Abstract
Background
Although the regression of symptomatic lumbar disc herniation (SLDH) has been widely reported, little data exist regarding the generalized incidence of regression (IR). We aimed to review the varying IRs and to synthesize the pooled IR of non-surgically-treated SLDH.
Methods
Four electronic databases were searched for relevant studies pertaining to the regression of SLDH after non-surgical treatment and for potential studies that may have reported morphological changes in lumbar disc herniation in the follow-up results of SLDH patients treated non-surgically. The main outcome was the regression of SLDH. A random effects model was used to determine the pooled IR of SLDH.
Results
We identified 13,672 articles, 38 of which were eligible for analysis. Our analysis included 2219 non-surgically treated SLDH patients, 1425 of whom presented regression. The pooled IR was 63% (95% CI 0.49–0.77). In subgroup analyses, studies that quantitatively measured the regression of SLDH yielded statistically higher pooled IRs than those that used qualitative methods. The pooled IRs gradually increased in randomized controlled trials and prospective and retrospective studies. The pooled IR varied from 62 to 66% after the sequential omission of any single study. Meta-regression showed that study types, herniation levels and regression measurements caused heterogeneity.
Conclusions
We report an overall IR of 63% among non-surgically treated SLDH patients, thus providing clinical decision makers with quantitative evidence of IR. Based on our systematic review, we suggest a follow-up timeline with time points 4 and 10.5 months after onset when deciding whether to perform surgery for SLDH.
Keywords: Lumbar disc herniation, Non-surgical treatment, Incidence of regression
Background
Symptomatic lumbar disc herniation (SLDH) can be treated non-surgically or surgically. Non-surgical treatment was shown to be effective for SLDH long ago [1], although surgery results in more rapid and effective short-term alleviation of symptoms than non-surgical treatment [2, 3]. However, the long-term effects of the two have not been consistently reported [2–4], and there is a risk of complications with surgery [5]. Thus, in many cases, there is not a clear correct decision regarding the use of surgical or non-surgical treatments for SLDH [6].
Since the first case of regression after the non-surgical treatment of SLDH was reported in 1984 [7], the phenomenon of SLDH regression has been widely reported [8–46], with the incidence of regression (IR) varying from study to study. Reports on the correlation between the regression of SLDH and clinical outcomes have been contradictory: an early study observed a connection between morphological changes in SLDH and clinical outcomes [41], while later studies found that the regression of SLDH does not correspond with the resolution of symptoms [9, 47]. However, we cannot ignore the physical decompression that occurs during regression in the acute context of SLDH, and the probable regression of SLDH still needs to be considered in clinical practice, according to the guidelines of the North American Spine Society [48]. Understanding the IR of SLDH is clearly of clinical importance. However, scant generalized data regarding the IR are currently available to serve as a reference. When making clinical decisions regarding SLDH, practitioners and patients have little high-level evidence regarding IR to which they can refer.
We therefore performed a systematic review and meta-analysis to provide a comprehensive examination of the IR of SLDH in patients who were treated non-surgically.
Methods
This systematic review and meta-analysis is reported in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [49]. We did not publish a prior protocol for this systematic review and meta-analysis.
Search strategy
For this systematic review and meta-analysis, we searched PubMed, Embase, the Cochrane Central Register of Controlled Trials, and the Web of Science (from inception to September 16, 2019). Search terms included those related to intervertebral disc herniation, regression, comparison, outcome, follow-up, image, and their variants. To avoid missing articles without information about the language in the database records, there was no language limitation in the literature search. A sample search strategy can be found in an additional file. We included studies identified from the references of included articles and other review articles on the topic. Two reviewers performed the searches. Disagreements were resolved by discussion with a third reviewer.
Eligibility and exclusion criteria
Relevant articles pertaining to the phenomenon of the regression of SLDH after non-surgical treatment and potential studies that may have reported morphological changes in lumbar disc herniation (LDH) among the follow-up results for non-surgically-treated SLDH patients were included, with the publication language restricted to English. Randomized controlled trials (RCTs) and nonrandomized studies were eligible for inclusion. The following studies were excluded: 1. Studies that only reported the follow-up results of surgery, including percutaneous endoscopic transforaminal discectomy, microendoscopic discectomy, microdiscectomy, fenestration discectomy, open discectomy, lumbar laminectomy, lumbar interbody fusion and radiofrequency ablation; 2. Studies on cervical discs; 3. Studies that did not report the morphological changes in SLDH; 4. Studies that did not report the number of patients exhibiting regression; 5. Studies on only intradiscal injections, including oxygen-ozone therapy, plasma injection and collagenase chemonucleolysis; 6. Studies on asymptomatic LDH; 7. Studies with less than 10 patients at follow-up; 8. Animal studies; 9. Reviews; and 10. Studies that did not report specific non-surgical treatment.
Quality assessment
The quality of the nonrandomized studies was assessed based on the Methodological Index for Nonrandomized Studies (MINORS) [50]. There is no consensus on when the regression of LDH occurs; thus, item six of the MINORS (follow-up period appropriate to the aim of the study) was not applicable, and the highest total score was 14 (high quality: 10–14; moderate quality: five-nine; and low quality: zero-four). The risk of bias of RCTs was evaluated using a tool from the Cochrane Collaboration [51]. Considering the nature of RCTs of the non-surgical treatment of SLDH, performance bias was generally not a particular concern and had a minor impact on the study quality. Thus, we considered all the included RCTs to have a low risk of performance bias. RCTs were categorized as having a high, low, or unclear risk according to the following criteria: high risk, any item presented a high risk; low risk, no more than 2 items presented an unclear risk; and unclear risk, more than 2 items presented an unclear risk. Two reviewers independently assessed the quality of the included studies and extracted the data. Disagreements were resolved by consensus with a third reviewer.
Data extraction and analysis
Relevant data were extracted using a standardized form that included the publication year, country, study type, study quality or risk of bias, LDH level, regression measurement, imaging method, patient count, total number of SLDH patients at follow-up and number of patients with SLDH regression, as well as age, symptom duration, nerve symptoms, whether regression was defined and follow-up duration. The primary outcome was the IR of SLDH after non-surgical treatment. The IR was estimated based on the total number of SLDH patients at follow-up and the number of patients that experienced regression. For studies that recorded the number of patients according to the regressed proportion or size interval but did not define the interval of non-regression or the number of patients without regression, we regarded the lowest interval as the no-regression range, and the number of patients outside of this interval was considered the number of patients with regression. For studies in which more than two imaging examinations were performed, we used the author’s final count, and if no final count was provided, the latest imaging examinations with enough information were compared to the baseline examinations. For studies reporting the same cohort or trial, only the latest study was included. For studies with overlapping data, we selected the study with the highest number of patients at the last follow-up. Herniations after baseline were not counted. For RCTs, we calculated the total number of occurrences in the two groups.
The I2 statistic was employed to evaluate the heterogeneity of pooled data, and the DerSimonian and Laird random effects model was used to pool the IRs with corresponding 95% confidence intervals (CIs). Incidences from studies with zero events were treated by adding 0.5 cases to both the numerator (number of patients with regression) and denominator (total number of SLDH patients), consistent with recommended practices [52]. Subgroup analysis was performed by stratifying the studies according to the time period, region, study type, LDH level, regression measurement, imaging method and method used to determine the patient count. Potential sources of heterogeneity were explored by meta-regression with a p value less than 0.1. Sensitivity analysis was performed by including only high-quality non-randomized studies and low-risk RCTs and by sequentially excluding each study. Publication bias was assessed using Egger’s test and was visualized with a funnel plot. All statistical analyses were performed using the Meta and metafor packages in R (V3.6.1) [53].
Results
Study selection and characteristics
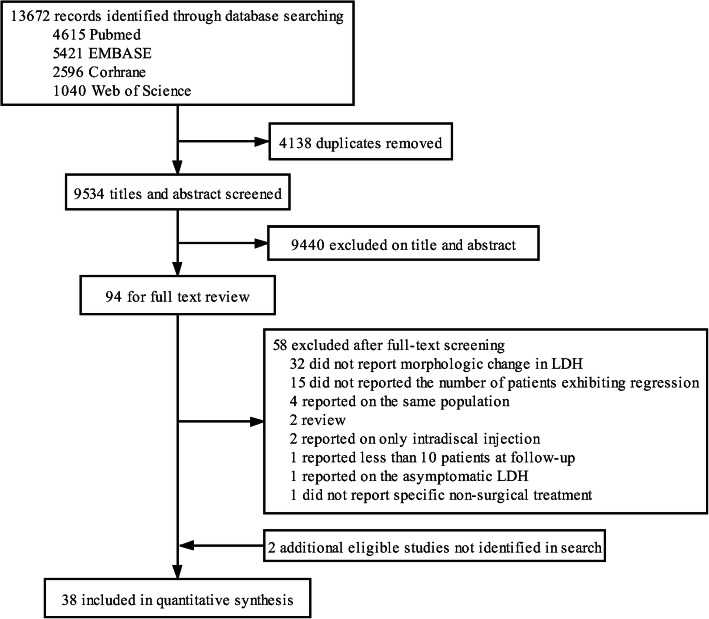
Our initial search yielded 13,672 articles, and two were hand-selected from reference lists. A total of 38 articles were included in the final meta-analysis (Fig. 1). The non-surgical treatment used in these studies included bed rest, lumbar support, traction, spinal manipulation, physical therapy, exercise, oral steroids, analgesics, nonsteroidal anti-inflammatory agents, epidural block, caudal epidural injections, traditional Chinese medicine and alternative medicine. These articles included 5 RCTs and 33 nonrandomized studies. The studies were from Asia, Europe and North America and were from a total of 13 countries. Japan contributed seven studies; Korea and the USA each contributed five; Turkey contributed four; China, the UK and Italy each contributed three; France and Finland each contributed two; and Denmark, Germany, the Netherlands and Sweden each contributed one. The imaging examinations used were magnetic resonance imaging (MRI) in 29 studies and computed tomography (CT) in eight studies, and one study used CT at baseline and MRI at follow-up. The characteristics of the included studies are summarized in Table 1. These studies reported patient age (14–78), symptom duration (one day-ten years) and follow-up time (20 days – 6.1 years) in different formats (Table 2). A total of 16 studies did not report symptom duration, five studies did not report whether nerve symptoms were experienced by all patients or by a subset of patients, and five studies did not describe or define regression (Table 2).
Fig. 1.
Study selection
Table 1.
Characteristics of the included studies
| Author | Year | Country | Study type | Qualitya | LDH Levelb | Measurement | Imaging method | Countingc | Number of patients | |
|---|---|---|---|---|---|---|---|---|---|---|
| Regression | Total | |||||||||
| El Barzouhi et al. [9] | 2013 | Netherlands | RCT | Low risk | single | Qualitative | MRI | A | 88 | 95 |
| Santilli et al. [10] | 2006 | Italy | RCT | Low risk | single/multiple | Qualitative | MRI | A | 0 | 102 |
| Fan et al. [11] | 2015 | China | RCT | Unclear risk | Unknow | Qualitative | MRI | A | 0 | 158 |
| Ahn et al. [12] | 2002 | Korea | Prospective | 11 | single | Qualitative | MRI | B | 13 | 17 |
| Maigne et al. [13] | 1992 | France | Prospective | 10 | single | Qualitative | CT | B | 39 | 48 |
| Benson et al. [14] | 2010 | UK | Prospective | 12 | single | Quantitative | MRI | B | 28 | 32 |
| Komori et al. [15] | 1998 | Japan | Retrospective | 12 | single | Qualitative | MRI | A | 19 | 22 |
| Modic et al. [16] | 1995 | USA | Prospective | 12 | single/multiple | Qualitative | MRI | B | 4 | 16 |
| Kamanli et al. [17] | 2010 | Turkey | Prospective | 8 | Unknown | Qualitative | MRI | A | 5 | 26 |
| Autio et al. [18] | 2006 | Finland | Prospective | 12 | single | Quantitative | MRI | A | 51 | 55 |
| Gallucci et al. [19] | 1995 | Italy | Prospective | 10 | Unknown | Qualitative | MRI | B | 11 | 15 |
| Ozturk et al. [20] | 2006 | Turkey | RCT | Low risk | single/multiple | Quantitative | CT | A | 19 | 46 |
| Ahn et al. [21] | 2000 | Korea | Prospective | 12 | single | Quantitative | MRI | A | 25 | 36 |
| Ilkko et al. [22] | 1993 | Finland | Unknown | 7 | single/multiple | Qualitative | CT | A | 15 | 18 |
| Delauche-Cavallier et al. [23] | 1992 | France | Prospective | 9 | single | Qualitative | CT | A | 14 | 21 |
| Buttermann et al. [24] | 2002 | USA | Prospective | 10 | single | Quantitative | MRI | A | 52 | 58 |
| Bozzao et al. [25] | 1992 | Italy | Prospective | 10 | Unknow | Qualitative | MRI | A | 41 | 65 |
| Broetz et al. [26] | 2008 | Germany | Prospective | 9 | Unknow | Qualitative | MRI | A | 0 | 10 |
| Jensen et al. [27] | 2006 | Denmark | Prospective | 10 | Unknow | Qualitative | MRI | A | 65 | 139 |
| Henmi et al. [28] | 2002 | Japan | Unknown | 11 | single | Quantitative | MRI | A | 4 | 10 |
| Takada et al. [29] | 2001 | Japan | Prospective | 9 | single | Qualitative | MRI | A | 37 | 42 |
| Cribb et al. [30] | 2007 | England | Unknown | 7 | single | Quantitative | MRI | A | 14 | 15 |
| Ellenberg et al. [31] | 1993 | USA | Prospective | 9 | single/multiple | Qualitative | CT | A | 11 | 14 |
| Demirel et al. [32] | 2017 | Turkey | RCT | Low risk | single/multiple | Quantitative | MRI | A | 18 | 20 |
| Hong et al. [33] | 2016 | Korea | Retrospective | 10 | single | Quantitative | MRI | A | 24 | 28 |
| Matsubara et al. [34] | 1995 | Japan | Unknown | 9 | single | Qualitative | MRI | B | 20 | 32 |
| Yukawa et al. [35] | 1996 | Japan | Unknown | 11 | single | Quantitative | MRI | A | 17 | 30 |
| Fagerlund et al. [36] | 1990 | Sweden | Prospective | 12 | single | Qualitative | CT | B | 22 | 30 |
| Kesikburun et al. [37] | 2019 | Turkey | Prospective | 12 | single | Quantitative | MRI | A | 36 | 40 |
| Teplick et al. [38] | 1985 | USA | Unknown | 7 | Unknown | Qualitative | CT | A | 11 | 55 |
| Shan et al. [39] | 2014 | China | Retrospective | 12 | single | Quantitative | MRI | A | 24 | 30 |
| Shin et al. [40] | 2017 | Korea | Prospective | 8 | Unknown | Qualitative | MRI | A | 42 | 73 |
| Komori et al. [41] | 1996 | Japan | Retrospective | 10 | single | Qualitative | MRI | A | 49 | 77 |
| Saal et al. [42] | 1990 | USA | Unknown | 8 | Unknown | Qualitative | CT/MRI | B | 9 | 11 |
| Bush et al. [43] | 1992 | UK | Prospective | 10 | single/multiple | Qualitative | CT | A | 71 | 111 |
| Iwabuchi et al. [44] | 2010 | Japan | Prospective | 10 | single | Qualitative | MRI | A | 21 | 34 |
| Yu et al. [45] | 2014 | China | Unknown | 10 | single | Qualitative | MRI | A | 20 | 83 |
| Lee et al. [46] | 2017 | Korea | Retrospective | 10 | single | Quantitative | MRI | A | 486 | 505 |
aQuality of non-randomized studies was assessed following the MINORS; the risk of bias of RCTs was evaluated using a tool from the Cochrane Collaboration
bSome studies included only single-level SLDH patients and some studies included both single- and multiple-level SLDH patients
cCounting. A. The number of patients with regression was reported and was extracted from the publication. B: For studies that recorded the number of patients by the regression proportion or size interval but did not define the interval of non-regression or report the number of patients without regression, we regarded the lowest interval as the no regression range, and the number of patients outside of this interval was considered the number of patients with regression
Table 2.
Other characteristics of the included studies
| Author | Year | Age | Duration of symptom | Nerve symptom | Regression defined | Follow-up |
|---|---|---|---|---|---|---|
| El Barzouhi et al. [9] | 2013 | 18–65 | 6–12 W | Yes | Yes | 1Y |
| Santilli et al. [10] | 2006 | 18–65 | Less than 10 D | Yes | Yes | 45D |
| Fan et al. [11] | 2015 | Unknown | Unknown | Yes | No | 20D |
| Ahn et al. [12] | 2002 | 19–73 (42.7) | 1-10 W(median 4.5 W) | Some | Yes | 3-11 M(11.9 M) |
| Maigne et al. [13] | 1992 | 26–75 (45.2) | Unknown | Yes | Yes | 1-48 M |
| Benson et al. [14] | 2010 | 25–62 (40.4) | More than 6 W | Yes | Yes | 3-42 M(13.2 M) |
| Komori et al. [15] | 1998 | 20–75 (41) | 2-359D(54D) | Yes | Yes | 27-856D(151D) |
| Modic et al. [16] | 1995 | 22–75 (49.2) | Less than 2 W | Yes | Yes | 6 W-6 M |
| Kamanli et al. [17] | 2010 | (37) | Unknown | Unknown | No | 4-6 W |
| Autio et al. [18] | 2006 | 19–78 | 3–28 W | Yes | Yes | 12 M |
| Gallucci et al. [19] | 1995 | 27–62 (37) | Unknown | Some | Yes | 6 M |
| Ozturk et al. [20] | 2006 | 16–70 | Less than 6 M | Some | Yes | 21D |
| Ahn et al. [21] | 2000 | 17–74 (39) | 1–28 M | Yes | Yes | 3-27 M(8.5 M) |
| Ilkko et al. [22] | 1993 | 35–74 (53) | Unknown | Some | Yes | 4.3–6.1Y(5.2Y) |
| Delauche-Cavallier et al. [23] | 1992 | 20–64 (43) | 15D-6 M(2 M) | Yes | Yes | 6-27 M(12.9 M) |
| Buttermann et al. [24] | 2002 | 18–70 | Unknown | Yes | Yes | 18 ± 10&19 ± 9 M |
| Bozzao et al. [25] | 1992 | 23–65 (52) | 1 M-1Y | Some | Yes | 6-15 M(11 M) |
| Broetz et al. [26] | 2008 | 18–65 | 5D-2Y(median 5 W) | Yes | No | 3-7D(5D) |
| Jensen et al. [27] | 2006 | 18–65 (45) | 1-3 M | Yes | Yes | 12 M |
| Henmi et al. [28] | 2002 | 20–50 | 1-200D | Yes | Yes | 6-12 M |
| Takada et al. [29] | 2001 | 16–64 (42) | 1-14 W | Yes | Yes | 3-24 M(10.3 M) |
| Cribb et al. [30] | 2007 | 24–73 (45) | Unknown | Yes | No | 5-56 M(24 M) |
| Ellenberg et al. [31] | 1993 | 28–67 (42) | Unknown | Yes | Yes | 6-18 M(9.8 M) |
| Demirel et al. [32] | 2017 | 50.7 | Unknown | Unknown | Yes | 3 M |
| Hong et al. [33] | 2016 | 26–78 (50.2) | Unknown | Unknown | Yes | 2-31 M(8.8 M) |
| Matsubara et al. [34] | 1995 | 16–52 (36) | Unknown | Yes | Yes | 3-18 M(9.7 M) |
| Yukawa et al. [35] | 1996 | 14–69 (39) | Unknown | Yes | Yes | 24-42 M(30 M) |
| Fagerlund et al. [36] | 1990 | 14–49 (35) | 6 ± 3 M | Unknown | Yes | 24 M |
| Kesikburun et al. [37] | 2019 | 39.7–71.5 (54.4) | 4.7–7.4 M(6 M) | No | Yes | 12-19 M(17 M) |
| Teplick et al. [38] | 1985 | Unknown | Unknown | Unknown | No | 3 M-5Y |
| Shan et al. [39] | 2014 | 20–66 (40) | 2 W-6 M | Yes | Yes | 6 M |
| Shin et al. [40] | 2017 | (35.8) | (2.7 M) | Yes | Yes | 3Y |
| Komori et al. [41] | 1996 | 18–86 (41) | 0.1–8.6 M(1.8 M) | Yes | Yes | 62-1208D(262D) |
| Saal et al. [42] | 1990 | Unknown | Unknown | Yes | Yes | 8-77 M(25) |
| Bush et al. [43] | 1992 | 17–72 (41) | 1-72 M(4.2 M) | Yes | Yes | 1Y |
| Iwabuchi et al. [44] | 2010 | −52 | Unknown | Yes | Yes | (4.1 M) |
| Yu et al. [45] | 2014 | 16–60 (38.7) | 3D-10Y(16.5 M) | Yes | Yes | 2-24 M |
| Lee et al. [46] | 2017 | 39.08 ± 10.19 | Unknown | Yes | Yes | 341.38 ± 306.83D |
Average values were presented in parentheses and “±”is connected to Mean and SD if available. D Day, M Month, Y Year
Quality assessment
Of the 5 included RCTs, 4 showed a low risk of bias, and 1 showed an unclear risk of bias. Of the 33 included non-randomized studies, 22 were of high quality, and 11 were of moderate quality (Table 1).
Incidence synthesis and data analysis
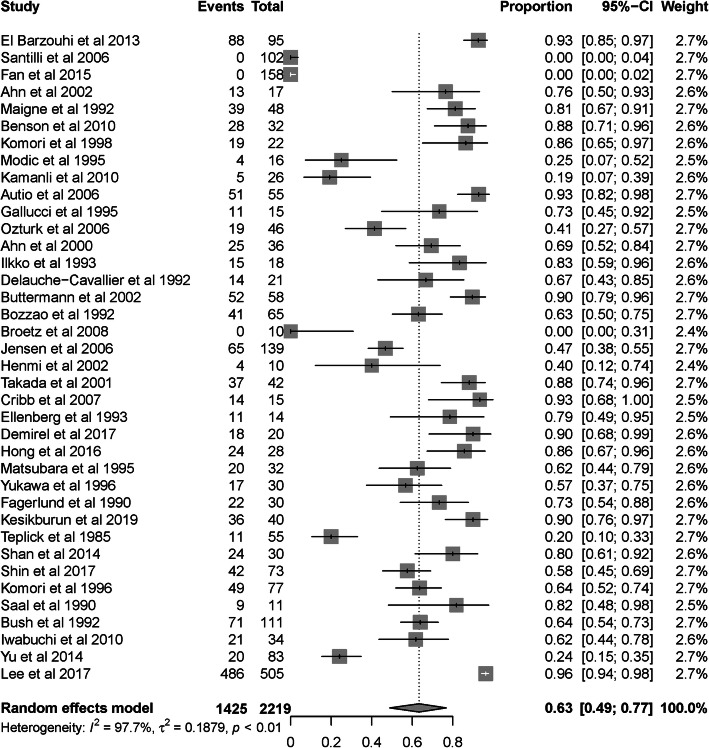
The pooled analysis for IR after the nonsurgical treatment of SLDH included 2219 patients, 1425 of whom presented regression. The pooled IR in our study was 63% (95% CI 0.49–0.77), with significant heterogeneity among the studies (I2 = 97.7%, p < 0.001; Fig. 2).
Fig. 2.
Overall IR after the non-surgical treatment of SLDH. Weights are from the random effects analysis. Grey squares represent the proportional weight of each study in the meta-analysis. The pooled incidence and CIs from studies with zero events were treated by adding 0.5 cases to both the numerator (number of patients with regression) and denominator (total number of SLDH patients)
Subgroup analyses (Table 3) showed that studies that quantitatively measured the regression of SLDH yielded statistically higher (p = 0.02) pooled IRs (81, 95% CI 0.69–0.91) than those that adopted qualitative methods (54, 95% CI 0.37–0.70). We repeated subgroup analyses based on the time period of the study and did not identify any secular trends in the IR of non-surgically treated patients before 2000 (65, 95% CI 0.55–0.75), from 2000 to 2009 (57%, 0.29–0.83), or from 2010 to 2019 (66%, 0.35–0.91). We found no significant regional variation within Asia (63, 95% CI 0.40–0.83), Europe (65%, 0.43–0.85), and North America (60%, 0.22–0.92). The pooled IR gradually increased in RCTs (37, 95% CI 0.00–0.88), prospective studies (67%, 0.57–0.77), and retrospective (84%, 0.65–0.97) studies. Studies of single-level SLDH patients (78, 95% CI 0.67–0.87) yielded higher pooled IRs than those that included both single- and multiple-level SLDH patients (51%, 0.17–0.86). Studies based on MRI yielded the same pooled IR (63, 95% CI 0.46–0.79) as those based on CT (63, 95% CI 0.45–0.79); the IR was calculated as 82% in Saal’s research [42], in which CT was used at baseline and MRI was used at follow-up. Studies that reported the number of patients without regression yielded lower pooled IRs (61, 95% CI 0.44–0.77) than those that did not define regression or reported the number of patients without regression (72%, 0.59–0.83).
Table 3.
Subgroup analyses of the regression measurement, time period, region, study type, LDH level, imaging method and patient count
| Included studies (n) | Number of patients | Incidence (95% CI) | P value* | ||
|---|---|---|---|---|---|
| With Regression | Total | ||||
| Measurement | 38 | 1425 | 2219 | 63%(0·49–0·77) | 0.01 |
| Qualitative | 25 | 627 | 1314 | 54% (0·37–0·70) | |
| Quantitative | 13 | 798 | 905 | 81% (0·69–0·91) | |
| Time period | 38 | 1425 | 2219 | 63%(0·49–0·77) | 0.87 |
| Before 2000 | 15 | 353 | 565 | 65% (0·55–0·75) | |
| 2000–2009 | 11 | 280 | 530 | 57% (0·29–0·83) | |
| 2010–2019 | 12 | 792 | 1124 | 66% (0·35–0·91) | |
| Region | 38 | 1425 | 2219 | 63%(0·49–0·77) | 0.97 |
| Asia | 19 | 879 | 1309 | 63% (0·40–0·83) | |
| Europe | 14 | 459 | 756 | 65% (0·43–0·85) | |
| North America | 5 | 87 | 154 | 60% (0·22–0·92) | |
| Study type | 30a | 1315 | 1965 | 65%(0·48–0·80) | 0.14 |
| RCT | 5 | 125 | 421 | 37% (0·00–0·88) | |
| Prospective | 20 | 588 | 882 | 67% (0·57–0·77) | |
| Retrospective | 5 | 602 | 662 | 84% (0·65–0·97) | |
| LDH level | 29b | 1241 | 1667 | 72% (0·58–0·84) | 0.17 |
| Single | 22 | 1103 | 1340 | 78% (0·67–0·87) | |
| Single/multiple | 7 | 138 | 327 | 51% (0·17–0·86) | |
| Imaging method | 37c | 1416 | 2208 | 63%(0·48–0·76) | 0.97 |
| CT | 8 | 202 | 343 | 63% (0·46–0·79) | |
| MRI | 29 | 1214 | 1865 | 63% (0·45–0·79) | |
| Countingd | 38 | 1425 | 2219 | 63%(0·49–0·77) | 0.33 |
| A | 30 | 1279 | 2018 | 61% (0·44–0·77) | |
| B | 8 | 146 | 201 | 72% (0·59–0·83) | |
*P value is from the test for subgroup differences (random effects model)
aEight studies did not report study type
bNine studies did not report LDH level
cOne study used CT at basline and MRI at follow-up
dCounting. A. The number of patients with regression was reported and was extracted from the publication. B. For studies that recorded the number of patients by the regression proportion or size interval but did not define the interval of non-regression or report the number of patients without regression, we regarded the lowest interval as the no regression range, and the number of patients outside of this interval was considered the number of patients with regression
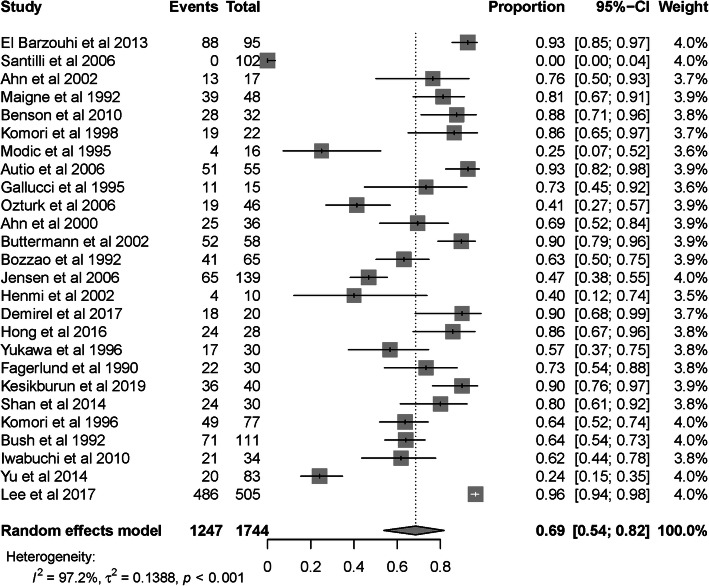
Meta-regression showed that study types (R2 = 41.94% p = 0.02), LDH levels (R2 = 31.53%, p = 0.05), and regression measurements (R2 = 41.94% p = 0.02) contributed to the heterogeneity. There was no significant change in the pooled IR (69, 95% CI 0.54–0.82) or heterogeneity (I2 = 97.2%, p < 0.001) when only high-quality non-randomized studies and low-risk RCTs were included (Fig. 3). The pooled IR varied from 62 to 66% after the sequential omission of any single study.
Fig. 3.
Forest plot for the meta-analysis of high-quality nonrandomized studies and low-risk RCTs
Publication bias
Egger’s test suggested that there was no publication bias (p = 0.46). No asymmetric patterns were seen in the funnel plot (Fig. 4).
Fig. 4.
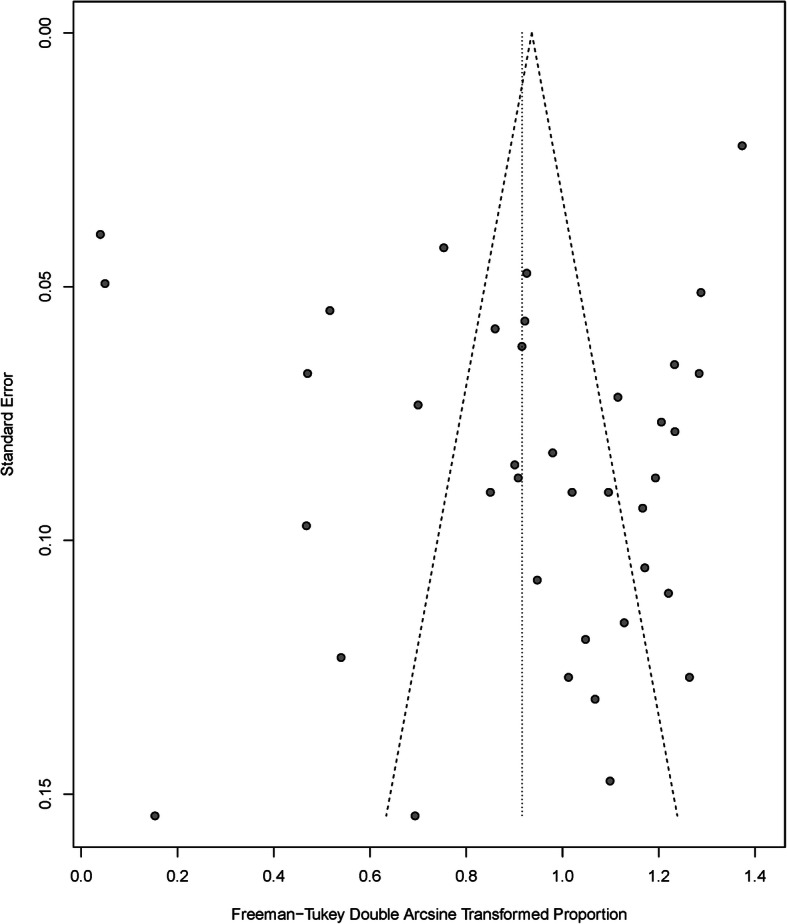
Funnel plot of incidence
Discussion
We found an IR of 63% after the non-surgical treatment of SLDH in the present systematic review and meta-analysis, with significant heterogeneity among the studies. Our pooled IR needs to be interpreted with caution.
We comprehensively searched for studies that potentially reported morphological changes in SLDH during clinical follow-up and that investigated the regression of SLDH. We conducted a wide database search, and a small number of articles were included. Because follow-up is a necessary step to study regression, “follow up” or “follow-up” or “outcome” or “result” was included in the search terms. The use of these search terms resulted in the retrieval of a large number of articles. However, there are so many non-surgical treatment methods for SLDH in the world that it is impossible to limit the specific non-surgical treatment methods in the literature search process. In addition, studies that compared the results of surgical and non-surgical treatment may have reported the morphological changes in herniated discs of the non-surgically treated patients, making it impossible to exclude studies on surgery. As a result, 13,672 articles were identified, more than half of which were studies on surgery for SLDH, and a small number of articles were included. Both RCTs and non-randomized studies were included in our study. The pooled IR in our study was similar to the IR of 66.66% that was reported in a previous review of 11 studies [54], and these IR values can be considered quantitative data that can inform clinical decisions regarding SLDH.
The highest IR (96%) was documented by Lee with an average follow-up of 341 days [46], suggesting that we should seriously consider the probability of SLDH regression. Three studies reported no regression with follow-ups of 45 days [10], 20 days [11], and a median of 5 days (3–7 days) [26], suggesting that SLDH regression should not be expected to occur within one and a half months of symptom onset. The average of the IRs reported in the included studies was 63%, which is the same as the pooled IR of the meta-analysis, and 7 studies reported IRs of approximately 63%: Ahn [21] reported an IR of 69% with an average follow-up time of 8.5 months, Delauche-Cavallier [23] reported an IR of 67% with an average follow-up time of 12.5 months, Bozzao [25] reported an IR of 63% with an average follow-up time of 11 months, Matsubara [34] reported an IR of 62% with an average follow-up time of 9.7 months, Komori [41] reported an IR of 64% with an average follow-up time of 262 days, Bush [43] reported an IR of 64% with an average follow-up time of 1 year, and Iwabuchi [44] reported an IR of 62% with an average follow-up time of 4.1 months. According to Iwabuchi’s report, which reported an IR that was consistent with the average of the IRs reported for the included studies and had a follow-up time of 4.1 months [44], we suggest that 4 months after onset is an important time point for imaging. The follow-up time of the other 6 studies with IRs of approximately 63% ranged from 8.5 to 12.9 months, with an average of 10.5 months. Therefore, we suggest that 10.5 months after onset is another important time point for imaging. There were 4 studies that reported long-term follow-up, with an average duration of more than 24 months: Fagerlund [36] reported an IR of 73% with a follow-up of 24 months, Yukawa [35] reported an IR of 57% with an average follow-up of 30 months, Shin [40] reported an IR of 58% with a follow-up of 3 years, and Ilkko [22] reported an IR of 83% with an average follow-up of 5.2 years. The IR trend reported by these articles over time was inconsistent; some reported that IR increased over time to above the average IR, some reported that IR decreased over time to fall below the average IR, and no secular trends were identified for long-term follow-up.
We did not classify SLDH during the data synthesis, as most of the studies included in our meta-analysis did not include classifications; this is in contrast to another review that calculated IR based on 9 articles reporting that sequestration, extrusion, protrusion and bulging were present in 96, 70, 41 and 13% of patients, respectively [55]. These IR classifications provide a more detailed reference. The probability of SLDH regression should be considered in clinical practice according to the guidelines of the North American Spine Society [48], and we provided an extensive summary of estimated IRs as evidence. Together with existing evidence, our research shows that the regression of SLDH should be fully considered by clinical decision makers. For patients without absolute indications for surgery, the regression of SLDH can be considered very likely, and surgery may be avoided for most patients. As some SLDH patients who were treated non-surgically did not experience regression, the effective prediction of SLDH regression should be explored in the future.
Our study revealed that the study types, LDH levels and regression measurements contributed to the heterogeneity. The increase in the risk of selection bias in the three study types (RCTs, prospective studies and retrospective studies [56, 57]) was consistent with the increase in the pooled IR of the three types of studies, explaining the heterogeneity observed among different study types. The pooled IR of studies that included only single-level SLDH patients was higher than that of those including both single- and multiple-level SLDH patients. Because there are usually more herniated disc tissues in single-level SLDH than in multiple-level SLDH, which induces a more robust inflammatory response, and the most likely mechanism underlying regression is an inflammatory response directed against the herniated disc tissues [58, 59], patients with single-level SLDH are more likely to experience regression than patients with multiple-level SLDH. We also found that studies that included quantitative measurements tended to report higher IRs for SLDH than studies that qualitatively measured LDH. The quantitative methods used in these included studies included 3D volume measurements and cross-sectional area measurements, while the qualitative methods used were visual estimations. Quantitative measurements were performed in millimetres or centimetres, and in some studies, they were accurate to one decimal place. In general, quantitative measurements are better for detecting small dimensional changes than visual assessments. In addition, intervertebral discs are three-dimensional irregularly shaped tissues, making it difficult to capture small changes in their volume on planar images using visual estimation. Quantitative measurements made it easier to record slight changes in the size of the discs on sagittal and cross-sectional views or changes in volume that are rarely detected by visual inspection due to the occurrence of slight changes in multiple directions. Both imaging methods have obvious defects that may cause inaccuracies. For quantitative measurements, it was impossible for the follow-up images to use the exact same slices that were initially scanned [60, 61]. For qualitative measurements, unclear borders and the three-dimensional characteristics of LDH made the judgement of regression inaccurate, especially for visual estimations. In the future, a more standardized and reliable method for determining the occurrence of SLDH regression needs to be established. Other factors, such as age, symptom duration, type of non-surgical treatment and follow-up time, may play a role in heterogeneity, these factors were documented in the included studies, but sufficient information was not available for determining whether these factors contributed to the heterogeneity.
Our study has limitations. We included studies in the meta-analysis without limiting the criteria or measurements for regression to ensure the robustness of IR synthesis, which inevitably led to the inclusion of sources of heterogeneity. Presently, there is no clear definition of the time frame for SLDH regression. The follow-up period of some of the included studies may not have been appropriate or long enough to observe the presence of SLDH regression, making the reported IR lower than the actual IR.
Conclusions
Our meta-analysis results supplement the guidelines of the North American Spine Society on the IR [48]. We revealed an overall IR of 63% among patients with SLDH who were treated non-surgically, thus providing clinical decision makers with quantitative evidence of IR. The probability of regression after the non-surgical treatment of SLDH should be fully considered before making decisions regarding surgery. Based on our systematic review, we suggest a follow-up timeline that consists of the time points 4 and 10.5 months after onset when deciding whether to perform surgery for SLDH. Surgery can be considered for patients with severe symptoms who do not experience regression after 4 months of onset, and we highly recommend surgery for those who do not experience regression after 10.5 months of onset.
Supplementary information
Acknowledgements
We thank Sawan Bopanna (Department of Gastroenterology, All India Institute of Medical Sciences, New Delhi, India) and Vineet Ahuja (Massachusetts General Hospital, Crohn’s and Colitis Center, Boston, MA, USA) for helping us with the quality assessment of non-controlled studies.
Abbreviations
- SLDH
Symptomatic lumbar disc herniation
- IR
Incidence of regression
- LDH
Lumbar disc herniation
- RCTs
Randomized controlled trials
- MINORS
Methodological Index for Non-Randomized Studies
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- CIs
Confidence intervals
Authors’ contributions
YW had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: YW, GD. Acquisition, analysis, or interpretation of data: all authors. Drafting of the manuscript: YW, LJ. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: YW, GD, SL. Obtained funding: YW. Administrative, technical, or material support: All authors. Supervision: GD. The author(s) read and approved the final manuscript.
Funding
This study was funded by Sichuan Provincial Orthopedics Hospital (grant number 2017–4).
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12891-020-03548-z.
References
- 1.Saal JA, Saal JS. Nonoperative treatment of herniated lumbar intervertebral disc with radiculopathy. An outcome study. Spine (Phila Pa 1976) 1989;14(4):431–437. doi: 10.1097/00007632-198904000-00018. [DOI] [PubMed] [Google Scholar]
- 2.Peul WC, van Houwelingen HC, van den Hout WB, Brand R, Eekhof JA, Tans JT, et al. Surgery versus prolonged conservative treatment for sciatica. N Engl J Med. 2007;356(22):2245–2256. doi: 10.1056/NEJMoa064039. [DOI] [PubMed] [Google Scholar]
- 3.Peul WC, van den Hout WB, Brand R, Thomeer RT, Koes BW. Leiden-the Hague spine intervention prognostic study G. prolonged conservative care versus early surgery in patients with sciatica caused by lumbar disc herniation: two year results of a randomised controlled trial. BMJ. 2008;336(7657):1355–1358. doi: 10.1136/bmj.a143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinstein JN, Lurie JD, Tosteson TD, Skinner JS, Hanscom B, Tosteson AN, et al. Surgical vs nonoperative treatment for lumbar disk herniation: the spine patient outcomes research trial (SPORT) observational cohort. JAMA. 2006;296(20):2451–2459. doi: 10.1001/jama.296.20.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shriver MF, Xie JJ, Tye EY, Rosenbaum BP, Kshettry VR, Benzel EC, et al. Lumbar microdiscectomy complication rates: a systematic review and meta-analysis. Neurosurg Focus. 2015;39(4):E6. doi: 10.3171/2015.7.FOCUS15281. [DOI] [PubMed] [Google Scholar]
- 6.Ramaswami R, Ghogawala Z, Weinstein JN. Management of Sciatica. N Engl J Med. 2017;376(12):1175–1177. doi: 10.1056/NEJMclde1701008. [DOI] [PubMed] [Google Scholar]
- 7.Guinto FC, Jr, Hashim H, Stumer M. CT demonstration of disk regression after conservative therapy. AJNR Am J Neuroradiol. 1984;5(5):632–633. [PMC free article] [PubMed] [Google Scholar]
- 8.Hong J, Ball PA. Images In Clinical Medicine. Resolution of lumbar disk herniation without surgery. N Engl J Med. 2016;374(16):1564. doi: 10.1056/NEJMicm1511194. [DOI] [PubMed] [Google Scholar]
- 9.El Barzouhi A, Vleggeert-Lankamp CLAM, Lycklama À, Nijeholt GJ, Van Der Kallen BF, Van Den Hout WB, Jacobs WCH, et al. Magnetic resonance imaging in follow-up assessment of sciatica. N Engl J Med. 2013;368(11):999–1007. doi: 10.1056/NEJMoa1209250. [DOI] [PubMed] [Google Scholar]
- 10.Santilli V, Beghi E, Finucci S. Chiropractic manipulation in the treatment of acute back pain and sciatica with disc protrusion: a randomized double-blind clinical trial of active and simulated spinal manipulations. Spine J. 2006;6(2):131–137. doi: 10.1016/j.spinee.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Fan Y, Zhao P. A randomized, placebo-controlled trial of vertebral mobilization treatment on patients with acute radiculopathy caused by lumbar disc herniation. Physiotherapy (united kingdom) 2015;101:eS1714–eS1eS5. [Google Scholar]
- 12.Ahn SH, Park HW, Byun WM, Ahn MW, Bae JH, Jang SH, et al. Comparison of clinical outcomes and natural morphologic changes between sequestered and large central extruded disc herniations. Yonsei Med J. 2002;43(3):283–290. doi: 10.3349/ymj.2002.43.3.283. [DOI] [PubMed] [Google Scholar]
- 13.Maigne JY, Rime B, Deligne B. Computed tomographic follow-up study of forty-eight cases of nonoperatively treated lumbar intervertebral disc herniation. Spine (Phila Pa 1976) 1992;17(9):1071–1074. doi: 10.1097/00007632-199209000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Benson RT, Tavares SP, Robertson SC, Sharp R, Marshall RW. Conservatively treated massive prolapsed discs: a 7-year follow-up. Ann R Coll Surg Engl. 2010;92(2):147–153. doi: 10.1308/003588410X12518836438840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Komori H, Okawa A, Haro H, Muneta T, Yamamoto H, Shinomiya K. Contrast-enhanced magnetic resonance imaging in conservative management of lumbar disc herniation. Spine (Phila Pa 1976) 1998;23(1):67–73. doi: 10.1097/00007632-199801010-00015. [DOI] [PubMed] [Google Scholar]
- 16.Modic MT, Ross JS, Obuchowski NA, Browning KH, Cianflocco AJ, Mazanec DJ. Contrast-enhanced MR imaging in acute lumbar radiculopathy: a pilot study of the natural history. Radiology. 1995;195(2):429–435. doi: 10.1148/radiology.195.2.7724762. [DOI] [PubMed] [Google Scholar]
- 17.Kamanli A, Karaca-Acet G, Kaya A, Koc M, Yildirim H. Conventional physical therapy with lumbar traction; clinical evaluation and magnetic resonance imaging for lumbar disc herniation. Bratisl Lek Listy. 2010;111(10):541–544. [PubMed] [Google Scholar]
- 18.Autio RA, Karppinen J, Niinimaki J, Ojala R, Kurunlahti M, Haapea M, et al. Determinants of spontaneous resorption of intervertebral disc herniations. Spine (Phila Pa 1976) 2006;31(11):1247–1252. doi: 10.1097/01.brs.0000217681.83524.4a. [DOI] [PubMed] [Google Scholar]
- 19.Gallucci M, Bozzao A, Orlandi B, Manetta R, Brughitta G, Lupattelli L. Does postcontrast MR enhancement in lumbar disk herniation have prognostic value? J Comput Assist Tomogr. 1995;19(1):34–38. doi: 10.1097/00004728-199501000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Ozturk B, Gunduz OH, Ozoran K, Bostanoglu S. Effect of continuous lumbar traction on the size of herniated disc material in lumbar disc herniation. Rheumatol Int. 2006;26(7):622–626. doi: 10.1007/s00296-005-0035-x. [DOI] [PubMed] [Google Scholar]
- 21.Ahn SH, Ahn MW, Byun WM. Effect of the transligamentous extension of lumbar disc herniations on their regression and the clinical outcome of sciatica. Spine (Phila Pa 1976) 2000;25(4):475–480. doi: 10.1097/00007632-200002150-00014. [DOI] [PubMed] [Google Scholar]
- 22.Ilkko E, Lahde S, Heikkinen ER. Late CT-findings in non-surgically treated lumbar disc herniations. Eur J Radiol. 1993;16(3):186–189. doi: 10.1016/0720-048x(93)90068-x. [DOI] [PubMed] [Google Scholar]
- 23.Delauche-Cavallier MC, Budet C, Laredo JD, Debie B, Wybier M, Dorfmann H, et al. Lumbar disc herniation: computed tomography scan changes after conservative treatment of nerve root compression. Spine. 1992;17(8):927–933. [PubMed] [Google Scholar]
- 24.Buttermann GR. Lumbar disc herniation regression after successful epidural steroid injection. J Spinal Disord Tech. 2002;15(6):469–476. doi: 10.1097/00024720-200212000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Bozzao A, Gallucci M, Masciocchi C, Aprile I, Barile A, Passariello R. Lumbar disk herniation: MR imaging assessment of natural history in patients treated without surgery. Radiology. 1992;185(1):135–141. doi: 10.1148/radiology.185.1.1523297. [DOI] [PubMed] [Google Scholar]
- 26.Broetz D, Hahn U, Maschke E, Wick W, Kueker W, Weller M. Lumbar disk prolapse: response to mechanical physiotherapy in the absence of changes in magnetic resonance imaging. Report of 11 cases. NeuroRehabilitation. 2008;23(3):289–294. [PubMed] [Google Scholar]
- 27.Jensen TS, Albert HB, Soerensen JS, Manniche C, Leboeuf-Yde C. Natural course of disc morphology in patients with sciatica: an MRI study using a standardized qualitative classification system. Spine (Phila Pa 1976) 2006;31(14):1605–1612. doi: 10.1097/01.brs.0000221992.77779.37. [DOI] [PubMed] [Google Scholar]
- 28.Henmi T, Sairyo K, Nakano S, Kanematsu Y, Kajikawa T, Katoh S, et al. Natural history of extruded lumbar intervertebral disc herniation. J Med Investig. 2002;49(1–2):40–43. [PubMed] [Google Scholar]
- 29.Takada E, Takahashi M, Shimada K. Natural history of lumbar disc hernia with radicular leg pain: spontaneous MRI changes of the herniated mass and correlation with clinical outcome. J Orthop Surg (Hong Kong) 2001;9(1):1–7. doi: 10.1177/230949900100900102. [DOI] [PubMed] [Google Scholar]
- 30.Cribb GL, Jaffray DC, Cassar-Pullicino VN. Observations on the natural history of massive lumbar disc herniation. J Bone Joint Surg (Br) 2007;89(6):782–784. doi: 10.1302/0301-620X.89B6.18712. [DOI] [PubMed] [Google Scholar]
- 31.Ellenberg MR, Ross ML, Honet JC, Schwartz M, Chodoroff G, Enochs S. Prospective evaluation of the course of disc herniations in patients with proven radiculopathy. Arch Phys Med Rehabil. 1993;74(1):3–8. [PubMed] [Google Scholar]
- 32.Demirel A, Yorubulut M, Ergun N. Regression of lumbar disc herniation by physiotherapy. Does non-surgical spinal decompression therapy make difference? Double-blind randomized controlled trial. J Back Musculoskelet Rehabil. 2017;30(5):1015–1022. doi: 10.3233/BMR-169581. [DOI] [PubMed] [Google Scholar]
- 33.Hong SJ, Kim DY, Kim H, Kim S, Shin KM, Kang SS. Resorption of massive lumbar disc herniation on MRI treated with epidural steroid injection: a retrospective study of 28 cases. Pain Physician. 2016;19(6):381–388. [PubMed] [Google Scholar]
- 34.Matsubara Y, Kato F, Mimatsu K, Kajino G, Nakamura S, Nitta H. Serial changes on MRI in lumbar disc herniations treated conservatively. Neuroradiology. 1995;37(5):378–383. doi: 10.1007/BF00588017. [DOI] [PubMed] [Google Scholar]
- 35.Yukawa Y, Kato F, Matsubara Y, Kajino G, Nakamura S, Nitta H. Serial magnetic resonance imaging follow-up study of lumbar disc herniation conservatively treated for average 30 months: relation between reduction of herniation and degeneration of disc. J Spinal Disord. 1996;9(3):251–256. [PubMed] [Google Scholar]
- 36.Fagerlund MKJ, Thelander U, Friberg S. Size of lumbar disc hernias measured using computed tomography and related to sciatic symptoms. Acta Radiol. 1990;31(6):555–558. [PubMed] [Google Scholar]
- 37.Kesikburun B, Eksioglu E, Turan A, Adiguzel E, Kesikburun S, Cakci A. Spontaneous regression of extruded lumbar disc herniation: correlation with clinical outcome. Pak J Med Sci. 2019;35(4):974–980. doi: 10.12669/pjms.35.4.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teplick JG, Haskin ME. Spontaneous regression of herniated nucleus pulposus. AJR Am J Roentgenol. 1985;145(2):371–375. doi: 10.2214/ajr.145.2.371. [DOI] [PubMed] [Google Scholar]
- 39.Shan Z, Fan S, Xie Q, Suyou L, Liu J, Wang C, et al. Spontaneous resorption of lumbar disc herniation is less likely when modic changes are present. Spine (Phila Pa 1976) 2014;39(9):736–744. doi: 10.1097/BRS.0000000000000259. [DOI] [PubMed] [Google Scholar]
- 40.Shin JS, Lee J, Kim MR, Shin BC, Lee MS, Ha IH. The long-term course of patients undergoing alternative and integrative therapy for lumbar disc herniation: 3-year results of a prospective observational study. BMJ Open. 2014;4:e005801.
- 41.Komori H, Shinomiya K, Nakai O, Yamaura I, Takeda S, Furuya K. The natural history of herniated nucleus pulposus with radiculopathy. Spine (Phila Pa 1976) 1996;21(2):225–229. doi: 10.1097/00007632-199601150-00013. [DOI] [PubMed] [Google Scholar]
- 42.Saal JA, Saal JS, Herzog RJ. The natural history of lumbar intervertebral disc extrusions treated nonoperatively. Spine (Phila Pa 1976) 1990;15(7):683–686. doi: 10.1097/00007632-199007000-00013. [DOI] [PubMed] [Google Scholar]
- 43.Bush K, Cowan N, Katz DE, Gishen P. The natural history of sciatica associated with disc pathology. A prospective study with clinical and independent radiologic follow-up. Spine (Phila Pa 1976) 1992;17(10):1205–1212. doi: 10.1097/00007632-199210000-00013. [DOI] [PubMed] [Google Scholar]
- 44.Iwabuchi M, Murakami K, Ara F, Otani K, Kikuchi S. The predictive factors for the resorption of a lumbar disc herniation on plain MRI. Fukushima J Med Sci. 2010;56(2):91–97. doi: 10.5387/fms.56.91. [DOI] [PubMed] [Google Scholar]
- 45.Yu PF, Jiang H, Liu JT, Li XC, Qian X, Han S, et al. Traditional Chinese medicine treatment for ruptured lumbar disc herniation: clinical observations in 102 cases. Orthop Surg. 2014;6(3):229–235. doi: 10.1111/os.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee J, Shin JS, Lee YJ, Kim J, Kim MR, Koh W, et al. Long-term course and predictive factors associated with disc resorption in lumbar disc herniation patients. J Neurol Sci. 2017;381:278. doi: 10.1155/2017/2147408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jensen TS, Albert HB, Sorensen JS, Manniche C, Leboeuf-Yde C. Magnetic resonance imaging findings as predictors of clinical outcome in patients with sciatica receiving active conservative treatment. J Manip Physiol Ther. 2007;30(2):98–108. doi: 10.1016/j.jmpt.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 48.Kreiner DS, Hwang SW, Easa JE, Resnick DK, Baisden JL, Bess S, et al. An evidence-based clinical guideline for the diagnosis and treatment of lumbar disc herniation with radiculopathy. Spine J. 2014;14(1):180–191. doi: 10.1016/j.spinee.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 49.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712–716. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 51.Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.TJ HJPT, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane handbook for systematic reviews of interventions version 6.0 (updated July 2019). Cochrane. :2019 Available from www.training.cochrane.org/handbook.
- 53.Team RC. R: a language and environment for statistical computing. R Foundation for statistical computing, Vienna, Austria URL https://www.Rproject.org/. Accessed 26 Oct 2019.
- 54.Zhong M, Liu JT, Jiang H, Mo W, Yu PF, Li XC, et al. Incidence of spontaneous Resorption of lumbar disc herniation: a Meta-analysis. Pain Physician. 2017;20(1):E45–E52. [PubMed] [Google Scholar]
- 55.Chiu CC, Chuang TY, Chang KH, Wu CH, Lin PW, Hsu WY. The probability of spontaneous regression of lumbar herniated disc: a systematic review. Clin Rehabil. 2015;29(2):184–195. doi: 10.1177/0269215514540919. [DOI] [PubMed] [Google Scholar]
- 56.Norvell DC. Study types and bias-Don't judge a study by the abstract's conclusion alone. Evid Based Spine Care J. 2010;1(2):7–10. doi: 10.1055/s-0028-1100908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Petrisor BA, Keating J, Schemitsch E. Grading the evidence: levels of evidence and grades of recommendation. Injury. 2006;37(4):321–327. doi: 10.1016/j.injury.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 58.Kato T, Haro H, Komori H, Shinomiya K. Sequential dynamics of inflammatory cytokine, angiogenesis inducing factor and matrix degrading enzymes during spontaneous resorption of the herniated disc. J Orthop Res. 2004;22(4):895–900. doi: 10.1016/j.orthres.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 59.Macki M, Hernandez-Hermann M, Bydon M, Gokaslan A, McGovern K, Bydon A. Spontaneous regression of sequestrated lumbar disc herniations: literature review. Clin Neurol Neurosurg. 2014;120:136–141. doi: 10.1016/j.clineuro.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 60.Seo JY, Roh YH, Kim YH, Ha KY. Three-dimensional analysis of volumetric changes in herniated discs of the lumbar spine: does spontaneous resorption of herniated discs always occur? Eur Spine J. 2016;25(5):1393–1402. doi: 10.1007/s00586-014-3587-1. [DOI] [PubMed] [Google Scholar]
- 61.Kawaji Y, Uchiyama S, Yagi E. Three-dimensional evaluation of lumbar disc hernia and prediction of absorption by enhanced MRI. J Orthop Sci. 2001;6(6):498–502. doi: 10.1007/s007760100004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.