Abstract
Background
The use of chest CT for COVID-19 diagnosis or triage in healthcare settings with limited SARS-CoV-2 PCR capacity is controversial. CO-RADS categorization of the level of COVID-19 suspicion might improve diagnostic performance.
Purpose
To investigate the value of chest CT with CO-RADS classification to screen for asymptomatic SARS-CoV-2 infections and to determine its diagnostic performance in individuals with COVID-19 symptoms during the exponential phase of viral spread.
Materials and Methods
In this secondary analysis of a prospective trial (Clinical Trial Number: IRB B1172020000008), from March 2020 to April 2020, we performed parallel SARS-CoV-2 PCR and CT with categorization of COVID-19 suspicion by CO-RADS, for individuals with COVID-19 symptoms and controls without COVID-19 symptoms admitted to the hospital for medical urgencies unrelated to COVID-19. CT-CORADS was categorized on a 5-point scale from 1 (very low suspicion) to 5 (very high suspicion). AUC were calculated in symptomatic versus asymptomatic individuals to predict positive SARS-CoV-2 positive PCR and likelihood ratios for each CO-RADS score were used for rational selection of diagnostic thresholds.
Results
859 individuals (median 70 years, IQR 52-81, 443 men) with COVID-19 symptoms and 1138 controls (median 68 years, IQR 52-81, 588 men) were evaluated. CT-CORADS had good diagnostic performance (P<.001) in both symptomatic (AUC=.89) and asymptomatic (AUC=.70) individuals. In symptomatic individuals (41.7% PCR+), CO-RADS ≥ 3 detected positive PCR with high sensitivity (89%, 319/358) and 73% specificity. In asymptomatic individuals (5.3% PCR+), a CO-RADS score ≥ 3 detected SARS-CoV-2 infection with low sensitivity (45%, 27/60) but high specificity (89%).
Conclusion
CT-CORADS had good diagnostic performance in symptomatic individuals, supporting its application for triage. Sensitivity in asymptomatic individuals was insufficient to justify its use as first-line screening approach. Incidental detection of CO-RADS ≥ 3 in asymptomatic individuals should trigger testing for respiratory pathogens.
Summary
Categorization of COVID-19 suspicion by CO-RADS CT has good diagnostic performance in individuals with or without symptoms. While CT screening for asymptomatic SARS-CoV-2 infections is not recommended, incidental findings of CO-RADS ≥ 3 in asymptomatic individuals have sufficient positive predictive value to trigger SARS-CoV-2 PCR reflex testing.
Key Results
■ CT with structured CO-RADS scoring has good diagnostic performance for COVID-19 pneumonia in both symptomatic (AUC=0.89) and asymptomatic (AUC=0.70) individuals (P<0.001).
■ In symptomatic individuals (42% PCR+), CO-RADS ≥ 3 detected positive PCR with acceptable sensitivity (89%) and specificity (73%) resulting in PPV of 70%.
■ In asymptomatic individuals (5% PCR+), CO-RADS ≥ 3 detected SARS-CoV-2 infection with low sensitivity (45%) but high specificity (89%) and PPV of 18%.
Introduction
Chest CT can help determine the temporal disease stage and severity of COVID-19 pneumonia 1-3. In the early stage of viral replication (day 0-4) ground-glass opacities are the predominant lesion. In the progressive stage (day 5-8), crazy paving patterns mark the increased recruitment of inflammatory cells to the lung interstitium. Peak stage (day 10-13) is marked by consolidation with fibrosis and diffuse alveolar damage. These radiological lesions are also observed in other viral pneumonia and non-infectious inflammatory lung diseases but in a pandemic context might harbor diagnostic potential for SARS-CoV-2 infection especially for patient triage. The reference method for COVID-19 diagnosis, SARS-CoV-2 PCR, is highly specific but has variable sensitivity as low as 70% 4. In health care settings with limited PCR capacity and long turnaround times, chest CT was proposed as alternative for COVID-19 diagnosis or triage 5. Studies supporting chest CT as first-line diagnostic tool for COVID-19 showed several methodological concerns 6-8. Most studies were underpowered, showed major selection biases including only individuals with COVID-19 symptoms and 40%-50% a priori risk of SARS-CoV-2 infection and used binary scoring of CT without standardized definition of COVID-19-compatible CT. Weighed against the cost and procedural risks of CT, this sparked a controversy 8,9 leading to consensus statements by the Centers for Disease Control and Prevention, the American College of Radiology, the Society of Thoracic Radiology, the American Society of Emergency Radiology, The Fleischner Society, and the Radiological Society of North America (RSNA), opposing CT as first-line COVID-19 diagnostic tool 10-13.
In this report, we studied the diagnostic power of chest CT versus SARS-CoV-2 PCR using COVID-19 Reporting and Data System classification system (CO-RADS) 14. CO-RADS was developed by the Dutch Radiological Society to categorize the level of suspicion for COVID-19 pneumonia. It generally aligns with the structured reporting recommended by the RNSA 13, scoring the level of COVID-19 suspicion on a scale of 1 to 5, with CO-RADS 1 corresponding to ‘negative’ category, CO-RADS 2 to ‘Atypical’, CO-RADS 3 and 4 corresponding to ‘Indeterminate’ with ‘lower’ or ‘higher likelihood’, and CO-RADS 5 equaling the RNSA ‘Typical’ category.
The purpose of this study was to investigate the value of chest CT with CO-RADS classification to screen for asymptomatic SARS-CoV-2 infections and to determine its diagnostic performance in individuals with COVID-19 symptoms during the exponential phase of viral spread. These data should allow a more evidence-based definition of the possible role of chest CT in COVID-19 triage.
Materials and Methods
Participants
This is a secondary analysis of a single-center prospective trial on consecutive individuals admitted to AZ Delta General Hospital in Roeselare, Belgium from March 19, 2020 to April 20, 2020. AZ Delta General Hospital is a central-network regional hospital that provides tertiary healthcare for a community of 500,000 inhabitants. Inclusion criteria: as part of the medical board-approved triage policy for COVID-19 quarantining, all individuals admitted to the hospital with clinical suspicion of COVID-19 pneumonia (hence ‘symptomatic individuals’) and individuals without COVID-19 symptoms but admitted for other medical urgencies, scheduled surgery or medical procedures and psychiatric or geriatric care (hence ‘asymptomatic individuals’), received a combined screening with chest CT and SARS-CoV-2 PCR within a 24-hour time frame. We used the COVID-19 case definition as specified by the World Health Organization (WHO) interim guidance of February 27, 202015 for classifying symptomatic individuals. Exclusion criteria: children < 14 years of age and pregnant individuals without COVID-19 symptoms did not receive standard chest CT. The study was approved by the AZ Delta Institutional Review Board with a waiver of written informed consent from study participants considering the study is based on secondary analysis of existing data (Clinical Trial Number: IRB B1172020000008, study protocol available through the registry of the Belgian Advisory Committee on Bioethics and email request to corresponding author). Authors received no specific funding for this study.
CT protocol
Within 24h from admission all individuals were imaged by multi-detector CT using either GE LightSpeed VCT scanner (1-mm slice thickness), Siemens Somatom AS (1-mm slice thickness) or the GE Optmima 660 scanner (1.25-mm slice thickness). All scans were performed without intravenous contrast with the patient in the supine position during end-inspiration.
Image evaluation
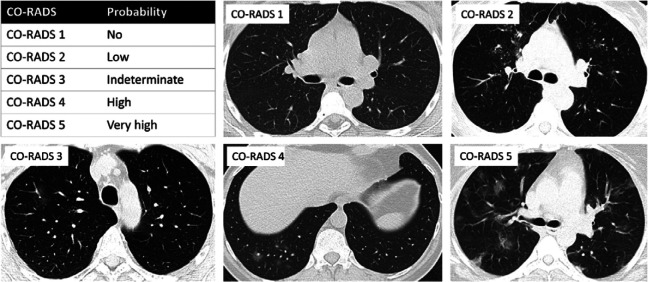
Two cardiothoracic radiologists with 24 and 9 years of experience (Gryspeerdt S., De Smet K.) retrospectively reviewed the CT exams on a PACS workstation (IDS7, Sectra) with multiplanar reconstruction tools. Reviewers were blinded to (a)symptomatic status and PCR result. Final CO-RADS scoring was always reached by consensus. The Dutch CO-RADS (COVID-19 Reporting and Data System) classification system was used to categorize the level of COVID-19 suspicion, exactly as described 14: CO-RADS score ranges from 1 (very low level of suspicion), 2 (low level), 3 (equivocal), 4 (high level of suspicion) to 5 (very high level of suspicion) (summarized and representative images in Fig. 3). See Appendix E1 for detailed CT protocol.
Figure 3:
Illustration of CO-RADS scoring system for level of COVID-19 pneumonia suspicion. COVID-19 Reporting and Data System classification system (CO-RADS) scores the level of COVID-19 pneumonia suspicion as summarized in the upper left panel. The other panels show representative scans for CO-RADS 1 (no suspicion: normal findings), CO-RADS 2 (low level of suspicion: absence of ground glass opacities (GGO), presence of tree-in-bud signs/endobronchial spread/bronchiolitis), CO-RADS 3 (indeterminate: unifocal GGO), CO-RADS 4 (high level of suspicion: unilateral multifocal GGO) and CO-RADS 5 (very high level of suspicion: multifocal bilateral GGO).
Comorbidities
were recorded by chest CT (chronic lung disease including emphysema, fibrosis and bronchiectasis and coronary artery disease as derived from coronary artery calcification scoring) or review of medical records (diabetes).
SARS-CoV-2 PCR
was done with multiplex RT-PCR (hence PCR) for E/N/RdRP genes using AllplexTM 2019-nCoV assay (Seegene Inc, Seoul, Korea) on nasopharyngeal swabs.
Statistical analysis
The diagnostic performance of categorical CT-assessment by CO-RADS classification (CT-CORADS) was evaluated by calculating area (AUC) under the receiver operating characteristics (ROC) curve compared to SARS-CoV-2 PCR positivity. Likelihood ratios (LR, 95%CI) were calculated for each CO-RADS score in the symptomatic versus the asymptomatic group and visualized in diagrams of pre/post-test probability. According to Bayes’ theorem, post-test probability (Ppost) can be derived from pre-test probability (Ppre) and LR according to the formula Ppost = (Ppre x LR) / (1 + Ppre x (LR-1)) where Ppre represents the prevalence of SARS-CoV-2 PCR-positivity in any population under study. Statistical differences in demographics and comorbidities were evaluated by Mann-Whitney test (age) and Chi-squared test (proportions). Statistical analyses were performed using MedCalc (version 12.2.1, MedCalc Software, Mariakerke, Belgium) and considered significant if P value was less than .05.
Results
Participant Characteristics
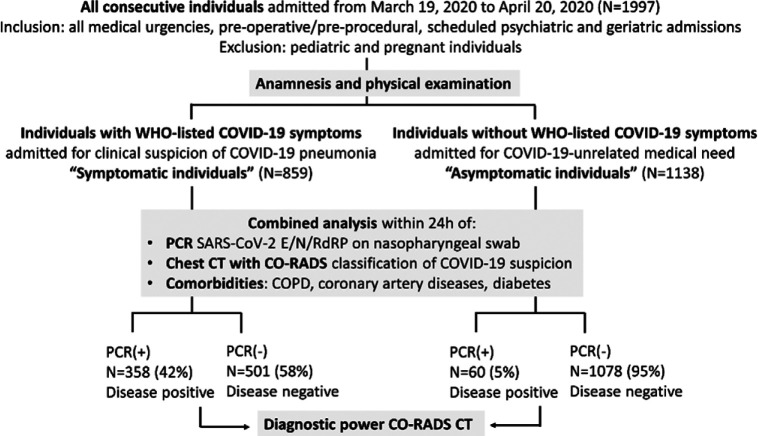
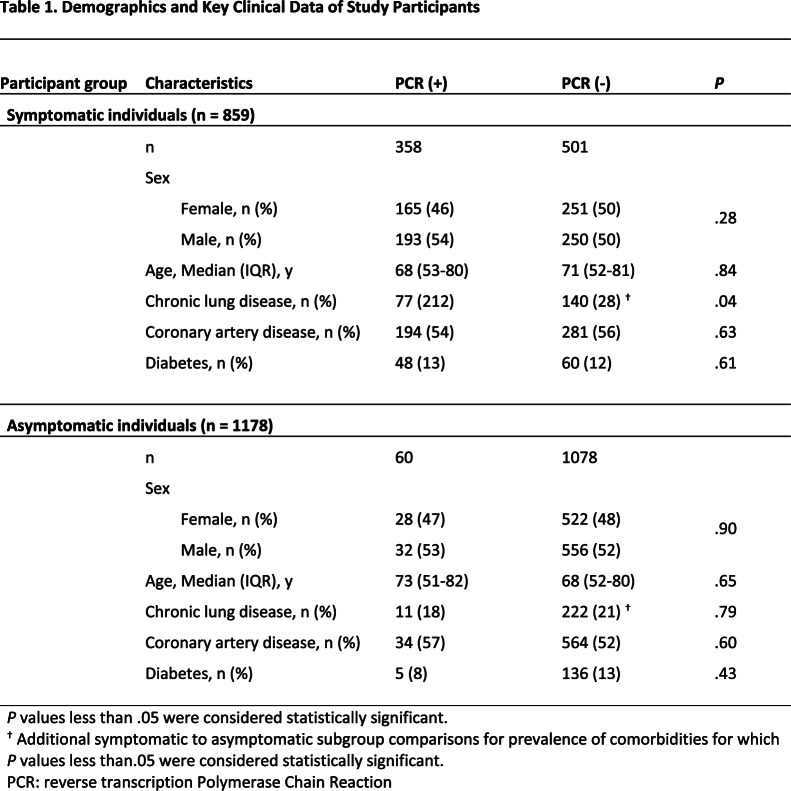
A total of 1997 consecutive individuals (flowchart Fig. 1) admitted to the hospital were allocated by physical examination and anamnesis into two groups. First, 859 individuals were admitted with WHO-listed symptoms of COVID-19 pneumonia (hence ‘symptomatic individuals’): 443 males (median age 71 years, IQR 54-80 years) and 416 females (median age 68 years, IQR 51-82 years) (Table 1). Second, 1138 individuals were admitted for medical needs unrelated to WHO-listed COVID-19 symptoms (hence ‘asymptomatic individuals’): 588 males (median age 66 years, IQR 53-78 years) and 550 females (median age 70 years, IQR 50-82 years). Demographics and key clinical comorbidities are shown in Table 1: individuals with or without COVID-19 symptoms showed a similar age- and sex-distribution and a similar prevalence of diabetes and coronary artery disease (Table 1). PCR-negative symptomatic individuals had higher rates of underlying chronic lung disease (27.9%, 140 of 501) than PCR-positive symptomatic (21.5%, 77 of 358, P<0.05) and PCR-negative asymptomatic individuals (20.6%, 222 of 1078, P<0.05).
Figure 1:
Flow diagram of study. Abbreviations: COVID-19 Reporting and Data System classification system; PCR: reverse phase Polymerase Chain Reaction test for SARS-CoV-2 viral RNA sequences E/N/RdRP; WHO: World Health Organization.
Table 1:
Demographics and Key Clinical Data of Study Participants
Diagnostic performance in symptomatic individuals
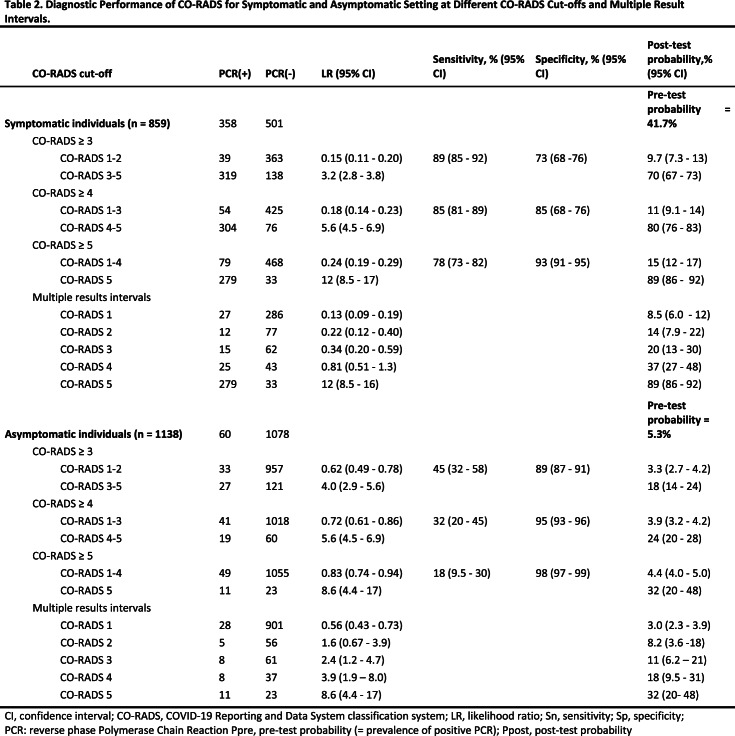
The overall prevalence of SARS-CoV-2 infection in symptomatic individuals was 41.7% (358 of 859). In symptomatic individuals with CO-RADS 5, 89.4% (279 of 312) were PCR+ as compared to only 8.6% (27 of 313) PCR+ cases in symptomatic individuals with CO-RADS 1. ROC analysis confirmed the diagnostic performance (P<0.001) of CT-CORADS with AUC = .89 (95%CI .87-.91) to predict SARS-CoV-2 PCR-positivity (Fig. 2A). Next we calculated likelihood ratios (LR) for each CO-RADS score in symptomatic individuals (Table 2): CORADS 1, 2 and also the ‘equivocal’ score CORADS 3 (LR=0.34, 95%CI 0.20-0.59) significantly lowered the odds of PCR positivity (confidence interval of LR excluding LR=1). CO-RADS 4 did not further increase post-test probability. CO-RADS 5, however, strongly increased the odds of a positive PCR (LR=11.8 95%CI 8.5-16.5) (Fig. 2B). A CO-RADS 5 score in symptomatic individuals identified SARS-CoV-2 PCR positivity with a sensitivity of 77.9% (95%CI 73.3-82.1) at high specificity of 93.4% (95%CI 90.9-95.4) and high overall accuracy of 87.0% (95%CI 84.5-89.1). Dichotomization of suspected CT at CO-RADS ≥ 4 and ≥ 3 increased sensitivity to 84.3% (95%CI 80.8-88.5) and 89.1% (95%CI 85.4-92.1) at a specificity of 84.8% (95%CI 68.3-76.3) and 72.5% (95%CI 68.3-76.3) respectively (Table 2). Table 2 and Fig.2B show the associated shift from pre-test probability (overall prevalence of positive PCR) to post-test probability (positive predictive value) of SARS-CoV-2 as function of individual CO-RADS scores or dichotomizations.
Figure 2a:
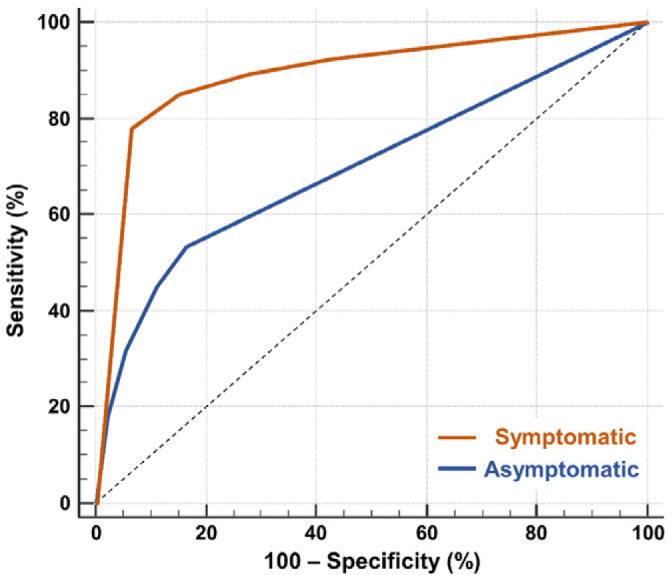
Diagnostic performance of CT-CORADS scoring in individuals with and without COVID-19 symptoms. A, The area under the receiver operating characteristics curve (AUC) of CT-CORADS to predict a positive SARS-CoV-2 PCR result in symptomatic (red line) and asymptomatic (blue line). The diagonal dashed line indicates no discrimination. B, Post-test probability of positive PCR as function of the pre-test probability for different likelihood ratios (LR) associated with the indicated CO-RADS score in 859 symptomatic individuals. The arrow indicates the pre-test probability as determined by overall prevalence of positive PCR (41.7%) in this sample. C, Post-test probability of positive PCR as function of the pre-test probability for different likelihood ratios (LR) associated with the indicated CO-RADS score in 1138 asymptomatic individuals. The arrow indicates the pre-test probability as determined by overall prevalence of positive PCR (5.2%) in this sample.
Table 2:
Diagnostic Performance of CO-RADS for Symptomatic and Asymptomatic Setting at Different CO-RADS Cut-offs and Multiple Result Intervals.
Figure 2b:
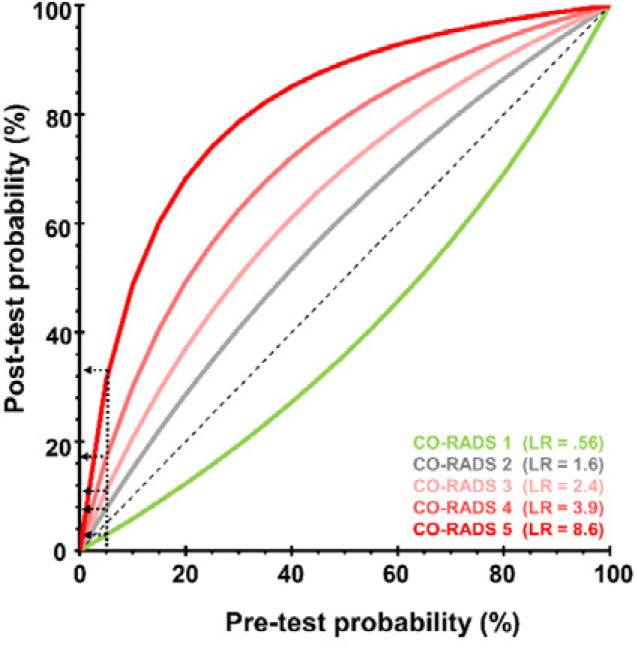
Diagnostic performance of CT-CORADS scoring in individuals with and without COVID-19 symptoms. A, The area under the receiver operating characteristics curve (AUC) of CT-CORADS to predict a positive SARS-CoV-2 PCR result in symptomatic (red line) and asymptomatic (blue line). The diagonal dashed line indicates no discrimination. B, Post-test probability of positive PCR as function of the pre-test probability for different likelihood ratios (LR) associated with the indicated CO-RADS score in 859 symptomatic individuals. The arrow indicates the pre-test probability as determined by overall prevalence of positive PCR (41.7%) in this sample. C, Post-test probability of positive PCR as function of the pre-test probability for different likelihood ratios (LR) associated with the indicated CO-RADS score in 1138 asymptomatic individuals. The arrow indicates the pre-test probability as determined by overall prevalence of positive PCR (5.2%) in this sample.
Screening potential of chest-CT in asymptomatic individuals in a SARS-CoV-2 pandemic setting
The prevalence of SARS-CoV-2 PCR-positivity (pre-test probability), in asymptomatic individuals was 5.3% (60 of 1138). 6.9% (79 of 1138) of asymptomatic individuals showed a CO-RADS score of 4 (high suspicion) or 5 (very high suspicion), 87.0% (990 of 1138) showed a CO-RADS score ≤ 2 with low to very low suspicion of COVID-19 (Table 2). ROC analysis indicated that CT-CORADS in asymptomatic individuals had diagnostic performance (P<0.001) to predict SARS-CoV-2 PCR-positivity with AUC = .70 (95%CI .67-.73) (Fig. 2A), albeit less than in symptomatic individuals. The percentage of PCR-positive cases was 3.0%, 8.2%, 11.6%, 17.8% and 32.4% in CO-RADS 1, 2, 3, 4 and 5, respectively. Analysis of LR (Table 2, Fig. 2C) indicated that only CO-RADS 1 could significantly lower the odds of a positive PCR (LR=0.56, 95%CI 0.43-0.73), that CO-RADS 2 had no diagnostic meaning with the 95% CI encompassing LR=1 and that CO-RADS 3 and higher, chest CT increased the odds of a positive PCR, resulting in a positive shift from pre- to post-test probability (Fig. 2C). In particular CO-RADS 5 had good diagnostic performance in asymptomatic individuals, with LR =8.6 (95%CI 4.4-17), predicting SARS-CoV-2 infection at high specificity of 97.9% (95%CI 96.8-98.6) but low sensitivity of 18.3% (95%CI 9.5-30). Dichotomization of suspected CT at CO-RADS ≥ 4 preserved a high specificity of 94.4% (95%CI 93-96) resulting in a post-test probability (positive predictive value) of 24.1% (95% CI 20-28) (Table 2, Fig. 2C). Its sensitivity was low at 31.7% (95%CI 20-45) resulting in a negligible shift in pre- to post-test probability in case of a negative test result (3.9%, 95% CI 3.2-4.2), arguing against the use of chest CT as a screening test for asymptomatic infections.
Figure 2c:
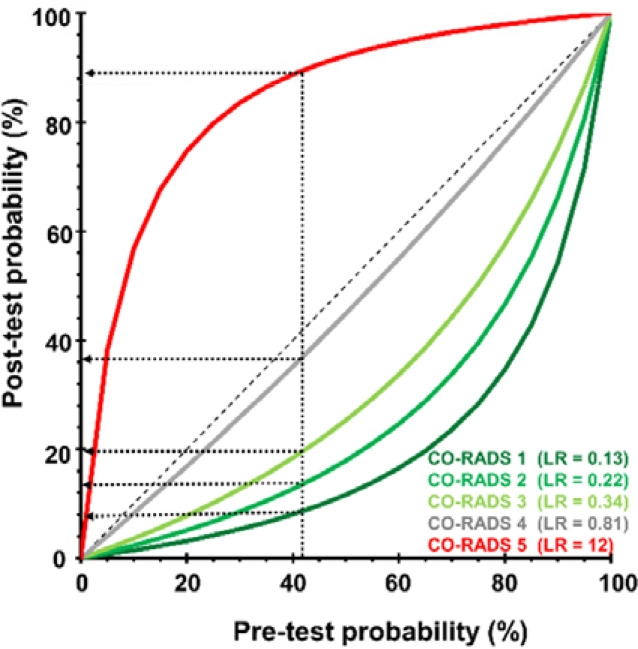
Diagnostic performance of CT-CORADS scoring in individuals with and without COVID-19 symptoms. A, The area under the receiver operating characteristics curve (AUC) of CT-CORADS to predict a positive SARS-CoV-2 PCR result in symptomatic (red line) and asymptomatic (blue line). The diagonal dashed line indicates no discrimination. B, Post-test probability of positive PCR as function of the pre-test probability for different likelihood ratios (LR) associated with the indicated CO-RADS score in 859 symptomatic individuals. The arrow indicates the pre-test probability as determined by overall prevalence of positive PCR (41.7%) in this sample. C, Post-test probability of positive PCR as function of the pre-test probability for different likelihood ratios (LR) associated with the indicated CO-RADS score in 1138 asymptomatic individuals. The arrow indicates the pre-test probability as determined by overall prevalence of positive PCR (5.2%) in this sample.
Representative clinical images
A summary of the CO-RADS scoring system and representative CT images for CO-RADS 1 to 5 are shown in Fig. 3. Fig. 4 to 6 highlight individual cases with a brief clinical summary of: (i) a false positive CO-RADS 5 in a PCR-negative symptomatic individual (Fig. 4), (ii) false negative CO-RADS 1 in a PCR-positive symptomatic individual (Fig. 5) and (iii) true positive CO-RADS 5 in a PCR-positive asymptomatic individual.
Figure 4:
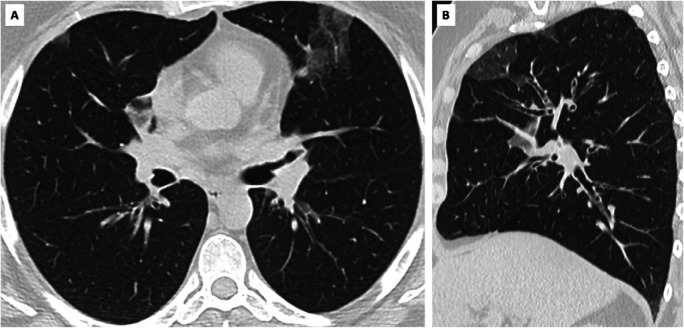
False positive CO-RADS 5 in SARS-CoV-2 PCR-negative symptomatic individual. A, axial and, B, sagittal CT scan of symptomatic individual with CO-RADS 5 but negative SARS-CoV-2 PCR test. Clinical summary: a 49 year old woman with medical history of haemochromatosis and psoriatic arthritis was admitted with wheezing, dry cough and increasing dyspnea since 2 weeks. She was subfebrile and hypoxic (89% SpO2). Blood testing showed increased CRP (32.8 mg/L) and leukocytosis with eosinophilia (1.1 x 10e3/µl). CT showed no pleural effusion but presence of multifocal bilateral ground glass opacities, scored as CO-RADS 5. SARS-CoV-2 PCR was repeatedly negative on nasopharyngeal swab. Extended syndromic PCR testing for 33 respiratory pathogens including 14 respiratory viruses was negative. Bronchoalveolar lavage was also repeatedly negative for SARS-CoV-2 PCR but showed high load of eosinophils (52 % of 65 x 10e4 nucleated cells/mL) supporting the diagnosis of acute eosinophilic pneumonia. The woman was successfully treated with corticosteroids.
Figure 5:
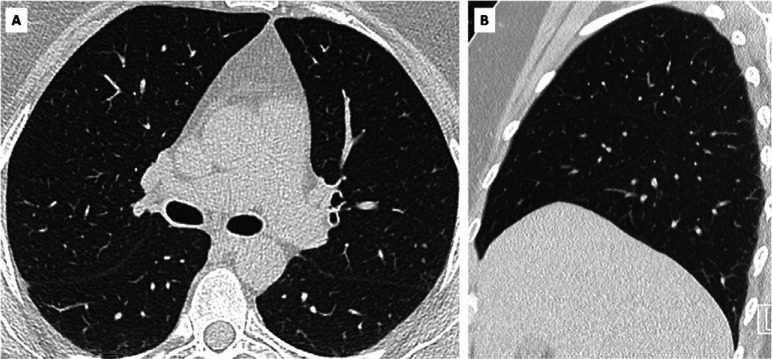
False negative CO-RADS 1 in SARS-CoV-2 PCR-positive symptomatic individual. A, axial and, B, sagittal CT scan of symptomatic individual with CO-RADS 1 and positive SARS-CoV-2 PCR test. Clinical summary: 57-year old woman presented headache, flu-like symptoms and dry cough for more than 10 days since returning from Hanoi, Vietnam. She was subfebrile and blood testing showed slightly increased CRP (5.3 mg/L), normal leukocyte count, no lymphocytopenia and normal D-dimers and LDH. Chest CT showed no abnormalities. PCR for Influenza A/B and RSV was negative but SARS-CoV-2 PCR was positive.
Figure 6:
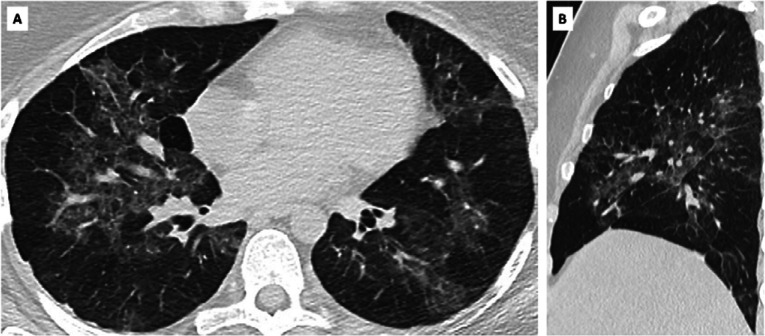
True positive CO-RADS 5 in SARS-CoV-2 PCR-positive asymptomatic individual. A, axial and, B, sagittal CT scan of asymptomatic individual with CO-RADS 5 and positive SARS-CoV-2 PCR. Clinical summary: a 31 year-old woman was admitted with diarrhea and left iliac fossa pain. She presented no respiratory symptoms, myalgia, loss of taste or smell or abnormal fatigue. Fever (39.4%) was attributed to suspected diverticulitis but a CT abdomen was negative. Standard chest CT scan as part of COVID-19 infection control policy showed multifocal bilateral ground glass opacities and crazy paving pattern, scored as CO-RADS 5. Blood testing showed increased CRP (48.4 mg/L), normal leukocyte count (6.8 x 10e3/μl) and no lymphocytopenia but increased D-dimers (1428 ng/mL) and increased LDH (669 U/L).
Discussion
We aimed to investigate the performance of CT-CORADS to diagnose SARS-CoV-2 PCR-positivity in individuals with COVID-19 symptoms and to screen for asymptomatic SARS-CoV-2 infection in control individuals in a setting with high prevalence of SARS-CoV-2 infections. In symptomatic patients, the pre-test probability of SARS-CoV-2 infection, as marked by the prevalence of PCR-positivity, was high at 41.7%. A CO-RADS score ≥ 3 strongly increased post-test probability to 69.8% and CO-RADS 5 even to 89.4%. For infection control policies, CO-RADS 5 could thus be used as triage tool to quarantine symptomatic individuals in settings with bottlenecks in PCR testing. Yet, CO-RADS < 3 was still associated with a post-test probability of 9.7% (corresponding to a 90.3% negative predictive value) indicating that chest CT cannot replace PCR as diagnostic test. In our asymptomatic controls, prevalence of SARS-CoV-2 PCR-positivity was 5.3%, in line with the secondary attack rate at population level of 6.6% during the exponential phase of viral spread 16. This control group was thus suitable to investigate if chest CT can screen for asymptomatic SARS-CoV-2 infection. Also in asymptomatic individuals CT-CORADS showed good diagnostic performance. However, various dichotomization scenarios failed to reach the high sensitivity required for a screening test. CO-RADS ≥ 4 attained only 31.7% sensitivity. A negative test (CO-RADS <4) shifted pre- to post-test probability only from 5.3% to 3.9%, insufficient to justify the procedural risk of CT. The specificity of CO-RADS ≥ 4 in asymptomatic individuals, however, was high (94.4%) and resulted in meaningful increase in post-test probability to 24.1%. In a pandemic setting, we propose that such incidental findings should be reported as ‘compatible with COVID-19 pneumonia’ rather than as ‘viral pneumonia’ as suggested by the RNSA 13 and should trigger SARS-CoV-2 PCR or syndromic panel-based PCR testing for other respiratory pathogens before exclusion of non-infectious inflammatory lung diseases.
The developers of CO-RADS reported a good diagnostic performance in a pilot study on 105 individuals with COVID-19 symptoms and 50.5% PCR-positivity with AUC under the ROC curve of 0.91 (95% CI 0.85-0.97) 14. Our study confirms this with similar AUC on a much larger sample. As compared with previous studies supporting chest CT for COVID-19 diagnosis or screening 3,17,18,19, our study answered the urgent call for well-powered data sets 18 and its prospective design on consecutive, unselected individuals with similar demographics, comorbidities and upfront clinical grouping according to absence or presence of COVID-19 symptoms, minimizes selection biases. Another strength is the use of structured reporting of chest CT data and the attribution of likelihood ratios (LR) to each level of suspicion. Most studies thus far used dichotomization of CT results as positive or negative, often without a clear definition of a positive CT. One large study in China reported a 97% sensitivity of chest CT for COVID-19 diagnosis but with a poor specificity of 25% 3, possibly explained by a low subjective interpretation threshold to maximize sensitivity 19. Like sensitivity and specificity, LR are test properties that, in defined patient populations, are independent of disease prevalence. The actual clinical values of a positive test result to confirm or negative test result to rule-out disease, the positive (PPV) and negative (NPV) predictive value respectively, strongly depend on disease prevalence 19. Using LR, the post-test probability as indicator of PPV can simply be calculated (formula in Methods) taking the observed prevalence of PCR-positivity as pre-test probability. Similarly, NPV is 1 minus the post-test probability. PPV is mathematically most influenced by specificity 19. Meta-analysis showed a low pooled specificity of dichotomic chest CT of 37% for COVID-19 diagnosis 20 with low associated PPV from 1.5%-8.3% in low prevalence (<10%) settings. Our data illustrate that CO-RADS categorization improves specificity and thus discloses higher PPV as LR increase. NPV is mostly influenced by sensitivity 19. In our data set, sensitivity of chest CT was insufficient to exclude SARS-CoV-2 infection both in symptomatic and asymptomatic patients. This supports the consensus statements that chest CT should not be used as diagnostic test.
Our study has limitations. It was conducted in a time frame with high rates of SARS-CoV-2 infections and low prevalence of other viral pneumonia. Higher incidence of seasonal respiratory viral infections will likely decrease specificity of CT-CORADS. Selection bias: study included mostly individuals older than 50 years attending the hospital and excluded pediatric and pregnant individuals. Paucisymptomatic infections in home-quarantined older individuals and asymptomatic infections in younger individuals are underrepresented in our data set.
In conclusion, our data show that CT with structured CO-RADS scoring had good diagnostic performance for COVID-19 pneumonia but cannot replace SARS-CoV-2 PCR as diagnostic test. It can be used as alternative triage tool in individuals with COVID-19 symptoms but not for the screening of asymptomatic SARS-CoV-2 infections.
Declaration of Interests and Source of Funding Statements
The authors declare no conflict of interest. This work was supported by a donation from board members of Fagron (Nazareth, Belgium), a healthcare company, to RADar, the teaching and education initiative of AZ Delta General Hospital, to be used as unconditional research grant for data collection and open access publication. The sponsor had no influence on the study design, data interpretation and drafting of the manuscript.
Acknowledgments
Acknowledgements
The authors thank Hendrik Verelst, Fien Trenson, Ludovic Cruyt and Jonas De Melio for expert interpretation of chest CT and data contribution to consensus CO-RADS scoring and Boris Keppens for helpful discussions.
K.D.S. and D.D.S. contributed equally to this article.
IRB Clinical Trial Number: B1172020000008
Study design: retrospective secondary analysis of prospective trial
Abbreviations:
- CORADS
- COVID-19 Reporting and Data System
- COVID-19
- Coronavirus disease 2019
- LR
- likelihood ratio
- RT-PCR
- reverse transcription polymerase chain reaction
References
- 1.Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis 2020; 20(4): 425-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernheim A, Mei X, Huang M, et al. Chest CT Findings in Coronavirus Disease-19 (COVID-19): Relationship to Duration of Infection. Radiology 2020: 200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ai T, Yang Z, Hou H, et al. Correlation of Chest CT and RT-PCR Testing in Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology 2020: 200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arevalo-Rodriguez I, Buitrago-Garcia D, Simancas-Racines D, et al. False-negative results of initial RT-PCR assays for COVID-19: a systematic review. medRXIV 10.1101/2020.04.16.20066787 [DOI] [PMC free article] [PubMed]
- 5.Chinese National Health Commission , Chinese Clinical Guidance for COVID-19 Pneumonia Diagnosis and Treatment (7th Edition). 2020. [Google Scholar]
- 6.Hope MD, Raptis CA, Henry TS. Chest Computed Tomography for Detection of Coronavirus Disease 2019 (COVID-19): Don't Rush the Science. Ann Intern Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raptis CA, Hammer MM, Short RG, et al. Chest CT and Coronavirus Disease (COVID-19): A Critical Review of the Literature to Date. AJR Am J Roentgenol 2020: 1-4. [DOI] [PubMed] [Google Scholar]
- 8.Hope MD, Raptis CA, Shah A, Hammer MM, Henry TS, six s. A role for CT in COVID-19? What data really tell us so far. Lancet 2020; 395(10231): 1189-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang Y, Cheng W, Zhao N, Qu H, Tian J. CT screening for early diagnosis of SARS-CoV-2 infection. Lancet Infect Dis 2020: 2020 Mar 26;S1473-3099(20)30241-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American College of Radiology (ACR). ACR recommendations for the use of chest radiography and computed tomography (CT) for suspected COVID-19 infection. ACR website. www.acr.org/Advocacy-and-Economics/ACR-Position-Statements/Recommendations-for-Chest-Radiography-and-CT-for-Suspected-COVID19-Infection. Updated March 22 2020. Accessed May 4, 2020. [Google Scholar]
- 11.American Society of Emergency Radiology (ASER). ASER COVID-19 Task Force: FAQs. ASER website. www.aser.org/covid-19-faqs/. Published March 11 2020. Accessed May 5, 2020.
- 12.Rubin GD, Ryerson CJ, Haramati LB, et al. The Role of Chest Imaging in Patient Management During the COVID-19 Pandemic: A Multinational Consensus Statement From the Fleischner Society. Chest 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simpson S, Kay FU, Abbara S, et al. Radiological Society of North America Expert Consensus Statement on Reporting Chest CT Findings Related to COVID-19. Endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA. J Thorac Imaging 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prokop M, van Everdingen W, van Rees Vellinga T, et al. CO-RADS - A categorical CT assessment scheme for patients with suspected COVID-19: definition and evaluation. Radiology 2020: 201473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization Global surveillance for COVID-19 disease caused by human infection with novel coronavirus (COVID-19): interim guidance, 27 February 2020. World Health (WHO/2019-nCoV/SurveillanceGuidance/2020.4). 2020. [Google Scholar]
- 16.Bi Q, Wu Y, Mei S, et al. Epidemiology and transmission of COVID-19 in 391 cases and 1286 of their close contacts in Shenzhen, China: a retrospective cohort study. Lancet Infect Dis 2020: 2020 Apr 27;S1473-3099(20)30287-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fang Y, Zhang H, Xie J, et al. Sensitivity of Chest CT for COVID-19: Comparison to RT-PCR. Radiology 2020: 200432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun Z, Zhang N, Li Y, Xu X. A systematic review of chest imaging findings in COVID-19. Quant Imaging Med Surg 2020; 10(5): 1058-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eng J, Bluemke DA. Imaging Publications in the COVID-19 Pandemic: Applying New Research Results to Clinical Practice. Radiology 2020: 201724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim H, Hong H, Yoon SH. Diagnostic Performance of CT and Reverse Transcriptase-Polymerase Chain Reaction for Coronavirus Disease 2019: A Meta-Analysis. Radiology 2020: 201343. [DOI] [PMC free article] [PubMed] [Google Scholar]