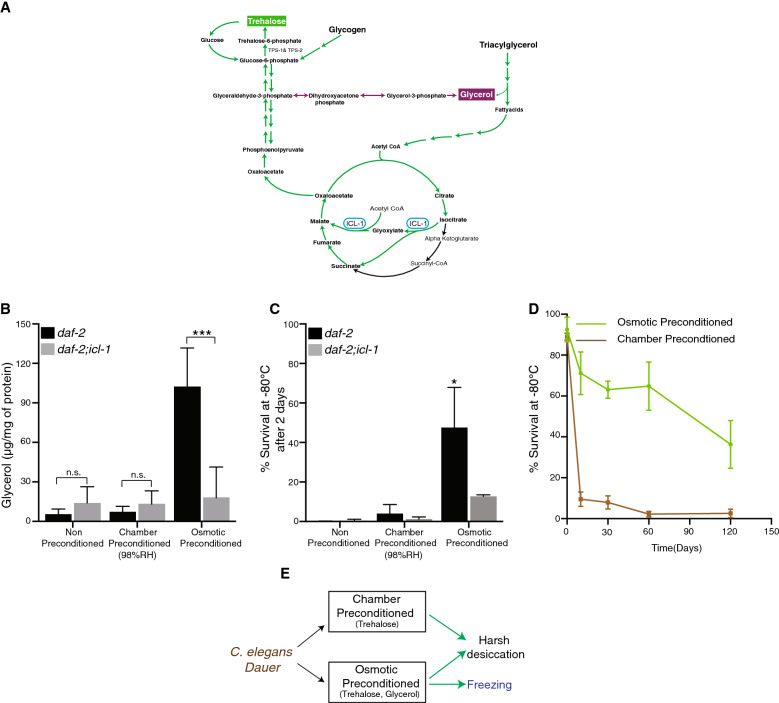
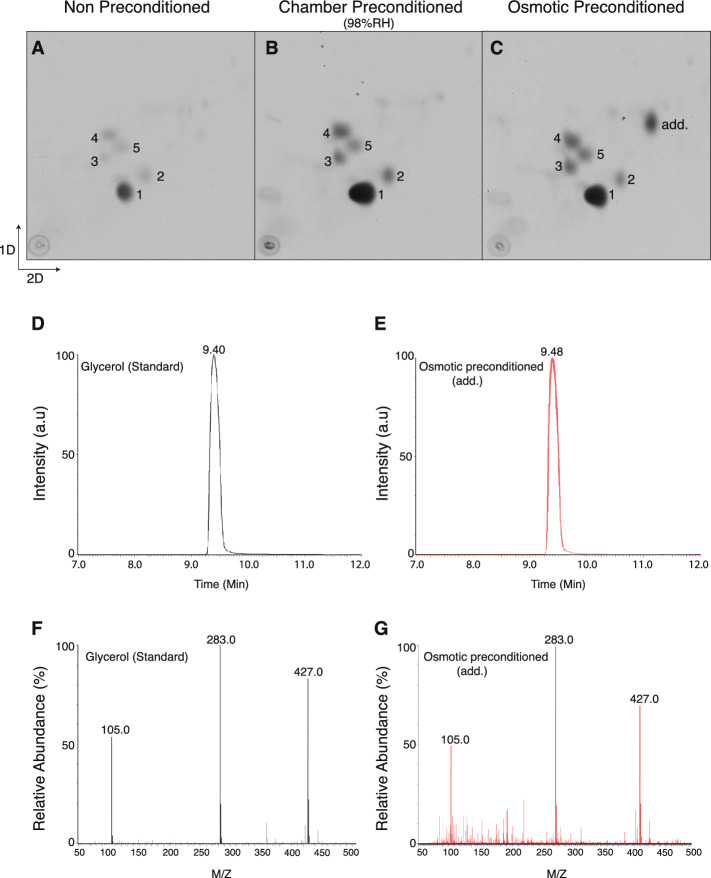
We previously showed that a major source of elevated trehalose during ChaP is acetyl-CoA, which is derived from the beta-oxidation of fatty acids18. Acetyl-CoA is diverted from the TCA (tricarboxylic acid cycle) via glyoxylate shunt and is used for gluconeogenesis, which culminates in trehalose synthesis (Fig. 4A). We therefore asked whether OsP treated dauer larvae display a similar metabolic trait. To address this question, we radioactively labelled metabolites derived from 14C-acetate. As shown by 2D-high performance thin layer chromatography (HPTLC), the most abundant radioactive labelled metabolite extracted was trehalose (Fig. 3A). Smaller amounts of glucose, glutamate, glutamine and alanine were also observed (Fig. 3A). ChaP increased trehalose levels (Fig. 3B) drastically, and the levels of glutamate, glutamine and alanine were also elevated, as reported previously18. OsP induced similar changes (Fig. 3C), although we observed an additional spot (add.) that was undetectable in non-preconditioned or under ChaP (Fig. 3C, add.) dauer larvae. This spot was also detected in TLC of non-radioactive samples upon OsP but not ChaP, suggesting that the corresponding compound is produced in large amounts (Supplementary Fig. S2C). This spot had similar mobility to glycerol on 2D-HPTLC (Supplementary Fig. S2C). We further resolved the spot on HPLC (Fig. 3D,E) and using mass spectrometry we confirmed the identity of this spot as glycerol (Fig. 3F,G).
Figure 4.
Glycerol upregulation upon osmotic preconditioning is essential for freezing tolerance. (A) Schematic diagram illustrating the gluconeogenic mode of dauer larvae. (B) Dauer larvae utilize glyoxylate shunt for elevation of glycerol upon osmotic preconditioning. Error bars indicate standard deviation from three independent experiments with three technical replicates. Statistical comparison was performed with unpaired t test with Welch correction. n.s. p > 0.05, *p < 0.05. (C) Glyoxylate shunt is essential for osmotic preconditioned dauer larvae to survive freezing. Survival rates of daf-2(1370), daf-2; icl-1(ok 531) dauer larvae to freezing. For daf-2(e1370) water n = 783, ChaP n = 1,331, OsP n = 1,802, daf-2; icl-1(ok 531) water n = 937, ChaP n = 2,082, OsP n = 2,684. Error bars indicate standard deviation of two biological replicates with two technical replicates. Statistical comparison was performed with two-way ANOVA with Sidak’s multiple comparisons test. *p < 0.05. (D) Osmotic preconditioned daf-2(e1370) dauer larvae survive to freezing for longer periods. For OsP n = 17,305, ChaP n = 7,763. Error bars indicate standard error of mean of three biological replicates with two technical replicates. (E) Schematic diagram illustrating that osmotic preconditioning enhances survival of C. elegans dauer larvae to desiccation and freezing.
Figure 3.
Osmotic preconditioning induces elevation of glycerol biosynthesis. (A–C) 2D-thin layer chromatography of 14C-acetate labelled metabolites from dauers that were non-preconditioned, chamber and osmotic preconditioned respectively. Enumerated spots indicate trehalose (1), glucose (2), glutamate (3), glutamine (4), and alanine (5). Representative images from at least two independent experiments performed on two different days. (D,E) Chromatogram of glycerol standard and spot add. scrapped out from osmotic preconditioned 2D-TLC. (F,G) Mass spectrum of glycerol standard and spot add. from osmotic preconditioned sample.
Osmotic preconditioning induced glycerol is essential for freezing tolerance
Measurement of glycerol levels during both types of preconditioning confirmed that OsP leads to an approximate 15-fold increase in glycerol compared to ChaP (Fig. 4B). Additionally, OsP upregulated glycerol levels in wild type (N2) dauer larvae (Supplementary Fig. S4D), indicating that the elevation is independent of daf-2(e1370) background. Since trehalose is derived from fatty acids via glyoxylate shunt (Fig. 4A), we asked whether the elevation of glycerol also depends on this pathway. To address this question, we quantified glycerol levels in dauer larvae from an icl-1(ok531) deletion mutant, which lacks a functional isocitrate lyase (ICL-1). This deletion leads to a frame-shit mutation that results in a non-functional glyoxylate shunt. As shown in Fig. 4B, compared to daf-2(e1370) dauers, daf-2;icl-1 dauers have significantly reduced amounts of glycerol. These results indicate that glycerol is also derived from acetyl-CoA, and that a functional glyoxylate shunt is instrumental in elevation of not only of trehalose and further survival at harsh desiccation (Supplementary Fig. S3A, S3B), but also for glycerol upon osmotic preconditioning.
Glycerol is widely used as a cryoprotectant in cells or organs30. We asked whether OsP induced glycerol elevation brings a survival advantage for dauer larvae upon freezing. Indeed, OsP treated dauer larvae when exposed directly to -80 °C for a brief period (2 days) survive significantly higher than non-preconditioned or ChaP dauer larvae (Fig. 4C). OsP treated wild type (N2) dauer larvae displayed a similar survival rate (Supplementary Fig. S4C). Contrarily, daf-2;icl-1 dauer larvae, which do not elevate glycerol upon OsP, displayed a significantly decreased survival rate upon freezing (Fig. 4C). We further asked whether OsP treated dauer larvae can survive to freezing for longer periods. As shown in Fig. 4D, a significant proportion of OsP treated larvae survive to freezing for longer periods up to 120 days in contrast to ChaP larvae. Taken together, these results suggest that metabolic adaptation during preconditioning can promote survival of dauer larvae under different abiotic stress conditions.
Discussion
Extreme environmental conditions present a constant threat to living organisms. In order to survive such extreme conditions, organisms need to have a general stress response program that activates similar pathways. It will be disadvantageous for them to activate specific pathways for withstanding each kind of stress they encounter. This would be demanding from aspects of energy consumption as well as of regulating the response. Thus, comprising a common survival program to resist various kinds of stress could provide an organism with significant advantage. In our work, we demonstrate that C. elegans dauer larvae utilize a combination of genetic and biochemical pathways that serves as a general program to enter into cryptobiosis. In particular, different stress factors such as decreased relative humidity or elevated osmolarity (Fig. 4E), can induce similar mechanisms (e.g. biosynthesis of trehalose, various isoforms of LEA-1) that enables the survival under diverse extreme conditions (desiccation, high osmolarity and freezing). We previously reported that TPS-1 and TPS-2 enzymes that catalyze the biosynthesis of trehalose are strongly regulated by insulin signaling via the FoxO transcription factor DAF-16 in the dauer larvae31. It was shown that osmotic stress induces DAF-16 dependent trehalose elevation in adult C. elegans29. Thus, DAF-16 might be a key regulator for trehalose elevation observed in dauer larvae upon ChaP and OsP treatment. In addition to trehalose elevation, ChaP and OsP also induces similar water loss in dauer larvae indicating most of the bulk water (intracellular and extracellular) is lost in similar fashion in both approaches.
Despite similarities in response to ChaP and OsP, we observed one pronounced difference: OsP leads to accumulation of glycerol. Interestingly, biosynthesis of glycerol similar to trehalose also occurs via the glyoxylate shunt and gluconeogenesis. The diverging point should be glyceraldehyde-3-phosphate (Fig. 4A). This provides the dauer larvae with an additional advantage to ChaP, namely the ability to survive freezing (Fig. 4E). Ice crystal formation is one of the factors that results in lethality upon freezing. Several strategies have been proposed to prevent the damage generated due to freezing, one of them is accumulation of polyhydroxy alcohols32. With 20% of body water (Fig. 1B), glycerol accumulated upon osmotic preconditioning confers freezing tolerance by preventing ice crystal formation. The synthesis of glycerol from glyceraldehyde-3-phosphate is catalysed by GPDH-1 and GDPH-233, the expression of these enzymes should be differently regulated upon OsP and ChaP. The dissimilarities in response to ChaP and OsP might be due to differences in subset of neurons that modulate the water loss response. TRP channels that mediate hygrosensation34 (changes in the humidity levels) might be specifically activated in response to ChaP, whereas tyraminergic neurons might mediate the response to OsP35. Future studies could address how these neurons activate differential biochemical response in ChaP and OsP.
OsP provided us a rare opportunity to demonstrate that the metabolic rate of an organism is significantly reduced while the anhydrobiotic program is initiated. As OsP occurs in fluid medium, it had a technical advantage over the conventional ChaP approach for measuring caloric output. It was hypothesized that the anhydrobiotic capacity of the dauer larvae is attributable to their hypometabolic state36. Under normal conditions, it would be extremely challenging for an organism to reduce its metabolic rate from an active state to undetectable levels. Dauer larvae in hypometabolic state possess an advantage, as it is thermodynamically more feasible to reduce their metabolism from hypometabolic to undetectable level. For the first time, these microcalorimetry experiments demonstrate the ability of the dauer larvae to transit from a non-preconditioned to anhydrobiotic state. This process is aided by a significant reduction in metabolic rate to promote survival during extreme conditions. Future studies could address how dauer metabolic rate is regulated by the central regulators, such as insulin, steroid hormone, and AMP-activated protein kinase signaling37. These pathways are known to reduce the metabolic rate and the transition from TCA cycle to gluconeogenesis at basal dauer state and thus may also modulate metabolism during preconditioning.
In summary, we report that C. elegans dauer larvae possess a program that is activated by osmotic pressure or mild desiccation enabling dauer larvae to survive various kinds of abiotic stress. Further understanding of how this program operates at a mechanistic level can provide insights into engineering novel preservative techniques for cells and tissues.
Methods
Materials and C. elegans strains
[1-14C]-acetate (sodium salt) was purchased from Hartmann Analytic (Braunschweig, Germany). All other chemicals were purchased from Sigma-Aldrich (Taufkirchen, Germany). The Caenorhabditis Genetic Centre (CGC) provided the C. elegans strain wild type (N2), daf-2(e1370) and the E. coli strain NA22. The compound mutant strains of daf-2(e1370)III;lea-1(tag1676)V, tps-2(ok526)II;daf-2(e1370)III;tps-1(ok373)X(daf-2;ΔΔtps), daf-2(e1370)III;icl-1(ok531)V were generated during this or previous studies as published18,38.
Osmotic preconditioning and desiccation survival assay
For osmotic preconditioning, dauer larvae were collected from complete S basal medium or NGM agar plates in 15 ml tubes. They were washed for at least 3–4 times with water to remove all the debris and distributed into 15 ml tubes. 7.5 ml of freshly prepared 0.3 M PEG1000 was added to dauer pellet and incubated on a horizontal shaker at 25 °C. After 18 h of incubation, dauers were centrifuged at 800g for 3 min and the supernatant was removed. 7.5 ml of 0.6 M PEG1000 was added to the dauer pellet and incubated on a horizontal shaker at 25 °C for 2–3 h. Dauer larvae treated with water were used as a control for the experiments. In order to remove any reminiscent PEG1000 adhered to the dauers, they were transferred to Isopore TETP membranes (8 μm pore size, Millipore, USA) washed under suction at least 5–6 times with water. Chamber preconditioning was performed according to previous protocol17. After osmotic and chamber preconditioning the dishes were transferred to harsh desiccation (60% RH) chamber for 1 day. After one day of harsh desiccation they were rehydrated with 500 μl of distilled water for 2–3 h. They were transferred to agar plates with food and kept at 15 °C for overnight. The following day, survivors and total number of worms in each plate were counted. Survival rate of each condition was calculated as the percentage of survivors in that plate. All the survival assays were performed on different days with at least two technical replicates.
Water loss and trehalose measurement
Water loss and trehalose measurements were performed according to previous report17.
Isothermal microcalorimetry measurements
To measure the heat production of dauer larvae during osmotic preconditioning with PEG1000, daf-2 dauers were collected from liquid culture and washed 3–4 times to remove bacteria and debris. After 18 h incubation in 0.3 M PEG1000, dauers were centrifuged at 800g for 3 min and the supernatant was removed and 0.6 M PEG1000 was added. Around 2,500 dauers in a volume of 200 μl per each condition were pipetted into a 4 ml glass ampoule (TA Instruments, New Castle, DE, USA) and these ampoules were then sealed with aluminum caps equipped with sealing discs (TA Instruments, New Castle, DE, USA). Isothermal calorimetric measurements were performed with a TAMIII (Thermal Activity Monitor) instrument (Waters GmbH, Eschborn, Germany) equipped with 12 microcalorimeters in twin configuration (one side for the sample the other for a steel reference) to continuously monitor the metabolic heat produced by dauers at 25 °C for up to 120–200 min. The samples were held in the TAM III in a waiting position for 15 min before complete insertion followed by 45 min equilibration. Thermograms were recorded at least in three biological replicates with four technical replicates. Mean metabolic rate was calculated as an average of the first 20 min of measurement and it represents the summation of heat flow of approximately 2,500 dauers per condition.
Radiolabeling of C. elegans dauer, metabolite extraction and 2D-TLC
The above-mentioned procedures were performed according to previous report19.
Measurement of glycerol in lysates and its identification from TLC plate
Glycerol measurements were performed using Glycerol assay kit (Sigma-Aldrich Chemie GmbH, Germany). The dauer samples per condition were immediately collected in 1 ml distilled water, flash frozen in liquid nitrogen, and stored at − 80 °C. They were homogenized by freezing in liquid nitrogen and subsequent thawing in a sonication bath, this cycle of freeze thawing was repeated 5 times. The debris was pelleted by centrifugation at 25,000g for 1 min at 4 °C. 30 μl of the supernatant was used for determining soluble protein amounts using a micro BCA assay kit (Thermo Fisher Scientific, Germany). All the reagents of the Glycerol assay kit except the enzyme mix were brought to room temperature. The protocol was optimized for dauer lysate by diluting them to 1:10 and incubating the plates for 25 min. Glycerol concentration in the lysates was calculated based on the absorbance (570 nm) in the samples and normalized to soluble protein amounts.
Aqueous fractions from the osmotic preconditioned sample was separated by high performance thin layer chromatography (HPTLC), using 1-propanol-methanol-ammonia (32%)-water (28:8:7:7 v/v/v/v) as first, dried for 15 min and 1-butanol-acetone-glacial acetic acid–water (35:35:7:23 v/v/v/v) second dimension respectively. Glycerol standard was run on both dimensions, only the region where the standards run was stained with 0.5% potassium permanganate in 1 N sodium hydroxide39 and the intersection region was scraped out from the TLC plate. The scraped-out silica was extracted with 10 ml of 50% methanol twice. The fractions were combined, dried under vacuum and dissolved in 100 μl of water.
For derivatization, 850 μl of 4 N sodium hydroxide, 500 μl of hexane, 100 μl of benzoyl chloride was added to 100 μl concentrated fraction in a 10 ml glass tube. The mixture was incubated at 40 °C for 4 h. 1 ml of water was added to this mixture and centrifuged at 2,500g for 2 min. The upper phase (organic phase) was transferred to a glass tube, dried under vacuum and dissolved in 100 μl water. The upper phase (organic phase) was filtered through the PTFE filter (GE healthcare) to remove particle debris. The filtered organic phase was subjected to LC–MS for separation purposes. 20 μl of the filtered organic phase was separated on Synergi 2.5 µm Fusion-RP 100 Ȧ LC Column 100 × 2 mm (Phenomenex). The mobile phase was composed of 0.01% Formic acid in water (eluent A) and 0.01% Formic acid in acetonitrile (eluent B). The following gradient program was used. Eluent B: from 50 to 100% within 12 min; 100% from 12 to 17 min; equilibration of 50% from 17 to 25 min. The flow rate was set at 0.3 ml/min. Mass spectrometry detection was carried out using G2-S QTof with electro spray ionization (ESI). MSe mode was used to detect the analytes in positive ionization mode. The spray voltage was set at 300 V and the source temperature was operated at 120 °C. Desolvation gas flow was set to 800 l/h with 450 °C. Sodium adduct of glycerol was detected in positive mode. Exact monoisotopic mass was 427.11521 and mass error in parts per million (ppm) was 4.0.
Freezing survival assay
Dauer larvae were collected from liquid culture and washed few times with water to remove debris and then resuspended in water. Non-preconditioned and osmotic preconditioned dauers were transferred to Isopore TETP membranes (8 μm pore size, Millipore, USA) washed under suction at least 5–6 times (minimum) with water. The membrane was immediately transferred to a 35 mm plastic petri dish, the lid was closed, wrapped with parafilm and transferred to − 80 °C. Several replicates were prepared per each condition at the beginning of the experiment and for each experimental time point two technical replicates were thawed. At an experimental time point the petri dishes were removed from − 80 °C, thawed for 5–10 min at room temperature and 500 μl of water was added to allow the worms to rehydrate for 2–3 h. Rehydrated dauers were transferred to NGM agar plates with food and allowed to recover. Survival rate of each condition was calculated as the percentage of survivors in that plate. All the survival assays were performed on different days with at least two technical replicates.
Detection of LEA-1 and generation of daf-2; lea-1
For detection of proteome changes, including upregulation of LEA-1 during preconditioning, 2D-DIGE was performed as previously described in19. daf-2;lea-1 strain was generated in the following way. Fifteen μl of master mix (AAATGAGAAGCCGATTGCGG) crRNA-IDT-sgl (5 μM), (CTGATAGTAAATATAGTTGG) crRNA-IDT-sg6 (5 μM), CAS9-Protein NLS (12.5 μM), tracerRNA-IDT (12.5 μM), dpy10 Oligo-IDT (733 nM), dpy-10-sgRNA-IDT (2.5 μM), 5 μl protein buffer stock (3 ×) was injected in young adults of N2 strain. Progeny of the rescued strains were genotyped for homozygous deletion with PCR primers for three generations. One of the several lines obtained was randomly selected and outcrossed twice with wild type strain. The outcrossed strain was crossed with daf-2(e1370) strain, daf-2(e1370) homozygosity was confirmed by 100% dauer formation and lea-1(tag1676) deletion was confirmed by PCR genotyping.
Supplementary information
Acknowledgements
We thank all the current and former members of the Kurzchalia lab for helpful discussions. V.R.G. is indebted to Sider Penkov for discussions and critical reading of the manuscript. We are thankful to Mihail Sarov and Dana Olbert from the TransgenOmics facility for generating the lea-1 Crispr mutant. We thank Jenny Philipp for technical support. We are grateful to the Caenorhabditis Genetics Centre (CGC) for providing the strains. Open access funding provided by Projekt DEAL.
Author contributions
V.R.G. and T.V.K. conceived and designed the experiments. V.R.G. performed survival assays, biochemistry experiments, water loss measurements and generated the strains. S.T. performed. HPLC and mass spectrometry for detection of glycerol. Isothermal calorimetry experiments were designed with the help of J.O. and K.F. J.O. performed the measurements. V.R.G. and T.V.K. wrote the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-70311-8.
References
- 1.Keilin D. The Leeuwenhoek lecture: the problem of anabiosis or latent life: history and current concept. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1959;150:149–191. doi: 10.1098/rspb.1959.0013. [DOI] [PubMed] [Google Scholar]
- 2.Cano RJ, Borucki MK. Revival and identification of bacterial spores in 25 to 40 million-year-old Domini, an amber. Science. 1995;268:1060–1064. doi: 10.1126/science.7538699. [DOI] [PubMed] [Google Scholar]
- 3.Shen-Miller J, Mudgett MB, Schopf JW, Clarke S, Berger R. Exceptional seed longevity and robust growth: ancient sacred lotus from China. Am. J. Bot. 1995;82:1367–1380. doi: 10.1002/j.1537-2197.1995.tb12673.x. [DOI] [Google Scholar]
- 4.Storey KB. Freeze tolerance in the frog, Rana sylvatica. Experientia. 1983;40:1261–1262. doi: 10.1007/BF01946664. [DOI] [Google Scholar]
- 5.Ramlov H, Westh P. Survival of the cryptobiotic eutardigrade Adorybiotus coronifer during cooling to 196 C: effect of cooling rate, trehalose level, and short-term acclimation. Cryobiology. 1992;29:125–130. doi: 10.1016/0011-2240(92)90012-Q. [DOI] [Google Scholar]
- 6.Aroian RV, Carta L, Kaloshian I, Sternberg PW. A free-living Panagrolaimus sp. from Armenia can survive in anhydrobiosis for 8.7 years. J. Nematol. 1993;25:500–502. [PMC free article] [PubMed] [Google Scholar]
- 7.Shatilovich AV, et al. Viable nematodes from Late Pleistocene permafrost of the Kolyma River Lowland. Dokl. Biol. Sci. 2018;480:100–102. doi: 10.1134/S0012496618030079. [DOI] [PubMed] [Google Scholar]
- 8.García AH. Anhydrobiosis in bacteria: from physiology to applications. J. Biosci. 2011;36:939–950. doi: 10.1007/s12038-011-9107-0. [DOI] [PubMed] [Google Scholar]
- 9.Calahan D, Dunham M, DeSevo C, Koshland DE. Genetic analysis of desiccation tolerance in Saccharomycescerevisiae. Genetics. 2011;189:507–519. doi: 10.1534/genetics.111.130369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tunnacliffe A, Lapinski J, McGee B. A putative LEA protein, but no trehalose, is present in anhydrobiotic bdelloid rotifers. Hydrobiologia. 2005;546:315–321. doi: 10.1007/s10750-005-4239-6. [DOI] [Google Scholar]
- 11.Wełnicz W, Grohme MA, Kaczmarek Ł, Schill RO, Frohme M. Anhydrobiosis in tardigrades—the last decade. J. Insect Physiol. 2011;57:577–583. doi: 10.1016/j.jinsphys.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 12.Kranner I, Beckett R, Hochman A, Nash TH., III Desiccation-tolerance in lichens: a review. Bryologist. 2008;111:576–593. doi: 10.1639/0007-2745-111.4.576. [DOI] [Google Scholar]
- 13.Rascio N, Rocca NL. Resurrection plants: the puzzle of surviving extreme vegetative desiccation. Crit. Rev. Plant Sci. 2005;24:209–225. doi: 10.1080/07352680591008583. [DOI] [Google Scholar]
- 14.Clegg JS. Desiccation tolerance in encysted embryos of the animal extremophile, Artemia. Integr. Comp. Biol. 2005;45:715–724. doi: 10.1093/icb/45.5.715. [DOI] [PubMed] [Google Scholar]
- 15.Perry RN. Desiccation survival of parasitic nematodes. Parasitology. 1999;119:S19–30. doi: 10.1017/S0031182000084626. [DOI] [PubMed] [Google Scholar]
- 16.Cornette R, Kikawada T. The induction of anhydrobiosis in the sleeping chironomid: current status of our knowledge. IUBMB Life. 2011;63:419–429. doi: 10.1002/iub.463. [DOI] [PubMed] [Google Scholar]
- 17.Erkut C, et al. Trehalose renders the dauer larva of Caenorhabditiselegans resistant to extreme desiccation. Curr. Biol. 2011;21:1331–1336. doi: 10.1016/j.cub.2011.06.064. [DOI] [PubMed] [Google Scholar]
- 18.Erkut C, Gade VR, Laxman S, Kurzchalia TV. The glyoxylate shunt is essential for desiccation tolerance in C. elegans and budding yeast. eLife. 2016;5:12897. doi: 10.7554/eLife.13614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erkut C, et al. Molecular strategies of the Caenorhabditiselegans dauer larva to survive extreme desiccation. PLoS ONE. 2013;8:e82473. doi: 10.1371/journal.pone.0082473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boothby TC, et al. Tardigrades use intrinsically disordered proteins to survive desiccation. Mol. Cell. 2017;65:975–984.e5. doi: 10.1016/j.molcel.2017.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boothby TC, Pielak GJ. Intrinsically disordered proteins and desiccation tolerance: elucidating functional and mechanistic underpinnings of anhydrobiosis. BioEssays. 2017;27:1700119–1700124. doi: 10.1002/bies.201700119. [DOI] [PubMed] [Google Scholar]
- 22.Jo Y, Jung Y. Interplay between intrinsically disordered proteins inside membraneless protein liquid droplets. Chem. Sci. 2020;11:1269–1275. doi: 10.1039/C9SC03191J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franzmann TM, et al. Phase separation of a yeast prion protein promotes cellular fitness. Science. 2018;359:eaa05654. doi: 10.1126/science.aao5654. [DOI] [PubMed] [Google Scholar]
- 24.Gems D, et al. Two pleiotropic classes of daf-2 mutation affect larval arrest, adult behavior, reproduction and longevity in Caenorhabditis elegans. Genetics. 1998;150:129–155. doi: 10.1093/genetics/150.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coleman JJ, Germann FEE. The theory of moderate deviations from van’t Hoff’s Law. J. Chem. Phys. 1934;2:396–399. doi: 10.1063/1.1749495. [DOI] [Google Scholar]
- 26.Money NP. Osmotic pressure of aqueous polyethylene glycols: relationship between molecular weight and vapor pressure deficit. Plant Physiol. 1989;91:766–769. doi: 10.1104/pp.91.2.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clegg JS. Cryptobiosis: a peculiar state of biological organization. Comp. Biochem. Physiol. B. 2001;128:613–624. doi: 10.1016/S1096-4959(01)00300-1. [DOI] [PubMed] [Google Scholar]
- 28.Robinson RHSRA. Standard solutions for humidity control at 25 °C. Ind. Eng. Chem. 1949;41:2013–2013. doi: 10.1021/ie50478a038. [DOI] [Google Scholar]
- 29.Braeckman BP, Houthoofd K, Vanfleteren JR. Assessing metabolic activity in aging Caenorhabditiselegans: concepts and controversies. Aging Cell. 2002;1:82–88. doi: 10.1046/j.1474-9728.2002.00021.x. [DOI] [PubMed] [Google Scholar]
- 30.Mazur P, Miller RH. Survival of frozen-thawed human red cells as a function of the permeation of glycerol and sucrose. Cryobiology. 1976;13:523–536. doi: 10.1016/0011-2240(76)90145-0. [DOI] [PubMed] [Google Scholar]
- 31.Penkov S, et al. Integration of carbohydrate metabolism and redox state controls dauer larva formation in Caenorhabditiselegans. Nat. Commun. 2015;6:1–10. doi: 10.1038/ncomms9060. [DOI] [PubMed] [Google Scholar]
- 32.Yancey PH. Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J. Exp. Biol. 2005;208:2819–2830. doi: 10.1242/jeb.01730. [DOI] [PubMed] [Google Scholar]
- 33.Lamitina ST. Adaptation of the nematode Caenorhabditiselegans to extreme osmotic stress. AJP Cell Physiol. 2004;286:785–791. doi: 10.1152/ajpcell.00381.2003. [DOI] [PubMed] [Google Scholar]
- 34.Russell J, Vidal-Gadea AG, Makay A, Lanam C, Pierce-Shimomura JT. Humidity sensation requires both mechanosensory and thermosensory pathways in Caenorhabditiselegans. Proc. Natl. Acad. Sci. 2014;111:8269–8274. doi: 10.1073/pnas.1322512111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghosh DD, et al. Neural architecture of hunger-dependent multisensory decision making in C. elegans. Neuron. 2016;92:1049–1062. doi: 10.1016/j.neuron.2016.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Erkut C, Kurzchalia TV. The C. elegans dauer larva as a paradigm to study metabolic suppression and desiccation tolerance. Planta. 2015 doi: 10.1007/s00425-015-2300-x. [DOI] [PubMed] [Google Scholar]
- 37.Penkov S, et al. A metabolic switch regulates the transition between growth and diapause in C. elegans. BMC Biol. 2020 doi: 10.1186/s12915-020-0760-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Penkov S, et al. Maradolipids: diacyltrehalose glycolipids specific to dauer larva in Caenorhabditiselegans. Angew. Chem. Int. Ed. 2010;49:9430–9435. doi: 10.1002/anie.201004466. [DOI] [PubMed] [Google Scholar]
- 39.Bansal K, McCrady J, Hansen A, Bhalerao K. Thin layer chromatography and image analysis to detect glycerol in biodiesel. Fuel. 2008;87:3369–3372. doi: 10.1016/j.fuel.2008.04.033. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.