Abstract
Prenatal testosterone (T)–treated sheep, similar to polycystic ovarian syndrome women, manifest reduced cyclicity, functional hyperandrogenism, and polycystic ovary (PCO) morphology. The PCO morphology results from increased follicular recruitment and persistence of antral follicles, a consequence of reduced follicular growth and atresia, and is driven by cell-specific gene expression changes that are poorly understood. Therefore, using RNA sequencing, cell-specific transcriptional changes were assessed in laser capture microdissection isolated antral follicular granulosa and theca cells from age 21 months control and prenatal T–treated (100 mg intramuscular twice weekly from gestational day 30 to 90; term: 147 days) sheep. In controls, 3494 genes were differentially expressed between cell types with cell signaling, proliferation, extracellular matrix, immune, and tissue development genes enriched in theca; and mitochondrial, chromosomal, RNA, fatty acid, and cell cycle process genes enriched in granulosa cells. Prenatal T treatment 1) increased gene expression of transforming growth factor β receptor 1 and exosome component 9, and decreased BCL6 corepressor like 1, BCL9 like, and MAPK interacting serine/threonine kinase 2 in both cells, 2) induced differential expression of 92 genes that included increased mitochondrial, ribosome biogenesis, ribonucleoprotein, and ubiquitin, and decreased cell development and extracellular matrix-related pathways in granulosa cells, and 3) induced differential expression of 56 genes that included increased noncoding RNA processing, ribosome biogenesis, and mitochondrial matrix, and decreased transcription factor pathways in theca cells. These data indicate that follicular function is affected by genes involved in transforming growth factor signaling, extracellular matrix, mitochondria, epigenetics, and apoptosis both in a common as well as a cell-specific manner and suggest possible mechanistic pathways for prenatal T treatment–induced PCO morphology in sheep.
Keywords: ovary, hyperandrogenic disorders, RNA sequencing
Considered a major health problem among women, polycystic ovarian syndrome (PCOS) is an infertility disorder characterized by oligoanovulation, clinical or biochemical hyperandrogenism, and polyfollicular ovary (PCO) morphology (1-3). Genetic predisposition and gene-environment interactions were previously thought to be the primary causes for PCOS (4). However, emerging evidence provides the basis for developmental origins of PCOS, including 1) virilizing congenital adrenal hyperplasia patients with PCOS-like phenotype (5); 2) daughters born to PCOS women with features of PCOS during adolescence (6); and 3) female offspring of gestational androgen-treated animal models that develop a PCOS phenotype (7-9). Specifically, female offspring born to gestational testosterone (T)–treated monkeys, sheep, rats, and mice develop oligoanovulation, hyperandrogenism, and multifollicular ovarian/PCO morphology (10), features that are characteristic of women with PCOS. Ovaries from PCOS women and in prenatal T-treated sheep have enhanced recruitment and follicular persistence, which are likely contributors to PCO morphology (11-15).
Follicular development is an intricately regulated process requiring synchrony between endocrine and various paracrine factors (15, 16). The growth of follicles from primordial to preantral stages is generally considered to be gonadotropin independent and promoted by various locally produced growth factors (17). From the late preantral follicle stage, follicular growth is driven by two gonadotropins: follicle-stimulating hormone (FSH) and luteinizing hormone (LH). Under the influence of FSH, preantral follicles grow and gain an antrum to form the antral follicle. A cohort of antral follicles continues to grow under the process defined as follicle selection, and one among these becomes dominant in women. The dominant follicle becomes responsive to LH, developing into a preovulatory follicle, which undergoes ovulation following the preovulatory LH surge. The remaining antral follicles undergo atresia through apoptosis (17). In ovaries from PCOS women, advanced ultrasound scanning shows an increased presence of antral follicles both less than 2 mm and 2 to 9 mm in range (11, 18, 19). In the prenatal T–treated sheep, histological examination of ovarian serial sections has shown an increase in the number of antral follicles of both less than 1 mm and greater than 1 mm in size (20), indicating that changes in the antral follicle count in prenatal T–treated sheep resemble those observed in women with PCOS. These findings are suggestive of disruptions in folliculogenesis that include FSH action and/or follicular dominance, atresia as well as steroidogenesis.
The ovarian antral follicle consists of somatic cells—granulosa and theca cells—of which theca cells are found on the exterior, receive vascular supply, and produce androgens, whereas granulosa cells are in the interior, are avascular, and aromatize theca-produced androgens into estrogens. In prenatal T–treated sheep, ovarian and cell-specific disruptions in the somatic cells of the antral follicles are evident (16). These are driven by altered gene expression in ovaries (21), some of which are cell specific (22). The changes at the level of protein expression observed are an altered balance of proteins that regulate FSH action (23, 24), extracellular matrix (25), and apoptosis (26) along with changes in steroidogenic enzymes (27), steroid receptors (28), insulin, and gonadotropin sensitizer adiponectin (29), angiogenic vascular endothelial growth factor (VEGF) receptor 3 (30), and gap junction protein connexin 43 (25) in prenatal T–treated Suffolk sheep. In addition, in Scottish Greyface sheep, prenatal T treatment increased theca cell gene expression of steroidogenic genes including steroidogenic acute regulatory protein, 11α hydroxylase, 3β hydroxysteroid dehydrogenase, and 17α hydroxylase (CYP17A1) (31). These paracrine changes likely affect follicular development, function, and fate. For example, changes in proteins that regulate FSH bioavailability, such as increased antimüllerian hormone (AMH) and follistatin with reduced activin and gonadotropin sensitizer adiponectin, may reduce FSH action in antral follicles, curbing growth and differentiation (16). Reduced proapoptotic protein caspase-3 may also impair atresia of follicles, allowing more of them to persist. These findings support the premise that prenatal T treatment adversely affects several cell-specific key genes involving folliculogenesis, but how such perturbed gene interactions promote the PCO morphology phenotype requires knowledge of the crucial gene pathways affected.
Although antral follicular somatic cells are involved in the development of PCO morphology, their cell-specific gene expression changes have not been well documented. High throughput quantitative gene expression analysis via RNA-sequencing of pure granulosa and theca cells, obtained via laser capture microdissection (22), allows whole-cell gene expression profiling without prior probe selection or biases from hybridization of microarrays (32, 33). Bioinformatic analysis of transcriptomes identifies disruptions in gene networks induced by prenatal T treatment both within and between cell types (33). Capitalizing on these approaches, the goals of the present study were to 1) determine altered gene expression in antral follicle granulosa and theca cells induced by prenatal T treatment, 2) identify common and divergent genes and gene family networks involved in programming antral follicle defects in prenatal T–treated sheep, and 3) gain mechanistic insights into the development of the PCO morphology phenotype.
Methods
Animals and prenatal treatment
Ovaries obtained from control and prenatal T–treated sheep generated as described previously were used in this study (34). The protocol was approved by the institutional animal care and use committee of the University of Michigan. Briefly, prenatal T–treated animals were generated by intramuscular administration of 100 mg T propionate (1.2 mg/kg; Sigma-Aldrich) to pregnant ewes twice weekly from days 30 to 90 of gestation (35). Control animals were generated by administration of vehicle for the same duration. Ovaries were collected from control and prenatal T–treated animals at age 21 months (end of second breeding season). Only one offspring was used in the study if twins were born. Estrus was synchronized with prostaglandin F2α (10 mg, intramuscular; Lutalyse, Pfizer) administered twice, 11 days apart, and ovaries were collected 18 hours after the second injection. One ovary was cryopreserved in optimal cutting temperature medium (Thermo Fisher) and stored at –80 °C until used. Developmental changes in ovarian follicular distribution and changes in gene expression of apoptotic factors, steroid receptors, steroidogenic enzymes, matrix proteins, ovarian VEGF, AMH, and epigenetic enzymes from animals used in this study have been previously published (20, 22, 25-30).
Laser capture microdissection
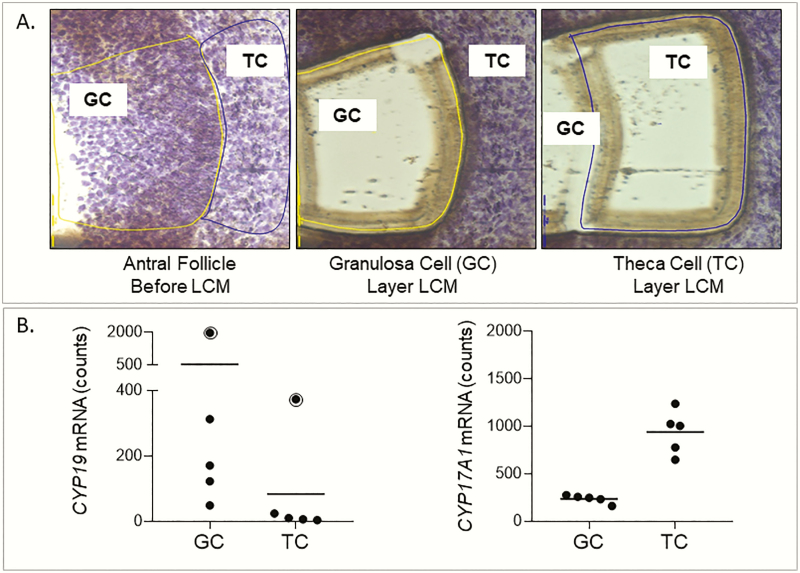
Ovaries cryopreserved in optimal cutting temperature medium were sectioned at 14-µm thickness using a Leica CM1950 cryotome onto polyethylene naphthalate membrane glass slides (Leica Microsystems). Two to three slides from each animal were stained with 1% cresyl violet solution to aid in visualization of follicular structures, air dried, and subject to laser capture microdissection using an LMD7000 system (Leica). Antral follicle granulosa and theca cells were visualized under microscope (Fig. 1) and captured with the following parameters of power: 30, aperture: 20, speed: 10, specimen balance: 25, head current: 100, pulse frequency: 800. Granulosa and theca cells were microdissected (Fig. 1A) from multiple antral follicles from each animal and pooled in Qiagen RLT buffer and stored at −80 °C. The purity of the isolated cells were ascertained through real-time polymerase chain reaction detection of high levels of aromatase (CYP19) and CYP17A1 expression in granulosa and theca cells, respectively, as reported previously (22).
Figure 1.
Purity of laser capture microdissected granulosa (GC) and theca (TC) cells from sheep ovary. A, Images of antral follicular wall with GC and TC layer before and after laser capture microdissection of respective cell layers. B, Normalized counts of CYP19 and CYP17A1 in GCs and TCs are shown. CYP19 expression in GCs and TCs of one animal was higher and is shown with a dot symbol enclosed in a circle.
RNA isolation, library preparation, and sequencing
RNA extraction was performed using the Qiagen RNeasy Micro isolation kit. RNA was submitted to the University of Michigan Advanced Genomics Core for preparation of complementary DNA libraries and RNA sequencing on matched granulosa and theca cell samples from control (n = 5) and prenatal T–treated animals (n = 5). Ribosomes were depleted and libraries were prepared using a SMARTer Stranded Total RNA-Seq Kit v2–Pico Input Mammalian kit (Takara). Libraries were sequenced on the HiSeq NovaSeq 6000 platform (Illumina). Paired-end sequencing was performed for 51 cycles. Raw and processed data from this study are available at the Genome Expression Omnibus (GEO, accession number GSE150236).
Data processing and quality control
Quality control metrics on raw fastq files for the 22 samples were examined using fastQC (version 0.11.5) (36) and multiQC (version 0.9) (37). Mean read quality scores were high across base positions, except for reduced quality observed in the first 3 base pairs in the second read of the paired end reads. Samples that had granulosa cell content ranging from 54% to 62%, and between 46% to 75% of reads were duplicated. Paired end reads were mapped to a sheep reference genome (Oar_rambouillet_v1.0) using the Spliced Transcripts Alignment to a Reference (version 2.6.0c) program (38). The first 3 base pairs of the second read of each paired read were clipped because of the decrease in quality score observed. QoRTs (version 1.3.6) (39) was used to examine quality control metrics postalignment. Alignment soft clipping rate was approximately 40% at the start of read 1. Approximately 35% to 55% of reads per sample were dropped for multimapping and 60% to 70% of reads were mapped to gene regions. Following mapping, featureCounts (version 1.6.1) (40) was used to quantify aligned reads mapping to exons. The ovary of one prenatal T–treated animal contained a large flocculent fluid-filled follicle and differed from all other prenatal animals that had multiple antral follicles and hence was excluded from further analysis.
Differential expression analysis
Codes to conduct the analyses presented in this manuscript are available through GitHub (https://github.com/bakulskilab). Processed data were analyzed in R statistical software (version 3.6.0) with the DESeq2 package (version 1.24.0) (41). Principal component analysis was performed, calculated on variance-stabilizing transformed values of the expression data. Principal components were plotted to visualize clustering by cell type and treatment. As a positive control check of laser capture microdissection purity, known cell type–specific genes (CYP19 gene in granulosa cells and CYP17A1 in theca cells) were tested in controls for differential expression by cell type.
Genome-wide differentially expressed genes were first examined by cell type (theca vs granulosa cells) among the control and prenatal T–treated animals, separately, with correction for individual sheep. Second, differentially expressed genes were tested within granulosa cells and by prenatal T treatment (T treated vs control). Third, expression in the prenatal T–treated group was compared to the control group in theca cells. Default settings for DESeq2 were used for filtering of genes with low normalized mean counts. Log2 fold-change shrinkage was performed using the “apeglm” prior (42). Volcano plots of log fold-change estimates were created with the EnhancedVolcano (version 1.2.0) package. To account for multiple comparisons, an adjusted P value of less than the .05 threshold was applied. We considered genes with a fold change greater than 2 or less than 0.5 (absolute log2 fold change greater than 2). Genes meeting both the statistical and fold change criteria were prioritized.
Common differences in gene expression by prenatal T treatment across cell types were assessed. Differentially expressed genes by prenatal T treatment meeting statistical criteria (adjusted P < .05) in granulosa or theca cells individually were compared across cell types.
Gene set enrichment
Differentially expressed genes were tested for enrichment of gene set terms with RNA-enrich (43). First, sheep genes were annotated to human orthologs using Ensembl identifiers and the BioMart package (44). Three pathway analyses were performed using the directional RNA-Enrich test for 1) cell type comparison, 2) prenatal T treatment in granulosa cells, and 3) prenatal T treatment in theca cells. For the tests, the Biocarta Pathway, EHMN metabolic pathways, Gene Ontology, KEGG Pathway, Panther Pathway, and transcription factors databases were selected. Maximum concept size was set to 1000 genes, and other options left at default values. To account for multiple comparisons, a false discovery rate (FDR) of less than a .01 threshold was applied.
Common enriched pathways by prenatal T treatment across cell types were assessed. Prenatal T treatment enriched pathways meeting statistical criteria (FDR < 0.01) in granulosa or theca cells individually were compared across cell types. Enriched pathways unique to a cell type were identified.
Gene interaction networks were visualized using the STRING database of protein-protein interactions (45). Gene interactions required a minimum interaction score of 0.3 for inclusion as edges. Genes with P less than .05 were shown as nodes.
Results
Descriptive statistics
Each sample from control and prenatal T treated animals produced a range of 12 million to 26 million reads that were assigned to between 18 031 and 21 668 genes (Supplemental Table 1 [46]). Samples clustered by cell type in principal component analysis (Supplemental Figure 1 [46]).
Purity of laser capture microdissection isolated granulosa and theca cells
To confirm the purity of cells isolated by laser capture microdissection, known cell type marker genes were tested for gene expression differences in control animals. CYP19 expression from one animal was higher both in granulosa and theca cells (Fig. 1B). Granulosa cells had a 10.5-fold higher expression (adjusted P = 3.1 × 1015) of the CYP19 gene. In one animal, very high levels of CYP19 expression were evident in granulosa and theca cells; however, the difference in the expression was still about 5.2-fold higher in the granulosa cells (Fig. 1B). Theca cells had a 3.9-fold higher expression (adjusted P = 1.3 × 10–26) of CYP17A1 (Fig. 1B; Supplemental Table 2 [46]). In addition, several other genes known to be highly expressed in granulosa and theca cells showed an expected direction of higher expression in the respective cell types (Table 1). Granulosa cells had higher expression of AMH and its receptor (AMHR2), follicle-stimulating hormone receptor (FSHR), follistatin (FST), inhibin and activin (INHA, INHBA, and INHBB), Indian hedgehog (IHH), and versican (VCAN). In contrast, theca cells showed expected higher expression of actin alpha 2, smooth muscle (ACTA2), collagen (COL1A1, COL1A2, and COL14A1), nerve growth factor (NGF), and ret proto-oncogene (RET).
Table 1.
Differentially Expression of Known Genes Between Granulosa (Gray Shaded Rows) and Theca Cells Among Control and Prenatal T-treatment Groups
| Control animals | Prenatal T-treated animals | |||||
|---|---|---|---|---|---|---|
| Gene | Mean expression granulosa | Mean expression theca | P | Mean expression granulosa | Mean expression theca | P |
| ACTA2 | 1589.1 | 41566.56 | 0.000 | 821.99 | 35744.89 | 0.000 |
| AMH | 4624.97 | 692.65 | 0.000 | 4650.43 | 862 | 0.000 |
| AMHR2 | 158.45 | 52.49 | 0.000 | 105.97 | 40.79 | 0.000 |
| COL14A1 | 1742.6 | 13995.48 | 0.000 | 623.96 | 10776.29 | 0.000 |
| CYP17A1 | 234.56 | 937.93 | 0.000 | 138.93 | 1338.37 | 0.000 |
| CYP19 | 523.76 | 83.17 | 0.000 | 1065.79 | 69.27 | 0.000 |
| FOXO1 | 15483.75 | 4919.47 | 0.000 | 11877.31 | 3481.16 | 0.000 |
| FRZB | 155.27 | 1255.75 | 0.000 | 38.85 | 915 | 0.000 |
| FSHR | 2108.8 | 337.73 | 0.000 | 2025.51 | 384.82 | 0.000 |
| FST | 14684.45 | 2145.9 | 0.000 | 13319.58 | 2361.29 | 0.000 |
| GJA1 | 16622.82 | 6599.32 | 0.000 | 19601.03 | 8802.21 | 0.000 |
| IHH | 4196.33 | 309.48 | 0.000 | 3053.75 | 346.24 | 0.000 |
| INHA | 19194.39 | 2921.61 | 0.000 | 20306.35 | 3374.95 | 0.000 |
| INHBA | 18949.94 | 3483.16 | 0.000 | 22319.3 | 4207.14 | 0.000 |
| INHBB | 9348.65 | 1583.37 | 0.000 | 8810.38 | 1522.57 | 0.000 |
| LHCGR | 544.12 | 1817.74 | 0.000 | 633.47 | 2487.79 | 0.002 |
| NGF | 13.89 | 86.59 | 0.000 | 4.56 | 90.28 | 0.000 |
| RET | 21.45 | 68.35 | 0.000 | 19.77 | 16.58 | 0.82 |
| RUNX1 | 1626.18 | 449.47 | 0.000 | 1537.59 | 402.42 | 0.000 |
| VCAN | 24038.22 | 7103.77 | 0.000 | 31599.66 | 9532.22 | 0.000 |
Genes highly expressed in granulosa cells are shaded in grey.
Gene expression differences between granulosa and theca cells
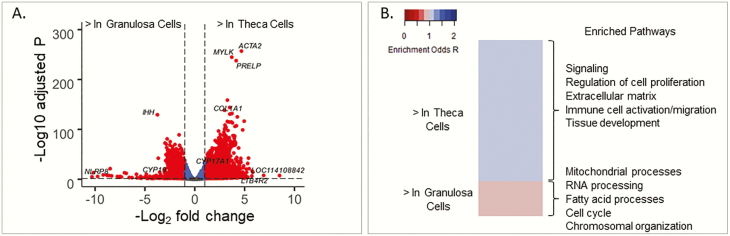
In control animals, 19 331 genes were eligible for analysis. Comparing granulosa and theca cells, 8151 genes statistically differed (adjusted P < .05) and 3494 gene magnitudes differed by at least 2-fold higher or 0.5-fold lower. Combining both of those metrics, 3447 genes met statistical and magnitude criteria (Fig. 2A; Supplemental Table 2 [46]). The genes with the most increased differential expression in the granulosa cells included NLR family pyrin domain containing 8 (NLRP8; 1269.5-fold higher in granulosa; adjusted P = 9.4 × 10–6), WEE2 oocyte meiosis inhibiting kinase (WEE2; 1176.3-fold higher in granulosa; adjusted P = 5.0 × 10–14), UNC homeobox (UNCX, 861.1-fold higher in granulosa; adjusted P = 3.4 × 10–6), as well as the uncharacterized genes LOC105609869 (soma ferritin-like; 588.1-fold higher in granulosa; adjusted P = 2.0 × 10–9) and LOC101102259 (NACHT, LRR, and PYD domains containing protein 7; 445.7-fold higher in granulosa; adjusted P = 1.2 × 10-8) (Table 2). The genes with the most increased differential expression in theca cells included the uncharacterized genes LOC114108842 (immunoglobulin λ variable 1-40-like; 362.0-fold higher in theca; adjusted P = 3.3 × 10–8), LOC114109061 (immunoglobulin heavy constant γ 4-like; 55.7-fold higher in theca; adjusted P = 7.7 × 10–15), and LOC114109091 (immunoglobulin heavy variable 4-59-like; 52.0-fold higher in theca; adjusted P = 3.9 × 10–7), as well as leukotriene B4 receptor 2 (LTB4R2; 119.4-fold higher in theca; adjusted P = 1.8 × 10–8) and protein phosphatase 2 regulatory subunit Bγ (PPP2R2C; 55.7-fold higher in theca; adjusted P = 5.3 × 10–17) (Table 2). Among the prenatal T–treated animals, the directionality of differentially expressed genes between theca and granulosa cells was similar to that in control animals (Supplemental Table 3) with the correlation between the effect estimates in the 2 comparisons of 0.88.
Figure 2.
A, Differential gene expression comparing theca controls vs granulosa controls. Red points have absolute log fold change greater than 1 and adjusted P value less than .05. B, Heat map of pathways with enrichment false discovery rate less than .01 comparing theca and granulosa cells from control animals, with some key gene pathways highlighted as enriched in each cell type.
Table 2.
Differentially Expressed Genes for Theca Versus Granulosa Comparison
| Gene | Mean expression granulosa | Mean expression theca | Log fold-change estimate | SE | P | Adjusted P |
|---|---|---|---|---|---|---|
| NLRP8 | 132.51 | 0 | –10.31 | 3.77 | 0.000 | 0.000 |
| WEE2 | 73.61 | 0 | –10.16 | 3.01 | 0.000 | 0.000 |
| UNCX | 79.63 | 0 | –9.75 | 3.43 | 0.000 | 0.000 |
| LOC105609869 | 43.2 | 0 | –9.18 | 2.95 | 0.000 | 0.000 |
| LOC101102259 | 35.45 | 0 | –8.81 | 2.93 | 0.000 | 0.000 |
| ESRP1 | 34.98 | 0 | –8.8 | 2.91 | 0.000 | 0.000 |
| NLRP9 | 31.8 | 0 | –8.62 | 2.93 | 0.000 | 0.000 |
| LOC114108842 | 0 | 29.54 | 8.5 | 2.88 | 0.000 | 0.000 |
| ACCSL | 193.71 | 0.46 | –8.49 | 1.04 | 0.000 | 0.000 |
| ASTL | 27.62 | 0 | –8.34 | 2.91 | 0.000 | 0.000 |
| LOC101115661 | 20.62 | 0 | –7.76 | 2.87 | 0.000 | 0.000 |
| NPM2 | 18.98 | 0 | –7.54 | 2.91 | 0.000 | 0.000 |
| ZAR1 | 18.09 | 0 | –7.5 | 2.86 | 0.000 | 0.000 |
| ZGLP1 | 17.53 | 0 | –7.37 | 2.95 | 0.000 | 0.000 |
| ZP3 | 78.76 | 0.45 | –7.05 | 1.09 | 0.000 | 0.000 |
| LTB4R2 | 0.18 | 33.7 | 6.91 | 1.45 | 0.000 | 0.000 |
| KPNA7 | 63.06 | 0.43 | –6.88 | 1.11 | 0.000 | 0.000 |
| EIF4E1B | 12.32 | 0 | –6.57 | 2.92 | 0.000 | 0.000 |
| HDC | 10.71 | 0 | –6.3 | 2.94 | 0.000 | 0.000 |
| PADI6 | 52.89 | 0.7 | –5.85 | 0.97 | 0.000 | 0.000 |
| LOC114109061 | 17.88 | 663.78 | 5.81 | 0.81 | 0.000 | 0.000 |
| OOEP | 9.05 | 0 | –5.79 | 2.97 | 0.000 | 0.000 |
| PPP2R2C | 2.15 | 96.56 | 5.76 | 0.72 | 0.000 | 0.000 |
| LOC114109091 | 0.49 | 25.45 | 5.72 | 1.32 | 0.000 | 0.000 |
| C13H10orf113 | 7.85 | 0 | –5.53 | 2.93 | 0.000 | 0.000 |
Among genes with adjusted P-value less than .05, the top 25 by fold change magnitude have been selected.
Genes differentially expressed between granulosa and theca cells were enriched in 789 gene sets (FDR < .01) (Supplemental Table 4 [46]). Summarizing overall patterns, the theca cells had upregulated gene pathways involved in cell signaling, regulation of cell proliferation, extracellular matrix, immune cell activation/migration, and tissue development (including angiogenesis, vascular smooth muscle contraction, and cardiovascular and neuron development) (Fig. 2B). In contrast, granulosa cells had upregulated gene pathways involved in mitochondrial processes, chromosomal organization and segregation, RNA processing, and fatty acid and cell cycle process (Fig. 2B).
Prenatal T–induced gene expression changes in granulosa cells
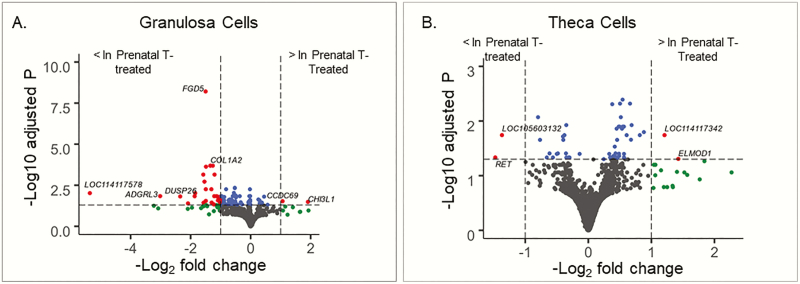
In granulosa cells, 16 207 genes were eligible for analysis. Granulosa cell samples clustered by treatment groups when genes differentially expressed by prenatal T treatment (n = 200, ordered by P value) (Supplemental Fig. 2A [46]) were considered. Comparing granulosa cell gene expression between controls and prenatal T–treated sheep, 92 genes met differential expression statistical criteria (adjusted P < .05), 54 met differential expression magnitude criteria (fold change > 2.0 or fold change < 0.5), and 29 genes met both thresholds (Fig. 3A; Supplemental Table 5 [46]). Of these 29 genes, only 2 genes had higher expression with prenatal T treatment and these were chitinase 3 like 1 (CHI3L1; 3.7-fold higher with prenatal T; adjusted P = .03) and coiled-coil domain containing 69 (CCDC69; 2.1-fold higher with prenatal T; adjusted P = .03). The remaining genes had lower expression with prenatal T treatment, including the uncharacterized genes LOC114117578 (42.2-fold higher in control; adjusted P = .01) and LOC101104028 (3.7-fold higher in control; adjusted P = .01), as well as adhesion G protein-coupled receptor L3 (ADGRL3; 8.0-fold higher in control; adjusted P = .01), dual specificity phosphatase 26 (DUSP26; 4.9-fold higher in control; adjusted P = .02), and reticulon 4 receptor like 1 (RTN4RL1; 4.3-fold higher in control; adjusted P = .04) (Table 3).
Figure 3.
Differential gene expression by prenatal testosterone (T) treatment in A, granulosa and B, theca cells. Blue points have an adjusted P value of less than .05, green points have a magnitude log fold change of greater than 1.0, and red points fit both cutoffs.
Table 3.
Prenatal Testosterone Induced Differentially Expressed Genes in the Granulosa Cells
| Gene | Mean expression control | Mean expression testosterone | Log fold-change estimate | SE | P | Adjusted P |
|---|---|---|---|---|---|---|
| LOC114117578 | 6.72 | 0 | –5.36 | 2.4 | 0.000 | 0.010 |
| ADGRL3 | 11.6 | 1.14 | –3.02 | 0.78 | 0.000 | 0.010 |
| DUSP26 | 16.01 | 2.45 | –2.35 | 0.67 | 0.000 | 0.020 |
| RTN4RL1 | 11.42 | 2.04 | –2.09 | 0.7 | 0.000 | 0.040 |
| CHI3L1 | 86.25 | 414.74 | 1.91 | 0.65 | 0.000 | 0.030 |
| LOC101104028 | 122.35 | 27.43 | –1.86 | 0.57 | 0.000 | 0.010 |
| LOC114114762 | 37.28 | 8.66 | –1.85 | 0.51 | 0.000 | 0.010 |
| SYT1 | 102.8 | 30.82 | –1.58 | 0.35 | 0.000 | 0.000 |
| RAB40B | 17.37 | 4.8 | –1.57 | 0.54 | 0.000 | 0.040 |
| FRZB | 141.58 | 43.37 | –1.54 | 0.37 | 0.000 | 0.000 |
| FGD5 | 497.55 | 168.05 | –1.5 | 0.22 | 0.000 | 0.000 |
| TMEM151B | 24.27 | 7.18 | –1.49 | 0.49 | 0.000 | 0.030 |
| CXXC4 | 57.23 | 17.71 | –1.49 | 0.39 | 0.000 | 0.010 |
| CTTNBP2 | 286.02 | 93.65 | –1.49 | 0.31 | 0.000 | 0.000 |
| COL1A2 | 16532.05 | 6052.64 | –1.34 | 0.28 | 0.000 | 0.000 |
| COL1A1 | 28787.45 | 10571.59 | –1.34 | 0.27 | 0.000 | 0.000 |
| COL3A1 | 20153.77 | 7298.35 | –1.31 | 0.34 | 0.000 | 0.010 |
| SLC1A6 | 62.97 | 21.29 | –1.3 | 0.46 | 0.000 | 0.040 |
| FBXW4 | 134.06 | 52.67 | –1.26 | 0.26 | 0.000 | 0.000 |
| LOC101111382 | 121.02 | 42.9 | –1.23 | 0.47 | 0.000 | 0.050 |
| LTBP4 | 671.5 | 255.46 | –1.21 | 0.36 | 0.000 | 0.010 |
| WDR87 | 59.8 | 23.87 | –1.2 | 0.27 | 0.000 | 0.000 |
| COL6A1 | 7917.28 | 3204.69 | –1.14 | 0.34 | 0.000 | 0.010 |
| SLC16A9 | 41.74 | 17.23 | –1.1 | 0.33 | 0.000 | 0.020 |
| TTC9 | 69.85 | 28.52 | –1.09 | 0.36 | 0.000 | 0.030 |
Among genes with adjusted P-value less than .05, the top 25 by fold change magnitude have been selected.
In granulosa cells, genes differentially expressed between controls and prenatal T treatment were enriched in 1024 pathways (FDR < .01). Prenatal T treatment upregulated pathways involved in mitochondrial inner membrane, envelope, matrix and membrane, mitochondrion organization, ribosome biogenesis, ribonucleoprotein related, ribosomal RNA (rRNA) processing and metabolic process, ubiquitin-related proteins, and antigen processing and presentation of peptide antigen via major histocompatibility complex class I. Downregulated pathways included regulation of cell development, and extracellular matrix components (Supplemental Table 6 [46).
Prenatal T–induced gene expression changes in theca cells
In theca cells, 14 298 genes were eligible for analysis. Theca cell samples clustered by treatment group when genes differentially expressed by prenatal T treatment (n = 200, ordered by P value) (Supplemental Fig. 2B [46]) were considered. Comparing theca cell gene expression differences between controls and prenatal T–treated sheep (Table 4), 56 genes met differential expression statistical criteria (adjusted P < .05), 19 genes met differential expression magnitude criteria (fold change > 2.0 or fold change < 0.5), and 4 genes met both criteria (Supplemental Table 7, Fig. 3B [46]). These 4 genes included 2 genes with higher expression with prenatal T treatment: ELMO domain containing 1 (ELMOD1; 2.6-fold higher with prenatal T; adjusted P = .049) and sterol O-acyltransferase 1-like (LOC114117342; 2.3-fold higher with prenatal T; adjusted P = .02). Two genes had lower gene expression with prenatal T treatment: RET (2.8-fold higher in control; adjusted P = .046) and uncharacterized gene (LOC105603132; 2.6-fold higher in control; adjusted P = .02).
Table 4.
Prenatal Testosterone Induced Differentially Expressed Genes in the Theca Cells
| Gene | Mean expression control | Mean expression testosterone | Log fold-change estimate | SE | P | Adjusted P |
|---|---|---|---|---|---|---|
| RET | 62.89 | 18.46 | –1.48 | 0.52 | 0.000 | 0.046 |
| ELMOD1 | 11.8 | 39.22 | 1.43 | 0.51 | 0.000 | 0.049 |
| LOC105603132 | 147.63 | 49.59 | –1.37 | 0.41 | 0.000 | 0.018 |
| LOC114117342 | 81.14 | 211.42 | 1.21 | 0.35 | 0.000 | 0.018 |
| ENPEP | 141.92 | 283.49 | 0.88 | 0.25 | 0.000 | 0.016 |
| SRGN | 2008.46 | 3855.93 | 0.82 | 0.24 | 0.000 | 0.018 |
| LENG9 | 152.35 | 82.56 | –0.8 | 0.2 | 0.000 | 0.009 |
| MAP3K9 | 208.99 | 113.58 | –0.77 | 0.23 | 0.000 | 0.022 |
| TTC27 | 1271.42 | 2162.96 | 0.69 | 0.18 | 0.000 | 0.012 |
| NMD3 | 612.76 | 1046.82 | 0.68 | 0.2 | 0.000 | 0.022 |
| MRPL1 | 239.99 | 396.55 | 0.66 | 0.15 | 0.000 | 0.005 |
| DAGLA | 147.15 | 85.49 | –0.66 | 0.23 | 0.000 | 0.046 |
| CACNB3 | 578.14 | 351.78 | –0.62 | 0.2 | 0.000 | 0.039 |
| GJA1 | 6069.48 | 9794.95 | 0.61 | 0.17 | 0.000 | 0.018 |
| LOC114108673 | 87.26 | 139.1 | 0.57 | 0.19 | 0.000 | 0.046 |
| MANEA | 153.17 | 242.02 | 0.57 | 0.18 | 0.000 | 0.040 |
| DCTN6 | 417.7 | 661.39 | 0.56 | 0.19 | 0.000 | 0.046 |
| PTGR2 | 345.58 | 535.01 | 0.56 | 0.15 | 0.000 | 0.013 |
| LOC101114597 | 100.61 | 63.41 | –0.56 | 0.19 | 0.000 | 0.046 |
| CDC40 | 605.59 | 925.25 | 0.55 | 0.15 | 0.000 | 0.013 |
| NOC3L | 751.45 | 1129.17 | 0.54 | 0.12 | 0.000 | 0.004 |
| VBP1 | 289.85 | 440.33 | 0.53 | 0.15 | 0.000 | 0.014 |
| SUCO | 1450.75 | 2150.78 | 0.51 | 0.13 | 0.000 | 0.009 |
| LOC101118051 | 337.71 | 494.31 | 0.5 | 0.12 | 0.000 | 0.005 |
| MCOLN1 | 321.06 | 214.75 | –0.5 | 0.16 | 0.000 | 0.040 |
Among genes with adjusted P-value less than .05, the top 25 by fold change magnitude have been selected.
In theca cells, genes differentially expressed between controls and prenatal T treatment were enriched in 195 pathways (FDR < .01). Among prenatal T–treated sheep, upregulated pathways included noncoding RNA processing, ribosome biogenesis, and mitochondrial matrix. Prenatal T treatment downregulated many transcription factor pathways, including ZID, OLF1, and RFX1 (Supplemental Table 8 [46]).
Prenatal T–induced common changes in both ovarian cell types
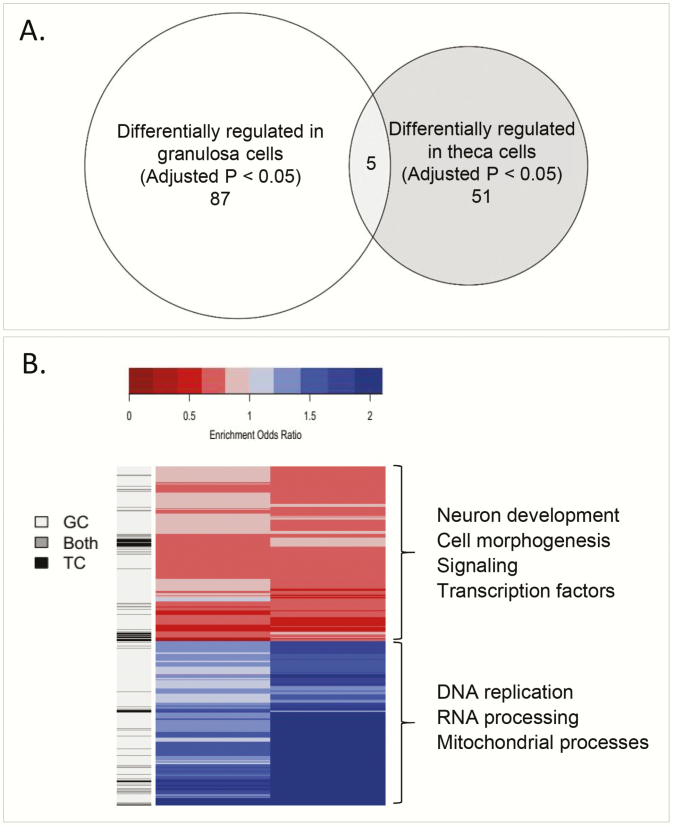
Only 5 genes (Fig. 4A) were found to be significantly modulated by prenatal T treatment in both cell types (adjusted P < .05). Two genes had higher expression with prenatal T treatment in both cell types: exosome component 9 (EXOSC9) and transforming growth factor β receptor 1 (TGFβR1). Three genes had lower expression with prenatal T treatment in both cell types: BCL6 corepressor like 1 (BCORL1), BCL9 like (BCL9L), and MAPK interacting serine/threonine kinase 2 (MKNK2).
Figure 4.
A, Venn diagram of genes in common between granulosa and theca cells at an adjusted P less than .05 level (BCORL1, EXOSC9, TGFBR1, BCL9L, MKNK2). B, Heat map of pathways with a false discovery rate of less than .01 in either granulosa or theca. (1079 pathways, 140 both, 55 theca cells only, 884 granulosa cells only), with some key pathways highlighted in either or both cell types.
There were 140 pathways differentially enriched by prenatal T treatment (FDR < .01) both in granulosa and theca cells. In general, these pathways were differentially enriched in the same direction in both cell types (both upregulated in 55, both downregulated in 43, opposite direction in 42). Pathways upregulated by prenatal T treatment in both tissues involved DNA replication, RNA processing, and mitochondrial processes. Some specific pathways in particular include noncoding RNA, rRNA, and transfer RNA (tRNA) metabolic process, tRNA modification, rRNA processing, ribosome and ribonucleoprotein complex biogenesis, mitochondrial matrix, cellular amino acid metabolic process and its regulation, and antigen processing and presentation of peptide antigen via major histocompatibility complex class I. Pathways downregulated by prenatal T treatment in both tissues included cell morphogenesis, signaling, and transcription factors (such as ZID, OLF1, RFX1, TAXCREB, and RREB1) (Fig. 4B, Supplemental Tables 6 and 8 [46]).
Gene networks
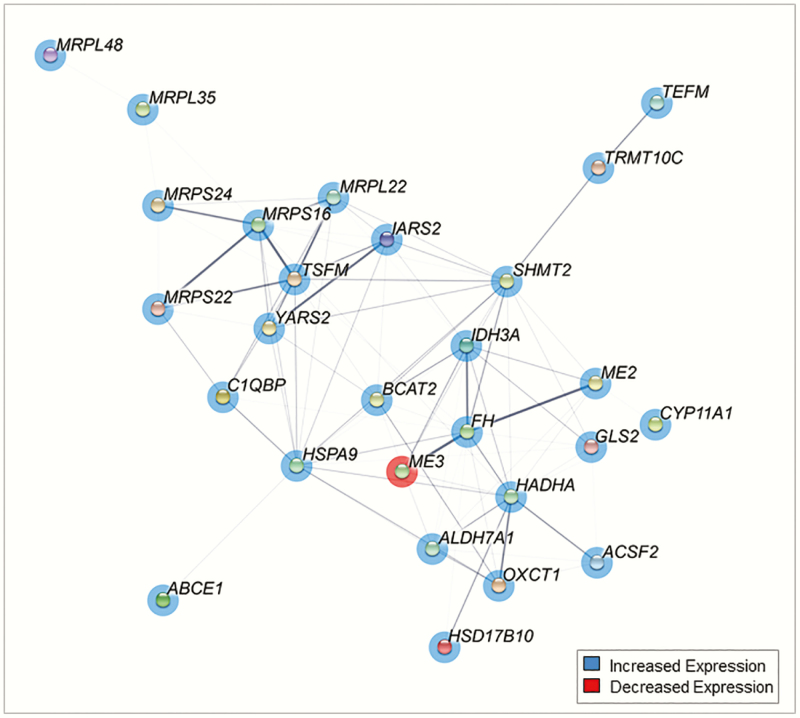
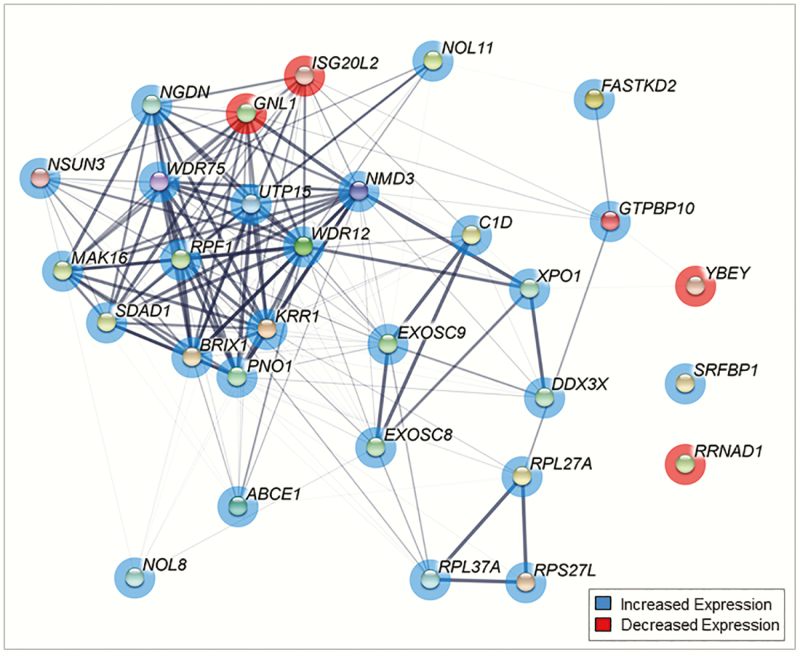
Prenatal T treatment differentially expressed a cluster of genes in granulosa cells (P < .05) involved in the mitochondrial matrix pathway (Fig. 5). Among these, key genes with many protein interactions include Ts Translation Elongation Factor, Mitochondrial (TSFM), and Hydroxyacyl-CoA Dehydrogenase Trifunctional Multienzyme Complex Subunit Alpha (HADHA). Prenatal T treatment differentially expressed a cluster of genes in theca cells involved in the ribosome biogenesis pathway (P < .05) (Fig. 6). These genes had dense networks of interaction with each other with many cross-protein interactions. The key genes in this interaction included ribosome production factor 1 homolog (RPF1), NMD3 ribosome export adaptor (NMD3), biogenesis of ribosomes BRX1 (BRIX1), and WD repeat domain 12 (WDR12) and 75 (WDR75).
Figure 5.
Network diagram of gene interactions of genes involved in mitochondrial matrix in granulosa cells from prenatal testosterone (T)-treated sheep. Minimum interaction score of 0.3. Genes with P less than .05 are shown as nodes. Genes in red are downregulated and blue are upregulated, and pink are both. Thickness of connection between nodes represent the level of evidence for interactions.
Figure 6.
Network diagram of gene interactions of genes involved in ribosomal biogenesis in theca cells from prenatal testosterone (T)-treated sheep. Minimum interaction score of 0.3. Genes with P less than .05 are shown as nodes. Genes in red are downregulated and blue are upregulated, and pink are both. Thickness of connection between nodes represent the level of evidence for interactions.
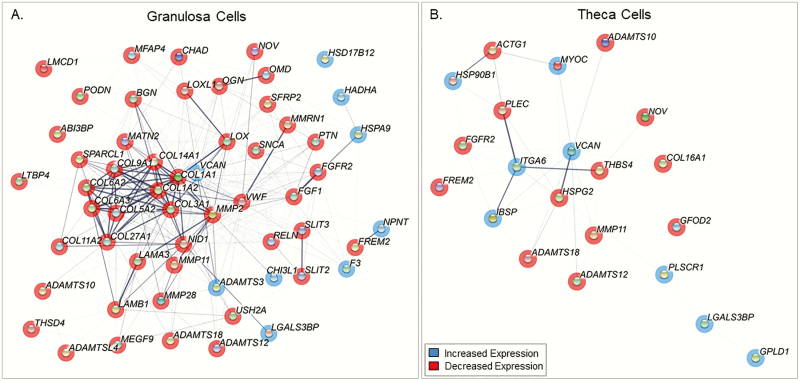
Prenatal T treatment differentially expressed extracellular matrix-related genes both in granulosa and theca cells (P < .05) (Fig. 7). Compared to theca cells, granulosa cells had denser and many cross-network interactions (Fig. 7A). The key extracellular matrix-related genes induced by prenatal T treatment in granulosa cells include collagen genes (COL1A1, COL3A1, COL14A1, COL1A2, COL6A3, COL6A2), VCAN, and biglycan. In contrast, theca cells showed few interactions and genes, including VCAN, thrombospondin 4, integrin subunit alpha 6, plectin, and heparan sulfate proteoglycan 2 (Fig. 7B).
Figure 7.
Network diagram of gene interactions of genes involved in extracellular matrix in A, granulosa and B, theca cells from prenatal testosterone (T)-treated sheep. Minimum interaction score of 0.3. Genes with P less than .05 are shown as nodes. Genes in red are downregulated and blue are upregulated, and pink are both. Thickness of connection between nodes represent the level of evidence for interactions.
Discussion
This study demonstrates that adult ovarian granulosa and theca cells show distinct gene expression profiles in sheep. Granulosa cells predominantly express genes involved in mitochondrial processes, chromosomal organization, and RNA processing, whereas theca cells predominantly express genes involved in cell signaling and proliferation, extracellular matrix, and immune cell migration. In both cell types, prenatal T treatment upregulated genes related to mitochondrial, ribosomal, ribonucleoprotein, and antigen processing, whereas various transcription factors were downregulated in both cell types. Cell-specific regulation following prenatal T treatment was also observed. Prenatal T–treated granulosa cells had upregulated regulators of ubiquitin activity, whereas prenatal T–treated theca cells had upregulated tRNA processing and amino acid metabolism. These findings provide the basis for gene expression changes that both are cell specific and common to both cell types, which may contribute to the development of PCOS-like ovarian morphology in prenatal T–treated female sheep.
Granulosa- and theca-specific gene expression in control sheep
Granulosa cells had high levels of CYP19 gene expression, whereas theca cells had high CYP17A1 messenger RNA levels, attesting to the purity of these cell types by laser capture microdissection, and confirming our earlier findings (22). In addition, in line with previous reports (24, 47, 48), several genes known to be highly expressed in the granulosa cells (AMH, AMHR2, FST, IHH, inhibin, and activin) are highly expressed. Similarly, higher expression of ACTA2 and NGF in theca cells (Table 1) is consistent with the higher levels of ACTA2 protein and NGF expression found in theca layers (49, 50). The expected directionality of cell-specific expression of key granulosa and theca genes attest to the efficacy of laser capture microdissection approach for obtaining pure granulosa and theca cells. The expected directionality of granulosa- and theca-specific genes in prenatal T–treated animals paralleled that of controls (Table 1)
Paradoxically, genes predominantly expressed in the oocyte, such as WEE2, NLRP8, ASTL, zygote arrest 1, and eukaryotic translation initiation factor 4E family member 1B (Table 1) (51-55), were highly expressed in the granulosa cells, although the significance of these findings relative to granulosa cell function is unclear. Many extracellular matrix-related genes were highly expressed in theca cells, including multiple genes belonging to the collagen superfamily. These findings agree with findings in humans and rodents of the presence of collagens in the basal lamina separating granulosa and theca cells, theca interna and externa, and perifollicular area (56-58). The higher expression of collagen genes in the theca cells indicate that these cells are likely the source of perifollicular and intrafollicular collagen. The changes in extracellular matrix-related genes were also accompanied by higher expression of matrix-regulating proteases and their inhibitors (Supplemental Table 2 [46]), which are required for extracellular matrix turnover and follicular growth (59). Theca cells also showed specific enrichment of genes related to angiogenesis, vascular smooth muscle contraction, cardiovascular, and vascular development. These findings concur with earlier reports that antral follicular vasculature is confined to the theca cell layer, with the granulosa cell layer being avascular (60). Granulosa cells were enriched with genes involving mitochondrial process, chromosomal organization and segregation, RNA processing, and fatty acid and cell cycle processes (Supplemental Table 4 [46]), which may represent the high proliferative/mitotic and metabolic activities in antral follicles (61, 62).
Effect of prenatal T treatment on granulosa and theca cell gene expression
Prenatal T treatment increased CHI3L1, also known as YKL-40, a gene highly upregulated in porcine granulosa cells during follicular growth (63) and found to be positively associated with metabolic features of PCOS (64, 65). Among PCOS women, plasma levels of CHI3L1 are positively associated with body mass index, total and free T, triglycerides, the inflammatory marker C-reactive protein (64), and with abnormal glucose tolerance (65). Because prenatal T–treated sheep also manifest metabolic characteristics of PCOS women (8), the prenatal T treatment–upregulated granulosa cell CHI3L1 expression suggests the ovary may be a potential source for increased plasma levels of CHI3L1 among PCOS women. In granulosa cells, both individual (Supplemental Table 5 [46]) and gene network analysis (Fig. 7) of extracellular matrix-related genes showed prenatal T treatment downregulated multiple genes encoding members of collagen family (COL1A1, COL1A2, COL3A1, COL6A1, and COL14A1), a finding consistent with the low levels of perifollicular collagen reported previously by us (25) and others (66) in ovaries from prenatal T–treated sheep. Changes in expression of these genes may lead to alterations in the extracellular matrix and contribute to the developmental arrest and accumulation of antral follicles and the consequent appearance of the PCO morphology phenotype in prenatal T–treated sheep.
In theca cells, only a small subset of genes changed more than 2-fold with prenatal T treatment. These included the upregulated genes ELMOD1, a protein coding gene with GTPase activator activity and sterol O-acyltransferase 1-like that catalyzes the formation of fatty acid cholesterol esters, and downregulated RET, another protein-coding gene, a member of the tyrosine protein kinase family of proteins that encodes a transmembrane receptor. The proto-oncogene RET is a receptor for neurotrophic factor glial cell line–derived neurotrophic factor (GDNF), which has a role in regulating follicular growth and oocyte maturation (67); therefore RET downregulation may reduce GDNF action and potentially impair follicular growth. Gene network analysis showed clustering of genes belonging to the ribosome biogenesis pathway that were mostly upregulated (Fig. 6), resembling increased expression of ribosomal transcripts involving ribosome biogenesis, as observed in cumulus cells of PCOS women (68). Ribosomes are macromolecular intracellular machinery involved in the translation of mRNA into proteins (69). How these gene changes alter theca cell function in prenatal T–treated sheep remains to be determined.
Among the 5 genes altered both in granulosa and theca cells, prenatal T treatment increased expression of TGFβR1, a receptor for transforming growth factor B (TGFB) (70), consistent with increases in various TGFB family members such as AMH and follistatin in the prenatal T–treated sheep (16). Interestingly, higher levels of TGFβR1 protein were also observed in granulosa cells from bovine cystic follicles (71). Although the presence of large cystic follicles differs from the multifollicular phenotype observed in prenatal T–treated sheep, both conditions arise from abnormal follicular development characterized by arrest in development and resistance from atresia. Although TGFβR1 signaling may contribute to abnormal follicle growth through TGFB-mediated cell proliferation, differentiation, and apoptosis (70), reduced apoptosis-related BCORL1 and BCL9L gene expression may lead to follicular persistence by reducing follicular atresia, thus providing a potential mechanism for PCO morphology in prenatal T–treated sheep (16). In line with this premise and the functional integrity of follicles in PCOS women with PCO morphology (12, 72, 73), ovarian tissue of women with PCOS shows a reduced proportion of atretic follicles compared to non-PCOS women (74). Granulosa cells from PCOS follicles, containing androgen-dominant follicular fluid, can function normally when removed from this microenvironment, with increased aromatase activity in response to FSH (75, 76). Furthermore, BCORL1 is a corepressor of BCL6 (77) that contributes to repression of antiapoptotic gene BCL2 expression (78), whereas BCL9L deficiency contributes to reduced levels of caspase 2 (79), agreeing with our earlier findings of low levels of caspase 3 protein in antral follicle cells from prenatal T–treated sheep (26). Lower BCL2 protein levels (26), which are inconsistent with this premise, may represent differences in gene transcription and protein turnover.
Part of the effect induced by prenatal T treatment may involve regulation of gene function at transcription, translation, and protein bioavailability and epigenetic alterations. Consistent with this, some transcriptional factors were downregulated, whereas gene pathways related to ribosomal and ribonucleoprotein processing were upregulated in both cell types. Other cell-specific changes included increases in regulators of ubiquitin activity in granulosa cells and tRNA processing and amino acid metabolism in theca cells. These changes induce their effects by affecting transcription both of coding (mRNA) and noncoding (small and microRNA) RNA (80-82) as well as influencing protein bioavailability (83). These transcriptional regulation–involving epigenetic changes are evident from increased expression of microRNA in fetal ovaries (84) and posttranslational histone modification associated with differential gene expression in the adult ovaries (21) from prenatal T–treated sheep. Because the histone modification may be cell specific (22), it is possible that the cell-specific gene expression and gene pathway changes observed in this study also involve epigenetic regulation.
Emerging data implicate mitochondrial dysfunction in PCOS women as a basis both for their underlying metabolic defects and ovarian dysfunction (85, 86). Therefore, the observations that genes involved in mitochondrial processing are upregulated by prenatal T treatment both in granulosa and theca cells suggest that changes in gene expression involving mitochondrial function contribute to the follicular abnormalities in this model.
Translational significance
Targeted ovarian analyses conducted to date in PCOS animal models and PCOS women have identified several gene and protein changes that could alter follicular growth, differentiation and function, contributing to development of PCO morphology. From a PCOS animal model perspective, altered gene expression profiles in prenatal T–treated sheep include increased granulosa cell androgen receptor and theca cell adiponectin expressions, along with decreased granulosa cell estrogen receptor 2 (ESR2), FSHR, and follistatin as well as reduced theca cell histone deacetylase 3 and histone demethylase KDM1A expressions (22), and these gene changes may be driven by epigenetic modifications evident both at the whole-ovary as well as the cell-specific level (21, 22). In a dihydrotestosterone-treated murine model of PCOS, furthermore, increased ovarian mRNA expression of FSHR, ESR2, and vascular cell adhesion molecule 1 occurs in combination with reduced expression of LHCGR, CYP17, CYP19, steroidogenic acute regulatory protein, and tissue inhibitor of matrix metalloproteinases 1 (87, 88). Additional protein changes in prenatal T–treated sheep include increased granulosa cell AMH content (24), reduced matrix proteins with increased enzymatic expression involving extracellular matrix turnover (25), decreased proapoptotic proteins (26), and altered steroidogenic enzymes and steroid receptors (27, 28). Many of these molecular changes resemble those found in small antral follicles of PCOS women, based on different diagnostic criteria for PCOS. In human PCOS antral follicles, gene abnormalities include overexpression of granulosa and theca cell LHCGR in National Institutes of Health–defined PCOS women (89), decreased granulosa cell FSHR and AR gene expression in female cancer patients with biochemical signs of PCOS (90), and increased gene expression levels for AMH, FSHR, and AR in hormone-stimulated PCOS women according to Rotterdam criteria (91). Due to these different PCOS criteria, it is not surprising that such variable PCOS follicles also can exhibit decreased inhibin α- and βA-subunit (92) or increased inhibin βA-subunit gene expressions in granulosa cells (90). Nor is it surprising that such variable PCOS follicles can exhibit increased granulosa cell CYP19 and theca cell CYP17 gene expressions, with either increased or decreased P450 side chain cleavage enzyme (CYP11A1) (89, 90, 93) and decreased ESR2 gene expressions in both cell types (94). Furthermore, PCOS as defined by the Rotterdam criteria represents different forms of phenotypic expression (95), in which the nonandrogenic PCOS phenotype of oligoanovulation with PCO morphology may not have its origin in prenatal androgen excess. Abnormal protein expression in these small PCOS antral follicles includes altered ESR1 and ESR2 levels in granulosa and theca cells (94), increased granulosa cell AMH production (96), and decreased apoptotic effector caspase-3 levels with reciprocally increased antiapoptotic inhibitor of apoptosis proteins-2 levels in granulosa cells (97). Potential reasons for the inability of the present study to replicate findings of the previous targeted analysis from the same cohort of prenatal T–treated sheep (22) include differences arising from transcriptional/translational regulation and/or protein turnover in studies assessing protein levels. For those studies assessing gene expression, differences may be due to cell-specific expression vs whole-ovary analysis, which includes follicles of different stages and other ovarian compartments such as stroma and vasculature (21, 88), developmental stage and health status of follicles selected for laser capture microdissection, variability in assay technologies for gene expression (ie, RNA sequencing vs in situ hybridization, real-time polymerase chain reaction, or oligonucleotide microarray), and/or statistical adjustments for large numbers of genes examined via RNA sequencing (98). Importantly, many of the reported changes in animal models of PCOS and women with PCOS contribute to the PCO morphology through reduced FSH sensitivity, altered extracellular matrix proteins, follicular arrest, and diminished follicular atresia (16, 99). Many of the changes observed in the present study agree with the gene/protein changes, reported previously, that contribute to the functional attributes of PCO morphology (Table 5). These include (1) increased expression of TGFβR1 consistent with increased TGFB superfamily members: AMH, FST, and inhibins (22, 24, 96, 100); (2) reduced extracellular matrix-related gene expression (COL1A1, COL1A2, COL3A1, COL6A1, and COL14A1) in line with diminished collagen and altered extracellular matrix enzyme content in antral follicles (25, 66); and (3) decreased expression of proapoptotic proteins BCORL1 and BCL9L consistent with reduced levels of proapoptotic proteins (26, 97) in prenatal T–treated sheep and also PCOS women.
Table 5.
Prenatal testosterone-treatment–induced changes in messenger RNA and protein levels
| Gene class | Gene expression changes reported by RT-PCR | Protein changes reported by immunohistochemistry | Gene expression changes in present study by RNA-Seq |
|---|---|---|---|
| Transforming growth factor family | Granulosa cell AMH and INHBB No change (22) FST ↑ in antral follicle by in situ hybridization (23); ↓ in granulosa cells by RT-PCR (22) | ↑ AMH in granulosa cells (24) | ↑ TGFβR1 both in granulosa and theca cells |
| Proapoptotic proteins | ↓ Antral follicular BAX and activated caspase3 (26) | ↓ BCORL1 and BCL9L both in granulosa and theca cells | |
| Matrix proteins | ↓ TIMP1, LAMB, collagen in antral follicles (25) | ↓ COL1A1, COL1A2, COL3A1, COL6A1, and COL14A1 in granulosa cells | |
| Gap junction proteins | ↑ GJA1 (25) | ↑ GJA1 both in granulosa and theca cells |
Abbreviations: AMH, antimüllerian hormone; FST, follistatin; RNA-Seq, RNA sequencing; RT-PCT, reverse transcriptase–polymerase chain reaction; TGFβR1, transforming growth factor β receptor 1; TIMP1, tissue inhibitor of matrix metalloproteinases 1.
A major strength of this study is the utilization of laser capture microdissection to isolate pure populations of unstimulated ovarian granulosa and theca cells. This approach advances prior studies that assessed ovarian changes in whole ovaries or ovarian cells from stimulated cycles. Ovaries are made up of follicles of different developmental stages, each with a distinct pattern of gene expression, and hormone stimulations can influence gene changes, but they pose their own problems. Another advantage is that this study was carried out in sheep, a precocial large animal model of PCOS with a similar developmental timeline and ovarian physiology to humans (101) and therefore of direct translational value to women with PCOS. A limitation is that we were unable to confirm several of the gene expression changes at the protein level because of the limited availability of captured cells. However, our previous observations with whole ovaries from a different cohort of animals (21) and theca cell- and granulosa cell-specific changes in epigenetic modifying enzymes from the same ovaries (22) are supportive of differential gene expression changes, particularly cell-specific differences as a basis for PCO morphology. A potential issue to consider is that these cell-specific changes may be modulated via epigenetic mechanisms, contributing to the development of multifollicular ovarian phenotype in prenatal T–treated sheep. Thus, this research lays the groundwork for future mechanistic and interventional studies to understand the development of PCO morphology and advance therapeutic approaches.
Acknowledgments
We are grateful to Mr Douglas Doop for his expert animal care, facility management, and help with generation of the experimental lambs; and to Drs Mohan Manikkam and Teresa Steckler, Ms Olga Astapova, Ms Carol Herkimer, and Mr James Lee for their assistance with prenatal steroid treatment and help during collection of ovaries.
Financial Support: This work was supported by Eunice Kennedy Shriver National Institute of Child Health & Human Development, National Institutes of Health (awards P01HD44232 to V.P. and P50HD071836 to D.D.)
Glossary
Abbreviations
- ACTA2
actin alpha 2, smooth muscle
- AMH
antimüllerian hormone
- BCL9L
BCL9 like
- BCORL1
BCL6 corepressor like 1
- CHI3L1
chitinase 3 like 1
- FDR
false discovery rate
- FSH
follicle-stimulating hormone
- FSHR
follicle-stimulating hormone receptor
- FST
follistatin
- LH
luteinizing hormone
- NGF
nerve growth factor
- PCO
polyfollicular ovary
- PCOS
polycystic ovarian syndrome
- RET
ret proto-oncogene
- T
testosterone
- TGFβR1
transforming growth factor β receptor 1
- VCAN
versican
- VEGF
vascular endothelial growth factor
- WEE2
WEE2 oocyte meiosis inhibiting kinase
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References
- 1. Dumesic DA, Oberfield SE, Stener-Victorin E, Marshall JC, Laven JS, Legro RS. Scientific statement on the diagnostic criteria, epidemiology, pathophysiology, and molecular genetics of polycystic ovary syndrome. Endocr Rev. 2015;36(5):487-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Conway G, Dewailly D, Diamanti-Kandarakis E, et al. ; ESE PCOS Special Interest Group The polycystic ovary syndrome: a position statement from the European Society of Endocrinology. Eur J Endocrinol. 2014;171(4):P1-P29. [DOI] [PubMed] [Google Scholar]
- 3. Azziz R, Carmina E, Chen Z, et al. . Polycystic ovary syndrome. Nat Rev Dis Primers. 2016;2:16057. [DOI] [PubMed] [Google Scholar]
- 4. Puttabyatappa M, Cardoso RC, Padmanabhan V. Effect of maternal PCOS and PCOS-like phenotype on the offspring’s health. Mol Cell Endocrinol. 2016;435:29-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barnes RB, Rosenfield RL, Ehrmann DA, et al. . Ovarian hyperandrogynism as a result of congenital adrenal virilizing disorders: evidence for perinatal masculinization of neuroendocrine function in women. J Clin Endocrinol Metab. 1994;79(5):1328-1333. [DOI] [PubMed] [Google Scholar]
- 6. Sir-Petermann T, Codner E, Pérez V, et al. . Metabolic and reproductive features before and during puberty in daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2009;94(6):1923-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dumesic DA, Padmanabhan V, Abbott DH. Polycystic ovary syndrome and oocyte developmental competence. Obstet Gynecol Surv. 2008;63(1):39-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Padmanabhan V, Veiga-Lopez A. Sheep models of polycystic ovary syndrome phenotype. Mol Cell Endocrinol. 2013;373(1-2):8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maliqueo M, Benrick A, Stener-Victorin E. Rodent models of polycystic ovary syndrome: phenotypic presentation, pathophysiology, and the effects of different interventions. Semin Reprod Med. 2014;32(3):183-193. [DOI] [PubMed] [Google Scholar]
- 10. Cardoso RC, Puttabyatappa M, Padmanabhan V. Steroidogenic versus metabolic programming of reproductive neuroendocrine, ovarian and metabolic dysfunctions. Neuroendocrinology. 2015;102(3):226-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dewailly D, Lujan ME, Carmina E, et al. . Definition and significance of polycystic ovarian morphology: a task force report from the Androgen Excess and Polycystic Ovary Syndrome Society. Hum Reprod Update. 2014;20(3):334-352. [DOI] [PubMed] [Google Scholar]
- 12. Maciel GA, Baracat EC, Benda JA, et al. . Stockpiling of transitional and classic primary follicles in ovaries of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89(11):5321-5327. [DOI] [PubMed] [Google Scholar]
- 13. Steckler T, Manikkam M, Inskeep EK, Padmanabhan V. Developmental programming: follicular persistence in prenatal testosterone-treated sheep is not programmed by androgenic actions of testosterone. Endocrinology. 2007;148(7):3532-3540. [DOI] [PubMed] [Google Scholar]
- 14. Steckler T, Wang J, Bartol FF, Roy SK, Padmanabhan V. Fetal programming: prenatal testosterone treatment causes intrauterine growth retardation, reduces ovarian reserve and increases ovarian follicular recruitment. Endocrinology. 2005;146(7):3185-3193. [DOI] [PubMed] [Google Scholar]
- 15. Franks S, Stark J, Hardy K. Follicle dynamics and anovulation in polycystic ovary syndrome. Hum Reprod Update. 2008;14(4): 367-378. [DOI] [PubMed] [Google Scholar]
- 16. Puttabyatappa M, Padmanabhan V. Ovarian and extra-ovarian mediators in the development of polycystic ovary syndrome. J Mol Endocrinol. 2018;61(4):R161-R184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Puttabyatappa M, Padmanabhan V. Developmental programming of ovarian functions and dysfunctions. Vitam Horm. 2018;107:377-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jarrett BY, Vantman N, Mergler RJ, et al. . Dysglycemia, not altered sex steroid hormones, affects cognitive function in polycystic ovary syndrome. J Endocr Soc. 2019;3(10):1858-1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jonard S, Robert Y, Cortet-Rudelli C, Pigny P, Decanter C, Dewailly D. Ultrasound examination of polycystic ovaries: is it worth counting the follicles? Hum Reprod. 2003;18(3):598-603. [DOI] [PubMed] [Google Scholar]
- 20. Smith P, Steckler TL, Veiga-Lopez A, Padmanabhan V. Developmental programming: differential effects of prenatal testosterone and dihydrotestosterone on follicular recruitment, depletion of follicular reserve, and ovarian morphology in sheep. Biol Reprod. 2009;80(4):726-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sinha N, Roy S, Huang B, Wang J, Padmanabhan V, Sen A. Developmental programming: prenatal testosterone-induced epigenetic modulation and its effect on gene expression in sheep ovary. Biol Reprod. 2020;102(5):1045-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guo X, Puttabyatappa M, Thompson RC, Padmanabhan V. Developmental programming: contribution of epigenetic enzymes to antral follicular defects in the sheep model of PCOS. Endocrinology. 2019;160(10):2471-2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. West C, Foster DL, Evans NP, Robinson J, Padmanabhan V. Intra-follicular activin availability is altered in prenatally-androgenized lambs. Mol Cell Endocrinol. 2001;185(1-2):51-59. [DOI] [PubMed] [Google Scholar]
- 24. Veiga-Lopez A, Ye W, Padmanabhan V. Developmental programming: prenatal testosterone excess disrupts anti-Müllerian hormone expression in preantral and antral follicles. Fertil Steril. 2012;97(3):748-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Puttabyatappa M, Lu C, Martin JD, Chazenbalk G, Dumesic D, Padmanabhan V. Developmental programming: impact of prenatal testosterone excess on steroidal machinery and cell differentiation markers in visceral adipocytes of female sheep. Reprod Sci. 2018;25(7):1010-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Salvetti NR, Ortega HH, Veiga-Lopez A, Padmanabhan V. Developmental programming: impact of prenatal testosterone excess on ovarian cell proliferation and apoptotic factors in sheep. Biology of reproduction. 2012;87(1):22, 1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Padmanabhan V, Salvetti NR, Matiller V, Ortega HH. Developmental programming: prenatal steroid excess disrupts key members of intraovarian steroidogenic pathway in sheep. Endocrinology. 2014;155(9):3649-3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ortega HH, Salvetti NR, Padmanabhan V. Developmental programming: prenatal androgen excess disrupts ovarian steroid receptor balance. Reproduction. 2009;137(5):865-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ortega HH, Rey F, Velazquez MM, Padmanabhan V. Developmental programming: effect of prenatal steroid excess on intraovarian components of insulin signaling pathway and related proteins in sheep. Biol Reprod. 2010;82(6):1065-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ortega HH, Veiga-Lopez A, Sreedharan S, del Luján Velázquez MM, Salvetti NR, Padmanabhan V. Developmental programming: does prenatal steroid excess disrupt the ovarian VEGF system in sheep? Biol Reprod. 2015;93(3):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hogg K, Young JM, Oliver EM, Souza CJ, McNeilly AS, Duncan WC. Enhanced thecal androgen production is prenatally programmed in an ovine model of polycystic ovary syndrome. Endocrinology. 2012;153(1):450-461. [DOI] [PubMed] [Google Scholar]
- 32. Zhao S, Fung-Leung WP, Bittner A, Ngo K, Liu X. Comparison of RNA-seq and microarray in transcriptome profiling of activated T cells. PloS One. 2014;9(1):e78644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet. 2009;10(1):57-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Manikkam M, Steckler TL, Welch KB, Inskeep EK, Padmanabhan V. Fetal programming: prenatal testosterone treatment leads to follicular persistence/luteal defects; partial restoration of ovarian function by cyclic progesterone treatment. Endocrinology. 2006;147(4):1997-2007. [DOI] [PubMed] [Google Scholar]
- 35. Manikkam M, Thompson RC, Herkimer C, et al. . Developmental programming: impact of prenatal testosterone excess on pre- and postnatal gonadotropin regulation in sheep. Biol Reprod. 2008;78(4):648-660. [DOI] [PubMed] [Google Scholar]
- 36. Andrews S. FastQC: A quality control tool for high throughput sequence data. 2010. https://www.bioinformatics.babraham.ac.uk/projects/fastqc/. Accessed June 14, 2020. [Google Scholar]
- 37. Ewels P, Magnusson M, Lundin S, Käller M. MultiQC: summarize analysis results for multiple tools and samples in a single report. Bioinformatics. 2016;32(19):3047-3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dobin A, Davis CA, Schlesinger F, et al. . STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29(1):15-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hartley SW, Mullikin JC. QoRTs: a comprehensive toolset for quality control and data processing of RNA-Seq experiments. BMC Bioinformatics. 2015;16:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30(7):923-930. [DOI] [PubMed] [Google Scholar]
- 41. Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhu A, Ibrahim JG, Love MI. Heavy-tailed prior distributions for sequence count data: removing the noise and preserving large differences. Bioinformatics. 2019;35(12):2084-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lee C, Patil S, Sartor MA. RNA-Enrich: a cut-off free functional enrichment testing method for RNA-seq with improved detection power. Bioinformatics. 2016;32(7):1100-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Durinck S, Spellman PT, Birney E, Huber W. Mapping identifiers for the integration of genomic datasets with the R/Bioconductor package biomaRt. Nat Protoc. 2009;4(8):1184-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Franceschini A, Szklarczyk D, Frankild S, et al. . STRING v9.1: protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res. 2013;41(Database issue):D808-D815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Puttabyatappa M, Bakulski K, Dou J, Guo X, Dumesic DA, Padmanabhan V.. Supplemental File for Developmental Programming: Granulosa and Theca Cell-Specific Transcriptional Regulation by Prenatal Testosterone in Sheep. figshare. Deposited April 12, 2020. 10.6084/m9.figshare.12116688.v3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Minegishi T, Tano M, Igarashi M, et al. . Expression of follicle-stimulating hormone receptor in human ovary. Eur J Clin Invest. 1997;27(6):469-474. [DOI] [PubMed] [Google Scholar]
- 48. Yamoto M, Minami S, Nakano R, Kobayashi M. Immunohistochemical localization of inhibin/activin subunits in human ovarian follicles during the menstrual cycle. J Clin Endocrinol Metab. 1992;74(5):989-993. [DOI] [PubMed] [Google Scholar]
- 49. Czernobilsky B, Shezen E, Lifschitz-Mercer B, et al. . Alpha smooth muscle actin (alpha-SM actin) in normal human ovaries, in ovarian stromal hyperplasia and in ovarian neoplasms. Virchows Arch B Cell Pathol Incl Mol Pathol. 1989;57(1):55-61. [DOI] [PubMed] [Google Scholar]
- 50. Dissen GA, Parrott JA, Skinner MK, Hill DF, Costa ME, Ojeda SR. Direct effects of nerve growth factor on thecal cells from antral ovarian follicles. Endocrinology. 2000;141(12):4736-4750. [DOI] [PubMed] [Google Scholar]
- 51. Hanna CB, Yao S, Patta MC, Jensen JT, Wu X. WEE2 is an oocyte-specific meiosis inhibitor in rhesus macaque monkeys. Biol Reprod. 2010;82(6):1190-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Minshall N, Reiter MH, Weil D, Standart N. CPEB interacts with an ovary-specific eIF4E and 4E-T in early Xenopus oocytes. J Biol Chem. 2007;282(52):37389-37401. [DOI] [PubMed] [Google Scholar]
- 53. Pires ES, Hlavin C, Macnamara E, et al. . SAS1B protein [ovastacin] shows temporal and spatial restriction to oocytes in several eutherian orders and initiates translation at the primary to secondary follicle transition. Dev Dyn. 2013;242(12):1405-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Romar R, De Santis T, Papillier P, et al. . Expression of maternal transcripts during bovine oocyte in vitro maturation is affected by donor age. Reprod Domest Anim. 2011;46(1):e23-e30. [DOI] [PubMed] [Google Scholar]
- 55. Sánchez F, Adriaenssens T, Romero S, Smitz J. Quantification of oocyte-specific transcripts in follicle-enclosed oocytes during antral development and maturation in vitro. Mol Hum Reprod. 2009;15(9):539-550. [DOI] [PubMed] [Google Scholar]
- 56. Bagavandoss P. Temporal expression of tenascin-C and type I collagen in response to gonadotropins in the immature rat ovary. Acta Histochem. 2014;116(7):1125-1133. [DOI] [PubMed] [Google Scholar]
- 57. Berkholtz CB, Lai BE, Woodruff TK, Shea LD. Distribution of extracellular matrix proteins type I collagen, type IV collagen, fibronectin, and laminin in mouse folliculogenesis. Histochem Cell Biol. 2006;126(5):583-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lind AK, Weijdegård B, Dahm-Kähler P, Mölne J, Sundfeldt K, Brännström M. Collagens in the human ovary and their changes in the perifollicular stroma during ovulation. Acta Obstet Gynecol Scand. 2006;85(12):1476-1484. [DOI] [PubMed] [Google Scholar]
- 59. Curry TE Jr, Osteen KG. The matrix metalloproteinase system: changes, regulation, and impact throughout the ovarian and uterine reproductive cycle. Endocr Rev. 2003;24(4):428-465. [DOI] [PubMed] [Google Scholar]
- 60. Robinson RS, Woad KJ, Hammond AJ, Laird M, Hunter MG, Mann GE. Angiogenesis and vascular function in the ovary. Reproduction. 2009;138(6):869-881. [DOI] [PubMed] [Google Scholar]
- 61. Collado-Fernandez E, Picton HM, Dumollard R. Metabolism throughout follicle and oocyte development in mammals. Int J Dev Biol. 2012;56(10-12):799-808. [DOI] [PubMed] [Google Scholar]
- 62. Robker RL, Richards JS. Hormonal control of the cell cycle in ovarian cells: proliferation versus differentiation. Biol Reprod. 1998;59(3):476-482. [DOI] [PubMed] [Google Scholar]
- 63. Chermuła B, Brązert M, Iżycki D, et al. . New gene markers of angiogenesis and blood vessels development in porcine ovarian granulosa cells during short-term primary culture in vitro. Biomed Res Int. 2019;2019:6545210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Aziz M, Wissing ML, Naver KV, Faber J, Skouby SO. Polycystic ovary syndrome and low-grade inflammation with special reference to YKL-40. Gynecol Endocrinol. 2014;30(4): 311-315. [DOI] [PubMed] [Google Scholar]
- 65. Celik C, Abali R, Guzel S, Bastu E, Kucukyalcin V, Yilmaz M. Elevated circulating levels of YKL-40 are a marker of abnormal glucose tolerance in women with polycystic ovary syndrome. Clin Endocrinol (Oxf). 2012;77(6):893-897. [DOI] [PubMed] [Google Scholar]
- 66. Monniaux D, Genêt C, Maillard V, et al. . Prenatal programming by testosterone of follicular theca cell functions in ovary. Cell Mol Life Sci. 2020;77(6):1177-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chang HM, Wu HC, Sun ZG, Lian F, Leung PCK. Neurotrophins and glial cell line-derived neurotrophic factor in the ovary: physiological and pathophysiological implications. Hum Reprod Update. 2019;25(2):224-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Polzikov M, Yakovenko S, Voznesenskaya J, Troshina M, Zatsepina O. Overexpression of ribosomal RNA in cumulus cells of patients with polycystic ovary syndrome. J Assist Reprod Genet. 2012;29(10):1141-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Thomson E, Ferreira-Cerca S, Hurt E. Eukaryotic ribosome biogenesis at a glance. J Cell Sci. 2013;126(Pt 21):4815-4821. [DOI] [PubMed] [Google Scholar]
- 70. Clark DA, Coker R. Transforming growth factor-beta (TGF-beta). Int J Biochem Cell Biol. 1998;30(3):293-298. [DOI] [PubMed] [Google Scholar]
- 71. Matiller V, Hein GJ, Stassi AF, et al. . Expression of TGFBR1, TGFBR2, TGFBR3, ACVR1B and ACVR2B is altered in ovaries of cows with cystic ovarian disease. Reprod Domest Anim. 2019;54(1):46-54. [DOI] [PubMed] [Google Scholar]
- 72. Webber LJ, Stubbs S, Stark J, et al. . Formation and early development of follicles in the polycystic ovary. Lancet. 2003;362(9389):1017-1021. [DOI] [PubMed] [Google Scholar]
- 73. Hughesdon PE. Morphology and morphogenesis of the Stein-Leventhal ovary and of so-called “hyperthecosis”. Obstet Gynecol Surv. 1982;37(2):59-77. [DOI] [PubMed] [Google Scholar]
- 74. Webber LJ, Stubbs SA, Stark J, et al. . Prolonged survival in culture of preantral follicles from polycystic ovaries. J Clin Endocrinol Metab. 2007;92(5):1975-1978. [DOI] [PubMed] [Google Scholar]
- 75. Almahbobi G, Anderiesz C, Hutchinson P, McFarlane JR, Wood C, Trounson AO. Functional integrity of granulosa cells from polycystic ovaries. Clin Endocrinol (Oxf). 1996;44(5):571-580. [DOI] [PubMed] [Google Scholar]
- 76. Mason HD, Willis DS, Beard RW, Winston RM, Margara R, Franks S. Estradiol production by granulosa cells of normal and polycystic ovaries: relationship to menstrual cycle history and concentrations of gonadotropins and sex steroids in follicular fluid. J Clin Endocrinol Metab. 1994;79(5):1355-1360. [DOI] [PubMed] [Google Scholar]
- 77. Pagan JK, Arnold J, Hanchard KJ, et al. . A novel corepressor, BCoR-L1, represses transcription through an interaction with CtBP. J Biol Chem. 2007;282(20):15248-15257. [DOI] [PubMed] [Google Scholar]
- 78. Saito M, Novak U, Piovan E, et al. . BCL6 suppression of BCL2 via Miz1 and its disruption in diffuse large B cell lymphoma. Proc Natl Acad Sci U S A. 2009;106(27):11294-11299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. López-García C, Sansregret L, Domingo E, et al. . BCL9L dysfunction impairs caspase-2 expression permitting aneuploidy tolerance in colorectal cancer. Cancer Cell. 2017;31(1):79-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lambert SA, Jolma A, Campitelli LF, et al. . The human transcription factors. Cell. 2018;172(4):650-665. [DOI] [PubMed] [Google Scholar]
- 81. Schmidt CA, Matera AG. tRNA introns: presence, processing, and purpose. Wiley Interdiscip Rev RNA. 2020;11(3):e1583. [DOI] [PubMed] [Google Scholar]
- 82. Aubert M, O’Donohue MF, Lebaron S, Gleizes PE. Pre-ribosomal RNA processing in human cells: from mechanisms to congenital diseases. Biomolecules. 2018;8(4):123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Dougherty SE, Maduka AO, Inada T, Silva GM. Expanding role of ubiquitin in translational control. Int J Mol Sci. 2020;21(3):1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Luense LJ, Veiga-Lopez A, Padmanabhan V, Christenson LK. Developmental programming: gestational testosterone treatment alters fetal ovarian gene expression. Endocrinology. 2011;152(12):4974-4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ilie IR. Advances in PCOS pathogenesis and progression-mitochondrial mutations and dysfunction. Adv Clin Chem. 2018;86:127-155. [DOI] [PubMed] [Google Scholar]
- 86. Shukla P, Mukherjee S. Mitochondrial dysfunction: an emerging link in the pathophysiology of polycystic ovary syndrome. Mitochondrion. 2020;52:24-39. [DOI] [PubMed] [Google Scholar]
- 87. Candelaria NR, Padmanabhan A, Stossi F, et al. . VCAM1 is induced in ovarian theca and stromal cells in a mouse model of androgen excess. Endocrinology. 2019;160(6):1377-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ma Y, Andrisse S, Chen Y, et al. . Androgen receptor in the ovary theca cells plays a critical role in androgen-induced reproductive dysfunction. Endocrinology. 2017;158(1):98-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Jakimiuk AJ, Weitsman SR, Navab A, Magoffin DA. Luteinizing hormone receptor, steroidogenesis acute regulatory protein, and steroidogenic enzyme messenger ribonucleic acids are overexpressed in thecal and granulosa cells from polycystic ovaries. J Clin Endocrinol Metab. 2001;86(3):1318-1323. [DOI] [PubMed] [Google Scholar]
- 90. Owens LA, Kristensen SG, Lerner A, et al. . Gene expression in granulosa cells from small antral follicles from women with or without polycystic ovaries. J Clin Endocrinol Metab. 2019;104(12):6182-6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Catteau-Jonard S, Jamin SP, Leclerc A, Gonzalès J, Dewailly D, di Clemente N. Anti-Mullerian hormone, its receptor, FSH receptor, and androgen receptor genes are overexpressed by granulosa cells from stimulated follicles in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93(11):4456-4461. [DOI] [PubMed] [Google Scholar]
- 92. Fujiwara T, Sidis Y, Welt C, et al. . Dynamics of inhibin subunit and follistatin mRNA during development of normal and polycystic ovary syndrome follicles. J Clin Endocrinol Metab. 2001;86(9):4206-4215. [DOI] [PubMed] [Google Scholar]
- 93. Wood JR, Ho CK, Nelson-Degrave VL, McAllister JM, Strauss JF III. The molecular signature of polycystic ovary syndrome (PCOS) theca cells defined by gene expression profiling. J Reprod Immunol. 2004;63(1):51-60. [DOI] [PubMed] [Google Scholar]
- 94. Jakimiuk AJ, Weitsman SR, Yen HW, Bogusiewicz M, Magoffin DA. Estrogen receptor alpha and beta expression in theca and granulosa cells from women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2002;87(12):5532-5538. [DOI] [PubMed] [Google Scholar]
- 95. Dumesic DA, Abbott DH, Sanchita S, Chazenbalk GD. Endocrine-Metabolic dysfunction in polycystic ovary syndrome: an evolutionary perspective. Curr Opin Endocr Metab Res. 2020;12:41-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Pellatt L, Hanna L, Brincat M, et al. . Granulosa cell production of anti-Müllerian hormone is increased in polycystic ovaries. J Clin Endocrinol Metab. 2007;92(1):240-245. [DOI] [PubMed] [Google Scholar]
- 97. Das M, Djahanbakhch O, Hacihanefioglu B, et al. . Granulosa cell survival and proliferation are altered in polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93(3): 881-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Chen SY, Feng Z, Yi X. A general introduction to adjustment for multiple comparisons. J Thorac Dis. 2017;9(6): 1725-1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Dewailly D, Robin G, Peigne M, Decanter C, Pigny P, Catteau-Jonard S. Interactions between androgens, FSH, anti-Müllerian hormone and estradiol during folliculogenesis in the human normal and polycystic ovary. Hum Reprod Update. 2016;22(6):709-724. [DOI] [PubMed] [Google Scholar]
- 100. Norman RJ, Milner CR, Groome NP, Robertson DM. Circulating follistatin concentrations are higher and activin concentrations are lower in polycystic ovarian syndrome. Hum Reprod. 2001;16(4):668-672. [DOI] [PubMed] [Google Scholar]
- 101. Padmanabhan V, Veiga-Lopez A. Reproduction symposium: developmental programming of reproductive and metabolic health. J Anim Sci. 2014;92(8):3199-3210. [DOI] [PMC free article] [PubMed] [Google Scholar]