Abstract
Over the past 10 to 15 years, intermittent fasting has emerged as an unconventional approach to reduce body weight and improve metabolic health beyond simple calorie restriction. In this review, we summarize findings related to Ramadan and Sunnah fasting. We then discuss the role of caloric restriction not only as an intervention for weight control, but importantly, as a strategy for healthy aging and longevity. Finally, we review the four most common intermittent fasting (IF) strategies used to date for weight management and to improve cardiometabolic health. Weight loss is common after IF, but does not appear to be different than daily caloric restriction when compared directly. IF may also provide additional cardiometabolic benefit, such as insulin sensitization, that is independent from weight loss. While no specific fasting regimen stands out as superior at this time, there is indeed heterogeneity in responses to these different IF diets. This suggests that one dietary regimen may not be ideally suited for every individual. Future studies should consider strategies for tailoring dietary prescriptions, including IF, based on advanced phenotyping and genotyping prior to diet initiation.
Keywords: Caloric restriction, alternate-day fasting, intermittent fasting, time-restricted feeding, metabolic health
Introduction
Physiological adaptations, such as the metabolic flexibility to food availability and the priority for energy storage, were needed to survive periodic food shortages throughout human evolution.1,2 Obesity appears to be the hallmark of modernization based on many case studies documenting the adaptation of western lifestyles.2 The precise temporal feeding patterns of our ancestors are unknown, but they were likely very different than the post-industrial revolution feeding patterns characterized by increased meal frequency3 and extended eating intervals with intake shifted to later in the day.4–6 Unfortunately, modern feeding habits often contrast with circadian physiology, which requires environmental cues (e.g., meal timing) to promote circadian synchrony.7 While circadian desynchrony has been linked to poor metabolic health and obesity, meal timing has only recently been postulated to contribute to metabolic dysfunction.8
Intermittent fasting (IF) has emerged over the past 10 to 15 years as an unconventional approach to potentially reduce body weight and improve metabolic health beyond simple calorie restriction (CR). There are a variety of IF regimens with regards to feed-and-fast cycles, meal timing, and energy intake.9,10 Clinical trials from the most common IF regimens will be discussed. These trials share similar characteristics of prolonged periods of fasting. Alternate-day fasting (ADF) and time-restricted feeding (TRF) are characterized by extending nocturnal fasting. While ADF incorporates a complete fast every other day, TRF simply shortens the daily feeding window. Both ADF and TRF may inadvertently restrict energy intake and, therefore, cause weight loss. In contrast, most alternate-day modified fasting (ADMF) and 5:2 diet regimens utilize very low-calorie diets occurring intermittently throughout a 7-d period. These four IF regimens are summarized in Table 1. The extent that each of these feeding regimens affect total body weight, body composition, and metabolic health are discussed below.
Table 1.
Description of different approaches to intermittent fasting
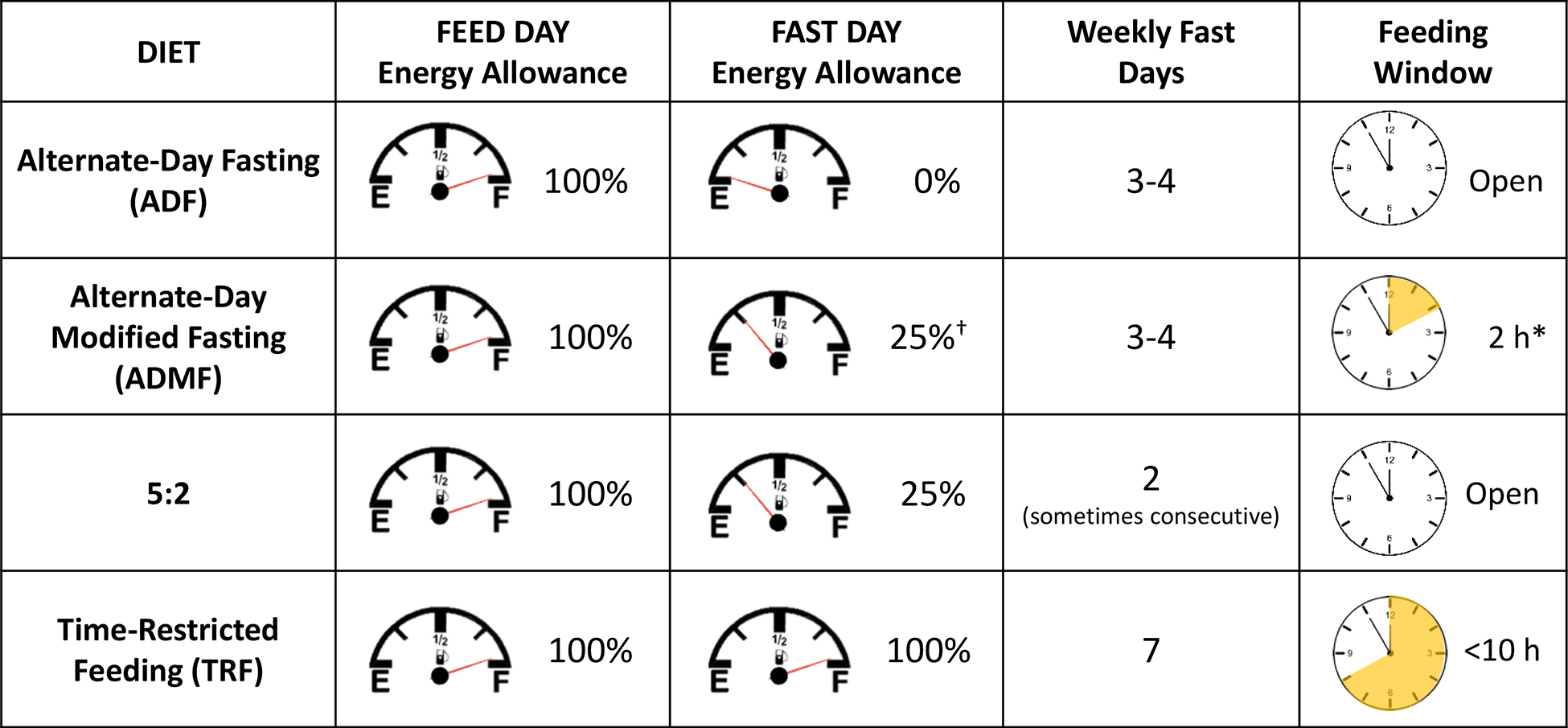
|
Halberg et al., 2005 and Soeters et al., 2009 did not restrict calorie intake.
Most trials have limited intake to a shortened window of time between 2 to 4 hours, and meals often occur mid-day.
Religious origins of IF
Many forms of religious fasting can be found in Christianity, Judaism, and Islam. While specific regimens vary by duration and dietary patterns, many fasts reduce animal protein, refined foods, and other indulgences while promoting intake of fruits and vegetables, increased social engagement, and prayer.11 Sunnah and Ramadan, fasts within Islam, will be discussed as these approaches bear similarities to the secular IF approaches.
Ramadan fasting
Muslims observing Ramadan traditionally are not allowed to eat, drink, smoke, or engage in any sensual activity between dawn and sunset during the Holy month.12 It is a common practice to wake early to have breakfast before dawn.12,13 Thus, energy intake is restricted to evening, nighttime, and very early morning in a bimodal pattern. Concern has been raised regarding this practice in those with diabetes.13 Here, we summarize the cardiometabolic effects of Ramadan fasting within the general adult population.
Changes in weight, body composition, and energy metabolism.
Several meta-analyses have reported weight loss following Ramadan fasting14–16 with initial body mass index (BMI) positively correlated with the magnitude of weight loss.17 However, complete weight regain is often observed 2 to 5 weeks after Ramadan.14,16 Several studies have reported no change in body weight,14–16 but two of these meta-analyses highlighted more pronounced weight loss in males compared with females.14,15 Considering the positive associations between the number of fasting days and the observed magnitude of weight loss,17 blunted weight loss is expected among females since fasting is not allowed during menstruation.14 As often experienced with weight loss, improvements in body composition such as decreased fat mass16 and reduced waist circumference18 are observed with Ramadan fasting. Decreases in fat-free mass are also observed but to a lesser degree than fat mass.16 Furthermore, recent studies report no effect of Ramadan fasting on resting metabolic rate or energy expenditure,19,20 yet increased fat oxidation and decreased carbohydrate oxidation have been reported.20,21
Changes in cardiometabolic health.
Meta-analyses of Ramadan fasting have reported cardiometabolic benefits among healthy individuals with inconsistencies regarding improvements in lipid profiles.15,18,22 Kul et al.15 reported a slight decrease in total cholesterol and triglycerides, a large decrease in low-density lipoprotein (LDL) cholesterol, and no change in high-density lipoprotein (HDL) cholesterol. Faris et al.18 similarly noted modest improvements in triglycerides, but reported an increase in HDL cholesterol. Mirmiran et al.22 included studies in athletes and pregnant women. They too reported an increase in HDL cholesterol, but were the only study to report an increase in LDL cholesterol.22 All reviews and meta-analyses suggest sex-specific effects of Ramadan fasting, but they do not seem to agree. For example, a subgroup analysis from Kul et al.15 indicates larger increases in HDL cholesterol in females and more pronounced decreases in triglycerides in males. Conversely, other meta-analyses indicate an increase in HDL cholesterol in males18,22 and a decrease in triglycerides in females.18 The reason for such discrepancies is unknown, but it could stem from a disproportionately higher male sample and different criteria used for study selection. Additional subgroup analyses indicates greater health improvements relative to baseline health, age, and reduction in body weight.22 Two meta-analyses reported minimal improvements in fasting glucose among both men and women.15,18 To date, it remains unclear how Ramadan fasting may reduce fasting insulin and improve insulin resistance. Some studies have shown a reduction in insulin concentrations and an improvement in insulin resistance (HOMA-IR),23,24 while others report an increase in insulin and insulin resistance25,26 or no change at all.27,28 Studies investigating the effect of Ramadan fasting on metabolic health using more robust techniques, such as the hyperinsulinemic euglycemic clamp, are warranted to further our scientific understanding of the effect of Ramadan fasting on metabolic health.
Sunnah fasting
Sunnah fasting is practiced year-round and includes fasting weekly on Mondays and Thursdays with an additional 6 days of fasting in the Shawwal month.29 Two studies by the same research group assessed 12 weeks of weekly Sunnah fasting combined with daily CR in older males from Malaysia.29,30 Sunnah fasting consisted of a small meal prior to sunrise and a full meal after sunset; additionally, participants were asked to restrict calories by 300 to 500 kcal/d and to increase intake of healthy foods.30 Both studies indicated an overall energy deficit of 18% and weight loss of ~3%.29,30 Fat mass decreased by about 6% to 8%.29,30 Fat mass decreased by about 6% to 8%.29,30 While fat-free mass was unchanged in one study,30 another study showed a slight decrease (−0.9%) that was not significantly different from the control group.29 Only Teng et al.29 assessed cardiometabolic outcomes and noted decreases in both total cholesterol and LDL cholesterol by 8% and a decrease in both systolic and diastolic blood pressures by 4.5% and 2.6%, respectively. There was no change in glucose, and insulin was not reported.
Additional work by Ismail et al.31 considered whether an emphasis on faith-based dietary practices could promote health following Ramadan for 12 weeks. The control group was assigned to standard dietary advice, while the intervention group was also provided faith-based dietary advice, such as the inclusion of Sunnah fasting. The occurrence of fasting twice weekly (Monday and Thursday) was unchanged; however, fasting once weekly (Monday or Thursday) increased in the intervention group. There was no change in fasting frequency in the control group. A decrease in BMI from baseline was observed in the intervention group, but was not significantly different from the control group. The intervention group also experienced modest improvements in cardiometabolic health, such as decreases in diastolic blood pressure and increases in HDL cholesterol. However, this could also be an outcome of enhanced vegetable intake occurring in the intervention group. Together, these studies suggest that the practice of Sunnah fasting may promote cardiometabolic health. A continuation of Ismail et al. on a larger and more generalizable scale could have public health implications within Muslim communities.
Caloric restriction (CR)
Naturally occurring episodes of CR suggest that prolonged CR with high-quality diets may improve both health and longevity in humans. Quite a few years ago, researchers reported that the greatest number of centenarians resided on the Japanese island of Okinawa.32 Importantly, Okinawans were reported to consume 15 to 20% less energy than the average Japanese individual consumed on mainland Japan.33 These observations suggest that CR may be a physiological driver of longevity. Another study in non-obese Spanish monks showed that assignment to a CR diet (only 1 L of milk and 500 g of fruit every other day) was associated with less time in the infirmary and a non-significant decrease in death rate.34,35 Results from the Biosphere 2 experiment also provided insights into the effect of long-term CR (almost 2 years) in humans with normal weight. After experiencing a shortage of homegrown food, the 8 individuals that lived within Biosphere 2 not only lost ~15% of their body weight, but they also experienced clear improvements in physiologic, hematologic, hormonal, and biochemical health indicators.36
Large-scale interventional studies emphasizing CR and increased physical activity (e.g., the Diabetes Prevention Program (DPP) and LookAHEAD [Action for Health in Diabetes]) have clearly demonstrated the ability to elicit weight loss and reduce diabetes risk.37,38 Even CR-induced weight loss of ~5 to 10% has definitive cardiometabolic health benefits.39 A more recent randomized clinical trial (the Comprehensive Assessment of Long-term Effects of Reducing Intake on Energy [CALERIE]) showed that CR significantly improved several cardiovascular risk factors, insulin sensitivity, and mitochondrial function.40–43 Such improvements may indeed compress mortality by delaying the incidence of aging-related chronic disease. Importantly, the health benefits of CR seem to be mediated by decreased energy expenditure, improved mitochondrial function, and decreased oxidative stress and inflammation.42 Unfortunately, maintaining CR and weight loss and their respective health benefits is challenging. Weight regain is a common occurrence during weight maintenance44,45 and even a mild degree of weight regain may negate initial cardiometabolic benefits of weight loss.45 More specifically, a 2-year follow-up from CALERIE reported only ~50% of the initial weight loss was maintained after the intervention.46
Much research is currently underway to better understand the inter-subject variability in weight gain and the interaction between the biology, psychology, prescribed diet, weight loss, or even more importantly, weight loss maintenance. Indeed, one diet does not fit all. As a result, IF approaches, including TRF, have been offered as alternative dietary strategies that may have beneficial effects on weight control and healthy aging.
Intermittent fasting: New strategies to improve weight management and metabolic health
In this section, we review the different IF strategies that have been studied for weight management, with added emphasis on their cardiometabolic health impact. These include the 5:2 diet, ADF, ADMF, and TRF (see Table 1).
5:2 diet
The 5:2 diet is a secular diet that shares some similarities with Sunnah fasting, as both regimens call for modified fasting to occur twice weekly. To date, all 5:2 regimens have allowed ~25% energy intake on the “fast day” rather than a complete fast. This feeding regimen, combined with ad libitum feeding on the remaining 5 days, may elicit a weekly energy deficit of ~20 to 25%. Meals consumed during the modified fast days are typically eaten at any time. About half of the trials permit participants to select two non-consecutive fast days, whereas others require a more aggressive approach with consecutive fast days.47–52
Changes in body weight, body composition, appetite, and energy intake.
All 5:2 diet studies have been designed as weight loss interventions, and many of these studies compare 5:2 versus daily CR. Short-term studies lasting ~8 weeks have reported high adherence (>90%) to 5:2 diets.51–53 Overall adherence does not appear to differ between 5:2 and daily CR.47,48,50,53 Interventions ranging from 4 to 24 weeks have reported weight loss of 4 to 8%.48,49,54–56 Weight loss of at least 5% is possible within 8 weeks.51 The efficacy of attaining at least 5% weight loss is similar between 5:2 and daily CR.51,55 Decreases in fat mass typically range from 9 to 13% with only a 1 to 4% reduction in fat-free mass and is similar to daily CR. Participants also self-report lower caloric intake during 5:2 compared to daily CR.47,51,54,57 Decreased intake could be the result of transient ketosis,58 which may partly explain the observed suppression of appetite ratings47,48 and maintenance of baseline ghrelin concentration during 5:2,47 a hormone which usually increases with weight loss and signals hunger. Initial ratings of hunger are higher during 5:2 compared to daily CR but appear to be mitigated by higher protein intake and normalize over time.48,55,59 Further investigation should decipher feed and fast day interactions on hunger, fullness, and satiation, while pairing perceptions with biomarkers of appetite.
Changes in cardiometabolic health.
Similar weight loss outcomes make it possible to compare weight loss-independent cardiometabolic differences between 5:2 and daily CR. Decreases in total cholesterol, LDL cholesterol, and triglycerides as high as 13%, 15%, and 22%, respectively, have been reported with 5:2.49,54 Modest improvements in lipids are similar between 5:2 and daily CR.48,54,60 The 5:2 diet, however, appears to have a superior insulin-sensitizing impact. Harvie et al.47 first observed improvements in insulin sensitivity after 6 months of 5:2, which was slightly better than daily CR. Similar findings were replicated in two other studies from the same investigators.48,49 These observations could be explained by the dramatic increase in insulin sensitivity immediately following 2 days of consecutive fasting that lingered throughout the remaining feed days.47–49 Additionally, differential adipokine responses between 5:2 and daily CR could also play a role.48,49 Among individuals with type 2 diabetes, reduced medication use (i.e., insulin) and lowered hemoglobin A1c (HbA1c) were observed with 5:2,50,59,60 thus supporting the theory that 5:2 promotes insulin sensitization. Future research should address whether baseline characteristics can predict which dietary intervention (5:2 versus daily CR) is the most effective for an individual based on their metabolic health status.
Followup and weight loss maintenance studies.
Following completion of a 5:2 diet, weight regain is highly variable and similar to daily CR.48,53,54,61 Carter et al.61 reported a 33% weight regain in returning participants upon a 2-year follow-up. In the same study, HbA1c increased, while total cholesterol and LDL cholesterol returned to baseline. Importantly, other studies curtailed weight regain while maintaining reductions in adiposity and cardiometabolic disease risk through continued engagement in weight loss maintenance programs lasting 4 to 24 weeks.48,53,54 In studies lacking continued counseling, weight regain occurred and cardiometabolic risk rebounded during follow-ups lasting approximately 6 months.53,54
Alternate-day fasting (ADF) and alternate-day modified fasting (ADMF)
Both ADF and ADMF switch back-and-forth between days of fasting and feeding. Fasting days can range from ~75 to 100% CR depending on the fasting regimen, and feeding days are usually ad libitum. Seminal work in this field utilized either a complete fast (ADF)62 or a 20-h fast every other day (ADMF).63 The extended daytime fasting has been studied less frequently due to poor tolerability of a complete fast every other day.62 Consequently, most ADMF protocols now include a single mid-day meal, which typically falls within a 2-h feeding window and allocates ~25% of energy requirements.
Changes in body weight, body composition, and appetite.
ADF and ADMF trials in humans often result in weight loss either by design or unintentionally, which is in contrast to mouse models demonstrating weight maintenance.64 Three short-term trials in male participants were successful at maintaining body weight.63,65,66 Other trials encouraged participants to consume 145 to 200% of their energy needs on the feed days, yet participants still experienced unintentional weight loss.62,67 These reports suggest that ADF and ADMF regimens may have therapeutic value as obesity treatments. Interventions lasting 4 to 16 weeks report weight loss between 3 to 13%,67–81 while trials extending to 24 weeks report weight loss of 6 to 11%.81–84 Comparisons of daily CR against ADF and ADMF demonstrate comparable decreases in body weight70,77,78,83 and fat mass.77–79,83 Some of which report trends in favor of greater weight loss during ADF or ADMF.77,79 Other inconsistencies exist. For example, Viegener et al.81 ADMF outperformed daily CR for weight loss for the first 4 months, but not at subsequent timepoints extending to 6 months. In other cases, ADMF elicited superior reductions in weight67,85 and fat mass67 compared to daily CR. Likewise, there is no consensus with respect to fat-free mass alterations. Several non-randomized trials of ADMF suggest fat-free mass retention.69,71– 72,74,76,86 This effect on fat-free mass is not repeated across studies67,75,77,79,82 and is often no different compared to daily CR.67,77–79,83 Divergent conclusions could stem from varying degrees of weight loss, duration of interventions, and discrepancies between body composition assessment methods (i.e., bioelectrical impedance versus dual-energy X-ray absorptiometry).
Despite overall high dietary adherence,69,71,72,75,85 generally good study retention,72,74,77,85 and minimal reported side effects,77,80,87 several ADF or ADMF studies have reported dropout rates greater than 20%.71,76,79,82,83 As expected, dropouts increased when the duration of the intervention was 12 weeks or longer. It also remains unclear how well-tolerated these types of IF diets actually are compared to daily CR. Small studies (<20 participants per group) appear to report similar dropout rates,70,77,79 whereas, larger studies (>40 participants) are equivocal.81,83 These findings coupled with inherent inter-individual variability in observed weight loss88 and the growing demand for precise medical treatment, suggest that individualized weight loss approaches may be beneficial. In support of this, self-identification as a “big eater” was negatively associated with weight loss likely due to the ability to better compensate for the fast day energy deficit.62 There also appears to be differential responses among demographic groups upon pooling several ADMF studies in a secondary analysis. Caucasians and older individuals experienced larger weight loss,89 which is consistent with other daily CR studies.90–92 Reasons for these differences remain unknown, but both cultural and physiological underpinnings likely play a role.93,94
The Varady group has explored several ADMF variations that could increase acceptability and adherence. The standard ADMF approach allows for only a single mid-day meal to be consumed on fasting days. Typically, a low-fat dietary pattern is recommended or provided. This may be difficult for dieters preferring to eat later in the day or those desiring higher fat meals. Hoddy et al.75 examined meal timing and frequency during ADMF and noted no influence with respect to weight loss or changes in body composition when the fast day meal was provided at different times or split into three small meals. Additionally, providing participants with high-fat meals during ADMF also did not hinder weight loss.72 Other ADMF variations have considered whether weight loss may be enhanced by incorporating other healthful behaviors, such as consuming higher dietary protein78,82 or including exercise.71,76,95 Kalam et al.82 noted fat-free mass retention from baseline with a higher protein diet. Another study demonstrated similar reductions in body weight, fat mass, fat-free mass, and visceral adiposity between high-protein variations of ADMF and daily CR.78 Neither study directly compared a high-protein ADMF approach to a standard ADMF approach. There have been direct comparisons between ADMF and ADMF with exercise. Bhutani et al.71 reported that the inclusion of exercise augmented weight and fat mass loss compared to ADMF alone. Similarly, Oh et al.76 reported improvements from baseline in body weight and body composition in both ADMF and ADMF with exercise conditions, but only the exercising ADMF group attained statistically higher fat mass loss from the control. Interestingly, exercise during ADMF appears to attenuate dropout rates.76,95 This body of work points to the ability to tailor ADMF according to individual preferences and weight loss goals.
Appetite, which is commonly an exploratory outcome, appears to be curbed and may explain the reduced self-reported caloric intake on feed days.67,77,83 Only one study compared appetite during ADMF to daily CR and reported no difference between the diets.79 Appetitive responses to ADF or ADMF vary by study and assessment method. Three weeks of ADF enhanced daily feelings of fullness; yet, hunger remained.62 With ADMF, hunger sensations appear to subside after only 2 weeks.96 This indicates that tolerability may be enhanced with the inclusion of a small fast day meal, but this comparison has not been made experimentally. Reduced feelings of hunger and increased feelings of fullness have also been observed after longer exposure to ADF and ADMF interventions (up to 10 weeks).67,68,74,95,96 This suggests appetite normalization with time. Responses to standardized meal tests, representing physiological appetite responses, have been inconsistent across studies.79,97,98 Heilbronn et al.97 reported no change in ghrelin after 3 weeks of ADF. Conversely, hunger was unchanged following 8 to 12 weeks of ADMF and a slight increase in ghrelin was observed.79,98 Findings related to fullness and peptide YY (PYY), a gut peptide which signals fullness, are inconclusive as well. Hoddy et al.98 observed a moderate increase in PYY (16%) coupled with increased feelings of fullness (10%) after ADMF, but Coutinho et al.79 showed no change in PYY or fullness. ADMF and ADF may, therefore, placate appetite over time, yet the mechanisms explaining are unclear and inconsistent.
Changes in cardiometabolic health.
ADF and ADMF have beneficial effects on overall cardiometabolic health, but disagreement exists when comparing specific outcomes. For example, fasting glucose has been shown to both increase (5%)84 and decrease (2 to 5%)78,85,98 during ADMF while others show no change73,76 in trials lasting at least 6 weeks. Additionally, fasting insulin may decrease 21 to 42% in studies lasting 8 to 24 weeks,78,83,98 thus improving insulin resistance (HOMA-IR).83 Insulin sensitivity, on the other hand, is unchanged when assessed via intravenous glucose tolerance test (IVGTT)77 and shows differential responses to mixed meal tolerance testing by sex.97 In response to a hyperinsuliemic-euglycemic clamp after 8 weeks of ADMF or daily CR, Hutchison et al.67 reported no difference in insulin sensitivity. The effect of ADF on lipid concentrations appears to also depend on sex and may require a longer exposure period to observe differences.62,65 Several studies lasting at least 8 weeks have also shown collective improvements in lipid profiles characterized by decreased triglycerides, LDL cholesterol, and total cholesterol.67,69,70,72,74,77,78,82 Benefits in total lipid concentrations are not universal and may not be different from daily CR. Three studies report no differences from daily CR for total or LDL cholesterol after weight loss,77–78,83 while another points to body weight independent improvements favoring ADMF.67 Importantly, the improvement in the atherosclerotic profile of lipid particles appears to consistently improve,70,71,75,99,100 and initial findings suggest it may outperform daily CR.70
Response according to metabolic health status is perhaps more compelling. A clinical trial in participants with metabolic syndrome showed a greater decrease in fasting glucose after ADMF compared to daily CR, but no difference in insulin resistance or fasting inslulin concentrations were observed.85 Other secondary analyses provide preliminary evidence that ADMF may be more effective than daily CR for attenuating diabetes risk. Participants with the highest degree of insulin resistance experienced the greatest improvement in HOMA-IR after ADMF.101 In a different analysis of only insulin resistant participants, there were marked decrease after ADMF compared to daily CR for fasting insulin (52% vs. 14%, respectively) and insulin resistance (53% vs. 17%, respectively).102 Long-term randomized clinical trials are needed in populations with diabetes or prediabetes to draw specific conclusions regarding disease outcomes after ADF or ADMF.
Followup and weight loss maintenance studies.
Weight loss maintenance following ADF and ADMF is questionable. To date, four studies have evaluated weight loss maintenance—all employing vastly different approaches.77,78,82,83 Following 8 weeks of ADF or daily CR, Cattenacci et al.77 reported significant weight regain (~30% of initial loss) in both diet groups during a 24-week unsupervised follow-up period. Both diet groups maintained absolute losses in fat and fat-free mass. The proportional modulations, however, in percent fat mass and percent fat-free mass were better with ADF compared to daily CR. This could be due to some particiapnts maintaining ADF during the undersupervised follow-up.77 In the longest weight maintenance comparison between ADMF (50% CR on the fast day) and daily CR, Trepanowski et al.83 reported similar weight regain trajectories between daily CR and ADMF and no difference in body composition. Interestingly, those individuals achieving at least 5% weight loss, self-selected higher protein intake throughout the trial.88 Other studies paired ADMF with daily CR during the weight loss phase while incorporating intake of higher dietary protein as part of the weight maintenance apprach.78,82 Kalam et al.82 used high-protein meal replacements on feed (1000 kcal/d) and fast (600 kcal/d) days during weight loss. During weight maintenance, the same fast day meal replacements were used while the feed days reduced reliance on meal replacements.82 Participants experienced small amounts of continued, but statistically insignificant, weight loss (<1 kg) over 12 weeks of weight loss maintenance. Cardiometabolic improvements only reached significance after weight maintenance, even though there was clinically significant weight loss (>5%) during the weight loss phase.82 Bowden et al.78 compared ADMF with calorie-restricted feed days to daily CR alone. Following the weight loss phase, diets equally maintained weight loss by following recommendations for higher protein diets for an additional 8 weeks.78 Regardless of approach, lifestyle modifications causing a sustained energy deficit are required to achieve successful weight loss maintenance. Further research is needed to determine whether protein intake during ADF of ADMF influences compliance, appetite, or body composition.
Time-restricted feeding (TRF)
In contrast to 5:2, ADF, and ADMF regimens, TRF does not purposely impose CR, rather it extends the daily fasting period by restricting food intake to a reduced window of time. To date, dozens of animal studies have reported that TRF infers definite health benefits, including reductions in body weight, food intake, hyperlipidemia, ectopic fat, and markers of inflammation, as well as improvements in heart health, cancer outcomes, and lifespan extension.103 Recent TRF trials in humans have allowed a feeding window between 4 to 12 hours, although a window of <10 hours is thought to be optimal based on glycogenolysis, fatty acid oxidation, and gluconeogenesis modulations occurring in the absence of dietary glucose availability.104
Single-arm TRF trials have reported 2 to 3% weight loss over 1 to 4 months.5,105 Gill and Panda5 enrolled 8 individuals with overweight and obesity who reported an eating duration of more than 14-h to choose a 10-h ad libitum eating window of their choice for 16 weeks. Importantly, the participants experienced weight loss, reduced hunger at night, increased overall energy levels, and improved sleep satisfaction, which persisted for 1-year after the intervention began.5 Another recent study by Anton et al.105 enrolled older, sedentary adults with overweight and obesity and restricted their eating window to an 8-h ad libitum flexible feeding window; study volunteers also experienced significant weight reduction.
In more controlled trials, eating earlier in the day (“early TRF”) or in the middle of the day (“mid-day TRF”) outperformed eating later in the day (“late TRF”) for cardiometabolic health. It is possible that eating in synchronization with the natural circadian endocrine rhythm—early in the morning or around noon-time—may hold the key to naturally reduce energy intake. In just 4 days, early TRF (6-h window: 8:00 AM – 2:00 PM) versus control (12-h window: 8:00 AM – 8:00 PM) altered expression in 6 out of 8 circadian genes.106 This was accompanied by temporal differences characterized by a nocturnal blunting of glucose assessed by continuious glucose monitoring along with altered perceptions of appetite and body temperature.106,107 Along with this, there was an overall decrease in appetite and increased fat oxidation without impacting 24-h energy expenditure.107 A longer 5-week crossover trial in males with overweight and prediabetes reported that early TRF (6-h window: 8:00 AM – 2:00 PM) actually improved insulin sensitivity by 24% and β-cell function by 13% when compared to a 12-h energy matched eating window (8:00 AM – 8:00 PM).108 There were also impressive declines in blood pressure and oxidative stress (8-Isoprostanes).108 Hutchinson et al.109 directly compared early TRF (8:00 AM – 5:00 PM) with late TRF (12:00 PM – 9:00 PM) in 15 overweight males in a crossover design. TRF reduced body weight, plasma triglycerides, and postprandial glucose levels in both groups, and a decrease in fasting glucose via continuous glucose monitoring was observed only with early TRF. Mid-day TRF approaches have reported similar improvements in cardiometabolic health. For example, a randomized controlled study by Antoni et al.110 found that delaying breakfast and advancing dinner by 1.5-h each (or mid-day TRF) for 10 weeks reduced body fat and energy intake in a cohort of mostly female participants. Another trial of mid-day TRF (8-h window: 10:00 AM – 6:00 PM) in 23 adults with obesity confirmed such improvements in cardiometabolic health, including reduced body weight, energy intake, and systolic blood pressure.111
A few studies suggest that late-TRF may worsen cardiometabolic health. Compared to consuming 3 meals/d of a eucaloric diet, consuming those same calories within a single 4-h evening meal (1 meal/d) increased systolic blood pressure, cholesterol, hunger, early morning fasting glucose, as well as delayed insulin response (via oral glucose tolerance test).112,113 However, one 8-week randomized controlled trial in 34 resistance-trained athletes did report that late-TRF (8-h window: 1:00 – 9:00 PM) reduced fat mass while maintaining fat-free mass, muscle area, and maximum strength when compared to consuming the same calories across the entire day.114 This trial also triggered a reduction in triglycerides, testosterone, IGF-1, and IL-1β with no changes in total cholesterol or fasting glycemic markers.114
Perspective and future directions
Our review indicates that IF approaches may provide health benefits independent of weight loss. This is in agreement with meta-analyses showing decreased fasting insulin9,115 despite no difference in weight loss between IF regimens and CR.9,10,86,115 It is possible that one IF regimen could outperform another, but this remains to be directly tested. Furthermore, compliance to IF and daily CR has often been comparable and demonstrates that one may not be superior to the other when trying to achieve a negative energy balance. Nonetheless, repeated examples across every type of IF regimen do indicate an insulin sensitizing effect occurring almost immediately upon diet initiation. It is possible that this IF effect could be mediated through circadian biology as diurnal variations in glucose,116 energy expenditure,117,118 and substrate utilization119 favor eating earlier in the day and fasting at night.
Importantly, the heterogeneity in the response to different IF strategies suggests that personalized approaches will improve weight loss and enhance cardiometabolic health. Therefore, the next phase of IF research should tailor dietary prescriptions based on factors related to an individual’s unique physiology, current health status, dietary preferences, social influences, and built environments. This new chapter of research has the potential to identify ideal candidates for a particular weight loss treatment. Advanced phenotyping and genotyping to identify the molecular transducers of dietary interventions may one day provide the basis for better customized care.120
Modern feeding behaviors include eating across extended periods of time (~15-h) with the largest proportion of calories consumed in the evening.4–6 Evidence from animal models of IF indicate its circadian resetting ability.121–125 Conversely, mistimed food intake during IF may contribute to chronobiological desynchrony.125 Continued examination of IF has the potential to clarify the interplay between diet, metabolism, and circadian physiology. Ramadan research provides insight into delayed IF in humans as Ramadan fasting confines energy intake to predominately evening hours and has been shown to decrease sleep and disrupt circadian rhythm.25,126–128 Limiting nighttime energy intake during simulated night-shift seems to offset some of the cardiometabolic dysfunction noted in night shift workers.129,130 Such findings posit that the metabolic effect of fasting may outweigh that of circadian disruption. As such, utilizing IF as a countermeasure to known and often unavoidable circadian disruptors, including sleep restriction, social jet lag, and nightshift, is an unexplored area of interventional research requiring attention.
Funding:
This work was partially funded by a NORC Center Grant P30DK072476; KLM is supported by the NIDDK sponsored Ruth L. Kirschstein National Research Service T32 Research Training Grant (T32-DK064584).
Footnotes
Disclosure Statement: KKH, KLM, HC, and ER have nothing to declare.
REFERENCES
- 1.Mattson MP, Allison DB, Fontana L, et al. Meal frequency and timing in health and disease. Proceedings of the National Academy of Sciences. 2014:201413965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown PJ, Konner M. An anthropological perspective on obesity. Ann N Y Acad Sci. 1987;499:29–46. [DOI] [PubMed] [Google Scholar]
- 3.Popkin BM, Duffey KJ. Does hunger and satiety drive eating anymore? Increasing eating occasions and decreasing time between eating occasions in the United States. The American Journal of Clinical Nutrition. 2010;91(5):1342–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang JB, Patterson RE, Ang A, Emond JA, Shetty N, Arab L. Timing of energy intake during the day is associated with the risk of obesity in adults. Journal of Human Nutrition and Dietetics. 2014;27:255–262. [DOI] [PubMed] [Google Scholar]
- 5.Gill S, Panda S. A Smartphone App Reveals Erratic Diurnal Eating Patterns in Humans that Can Be Modulated for Health Benefits. Cell Metab. 2015;22(5):789–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta NJ, Kumar V, Panda S. A camera-phone based study reveals erratic eating pattern and disrupted daily eating-fasting cycle among adults in India. PLoS One. 2017;12(3):e0172852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roenneberg T, Merrow M. The Circadian Clock and Human Health. Current Biology. 2016;26(10):R432–R443. [DOI] [PubMed] [Google Scholar]
- 8.Qian J, Scheer FAJL. Circadian System and Glucose Metabolism: Implications for Physiology and Disease. Trends in Endocrinology & Metabolism. 2016;27(5):282–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris L, Hamilton S, Azevedo LB, et al. Intermittent fasting interventions for treatment of overweight and obesity in adults: a systematic review and meta-analysis. JBI Database System Rev Implement Rep. 2018;16(2):507–547. [DOI] [PubMed] [Google Scholar]
- 10.Harris L, McGarty A, Hutchison L, Ells L, Hankey C. Short-term intermittent energy restriction interventions for weight management: a systematic review and meta-analysis. Obes Rev. 2018;19(1):1–13. [DOI] [PubMed] [Google Scholar]
- 11.Trepanowski JF, Bloomer RJ. The impact of religious fasting on human health. Nutr J.2010;9:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alghafli Z, Hatch TG, Rose AH, Abo-Zena MM, Marks LD, Dollahite DC. A Qualitative Study of Ramadan: A Month of Fasting, Family, and Faith. Religions. 2019;10(2). [Google Scholar]
- 13.Al-Arouj M, Assaad-Khalil S, Buse J, et al. Recommendations for management of diabetes during Ramadan: update 2010. Diabetes Care. 2010;33(8):1895–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sadeghirad B, Motaghipisheh S, Kolahdooz F, Zahedi MJ, Haghdoost AA. Islamic fasting and weight loss: a systematic review and meta-analysis. Public Health Nutr. 2014;17(2):396–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kul S, Savas E, Ozturk ZA, Karadag G. Does Ramadan fasting alter body weight and blood lipids and fasting blood glucose in a healthy population? A meta-analysis. J Relig Health. 2014;53(3):929–942. [DOI] [PubMed] [Google Scholar]
- 16.Fernando HA, Zibellini J, Harris RA, Seimon RV, Sainsbury A. Effect of Ramadan Fasting on Weight and Body Composition in Healthy Non-Athlete Adults: A Systematic Review and Meta Analysis. Nutrients. 2019;11(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan N, Rasheed A, Ahmed H, Aslam F, Kanwal F. Effect of Ramadan fasting on glucose level, lipid profile, HbA1c and uric acid among medical students in Karachi, Pakistan. East Mediterr Health J. 2017;23(4):274–279. [DOI] [PubMed] [Google Scholar]
- 18.Faris MeA-IE, Jahrami HA, Alsibai J, Obaideen AA. Impact of Ramadan diurnal intermittent fasting on the metabolic syndrome components in healthy, non-athletic Muslim people aged over 15 years: a systematic review and meta-analysis. British Journal of Nutrition. 2020;123(1):1–22. [DOI] [PubMed] [Google Scholar]
- 19.Lessan N, Saadane I, Alkaf B, et al. The effects of Ramadan fasting on activity and energy expenditure. Am J Clin Nutr. 2018;107(1):54–61. [DOI] [PubMed] [Google Scholar]
- 20.Alsubheen SA, Ismail M, Baker A, et al. The effects of diurnal Ramadan fasting on energy expenditure and substrate oxidation in healthy men. Br J Nutr. 2017;118(12):1023–1030. [DOI] [PubMed] [Google Scholar]
- 21.el Ati J, Beji C, Danguir J. Increased fat oxidation during Ramadan fasting in healthy women: an adaptative mechanism for body-weight maintenance. Am J Clin Nutr. 1995;62(2):302–307. [DOI] [PubMed] [Google Scholar]
- 22.Mirmiran P, Bahadoran Z, Gaeini Z, Moslehi N, Azizi F. Effects of Ramadan intermittent fasting on lipid and lipoprotein parameters: An updated meta-analysis. Nutr Metab Cardiovasc Dis. 2019;29(9):906–915. [DOI] [PubMed] [Google Scholar]
- 23.Gnanou JV, Caszo BA, Khalil KM, Abdullah SL, Knight VF, Bidin MZ. Effects of Ramadan fasting on glucose homeostasis and adiponectin levels in healthy adult males. J Diabetes Metab Disord. 2015;14:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kassab S, Abdul-Ghaffar T, Nagalla DS, Sachdeva U, Nayar U. Interactions between leptin, neuropeptide-Y and insulin with chronic diurnal fasting during Ramadan. Ann Saudi Med. 2004;24(5):345–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ajabnoor GM, Bahijri S, Borai A, Abdulkhaliq AA, Al-Aama JY, Chrousos GP. Health impact of fasting in Saudi Arabia during Ramadan: association with disturbed circadian rhythm and metabolic and sleeping patterns. PLoS One. 2014;9(5):e96500–e96500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nachvak SM, Pasdar Y, Pirsaheb S, et al. Effects of Ramadan on food intake, glucose homeostasis, lipid profiles and body composition composition. Eur J Clin Nutr. 2019;73(4):594–600. [DOI] [PubMed] [Google Scholar]
- 27.Nematy M, Alinezhad-Namaghi M, Rashed MM, et al. Effects of Ramadan fasting on cardiovascular risk factors: a prospective observational study. Nutr J. 2012;11:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faris MAE, Madkour MI, Obaideen AK, et al. Effect of Ramadan diurnal fasting on visceral adiposity and serum adipokines in overweight and obese individuals. Diabetes Res Clin Pract. 2019;153:166–175. [DOI] [PubMed] [Google Scholar]
- 29.Teng NI, Shahar S, Manaf ZA, Das SK, Taha CS, Ngah WZ. Efficacy of fasting calorie restriction on quality of life among aging men. Physiol Behav. 2011;104(5):1059–1064. [DOI] [PubMed] [Google Scholar]
- 30.Teng NI, Shahar S, Rajab NF, Manaf ZA, Johari MH, Ngah WZ. Improvement of metabolic parameters in healthy older adult men following a fasting calorie restriction intervention. Aging Male. 2013;16(4):177–183. [DOI] [PubMed] [Google Scholar]
- 31.Ismail S, Shamsuddin K, Latiff KA, Saad HA, Majid LA, Othman FM. Voluntary Fasting to Control Post-Ramadan Weight Gain among Overweight and Obese Women. Sultan Qaboos Univ Med J. 2015;15(1):e98–e104. [PMC free article] [PubMed] [Google Scholar]
- 32.Willcox BJ, Willcox DC, Todoriki H, et al. Caloric restriction, the traditional Okinawan diet, and healthy aging: the diet of the world’s longest-lived people and its potential impact on morbidity and life span. Ann N Y Acad Sci. 2007;1114:434–455. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki M, Wilcox BJ, Wilcox CD. Implications from and for food cultures for cardiovascular disease: longevity. Asia Pac J Clin Nutr. 2001;10(2):165–171. [DOI] [PubMed] [Google Scholar]
- 34.Vallejo EA. [Hunger diet on alternate days in the nutrition of the aged]. Prensa Med Argent. 1957;44(2):119–120. [PubMed] [Google Scholar]
- 35.Stunkard AJ. Nutrition, aging and obesity In: Rockstein M, Sussman ML, eds Nutrition, longevity, and aging New York: Academic Press; 1976; :253–284. [Google Scholar]
- 36.Walford RL, Mock D, Verdery R, MacCallum T. Calorie restriction in biosphere 2: alterations in physiologic, hematologic, hormonal, and biochemical parameters in humans restricted for a 2 year period. J Gerontol A Biol Sci Med Sci. 2002;57(6):B211–224. [DOI] [PubMed] [Google Scholar]
- 37.Look AHEAD Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. New England journal of medicine. 2013;369(2):145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mudaliar U, Zabetian A, Goodman M, et al. Cardiometabolic Risk Factor Changes Observed in Diabetes Prevention Programs in US Settings: A Systematic Review and Meta-analysis. PLoS medicine. 2016;13(7):e1002095–e1002095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Magkos F, Fraterrigo G, Yoshino J, et al. Effects of Moderate and Subsequent Progressive Weight Loss on Metabolic Function and Adipose Tissue Biology in Humans with Obesity. Cell Metab. 2016;23(4):591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ravussin E, Redman LM, Rochon J, et al. A 2-Year Randomized Controlled Trial of Human Caloric Restriction: Feasibility and Effects on Predictors of Health Span and Longevity. J Gerontol A Biol Sci Med Sci. 2015;70(9):1097–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kraus WE, Bhapkar M, Huffman KM, et al. 2 years of calorie restriction and cardiometabolic risk (CALERIE): exploratory outcomes of a multicentre, phase 2, randomised controlled trial. Lancet Diabetes Endocrinol. 2019;7(9):673–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Redman LM, Smith SR, Burton JH, Martin CK, Il’yasova D, Ravussin E. Metabolic Slowing and Reduced Oxidative Damage with Sustained Caloric Restriction Support the Rate of Living and Oxidative Damage Theories of Aging. Cell Metab. 2018;27(4):805–815 e804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Civitarese AE, Carling S, Heilbronn LK, et al. Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med. 2007;4(3):e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nordmo M, Danielsen YS, Nordmo M. The challenge of keeping it off, a descriptive systematic review of high-quality, follow-up studies of obesity treatments. Obesity Reviews. 2020;21(1):e12949. [DOI] [PubMed] [Google Scholar]
- 45.Kroeger CM, Hoddy KK, Varady KA. Impact of weight regain on metabolic disease risk: a review of human trials. J Obes. 2014;2014:614519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marlatt KL, Redman LM, Burton JH, Martin CK, Ravussin E. Persistence of weight loss and acquired behaviors 2 y after stopping a 2-y calorie restriction intervention. Am J Clin Nutr. 2017;105(4):928–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harvie MN, Pegington M, Mattson MP, et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int J Obes (Lond). 2011;35(5):714–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harvie M, Wright C, Pegington M, et al. The effect of intermittent energy and carbohydrate restriction v. daily energy restriction on weight loss and metabolic disease risk markers in overweight women. The British journal of nutrition. 2013;110(8):1534–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harvie MN, Sims AH, Pegington M, et al. Intermittent energy restriction induces changes in breast gene expression and systemic metabolism. Breast Cancer Res. 2016;18(1):57–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Corley BT, Carroll RW, Hall RM, Weatherall M, Parry-Strong A, Krebs JD. Intermittent fasting in Type 2 diabetes mellitus and the risk of hypoglycaemia: a randomized controlled trial. Diabetic Medicine. 2018;35(5):588–594. [DOI] [PubMed] [Google Scholar]
- 51.Antoni R, Johnston KL, Collins AL, Robertson MD. Intermittent v. continuous energy restriction: differential effects on postprandial glucose and lipid metabolism following matched weight loss in overweight/obese participants. British Journal of Nutrition. 2018;119(5):507–516. [DOI] [PubMed] [Google Scholar]
- 52.Hirsh SP, Pons M, Joyal SV, Swick AG. Avoiding holiday seasonal weight gain with nutrient supported intermittent energy restriction: a pilot study. Journal of Nutritional Science. 2019;8:e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sundfør TM, Svendsen M, Tonstad S. Effect of intermittent versus continuous energy restriction on weight loss, maintenance and cardiometabolic risk: A randomized 1-year trial. Nutrition, Metabolism and Cardiovascular Diseases. 2018;28(7):698–706. [DOI] [PubMed] [Google Scholar]
- 54.Schübel R, Nattenmüller J, Sookthai D, et al. Effects of intermittent and continuous calorie restriction on body weight and metabolism over 50 wk: a randomized controlled trial. The American Journal of Clinical Nutrition. 2018;108(5):933–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Conley M, Le Fevre L, Haywood C, Proietto J. Is two days of intermittent energy restriction per week a feasible weight loss approach in obese males? A randomised pilot study. Nutr Diet. 2018;75(1):65–72. [DOI] [PubMed] [Google Scholar]
- 56.Headland ML, Clifton PM, Keogh JB. Effect of intermittent compared to continuous energy restriction on weight loss and weight maintenance after 12 months in healthy overweight or obese adults. International Journal of Obesity. 2018. [DOI] [PubMed] [Google Scholar]
- 57.Harvey J, Howell A, Morris J, Harvie M. Intermittent energy restriction for weight loss: Spontaneous reduction of energy intake on unrestricted days. Food science & nutrition. 2018;6(3):674–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paoli A, Bosco G, Camporesi EM, Mangar D. Ketosis, ketogenic diet and food intake control: a complex relationship. Front Psychol. 2015;6:27–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carter S, Clifton PM, Keogh JB. The effects of intermittent compared to continuous energy restriction on glycaemic control in type 2 diabetes; a pragmatic pilot trial. Diabetes Research and Clinical Practice. 2016;122:106–112. [DOI] [PubMed] [Google Scholar]
- 60.Carter S, Clifton PM, Keogh JB. Effect of Intermittent Compared With Continuous Energy Restricted Diet on Glycemic Control in Patients With Type 2 Diabetes: A Randomized Noninferiority Trial. JAMA Network Open. 2018;1(3):e180756–e180756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carter S, Clifton PM, Keogh JB. The effect of intermittent compared with continuous energy restriction on glycaemic control in patients with type 2 diabetes: 24-month follow-up of a randomised noninferiority trial. Diabetes Research and Clinical Practice. 2019;151:11–19. [DOI] [PubMed] [Google Scholar]
- 62.Heilbronn LK, Smith SR, Martin CK, Anton SD, Ravussin E. Alternate-day fasting in nonobese subjects: effects on body weight, body composition, and energy metabolism. Am J Clin Nutr. 2005;81(1):69–73. [DOI] [PubMed] [Google Scholar]
- 63.Halberg N, Henriksen M, Soderhamn N, et al. Effect of intermittent fasting and refeeding on insulin action in healthy men. J Appl Physiol (1985). 2005;99(6):2128–2136. [DOI] [PubMed] [Google Scholar]
- 64.Varady KA, Hellerstein MK. Alternate-day fasting and chronic disease prevention: a review of human and animal trials. Am J Clin Nutr. 2007;86(1):7–13. [DOI] [PubMed] [Google Scholar]
- 65.Harder-Lauridsen NM, Nielsen ST, Mann SP, et al. The effect of alternate-day caloric restriction on the metabolic consequences of 8 days of bed rest in healthy lean men: a randomized trial. Journal of Applied Physiology. 2017;122(2):230–241. [DOI] [PubMed] [Google Scholar]
- 66.Soeters MR, Lammers NM, Dubbelhuis PF, et al. Intermittent fasting does not affect whole-body glucose, lipid, or protein metabolism. American Journal of Clinical Nutrition. 2009;90(5):1244–1251. [DOI] [PubMed] [Google Scholar]
- 67.Hutchison AT, Liu B, Wood RE, et al. Effects of Intermittent Versus Continuous Energy Intakes on Insulin Sensitivity and Metabolic Risk in Women with Overweight. Obesity. 2019;27(1):50–58. [DOI] [PubMed] [Google Scholar]
- 68.Johnson JB, Summer W, Cutler RG, et al. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radical Biology and Medicine. 2007;42(5):665–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Varady KA, Bhutani S, Church EC, Klempel MC. Short-term modified alternate-day fasting: a novel dietary strategy for weight loss and cardioprotection in obese adults. Am J Clin Nutr. 2009;90(5):1138–1143. [DOI] [PubMed] [Google Scholar]
- 70.Varady KA, Bhutani S, Klempel MC, Kroeger CM. Comparison of effects of diet versus exercise weight loss regimens on LDL and HDL particle size in obese adults. Lipids Health Dis. 2011;10:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bhutani S, Klempel MC, Kroeger CM, Trepanowski JF, Varady KA. Alternate day fasting and endurance exercise combine to reduce body weight and favorably alter plasma lipids in obese humans. Obesity. 2013;21(7):1370–1379. [DOI] [PubMed] [Google Scholar]
- 72.Klempel MC, Kroeger CM, Varady KA. Alternate day fasting (ADF) with a high-fat diet produces similar weight loss and cardio-protection as ADF with a low-fat diet. Metabolism: clinical and experimental. 2013;62(1):137–143. [DOI] [PubMed] [Google Scholar]
- 73.Eshghinia S, Mohammadzadeh F. The effects of modified alternate-day fasting diet on weight loss and CAD risk factors in overweight and obese women. J Diabetes Metab Disord. 2013;12(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Varady KA, Bhutani S, Klempel MC, et al. Alternate day fasting for weight loss in normal weight and overweight subjects: a randomized controlled trial. Nutr J. 2013;12(1):146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hoddy KK, Kroeger CM, Trepanowski JF, Barnosky A, Bhutani S, Varady KA. Meal timing during alternate day fasting: Impact on body weight and cardiovascular disease risk in obese adults. Obesity (Silver Spring). 2014;22(12):2524–2531. [DOI] [PubMed] [Google Scholar]
- 76.Oh M, Kim S, An K-Y, et al. Effects of alternate day calorie restriction and exercise on cardiometabolic risk factors in overweight and obese adults: an exploratory randomized controlled study. BMC public health. 2018;18(1):1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Catenacci VA, Pan Z, Ostendorf D, et al. A randomized pilot study comparing zero-calorie alternate-day fasting to daily caloric restriction in adults with obesity. Obesity (Silver Spring). 2016;24(9):1874–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bowen J, Brindal E, James-Martin G, Noakes M. Randomized trial of a high protein, partial meal replacement program with or without alternate day fasting: similar effects on weight loss, retention status, nutritional, metabolic, and behavioral outcomes. Nutrients. 2018;10(9):1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Coutinho SR, Halset EH, Gåsbakk S, et al. Compensatory mechanisms activated with intermittent energy restriction: A randomized control trial. Clinical Nutrition. 2018;37(3):815–823. [DOI] [PubMed] [Google Scholar]
- 80.Stekovic S, Hofer SJ, Tripolt N, et al. Alternate day fasting improves physiological and molecular markers of aging in healthy, non-obese humans. Cell metabolism. 2019;30(3):462–476. e465. [DOI] [PubMed] [Google Scholar]
- 81.Viegener BJ RD, McKelvey WF, Schein RL, Perri MG, Nezu AM. Effects of an intermittent, low-fat, low-calorie diet in the behavioral treatment of obesity. Behavior Therapy. 1990;21(4):499–509. [Google Scholar]
- 82.Kalam F, Gabel K, Cienfuegos S, et al. Alternate day fasting combined with a low-carbohydrate diet for weight loss, weight maintenance, and metabolic disease risk reduction. Obesity Science & Practice. 2019;0(0). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Trepanowski JF, Kroeger CM, Barnosky A, et al. Effect of Alternate-Day Fasting on Weight Loss, Weight Maintenance, and Cardioprotection Among Metabolically Healthy Obese Adults: A Randomized Clinical Trial. JAMA Intern Med. 2017;177(7):930–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Trepanowski JF, Kroeger CM, Barnosky A, et al. Effects of alternate-day fasting or daily calorie restriction on body composition, fat distribution, and circulating adipokines: Secondary analysis of a randomized controlled trial. Clinical Nutrition. 2018(In Press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Parvaresh A, Razavi R, Abbasi B, et al. Modified alternate-day fasting vs. calorie restriction in the treatment of patients with metabolic syndrome: A randomized clinical trial. Complement Ther Med. 2019;47:102187. [DOI] [PubMed] [Google Scholar]
- 86.Alhamdan BA, Garcia-Alvarez A, Alzahrnai AH, et al. Alternate-day versus daily energy restriction diets: which is more effective for weight loss? A systematic review and meta-analysis. Obesity Science & Practice. 2016;2(3):293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hoddy KK, Kroeger CM, Trepanowski JF, Barnosky AR, Bhutani S, Varady KA. Safety of alternate day fasting and effect on disordered eating behaviors. Nutrition Journal. 2015;14(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kroeger CM, Trepanowski JF, Klempel MC, et al. Eating behavior traits of successful weight losers during 12 months of alternate-day fasting: An exploratory analysis of a randomized controlled trial. Nutrition and health. 2018;24(1):5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Varady KA, Hoddy KK, Kroeger CM, et al. Determinants of weight loss success with alternate day fasting. Obes Res Clin Pract. 2016;10(4):476–480. [DOI] [PubMed] [Google Scholar]
- 90.Davis KK, Tate DF, Lang W, et al. Racial Differences in Weight Loss Among Adults in a Behavioral Weight Loss Intervention: Role of Diet and Physical Activity. J Phys Act Health. 2015;12(12):1558–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Svetkey LP, Clark JM, Funk K, et al. Greater weight loss with increasing age in the weight loss maintenance trial. Obesity (Silver Spring). 2014;22(1):39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mitchell NS, Furniss AL, Helmkamp LJ, Van Pelt RE. Factors Associated with Achievement of Clinically Significant Weight Loss by Women in a National Nonprofit Weight Loss Program. Journal of Women’s Health. 2017;26(8):911–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Marquez B, Murillo R. Racial/Ethnic Differences in Weight-Loss Strategies among US Adults: National Health and Nutrition Examination Survey 2007–2012. J Acad Nutr Diet. 2017;117(6):923–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.DeLany JP, Jakicic JM, Lowery JB, Hames KC, Kelley DE, Goodpaster BH. African American women exhibit similar adherence to intervention but lose less weight due to lower energy requirements. Int J Obes (Lond). 2014;38(9):1147–1152. [DOI] [PubMed] [Google Scholar]
- 95.Bhutani S, Klempel MC, Kroeger CM, et al. Effect of exercising while fasting on eating behaviors and food intake. Journal of the International Society of Sports Nutrition. 2013;10(1):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Klempel MC, Bhutani S, Fitzgibbon M, Freels S, Varady KA. Dietary and physical activity adaptations to alternate day modified fasting: implications for optimal weight loss. Nutr J. 2010;9:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Heilbronn LK, Civitarese AE, Bogacka I, Smith SR, Hulver M, Ravussin E. Glucose tolerance and skeletal muscle gene expression in response to alternate day fasting. Obes Res. 2005;13(3):574–581. [DOI] [PubMed] [Google Scholar]
- 98.Hoddy KK, Gibbons C, Kroeger CM, et al. Changes in hunger and fullness in relation to gut peptides before and after 8 weeks of alternate day fasting. Clinical Nutrition. 2016;35(6):1380–1385. [DOI] [PubMed] [Google Scholar]
- 99.Varady KA, Bhutani S, Klempel MC, Lamarche B. Improvements in LDL particle size and distribution by short-term alternate day modified fasting in obese adults. British journal of nutrition. 2011;105(4):580–583. [DOI] [PubMed] [Google Scholar]
- 100.Klempel MC, Kroeger CM, Varady KA. Alternate day fasting increases LDL particle size independently of dietary fat content in obese humans. Eur J Clin Nutr. 2013;67(7):783–785. [DOI] [PubMed] [Google Scholar]
- 101.Hoddy KK, Bhutani S, Phillips SA, Varady KA. Effects of different degrees of insulin resistance on endothelial function in obese adults undergoing alternate day fasting. Nutr Healthy Aging. 2016;4(1):63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gabel K, Kroeger CM, Trepanowski JF, et al. Differential Effects of Alternate-Day Fasting Versus Daily Calorie Restriction on Insulin Resistance. Obesity (Silver Spring). 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chaix A, Manoogian ENC, Melkani GC, Panda S. Time-Restricted Eating to Prevent and Manage Chronic Metabolic Diseases. Annu Rev Nutr. 2019;39:291–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ruderman NB AT, Cahill GF. Gluconeogenesis and its disorders in man In Gluconeogenesis: Its Regulation in Mammalian New York: Wiley; 1976. [Google Scholar]
- 105.Anton SD, Lee SA, Donahoo WT, et al. The Effects of Time Restricted Feeding on Overweight, Older Adults: A Pilot Study. Nutrients. 2019;11(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jamshed H, Beyl RA, Della Manna DL, Yang ES, Ravussin E, Peterson CM. Early Time-Restricted Feeding Improves 24-Hour Glucose Levels and Affects Markers of the Circadian Clock, Aging, and Autophagy in Humans. Nutrients. 2019;11(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ravussin E, Beyl RA, Poggiogalle E, Hsia DS, Peterson CM. Early Time-Restricted Feeding Reduces Appetite and Increases Fat Oxidation But Does Not Affect Energy Expenditure in Humans. Obesity. 2019;27(8):1244–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab. 2018;27(6):1212–1221 e1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hutchison AT, Regmi P, Manoogian ENC, et al. Time-Restricted Feeding Improves Glucose Tolerance in Men at Risk for Type 2 Diabetes: A Randomized Crossover Trial. Obesity (Silver Spring). 2019;27(5):724–732. [DOI] [PubMed] [Google Scholar]
- 110.Antoni R, Robertson TM, Robertson MD, Johnston JD. A pilot feasibility study exploring the effects of a moderate time-restricted feeding intervention on energy intake, adiposity and metabolic physiology in free-living human subjects. Journal of Nutritional Science. 2018;7:e22. [Google Scholar]
- 111.Gabel K, Hoddy KK, Haggerty N, et al. Effects of 8-hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: A pilot study. Nutr Healthy Aging. 2018;4(4):345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Carlson O, Martin B, Stote KS, et al. Impact of reduced meal frequency without caloric restriction on glucose regulation in healthy, normal-weight middle-aged men and women. Metabolism: clinical and experimental. 2007;56(12):1729–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Stote KS, Baer DJ, Spears K, et al. A controlled trial of reduced meal frequency without caloric restriction in healthy, normal-weight, middle-aged adults. Am J Clin Nutr. 2007;85(4):981–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Moro T, Tinsley G, Bianco A, et al. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J Transl Med. 2016;14(1):290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cioffi I, Evangelista A, Ponzo V, et al. Intermittent versus continuous energy restriction on weight loss and cardiometabolic outcomes: a systematic review and meta-analysis of randomized controlled trials. J Transl Med. 2018;16(1):371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Van Cauter E, Blackman JD, Roland D, Spire JP, Refetoff S, Polonsky KS. Modulation of glucose regulation and insulin secretion by circadian rhythmicity and sleep. J Clin Invest. 1991;88(3):934–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Krauchi K, Wirz-Justice A. Circadian rhythm of heat production, heart rate, and skin and core temperature under unmasking conditions in men. Am J Physiol. 1994;267(3 Pt 2):R819–829. [DOI] [PubMed] [Google Scholar]
- 118.Zitting K-M, Vujovic N, Yuan RK, et al. Human Resting Energy Expenditure Varies with Circadian Phase. Current Biology. 2018;28(22):3685–3690.e3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Morris CJ, Yang JN, Garcia JI, et al. Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proc Natl Acad Sci U S A. 2015;112(17):E2225–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Acosta A, Camilleri M. A working paradigm for the treatment of obesity in gastrointestinal practice. Tech Gastrointest Endosc. 2017;19(1):52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chaix A, Lin T, Le HD, Chang MW, Panda S. Time-Restricted Feeding Prevents Obesity and Metabolic Syndrome in Mice Lacking a Circadian Clock. Cell Metab. 2019;29(2):303–319 e304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chaix A, Zarrinpar A, Miu P, Panda S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell metabolism. 2014;20(6):991–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hatori M, Vollmers C, Zarrinpar A, et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15(6):848–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kentish SJ, Hatzinikolas G, Li H, Frisby CL, Wittert GA, Page AJ. Time-Restricted Feeding Prevents Ablation of Diurnal Rhythms in Gastric Vagal Afferent Mechanosensitivity Observed in High-Fat Diet-Induced Obese Mice. The Journal of Neuroscience. 2018;38(22):5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Froy O, Chapnik N, Miskin R. Effect of intermittent fasting on circadian rhythms in mice depends on feeding time. Mechanisms of Ageing and Development. 2009;130(3):154–160. [DOI] [PubMed] [Google Scholar]
- 126.Roky R, Iraki L, HajKhlifa R, Lakhdar Ghazal N, Hakkou F. Daytime alertness, mood, psychomotor performances, and oral temperature during Ramadan intermittent fasting. Ann Nutr Metab. 2000;44(3):101–107. [DOI] [PubMed] [Google Scholar]
- 127.Bogdan A, Bouchareb B, Touitou Y. Ramadan fasting alters endocrine and neuroendocrine circadian patterns. Meal-time as a synchronizer in humans? Life Sci. 2001;68(14):1607–1615. [DOI] [PubMed] [Google Scholar]
- 128.Faris MeA-IE, Jahrami HA, Alhayki FA, et al. Effect of diurnal fasting on sleep during Ramadan: a systematic review and meta-analysis. Sleep and Breathing. 2019. [DOI] [PubMed] [Google Scholar]
- 129.Centofanti S, Dorrian J, Hilditch C, Grant C, Coates A, Banks S. Eating on nightshift: A big vs small snack impairs glucose response to breakfast. Neurobiol Sleep Circadian Rhythms. 2018;4:44–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Grant CL, Coates AM, Dorrian J, et al. Timing of food intake during simulated night shift impacts glucose metabolism: A controlled study. Chronobiology International. 2017;34(8):1003–1013. [DOI] [PubMed] [Google Scholar]


