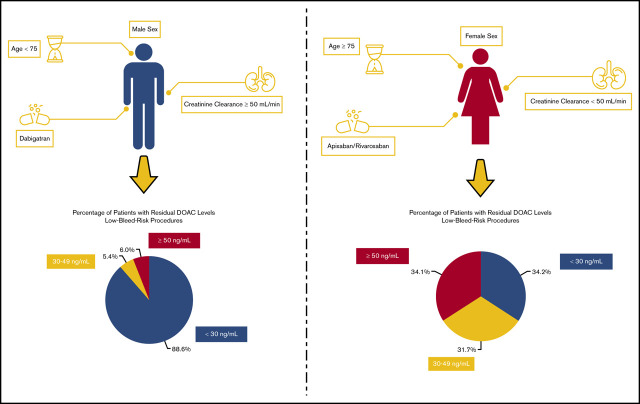
Key Points
Older age, female sex, low weight, renal dysfunction, and shorter interruption are associated with risk of preprocedural DOAC levels.
Apixaban and rivaroxaban were more likely than dabigatran to be associated with residual preprocedural levels using the PAUSE protocol.
Abstract
The Perioperative Anticoagulation Use for Surgery Evaluation (PAUSE) study prospectively evaluated a prespecified periprocedural-interruption strategy of direct oral anticoagulants (DOACs) among patients with atrial fibrillation. Logistic regression analyses were performed to identify clinical parameters associated with residual DOAC levels ≥30 ng/mL or ≥50 ng/mL. Patients undergoing low-bleed-risk procedures were more likely to have residual levels of ≥30 ng/mL and ≥50 ng/mL. For low-risk procedures, age ≥75 years, female sex, a creatinine clearance (CrCl) <50 mL/min, and an interruption of <36 hours were associated with a greater likelihood of levels ≥30 ng/mL, whereas age ≥75 years, female sex, a CrCl of <50 mL/min, and standard DOAC dosing were associated with levels ≥50 ng/mL. For high-risk procedures, weight of <70 kg, CrCl <50 mL/min, and standard DOAC dosing were associated with residual levels ≥30 ng/mL, whereas female sex was associated with levels ≥50 ng/mL. For low-risk procedures, apixaban was associated with a higher likelihood of levels ≥30 ng/mL as compared with dabigatran (P = .0019) and of levels ≥50 ng/mL when compared with rivaroxaban (P = .0003). For high-risk procedures, apixaban was marginally associated with a higher likelihood of residual levels ≥30 ng/mL when compared with dabigatran (P = .05), whereas rivaroxaban was associated with a higher likelihood of levels ≥30 ng/mL as compared with apixaban. Further study is required to determine whether adjustments to perioperative plans based on these clinical parameters could result in a lower risk of residual DOAC levels. The PAUSE trial was registered at www.clinicaltrials.gov as #NCT2228798.
Visual Abstract
Introduction
Direct oral anticoagulants (DOACs) are being used increasingly for stroke prevention in atrial fibrillation (AF),1 a condition whose prevalence will more than double in the next 30 years.2 The need for surgery or invasive procedures increases with age3 and ∼6 million patients with AF worldwide will require perioperative management of anticoagulants each year.4 DOACs have a rapid onset and offset of action as well as short half-lives,5 pharmacokinetic properties that simplify perioperative management and obviate the need for bridging anticoagulation.6
There was previous uncertainty about optimal periprocedural DOAC management, with some experts advocating for DOAC interruption followed by preoperative DOAC-level measurements to ensure that DOAC levels are below a threshold that is considered safe for a surgery/procedure.7 Others advocated an approach based on surgical bleed risk and DOAC pharmacokinetic properties that did not incorporate preoperative DOAC measurements.8 Retrospective analyses of randomized trials of DOACs in AF found that a pharmacokinetic approach to periprocedural DOAC management is associated with low 30-day postoperative rates of major bleeding and thromboembolism but these studies did not measure preoperative DOAC levels.9-13 The Perioperative Anticoagulation Use for Surgery Evaluation (PAUSE) study was the first to prospectively assess a standardized perioperative management strategy in 3 cohorts of patients with AF who were receiving apixaban, dabigatran, or rivaroxaban and required DOAC interruption for an elective surgery/procedure.4 PAUSE assessed a simple management approach with DOAC-specific interruption and resumption intervals while foregoing the use of heparin bridging and preoperative coagulation function testing. Preoperative DOAC levels were measured as part of the study but not used to manage patients.
For the PAUSE perioperative DOAC management approach to be widely adopted in clinical practice, it would need to have been associated with acceptably low rates of perioperative bleeding and thromboembolic outcomes. The PAUSE protocol would also gain adoption if residual DOAC levels at the time of a surgery/procedure were not clinically important, that is, not associated with perioperative bleeding. In PAUSE, these requirements were satisfied as patients in the apixaban, dabigatran, and rivaroxaban cohorts had 30-day postoperative rates of major bleeding of 1.35%, 0.90%, and 1.85%, respectively, which are acceptably low and comparable to major bleeding rates in other perioperative management studies.14,15 Moreover, in a prespecified analysis of the PAUSE database, there was no association between preoperative residual DOAC levels ≥50 ng/mL, or 30 to 49.9 ng/mL and the occurrence of perioperative major bleeding or clinically relevant nonmajor bleeding.16
Although the PAUSE study showed that the use of a simple standard protocol was associated with low bleeding rates, it is possible that specific patients would benefit from a more personalized approach. To evaluate whether clinical- or protocol-related factors could be modified to lower the likelihood of elevated residual DOAC levels, we did a prespecified subanalysis of PAUSE to identify factors associated with residual preoperative DOAC levels that were classified empirically as undetectable or negligible (<30 ng/mL), mildly elevated (30-49.9 ng/mL), or moderately elevated (≥50 ng/mL). Our aim was to assess whether there were any patient characteristics that resulted in an elevated preprocedural DOAC level using the PAUSE management protocol.
Methods
Study population and periprocedural DOAC management
The study population for this analysis consists of 2541 patients (84.5% of total PAUSE study population) for whom preprocedural DOAC levels were available. PAUSE was a prospective management study with patients separated into 3 cohorts, according to the DOAC taken: apixaban, dabigatran, or rivaroxaban. Patients received standardized perioperative management according to DOAC, procedural bleed risk, and estimated creatinine clearance (CrCl).4,17 The surgery/procedure was classified as high or low bleed risk based on a prespecified scheme (supplemental Appendix 1). In a recent surgery/procedure bleeding-risk classification by the International Society on Thrombosis and Haemostasis, low-bleed-risk patients in PAUSE equate to low/moderate-bleed-risk in their classification scheme.18 The bleed-risk classification of the surgery/procedure was left to physician discretion to account for real-life situations (eg, colonoscopy would be, in general, considered low risk but if polyps were resected, this could be managed as a high-bleed-risk procedure). DOACs were omitted for 1 day prior to a low-bleed-risk surgery/procedure and for 2 days prior to a high-bleed-risk surgery/procedure. Patients on dabigatran with a CrCl <50 mL/min had a 1- to 2-day longer interruption to account for dabigatran’s greater dependence on renal elimination. Blood samples were obtained from patients just before the surgery/procedure to measure residual DOAC levels; however, these DOAC levels were not available for clinical use and did not influence perioperative DOAC management. DOACs were resumed 1 day (∼24 hours) after a low-bleed-risk surgery/procedure and 2 to 3 days (48-72 hours) after a high-bleed-risk surgery/procedure, provided adequate hemostasis was achieved.
Patient characteristics
Patient characteristics collected as part of the PAUSE study included age, sex, weight, body mass index, CrCl, and concurrent use of strong P-glycoprotein and cytochrome P450 3A4 inhibitors (amiodarone, clarithromycin, cyclosporine, itraconazole, ketoconazole, quinidine, tacrolimus, tamoxifen, ritonavir, verapamil) and inducers (carbamazepine, phenytoin, St. John’s wort, rifampicin).
Preprocedural DOAC-level measurement
Preoperative plasma samples were collected in citrated Vacutainer tubes, centrifuged for 15 minutes at 1500g, then transferred and double-spun at 1500g to ensure platelet-poor plasma. Samples were analyzed at the McMaster University Special Coagulation Laboratory by technologists who were blinded to patient characteristics. All testing was done on a STAr-Evolution analyzer (Diagnostica Stago, Asnières-sur-Seine, France). Rivaroxaban and apixaban levels were measured by calibrated anti-Xa levels (anti-Xa reagent: BIOPHEN DiXaI; BIOPHEN Rivaroxaban Calibrator Low; BIOPHEN Apixaban Calibrator Low, BIOPHEN Dabigatran Calibrator Low, Hyphen BioMed, Neuville-sur-Oise, France); dabigatran levels were measured by the dilute thrombin time (Hemoclot; Hyphen BioMed, Neuville-sur-Oise, France). The lower limit of detection reported for anti-Xa and dilute thrombin time assays is <20 ng/mL.
Statistical analyses
Categorical variables are presented as numbers and percentages, continuous variables are described by mean values and standard deviations (SDs), or medians and interquartile range (IQR). Descriptive analyses were done to determine the proportion of patients with preprocedural residual DOAC levels ≥30 ng/mL and ≥50 ng/mL. Both thresholds were considered relevant for our analyses as there is uncertainty as to which DOAC level is considered safe for surgery, and current guidance statements are expert opinion-based, although no clinical data support the use of either threshold.8,19,20
We aimed to determine whether patient characteristics, DOAC type and dose, and the duration of DOAC interruption were associated with residual DOAC levels. We generated plots to characterize the distribution of preprocedural DOAC levels according to DOAC-interruption intervals and DOAC type. Univariate and multivariate logistic regression analyses, stratified according to procedural bleed-risk management and study region (Hamilton vs non-Hamilton), were done to determine whether demographic and pharmacokinetic factors were associated with an increased probability of residual DOAC levels ≥30 ng/mL and ≥50 ng/mL. P <.05 was considered statistically significant. All analyses were performed using R 3.5.2 (R Core Team 2018).
Results
Patient characteristics and periprocedural management
A total of 2541 of 3007 patients (84.5%) in the PAUSE study had preprocedural DOAC levels measured. The characteristics of patients with residual DOAC levels are shown in Table 1, and are comparable to patients in the full study population. Their mean age was 72.5 years (SD, 9.4), and approximately two-thirds (66.2%) were male. Periprocedural DOAC-interruption intervals and adherence to interruption protocols are shown in supplemental Table 1.
Table 1.
Baseline characteristics (N = 2541)
| Variable | Apixaban cohort, n = 1086 | Dabigatran cohort, n = 535 | Rivaroxaban cohort, n = 920 |
|---|---|---|---|
| Age, mean ± SD, y | 73.1 ± 9.2 | 72.5 ± 10.0 | 71.9 ± 9.4 |
| Male sex, n (%) | 706 (65.0) | 366 (68.4) | 611 (66.4) |
| BMI, mean ± SD | 29.4 ± 6.0 | 30.2 ± 6.9 | 29.6 (6.4) |
| Race, n (%) | |||
| White | 1043 (96.8) | 524 (98.3) | 890 (97.7) |
| Nonwhite | 35 (3.3) | 9 (1.7) | 21 (2.3) |
| Unknown | 8 (0.7) | 2 (0.4) | 9 (1.0) |
| CHADS2,* mean ± SD | 2.1 ± 1.3 | 2.2 ± 1.3 | 2.0 ± 1.3 |
| CHADS2VA2Sc,† mean ± SD | 3.5 ± 1.7 | 3.5 ± 1.6 | 3.3 ± 1.7 |
| Modified HASBLED,‡ mean ± SD | 2.0 ± 0.9 | 2.0 ± 0.9 | 1.9 ± 0.9 |
| Medical condition, n (%) | |||
| Congestive heart failure | 216 (19.9) | 92 (17.3) | 120 (13.2) |
| Hypertension | 809 (74.7) | 402 (75.4) | 663 (72.3) |
| Diabetes | 291 (26.8) | 151 (28.2) | 242 (26.3) |
| Stroke | 85 (7.9) | 50 (9.4) | 64 (7.0) |
| Transient ischemic attack | 101 (9.3) | 70 (13.1) | 80 (8.7) |
| Coronary artery disease | 205 (19.0) | 94 (17.6) | 155 (16.9) |
| Peripheral artery disease | 7 (0.7) | 5 (0.9) | 10 (1.1) |
| Bioprosthetic heart valve | 30 (2.8) | 7 (1.3) | 18 (2.0) |
| Mitral valve disease | 116 (10.7) | 43 (8.0) | 74 (8.0) |
| Venous thromboembolism | 66 (6.1) | 29 (5.4) | 72 (7.8) |
| Cancer | 289 (26.6) | 133 (24.9) | 256 (27.8) |
| Active cancer§ | 88 (8.1) | 47 (8.8) | 91 (9.9) |
| Laboratory values | |||
| Hemoglobin, mean ± SD, g/L | 134.4 ± 17.5 | 137.2 ± 16.5 | 135.6 ± 17.6 |
| Platelets <100 × 109/L, n (%) | 5 (2.5) | 2 (2.2) | 3 (1.8) |
| Serum creatinine, mean ± SD, μmol/L | 93.7 ± 28.6 | 87.7 ± 22.0 | 90.2 ± 23.0 |
| CrCl, mean ± SD, mL/min | 78.0 ± 31.4 | 86.2 ± 36.5 | 82.1 ± 32.7 |
| Medication use, n (%) | |||
| Lower-dose DOAC regimen | 209 (19.2) | 204 (38.1) | 156 (17.0) |
| Aspirin | 137 (12.6) | 81 (15.1) | 89 (9.7) |
| P2Y12 inhibitor | 10 (0.9) | 7 (1.3) | 10 (1.1) |
| P-glycoprotein/CYP450 3A4 inhibitor or inducer | 64 (5.9) | 47 (8.8) | 47 (5.1) |
| Surgery type, n (%) | |||
| High bleeding risk | 335 (30.9) | 183 (34.2) | 314 (34.1) |
| Low bleeding risk | 751 (69.2) | 352 (65.8) | 606 (65.9) |
| Anesthesia type, n (%) | |||
| General | 358 (33.0) | 163 (30.5) | 330 (38.0) |
| Neuraxial | 85 (7.8) | 44 (8.2) | 52 (5.7) |
| Other | 603 (55.5) | 298 (55.7) | 504 (54.8) |
BMI, body mass index; CHADS2, congestive heart failure, hypertension, age ≥75 years, stroke; CHADS2-VA2Sc, congestive heart failure, hypertension, age ≥75 years, stroke, vascular disease, age 65-74 years, sex category; CYP450, cytochrome P450; HASBLED, hypertension, abnormal renal and liver function, stroke, bleeding, labile international normalized ratio, elderly, drugs or alcohol.
CHADS2 risk score range = 0-6. Risks include congestive heart failure, hypertension, age 75 years or older, diabetes, and previous stroke or transient ischemic attack.
CHADS2-VA2Sc risk score range = 0-9. Risks include congestive heart failure, hypertension, age 75 years or older or 65 years or older, diabetes, previous stroke or transient ischemic attack, female sex, and vascular disease.
HASBLED bleeding risk score range = 0-7. Risks include hypertension, abnormal renal or liver function, previous stroke, previous bleed or bleed predisposition, labile international normalized ratio (omitted), age 65 years or older, and drug use that affects hemostasis or alcohol use (omitted).
Cancer diagnosed within 3 months or treated within 6 months or metastatic. The percentage of patients according to each variable may not correspond to the exact proportion of total patients according to each DOAC as data on each variable was not available on all patients.
Residual DOAC levels according to periprocedural management and DOAC dosing
The relation between DOAC-interruption interval and residual DOAC levels is shown in Figure 1. The proportions of patients with residual DOAC levels ≥30 ng/mL and ≥50 ng/mL according to interruption intervals and surgery/procedure bleed risk are shown in Figure 2 and supplemental Table 2. The proportion of patients with residual DOAC levels ≥30 ng/mL and ≥50 ng/mL gradually decreased with increasing DOAC-interruption duration. The proportion of patients with interruption intervals <48 hours that had residual levels ≥30 ng/mL was 30.7% for apixaban, 16.8% for dabigatran, and 31.6% for rivaroxaban. The proportion of patients with interruption intervals ≥48 hours with residual levels ≥30 ng/mL was 10.0% for apixaban, 6.3% for dabigatran, and 18.0% for rivaroxaban.
Figure 1.
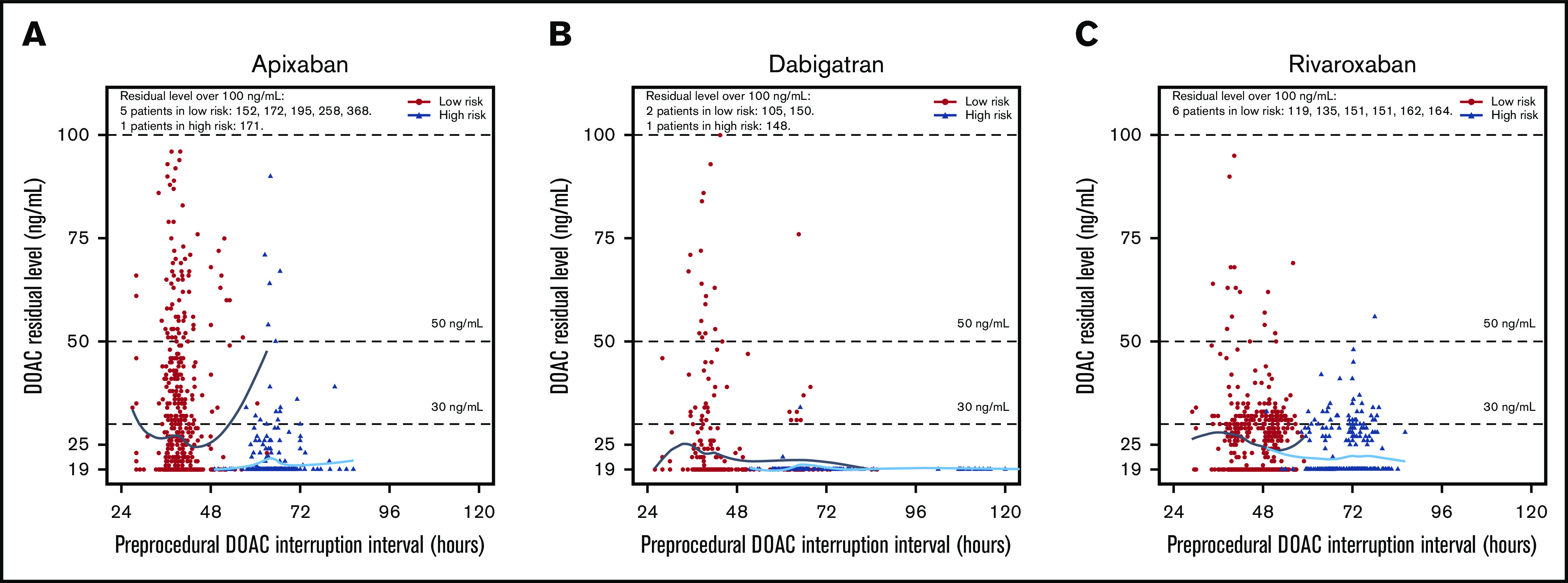
Residual DOAC levels according to preprocedural DOAC-interruption interval in hours. Patients undergoing low- and high-bleeding-risk procedures had their DOAC stopped 1 and 2 days prior to the procedure, respectively. Patients undergoing low-bleeding-risk procedures are indicated in black, whereas patients undergoing high-bleeding-risk procedures are indicated in blue. (A) The median preoperative-interruption intervals for low- and high-bleeding-risk procedures for apixaban were 39.4 hours (IQR, 37.5, 41.5) and 63.8 hours (IQR, 61.0, 67.0), respectively. (B) The median preoperative-interruption intervals for dabigatran for low- and high-bleeding-risk procedures were 40.5 (IQR, 38.4, 44.7) and 63.8 (IQR, 61.7, 74.3), respectively. (C) The median preoperative-interruption intervals for rivaroxaban for low- and high-bleeding-risk procedures were 48.0 (IQR, 40.8, 51.0) and 72.0 (IQR, 66.1, 75.0), respectively.
Figure 2.
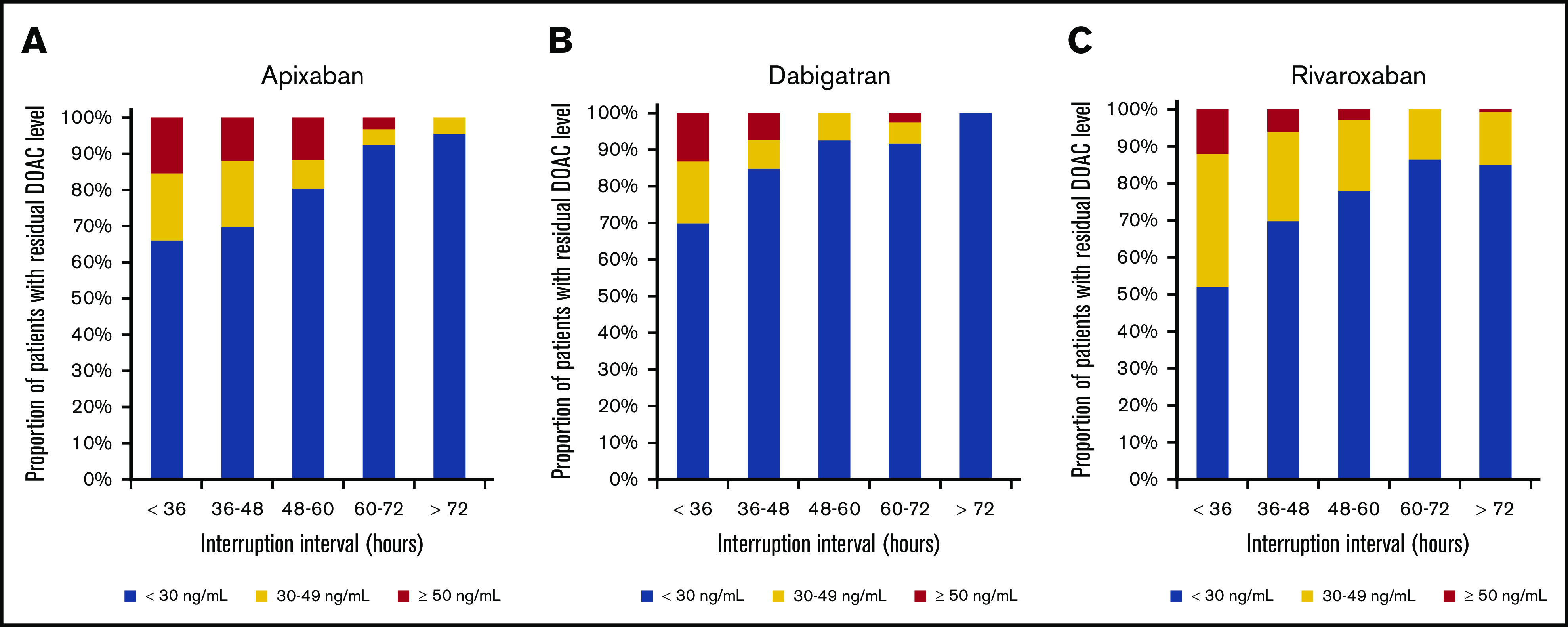
Residual DOAC levels according to interruption interval. The proportion of patients with residual preprocedural levels ≥30 ng/mL and ≥50 ng/mL declines with increasing interruption duration. (A) Apixaban. (B) Dabigatran. (C) Rivaroxaban. Apixaban was associated with a higher likelihood of levels ≥30 ng/mL compared with dabigatran and of levels ≥50 ng/mL when compared with rivaroxaban for low-risk procedures with shorter interruption intervals. Rivaroxaban was associated with a higher likelihood of levels ≥30 ng/mL as compared with apixaban for high-risk procedures with longer interruption intervals.
Patients undergoing low-bleed-risk procedures (with shorter preprocedural DOAC-interruption intervals) were more likely to have residual DOAC levels ≥30 and ≥50 ng/mL (supplemental Table 2); this effect was consistent across all DOACs. Among patients having low-bleed-risk procedures, 30.6% (apixaban), 17.1% (dabigatran), and 26.4% (rivaroxaban) had residual DOAC levels ≥30 ng/mL, whereas 12.8% (apixaban), 7.1% (dabigatran), and 4.5% (rivaroxaban) had residual levels ≥50 ng/mL. The proportions of patients with DOAC levels ≥30 and ≥50 ng/mL according to DOAC dose are shown in supplemental Table 3.
Predictors of residual DOAC levels
All univariate analyses are shown in supplemental Table 4. Multivariate analyses found that in patients having a low-bleed-risk surgery/procedure, age ≥75 years (P = .0127), female sex (P = .004), CrCl <50 mL/min (P = .0024), and DOAC-interruption interval <36 hours were associated with residual DOAC levels ≥30 ng/mL (P = .0262; Table 2). In low-bleed-risk patients, age ≥75 years (P = .0367), female sex (P = .0034), CrCl <50 mL/min (P = .0193), and use of standard- vs low-dose DOAC regimens (P = .0106) were associated with residual levels ≥50 ng/mL. In patients having a high-bleed-risk surgery/procedure, weight <70 kg (P = .0304), CrCl <50 mL/min (P = .0139), and standard DOAC dosing (P = .0442) were associated with residual levels ≥30 ng/mL. Female sex was the only factor associated with residual DOAC levels ≥50 ng/mL (P = .0469). In terms of the effect of DOAC type on residual DOAC levels, in patients having a low-bleed-risk surgery/procedure, those on apixaban had a higher likelihood of having levels ≥30 ng/mL than patients on dabigatran (P = .0019), and of having levels ≥50 ng/mL than patients on rivaroxaban (P = .0003). In patients having a high-bleed-risk surgery/procedure, those on apixaban were marginally more likely to have residual levels ≥30 ng/mL than those on dabigatran (P = .0504), whereas those on rivaroxaban were more likely to have levels ≥30 ng/mL than patients on apixaban (P = .0005).
Table 2.
Clinical parameters associated with residual DOAC levels: multivariate logistic regression analyses stratified according to region
| Clinical parameter | Comparison | ≥30 ng/mL | ≥50 ng/mL | ||
|---|---|---|---|---|---|
| OR [95% CI] | P | OR [95% CI] | P | ||
| Low-bleeding-risk procedures | |||||
| DOAC | Dabigatran vs apixaban | 0.63 [0.47-0.84] | .0019 | 0.71 [0.45-1.14] | .1553 |
| Rivaroxaban vs apixaban | 1.0 [0.8-1.25] | .9849 | 0.42 [0.26-0.67] | .0003 | |
| Age, y | ≥75 vs <75 | 1.31 [1.06-1.62] | .0127 | 1.49 [1.03-2.17] | .0367 |
| Sex | Female vs male | 1.36 [1.1-1.68] | .004 | 1.73 [1.2-2.49] | .0034 |
| Weight, kg | 70-90 vs <70 | 0.97 [0.74-1.27] | .8122 | 0.96 [0.6-1.55] | .881 |
| >90 vs <70 | 1.1 [0.82-1.48] | .5042 | 1.33 [0.81-2.21] | .2637 | |
| CrCl, mL/min | ≥50 vs <50 | 0.65 [0.49-0.86] | .0024 | 0.56 [0.35-0.91] | .0193 |
| P-glycoprotein or CYP3A4 inhibitor | Presence vs absence | 1.08 [0.75-1.55] | .6712 | 1.15 [0.64-2.1] | .6378 |
| Active cancer | Presence vs absence | —* | —* | 1.69 [0.88-3.27] | .1167 |
| DOAC dosing | Low dose vs standard dose | 0.77 [0.59-1.01] | .0606 | 0.53 [0.32-0.86] | .0106 |
| DOAC interruption, h | <36 vs 36-48 | 1.44 [1.04-1.99] | .0262 | 1.57 [0.93-2.62] | .0888 |
| >48 vs 36-48 | 0.78 [0.8-1.01] | .0624 | 0.75 [0.44-1.28] | .2919 | |
| High-bleeding-risk procedures | |||||
| DOAC | Dabigatran vs apixaban | 0.23 [0.05-1.0] | .0504 | 0.39 [0.04-3.5] | .4027 |
| Rivaroxaban vs apixaban | 2.73 [1.56-4.8] | .0005 | 0.43 [0.08-2.36] | .3328 | |
| Age, y | ≥75 vs <75 | 0.84 [0.49-1.45] | .5411 | 1.7 [0.38-7.55] | .4838 |
| Sex | Female vs male | 1.32 [0.77-2.27] | .3137 | 5.55 [1.02-30.05] | .0469 |
| Weight, kg | 70-90 vs <70 | 0.61 [0.33-1.1] | .1014 | 0.95 [0.21-4.29] | .9476 |
| >90 vs <70 | 0.43 [0.2-0.92] | .0304 | 0.51 [0.04-6.21] | .5979 | |
| CrCl, mL/min | ≥50 vs <50 | 0.45 [0.24-0.85] | .0139 | 0.42 [0.09-1.94] | .2664 |
| P-glycoprotein or CYP3A4 inhibitor | Presence vs absence | 1.33 [0.48-3.71] | .583 | —* | —* |
| Active cancer | Presence vs absence | —* | —* | —* | —* |
| DOAC dosing | Low dose vs standard dose | 0.52 [0.28-0.98] | .0442 | 0.51 [0.11-2.41] | .3968 |
| DOAC interruption, h | <60 vs 60-72 | 1.51 [0.66-3.42] | .3279 | 0.77 [0.09-6.31] | .8054 |
| >72 vs 60-72 | 0.83 [0.47-1.46] | .5116 | 0.44 [0.05-4.16] | .4712 | |
Bold values in the table represent results that were statistically significant (P < .05).
CI, confidence interval; OR, odds ratio.
Dashes represent variables that were omitted from the multivariate model due to small sample sizes and minimal impact on the model.
Among male patients on dabigatran undergoing low-bleed-risk procedures lacking identified risk factors (age <75 years, CrCl ≥50 mL/min), the proportion of patients with residual levels 30 to 49 ng/mL and ≥50 ng/mL was 8 of 149 (5.4%) and 9 of 149 (6.0%), respectively. The proportion of these male patients lacking risk factors undergoing high-bleed-risk procedures (with longer interruptions) with residual levels 30 to 49 ng/mL and ≥50 ng/mL was 0 of 45 (0%) and 0 of 45 (0%), respectively. On the other hand, for female patients undergoing low-bleed-risk procedures on apixaban/rivaroxaban (standard dosing) with a few of the identified risk factors (age ≥75 years, CrCl <50 mL/min), the proportion of patients with residual levels 30 to 49 ng/mL and ≥50 ng/mL was 13 of 41 (31.7%) and 14 of 41 (34.1%). Similarly, for female patients with these risk factors undergoing high-bleed-risk procedures, the proportion of patients with residual levels 30 to 49 ng/mL and ≥50 ng/mL was 5 of 17 (29.4%) and 2 of 17 (11.8%).
Discussion
This study is the first, to our knowledge, to assess an association between clinical factors and residual preprocedural DOAC levels in patients undergoing standardized DOAC interruption before an elective surgery/procedure. There are 2 main findings from our analysis. First, patients having a low-bleed-risk surgery/procedure who had a shorter, 1-day (or 30-36 hour) DOAC interruption were more likely to have residual DOAC levels ≥30 ng/mL and ≥50 ng/mL than patients having a high-bleed-risk procedure who had a longer, 2-day (or 60-68 hours) DOAC interruption; this finding was observed irrespective of the DOAC. Other factors associated with residual DOAC levels ≥30 ng/mL and ≥50 ng/mL were age ≥75 years, female sex, CrCl <50 mL/min, and use of standard DOAC dosing. Second, in patients having a high-bleed-risk surgery/procedure in whom the PAUSE DOAC-interruption protocol aimed to minimize DOAC levels at the time of surgery/procedure, there were no protocol-related factors identified that predicted DOAC levels >50 ng/mL; this suggests that the PAUSE protocol “worked” to result in a minimal-to-no residual DOAC level for high-bleed-risk procedures.
Our first finding is not unexpected as the PAUSE protocol was designed so that patients undergoing low-bleed-risk procedures were allowed some anticoagulant effect at the time of the procedure, whereas the protocol adjusted for high-bleed-risk procedures to allow for a minimal-to-no residual preprocedural anticoagulant effect. Unsurprisingly, a substantial proportion of patients having low-bleed-risk procedures had residual DOAC levels ≥30 ng/mL (30.6% on apixaban, 17.1% on dabigatran, and 26.4% on rivaroxaban) and ≥50 ng/mL (12.8% on apixaban, 7.1% on dabigatran, 4.5% on rivaroxaban).
With regard to our second finding in patients having a high-bleed-risk surgery/procedure, only 1 factor (female sex) was associated with a residual DOAC level ≥50 ng/mL, and only 2 factors (weight <70 kg and CrCl <50 mL/min) were associated with DOAC levels ≥30 ng/mL. Although it is reassuring that there were no factors related to the PAUSE protocol associated with an increased residual DOAC level in the most vulnerable high-bleed-risk patients, further study is needed to determine whether it is clinically important to adjust DOAC interruption in high-bleed-risk patients with 1 or more of these characteristics.
Our finding that a CrCl <50 mL/min was associated with higher residual DOAC levels is not unexpected. In addition, female sex has been associated with higher area under the curve (AUC) values or plasma levels for apixaban,21 dabigatran,22 and rivaroxaban.23 These findings were partially attributed to sex-related differences in renal function, although no formal analyses to address this have been done. Our results indicate that sex may play a role independently of renal function in determining residual DOAC levels following preprocedural interruption. Similarly, several studies have found higher steady state AUC values in elderly as compared with younger patients with respect to apixaban, dabigatran, and rivaroxaban.21,22,24 These differences were attributed to changes in renal function with age, although the correlation between total clearance and renal clearance with respect to apixaban was weak.21 Increased peak DOAC levels, AUC values, or prolonged half-lives have been associated with low body weight in pharmacokinetic analyses for rivaroxaban and apixaban.21,25,26 These studies provide some pharmacokinetic rationale for our findings and could explain why we observed these parameters to be significantly associated with higher residual levels following DOAC interruption.
Another noteworthy finding is that patients on dabigatran were the least likely to have residual levels ≥30 ng/mL and ≥50 ng/mL, compared with the other DOACs. Dabigatran differs pharmacokinetically from apixaban and rivaroxaban in that it is mainly renally eliminated; the PAUSE management accounted for this by extending dabigatran interruption by 1 to 2 days in patients with a CrCl <50 mL/min. As shown in Figure 1, patients on dabigatran appear to be less likely to have elevated residual levels, particularly among patients having high-bleed-risk procedures. This might suggest that adjustments based on pharmacokinetic principles to dabigatran-interruption intervals according to the PAUSE protocol may have reduced the likelihood of residual dabigatran levels. These findings indicate that the risk of residual apixaban/rivaroxaban levels might be reduced by extending interruption intervals for patients on apixaban and rivaroxaban based on the presence of 1 or several of the identified clinical risk factors. Provided both apixaban and rivaroxaban are less dependent on renal clearance, it is possible that adjustments to preprocedural-interruption intervals might need to take age, sex, and weight into account, in additional to renal dysfunction. Further study is warranted to determine whether adjustments to DOAC-interruption intervals based on these clinical parameters will confer a lower likelihood for residual DOAC levels and whether this would have a clinically meaningful impact on bleeding event rates. In a companion analysis of the PAUSE database, residual DOAC levels ≥30 ng/mL or ≥50 ng/mL were not associated with major or any clinically important (major and clinically relevant nonmajor) bleeding.16 However, PAUSE was not powered to assess predictors of major bleeding. As such, this companion analysis evaluated correlations with overall bleeding (major bleeding plus clinically relevant nonmajor bleeding) to increase analysis power. Although there was no signal that residual DOAC levels predict bleeding, a twofold risk increase could not be excluded.
Few studies have assessed preprocedural DOAC levels and clinical factors associated with higher residual levels. The COncentration of Rivaroxaban, Dabigatran and Apixaban (CORIDA) study was a prospective observational study that measured preprocedural DOAC levels in 422 DOAC-treated patients undergoing elective procedures but, unlike the PAUSE study, perioperative DOAC management was not standardized and the duration of DOAC interruption varied widely (1-218 hours).27 In this study, the duration of DOAC interruption, a CrCl <50 mL/min, and antiarrhythmic drug use (but not patient age, sex, and weight) were predictors of preprocedural DOAC levels ≥30 ng/mL. Several additional studies have evaluated the impact of DOAC levels on bleeding and thrombotic outcomes, although these were among patients during ongoing treatment and did not specifically evaluate preprocedural DOAC levels or their impact on periprocedural outcomes.28,29 It should be noted that among patients in PAUSE who had >72 hours of preprocedural interruption, only small proportions of patients had residual DOAC levels (Figure 2). This was found across all 3 DOAC types. These findings are consistent with the results of the CORIDA study, and are in agreement with current American Society of Regional Anesthesia and Pain Medicine (ASRA) guidelines recommending that DOACs be discontinued 72 hours prior to neuraxial anesthesia.30
Our study has limitations. First, given the small number of patients with residual DOAC levels ≥50 ng/mL, our analyses may have been underpowered to detect statistically significant associations between clinical parameters and DOAC levels ≥50 ng/mL. On the other hand, the fact that few patients had residual DOAC levels ≥50 ng/mL supports the premise that the PAUSE protocol met its key secondary outcome, namely that few patients had what was a priori defined to be an excessive preoperative anticoagulant effect. Second, 85% of patients in PAUSE had preprocedural DOAC levels drawn. We cannot exclude potential selection bias toward which patients had levels drawn, and we cannot rule out that the patients without preprocedural levels were different in some respect. However, among patients who had a DOAC level measured, 66% were male and 33% had a high-bleed-risk procedure, which is similar to the proportion of males (66%) and high-bleed-risk procedures (33%) in the entire study population, thereby suggesting that there was no selection bias toward DOAC-level measurement.
In summary, we found that nonmodifiable factors, comprising age ≥75 years, female sex, weight <70 kg, and a CrCl <50 mL/min, were predictive of residual DOAC levels ≥30 ng/mL or ≥50 ng/mL. The lack of an association between the DOAC-interruption intervals in high-bleed-risk patients and an elevated residual DOAC level (≥30 ng/mL or ≥50 ng/mL) supports the safety of the PAUSE protocol in this vulnerable patient group. Further study is required to determine whether adjustments to perioperative management plans based on the clinical parameters identified in this analysis could result in a lower rate of residual DOAC levels, and whether this approach could ultimately further reduce postoperative bleeding rates in vulnerable patient populations.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
This work was supported by the Canadian Institutes of Health Research and the Heart and Stroke Foundation of Canada.
Footnotes
Data-sharing requests may be e-mailed to the corresponding author, James D. Douketis, at jdouket@mcmaster.ca.
Authorship
Contribution: J.R.S. contributed to study design, data analysis, manuscript write-up, and manuscript revisions; N.L. performed the statistical analyses and provided critical revisions to the manuscript; T.V., A.C.S., S. Syed, M.C., M.R., and S. Schulman provided critical revisions to the manuscript; J.D. contributed to study design; and J.D.D. contributed to study design and data analysis and provided critical revisions to the manuscript.
Conflict-of-interest disclosure: T.V. has participated in advisory boards and/or as a speaker for Bayer AG, Boehringer Ingelheim, Daiichi Sankyo, Bristol-Myers Squibb/Pfizer, and Sanofi. M.C. received research support or lecturing and consultancy fees from Boehringer Ingelheim, Bayer, Bristol-Myers Squibb, Pfizer, Daiichi Sankyo, Sanquin Blood Supply, and Portola. A.C.S. has worked as a consultant for Bayer, Boehringer Ingelheim, Janssen, Bristol-Myers Squibb, Pfizer, Portola, and the ATLAS Group; and reports research grants from Boehringer Ingelheim and Janssen. S. Schulman reports research grants from Octapharma and Boehringer Ingelheim and honoraria from Alnylam, Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, and Sanofi. J.D.D. reports personal fees from Janssen, Pfizer, Bayer, Bristol-Myers Squibb, Sanofi, Servier Canada, and Portola. The remaining authors declare no competing financial interests.
Correspondence: James D. Douketis, Department of Medicine, St. Joseph’s Healthcare Hamilton, and McMaster University, 50 Charlton Ave East, Hamilton, ON L8N 4A6, Canada; e-mail: jdouket@mcmaster.ca.
References
- 1.Yu AYX, Malo S, Svenson LW, Wilton SB, Hill MD. Temporal trends in the use and comparative effectiveness of direct oral anticoagulant agents versus warfarin for nonvalvular atrial fibrillation: a Canadian population-based study. J Am Heart Assoc. 2017;6(11):e007129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285(18):2370-2375. [DOI] [PubMed] [Google Scholar]
- 3.Omling E, Jarnheimer A, Rose J, Björk J, Meara JG, Hagander L. Population-based incidence rate of inpatient and outpatient surgical procedures in a high-income country. Br J Surg. 2018;105(1):86-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Douketis JD, Spyropoulos AC, Duncan J, et al. Perioperative management of patients with atrial fibrillation receiving a direct oral anticoagulant. JAMA Intern Med. 2019;179(11):1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spyropoulos AC, Douketis JD. How I treat anticoagulated patients undergoing an elective procedure or surgery. Blood. 2012;120(15):2954-2962. [DOI] [PubMed] [Google Scholar]
- 6.Spyropoulos AC, Al-Badri A, Sherwood MW, Douketis JD. Periprocedural management of patients receiving a vitamin K antagonist or a direct oral anticoagulant requiring an elective procedure or surgery. J Thromb Haemost. 2016;14(5):875-885. [DOI] [PubMed] [Google Scholar]
- 7.Tripodi A. To measure or not to measure direct oral anticoagulants before surgery or invasive procedures. J Thromb Haemost. 2016;14(7):1325-1327. [DOI] [PubMed] [Google Scholar]
- 8.Spyropoulos AC, Al-Badri A, Sherwood MW, Douketis JD. To measure or not to measure direct oral anticoagulants before surgery or invasive procedures: comment. J Thromb Haemost. 2016;14(12):2556-2559. [DOI] [PubMed] [Google Scholar]
- 9.Garcia D, Alexander JH, Wallentin L, et al. Management and clinical outcomes in patients treated with apixaban vs warfarin undergoing procedures. Blood. 2014;124(25):3692-3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sherwood MW, Douketis JD, Patel MR, et al. ; ROCKET AF Investigators . Outcomes of temporary interruption of rivaroxaban compared with warfarin in patients with nonvalvular atrial fibrillation: results from the rivaroxaban once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation (ROCKET AF). Circulation. 2014;129(18):1850-1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Healey JS, Eikelboom J, Douketis J, et al. ; RE-LY Investigators . Periprocedural bleeding and thromboembolic events with dabigatran compared with warfarin: results from the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) randomized trial [published correction appears in Circulation. 2012;126(10):e160]. Circulation. 2012;126(3):343-348. [DOI] [PubMed] [Google Scholar]
- 12.Douketis JD, Murphy SA, Antman EM, et al. Peri-operative adverse outcomes in patients with atrial fibrillation taking warfarin or edoxaban: analysis of the ENGAGE AF-TIMI 48 Trial. Thromb Haemost. 2018;118(6):1001-1008. [DOI] [PubMed] [Google Scholar]
- 13.Shaw JR, Woodfine JD, Douketis J, Schulman S, Carrier M. Perioperative interruption of direct oral anticoagulants in patients with atrial fibrillation: a systematic review and meta-analysis. Res Pract Thromb Haemost. 2018;2(2):282-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Douketis JD, Spyropoulos AC, Kaatz S, et al. ; BRIDGE Investigators . Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373(9):823-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siegal D, Yudin J, Kaatz S, Douketis JD, Lim W, Spyropoulos AC. Periprocedural heparin bridging in patients receiving vitamin K antagonists: systematic review and meta-analysis of bleeding and thromboembolic rates. Circulation. 2012;126(13):1630-1639. [DOI] [PubMed] [Google Scholar]
- 16.Tafur A, Li N, Clark N, et al. Predictors of bleeding in the Perioperative Anticoagulant Use for Surgery Evaluation (PAUSE) study [abstract]. Blood. 2019;134(suppl 1). Abstract 710. [Google Scholar]
- 17.Douketis JD, Spyropoulos AC, Anderson JM, et al. The Perioperative Anticoagulant Use for Surgery Evaluation (PAUSE) Study for patients on a direct oral anticoagulant who need an elective surgery or procedure: design and rationale. Thromb Haemost. 2017;117(12):2415-2424. [DOI] [PubMed] [Google Scholar]
- 18.Spyropoulos AC, Brohi K, Caprini J, et al. ; SSC Subcommittee on Perioperative and Critical Care Thrombosis and Haemostasis of the International Society on Thrombosis and Haemostasis . Scientific and Standardization Committee Communication: guidance document on the periprocedural management of patients on chronic oral anticoagulant therapy: recommendations for standardized reporting of procedural/surgical bleed risk and patient-specific thromboembolic risk. J Thromb Haemost. 2019;17(11):1966-1972. [DOI] [PubMed] [Google Scholar]
- 19.Godier A, Martin AC, Leblanc I, et al. Peri-procedural management of dabigatran and rivaroxaban: duration of anticoagulant discontinuation and drug concentrations. Thromb Res. 2015;136(4):763-768. [DOI] [PubMed] [Google Scholar]
- 20.Pernod G, Albaladejo P, Godier A, et al. ; Working Group on Perioperative Haemostasis . Management of major bleeding complications and emergency surgery in patients on long-term treatment with direct oral anticoagulants, thrombin or factor-Xa inhibitors: proposals of the working group on perioperative haemostasis (GIHP) - March 2013. Arch Cardiovasc Dis. 2013;106(6-7):382-393. [DOI] [PubMed] [Google Scholar]
- 21.Frost CE, Song Y, Shenker A, et al. Effects of age and sex on the single-dose pharmacokinetics and pharmacodynamics of apixaban. Clin Pharmacokinet. 2015;54(6):651-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stangier J, Stähle H, Rathgen K, Fuhr R. Pharmacokinetics and pharmacodynamics of the direct oral thrombin inhibitor dabigatran in healthy elderly subjects. Clin Pharmacokinet. 2008;47(1):47-59. [DOI] [PubMed] [Google Scholar]
- 23.Mueck W, Eriksson BI, Bauer KA, et al. Population pharmacokinetics and pharmacodynamics of rivaroxaban–an oral, direct factor Xa inhibitor–in patients undergoing major orthopaedic surgery. Clin Pharmacokinet. 2008;47(3):203-216. [DOI] [PubMed] [Google Scholar]
- 24.Kubitza DBM, Mueck W, Zuehlsdorf M. The effect of extreme age, and gender, on the pharmacology and tolerability of rivaroxaban – an oral, direct Factor Xa inhibitor [abstract]. Blood. 2006;108(11). Abstract 905. [Google Scholar]
- 25.Kubitza D, Becka M, Zuehlsdorf M, Mueck W. Body weight has limited influence on the safety, tolerability, pharmacokinetics, or pharmacodynamics of rivaroxaban (BAY 59-7939) in healthy subjects. J Clin Pharmacol. 2007;47(2):218-226. [DOI] [PubMed] [Google Scholar]
- 26.Upreti VV, Wang J, Barrett YC, et al. Effect of extremes of body weight on the pharmacokinetics, pharmacodynamics, safety and tolerability of apixaban in healthy subjects. Br J Clin Pharmacol. 2013;76(6):908-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Godier A, Dincq AS, Martin AC, et al. Predictors of pre-procedural concentrations of direct oral anticoagulants: a prospective multicentre study. Eur Heart J. 2017;38(31):2431-2439. [DOI] [PubMed] [Google Scholar]
- 28.Testa S, Paoletti O, Legnani C, et al. Low drug levels and thrombotic complications in high-risk atrial fibrillation patients treated with direct oral anticoagulants. J Thromb Haemost. 2018;16(5):842-848. [DOI] [PubMed] [Google Scholar]
- 29.Testa S, Legnani C, Antonucci E, et al. ; Coordinator of START2-Register . Drug levels and bleeding complications in atrial fibrillation patients treated with direct oral anticoagulants. J Thromb Haemost. 2019;17(7):1064-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horlocker TT, Vandermeuelen E, Kopp SL, Gogarten W, Leffert LR, Benzon HT. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine evidence-based guidelines (fourth edition). Reg Anesth Pain Med. 2018;43(3):263-309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.