Abstract
Anhedonia remains a major clinical issue for which there is few effective interventions. Untreated or poorly controlled anhedonia has been linked to worse disease course and increased suicidal behavior across disorders. Taking a proof-of-mechanism approach under the auspices of the National Institute of Mental Health FAST-FAIL initiative, we were the first to show that, in a transdiagnostic sample screened for elevated self-reported anhedonia, 8 weeks of treatment with a kappa-opioid receptor (KOR) antagonist resulted in significantly higher reward-related activation in one of the core hubs of the brain reward system (the ventral striatum), better reward learning in the Probabilistic Reward Task (PRT), and lower anhedonic symptoms, relative to 8 weeks of placebo. Here, we performed secondary analyses of the PRT data to investigate the putative effects of KOR antagonism on anhedonic behavior with more precision by using trial-level model-based Bayesian computational modeling and probability analyses. We found that, relative to placebo, KOR antagonism resulted in significantly higher learning rate (i.e., ability to learn from reward feedback) and a more sustained preference toward the more frequently rewarded stimulus, but unaltered reward sensitivity (i.e., the hedonic response to reward feedback). Collectively, these findings provide novel evidence that in a transdiagnostic sample characterized by elevated anhedonia, KOR antagonism improved the ability to modulate behavior as a function of prior rewards. Together with confirmation of target engagement in the primary report (Krystal et al., Nat Med, 2020), the current findings suggest that further transdiagnostic investigation of KOR antagonism for anhedonia is warranted.
Subject terms: Depression, Human behaviour
Introduction
Despite substantial efforts, Major Depressive Disorder (MDD) remains a common, recurrent, and, for many, difficult-to-treat disorder. In the USA, large studies have shown that up to 50% of patients fail to respond to first-line antidepressant medications (e.g., selective serotonin reuptake inhibitors) [1] or evidence-based psychotherapy [2]. There are several reasons for this modest progress. First, our understanding of the etiology and pathophysiology of depression remains incomplete. Second, MDD—as defined by current nosology [3]–is highly heterogenous in its clinical presentation, which points to neurobiological heterogeneity.
To address these unmet needs, in 2010 the National Institute of Mental Health launched the Research Domain Criteria initiative [4], which proposed to focus on transdiagnostic dimensions of behavior expected to map onto neurobiological substrates more closely than psychiatric syndromes. Among such dimensions, anhedonia has attracted substantial attention, particularly since it has been implicated in numerous neuropsychiatric conditions [5–9]. Moreover, anhedonia has been linked to worse treatment response and disease course as well as increased risk for suicide [10–13].
Critically, mounting evidence indicates that anhedonia can be parsed into subdomains, which points to partially dissociable neurobiological abnormalities [10, 14, 15]. For example, findings across species indicate that reward learning, incentive motivation, and effort-based decision making strongly rely on dopaminergic signaling, whereas the experience of pleasure is more strongly related to GABA and mu opioid signaling. When probing reward learning, several laboratories have used the Probabilistic Reward Task (PRT), which provides an objective assessment of the ability to modulate behavior as a function of prior reinforcement [16–18]. Relevant here, findings across laboratories and species have shown that reward learning in the PRT was bi-directionally modulated by dopaminergic manipulations [17–19] and related to dopaminergic markers and neural functioning along mesocorticolimbic pathways [20–22]. Moreover, reward learning was inversely related to current anhedonic symptoms among (unselected) children [23] and adults [16, 24], individuals with MDD [25] and relatives of patients with MDD [26], and predicted future anhedonic symptoms [16, 27]. Finally, reward learning was reduced in individuals with increased depressive symptoms [16], current MDD [25, 28, 29], and past MDD [30, 31] (cf. [32]), with effects particularly pronounced in those with elevated anhedonic symptoms [29] or melancholic depression [33], and among transdiagnostic youth samples with heightened anhedonia [34].
Importantly, the observation that up to 50% of patients fail to respond to monoaminergic antidepressants and the modest success in treating anhedonia suggest that additional neurobiological abnormalities might be implicated in MDD and anhedonia. In light of their pivotal role in regulating reward processing and stress (among other functions), kappa-opioid receptors (KOR) are emerging as a promising target for MDD and, especially, anhedonia. Converging lines of evidence support this assumption (for review, see [35]). First, rodent studies show that KOR antagonists have antidepressant effects (as assessed by reduced stress-induced immobility in the forced swim test) [36, 37] and reduced learned helplessness [38], whereas KOR agonists induce depressive-like effects [39]. Second, administration of KOR antagonists in the ventral striatum (nucleus accumbens)—a region where dopaminergic and reward-related activation is often blunted in MDD [40–42]–leads to 175% increase in dopamine release in this region [43], whereas KOR agonists reduce accumbal dopamine release by 50% [43] and foster the emergence of anhedonic behavior [39].
Mechanistically, there is evidence that stress leads to a release of dynorphin, which binds to KOR receptors and inhibits the release of dopamine into the nucleus accumbens by ventral tegmental area neurons [44–48]. Accordingly, KOR antagonists may produce anti-anhedonic effects by blocking the consequences of cAMP response element binding-mediated upregulation of dynorphin function, which in turn might restore function within the mesolimbic dopaminergic system [48]. Collectively, these findings suggest that KOR antagonism may be effective at ameliorating anhedonic behavior.
To test this hypothesis, we performed secondary analyses on the recently published FAST-MAS dataset [49]. Supported by the NIMH’s New Experimental Medicine Studies: Fast-Fail Trials Program (https://www.nimh.nih.gov/research-priorities/research-initiatives/fast-fast-fail-trials.shtml), the study took a “proof-of-mechanism” approach to evaluate whether KOR antagonism would have anti-anhedonic effects in a transdiagnostic sample by improving reward-related brain circuitry (specifically, ventral striatal activation to reward-predicting stimuli). This hypothesis was recently confirmed [49], along with evidence that KOR antagonism also positively affected the secondary outcome measures—self-reported anhedonia (as assessed by the Snaith Hamilton Pleasure Scale [50]) and reward learning (as assessed by the PRT [16]). In the current secondary analyses we provide novel evidence that significant group differences in posttreatment reward learning were driven by increased probability of selecting the stimulus previously paired with more frequent rewards, as well as a higher learning rate (as assessed using computational modeling), in the KOR antagonist relative to placebo group. This latter finding was hypothesized owing to prior reports that learning rate is sensitive to dopaminergic manipulations [51–54]. In line with this hypothesis, and highlighting specificity, groups did not differ in the second parameter, reward sensitivity. Collectively, these findings pinpoint precise reward subdomains that are ameliorated by KOR antagonism and further underscore the promise of KOR antagonism in alleviating anhedonia in transdiagnostic samples.
Materials and methods
Participants
Participants were recruited at six US centers (ClinicalTrials.gov Identifier: NCT02218736; see Supplementary Material). Participants were enrolled after providing informed written consent to a protocol approved by each local institutional review board. In total, 163 patients were screened and 94 met eligibility criteria (Supplementary Fig. 1). Among these 94, 5 did not complete baseline assessments, leaving 89 participants for randomization (45 KOR, 44 placebo). Two subjects (both in the KOR group) did not perform the PRT; among the remaining 87, 76 participants (35 KOR, 41 placebo) had usable pretreatment PRT data after quality control checks, which were performed blindly to treatment arm using predefined cutoff scores (Supplementary Material); among these 76, 16 participants (9 KOR, 7 placebo) did not perform the PRT at posttreatment and 5 (2 KOR, 3 placebo) failed QC evaluations at posttreatment. As a result, 55 participants (24 KOR, 31 placebo) had both pre- and posttreatment PRT data that passed QC and were included in the analyses. Highlighting the translational nature of the sample, DSM diagnoses included MDD, bipolar disorder, generalized anxiety disorder, social anxiety disorder, panic disorder, and posttraumatic stress disorder (Table 1).
Table 1.
Clinical and sociodemographic variables.
| Kappa-opioid receptor N = 24 | Placebo N = 31 | t value | p value | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Age | 39.17 | 13.87 | 40.81 | 13.68 | −0.44 | >0.66 |
| Baseline | ||||||
| HRSD | 15.13 | 5.04 | 14.32 | 5.91 | 0.54 | >0.93 |
| SHAPS | 37.29 | 8.89 | 33.03 | 5.54 | 2.18 | 0.034 |
| CGI i | 3.96 | 0.36 | 3.97 | 0.48 | −0.10 | >0.93 |
| CGI s | 3.79 | 0.51 | 3.84 | 0.52 | −0.34 | >0.73 |
| Posttreatment | ||||||
| HRSD | 11.96 | 7.89 | 10.42 | 7.46 | 0.74 | >0.46 |
| SHAPS | 32.86 | 8.13 | 30.76 | 6.75 | 1.01 | >0.31 |
| CGI i | 3.50 | 0.96 | 3.21 | 1.11 | 0.99 | >0.32 |
| CGI s | 3.23 | 0.87 | 3.21 | 1.01 | 0.08 | >0.94 |
| N (%) | N (%) | χ2 value | p value | |||
| Sex | 0.049 | >0.80 | ||||
| Females | 14 | 19 | ||||
| Males | 10 | 12 | ||||
| DSM diagnosis | 7.98 | >0.24 | ||||
| MDDa | 16 (66.7%) | 18 (58.1%) | ||||
| Bipolar Ib | 2 (8.3%) | 2 (6.5%) | ||||
| Bipolar IIb | 0 (0%) | 4 (12.9%) | ||||
| GAD | 2 (8.3%) | 5 (16.1%) | ||||
| SAD | 2 (8.3%) | 0 (0%) | ||||
| Panic disorder | 1 (4.2%) | 2 (6.5%) | ||||
| PTSD | 1 (4.2%) | 0 (0%) | ||||
| Race | 2.34 | >0.66 | ||||
| White | 18 (75%) | 19 (61.3%) | ||||
| African American | 3 (12.5%) | 5 (16.1%) | ||||
| Asian | 0 (0%) | 2 (6.5%) | ||||
| More than 1 race | 2 (8.3%) | 4 (12.9%) | ||||
| Unknown | 1 (4.2%) | 1 (3.2%) | ||||
The table summarizes primary diagnosis. Fourteen of the 25 patients randomized to the KOR and 18 of the 31 patients randomized to placebo had at least a secondary DSM diagnosis (χ2(1) = 0.02, ns). For control analyses of the PRT data within the MDD subsample, see Supplementary.
HRSD Hamilton Rating Scale for depression, SHAPS Snaith Hamilton Pleasure Scale. CGI i Clinical Global Impression—improvement scale, CGI s Clinical Global Impression—severity scale, GAD generalized anxiety disorder, SAD social anxiety disorder, PTSD Posttraumatic stress disorder.
aMajor Depressive Disorder (MDD) current 2 weeks or recurrent.
bBipolar disorder current or past.
The two groups did not differ in any sociodemographic variable or baseline depression severity (Table 1). However, relative to patients eventually randomized to placebo, the KOR group unexpectedly had higher baseline SHAPS scores (mean ± SD: 37.29 ± 8.89 vs. 33.03 ± 5.54, t(53) = 2.18, p = 0.034). Owing to prior PRT findings showing that anhedonic symptoms negatively correlated with response bias [16, 23, 24, 26, 29], all analyses included baseline (pretreatment) SHAPS score as a covariate.
Note that in the original analysis [49] we did not control for baseline anhedonia because (1) SHAPS scores did not significantly differ between treatment groups in the overall sample, and (2) it was not part of our prespecified analysis plan. Accordingly, the prespecified three-way interaction analysis (Treatment Arm × Time × Block) was repeated by controlling for baseline SHAPS scores to confirm the original findings. In addition, we performed novel analyses (computational modeling and probability analyses) to probe putative dysfunction in more depth.
Randomized clinical trial
A full description of the randomized clinical trial has been provided [49]. Briefly, eligibility was determined using the Mini-International Neuropsychiatric Interview for DSM 4 [55, 56], and clinical scales, including the SHAPS to assess anhedonia. Eligible patients scoring ≥20 on the SHAPS [57] returned for a baseline visit, which included: a second administration of the SHAPS and other clinical scales (Hamilton Depression Rating Scale [58], Hamilton Rating Scale for Anxiety [59]); an MRI session; an EEG recording; and the PRT. Next, patients were randomized to a KOR antagonist or placebo (1:1 ratio) for 8 weeks. After 8 weeks, patients were re-assessed with the same procedures. For the active condition, we selected JNJ-67953964 (Aticaprant) (formerly, CERC-501 and LY2456302), a high-affinity, selective KOR antagonist with favorable pharmacologic and safety profiles. A 10-mg dose was selected based on preclinical toxicology and human single ascending dose and multiple ascending dose studies [60, 61] as well as positron emission tomography evidence of robust KOR engagement at this dose [62].
Probabilistic reward task
The PRT is a computerized task rooted in signal detection theory [16, 19, 25]. On each trial, participants are asked to determine, via key press, whether one of two difficult-to-differentiate stimuli had been presented. Unlike earlier PRT papers [16, 19, 25], this study used two blocks of 100 trials to limit task duration. For each trial, participants had to decide whether a brief visual stimulus (a mouth presented on a cartoon face for 100 ms) was “long” or “short”, by pressing one of two computer keys (“z” or “/”, counterbalanced). Per design, the brief stimulus presentation time (100 ms) and small physical difference between the mouth stimuli (11.5 vs. 13 mm) make discrimination challenging. Critically, and unbeknownst to participants, the task includes an asymmetrical reinforcement schedule such that one of the two stimuli (the “rich” stimulus) is rewarded (“Correct!! You Won 20 Cents”) three times more frequently than the “lean” stimulus (30 vs. 10 times per block). Participants were instructed that not all correct responses would be followed by rewards and to respond as quickly and accurately as possible in order to maximize task earnings.
Data reduction
Following established procedures [16, 19, 25], PRT data were analyzed using signal detection theory. First, QC evaluations were performed using a priori defined criteria and blind to treatment arm (Supplementary Material). Next, response bias (log b) and discriminability (log d) were computed as:
In secondary analyses, accuracy and reaction time in response to the rich and lean stimulus were computed for each block.
Statistics
The main variable of interest was response bias, which was analyzed using a mixed analysis of covariance (ANCOVA) with the between-subject factor of Treatment Arm (KOR, placebo) and the repeated measures of Time (pre-, posttreatment) and Block (1, 2), with pretreatment SHAPS scores entered as covariate. To evaluate with more precision response bias differences, two additional analyses were performed. In the first, we computed the probability of a given response (e.g., “rich”) as a function of whether the preceding trial (rich vs. lean) had been rewarded or not. Specifically, ANCOVA analyses were performed on the probability of both a rich hit (i.e., a rich stimulus was presented, and the participant responded “rich”) and a lean miss (i.e., a lean stimulus was presented, and the participant responded “rich”), as a function of whether the immediately preceding trial had been rich or lean, and had been rewarded or not.
In the second analysis, a computational model of trial-level performance was implemented (Supplementary Methods). This model-based Bayesian modeling [51] allows parsing of the contribution of two subconstructs on PRT performance: reward sensitivity (which operationalizes consummatory pleasure) and learning rate (which operationalizes participants’ ability to learn from reward feedback). For each parameter, a Time (pre-, posttreatment) × Treatment Arm (KOR, placebo) ANCOVA was run (covariate: pretreatment SHAPS scores). Relations among variables are summarized in the Supplementary Table 1 (see also Supplementary Fig. 2).
Finally, control analyses comparing groups in their rich-to-lean reward ratio and discriminability scores were performed to confirm that possible differences in response bias were not affected by group differences in the reinforcement schedule received, or by overall task difficulty. For these analyses, a Time × Block × Treatment Arm ANCOVA was run. Analyses on accuracy and reaction time were performed to confirm that the task elicited the intended behavioral effects, and results are reported in the Supplementary Results (see Supplementary Fig. 3). Across all analyses, variables were normally distributed, pretreatment SHAPS scores were entered as covariates, and significant ANCOVA effects were followed up with Bonferroni-corrected post-hoc tests of simple effects.
Results
Response bias
The Time (pre-, posttreatment) × Block (1, 2) × Treatment Arm (KOR, placebo) ANCOVA on response bias revealed a significant Time × Treatment Arm interaction, F(1, 52) = 4.69, p = 0.035, ηp2 = 0.083 (Fig. 1a). Bonferroni-corrected post-hoc tests of simple effects indicated that this interaction was driven by significantly higher posttreatment response bias in the KOR relative to placebo group (p = 0.017; Cohen’s d = 0.69), whereas groups did not differ pretreatment (p > 0.60). Moreover, the KOR group (p = 0.074) showed a trend for an increase in response bias from pre- to posttreatment (placebo: p > 0.17). At an individual level, 16 of the 24 patients randomized to KOR group (66.7%) showed an increase in response bias from pre- to posttreatment (binomial p(16/24) = 0.044), whereas only 13 of the 31 patients randomized to placebo (41.4%) showed this pattern (binomial p(13/31) > 0.09; χ2 = 7.73, df = 1, p < 0.005). As summarized in the Supplementary Results, secondary analyses on accuracy scores clarified that the KOR group had significantly higher rich accuracy at posttreatment relative to the placebo group (p = 0.002; Cohen’s d = 0.88), whereas groups did not differ in their pretreatment rich accuracy (p = 0.50) nor in their pre- or posttreatment lean accuracy. Moreover, the KOR and placebo groups showed a significant increase (p = 0.026) and decrease (p = 0.003), respectively, in rich accuracy from pre- to posttreatment (Supplementary Fig. 3).
Fig. 1. Pre- to Post-treatment changes in Response Bias and Rich Hit Rates as a Function of Treatment.
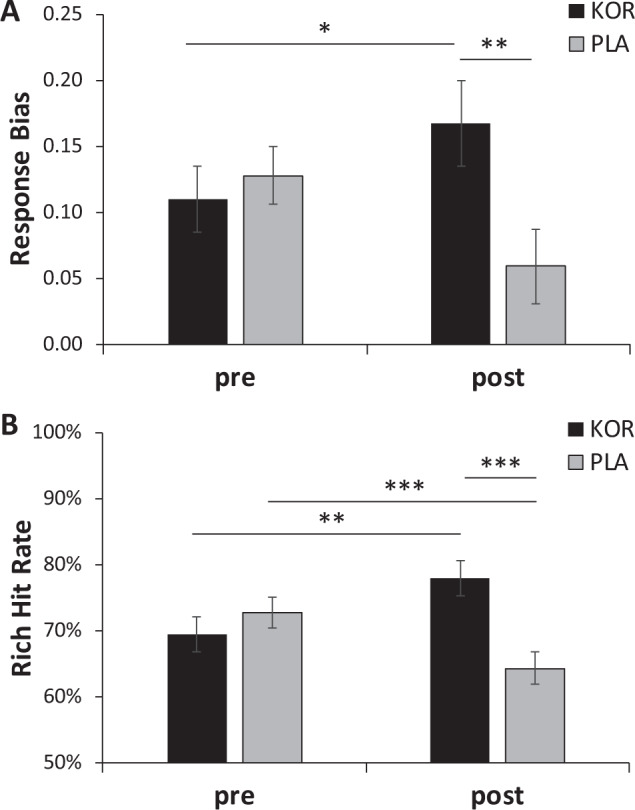
a Response bias and b probability of Rich Hits in the KOR (n = 24) and Placebo (N = 31) group pre- and posttreatment. Error bars denote standard errors. Estimated means are plotted (covariate: pretreatment SHAPS scores). ***p < 0.01, **p < 0.05, *p < 0.10.
Probability analyses
Rich hit rates
A Preceding Trial (rich, lean) × Rewarded (yes, no) × Time (pre, post) × Treatment Arm ANCOVAs was run on the probability of a rich hit (i.e., the next trial was rich and the subject responded “rich”). The only significant findings involving Treatment Arm were the Time × Treatment Arm interaction, F(1, 52) = 13.13, p < 0.001, ηp2 = 0.202, as well as the four-way interaction, F(1, 52) = 5.41, p = 0.024, ηp2 = 0.202. Post-hoc tests indicated that the Time × Treatment Arm interaction was driven by significantly higher posttreatment rich hits for the KOR relative to the placebo group (Fig. 1b; p < 0.001; Cohen’s d = 1.03), whereas groups did not differ in their pretreatment scores (p > 0.35). Moreover, the KOR group showed significantly higher rich hits at postrelative to pretreatment (p = 0.017), whereas the placebo group showed a significant reduction from pre- to posttreatment in rich hit rates (p = 0.007).
To follow-up the four-way interaction, we ran a Preceding Trial (rich, lean) × Time (pre, post) × Treatment Arm ANCOVA on the probability of a rich hit separately depending on whether the preceding trials had been rewarded or not. For both analyses, the Time × Treatment Arm interaction was significant (rewarded preceding trial: F(1, 52) = 8.13, p = 0.006, ηp2 = 0.135; nonrewarded preceding trial: F(1, 52) = 8.05, p = 0.006, ηp2 = 0.134). Post-hoc tests confirmed that, irrespective of whether the preceding trial had been rewarded or not, the KOR group had significantly higher rich hit rates relative to the placebo group at posttreatment (both ps < 0.011), whereas groups did not differ at pretreatment (both ps > 0.44).
Lean miss rates
An identical ANCOVA was run on the probability of a lean miss (i.e., the next trial was lean and the subject responded “rich”). No effects involving Treatment Arm emerged (all Fs < 1.63, ps > 0.20).
Computational model
Learning rate
A Time × Treatment Arm ANCOVA on the learning rate parameter indicated that the Time × Treatment Arm interaction was significant, F(1, 52) = 7.52, p < 0.008, ηp2 = 0.126. Post-hoc tests revealed that, relative to the placebo group, the KOR group had significantly higher posttreatment learning rate (p < 0.005; Cohen’s d = 0.81), whereas groups did not differ at pretreatment (p > 0.20) (Fig. 2a). For the placebo group (p < 0.025)—but not KOR group (p > 0.10)—learning rates significantly decreased from pre- to posttreatment. When considering individual scores, 16 of the 24 participants (66.7%) randomized to the KOR antagonist (binomial p(16/24) < 0.044) but only 9 of the 31 participants (29.0%) randomized to placebo (binomial p(9/31) < 0.01) showed an increase from pre- to posttreatment in learning rates (χ2 = 7.73, df = 1, p < 0.005).
Fig. 2. Pre- to Post-treatment changes in Computational Modeling Parameters as a Function of Treatment.
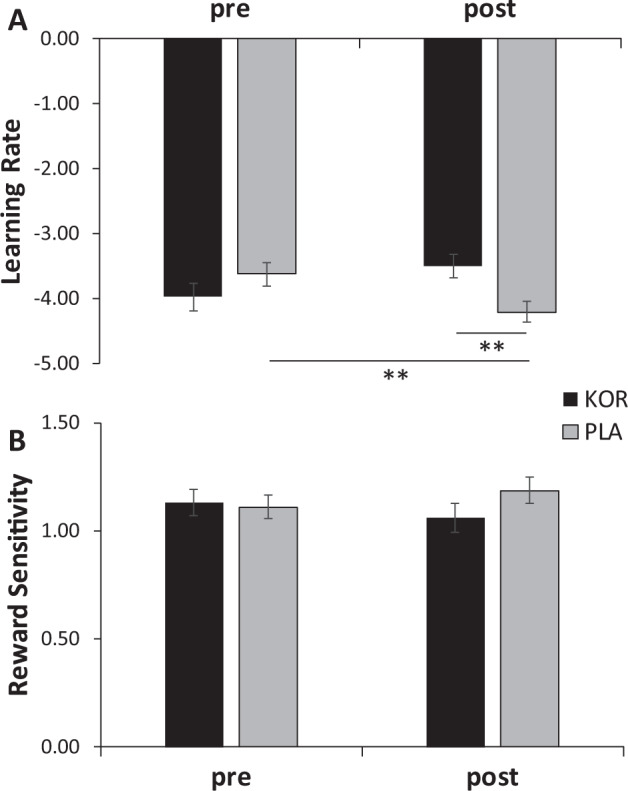
a Learning rate and b reward sensitivity in the KOR (n = 24) and Placebo (N = 31) group pre- and posttreatment. Error bars denote standard errors. Estimated means are plotted (covariate: pretreatment SHAPS scores). Note that the learning rate and reward sensitivity parameters were transformed to prevent issues with nonnormal distribution. ***p < 0.01, **p < 0.05, *p < 0.10.
Reward sensitivity
A Time (pre-, posttreatment) × Treatment Arm (KOR, placebo) ANCOVA on reward sensitivity revealed no significant effects involving Treatment Arm (all Fs < 1.45, all ps > 0.23; Fig. 2b).
Control analyses
Two separate Time × Block × Treatment Arm ANCOVAs revealed no effects involving Treatment Arm on rich-to-lean rewards or discriminability (Supplementary Results), indicating that the main findings were not affected by group differences in reinforcement schedule or task difficulty.
Discussion
Anhedonia is a major clinical issue across multiple neuropsychiatric disorders. In MDD, anhedonia is typically poorly addressed by first-line treatments (e.g., SSRI) and predicts worse disease trajectory [10–13]. Moreover, anhedonia has been found to precede fully symptomatic syndromes in MDD [63], Parkinson’s disease [64, 65], and substance abuse [66], and has long been identified as a core vulnerability factor for schizophrenia [8]. Accordingly, identifying novel treatment targets for anhedonia is a major priority. Supported by compelling preclinical evidence that KOR antagonism has anti-anhedonic effects, normalizes DA signaling in the nucleus accumbens (one of the core hubs of the brain reward system), and removes the inhibiting effects of dynorphin on DA neurons [35, 48], the FAST-MAS study was specifically designed to test the hypothesis that a KOR antagonist would ameliorate anhedonia across three units of analysis: brain (as operationalized as ventral striatal activation to reward-predicting cues), behavior (i.e., reward learning in the PRT), and self-report (i.e., SHAPS scores). As recently described, this hypothesis was supported [49]. Highlighting the specificity of the effects to anhedonia, posttreatment differences in ventral striatal activation during reward anticipation, reward learning abilities, and self-reported anhedonia between the KOR antagonist and placebo group emerged in the context of no changes in overall depression severity. This indicates that the use of broad syndrome-based clinical scales might cloud identification of treatment targets for anhedonia.
The overarching goal of the current secondary analyses was twofold. First, we aimed to confirm that posttreatment group differences in reward learning were not unduly affected by the unexpected observation that, before randomization, patients with usable PRT data who went on to receive the KOR antagonist had significantly higher pretreatment SHAPS scores relative to patients in the placebo group. This control analysis was motivated by prior findings that self-reported anhedonia is inversely related to reward learning in the PRT [16, 23, 24, 26, 29]. Of note, the Time × Treatment Arm interaction was confirmed, and post-hoc tests clarified that this interaction was driven by significantly higher posttreatment response bias in the KOR relative to the placebo group, with no pretreatment differences. Additional analyses on accuracy scores clarified that response bias findings were driven by posttreatment group differences in rich—but not lean—accuracy, and a significant increase from pre- to posttreatment in rich accuracy for the KOR group, whereas the placebo group showed a significant reduction in rich accuracy from pre- to posttreatment.
Second, using computational modeling and trial-level analyses probing the probabilities of specific behavioral responses as a function of prior trials, we sought to pinpoint the putative sources of group differences in posttreatment response bias. Building on prior work, we implemented a model-based Bayesian modeling approach [51] to disentangle the contribution of two subconstructs on PRT performance: reward sensitivity (which captures hedonic response to the reward feedback) and learning rate (which captures the ability to learn from reward feedback). In light of vast evidence implicating DA in learning rate [52–54] and prior PRT findings indicating that a DA challenge modulated learning rate [51], we expected that the KOR and placebo groups would differ in their posttreatment learning rate, but not posttreatment reward sensitivity. This hypothesis was confirmed. Higher learning rate reflects better ability to choose by integrating reinforcement history into modulating option values. Finally, and further highlighting that a KOR antagonist boosted patients’ ability to sustain a preference toward a more frequently rewarded stimulus, probability analyses clarified that, relative to placebo, KOR antagonism was associated with a higher likelihood of selecting “rich” irrespective of whether the prior trial had been a rewarded or nonrewarded rich or lean stimulus. Thus, the preference for the rich stimulus was boosted regardless of the immediately preceding stimulus and whether it had been rewarded or not. Overall, this points to a better ability to sustain reward-related behavior after KOR antagonism. These findings represent an important contribution as they allow us to increase the precision with which we can define the specific type of reward-related deficit that is ameliorated by KOR antagonism. The evidence suggests that KOR antagonism specifically impacts learning rate but not reward sensitivity and improves a specific element of impaired reward learning: the ability to express a response bias toward a more frequently rewarded stimulus irrespective of the outcome of the immediately preceding trial.
The current findings, particularly the KOR-related effects on response bias and learning rates, are consistent with a large body of preclinical research indicating that KOR receptors modulate brain reward function [44], influence positive reinforcement [47], and are implicated in the acquisition and consolidation of learned associations. Directly relevant to the task used here, prior rodent studies have shown that KOR modulation shape learning on a variety of tasks [67–69]. These pharmacological findings are consistent with the anatomical distribution of KOR, which are prominently represented in brain regions critically implicated in reinforcement learning and reward prediction coding, including the ventral tegmental area, nucleus accumbens, caudate, and putamen in both the rat [70] and human [71, 72] brain. Critically, these regions show abnormal reward-related activation and functional connectivity (including during reinforcement learning tasks) in MDD [40, 41, 73]. Thus, we speculate that the current behavioral findings might reflect some degree of normalization within this brain circuitry.
The current study has several important strengths. First, the FAST-FAIL initiative represents a novel conceptualization for clinical trials, whereby the primary outcome variable was not a clinical scale but rather a neural marker of target engagement (with two additional secondary outcome measures, which were also narrowly selected: a self-reported and a behavioral measure of anhedonia). Second, the multi-site FAST-MAS study used a promising target (KOR antagonism), which has received compelling support from the preclinical literature [35, 48] but has not been thoroughly investigated in humans. Third, patients were selected transdiagnostically with the common feature of reporting some degree of anhedonia (baseline SHAPS score ≥ 20). Fourth, Bayesian-based computational modeling was used to disentangle different subconstructs that could contribute to blunted response bias—a reduced hedonic responsiveness to reward vs. preserved hedonic responsiveness but reduced ability to learn from reward.
Despite these significant strengths and high degree of conceptual and methodological innovation, the study had some limitations. First, relative to the original sample (N = 89), only a smaller subsample had PRT data at both sessions (N = 55). Although the medium-to-large effect sizes we observed speak against low power, it will be important to replicate the current findings in larger samples. Second, the smaller size for the subsample with usable PRT data led to unexpected group differences in baseline SHAPS scores before the randomization not seen in the total study sample, which required that all analyses controlled for pretreatment SHAPS scores. Third, in the original analyses [49], we had hypothesized that the Time × Treatment Arm × Block interaction would be significant, driven by significant posttreatment group differences in the change in response bias from block 1 to block 2. In retrospect, this assumption was ill informed given the implementation of a version of the PRT that included only two blocks instead of the usual three blocks (in order to decrease patients’ burden). Based on similar experiences in other large studies using a 2-block version of the PRT [74, 75], we believe that the average response bias across the two blocks (rather than the difference between block 1 and 2) is a better metric for PRT studies unable to implement three blocks. Finally, the lack of a psychiatrically healthy control group prevented us to test whether the KOR antagonist normalized reward learning to the level of healthy controls.
These limitations notwithstanding, the current findings provide novel evidence that, relative to placebo, KOR antagonism was associated with better ability to learn from reward feedback and express a behavioral preference toward a more advantageous stimulus, which overall points to better ability to integrate reinforcement over time. Moreover, we were able to confirm that the recently reported effects on response bias [49] remained when controlling for baseline anhedonia. Thus, the findings of this study build on our prior study [49] and strengthen the evidence that KOR antagonism has a therapeutic effect on a behavioral test reflecting a dimension of anhedonia, reward learning. They also increase the precision of the identification of the reward subdomains that are ameliorated by KOR antagonism. Together with the clinical outcome and fMRI data confirming target engagement, the current findings speak for further investigations of KOR antagonism for addressing the unmet needs associated with anhedonia across neuropsychiatric disorders.
Funding and disclosure
This project was supported by Contract HHS-N271-2012-000006-I from the National Institute of Mental Health. DAP was partially supported by R37 MH068376. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. DAP: Consulting: Akili Interactive Labs, BlackThorn Therapeutics, Boehringer Ingelheim, Compass Pathway, Otsuka Pharmaceuticals, and Takeda Pharmaceuticals. Honoraria: Alkermes. Stock options: BlackThorn Therapeutics. Research Support: NIMH, Dana Foundation, Brain and Behavior Research Foundation, Millennium Pharmaceuticals. DAP has a financial interest in BlackThorn Therapeutics, which has licensed the copyright to the Probabilistic Reward Task through Harvard University. DAP’s interests were reviewed and are managed by McLean Hospital and Partners HealthCare in accordance with their conflict of interest policies. No funding from these entities was used to support the current work, and all views expressed are solely those of the authors. MS: Research support: NIMH. GS: Consulting: Allergan, Alkermes, AstraZeneca, Avanier, Axsome Therapeutics, Pharmaceuticals, Biohaven Pharmaceuticals, Bristol-Myers Squibb, Boehringer Ingelheim, Clexio Biosciences, EMA Wellness, Epiodyne, Intra-Cellular Therapies, Janssen, Merck & Co., Naurex, Navitor, NeruoRx, Novartis, Noven Pharmaceuticals, Otsuka, Perception Neuroscience, Praxis Therapeutics, Sage Pharmaceuticals, Seelos Therapeutics, Taisho Pharmaceuticals, Teva, Valeant, and Vistagen therapeutics. Research support: AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Johnson & Johnson, Hoffman La-Roche, Merck, Naurex, and Usona. Free medication was provided for an NIH-sponsored study by Sanofi-Aventis. In addition, GS holds shares in BioHaven Pharmaceuticals Holding Company and is a co-inventor on a patent “Glutamate agents in the treatment of mental disorders” (Patent number: 8778979), and a U.S. Provisional Patent Application No. 047162-7177P1 (00754) filed on August 20, 2018 by Yale University Office of Cooperative Research OCR 7451 US01. SJM: Consulting: Allergan, Clexio Biosciences, EMA Wellness, Greenwich Biosciences, Intra-Cellular Therapies, Janssen, Perception Neuroscience, Seelos Therapeutics, and Signant Health. Research Support: Biohaven Pharmaceuticals and VistaGen Therapeutics. SJM is supported through the use of facilities and resources at the Michael E. Debakey VA Medical Center, Houston, Texas. SJM served as a consultant to Alkermes. JN: Research support: Janssen. SHL: contributed to this article while at Duke University, prior to joining NIMH. The views expressed are her own and do not necessarily represent the views of the National Institutes of Health or the United States Government. SHL is a co-inventor on a patent for TMS technology, unrelated to this paper. DVL: Consulting: Alkermes, Axsome, Centers of Psychiatric Excellence, Jazz Pharmaceuticals, Lundbeck, MyndAnalytics (CNS Response), Precision Neuroscience, Sage, Sunovion. Research Support (through his academic institution): LiteCure, Neosync, Otsuka. JWM: Consulting: Otsuka, Clexio Biosciences, FSV7, Boehringer Ingelheim, Sage Therapeutics, Novartis, Allergan, Fortress Biotech, Janssen Research and Development, Genentech, Medavante-Prophase, and Global Medical Education (GME). Research support: Avanir Pharmaceuticals, Inc. JWM is named on a patent filed for neuropeptide Y as a treatment for mood and anxiety disorders and on a patent filed for the use of KCNQ channel modulators for the treatment of depression and other disorders. WG: Consulting: Biohaven Pharmaceuticals. Research funding: NIH, Simons Foundation and Biohaven Pharmaceuticals; Device Donation: Medtronic. WZP: Advisory Board/Consultant: Takeda, Lilly, Praxis, Astellas, Otsuka and Noven. DSMB: Agene-Bio; Stock Ownership: Merck. ADK: Consulting: Adare, Eisai, Ferring, Galderma, Idorsia, Jazz, Janssen, Takeda, Merck, Neurocrine, Pernix, Physician’s Seal. Research support: NIH, Janssen, Jazz. Axsome, Reveal Biosensors. Y-SA, AEW, HY, RDW, JRC: no disclosures.
Supplementary information
Author contributions
Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work: all authors. Drafting the work or revising it critically for important intellectual content: DAP, MS, Y-SA, AEW, GS, SJM, DVI, JWN, ADK. Final approval of the version to be published: all authors. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: DAP, ADK.
Footnotes
The original online version of this article was revised due to missing conflict of interest of the co-author Sanjay J. Mathew.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
8/13/2021
A Correction to this paper has been published: 10.1038/s41386-021-01145-9
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41386-020-0738-4).
References
- 1.Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 2.DeRubeis RJ, Hollon S, Amsterdam J, et al. Cognitive therapy vs medications in the treatment of moderate to severe depression. Arch Gen Psychiatry. 2005;62:409–16. doi: 10.1001/archpsyc.62.4.409. [DOI] [PubMed] [Google Scholar]
- 3.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
- 4.Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–51. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 5.Loas G, Krystkowiak P, Godefroy O. Anhedonia in Parkinson’s disease: an overview. J Neuropsychiatry Clin Neurosci. 2012;24:444–51. doi: 10.1176/appi.neuropsych.11110332. [DOI] [PubMed] [Google Scholar]
- 6.Whitton AE, Treadway MT, Pizzagalli DA. Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Curr Opin Psychiatry. 2015;28:7–12. doi: 10.1097/YCO.0000000000000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nawijn L, van Zuiden M, Frijling JL, Koch SBJ, Veltman DJ, Olff M. Reward functioning in PTSD: a systematic review exploring the mechanisms underlying anhedonia. Neurosci Biobehav Rev. 2015;51:189–204. doi: 10.1016/j.neubiorev.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 8.Meehl PE. Hedonic capacity: some conjectures. Bull Menn Clin. 1975;39:295–307. [PubMed] [Google Scholar]
- 9.Klein DF. Endogenomorphic depression. A conceptual and terminological revision. Arch Gen Psychiatry. 1974;31:447–54. doi: 10.1001/archpsyc.1974.01760160005001. [DOI] [PubMed] [Google Scholar]
- 10.Pizzagalli DA. Depression, stress, and anhedonia: toward a synthesis and integrated model. Annu Rev Clin Psychol. 2014;10:393–423. doi: 10.1146/annurev-clinpsy-050212-185606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spijker J, Bijl RV, de Graaf R, Nolen WA. Determinants of poor 1-year outcome of DSM-III-R major depression in the general population: results of the Netherlands Mental Health Survey and Incidence Study (NEMESIS) Acta Psychiatr Scand. 2001;103:122–30. doi: 10.1034/j.1600-0447.2001.103002122.x. [DOI] [PubMed] [Google Scholar]
- 12.Moos RH, Cronkite RC. Symptom-based predictors of a 10-year chronic course of treated depression. J Nerv Ment Dis. 1999;187:360–8. doi: 10.1097/00005053-199906000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Fawcett J, Scheftner WA, Fogg L, Clark DC, Young MA, Hedeker D, et al. Time-related predictors of suicide in major affective disorder. Am J Psychiatry. 1990;147:1189–94. doi: 10.1176/ajp.147.9.1189. [DOI] [PubMed] [Google Scholar]
- 14.Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–69. doi: 10.1016/S0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 15.Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev. 2011;35:537–55. doi: 10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pizzagalli DA, Jahn AL, O’Shea JP. Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biol Psychiatry. 2005;57:319–27. doi: 10.1016/j.biopsych.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Der-Avakian A, D’Souza MSS, Pizzagalli DAA, Markou A. Assessment of reward responsiveness in the response bias probabilistic reward task in rats: implications for cross-species translational research. Transl Psychiatry. 2013;3:e297. doi: 10.1038/tp.2013.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lamontagne SJ, Melendez SI, Olmstead MC. Investigating dopamine and glucocorticoid systems as underlying mechanisms of anhedonia. Psychopharmacology. 2018;235:3103–13. doi: 10.1007/s00213-018-5007-4. [DOI] [PubMed] [Google Scholar]
- 19.Pizzagalli DA, Evins AE, Schetter EC, Frank MJ, Pajtas PE, Santesso DL, et al. Single dose of a dopamine agonist impairs reinforcement learning in humans: behavioral evidence from a laboratory-based measure of reward responsiveness. Psychopharmacology. 2008;196:221–32. doi: 10.1007/s00213-007-0957-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaiser RH, Treadway MT, Wooten DW, Kumar P, Goer F, Murray L, et al. Frontostriatal and dopamine markers of individual differences in reinforcement learning: a multi-modal investigation. Cereb Cortex. 2018;28:4281–90. doi: 10.1093/cercor/bhx281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vrieze E, Ceccarini J, Pizzagalli DA, Bormans G, Vandenbulcke M, Demyttenaere K, et al. Measuring extrastriatal dopamine release during a reward learning task. Hum Brain Mapp. 2013;34:575–86. doi: 10.1002/hbm.21456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santesso DL, Dillon DG, Birk JL, Holmes AJ, Goetz E, Bogdan R, et al. Individual differences in reinforcement learning: behavioral, electrophysiological, and neuroimaging correlates. Neuroimage. 2008;42:807–16. doi: 10.1016/j.neuroimage.2008.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luking KR, Neiman JS, Luby JL, Barch DM. Reduced hedonic capacity/approach motivation relates to blunted responsivity to gain and loss feedback in children. J Clin Child Adolesc Psychol. 2017;46:450–62. doi: 10.1080/15374416.2015.1012721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bogdan R, Pizzagalli DA. Acute stress reduces reward responsiveness: implications for depression. Biol Psychiatry. 2006;60:1147–54. doi: 10.1016/j.biopsych.2006.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, Fava M. Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. J Psychiatr Res. 2008;43:76–87. doi: 10.1016/j.jpsychires.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu W, Roiser JP, Wang L-Z, Zhu Y-H, Huang J, Neumann DL, et al. Anhedonia is associated with blunted reward sensitivity in first-degree relatives of patients with major depression. J Affect Disord. 2016;190:640–8. doi: 10.1016/j.jad.2015.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan A, Costi S, Morris LS, Van Dam NT, Kautz M, Whitton AE, et al. Effects of the KCNQ channel opener ezogabine on functional connectivity of the ventral striatum and clinical symptoms in patients with major depressive disorder. Mol Psychiatry. 2018. 10.1038/s41380-018-0283-2. [DOI] [PMC free article] [PubMed]
- 28.Liu W, Chan RCK, Wang L, Huang J, Cheung EFC, Gong Q, et al. Deficits in sustaining reward responses in subsyndromal and syndromal major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1045–52. doi: 10.1016/j.pnpbp.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 29.Vrieze E, Pizzagalli DA, Demyttenaere K, Hompes T, Sienaert P, De Boer P, et al. Reduced reward learning predicts outcome in major depressive disorder. Biol Psychiatry. 2013;73:639–45. doi: 10.1016/j.biopsych.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pechtel P, Dutra SJ, Goetz EL, Pizzagalli DA. Blunted reward responsiveness in remitted depression. J Psychiatr Res. 2013;47:1864–9. doi: 10.1016/j.jpsychires.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whitton AE, Kakani P, Foti D, Veer AV, Haile A, Crowley DJ, et al. Blunted neural responses to reward in remitted major depression: a high-density event-related potential study. Biol Psychiatry Cognit Neurosci Neuroimaging. 2016;1:87–95. doi: 10.1016/j.bpsc.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Audrain-McGovern J, Wileyto EP, Ashare R, Cuevas J, Strasser AA. Reward and affective regulation in depression-prone smokers. Biol Psychiatry. 2014;76:689–97. doi: 10.1016/j.biopsych.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fletcher K, Parker G, Paterson A, Fava M, Iosifescu D, Pizzagalli DA. Anhedonia in melancholic and non-melancholic depressive disorders. J Affect Disord. 2015;184:81–8. doi: 10.1016/j.jad.2015.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morris BH, Bylsma LM, Yaroslavsky I, Kovacs M, Rottenberg J. Reward learning in pediatric depression and anxiety: preliminary findings in a high-risk sample. Depression Anxiety. 2015;32:373–81. doi: 10.1002/da.22358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jacobson ML, Browne CA, Lucki I. Kappa opioid receptor antagonists as potential therapeutics for stress-related disorders. Annu Rev Pharmacol Toxicol. 2020;60:615–36. doi: 10.1146/annurev-pharmtox-010919-023317. [DOI] [PubMed] [Google Scholar]
- 36.McLaughlin JP, Li S, Valdez J, Chavkin TA, Chavkin C. Social defeat stress-induced behavioral responses are mediated by the endogenous kappa opioid system. Neuropsychopharmacology. 2006;31:1241–8. doi: 10.1038/sj.npp.1300872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reindl JD, Rowan K, Carey AN, Peng X, Neumeyer JL, McLaughlin JP. Antidepressant-like effects of the novel kappa opioid antagonist MCL-144B in the forced-swim test. Pharmacology. 2008;81:229–35. doi: 10.1159/000112867. [DOI] [PubMed] [Google Scholar]
- 38.Shirayama Y, Ishida H, Iwata M, Hazama GI, Kawahara R, Duman RS. Stress increases dynorphin immunoreactivity in limbic brain regions and dynorphin antagonism produces antidepressant-like effects. J Neurochem. 2004;90:1258–68. doi: 10.1111/j.1471-4159.2004.02589.x. [DOI] [PubMed] [Google Scholar]
- 39.Carlezon JrWA, Beguin C, DiNieri JA, Baumann MH, Richards MR, Todtenkopf MS, et al. Depressive-like effects of the kappa-opioid receptor agonist salvinorin A on behavior and neurochemistry in rats. J Pharmacol Exp Ther. 2006;316:440–7. doi: 10.1124/jpet.105.092304. [DOI] [PubMed] [Google Scholar]
- 40.Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, et al. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry. 2009;166:702–10. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Admon R, Kaiser RH, Dillon DG, Beltzer M, Goer F, Olson DP, et al. Dopaminergic enhancement of striatal response to reward in major depression. Am J Psychiatry. 2017;174:378–86. doi: 10.1176/appi.ajp.2016.16010111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Forbes EE, Hariri AR, Martin SL, Silk JS, Moyles DL, Fisher PM, et al. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. Am J Psychiatry. 2009;166:64–73. doi: 10.1176/appi.ajp.2008.07081336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maisonneuve IM, Archer S, Glick SD. U50,488, a kappa opioid receptor agonist, attenuates cocaine-induced increases in extracellular dopamine in the nucleus accumbens of rats. Neurosci Lett. 1994;181:57–60. doi: 10.1016/0304-3940(94)90559-2. [DOI] [PubMed] [Google Scholar]
- 44.Bruijnzeel AW. kappa-Opioid receptor signaling and brain reward function. Brain Res Rev. 2009;62:127–46. doi: 10.1016/j.brainresrev.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muschamp JW, Van’t Veer A, Carlezon WA. Tracking down the molecular substrates of stress: new roles for p38α mapk and kappa-opioid receptors. Neuron. 2011;71:383–5. doi: 10.1016/j.neuron.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carlezon WA, Krystal AD. Kappa-opioid antagonists for psychiatric disorders: from bench to clinical trials. Depression Anxiety. 2016;33:895–906. doi: 10.1002/da.22500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wee S, Koob GF. The role of the dynorphin-kappa opioid system in the reinforcing effects of drugs of abuse. Psychopharmacology. 2010;210:121–35. doi: 10.1007/s00213-010-1825-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carlezon JrWA, Beguin C, Knoll AT, Cohen BM. Kappa-opioid ligands in the study and treatment of mood disorders. Pharmacol Ther. 2009;123:334–43. doi: 10.1016/j.pharmthera.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krystal AD, Pizzagalli DA, Smoski M, Mathew SJ, Nurnberger J, Lisanby SH, et al. A randomized proof-of-mechanism trial applying the ‘fast-fail’ approach to evaluating κ-opioid antagonism as a treatment for anhedonia. Nat Med. 2020. 10.1038/s41591-020-0806-7. [DOI] [PMC free article] [PubMed]
- 50.Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. Br J Psychiatry. 1995;167:99–103. doi: 10.1192/bjp.167.1.99. [DOI] [PubMed] [Google Scholar]
- 51.Huys QJ, Pizzagalli DA, Bogdan R, Dayan P. Mapping anhedonia onto reinforcement learning: a behavioural meta-analysis. Biol Mood Anxiety Disord. 2013;3:12. doi: 10.1186/2045-5380-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Waelti P, Dickinson A, Schultz W. Dopamine responses comply with basic assumptions of formal learning theory. Nature. 2001;412:43–8. doi: 10.1038/35083500. [DOI] [PubMed] [Google Scholar]
- 53.Montague PR, Hyman SE, Cohen JD. Computational roles for dopamine in behavioural control. Nature. 2004;431:760–7. doi: 10.1038/nature03015. [DOI] [PubMed] [Google Scholar]
- 54.Bayer HM, Glimcher PW. Midbrain dopamine neurons encode a quantitative reward prediction error signal. Neuron. 2005;47:129–41. doi: 10.1016/j.neuron.2005.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- 56.Diagnostic and statistical manual of mental disorders. 4th ed. text revision. Washington, DC: American Psychiatric Press; 2000.
- 57.Franken IH, Rassin E, Muris P. The assessment of anhedonia in clinical and non-clinical populations: further validation of the Snaith-Hamilton Pleasure Scale (SHAPS) J Affect Disord. 2007;99:83–9. doi: 10.1016/j.jad.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 58.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maier W, Buller R, Philipp M, Heuser I. The Hamilton Anxiety Scale: reliability, validity and sensitivity to change in anxiety and depressive disorders. J Affect Disord. 1988;14:61–8. doi: 10.1016/0165-0327(88)90072-9. [DOI] [PubMed] [Google Scholar]
- 60.Rorick-Kehn LM, Witkin JM, Statnick MA, Eberle EL, McKinzie JH, Kahl SD, et al. LY2456302 is a novel, potent, orally-bioavailable small molecule kappa-selective antagonist with activity in animal models predictive of efficacy in mood and addictive disorders. Neuropharmacology. 2014;77:131–44. doi: 10.1016/j.neuropharm.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 61.Lowe SL, Wong CJ, Witcher J, Gonzales CR, Dickinson GL, Bell RL, et al. Safety, tolerability, and pharmacokinetic evaluation of single- and multiple-ascending doses of a novel kappa opioid receptor antagonist LY2456302 and drug interaction with ethanol in healthy subjects. J Clin Pharmacol. 2014;54:968–78. doi: 10.1002/jcph.286. [DOI] [PubMed] [Google Scholar]
- 62.Zheng M-Q, Nabulsi N, Kim SJ, Tomasi G, Lin S-F, Mitch C, et al. Synthesis and evaluation of 11C-LY2795050 as a κ-opioid receptor antagonist radiotracer for PET imaging. J Nucl Med. 2013;54:455–63. doi: 10.2967/jnumed.112.109512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nelson BD, Perlman G, Klein DN, Kotov R, Hajcak G. Blunted neural response to rewards as a prospective predictor of the development of depression in adolescent girls. Am J Psychiatry. 2016;173:1223–30. doi: 10.1176/appi.ajp.2016.15121524. [DOI] [PubMed] [Google Scholar]
- 64.Lemke MR. Depressive symptoms in Parkinson’s disease. Eur J Neurol. 2008;15 Suppl 1:21–5. doi: 10.1111/j.1468-1331.2008.02058.x. [DOI] [PubMed] [Google Scholar]
- 65.Poewe W. Non-motor symptoms in Parkinson’s disease. Eur J Neurol. 2008;15:14–20. doi: 10.1111/j.1468-1331.2008.02056.x. [DOI] [PubMed] [Google Scholar]
- 66.Stone MD, Audrain-McGovern J, Leventhal AM. Association of anhedonia with adolescent smoking susceptibility and initiation. Nicotine Tob Res. 2017;19:738–42. doi: 10.1093/ntr/ntw177. [DOI] [PubMed] [Google Scholar]
- 67.Ilyutchenok RY, Dubrovina NI. Memory retrieval enhancement by kappa opioid agonist and mu, delta antagonists. Pharmacol Biochem Behav. 1995;52:683–7. doi: 10.1016/0091-3057(95)00099-I. [DOI] [PubMed] [Google Scholar]
- 68.Tejeda HA, Counotte DS, Oh E, Ramamoorthy S, Schultz-Kuszak KN, Bäckman CM, et al. Prefrontal cortical kappa-opioid receptor modulation of local neurotransmission and conditioned place aversion. Neuropsychopharmacology. 2013;38:1770–9. doi: 10.1038/npp.2013.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kelsey JE, Verhaak AMS, Schierberl KC. The kappa-opioid receptor antagonist, nor-binaltorphimine (nor-BNI), decreases morphine withdrawal and the consequent conditioned place aversion in rats. Behav Brain Res. 2015;283:16–21. doi: 10.1016/j.bbr.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 70.Meng F, Xie GX, Thompson RC, Mansour A, Goldstein A, Watson SJ, et al. Cloning and pharmacological characterization of a rat κ opioid receptor. Proc Natl Acad Sci USA. 1993;90:9954–8. doi: 10.1073/pnas.90.21.9954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Simonin F, Gavériaux-Ruff C, Befort K, Matthes H, Lannes B, Micheletti G, et al. κ-Opioid receptor in humans: cDNA and genomic cloning, chromosomal assignment, functional expression, pharmacology, and expression pattern in the central nervous system. Proc Natl Acad Sci USA. 1995;92:7006–10. doi: 10.1073/pnas.92.15.7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhu J, Chen C, Xue JC, Kunapuli S, DeRiel JK, Liu-Chen LY. Cloning of a human κ opioid receptor from the brain. Life Sci. 1995;56:PL201–7. doi: 10.1016/0024-3205(94)00507-o. [DOI] [PubMed] [Google Scholar]
- 73.Kumar P, Goer F, Murray L, Dillon DG, Beltzer ML, Cohen AL, et al. Impaired reward prediction error encoding and striatal-midbrain connectivity in depression. Neuropsychopharmacology. 2018;43:1581–8. doi: 10.1038/s41386-018-0032-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Trivedi MH, South C, Jha MK, Rush AJ, Cao J, Kurian B, et al. A novel strategy to identify placebo responders: prediction index of clinical and biological markers in the EMBARC trial. Psychother Psychosom. 2018;87:285–95. doi: 10.1159/000491093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Webb CA, Dillon DG, Pechtel P, Goer FK, Murray L, Huys QJQJM, et al. Neural correlates of three promising endophenotypes of depression: evidence from the EMBARC study. Neuropsychopharmacology. 2016;41:454–63. doi: 10.1038/npp.2015.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


