Abstract
Alkyl chlorides and aryl chlorides are among the most abundant and stable carbon electrophiles. Although their coupling with carbon nucleophiles is well developed, the cross-electrophile coupling of aryl chlorides with alkyl chlorides has remained a challenge. We report here the first general approach to this transformation. The key to productive, selective cross-coupling is the use of a small amount of iodide or bromide along with a recently reported ligand, pyridine-2,6-bis(N-cyanocarboxamidine) (PyBCamCN). The scope of the reaction is demonstrated with 35 examples (63%±16% ave yield) and we show that the Br− and I− additives act as co-catalysts, generating a low, steady-state concentration of more-reactive alkyl bromide/iodide.
Graphical Abstract
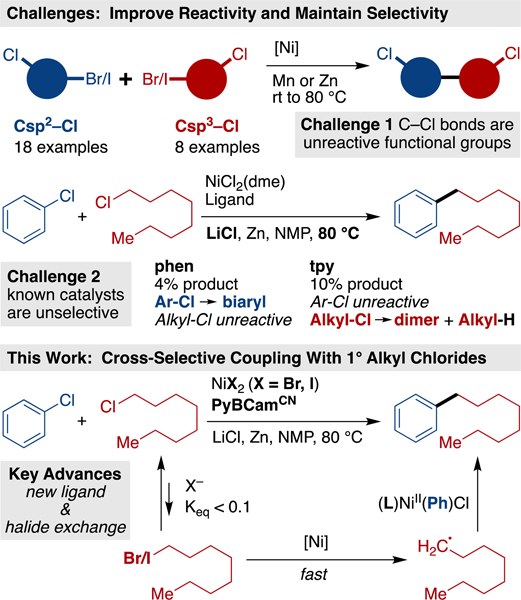
Cross-electrophile coupling has rapidly become an important approach to the synthesis of Csp2-Csp3 bonds,1 but engaging less reactive C-Cl bonds, outside of activated systems2 or intramolecular reactions,3 has proven challenging. Indeed, unactivated C-Cl bonds are well-tolerated functional groups4 in cross-electrophile coupling methods (Scheme 1).5,6 The ability to cross-couple with organic chlorides is valuable for several reasons – first, organic chlorides are more abundant than organic bromides or organic iodides;7 second, the low reactivity of the C-Cl bond allows it to be introduced early in a synthesis and later diversified.8,9,10
Scheme 1. Challenges in the Cross-Electrophile Coupling Organic Chlorides.
The central challenge presented by C-Cl bonds in cross-electrophile coupling is the need for higher reactivity without sacrificing selectivity (Scheme 1). While the homodimerization of alkyl chlorides11 and aryl chlorides8c has been reported, no general cross-selective approach has yet been found.12 Recently, Zhang reported couplings of a variety of aryl chlorides, but only with an excess of ClCF2R reagents.13 Several groups have reported on the coupling of aryl chlorides with alkyl bromides14 or tertiary alkyl oxalate esters.15 However, the coupling of chlorobenzene with a simple alkyl bromide provided less than 25% yield of cross-coupled product.14a Switching to an alkyl chloride further diminishes selectivity and yield using our standard conditions (Scheme 1).16
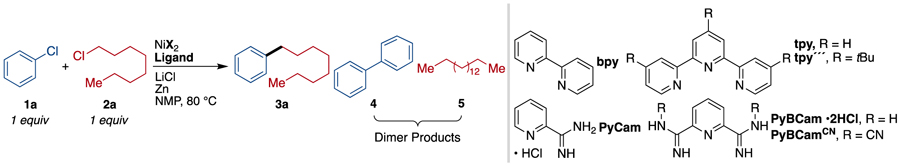
Based upon our proposed mechanism for the coupling of aryl iodides with alkyl iodides,17–18,19, overcoming this dual reactivity-selectivity challenge requires a catalyst that selectively reacts with the Ar-Cl over the Alkyl-Cl, yet can slowly generate an alkyl radical from the Alkyl-Cl starting material. Herein we show that this can be accomplished through the use of salt additives to maintain a very low, steady-state concentration of an alkyl bromide/iodide and a uniquely selective pyridine-2,6-bis(N-cyanocarboxamidine) (PyBCamCN)20,21 ligated nickel catalyst (Scheme 1).
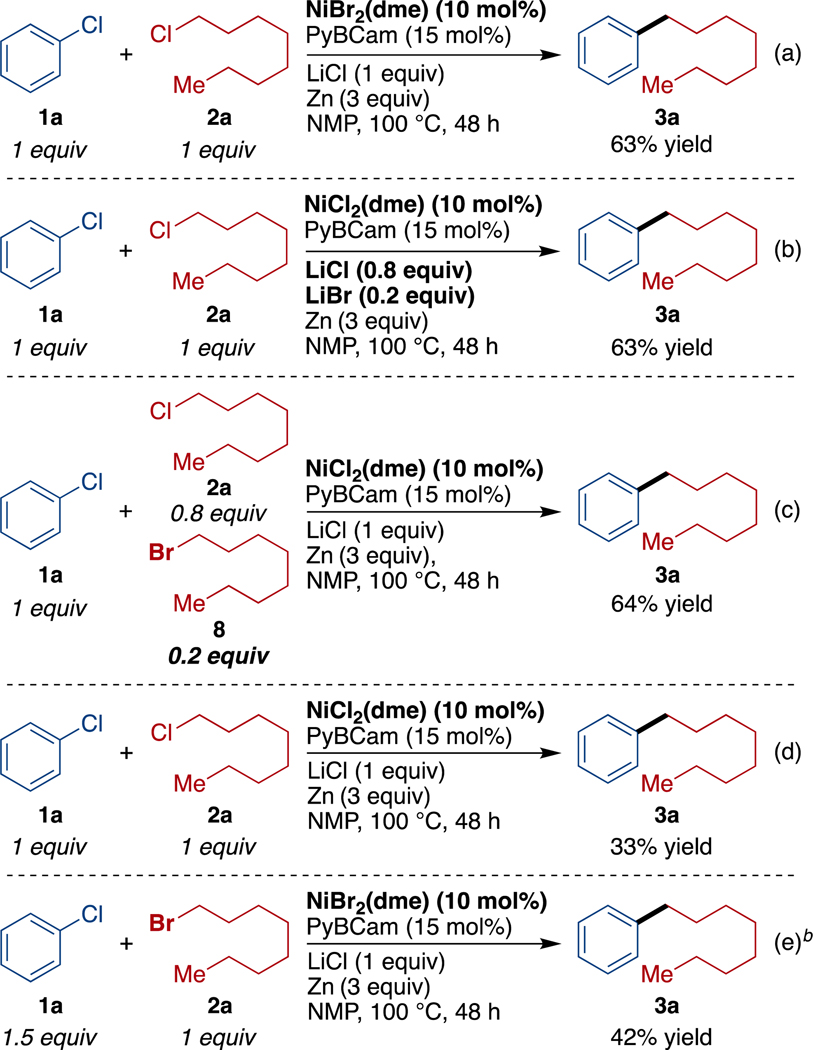
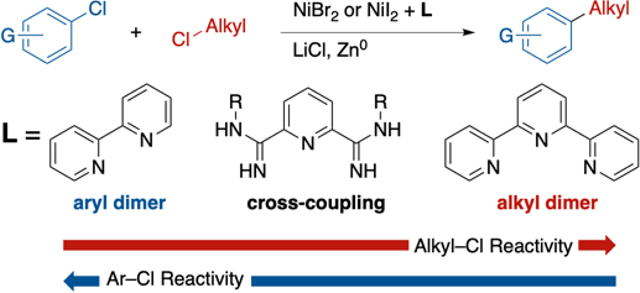
During reaction development, we observed a strong synergistic effect between the catalyst and the presence of substoichiometric amounts (10–30 mol%) of bromide or iodide (Table 1 and Supporting Information Figures S1, S4-S7). While no catalysts were found that provided high yields of product in the absence of bromide or iodide, high selectivity could be achieved in reactions with PyBCamCN ligand and NiBr2(dme) or NiI2•4H2O; and in reactions with PyBCam ligand and NiBr2(dme) (Table 1, bold-faced entries). Reactions with bipyridine (bpy) or pyridine 2-carboxamidine (PyCam) ligands, which are optimal for the coupling of aryl bromides with alkyl bromides,20,22 favored formation of aryl dimer products (bpy) or hydrodehalogenated arene (PyCam) without consuming the alkyl chloride. Reactions with terpyridine (tpy), which is useful for the dimerization of alkyl halides,23 converted alkyl chloride to dimeric and hydrodehalogenated products without consuming aryl chloride. In contrast to tpy, reactions with 4,4′,4″-tri-tert-butyl-2,2′:6′,2″-terpyridine (tpy‴), which is useful in Negishi cross-coupling reactions of alkyl halides,24 consumed both substrates but formed approximately 1:1:1 product/alkyl dimer/aryl dimer.25 See also Chart S1 in the Supporting Information.
Table 1.
The Effect of Ligands and Additives on the Cross-Electrophile Coupling of Chlorobenzene with Chlorooctane.a
 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligand | X | Yield 3a (%)b | Yield 4 (%)b | Yield 5 (%)b | Ligand | X | Yield 3a (%)b | Yield 4 (%)b | Yield 5 (%)b |
| bpy | Cl | 2 | 48 | 1 | PyCam | Cl | 16 | 19 | 6 |
| Br | 9 | 43 | 4 | Br | 43 | 9 | 5 | ||
| I | 17 | 39 | 17 | I | 19 | 2 | 3 | ||
| tpy | Cl | 10 | 0 | 25 | PyBCam•2HCl | Cl | 11 | 0 | 0 |
| Br | 4 | 2 | 40 | Br | 53 | 0 | 2 | ||
| I | 1 | 0 | 16 | I | 18 | 0 | 23 | ||
| tpy‴ | Cl | 38 | 28 | 16 | PyBCamCN | Cl | 46 | 1 | 7 |
| Br | 22 | 26 | 19 | Br | 65 | 0 | 9 | ||
| I | 4 | 33 | 8 | I | 87 (82)c | 0 | 6 | ||
Reaction conditions: chlorobenzene (0.5 mmol), 1-chlorooctane (0.5 mmol), NiX2 = NiI2•4H2O/NiBr2(dme)/NiCl2(dme) (0.05 mmol), ligand (0.05 mmol), LiCl (0.5 mmol), Zn (1.0 mmol), and NMP (1 mL) were assembled in a N2 filled glovebox and heated for 24 h. PyCam and PyBCam were added as their HCl salts.
Yields were determined by GC analysis calibrated against 1,3,5-trimethoxybenzene as an internal standard.
Isolated yield after column chromatography.
Routine optimization with PyBCam and PyBCamCN demonstrated that PyBCamCN was superior, that reactions were best conducted at 60–80 °C, and that a variety of iodide and bromide additives provide similar results.25 Reactions with bromide additive provided the highest yields when the alkyl chloride was added slowly, either portionwise via syringe or dropwise through an addition funnel. Reactions with iodide additive did not benefit from slow addition. The primary side products in both cases are the alkyl dimer and aryl hydrodehalogenated product.
The optimized conditions were then applied to a variety of primary alkyl chlorides and chloroarenes (Scheme 2). Electron-rich aryl chlorides, which were unreactive under our previously published conditions, coupled in 69–72% yield (3b, 3f, 3g, 3r). However, a more sterically hindered aryl chloride, 2-chlorotoluene, coupled poorly (3e, 15% yield). While we had coupled electron-poor aryl chlorides with alkyl bromides previously,14 under these conditions electron-poor aryl chlorides could be coupled with alkyl chlorides for the first time, with yields ranging from 53–73% yield (3c, 3h, 3i, 3s, 3u, 3v). As expected with PyBCam ligands,20 a variety of heterocycles could be coupled, including both electron-poor quinoline (3s, 63%) and pyridine (3u, 66% and 3v, 73%); and electron-rich indole (3r, 71%) and thiophene (3t, 33%). A particular advantage of cross-electrophile coupling is tolerance for alkyl halides with β-leaving groups (3z-3ad). The analogous organometallic reagents would be prone to elimination. Finally, secondary alkyl chlorides do couple under these conditions, but in lower yield (3ai, 44%).
Scheme 2. Reaction Scope for the Nickel-Catalyzed Coupling of Aryl Chlorides with Alkyl Chlorides.a.
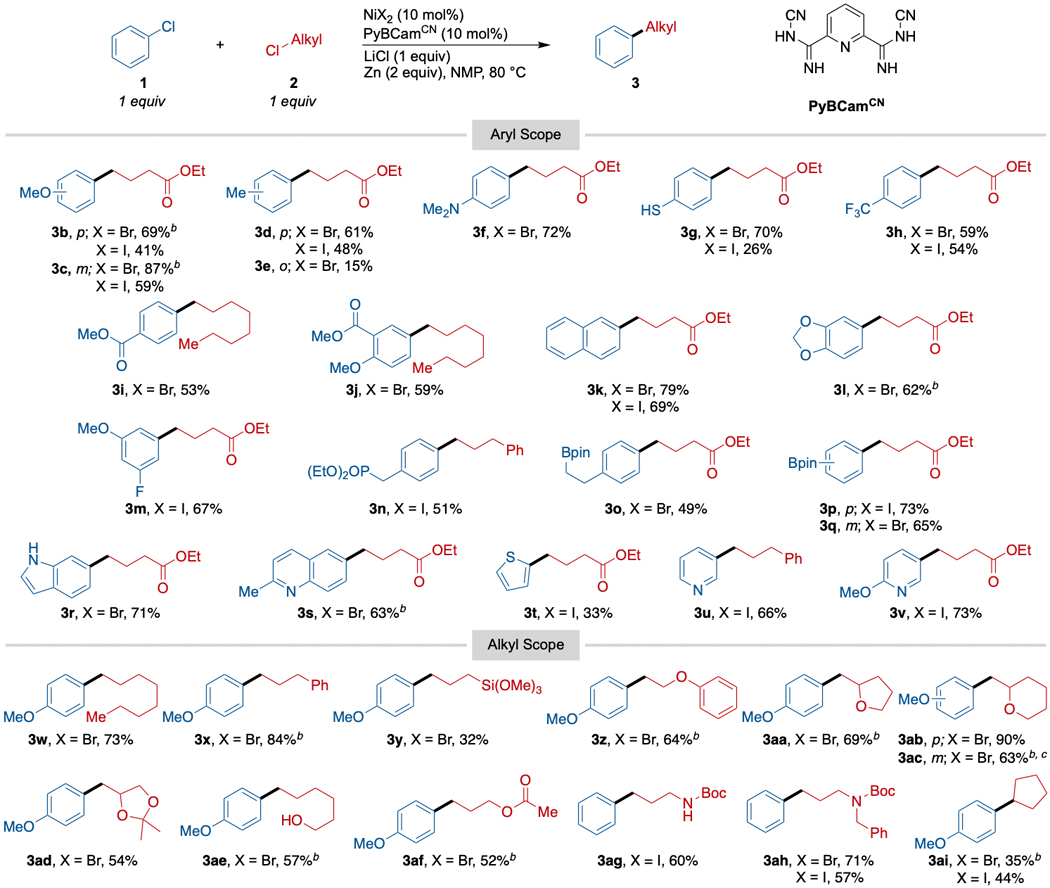
aReactions run on 0.5 mmol scale in 1 mL NMP for 18–24 h. NiX2 was either NiBr2(dme) or NiI2•4H2O. For reactions with X = Br, alkyl-Cl was added in portions. bReaction was conducted with 1.25 equiv of alkyl chloride (0.75 mmol). cReaction was run on a 7.0 mmol scale.
Despite the higher temperatures, functional group compatibility remained broad. The low basicity of the conditions allowed us to tolerate both aryl and alkyl pinacol boronic acid esters (3o-3q, 49–73% yield), providing opportunities for further elaboration of the products. Acidic N-H (3ag, 60%) and O-H (3ae, 57%) groups are tolerated, which would be a challenge for organomagnesium or organozinc reagents.26 As a testament to the low basicity of the conditions, a free thiol was tolerated (3g, 70% yield), avoiding competing SN2 with the alkyl electrophile and S-arylation (pKa of thiophenol in DMSO is 10.3,27 which makes it more acidic than acetic acid).28 On the other hand, despite the presence of Lewis acids (ZnII salts, Li+ salts) at 60–80 °C, Boc groups on nitrogen were still tolerated (3ag, 60%; 3ah, 71%). While esters were tolerated, we did observe scrambling when two different esters were present due to transesterification (for example, methyl and ethyl ester exchange). For this reason, we coupled chloroarenes bearing esters (3i, 3j) with 1-chlorooctane. Other functional group highlights include a benzylic diethylphosphonate ester (3n, 51%) and a trimethoxysilane (3y, 32%). Despite the low yield, the cross-coupling to form trimethoxysilane product 3y is notable because it is a different approach29,30 to forming functionalized silanes that could be useful in attaching molecules to glass or silica.31 As in our previous studies on cross-electrophile coupling reactions with less reactive substrates, this chemistry can be scaled up using standard techniques (3ac).32
The distinctive feature of this reaction, when compared to other cross-electrophile couplings of aryl halides with alkyl halides, is the ability to engage two relatively unreactive substrates in a selective manner (Scheme 1). There are three keys to the success of this method.
First, LiCl was essential for efficient reduction of the nickel catalyst by the zinc surface. We have recently noted that ZnCl2 can have an inhibitory effect on reduction of nickel catalysts and that lithium chloride is among the best agents for overcoming inhibition,33 consistent with previous reports on reduction of organic molecules.34 Here too, reactions conducted without LiCl resulted in 3% formation of the cross-coupled product and primarily returned both substrates (Supporting Information Figure S2). We also verified that neither organic chloride reacts directly with zinc to form an organozinc reagent (Supporting Information Figure S2).
Second, halide exchange plays a key role by increasing the reactivity of the alkyl chloride. We found that 10–30% of bromide or iodide, regardless of how it was introduced, was essential for reasonable reaction rates (Scheme 3 and Supporting Information Figures S4-S7). Importantly, the low concentration of bromide was essential; reactions run without any bromide (Scheme 3d) or with only alkyl bromide (Scheme 3e) provided lower yields than reactions with a catalytic amount of bromide (Scheme 3a – Scheme 3c and Table 1).
Scheme 3. Evidence for Bromide Co-Catalysis.a.
aReactions were run on a 0.5 mmol scale. Yields were determined by GC analysis calibrated against 1,3,5-trimethoxybenzene as an internal standard. bReaction run with DIPEA (20 mol%). DIPEA had no effect on reaction outcome.
Studies on halide exchange showed that it is fast compared to the rate of reaction (reaching equilibrium in 1–2 h vs 24 h for reaction time) and unfavorable (Supporting Information Figure S8-S16). Significantly, the presence of zinc and lithium salts altered the equilibrium to more strongly favor alkyl iodide/bromide. This led to the counterintuitive outcome that increasing total chloride concentration increased alkyl iodide concentration. Under concentrations of salts chosen to mimic those present catalytic reactions, we found that the amount of alkyl iodide increased as the concentration of ZnCl2 increased, although the ratio of alkyl-Cl/alkyl-I remained large in all cases (≥98:2, Figure S10 and S16). We tentatively attribute this phenomenon to the favorable formation of LiZnCl3 over LiZnCl2Br or LiZnCl2I, resulting in sequestration of chloride as the concentration of Zn2+ increases at later reaction times.35 The halogen exchange is also somewhat faster than reported for exchanges in amide solvents with only sodium bromide, but this process could be catalyzed by zinc: catalysis of alkyl halogen exchange by titanium, zirconium, rhodium, and iron salts has been reported.36
While iodide exchange to enhance the reactivity of alkyl bromides,14 sulfonic acid esters,37 epoxides,38 and chlorides11 in cross-coupling reactions is now well established, the use of bromide is more rare.39 In cases where iodide co-catalysis isn’t practical, the use of bromide co-catalysis should be considered.
Finally, studies with a variety of ligands revealed that PyBCam nickel catalysts are unique in being able to react with both substrates at similar rates, even with activation by halide exchange (Table 1 and Supporting Information Figure S1). Compared to nickel complexes of tpy‴, which could also react with both substrates but formed both biaryl and bialkyl, nickel PyBCam catalysts avoid biaryl formation entirely and form only small amounts of alkyl dimer. The origin of these differences in reactivity are not yet clear and are the subject of ongoing studies, but it is clear that PyBCam and PyBCamCN are a distinctive, new class of tridentate ligands for nickel catalysis.40
In conclusion, the first selective cross-electrophile coupling reaction of aryl chlorides with primary alkyl chlorides has been developed by the synergistic effect of three changes: a new, selective ligand (PyBCamCN), LiCl to enhance catalyst turnover, and bromide/iodide co-catalysis. The mechanism by which PyBCamCN improves yields is under investigation and will be reported in due course. We expect that the generally unreactive nature of alkyl and aryl chlorides should make this new method to functionalize them a useful addition to synthesis.
Supplementary Material
ACKNOWLEDGMENT
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number R01GM097243 (DJW) and F32GM122362 (MJG). Analytical data were obtained from the CENTC Elemental Analysis Facility at the University of Rochester, funded by NSF CHE-0650456. The following instrumentation in the PBCIC was supported by: Thermo Q Exactive™ Plus by NIH 1S10 OD020022; Shimadzu GCMS-QP2010S by the Department of Chemistry; Bruker Avance III 400 by NSF CHE-1048642; Bruker Avance III 500 by a generous gift from Paul J. and Margaret M. Bender.
Footnotes
ASSOCIATED CONTENT
Supporting Information. The Supporting Information is available free of charge on the ACS Publications website. Additional tables of optimization data, mechanistic studies, detailed experimental procedures, characterization of products, copies of product NMR spectra (PDF)
REFERENCES
- (1).(a) Goldfogel MJ; Huang L; Weix DJ: Cross-Electrophile Coupling. In Nickel Catalysis in Organic Synthesis; Ogoshi S, Ed.; Wiley-VCH: Weinheim, 2020; pp 183–222. [Google Scholar]; (b) Wang X; Dai Y; Gong H. Nickel-Catalyzed Reductive Couplings. Top. Curr. Chem 2016, 374, 43. [DOI] [PubMed] [Google Scholar]; (c) Everson DA; Weix DJ. Cross-Electrophile Coupling: Principles of Reactivity and Selectivity. J. Org. Chem 2014, 79, 4793–4798. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Knappke CEI; Grupe S; Gärtner D; Corpet M; Gosmini C; Jacobi von Wangelin A. Reductive Cross-Coupling Reactions between Two Electrophiles. Chem.–Eur. J 2014, 20, 6828–6842. [DOI] [PubMed] [Google Scholar]
- (2).(a) For example, benzylic chlorides, allylic chlorides, and α-chloroesters react rapidly under conditions that were effective with unactivated alkyl bromides. See the following examples: Durandetti M; Nédélec J-Y; Périchon J. Nickel-Catalyzed Direct Electrochemical Cross-Coupling between Aryl Halides and Activated Alkyl Halides. J. Org. Chem 1996, 61, 1748–1755. [DOI] [PubMed] [Google Scholar]; (b) Poremba KE; Kadunce NT; Suzuki N; Cherney AH; Reisman SE, Nickel-Catalyzed Asymmetric Reductive Cross-Coupling To Access 1,1-Diarylalkanes. J. Am. Chem. Soc 2017, 139, 5684–5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Erickson LW; Lucas EL; Tollefson EJ; Jarvo ER Nickel-Catalyzed Cross-Electrophile Coupling of Alkyl Fluorides: Stereospecific Synthesis of Vinylcyclopropanes. J. Am. Chem. Soc 2016, 138, 14006–14011. [DOI] [PubMed] [Google Scholar]
- (4). A survey of the literature discussed in reference 1a had 18 examples of Csp2–Cl and 8 examples of Csp3–Cl bonds being tolerated as functional groups in reactions to form Csp2–Csp3 bonds.
- (5).(a) The coupling of aryl C-Cl bonds and alkyl C-Cl bonds with CO2 and related π-electrophiles has been reported. See the following lead references: Fujihara T; Nogi K; Xu T; Terao J; Tsuji Y. Nickel-Catalyzed Carboxylation of Aryl and Vinyl Chlorides Employing Carbon Dioxide. J. Am. Chem. Soc 2012, 134, 9106–9109; [DOI] [PubMed] [Google Scholar]; (b) Börjesson M; Moragas T; Martin R. Ni-Catalyzed Carboxylation of Unactivated Alkyl Chlorides with CO2. J. Am. Chem. Soc 2016, 138, 7504–7507. [DOI] [PubMed] [Google Scholar]
- (6).See also a recent report on the coupling of Ar-Cl with C-H bonds via arylnickel(II) intermediates: Shields BJ; Doyle AG Direct C(sp3)–H Cross Coupling Enabled by Catalytic Generation of Chlorine Radicals. J. Am. Chem. Soc 2016, 138, 12719–12722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7). About 1.8 million organic chlorides are commercially available compared to about 841,000 organic bromides. Organic iodides and organometal reagents each have <100,000 examples. Notably, aryl chlorides represent about ½ of all commercially available aryl-X (X ≠ H).
- (8).(a) Aryl chlorides are relatively unreactive under conditions used for aryl bromides and usually require specialized conditions See: Grushin VV; Alper H. Transformations of Chloroarenes, Catalyzed by Transition-Metal Complexes. Chem. Rev 1994, 94, 1047–1062. [Google Scholar]; (b) Littke AF; Fu GC Palladium‐Catalyzed Coupling Reactions of Aryl Chlorides. Angew. Chem., Int. Ed 2002, 41, 4176–4211. [DOI] [PubMed] [Google Scholar]; (c) Hassan J; Sévignon M; Gozzi C; Schulz E; Lemaire M. Aryl-Aryl Bond Formation One Century after the Discovery of the Ullmann Reaction. Chem. Rev 2002, 102, 1359–1470. [DOI] [PubMed] [Google Scholar]
- (9).(a) Alkyl chlorides often require different conditions than alkyl bromides for success See: Frisch AC; Beller M. Catalysts for Cross-Coupling Reactions with Non-activated Alkyl Halides. Angew. Chem., Int. Ed 2005, 44, 674–688. [DOI] [PubMed] [Google Scholar]; (b) Zhou J; Fu GC Palladium-Catalyzed Negishi Cross-Coupling Reactions of Unactivated Alkyl Iodides, Bromides, Chlorides, and Tosylates. J. Am. Chem. Soc 2003, 125, 12527–12530. [DOI] [PubMed] [Google Scholar]
- (10).(a) Substitution reactions of alkyl bromides vs alkyl chlorides have krel of about 20. For oxidative addition, krel can be as high as 103 See: Richard JP; Jencks WP Concerted bimolecular substitution reactions of 1-phenylethyl derivatives. J. Am. Chem. Soc 1984, 106, 1383–1396. [Google Scholar]; (b) Labinger JA. Tutorial on Oxidative Addition. Organometallics 2015, 34, 4784–4795. [Google Scholar]; (c) Labinger JA; Osborn JA; Coville NJ Mechanistic studies of oxidative addition to low-valent metal complexes. Mechanisms for addition of alkyl halides to iridium(I). Inorg. Chem 1980, 19, 3236–3243. [Google Scholar]
- (11).Prinsell MR; Everson DA; Weix DJ Nickel-Catalyzed, Sodium Iodide-Promoted Reductive Dimerization of Alkyl Halides, Alkyl Pseudohalides, and Allylic Acetates. Chem. Commun 2010, 46, 5743–5745. [DOI] [PubMed] [Google Scholar]
- (12). Martin has reported on the coupling of unactivated alkyl chlorides with CO2, see reference 5b. In collaboration with Pfizer and Asymchem, we had noted a selective coupling with an alkyl chloride for the first time, but it was not general, see reference 20b. Finally, couplings with activated alkyl chlorides are well known, see reference 2.
- (13).Xu C; Guo W-H; He X; Guo Y-L; Zhang X-Y; Zhang X. Difluoromethylation of (hetero)aryl chlorides with chlorodifluoromethane catalyzed by nickel. Nat. Commun 2018, 9, 1170. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Liu J; Zhang J; Li X; Wu C; Liu H; Liu H; Sun F; Li Y; Liu Y; Li X. Nickel-Catalyzed 1,1-Difluoroethylation of (Hetero)aryl Halides with 1,1-Difluoroethyl Chloride (CH3CF2Cl). Asian J. Org. Chem 2020, 9, 391–394. [Google Scholar]
- (14).Czaplik WM; Mayer M; Jacobi von Wangelin A. Domino Iron Catalysis: Direct Aryl-Alkyl Cross-Coupling. Angew. Chem., Int. Ed 2009, 48, 607–610. [DOI] [PubMed] [Google Scholar]; (b) Everson DA; Jones BA; Weix DJ Replacing Conventional Carbon Nucleophiles with Electrophiles: Nickel-Catalyzed Reductive Alkylation of Aryl Bromides and Chlorides. J. Am. Chem. Soc 2012, 134, 6146–6159. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Yang Y; Luo G; Li Y; Tong X; He M; Zeng H; Jiang Y; Liu Y; Zheng Y. Nickel‐Catalyzed Reductive Coupling for Transforming Unactivated Aryl Electrophiles into β‐Fluoroethylarenes. Chem. – Asian J 2020, 15, 156–162. [DOI] [PubMed] [Google Scholar]
- (15).Ye Y; Chen H; Sessler JL; Gong H. Zn-Mediated Fragmentation of Tertiary Alkyl Oxalates Enabling Formation of Alkylated and Arylated Quaternary Carbon Centers. J. Am. Chem. Soc 2019, 141, 820–824. [DOI] [PubMed] [Google Scholar]
- (16). In reference 14a Jacobi von Wangelin reported the coupling of chlorocyclohexane with 2-bromoanisole (63% yield) and bromobenzene (25% yield) using iron catalysis and Mg as the terminal reductant.
- (17).Biswas S; Weix DJ Mechanism and Selectivity in Nickel-Catalyzed Cross-Electrophile Coupling of Aryl Halides with Alkyl Halides. J. Am. Chem. Soc 2013, 135, 16192–16197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).(a) For related studies, see: Breitenfeld J; Ruiz J; Wodrich MD; Hu X. Bimetallic Oxidative Addition Involving Radical Intermediates in Nickel-Catalyzed Alkyl-Alkyl Kumada Coupling Reactions. J. Am. Chem. Soc 2013, 135, 12004–12012; [DOI] [PubMed] [Google Scholar]; (b) Schley ND; Fu GC Nickel-Catalyzed Negishi Arylations of Propargylic Bromides: A Mechanistic Investigation. J. Am. Chem. Soc 2014, 136, 16588–16593; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Zhao C; Jia X; Wang X; Gong H. Ni-Catalyzed Reductive Coupling of Alkyl Acids with Unactivated Tertiary Alkyl and Glycosyl Halides. J. Am. Chem. Soc 2014, 136, 17645–17651. [DOI] [PubMed] [Google Scholar]
- (19).(a) For alternative mechanistic hypotheses for related reactions, see: Lin Q; Diao T. Mechanism of Ni-Catalyzed Reductive 1,2-Dicarbofunctionalization of Alkenes. J. Am. Chem. Soc 2019, 141, 17937–17948. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Gutierrez O; Tellis JC; Primer DN; Molander GA; Kozlowski MC Nickel-Catalyzed Cross-Coupling of Photoredox-Generated Radicals: Uncovering a General Manifold for Stereoconvergence in Nickel-Catalyzed Cross-Couplings. J. Am. Chem. Soc 2015, 137, 4896–4899. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Mohadjer Beromi M; Brudvig GW; Hazari N; Lant HMC; Mercado BQ Synthesis and Reactivity of Paramagnetic Nickel Polypyridyl Complexes Relevant to C(sp2)–C(sp3)Coupling Reactions. Angew. Chem., Int. Ed 2019, 58, 6094–6098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).(a) Hansen EC; Pedro DJ; Wotal AC; Gower NJ; Nelson JD; Caron S; Weix DJ New ligands for nickel catalysis from diverse pharmaceutical heterocycle libraries. Nat. Chem 2016, 8, 1126–1130; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Hansen EC; Li C; Yang S; Pedro D; Weix DJ Coupling of Challenging Heteroaryl Halides with Alkyl Halides via Nickel-Catalyzed Cross-Electrophile Coupling. J. Org. Chem 2017, 82, 7085–7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21). PyBCam (Order No. 902047) and PyBCamCN (Order No. 902063) are commercially available from Sigma-Aldrich.
- (22).Johnson KA; Biswas S; Weix DJ Cross-Electrophile Coupling of Vinyl Halides with Alkyl Halides. Chem.–Eur. J 2016, 22, 7399–7402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Goldup SM; Leigh DA; McBurney RT; McGonigal PR; Plant A. Ligand-assisted nickel-catalysed sp3–sp3 homocoupling of unactivated alkyl bromides and its application to the active template synthesis of rotaxanes. Chem. Sci 2010, 383–386. [Google Scholar]
- (24).(a) Anderson TJ; Jones GD; Vicic DA Evidence for a NiI Active Species in the Catalytic Cross-Coupling of Alkyl Electrophiles. J. Am. Chem. Soc 2004, 126, 8100–8101. [DOI] [PubMed] [Google Scholar]; (b) Jones GD; Martin J; McFarland C; Allen O; Hall R; Haley A; Brandon R; Konovalova T; Desrochers P; Pulay P; Vicic D. Ligand Redox Effects in the Synthesis, Electronic Structure, and Reactivity of an Alkyl–Alkyl Cross-Coupling Catalyst. J. Am. Chem. Soc 2006, 128, 13175–13183. [DOI] [PubMed] [Google Scholar]
- (25). [See the Supporting Information for extensive ligand reactivity and selectivity data (Figure S1) as well as additional optimization data (Figures S4, S5, S7).]
- (26).(a) While Csp3–Csp2 bond-forming Negishi reactions with aryl iodides and bromides have been shown to tolerate O-H and N-H bonds in many cases, fewer examples exist for couplings with C-Cl bonds Leroux M; Vorherr T; Lewis I; Schaefer M; Koch G; Karaghiosoff K; Knochel P. Late‐Stage Functionalization of Peptides and Cyclopeptides Using Organozinc Reagents. Angew. Chem., Int. Ed 2019, 58, 8231–8234. [DOI] [PubMed] [Google Scholar]; (b) Pompeo M; Froese RDJ; Hadei N; Organ MG Pd‐PEPPSI‐IPentCl: A Highly Effective Catalyst for the Selective Cross‐Coupling of Secondary Organozinc Reagents. Angew. Chem., Int. Ed 2012, 51, 11354–11357. [DOI] [PubMed] [Google Scholar]
- (27).Bordwell FG; Hughes DL Thiol acidities and thiolate ion reactivities toward butyl chloride in dimethyl sulfoxide solution. The question of curvature in Brøensted plots. J. Org. Chem 1982, 47, 3224–3232. [Google Scholar]
- (28).(a) Nickel is known to catalyze C-S bond formation of aryl thiols with aryl chlorides. Arylzinc reagents and zinc powder have been used as promoters. See: Jones KD; Power DJ; Bierer D; Gericke KM; Stewart SG Nickel Phosphite/Phosphine-Catalyzed C–S Cross-Coupling of Aryl Chlorides and Thiols. Org. Lett 2018, 20, 208–211. [DOI] [PubMed] [Google Scholar]; (b) Gehrtz PH; Geiger V; Schmidt T; Sršan L; Fleischer I. Cross-Coupling of Chloro(hetero)arenes with Thiolates Employing a Ni(0)-Precatalyst. Org. Lett 2019, 21, 50–55. [DOI] [PubMed] [Google Scholar]; (c) Gogoi P; Hazarika S; Sarma MJ; Sarma K; Barman P. Nickel–Schiff base complex catalyzed C–S cross-coupling of thiols with organic chlorides. Tetrahedron 2014, 70, 7484–7489. [Google Scholar]
- (29).(a) Generally, these compounds are made by hydrosilylation of olefins (with HSiCl3 or HSi(OMe)3) or Grignard reactions (with tetrachlorosilane). Hydrosilylation to form trimethoxysilanes requires (MeO)3SiH, which can form pyrophoric SiH4. A workaround is to use HSiCl3, but this is also prone to rapid decomposition Buslov I; Song F; Hu X. An Easily Accessed Nickel Nanoparticle Catalyst for Alkene Hydrosilylation with Tertiary Silanes. Angew. Chem., Int. Ed 2016, 55, 12295–12299. [DOI] [PubMed] [Google Scholar]; (b) Katoh K; Ito S; Wada Y; Higashi E; Suzuki Y; Kubota K; Nakano K; Wada Y. Investigation of thermal hazard during the hydrosilylation of 1,6-divinyl(perfluorohexane) with trichlorosilane. J. Chem. Thermodyn 2011, 43, 1229–1234. [Google Scholar]
- (30).The Kumada coupling of trimethoxysilanes is reported to be impractical, but triethoxysilanes can be cross-coupled. See: Brondani DJ; Corriu RJP; El Ayoubi S; Moreau JJE; Wong Chi Man M. Polyfunctional carbosilanes and organosilicon compounds. Synthesis via grignard reactions. Tetrahedron Lett. 1993, 34, 2111–2114. [Google Scholar]
- (31).(a) Sagiv J. Organized monolayers by adsorption. 1. Formation and structure of oleophobic mixed monolayers on solid surfaces. J. Am. Chem. Soc 1980, 102, 92–98. [Google Scholar]; (b) Wasserman SR; Tao YT; Whitesides GM Structure and reactivity of alkylsiloxane monolayers formed by reaction of alkyltrichlorosilanes on silicon substrates. Langmuir 1989, 5, 1074–1087. [Google Scholar]; (c) Ulman A. Formation and Structure of Self-Assembled Monolayers. Chem. Rev 1996, 96, 1533–1554. [DOI] [PubMed] [Google Scholar]; (d) Haensch C; Hoeppener S; Schubert US Chemical modification of self-assembled silane based monolayers by surface reactions. Chem. Soc. Rev 2010, 39, 2323–2334. [DOI] [PubMed] [Google Scholar]
- (32).See Supporting Information for a detailed procedure. For a recent publication on scale-up of related chemistry in flow, see:Watanabe E; Chen Y; May O; Ley SV A Practical Method for Continuous Production of sp3-Rich Compounds from (Hetero)Aryl Halides and Redox-Active Esters. Chem.–Eur. J 2020, 26, 186–191. [DOI] [PubMed] [Google Scholar]
- (33).Huang L; Ackerman LKG; Kang K; Parsons AM; Weix DJ LiCl-Accelerated Multimetallic Cross-Coupling of Aryl Chlorides with Aryl Triflates. J. Am. Chem. Soc 2019, 141, 10978–10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).(a) Krasovskiy A; Malakhov V; Gavryushin A; Knochel P. Efficient Synthesis of Functionalized Organozinc Compounds by the Direct Insertion of Zinc into Organic Iodides and Bromides. Angew. Chem., Int. Ed 2006, 45, 6040–6044. [DOI] [PubMed] [Google Scholar]; (b) Koszinowski K; Böhrer P. Formation of Organozincate Anions in LiCl-Mediated Zinc Insertion Reactions. Organometallics 2009, 28, 771−779. [Google Scholar]; (c) Feng C; Cunningham DW; Easter QT; Blum SA Role of LiCl in Generating Soluble Organozinc Reagents. J. Am. Chem. Soc 2016, 138, 11156−11159. [DOI] [PubMed] [Google Scholar]; (d) Jess K; Kitagawa K; Tagawa TKS; Blum SA Microscopy Reveals: Impact of Lithium Salts on Elementary Steps Predicts Organozinc Reagent Synthesis and Structure. J. Am. Chem. Soc 2019, 141, 9879−9884. [DOI] [PubMed] [Google Scholar]
- (35).(a) Anderson TJ; Vicic DA Direct Observation of Noninnocent Reactivity of ZnBr2 with Alkyl Halide Complexes of Nickel. Organometallics 2004, 23, 623–625. [Google Scholar]; (b) Koszinowski K; Böhrer P. Formation of Organozincate Anions in LiCl-Mediated Zinc Insertion Reactions. Organometallics 2009, 28, 771–779. [Google Scholar]; (c) Achonduh GT; Hadei N; Valente C; Avola S; O’Brien CJ; Organ MG On the role of additives in alkyl-alkyl Negishi cross-couplings. Chem. Commun 2010, 46, 4109–4111. [DOI] [PubMed] [Google Scholar]; (d) Hunter HN; Hadei N; Blagojevic V; Patschinski P; Achonduh GT; Avola S; Bohme DK; Organ MG Identification of a Higher-Order Organozincate Intermediate Involved in Negishi Cross-Coupling Reactions by Mass Spectrometry and NMR Spectroscopy. Chem.–Eur. J 2011, 17, 7845–7851. [DOI] [PubMed] [Google Scholar]; (e) McCann LC; Hunter HN; Clyburne JAC; Organ MG Higher-Order Zincates as Transmetalators in Alkyl–Alkyl Negishi Cross-Coupling. Angew. Chem., Int. Ed 2012, 51, 7024–7027. [DOI] [PubMed] [Google Scholar]; (f) Charboneau DJ; Brudvig GW; Hazari N; Lant HMC; Saydjari AK Development of an Improved System for the Carboxylation of Aryl Halides through Mechanistic Studies. ACS Catalysis 2019, 9, 3228–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).(a) Yoon KB; Kochi JK Catalytic bromide and iodide exchange of alkyl chlorides with hydrogen bromide and hydrogen iodide. J. Org. Chem 1989, 54, 3028–3036. [Google Scholar]; (b) Willy WE; McKean DR; Garcia BA Conversion of Alkyl Chlorides to Bromides, Selective Reactions of Mixed Bromochloroalkanes, and Halogen Exchange. Bull. Chem. Soc. Jpn 1976, 49, 1989–1995. [Google Scholar]; (c) Wang J; Tong X; Xie X; Zhang Z. Rhodium-Catalyzed Activation of C(sp3)−X (X = Cl, Br) Bond: An Intermolecular Halogen Exchange Case. Org. Lett 2010, 12, 5370–5373. [DOI] [PubMed] [Google Scholar]; (d) Mizukami Y; Song Z; Takahashi T. Halogen Exchange Reaction of Aliphatic Fluorine Compounds with Organic Halides as Halogen Source. Org. Lett 2015, 17, 5942–5945. [DOI] [PubMed] [Google Scholar]; (e) Petrone DA; Ye J; Lautens M. Modern Transition-Metal-Catalyzed Carbon–Halogen Bond Formation. Chem. Rev 2016, 116, 8003–8104. [DOI] [PubMed] [Google Scholar]
- (37).(a) Liang Z; Xue W; Lin K; Gong H. Nickel-Catalyzed Reductive Methylation of Alkyl Halides and Acid Chlorides with Methyl p-Tosylate. Org. Lett 2014, 16, 5620–5623. [DOI] [PubMed] [Google Scholar]; (b) Molander GA; Traister KM; O’Neill BT Engaging Nonaromatic, Heterocyclic Tosylates in Reductive Cross-Coupling with Aryl and Heteroaryl Bromides. J. Org. Chem 2015, 80, 2907–2911. [DOI] [PubMed] [Google Scholar]; (c) Do H-Q; Chandrashekar ERR; Fu GC Nickel/Bis(oxazoline)-Catalyzed Asymmetric Negishi Arylations of Racemic Secondary Benzylic Electrophiles to Generate Enantioenriched (37) 1,1-Diarylalkanes. J. Am. Chem. Soc 2013, 135, 16288–16291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Zhao Y; Weix DJ Nickel-Catalyzed Regiodivergent Opening of Epoxides with Aryl Halides: Co-Catalysis Controls Regioselectivity. J. Am. Chem. Soc 2014, 136, 48–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39). While several reports on the conversion of alkyl chlorides to bromides are known (reference 36), we could not find explicit examples of this strategy for transition-metal catalysis. Given the frequent use of metal bromide salts as catalyst precursors, we suspect this phenomenon is common.
- (40).Hughes JME; Fier PS Desulfonylative Arylation of Redox-Active Alkyl Sulfones with Aryl Bromides. Org. Lett 2019, 21, 5650–5654. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.