Abstract
The classification of the central disorders of hypersomnolence has undergone multiple iterations in an attempt to capture biologically meaningful disease entities in the absence of known pathophysiology. Accumulating data suggests that further refinements may be necessary. At the 7th International Symposium on Narcolepsy, a group of clinician-scientists evaluated data in support of keeping or changing classifications, and as a result suggest several changes. First, idiopathic hypersomnia with long sleep durations appears to be an identifiable and meaningful disease subtype. Second, idiopathic hypersomnia without long sleep time and narcolepsy without cataplexy share substantial phenotypic overlap and cannot reliably be distinguished with current testing, and so combining them into a single disease entity seems warranted at present. Moving forward, it is critical to phenotype patients across a wide variety of clinical and biological features, to aid in future refinements of disease classification.
Keywords: narcolepsy, idiopathic hypersomnia, hypersomnolence, classification
Statement of Significance.
This expert review discusses the current limitations of disease classification for the central disorders of hypersomnolence. We propose a new classification, once again separating out idiopathic hypersomnia with long sleep time as a separate entity. We further propose combining the currently indistinguishable entities of narcolepsy without cataplexy and idiopathic hypersomnia without long sleep time. Better phenotyping of ancillary symptoms, sleep durations, quality of sleepiness, biological fingerprints, and momentary versus perpetual features is needed.
Introduction
Classification of disease is an iterative process based on evolving scientific understanding and early classifications often turn out to be flawed in the light of newer data. This has been as true for the field of sleep medicine as other medical specialties, and for some disorders within sleep medicine, robust debate still exists about ideal classification and the biological meaning of certain disease labels. In particular, appropriate classification of disorders under the category of “central disorders of hypersomnolence (CDH),” i.e. disorders of excessive sleepiness likely to represent a central nervous system cause, remains controversial [1, 2]. At the 7th International Symposium on Narcolepsy held in Massachusetts, United States, in late 2018, the authors of this consensus review, a group of experienced clinician-scientists, convened to reassess the current classification of narcolepsy and idiopathic hypersomnia (IH). This manuscript summarizes the outcome of their discussion and represents their opinions.
Current Classification: The International Classification of Sleep Disorders, Third Edition
Currently, the International Classification of Sleep Disorders, third edition (ICSD-3) classifies three primary, non-recurrent CDH: narcolepsy type 1 (NT1), narcolepsy type 2 (NT2), and IH [3]. Of note, diagnostic criteria are the same for adults and children, despite much more limited research into the pediatric phenotype of these disorders. All three diagnoses require at least 3 months of daily sleepiness, defined as either daytime sleep episodes or an “irrepressible” need to sleep.
In addition to sleepiness, NT1 is defined by either (1) documentation of cerebrospinal fluid (CSF) hypocretin (orexin) deficiency, as this biomarker reflects current understanding of NT1 as a likely T-cell-mediated autoimmune attack on hypocretin-producing hypothalamic neurons; or (2) the combination of clear-cut cataplexy and polysomnography (PSG)/multiple sleep latency test (MSLT) showing an MSLT mean sleep latency (MSLT-SL) less than or equal to 8 min with at least two sleep-onset rapid eye movement periods (SOREMs) between PSG and MSLT recordings. The latter criterion is a practical and reasonably accurate way of identifying individuals who are likely to have hypocretin deficiency, given the pathognomonic value of cataplexy and again emphasizing that the diagnostic category of NT1 captures a relatively homogenous group with single, well-known pathophysiology [3].
NT2 is defined by a combination of positive and negative features; that is, for a diagnosis of NT2, individuals must have an MSLT-SL less than or equal to 8 min and at least two SOREMs, but cannot have cataplexy, hypocretin deficiency, or other conditions that better explain the symptoms [3].
IH requires the same exclusions of cataplexy, hypocretin deficiency, and other explanatory conditions, but is distinct from NT2 on the objective confirmation of hypersomnolence. This can be made in one of three ways: (1) an MSLT-SL less than or equal to 8 min, but without at least two SOREMs that would instead result in an NT2 diagnosis; (2) at least 660 min (11 h) of sleep time measured over up to 24 h of PSG monitoring after correcting chronic sleep deprivation; or (3) at least 660 min of sleep time per 24 h, estimated by wrist actigraphy, averaged over at least 1 week of ad libitum sleep [3].
Challenges With Current Classification
The evidence that NT1 should be classified as a distinct disorder, as it is in the ICSD-3, is now compelling. From early recognition that immune system regulation could be involved with the development of this form of narcolepsy, based on strong associations with HLA subtypes, especially DQB1*0602 [4], data now convincingly support (although still do not fully prove) narcolepsy with hypocretin deficiency as an autoimmune attack on hypocretin neurons in the lateral hypothalamus, occurring in genetically susceptible individuals [5, 6]. Most recently, the identification of CD4 autoreactive T cells against hypocretin using sensitive methods [7] most directly points to the autoimmune nature of this disorder. As a surrogate for hypocretin deficiency, the current PSG/MSLT protocol plus cataplexy is sufficient to diagnose NT1 in most cases. The retest reliability of the PSG/MSLT for NT1 is high at 78%–81.3% [8, 9].
In contrast to NT1, however, NT2 and IH remain as disorders without known pathophysiology and lacking validated, reliable biomarkers. As such, multiple methods of classifying patients with symptoms of these disorders may be reasonable, and it is quite difficult to argue that any one classification will necessarily turn out to be correct or incorrect. However, we believe that the current structure of the ICSD-3 does not fully capture the phenotypic features of NT2 and IH.
NT2: a heterogeneous group of NT1 and IH?
In the current classification, PSG/MSLT is critical for the diagnosis of NT2, as hypocretin levels are normal by definition (if measured) and the presence of two or more SOREMs differentiates NT2 and IH. Historically, it has been taught that NT2 exists as a specific disease because patients lack sleep inertia and habitual long sleep times and have chronic excessive daytime sleepiness (EDS), refreshing brief naps, and more specific reporting of hypnagogic hallucinations and sleep paralysis that reflect underlying REM dysregulation [10]. However, it is clear that this description of NT2 is far from universal among people with NT2. REM-related symptoms of sleep paralysis and sleep-related hallucinations are reported by only 21%–47% and 14%–37% of NT2 patients, respectively [11–14]. Long sleep times, classically described as a characteristic of IH, are seen in 18% of people with narcolepsy, the majority of whom have NT2 [15]. Sleep drunkenness has not been well-quantified among people with NT2 but is reported to occur in 47% of those with NT2 [16]. Objective testing in NT2 also shows substantial heterogeneity, particularly in rates of the objective REM features, including nocturnal REM sleep-onset latency [17], number of daytime SOREMs [18], and REM without atonia [18, 19].
Some of the variability in NT2 symptomatology and objective findings likely reflects heterogeneity within the diagnosis. Some patients diagnosed with NT2 likely have unrecognized hypocretin deficiency, because they lack cataplexy or because their disease process is still evolving at the time of clinical evaluation [20–23]. Although hypocretin levels cannot be low, by definition, to maintain a diagnosis of NT2, low or indeterminate hypocretin levels are found in 10%–30% of those initially diagnosed as narcolepsy without cataplexy [17, 24]. Nearly half of patients with low hypocretin and narcolepsy without cataplexy at presentation will develop cataplexy over time [17]. Cutoff values for hypocretin depend on the assay being used, but are considered low if less than 110 pg/mL and intermediate if less than 200 pg/mL when using the Stanford reference standard [3]. There is also marked, but largely unexplained, variation in the likelihood of hypocretin deficiency in the absence of diagnosed cataplexy by racial classification, i.e. African American narcolepsy patients are more likely to have no cataplexy despite being hypocretin-deficient [25]. After excluding individuals with occult or evolving hypocretin deficiency, the pathophysiology of the remaining cases of NT2 is currently unknown. The association with DBQ1*0602, and therefore evidence for an autoimmune process, is considerably weaker in NT2 than in NT1 [26] and may be regarded as a biomarker of possible disease evolution. Although it is possible that NT2 is caused by an abnormality of the hypocretin system other than autoimmune loss of hypocretin-producing neurons, there is presently insufficient evidence to support or refute this hypothesis.
IH: decreased alertness and more heterogeneity
IH is clinically characterized by long and unrefreshing naps, prolonged and undisturbed nocturnal sleep, impaired daytime alertness and focus, and sleep inertia, in addition to EDS [1, 2]. Current diagnostic criteria, however, focus on daytime sleepiness and sleep duration. Despite this emphasis on daytime sleepiness, it is important to note that the experience of EDS may differ qualitatively between people with IH and those with NT1. In contrast to patients with NT1, many IH patients (and more in those with habitual long sleep durations) describe no or rare daytime sleep attacks [2]. Rather, they report having continuous nonimperative sleepiness, which leads them to never feel fully awake during the daytime, to feel “foggy,” and to lack clear alertness. In patients with sleep drunkenness, this fogginess is maximal upon awakening and may fade in the evening. The use of multiple alarm clocks or assistance from a family member is often needed for awakening at a particular time. In a group of 62 patients with IH, patients insisted on making a difference between sleepiness (as estimated by their ability to fall asleep in passive conditions, e.g. using the MSLT or the Epworth Sleepiness Score) and decreased alertness [2]. However, the same stimuli affected the sleepiness and the decreased alertness, in the same direction. During the daytime, the alertness was modulated by the same external conditions (e.g. higher during a sunny than a rainy day, lower with artificial than natural light) in controls and in patients. In addition, patients described that they could not sustain attention for more than 1 h (vs almost 4 h in the controls), suggesting a cognitive fatigability. The current classification does not distinguish this IH decreased alertness from classic sleep attacks, and optimal measurement of this symptom is yet to be determined.
Furthermore, the diagnosis of IH still relies heavily on the MSLT, which operationalizes sleepiness as the tendency to fall asleep when attempting to do so, which is not necessarily the best way to operationalize decreased alertness. Several groups have assessed the sensitivity of MSLT MSL less than 8 min for IH diagnosis. Multiple approaches have been used to define the “gold standard” for IH diagnosis against which the MSLT can be compared, including clinical hypersomnia diagnosis and clinical hypersomnia diagnosis plus long sleep time.
Using expert clinical judgment to define IH, an MSL less than 8 is seen in approximately half of the patients. In the Cambridge group, only 51% of 72 people with a typical clinical phenotype of IH met this MSLT criterion [27]. Similarly, among 105 participants evaluated for IH by the Paris group, 45% had an MSL less than 8 min, although this group then excluded participants with normal sleep durations and MSL more than 8 min from a research diagnosis of IH for their remaining analyses [28]. In a series of 61 patients with “clear-cut clinical EDS” but without cataplexy, 31% met MSLT criteria for IH, 3% had IH with long sleep time, and 11% met MSLT criteria for narcolepsy without cataplexy [29]. The remaining 54% had a normal MSLT, closely approximating the rate of normal MSLT seen in other studies using clinical IH diagnosis.
Using a combination of expert clinical judgment and objectively measured long sleep time to define IH, the MSLT cutoff of 8 min performs similarly poorly. In the Paris group, those with clinical IH and nocturnal sleep of more than 10 h had an MSL more than 8 min in 71% of cases; of these normal MSLTs, only 17% were considered borderline abnormal, i.e. between 8 and 10 min [28]. The Montpellier group performed both a standard and a modified MSLT (the latter interrupting each nap after 1 min of sleep) and found that those with clinical IH had an MSL less than 8 min in 32% of cases and those with long sleep time (at least 19 h out of 32) had an MSL less than 8 min in 58% of cases [30]. Conversely, a short sleep latency at the MSLT can occur also in the absence of any sleepiness complaint in otherwise healthy participants, pointing to the need to merge clinical and polysomnographic evidence to reach a definite diagnosis [31].
These results highlight the importance of measures outside the MSLT to capture IH sleep/sleepiness characteristics. Long sleep durations are currently used as the non-MSLT objective measure for IH diagnosis. The ICSD-3 permits IH diagnosis on the basis of total sleep time in excesses of 660 min (as documented by 24-h PSG or average sleep duration on more than 7 days of actigraphy) [3]. Actigraphy was included in the ICSD-3, despite the absence of validation studies [3], although recent work to validate this measure has begun [32]. Incorporating long sleep durations into IH diagnosis is compatible with Bedrich Roth’s early descriptions of this syndrome, in which he reported a subgroup with long sleep time [1, 33], and with earlier versions of the ICSD that emphasized this distinction [10, 34]. Indeed, the very word hypersomnia comes from the Greek root “hyper” for excessive and the Latin root “somnius” for sleep. Consequently, the word means “sleep excess,” even though its meaning has broadened over time to also include EDS.
A striking example in which MSLT was normal in spite of obviously excessive sleep duration was reported by Voderholzer et al. [35]. This 16-year old man had a 4-year history of severely increased sleep need, daytime fatigue, and great difficulty waking up after 9 h of nighttime sleep, with hypotonia and dizziness in the morning, and no cataplexy or sleep attacks during the daytime. During the MSLT, the mean sleep latency was 10.8 min (i.e. normal). During long-term monitoring, he slept 1162 min (from 11:00 pm to 6:30 pm the next day, with a 97% sleep efficiency). This extreme case illustrates the contrast between a measure of daytime sleep propensity (MSLT) and a measure of ad libitum sleep (long-term sleep monitoring). However, despite the need to measure sleep duration in IH, not all laboratories have the protocols, staff, and/or equipment to conduct such diagnostic testing. This is particularly problematic in regions where extended PSG is not typically reimbursed by payors, such as the United States.
Furthermore, a number of ad lib PSG protocols for capturing excessive sleep durations are currently in use, without consensus about the optimal procedure. In IH, the sleep excess is best expressed in unrestricted conditions, such as during the weekend, holidays, and in the sleep laboratory, with on average three additional hours slept than during weekdays [2]. The duration of spontaneous sleep must capture both nighttime sleep and daytime sleep during naps, either or both which may be very prolonged in IH. Monitoring should follow 1–2 weeks without sleep restriction, although this can be difficult to achieve in employed IH patients, unless the sleep testing is performed directly after vacation with unrestrained sleep.
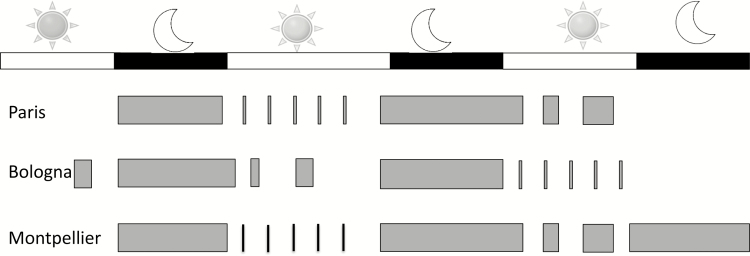
So far, three different procedures have been developed to capture sleep excess (Figure 1) [28–30]. The shortest procedure is the Paris procedure (48 h) and the longest is the Montpellier procedure (80 h, in two separated periods of 24 h and 58 h, respectively). The Paris procedure is routinely used in cases of central hypersomnolence in French competence centers for hypersomnia (Figure 1, top) [15, 28, 36]. The procedure allows assessment of both the “narcoleptic” phenotype (i.e. short MSLT-SL and multiple SOREMs) and long sleep phenotype (measured by the time slept during the second night and day). Television, computer, and a visit from friends are forbidden, but books, newspapers, watches, and daylight are allowed. The first night is truncated at 6:30 am, in order to start a five-nap MSLT at 08:00 am. The second night starts ad libitum (and no later than 11:00 pm) and is uninterrupted. The participants sleep until spontaneous awakening (and lights on) the next day. Then, all participants are offered two naps, one during the morning and one during the afternoon, lying in the dark. The nap attempts continue for as long as the participant remains asleep but are discontinued after 30 min if participants cannot sleep. Between the naps, natural light and activities are allowed. Tests are stopped at 05:00 pm. All in all, this procedure provides 18–20 h for sleep. The sleep time obtained during this 18 h monitoring in 75 IH patients was very similar to their reported usual sleep time during holidays and weekends, suggesting it is an objective measure with real-world relevance in people with IH [2]. In comparing people with IH to healthy controls, a cutoff of 662 min (roughly 11 h of sleep) has the best sensitivity (72%) and specificity (97%). In contrast, a lower cutoff of 600 min (10 h) has a sensitivity of 55% and a specificity of 77%; 691 min (11h 30m) is highly specific (100%) but poorly sensitive (53%) [36].
Figure 1.
Protocols for measuring sleep time in idiopathic hypersomnia. Several protocols are currently in use for measurement of sleep time in people with idiopathic hypersomnia. Gray bars indicate periods where participants are asked or allowed to sleep, with the size of the bars indicating relative duration (see text for details).
The Bologna procedure lasts 60 h in total and includes an ad libitum monitoring of sleep during the first 48 h, with a patient allowed to move around, read, and watch TV, followed by an MSLT the last day (Figure 1, middle) [29]. Consequently, the MSLT performed after sleep has been sufficient during 48 h in the laboratory setting, limiting the risk of sleep deprivation. Daytime sleep is not imposed in darkness but is spontaneous. Spontaneous naps and SOREMs during these naps correlate with MSLT results [29]. However, normative data from controls were not provided for this procedure.
The Montpellier procedure lasts in total 80 h and starts with a classical nighttime PSG followed by MSLT (Figure 1, bottom) [30]. Then, depending on clinical features, patients may be referred for a second procedure lasting 58 h. It starts with a nighttime PSG, followed by a modified MSLT. The five tests of the modified MSLT are interrupted after 1 min of sleep, in order to avoid decreasing the homeostatic sleep pressure. The sleep is then monitored during a 32-h bed rest procedure, including a second night, a second day, and then a third night. Participants are invited to sleep as long as possible, ad libitum. Daylight, television, computer, newspapers, phones, watches, and visits from family or friends are forbidden. Communication with hospital staff is limited to emergencies and meal delivery. The sleep room is maintained in dim light (10 lux). The authors determined the sleep duration during the 32-h bed rest in 32 patients with clinical IH and MSL lower than 10 min during the first MSLT and compared it to 21 healthy controls. A cutoff of 19 h/32 h reaches the highest sensitivity (92%) and specificity (86%) to distinguish the groups. When restraining the analysis to the first 24 h of the 32-h bed rest, a surrogate cutoff of 12 h had the highest sensitivity (100%)/specificity (86%).
Each procedure has its advantages (measuring ad libitum, unrestrained sleep during 18–48 h, being simple or not), limits (long or very long duration, reduced number of healthy controls, presence or absence of zeitgebers, price), and normative measures. An important, unresolved question regards the best cutoff in defining long sleep times. The Diagnostic and Statistical Manual, version 5, defines long sleep in hypersomnia disorder as longer than 9 h during the night, the immediate prior version of the ICSD (i.e. ICSD2) used a cutoff of 10 h during the night to separate IH with and without long sleep, the Paris procedure suggests a cutoff of 11 h (including sleep during the day and night), and the Montpellier procedure suggests a cutoff of 12 h (including sleep during the day and night) [10, 28, 30]. In the general population, sleep durations in excess of 9 h per night are endorsed by 8% of the population [37]. Additionally, whether home studies may replace these in lab procedures is yet questionable, as normative measures have been established in lab-controlled conditions, but not for home studies.
The clinical phenotype of IH without long sleep time may be more difficult to capture, because it relies only on the MSLT, without the benefit of in-laboratory measurement or ambulatory estimation of prolonged sleep times. There is an ongoing debate about whether IH should be subclassified into that with versus without prolonged sleep times, as evidenced by the change in this characterization from the second to the third version of the ICSD [3, 10]. In our experience, people with IH with long sleep time are more likely to demonstrate other classical findings of IH, including sleep inertia and decreased alertness. However, to date, published studies reported only borderline relationship between long sleep and other features such as sleep drunkenness. Among 75 IH cases [28], sleep drunkenness tended to be more frequent in the IH subgroup with than without long sleep time (but p = 0.08). In another series of 62 cases the association between prolonged nighttime sleep and sleep drunkenness was also borderline (but p = 0.083) [27]. Reduced sample sizes may have limited the ability to show a significant difference here.
International Classification of Sleep Disorders—Ideas for the Fourth edition
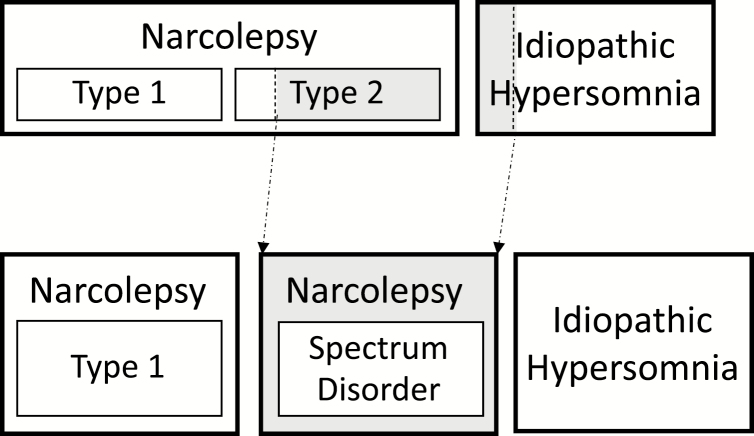
Our clinical and research expertise with these disorders leads us to propose a somewhat different classification than that codified in the ICSD-3 (Figure 2). We propose that NT1 remains a distinct disorder, that IH with long sleep time once again be considered a distinct disorder, and that NT2 and IH without long sleep time be merged into a single disorder, always after careful exclusion of insufficient sleep syndrome which can produce typical MSLT results with or without multiple SOREMs. The arguments for separating NT1 and IH with long sleep time are similar—each has a phenotype that is distinct and measurable, i.e. the combination of cataplexy and hypocretin deficiency in NT1 and extended 24-h sleep durations in IH.
Figure 2.
Current International Classification of Sleep Disorders, third edition, versus proposed grouping. The ICSD-3 lists eight central disorders of hypersomnolence, including narcolepsy type 1, narcolepsy type 2, and idiopathic hypersomnia (top row). Based on current data, the authors propose combining those with narcolepsy type 2 and those with idiopathic hypersomnia without long sleep time into a single, new diagnosis called “Narcolepsy spectrum disorder” (bottom row).
Lumping IH (short sleep time) with NT2
Once those individuals with hypocretin deficiency and those with long sleep time, sleep inertia, and a hypoarousal phenotype are separately classified, and after exclusion of chronic sleepiness due to any other comorbidity or insufficient sleep, there is presently little if any justification for further separating the remaining individuals into NT2 versus IH. We favor lumping them in the same classification for several reasons. First, there is a clear phenotypic overlap between these two conditions (Table 1). For many measures, such as sleep paralysis, sleep-related hallucinations, and possibly disrupted nocturnal sleep, NT2 demonstrates a frequency of symptoms that are intermediate between that seen in NT1 and that seen in IH. That some patients classified as NT2 have unrecognized hypocretin deficiency likely accounts for some of this phenotypic overlap with NT1, while those without hypocretin deficiency likely more resemble those with IH without long sleep time. In a longitudinal study assessing clinical remission of EDS, NT1 patients did not show any remission, as expected, but both the NT2 and IH group (measured with a short MSLT) had similar rates of remission (44.6% at 5 years and 32.5% at 5.5 years after diagnosis) [38]. While other studies have suggested remission rates for IH that are somewhat lower (14%–26%), the absence of spontaneous remissions is a likely clinical feature that distinguishes NT1 from the other two disorders [14, 27].
Table 1.
Required Symptoms, Findings, and/or Comorbidities for Central Disorders of Hypersomnolence, According to the ICSD-3
| NT1 | NT2 | IH | |
|---|---|---|---|
| Daily periods of irrepressible sleep need or daytime lapses into sleep | |||
| X | X | X | |
| Objective measures: Laboratory | |||
| - Hypocretin (orexin) deficiency | (X) | – | – |
| Objective measures: PSG or actigraphy | |||
| - ≥11 h sleep per 24 h | (P) | (X) | |
| - Sleep efficiency ≥90% | (S) | ||
| Objective measures: MSLT | |||
| - Low mean sleep latency (≤8 min) | (X) | X | (X) |
| - <2 SOREMs (including PSG) | X | ||
| - ≥2 SOREMs (including PSG) | (X) | X | |
| Reported measures: Symptoms | |||
| - Cataplexy | (X) | – | – |
| - Sleep inertia, unrefreshing naps | (P) | (S) | |
| Reported measures: Sleep–wake behavior | |||
| - Sleep time shorter than expected | – | – | |
| - Sleep time curtailed by an alarm clock, etc. | (P) | ||
| - Longer sleep during weekend/vacation | (P) | ||
| - Sleepiness remits with longer sleep | – | – |
NC1 and NC2 = narcolepsy type 1 and 2; IH = idiopathic hypersomnia; X = mandatory criterion; (X) = either/or criterion, or facultative criterion; (S) = supportive criterion; – = must be absent for the diagnosis; (P) = not considered a classic feature but sometimes present.
Presently, the only clinical feature that allows differentiation of NT2 and IH without long sleep time is the number of SOREMs on PSG/MSLT. Thus, the second reason for lumping NT2 and IH without long sleep time springs from the poor retest reliability of MSLT for IH or NT2 diagnosis. Patients initially diagnosed with these disorders who undergo repeat testing will frequently change the diagnosis, either because of a change in SOREMs or a change in sleep latency [8, 9, 39]. This may be because of inconsistent MSLT testing protocols, unstable symptom characteristics, and/or that number of SOREMs on MSLT is not a stable feature of these disorders. Whether nocturnal PSG SOREMs may add to reliable differentiation between NT2 and IH is currently unknown. Nocturnal SOREMs may be more specific for narcolepsy with cataplexy/hypocretin deficiency than without [40] and so are anticipated to add limited discriminative power to distinguishing NT2 and IH. Furthermore, the test–retest reliability of nocturnal PSG SOREMs in people with hypersomnolence disorders has not been studied.
Third and finally, in a data-driven cluster analysis based on MSLT findings and clinical features of cataplexy, sleep times, and nap characteristics, three well-differentiated clusters of central hypersomnia disorders became apparent: narcolepsy with cataplexy, IH with long sleep time, and a combined group of narcolepsy without cataplexy and IH without long sleep time [41].
On the basis of all of these factors, it is difficult at present to justify a clear differentiation between NT2 and IH without long sleep time, and hence we recommend lumping these two disorders as presently defined into a single entity. Of course, we make this recommendation with the understanding that, like all classifications based on phenomenology, we also may turn out to be wrong. It is perfectly plausible that NT2 and IH without long sleep time represent two (or more) different pathophysiologic entities. We argue only that current diagnostic tools do not allow convincing separation of such. Continued careful phenotyping of this combined group will be critical, to identify any within-group differences that may emerge.
How Do We Further Refine Disease Definition?
Until the pathophysiology of these diseases is understood, the diagnosis will continue to rely on clinical phenotyping. As such, continued research work is needed to operationalize and test the discriminant validity of currently identified phenotypic features. For instance, disrupted nocturnal sleep is common in people with NT1 [42]. Formal measurement of this feature, whether by sleep efficiency or more detailed analysis of nocturnal state transitions, may add to the specificity of an NT1 diagnosis [26, 43, 44]. In contrast, sleep efficiency is generally considered to be higher than average in people with IH, such that assessment of the stability of sleep state overnight might be revealing in this group [45]. The refreshing or unrefreshing nature of naps is a commonly cited difference between narcolepsy and IH that may be difficult to operationalize [42], but typical nap duration may be easier to quantify with ambulatory monitoring. Tools to measure sleep drunkenness are in development, both self-report and measurement of cognitive performance upon awakening [46], which may help distinguish IH subtypes and clarify the frequency of this symptom in people with NT2. The development of novel wearables measuring EEG over a long time will likely improve the measurement of both habitual sleep durations and nightly variability of sleep–wake behavior.
Novel biomarkers are also needed. There is growing evidence of altered functional imaging findings in a variety of CDH [50, 51], which might eventually allow differentiation of hypersomnia disorders. Novel fluid biomarkers, be it hypocretin (orexin) in serum or histamine in CSF, might improve diagnostic accuracy in disorders with proven or suspected neurotransmitter-related pathophysiology [52, 53]. All clinical, electrophysiological, chemical, or imaging biomarkers of hypersomnia phenotypes must be validated before widespread application and inclusion in a future classification of sleep disorders. Validation procedures can be done against well-understood biomarkers of disease, e.g. hypocretin deficiency in NT1. For disorders with largely unknown pathophysiology such as NT2 or IH, cluster analyses of raw data from novel chronic measurements in large samples may be followed by validation studies in independent samples, ultimately leading to better-defined phenotypes, enhanced understanding of pathophysiology, and improved diagnoses of hypersomnolence disorders. Finally, research on the potential need for different diagnostic criteria based on age and age-related evolution of disease phenotypes is warranted.
What Should the Clinician Do Now?
Regardless of what will be the next change in the classification of “CDH,” it remains of vital importance for clinicians to carefully phenotype their patients. This includes assessing comorbid disorders which might be related to complaints of hypersomnolence. Secondary causes should be carefully explored as well, e.g. a recent viral infection or traumatic brain injury. In those cases, complaints often follow a different time-course. Furthermore, mood disorders should be evaluated and if needed diagnosed by a psychiatrist, to distinguish depressive symptoms caused by EDS from a primary, major depression leading to complaints of hypersomnolence.
There are some practical points that can already be implemented in daily practice. First, sleep deprivation and circadian misalignment should always be assessed and ruled out as the cause of EDS. Long sleepers may experience sleepiness in mild chronic deprivation conditions (e.g. sleeping “only” 8–9 h). They can be identified by their need to sleep more than 10 h since birth, the frequent presence of long sleepers among parents and siblings, and a frank disappearance of sleepiness and sleep inertia when sleeping 10–11 h. This also holds true for shift work. Second, the clinical assessment should not only focus on sleep itself and sleepiness, but also on the quality of wakefulness related to daytime performance in school, work, and life. Third, if patients report long sleep times and prominent sleep inertia, an extended PSG allowing for ad libitum sleep should be considered if available.
Conclusions
IH with long sleep time and NT1 are both disorders that are distinct based on their clinical phenotype and, in the case of the latter, by our knowledge about the pathophysiology. However, the clinical phenotype of NT2 is indistinguishable from that of IH without long sleep time. We propose that in a new classification of “CDH” these two disorders be “lumped” into one category and propose the name “narcolepsy spectrum disorder” as an acknowledgment of the potential heterogeneity within the group. Following this, it is not justifiable to exclude patients having IH, especially those without a long sleep time, from pharmacological treatments currently approved or being tested for NT2.
Acknowledgments
We gratefully acknowledge the organizers and participants of the Seventh International Narcolepsy Symposium. We also thank Professor Giuseppe Plazzi for helpful discussions regarding this work.
Funding
This work was supported by the National Institutes of Health (K23 NS083748 to LMT).
Financial Disclosure: RF reports lecture fees and travel support from Bioproject and travel support from UCB Europe. IA reports lecture fees from UCB Pharma and consultancy from Ono Pharma and Roche Pharma. KM reports research support from Jazz Pharmaceuticals and has received consulting fees from Harmony Biosciences. CRB, FP, and LMT report no conflicts.
Nonfinancial Disclosure Statement: The authors report no nonfinancial conflicts.
References
- 1. Roth B. Narcolepsy and hypersomnia: review and classification of 642 personally observed cases. Schweiz Arch Neurol Neurochir Psychiatr. 1976;119(1):31–41. [PubMed] [Google Scholar]
- 2. Vernet C, et al.. Subjective symptoms in idiopathic hypersomnia: beyond excessive sleepiness. J Sleep Res. 2010;19(4):525–534. [DOI] [PubMed] [Google Scholar]
- 3. American Academy of Sleep Medicine. International Classification of Sleep Disorders— 3rd ed. 2014. [Google Scholar]
- 4. Mignot E, et al.. HLA DQB1*0602 is associated with cataplexy in 509 narcoleptic patients. Sleep. 1997;20(11):1012–1020. [PubMed] [Google Scholar]
- 5. Hallmayer J, et al.. Narcolepsy is strongly associated with the T-cell receptor alpha locus. Nat Genet. 2009;41(6):708–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mahlios J, et al.. The autoimmune basis of narcolepsy. Curr Opin Neurobiol. 2013;23(5):767–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Latorre D, et al.. T cells in patients with narcolepsy target self-antigens of hypocretin neurons. Nature. 2018;562(7725):63–68. [DOI] [PubMed] [Google Scholar]
- 8. Lopez R, et al. . Test–retest reliability of the multiple sleep latency test in central disorders of hypersomnolence. Sleep. 2017;40(12). doi: 10.1093/sleep/zsx164 [DOI] [PubMed] [Google Scholar]
- 9. Ruoff C, et al.. The MSLT is repeatable in narcolepsy type 1 but not narcolepsy type 2: a retrospective patient study. J Clin Sleep Med. 2018;14(1):65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. American Academy of Sleep Medicine. International Classification of Sleep Disorders— 2nd ed. 2005. [Google Scholar]
- 11. Sasai T, et al.. Comparison of clinical characteristics among narcolepsy with and without cataplexy and idiopathic hypersomnia without long sleep time, focusing on HLA-DRB1(*)1501/DQB1(*)0602 finding. Sleep Med. 2009;10(9):961–966. [DOI] [PubMed] [Google Scholar]
- 12. Dodet P, et al.. Lucid dreaming in narcolepsy. Sleep. 2015;38(3):487–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Leu-Semenescu S, et al.. Hallucinations in narcolepsy with and without cataplexy: contrasts with Parkinson’s disease. Sleep Med. 2011;12(5):497–504. [DOI] [PubMed] [Google Scholar]
- 14. Bassetti C, et al.. Idiopathic hypersomnia. A series of 42 patients. Brain. 1997;120(Pt 8):1423–1435. [DOI] [PubMed] [Google Scholar]
- 15. Vernet C, et al.. Narcolepsy with long sleep time: a specific entity? Sleep. 2009;32(9):1229–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Trotti LM. Waking up is the hardest thing I do all day: sleep inertia and sleep drunkenness. Sleep Med Rev. 2017;35:76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Andlauer O, et al.. Predictors of hypocretin (orexin) deficiency in narcolepsy without cataplexy. Sleep. 2012;35(9):1247–155F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sasai-Sakuma T, et al.. Differences in electroencephalographic findings among categories of narcolepsy-spectrum disorders. Sleep Med. 2015;16(8):999–1005. [DOI] [PubMed] [Google Scholar]
- 19. Bin-Hasan S, et al.. Nocturnal REM sleep without atonia is a diagnostic biomarker of pediatric narcolepsy. J Clin Sleep Med. 2018;14(2):245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pizza F, et al. . Primary progressive narcolepsy type 1. Neurology. 2014;83(23):2189–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lopez R, et al. . Temporal changes in the cerebrospinal fluid level of hypocretin-1 and histamine in narcolepsy. Sleep. 2017;40(1). doi: 10.1093/sleep/zsw010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bonakis A, et al. . REM sleep behaviour disorder (RBD) and its associations in young patients. Sleep Med. 2009;10(6):641–645. [DOI] [PubMed] [Google Scholar]
- 23. Luca G, et al.; European Narcolepsy Network Clinical, polysomnographic and genome-wide association analyses of narcolepsy with cataplexy: a European Narcolepsy Network study. J Sleep Res. 2013;22(5):482–495. [DOI] [PubMed] [Google Scholar]
- 24. Baumann CR, et al.. Challenges in diagnosing narcolepsy without cataplexy: a consensus statement. Sleep. 2014;37(6):1035–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kawai M, et al.. Narcolepsy in African Americans. Sleep. 2015;38(11):1673–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pizza F, et al.. Nocturnal sleep dynamics identify narcolepsy type 1. Sleep. 2015;38(8):1277–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Anderson KN, et al.. Idiopathic hypersomnia: a study of 77 cases. Sleep. 2007;30(10):1274–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vernet C, et al.. Idiopathic hypersomnia with and without long sleep time: a controlled series of 75 patients. Sleep. 2009;32(6):753–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pizza F, et al.. Daytime continuous polysomnography predicts MSLT results in hypersomnias of central origin. J Sleep Res. 2013;22(1):32–40. [DOI] [PubMed] [Google Scholar]
- 30. Evangelista E, et al. . Alternative diagnostic criteria for idiopathic hypersomnia: a 32‐hour protocol. Ann Neurol. 2018;83(2):235–247. [DOI] [PubMed] [Google Scholar]
- 31. Harrison Y, et al.. “High sleepability without sleepiness.” The ability to fall asleep rapidly without other signs of sleepiness. Neurophysiol Clin. 1996;26(1):15–20. [DOI] [PubMed] [Google Scholar]
- 32. Cook JD, et al.. Optimizing actigraphic estimation of sleep duration in suspected idiopathic hypersomnia. J Clin Sleep Med. 2019;15(04):597–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Roth B, et al.. Hypersomnia with sleep drunkenness. Arch Gen Psychiat. 1972;26(5):456–462. [DOI] [PubMed] [Google Scholar]
- 34. American Academy of Sleep Medicine. International Classification of Sleep Disorders. 1990. [Google Scholar]
- 35. Voderholzer U, et al.. A 19-h spontaneous sleep period in idiopathic central nervous system hypersomnia. J Sleep Res. 1998;7(2):101–103. [DOI] [PubMed] [Google Scholar]
- 36. Vernet C, et al.. Residual sleepiness in obstructive sleep apnoea: phenotype and related symptoms. Eur Respir J. 2011;38(1):98–105. [DOI] [PubMed] [Google Scholar]
- 37. Ohayon MM, et al.. Excessive sleep duration and quality of life. Ann Neurol. 2013;73(6):785–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kim T, et al.. Different fates of excessive daytime sleepiness: survival analysis for remission. Acta Neurol Scand. 2016;134(1):35–41. [DOI] [PubMed] [Google Scholar]
- 39. Trotti LM, et al.. Test-retest reliability of the multiple sleep latency test in narcolepsy without cataplexy and idiopathic hypersomnia. J Clin Sleep Med. 2013;9(8):789–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Andlauer O, et al.. Nocturnal rapid eye movement sleep latency for identifying patients with narcolepsy/hypocretin deficiency. JAMA Neurol. 2013;70(7):891–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Šonka K, et al.. Narcolepsy with and without cataplexy, idiopathic hypersomnia with and without long sleep time: a cluster analysis. Sleep Med. 2015;16(2):225–231. [DOI] [PubMed] [Google Scholar]
- 42. Bassetti CLA, et al.. Narcolepsy—clinical spectrum, aetiopathophysiology, diagnosis and treatment. Nat Rev Neurol. 2019;15(9):519–539. [DOI] [PubMed] [Google Scholar]
- 43. Ferri R, et al.. Decreased sleep stage transition pattern complexity in narcolepsy type 1. Clin Neurophysiol. 2016;127(8):2812–2819. [DOI] [PubMed] [Google Scholar]
- 44. Sorensen GL, et al.. Sleep transitions in hypocretin-deficient narcolepsy. Sleep. 2013;36(8):1173–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pizza F, et al.. Polysomnographic study of nocturnal sleep in idiopathic hypersomnia without long sleep time. J Sleep Res. 2013;22(2):185–196. [DOI] [PubMed] [Google Scholar]
- 46. Kanady JC, et al.. Development and validation of the Sleep Inertia Questionnaire (SIQ) and assessment of sleep inertia in analogue and clinical depression. Cognit Ther Res. 2015;39(5):601–612. [DOI] [PMC free article] [PubMed] [Google Scholar]