Abstract
Background:
Several herbal mouth rinses are assessed in the literature as an adjunct to scaling and root planning (SRP) for the treatment of periodontal diseases. The objective of this study was to evaluate and compare the clinical and microbiological effects of Matricaria chamomilla (MTC) mouth rinse with chlorhexidine (CHX) and placebo mouth rinse for the management of chronic periodontitis.
Materials and Methods:
This double-blind, randomized, placebo controlled, clinical trial involved seventy five patients, suffering from chronic periodontitis, which were randomly divided into three groups: negative control (SRP + placebo), positive control (SRP + 0.12% CHX), and test group (SRP + 1% MTC mouth rinse). Mouth rinsing (adjunctive therapy) was continued for 1 month while clinical parameters (plaque index, gingival index, sulcus bleeding index, probing pocket depth [PPD], clinical attachment level, gingival recession [GR], stain index) and microbial colony forming units were evaluated at base line, 6 weeks, and 3 months.
Results:
All groups showed a significant change in parameters (except GR for placebo group) between base line and 3 months. MTC mouth rinse suggested added significant benefits over placebo group over the study period. However, it determined more but nonsignificant improvement in PPD (3.68 mm vs. 3.36 mm) and CAL (3.00 mm vs. 2.72 mm) as compared to CHX rinse at 3 months’ period as compared to baseline.
Conclusion:
Advantages of using test group were comparable to CHX associated group; therefore, MTC mouth rinse can be used as an effective adjunct during nonsurgical periodontal therapy for chronic periodontitis.
Keywords: Chronic periodontitis, mouth rinse, nonsurgical periodontal therapy, scaling and root planning
INTRODUCTION
Chronic periodontitis is a common destructive inflammatory disease of the periodontium. Biofilm is a critical factor for the initiation and development of destructive periodontal diseases. Periodontal microorganisms in biofilm are responsible for the immune inflammatory cascade in host tissue that lead to both soft and hard tissue loss.[1,2]
Removal of etiological agent, i.e., bacterial plaque is the main goal for the management of periodontal diseases. Although mechanical plaque control is of prime importance, but chemical plaque control forms an ideal adjunct to mechanical therapy.[3,4,5] Several chemical agents in adjunction with scaling and root planning (SRP) are used in the literature for the prevention, treatment and maintenance of periodontal destruction. Mouth rinsing with chlorhexidine (CHX), an extensively studied chemical agent for plaque control, considered as the gold standard for reducing the pathogenic microbial load and maintaining the periodontal health due to its antimicrobial and anti-plaque properties. However, tooth staining and taste alteration are the common reversible side effects of the long-term use of CHX rinse.[6,7,8]
Recent oral researches pull the attention toward the possible role of phytomedicines for combating with oral and periodontal inflammatory conditions.[9,10,11,12] Matricaria chamomilla (MTC) (commonly known as chamomile), an ancient medicinal plant of daisy (Asteraceae) family, whose flower extracts and oil are commonly known for its wide therapeutic properties. Active components of this plant extract are terpenoids (α-bisabol, chamazulene, and sesquiterpenes), coumarins (umbelliferone), flavonoids (luteolin, apigenin, and quercetin), and spiroether.[13,14,15] Previous literature proposed anti-inflammatory, antimicrobial, antiseptic, antioxidant, and wound healing properties of MTC.[13,16,17] The MTC oral rinse showed improvement in plaque accumulation, gingival inflammation, recurrent stomatitis, and oral mucositis.[18,19,20,21,22] However, no study in literature is found regarding the use of MTC mouth rinse for the management of chronic periodontitis.
The objective of this randomized clinical trial was to evaluate the null hypothesis that there are no added clinical advantages of 1% MTC mouth rinse as an adjunct to SRP compared with 0.12% CHX mouth rinse for the management of chronic periodontitis.
MATERIALS AND METHODS
Seventy-five individuals (39 males and 36 females) suffering from chronic periodontitis (age group of 30–65 years) were assigned in this randomized controlled clinical trial from the outpatient department of Periodontics. The research protocol was approved by the institutional ethical committee and review board. All examinations, treatments and procedures associated with this study followed the Helsinki Declaration of 1975, as revised in 2000. Written informed consent was taken from all participants recruited in the study.
Inclusion criteria included systemically healthy individuals of >30 years of age with previously untreated generalized chronic periodontitis,[23] having minimum 15 teeth, minimum of six teeth with at least one interproximal site with probing pocket depth (PPD) between 5 and 7 mm, and clinical attachment level (CAL) between 5 and 10 mm, at least 30% of the sites with PPD and CAL ≥5 mm and presence of bleeding on probing.
Exclusion criteria were any systemic disease, use of any medication in the previous 6 months, subjects wearing partial removal prosthesis or orthodontic appliance, any known allergy, alcoholics, smokers or tobacoo users in any form, mentally retarded individual, pregnant, or lactating females.
This prospective study was designed as a randomized, double-blind, three arms longitudinal, placebo-controlled clinical trial. One hundred and thirty-five individuals were examined for eligibility, out of which 75 individuals satisfying the inclusion criteria were randomly recruited to one of the three groups using a computer generated random allocation sequence. Group sample sizes were decided by power analysis with 95% power and a significance level of 0.05.
Group 1 – Twenty-five individuals (14 males and 11 females): SRP + placebo mouth rinsing (negative control group)
Group 2 – Twenty-five individuals (12 males and 13 females): SRP + 0.12% CHX mouth rinsing (positive control group)
Group 3 – Twenty-five individuals (13 males and 12 females): SRP + 1% MTC mouth rinsing (test group).
The primary outcome of this study was CAL, while PPD and microbial colony forming units (CFUs) were the secondary outcome measures. A single clinician who was blinded to the groups assigned to the individuals, recorded all the parameters, i.e., plaque index (PI),[24] gingival index (GI),[25] sulcus bleeding index,[26] PPD, CAL, gingival recession (GR), stain index (SI)[27] at base line (prior to the treatment), 6 weeks and 3 months after therapy. PPD, CAL, and GR were recorded at the six sites per tooth in every tooth, except third molar, with a manual UNC-15 periodontal probe (Hu-Friedy, Leimen, Germany) to the nearest millimeter. For recording of parameters at different periods, patients were instructed to refrain from any oral hygiene procedure for 8 h prior to the evaluation. The staining of the six maxillary anterior teeth was assessed using the Lobene index.[27,28]
For subgingival plaque collection, the teeth were isolated with cotton rolls, and a plaque sample was obtained by the introduction of sterile paper cones inside the pocket for 30 s. The sample was placed in a vial containing 10 ml of transport medium. The samples were placed into the petridishes containing blood agar under anaerobic environment (5%–10% carbon dioxide) at 35°C–37°C. After 5–7 days of incubation period colonies of the selected anaerobic microorganism (Porphyromonas gingivalis, Tannerella forsythia, Treponema denticola) were identified and counted. The results were converted into logarithm values for better understanding and statistical analysis. Plaque samples were collected at baseline, 6 weeks, and 3 months of postoperative period.
The mouth rinse was prepared in the laboratory of the Department of Pharmacy, MJP Rohilkhand University, Bareilly, by an experienced pharmacologist. The batch number for this mouth rinse is CMA-0801–1001. 50 g dried CML flower [Figure 1] was taken and was crushed with the help of mortar and pestle to powder form. This powder was mixed with 500 ml of boiled water in a conical flask and suspension was prepared by shaking flask in a shaking incubator (200 rpm) at 37° temperature for 4 h. This flask was then brought to room temperature and was filtered using Whatman paper under suction and then passed through 0.22 μ filter paper (Millipore, Billerica, MA) to obtain uniform acqu1eous extract. After discarding residual flash and evaporation, the remaining extract was 250 ml consisting of appropriate concentration. This extract was freeze dried at −20°C until utilized. The standardization of concentration for mouth rinse was determined by titration. From that extract, 2.2 ml volume of the was diluted using pipette with 220 ml of distilled water to prepare 1% v/v mouthwash solution and 0.05% peppermint oil was also add as a flavoring agent.
Figure 1.

Dried Matricaria chamomilla flowers
All participants received full mouth SRP by manual and ultrasonic scalers and dental polishing by a periodontist, unaware to the study protocol, in a single visit [Figures 2 and 3]. According to their groups, patients were asked to rinse with 15 ml of assigned medication two time in a day till 30 days for 1 min, i.e., in the morning 30–45 min after brushing and were instructed not to rinse or eat anything for 30 min after using mouthwash and at night before going to sleep. Mouthwashes were put in three identical opaque bottles coded with A, B, or C [Figure 4]. Another clinician distributed the bottles to the patients according to the randomization and allocation list.
Figure 2.
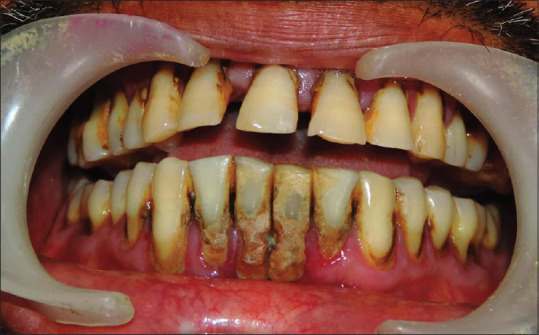
Patient's intraoral view (prior to scaling and root planning)
Figure 3.
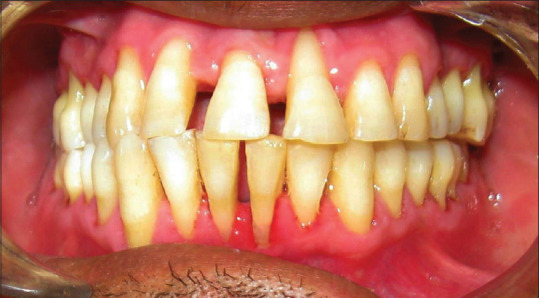
Patient's intraoral view (postoperative after 3 months)
Figure 4.

Three mouth rinse in different bottles
No professional supra gingival debridement and polishing was performed after baseline scaling or before the end of the study. All patients were prescribed by the same dentifrices (Colgate Strong teeth, Colgate-Palmolive, India), toothbrushes (Colgate Sensitive Ultra Soft, Colgate-Palmolive, India) and were instructed in the use of the dentifrice to use during the period of the study. Patients were advised to minimize colored beverage (i.e., tea, coffee, and fruit juice) consumption during the study.
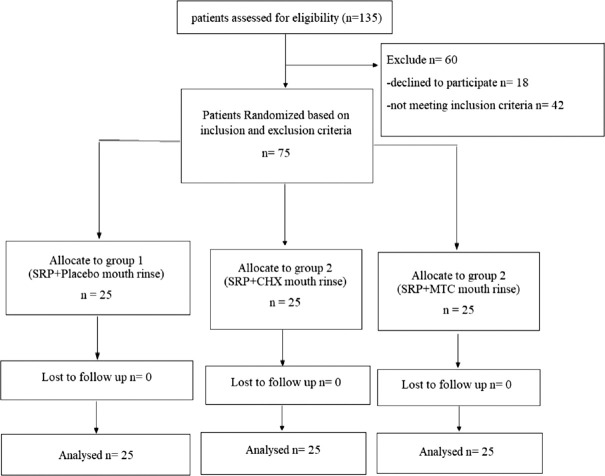
The patient's compliance was assessed every week at the time of recall visits. In these visits, patients exchanged their old bottles with new bottles of medication/placebo. Each bottle contained 220 ml of solution, sufficient for 7 days of rinsing. Subjects were also asked about the self-reported complications. The entire study design is shown with the help of a consort flowchart in Figure 5.
Figure 5.
Consort flowchart of study. n – Patients analysed after followup
Descriptive statistics were computed for each analyzed variable within each study group. The normality of the distributions was assessed by Shapiro–Wilk test. As the data were not normally distributed, nonparametric statistical test were used for analysis. Friedman test was used to compare the periodontal parameters at different time intervals within each of the study group. After applying the Bonferroni correction, Post hoc comparison was done using Wilcoxon signed rank test. Krystal–Walli's anova was used for intergroup comparison.
RESULTS
Demographical data for the study are in Table 1. For all the three rinses, on intragroup comparison, mean values of PPD, PI, GI, GBI, SI demonstrated significant (P < 0.05) improvement (throughout the study) between any two prospective time period [Table 2]. While for CAL all groups proposed significant benefit (within groups) at all time frame except for CHX and MTC between 6 weeks and 3 months [Table 3].
Table 1.
Base line demographical data of study population
| Parameter | Placebo (n=25) | CHX (n=25) | MTC (n=25) | Total (n=75) |
|---|---|---|---|---|
| Male/female | 15/10 | 12/13 | 12/13 | 39/36 |
| Mean age±SD (years) | 54.76±8.13 | 49.12±9.77 | 51.43±9.88 | 51.70±9.50 |
| Age range (years) | 32-65 | 34-65 | 33-63 | 32-65 |
CHX – Chlorhexidine mouth rinse group; MTC – Matricaria chamomilla (MTC) Mouth Rinse Group; SD – Standard deviation; n – number of patients
Table 2.
Values (mean±standard deviation in mm) of clinical parameters at different time intervals
| Parameters | Baseline (n=25) | 6 weeks (n=25) | 3 months (n=25) | P | Mean change (%) |
|---|---|---|---|---|---|
| Placebo | |||||
| PPD | 7.32±1.41 | 5.48±0.96 | 4.84±0.80 | 0.000* | 33.88 |
| CAL | 8.48±1.90 | 6.92±1.26 | 6.32±1.11 | 0.000* | 25.47 |
| GR | 1.20±0.71 | 1.44±0.71 | 1.48±0.77 | 0.074 | 23.33 |
| GI | 2.23±0.40 | 0.55±0.41 | 0.97±0.34 | 0.000* | 56.50 |
| PI | 2.31±0.22 | 0.56±0.15 | 0.68±0.17 | 0.000* | 70.56 |
| GBI | 2.36±0.18 | 0.58±0.16 | 0.76±0.17 | 0.000* | 67.80 |
| SI | 1.81±0.07 | 0.12±0.04 | 0.16±0.04 | 0.000* | 91.16 |
| CHX | |||||
| PPD | 7.12±1.36 | 4.88±0.67 | 3.76±0.44 | 0.000* | 47.19 |
| CAL | 8.44±1.96 | 5.88±1.09 | 5.72±1.24 | 0.000* | 32.23 |
| GR | 1.32±0.69 | 0.88±0.78 | 1.96±1.02 | 0.000* | 48.48 |
| GI | 2.31±0.43 | 0.27±0.10 | 0.47±0.11 | 0.000* | 79.65 |
| PI | 2.32±0.25 | 0.36±0.10 | 0.48±0.39 | 0.000* | 79.31 |
| GBI | 2.33±0.30 | 0.39±0.08 | 0.48±0.10 | 0.000* | 79.40 |
| SI | 1.88±0.09 | 0.25±0.05 | 0.30±0.05 | 0.000* | 84.04 |
| MTC | |||||
| PPD | 7.16±1.40 | 4.76±0.83 | 3.48±0.65 | 0.000* | 51.40 |
| CAL | 8.36±1.80 | 5.76±0.97 | 5.36±1.11 | 0.000* | 35.89 |
| GR | 1.20±0.50 | 1.00±0.50 | 1.88±0.83 | 0.000* | 56.67 |
| GI | 2.41±0.43 | 0.25±0.10 | 0.40±0.08 | 0.000* | 83.40 |
| PI | 2.35±0.23 | 0.36±0.09 | 0.40±0.09 | 0.000* | 82.98 |
| GBI | 2.28±0.18 | 0.38±0.10 | 0.44±0.10 | 0.000* | 80.70 |
| SI | 1.89±0.09 | 0.22±0.06 | 0.27±0.06 | 0.000* | 85.71 |
Friedman test, *P<0.05 – statistically significant. PPD – Probing pocket depth; CAL – Clinical attachment level; GR – Gingival recession; GI – Gingival index; PI – Plaque index; SBI – Sulcus bleeding index; SI – Stating index; CHX – Chlorhexidine mouth rinse group; MTC – Matricaria chamomilla (MTC) Mouth Rinse Group; SD – Standard deviation; n – Number of patients; P – Significance level; GBI – Gingival bleeding index
Table 3.
Significance value for difference in mean of parameters between the time periods (within groups)
| Comparison | PPD (n=25) | CAL (n=25) | GR (n=25) | GI (n=25) | Pl (n=25) | GBI (n=25) | SI (n=25) |
|---|---|---|---|---|---|---|---|
| Placebo | |||||||
| Baseline - 6 weeks | 0.000* | 0.000* | 0.034 | 0.000* | 0.000* | 0.000* | 0.000* |
| Baseline - 3 months | 0.000* | 0.000* | 0.053 | 0.000* | 0.000* | 0.000* | 0.000* |
| 6 weeks - 3 months | 0.001* | 0.000* | 0.739 | 0.000* | 0.000* | 0.000* | 0.000* |
| CHX | |||||||
| Baseline - 6 weeks | 0.000* | 0.000* | 0.001* | 0.000* | 0.000* | 0.000* | 0.000* |
| Baseline - 3 months | 0.000* | 0.000* | 0.000* | 0.000* | 0.000* | 0.000* | 0.000* |
| 6 weeks - 3 months | 0.000* | 0.305 | 0.000* | 0.000* | 0.000* | 0.000* | 0.000* |
| MTC | |||||||
| Baseline - 6 weeks | 0.000* | 0.000* | 0.059 | 0.000* | 0.000* | 0.000* | 0.000* |
| Baseline - 3 months | 0.000* | 0.000* | 0.000* | 0.000* | 0.000* | 0.000* | 0.000* |
| 6 weeks - 3 months | 0.000* | 0.004 | 0.000* | 0.000* | 0.000* | 0.000* | 0.000* |
Wilcoxon signed-ranks test, *P<0.002 – statistically significant. PPD – Probing pocket depth; CAL – Clinical attachment level; GR – Gingival recession; GI – Gingival index; PI – Plaque index; SBI – Sulcus bleeding index; SI – Stating index; CHX – Chlorhexidine mouth rinse group; MTC – Matricaria chamomilla (MTC) Mouth Rinse Group; SD – Standard deviation; n – Number of patients; P – Significance level; GBI – Gingival bleeding index
On intergroup comparison, mean values of all parameters except SI showed nonsignificant (P > 0.05) difference at base line. Both CHX and MTC showed significant difference in mean values of all the parameters (except GR) as compared to placebo group at 6 weeks’ period. While between CHX and MTC, mean values of all the parameters investigated in nonsignificant difference at 6 weeks after base line [Table 4].
Table 4.
Significance value (P) for difference in mean of parameters between the groups (within time period)
| Parameters | Time periods | Placebo - CHX | Placebo - MTC | CHX - MTC |
|---|---|---|---|---|
| PPD | 6 weeks | 0.091 | 0.018* | NS |
| 3 months | 0.000* | 0.000* | NS | |
| CAL | 6 weeks | 0.017* | 0.004* | NS |
| 3 months | 0.041* | 0.017* | NS | |
| GR | 6 weeks | 0.018* | 0.066 | NS |
| GI | 6 weeks | 0.001* | 0.000* | NS |
| 3 months | 0.000* | 0.000* | NS | |
| PI | 6 weeks | 0.000* | 0.000* | NS |
| 3 months | 0.000* | 0.000* | NS | |
| GBI | 6 weeks | 0.000* | 0.000* | NS |
| 3 months | 0.000* | 0.000* | NS | |
| SI | Baseline | 0.008* | 0.005* | NS |
| 6 weeks | 0.000* | 0.000* | NS | |
| 3 months | 0.000* | 0.000* | NS |
*P<0.05 – statistically significant. NS – Non significant; PPD – Probing pocket depth; CAL – Clinical attachment level; GR – Gingival recession; GI – Gingival index; PI – Plaque index; SBI – Sulcus bleeding index; SI – Stating index; CHX – Chlorhexidine mouth rinse group; MTC – Matricaria chamomilla (MTC) Mouth Rinse Group; P – Significance level; GBI – Gingival bleeding index
For mean change in parameters both CHX and MTC showed significant more improvement (except in GR, SI) as compared to placebo group between base line-6 weeks. However, between CHX and MTC, all parameters improved in nonsignificant manner [Table 5]. Table 6 shows the frequency distribution of CAL gain.
Table 5.
Significance value (P) for change in parameters from baseline to 3 months (inter group)
| Parameters | Placebo - CHX | Placebo - MTC | CHX - MTC |
|---|---|---|---|
| PPD | 0.027* | 0.001* | NS |
| CAL | 0.044* | 0.011* | NS |
| GR | NS | NS | NS |
| GI | 0.001* | 0.000* | NS |
| PI | 0.004* | 0.000* | NS |
| GBI | 0.003* | 0.006* | NS |
| SI | NS | NS | NS |
*P<0.05 – statistically significant. NS – Non significant; PPD – Probing pocket depth; CAL – Clinical attachment level; GR – Gingival recession; GI – Gingival index; PI – Plaque index; SBI – Sulcus bleeding index; SI – Stating index; CHX – Chlorhexidine mouth rinse group; MTC – Matricaria chamomilla (MTC) Mouth Rinse Group; P – Significance level; GBI – Gingival bleeding index
Table 6.
Frequency distribution of clinical attachment level gain over the study
| CAL gain (mm) | Placebo, n (%) | CHX, n (%) | MTC, n (%) |
|---|---|---|---|
| ≤1 | 4 (16) | 2 (8) | 0 (0) |
| 2 | 13 (52) | 7 (28) | 8 (32) |
| 3 | 6 (24) | 13 (52) | 10 (40) |
| ≥4 | 2 (8) | 3 (12) | 7 (28) |
CAL – Clinical attachment level; CHX – Chlorhexidine mouth rinse group; MTC – Matricaria chamomilla (MTC) Mouth Rinse Group; n – Number of patients
Three patients in CHX group evident with teeth staining and one patient with taste alteration. Apart from this, no adverse effects or complications were reported by any group during the study.
Microbiological parameter was improved for all three groups during the study. MTC group showed significantly less (P < 0.05) CFUs than placebo but comparable (P > 0.05) to CHX group [Tables 7 and 8].
Table 7.
Microbiological mean±standard deviation (colony forming units in log) for groups
| Parameters | Baseline (n=25) | 6 weeks (n=25) | 3 months (n=25) | P | Mean change (%) |
|---|---|---|---|---|---|
| Placebo | |||||
| P. gingivalis | 1.62±0.44 | 1.42±0.48 | 1.41±0.80 | 0.02* | 12.3 |
| T. forsythia | 1.32±0.80 | 1.21±0.36 | 1.19±0.11 | 0.02* | 9.9 |
| T. denticola | 0.95±0.81 | 0.81±0.71 | 0.85±0.77 | 0.03* | 10.7 |
| CHX | |||||
| P. ginigvalis | 1.58±0.56 | 1.20±0.67 | 1.17±0.64 | 0.00* | 25.5 |
| T. forsythia | 1.35±0.46 | 1.08±0.79 | 1.07±0.24 | 0.00* | 20.9 |
| T. denticola | 0.98±0.69 | 0.76±0.79 | 0.72±0.72 | 0.00* | 26.6 |
| MTC | |||||
| P. gingivalis | 1.59±0.50 | 1.18±0.85 | 1.16±0.69 | 0.00* | 27.1 |
| T. forsythia | 1.31±0.69 | 1.09±0.67 | 1.09±0.52 | 0.00* | 17.1 |
| T. denticola | 0.97±0.50 | 0.73±0.70 | 0.75±0.63 | 0.00* | 22.2 |
Friedman test, *P<0.05 – statistically significant. CFUs – Colony forming units; CHX – Chlorhexidine mouth rinse group; MTC – Matricaria chamomilla (MTC) Mouth Rinse Group; SD – Standard deviation; n – Number of patients; P – Significance level; P. gingivalis – Porphyromonas gingivalis; T. forsythia – Tannerella forsythia; T. denticola – Treponema denticola
Table 8.
Significance value (P) for mean difference in parameters between the groups (within time period)
| Parameters | Time periods | Placebo - CHX | Placebo - MTC | CHX - MTC |
|---|---|---|---|---|
| P. gingivalis | 6 weeks | 0.00* | 0.00* | NS |
| 3 months | 0.00* | 0.00* | NS | |
| T. forsythia | 6 weeks | 0.00* | 0.00* | NS |
| 3 months | 0.00* | 0.00* | NS | |
| T. denticola | 6 weeks | 0.00* | 0.00* | NS |
| 3 months | 0.00* | 0.00* | NS |
*P<0.05 – statistically significant. NS – Non significant; CHX – Chlorhexidine mouth rinse group; MTC – Matricaria chamomilla (MTC) Mouth Rinse Group; P – Significance level; P. gingivalis – Porphyromonas gingivalis; T. forsythia – Tannerella forsythia; T. denticola – Treponema denticola
DISCUSSION
Our findings proposed that all three treatment groups presented with significant changes in parameters at different time periods (except GR for placebo and MTC, and CAL for CHX and MTC). However, on intergroup comparison, mean of parameters determined nonsignificant differences between CHX and MTC at 6 weeks and 3 months’ period. Both combined therapies showed significant differences, in mean of parameter, as compared to placebo at 6 weeks and 3 months’ period (except PPD at 6 weeks, and GR at 3 months between CHX and placebo and GR at 6 weeks and 3 months between MTC and placebo).
In addition to above, observation of this trial also demonstrated that both MTC and CHX rinses suggested similar advantages in reduction of PPD, CAL, PI, GI, and MBI after 3 months of base line period, while for same comparison both oral rinses were significantly more beneficial than SRP/placebo group. Ample amount of evidence is present in the literature regarding the positive outcomes of CHX in adjunction with SRP for the management of periodontal disease.[6,7,8,11] Therefore CHX mouth rinse was taken as a positive control in this study. However, certain disadvantages of CHX like unpleasant taste and staining of teeth always seeking for the use of any new herbal mouth rinse. The placebo group was included as the negative control in this study because it was supposed to show no additional benefit with SRP. These two well-design controls curtailed the effects of variables other than the independent variables.
In the best of our knowledge, MTC and CHX rinses were not compared before for the management of chronic periodontitis. Therefore, direct verification of the results was not possible. The observations for placebo and CHX groups of our study are in the accordance with previous studies.[7,29,30] Our study follows the results of other trials involving herbal rinse in which test group demonstrated with no additional benefits as compared to CHX oral rinse.[12,31,32] Variability in the magnitude of results was observed in the literature for the adjunctive use of CHX rinse with SRP in the management of chronic periodontitis. The disparity in patient selection, base line parameters, and follow-up period might influence the observed heterogeneity in the findings.
We use chamomile rinse for the management of chronic periodontitis due to its antimicrobial, anti-inflammatory, antioxidant, immune regulatory, and superior healing properties.[14,15] MTC is an annual plant indigenous to Asia and Europe, possessing hollow, bright gold cones of the flowers that are packed with disc or tubular florets, and are ringed with white ray or ligulate florets. It is also known as chamomile or camomile, Italian camomilla, German chamomile, wild chamomile, and Hungarian chamomile. Chamomile is used in several countries for commercial purposes as herbal tea and for pharmaceutical and cosmeceutical uses.[13,16,17] Chamomile is listed on the FDAs Generally Recognized as Safe List; however, persons with known hypersensitivity to plants of the Asteraceae (Compositae) family such as arnica flower, marigold, ragweed, should avoid its use.
The main mechanism of action is anti-inflammatory property due to apigenin, chamazulene, and bisabolol present in MTC extract that inhibit nitric oxide (NO) production, activities of hyaluronidase, collagenase, cyclooxygenases enzymes, prostaglandin E2, interleukin-1 β, -6, -12, and tumor necrosis factor-alpha.[16,17]
Goes et al.[22] evaluated a significant improvement in PI, and GBI for both CHX and MTC mouth rinse after 15 days of the study in gingivitis patients. Both CHX and MTC groups determined more significant benefits as compared to placebo. However, no significant differences found between the above said two medicated mouth rinses. No adverse event presented with MTC rinse as similar to our study. An another cross over design study also elucidated significantly more improvement in mean plaque and gingival score after 4 weeks of chamomile mouth rinse intervention, as compared to control, in gingivitis patients with no adverse effects throughout the study.[18] A previous double-blind, placebo-controlled clinical trial detected nonsignificant benefits of chamomile mouth rinse for chemotherapy induced stomatitis.[33] Please change it as Mazokopakis EE et al also advocated the role of CMT m/w for the management of methotrexate-induced oral mucositis.[34]
Whenever microbial assessment used in conjunction with clinical trials, it tributes and nourishes the inferences of the research because certain pathogens have a positive correlation with clinical condition or parameters. In this study, we used microbiological culture because it is not only the gold standard method for identification and counting of the viable microbial colonies but also has the economical advantage in comparison to other techniques.[35,36] Culture was performed using selective means for three red complex pathogens, T. forsythia, P. gingivalis, and Treponema denticola, which are strongly related as main culprit to chronic periodontitis.[37,38] The rationale for testing oral rinse with SRP is that the previous reports demonstrated the limited effect of nonsurgical periodontal therapy for clinical and microbiological parameters.[39,40] Therefore, there is always the requirement for such adjunctive that has the ability of combat to periopathogens as well as improves the clinical picture. Our microbial counting results for placebo and CHX were corroborated the previous findings.[29,30] Improvement in the colonies of the red complex bacteria also reflects in clinical observations of this study.
The person with known hypersensitivity to Asteraceae family (e.g., Marigold) should avoid the use of MTC mouth rinse. Apart from this contact dermatitis, eye irritation, vomiting, and uterus contraction are also documented in some patients if taken in large amount. Rare cases of anaphylactic reactions due to chamomile also documented.[14,16,17]
More recently, we have demonstrated that aqueous and methanolic chamomile extracts possess anticancer and cytotoxic effects; specifically, reduced proliferation and enhanced induction of apoptosis in various human cancer cells.[16] The tea obtained from chamomile flowers revealed the cytotoxic action on malignant cells of the skin (mainly melanoma).
In our study, allocation of individuals in different groups was made with computer based software but base line data in CHX and MTC groups still showed significantly more mean values in SI as compared to placebo. At 6 weeks and 3 months, means of SI were also significantly greater in the above said group. However, it is quite interesting to note that change in SI suggested nonsignificant difference at any time period between groups. In our study, postoperative change of tooth staining in 0.12% CHX rinse was comparable to placebo group, a possible reason of this may be that lower concentration of CHX revealed with less staining in contrast to higher concentration (0.2%) of CHX.[41]
CONCLUSION
The MTC mouth rinse was found to improve the clinical and microbiological picture of chronic periodontitis. Its outcomes commensurate the gold standard mouth wash CHX; therefore, Chamomile mouth rinse can be a potential therapeutic agent for chronic periodontitis.
Financial support and sponsorship
Self.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We (authors) are highly thankful to Dr. Shiv Dev Singh, Associate Professor, Dept of Pharmacy, MJP Rohilkhand university, Bareilly for helping in the preparation of chamomile mouth rinse.
REFERENCES
- 1.Cekici A, Kantarci A, Hasturk H, Van Dyke TE. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontol 2000. 2014;64:57–80. doi: 10.1111/prd.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanz M, Beighton D, Curtis MA, Cury JA, Dige I, Dommisch H, et al. Role of microbial biofilms in the maintenance of oral health and in the development of dental caries and periodontal diseases. Consensus report of group 1 of the Joint EFP/ORCA workshop on the boundaries between caries and periodontal disease. J Clin Periodontol. 2017;44(Suppl 18):S5–11. doi: 10.1111/jcpe.12682. [DOI] [PubMed] [Google Scholar]
- 3.Figuero E, Nóbrega DF, García-Gargallo M, Tenuta LM, Herrera D, Carvalho JC. Mechanical and chemical plaque control in the simultaneous management of gingivitis and caries: A systematic review. J Clin Periodontol. 2017;44(Suppl 18):S116–34. doi: 10.1111/jcpe.12674. [DOI] [PubMed] [Google Scholar]
- 4.Drisko CH. Nonsurgical periodontal therapy. Periodontol 2000. 2001;25:77–88. doi: 10.1034/j.1600-0757.2001.22250106.x. [DOI] [PubMed] [Google Scholar]
- 5.Duarte PM, da Rocha M, Sampaio E, Mestnik MJ, Feres M, Figueiredo LC, et al. Serum levels of cytokines in subjects with generalized chronic and aggressive periodontitis before and after non-surgical periodontal therapy: A pilot study. J Periodontol. 2010;81:1056–63. doi: 10.1902/jop.2010.090732. [DOI] [PubMed] [Google Scholar]
- 6.da Costa LF, Amaral CD, Barbirato DD, Leão AT, Fogacci MF. Chlorhexidine mouthwash as an adjunct to mechanical therapy in chronic periodontitis: A meta-analysis. J Am Dent Assoc. 2017;148:308–18. doi: 10.1016/j.adaj.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 7.Fonseca DC, Cortelli JR, Cortelli SC, Miranda Cota LO, Machado Costa LC, Moreira Castro MV, et al. Clinical and microbiologic evaluation of scaling and root planing per quadrant and one-stage full-mouth disinfection associated with azithromycin or chlorhexidine: A clinical randomized controlled trial. J Periodontol. 2015;86:1340–51. doi: 10.1902/jop.2015.150227. [DOI] [PubMed] [Google Scholar]
- 8.Berchier CE, Slot DE, Van der Weijden GA. The efficacy of 0.12% chlorhexidine mouthrinse compared with 0.2% on plaque accumulation and periodontal parameters: A systematic review. J Clin Periodontol. 2010;37:829–39. doi: 10.1111/j.1600-051X.2010.01575.x. [DOI] [PubMed] [Google Scholar]
- 9.Samuels N, Grbic JT, Saffer AJ, Wexler ID, Williams RC. Effect of an herbal mouth rinse in preventing periodontal inflammation in an experimental gingivitis model: A pilot study. Compend Contin Educ Dent. 2012;33:204–6. [PubMed] [Google Scholar]
- 10.Dhingra K, Vandana KL. Effectiveness of Azadirachta indica (neem) mouthrinse in plaque and gingivitis control: A systematic review. Int J Dent Hyg. 2017;15:4–15. doi: 10.1111/idh.12191. [DOI] [PubMed] [Google Scholar]
- 11.Manipal S, Hussain S, Wadgave U, Duraiswamy P, Ravi K. The mouthwash war – Chlorhexidine vs. herbal mouth rinses: A meta-analysis. J Clin Diagn Res. 2016;10:ZC81–3. doi: 10.7860/JCDR/2016/16578.7815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pradeep AR, Suke DK, Martande SS, Singh SP, Nagpal K, Naik SB. Triphala, a new herbal mouthwash for the treatment of gingivitis: A randomized controlled clinical trial. J Periodontol. 2016;87:1352–9. doi: 10.1902/jop.2016.130406. [DOI] [PubMed] [Google Scholar]
- 13.Srivastava JK, Shankar E, Gupta S. Chamomile: A herbal medicine of the past with bright future. Mol Med Rep. 2010;3:895–901. doi: 10.3892/mmr.2010.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKay DL, Blumberg JB. A review of the bioactivity and potential health benefits of chamomile tea (Matricaria recutita L.) Phytother Res. 2006;20:519–30. doi: 10.1002/ptr.1900. [DOI] [PubMed] [Google Scholar]
- 15.Avallone R, Zanoli P, Puia G, Kleinschnitz M, Schreier P, Baraldi M. Pharmacological profile of apigenin, a flavonoid isolated from Matricaria chamomilla. Biochem Pharmacol. 2000;59:1387–94. doi: 10.1016/s0006-2952(00)00264-1. [DOI] [PubMed] [Google Scholar]
- 16.Srivastava JK, Pandey M, Gupta S. Chamomile, a novel and selective COX-2 inhibitor with anti-inflammatory activity. Life Sci. 2009;85:663–9. doi: 10.1016/j.lfs.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martins MD, Marques MM, Bussadori SK, Martins MA, Pavesi VC, Mesquita-Ferrari RA, et al. Comparative analysis between Chamomilla recutita and corticosteroids on wound healing. An in vitro and in vivo study. Phytother Res. 2009;23:274–8. doi: 10.1002/ptr.2612. [DOI] [PubMed] [Google Scholar]
- 18.Pourabbas R, Delazar A. The effect of German chamomile mouthrinse on dental plaque and gingival inflammation. Iran J Pharm Res. 2005;4:105–9. [Google Scholar]
- 19.Willershausen B, Kasaj A, Sculean A, Wehrbein H. Influence of an herbal mouthwash on inflammatory changes of the gingiva in patients with fixed orthodontic appliance. PERIO Periodontal Pract Today. 2004;1:255–62. [Google Scholar]
- 20.Batista AL, Lins RD, de Souza Coelho R, do Nascimento Barbosa D, Moura Belém N, Alves Celestino FJ. Clinical efficacy analysis of the mouth rinsing with pomegranate and chamomile plant extracts in the gingival bleeding reduction. Complement Ther Clin Pract. 2014;20:93–8. doi: 10.1016/j.ctcp.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Tiemann P, Toelg M, Ramos FM. Administration of Ratanhia-based herbal oral care products for the prophylaxis of oral mucositis in cancer chemotherapy patients: A clinical trial. Evid Based Complement Alternat Med. 2007;4:361–6. doi: 10.1093/ecam/nel070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goes P, Dutra CS, Lisboa MR, Gondim DV, Leitão R, Brito GA, et al. Clinical efficacy of a 1% Matricaria chamomile L. mouthwash and 0.12% chlorhexidine for gingivitis control in patients undergoing orthodontic treatment with fixed appliances. J Oral Sci. 2016;58:569–74. doi: 10.2334/josnusd.16-0280. [DOI] [PubMed] [Google Scholar]
- 23.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 24.Silness J, Loe H. Periodontal disease in pregnancy. II Correlation between oral hygiene and periodontal condtion. Acta Odontol Scand. 1964;22:121–35. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 25.Loe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand. 1963;21:533–51. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 26.Mühlemann HR, Son S. Gingival sulcus bleeding – A leading symptom in initial gingivitis. Helv Odontol Acta. 1971;15:107–13. [PubMed] [Google Scholar]
- 27.Lobene RR. Effect of dentifrices on tooth stains with controlled brushing. J Am Dent Assoc. 1968;77:849–55. doi: 10.14219/jada.archive.1968.0298. [DOI] [PubMed] [Google Scholar]
- 28.Duss C, Lang NP, Cosyn J, Persson GR. A randomized, controlled clinical trial on the clinical, microbiological, and staining effects of a novel 0.05% chlorhexidine/herbal extract and a 0.1% chlorhexidine mouthrinse adjunct to periodontal surgery. J Clin Periodontol. 2010;37:988–97. doi: 10.1111/j.1600-051X.2010.01609.x. [DOI] [PubMed] [Google Scholar]
- 29.Faveri M, Gursky LC, Feres M, Shibli JA, Salvador SL, de Figueiredo LC. Scaling and root planing and chlorhexidine mouthrinses in the treatment of chronic periodontitis: A randomized, placebo-controlled clinical trial. J Clin Periodontol. 2006;33:819–28. doi: 10.1111/j.1600-051X.2006.00994.x. [DOI] [PubMed] [Google Scholar]
- 30.Feres M, Gursky LC, Faveri M, Tsuzuki CO, Figueiredo LC. Clinical and microbiological benefits of strict supragingival plaque control as part of the active phase of periodontal therapy. J Clin Periodontol. 2009;36:857–67. doi: 10.1111/j.1600-051X.2009.01471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Radafshar G, Ghotbizadeh M, Saadat F, Mirfarhadi N. Effects of green tea (Camellia sinensis) mouthwash containing 1% tannin on dental plaque and chronic gingivitis: A double-blinded, randomized, controlled trial. J Investig Clin Dent. 2017;8:e12184–91. doi: 10.1111/jicd.12184. [DOI] [PubMed] [Google Scholar]
- 32.Al-Maweri SA, Nassani MZ, Alaizari N, Kalakonda B, Al-Shamiri HM, Alhajj MN, et al. Efficacy of Aloe vera mouthwash versus chlorhexidine on plaque and gingivitis: A systematic review. Int J Dent Hyg. 2020;18:44–51. doi: 10.1111/idh.12393. [DOI] [PubMed] [Google Scholar]
- 33.Fidler P, Loprinzi CL, O’Fallon JR, Leitch JM, Lee JK, Hayes DL, et al. Prospective evaluation of a chamomile mouthwash for prevention of 5-FU-induced oral mucositis. Cancer. 1996;77:522–5. doi: 10.1002/(SICI)1097-0142(19960201)77:3<522::AID-CNCR14>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 34.Mazokopakis EE, Vrentzos GE, Papadakis JA, Babalis DE, Ganotakis ES. Wild chamomile (Matricaria recutita L.) mouthwashes in methotrexate-induced oral mucositis. Phytomedicine. 2005;12:25–7. doi: 10.1016/j.phymed.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 35.Jervøe-Storm PM, Koltzscher M, Falk W, Dörfler A, Jepsen S. Comparison of culture and real-time PCR for detection and quantification of five putative periodontopathogenic bacteria in subgingival plaque samples. J Clin Periodontol. 2005;32:778–83. doi: 10.1111/j.1600-051X.2005.00740.x. [DOI] [PubMed] [Google Scholar]
- 36.Atieh MA. Accuracy of real-time polymerase chain reaction versus anaerobic culture in detection of Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis: A meta-analysis. J Periodontol. 2008;79:1620–9. doi: 10.1902/jop.2008.070668. [DOI] [PubMed] [Google Scholar]
- 37.Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–44. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 38.Kigure T, Saito A, Seida K, Yamada S, Ishihara K, Okuda K. Distribution of Porphyromonas gingivalis and Treponema denticola in human subgingival plaque at different periodontal pocket depths examined by immunohistochemical methods. J Periodontal Res. 1995;30:332–41. doi: 10.1111/j.1600-0765.1995.tb01284.x. [DOI] [PubMed] [Google Scholar]
- 39.Colombo AP, Teles RP, Torres MC, Rosalém W, Mendes MC, Souto RM, et al. Effects of non-surgical mechanical therapy on the subgingival microbiota of Brazilians with untreated chronic periodontitis: 9-month results. J Periodontol. 2005;76:778–84. doi: 10.1902/jop.2005.76.5.778. [DOI] [PubMed] [Google Scholar]
- 40.Cugini MA, Haffajee AD, Smith C, Kent RL, Jr, Socransky SS. The effect of scaling and root planing on the clinical and microbiological parameters of periodontal diseases: 12-month results. J Clin Periodontol. 2000;27:30–6. doi: 10.1034/j.1600-051x.2000.027001030.x. [DOI] [PubMed] [Google Scholar]
- 41.Jenkins S, Addy M, Newcombe R. Comparison of two commercially available chlorhexidine mouthrinses: II. Effects on plaque reformation, gingivitis, and tooth staining. Clin Prev Dent. 1989;11:12–6. [PubMed] [Google Scholar]