Single-male polygyny with reproductive fidelity is documented across animals, but it was not fully demonstrated for any amphibian.
Abstract
Polygynous mating systems with group fidelity are a common animal organization, typically consisting of multiple females in a mated group with a single male for an extended period (sometimes referred to as harem polygyny). Single-male polygyny with reproductive fidelity occurs in invertebrates, bony fishes, and some tetrapods, such as lizards, mammals, and birds. In amphibians, reproductive fidelity in polygynous groups is not fully demonstrated. Combining data on larval development, molecular paternity assignment, and in situ behavioral observations, we reveal high fidelity during a prolonged breeding season in a Neotropical polygynous frog. Males dominate scarce breeding sites, guarding offspring, and mating exclusively with multiple females that exhibit dominance rank. This system likely evolved in response to intense competition for breeding sites and intrasexual competition for mates.
INTRODUCTION
Animal social organizations are characterized by mating systems with different levels of cohesiveness, along a continuum from polygamy by both sexes (promiscuous mating) to monogamy (1, 2). Polygamy is the evolutionarily ancestral condition in vertebrates, with monogamy evolving in many clades, including amphibians (3, 4). The evolution of monogamy is typically driven by the need for parental investment and reinforced by establishment of strong mate bonds (1, 2, 5). The most common social organization among vertebrates is polygyny, with males maximizing their fitness by mating with several females (2, 5). Across polygynous species, variable levels of mate fidelity and/or bonding have evolved (1–4, 6). In cases of extreme resource limitation, polygynous males often defend mates or resources or increase parental investment to guarantee offspring survival, leading to evolution of mate bonding and sometimes parental care (1, 2). This general pattern is not evident in frogs, where male defense of resources or females is common, but mate fidelity within polygynous groups has not yet been documented (7–10) as in other animal groups (11–15). Therefore, frogs, even those with prolonged breeding seasons, are not known to couple resource or female defense with any form of fidelity or pair bonding, making them an exception among tetrapods (16–18).
Here, combining data on larval development, molecular paternity assignment, and in situ behavioral observations, we reveal unexpected high fidelity in the saxicolous frog Thoropa taophora (family Cycloramphidae). Endemic to the Brazilian Atlantic rainforest, this frog has a peculiar semiterrestrial tadpole that feeds and develops until metamorphosis in freshwater seeps flowing on the surfaces of outcrops and rocky shores (19, 20). During the night, males are markedly aggressive and defend seeps as breeding territories for egg deposition by females (19). Throughout the 10-month breeding season (19, 21), males defend their territories against potential predators, including conspecifics, which can cannibalize eggs and tadpoles (19). We found that monopolist males mate with two genetically unrelated females (a dominant and a secondary) recurrently and exclusively during the prolonged breeding season. Therefore, this species represents the first case of single-male polygyny with reproductive fidelity in amphibians documented with parentage analyses and behaviors that potentially maintain bonds among members of the polygynous group.
RESULTS
Territorial defense and mating behaviors
To examine the possibility of pair bonding in this polygynous species, we video-recorded individual interactions among males and females under natural conditions and described specific interaction types and their frequencies (hereafter indicated by n; table S1). Monopolist males remain close to their eggs and tadpoles, protecting them and occasionally patrolling territorial boundaries. They discourage intruders by emitting aggressive calls (n = 807), sometimes also by sparsely adding advertisement calls (n = 103), and by displaying body-raising postures (n = 105). Monopolist males use vigorous jump attacks (n = 148), kicks (n = 16), and embraces that press keratinized spines on their thumbs against the intruder’s body (n = 9) to repel conspecific males that invade their territory or begin to cannibalize eggs (movies S1 and S2).
We also video-captured mating behaviors, which, combined with parentage studies, confirm that T. taophora is polygynous, with one monopolist male mating with two females, simultaneously sharing the same breeding seep for both females’ egg clutches (22, 23) over a prolonged breeding season with multiple reproductive bouts. Monopolist males interact in courtship with two or three females at the same time, with distinct types and levels of interactions between male and each female, indicating a hierarchy among females (Fig. 1, table S1, and movies S3 and S4). In a typical mating interaction, a dominant female mates with the male (n = 3) and emits female reciprocal calls (24) in response to courtship behaviors (n = 10). A secondary female, and sometimes a third peripheral female, is mostly motionless during mating activities of the dominant female. Our field videos revealed three behavioral tactics in T. taophora courtship: (i) A female approaches the male, turns her back toward him, and positions herself under him, thus stimulating amplexus (this behavior was only observed for dominant females, n = 35); (ii) a female moves within the territory, the male approaches the female (observed for dominant, n = 110, secondary, n = 19, and peripheral females, n = 13) and sometimes amplects her (amplexus only observed for dominant females, n = 5); or (iii) a female begins to cannibalize eggs, and the male immediately stops the behavior by chasing her away (observed for dominant, n = 14, secondary, n = 3, and peripheral females, n = 2), by briefly embracing her (observed for secondary, n = 2, and peripheral females, n = 2), or by effectively amplecting her (only observed for dominant females, n = 2). These behavioral tactics show that female-female competition is acting within the mated group, with clear social ranks and different fitness outcomes for females.
Fig. 1. Mating in T. taophora occurs in single-male polygynous groups with reproductive fidelity, a system that is well documented among amniote tetrapods but only now fully demonstrated in amphibians.
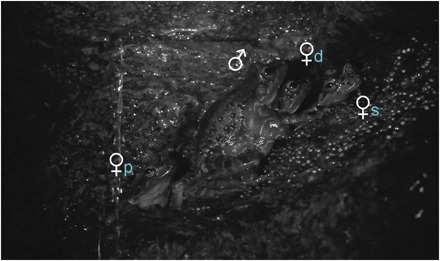
Males (♂; note: enlarged male forearm, a sexually dimorphic trait) monopolize scarce breeding seeps necessary for egg deposition and larval development. In this photo, the male is in his territory with eggs adhered to the humid rock surface and amplecting the dominant female (♀d). A secondary female (♀s) and a peripheral female (♀p) are near the amplecting pair. Municipality of São de Sebastião, São Paulo, Brazil. Photo credit: Rafael Consolmagno (Universidade Federal de São Paulo, Brazil)
Males respond differently to behaviors by females with different ranks. Amplexus by the monopolist male precludes further cannibalism by the dominant female and results in new eggs (deposited by the monopolist male and the dominant female). When a secondary or a peripheral female cannibalizes eggs, the monopolist male immediately approaches and briefly embraces the female, which stops cannibalism. This embracing behavior with secondary and peripheral females does not resemble amplexus and does not result in mating but interrupts female cannibalism of eggs. In our video recordings, we never observed mating with secondary or peripheral females; however, molecular parentage analyses of offspring in seeps confirm that secondary females also mate and deposit eggs that are fertilized by the monopolist male (23).
Tadpole cohorts and reproductive fidelity
We paired parentage analyses (23) with tadpole cohort analyses to measure the reproductive success of dominant, secondary, and peripheral females in seeps. Reconstructed parental genotypes for tadpoles collected at seven breeding seeps indicate a polygynous mating system in T. taophora, with larval half-sibships within each seep coming from a father and two mothers (23). On the basis of the parentage data, we separated tadpoles per mother from each site sampled and showed that the two females contribute unevenly to the total number of tadpoles. Each one of the seven breeding seeps analyzed included 56 to 97% (mean, 86 ± 14.8%) of tadpoles from a dominant female and 3 to 44% of tadpoles (mean, 14 ± 14.8%) from a secondary female (Table 1 and table S2). Tadpoles sampled from each one of 14 mothers (two mothers per breeding seep) varied in size from 4.3 ± 1.6 mm to 10.4 ± 1.7 mm and ranged in developmental Gosner stages (25) from 27 ± 3 to 39 ± 2 (n = 563; table S2). This range of developmental stages includes individuals recently hatched to stages immediately preceding metamorphosis (Table 1) and indicates that the tadpoles belong to different cohorts, hatched from eggs laid at different times by the same mating pair. Overall, developmental stages of tadpoles assigned to the two females overlap entirely, indicating that the dominant and secondary females mate multiply with the same male several times during the breeding season. We define this multiple mating by mated pairs as reproductive fidelity (Fig. 2) (1, 7, 16–18). We found no evidence of females or males mating in more than one breeding seep, even when neighboring seeps were only a few meters apart. Therefore, the genetic parentage and tadpole cohort analyses corroborate our field behavior observations of male monopoly of breeding seeps and repeated mating with the same two females.
Table 1. T. taophora tadpole body sizes and developmental stages for offspring of dominant and secondary females at each breeding seep analyzed in the parentage study.
| Females (n = 14) | Tadpoles (n = 563) | ||||||||||||||||||||
| n | Body sizes (mm) | Gosner developmental stages from recently hatched (25) to near metamorphosis (41) | |||||||||||||||||||
| Smallest | Largest | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | 40 | 41 | |||
| First seep |
Dominant | 29 | 5.5 | 8.8 | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| Secondary | 23 | 3.4 | 7.1 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| Second seep |
Dominant | 94 | 3.3 | 11.8 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Secondary | 7 | 3.5 | 7.6 | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||||
| Third seep |
Dominant | 55 | 3 | 11.8 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Secondary | 6 | 7.5 | 11.6 | ✓ | ✓ | ✓ | ✓ | ||||||||||||||
| Fourth seep |
Dominant | 59 | 3.2 | 11.6 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Secondary | 6 | 5.8 | 10.6 | ✓ | ✓ | ✓ | ✓ | ||||||||||||||
| Fifth seep |
Dominant | 37 | 3.3 | 10.8 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Secondary | 12 | 3.8 | 9.9 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Sixth seep |
Dominant | 145 | 3.5 | 12.7 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Secondary | 5 | 4.8 | 10.9 | ✓ | ✓ | ✓ | ✓ | ||||||||||||||
| Seventh seep |
Dominant | 81 | 3.4 | 9.9 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Secondary | 4 | 7.2 | 9.2 | ✓ | ✓ | ✓ | ✓ | ||||||||||||||
Fig. 2. Larval developmental stages within seven breeding seeps show recurrent mating by two females with the same monopolist male throughout the breeding season.
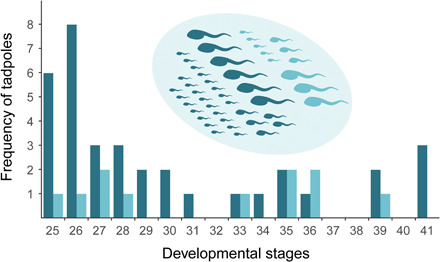
Frequencies of larvae mothered by two females (dominant and secondary) from one of the seeps sampled. Postembryonic tadpoles ranged from developmental stage 25 (immediately after eclosion) through stage 41 (immediately preceding emergence of all four limbs). Tadpoles of the dominant female are in the darker color (n = 35) and those of the secondary female are in the lighter color (n = 11). Inset scheme shows the proportion of offspring sampled from each mother. The same pattern was found in all breeding seeps sampled (Table 1).
DISCUSSION
Our findings extend female-defense polygynous mating systems with reproductive fidelity (harem polygyny) (1, 16–18) to all tetrapod groups and raise some interesting questions about the contexts that favor fidelity over promiscuous mating systems. The scarcity of breeding sites, as well as their discrete and monopolisable nature, promotes intense competition among males for sites and among females for access to those sites. Males reach higher reproductive success not only by monopolizing scarce breeding resources but also by maintaining females and aggressively excluding all other conspecific intruder males from their territories (1, 2, 5). The fitness benefits of breeding site monopoly must be extremely high, explaining male investment in intense and, likely costly, aggressive behaviors (1, 5). High male intrasexual aggression can also select for sexual dimorphism in weaponry that females lack (26). In T. taophora, this is reflected in the enlarged male forearms and keratinized thumb spines (19, 27) that, we observed, were being used in male-male agonistic interactions.
When critical resources are unevenly distributed, a female mating with an already paired male at a superior-quality breeding site will most likely have equal or higher reproductive success than a female mating with an unpaired male at a poorer-quality site, thus promoting polygyny (1, 5). Females within each single-male group also exhibited a social rank, with dominant females having higher reproductive success (28). The cost of polygyny in this system is the loss of eggs due to cannibalism by females in the group. We do not currently know whether females preferentially eat the eggs of others, but we previously showed that females sharing a seep are less related to each other than expected given levels of kinship in the overall population (23). We hypothesize that the low female-female relatedness in the polygynous group offsets the potential cost of cannibalism of closely related tadpoles. Low relatedness between reproductive T. taophora females in each breeding seep also results in more diverse offspring for the monopolist male, compensating for inbreeding effects inherent in polygyny (5). Our behavioral observations complement earlier parentage analyses and show that cannibalism of eggs by females is part of the mating repertoire and can elicit male amplexus, thus promoting complex costs and benefits in T. taophora. Cannibalism within the same group is unfavorable for the monopolist males but advantageous for females, who acquire food energy, reduce competition from embryos produced by the other females, increase resource availability for their own embryos (especially if females can distinguish their own eggs), and lastly gain new fertilized eggs through new mating opportunities.
The constraints imposed by limited breeding sites, coupled with the risks to offspring survival, have imposed strong sexual selection on T. taophora males and females, leading to a diversity of mating behaviors that were previously unknown. In amphibians, the existence of long-term fidelity between mated individuals, as well as the behaviors that maintain it, has been studied primarily in monogamous species (3, 4), where participation of both parents is required for successful reproduction. We propose that mating systems in amphibians are shaped by the same evolutionary selective pressures observed in other animals, with resource or female defense leading to reproductive fidelity in species that can monopolize those resources. Therefore, among polygynous frogs, other cases of reproductive fidelity will most likely be found in reproductively specialized species that depend on specific breeding habitats that are not ubiquitous in the environment.
MATERIALS AND METHODS
Focal populations and sampling
We studied the mating system of T. taophora at two different Atlantic rainforest sites in the state of São Paulo, Brazil. Both study sites were coastal rocky outcrops, with few freshwater seeps of small dimensions (1 to 4 m2, n = 17), which are the ideal breeding sites for this species. All field research was approved by the Committee on Animal Care Ethics at Universidade Estadual Paulista, Rio Claro, which agrees with the guidelines from the Institutional Animal Care and Use Committee.
In January 2004, we sampled T. taophora larvae on a 664-m-long rock shore outcrop at Itaguá beach (23o27′ S, 45o03′ W), Municipality of Ubatuba, São Paulo state, Brazil. We randomly collected tadpoles from seven freshwater seeps (ranging from 52 to 154 larvae per seep; mean, 85.4 ± 37.6; n = 598) and stored them in 95% ethanol. Freshwater seeps were separated by 10 to 95 m (mean, 44 ± 39.07 m) of unsuitable dry rock surfaces, which prevents larval migration from their birthplaces. Using a stereomicroscope, we examined all collected tadpoles, classifying them into developmental stages (25). Because tadpoles from Gosner stage 42 onward have four developed limbs and can migrate from one seep to another, we only selected individuals ranging between Gosner stages 25 and 41. Using a digital caliper, we measured total body lengths (including tail) of all tadpoles to the nearest millimeter. All data on tadpole developmental stages and body sizes, as well as their original breeding seep, are listed in table S2. To measure the strength of the correlation between developmental stages and body sizes for tadpoles, we applied the nonparametric Spearman rank correlation coefficient using R package stats (function correlation test) (29, 30). As expected, body size of tadpoles in T. taophora is strongly correlated with developmental stage (r = 0.965, P = 2.2−16, n = 553; fig. S1). The variance in body sizes and developmental stages among sibs can be used to infer whether full-sib tadpoles belong to the same clutch or clutches laid at different time points. This is corroborated by developmental timelines for closely related species (31, 32) that indicate that plasticity alone is insufficient to explain the range of T. taophora tadpole body sizes within each breeding seep.
Between October 2012 and February 2013, we carried out behavioral studies of T. taophora on a 600-m2 rocky shore outcrop in a nearby population at Toque-Toque Grande beach, Municipality of São Sebastião (23o50′ S, 45o31′ W), São Paulo state, Brazil. Over a period of 5 months, we spent 53 nights in the field, totaling 138 hours, of direct observations under natural conditions and 59 hours and 45 min of video recordings under natural conditions at 10 freshwater seeps. We observed territorial defense and courtship behaviors and counted the number of each behavior in the video footage (table S1) using focal animal and all occurrence sampling methods (33, 34). We performed video recordings at night using a Sony DCRSR85 camera in nightshot mode. Between 18:00 and 6:00, we placed the camera next to male territories to record behaviors without the presence of researchers whenever possible. Together, we observed 17 events of males agonistically interacting (from 55 hours and 38 min of videos) and two reproductive events (entailing courtship, amplexus, and oviposition; from 4 hours and 7 min of videos), with the presence of up to three females simultaneously at the same reproductive site. From these videos, we characterized types and quantified frequencies of behaviors performed by males and females before and during courtship in polygynous groups (table S1).
Parentage analyses
In a previous study, we genotyped T. taophora tadpoles for parentage analyses using microsatellites (23). We reconstructed parental genotypes and showed that tadpoles from each of the same seven breeding seeps are always half-siblings, sharing a single father and one of two genetically unrelated mothers (23). At the time, we hypothesized that females might visit the same breeding seep at different times to oviposit partial clutches and pointed out the necessity of direct behavioral observations to determine behaviors that might promote potential female breeding site fidelity. In this study, we grouped all T. taophora sampled tadpoles by genetic kinship and examined the temporal spread of tadpoles (according to Gosner developmental stages) within each breeding seep. Reinforced by in situ behavioral observations, our study reveals aspects of mating system dynamics not previously documented for amphibians.
Supplementary Material
Acknowledgments
We thank A. M. Haddad for collection of larvae and G. Machado for help with behavioral observations. Funding: Our work was supported by grants from the São Paulo Research Foundation (FAPESP; #2012/00205-9, #2014/24972-4, #2016/06876-3, and #2018/17993-6 to F.P.d.S. and #2008/50928-1, #2012/17220-0, and #2013/50741-7 to C.F.B.H.), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; to C.F.B.H., R.C.C., and C.A.B.), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES–Finance Code 001; to F.P.d.S., R.C.C., and C.A.B.), and NSF (to K.R.Z.). Author contributions: F.P.d.S., K.R.Z., and C.F.B.H. conceived the project and designed the analyses. C.F.B.H. collected field samples for paternity analyses. F.P.d.S. and C.F.B.H. analyzed variation in larval development. F.P.d.S. and P.M. genotyped larvae and performed parentage analyses. R.C.C. and C.A.B. recorded field behavioral data. F.P.d.S. and R.C.C. analyzed behavioral data. F.P.d.S., R.C.C., C.F.B.H., and K.R.Z. wrote the manuscript with contributions from all authors. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/33/eaay1539/DC1
REFERENCES AND NOTES
- 1.Emlen S. T., Oring L. W., Ecology, sexual selection, and the evolution of mating systems. Science 197, 215–223 (1977). [DOI] [PubMed] [Google Scholar]
- 2.S. M. Shuster, M. J. Wade, Mating Systems and Strategies (Princeton Univ. Press, 2003). [Google Scholar]
- 3.Caldwell J. P., Pair bonding in spotted poison frogs. Nature 385, 211 (1997). [Google Scholar]
- 4.Caldwell J. P., Oliveira V. R. L., Determinants of biparental care in the spotted poison frog, Dendrobates vanzolinii (Anura: Dendrobatidae). Copeia 3, 565–575 (1999). [Google Scholar]
- 5.Kvarnemo C., Why do some animals mate with one partner rather than many? A review of causes and consequences of monogamy. Biol. Rev. 93, 1795–1812 (2018). [DOI] [PubMed] [Google Scholar]
- 6.O’Brien D. M., Keogh J. S., Silla A. J., Byrne P. G., The unexpected genetic mating system of the red-backed toadlet (Pseudophryne coriacea): A species with prolonged terrestrial breeding and cryptic reproductive behaviour. Mol. Ecol. 27, 3001–3015 (2018). [DOI] [PubMed] [Google Scholar]
- 7.B. K. Sullivan, M. J. Ryan, P. A. Verrel, Female choice and mating system structure, in Amphibian Biology: Social Behaviour, H. Heatwole, B. K. Sullivan, Eds. (Surrey Beatty, 1995), vol. 2, pp. 490–507. [Google Scholar]
- 8.Stamps J. A., Territorial behavior: Testing the assumptions. Adv. Study Behav. 23, 173–232 (1994). [Google Scholar]
- 9.Crump M. L., Parental care among the Amphibia. Adv. Study Behav. 25, 109–144 (1996). [Google Scholar]
- 10.K. D. Wells, Mating systems and sexual selection in anurans, in The Ecology and Behavior of Amphibians (University of Chicago Press, 2007), pp. 338–402. [Google Scholar]
- 11.D. M. Shuker, L. W. Simmons, The Evolution of Insect Mating Systems (Oxford Univ. Press, 2014). [Google Scholar]
- 12.Kadota T., Osato J., Hashimoto H., Sakai Y., Harem structure and female territoriality in the dwarf hawkfish Cirrhitichthys falco (Cirrhitidae). Environ. Biol. Fish. 92, 79–88 (2011). [Google Scholar]
- 13.Brattstrom B. H., The evolution of reptilian social behavior. Am. Zool. 14, 35–49 (1974). [Google Scholar]
- 14.Terborgh J., Janson C. H., The socioecology of primate groups. Annu. Rev. Ecol. Syst. 17, 111–136 (1986). [Google Scholar]
- 15.Ridley M. W., Hill D. A., Social organization in the pheasant (Phasianus colchicus): Harem formation, mate selection and the role of mate guarding. J. Zool. 211, 619–630 (1987). [Google Scholar]
- 16.J. Berger, Social systems, resources, and phylogenetic inertia: An experimental test and its limitations, in The Ecology of Social Behavior, C. N. Slobodchikoff, Ed. (Academic Press, 1988), pp. 157–186. [Google Scholar]
- 17.E. O. Wilson, Sociobiology: The New Synthesis (Harvard Univ. Press, twenty-fifth anniversary ed., 2000). [Google Scholar]
- 18.E. M. Barrows, Animal Behavior Desk Reference: A Dictionary of Animal Behavior, Ecology, and Evolution (CRC Press, ed. 3, 2011). [Google Scholar]
- 19.Giaretta A. A., Facure K. G., Reproductive ecology and behavior of Thoropa miliaris (Spix, 1824) (Anura, Leptodactylidae, Telmatobiinae). Biota Neotrop. 4, 1–10 (2004). [Google Scholar]
- 20.Bokermann W. C. A., Notas sôbre as espécies de Thoropa Fitzinger (Amphibia, Leptodactylidae). An. Acad. Bras. Ciênc. 37, 525–537 (1965). [Google Scholar]
- 21.M. T. Hartmann, “Biologia reprodutiva de uma comunidade de anuros na Mata Atlântica (Picinguaba, Ubatuba, SP),” thesis, Universidade Estadual Paulista, Rio Claro (2004). [Google Scholar]
- 22.Consolmagno R. C., Requena G. S., Machado G., Brasileiro C. A., Costs and benefits of temporary egg desertion in a rocky shore frog with male-only care. Behav. Ecol. Sociobiol. 70, 785–795 (2016). [Google Scholar]
- 23.Muralidhar P., de Sá F. P., Haddad C. F. B., Zamudio K. R., Kin-bias, breeding site selection and female fitness in a cannibalistic Neotropical frog. Mol. Ecol. 23, 453–463 (2014). [DOI] [PubMed] [Google Scholar]
- 24.Toledo L. F., Martins I. A., Bruschi D. P., Passos M. A., Alexandre C., Haddad C. F. B., The anuran calling repertoire in the light of social context. Acta Ethol. 18, 87–99 (2015). [Google Scholar]
- 25.Gosner K. L., A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16, 183–190 (1960). [Google Scholar]
- 26.Emlen D. J., The evolution of animal weapons. Annu. Rev. Ecol. Evol. Syst. 39, 387–413 (2008). [Google Scholar]
- 27.Cocroft R. B., Heyer W. R., Notes on the frog genus Thoropa (Amphibia: Leptodactylidae) with description of a new species (Thoropa saxatilis). Proc. Biol. Soc. Wash. 101, 209–220 (1988). [Google Scholar]
- 28.Rosvall A. K., Intrasexual competition in females: Evidence for sexual selection? Behav. Ecol. 22, 1131–1140 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.R Core Team, “R: A language and environment for statistical computing” (R Foundation for Statistical Computing, 2018).
- 30.M. Hollander, D. A. Wolfe, E. Chicken, Nonparametric Statistical Methods (Wiley, ed. 3, 2014). [Google Scholar]
- 31.Candioti M. F. V., Nuñez J. J., Úbeda C., Development of the nidicolous tadpoles of Eupsophus emiliopugini (Anura: Cycloramphidae) until metamorphosis, with comments on systematic relationships of the species and its endotrophic developmental mode. Acta Zool. 92, 27–45 (2011). [Google Scholar]
- 32.Desnitskiy A. G., Ontogenetic diversity and early development of frogs in the South American family Cycloramphidae. Phyllomedusa 10, 7–13 (2011). [Google Scholar]
- 33.Altmann J., Observational study of behavior: Sampling methods. Behaviour 49, 227–266 (1974). [DOI] [PubMed] [Google Scholar]
- 34.P. N. Lehner, Handbook of Ethological Methods (Garland STPM, ed. 2, 1979). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/33/eaay1539/DC1


