Supplemental Digital Content is available in the text.
Keywords: biomarker, cachexia, intensive care unit, prognosis, sarcopenia
Objectives:
Parameters of patients’ body composition have been suggested as prognostic markers in several clinical conditions including cancer and liver transplantation, but only limited data on its value in critical illness exist to date. In this study, we aimed at evaluating a potential prognostic value of the skeletal muscle mass and skeletal muscle myosteatosis of critically ill patients at admission to the ICU.
Design:
Exploratory observational cohort study.
Setting:
An urban, academic medical institution.
Patients:
One-hundred fifty-five patients treated for critical illness on a medical ICU.
Interventions:
None.
Measurements and Main Results:
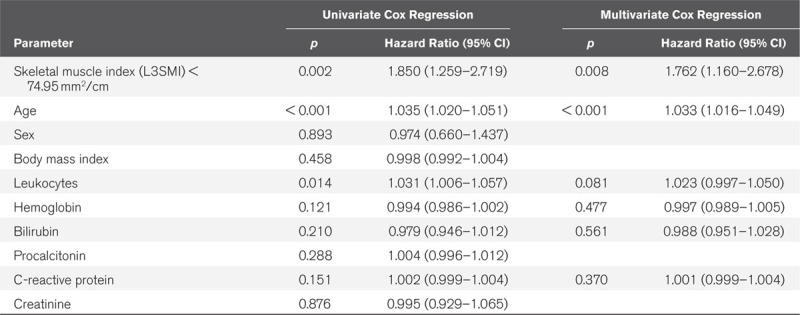
We used routine CT scans to assess the patients’ individual body composition. The skeletal muscle index as a surrogate for sarcopenia was defined as the total skeletal muscle area at the level of the third lumbar vertebra on axial CT scan, normalized for the patient’s height. Myosteatosis was evaluated by assessing the mean skeletal muscle attenuation measured in Hounsfield unit at the same sectional plane. The skeletal muscle index and mean skeletal muscle attenuation at admission to the ICU were significantly higher in patients with long-term survival (180-day or 1-year mortality), while both parameters were comparable between short-term survivors and nonsurvivors (ICU mortality or 30-d mortality). Patients with a skeletal muscle index or mean skeletal muscle attenuation below our established ideal cutoff values (74.95 mm2/cm and 29 Hounsfield unit) showed a significantly reduced overall survival. These findings were confirmed in univariate and multivariate Cox regression analyses. Furthermore, myosteatosis significantly correlated with the time of mechanical ventilation, the duration of hospital stay, and the presence of sepsis.
Conclusions:
Our data suggest that sarcopenia and myosteatosis represent important prognostic factors in critically ill patients that can be easily obtained from routine CT scans. Both parameters at admission to the ICU yield important information on the patients’ long-term outcome and might be used for early clinical decision-making in these patients.
Despite intensive research efforts, prediction of long-term prognosis in critically ill patients has remained poor. In this context, the body composition was identified as a potential factor determining the outcome of patients treated on an ICU. Different authors have suggested a link between low skeletal muscle mass and patients´ ICU mortality (1–3). However, the term “low skeletal muscle mass” is only poorly defined and often used synonymous to sarcopenia. The latter is defined as the “progressive loss of muscle mass and strength with a risk of adverse outcomes such as disability, poor quality of life and death” by the Special Interest Group of the European Sarcopenia Working Group in 2010 (4). Since both muscle strength and muscle mass are difficult to measure in clinical routine, many authors have defined sarcopenia as a skeletal muscle area index below the 5th percentile of the of a healthy control population (5). However, using this definition, the role of sarcopenia as a prognostic marker for critically ill patients remains controversial (5), which might be due to the fact that functional aspects such as muscle strength and muscle composition are not properly reflected. Recently, Faron et al (6) showed the clinical potential of fat-free muscle area (FFMA) in MRI scans to predict outcome in patients with colorectal cancer, suggesting that FFMA might be a new and easily accessible prognostic biomarker for prognosis.
In this study, we aimed at analyzing whether sarcopenia, assessed by the skeletal muscle index (L3SMI), and the mean skeletal muscle attenuation (MMA) as a surrogate for muscular fat deposition, represent prognostic factors in critically ill patients treated on a medical ICU.
PATIENT AND METHODS
Study Design and Patient Characteristics
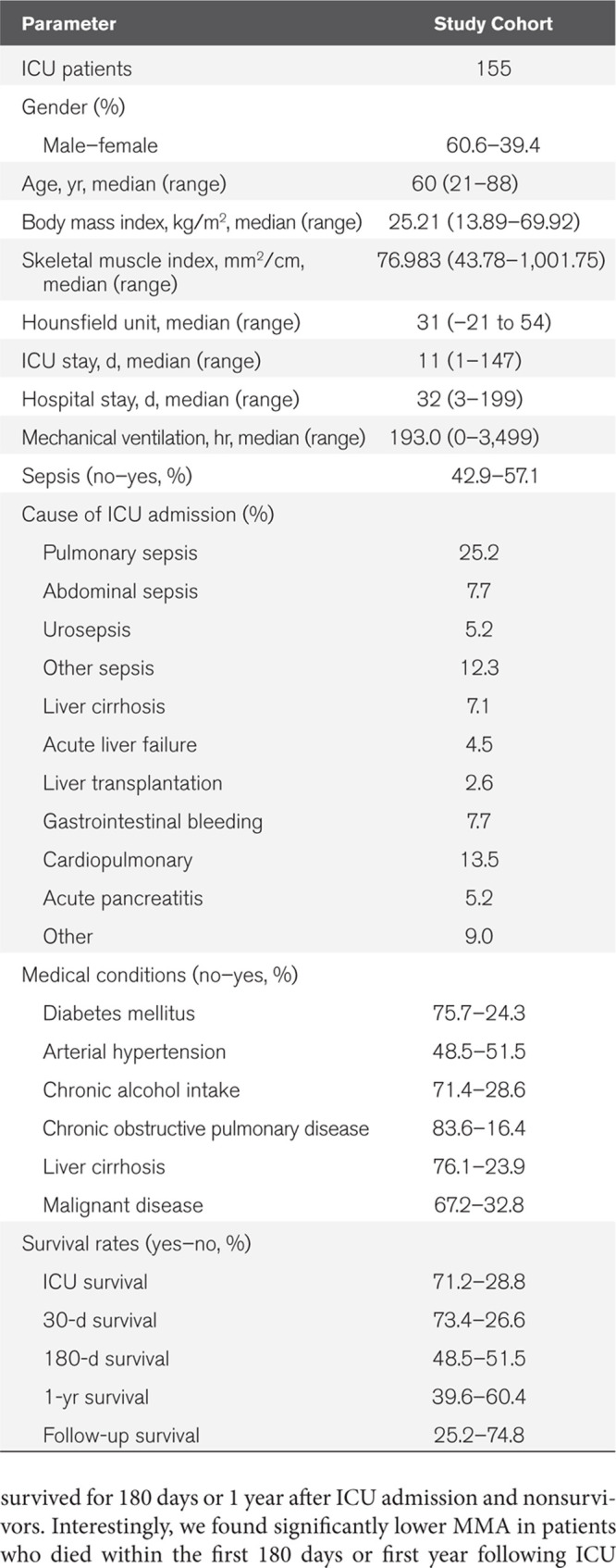
A total of 155 patients who were admitted to the medical ICU at the Department of Medicine III at University Hospital Rheinisch-Westfälische Technische Hochschule (RWTH) Aachen between 2006 and 2015 were enrolled in this study (detailed patient characteristics are given in Table 1). The study was approved by the local ethics committee (EK 150/06) of the University Hospital RWTH Aachen, Germany, and written informed consent was obtained from every participant or authorized relatives in the case of unconsciousness.
Table 1.
Characteristics of Study Cohort
Assessment of Body Composition and Definition of the Skeletal Muscle Index
We only used CT scans in venous phase with a slice thickness of 5 mm for this study. We included CT scans that were performed upon ICU admission. The total skeletal muscle area, as well as the MMA, were segmented at the center plane of the third lumbar vertebra on axial CT scans. We used this approach as it was recently shown that single-slice measurements of the skeletal muscle area and adipose tissue at anatomical landmarks are strongly associated with total compartment volumes and therefore provide comparability of results (7). All parameters were assessed manually using a semi-automatically segmentation tool, 3D slicer, an open source software platform for medical image informatics (8). The following muscle groups were segmented: rectus abdominis, external and internal obliques, transversus abdominis, quadratus lumborum, as well as the psoas major and erector spinae (Supplemental Fig. 1, Supplemental Digital Content 1, http://links.lww.com/CCX/A240; legend, Supplemental Digital Content 8, http://links.lww.com/CCX/A247). All muscles were identified and quantified with cutoff values of –29 to 150 Hounsfield units (HUs). The MMA was automatically calculated by the software. The L3SMI was further normalized for the patients’ height as follows:
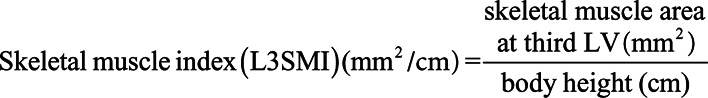 |
Statistical Analysis
Statistical analyses were performed as recently described (9) (Supplemental Patients and Methods, Supplemental Digital Content 2, http://links.lww.com/CCX/A241). A p value of less than 0.05 was considered statistically significant (*p < 0.05; **p < 0.01; ***p < 0.001).
RESULTS
The Individual Body Composition Does Not Predict Short-Term Mortality or ICU Survival in Critically Ill Patients
A total of n = 155 patients who were admitted to the medical ICU for treatment of critical illness were included in this study (Table 1). The median L3SMI that we used as a surrogate for sarcopenia was 76.983 mm2/cm (range, 43.78–1,001.75 mm2/cm). The median skeletal muscle attenuation (MMA) that we used a surrogate parameter for muscular fat deposition was 30 HU (range, –21 to 54 HU). We hypothesized that the patients’ individual body composition might predict short-term mortality in ICU patients. We therefore compared the L3SMI between patients who survived the ICU stay and were admitted to standard care (ICU survivors) and patients who deceased during the ICU stay (ICU nonsurvivors). Interestingly, we did not see a significant difference between these two groups (Supplemental Fig. 2, A and B, Supplemental Digital Content 3, http://links.lww.com/CCX/A242; legend, Supplemental Digital Content 8, http://links.lww.com/CCX/A247). In a second step, we evaluated whether or not an increased fat deposition in the skeletal muscle might be a negative predictor for short-term outcomes. Therefore, we determined the MMA of the skeletal muscle area that was used to calculate the L3SMI as a surrogate for fatty muscle depositions. However, when comparing the MMA of patients who did or did not survive the ICU stay, we did not observe a significant difference between ICU survivors and nonsurvivors (Supplemental Fig. 2C, Supplemental Digital Content 3, http://links.lww.com/CCX/A242; legend, Supplemental Digital Content 8, http://links.lww.com/CCX/A247). In line, there was no significant association between the MMA and the 30-day mortality (Supplemental Fig. 2D, Supplemental Digital Content 3, http://links.lww.com/CCX/A242; legend, Supplemental Digital Content 8, http://links.lww.com/CCX/A247).
The Individual Body Composition Is Associated With Long-Term Outcome of ICU Patients
Hypothesizing that an unfavorable body composition (low amount of skeletal muscle, high amount of muscular fat deposition) might be associated with an impaired long-term survival rather than predicting short-term mortality, we next compared the L3SMI between patients who did or did not survive for 180 days and 1 year, respectively. Strikingly, we observed significantly higher L3SMI values in patients who survived for 180 days compared to patients who deceased during this time (Fig. 1A). The median L3SMI was 79.05 mm2/cm for the 180 days survivors and 72.65 mm2/cm for nonsurvivors. In line, the L3SMI was significantly associated with the 1-year mortality rate of ICU patients as patients who survived for at least 1 year had significantly higher L3SMI values compared to patients who died within the first year after ICU admission (79.81 vs 74.05 mm2/cm; Fig. 1B). Subsequently, we compared the MMA between patients who survived for 180 days or 1 year after ICU admission and nonsurvivors. Interestingly, we found significantly lower MMA in patients who died within the first 180 days or first year following ICU admission (Fig. 1, C and D) compared to patients who survived this time period, suggesting that a higher amount of muscular fat deposition (low MMA) also represents a negative predictor of long-term ICU survival. In line, binary logistic-regression analysis revealed both the L3SMI and the MMA as prognostic factors for 180-day mortality (odds ratio [ORL3SMI], 0.979; 0.961–0.997; p = 0.025 and ORHU, 0.964; 0.937–0.993; p = 0.014).
Figure 1.
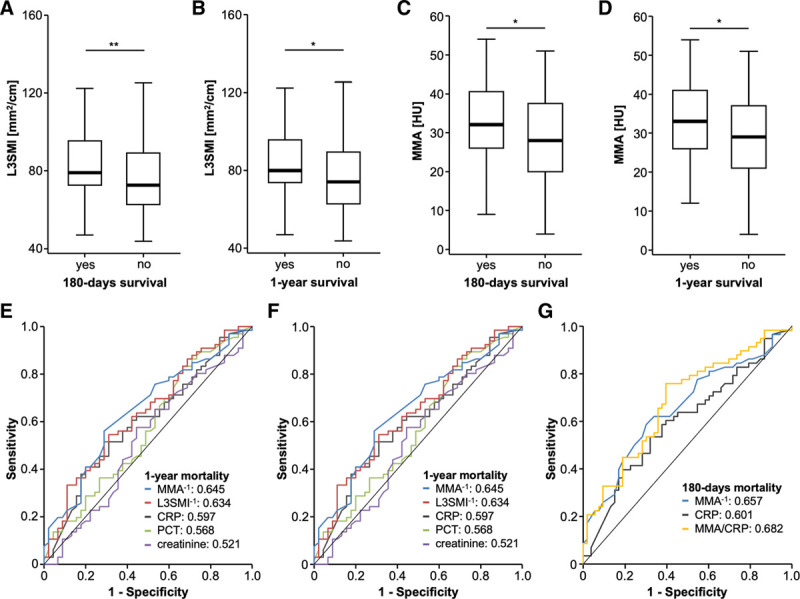
The ICU patients’ body composition predicts long-term outcome. A, Patients with 180-d survival have significantly higher skeletal muscle index (L3SMI) values compared to patients who deceased during this time. B, The L3SMI is significantly higher in patients who survive for at least one after ICU admission. Patients who die within the first 180 d (C) or first year (D) following ICU admission have significantly lower mean skeletal muscle attenuation (MMA). The area under the curve values for the L3SMI and Hounsfield unit (HU) for prediction of 180-d (E) and 1-yr (F) mortality are higher compared to established predictive serum parameters. G, The prediction of 180-d mortality is highest when the MMA and C-reactive protein (CRP) levels are combined. PCT = procalcitonin.
Finally, we compared the predictive power of the L3SMI and the MMA regarding long-term survival with circulating predictive markers that have previously been associated with ICU survival, such as the C-reactive protein (CRP), procalcitonin, and creatinine using receiver operating characteristic curve analyses. Here, we observed higher area under the curve (AUC) values for the L3SMI and the MMA for both prediction of 180-day (AUCL3SMI: 0.654, AUCMMA: 0.657) and 1-year (AUCL3SMI: 0.654, AUCMMA: 0.657) mortality when compared to established predictive serum parameters (Fig. 1, E and F). Importantly, the predictive power for long-term mortality was even higher when we combined parameters of body composition with established prognostic serum markers such as the MMA and CRP levels, revealing a higher AUC of 0.682 (Fig. 1G).
The L3SMI and Myosteatosis Are Predictors of Overall Survival in ICU Patients
We subsequently evaluated if L3SMI and the MMA are also indicative for the patients’ overall survival (OS). Therefore, we subdivided our cohort of patients into two groups with either high or low L3SMI or MMA values (50th percentile cutoff) and compared the OS between groups using Kaplan-Meier curve analysis. This analysis revealed a significantly impaired OS for patients with a L3SMI below 76.98 mm2/cm compared to patients with an L3SMI value above this cutoff (Fig. 2A). In line, patients showing a larger amount of muscular fat deposition with an MMA below 31 HU had a significantly impaired OS compared to patients with an MMA above 31 HU (Fig. 2B). When using ideal cutoff values (L3SMI: 74.95 mm2/cm and MMA: 29 HU), the L3SMI and the MMA both showed a highly significant discriminatory potential for the identification of ICU patients with an unfavorable long-term prognosis (Fig. 2, C and D). The median OS both in the low L3SMI and in low MMA group was only 8.4 weeks compared with 64.8 weeks in the L3SMI high and 62.1 weeks in the MMA high group. In line, Cox regression analyses revealed a L3SMI (hazard ratio [HR], 1.850; 1.259–2.719; p = 0.002) as well as an MMA (HR, 1.690; 1.167–2.446; p = 0.005) below these cutoff values as negative prognostic factors for OS. Importantly, multivariate Cox regression analysis showed that the prognostic potential of the L3SMI was independent of various clinicopathological parameters such as the patients’ age, markers of systemic inflammation (leucocyte count and CRP), and parameters of organ dysfunction (bilirubin, hemoglobin) (Table 2). Importantly, the combinational use of the L3SMI and the MMA (low L3SMI/low MMA vs high L3SMI/high MMA) revealed an even better prognostic potential to predict OS in critically ill patients (Fig. 2E). Finally, the association of the patients’ body composition with outcome is further supported by quartile analyses, showing, for example, that a gradually decreasing skeletal muscle density leads to increasingly reduced OS (median OSMMA: 132.6 wk if MMA > 75th percentile [39 HU], 47.0 wk if MMA between 75th and 50th percentile [31–39 HU], 31.4 wk if MMA between 50th and 25th percentile [21–31 HU], and 13.1 wk if MMA < 25th percentile [< 21 HU]).
Figure 2.
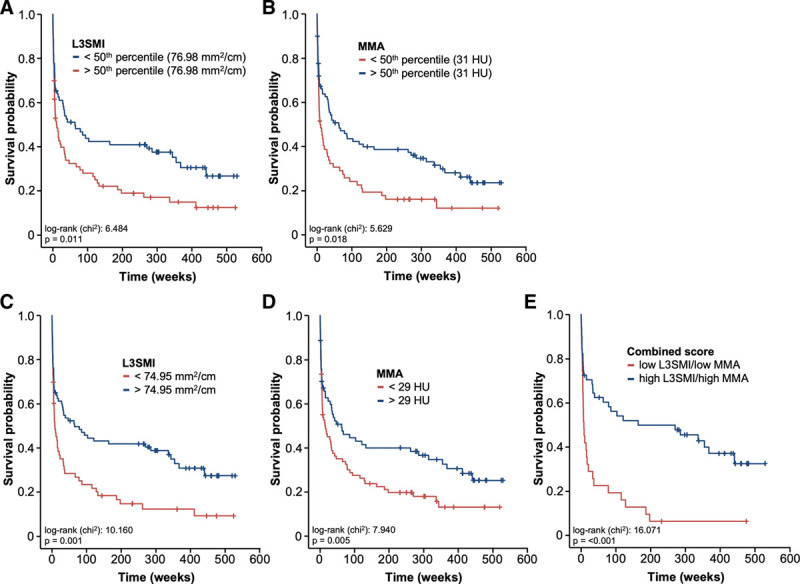
The body composition correlates with the patients’ overall survival (OS). A, ICU patients with a low skeletal muscle index (L3SMI) (below 50th percentile) show a significantly reduced OS. B, A low mean skeletal muscle attenuation (MMA) (below 50th percentile) is associated with a significantly reduced OS. When using the optimal prognostic cutoff values, both the L3SMI (C) and the mean skeletal Hounsfield unit (HU) (D) show a highly significant prediction of OS. E, The combinational use of the L3SMI and the MMA (L3SMI low/MMA low vs L3SMI high/MMA high) reveals the highest prognostic potential.
Table 2.
Univariate and Multivariate Cox Regression Analysis of the Skeletal Muscle Index and Several Clinicopathological Parameters for the Prediction of Overall Survival
Subsequently, we aimed at identifying potential underlying factors which contributed to an impaired OS in patients with an unfavorable body composition. As the time of mechanical ventilation on the ICU is a well-established prognostic factor for OS (10), we correlated the MMA with the duration of ventilation and observed a significant negative correlation between the HU and the total hours of mechanical ventilation on the ICU (Fig. 3A). Thus, a higher degree of myosteatosis (lower MMA) was associated with a longer necessity of mechanical ventilation, potentially leading to an impaired OS. Besides the duration of mechanical ventilation, we also found a significant negative correlation between the MMA and the duration of hospital stay, including the stay on the ICU and standard care (Fig. 3B). Finally, we compared the MMA in patients who did or did not fulfill the criteria of sepsis, representing another well-established prognostic parameter in ICU patients. Here, we observed significantly lower MMA values in septic patients compared with patients with nonseptic disease (Fig. 3C). On the contrary, the L3SMI did not correlate with any of these parameters (Fig. 3D–F).
Figure 3.
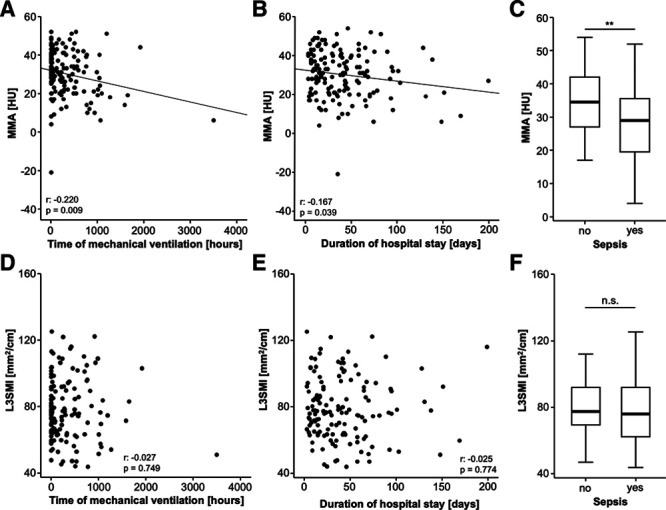
Increased levels of myosteatosis are associated with sepsis and duration of mechanical ventilation. A, There is a significant negative correlation between the mean skeletal muscle attenuation (MMA) and the duration of mechanical ventilation. B, The duration of hospital stay negatively correlates with the degree of myosteatosis (MMA). C, Patients who fulfill the criteria of sepsis have significantly lower MMA. D–F, The skeletal muscle index (L3SMI) does not correlate with any of these parameters. HU = Hounsfield unit, n.s. = not significant.
Body Composition and Patient Characteristics
To evaluate a potential association between the patients’ body composition and clinicopathological parameters, we compared the L3SMI and the MMA between patients with different medical conditions (diabetes, arterial hypertension, chronic obstructive pulmonary disease [COPD], liver cirrhosis, chronic alcohol intake, and malignant disease). While patients with diabetes mellitus showed significantly higher L3SMI values than nondiabetic patients, we did not observe a significant difference of the L3SMI between patients with or without arterial hypertension, COPD, liver cirrhosis, chronic alcohol intake, or malignant disease. The L3SMI was also not significantly altered when patients were stratified with respect to their acute medical condition that led to ICU admission (Supplemental Fig. 3 A–G, Supplemental Digital Content 4, http://links.lww.com/CCX/A243; legend, Supplemental Digital Content 8, http://links.lww.com/CCX/A247). Similarly, we observed significantly lower MMA levels in patients with preexisting diabetes mellitus. We also found higher grades of myosteatosis (low MMA) in patients with arterial hypertension, while patients with or without COPD, liver cirrhosis, chronic alcohol intake, or malignant disease had similar MMA values. The MMA was also not altered between the different disease etiologies that led to ICU admission (Supplemental Fig. 4 A–G, Supplemental Digital Content 5, http://links.lww.com/CCX/A244; legend, Supplemental Digital Content 8, http://links.lww.com/CCX/A247).
Furthermore, we performed extensive correlation analysis between the L3SMI as well as the MMA and various clinical laboratory parameters of organ dysfunction (Supplemental Table 1, Supplemental Digital Content 6, http://links.lww.com/CCX/A245). Here, we found a significant negative correlation between the MMA and serum cholesterol levels (rS: –0.185; p = 0.040), arguing that hypercholesterolemia is associated with a higher degree of fatty muscle deposition and myosteatosis. Furthermore, we observed a negative correlation between the MMA and the creatinine as well as NT-pro brain natriuretic peptide (NT-proBNP). Circulating interleukin (IL)–6 levels positively correlated with the L3SMI (rS: 0.182; p = 0.047), which corroborates the finding that IL-6 is released by skeletal muscle tissue during systemic stress response (11). In addition, the L3SMI negatively correlated with serum levels of alkaline phosphatase (alkaline phosphatase [rS: –0.275; p = 0.001] and NT-proBNP [rS: –0.242; p = 0.011]).
DISCUSSION
The body composition of an individual patient might be determined by very different techniques such as bioelectrical impedance analysis or air displacement plethysmography, just to name a few (12, 13). However, in case of critically ill patients often receiving extensive hydration, the value of these methods to determine skeletal muscle quantity and quality is hampered by consecutive fluid overload (13). Furthermore, in such patients, classical functional tests for estimating body composition and physical strength (body weight, waist circumference, body mass index, ability to walk or physical activity, and handgrip) do not provide reliable results (13). Therefore, CT, available for almost all ICU patients, represents the gold standard for assessing muscle mass and muscle quality in critically ill patients (13–15). So far, the role of sarcopenia and myosteatosis as prognostic factors in critical illness is unclear. Within this study, we quantified both the skeletal muscle mass and the amount of skeletal muscle fat deposition and evaluated the prognostic value of these parameters. At the respective optimal cutoff values that we established using a recently described biometric software (16), both a low L3SMI and a low MMA turned out as powerful predictors of OS. Importantly, when both markers were combined (e.g., L3SMI low/MMA low vs L3SMI high/MMA high patients), the prognostic potential was even further increased (Fig. 2).
Sarcopenia is a common characteristic of elderly and moribund patients. Muscle wasting can be triggered by manifold disease conditions, including disuse, denervation, fasting, cancer, cardiac failure, and renal dysfunction (17). As all of these factors are frequently found in critically ill patients and might themselves limit patients’ prognosis, systematic research on the role of an impaired muscle mass and strength bears a risk of important bias. In our analysis, however, L3SMI/MMA values were comparable within the different etiologies of critical illness (Supplemental Fig. 3, Supplemental Digital Content 4, http://links.lww.com/CCX/A243; and Supplemental Fig. 4, Supplemental Digital Content 5, http://links.lww.com/CCX/A244 [legend, Supplemental Digital Content 8, http://links.lww.com/CCX/A247]). Furthermore, multivariate analysis including the patients’ age as well as parameters of systemic inflammation (leucocyte count and CRP) and organ dysfunction (bilirubin and hemoglobin), identified the L3SMI as an independent prognostic factor in critically ill patients (Table 2). Nevertheless, both L3SMI, as well as the MMA, were linked to metabolic and/or cardiovascular diseases since diabetics demonstrated higher L3SMI and lower MMA values and the L3SMI/MMA were correlated with cholesterol serum concentrations and NT-proBNP serum levels. While our data clearly argue for a role of the L3SMI and the MMA as prognostic markers in patients undergoing ICU treatment, it remains unclear if these factors might also have a predictive value in ICU patients of different disease etiologies (e.g., trauma patients) or ICU patients receiving different treatment modalities (e.g., extracorporeal membrane oxygenation therapy). Thus, further studies are warranted to not only confirm their prognostic role in the context of critical illness but also to assess if the L3SMI and the MMA could support future biomarker-driven clinical decision algorithms in the multimodal treatment of critical illness. Importantly, our analysis only gave information on the prognosis of these patients but had no predictive value, meaning that it is unclear if those patients with an unfavorable prognosis in terms of their individual L3SMI/MMA levels might have benefitted to a greater extent from a specific therapy or even represent candidates for active symptom control who should not been admitted to the ICU. Furthermore, we only evaluated L3SMI/HU values at ICU admission but longitudinal measurements of L3SMI/MMA could also be important to answer the question whether therapeutic interventions affecting body composition might influence patients’ outcome.
Our data are in line with previous studies demonstrating an association of sarcopenia and an impaired prognosis in patients with nonalcoholic liver disease (18), liver cirrhosis (19), cardiovascular (20), and lung diseases (21). Furthermore, it was demonstrated that sarcopenia might predict outcome of patients undergoing therapeutic interventions such as transcatheter arterial chemoembolization (TACE) (22), liver transplantation (23), or colorectal cancer surgery (24). Within our study, we not only analyzed the association between sarcopenia and patient’s outcome but also of myosteatosis and patients´ outcome. Low skeletal muscle radiodensity is related to the accumulation of fat deposits within muscle (25, 26). It was recently demonstrated that sarcopenia and myosteatosis are independent abnormalities that represent two separate biological processes and that at least in patients with pancreatic and periampullary adenocarcinomas they frequently do not occur coincidentally (27). In contrast, we observed that the L3SMI and the MMA significantly correlated with each other in patients with critical illness (r: 0.168; p = 0.044; Supplemental Fig. 5, Supplemental Digital Content 7, http://links.lww.com/CCX/A246 [legend, Supplemental Digital Content 8, http://links.lww.com/CCX/A247]). Furthermore, the combinational use of the L3SMI and MMA was superior to either marker alone (Fig. 2), highlighting that sarcopenia and myosteatosis might not represent simple prognostic markers but might reflect specific aspects within different diseases.
The pathophysiological relation between sarcopenia/myosteatosis and impaired patients’ prognosis is not fully clear at present. Stretch et al (27) used microarray analysis to identify canonical pathways deregulated in samples from patients with sarcopenia and myosteatosis. Interestingly, in patients with sarcopenia, most differentially regulated genes were part of the antigen presentation pathway, while in patients with myosteatosis genes of the oxidative phosphorylation pathway were most regulated. Additionally, genes of the lipid metabolism pathway, potentially contributing to lipid accumulation (e.g., adiponectin receptor 2, apolipoprotein L1, apolipoprotein L2, apolipoprotein O, and paraoxonase 3), were regulated in myosteatosis but not (or to a much lesser extent) in sarcopenia (27).
As the direct association between sarcopenia and outcome is gaining increasing attention in different clinical conditions, a reliable, cost-effective, and easily applicable method for the evaluation of sarcopenia is essential. Although our method of sarcopenia assessment is easily performed in patients undergoing CT scans for clinical indications, the mere determination of the patients’ skeletal muscle status would not justify a CT scan due to radiation exposure and costs. In this line of thinking, assessment of sarcopenia using ultrasound might represent a noninvasive, radiation-free, and inexpensive alternative. As such, ultrasound measurement of rectus femoris muscle thickness has been suggested as a quick screening test for sarcopenia assessment (28). In addition, different dynamic features of muscle quality such as microcirculation, perfusion during rest and activity, as well as the muscle elasticity could be assessed using ultrasound techniques (29).
Our study was limited by some points. First of all, the study was conducted in an exploratory, single-center design, including a total of n = 155 patients treated a single ICU. Although this design might reduce the inter-hospital bias, our data clearly need further validation from larger multicenter studies. Second, our study included more than 50% of patients fulfilling the criteria for sepsis, which might represent a confounder of results. In addition, the heterogeneity regarding the underlying disease etiology could also influence the results on OS in our study cohort. Finally, our study cohort was heterogeneous in terms of duration of ICU stay, duration of hospital stay, and duration of mechanical ventilation. Although this aspect argues for a rather general validity of results among different ICU patients, larger control studies including further multivariate analyses on confounding parameters on OS are warranted to fully elucidate the role of sarcopenia in better-defined cohorts of ICU patients. Nevertheless, our study is the first to demonstrate a potential prognostic value of myosteatosis in the context of critically ill patients. Furthermore, our study for the first time demonstrates that simultaneous assessment and combined analysis of different parameters of the patients’ individual body composition in critically ill patients might be superior over a single marker for estimating the long-term outcome of these ICU patients. Such data might provide important information for early clinical decision-making on patients in emergency departments or ICUs.
Supplementary Material
Footnotes
Drs. Loosen, Schulze-Hagen, and Püngel shared first authorship. Drs. Tacke, Luedde, Koch, and Roderburg shared last authorship.
Drs. Loosen, Tacke, Koch, and Roderburg designed the study. Dr. Schulze-Hagen performed analysis of body composition. Drs. Loosen, Püngel, Bündgens, and Wirtz performed data acquisition. Drs. Loosen and Püngel performed statistical analysis. Dr. Loosen generated figures and tables. Drs. Loosen and Roderburg drafted the article and performed its revision. Drs. Wirtz, Vucur, Paffenholz, Bruners, Kuhl, Trautwein, Tacke, Luedde, and Koch provided intellectual input. All authors approved the final version of the article.
Supplemental digital content is available for this article. Direct URL citations appear in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Dr. Luedde was funded from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program through the ERC Consolidator Grant Phase Control (Grant Agreement n° 771083). Dr. Luedde was further supported by the German Cancer Aid (Deutsche Krebshilfe 110043 and a Mildred-Scheel-Professorship), the German-Research-Foundation (SFB-TRR57/P06 and LU 1360/3-1), the Ernst-Jung-Foundation Hamburg, the Interdisciplinary Center of Clinical Research (IZKF) Aachen, and a grant from the medical faculty of the Rheinisch-Westfälische Technische Hochschule Aachen.
REFERENCES
- 1.Jaitovich A, Khan MMHS, Itty R, et al. ICU Admission muscle and fat mass, survival, and disability at discharge: A prospective cohort study. Chest. 2019; 155:322–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moisey LL, Mourtzakis M, Cotton BA, et al. ; Nutrition and Rehabilitation Investigators Consortium (NUTRIC). Skeletal muscle predicts ventilator-free days, ICU-free days, and mortality in elderly ICU patients. Crit Care. 2013; 17:R206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ji Y, Cheng B, Xu Z, et al. Impact of sarcopenic obesity on 30-day mortality in critically ill patients with intra-abdominal sepsis. J Crit Care. 2018; 46:50–54 [DOI] [PubMed] [Google Scholar]
- 4.Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: An undiagnosed condition in older adults. Current consensus definition: Prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011; 12:249–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Werf A, Langius JAE, de van der Schueren MAE, et al. Percentiles for skeletal muscle index, area and radiation attenuation based on computed tomography imaging in a healthy Caucasian population. Eur J Clin Nutr. 2018; 72:288–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faron A, Pieper CC, Schmeel FC, et al. Fat-free muscle area measured by magnetic resonance imaging predicts overall survival of patients undergoing radioembolization of colorectal cancer liver metastases. Eur Radiol. 2019; 29:4709–4717 [DOI] [PubMed] [Google Scholar]
- 7.Faron A, Luetkens JA, Schmeel FC, et al. Quantification of fat and skeletal muscle tissue at abdominal computed tomography: Associations between single-slice measurements and total compartment volumes. Abdom Radiol (NY). 2019; 44:1907–1916 [DOI] [PubMed] [Google Scholar]
- 8.Fedorov A, Beichel R, Kalpathy-Cramer J, et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging. 2012; 30:1323–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loosen SH, Roderburg C, Kauertz KL, et al. Elevated levels of circulating osteopontin are associated with a poor survival after resection of cholangiocarcinoma. J Hepatol. 2017; 67:749–757 [DOI] [PubMed] [Google Scholar]
- 10.Herer B. Outcomes of prolonged mechanical ventilation before and after implementation of a respiratory ICU. Respir Care. 2020; 65:1011–1018 [DOI] [PubMed] [Google Scholar]
- 11.Welc SS, Clanton TL. The regulation of interleukin-6 implicates skeletal muscle as an integrative stress sensor and endocrine organ. Exp Physiol. 2013; 98:359–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eslamparast T, Montano-Loza AJ, Raman M, et al. Sarcopenic obesity in cirrhosis-the confluence of 2 prognostic titans. Liver Int. 2018; 38:1706–1717 [DOI] [PubMed] [Google Scholar]
- 13.Montano-Loza AJ, Meza-Junco J, Baracos VE, et al. Severe muscle depletion predicts postoperative length of stay but is not associated with survival after liver transplantation. Liver Transpl. 2014; 20:640–648 [DOI] [PubMed] [Google Scholar]
- 14.Montano-Loza AJ, Angulo P, Meza-Junco J, et al. Sarcopenic obesity and myosteatosis are associated with higher mortality in patients with cirrhosis. J Cachexia Sarcopenia Muscle. 2016; 7:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merli M, Berzigotti A, Zelber-Sagi S, et al. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J Hepatol. 2019; 70:172–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Budczies J, Klauschen F, Sinn BV, et al. Cutoff Finder: A comprehensive and straightforward Web application enabling rapid biomarker cutoff optimization. PLoS One. 2012; 7:e51862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schefold JC, Bierbrauer J, Weber-Carstens S. Intensive care unit-acquired weakness (ICUAW) and muscle wasting in critically ill patients with severe sepsis and septic shock. J Cachexia Sarcopenia Muscle. 2010; 1:147–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koo BK, Kim D, Joo SK, et al. Sarcopenia is an independent risk factor for non-alcoholic steatohepatitis and significant fibrosis. J Hepatol. 2017; 66:123–131 [DOI] [PubMed] [Google Scholar]
- 19.Buchard B, Boirie Y, Cassagnes L, et al. Assessment of malnutrition, sarcopenia and frailty in patients with cirrhosis: Which tools should we use in clinical practice? Nutrients. 2020; 12:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santana NM, Mendes RML, Silva NFD, et al. Sarcopenia and sarcopenic obesity as prognostic predictors in hospitalized elderly patients with acute myocardial infarction. Einstein (Sao Paulo). 2019; 17:eAO4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirai K, Tanaka A, Homma T, et al. Comparison of three frailty models and a sarcopenia model in elderly patients with chronic obstructive pulmonary disease. Geriatr Gerontol Int. 2019; 19:896–901 [DOI] [PubMed] [Google Scholar]
- 22.Loosen SH, Schulze-Hagen M, Bruners P, et al. Sarcopenia is a negative prognostic factor in patients undergoing transarterial chemoembolization (TACE) for hepatic malignancies. Cancers (Basel). 2019; 11:1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Czigany Z, Kramp W, Bednarsch J, et al. Myosteatosis to predict inferior perioperative outcome in patients undergoing orthotopic liver transplantation. Am J Transplant. 2020; 20:493–503 [DOI] [PubMed] [Google Scholar]
- 24.Nakanishi R, Oki E, Sasaki S, et al. Sarcopenia is an independent predictor of complications after colorectal cancer surgery. Surg Today. 2018; 48:151–157 [DOI] [PubMed] [Google Scholar]
- 25.Stephens NA, Skipworth RJ, Macdonald AJ, et al. Intramyocellular lipid droplets increase with progression of cachexia in cancer patients. J Cachexia Sarcopenia Muscle. 2011; 2:111–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aubrey J, Esfandiari N, Baracos VE, et al. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol (Oxf). 2014; 210:489–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stretch C, Aubin JM, Mickiewicz B, et al. Sarcopenia and myosteatosis are accompanied by distinct biological profiles in patients with pancreatic and periampullary adenocarcinomas. PLoS One. 2018; 13:e0196235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rustani K, Kundisova L, Capecchi PL, et al. Ultrasound measurement of rectus femoris muscle thickness as a quick screening test for sarcopenia assessment. Arch Gerontol Geriatr. 2019; 83:151–154 [DOI] [PubMed] [Google Scholar]
- 29.Ivanoski S, Vasilevska Nikodinovska V. Future ultrasound biomarkers for sarcopenia: Elastography, contrast-enhanced ultrasound, and speed of sound ultrasound imaging. Semin Musculoskelet Radiol. 2020; 24:194–200 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


