Abstract
Background:
Bisphenol-A (BPA) exposure is widespread and early life exposure is associated with metabolic syndrome. While visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) are implicated in the development of metabolic syndrome, the adipose depot-specific effects of prenatal BPA treatment are poorly understood.
Objective:
To determine the impact of prenatal BPA exposure on genome-wide gene expression of VAT and SAT depots.
Methods:
RNA sequencing was performed on SAT and VAT from 21-month old control and prenatal BPA-treated female sheep. Gene expression and pathway differences between SAT and VAT depots with or without prenatal BPA-treatment and the effect of prenatal BPA treatment on each depot were tested.
Results:
There were 179 differentially expressed genes (padjusted<0.05, log2-fold change >2.5) between SAT and VAT. Development and immune response pathways were upregulated in SAT, while metabolic pathways were upregulated in VAT. These adipose depot-specific genes and pathways were consistent with prenatal BPA-treatment. In SAT, BPA-treatment resulted in differential expression of 108 genes (78% upregulated with BPA) and altered pathways (immune response downregulated, RNA processing upregulated). In contrast in VAT, BPA-treatment differentially expressed 4 genes and upregulated chromatin and RNA processing pathways.
Conclusion:
Prenatal BPA-treatment induces adult depot-specific alterations in RNA expression in inflammation, RNA processing, and chromatin pathways, reflecting the diverse roles of SAT and VAT in regulating lipid storage and insulin sensitivity. These adipose tissue transcriptional dysregulations may contribute to the metabolic disorders observed in prenatal BPA-treated female sheep.
Keywords: Adipose depots, Bisphenol A, Endocrine disruptor, RNA sequencing
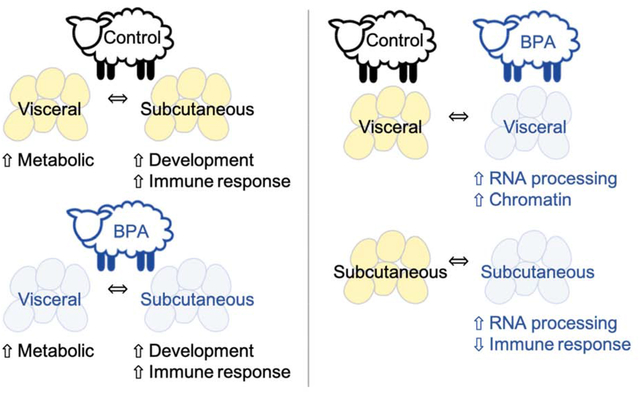
Graphical abstract.
Graphical abstract for “Developmental programming: Transcriptional regulation of visceral and subcutaneous adipose by prenatal bisphenol-A in female sheep”
INTRODUCTION
The worldwide prevalence of obesity tripled between 1975 and 2016, reaching 650 million obese adults and 1.25 billion overweight adults (WHO, 2018). Obesity and associated health complications have an annual economic burden estimated between $147 and $210 billion in the United States (Levi et al., 2010; Regnier and Sargis, 2014). Primary risk factors for the development of obesity are genetic predisposition and lifestyle factors, including increased caloric intake and decreased energy expenditure (Williams et al., 2015). However, these factors alone fail to explain the global rise in obesity prevalence. Accumulating evidence from epidemiological and animal studies demonstrate early developmental exposures to environmental endocrine disrupting chemicals, such as bisphenol A (BPA), are also risk factors for obesity (Zimmet and Alberti, 2006; Holtcamp, 2012; Kelishadi et al., 2013; Heindel et al., 2015). BPA is used in the production of epoxy and polycarbonate resins, and found in common household plastics. Over 90% of US adults and 96% of pregnant women have detectable urinary concentrations of BPA (Calafat et al., 2008; Woodruff et al., 2011), indicating widespread exposure including during the prenatal period. BPA affects mouse pancreatic cell function and adipogenesis (Le Magueresse-Battistoni et al., 2018; Rubin et al., 2019). While the endocrine disrupting potential of BPA (Vogel, 2009; Vom Saal, 2016) is well known and epidemiological evidence point to positive associations between urinary BPA levels and obesity (Carwile and Michels, 2011) a causal role for BPA as an obesogen is yet to be unequivocally established (Whaley, 2014; Legeay and Faure, 2017). Understanding the gene expression and gene networks programmed by developmental BPA exposure at the adipose tissue level is critical to obtain mechanistic insights.
BPA exposure in humans is linked to metabolic defects, such as insulin resistance, diabetes, obesity and metabolic syndrome (Newbold, 2010; Teppala et al., 2012; Wang et al., 2012). Developmental BPA exposure may be associated with reduced birth weight (Veiga-Lopez et al., 2015a), a major risk factor for adult onset cardio-metabolic disorders (Barker, 2005). Developmental BPA exposure induces metabolic defects in animal studies (vom Saal et al., 2007; Alonso-Magdalena et al., 2010; Veiga-Lopez et al., 2016; Wassenaar et al., 2017), and early-life exposure to BPA causes adipose tissue disruptions (Wassenaar et al., 2017). In sheep, the animal model used in this study, prenatal BPA-treated female offspring kept on maintenance diet to avoid confound from postnatal obesity develop metabolic defects, manifested as peripheral insulin resistance with compensatory hyperinsulinemia and adipose tissue disruptions in the absence of them becoming obese (Veiga-Lopez et al., 2015b; Veiga-Lopez et al., 2016). These sheep develop adipocyte hypertrophy with visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) specific changes in markers of inflammation, oxidative stress and adipose differentiation (Supplemental Table 1) (Veiga-Lopez et al., 2015b; Veiga-Lopez et al., 2016). Although both VAT and SAT are predominantly white adipose depots, they are morphologically and functionally different. The VAT depot has increased proinflammatory cytokine expression, is more responsive to catecholamine-induced lipolysis, less responsive to the antilipolytic effect of insulin, and therefore has a higher rate of fatty acid turnover and lipolysis (Engfeldt and Arner, 1988; Ibrahim, 2010). In contrast, SAT adipocytes are responsive to insulin action and favor uptake and storage of free fatty acids and triglycerides (Ibrahim, 2010). Given these functional differences, accumulation of VAT is a risk factor for cardiometabolic defects (Lemieux and Despres, 1994; Bjorntorp, 2000; Dobbelsteyn et al., 2001). Understanding the adipose depot-specific effects of prenatal BPA treatment can aid in delineating the poorly understood mechanisms of BPA induced adipose and metabolic defects.
In adipose tissues, BPA activates transcription factors (Wetherill et al., 2007; Boucher et al., 2014a; Ahmed, 2016; Ahmed and Atlas, 2016). These transcription factors influence various aspects of adipose tissue function including adipogenesis, adipocyte proliferation and differentiation, and glucose and lipid metabolism (Moreno-Navarrete and Fernández-Real, 2017; Newell-Fugate, 2017; Corrales et al., 2018) by changing gene expression patterns (Polvani et al., 2016; Mota de Sa et al., 2017). BPA treatment in adipose tissue influences gene families including adipokines and inflammatory cytokines, mediators of lipogenesis, and adipogenesis (Masuno et al., 2005; Ben-Jonathan et al., 2009; Melzer et al., 2011). In sheep, prenatal BPA treatment alters VAT expression of adiponectin, oxidative stress markers, steroidogenic enzymes and steroid receptors in a dose-dependent manner (Veiga-Lopez et al., 2015b; Puttabyatappa et al., 2019b). Treatment also induces VAT and SAT expression changes in adiponectin and a macrophage marker (Veiga-Lopez et al., 2016). Considering VAT and SAT have different roles in the maintenance of metabolic homeostasis (Zhang et al., 2014) and BPA influences transcriptional regulation, testing the impact of developmental exposure to BPA in mediating depot-specific gene expression differences is of interest.
Prenatal BPA treatment affects adipose tissue function in a depot-specific manner through multiple mechanisms, thus it is challenging to comprehensively understand the underlying signaling pathways. High throughput quantitative gene expression profiling through whole adipose tissue RNA-sequencing to identify depot-specific gene and gene networks disrupted by prenatal BPA exposure can therefore be advantageous. Importantly, sheep like humans are precocial; there are marked similarities in adipose tissue development and distribution between sheep and human (Symonds et al., 2015). Utilizing a female sheep model of prenatal BPA treatment with well documented metabolic defects, the goals of the present study were 1) to compare gene expression differences between VAT and SAT depots in control and prenatal BPA-treated female sheep, 2) determine genes and gene networks disrupted by prenatal BPA treatment in each depot and 3) to identify pathways that underlie lasting adipose-depot specific functional changes stemming from developmental exposure to BPA in the female sheep.
METHODS
Animals
All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Michigan and are consistent with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals. These studies were conducted at the University of Michigan Sheep Research Facility (Ann Arbor, MI) using the Suffolk sheep breed acquired from local farmers. The maintenance, breeding, prenatal treatments and lambing were performed as described previously (Manikkam et al., 2004; Veiga-Lopez et al., 2016). All animals including the control group were maintained together and following weaning lambs were provided with commercial feed pellets (Shur-Gain, Elma, NY) containing 18% crude protein and alfalfa hay. When the lambs reached a weight of about 40 kg, commercial pellet feed was switched to one containing 15% crude protein and alfalfa hay and lambs maintained on this diet until tissue collection.
Prenatal BPA Treatment
Pregnant sheep were randomly assigned to control and BPA groups. Control mothers received only the vehicle (corn oil) and BPA animals received 0.5 mg/kg /day of BPA (purity ≥99%, cat. no. 239658; Aldrich Chemical, Milwaukee, WI) dissolved in corn oil. Both treatments were administered daily through subcutaneous injections from gestational days 30 through 90 (term: ~147 days). This BPA dosing resulted in 2.62 ± 0.52 ng/ml of free BPA measured in the umbilical artery on day 90 of fetal life (Veiga-Lopez et al., 2013a), which are within the range found in human mid-gestation umbilical cord blood concentrations of free BPA (Gerona et al., 2013; Veiga-Lopez et al., 2015b; Lee et al., 2018). The female lambs were weaned at ~8 weeks of age and provided with a maintenance diet consisting of 0.64 kg of corn, 0.64 kg hay/lamb/day, and 0.014 kg of supplement (36% crude protein) to distinguish programmed events from confounding due to postnatal development of obesity. If twin pregnancies were involved, only one female offspring from each dam was used. The effects of prenatal BPA exposure on insulin sensitivity, adiposity, adipocyte size, and mediators of insulin sensitivity in the animals used in this study have been previously reported (Veiga-Lopez et al., 2013b; Veiga-Lopez et al., 2015b; Puttabyatappa et al., 2019b) (Supplemental Table 1).
Tissue Collection
Both VAT and SAT were collected during the second breeding season at ~21 months of age. Before collection, estrus was synchronized with two injections of prostaglandin F2α (PGF2α, 10 mg, i.m.; Lutalyse, Pfizer Animal Health, Florham Park, NJ) administered 11 days apart. Tissues were harvested 24 hours after the second PGF2α injection during the follicular phase to maintain comparable steroid environment. All animals were euthanized by barbiturate overdose (Fatal Plus; Vortech Pharmaceuticals, Dearborn, MI). VAT from the mesenteric fat surrounding the ventral sac of the rumen and SAT from the sternal region were obtained. Tissues were flash-frozen and stored at −80°C until processing.
RNA Sequencing
Total RNA was isolated from both SAT and VAT depots using Trizol reagent (Invitrogen Technologies, Carlsbad, CA) and then DNAse treated and purified using the RNeasy mini kit (Qiagen, Germantown, MD) as per manufacturer’s instruction. The University of Michigan Advanced Genomics Core analyzed the RNA quality using Agilent Bioanalyzer (Agilent Technologies, Santa Clara, CA), prepared cDNA libraries and performed RNA sequencing on VAT and SAT tissues from randomly selected control and prenatal BPA-treated animals (n=4 / treatment group). Libraries were prepared with SMARTer universal low input RNA kit (Takara, Mountain View, CA) following ribosome depletion. Libraries were split across four lanes and sequenced on the HiSeq NovaSeq 6000 platform (Illumina, San Diego, CA). Single end sequencing was performed for 76 cycles. Raw and processed data from this experiment are available at the Genome Expression Omnibus (GEO, accession number GSE142222).
Data Processing and Quality Control
Raw fastq files were examined using fastQC (version 0.11.5) (Andrews, 2010). Reports generated for the 16 samples across 4 lanes were summarized using multiQC (version 0.9) (Ewels et al., 2016). Across samples and at all base positions, mean quality scores were high. GC content ranged from 49% to 58%. There were between 32% and 64% of reads duplicated. Reads were mapped to a sheep reference genome (Oar_rambouillet_v1.0) using the Spliced Transcripts Alignment to a Reference (STAR) (version 2.6.0c) program (Dobin et al., 2013). Post alignment, QoRTs (version 1.3.6) (Hartley and Mullikin, 2015) was used to examine further quality control metrics. Sample distributions in quality metrics were clustered by the subcutaneous and visceral adipose tissue types. Alignment soft clipping rate was highest at the start and end of read cycles, with a larger spike of approximately 30–40% at the start. Approximately 15–20% of reads per sample were dropped for multi-mapping. A large proportion of reads (approximately 50–70%) mapped to non-gene regions, which may reflect lack of annotation in the sheep genome. Following mapping, featureCounts (version 1.6.1) (Liao et al., 2014) was used to quantify aligned reads. Default behavior was used to drop multi-mapping reads and count features mapping to exons.
Differential Expression Analysis
Aligned and quantified reads were analyzed in R statistical software (version 3.6.0) with the DESeq2 package (version 1.24.0) (Love et al., 2014). Lanes were collapsed to single samples. Default settings for DESeq2 were used for filtering of genes with low normalized mean counts. Principal components analysis was performed, calculated on variance stabilizing transformed values of the expression data. Principal components were plotted to examine clustering by sample type.
Differentially expressed genes were examined by tissue type (VAT versus SAT among control and prenatal BPA-treated animals) and by treatment (control versus prenatal BPA). To investigate tissue type differences, samples from SAT and VAT of control and prenatal BPA treated animals were compared within the respective treatment groups. To investigate the effects of prenatal BPA treatment, the BPA-treated group was compared to the control, separately for SAT and VAT. Volcano plots of results were created using the EnhancedVolcano (version 1.2.0) package, after applying log fold change shrinkage using the “apeglm” prior (Zhu et al., 2019). To account for multiple comparisons, an adjusted p-value < 0.05 was set as the threshold. To prioritize genes for further investigation, an absolute log2 fold-change > 2.5 in the tissue type comparison, and an absolute log2 fold-change > 1.5 in BPA treatment comparisons were considered.
Gene Set Enrichment
Enriched gene set terms were tested using RNA-Enrich (Lee et al., 2016). Sheep genes were annotated to human orthologs using Ensembl identifiers and the BioMart package (Durinck et al., 2009). Three pathway analyses were performed using the directional RNA-Enrich test for 1) the tissue type comparison, 2) for BPA exposure in SAT, and 3) for BPA exposure in VAT. RNA-Enrich uses logistic regression to test for enrichment, which allows the expression data to remain on a continuous scale and does not require a significance cutoff for genes (Sartor et al., 2009). RNA-Enrich accounts for the relationship between gene read count and differential expression statistical significance in the enrichment test. Enrichment z-scores were back calculated from RNA-Enrich results. We ran tests with the Biocarta Pathway, EHMN metabolic pathways, Gene Ontology, KEGG Pathway, Panther Pathway, and transcription factors databases selected. Maximum concept size was set to 1000 genes, with other options at default values. For all differentially expressed genes (P<0.05) annotated to the gene set, the number of genes and their Entrez gene IDs were reported.
Network Analysis
Gene interaction networks were visualized using the STRING database of protein-protein interactions (Franceschini et al., 2013). Genes with p<0.05 in both fat deposits in relation to BPA exposure were used to build network diagrams. A score threshold of 700 was set for inclusion of gene interactions.
Code to conduct the analyses presented in this manuscript are available through GitHub (https://github.com/bakulskilab).
RESULTS
Descriptive Statistics
The study consisted of two adipose tissue types and two treatment groups. Each adipose depot and treatment combination had four samples. Following RNA sequence alignment and quantification, samples had between 3,046,126 to 14,305,764 reads assigned to features numbering from 16,926 to 18,914 (Supplemental Table 2). In principal component plots, clustering by adipose tissue type and treatment group were observed (Supplemental Figure 1).
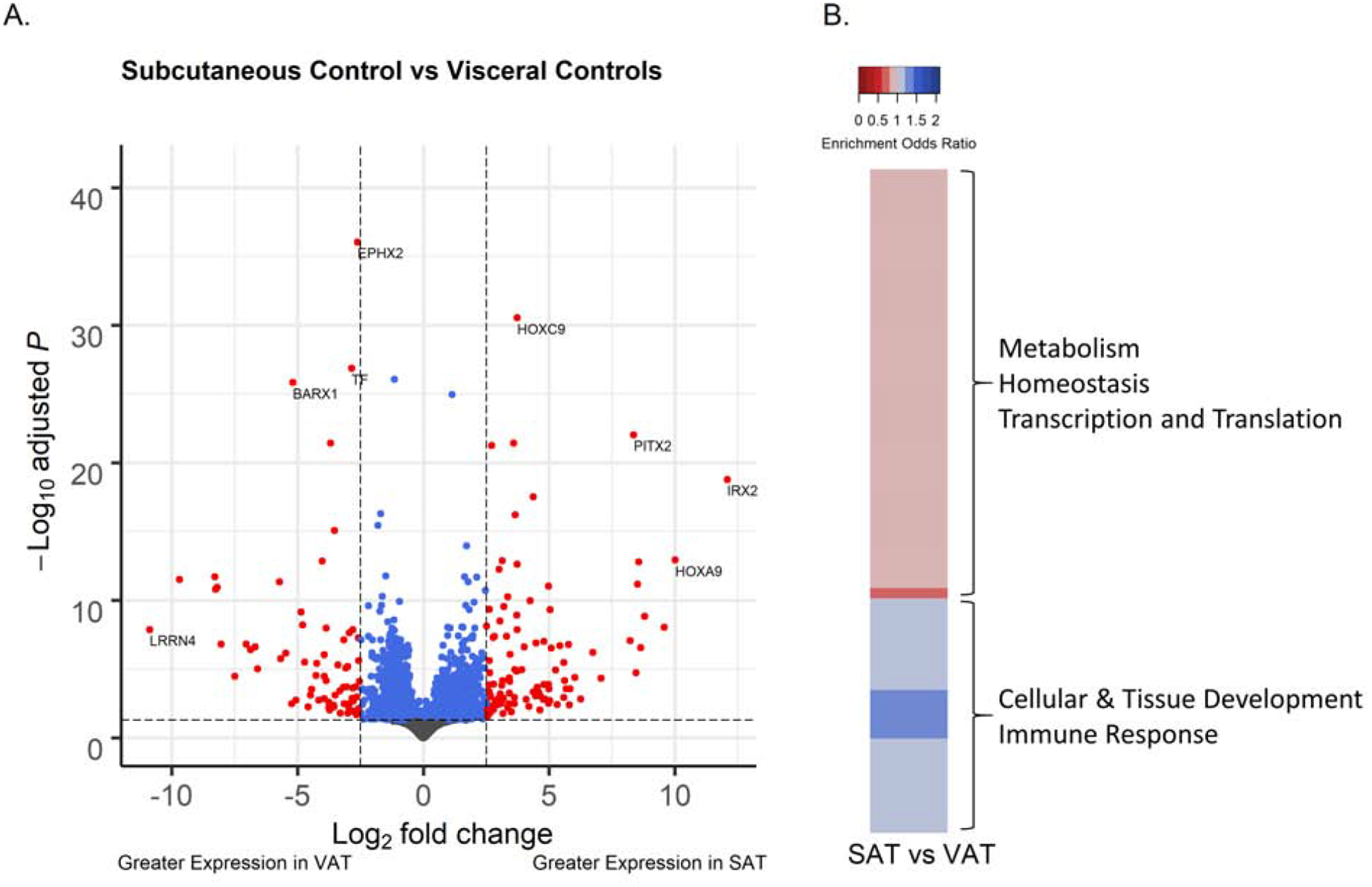
Gene Expression Differences Between VAT and SAT Depots
Among control animals, following filtering of genes with low normalized mean counts and extreme count outliers, 17,242 genes were analyzed. There were 2,196 genes differentially expressed (adjusted p<0.05), 180 genes with a log2-fold change magnitude difference > 2.5, and 179 genes met both criteria between SAT and VAT depots from control animals (Figure 1A). Of the genes meeting these statistical and magnitude criteria, 61% (109 genes) had higher expression in SAT from control animals. The top statistically differentially expressed genes in SAT relative to VAT by adjusted p-value were epoxide hydrolase 2 (EPHX2) (−2.61 lower log2-fold change expression, adjusted-p=9.1×10−37), homeobox C9 (HOXC9) (3.7 higher log2-fold change expression, adjusted-p=2.9×10−31) (Supplemental Figure 2A), transferrin (TF) (−2.8 lower log2-fold change expression, adjusted-p=1.4×10−27) and cut like homeobox 1 (CUX1) (−1.2 lower log2-fold change expression, adjusted-p=8.9×10−27) (Table 1, Supplemental Table 3). The highest magnitude differentially expressed genes between SAT and VAT ordered by shrunk log2-fold change values were Iroquois homeobox 2 (IRX2) (12.1 higher log2-fold change, p-adjusted=1.7×10−19) (Supplemental Figure 2B), leucine rich repeat neuronal 4 (LRRN4) (−10.9 lower log2-fold change, adjusted-p=1.3×10−8), HOXA9 (10.0 higher log2-fold change, adjusted-p=1.2×10−13) and transcription factor 21 (TCF21) (−9.7 lower log2-fold change, adjusted-p=3.2×10−12). Among prenatal BPA-treated animals, the genes that were differentially expressed between SAT and VAT depots were similar to the changes observed among control animals (Supplemental Table 4) such that the differentially expressed genes were highly correlated between both treatment groups (r=0.68, p<2.2×10−16).
Figure 1.
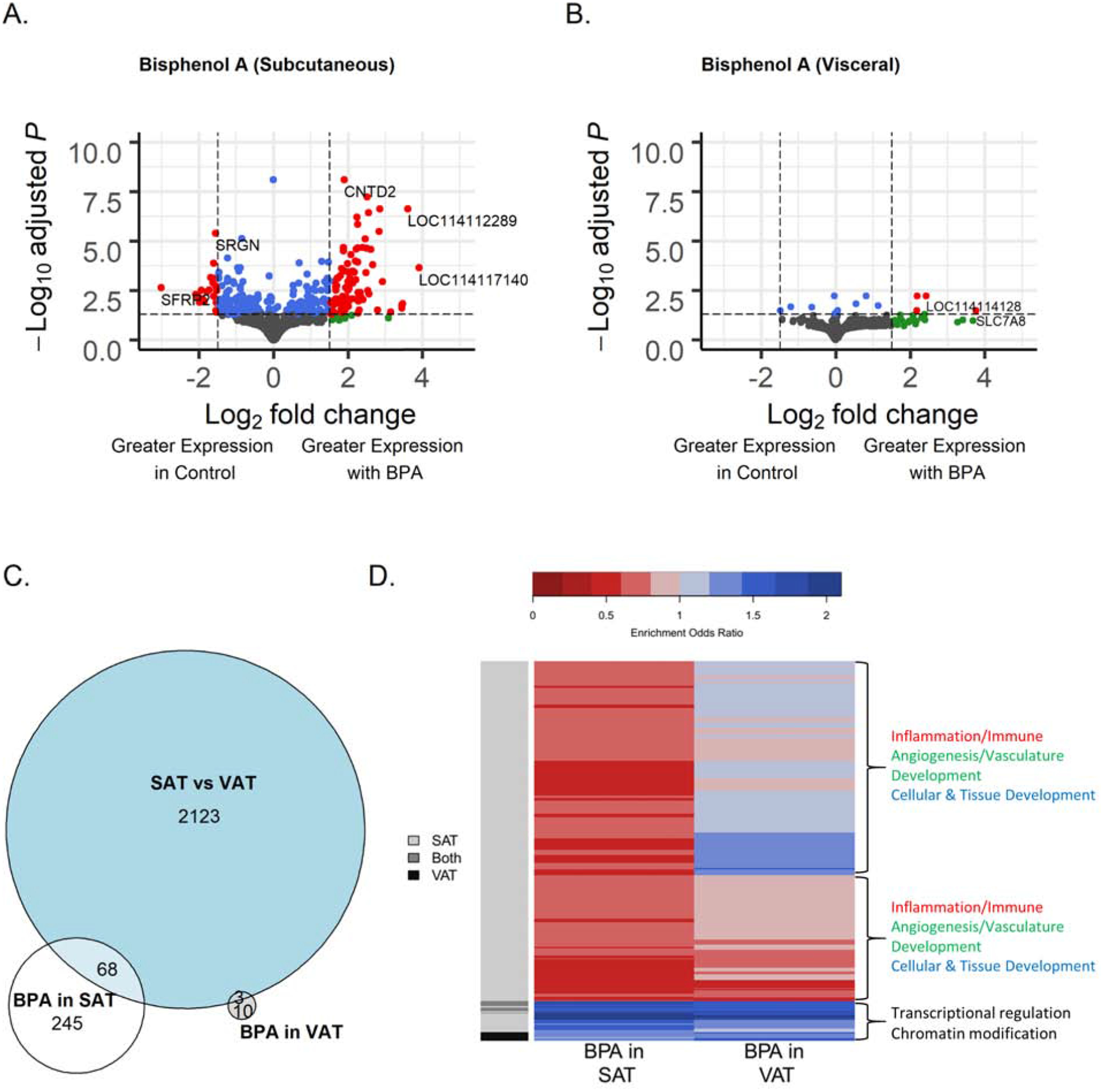
Comparison of the gene expression of 21-month old female sheep in subcutaneous adipose tissue (SAT) relative to visceral adipose tissue (VAT). (A) Volcano plot of differential gene expression by adipose tissue type, comparing the SAT versus VAT from control sheep. Genes are plotted by log-fold change, using “apeglm” for shrinkage, and -log adjusted p-values. Blue points have adjusted p-values < 0.05. Red points also have log-fold change > 2.5. (B) Heatmap of pathways with FDR < 0.05 in the comparison of SAT versus VAT tissue differences. Upregulated gene sets are in blue, downregulated gene sets in red.
Table 1.
Differential gene expression between subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT) depots from 21-month old female sheep. Among genes with adjusted p-value < 0.05, the top 20 have been selected and ordered by fold change magnitude. Genes lacking conventional symbols (with names starting with LOC) include inactive heparinase-2 (LOC114110341), mucin-16 (LOC101103644), mucin-16-like (LOC114114799) and non-coding RNA (LOC114113970).
| Gene | Mean Expression VAT | Mean Expression SAT | Fold Change Estimate | Log2 Fold Change Estimate | Standard Error | P-Value | Adjusted P-Value |
|---|---|---|---|---|---|---|---|
| IRX2 | 0 | 189.65 | 4389.98 | 12.1 | 2.85 | 1.08E-22 | 1.69E-19 |
| LRRN4 | 173.69 | 0 | 0.00052 | −10.9 | 3.25 | 5.14E-11 | 1.34E-08 |
| HOXA9 | 0.17 | 232.64 | 1024 | 10 | 1.45 | 1.22E-16 | 1.17E-13 |
| TCF21 | 74.89 | 0 | 0.00121 | −9.69 | 2.89 | 5.17E-15 | 3.18E-12 |
| HOXA10 | 0 | 41.87 | 760.08 | 9.57 | 2.81 | 3.05E-11 | 9.07E-09 |
| LOC114110341 | 0 | 25.35 | 445.72 | 8.8 | 2.63 | 4.21E-12 | 1.42E-09 |
| LGR5 | 0 | 26.49 | 401.71 | 8.65 | 2.65 | 1.79E-09 | 2.81E-07 |
| IRX1 | 0.84 | 373.05 | 380.04 | 8.57 | 1.09 | 1.95E-16 | 1.60E-13 |
| PITX1 | 0.15 | 80.28 | 364.56 | 8.51 | 1.35 | 1.23E-14 | 6.84E-12 |
| CD300LB | 0 | 25.11 | 349.71 | 8.45 | 2.83 | 2.50E-07 | 1.91E-05 |
| PITX2 | 0.62 | 226.29 | 328.56 | 8.36 | 0.785 | 3.87E-26 | 9.54E-23 |
| NKX3–2 | 128.15 | 0.27 | 0.00319 | −8.29 | 1.32 | 2.82E-15 | 1.94E-12 |
| WT1 | 128.99 | 0.36 | 0.00328 | −8.25 | 1.36 | 3.12E-14 | 1.58E-11 |
| METTL21C | 0 | 18.5 | 298.17 | 8.22 | 2.62 | 4.62E-10 | 8.75E-08 |
| LOC101103644 | 325.38 | 0.91 | 0.00342 | −8.19 | 1.1 | 2.22E-14 | 1.16E-11 |
| KRT23 | 29.71 | 0 | 0.0038 | −8.04 | 2.74 | 9.00E-10 | 1.58E-07 |
| LOC114114799 | 24.04 | 0 | 0.00556 | −7.49 | 2.82 | 5.11E-07 | 3.44E-05 |
| LOC114113970 | 0 | 9.95 | 134.36 | 7.07 | 2.63 | 7.41E-07 | 4.58E-05 |
| SCEL | 59.88 | 0.28 | 0.0076 | −7.04 | 1.42 | 8.78E-10 | 1.56E-07 |
| ADGRB3 | 53.44 | 0.28 | 0.00861 | −6.86 | 1.43 | 2.59E-09 | 3.99E-07 |
Several pathways were differentially regulated in SAT compared to VAT (Supplemental Table 5). A total of 260 pathways had FDR<0.05. Pathways upregulated in SAT were involved in cellular and tissue development, and immune response (Figure 1B). Representative upregulated pathways in these categories include skeletal system development (enrichment odds ratio = 1.16, adjusted-p=1.4×10−7), adaptive immune response (enrichment odds ratio = 1.09, adjusted-p=0.036) and leukocyte cell-cell adhesion (enrichment odds ratio = 1.11, adjusted-p=0.0008). Pathways involved in metabolism, mitochondria, homeostasis, transcription, and translation were downregulated in SAT (Figure 1B). Representative downregulated pathways in these categories include xenobiotic metabolic process (enrichment odds ratio = 0.83, adjusted-p=6.5×10−5), mitochondrial protein complex (enrichment odds ratio = 0.88, adjusted-p=0.003), lipid homeostasis (enrichment odds ratio = 0.87, adjusted-p=0.001), RNA polymerase complex (enrichment odds ratio = 0.9, adjusted-p=0.02), and translational initiation (enrichment odds ratio = 0.9, adjusted-p=0.01). Pathways enriched among genes differentially expressed between SAT and VAT tissues in prenatal BPA-treated animals (Supplemental Table 6) were also consistent with the SAT vs. VAT comparison among control animals.
Gene Expression Differences Between Control and BPA-Treated Adipose Depots
In SAT, after filtering for low normalized mean counts, 16,388 genes remained in analysis. There were 319 genes differentially expressed between control and BPA-treated groups with adjusted p-value < 0.05, 118 genes with an absolute log2-fold change > 1.5, and 108 genes met both these conditions (Figure 2A). Of the genes meeting these statistical and magnitude criteria, 78% (84 genes) had higher expression in the BPA-treated group. The top statistically differentially expressed genes with BPA treatment relative to control treatment ordered by adjusted p-value were uncharacterized gene (LOC114116052) (−0.006 lower log2-fold change expression adjusted-p=7.7×10−9), cyclin N-terminal domain containing 2 (CNTD2) (1.9 higher log2-fold change expression, adjusted-p=7.7×10−9) (Supplemental Figure 3A), 5S ribosomal RNA LOC114116728 (2.5 higher log2-fold change expression, adjusted-p=5.9×10−8), and LOC114116239 (2.8 higher log2-fold change expression, adjusted-p=2.3×10−7) (Table 2, Supplemental Table 7). The highest magnitude differentially expressed genes between BPA-treated and control SAT ordered by log2-fold change were U6 spliceosomal RNA LOC114117140 (3.9 higher log2-fold change expression, adjusted-p=2.2×10−4), 5S ribosomal RNA LOC114112289 (3.6 higher log2-fold change expression, adjusted-p=2.3×10−7) (Supplemental Figure 3B), small nucleolar RNA SNORD61 LOC114111819 (3.5 higher log2-fold change expression, adjusted-p=0.01), and troponin C2 (TNNC2) (3.4 higher log2-fold change expression, adjusted-p=0.02). Hierarchical clustering based on gene expression profiles showed clear clustering in SAT samples by BPA treatment (Supplementary Figure 4).
Figure 2.
Comparison of the gene expression of 21-month old female sheep in subcutaneous adipose tissue from prenatal bisphenol-A (BPA) treated and control t groups. (A) Volcano plot of differential gene expression by prenatal BPA treatment in subcutaneous adipose tissue (SAT). (B). Volcano plot of differential gene expression by prenatal BPA treatment in visceral adipose tissue (VAT). Genes are plotted by log-fold change, using “apeglm” for shrinkage, and -log adjusted p-values. Blue points have adjusted p-values < 0.05. Green points have shrunk log fold change > 1.5. Red points have both adjusted p-value < 0.05 and log fold change > 1.5. (C). Venn diagram of number of FDR significant genes in tissue comparison, BPA effect in SAT, and BPA effect in VAT. (D). Heatmap of clustering of significant genes sets from RNA-Enrich with FDR < 10−6 in either the BPA comparison in SAT or the BPA comparison in VAT. Upregulated gene sets are in blue, downregulated gene sets in red.
Table 2.
Prenatal BPA treatment induced differential gene expression in the subcutaneous adipose tissue depot in 21-month old female sheep. Among genes with adjusted p-value < 0.05, the top 20 have been selected and ordered by fold change magnitude. Genes lacking conventional symbols (with names starting with LOC) include small nucleolar RNA (LOC114111819, LOC114116239, LOC114116240, LOC114114237, LOC114116235, LOC114111821, LOC114118580), ribosomal RNA (LOC114112289, LOC114112194, LOC114112366, LOC114116728, LOC114110878), and splicesomal RNA (LOC114117140, LOC114110989, LOC114111012).
| Gene | Mean Expression BPA | Mean Expression Control | Fold Change Estimate | Log2 Fold Change Estimate | Standard Error | P-Value | Adjusted P-Value |
|---|---|---|---|---|---|---|---|
| LOC114117140 | 33.67 | 1.64 | 14.93 | 3.9 | 0.852 | 4.40E-07 | 0.000225 |
| LOC114112289 | 1051.92 | 76.08 | 12.13 | 3.6 | 0.596 | 6.53E-11 | 2.33E-07 |
| LOC114111819 | 15.09 | 0.85 | 11.08 | 3.47 | 1.1 | 0.000152 | 0.0145 |
| TNNC2 | 43.53 | 2.45 | 10.93 | 3.45 | 1.26 | 0.000193 | 0.017 |
| LOC114110989 | 26.9 | 1.4 | 10.85 | 3.44 | 1.3 | 0.000312 | 0.0246 |
| LOC114112194 | 14.46 | 1 | 8.82 | 3.14 | 1.26 | 0.000612 | 0.0386 |
| SFRP2 | 2.75 | 29.83 | 0.12 | −3.02 | 0.794 | 1.07E-05 | 0.00228 |
| LOC114112366 | 53.29 | 5.51 | 7.57 | 2.92 | 0.747 | 4.13E-06 | 0.00113 |
| LOC114116239 | 330.53 | 41.54 | 7.21 | 2.85 | 0.472 | 7.11E-11 | 2.33E-07 |
| LOC114116240 | 60.87 | 7.65 | 7.11 | 2.83 | 0.513 | 1.79E-09 | 3.26E-06 |
| LOC114111012 | 12.89 | 1.19 | 6.87 | 2.78 | 1.03 | 0.000427 | 0.0304 |
| TRNAR-CCU_7 | 54.68 | 7.35 | 6.32 | 2.66 | 0.589 | 2.71E-07 | 0.000153 |
| LOC114114237 | 48.26 | 6.87 | 6.11 | 2.61 | 0.521 | 2.82E-08 | 2.57E-05 |
| LOC114116235 | 37.61 | 4.99 | 5.9 | 2.56 | 0.756 | 2.75E-05 | 0.00464 |
| TRNAK-CUU_8 | 315.71 | 48.94 | 5.86 | 2.55 | 0.43 | 1.34E-10 | 3.66E-07 |
| TRNAM-CAU_7 | 121.14 | 16.44 | 5.78 | 2.53 | 0.728 | 1.77E-05 | 0.00318 |
| LOC114116728 | 949.93 | 153.46 | 5.7 | 2.51 | 0.396 | 1.08E-11 | 5.88E-08 |
| LOC114110878 | 185.67 | 28.92 | 5.66 | 2.5 | 0.499 | 2.23E-08 | 2.28E-05 |
| LOC114111821 | 102.07 | 15.46 | 5.58 | 2.48 | 0.586 | 9.53E-07 | 0.000391 |
| LOC114118580 | 759.74 | 123.45 | 5.5 | 2.46 | 0.467 | 5.58E-09 | 7.63E-06 |
In VAT, following filtering of genes with low normalized mean counts and extreme count outliers, 15,537 genes were analyzed. Comparing BPA-treated and control VAT, there were 13 genes differentially expressed at adjusted p-value<0.05, 27 genes differentially expressed with an absolute log2-fold change > 1.5, and 4 genes met both criteria (Figure 2B). These 4 genes were small nucleolar RNA SNORA61 LOC114113403 (2.2 higher log2-fold-change expression with BPA treatment, adjusted-p=0.006), an uncharacterized gene (LOC114114128) (2.4 higher log2-fold-change expression, adjusted-p=0.006), solute carrier family 7 member 8 (SLC7A8) (3.8 higher log2-fold change expression, adjusted-p=0.03), and cuticle collagen dpy-13-like (LOC114110120) (2.2 higher log2-fold change expression, adjusted-p=0.03) (Table 3, Supplemental Table 8).
Table 3.
Prenatal BPA treatment induced differential gene expression in the visceral adipose tissue depot in 21-month old female sheep. The 9 genes with adjusted p-value < 0.05 have been selected and ordered by fold change magnitude. Genes lacking conventional symbols (with names starting with LOC) include non-coding RNA (LOC114114128), small nucleolar RNA (LOC114113403), cuticle collagen dpy-13-like (LOC114110120), and multidrug resistance-associated protein 4-like (LOC114116716, LOC105605961).
| Gene | Mean Expression BPA | Mean Expression Control | Fold Change Estimate | Log2 Fold Change Estimate | Standard Error | P-Value | Adjusted P-Value |
|---|---|---|---|---|---|---|---|
| SLC7A8 | 64.48 | 3.22 | 13.55 | 3.76 | 1.12 | 2.26E-05 | 0.0329 |
| LOC114114128 | 73.6 | 11.58 | 5.39 | 2.43 | 0.55 | 3.95E-07 | 0.00598 |
| LOC114113403 | 257.8 | 48.61 | 4.53 | 2.18 | 0.518 | 9.24E-07 | 0.00598 |
| LOC114110120 | 85.28 | 15.01 | 4.53 | 2.18 | 0.645 | 2.45E-05 | 0.0329 |
| HTR1B | 13.15 | 43.92 | 0.36 | −1.49 | 0.435 | 2.54E-05 | 0.0329 |
| PSTPIP2 | 54.79 | 141.18 | 0.44 | −1.2 | 0.328 | 9.82E-06 | 0.0218 |
| FUK | 235.24 | 96.39 | 2.2 | 1.14 | 0.305 | 7.30E-06 | 0.0189 |
| ARID3A | 545.62 | 293.32 | 1.76 | 0.813 | 0.193 | 1.16E-06 | 0.00598 |
| DDR2 | 1316.92 | 2181.52 | 0.64 | −0.645 | 0.176 | 1.16E-05 | 0.0225 |
The number of genes impacted by BPA in VAT was smaller than the effects in SAT, and there was no overlap in FDR significant genes between the two tissue types (Figure 2C). Examining gene network interactions between genes at a more liberal p-value < 0.05 level revealed groups of genes involved in RNA processing with similar BPA effects in both SAT and VAT (Figure 3). A set of genes involved in ribosomes and RNA binding were downregulated by BPA in both tissues, including Ribosome Biogenesis Regulator 1 Homolog (RRS1), Ribosomal RNA Processing 1 (RRP1), RNA Binding Motif Protein 19 (RBM19), and Biogenesis of Ribosomes BRX1 (BRX1). Another set similarly downregulated in both are involved in splicing such as Pre-MRNA Processing Factor 6 (PRPF6) and Spliceosome Associated Factor 1, Recruiter Of U4/U6.U5 Tri-SnRN (SART1). Interacting chromosome modification related genes were also downregulated by BPA, including DNA Methyltransferase 1 Associated Protein 1 (DMAP1), Histone Deacetylase 4 (HDAC4), and SWI/SNF Related, Matrix Associated, Actin Dependent Regulator of Chromatin, Subfamily A, Member 4 (SMARCA4).
Figure 3.
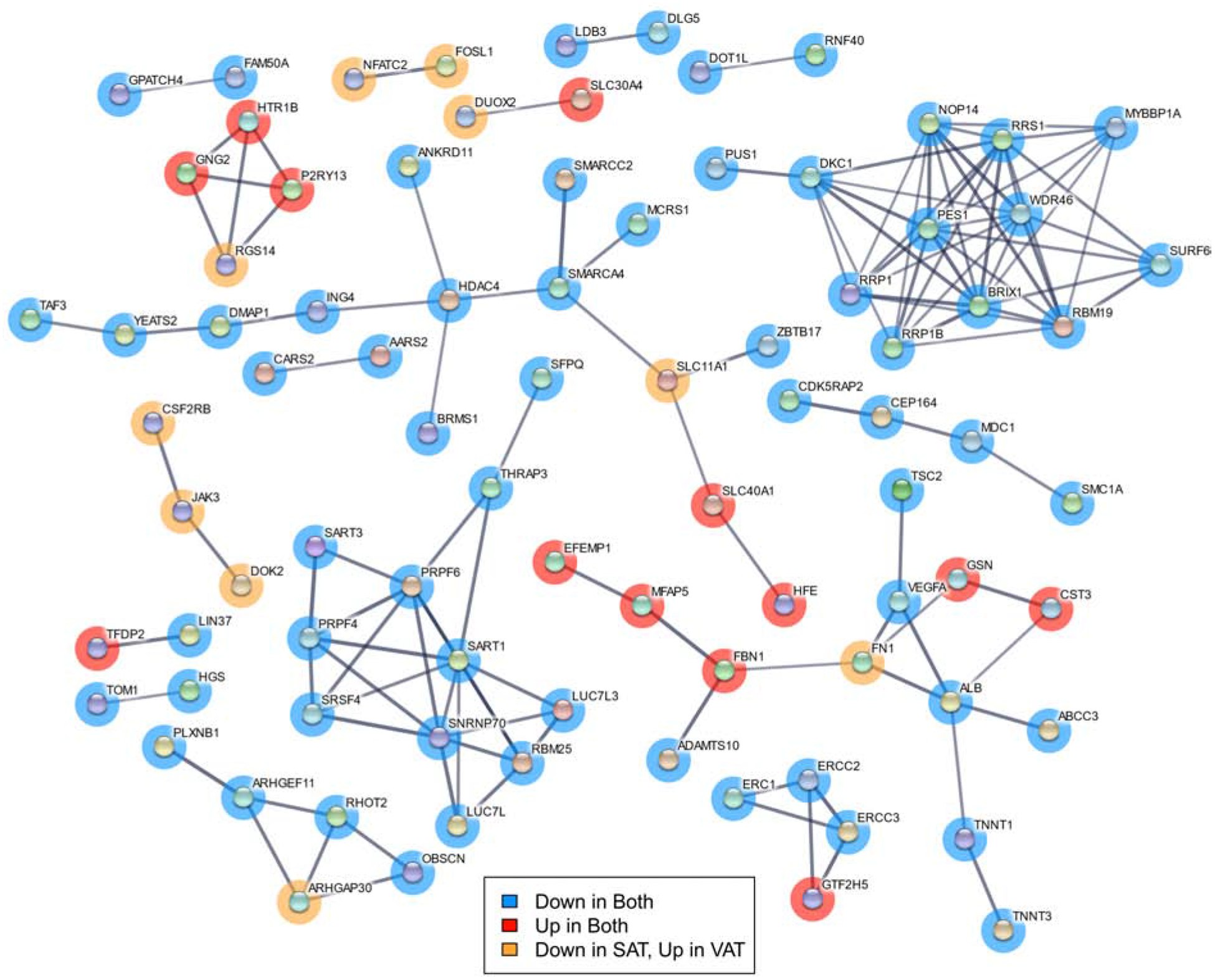
Network diagram of gene interactions among genes associated with BPA treatment at p-value < 0.05 in both SAT and VAT. Minimum interaction score of 0.7. Genes in blue were downregulated by BPA exposure in both adipose depots, those in red upregulated in both, and those in orange downregulated in SAT but upregulated in VAT.
Several up and downregulated pathways in relation to differential gene expression by BPA were identified through RNA-Enrich. In SAT, BPA downregulated several immune cell pathways such as leukocyte activation (enrichment odds ratio = 0.6, adjusted-p=3.4×10−32), inflammatory response (enrichment odds ratio = 0.61, adjusted-p=1.6×10−26), and cytokine production (enrichment odds ratio = 0.65, adjusted-p=9.4×10−23). In SAT, BPA upregulated RNA processing pathways including RNA splicing (enrichment odds ratio = 1.65, adjusted-p=1.7×10−15) and mRNA metabolic process (enrichment odds ratio = 1.48, adjusted-p=3.5×10−13) (Figure 2D; Supplemental Table 9). BPA in VAT was associated with upregulation of chromatin modification pathway (enrichment odds ratio = 1.58, adjusted-p=3.2×10−13) and RNA processing pathways, such as mRNA processing (enrichment odds ratio = 1.48, adjusted-p=6.3×10−8) (Figure 2D; Supplemental Table 10). Summarizing the overall pattern of pathways affected by BPA, transcriptional regulation and chromatin modification pathways were upregulated in response to BPA in both SAT and VAT (Figure 2D). Inflammation, immune, angiogenesis/vasculature development, and cellular and tissue development related pathways were downregulated in SAT, with more mixed or smaller impacts in VAT (Figure 2D).
DISCUSSION
This study demonstrates SAT and VAT depots have distinct transcriptional profiles in female sheep, similar to that observed in humans. These differences reflect the diverse roles of SAT and VAT in regulating lipid storage and insulin sensitivity. Importantly, the results also show that prenatal BPA treatment induces depot-specific RNA expression differences at genes involved in immune response, RNA processing, and chromatin remodeling. These findings suggest adipose tissue transcriptional dysregulations may contribute to the metabolic disorders observed in prenatal BPA-treated female sheep.
This study used RNA sequencing to quantify gene expression differences across tissue and treatment groups. The major advantage of RNA sequencing is that it measures the whole cell or tissue gene expression profile, including mRNA and long non-coding RNAs. A potential alternative is microarray technology, where prior probe selection depends on genome annotation and biases may be introduced during hybridization (Wang et al., 2009; Zhao et al., 2014). In addition, RNA sequencing allows for identification of affected gene networks (Wang et al., 2009). Use of RNA sequencing to comprehensively assess gene expression is a strength of this study.
Depot-Specific Transcriptional Changes in SAT and VAT
This study benchmarks physiologic gene expression differences between SAT and VAT in control female sheep. The divergent transcriptional profiles of SAT and VAT revealed by RNA-sequencing analysis likely reflect differences in their roles in lipid storage, inflammatory status and distribution of thermogenic brown/beige adipocytes. These findings parallel earlier reports in other species, which include both sexes. In human (Gesta et al., 2006; Hocking et al., 2010; Saely et al., 2012) . SAT favors lipid storage, which is concordant with the observed increase in expression of HOXC9, HOXA9, IRX2 and engrailed homeobox 1 (EN1). These genes promote white adipocyte development and differentiation (Yamamoto et al., 2010; Karpe and Pinnick, 2015), the adipose cell type specializing in lipid/triglyceride storage. Relative to VAT depots, SAT depots showed increased expression of the transcription factor, T-box transcription factor 15 (TBX15), which participates in thermogenic brown/beige adipocyte development (Gburcik et al., 2012). This is consistent with the previously reported observation (Puttabyatappa et al., 2019a) that SAT depots from female sheep have higher expression of the thermogenic adipocyte marker, uncoupled protein 1 (UCP1). SAT plays a role in promoting metabolic homeostasis through sequestering lipids from entering circulation and deposition in ectopic locations (Frayn, 2002; Manolopoulos et al., 2010) and/or utilization of lipids by thermogenic adipocytes (Ishibashi and Seale, 2010). The SAT-specific gene expression profiles observed in this study are in line with known SAT physiology.
Similar to SAT, VAT is also rich in white adipocytes. Unlike SAT however, VAT depots are less responsive to anabolic effects of insulin, they secrete proinflammatory cytokines, and they attract inflammatory cells (Hajer et al., 2008). VAT is lipolytically active, promoting increases in circulating lipids and ectopic lipid deposition in insulin target tissues (Wajchenberg, 2000; Hajer et al., 2008; Bjorndal et al., 2011). Therefore, increased VAT depot adiposity in particular is a risk factor for development of metabolic disorders (Wajchenberg, 2000; Bjorndal et al., 2011). Consistent with VAT physiology, VAT was characterized by increased expression of the proinflammatory gene EPHX2 and adiposity associated transcription factor CUX1. Increased EPHX2 expression would likely provoke an inflammatory state in VAT as it converts anti-inflammatory arachidonic acid metabolites into inactive diols (Newman et al., 2005). In the male mouse, EPHX2 gene silencing ameliorated high fat diet induced obesity complications (Bettaieb et al., 2013), supporting the role for this gene in mitigating metabolic defects. CUX1 is involved in expression of FTO alpha-ketoglutarate dependent dioxygenase and retinitis pigmentosa GTPase regulator-interacting protein-1 like (RPGRIP1L) (Stratigopoulos et al., 2011), which is strongly associated with adiposity among both sexes in humans (Frayling et al., 2007). These gene expression patterns are consistent with the negative role VAT plays in regulating lipid storage and metabolic homeostasis. Paradoxically, genes involved in maintaining insulin sensitivity, specifically TF (McClain et al., 2018) and adipogenesis, such as glycerol-3-phosphate acyltransferase 3 (GPAT3) (Shan et al., 2010), were also upregulated in VAT in this study. GPAT3 knockout female mice were protected from diet induced obesity (Cao et al., 2014). In the current study, increased expression of GPAT3 and CUX1 in VAT raise the possibility that the gene expression profile would favor increased visceral adiposity under conditions of positive energy, such as an overfed state. In contrast, the increase in TF may be indicative of a compensatory process to overcome the potential proinflammatory state to maintain VAT insulin sensitivity, a possibility that needs to be tested further.
Comparing gene expression pathways between SAT and VAT, skeletal system morphogenesis pathways were upregulated in SAT. Genes involved in skeletal system development were downregulated in VAT from diet-induced obese male mouse (Choi et al., 2015). How changes in these gene pathways in SAT and upregulation of toxic/xenobiotic metabolism pathways, mitochondrial membrane pathways, and vasodilation pathways in VAT contribute towards the differing SAT and VAT functions in maintaining metabolic homeostasis needs further exploration. Interestingly prenatal BPA-treatment did not change the differentially expressed genes or gene-pathways between the two depots indicating that depot-specific differences are maintained.
Effect of Prenatal BPA-Treatment on SAT Gene Expression
Prenatal BPA treatment induced marked changes in gene expression in the SAT depot of the adult female sheep. Specifically, BPA treatment increased expression of multiple uncharacterized genes, ribosomal RNA, cyclin N-terminal domain containing 2 (CNTD2), transfer RNA lysine (anticodon CUU) (TRNAK-CUU_8), serglycin (SRGN), and serine/threonine kinase 17a (STK17A). Their roles in modulating SAT function remain to be determined. Gene network analysis showed large number of genes modulated by prenatal BPA in SAT related to inflammation and immune functions. BPA treatment down regulated multiple gene sets related to immune function: leukocyte activation, migration, aggregation and cell-cell adhesion, lymphocyte activation and aggregation, T cell activation and aggregation, inflammatory response, cytokine production, positive regulation and activation of immune response (Supplemental Table 7). The physiologic basis for downregulation of genes involved in immune function with BPA treatment in SAT is not known. The SAT depot maintains a low inflammatory profile and promotes healthy metabolic state (McLaughlin et al., 2011). These findings may reflect compensatory processes in SAT to overcome systemic insulin resistance observed in prenatal BPA-treated female sheep (Veiga-Lopez et al., 2016). Similar changes in proinflammatory gene expression in SAT were observed in female sheep treated during the same susceptibility window with native steroid, testosterone, which also manifested peripheral insulin resistance (Puttabyatappa et al., 2017). Interestingly, SAT from prenatal testosterone-treated female sheep maintained insulin responsiveness (Lu et al., 2016). Since the effects of testosterone may be mediated in part by estradiol, the result of aromatization of testosterone (Abi Salloum et al., 2015), and BPA is a xenoestrogen (Laws et al., 2000), it is possible that the changes in SAT may be similarly programmed in prenatal BPA-treated female sheep.
Effect of Prenatal BPA-Treatment on VAT Gene Expression
Prenatal BPA-treatment induced modest gene expression changes in the adult VAT depot. Specifically, BPA-treatment increased expression of transcriptional factors that participate in adipogenesis, such as AT-rich interaction domain 3A (ARID3A), zinc finger protein 565 (ZNF565) and RecQ like helicase 4 (RECQL4). These findings are consistent with increased adipogenesis following gestational BPA treatment in fetal female sheep (Pu et al., 2017) and in BPA-treated human adipose stromal/stem cells, human omental fat cells, and 3T3-L1 preadipocytes (Sargis et al., 2010; Wang et al., 2013; Ohlstein et al., 2014). The zinc finger protein family is well known for its role in the promotion of adipogenesis and determination of adipogenic lineage (Wei et al., 2013), although the specific role of ZFP565 still needs to be elucidated. Both RECQL4 and ARID3A are involved in regulation of adipogenesis. They seem to have contrasting roles as knockdown of RECQL4 reduces adipogenesis (Chacko, 2017), while depletion of ARID3A expression in adipose stromal/stem cells promotes adipocyte formation (Webb, 2008). These changes are indicative that programming effects of prenatal BPA treatment on adipogenesis may reflect net effect of multiple transcription factors (Boucher et al., 2014b). We did not observe differences in key markers of adipogenesis and adipocyte differentiation, such as ZNF423, PPARG, LPL, FASN, and ADIPOQ (Ghaben and Scherer, 2019) (Supplemental Tables 5 and 6). Earlier studies profiled isolated adipose tissue derived mesenchymal stem cells or adipocytes. The current study expands the scope to whole adipose tissue gene expression.
Interestingly prenatal BPA-treatment upregulated multiple small nucleolar RNAs (LOC114113403, LOC114117716 and LOC114116238). These are noncoding RNAs, transcribed by RNA Polymerase II and are usually involved in processing and modification of other RNAs and can occasionally act like microRNA to induce epigenetic alterations (Ender et al., 2008; Kufel and Grzechnik, 2019). Consistent with the increase in these small nucleolar RNA transcripts, gene network and gene enrichment analysis revealed BPA treatment upregulated multiple gene sets related to RNA splicing, such as mRNA processing, negative regulation of transcription from RNA polymerase II promoter, transcription factor activity, binding and cofactor activity, and RNA splicing in the SAT depot (Figure 2D; Supplemental Table 7). In addition, BPA treatment upregulated gene sets involved in chromatin, histone and peptidyl-lysine modification that were enriched in VAT and SAT depots (Figure 2D). These results are consistent with BPA exposure associated epigenetic alterations through both histone modification and noncoding RNA expression, and epigenetics as a BPA programming mechanism (Alonso-Magdalena et al., 2016).
Conclusions
The present study demonstrates prenatal BPA treatment causes adipose depot-specific gene expression differences in female sheep. Pregnant women are ubiquitously exposed to BPA (Woodruff et al., 2011) and BPA disrupts endocrine signaling (Le Magueresse-Battistoni et al., 2018) and likely promotes adipogenesis (Rubin et al., 2019). Together, these issues highlight the clinical and public health significance of this study. Endocrine disrupting chemicals contribute to significant increases in adipose defects which are suggested to lead to abdominal obesity (Trasande et al., 2012; Liu et al., 2017). Prenatal BPA treatment highly upregulated genes associated with adipogenesis in VAT, and the depot-specific differences with higher expression of genes that promote adiposity in the VAT was maintained by prenatal BPA treatment. How these gene changes impact the development of obesity under overfed state needs further exploration. The experimental animals in the current study were raised on a maintenance nutritional regimen to prevent them from becoming obese and avoid confounding from the impact of postnatal obesity on programmed effects of BPA. Since this nutritional regimen prevented adiposity (Veiga-Lopez et al., 2016), it remains to be determined whether these gene changes evident in this study increases the risk for development of obesity due to a postnatal secondary hit such as overfeeding.
Epigenetic reprogramming contributes to BPA-associated adult onset diseases, however for the most part such evidence in human tissues comes from non-adipose tissues (Doherty et al., 2010). The present study identified changes in genes involved in chromatin modification in both VAT and SAT, which is in line with epigenetic contributions to adipose defects. These gene sets were highly upregulated in the VAT (Supplemental Table 8), a depot with higher risk for development of obesity. Importantly, the pathways impacted by prenatal BPA treatment, such as the inflammatory and epigenetic pathways, provide future targets for developing interventions to prevent prenatal BPA-induced pathology.
Major strengths of this study are the assessment of the depot-specific gene expression changes using the robust RNA-sequencing approach in an animal model of translational significance. The human translational significance of the sheep model relates to species similarity in fetal developmental timeline, precociality at birth, the homology in adipogenesis and distribution of brown/beige fat (Padmanabhan and Veiga-Lopez, 2014; Symonds et al., 2015; Gonzalez-Bulnes and Chavatte-Palmer, 2017; Fuller-Jackson and Henry, 2018), and development of insulin resistance with adiposity (Clarke, 2008). These data were generated using a BPA dose that resulted in fetal exposure typical of environmental exposure to a pregnant woman (Gerona et al., 2013; Veiga-Lopez et al., 2013a; Veiga-Lopez et al., 2015b; Lee et al., 2018). Findings from this study should also be interpreted taking into account the limitation – gene expression assessment in whole adipose tissues; adipose tissues are made up of diverse cell types including adipocytes, immune and vascular cells. Another limitation of the study is that this study was carried out using only female offspring; adiposity and distribution of white and brown fats show sexual dichotomy (Bloor and Symonds, 2014; Chang et al., 2018). Nonetheless, the findings are in agreement with the dimorphic role of VAT and SAT on lipid metabolism and insulin sensitivity lending support on the validity of the experimental approach that helps identify novel gene targets disrupted by gestational BPA exposure. Other limitations include lack of inclusion of interventional studies to overcome the pathology, an aspect to be considered in future investigations. In conclusion, the results from this study show that prenatal BPA treatment in the female sheep has adipose-depot specific impact on genes involved in inflammation, adipogenesis, adipocyte differentiation, and chromatin remodeling, which provides mechanistic cues that could be exploited to understand pathogenesis and in developing therapeutic strategies.
Supplementary Material
Highlights.
Distinct subcutaneous and visceral adipose depot transcripts in adult female sheep
Metabolic pathways upregulated in visceral compared to subcutaneous adipose tissue
Prenatal bisphenol A treatment changes adipose RNA expression at 21-months
Prenatal bisphenol A downregulates immune response in subcutaneous adipose tissue
Prenatal bisphenol A upregulates chromatin pathways in visceral adipose tissue
Acknowledgements
We thank Mr. Douglas Doop and Gary McCalla for their valuable assistance in breeding, lambing, and careful animal care; Dr. Almudena Veiga-Lopez, Dr. Bachir Abi Salloum, Mr. Evan Beckett, Mrs. Carol Herkimer and students supported through the Undergraduate Research Opportunity Program (University of Michigan) for the help provided with administration of treatments and tissue harvest.
Research reported in this publication was supported by R01 ES016541 (VP) and P30 ES017885. KB and JD are supported by R01 ES025574, R01 ES025531, R01 MD013299, R01 AG055406, UG3 OD023285, UH3 OD023285, and P30 AG053760. MP is supported via Ruth L. Kirschstein Institutional Training Grant T32 ES007062.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement: Authors have nothing to disclose.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abi Salloum B, Veiga-Lopez A, Abbott DH, Burant CF, Padmanabhan V, 2015. Developmental programming: exposure to testosterone excess disrupts steroidal and metabolic environment in pregnant sheep. Endocrinology 156, 2323–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed RG, 2016. Maternal bisphenol A alters fetal endocrine system: Thyroid adipokine dysfunction. Food Chem Toxicol 95, 168–174. [DOI] [PubMed] [Google Scholar]
- Ahmed S, Atlas E, 2016. Bisphenol S- and bisphenol A-induced adipogenesis of murine preadipocytes occurs through direct peroxisome proliferator-activated receptor gamma activation. Int J Obes (Lond) 40, 1566–1573. [DOI] [PubMed] [Google Scholar]
- Alonso-Magdalena P, Rivera FJ, Guerrero-Bosagna C, 2016. Bisphenol-A and metabolic diseases: epigenetic, developmental and transgenerational basis. Environ Epigenet 2, dvw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Magdalena P, Vieira E, Soriano S, Menes L, Burks D, Quesada I, Nadal A, 2010. Bisphenol A exposure during pregnancy disrupts glucose homeostasis in mothers and adult male offspring. Environ Health Perspect 118, 1243–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews S, 2010. FastQC: A quality control tool for high throughput sequence data. [Google Scholar]
- Barker DJ, 2005. The developmental origins of insulin resistance. Horm Res 64 Suppl 3, 2–7. [DOI] [PubMed] [Google Scholar]
- Ben-Jonathan N, Hugo ER, Brandebourg TD, 2009. Effects of bisphenol A on adipokine release from human adipose tissue: Implications for the metabolic syndrome. Mol Cell Endocrinol 304, 49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettaieb A, Nagata N, AbouBechara D, Chahed S, Morisseau C, Hammock BD, Haj FG, 2013. Soluble epoxide hydrolase deficiency or inhibition attenuates diet-induced endoplasmic reticulum stress in liver and adipose tissue. J Biol Chem 288, 14189–14199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorndal B, Burri L, Staalesen V, Skorve J, Berge RK, 2011. Different adipose depots: their role in the development of metabolic syndrome and mitochondrial response to hypolipidemic agents. J Obes 2011, 490650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorntorp P, 2000. [Metabolic difference between visceral fat and subcutaneous abdominal fat]. Diabetes Metab 26 Suppl 3, 10–12. [PubMed] [Google Scholar]
- Bloor ID, Symonds ME, 2014. Sexual dimorphism in white and brown adipose tissue with obesity and inflammation. Horm Behav 66, 95–103. [DOI] [PubMed] [Google Scholar]
- Boucher JG, Boudreau A, Atlas E, 2014a. Bisphenol A induces differentiation of human preadipocytes in the absence of glucocorticoid and is inhibited by an estrogen-receptor antagonist. Nutr Diabetes 4, e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher JG, Husain M, Rowan-Carroll A, Williams A, Yauk CL, Atlas E, 2014b. Identification of mechanisms of action of bisphenol a-induced human preadipocyte differentiation by transcriptional profiling. Obesity (Silver Spring) 22, 2333–2343. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL, 2008. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ Health Perspect 116, 39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Perez S, Goodwin B, Lin Q, Peng H, Qadri A, Zhou Y, Clark RW, Perreault M, Tobin JF, Gimeno RE, 2014. Mice deleted for GPAT3 have reduced GPAT activity in white adipose tissue and altered energy and cholesterol homeostasis in diet-induced obesity. Am J Physiol Endocrinol Metab 306, E1176–1187. [DOI] [PubMed] [Google Scholar]
- Carwile JL, Michels KB, 2011. Urinary bisphenol A and obesity: NHANES 2003–2006. Environ Res 111, 825–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacko L, 2017. RECQL4: linking DNA replication to bone tumorigenesis. University of Lincoln. [Google Scholar]
- Chang E, Varghese M, Singer K, 2018. Gender and Sex Differences in Adipose Tissue. Curr Diab Rep 18, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi MS, Kim YJ, Kwon EY, Ryoo JY, Kim SR, Jung UJ, 2015. High-fat diet decreases energy expenditure and expression of genes controlling lipid metabolism, mitochondrial function and skeletal system development in the adipose tissue, along with increased expression of extracellular matrix remodelling- and inflammation-related genes. Br J Nutr 113, 867–877. [DOI] [PubMed] [Google Scholar]
- Clarke IJ, 2008. Models of ‘obesity’ in large animals and birds. Front Horm Res 36, 107–117. [DOI] [PubMed] [Google Scholar]
- Corrales P, Vidal-Puig A, Medina-Gomez G, 2018. PPARs and Metabolic Disorders Associated with Challenged Adipose Tissue Plasticity. Int J Mol Sci 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbelsteyn CJ, Joffres MR, MacLean DR, Flowerdew G, 2001. A comparative evaluation of waist circumference, waist-to-hip ratio and body mass index as indicators of cardiovascular risk factors. The Canadian Heart Health Surveys. Int J Obes Relat Metab Disord 25, 652–661. [DOI] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR, 2013. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty LF, Bromer JG, Zhou Y, Aldad TS, Taylor HS, 2010. In utero exposure to diethylstilbestrol (DES) or bisphenol-A (BPA) increases EZH2 expression in the mammary gland: an epigenetic mechanism linking endocrine disruptors to breast cancer. Horm Cancer 1, 146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durinck S, Spellman PT, Birney E, Huber W, 2009. Mapping identifiers for the integration of genomic datasets with the R/Bioconductor package biomaRt. Nat Protoc 4, 1184–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ender C, Krek A, Friedlander MR, Beitzinger M, Weinmann L, Chen W, Pfeffer S, Rajewsky N, Meister G, 2008. A human snoRNA with microRNA-like functions. Mol Cell 32, 519–528. [DOI] [PubMed] [Google Scholar]
- Engfeldt P, Arner P, 1988. Lipolysis in human adipocytes, effects of cell size, age and of regional differences. Horm Metab Res Suppl 19, 26–29. [PubMed] [Google Scholar]
- Ewels P, Magnusson M, Lundin S, Käller M, 2016. MultiQC: summarize analysis results for multiple tools and samples in a single report. Bioinformatics 32, 3047–3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschini A, Szklarczyk D, Frankild S, Kuhn M, Simonovic M, Roth A, Lin J, Minguez P, Bork P, von Mering C, Jensen LJ, 2013. STRING v9.1: protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res 41, D808–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, Perry JR, Elliott KS, Lango H, Rayner NW, Shields B, Harries LW, Barrett JC, Ellard S, Groves CJ, Knight B, Patch AM, Ness AR, Ebrahim S, Lawlor DA, Ring SM, Ben-Shlomo Y, Jarvelin MR, Sovio U, Bennett AJ, Melzer D, Ferrucci L, Loos RJ, Barroso I, Wareham NJ, Karpe F, Owen KR, Cardon LR, Walker M, Hitman GA, Palmer CN, Doney AS, Morris AD, Smith GD, Hattersley AT, McCarthy MI, 2007. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 316, 889–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frayn KN, 2002. Adipose tissue as a buffer for daily lipid flux. Diabetologia 45, 1201–1210. [DOI] [PubMed] [Google Scholar]
- Fuller-Jackson JP, Henry BA, 2018. Adipose and skeletal muscle thermogenesis: studies from large animals. J Endocrinol 237, R99–R115. [DOI] [PubMed] [Google Scholar]
- Gburcik V, Cawthorn WP, Nedergaard J, Timmons JA, Cannon B, 2012. An essential role for Tbx15 in the differentiation of brown and “brite” but not white adipocytes. Am J Physiol Endocrinol Metab 303, E1053–1060. [DOI] [PubMed] [Google Scholar]
- Gerona RR, Woodruff TJ, Dickenson CA, Pan J, Schwartz JM, Sen S, Friesen MW, Fujimoto VY, Hunt PA, 2013. Bisphenol-A (BPA), BPA glucuronide, and BPA sulfate in midgestation umbilical cord serum in a northern and central California population. Environ Sci Technol 47, 12477–12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesta S, Bluher M, Yamamoto Y, Norris AW, Berndt J, Kralisch S, Boucher J, Lewis C, Kahn CR, 2006. Evidence for a role of developmental genes in the origin of obesity and body fat distribution. Proc Natl Acad Sci U S A 103, 6676–6681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaben AL, Scherer PE, 2019. Adipogenesis and metabolic health. Nat Rev Mol Cell Biol 20, 242–258. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Bulnes A, Chavatte-Palmer P, 2017. Contribution of Large Animals to Translational Research on Prenatal Programming of Obesity and Associated Diseases. Curr Pharm Biotechnol 18, 541–551. [DOI] [PubMed] [Google Scholar]
- Hajer GR, van Haeften TW, Visseren FL, 2008. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J 29, 2959–2971. [DOI] [PubMed] [Google Scholar]
- Hartley SW, Mullikin JC, 2015. QoRTs: a comprehensive toolset for quality control and data processing of RNA-Seq experiments. BMC Bioinformatics 16, 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindel JJ, Newbold R, Schug TT, 2015. Endocrine disruptors and obesity. Nat Rev Endocrinol 11, 653–661. [DOI] [PubMed] [Google Scholar]
- Hocking SL, Wu LE, Guilhaus M, Chisholm DJ, James DE, 2010. Intrinsic depot-specific differences in the secretome of adipose tissue, preadipocytes, and adipose tissue-derived microvascular endothelial cells. Diabetes 59, 3008–3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtcamp W, 2012. Obesogens: an environmental link to obesity. Environ Health Perspect 120, a62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim MM, 2010. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev 11, 11–18. [DOI] [PubMed] [Google Scholar]
- Ishibashi J, Seale P, 2010. Medicine. Beige can be slimming. Science 328, 1113–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpe F, Pinnick KE, 2015. Biology of upper-body and lower-body adipose tissue--link to whole-body phenotypes. Nat Rev Endocrinol 11, 90–100. [DOI] [PubMed] [Google Scholar]
- Kelishadi R, Poursafa P, Jamshidi F, 2013. Role of environmental chemicals in obesity: a systematic review on the current evidence. J Environ Public Health 2013, 896789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufel J, Grzechnik P, 2019. Small Nucleolar RNAs Tell a Different Tale. Trends Genet 35, 104–117. [DOI] [PubMed] [Google Scholar]
- Laws SC, Carey SA, Ferrell JM, Bodman GJ, Cooper RL, 2000. Estrogenic activity of octylphenol, nonylphenol, bisphenol A and methoxychlor in rats. Toxicol Sci 54, 154–167. [DOI] [PubMed] [Google Scholar]
- Le Magueresse-Battistoni B, Multigner L, Beausoleil C, Rousselle C, 2018. Effects of bisphenol A on metabolism and evidences of a mode of action mediated through endocrine disruption. Mol Cell Endocrinol 475, 74–91. [DOI] [PubMed] [Google Scholar]
- Lee C, Patil S, Sartor MA, 2016. RNA-Enrich: a cut-off free functional enrichment testing method for RNA-seq with improved detection power. Bioinformatics 32, 1100–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Choi K, Park J, Moon HB, Choi G, Lee JJ, Suh E, Kim HJ, Eun SH, Kim GH, Cho GJ, Kim SK, Kim S, Kim SY, Kim S, Eom S, Choi S, Kim YD, Kim S, 2018. Bisphenol A distribution in serum, urine, placenta, breast milk, and umbilical cord serum in a birth panel of mother-neonate pairs. Sci Total Environ 626, 1494–1501. [DOI] [PubMed] [Google Scholar]
- Legeay S, Faure S, 2017. Is bisphenol A an environmental obesogen? Fundam Clin Pharmacol 31, 594–609. [DOI] [PubMed] [Google Scholar]
- Lemieux S, Despres JP, 1994. Metabolic complications of visceral obesity: contribution to the aetiology of type 2 diabetes and implications for prevention and treatment. Diabete Metab 20, 375–393. [PubMed] [Google Scholar]
- Levi J, Vinter S, St Laurent R, Segal L, 2010. Issue report: F as in fat: How obesity threatens America‟ s future, 2011. [Google Scholar]
- Liao Y, Smyth GK, Shi W, 2014. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930. [DOI] [PubMed] [Google Scholar]
- Liu B, Lehmler HJ, Sun Y, Xu G, Liu Y, Zong G, Sun Q, Hu FB, Wallace RB, Bao W, 2017. Bisphenol A substitutes and obesity in US adults: analysis of a population-based, cross-sectional study. Lancet Planet Health 1, e114–e122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S, 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15, 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Cardoso RC, Puttabyatappa M, Padmanabhan V, 2016. Developmental Programming: Prenatal Testosterone Excess and Insulin Signaling Disruptions in Female Sheep. Biol Reprod 94, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manikkam M, Crespi EJ, Doop DD, Herkimer C, Lee JS, Yu S, Brown MB, Foster DL, Padmanabhan V, 2004. Fetal programming: prenatal testosterone excess leads to fetal growth retardation and postnatal catch-up growth in sheep. Endocrinology 145, 790–798. [DOI] [PubMed] [Google Scholar]
- Manolopoulos KN, Karpe F, Frayn KN, 2010. Gluteofemoral body fat as a determinant of metabolic health. Int J Obes (Lond) 34, 949–959. [DOI] [PubMed] [Google Scholar]
- Masuno H, Iwanami J, Kidani T, Sakayama K, Honda K, 2005. Bisphenol a accelerates terminal differentiation of 3T3-L1 cells into adipocytes through the phosphatidylinositol 3-kinase pathway. Toxicol Sci 84, 319–327. [DOI] [PubMed] [Google Scholar]
- McClain DA, Sharma NK, Jain S, Harrison A, Salaye LN, Comeau ME, Langefeld CD, Lorenzo FR, Das SK, 2018. Adipose Tissue Transferrin and Insulin Resistance. J Clin Endocrinol Metab 103, 4197–4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin T, Lamendola C, Liu A, Abbasi F, 2011. Preferential fat deposition in subcutaneous versus visceral depots is associated with insulin sensitivity. J Clin Endocrinol Metab 96, E1756–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer D, Harries L, Cipelli R, Henley W, Money C, McCormack P, Young A, Guralnik J, Ferrucci L, Bandinelli S, Corsi AM, Galloway T, 2011. Bisphenol A exposure is associated with in vivo estrogenic gene expression in adults. Environ Health Perspect 119, 1788–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Navarrete JM, Fernández-Real JM, 2017. Adipocyte Differentiation in: Symonds ME (Ed.). Adipose Tissue Biology. Springer International Publishing, Cham, pp. 69–90. [Google Scholar]
- Mota de Sa P, Richard AJ, Hang H, Stephens JM, 2017. Transcriptional Regulation of Adipogenesis. Compr Physiol 7, 635–674. [DOI] [PubMed] [Google Scholar]
- Newbold RR, 2010. Impact of environmental endocrine disrupting chemicals on the development of obesity. Hormones (Athens) 9, 206–217. [DOI] [PubMed] [Google Scholar]
- Newell-Fugate AE, 2017. The role of sex steroids in white adipose tissue adipocyte function. Reproduction 153, R133–R149. [DOI] [PubMed] [Google Scholar]
- Newman JW, Morisseau C, Hammock BD, 2005. Epoxide hydrolases: their roles and interactions with lipid metabolism. Prog Lipid Res 44, 1–51. [DOI] [PubMed] [Google Scholar]
- Ohlstein JF, Strong AL, McLachlan JA, Gimble JM, Burow ME, Bunnell BA, 2014. Bisphenol A enhances adipogenic differentiation of human adipose stromal/stem cells. J Mol Endocrinol 53, 345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan V, Veiga-Lopez A, 2014. Reproduction Symposium: developmental programming of reproductive and metabolic health. J Anim Sci 92, 3199–3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polvani S, Tarocchi M, Tempesti S, Bencini L, Galli A, 2016. Peroxisome proliferator activated receptors at the crossroad of obesity, diabetes, and pancreatic cancer. World J Gastroenterol 22, 2441–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu Y, Gingrich JD, Steibel JP, Veiga-Lopez A, 2017. Sex-Specific Modulation of Fetal Adipogenesis by Gestational Bisphenol A and Bisphenol S Exposure. Endocrinology 158, 3844–3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puttabyatappa M, Andriessen V, Mesquitta M, Zeng L, Pennathur S, Padmanabhan V, 2017. Developmental Programming: Impact of Gestational Steroid and Metabolic Milieus on Mediators of Insulin Sensitivity in Prenatal Testosterone-Treated Female Sheep. Endocrinology 158, 2783–2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puttabyatappa M, Ciarelli JN, Chatoff AG, Singer K, Padmanabhan V, 2019a. Developmental programming: Adipose depot-specific changes and thermogenic adipocyte distribution in the female sheep. Mol Cell Endocrinol 503, 110691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puttabyatappa M, Martin JD, Andriessen V, Stevenson M, Zeng L, Pennathur S, Padmanabhan V, 2019b. Developmental programming: Changes in mediators of insulin sensitivity in prenatal bisphenol A-treated female sheep. Reprod Toxicol 85, 110–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regnier SM, Sargis RM, 2014. Adipocytes under assault: environmental disruption of adipose physiology. Biochim Biophys Acta 1842, 520–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin BS, Schaeberle CM, Soto AM, 2019. The Case for BPA as an Obesogen: Contributors to the Controversy. Front Endocrinol (Lausanne) 10, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saely CH, Geiger K, Drexel H, 2012. Brown versus white adipose tissue: a mini-review. Gerontology 58, 15–23. [DOI] [PubMed] [Google Scholar]
- Sargis RM, Johnson DN, Choudhury RA, Brady MJ, 2010. Environmental endocrine disruptors promote adipogenesis in the 3T3-L1 cell line through glucocorticoid receptor activation. Obesity (Silver Spring) 18, 1283–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor MA, Leikauf GD, Medvedovic M, 2009. LRpath: a logistic regression approach for identifying enriched biological groups in gene expression data. Bioinformatics 25, 211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan D, Li JL, Wu L, Li D, Hurov J, Tobin JF, Gimeno RE, Cao J, 2010. GPAT3 and GPAT4 are regulated by insulin-stimulated phosphorylation and play distinct roles in adipogenesis. J Lipid Res 51, 1971–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratigopoulos G, LeDuc CA, Cremona ML, Chung WK, Leibel RL, 2011. Cut-like homeobox 1 (CUX1) regulates expression of the fat mass and obesity-associated and retinitis pigmentosa GTPase regulator-interacting protein-1-like (RPGRIP1L) genes and coordinates leptin receptor signaling. J Biol Chem 286, 2155–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symonds ME, Pope M, Budge H, 2015. The Ontogeny of Brown Adipose Tissue. Annu Rev Nutr 35, 295–320. [DOI] [PubMed] [Google Scholar]
- Teppala S, Madhavan S, Shankar A, 2012. Bisphenol A and Metabolic Syndrome: Results from NHANES. Int J Endocrinol 2012, 598180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trasande L, Attina TM, Blustein J, 2012. Association between urinary bisphenol A concentration and obesity prevalence in children and adolescents. JAMA 308, 1113–1121. [DOI] [PubMed] [Google Scholar]
- Veiga-Lopez A, Kannan K, Liao C, Ye W, Domino SE, Padmanabhan V, 2015a. Gender-Specific Effects on Gestational Length and Birth Weight by Early Pregnancy BPA Exposure. J Clin Endocrinol Metab 100, E1394–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga-Lopez A, Luense LJ, Christenson LK, Padmanabhan V, 2013a. Developmental programming: gestational bisphenol-A treatment alters trajectory of fetal ovarian gene expression. Endocrinology 154, 1873–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga-Lopez A, Moeller J, Patel D, Ye W, Pease A, Kinns J, Padmanabhan V, 2013b. Developmental programming: impact of prenatal testosterone excess on insulin sensitivity, adiposity, and free fatty acid profile in postpubertal female sheep. Endocrinology 154, 1731–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga-Lopez A, Moeller J, Sreedharan R, Singer K, Lumeng C, Ye W, Pease A, Padmanabhan V, 2016. Developmental programming: interaction between prenatal BPA exposure and postnatal adiposity on metabolic variables in female sheep. Am J Physiol Endocrinol Metab 310, E238–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga-Lopez A, Pennathur S, Kannan K, Patisaul HB, Dolinoy DC, Zeng L, Padmanabhan V, 2015b. Impact of gestational bisphenol A on oxidative stress and free fatty acids: Human association and interspecies animal testing studies. Endocrinology 156, 911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel SA, 2009. The politics of plastics: the making and unmaking of bisphenol a “safety”. Am J Public Health 99 Suppl 3, S559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vom Saal FS, 2016. TRIENNIAL REPRODUCTION SYMPOSIUM: Environmental programming of reproduction during fetal life: Effects of intrauterine position and the endocrine disrupting chemical bisphenol A. J Anim Sci 94, 2722–2736. [DOI] [PubMed] [Google Scholar]
- vom Saal FS, Akingbemi BT, Belcher SM, Birnbaum LS, Crain DA, Eriksen M, Farabollini F, Guillette LJ Jr., Hauser R, Heindel JJ, Ho SM, Hunt PA, Iguchi T, Jobling S, Kanno J, Keri RA, Knudsen KE, Laufer H, LeBlanc GA, Marcus M, McLachlan JA, Myers JP, Nadal A, Newbold RR, Olea N, Prins GS, Richter CA, Rubin BS, Sonnenschein C, Soto AM, Talsness CE, Vandenbergh JG, Vandenberg LN, Walser-Kuntz DR, Watson CS, Welshons WV, Wetherill Y, Zoeller RT, 2007. Chapel Hill bisphenol A expert panel consensus statement: integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reprod Toxicol 24, 131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wajchenberg BL, 2000. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev 21, 697–738. [DOI] [PubMed] [Google Scholar]
- Wang J, Sun B, Hou M, Pan X, Li X, 2013. The environmental obesogen bisphenol A promotes adipogenesis by increasing the amount of 11beta-hydroxysteroid dehydrogenase type 1 in the adipose tissue of children. Int J Obes (Lond) 37, 999–1005. [DOI] [PubMed] [Google Scholar]
- Wang T, Li M, Chen B, Xu M, Xu Y, Huang Y, Lu J, Chen Y, Wang W, Li X, Liu Y, Bi Y, Lai S, Ning G, 2012. Urinary bisphenol A (BPA) concentration associates with obesity and insulin resistance. J Clin Endocrinol Metab 97, E223–227. [DOI] [PubMed] [Google Scholar]
- Wang Z, Gerstein M, Snyder M, 2009. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet 10, 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassenaar PNH, Trasande L, Legler J, 2017. Systematic Review and Meta-Analysis of Early-Life Exposure to Bisphenol A and Obesity-Related Outcomes in Rodents. Environ Health Perspect 125, 106001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb CK, P., 2008. Production of pluripotent cells through inhibition of bright/ARID3a function in: Office, U.S.P.a.T (Ed.). Oklahoma Medical Research Foundation, United States of America. [Google Scholar]
- Wei S, Zhang L, Zhou X, Du M, Jiang Z, Hausman GJ, Bergen WG, Zan L, Dodson MV, 2013. Emerging roles of zinc finger proteins in regulating adipogenesis. Cell Mol Life Sci 70, 4569–4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill YB, Akingbemi BT, Kanno J, McLachlan JA, Nadal A, Sonnenschein C, Watson CS, Zoeller RT, Belcher SM, 2007. In vitro molecular mechanisms of bisphenol A action. Reprod Toxicol 24, 178–198. [DOI] [PubMed] [Google Scholar]
- Whaley PA, 2014. Childhood obesity and the environment Controversies in Obesity. Springer, pp. 97–102. [Google Scholar]
- WHO, 2018. Obesity and overweight. [Google Scholar]
- Williams EP, Mesidor M, Winters K, Dubbert PM, Wyatt SB, 2015. Overweight and Obesity: Prevalence, Consequences, and Causes of a Growing Public Health Problem. Curr Obes Rep 4, 363–370. [DOI] [PubMed] [Google Scholar]
- Woodruff TJ, Zota AR, Schwartz JM, 2011. Environmental chemicals in pregnant women in the United States: NHANES 2003–2004. Environ Health Perspect 119, 878–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Gesta S, Lee KY, Tran TT, Saadatirad P, Kahn CR, 2010. Adipose depots possess unique developmental gene signatures. Obesity (Silver Spring) 18, 872–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Cong J, Shen H, Wu Q, Wu X, 2014. Genome-wide identification of aberrantly methylated promoters in ovarian tissue of prenatally androgenized rats. Fertil Steril 102, 1458–1467. [DOI] [PubMed] [Google Scholar]
- Zhao S, Fung-Leung WP, Bittner A, Ngo K, Liu X, 2014. Comparison of RNA-Seq and microarray in transcriptome profiling of activated T cells. PLoS One 9, e78644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu A, Ibrahim JG, Love MI, 2019. Heavy-tailed prior distributions for sequence count data: removing the noise and preserving large differences. Bioinformatics 35, 2084–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmet PZ, Alberti KG, 2006. Introduction: Globalization and the non-communicable disease epidemic. Obesity (Silver Spring) 14, 1–3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


