Abstract
Background
Since a national lockdown was introduced across the UK in March, 2020, in response to the COVID-19 pandemic, cancer screening has been suspended, routine diagnostic work deferred, and only urgent symptomatic cases prioritised for diagnostic intervention. In this study, we estimated the impact of delays in diagnosis on cancer survival outcomes in four major tumour types.
Methods
In this national population-based modelling study, we used linked English National Health Service (NHS) cancer registration and hospital administrative datasets for patients aged 15–84 years, diagnosed with breast, colorectal, and oesophageal cancer between Jan 1, 2010, and Dec 31, 2010, with follow-up data until Dec 31, 2014, and diagnosed with lung cancer between Jan 1, 2012, and Dec 31, 2012, with follow-up data until Dec 31, 2015. We use a routes-to-diagnosis framework to estimate the impact of diagnostic delays over a 12-month period from the commencement of physical distancing measures, on March 16, 2020, up to 1, 3, and 5 years after diagnosis. To model the subsequent impact of diagnostic delays on survival, we reallocated patients who were on screening and routine referral pathways to urgent and emergency pathways that are associated with more advanced stage of disease at diagnosis. We considered three reallocation scenarios representing the best to worst case scenarios and reflect actual changes in the diagnostic pathway being seen in the NHS, as of March 16, 2020, and estimated the impact on net survival at 1, 3, and 5 years after diagnosis to calculate the additional deaths that can be attributed to cancer, and the total years of life lost (YLLs) compared with pre-pandemic data.
Findings
We collected data for 32 583 patients with breast cancer, 24 975 with colorectal cancer, 6744 with oesophageal cancer, and 29 305 with lung cancer. Across the three different scenarios, compared with pre-pandemic figures, we estimate a 7·9–9·6% increase in the number of deaths due to breast cancer up to year 5 after diagnosis, corresponding to between 281 (95% CI 266–295) and 344 (329–358) additional deaths. For colorectal cancer, we estimate 1445 (1392–1591) to 1563 (1534–1592) additional deaths, a 15·3–16·6% increase; for lung cancer, 1235 (1220–1254) to 1372 (1343–1401) additional deaths, a 4·8–5·3% increase; and for oesophageal cancer, 330 (324–335) to 342 (336–348) additional deaths, 5·8–6·0% increase up to 5 years after diagnosis. For these four tumour types, these data correspond with 3291–3621 additional deaths across the scenarios within 5 years. The total additional YLLs across these cancers is estimated to be 59 204–63 229 years.
Interpretation
Substantial increases in the number of avoidable cancer deaths in England are to be expected as a result of diagnostic delays due to the COVID-19 pandemic in the UK. Urgent policy interventions are necessary, particularly the need to manage the backlog within routine diagnostic services to mitigate the expected impact of the COVID-19 pandemic on patients with cancer.
Funding
UK Research and Innovation Economic and Social Research Council.
Introduction
A national lockdown was introduced across the UK on March 23, 2020, as part of the national strategy to flatten the curve of the COVID-19 pandemic and reduce the potential impact on the UK National Health Service (NHS).1 The lockdown has been associated with a decrease in, or cessation of, most non-COVID-19 NHS services, and increasing concern about the effect on other patient groups requiring time-critical access to health-care services. These patient groups include patients with cancer for whom timely diagnosis and the prompt initiation of treatment is vital for ensuring optimal outcomes.2, 3
Since the beginning of the pandemic, multiple changes in the provision of cancer care from the point of diagnosis, including modification of treatment schedules (change in therapy, deferral, or omission), have been advised by professional bodies and commissioners of services globally.4, 5, 6, 7 However, substantial heterogeneity has been seen in the implementation of these recommendations across providers nationally and internationally and for individual patients. Such variations in the extent of treatment delay, and in changes to treatment doses and schedules (including new treatment techniques) mean that modelling of these variations in practice on cancer outcomes at a population level is challenging.
Research in context.
Evidence before this study
In the UK, national COVID-19 pandemic measures since March 16, 2020, have resulted in the suspension of cancer screening and deferral of routine diagnostic investigations. Additionally, urgent 2-week wait referrals for patients with suspected cancer initiated by general practitioners (GPs) have decreased by up to 80% in response to physical distancing. To identify studies reporting on the current or predicted impact of diagnostic delay on cancer mortality during the COVID-19 pandemic, we searched PubMed for articles in English published between Jan 1 and April 30, 2020, to identify national estimates and methods of estimation using the search terms (“COVID-19” OR “coronavirus” OR “SARS-CoV-2”) AND “cancer” AND (“diagnosis” OR “diagnostic”) AND “delay”. To date, no study has attempted to model the impact of changes in health-seeking behaviour and in the availability of and access to diagnostic services in the UK as a result of the COVID-19 lockdown on cancer survival and the additional number of deaths expected.
Added value of this study
To our knowledge, this study is the first of its kind to estimate the impact of delays in diagnostic pathways due to pandemic lockdown measures on cancer survival for four major tumour types. We use linked national cancer registration and hospital datasets, which provide a robust template for understanding the impact of current and predicted changes in availability, access, and health-seeking behaviour in response to the COVID-19 pandemic on cancer survival. We used a routes-to-diagnosis framework, which is novel and provides a transparent approach to understanding what components of the diagnostic pathway need to be targeted as part of health service mitigation and recovery programmes. Additionally, this method does not require any new estimation of changes in cancer outcomes, but derives this from previous real-world observations. We also estimated the years of life lost to understand the wider welfare effects resulting from avoidable cancer deaths, and how this varies according to tumour type and the age profile of men and women diagnosed with these cancers.
Implications of all the available evidence
Our results are conservative estimates of the number of additional deaths and years of life lost because we do not consider the effect of suboptimal or delayed cancer treatment. These data are essential for policy makers to drive changes in national lockdown and stay-at-home messaging, and to urgently reduce diagnostic delays, particularly for routine investigations, through outreach and accessibility programmes. Our model can also be used by other countries in their unique health-care settings to understand the impact of delays in diagnosis on cancer outcomes.
Instead, in this study, we focused on analysing the impact of changes in cancer diagnostic pathways and subsequent delays in diagnosis during the COVID-19 pandemic. Routine non-urgent diagnostic work initiated by referral from both primary care (general practitioners [GPs]) and secondary care teams (eg, for radiology or endoscopic procedures8) has been deferred across the UK. Cancer screening services have been suspended, and patients' only routes to diagnosis since lockdown began have been via urgent 2-week-wait referral pathways for suspected cancer initiated by the GP or through direct presentation to an emergency department.9 Patients are eligible for these rapid access 2-week-wait pathways to access diagnostic investigations, on the basis of their age, symptom profile (eg, dysphagia), signs (eg, breast lump), or results of investigations (eg, iron deficiency anaemia) as specified by guidelines developed by the National Institute for Health and Care Excellence.9
However, since March, 2020, changes in health-seeking behaviour have been observed, with urgent 2-week-wait cancer referrals decreasing by up to 80% in response to physical distancing and concerns about contracting severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).10 Additionally, some form of physical distancing is expected to continue for up to 12 months, which will probably further affect presentations to health-care services.11, 12
Quantifying the impact of delays in diagnosis on stage and prognosis is complex, but a routes-to-diagnosis approach provides a validated methodological framework for understanding their effect. Work by Elliss-Brookes and colleagues13 showed that referral routes to diagnosis are characterised by differences in both stage at presentation and survival. For example, urgent 2-week-wait referrals for suspected cancer and emergency presentations are associated with later stage of disease at diagnosis than diagnoses via routine GP and secondary care referral routes and screening. Additionally, diagnosis after initial presentation to an emergency department is consistently associated with the worst survival outcomes compared with all other routes.13
Given the changes in health-seeking behaviour and availability and access to diagnostic services as a result of the COVID-19 lockdown, these routes to diagnosis provide a framework for estimating the impact of these changes on stage migration and excess cancer mortality on the basis of patients moving to different referral routes during the pandemic.
The effect of delayed presentation on patients with cancer is not immediate, and premature death as a result might occur up to 5 years later and will differ according to tumour type. In this study, using national population datasets of patients diagnosed and treated in the English NHS, we estimated the impact of delays in diagnosis that are attributed to the lockdown measures put in place in the UK in March, 2020, for four major tumour types: breast, colorectal, lung, and oesophageal. We chose these tumour types because they differ in their predominant routes to referral (including screening), stage at presentation, and both short-term and long-term prognoses according to stage. We estimated the effect on patient survival and the number of additional deaths expected due to these cancers, and the additional years of life lost (YLLs).
Methods
Study design and population
In this national, population-based, modelling study, we obtained information on adults in England, UK, with non-small-cell lung cancer (hereafter referred to as lung cancer: International Classification of Diseases 10th edition C33, C34), cancers of the colon (C18) and rectum (C19), cancers of the oesophagus and gastro-oesophageal junction (C15, C16.0), and women with breast cancer (C50) from the National Cancer Registration Service. The pre-pandemic cohort refers to patients diagnosed between Jan 1, 2010, and Dec 31, 2010, with follow-up data until Dec 31, 2014, for cancers of the colon, rectum, oesophagus, and breast, and to patients diagnosed between Jan 1, 2012, and Dec 31, 2012, with follow-up data until Dec 31, 2015, for lung cancer. We restricted the analyses to patients aged 15–84 years at diagnosis and those who had a known route of diagnosis coded (ie, 91% of patients for colorectal cancer, 93% of patients for oesophageal cancer, 94% of patients for breast cancer, and 97% of patients for lung cancer).
The National Cancer Registration Service records and updates patient and tumour characteristics for almost all cancers diagnosed in England (98–100%).14 We derived information on referral pathways from linkages of the cancer registrations with secondary care data (Hospital Episode Statistics), screening records, and data on cancer waiting times.13 We did these linkages using deterministic linkage methods using each individual patient's NHS Number, with a linkage success of 99–100%.14 We derived information on patient's comorbidity status from Hospital Episode Statistics diagnostic codes when patients attend hospital.15 We determined levels of deprivation via the quintiles of the Index of Multiple Deprivation income domain for the patients' residential postcodes, measured at Lower Super Output Area level.16 We used this information to link each patient with their expected mortality according to age, sex, deprivation, and region of residence using general population life tables.
This study was done in accordance with existing statutory and ethical approvals from the Confidentiality Advisory Group and Research Ethics Committee (PIAG 1–05(c)/2007 and REC 13/LO/0610).
Conceptual framework
We assumed that the incidence of each of the four tumour types of interest will remain relatively stable year on year on the basis of trends in previous years (2010–18),17 and that the ongoing COVID-19 pandemic and UK lockdown will mean patients are more likely to delay presentation. We estimated the subsequent impact on survival by reallocating patients from screening and non-urgent routine referral pathways (from GPs and secondary care) to urgent pathways—namely, 2-week wait referral routes and presentation at an emergency department. Both of these urgent pathways are associated with later stage of diagnosis and enabled us to estimate the impact of diagnostic delay on stage migration and survival outcome.
We justified our reallocation model on four assumed factors. First, 2-week-wait and emergency pathways are the only referral routes at the present time. Second, although routine diagnostic work and non-urgent referral pathways are delayed and screening suspended, some patients awaiting investigation will become symptomatic as their cancer progresses and will meet the criteria for urgent 2-week wait referral for suspected cancer or present as emergencies direct to secondary care. Third, for patients awaiting routine diagnostic investigations from their GP and secondary care referrals, substantial delays are expected (>6 months)12 due to the backlogs of routine work across all medical and surgical services increasing the likelihood of disease progression, which we estimated via reallocation to 2-week wait and emergency pathways. Finally, changes in health-seeking behaviour as a result of the pandemic means that some patients will delay presentation until more prominent symptoms develop, and these patients will be more likely to present through 2-week-wait and emergency pathways.
The starting point for our estimation is from March 16, 2020, which is the date physical distancing measures were introduced in the UK, and the impact is modelled over a 12-month period to account for the expected duration of disruption to services and patterns of referral. This period defines our cohort of expected number of cancer diagnoses for each tumour type, but we acknowledge that patients might present and be diagnosed beyond this period because of diagnostic delay. Our model reallocates patients on the basis of pre-pandemic ratios. For example, if 10% of new diagnoses for a given tumour type are after an emergency department presentation, and 90% are via an outpatient referral, our simulation analysis will maintain these proportions when reallocating patients from screening and routine referral pathways.
For patients with breast cancer diagnosed via the screening referral pathway, we accounted for the fact that many are diagnosed with pre-invasive disease18 or disease that is unlikely to progress even within a 12-month period. Therefore, we reallocated only 25% of patients diagnosed with breast cancer through the NHS breast cancer screening programme. This percentage reflects the proportion of patients who are diagnosed with breast cancer through screening referral pathways with tumours at stage III–IV, node-positive, or metastatic disease at the time of diagnosis.
We used reallocation to estimate the excess mortality compared with the pre-pandemic period. For colorectal cancer, we undertook reallocation separately for colon and rectal cancer because the proportion of patients presenting via the different referral routes differed between the two cancers, as did the cancer stage at the time of diagnosis.
Scenarios
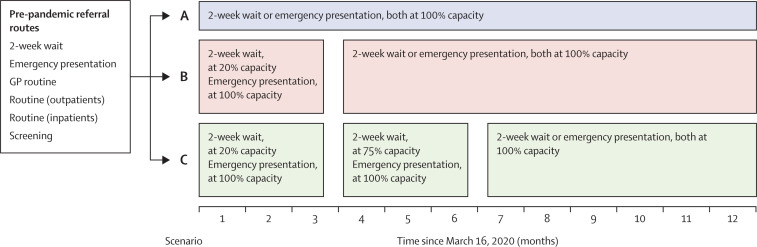
We based our analysis on three sets of predictions according to possible changes in referral patterns (figure 1 ) representing the best and worst case scenarios. For scenario A, we estimated survival outcomes for patients by reallocating those who are expected to be diagnosed through screening and routine referral pathways (GP or secondary care) to 2-week-wait and emergency presentation pathways, from March 16, 2020. Scenario B is the same as scenario A, but from March 16, we simulated the effect of an 80% reduction in 2-week-wait referrals, which has already been observed during the lockdown period,10 and assumed that this reduction will continue (due to COVID-19-related concerns) for up to 3 months. Emergency presentations are assumed to continue at their usual rate. Therefore, we re-allocated the backlog of patients in months 4–12 to 2-week-wait pathways and emergency presentations. And scenario C is the same as scenario B, but we simulated the effect of 2-week-wait referrals continuing to be reduced beyond the first 3-month period by 25% for a further 3-month period—ie, until month 6 after introduction of physical distancing measures. Under this scenario, emergency presentations are assumed to continue at the usual rate. Therefore, we re-allocated the backlog of patients in months 7–12 to 2-week-wait pathways and emergency presentations.
Figure 1.
Conceptual framework for reallocation of pre-pandemic referral routes in three modelling scenarios (A, B, and C)
For breast cancer, in addition to patients on routine pathways, only 25% of patients diagnosed through screening (ie, the proportion of patients with tumour stage III or IV, node-positive, or metastatic disease) were reallocated to 2-week wait or emergency presentation in the pandemic scenarios. GP=general practitioner.
Statistical analysis
We randomly modified the mode of presentation and dates of diagnosis of the pre-pandemic cohorts according to scenarios A–C. We reallocated patients diagnosed through screening and routine referral pathways (outpatient or inpatient) to either emergency presentation or 2-week-wait referral routes. For scenarios B and C, we reallocated a proportion of patients diagnosed through the 2-week-wait pathway because under these scenarios this referral route was assumed to operate at 20% (scenario B) and 75% (scenario C) of its usual capacity.
We estimated the reallocation of patients from routine and screening pathways to the emergency presentation route at the same proportion observed in the pre-pandemic cohorts (table 1 ).
Table 1.
Distribution of patients by referral pathway, stage of cancer, and 1-year, 3-year, and 5-year net survival in the pre-pandemic period and by each pandemic scenario
| Patients | Stage III–IV* |
Net survival |
||||||
|---|---|---|---|---|---|---|---|---|
| 1 year | 3 years | 5 years | ||||||
| Breast cancer | ||||||||
| Pre-pandemic period | ||||||||
| Emergency presentation | 930 (2·9%) | 245/356 (68·8%) | 56·3% (53·9–58·6) | 39·0% (37·0–41·0) | 33·4% (31·8–35·1) | |||
| GP referral | 5136 (15·8%) | 566/2836 (20·0%) | 96·3% (96·2–96·3) | 90·0% (89·9–90·1) | 86·2% (86·2–86·3) | |||
| Other routine† | 887 (2·7%) | 93/418 (22·2%) | 94·0% (93·8–94·2) | 85·8% (85·5–86·1) | 81·3% (81·0–81·7) | |||
| Screening | 10 795 (33·1%) | 406/6789 (6·0%) | 100·0% (100–100) | 99·6% (99·6–99·6) | 98·8% (98·8–98·8) | |||
| 2-week wait | 14 835 (45·5%) | 1821/8934 (20·4%) | 97·9% (97·9–97·9) | 91·3% (91·3–91·4) | 86·3% (86·2–86·3) | |||
| Overall | 32 583 (100%) | .. | 97·0% (97·0–97·1) | 92·2% (92·2–92·3) | 88·8% (88·7–88·8) | |||
| Pandemic period | ||||||||
| Scenario A | .. | .. | 96·0% (95·9–96·1) | 89·0% (88·9–89·1) | 83·9% (83·9–84·0) | |||
| Emergency presentation | 1149 (4·7%) | .. | .. | .. | .. | |||
| 2-week wait | 23 357 (95·3%) | .. | .. | .. | .. | |||
| Scenario B | .. | .. | 95·9% (95·9–96·0) | 88·8% (88·7–88·9) | 83·6% (83·6–83·7) | |||
| Emergency presentation | 1225 (5·0%) | .. | .. | .. | .. | |||
| 2-week wait | 23 286 (95·0%) | .. | .. | .. | .. | |||
| Scenario C | .. | .. | 95·9% (95·8–96·0) | 88·7% (88·6–88·8) | 83·6% (83·5–83·6) | |||
| Emergency presentation | 1249 (5·1%) | .. | .. | .. | .. | |||
| 2-week wait | 23 240 (94·9%) | .. | .. | .. | .. | |||
| Colorectal cancer‡ | ||||||||
| Pre-pandemic period | ||||||||
| Emergency presentation | .. | .. | 54·8% (54·6–55·1) | 40·3% (40·1–40·4) | 35·1% (34·9–35·2) | |||
| Colon | 4143 (26·1%) | 1753/2263 (77·5%) | .. | .. | .. | |||
| Rectum | 1040 (11·4%) | 459/584 (78·6%) | .. | .. | .. | |||
| GP referral | .. | .. | 83·5% (83·4–83·5) | 70·6% (70·5–70·7) | 64·4% (64·3–64·4) | |||
| Colon | 3769 (23·8%) | 1262/2082 (60·6%) | .. | .. | .. | |||
| Rectum | 2538 (27·9%) | 903/1531 (59·0%) | .. | .. | .. | |||
| Other routine† | .. | .. | 83·7% (83·6–83·8) | 71·3% (71·2–71·4) | 65·4% (65·3–65·5) | |||
| Colon | 2063 (13·0%) | 666/1112 (59·9%) | .. | .. | .. | |||
| Rectum | 1001 (11·0%) | 365/587 (62·2%) | .. | .. | .. | |||
| Screening | .. | .. | 97·5% (97·5–97·5) | 92·9% (92·9–93·0) | 89·6% (89·6–89·7) | |||
| Colon | 1922 (12·1%) | 431/985 (43·8%) | .. | .. | .. | |||
| Rectum | 1102 (12·1%) | 307/677 (45·3%) | .. | .. | .. | |||
| 2-week wait | .. | .. | 85·0% (85·0–85·1) | 71·2% (71·2–71·3) | 64·2% (64·1–64·2) | |||
| Colon | 3970 (25·0%) | 1493/2444 (61·1%) | .. | .. | .. | |||
| Rectum | 3427 (37·6%) | 1449/2344 (61·8%) | .. | .. | .. | |||
| Overall | .. | .. | 79·7% (79·7–79·8) | 67·3% (67·2–67·3) | 61·4% (61·4–61·5) | |||
| Colon | 15 867 (100%) | .. | .. | .. | .. | |||
| Rectum | 9108 (100%) | .. | .. | .. | .. | |||
| Pandemic period | .. | .. | .. | .. | .. | |||
| Scenario A | .. | .. | 76·0% (75·9–76·0) | 61·9% (61·8–61·9) | 55·3% (55·3–55·3) | |||
| Emergency presentation | ||||||||
| Colon | 6166 (38·9%) | .. | .. | .. | .. | |||
| Rectum | 1570 (17·2%) | .. | .. | .. | .. | |||
| 2-week wait | ||||||||
| Colon | 9700 (61·1%) | .. | .. | .. | .. | |||
| Rectum | 7538 (82·8%) | .. | .. | .. | .. | |||
| Scenario B | ||||||||
| Emergency presentation | .. | .. | 75·7% (75·6–75·7) | 61·6% (61·6–61·7) | 55·1% (55·1–55·2) | |||
| Colon | 6384 (40·2%) | .. | .. | .. | .. | |||
| Rectum | 1654 (18·2%) | .. | .. | .. | .. | |||
| 2-week wait | ||||||||
| Colon | 9482 (59·8%) | .. | .. | .. | .. | |||
| Rectum | 7454 (81·8%) | .. | .. | .. | .. | |||
| Scenario C | ||||||||
| Emergency presentation | .. | .. | 75·5% (75·5–75·6) | 61·5% (61·4–61·5) | 55·0% (55·0–55·0) | |||
| Colon | 6456 (40·7%) | .. | .. | .. | .. | |||
| Rectum | 1678 (18·4%) | .. | .. | .. | .. | |||
| 2-week wait | ||||||||
| Colon | 9410 (59·3%) | .. | .. | .. | .. | |||
| Rectum | 7430 (81·6%) | .. | .. | .. | .. | |||
| Lung cancer | ||||||||
| Pre-pandemic period | ||||||||
| Emergency presentation | 9636 (32·9%) | 7674/8690 (88·3%) | 15·9% (15·9–15·9) | 6·6% (6·6–6·6) | 4·6% (4·6–4·6) | |||
| GP referral | 6549 (22·3%) | 4158/6108 (68·1%) | 46·4% (46·4–46·4) | 26·1% (26·1–26·1) | 19·6% (19·6–19·6) | |||
| Other routine† | 4003 (13·7%) | 2483/3732 (66·5%) | 50·3% (50·3–50·4) | 29·1% (29·1–29·1) | 22·0% (22·0–22·0) | |||
| 2-week wait | 9117 (31·1%) | 6806/8917 (76·3%) | 48·7% (48·7–48·7) | 21·9% (21·9–21·9) | 13·6% (13·6–13·6) | |||
| Overall | 29 305 (100%) | .. | 37·6% (37·6–37·6) | 18·8% (18·8–18·8) | 13·1% (13·1–13·1) | |||
| Pandemic period | ||||||||
| Scenario A | .. | .. | 34·1% (34·0–34·1) | 15·1% (15·1–15·1) | 9·6% (9·6–9·6) | |||
| Emergency presentation | 12 802 (43·7%) | .. | .. | .. | .. | |||
| 2-week wait | 16 503 (56·3%) | .. | .. | .. | .. | |||
| Scenario B | .. | .. | 33·3% (33·3–33·3) | 14·7% (14·7–14·7) | 9·4% (9·4–9·4) | |||
| Emergency presentation | 13 715 (46·8%) | .. | .. | .. | .. | |||
| 2-week wait | 15 590 (53·2%) | .. | .. | .. | .. | |||
| Scenario C | .. | .. | 33·1% (33·1–33·1) | 14·6% (14·6–14·6) | 9·3% (9·3–9·3) | |||
| Emergency presentation | 13 538 (46·2%) | .. | .. | .. | .. | |||
| 2-week wait | 15 767 (53·8%) | .. | .. | .. | .. | |||
| Oesophageal cancer | ||||||||
| Pre-pandemic period | ||||||||
| Emergency presentation | 1228 (18·2%) | 258/283 (91·2%) | 20·7% (20·3–21·1) | 9·5% (9·4–9·7) | 7·9% (7·8–8·1) | |||
| GP referral | 1410 (20·9%) | 215/300 (71·7%) | 54·8% (54·6–55·0) | 27·3% (27·2–27·4) | 21·2% (21·0–21·3) | |||
| Other routine† | 1303 (19·3%) | 196/268 (73·1%) | 55·7% (55·6–55·9) | 29·7% (29·6–29·9) | 23·9% (23·7–24·0) | |||
| 2-week wait | 2803 (41·6%) | 629/755 (83·3%) | 48·2% (48·1–48·3) | 19·1% (19·0–19·2) | 13·4% (13·3–13·5) | |||
| Overall | 6744 (100%) | .. | 46·0% (45·9–46·1) | 21·1% (21·1–21·2) | 16·1% (16·0–16·1) | |||
| Pandemic period | ||||||||
| Scenario A | .. | .. | 41·3% (41·2–41·4) | 16·7% (16·7–16·8) | 12·0% (12·0–12·1) | |||
| Emergency presentation | 1690 (25·1%) | .. | .. | .. | .. | |||
| 2-week wait | 5054 (74·9%) | .. | .. | .. | .. | |||
| Scenario B | .. | .. | 39·9% (39·7–40·0) | 15·8% (15·7–15·8) | 11·3% (11·3–11·4) | |||
| Emergency presentation | 1783 (26·4%) | .. | .. | .. | .. | |||
| 2-week wait | 4961 (73·6%) | .. | .. | .. | .. | |||
| Scenario C | .. | .. | 39·7% (39·6–39·8) | 15·7% (15·7–15·8) | 11·3% (11·2–11·3) | |||
| Emergency presentation | 1812 (26·9%) | .. | .. | .. | .. | |||
| 2-week wait | 4932 (73·1%) | .. | .. | .. | .. | |||
Data are n (%), n/N (%), or net survival with 95% CI in parentheses. For breast cancer, in addition to patients on routine pathways, only 25% (n=2700) of patients diagnosed through screening (ie, the proportion of patients with T3, T4, node positive, or metastatic disease) were reallocated to 2-week wait and emergency presentation in the pandemic scenarios.
The proportion of patients diagnosed with stage III or IV disease is based on patients with available staging information in the cancer registry dataset and has been reported to show the stage variation according to diagnostic referral route; information on cancer stage is not used in the modelling of net survival.
Includes referrals within secondary care.
Net survival for colorectal cancer is for both colon and rectum tumour type combined. However, allocation of patients to 2-week wait and emergency presentation diagnostic routes was done separately for each tumour type.
To estimate the impact that the response to the COVID-19 pandemic could have on cancer survival, we compared the net survival of pre-pandemic cohorts of patients with cancer to that of patients diagnosed according to the postulated scenarios A–C. Notably, for colorectal cancer, even though the reallocation from routine to urgent pathways was done separately for patients with rectal and colon cancer, the survival estimates are for the combined colorectal cancer population. We translated the differences in net survival between pre-pandemic and pandemic cohorts into the number of deaths due to cancer for each scenario. Compared with the number of deaths due to cancer in the pre-pandemic cohorts, we derived the additional number of deaths due to cancer and additional number of YLLs. We calculated point estimates and 95% CIs using bootstrap resampling.
We obtained our estimates via multivariable excess hazard models. For these population-based data, we retrieved the measure of interest (excess mortality due to cancer) by removing the effect of competing risks of death (ie, deaths due to causes other than the cancer of interest).19 We derived these competing risks from general population life tables defined by sex, single years of age, calendar years, deprivation quintile, and Government Office Regions. All-cause mortality from general population life tables includes cancer-related mortality. Since each cancer site-specific mortality is a negligible cause of death, all-cause mortality, as estimated from population life tables, is the mortality that patients with cancer would experience, had they not been diagnosed with cancer.20, 21 Further details and mathematical formulae are in the appendix (pp 1–3). We did all statistical analyses using Stata (version 16.1).
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. CM, BR, and AA had full access to all the data in the study and had final responsibility for the decision to submit to publication.
Results
We analysed data on 32 583 patients with breast cancer, 24 975 with colorectal cancer, 29 305 with lung cancer, and 6744 with oesophageal cancer (table 1). Patients were all diagnosed in England. Patients were aged 15–84 years and the mean age at diagnosis was 60·5 years (SD 12·6) for breast cancer, 68·5 years (10·7) for colorectal cancer, 68·5 years (10·3) for oesophageal cancer, and 69·8 years (9·3) for lung cancer. 10 441 (41·8%) of 24 975 patients diagnosed with colorectal cancer, 13 211 (45·1%) of 29 305 diagnosed with lung cancer, and 1894 (28·1%) of 6744 diagnosed with oesophageal cancer were women. In the pre-pandemic period, survival varied substantially by tumour type and referral pathway, with the worst prognosis evident for oesophageal and lung cancers and for patients diagnosed after an emergency presentation. The proportion of patients diagnosed through emergency presentation pathways varied substantially across tumour types. These differences in survival between referral pathways correlated with increased proportions of patients diagnosed at stages III and IV, irrespective of tumour type (table 1; appendix p 4). Notably, 2-week-wait referral pathways are not associated with substantial differences in stage or survival compared with non-urgent referral routes.
We estimated the impact of diagnostic delay for the 12-month period from March 16, 2020, to March 15, 2021. Across scenarios A–C, we estimated an absolute decrease in cancer survival ranging between 1·0–1·1% (breast, all scenarios) and 6·1–6·3% (oesophageal, scenarios B and C) at 1 year after diagnosis, and between 3·5% (lung, scenario A) and 6·4% (colorectal, scenario C) at 5 years after diagnosis (table 1).
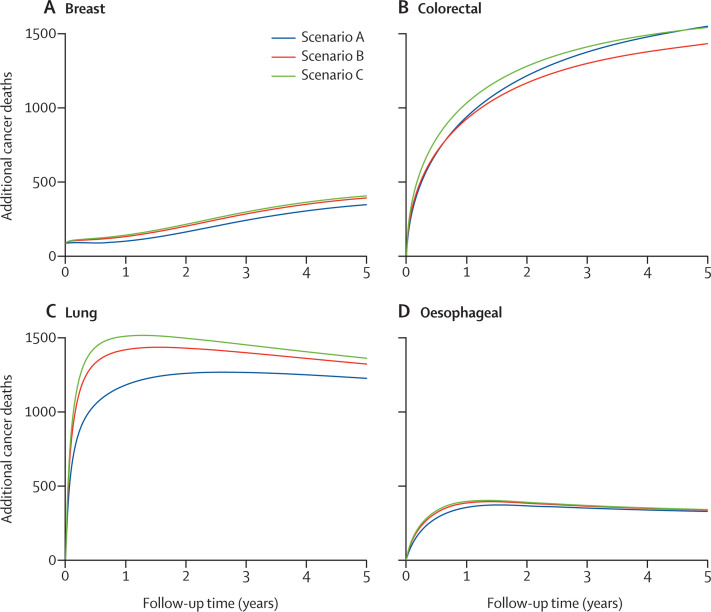
The differences in survival translate into substantial additional numbers of deaths due to cancer in the first 5 years of follow-up. The estimated number of deaths due to each cancer up to 1, 3, and 5 years after diagnosis in the pre-pandemic period and across scenarios A–C are shown in table 2 . The number of additional cancer deaths estimated across the scenarios are shown as cumulative estimates up to year 5 (table 2, figure 2 ).
Table 2.
Estimated cumulative number of deaths due to cancer up to 1 year, 3 years, and 5 years after diagnosis, in the pre-pandemic period and for each pandemic scenario A–C (also presented as additional number of deaths)
|
Number of deaths due to cancer |
Additional number of deaths due to cancer |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 year | 3 years | 5 years | 1 year |
3 years |
5 years |
||||
| n | Percentage increase | n | Percentage increase | n | Percentage increase | ||||
| Breast cancer (n=32 583) | |||||||||
| Pre-pandemic period | 965 (958–972) | 2495 (2484–2505) | 3565 (3554–3577) | ·· | ·· | ·· | ·· | ·· | ·· |
| Scenario A | 985 (977–993) | 2664 (2651–2676) | 3846 (3831–3861) | 20 (15–25) | 2·1% (1·6–2·6) | 169 (159–179) | 6·8% (6·4–7·2) | 281 (266–295) | 7·9% (7·5–8·3) |
| Scenario B | 1018 (1009–1026) | 2709 (2696–2722) | 3894 (3876–3911) | 53 (47–59) | 5·5% (4·9–6·2) | 214 (202–226) | 8·6% (8·1–9·0) | 329 (313–344) | 9·2% (8·8–9·7) |
| Scenario C | 1028 (1019–1036) | 2723 (2709–2737) | 3908 (3890–3926) | 63 (57–70) | 6·6% (5·9–7·2) | 228 (218–239) | 9·1% (8·7–9·6) | 344 (329–358) | 9·6% (9·2–10·1) |
| Colorectal cancer (n=24 975) | |||||||||
| Pre-pandemic period | 5051 (5004–5099) | 8056 (8007–8109) | 9417 (9367–9470) | ·· | ·· | ·· | ·· | ·· | ·· |
| Scenario A | 5986 (5943–6025) | 9436 (9391–9475) | 10 980 (10 940–11 020) | 935 (918–953) | 18·5% (18·0–19·0) | 1379 (1354–1405) | 17·1% (16·8–17·5) | 1563 (1534–1592) | 16·6% (16·2–17·0) |
| Scenario B | 5972 (5929–6028) | 9357 (9299–9459) | 10 862 (10 797–10 995) | 921 (894–970) | 18·2% (17·6–19·2) | 1301 (1257–1411) | 16·1% (15·6–17·5) | 1445 (1392–1591) | 15·3% (14·8–16·9) |
| Scenario C | 6078 (6032–6140) | 9470 (9409–9613) | 10 972 (10 903–11 162) | 1027 (999–1094) | 20·3% (19·7–21·6) | 1414 (1371–1568) | 17·6% (17·0–19·4) | 1555 (1498–1760) | 16·5% (15·9–18·7) |
| Lung cancer (n=29 305) | |||||||||
| Pre-pandemic period | 18 443 (18 388–18 503) | 24 138 (24 097–24 172) | 25 934 (25 901–25 963) | ·· | ·· | ·· | ·· | ·· | ·· |
| Scenario A | 19 545 (19 497–19 594) | 25 369 (25 339–25 398) | 27 170 (27 148–27 191) | 1102 (1087–1117) | 6·0% (5·9–6·1) | 1231 (1216–1249) | 5·1% (5·0–5·2) | 1235 (1220–1254) | 4·8% (4·7–4·8) |
| Scenario B | 19 769 (19 721–19 817) | 25 498 (25 464–25 531) | 27 267 (27 240–27 297) | 1326 (1295–1362) | 7·2% (7·0–7·4) | 1360 (1331–1389) | 5·6% (5·5–5·8) | 1332 (1306–1360) | 5·1% (5·0–5·2) |
| Scenario C | 19 855 (19 804–19 901) | 25 549 (25 519–25 582) | 27 306 (27 280–27 334) | 1412 (1379–1447) | 7·7% (7·5–7·9) | 1412 (1381–1442) | 5·8% (5·7–6·0) | 1372 (1343–1401) | 5·3% (5·2–5·4) |
| Oesophageal cancer (n=6744) | |||||||||
| Pre-pandemic period | 3656 (3642–3670) | 5359 (5349–5369) | 5730 (5720–5741) | ·· | ·· | ·· | ·· | ·· | ·· |
| Scenario A | 3995 (3978–4012) | 5701 (5690–5714) | 6060 (6049–6073) | 339 (334–343) | 9·3% (9·2–9·4) | 343 (337–348) | 6·4% (6·3–6·5) | 330 (324–335) | 5·8% (5·7–5·8) |
| Scenario B | 4024 (4006–4041) | 5714 (5703–5726) | 6069 (6058–6081) | 367 (362–373) | 10·1% (9·9–10·2) | 355 (350–361) | 6·6% (6·5–6·7) | 339 (333–345) | 5·9% (5·8–6·0) |
| Scenario C | 4034 (4017–4050) | 5718 (5707–5731) | 6072 (6061–6084) | 377 (372–383) | 10·3% (10·2–10·5) | 359 (354–365) | 6·7% (6·6–6·9) | 342 (336–348) | 6·0% (5·9–6·1) |
Data are cumulative number of deaths or percentage change in number of deaths, with 95% CIs in parentheses. Point estimates and 95% CIs were calculated from bootstrap samples of the original data.
Figure 2.
Estimated additional number of cancer deaths for each pandemic scenario A–C, for breast cancer (A), colorectal cancer (B), lung cancer (C), and oesophageal cancer (D)
We estimated across scenarios A–C, compared with the pre-pandemic period, a 2·1–6·6% increase in the number of deaths due to breast cancer up to year 1 (corresponding to between 20 [95% CI 15–25] and 63 [57–70] additional deaths), a 6·8–9·1% increase up to year 3 (169 [159–179] to 228 [218–239] additional deaths), and a 7·9–9·6% increase up to year 5 (281 [266–295] to 344 [329–358] additional deaths). For colorectal cancer across scenarios A–C, we estimated an 18·2–20·3% increase in deaths due to cancer up to year 1 (921 [894–970] to 1027 [999–1094] additional deaths), a 16·1–17·6% increase up to year 3 (1301 [1257–1411] to 1414 [1371–1568] additional deaths), and a 15·3–16·6% increase up to year 5 (1445 [1392–1591) to 1563 [1534–1592] additional deaths). For lung cancer across scenarios A–C, we estimated a 6·0–7·7% increase in the number of deaths due to cancer up to year 1 (1102 [1087–1117] to 1412 [1379–1447] additional deaths), a 5·1–5·8% increase up to year 3 (1231 [1216–1249] to 1412 [1381–1442] additional deaths), and a 4·8–5·3% increase up to year 5 (1235 [1220–1254] to 1372 [1343–1401] additional deaths). For oesophageal cancer, across scenarios A–C, we estimated a 9·3–10·3% increase in deaths due to cancer up to year 1 (339 [334–343] to 377 [372–383] additional deaths), a 6·4–6·7% increase up to year 3 (343 [337–348] to 359 [354–365] additional deaths) and a 5·8–6·0% increase up to year 5 (330 [324–335] to 342 [336–348] additional deaths).
The plateau in additional deaths due to cancer over the 5-year period for lung and oesophageal cancer (figure 2) reflects relatively higher proportions of early cancer deaths at year 1 due to more advanced stage at presentation in our scenarios. In the pre-pandemic period, some of these patients would have been expected to die beyond year 1 as a result of less advanced disease at presentation compared with the pandemic scenarios.
Overall, in comparison with the pre-pandemic period, the estimated number of additional deaths attributable to these four cancers at 5 years is between 3291 and 3621 deaths across the scenarios due to delays in cancer diagnosis (table 2, figure 2). These additional cancer deaths in the first few years after diagnosis translate into expected YLLs in the entire cohort of patients. At 5 years, across scenarios A–C, the total additional YLLs for each cancer type is shown in table 3 . Colorectal cancer and lung cancer are associated with the largest number of YLLs due to delays in the diagnostic pathways. We estimated the expected YLLs to be between 59 204 and 63 229 years because of additional deaths due to these four cancers in the first 5 years after diagnosis.
Table 3.
Estimated years of life lost from additional deaths due to cancer, at 5 years from diagnosis, for each pandemic scenario
| Years of life lost (95% CI) | |
|---|---|
| Breast cancer (n=32 583) | |
| Scenario A | 8181 (7797–8535) |
| Scenario B | 9033 (8638–9390) |
| Scenario C | 9261 (8843–9631) |
| Colorectal cancer (n=24 975) | |
| Scenario A | 27 735 (27 188–28 241) |
| Scenario B | 25 583 (24 792–27 744) |
| Scenario C | 27 043 (26 234–29 968) |
| Lung cancer (n=29 305) | |
| Scenario A | 20 537 (20 184–20 947) |
| Scenario B | 20 860 (20 250–21 277) |
| Scenario C | 20 413 (19 833–20 909) |
| Oesophageal cancer (n=6744) | |
| Scenario A | 5373 (5227–5530) |
| Scenario B | 5152 (5006–5301) |
| Scenario C | 5027 (4861–5213) |
Point estimates and 95% CIs were calculated from bootstrap samples of the original data.
Discussion
We estimated that across the four major tumour types, breast, colorectal, lung, and oesophageal, 3291 to 3621 avoidable deaths and an additional 59 204 to 63 229 YLLs will be attributable to delays in cancer diagnosis alone as a result of the COVID-19 lockdown in the UK. The increase in deaths due to cancer up to 5 years after diagnosis ranged from 4·8% for lung cancer to 16·6% for colorectal cancer. These additional deaths are projected to occur as a consequence of the national COVID-19 pandemic measures, which have reduced the number of people seeking health care and access to and availability of diagnostic services. Our findings complement those from a study by Sud and colleagues22 showing the impact of treatment delay, predominantly surgical, on excess mortality.
From the onset of the lockdown, essential diagnostic services (eg, endoscopy) were suspended or operating at substantially reduced capacity, even through the urgent 2-week-wait referral pathway. The number of endoscopies done in April, 2020, was 90% fewer than the number done in each of the first three months of 2020.23 As of June, 2020, these diagnostic services had restarted but at reduced capacities.24 These suspensions were due to the perceived risk of exposure to SARS-CoV-2 for patients and clinicians, and because of re-deployment of staff towards critical care to manage patients with COVID-19. This combination of perceived risk and redeployment of staff will result in further delays, which could also affect survival, that are not included in our model. Our results also highlight the substantial proportion of patients diagnosed with cancer through routine outpatient referral pathways (30–40%) and the subsequent effect of deferral and delay in these referral pathways during the pandemic. Even when routine diagnostic services are re-initiated, substantial delays in routine and 2-week-wait referral pathways are to be expected due to backlogs currently building up across all benign and malignant medical and surgical subspecialities.
Changes in health-seeking behaviour have meant that routine referrals from GPs have reduced in volume because patients are being asked to only present if they have major or urgent concerns.12 Additionally, whether the increasing number of remote consultations via telephone or videoconferencing will result in an increased proportion of missed diagnoses, without the ability to examine and triage the patient directly, is unknown.
Conversely, increased diagnostic efficiency has potentially been introduced into the system as a result of the pandemic. For example, patients who now report a symptom to their GP are an enriched population compared with those who reported in the pre-pandemic period and are potentially more likely to have cancer. Similarly, selection by GPs of patients for further investigation is likely to yield an increased proportion of cancer diagnoses. However, these effects are likely to be small when considering concerns about the overall shortfall in the number of new cancer diagnoses. Additionally, as of June 18, 2020, 2-week-wait referrals are still not operating at their usual pre-pandemic level, particularly for endoscopic intervention.25
Our findings reflect the urgent need for policy interventions to mitigate the predicted additional cancer deaths resulting from delays in diagnosis. Key areas to consider include public health messaging, the public's perception of their personal risk of severe illness from COVID-19 versus the risks of not seeking health-care advice if they are experiencing symptoms suggestive of cancer, provision of evidence-based information to enable health-care workers to adequately manage the risks for patients with suspected cancer during the pandemic with respect to the balance of risks and benefits of procedures, and to consider options and opportunities for increasing diagnostic capacity.
In the UK, the Stay at Home and subsequent Stay Alert public health messaging has had a substantial effect on health-seeking behaviour.26 Even as lockdown measures are being relaxed, presentation to primary care services continues to be much lower than pre-pandemic levels,25 and we cannot assume that, once all restrictions have been lifted, presentations will return to pre-pandemic levels in the next 3–6 months. Any exit strategy from lockdown27 therefore needs to include accurate and measured public health messaging that is tailored towards patients, GPs, and secondary care services that puts into perspective the risk of death from COVID-19 compared with other serious illnesses. Dedicated cancer awareness programmes will need to consider a range of media channels to reach their target groups, including direct messaging from GPs to their patients to seek attention if they are having new or worrying symptoms.
Increasing diagnostic capacity is complex because it necessitates effective coordination across all hospital subspecialities and not just in specialist cancer teams. Additionally, the requirement for full personal protective equipment when doing procedures and the initiation of robust cleaning protocols between patients has reduced capacity compared with pre-pandemic levels. In the short term, diagnostic capacity can be increased through changes in working patterns—eg, increased working hours and 7 days-a-week working. Furthermore, a central coordinating system for diagnostic investigations in a similar vein to a choose-and-book approach, whereby primary care physicians are able to refer patients to any NHS hospital, will optimise use of capacity.28 For detection of bowel cancer, surgeons are increasingly using new tools such as the faecal immunochemical test29 to triage their patients for investigation to avoid unnecessary colonoscopy and CT imaging and therefore improving capacity in this diagnostic pathway.
The paucity of information for health-care workers and patients regarding their risk of contracting COVID-19 from different health-care interactions remains a challenge as hospitals plan for restarting routine services. Antibody testing would increase confidence in clinicians doing procedures if immunity to SARS-CoV-2 does exist for even a short period.30 The health-care community needs accurate data on the true nosocomial risk of COVID-19 depending on the type of diagnostic procedure being done—eg, colonoscopy versus CT scan. When rapid antigen testing becomes routinely available, patients requiring investigation can receive testing on the day of the procedure and their risks can be managed accordingly. Equally, the implication of contracting COVID-19 needs to be considered—specifically, to be able to counsel patients effectively on the true risk of life-threatening illness and death.
A strength of this study is the use of linked national administrative health records of actual patients diagnosed and treated in the NHS for the four tumour types. These records provide a robust template for understanding the impact of current and predicted changes in availability, access, and health-seeking behaviour in response to the COVID-19 pandemic on cancer survival. This method does not require any de-novo estimation of changes in cancer outcomes but derives this estimation from previous real-world observations.
We chose the routes-to-diagnosis concept as our method to analyse diagnostic delay to overcome some of the challenges that have been raised in the scientific literature regarding the relative risk of death from diagnostic delay across tumours.2, 31, 32 Inconsistencies in the evidence are primarily associated with flaws in study design in which the true onset of symptoms remains unclear. Additionally, recent work has pointed to a waiting time paradox, whereby quicker diagnosis is associated with later stage of presentation; this paradox confounds assessment of the impact of diagnostic delay on outcomes.33, 34 Modelling the extent and duration of diagnostic delay at the population level is challenging because diagnostic delays are predicated on health system factors, such as access and availability of diagnostic capacity, and patient-level factors (awareness, symptoms, health-seeking behaviour). Our model accounts for both of these factors and is grounded in the reality of current service levels in the English NHS by providing best and worst case estimates. We acknowledge that our approach might underestimate or overestimate the impact of diagnostic delay on survival, and retrospective evaluation will be necessary to further appraise this modelling approach.
Our model assumes that disruptions due to the COVID-19 pandemic will affect timely access to routine and urgent diagnostic services and alter health-seeking behaviour for a 12-month period. These assumptions are likely given the changes in patterns of patient presentation and availability of diagnostic services observed since the start of lockdown.1, 11, 12, 24, 25 Since beginning our modelling study, substantial reduction in 2-week-wait referrals have indeed been seen in the first 3 months, as we predicted in scenarios B and C. Scenario A conservatively considers no reduction in 2-week-wait referrals. Given the ongoing reductions in the volume of 2-week-wait referrals (estimates suggest a 40–50% reduction),35 reductions are expected to continue for up to 6 months as predicted in scenario C because of the effects of pandemic lockdown measures on patients presenting to their GP or health-care provider. These measures include advice to minimise non-essential travel and the continued shielding of high-risk groups.1, 12 Cancer Research UK has estimated that the first 10 weeks of the UK lockdown has already resulted in 2·1 million deferred cancer screening investigations with 290 000 fewer people being referred on 2-week-wait pathways.35
6 months after the implementation of physical distancing measures the backlog of patients with potential cancers awaiting investigation will be considerable, and health-care presentations will continue to be affected due to physical distancing measures that are expected to continue until 2021.11, 12 Additionally, NHS hospital trusts suspended their routine diagnostic services at the start of lockdown, which is concerning because routine referral routes account for 30–40% of cancer diagnoses and the backlog in this pathway once routine services restart will include all patients still awaiting diagnostic investigations both from before and after lockdown started. Further competition for capacity will subsequently come from the increase in new referrals for suspected cancers on 2-week-wait referrals and those referred for investigation or follow up of seemingly benign health conditions. At the same time, diagnostic capacity has decreased for some procedures due the increased time taken per case since the introduction of new infection control measures.36 Together, all these factors will increase the likelihood of patients becoming symptomatic and presenting via 2-week-wait referral or emergency pathways. Alternatively, if or when patients are diagnosed through routine pathways, the likelihood of stage migration and associated worse prognosis due to delays in diagnosis is increased.
Our analysis used a retrospective population cohort, therefore, the predicted survival for patients as of 2020 presenting via the different referral pathways, even for patients with stage IV disease, has slightly improved37 in the past decade because of improvements in treatments and processes of care. However, our analysis focuses on the differences in cancer deaths between two situations (pre-pandemic and pandemic periods) and not on the absolute numbers of cancer deaths. Additionally, these estimates do not consider the impact of treatment delay or suboptimal treatment on survival during the pandemic.22 The proportions of patients presenting through different referral pathways has changed over time, with decreasing proportions of patients presenting via emergency departments in the past decade, which might also affect our results.38 However, we consider the probable effect on the overall results to be small given the steady trajectories of improvements in patterns of presentation we have seen over the past 5 years.37, 38
We did not analyse patients aged 85 years or older at diagnosis because competing events, such as deaths from other causes, are predominant in this population. Although delays in cancer diagnosis in older populations will lead to excess short-term cancer mortality, the effect on national population-levels is less likely to be affected. Furthermore, because we report survival up to 5 years after diagnosis, such an estimate is less reliable in patients aged 90 years and older.
In the screening population, we recognise that not all patients diagnosed with breast cancer through this route would have progressed or developed symptomatic disease. As a result, we included only 25% of this cohort in our reallocation. For colorectal cancer, 10% of patients are diagnosed through the screening route, of whom 45% are diagnosed with stage III–IV disease (70% stage II–IV) compared with 6% diagnosed with stage III–IV tumours through breast cancer screening; hence, we showed that more patients with colorectal cancer are diagnosed with advanced disease at screening compared with those diagnosed with breast cancer. Overdiagnosis and overtreatment are not specific concerns associated with the bowel screening programme,39 whereas they are concerns associated with breast screening, and the suspension of the bowel screening programme is likely to result in delayed presentation and stage migration if cancers remain untreated.40
Our model also considered the English NHS as a whole, and therefore assigned blanket reallocation across the country. However, we recognise that variation is probable across the country in terms of GP access, the burden of COVID-19, and the extent of discontinuation of critical diagnostic services in secondary care settings. In this regard, we acknowledge that 2-week-wait referrals have not decreased uniformly by 80% across all tumour types and UK regions, as per our estimations in scenarios B and C. Additionally, variation will exist in the recovery of services across regions and individual hospitals, which are not included in our estimations.
In summary, we estimated that changes in health-seeking behaviour and the availability of and access to essential diagnostic services resulting from national pandemic measures will result in a large number of additional deaths from breast, colorectal, lung, and oesophageal cancer in the medium (1 year) and longer term (5 years). Our study results do not consider the effect of delay on other cancer types, or the additional effect of changes in treatment pathways for these cancers that are likely to substantially increase the expected avoidable deaths beyond what we have estimated. Urgent policy interventions are necessary to mitigate the indirect effects of the COVID-19 pandemic on patients with cancer. These interventions should focus on increasing routine diagnostic capacity through which up to 40% of patients with cancer are diagnosed, public health messaging that accurately conveys the risk of severe illness from COVID-19 versus the risks of not seeking health-care advice if patients are symptomatic, and the provision of evidence-based information for clinicians to adequately manage the risks of patients to the risk and benefits of procedures during the pandemic.
This online publication has been corrected. The corrected version first appeared at thelancet.com/oncology on December 30, 2020
Data sharing
This study was based on English national cancer registry data. We do not own these data and hence are not permitted to share them in the original form. The data are available from the Office for Data Release at Public Health England. For access please email odr@phe.gov.uk.
Acknowledgments
Acknowledgments
This study was funded by the UK Research and Innovation Economic and Social Research Council (ES/P010962/1). In this study, we used data provided by patients and collected by the NHS as part of their care and support. CM and BR are funded through the Cancer Research UK Population Research Committee Funding Scheme: Cancer Research UK Population Research Committee - Programme Award (C7923/A20987 and C7923/A29018). RS is funded through the UK Research and Innovation GCRF Research for Health in Conflict (R4HC-MENA) ES/P010962/1. AA is supported by a National Institute for Health Research (NIHR) Advanced Fellowship (NIHR300599). JS is supported by the NIHR Biomedical Research Centre based at Guy's and St Thomas' NHS Foundation Trust and King's College London (IS-BRC-1215-20006), and the Cancer Research UK King's Health Partners Centre at King's College London (C604/A25135). The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research, or the Department of Health and Social Care.
Contributors
AA, MM, EN, RS, and BR conceived and designed the study. CM and BR analysed the data. AA, BR, JS, RS, and CM were involved in data interpretation. AA and CM wrote the first draft of the paper. CM produced the manuscript figures and tables. CM, JS, MM, AP, EN, RS, BR, and AA were involved in reviewing and editing drafts of the paper and approving the manuscript.
Declaration of interests
We declare no competing interests.
Supplementary Material
References
- 1.UK Government Coronavirus (COVID-19) guidance. 2020. https://www.gov.uk/government/collections/coronavirus-covid-19-list-of-guidance
- 2.Neal RD, Tharmanathan P, France B. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br J Cancer. 2015;112(suppl 1):S92–S107. doi: 10.1038/bjc.2015.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tørring ML, Murchie P, Esteva M. The signal and the noise in colorectal cancer diagnosis: exploring and explaining the relationship between diagnostic delays and stage at diagnosis using the Ca-PRI Colorectal Cancer Collaboration dataset. Eur J Cancer Care. 2015;24(suppl 2):22. [Google Scholar]
- 4.NHS England . NHS England and NHS Improvement; April 7, 2020. Publication approval reference 001559. Specialty guides for patient management during the coronavirus pandemic. Clinical guide for the management of essential cancer surgery for adults during the coronavirus pandemic. [Google Scholar]
- 5.National Institute for Health and Care Excellence . National Institute for Health and Care Excellence; March 20, 2020. COVID-19 rapid guidelines: delivery of systemic anticancer treatments.https://www.nice.org.uk/guidance/ng161/resources/covid19-rapid-guideline-delivery-of-systemic-anticancer-treatments-pdf-66141895710661 [PubMed] [Google Scholar]
- 6.Coles CE, Aristei D, Bliss J. Guidelines on radiation therapy for breast cancer during the COVID-19 pandemic. Clin Oncol. 2020;32:279–281. doi: 10.1016/j.clon.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Royal College of Radiologists . Royal College of Radiologists; London: 2020. Coronavirus (COVID-19): cancer treatment documents.https://www.rcr.ac.uk/college/coronavirus-covid-19-what-rcr-doing/clinical-information/coronavirus-covid-19-cancer [Google Scholar]
- 8.British Society of Gastroenterology . British Society of Gastroenterology; April 3, 2020. Endoscopy activity and COVID-19: BSC and JAG guidance.https://www.bsg.org.uk/covid-19-advice/endoscopy-activity-and-covid-19-bsg-and-jag-guidance/ [Google Scholar]
- 9.National Institute for Health and Care Excellence . National Institute for Health and Care Excellence; July 26, 2017. Suspected Cancer: recognition and referral.https://www.nice.org.uk/guidance/ng12 [Google Scholar]
- 10.Bodkin H. The Telegraph; London: April 15, 2020. Cancer referrals down by the 80 per cent in some areas as coronavirus fears keep patients from hospitals.https://www.telegraph.co.uk/news/2020/04/15/cancer-referrals-80-per-cent-areas-coronavirus-fears-keep-patients/ [Google Scholar]
- 11.Mason R, Proctor K. The Guardian; London: April 22, 2020. UK will need social distancing until the end of year, says Whitty.https://www.theguardian.com/world/2020/apr/22/uk-will-need-social-distancing-until-at-least-end-of-year-says-whitty [Google Scholar]
- 12.Jones D, Neal RD, Duffy SRG, Scott SE, Whitaker KL, Brain K. Impact of the COVID-19 pandemic on the symptomatic diagnosis of cancer: the view from primary care. Lancet Oncol. 2020;21:748–750. doi: 10.1016/S1470-2045(20)30242-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elliss-Brookes L, McPhail S, Ives A. Routes to diagnosis for cancer - determining the patient journey using multiple routine data sets. Br J Cancer. 2012;107:1220–1226. doi: 10.1038/bjc.2012.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Office for National Statistics . Office for National Statistics; July 3, 2019. Quality assurance of administrative data used in cancer registrations and cancer survival statistics.https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/methodologies/inistrativedatausedincancerregistrationsandcancersurvivalstatistics [Google Scholar]
- 15.Maringe C, Fowler H, Rachet B, Luque-Fernandez MA. Reproducibility, reliability and validity of population-based administrative health data for the assessment of cancer non-related comorbidities. PLoS One. 2017;12 doi: 10.1371/journal.pone.0172814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noble M, McLennan D, Wilkinson K. Communities and Local Government Publications; Wetherby: 2008. The English Indices of Deprivation 2007.http://geoconvert.mimas.ac.uk/help/imd-2007-manual.pdf [Google Scholar]
- 17.Office for National Statistics . Office for National Statistics; 2019. Cancer registration statistics, England Statistical bulletins.https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/cancerregistrationstatisticsengland/previousReleases [Google Scholar]
- 18.Marmot MG, Altman DG, Cameron DA, Dewar JA, Thompson SG, Wilcox M. The benefits and harms of breast cancer screening: an independent review. Br J Cancer. 2013;108:2205–2240. doi: 10.1038/bjc.2013.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pohar Perme M, Estève J, Rachet B. Analysing population-based cancer survival - settling the controversies. BMC Cancer. 2016;16:933. doi: 10.1186/s12885-016-2967-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ederer F, Axtell LM, Cutler SJ. The relative survival rate: a statistical methodology. Natl Cancer Inst Monogr. 1961;6:101–121. [PubMed] [Google Scholar]
- 21.Hinchliffe SR, Dickman PW, Lambert PC. Adjusting for the proportion of cancer deaths in the general population when using relative survival: a sensitivity analysis. Cancer Epidemiol. 2012;36:148–152. doi: 10.1016/j.canep.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Sud A, Jones M, Broggio J. Collateral damage: the impact on outcomes from cancer surgery of the COVID-19 pandemic. Ann Oncol. 2020 doi: 10.1016/j.annonc.2020.05.009. published online May 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richards M, Anderson M, Carter P, Ebert BL, Mossialos E. The impact of the COVID-19 pandemic on cancer care. Nature Cancer. 2020;1:565–567. doi: 10.1038/s43018-020-0074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The Lancet Gastroentereology & Heptaology Resuming bowel cancer screening post-COVID-19. Lancet Gastroenterol Hepatol. 2020 doi: 10.1016/S2468-1253(20)30200-4. published online June 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morris J. Nuffield Trust; June 18, 2020. Chart of the week: the alarming drop in referrals from GPs to hospital services since the COVID-19 outbreak.https://www.nuffieldtrust.org.uk/resource/new-chart-of-the-week-the-alarming-drop-in-referrals-from-gps-to-hospital-services-since-the-covid-19-outbreak [Google Scholar]
- 26.Illman J. HSJ; April 5, 2020. Coronavirus response could create ‘very serious unintended consequences’.https://www.hsj.co.uk/policy-and-regulation/coronavirus-response-could-create-very-serious-unintended-consequences/7027321.article [Google Scholar]
- 27.Altmann DM, Douek DC, Boyton RJ. What policy makers need to know about COVID-19 protective immunity. Lancet. 2020;395:1527–1529. doi: 10.1016/S0140-6736(20)30985-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.NHS England Book an appointment using the NHS e-Referral Service. April 27, 2020. https://www.nhs.uk/using-the-nhs/nhs-services/hospitals/nhs-e-referral-service/
- 29.Mowat C, Digby J, Strachan JA. Impact of introducing a faecal immunochemical test (FIT) for haemoglobin into primary care on the outcome of patients with new bowel symptoms: a prospective cohort study. BMJ Open Gastroenterol. 2019;6 doi: 10.1136/bmjgast-2019-000293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Studdert DM, Hall MA. Disease control, civil liberties, and mass testing - calibrating restrictions during the COVID-19 pandemic. N Engl J Med. 2020 doi: 10.1056/NEJMp2007637. https://doi.org.10.1056/NEJMp2007637 published online April 9. [DOI] [PubMed] [Google Scholar]
- 31.Redaniel MT, Martin RM, Ridd MJ, Wade J, Jeffreys M. Diagnostic intervals and its association with breast, prostate, lung and colorectal cancer survival in England: historical cohort study using the Clinical Practice Research Datalink. PLoS One. 2015;10 doi: 10.1371/journal.pone.0126608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grotenhuis BA, van Hagen P, Wijnhoven BPL, Spaander MCW, Tilanus HW, van Lanschot JJB. Delay in diagnostic workup and treatment of esophageal cancer. J Gastrointest Surg. 2010;14:476–483. doi: 10.1007/s11605-009-1109-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neal RD. Do diagnostic delays in cancer matter? Br J Cancer. 2009;101(suppl 2):S9–S12. doi: 10.1038/sj.bjc.6605384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aggarwal A, Herz N, Campbell P, Arkush L, Short S, Rees J. Diagnostic delay and survival in high-grade gliomas - evidence of the ‘waiting time paradox’? Br J Neurosurg. 2015;29:520–523. doi: 10.3109/02688697.2015.1012050. [DOI] [PubMed] [Google Scholar]
- 35.Roberts K. Cancer Research UK; June 1, 2020. Over 2 million people waiting for cancer screening, tests and treatments.https://scienceblog.cancerresearchuk.org/2020/06/01/impact-of-coronavirus-on-cancer-services-revealed-over-2-million-people-waiting-for-screening-tests-and-treatments/ [Google Scholar]
- 36.Penman I, Rees C. British Society of Gastroenterology guidance on recommencing gastrointestinal endoscopy in the deceleration and early recovery phases of COVID-19 pandemic. June 30, 2020. https://wmcanceralliance.nhs.uk/images/Documents/Covid-19_2020/BSG_Endoscopy_early_recovery_guidance_-_FINAL_30.04.20.pdf [DOI] [PMC free article] [PubMed]
- 37.Office for National Statistics . Office for National Statistics; Aug 12, 2019. Cancer survival in England - adults diagnosed.https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/datasets/cancersurvivalratescancersurvivalinenglandadultsdiagnosed [Google Scholar]
- 38.National Bowel Cancer Audit . The Association of Coloproctology of Great Britain and Ireland, Royal College of Surgeons of England, NHS Digital, Healthcare Quality Improvement Partnership; 2018. Annual report 2018. 2018.https://www.nboca.org.uk/content/uploads/2018/12/NBOCA-annual-report2018.pdf [Google Scholar]
- 39.Kalager M, Wieszczy P, Lansdorp-Vogelaar I, Corley DA, Bretthauer M, Kaminski MF. Overdiagnosis in colorectal cancer screening: time to acknowledge a blind spot. Gastroenterology. 2018;155:592–595. doi: 10.1053/j.gastro.2018.07.037. [DOI] [PubMed] [Google Scholar]
- 40.Tørring ML, Murchie P, Hamilton W. Evidence of advanced stage colorectal cancer with longer diagnostic intervals: a pooled analysis of seven primary care cohorts comprising 11 720 patients in five countries. Br J Cancer. 2017;117:888–897. doi: 10.1038/bjc.2017.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study was based on English national cancer registry data. We do not own these data and hence are not permitted to share them in the original form. The data are available from the Office for Data Release at Public Health England. For access please email odr@phe.gov.uk.