Abstract
Background
General anaesthetics interact with the pathophysiological mechanisms of traumatic brain injury (TBI). We used a Drosophila melanogaster (fruit fly) model to test the hypothesis that ageing and genetic background modulate the effect of anaesthetics and hyperoxia on TBI-induced mortality in the context of blunt trauma.
Methods
We exposed flies to isoflurane or sevoflurane under normoxic or hyperoxic conditions and TBI, and subsequently quantified the effect on mortality 24 h after injury. To determine the effect of age on anaesthetic-induced mortality, we analysed flies at 1–8 and 43–50 days old. To determine the effect of genetic background, we performed a genome-wide association study (GWAS) analysis on a collection of young inbred, fully sequenced lines.
Results
Exposure to anaesthetics and hyperoxia differentially affected mortality in young and old flies. Pre-exposure of young but not old flies to anaesthetics reduced mortality. Post-exposure selectively increased mortality. For old but not young flies, hyperoxia enhanced the effect on mortality of post-exposure to isoflurane but not to sevoflurane. Post-exposure to isoflurane in hyperoxia increased the mortality of young fly lines in the Drosophila Genetic Reference Panel collection to different extents. GWAS analysis of these data identified single nucleotide polymorphisms in genes involved in cell water regulation and oxygen sensing as being associated with the post-exposure effect on mortality.
Conclusions
Ageing and genetic background influence the effects of volatile general anaesthetics and hyperoxia on mortality in the context of traumatic brain injury. Polymorphisms in specific genes are identified as potential causes of ageing and genetic effects.
Keywords: aquaporin, Drosophila, genetics, GWAS, hyperoxia, isoflurane, mortality, sevoflurane, toxicity
Editor's key points.
-
•
Invertebrate animal models provide powerful unbiased genetic tools for identifying novel molecular targets for drugs and disease.
-
•
A Drosophila melanogaster (fruit fly) model was used to test the hypothesis that ageing and genetic background modulate the effect of anaesthetics and hyperoxia on traumatic brain injury (TBI)-induced mortality in the context of blunt trauma.
-
•
Genetic analysis identified single nucleotide polymorphisms in genes involved in cell water regulation and oxygen sensing associated with the post-exposure effect of anaesthetics on mortality that are promising targets for further investigation.
-
•
Ageing and genetic background influenced the effects of volatile general anaesthetics on mortality after TBI.
Exposure to anaesthetics and supplemental oxygen (hyperoxia) causes collateral cell biological effects of largely unknown scope and significance,1, 2, 3, 4, 5 but anaesthetics and hyperoxia are usually considered benign in the care of traumatic brain injury (TBI) patients and in experimental models of TBI. Using a Drosophila melanogaster closed-head TBI model caused by blunt trauma,6 we have shown that the volatile general anaesthetics isoflurane (ISO) and sevoflurane (SEVO) are biologically active in the context of an injured brain.7 Because experimental logistics favour a focus on young subjects and animal welfare concerns limit research into the intrinsic effects of anaesthetics on TBI pathophysiology, substantial gaps remain in our understanding of the interaction between anaesthetics, ageing, and genetic background and the pathophysiology of TBI. Some of these gaps can be effectively addressed in flies because fundamental ageing and injury response pathways are conserved with higher animals. Furthermore, collections of isogenic and fully sequenced fly lines allow genome-wide association studies (GWAS)8 to identify genes associated with outcomes from TBI on a scale currently not feasible in mammalian models.9
We hypothesised that exposure to ISO, SEVO, and hyperoxia differentially influences mortality from TBI induced by blunt trauma in young and old flies. To test for genetic influence, we used 24 h mortality to determine the degree to which exposure to ISO and hyperoxia modulates outcomes of TBI among different fly lines from the Drosophila Genetic Reference Panel (DGRP). This study was also used to identify single nucleotide polymorphisms (SNPs) associated with differentially enhanced mortality. Although mortality in flies should not be literally translated to mortality in higher animals, the molecular events that lead to mortality in flies are likely to be conserved in higher animals and to contribute to the pathophysiology of the secondary injury in TBI. Results indicate that the outcome of the interaction between volatile general anaesthetics and their molecular targets is influenced by biological factors (i.e. age and genetic background) and clinically relevant environmental factors (i.e. oxygen concentration and the type of volatile general anaesthetic).
Methods
The experiments adhere to applicable ARRIVE (Animal Research: Reporting of In Vivo Experiments) reporting guidelines (preclinical animal research). Approval from the Institutional Animal Care and Use Committee has been waived.
Determination of anaesthetic potency
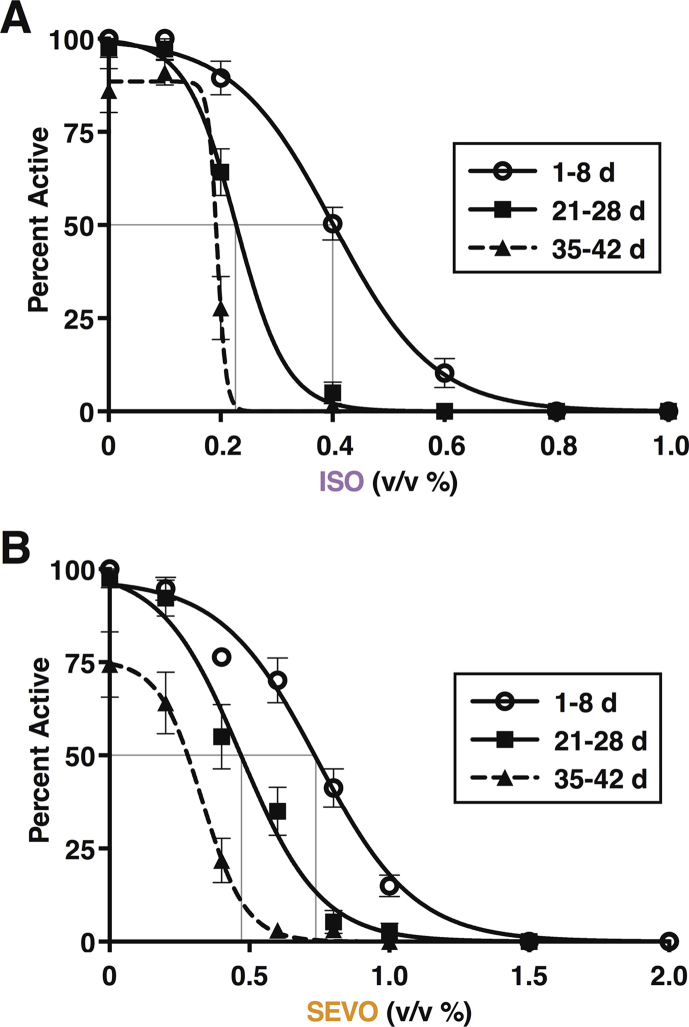
To determine the extent to which age affects anaesthetic potency in flies, we used a customised, negative geotaxis-based behavioural assay that we termed percent active (PA), as described.10 To calculate the half-maximal effective concentration (EC50), we exposed 10 flies simultaneously to one anaesthetic concentration for 10 min and determined the PA. We increased the anaesthetic concentration using the smallest steps possible with agent-specific vaporisers and repeated the PA determination until no flies met the climbing criterion. Each experiment was performed on a naïve group of flies and four or more biological replicates (i.e. at least 40 flies). We tested up to seven concentrations per age group and agent to derive the dose–response relationship (Supplementary Fig. 1) and calculated the EC50 (Fig. 1a).
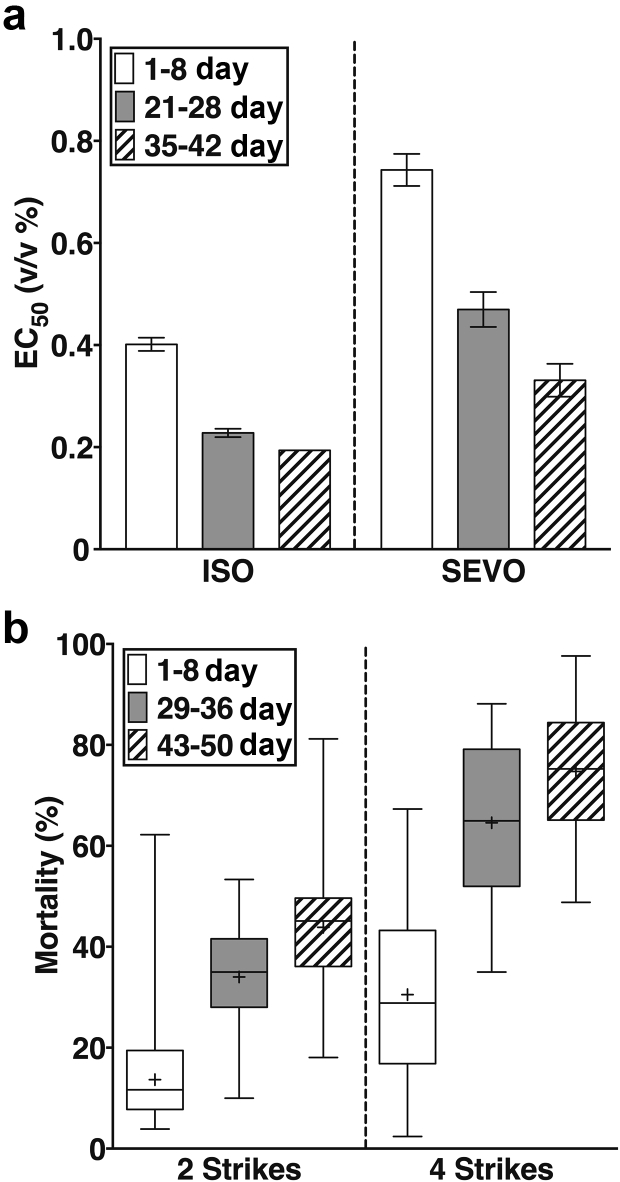
Fig 1.
Ageing sensitises to the behavioural effects of anaesthetics and to mortality from traumatic brain injury (TBI). (a) The EC50 values of volatile anaesthetics decreased from young to old adulthood. The EC50 values for isoflurane (ISO) determined in 1–8, 21–28, and 35–42 day old flies were 0.4% (0.01), 0.23% (0.01) (P<0.001), and 0.19%, respectively. Error bars are standard errors. The error for the 35- to 42-day-old group was not informative because the minimal concentration step on the ISO vaporiser was from 0.1% to 0.2%, which resulted in a large suppression of climbing behaviour (Supplementary Fig. 1). The EC50 values for sevoflurane (SEVO) were 0.74% (0.03) to 0.47% (0.03) and 0.33% (0.03), respectively. EC50 values were calculated based on seven to eight anaesthetic concentrations with an n of at least 4. (b) Mortality from TBI increases with age. 24 h mortality from two strikes (left panel) was 13.7% ([12.6, 14.9], n=136); 36.0% ([33.0, 39.2], n=60), and 43.8% ([42.1, 45.6], n=136) for young, middle-aged, and old w1118 flies, respectively (mean [95% confidence interval). The corresponding mortalities for four strikes (right panel) were 30.5% ([28.4, 32.7], n=78), 69.1% ([65.2, 73.4], n=26), and 74.7% ([69.8, 80.0], n= 24), respectively. Experiments in Fig 2, Fig 3 were performed using two strikes and standard doses of either 2% ISO or 3.5% SEVO.
Traumatic brain injury
TBI was inflicted in a standard laboratory strain (w1118) using a High-Impact Trauma (HIT) device acting on the whole fly.6 All flies were maintained on molasses food at 25°C and transferred weekly until the target age range was reached, 1–8, 29–36, or 43–50 days old. The experimental unit on which analysis is based is a vial that typically contained 20 flies. Experiments that were performed on different days were considered biological replicates. For the experiments in Fig 2, Fig 3 and Supplementary Fig. 2, we used 1088 vials. A detailed break-up of vial numbers per condition is summarised in Supplementary Table 1. On the day before an experiment, eight vials containing 20 mixed-sex flies of the same age (0–7, 28–35, or 42–49 days old) were incubated at 25°C in vials with molasses food. On the day of the experiment, flies were rapidly transferred into empty vials. Four of the vials were subjected to either two or four strikes from the HIT device with the spring deflected to 90° and 5 min between strikes. After injury and exposure to volatile general anaesthetics, hyperoxia, or both, flies were transferred to vials with molasses food and incubated at 25°C. Mortality was determined at 24 h from the time of TBI. At least eight replicates were performed for each experimental and control condition. Because percent mortality after TBI does not differ between male and female flies, we performed all experiments using mixed-sex groups.6
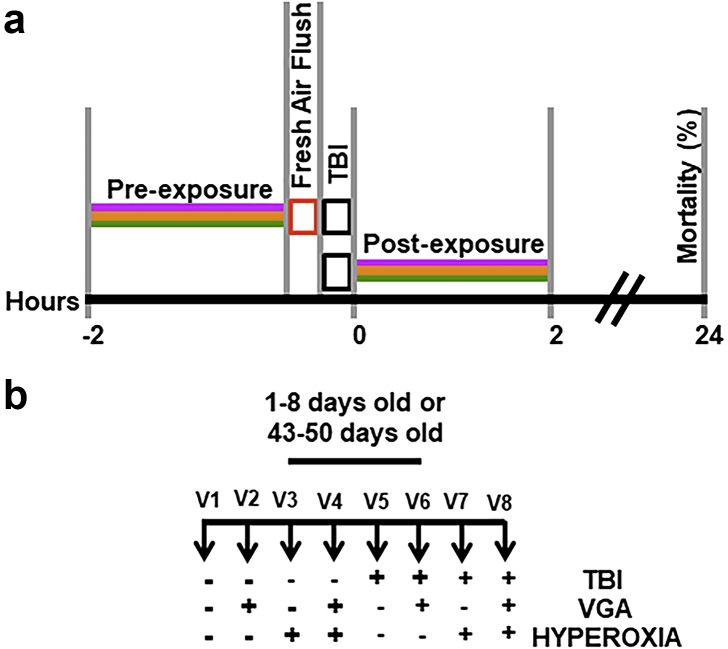
Fig 2.
Diagram of the workflow for experiments presented in Figs 2 and 3. (a) Timelines for Pre-exposure vs. Post-exposure to anaesthetics and hyperoxia relative to traumatic brain injury (TBI). The standard exposure duration was 2 h of 2% isoflurane (ISO), 3.5% sevoflurane (SEVO), and/or 100% O2 (purple, yellow, and green lines, respectively). After pre-exposure, the anaesthetic was flushed for 5 min (red rectangle). TBI was administered with 5 min intervals between strikes. (b) Experimental schematic illustrating all of the experimental and control conditions. Vials one through eight (V1eV8) were considered one experimental unit. Horizontal rows show exposure to interventions (þ) or lack thereof (e). For example, V1 undergoes no intervention and serves as control for natural attrition. V5 undergoes only TBI. See methods and statistical appendix in supplementary material for details.
Fig 3.
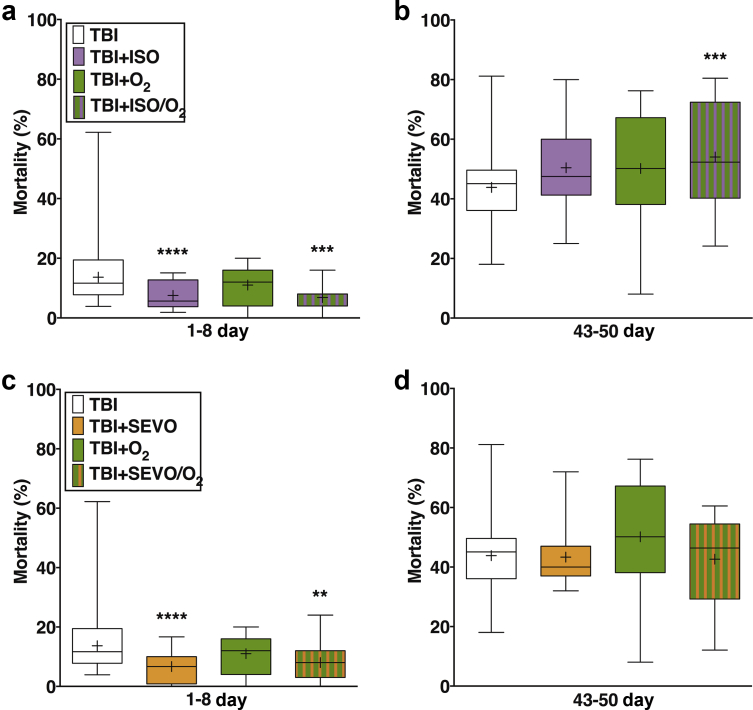
Ageing eliminates the protective effect of pre-exposure to volatile general anaesthetics and hyperoxia on mortality after traumatic brain injury (TBI). Effects on mortality of preexposure to isoflurane (ISO; purple), hyperoxia (green), and ISO in hyperoxia (striped) of 1- to 8-day-old (a) and 43- to 50-day-old (b) w1118 flies. Analogous experiments with sevoflurane (SEVO; yellow) in young (c) and old (d) flies. Note the significant reduction in mortality by both agents in young but not in old flies. Median is indicated by cross bar, mean by þ, and the range of values by whiskers. ∗∗P¼0.004, ∗∗∗P¼0.001, and ∗∗∗∗P<0.001. N¼12e14 for each group. Log-binomial regression analysis. Numeric values of means and confidence intervals are listed in Supplementary Table 6.
Traumatic brain injury, anaesthesia, and hyperoxia
We used a custom-built Serial Anaesthesia Array to simultaneously expose up to eight samples of at least 20 flies each to precise doses of volatile general anaesthetics and oxygen.7,10 Volatile general anaesthetics were administered through the Array using a Datex-Ohmeda Aestiva/5 anaesthesia machine equipped with commercial agent-specific vaporisers (Datex-Ohmeda Inc., Madison, WI, USA). Compressed gas cylinders (Airgas USA, LLC, Radnor, PA, USA) containing 100% oxygen (O2), 100% nitrogen (N2), or air (21% O2/79% N2) provided carrier gas of the desired composition. Anaesthetic exposure consisted of either 2 vol% ISO or 3.5 vol% SEVO for 2 h (i.e. 4% h and 7% h, respectively). The resulting anaesthetic doses of 4% h and 7% h for ISO and SEVO, respectively, are behaviourally equivalent and do not affect median and maximum lifespans.10 To compare anaesthetic pharmacodynamics between young and old flies, we used the same dose of anaesthetics despite the observed change in behavioural potency with age (Fig. 1a). The rationale for this decision is that the molecular targets underlying anaesthetic effects on mortality in the context of TBI are unknown, and there is no reason to assume that their responsiveness to volatile general anaesthetics undergoes the same age-dependent changes as that of complex neural circuits mediating behavioural effects (e.g. minimum alveolar concentration [MAC] in mammals or suppression of reflexive locomotion in flies). Anaesthetics were administered either before (pre-exposure) or after (post-exposure) TBI in either 21% O2 (normoxia) or in nominally 100% O2 (hyperoxia) (Fig. 4a). In experiments under hyperoxic conditions, 100% O2 was used as the carrier gas to administer 2% ISO or 3.5% SEVO. All flies, independent of age, resumed movement within <1 h after discontinuing ISO or SEVO, indicating that the doses were safe. A typical assay simultaneously tested three control conditions (i–iii) and one experimental condition (iv): (i) no treatment; (ii) TBI alone; (iii) anaesthesia, hyperoxia alone, or both; and (iv) TBI and anaesthesia, hyperoxia, or both in both young (1–8 days old) and old (43–50 days old) flies (Fig. 4b). All experiments were conducted under normobaric conditions.
Figure 4.
Ageing enhances the deleterious effect of post-exposure to volatile general anaesthetics and hyperoxia on mortality from traumatic brain injury (TBI). Effects on mortality of post-exposure to isoflurane (ISO; purple), hyperoxia (green), and ISO in hyperoxia (striped) of 1- to 8-day-old (a) and 43- to 50-day-old (b) w1118 flies. Analogous experiments with sevoflurane (SEVO; yellow) in young (c) and old (d) flies. Note the significant increase in mortality by ISO in young flies and by SEVO in old flies. Hyperoxia alone and in combination with ISO increased mortality in old flies. Median is indicated by cross bar, mean by þ, and range of values by whiskers. ∗P¼0.01e0.04, ∗∗P¼0.002, and ∗∗∗∗P<0.001 compared with control; n¼12e16 for each group. Log-binomial regression analysis. Numeric values of means and confidence intervals are listed in Supplementary Table 6.
Genetic background
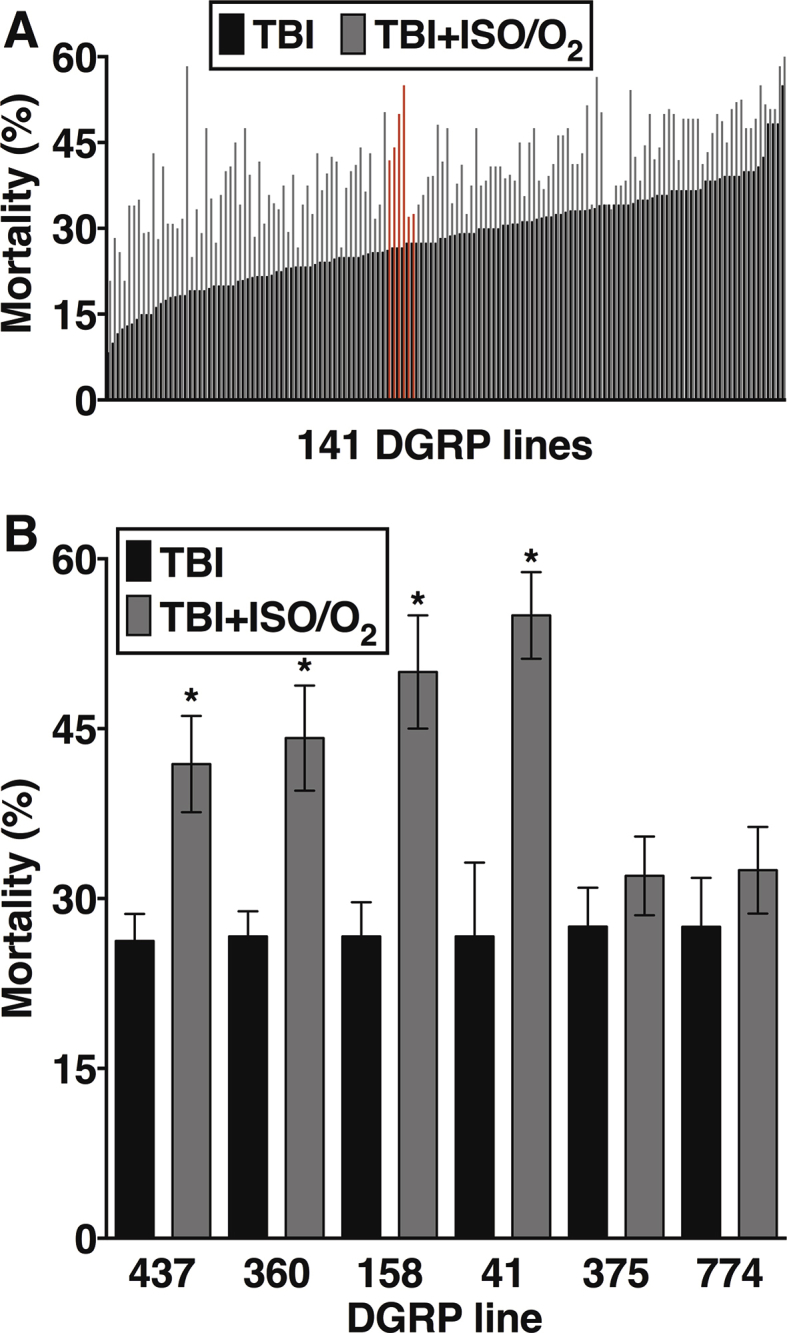
We treated 1- to 8-day-old flies from 141 isogenic lines from the DGRP with the standard four-strike injury protocol, with or without post-exposure to 1 h of ISO in hyperoxia. Each experiment included two vials with 20 flies in each (considered a single biological replicate) undergoing the following interventions: TBI only, 1 h of 2% ISO in 98% O2 only, TBI followed by 1 h of 2% ISO in 98% O2. Forty control flies were kept in room air. Every experiment consisted of at least three biological replicates. The difference in mortality between anaesthetised and unanaesthetised populations was determined 24 h after TBI. For each DGRP line, we controlled for natural mortality and mortality caused by exposure to ISO in hyperoxia in the absence of injury. There was no measurable mortality in flies not exposed to TBI or exposed to anaesthesia and hyperoxia only. Excess mortality (Δ mortality) caused by exposure for 1 h to 2% ISO in 98% O2 after TBI was calculated as Δ mortality (%)={([% mortality TBI and ISO/O2]–[% mortality TBI])/% mortality TBI}×100, and represents the average percent increase in mortality for flies with TBI followed by exposure to ISO in hyperoxia compared with the percent mortality for flies with TBI only.
GWAS analysis
GWAS analysis using Δ mortality (Fig. 5 and Supplementary Fig. 3) was carried out using publicly available web-based analysis tools at the DGRP website (http://dgrp2.gnets.ncsu.edu).
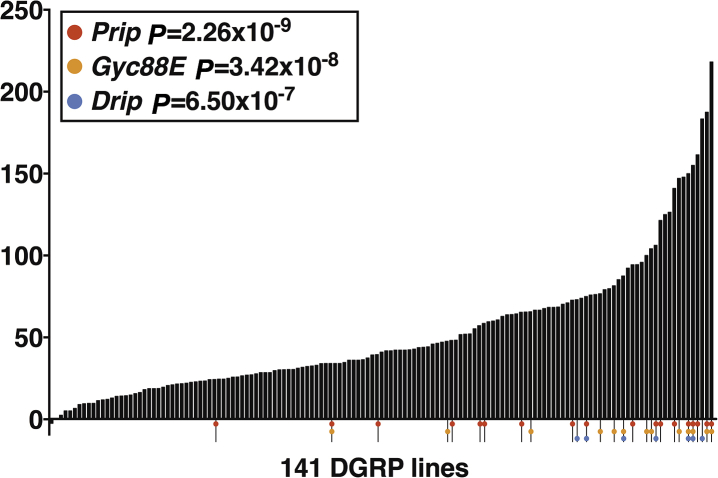
Figure 5.
Genetic background influences resilience to toxicity caused by isoflurane/O2 after traumatic brain injury (TBI). The percent change in mortality caused by post-exposure to isoflurane (ISO)/O2 for each of 141 fly lines from the Drosophila Genetic reference Panel (DGRP). Genome-wide association study (GWAS) identified single nucleotide polymorphisms (SNPs) in Drip, Prip, and Gyc88E as being associated with the change in percent mortality at the indicated level of significance. Coloured dots indicate DGRP lines that contain significant SNPs.
Statistical analysis
For results that report multiple experimental conditions, we used generalised linear models (GLM; either Poisson regression or log-binomial regression) to estimate mortality. This analysis underlies the statistical analyses for the results presented in Fig 2, Fig 3 and Supplementary Fig 2; all tests were conducted at the 0.05 level of significance and drawn in box and whisker plots, according to the Tukey method. The median is indicated by cross bar and the mean by +.
Data collected from each sample consisted of the number of flies alive at the start of a trial (m) and the number of flies that were dead at a trial's conclusion (y). Log-binomial regression was used to estimate and test whether mortality (y/m) differed as a function of anaesthesia, TBI, or the interaction between these two factors, and whether any of these elements differed among the 10 conditions defined by combinations of anaesthesia (ISO, SEVO, or none; each with and without hyperoxia) and timing (pre-vs post-exposure). These models were applied separately to each of two age groups (1–8 and 43–50 days old). For experimental conditions where mortality was especially low (y<<m), Poisson regression, with y as the response and log(m) as an offset, was used instead to estimate the frequency of mortality. Standard errors for these models were computed using a sandwich estimator of variance to account for having used an alternate distribution (i.e. Poisson approximation to binomial for rare events).11 Statistical significance was set to 0.05 with supporting 95% confidence intervals (CIs) for ratios involving mortality risk. All analyses were performed using R (version 3.5.1; R Foundation for Statistical Computing, Vienna, Austria) and the accompanying Sandwich package. We used Prism 6.0 (GraphPad Software Inc., La Jolla, CA, USA) for graphing. P-values reported in the figures refer to comparisons with mortality from TBI in normoxia and without volatile general anaesthetics. A detailed summary of the data used for the statistical analysis of the effects of volatile general anaesthetics and hyperoxia and the 95% CIs are available in the Supplementary Tables 1–6. Numerical results related to Fig 1, Fig 3, Figure 4 and Supplementary Fig 2 are reported as mean [95% CI]. All other results (Fig 1, Figure 5 and Appendix A, Appendix A) as mean (standard error). We used one-way analysis of variance (anova) and Tukey's multiple comparisons test for the data in Fig. 1.
We used Hedge's g to calculate effect size. Hedge's g, similar to Cohen's d, is used for comparing different sample sizes. We used four benchmarks to accommodate the range of data (0, no effect; 0.5, small effect; 0.8, medium effect; 1.1, large effect; https://www.statisticshowto.datasciencecentral.com/hedges-g/).
Results
Ageing increases behavioural sensitivity to anaesthetics and mortality after TBI
The MAC to prevent movement in response to a noxious stimulus undergoes age-dependent changes in mammals.12,13 To what extent this change translates to invertebrates is unknown. We determined the EC50 for suppression of reflexive locomotion in young (1–8 days old), middle-aged (21–28 days old), and old (35–42 days old) flies. The designation of ‘old’ is based on the median lifespan of 48 days for w1118 flies in our laboratory.6 As occurs in mammals, the EC50 values for both ISO and SEVO declined with age from 0.4% (0.01%) to 0.19% (error not determined; see legend for Fig. 1a and Supplementary Fig. 1) for ISO (P<0.0001 for 1–8 vs 21–28) and from 0.74% (0.03%) to 0.33% (0.03%) for SEVO (multiplicity adjusted P<0.0001 for 1–8 vs. 35–42; Fig. 1a). Despite the change in behavioural EC50, we used the same dose of anaesthetics in young and old flies because we were interested in determining the effect of a given dose of anaesthetic rather than the effect of doses that produce the same behavioural effect.
To investigate the effect of ageing on mortality after TBI, we examined w1118 flies at three age groups: young (1–8 days old), middle-aged (29–36 days old), and old (43–50 days old). To avoid levels of TBI-induced mortality that are too high to be altered by anaesthetics, we injured flies with either two or four strikes from the HIT device. Within each age group, mortality from four strikes was higher than from two strikes (multiplicity adjusted P<0.0001). Moreover, for both two and four strikes, mortality was higher for middle-aged and old flies than for young flies (multiplicity adjusted P<0.0001), but there was no difference between middle-aged and old flies (Fig. 1b). These data indicate that ageing-dependent processes exacerbate the secondary injury mechanisms that lead to mortality after TBI at different severities.
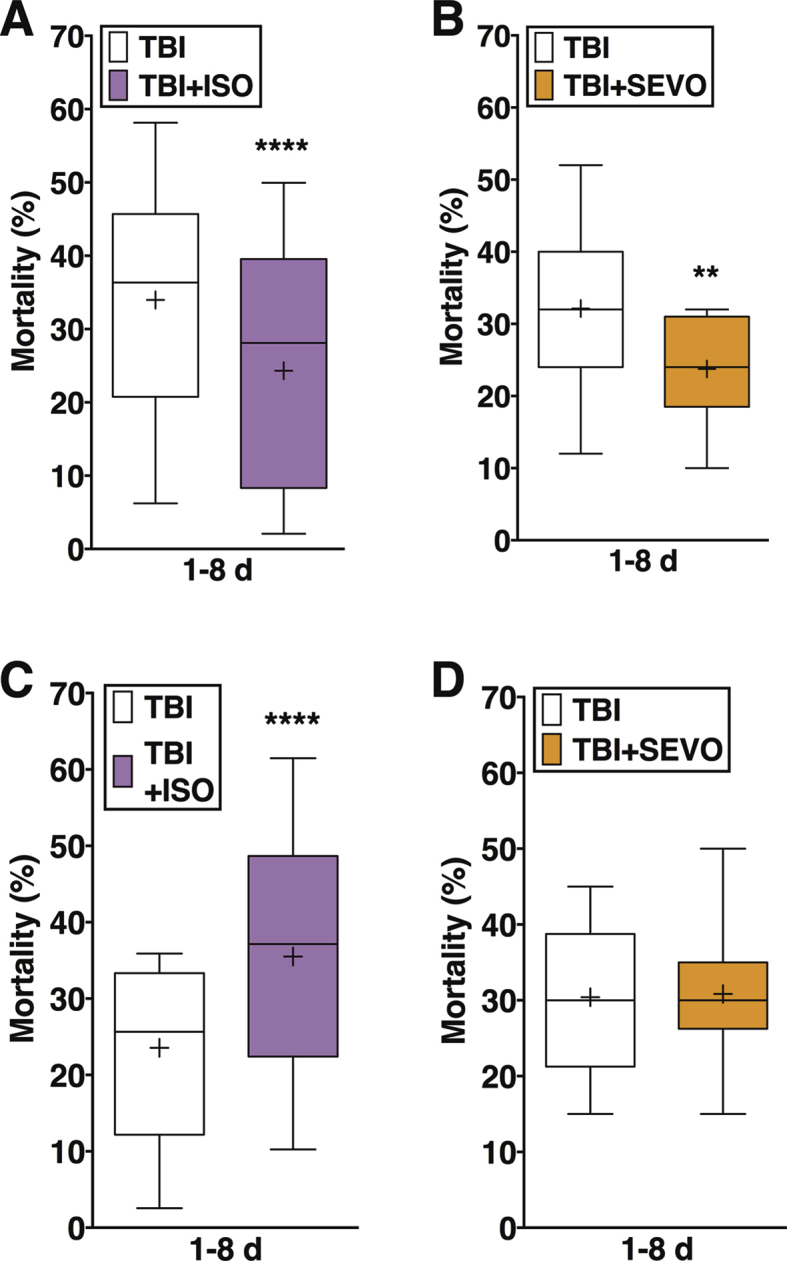
Ageing eliminates the protective effect of pre-exposure to anaesthetics on mortality after TBI
To test the hypothesis that ageing affects the ability of volatile general anaesthetics to alter TBI outcomes, we exposed young and old w1118 flies immediately before TBI (Fig. 3). Because the four-strike protocol resulted in very high mortality in old flies, we used the two-strike protocol to compare the volatile general anaesthetics in young and old flies. To facilitate comparison and because neither agent increased mortality in old flies in the absence of TBI, we decided to use the same dose of anaesthetics in both young and old flies despite the change in behavioural sensitivity (Fig. 1a).
As reported,7 pre-exposure to ISO or SEVO of young flies injured with the four-strike protocol reduced mortality (Supplementary Fig. 2). A similar anaesthetic-induced reduction in mortality was observed in young flies with the milder injury inflicted by the two-strike protocol (from 13.7% [12.6, 14.9] to 7.5% [5.7, 9.9], n=12, P<0.001, Fig. 3a for ISO and to 6.7% [4.5, 9.8], n=12, P<0.001, Fig. 3c for SEVO). In contrast, pre-exposure to ISO or SEVO of old flies injured with the two-strike protocol did not significantly affect mortality (from 43.8% [42.1, 45.6] to 50.4% [42.8, 59.4], n=12, P=0.069, Fig. 3b for ISO and to 43.3% [36.6, 51.4], n=12, P=0.885, Fig. 3d for SEVO). Table 1 summarises the directionality and magnitude of change under the different experimental conditions. Note that, contrary to in-figure symbols, arrows in Table 1 denote the magnitude of effect, not its statistical significance. We conclude that ageing-related processes degrade the protective potential of volatile general anaesthetics in the context of TBI independently of injury severity.
Table 1.
Summary of the magnitude and the directionality of effects on mortality derived from in Fig 2, Fig 3. We used Hedge's g to calculate effect size. Hedge's g, similar to Cohen's d, is used for comparing different sample sizes. We used four benchmarks to accommodate the range of our data (0, no effect; 0.5, small effect; 0.8, medium effect; 1.1, large effect; https://www.statisticshowto.datasciencecentral.com/hedges-g/). Large decrease/increase (↓↓↓/↑↑↑); medium decrease/increase (↓↓/↑↑); small decrease/increase (↓/↑); no effect (−).
| Agent(s) | 1–8 days |
43–50 days |
||
|---|---|---|---|---|
| Pre-exposure | Post-exposure | Pre-exposure | Post-exposure | |
| Isoflurane | ↓↓ | ↑ | ↑ | ↑ |
| Isoflurane/O2 | ↓↓ | ↑ | ↑↑ | ↑↑↑ |
| Sevoflurane | ↓↓ | − | − | ↑↑ |
| Sevoflurane/O2 | ↓ | − | − | ↑ |
| O2 | − | − | ↑ | ↑↑↑ |
Ageing reveals a toxic effect of post-exposure to SEVO on mortality after TBI
To further test the effect of ageing on the ability of volatile general anaesthetics to alter TBI outcomes, we exposed young and old w1118 flies to anaesthetics immediately after TBI. Post-exposure to ISO significantly increased mortality of young flies injured with either the two- or four-strike protocol (Fig. 4a and Supplementary Fig. 2c). In contrast, post-exposure of young flies to SEVO did not alter the mortality with either injury protocol (Fig. 4c and Supplementary Fig. 2d). Thus, as previously reported, in young flies, pre-exposure and post-exposure to ISO had opposite effects, and post-exposure to ISO vs. SEVO had different effects on mortality. In old flies, post-exposure to ISO caused a non-significant increase in mortality (from 43.8% [42.1, 45.6], n=136 to 49.9% [43.3, 57.4], n=16, P=0.054), and SEVO caused a 25% increase in mortality (from 43.8% [42.1, 45.6], n=136 to 54.7% [47.1, 63.5], n=12, P=0.002). Therefore, in the context of TBI, ageing-related processes enhance the damaging effect of post-exposure to SEVO to a greater extent than post-exposure to ISO (Table 1).
Ageing sensitises to damaging effects of hyperoxia on mortality after TBI
Concentrations of O2 higher than 21% are routinely administered in clinical care, and volatile general anaesthetics are usually administered under hyperoxic conditions. We tested whether ageing alters the effect on mortality of pre- and post-exposure to 100% O2 (hyperoxia) under normobaric conditions. In young w1118 flies, neither pre- nor post-exposure to hyperoxia altered mortality (Fig 3, Figure 4a). In old flies, pre- and post-exposure to hyperoxia increased mortality by 14% (from 43.8% [42.1, 45.6], n=136 to 50.2% [43.2, 58.2], n=12, P=0.054) and by 35% (from 43.8% [42.1, 45.6], n=136 to 59.0% [51.9, 67.1], n=14, P<0.001), respectively (Fig 3, Figure 4b). These data indicate that ageing sensitises flies to detrimental effects of hyperoxia (Table 1).
Ageing alters the interactive effects of volatile general anaesthetics and hyperoxia on mortality after TBI
We examined whether ageing alters the effect of exposure to volatile general anaesthetics in hyperoxia on mortality. Under the pre-exposure condition in young flies, ISO or SEVO in hyperoxia reduced mortality relative to unexposed flies (from 13.7% [12.6, 14.9] to 6.9% [4.7,10.1], n=12, P=0.001 for ISO and to 8.0 [5.6, 11.4], n=12, P=0.004 for SEVO) but to a slightly lesser extent than ISO or SEVO in normoxia (from 13.7% [12.6, 14.9], n=12 to 7.5% [5.7, 9.9] for ISO and to 6.7% [4.5, 9.8], n=12; for SEVO, P<0.001 for both; Fig. 3a and c). Under the post-exposure condition, exposure of young flies to ISO in hyperoxia increased mortality (from 13.7% [12.6, 14.9] to 18.1% [14.3, 22.9], n=14, P=0.029), whereas SEVO in hyperoxia had no significant effect (from 13.7% [12.6, 14.9] to 14.4% [11.0, 18.8], n=16, P=0.72). These results were similar to the effects of ISO (increase to 17.7% [14.1–22.1], n=16, P=0.035) and SEVO (no effect from 13.7% [12.6, 14.9] to 16.7% [12.6, 22.1], n=16, P=0.187) in normoxia (Fig. 4a and c). Therefore, in regard to mortality, young flies are resistant to the effects of hyperoxia alone or in combination with SEVO but not with ISO (Table 1).
Under both pre- and post-exposure conditions, exposure of old flies to ISO in hyperoxia increased mortality. Pre-exposure increased mortality by 23% (from 43.8% [42.1, 45.6], n=139 to 54.0% [47.6, 61.4], n=14, P=0.001, Fig. 3b). Post-exposure to ISO in hyperoxia increased mortality by 41% (from 43.8% [42.1, 45.6], n=136 to 61.7% [54.8, 69.3], n=14, P<0.001, Fig. 4b). In contrast, exposure of old flies to SEVO in hyperoxia did not affect mortality in the pre-exposure condition (43.3% [36.6, 51.4] and 43.8% [42.1, 45.6], n=14, P=0.715) but increased mortality by 17.4% in the post-exposure condition (from 43.8% [42.1, 45.6] to 51.4% [44.7, 59.1], n=16, P=0.017) Fig 3, Figure 4, respectively. Therefore, old flies are sensitive to the effects of hyperoxia in combination with volatile general anaesthetics on mortality, but the combined effects are anaesthetic-specific (Table 1).
Genetic background modulates the extent of post-exposure to ISO and hyperoxia on mortality after TBI
To investigate the role of genetic background in determining the effect of exposure to ISO/O2 on mortality after TBI, we examined 141 inbred, fully sequenced fly lines from the DGRP collection that was derived from a natural population. For each line, mortality was determined 24 h after injury at 1–8 days old using the four-strike protocol and either post-exposure to ISO/O2 or no exposure. We chose to examine post-exposure to ISO/O2 as opposed to post-exposure to SEVO/O2 because it had a more detrimental effect in w1118 flies (Table 1).
As reported, the percent mortality from TBI in the absence of exposure to ISO/O2 had a continuous distribution among DGRP lines, ranging from 6.7 to 57.5.9 Similarly, the percent change (Δ) in mortality after TBI caused by post-exposure to ISO/O2 had a continuous distribution (Supplementary Fig. 3a). The change in mortality ranged from no change (RAL332: 34.2 [2.2]% for TBI alone and 33.8 [0.8]% for TBI+ISO/O2) to a more than two-fold increase (RAL304: 19.7 [4.6]% for TBI alone and 47.5 [6.3]% for TBI+ISO/O2). Notably, fly lines with similar TBI-induced mortality varied in their susceptibility to the toxic effect from ISO/O2 exposure (Supplementary Fig. 3b). These data indicate that the interaction of anaesthetics and hyperoxia with TBI pathophysiology is influenced by genotype and that genetic variants affecting this interaction occur among natural populations of Drosophila.
GWAS analysis identifies genes associated with ISO/O2 toxicity after TBI
To identify genes that affected mortality from TBI and post-exposure to ISO/O2, we carried out a GWAS analysis using the mortality data shown in Fig 5 (derived from the data shown in Supplementary Fig. 3a). GWAS analysis using the DGRP collection has identified SNPs associated with many phenotypes, including TBI.9 Our analysis revealed that 17 SNPs located in or near 12 genes were associated with the change in Δ mortality at a discovery significance threshold of P<10−6 using the DGRP Freeze 2 algorithm that has a minor allele frequency cut-off of 10 lines.14 Three SNPs, including the most significantly associated SNP, were located in the 5′ upstream untranslated region (5′ UTR) of Prip, and one was located in the 5′ UTR of Drip. Two SNPs were in an intron of Gyc88E. Prip and Drip encode members of the aquaporin family of channels that regulate water permeability of cell membranes, and Gyc88E encodes a member of the guanylyl cyclase family and functions as a cellular oxygen sensor. Members of the aquaporin family have been implicated in the pathophysiology of brain injury in mammalian models15, 16, 17, 18, 19 and humans,20 but mammalian homologs of Gyc88E have not been implicated in TBI. We conclude that these genes represent plausible candidates for modulating ISO/O2 toxicity in the context of TBI.
Discussion
Age beyond the fourth decade of life is clearly associated with progressively worse outcomes after TBI.21 Ageing also affects an organism's response to xenobiotics, especially those acting on the central nervous system.22 We previously found that volatile general anaesthetics modulate mortality after TBI in young flies.7 Because victims of TBI of all ages are likely to be exposed to anaesthetics and hyperoxia, we investigated the effect of ageing on the interaction among anaesthetics, hyperoxia, and TBI in flies. Furthermore, we sought to identify genetic variants associated with the most striking phenotype in young flies, the toxic effect of exposure to ISO/O2 after TBI.
A fly model reproduces key characteristics of mammalian traumatic brain injury
Our fly model mimics TBI characteristics observed in mammalian models and humans. For example, mortality varies with the severity of injury and increases progressively with age.6 In flies that survive beyond 24 h, TBI is associated with neurodegeneration and reduced lifespan.6 9 Furthermore, TBI in flies causes a transient concussion-like state characterised by temporary immobility (analogous to transient loss of consciousness). Temporary immobility in flies correlates with early mortality,7 mimicking the correlation of loss of consciousness with mortality in humans.23 Here, we show that resilience to TBI in flies decreases with age, even for milder injuries.
Ageing reduces resilience to hyperoxia administered before or after traumatic brain injury
Prolonged hyperoxia causes oxidative stress in numerous models.24 In Drosophila, prolonged hyperoxia leads to neurodegeneration, which begins to be detectable after 6 days of constant exposure to 99.5% oxygen and results in a severely shortened lifespan.25 Much less is known about the consequences of short-term hyperoxia under conditions of traumatic stress. Constant exposure of 2-day-old male flies to 99.5% O2 for up to 48 h does not cause appreciable mortality.25 We found that exposure of young w1118 flies to 100% O2 for 2 h caused less than 0.1% mortality. Mortality attributable to hyperoxia in the oldest group was higher and included into the calculation of mortality caused by TBI when hyperoxia was part of the protocol. After controlling for the effects of hyperoxia in naïve flies, hyperoxia either before or after TBI was toxic in old w1118 flies. These results are consistent with the finding that hyperoxia is deleterious in humans after TBI.26,27
Ageing alters the modulation of mortality by anaesthetics after traumatic brain injury
A better understanding of the pathophysiology of secondary TBI injuries offers the best chance for improving outcomes. Our data indicate that ageing-related processes affect the profile of collateral effects of both volatile general anaesthetics and hyperoxia, suggesting that interventions may need adjustments between young and old individuals. In contrast to the robust mortality-reducing effect of exposure to anaesthetics before TBI in young flies, pre-exposure to neither ISO nor SEVO reduced mortality in old flies. These findings are consistent with attenuation of the protective effect of ISO-preconditioning reported for ageing human and rodent myocardial tissue,28,29 which was attributed to age-dependent changes in mitochondrial function.
Adding hyperoxia to a volatile general anaesthetic had no distinctive phenotype in young flies in either the pre- or the post-exposure protocol. In old flies, however, the results were more nuanced. Hyperoxia alone and in combination with SEVO had no significant effect, but pre-exposing old flies to a combination of ISO with hyperoxia increased mortality. In the post-exposure condition, hyperoxia with ISO increased mortality to a similar degree as hyperoxia alone. In contrast, combining SEVO with hyperoxia resulted in a lower mortality compared with either agent alone, indicating a mitigating effect of SEVO on the toxicity of hyperoxia. Overall, these data are consistent with differential interactions of volatile general anaesthetics with TBI pathophysiology, as suggested by our previous findings,7 which are further influenced by age and oxygen concentration.
Genetic background shapes ISO/O2 effects
Segregating variation in the DGRP collection mimics genetic variation in human populations8,14 allowing inferences on the involvement of conserved pathways. We tested 141 fly lines from the DGRP8 for changes in 24 h mortality attributable to ISO/O2. Genome-wide, we identified five SNPs in three biologically plausible genes (Prip, Drip, and Gyc88E) that were associated with variability in mortality at a P-value threshold <10−7. Prip and Drip are orthologous to mammalian water permeable channels (aquaporins) and Gyc88E is orthologous to the oxygen sensor GUCYB1. Variants in the aquaporin-4 channel are associated with outcomes from various types of brain injury in rodents16,19 and humans.20,30,31 The identification of these genes using GWAS analysis of a Drosophila collection attests to evolutionary conservation of key physiological pathways and to the potential for translatability of experimentally flexible model organisms. GUCYB1 has not yet been associated with outcome in mammalian TBI models. However, our experimental paradigm that includes hyperoxia makes a cellular oxygen sensor a plausible potential candidate gene for modulating outcome of TBI under clinically relevant circumstances.
Summary
Volatile general anaesthetics are highly promiscuous agents whose presence in the brain not only generates a state of profound unresponsiveness but also leads to numerous collateral effects on the cell biological level, which differ among individual agents and remain of largely unknown significance.32 Concerns over collateral effects of anaesthetics and of hyperoxia on the ageing brain have recently led to an interdisciplinary call for action with exceptionally wide coverage.33 Known molecular targets of collateral effects include members of the transient receptor potential (TRP) superfamily, TRPA1 and TRPV1, which not only are differentially affected by ISO and SEVO34 but also undergo an age-dependent functional transition from anti-to pro-inflammatory.35 On the organelle level, mitochondria are known to undergo age-dependent changes in their sensitivity to volatile general anaesthetics,28,29 whereas the endoplasmic reticulum (ER; a key player in the integrated stress response) goes through ageing-related deterioration.36 Modulation of ER function by volatile general anaesthetics37 can result in intracellular Ca2+ dysregulation with both cytotoxic and cytoprotective consequences.38
Weaknesses
A limitation of this study is that injuries to organs other than the brain may contribute to the observed effects of anaesthetics even though substantial evidence indicates that mortality is primarily determined by the TBI component. The principal weakness of the study is that we did not test whether polymorphisms in the identified genes are causally linked to increased mortality. Furthermore, we did not determine whether the association of the genes with mortality after TBI is attributable to ISO, hyperoxia, or both, and how these SNPs affect toxicity from other agents. Lastly, we did not investigate the underlying molecular mechanisms that link the genes to mortality. We believe that the interaction of two complicated processes (ageing and TBI) with promiscuous agents (volatile general anaesthetics) offers almost limitless molecular targets and pathways for interaction that will require a long-term commitment to address systematically. Nevertheless, we believe that these data can stimulate the design of targeted experiments in higher organisms.
Future studies
Tractability from gene to whole organism is a major strength of the fruit fly as a model organism. We plan to test Drip, Prip, and Gyc88E for causality in mediating the aggravating effects of volatile general anaesthetics and hyperoxia on mortality after TBI.
Conclusions
Disentangling the contribution of individual molecular players to beneficial and deleterious sequelae of anaesthetic exposure and linking them to biologically relevant outcomes is difficult in higher organisms. However, the identification of candidate genes and associated pathways that can lead to novel druggable targets can be accelerated by taking advantage of the experimental flexibility of flies and other model organisms.
Authors' contributions
Conduct of experiments and preparation of figures: HJS, ZPGO
Statistical data analysis: MRL
Design of experiments and writing of the manuscript: DAW, MP
Declaration of interest
The authors declare that they have no conflicts of interest.
Funding
University of Wisconsin Institute for Clinical and Translational Research (ICTR) via the Clinical and Translational Science Award (CTSA) program through the National Center for Advancing Translational Sciences (NCATS) (grant number 1UL1TR002373) and Department of Anesthesiology, University of Wisconsin–Madison, WI, USA (seed grant) to MP.
Acknowledgements
Rebeccah Katzenberger provided technical assistance and Mary Roth assisted with formatting the manuscript. Barry Ganetzky and Grace Boekhoff-Falk served as scientific advisors at the University of Wisconsin–Madison, WI, USA.
Handling editor: Hugh C Hemmings Jr
Footnotes
†This work has been presented in part at the annual meeting of the Institute of Aging of the University of Wisconsin in Madison, WI, USA, on October 25, 2018, the Annual National Neurotrauma Symposium in Pittsburgh, PA, USA, on July 2, 2019, and the Annual Meeting of the American Society of Anesthesiologists in Orlando, FL, USA, on October 21, 2019 within the framework of the Anesthesiology Journal Symposium ‘What's New in the Old’.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2020.03.029.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
figs2.
figs3.
References
- 1.Kitano H., Kirsch J.R., Hurn P.D., Murphy S.J. Inhalational anesthetics as neuroprotectants or chemical preconditioning agents in ischemic brain. J Cereb Blood Flow Metab. 2007;27:1108–1128. doi: 10.1038/sj.jcbfm.9600410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jevtovic-Todorovic V., Absalom A.R., Blomgren K. Anaesthetic neurotoxicity and neuroplasticity: an expert group report and statement based on the BJA Salzburg Seminar. Br J Anaesth. 2013;111:143–151. doi: 10.1093/bja/aet177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stollings L.M., Jia L.J., Tang P., Dou H., Lu B., Xu Y. Immune modulation by volatile anesthetics. Anesthesiology. 2016;125:399–411. doi: 10.1097/ALN.0000000000001195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yuki K., Astrof N.S., Bracken C. The volatile anesthetic isoflurane perturbs conformational activation of integrin LFA-1 by binding to the allosteric regulatory cavity. FASEB J. 2008;22:4109–4116. doi: 10.1096/fj.08-113324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tavare A.N., Perry N.J., Benzonana L.L., Takata M., Ma D. Cancer recurrence after surgery: direct and indirect effects of anesthetic agents. Int J Cancer. 2012;130:1237–1250. doi: 10.1002/ijc.26448. [DOI] [PubMed] [Google Scholar]
- 6.Katzenberger R.J., Loewen C.A., Wassarman D.R., Petersen A.J., Ganetzky B., Wassarman D.A. A Drosophila model of closed head traumatic brain injury. Proc Natl Acad Sci U S A. 2013;110:E4152–E4159. doi: 10.1073/pnas.1316895110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischer J.A., Olufs Z.P.G., Katzenberger R.J., Wassarman D.A., Perouansky M. Anesthetics influence mortality in a Drosophila model of blunt trauma with traumatic brain injury. Anesth Analg. 2018;126:1979–1986. doi: 10.1213/ANE.0000000000002906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mackay T.F., Richards S., Stone E.A. The Drosophila melanogaster genetic reference panel. Nature. 2012;482:173–178. doi: 10.1038/nature10811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katzenberger R.J., Chtarbanova S., Rimkus S.A. Death following traumatic brain injury in Drosophila is associated with intestinal barrier dysfunction. eLife. 2015;4 doi: 10.7554/eLife.04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olufs Z.P.G., Loewen C.A., Ganetzky B., Wassarman D.A., Perouansky M. Genetic variability affects absolute and relative potencies and kinetics of the anesthetics isoflurane and sevoflurane in Drosophila melanogaster. Sci Rep. 2018;8:2348. doi: 10.1038/s41598-018-20720-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeileis A. Object-oriented computation of sandwich estimators. J Stat Softw. 2006;16:16. [Google Scholar]
- 12.Rampil I.J., Lockhart S.H., Zwass M.S. Clinical characteristics of desflurane in surgical patients: minimum alveolar concentration. Anesthesiology. 1991;74:429–433. doi: 10.1097/00000542-199103000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Stevens W.C., Dolan W.M., Gibbons R.T. Minimum alveolar concentrations (MAC) of isoflurane with and without nitrous oxide in patients of various ages. Anesthesiology. 1975;42:197–200. doi: 10.1097/00000542-197502000-00014. [DOI] [PubMed] [Google Scholar]
- 14.Huang W., Massouras A., Inoue Y. Natural variation in genome architecture among 205 Drosophila melanogaster Genetic Reference Panel lines. Genome Res. 2014;24:1193–1208. doi: 10.1101/gr.171546.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manley G.T., Fujimura M., Ma T. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat Med. 2000;6:159–163. doi: 10.1038/72256. [DOI] [PubMed] [Google Scholar]
- 16.Ke C., Poon W.S., Ng H.K., Pang J.C., Chan Y. Heterogeneous responses of aquaporin-4 in oedema formation in a replicated severe traumatic brain injury model in rats. Neurosci Lett. 2001;301:21–24. doi: 10.1016/s0304-3940(01)01589-0. [DOI] [PubMed] [Google Scholar]
- 17.Kleffner I., Bungeroth M., Schiffbauer H., Schabitz W.R., Ringelstein E.B., Kuhlenbaumer G. The role of aquaporin-4 polymorphisms in the development of brain edema after middle cerebral artery occlusion. Stroke. 2008;39:1333–1335. doi: 10.1161/STROKEAHA.107.500785. [DOI] [PubMed] [Google Scholar]
- 18.Akdemir G., Ratelade J., Asavapanumas N., Verkman A.S. Neuroprotective effect of aquaporin-4 deficiency in a mouse model of severe global cerebral ischemia produced by transient 4-vessel occlusion. Neurosci Lett. 2014;574:70–75. doi: 10.1016/j.neulet.2014.03.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katada R., Akdemir G., Asavapanumas N., Ratelade J., Zhang H., Verkman A.S. Greatly improved survival and neuroprotection in aquaporin-4-knockout mice following global cerebral ischemia. FASEB J. 2014;28:705–714. doi: 10.1096/fj.13-231274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dardiotis E., Paterakis K., Tsivgoulis G. AQP4 tag single nucleotide polymorphisms in patients with traumatic brain injury. J Neurotrauma. 2014;31:1920–1926. doi: 10.1089/neu.2014.3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haring R.S., Narang K., Canner J.K. Traumatic brain injury in the elderly: morbidity and mortality trends and risk factors. J Surg Res. 2015;195:1–9. doi: 10.1016/j.jss.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 22.Turnheim K. Drug therapy in the elderly. Exp Gerontol. 2004;39:1731–1738. doi: 10.1016/j.exger.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 23.Alsulaim H.A., Smart B.J., Asemota A.O. Conscious status predicts mortality among patients with isolated traumatic brain injury in administrative data. Am J Surg. 2017;214:207–210. doi: 10.1016/j.amjsurg.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 24.Lee P.J., Choi A.M. Pathways of cell signaling in hyperoxia. Free Radic Biol Med. 2003;35:341–350. doi: 10.1016/s0891-5849(03)00279-x. [DOI] [PubMed] [Google Scholar]
- 25.Gruenewald C., Botella J.A., Bayersdorfer F., Navarro J.A., Schneuwly S. Hyperoxia-induced neurodegeneration as a tool to identify neuroprotective genes in Drosophila melanogaster. Free Radic Biol Med. 2009;46:1668–1676. doi: 10.1016/j.freeradbiomed.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 26.Rincon F., Kang J., Vibbert M., Urtecho J., Athar M.K., Jallo J. Significance of arterial hyperoxia and relationship with case fatality in traumatic brain injury: a multicentre cohort study. J Neurol Neurosurg Psychiatry. 2014;85:799–805. doi: 10.1136/jnnp-2013-305505. [DOI] [PubMed] [Google Scholar]
- 27.Kilgannon J.H., Jones A.E., Parrillo J.E. Relationship between supranormal oxygen tension and outcome after resuscitation from cardiac arrest. Circulation. 2011;123:2717–2722. doi: 10.1161/CIRCULATIONAHA.110.001016. [DOI] [PubMed] [Google Scholar]
- 28.Mio Y., Bienengraeber M.W., Marinovic J. Age-related attenuation of isoflurane preconditioning in human atrial cardiomyocytes: roles for mitochondrial respiration and sarcolemmal adenosine triphosphate-sensitive potassium channel activity. Anesthesiology. 2008;108:612–620. doi: 10.1097/ALN.0b013e318167af2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sniecinski R., Liu H. Reduced efficacy of volatile anesthetic preconditioning with advanced age in isolated rat myocardium. Anesthesiology. 2004;100:589–597. doi: 10.1097/00000542-200403000-00019. [DOI] [PubMed] [Google Scholar]
- 30.Sorani M.D., Zador Z., Hurowitz E., Yan D., Giacomini K.M., Manley G.T. Novel variants in human Aquaporin-4 reduce cellular water permeability. Hum Mol Genet. 2008;17:2379–2389. doi: 10.1093/hmg/ddn138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Appelboom G., Bruce S., Duren A. Aquaporin-4 gene variant independently associated with oedema after intracerebral haemorrhage. Neurol Res. 2015;37:657–661. doi: 10.1179/1743132815Y.0000000047. [DOI] [PubMed] [Google Scholar]
- 32.Berger M., Schenning K.J., Brown C.H. Best practices for postoperative brain health: recommendations from the fifth international perioperative neurotoxicity working group. Anesth Analg. 2018;127:1406–1413. doi: 10.1213/ANE.0000000000003841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Evered L., Silbert B., Knopman D.S. Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery-2018. Anesthesiology. 2018;129:872–879. doi: 10.1097/ALN.0000000000002334. [DOI] [PubMed] [Google Scholar]
- 34.Matta J.A., Cornett P.M., Miyares R.L., Abe K., Sahibzada N., Ahern G.P. General anesthetics activate a nociceptive ion channel to enhance pain and inflammation. Proc Natl Acad Sci U S A. 2008;105:8784–8789. doi: 10.1073/pnas.0711038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wanner S.P., Garami A., Pakai E. Aging reverses the role of the transient receptor potential vanilloid-1 channel in systemic inflammation from anti-inflammatory to proinflammatory. Cell Cycle. 2012;11:343–349. doi: 10.4161/cc.11.2.18772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naidoo N. The endoplasmic reticulum stress response and aging. Rev Neurosci. 2009;20:23–37. doi: 10.1515/revneuro.2009.20.1.23. [DOI] [PubMed] [Google Scholar]
- 37.Komita M., Jin H., Aoe T. The effect of endoplasmic reticulum stress on neurotoxicity caused by inhaled anesthetics. Anesth Analg. 2013;117:1197–1204. doi: 10.1213/ANE.0b013e3182a74773. [DOI] [PubMed] [Google Scholar]
- 38.Ren G., Zhou Y., Liang G. General anesthetics regulate autophagy via modulating the inositol 1,4,5-trisphosphate receptor: implications for dual effects of cytoprotection and cytotoxicity. Sci Rep. 2017;7:12378. doi: 10.1038/s41598-017-11607-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.