Abstract
In December 2019, a pneumonia outbreak was reported in Wuhan, Hubei province, China. Since then, the World Health Organization declared a public health emergency of international concern due to a growing number of deaths around the globe, as well as unparalleled economic and sociodemographic consequences. The disease called coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a novel form of human coronavirus. Although coronavirus infections have been associated with neurological manifestations such as febrile seizures, convulsions, change in mental status, and encephalitis, less is known about the impact of SARS-CoV-2 in the brain. Recently, emerging evidence suggests that SARS-CoV-2 is associated with neurological alterations in COVID-19 patients with severe clinical manifestations. The molecular and cellular mechanisms involved in this process, as well as the neurotropic and neuroinvasive properties of SARS-CoV-2, are still poorly understood. Glial cells, such as astrocytes and microglia, play pivotal roles in the brain response to neuroinflammatory insults and neurodegenerative diseases. Further, accumulating evidence has shown that those cells are targets of several neurotropic viruses that severely impact their function. Glial cell dysfunctions have been associated with several neuroinflammatory diseases, suggesting that SARS-CoV-2 likely has a primary effect on these cells in addition to a secondary effect from neuronal damage. Here, we provide an overview of these data and discuss the possible implications of glial cells as targets of SARS-CoV-2. Considering the roles of microglia and astrocytes in brain inflammatory responses, we shed light on glial cells as possible drivers and potential targets of therapeutic strategies against neurological manifestations in patients with COVID-19. The main goal of this review is to highlight the need to consider glial involvement in the progression of COVID-19 and potentially include astrocytes and microglia as mediators of SARS-CoV-2-induced neurological damage.
Keywords: Glial cells, Astrocyte, Microglia, SARS-CoV-2, COVID-19, Coronavirus, Neuroinflammation
Highlights
-
•
Due to ACE2 expression, glial cells are potential targets of SARS-CoV-2.
-
•
Glia cells are the major source of cytokines in SARS-CoV-2-triggered neuroinflammation.
-
•
Astrocytes/Microglia may be associated with SARS-CoV-2-triggered neurological damage.
-
•
Glial cells should be considered targets for therapeutic strategies to COVID-19.
Abbreviations
- ACE2
angiotensin converting enzyme 2
- ADEM
disseminated encephalomyelitis
- AIDS
acquired immunodeficiency syndrome
- APCs
antigen presenting cells
- BBB
blood-brain-barrier
- BCSFB
blood-cerebrospinal fluid barrier
- CNS
central nervous system
- CoV
coronaviruses
- COVID-19
coronavirus disease 2019
- CSFs
colony-stimulating factors
- DENV
dengue virus
- ECs
endothelial cells
- GFAP
glial fibrillary acidic protein
- HCoV
human coronavirus
- ICU
intense care unit
- IFN family
interferon family
- IFN-γ
interferon gamma
- IL-10
interleukin 10
- IL-12
interleukin 12
- IL-13
interleukin 13
- IL-15
interleukin 15
- IL-15
interleukin 15
- IL-17
interleukin 17
- IL-6
interleukin 6
- IL1-α
interleukin 1 alpha
- IL1-β
interleukin 1 beta
- iNOs
type 2 nitric oxide synthase
- MCP-1
monocyte chemoattractant protein-1
- MERS-CoV
Middle East respiratory syndrome coronavirus
- MERS
Middle East respiratory syndrome
- MHC
main histocompatibility complex
- MHV
mouse hepatitis virus
- MIF
macrophage migration inhibitory factor
- NfL
neurofilament light chain protein
- OPN
osteopontin
- RABV
rabies virus
- RANTES
chemokine (C–C motif) ligand 5 (also CCL5)
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- SARS
severe acute respiratory syndrome
- SASP
senescence-associated secretory phenotype
- TGF-β1
transforming growth factor beta 1
- TLR4
toll-like receptor 4
- TNF-α
tumor necrosis factor alpha
- VEGF
vascular endothelial growth factor
- WNV
West Nile Virus
- ZIKV
zika virus
1. Coronaviruses, SARS-CoV-2 and the central nervous system
Coronaviruses (CoV) belong to the Coronaviridae family, which is responsible for causing a broad spectrum of illnesses such as respiratory, enteric, and neurological diseases in animals and human. The human coronaviruses (HCoV) are known to cause common cold in immunocompetent individuals and, rarely, pneumonia. Meanwhile, SARS (severe acute respiratory syndrome) and MERS (Middle East respiratory syndrome) – CoV (SARS-CoV and MERS-CoV, respectively) were causes of epidemics in 2002 and 2012, respectively. The new virus, SARS-CoV-2, is the etiologic agent of the current coronavirus disease 2019 (COVID-19) pandemic, which originated in December 2019 in Wuhan, Hubei Province, China (Ciotti et al., 2020).
The coronaviruses are enveloped, pleomorphic viruses, with diameters ranging from 80 to 120 nm. The genome consists of a positive single-stranded RNA, the largest known RNA genome, with a length of up to 30 kb. At least four structural proteins are encoded by this genome, such as: S (spike), which gives the virus its crown aspect and allows binding to the host cells; E (envelope), a small membrane and hydrophobic protein; M (membrane), which plays a crucial role in the assembly and budding of virus particles together with E protein; and N (nucleocapsid), strongly associated with RNA (Weiss and Leibowitz, 2011).
Initially, SARS-CoV-2 was only considered as a zoonotic virus; however, the virus has crossed species to infect humans, and human-to-human transmission also occurs, mainly through direct contact and droplet spread (Li et al., 2020b). These features are facilitators for the rapid spread of the virus worldwide. As of July 31, 2020, regarding the COVID-19 situation, there were 17,106,007 confirmed cases and 668,910 deaths globally ((OPAS) 2020). The total number of reported COVID-19 infections is probably underestimated since there are mild or asymptomatic cases and considering the impossibility of performing population-wide laboratory diagnoses, especially in low- and middle-income countries.
At first, this virus was shown to cause only an acute lower tract respiratory infection, which could lead to pneumonia; however, multiple organ distress syndrome may occur, which may affect several organs, including the brain, provoking neurological manifestations (Dos Santos et al., 2020; Fotuhi et al., 2020). Although the mechanisms of brain damage in COVID-19 are poorly understood, other members of the coronavirus family have already been associated with neurological disease (Wu et al., 2020), which may give support to the neurotropic behavior of this virus.
In previous epidemics, SARS-CoV was detected in the brain and in the cerebrospinal fluid of patients who presented neurological manifestations (Xu et al., 2005). Some authors related CoV infections to acute disseminated encephalomyelitis (ADEM) (Algahtani et al., 2016). Four of twenty three patients with MERS-CoV reported neurological symptoms and were diagnosed with Bickerstaff’s encephalitis overlapping with Guillain-Barré syndrome, without any respiratory symptoms (Kim et al., 2017).
Increasing reports of COVID-19 patient cohorts, although still sparse, have shown a prevalence of neurologic signs and symptoms (Helms et al., 2020; Mao et al., 2020). The clear and predominant neurological symptom of COVID-19 patients is headache, in up to a third of all patients (Helms et al., 2020; Jin et al., 2020; Mao et al., 2020). Following headache, anosmia and ageusia were quickly described as early symptoms of SARS-CoV-2 infection, although the prevalence of these symptoms in studies is too variable to drawn any final conclusions (Giacomelli et al., 2020; Lechien et al., 2020; Mao et al., 2020; Vaira et al., 2020). Nevertheless, the presence of anosmia in COVID-19 patients led to the first hypothesis about the neurotrophic capacity of SARS-CoV-2, as it was already reported for other coronaviruses (as HCoV-OC43, SARS-CoV-1 and MERS-CoV) (Arbour et al., 2000; Jacomy and Talbot, 2003; Xu et al., 2005; Su et al., 2016; Dube et al., 2018).
A study suggested patients with more severe systemic presentations are more likely to have neurological impairment than those with milder forms of the infection, since 36.4% of 214 patients with COVID-19 presented neurological symptoms (Mao et al., 2020). Similarly, COVID-19 patients with central nervous system (CNS) symptoms exhibited lower lymphocyte levels and platelet counts (Mao et al., 2020; Yan et al., 2020). Given this information, it is very likely SARS-CoV-2 is associated with neurological alterations in COVID-19 patients with severe clinical manifestations; however, further studies are necessary to confirm this.
Among the neurological manifestations reported, delirium is suggested to be the most common psychiatric manifestation in the acute phase of SARS-CoV-2 infection, occurring in almost 30% of the patients (Rogers et al., 2020). Although delirium is considered to be an early clinical expression of acute brain dysfunction, it is important to note that in the context of infectious diseases, it can be caused by an exacerbated peripheral inflammatory response and/or by direct action of the pathogen in the brain (Tsuruta and Oda, 2016). Intensive production of inflammatory mediators (as IL-6, IL-10, TNFs, IL-1β) and changes in brain perfusion can lead to blood brain barrier (BBB) breakdown, microglial and astrocytic activation, thus leading to an imbalance of neurotransmitters and delirium (reviewed in (Tsuruta and Oda, 2016). Considering this, many factors can contribute to the delirium observed in COVID-19 patients, especially the ones from ICU (intense care unit), such as the prolonged sedation and choice of sedative strategies, immobilization, socio-environmental factors, the most advanced age of the patients presenting more severe forms of the disease and, finally, direct viral CNS infection and induction of neuroinflammation (Kotfis et al., 2020a, 2020b).
As will be discussed in the next sections, considering previous evidence of the neuroinvasive potential of coronaviruses and their ability to infect glial cells, this could be a major mechanism driving the development of delirium in COVID-19 patients. Microglial and astrocytic activation, either by direct infection or systemic inflammation, can lead to a neurotoxic response which translates into neuronal damage and is clinically manifested as delirium (van Gool et al., 2010; Khan et al., 2020). Furthermore, previous evidence suggests that glial cells in the aged brain are more prone to generate this neurotoxic response to external pathogens, which would explain the increased prevalence of delirium in the elder patients with SARS-CoV-2 infection.
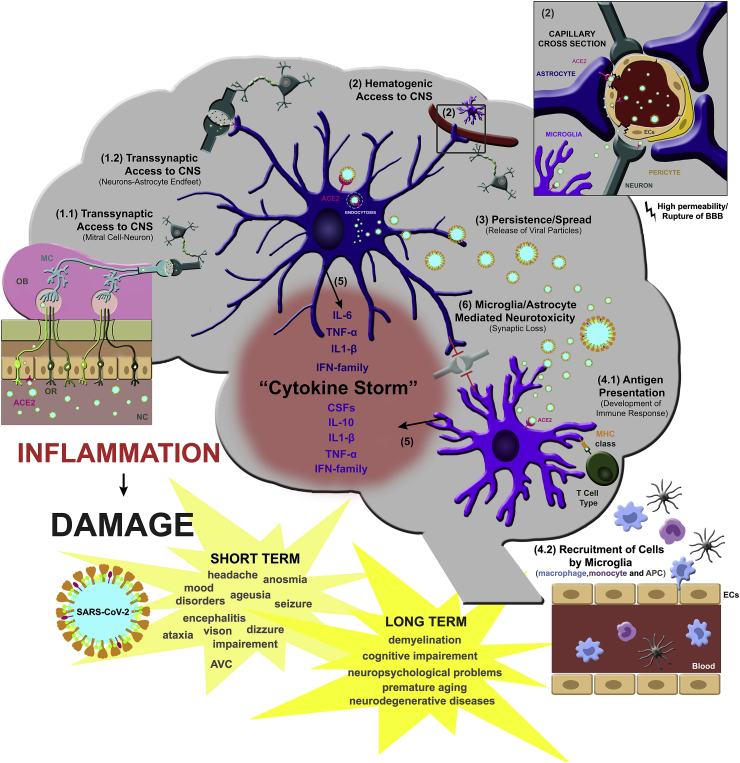
There are strong pieces of evidence of the neurotropism of coronaviruses, most of them based on the clinical manifestations, although it has not been completely elucidated how SARS-CoV-2 accesses the human CNS (for further review on this topic see (Alam et al., 2020; Dos Santos et al., 2020; Fotuhi et al., 2020). Two hypotheses to explain SARS-CoV-2 neuroinvasion have been proposed so far (Fig. 1): (i) via hematogenous access or (ii) via retrograde neuronal routes. In the first one (i), the virus seems to infect endothelial cells (ECs) from the blood-brain-barrier (BBB), epithelial cells of the blood-cerebrospinal fluid barrier (BCSFB) or leukocytes that would promote viral dissemination through the CNS. In (ii), because they are respiratory viruses, they seem to use the olfactory nerve and then the olfactory bulb to reach the brain (Li et al., 2020a).
Fig. 1.
Overview of the roles of glial cells, astrocytes and microglia in the brain response to SARS-CoV-2-triggered neuroinflammation and possible implications for COVID-19. Two hypotheses have been proposed to explain SARS-CoV-2 neuroinvasion: via retrograde neuronal routes (1.1 and 1.2) or via hematogenic access (2). In both cases, astrocyte involvement is suggested. In the first one, SARS-CoV-2 seems to use the olfactory nerve and then the olfactory bulb to reach the brain by transsynaptic contact between mitral cells and neurons (1.1) and then between neurons and astrocyte’s endfeet on the synaptic cleft (1.2). Second, SARS-CoV-2 may infect endothelial cells (ECs) and/or astrocytes from the blood-brain-barrier (BBB), leading to increased permeability and/or rupture of the BBB (2) and further infection of other cells around the neurovascular region (2). Both options lead to viral dissemination through the CNS. SARS-CoV-2 replication by glial cells is unclear, although astrocytes have been suggested as a reservoir of coronavirus and thus contribute to virus spread (3). In response to virus infection, microglial cells trigger T cell and APC activation (4.1) and recruitment/activation of innate immune cells (monocytes, macrophages and APC) (4.2). This scenario leads to release of several cytokines, chemokines and colony-stimulating factors (CSFs) by astrocytes and microglia (5), known as a “cytokine storm,” which leads to neurotoxicity and synapse loss (6) and consequently may contribute to short- and long-term brain damage in COVID-19 patients. OB, olfactory bub; OR, olfactory receptor; MC, mitral cells; ECs, endothelial cells; ACE2, angiotensin converting enzyme 2.
The early appearance of anosmia supports the hypothesis that coronavirus could reach the CNS via retrograde axonal transport from the cribriform plate (McCray et al., 2007; Li et al., 2016; Dube et al., 2018).
However, it remains unclear if transcribriform infection of neurons would be the actual mechanism of CNS entry or if the anosmia is caused by SARS-CoV-2 infection of the nasal neuroepithelium, especially after recent reports suggesting that unlike neuroepithelial cells, olfactory sensory neurons do not express angiotensin converting enzyme 2, ACE2, the main coronavirus receptor, and other coreceptors necessary for SARS-CoV-2 infection (Brann et al., 2020; Fodoulian et al., 2020).
Another possible mechanism of CNS infection by SARS-CoV-2 is through infection of vascular ECs, which have already been reported to be infected by SARS-CoV-2 (Varga et al., 2020). In addition, other neurovirulent viruses, such as some strains of Dengue Fever virus, are also capable of reaching the CNS by infecting ECs to cross the BBB (Calderon-Pelaez et al., 2019). Apart from direct EC infection, the important systemic inflammation observed in COVID-19 patients can also induce BBB disruption (Sankowski et al., 2015), allowing potential entry of infected leukocytes into the CNS. Previous studies with SARS-CoV-1 showed that the virus was capable of infecting several different types of ACE2-expressing immune cells (Spiegel et al., 2006; Wang et al., 2020). Rupture of the BBB, followed by the spread of SARS-CoV-2 by the capillary endothelium and access to the brain, is also suggested (Montalvan et al., 2020). This hematogenous route pathway for SARS-CoV-2 to the brain is further supported by findings of viral-like particles in brain capillary endothelium in the frontal lobe tissue of postmortem brain of COVID-19 patients (Chigr et al., 2020).
An additional route to be considered is through the lymphatic system. Mouse hepatitis virus (MHV) was reported to disseminate in the brain via cervical and mesenteric lymph nodes in addition to viremia (Barthold and Smith, 1992). The recently described glymphatic system (Plog and Nedergaard, 2018), where glial cells play an important role in the communication between blood and nervous system, suggests that infection of glial cells by SARS-CoV-2 might contribute to virus access to brain parenchyma. It is important to highlight that ACE2 binding affinity of the SARS-CoV-2 spike protein ectodomain is much higher than the SARS-CoV-1 spike protein, about 10-20-fold higher (Wrapp et al., 2020). These findings may be a possible explanation to the rapid spread of SARS-CoV-2 from human to human (Chan et al., 2020).
Thus, despite similarities of SARS-CoV-2 and other members of coronavirus family, further studies are required to investigate whether SARS-CoV-2 can access the brain through this pathway. Since it is a fairly new virus, this review is aimed at exposing some information about the neuropathogenesis of coronaviruses, with focus on glial cells, which may help in future studies in the field of SARS-CoV-2 and COVID-19 infections.
2. Glial cells, CoVs and other neurotropic viruses
Glial cells, such as astrocytes and microglia, have key functions in maintaining brain homeostasis and in the CNS response to insults, whether physical, infectious or neuropsychiatric and neurodegenerative disease-related. Commonly, the hallmark of these conditions is a general neuroinflammation scenario, characterized by activation of glial cells, production and release of pro- and/or anti-inflammatory cytokines and chemokines, antioxidants, free radicals, and neurotrophic factors. Whether this response collaborates in the development or delay of the disease is still a matter of discussion and is highly dependent on the insult, stage of the disease and brain region. Emerging evidence from our group and others supports the concept that reactive glial cells, astrocytes and microglia have a duality in their phenotype, neurotoxic or neuroprotective properties, depending on the age, infectious stimuli, and physiological/pathological condition (Moraes et al., 2015; Diniz et al., 2017; Diniz et al., 2019; Matias et al., 2019; do Amaral et al., 2020). The underlying mechanisms of their activation, cellular interplays and the impact of regional astrocytic and microglial heterogeneity are still a matter of discussion. Given the large number of functions performed by astrocytes and microglia in the neuroinflammatory response, it is to be expected that activation of these cells has a major impact on brain function in virus infection, such as the SARS-CoV-2 infection found in COVID-19 patients.
The rabies virus (RABV) is transmitted to humans through biting, scratching and/or licking of infected animals, which can lead to neurological signs, such as confusion, agitation, hallucinations and delirium (Consales and Bolzan, 2007). Despite neurons being primarily infected, RABV is able to infect microglia and astrocytes, possibly contributing to viral spread and persistence of the virus. In addition, they seem to affect neurons via the release of cytokines or neurotoxins (Ray et al., 1997).
In the arboviruses group, some members of the Flaviviridae family are known for their neurotropic activity, such as dengue virus (DENV), zika virus (ZIKV), and West Nile virus (WNV).
In 2009, the World Health Organization updated the dengue classifications. The categories are dengue without warning signs, dengue with warning signs and severe dengue. In the latter, CNS impairment was included, due to the increased number of cases reporting neurological manifestations. In this context, our group has studied the involvement of glial cells in DENV infection. Microglia cells are speculated to be the major target cells of the virus in the brain. In experimental mice and human fatal cases, they exhibit phenotypic changes suggestive of activation (Ramos et al., 1998). Although it is not fully understood, some authors defend the idea that microglia have pro-inflammatory and pathogenic roles leading to neurotoxicity, and consequently, to neurological complications. DENV NS3 antigen was detected in microglial cells of a fetus who died together with the mother, who presented severe complications due to a DENV infection. These cells were responsible for producing RANTES, IFN-γ, MCP-1, and VEGF (Nunes et al., 2019). In contrast, microglia were already reported as having an antiviral role, inducing CD8-positive cytotoxic T lymphocyte responses (Tsai et al., 2016). Meanwhile, astrocytes were not infected by DENV in vitro and in vivo, although they were altered in number, size and shape in murine models (Velandia-Romero et al., 2016), as well as in dengue fatal cases (Salomão et al., 2020), which could cause alterations in brain homeostasis.
Beyond that, ZIKV became known due to reports of newborns with microcephaly born of infected pregnant women in Brazil. The virus infects fetal microglia and induces high levels of pro-inflammatory mediators, which could be harmful to the fetus, evolving to congenital Zika syndrome (Rabelo et al., 2018). In addition, our group previously demonstrated that ZIKV viral antigen was detected in astrocytes, neurons, pyramidal neurons and microglia cells in the cortical region in severe syndrome outcome (Alves-Leon et al., 2019).
Fetal brain autopsy showed histopathological alterations related to microglia activation, leading to neuroinflammation and viral dissemination to the brain parenchyma (Mlakar et al., 2016). In in vitro experiments, microglia are highly susceptible to ZIKV infection. In contrast to what is seen in DENV infection, ZIKV is able to infect astrocytes, leading to morphologic changes (Stefanik et al., 2018), which may alter the maintenance and permeability of the BBB. Recently, Ledur and collaborators demonstrated that ZIKV infection leads to ROS imbalance, mitochondrial defects and DNA breakage in iPSC-derived human astrocytes. They also detected glial reactivity in mice and in post-mortem brains from infected neonates from the northeast of Brazil, indicating that astrocytes are targets and responsive to ZIKV (Ledur et al., 2020).
Astrocytes were also reported to be infected by WNV in some fatal cases, inducing neuroinflammatory genes (van Marle et al., 2007). Together with microglial cells, they were activated, which was evidenced by glial hypertrophy and increased number of activated glia, provided by immunohistochemical analyses (van Marle et al., 2007). Astrocytes, however, were suggested to play an important role in controlling WNV dissemination (Hussmann et al., 2013).
Out of the arboviruses group, the human immunodeficiency virus (HIV), the etiological agent of acquired immunodeficiency syndrome (AIDS), is also able to infect the brain. The encephalopathy caused by HIV is characterized by the formation of multinucleated giant cells and microglial nodules, which provoke neurological symptoms in more than 50% of patients not receiving antiretroviral therapy. The neurodegeneration seems to occur mainly due to microglial cell infection and activation, leading to production of cytokines and neurotoxic substances (Alirezaei et al., 2008) that stimulate astrocytes, contributing to neuronal injury. Otherwise, microglia also have some neuroprotective roles in the early stages of the disease (Gras et al., 2003). The astrocytes infected by HIV are particularly found in perivascular regions and are associated with macrophages, contributing to neuropathogenesis (Churchill et al., 2009).
Concerning the Coronaviridae family, several lines of evidence show that members of this family are able to infect the brain, including SARS-CoV, the human coronavirus (HCoV), MERS-CoV, and the mouse hepatitis virus (MHV). SARS-CoV-1 has been found in eight brain autopsies of patients by electron microscopy, immunohistochemistry, and real-time reverse transcription (Xu et al., 2005; Netland et al., 2008).
The human coronavirus (HCoV) OC43 strain seems to be able to infect primary cultures of human astrocytes and microglia. In some astrocytic cell lines, the infection is persistent (Arbour and Talbot, 1998). In MERS-CoV infections, the cerebrospinal fluid presented high levels of MIF (macrophage migration inhibitory factor) and OPN (osteopontin), two pro-inflammatory cytokines produced by activated microglia (Ichiyama et al., 2009).
The mouse hepatitis virus (MHV) is extensively used as an experimental model for the study of CNS viral infection. MHV causes different types of diseases, such as hepatitis, enteritis, and encephalomyelitis. In encephalomyelitis, the strain JHMV extensively infects brain cells, including astrocytes and microglia (Sun and Perlman, 1995), as discussed in subsequent sections.
Although several lines of evidence support infection of the CNS by coronaviruses, there is less data concerning the impact of these viruses, especially the newly identified SARS-Cov-2, on glial cells. In the next sections, we will present new results on the effects of SARS-CoV-2, mainly on microglia and astrocytes, and we will discuss the role of glial cells as modulators of the COVID-19 neuropathology.
2.1. Microglia and the cytokine storm in COVID-19
Microglial cells are the resident macrophages from the CNS. They are derived from embryonic erythro-myeloid yolk sac progenitors, which colonize the CNS at early stages of development and are maintained by prolonged cellular longevity and local proliferation rather than peripheral hematopoiesis (Ginhoux et al., 2010). As microglia are the key components of the innate immune response in the CNS, it is clear that any viral infection of the CNS will drive direct and indirect responses of these cells, which are essential for clearance of viral particles and dying neurons and can be responsible for the neurological manifestations of the infection (Klein et al., 2019).
Previous research with mouse models and neurovirulent strains of virus from the coronavirus family have shown that microglial cells are infected by these viruses and, given their central position in the neurovascular niche and the possible routes of CNS entry of the viral particles, are important for spreading the infection to other cells in the CNS (Nakagaki et al., 2005; Lannes et al., 2017).
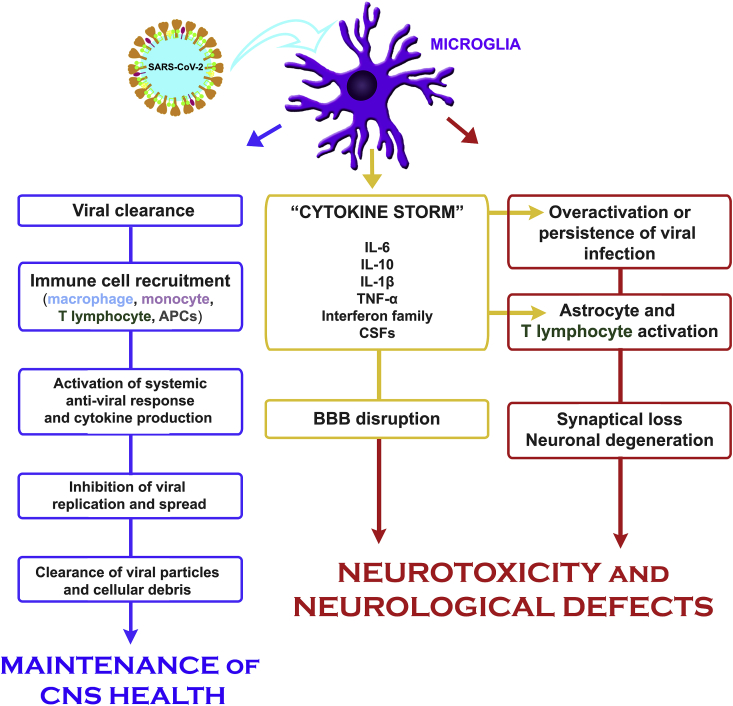
Nevertheless, studies with different viruses sometimes show antagonistic responses of microglial cells (Fig. 2). On one hand, microglial cells act in the innate immune response in the CNS and are essential for restricting viral replication and activating proper systemic anti-viral responses (Wheeler et al., 2018; Klein et al., 2019; Mangale et al., 2020). On the other hand, these cells can also have detrimental effects, indirectly by activating astrocyte-mediated neurotoxicity (Tremblay et al., 2011) and directly by inducing synapse loss (Klein et al., 2019; Trzeciak et al., 2019) (Fig. 1).
Fig. 2.
Microglia and SARS-CoV-2 infection. In less severe cases of COVID-19, microglial cells act as the innate immune response in the CNS and are essential for proper viral clearance and activation of proper systemic anti-viral response. This event involves recruitment and activation of peripheral monocytes/macrophages, enhancement of innate immunity response, increased production of cytokines and activation of both APCs in the periphery and viral-specific T cells, which untimely leads to control of viral spread. On the other hand, in more severe COVID-19 cases, overactivation of microglial cells can promote detrimental effects, indirectly, by activating astrocytes or T lymphocyte -mediated neurotoxicity and/or, directly, by inducing synapse loss, further contributing to neuronal degeneration in response to viral infection. In addition, the cytokine storm, largely produced by microglia, leads to increased BBB permeability and may be responsible for several of the neurological symptoms of COVID-19.
For example, in an in vivo murine model of infection with the neurotropic JHM strain of mouse hepatitis virus (JHMV), microglial cells are necessary for effective restriction of viral replication in the CNS. Furthermore, microglial depletion disturbs the host systemic T cell anti-viral response and leads to increased neurological manifestations (demyelination), morbidity and mortality (Wheeler et al., 2018; Mangale et al., 2020).
As shown by these studies, microglial cells are important not only for the intrinsic anti-viral response in the CNS tissue but also as powerful activators of the systemic anti-viral immunity in the case of neurotrophic coronavirus (Wheeler et al., 2018; Mangale et al., 2020). In this sense, in vivo microglial cell interactions with the immune system and with the microbiota have proven to be important in an effective anti-viral response, both on the systemic level and at the CNS (Brown et al., 2019). Both the presence of microglial cells, their intrinsic TLR4 signaling through the microbiota, and the crosstalk between microglia and T lymphocytes are important for the assembly of an effective response to neurotrophic coronavirus (Brown et al., 2019). Importantly, this study showed that isolate depletion of either microglial cells or the host microbiota was sufficient to impact both the local (CNS) or systemic response to coronavirus infection (Brown et al., 2019).
Furthermore, the crosstalk between microglial cells and the immune system outside of the CNS involves several steps, from the recruitment and activation of peripheral monocytes/macrophages to enhancement of the innate immune response (Templeton et al., 2008; Fekete et al., 2018) and to the activity of antigen presenting cells (APCs) and cytokine producers activating both APCs in the periphery and viral-specific T cells and controlling viral spread (Suzumura et al., 1988; Malone et al., 2006; Zimmermann et al., 2017; Wheeler et al., 2018; Brown et al., 2019; Mangale et al., 2020). Also, systemic responses are important to clear infected APC-like microglial cells (Herz et al., 2015).
However, activation of microglial cells following viral infection in the CNS can also have deleterious effects as we previously showed (Amorim et al., 2019; Salomão et al., 2020). Although both in physiological and pathological contexts, microglial cells are supposed to support neuronal development and survival and clear debris and dysfunctional synapses (Tremblay et al., 2011; Yanuck, 2019), when overactivated during viral or bacterial infection, those cells can directly induce neuronal damage (Chen et al., 2010; Klein et al., 2019; Mukherjee et al., 2019; Trzeciak et al., 2019). Also, microglial overactivation can lead to indirect neuronal death by activating astrocytes (Tremblay et al., 2011) or T lymphocytes (Zimmermann et al., 2017; Garber et al., 2019), further contributing to neuronal degeneration after viral infection (Fig. 2).
Finally, a key life-threatening clinical presentation of patients with severe COVID-19 is the cytokine storm (Coperchini et al., 2020; Huang et al., 2020; Mehta et al., 2020). This term was first employed to describe the life-threatening exaggerated and uncontrolled general activation of the immune system observed in severe forms of graft-versus-host disease (Tisoncik et al., 2012) and can be observed in several infectious diseases (Tisoncik et al., 2012). This phenomenon is characterized by the production of high levels of several inflammatory mediators (such as interferons, TNFα, interleukins (particularly IL-1β, IL-6 and IL-10), chemokines and colony-stimulating factors (CSFs) (Tisoncik et al., 2012; Coperchini et al., 2020), which are increased in patients with severe forms of COVID-19, and is capable of predicting prognosis (Henry et al., 2020; Huang et al., 2020; McGonagle et al., 2020). In this context, inhibiting IL-6 signaling using neutralizing monoclonal anti-IL-6 antibodies (Tocilizumab) is a strategy currently being tested in several clinical trials with severe COVID-19 patients and showing promising preliminary results (Xu et al., 2020).
In this sense, the cytokine storm could also be responsible for some of the neurological symptoms of COVID-19, generating BBB permeability and neuronal damage independent of direct viral infection. At this stage of the disease, viral replication is thought to be low or nonexistent, and the deleterious effects observed in several organs result from the high levels of cytokines and not from direct viral action.
Previous studies with animal models of CNS infection by coronavirus have shown that both astrocytes and microglial cells produce important levels of cytokines after infection (Savarin and Bergmann, 2018; Lavi and Cong, 2020). Nevertheless, the most important source of the cytokines involved in the cytokine storm in the CNS (as IL-6, interferons and IL-10) is microglial cells (Savarin and Bergmann, 2018; Lavi and Cong, 2020), and these cytokines produced by microglial cells are an important source of direct neuronal damage (Zimmermann et al., 2017) (Fig. 2). This also leads us to believe that microglial activation during cytokine storm could be involved in the neurological manifestations of SARS-CoV-2 infection, independent of the neurotropism of the virus itself.
Taken together, clinical evidence from COVID-19 patient cohorts and previous studies on other coronaviruses suggests a potential neurotropism of SARS-CoV-2. Also, based on the expression of ACE2 and other coreceptors necessary for viral infection, glial cells could be potential targets of SARS-CoV-2 and could help the spread of the virus in the CNS after transcribiform or hematogenic entry in the CNS. Furthermore, microglial cells could be especially relevant in several phases of the neuropathogenesis of SARS-CoV-2. In the less severe cases, they would be important for proper viral clearance and activation of systemic anti-viral responses. In the more severe cases, with important neurological signs and symptoms, microglial cells could be responsible for either direct neurological damage or for astrocyte- or T lymphocyte-mediated neurotoxicity (Fig. 1). In this sense, strategies aiming at those cells, especially for patients experiencing cytokine storm, could be a valuable tool for treating the neurological manifestations of COVID-19. Finally, further studies in preclinical models and long-term follow-up of SARS-CoV-2 infected patients will be important for understanding the possible deleterious long-term effects of excessive microglial activation after viral infection in the CNS.
2.2. Astrocytes as mediators of SARS-CoV-2 effects in the brain
Astrocytes, one of the largest glial cell populations in the brain, are responsible for controlling several steps of brain development and function in the formation and maturation of synapses (Diniz et al., 2012; Diniz et al., 2014a; Diniz et al., 2014b) and maintenance, pruning and remodeling of synapses in development, aging and diseases (Matias et al., 2016, 2019). Further, they control neurotransmitter release and uptake and production of trophic factors essential for neuronal differentiation and survival. In addition, astrocytes maintain intimate contact along with the vasculature, thus contributing to the formation and function of the BBB (da Silva et al., 2019) and the recently described glymphatic system, through which compounds such as glucose and amino acids are distributed, and the excess of toxic waste products is removed (Plog and Nedergaard, 2018).
Further, astrocytes play a key role in brain injury by triggering a response known as astrocyte reactivity. This is characterized by changes in the profile of astrocytes’ gene expression, leading to both morphological and functional changes that lead to the production of several pro- and anti-inflammatory signals. The extension of the astrocytic reaction may vary depending on the nature of the insult, such as stroke, neurodegenerative disorders, tumors, trauma, infection, ischemia and aging; size of the affected area; the intensity of BBB disruption; and the inflammatory response (Sofroniew, 2009; Matias et al., 2019).
Given the large number of functions performed by astrocytes in the healthy and injured brain, it is to be expected that these cells have a major impact on brain damage caused by SARS-CoV-2, either as direct targets of the virus or by controlling the inflammatory response to the virus and BBB rupture.
Recently, a biochemical analysis of the plasma from severe and moderate cases of COVID-19 patients demonstrated enhancement of biomarkers of CNS injury, such as GFAP (glial fibrillary acidic protein) and NfL (neurofilament light chain protein), suggesting astrocyte activation and neuronal injury in these patients (Kanberg et al., 2020). In the following paragraphs, we will discuss evidence that suggests how astrocytes can contribute to different aspects of SARS-CoV-2 damage: access and spread to the brain, persistence in the organism and inflammatory response.
In order to address SARS-CoV-2 effects on the brain, several studies have taken advantage of experimental models that use other members of the coronavirus family, such as the human respiratory coronavirus (HCV) (Lachance et al., 1998) and mouse hepatitis virus (MHV) (Savarin and Bergmann, 2018). Infection with these viruses triggers encephalitis and anosmia associated with IL-6 exacerbation in vivo, features also observed in SARS-CoV-2 infection (Lavi and Cong, 2020).
Although neurotropism has been shown for other coronavirus, so far, infection of glial cells by SARS-CoV-2 still awaits strong evidence, especially in vivo.
As previously discussed, entrance of SARS-CoV-2 into human cells is dependent on ACE2, which triggers the start of the infectious process (Butowt and Bilinska, 2020; Galougahi et al., 2020; Ou et al., 2020; Zhou et al., 2020). Recently, Chen and collaborators, using transcriptome databases, showed that most ACE2 was found in neuron and non-neuron cells in the human middle temporal gyrus and posterior cingulate cortex (Chen et al., 2020). From non-neuron cells, glial cells, mostly astrocytes and oligodendrocytes, were positive for ACE2 in the human brain, while microglia were positive only in the human middle temporal gyrus (Chen et al., 2020). The authors also found high expression of ACE2 in the olfactory bulb and ECs, supporting the hypothesis of access of SARS-CoV-2 into the brain by the olfactory bulb or BBB.
Astrocytes are described as the major CNS cell for coronavirus MHV (Cai et al., 2003, 2006) and human respiratory coronavirus (HCoV) (Pearson and Mims, 1985) persistence. Recently, however, by using SARS-CoV-2 infected iPSC-derived human brain organoids, Mesci and collaborators described that astrocytes were infected and showed a 4-fold increase in death, but no viral accumulation was observed. This suggests that although astrocytes might be targets of SARS-CoV-2, these cells may not replicate the virus (Mesci et al., 2020) as suggested for other coronavirus family members (Cai et al., 2003, 2006). Further, the authors also observed that excitatory synaptogenesis was highly impaired in SARS-CoV-2 infected organoids. Since astrocytes have key roles in synaptogenesis by secreting several synaptogenic molecules (Diniz et al., 2012; Diniz et al., 2014a), it is likely that SARS-CoV-2 may affect astrocyte synaptogenic properties (Fig. 1). However, this remains to be investigated.
Together, these results suggest that, according to cell-type distribution analysis of ACE2 in the human and mouse brain and glial response to murine and human coronavirus, SARS-CoV-2 might be capable of directly infecting several neural cells, including astrocytes, thus contributing to the neurological manifestations in COVID-19 patients (Fig. 1). Confirmation of glial infection by SARS-CoV-2 in patients, though, awaits further evidence, including in vivo demonstration.
As previously discussed, two pathways are suggested to allow access of SARS-CoV-2 to the CNS: from the nasal cavity or by crossing BBB.
Astrocyte endfeet form the glial surface as part of the BBB. In MHV infections, intracellular distribution of connexin 43 (Cx43) leads to loss of glial-pial gap junction communication and indicates a possible contribution to disruption of the BBB integrity, thus contributing to viral entrance (Basu et al., 2015; Bose et al., 2018). Furthermore, it is suggested that astrocytes may act as a conduit for the spread of MHV virus between neurons through their synapses (Sun and Perlman, 1995). Taken together, those observations suggest that through their role in BBB structure and function, astrocytes may contribute to coronavirus brain access and infection spread (Fig. 1).
Besides astrocyte contributions to SARS-CoV-2 infection and/or persistence, the main involvement of astrocytes in COVID-19 is certainly controlling brain inflammation (Fig. 1). Although astrocytes can present protective roles by producing anti-inflammatory and survival factors, these cells can also acquire a toxic reactive phenotype, thus producing cytokines that exacerbate injuries or presenting impaired/loss of functions, as we will discuss shortly (Diniz et al., 2017).
By using a sepsis model, our group previously demonstrated that LPS-stimulated microglia induced synaptic elimination, whereas activated astrocytes increased synapse numbers. Both cell types showed increased production of TNF-α and IL-6, and while astrocytes had increased production of TGF-β1, an anti-inflammatory and synaptogenic cytokine, microglia showed elevated secretion of interleukin-1β (IL-1β), a pro-inflammatory cytokine (Moraes et al., 2015).
Further, the profile of cytokines secreted by astrocytes also varies within different contexts of neurodegenerative diseases. We previously demonstrated that astrocyte functions are impaired in an Alzheimer’s disease model mainly due to decreased production of the anti-inflammatory and synaptogenic molecule, TGF- β1 (Diniz et al., 2017). Conversely, increased production of this cytokine by astrocytes is observed in a Parkinson’s disease experimental model (Diniz et al., 2019). These data shed light on the heterogeneity of glial responses to different insults.
In this context, production of cytokines by astrocytes seems to be a determinant of coronavirus neurovirulence and disease behavior. Highly neurovirulent MHV strains induce astrocyte release of pro-inflammatory cytokines, such as interleukin 12 (IL-12), p40, tumor necrosis factor alpha (TNF-α), interleukin 6 (IL-6), interleukin-15 (IL-15), and interleukin one beta (IL-1β) (Li et al., 2004). Further, spinal cord astrocytes also express those major cytokines, TNF-α, IL-6 and IL-1β, as well type 2 nitric oxide synthase (iNOs) and major histocompatibility complex (MHC) class I and II, due to the chronic demyelinating process in virus-infected mice (Sun et al., 1995). Also, HCV-OC43 induces upregulation of IL-6, TNF-α, and monocyte chemoattractant protein-1 (MCP-1) mRNA expression in an in vitro model with an astrocytic cell line, U-373MG (Edwards et al., 2000). Together, these findings suggest that coronavirus neurovirulence may depend on the inflammatory profile triggered by astrocyte activation in response to virus infection in the CNS (Fig. 1).
Recently, by using tissue cultures of microglia and clonal populations of astrocytes, Lavi and Cong found that microglia and type I astrocytes produced pro-inflammatory cytokines in response to MHV-A59 infection, including IL- α and β, IL-2, IL-15, IL-13, IL-17, IFN family, IL-6, and TNF-α (Lavi and Cong, 2020).
Notably, IL-6-related cytokine storm described in the COVID-19 pathology is highly associated with severity, criticality, viral load, and prognosis of patients (Magro, 2020). Severe inflammation based on release of IL-6 was associated with higher mortality in mice infected with SARS-CoV-1 and also with SARS-CoV-2 in COVID-19 patients (Magro, 2020). Based on this data, the use of a monoclonal antibody against IL-6 (Tocilizumab) has been proposed as a therapeutic alternative to COVID-19.
Taken together, data from MHV models and in vitro assays suggest that astrocytes may be a target and respond to SARS-CoV-2. It is likely that cross talk between astrocytes, microglia and ECs plays a key role in the control of the cytokine microenvironment and brain function in COVID-19. Whether astrocytic activation is beneficial or harmful to COVID-19 pathology is still a matter of investigation. Further, it remains to be determined if SARS-CoV-2 infection impairs astrocyte functions, such as synaptogenesis and neuronal trophic support. Lastly, it is essential to investigate if astrocyte infection is observed in COVID-19 patients and, if so, what are the consequences of the brain inflammatory response elicited by SARS-CoV-2 infection in the long term.
3. Concluding remarks and perspectives
Astrocytes and microglia play key roles in several events of brain function and in response to injury. As extensively discussed in this review, due to the broad participation of these cells in brain homeostasis and viral infections, it is likely that impaired functions of glial cells may directly or indirectly impact COVID-19 development.
Aging is considered a main risk factor for higher mortality in COVID-19 patients, although the correlation between SARS-CoV-2 and aging is still unclear (Hascup and Hascup, 2020). Age-dependent remodeling is observed in glial cells, which ultimately leads to impairment/loss of functional properties and may contribute in certain cases to the development of neurodegenerative diseases. Some of these alterations include the appearance of A1, a toxic astrocyte phenotype (Clarke et al., 2018); upregulation of genes that eliminate synapses and partially resemble reactive astrocytes (Boisvert et al., 2018); exacerbation of neuroinflammation; loss of proteostasis and reduction of stress response mechanisms and several other senescent markers (Verkhratsky and Nedergaard, 2018; Steardo et al., 2020).
Similarly, microglial cells also present several morphological and functional disabilities in the aged brain such as reduced phagocytic capacity; increased ROS and pro-inflammatory cytokine production (Koellhoffer et al., 2017); loss of dendritic branching and reduced motility (Damani et al., 2011) and senescence-related changes such as increased DNA and mitochondrial damage and telomere shortening (von Bernhardi et al., 2015; Angelova and Brown, 2019).
Further, as consequence of the aging process, inflammaging, a process characterized by an increase of systemic cytokine levels, namely IL-1β, IL-6, and TNF-α, and the senescence-associated secretory phenotype (SASP) is started (Akbar and Gilroy, 2020; Mauvais-Jarvis, 2020). All these changes result in a phenotype of aged astrocytes and microglial cells which not only lose their ‘normal’ neuroprotective role, but they are also more prone to induce neurodegeneration and neurotoxicity (Dilger and Johnson, 2008; Norden and Godbout, 2013; Lana et al., 2016). Although this event has not been clearly demonstrated in elderly COVID-19 patients, it is well known that exaggerated release of proinflammatory cytokines causes an amplified neuroinflammation and constitutes the main trigger of systemic symptoms and neurological impairment in COVID-19 patients (Hascup and Hascup, 2020; Mao et al., 2020; Montalvan et al., 2020).
Since glial cells present a molecular signature during aging, it would be of interest to investigate if this signature contributes to the higher vulnerability of the elderly in COVID-19. Interestingly, a database analysis revealed that SARS-CoV-2 proteins interact with human proteins associated with several aging-related processes such as vesicle trafficking, lipid modifications, RNA processing and regulation, ubiquitin ligases, and mitochondrial activity (Gordon et al., 2020). Several of these pathways are also associated with neurodegenerative diseases such as Alzheimer’s and Parkinson’s (Lippi et al., 2020). Thus, this data together with the fact that glial cell dysfunctions are highly associated with aging and neurodegenerative diseases (Diniz et al., 2017, 2019) highlight the need to consider the long-term consequences of glial activation and neuroinflammation triggered by SARS-CoV-2. Interestingly, recently, Viel and colleagues showed that low-dose of lithium suppresses IL-6 and reduces SASP (senescence-associated secretory phenotype) in senescent human astrocytes, suggesting that is a potential therapeutic strategy to COVID-19 in elderly patients (Viel et al., 2020).
Further, several recent studies showed that astrocytes and microglia are very heterogeneous populations of cells both in the healthy brain as well as in response to different insults and upon aging (Soreq et al., 2017; Boisvert et al., 2018; Buosi et al., 2018; Masuda et al., 2020). The underlying mechanisms of their activation, cellular interplays and the impact of regional glial heterogeneity are still a matter of discussion. Emerging data have correlated glial diversity to brain region-specific susceptibility to aging and neurodegenerative diseases (Soreq et al., 2017; Angelova and Brown, 2019). Whether glial heterogeneity and diversity in the CNS contribute to the distinct vulnerability of different brain regions to SARS-CoV-2 remains to be established.
Whether the involvement of glial cells in COVID-19 is directly due to their infection by SARS-CoV-2, thus impairing their regular biological functions, or indirectly, by controlling neuroinflammation, BBB integrity, and virus spread, remains to be investigated. Based on other neurotropic viruses and neurodegenerative diseases where glial involvement is relevant, SARS-CoV-2 likely has direct and indirect effects on glia that play a role in COVID-19. Whether glial activation is beneficial or harmful to the brain in COVID-19 pathology is still a matter of investigation. Thus, there are still many uncertainties and open questions to be addressed, listed in Box 1. Addressing these questions will certainly not only provide a better understanding of glial involvement in SARS-CoV-2 infection but ultimately may contribute to developing glia-based therapeutic strategies for the treatment of COVD-19.
Table 1.
The main goal of this review is to highlight the need to consider glial involvement in the progression of COVID-19 and potentially include astrocytes and microglia as mediators of the neurological damage triggered by SARS-CoV-2. We would like to shed light on the possibility of glial cells as targets for drug development to treat the neurological manifestations of COVID-19.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgements
Work in the laboratories of the authors was supported by grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (FCAG, FRSL), Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) (FCAG, FRSL, MVP), European Society of Cardiology (ESC) Basic Research Fellowship (LHMG) and Ministério da Saúde (MS-Decit) (FCAG, GV).
References
- Akbar A.N., Gilroy D.W. Aging immunity may exacerbate COVID-19. Science. 2020;369(6501):256–257. doi: 10.1126/science.abb0762. [DOI] [PubMed] [Google Scholar]
- Alam S.B., Willows S., Kulka M., Sandhu J.K. Sever acute respiratory syndrome coronavirus-2 may be an underappreciated pathogen of the central nervous system. Eur. J. Neurol. 2020 doi: 10.1111/ene.14442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Algahtani H., Subahi A., Shirah B. Neurological complications of Middle East respiratory syndrome coronavirus: a report of two cases and review of the literature. Case Rep. Neurol. Med. 2016:3502683. doi: 10.1155/2016/3502683. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alirezaei M., Kiosses W.B., Fox H.S. Decreased neuronal autophagy in HIV dementia: a mechanism of indirect neurotoxicity. Autophagy. 2008;4(7):963–966. doi: 10.4161/auto.6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves-Leon S.V., Lima M.D.R., Nunes P.C.G., Chimelli L.M.C., Rabelo K., Nogueira R.M.R., de Bruycker-Nogueira F., de Azeredo E.L., Bahia P.R., Rueda Lopes F.C., Marcondes de Souza J.P.B., Fontes-Dantas F.L., Paes M.V., Lemos E.R., Santos F.B. Zika virus found in brain tissue of a multiple sclerosis patient undergoing an acute disseminated encephalomyelitis-like episode. Mult. Scler. 2019;25(3):427–430. doi: 10.1177/1352458518781992. [DOI] [PubMed] [Google Scholar]
- Amorim J.F.S., Azevedo A.S., Costa S.M., Trindade G.F., Basilio-de-Oliveira C.A., Goncalves A.J.S., Salomao N.G., Rabelo K., Amaral R., Geraldo L.H.M., Lima F.R.S., Mohana-Borges R., Paes M.V., Alves A.M.B. Dengue infection in mice inoculated by the intracerebral route: neuropathological effects and identification of target cells for virus replication. Sci. Rep. 2019;9(1):17926. doi: 10.1038/s41598-019-54474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelova D.M., Brown D.R. Microglia and the aging brain: are senescent microglia the key to neurodegeneration? J. Neurochem. 2019;151(6):676–688. doi: 10.1111/jnc.14860. [DOI] [PubMed] [Google Scholar]
- Arbour N., Talbot P.J. Persistent infection of neural cell lines by human coronaviruses. Adv. Exp. Med. Biol. 1998;440:575–581. doi: 10.1007/978-1-4615-5331-1_75. [DOI] [PubMed] [Google Scholar]
- Arbour N., Day R., Newcombe J., Talbot P.J. Neuroinvasion by human respiratory coronaviruses. J. Virol. 2000;74(19):8913–8921. doi: 10.1128/jvi.74.19.8913-8921.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthold S.W., Smith A.L. Viremic dissemination of mouse hepatitis virus-JHM following intranasal inoculation of mice. Arch. Virol. 1992;122(1–2):35–44. doi: 10.1007/BF01321116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu R., Banerjee K., Bose A., Das Sarma J. Mouse hepatitis virus infection remodels connexin43-mediated gap junction intercellular communication in vitro and in vivo. J. Virol. 2015;90(5):2586–2599. doi: 10.1128/JVI.02420-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisvert M.M., Erikson G.A., Shokhirev M.N., Allen N.J. The aging astrocyte transcriptome from multiple regions of the mouse brain. Cell Rep. 2018;22(1):269–285. doi: 10.1016/j.celrep.2017.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose A., Basu R., Maulik M., Das Sarma J. Loss of cx43-mediated functional gap junction communication in meningeal fibroblasts following mouse hepatitis virus infection. Mol. Neurobiol. 2018;55(8):6558–6571. doi: 10.1007/s12035-017-0861-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brann D.H., Tsukahara T., Weinreb C., Logan D.W., Datta S.R. 2020. Non-neural expression of SARS-CoV-2 entry genes in the olfactory epithelium suggests mechanisms underlying anosmia in COVID-19 patients. bioRXiv THE PREPRINTS SERVER FOR BIOLOGY. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D.G., Soto R., Yandamuri S., Stone C., Dickey L., Gomes-Neto J.C., Pastuzyn E.D., Bell R., Petersen C., Buhrke K., Fujinami R.S., O’Connell R.M., Stephens W.Z., Shepherd J.D., Lane T.E., Round J.L. The microbiota protects from viral-induced neurologic damage through microglia-intrinsic TLR signaling. Elife. 2019;8 doi: 10.7554/eLife.47117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buosi A.S., Matias I., Araujo A.P.B., Batista C., Gomes F.C.A. Heterogeneity in synaptogenic profile of astrocytes from different brain regions. Mol. Neurobiol. 2018;55(1):751–762. doi: 10.1007/s12035-016-0343-z. [DOI] [PubMed] [Google Scholar]
- Butowt R., Bilinska K. SARS-CoV-2: olfaction, brain infection, and the urgent need for clinical samples allowing earlier virus detection. ACS Chem. Neurosci. 2020;11(9):1200–1203. doi: 10.1021/acschemneuro.0c00172. [DOI] [PubMed] [Google Scholar]
- Cai Y., Liu Y., Yu D., Zhang X. Down-regulation of transcription of the proapoptotic gene BNip3 in cultured astrocytes by murine coronavirus infection. Virology. 2003;316(1):104–115. doi: 10.1016/j.virol.2003.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y., Liu Y., Zhang X. Induction of transcription factor Egr-1 gene expression in astrocytoma cells by Murine coronavirus infection. Virology. 2006;355(2):152–163. doi: 10.1016/j.virol.2006.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon-Pelaez M.A., Velandia-Romero M.L., Bastidas-Legarda L.Y., Beltran E.O., Camacho-Ortega S.J., Castellanos J.E. Dengue virus infection of blood-brain barrier cells: consequences of severe disease. Front. Microbiol. 2019;10:1435. doi: 10.3389/fmicb.2019.01435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.F., Yuan S., Kok K.H., To K.K., Chu H., Yang J., Xing F., Liu J., Yip C.C., Poon R.W., Tsoi H.W., Lo S.K., Chan K.H., Poon V.K., Chan W.M., Ip J.D., Cai J.P., Cheng V.C., Chen H., Hui C.K., Yuen K.Y. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.J., Ou Y.C., Lin S.Y., Raung S.L., Liao S.L., Lai C.Y., Chen S.Y., Chen J.H. Glial activation involvement in neuronal death by Japanese encephalitis virus infection. J. Gen. Virol. 2010;91(Pt 4):1028–1037. doi: 10.1099/vir.0.013565-0. [DOI] [PubMed] [Google Scholar]
- Chen R., Wang K., Yu J., Howard D., French L., Chen Z., Wen C.V., Xu Z. 2020. The spatial and cell-type distribution of SARS-CoV-2 receptor ACE2 in human and mouse brain. bioRXiv THE PREPRINTS SERVER FOR BIOLOGY. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chigr F., Merzouki M., Najimi M. Comment on "The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J. Med. Virol. 2020 doi: 10.1002/jmv.25960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill M.J., Wesselingh S.L., Cowley D., Pardo C.A., McArthur J.C., Brew B.J., Gorry P.R. Extensive astrocyte infection is prominent in human immunodeficiency virus-associated dementia. Ann. Neurol. 2009;66(2):253–258. doi: 10.1002/ana.21697. [DOI] [PubMed] [Google Scholar]
- Ciotti M., Angeletti S., Minieri M., Giovannetti M., Benvenuto D., Pascarella S., Sagnelli C., Bianchi M., Bernardini S., Ciccozzi M. COVID-19 outbreak: an overview. Chemotherapy. 2020:1–9. doi: 10.1159/000507423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke L.E., Liddelow S.A., Chakraborty C., Munch A.E., Heiman M., Barres B.A. Normal aging induces A1-like astrocyte reactivity. Proc. Natl. Acad. Sci. U. S. A. 2018;115(8):E1896–E1905. doi: 10.1073/pnas.1800165115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consales C.A., Bolzan V.L. Rabies review: immunopathology, clinical aspects and treatment. J. Venom. Anim. Toxins Incl. Trop. Dis. 2007;13(1):34. doi: 10.1590/S1678-91992007000100002. [DOI] [Google Scholar]
- Coperchini F., Chiovato L., Croce L., Magri F., Rotondi M. The cytokine storm in COVID-19: an overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020;53:25–32. doi: 10.1016/j.cytogfr.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva S.M., Campos G.D., Gomes F.C.A., Stipursky J. Radial glia-endothelial cells’ bidirectional interactions control vascular maturation and astrocyte differentiation: impact for blood-brain barrier formation. Curr. Neurovascular Res. 2019;16(4):291–300. doi: 10.2174/1567202616666191014120156. [DOI] [PubMed] [Google Scholar]
- Damani M.R., Zhao L., Fontainhas A.M., Amaral J., Fariss R.N., Wong W.T. Age-related alterations in the dynamic behavior of microglia. Aging Cell. 2011;10(2):263–276. doi: 10.1111/j.1474-9726.2010.00660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilger R.N., Johnson R.W. Aging, microglial cell priming, and the discordant central inflammatory response to signals from the peripheral immune system. J. Leukoc. Biol. 2008;84(4):932–939. doi: 10.1189/jlb.0208108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz L.P., Almeida J.C., Tortelli V., Vargas Lopes C., Setti-Perdigao P., Stipursky J., Kahn S.A., Romao L.F., de Miranda J., Alves-Leon S.V., de Souza J.M., Castro N.G., Panizzutti R., Gomes F.C. Astrocyte-induced synaptogenesis is mediated by transforming growth factor beta signaling through modulation of D-serine levels in cerebral cortex neurons. J. Biol. Chem. 2012;287(49):41432–41445. doi: 10.1074/jbc.M112.380824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz L.P., Tortelli V., Garcia M.N., Araujo A.P., Melo H.M., Silva G.S., Felice F.G., Alves-Leon S.V., Souza J.M., Romao L.F., Castro N.G., Gomes F.C. Astrocyte transforming growth factor beta 1 promotes inhibitory synapse formation via CaM kinase II signaling. Glia. 2014 a;62(12):1917–1931. doi: 10.1002/glia.22713. [DOI] [PubMed] [Google Scholar]
- Diniz B.S., Reynolds C.F., 3rd, Begley A., Dew M.A., Anderson S.J., Lotrich F., Erickson K.I., Lopez O., Aizenstein H., Sibille E.L., Butters M.A. Brain-derived neurotrophic factor levels in late-life depression and comorbid mild cognitive impairment: a longitudinal study. J. Psychiatr. Res. 2014 b;49:96–101. doi: 10.1016/j.jpsychires.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz L.P., Tortelli V., Matias I., Morgado J., Bergamo Araujo A.P., Melo H.M., Seixas da Silva G.S., Alves-Leon S.V., de Souza J.M., Ferreira S.T., De Felice F.G., Gomes F.C.A. Astrocyte transforming growth factor beta 1 protects synapses against abeta oligomers in alzheimer’s disease model. J. Neurosci. 2017;37(28):6797–6809. doi: 10.1523/JNEUROSCI.3351-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz L.P., Matias I., Siqueira M., Stipursky J., Gomes F.C.A. Astrocytes and the TGF-beta1 pathway in the healthy and diseased brain: a double-edged sword. Mol. Neurobiol. 2019;56(7):4653–4679. doi: 10.1007/s12035-018-1396-y. [DOI] [PubMed] [Google Scholar]
- do Amaral R.F., Geraldo L.H.M., Einicker-Lamas M., TCLS E.S., Mendes F., Lima F.R.S. Microglial lysophosphatidic acid promotes glioblastoma proliferation and migration via LPA1 receptor. J. Neurochem. 2020 doi: 10.1111/jnc.15097. [DOI] [PubMed] [Google Scholar]
- Dos Santos M.F., D’evalle S., Aran V., Capra D., Roque N.R., Coelho-Aguiar J.M., Spohr T.C.L.S., Subilhaga J.G., Moura-Neto V. Neuromechanisms of SARS-CoV-2: a review. Front. Neuroanat. 2020;14(37):12. doi: 10.3389/fnana.2020.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube M., Le Coupanec A., Wong A.H.M., Rini J.M., Desforges M., Talbot P.J. Axonal transport enables neuron-to-neuron propagation of human coronavirus OC43. J. Virol. 2018;92(17) doi: 10.1128/JVI.00404-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J.A., Denis F., Talbot P.J. Activation of glial cells by human coronavirus OC43 infection. J. Neuroimmunol. 2000;108(1–2):73–81. doi: 10.1016/s0165-5728(00)00266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete R., Cserep C., Lenart N., Toth K., Orsolits B., Martinecz B., Mehes E., Szabo B., Nemeth V., Gonci B., Sperlagh B., Boldogkoi Z., Kittel A., Baranyi M., Ferenczi S., Kovacs K., Szalay G., Rozsa B., Webb C., Kovacs G.G., Hortobagyi T., West B.L., Kornyei Z., Denes A. Microglia control the spread of neurotropic virus infection via P2Y12 signalling and recruit monocytes through P2Y12-independent mechanisms. Acta Neuropathol. 2018;136(3):461–482. doi: 10.1007/s00401-018-1885-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodoulian L., Tuberosa J., Rossier D., Landis B.N., Carleton A., Rodriguez I. 2020. SARS-CoV-2 receptor and entry genes are expressed by sustentacular cells in the human olfactory neuroepithelium. bioRXiv THE PREPRINTS SERVER FOR BIOLOGY. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotuhi M., Mian A., Meysami S., Raji C.A. Neurobiology of COVID-19. J. Alzheim Dis. 2020 doi: 10.3233/JAD-200581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galougahi M.K., Ghorbani J., Bakhshayeshkaram M., Naeini A.S., Haseli S. Olfactory bulb magnetic resonance imaging in SARS-CoV-2-induced anosmia: the first report. Acad. Radiol. 2020 doi: 10.1016/j.acra.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber C., Soung A., Vollmer L.L., Kanmogne M., Last A., Brown J., Klein R.S. T cells promote microglia-mediated synaptic elimination and cognitive dysfunction during recovery from neuropathogenic flaviviruses. Nat. Neurosci. 2019;22(8):1276–1288. doi: 10.1038/s41593-019-0427-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomelli A., Pezzati L., Conti F., Bernacchia D., Siano M., Oreni L., Rusconi S., Gervasoni C., Ridolfo A.L., Rizzardini G., Antinori S., Galli M. Self-reported olfactory and taste disorders in SARS-CoV-2 patients: a cross-sectional study. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F., Greter M., Leboeuf M., Nandi S., See P., Gokhan S., Mehler M.F., Conway S.J., Ng L.G., Stanley E.R., Samokhvalov I.M., Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330(6005):841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D.E., Jang G.M., Bouhaddou M., Xu J., Obernier K., White K.M., O’Meara M.J., Rezelj V.V., Guo J.Z., Swaney D.L., Tummino T.A., Huettenhain R., Kaake R.M., Richards A.L., Tutuncuoglu B., Foussard H., Batra J., Haas K., Modak M., Kim M., Haas P., Polacco B.J., Braberg H., Fabius J.M., Eckhardt M., Soucheray M., Bennett M.J., Cakir M., McGregor M.J., Li Q., Meyer B., Roesch F., Vallet T., Mac Kain A., Miorin L., Moreno E., Naing Z.Z.C., Zhou Y., Peng S., Shi Y., Zhang Z., Shen W., Kirby I.T., Melnyk J.E., Chorba J.S., Lou K., Dai S.A., Barrio-Hernandez I., Memon D., Hernandez-Armenta C., Lyu J., Mathy C.J.P., Perica T., Pilla K.B., Ganesan S.J., Saltzberg D.J., Rakesh R., Liu X., Rosenthal S.B., Calviello L., Venkataramanan S., Liboy-Lugo J., Lin Y., Huang X.P., Liu Y., Wankowicz S.A., Bohn M., Safari M., Ugur F.S., Koh C., Savar N.S., Tran Q.D., Shengjuler D., Fletcher S.J., O’Neal M.C., Cai Y., Chang J.C.J., Broadhurst D.J., Klippsten S., Sharp P.P., Wenzell N.A., Kuzuoglu D., Wang H.Y., Trenker R., Young J.M., Cavero D.A., Hiatt J., Roth T.L., Rathore U., Subramanian A., Noack J., Hubert M., Stroud R.M., Frankel A.D., Rosenberg O.S., Verba K.A., Agard D.A., Ott M., Emerman M., Jura N., von Zastrow M., Verdin E., Ashworth A., Schwartz O., d’Enfert C., Mukherjee S., Jacobson M., Malik H.S., Fujimori D.G., Ideker T., Craik C.S., Floor S.N., Fraser J.S., Gross J.D., Sali A., Roth B.L., Ruggero D., Taunton J., Kortemme T., Beltrao P., Vignuzzi M., Garcia-Sastre A., Shokat K.M., Shoichet B.K., Krogan N.J. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020 doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gras G., Chretien F., Vallat-Decouvelaere A.V., Le Pavec G., Porcheray F., Bossuet C., Leone C., Mialocq P., Dereuddre-Bosquet N., Clayette P., Le Grand R., Creminon C., Dormont D., Rimaniol A.C., Gray F. Regulated expression of sodium-dependent glutamate transporters and synthetase: a neuroprotective role for activated microglia and macrophages in HIV infection? Brain Pathol. 2003;13(2):211–222. doi: 10.1111/j.1750-3639.2003.tb00020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascup E.R., Hascup K.N. Does SARS-CoV-2 infection cause chronic neurological complications? Geroscience. 2020 doi: 10.1007/s11357-020-00207-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms J., Kremer S., Merdji H., Clere-Jehl R., Schenck M., Kummerlen C., Collange O., Boulay C., Fafi-Kremer S., Ohana M., Anheim M., Meziani F. Neurologic features in severe SARS-CoV-2 infection. N. Engl. J. Med. 2020;382(23):2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry B.M., de Oliveira M.H.S., Benoit S., Plebani M., Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin. Chem. Lab. Med. 2020 doi: 10.1515/cclm-2020-0369. [DOI] [PubMed] [Google Scholar]
- Herz J., Johnson K.R., McGavern D.B. Therapeutic antiviral T cells noncytopathically clear persistently infected microglia after conversion into antigen-presenting cells. J. Exp. Med. 2015;212(8):1153–1169. doi: 10.1084/jem.20142047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussmann K.L., Samuel M.A., Kim K.S., Diamond M.S., Fredericksen B.L. Differential replication of pathogenic and nonpathogenic strains of West Nile virus within astrocytes. J. Virol. 2013;87(5):2814–2822. doi: 10.1128/JVI.02577-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiyama T., Ito Y., Kubota M., Yamazaki T., Nakamura K., Furukawa S. Serum and cerebrospinal fluid levels of cytokines in acute encephalopathy associated with human herpesvirus-6 infection. Brain Dev. 2009;31(10):731–738. doi: 10.1016/j.braindev.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Jacomy H., Talbot P.J. Vacuolating encephalitis in mice infected by human coronavirus OC43. Virology. 2003;315(1):20–33. doi: 10.1016/s0042-6822(03)00323-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X., Lian J.S., Hu J.H., Gao J., Zheng L., Zhang Y.M., Hao S.R., Jia H.Y., Cai H., Zhang X.L., Yu G.D., Xu K.J., Wang X.Y., Gu J.Q., Zhang S.Y., Ye C.Y., Jin C.L., Lu Y.F., Yu X., Yu X.P., Huang J.R., Xu K.L., Ni Q., Yu C.B., Zhu B., Li Y.T., Liu J., Zhao H., Zhang X., Yu L., Guo Y.Z., Su J.W., Tao J.J., Lang G.J., Wu X.X., Wu W.R., Qv T.T., Xiang D.R., Yi P., Shi D., Chen Y., Ren Y., Qiu Y.Q., Li L.J., Sheng J., Yang Y. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69(6):1002–1009. doi: 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanberg N., Ashton N.J., Andersson L.M., Yilmaz A., Lindh M., Nilsson S., Price R.W., Blennow K., Zetterberg H., Gisslen M. Neurochemical evidence of astrocytic and neuronal injury commonly found in COVID-19. Neurology. 2020 doi: 10.1212/WNL.0000000000010111. [DOI] [PubMed] [Google Scholar]
- Khan B.A., Perkins A.J., Prasad N.K., Shekhar A., Campbell N.L., Gao S., Wang S., Khan S.H., Marcantonio E.R., Twigg H.L., 3rd, Boustani M.A. Biomarkers of delirium duration and delirium severity in the ICU. Crit. Care Med. 2020;48(3):353–361. doi: 10.1097/CCM.0000000000004139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.E., Heo J.H., Kim H.O., Song S.H., Park S.S., Park T.H., Ahn J.Y., Kim M.K., Choi J.P. Neurological complications during treatment of Middle East respiratory syndrome. J. Clin. Neurol. 2017;13(3):227–233. doi: 10.3988/jcn.2017.13.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R.S., Garber C., Funk K.E., Salimi H., Soung A., Kanmogne M., Manivasagam S., Agner S., Cain M. Neuroinflammation during RNA viral infections. Annu. Rev. Immunol. 2019;37:73–95. doi: 10.1146/annurev-immunol-042718-041417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koellhoffer E.C., McCullough L.D., Ritzel R.M. Old maids: aging and its impact on microglia function. Int. J. Mol. Sci. 2017;18(4) doi: 10.3390/ijms18040769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotfis K., Williams Roberson S., Wilson J., Pun B., Ely E.W., Jezowska I., Jezierska M., Dabrowski W. COVID-19: what do we need to know about ICU delirium during the SARS-CoV-2 pandemic? Anaesthesiol. Intensive Ther. 2020;52(2):132–138. doi: 10.5114/ait.2020.95164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotfis K., Williams Roberson S., Wilson J.E., Dabrowski W., Pun B.T., Ely E.W. COVID-19: ICU delirium management during SARS-CoV-2 pandemic. Crit. Care. 2020;24(1):176. doi: 10.1186/s13054-020-02882-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachance C., Arbour N., Cashman N.R., Talbot P.J. Involvement of aminopeptidase N (CD13) in infection of human neural cells by human coronavirus 229E. J. Virol. 1998;72(8):6511–6519. doi: 10.1128/jvi.72.8.6511-6519.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lana D., Iovino L., Nosi D., Wenk G.L., Giovannini M.G. The neuron-astrocyte-microglia triad involvement in neuroinflammaging mechanisms in the CA3 hippocampus of memory-impaired aged rats. Exp. Gerontol. 2016;83:71–88. doi: 10.1016/j.exger.2016.07.011. [DOI] [PubMed] [Google Scholar]
- Lannes N., Neuhaus V., Scolari B., Kharoubi-Hess S., Walch M., Summerfield A., Filgueira L. Interactions of human microglia cells with Japanese encephalitis virus. Virol. J. 2017;14(1):8. doi: 10.1186/s12985-016-0675-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavi E., Cong L. Type I astrocytes and microglia induce a cytokine response in an encephalitic murine coronavirus infection. Exp. Mol. Pathol. 2020:104474. doi: 10.1016/j.yexmp.2020.104474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechien J.R., Chiesa-Estomba C.M., De Siati D.R., Horoi M., Le Bon S.D., Rodriguez A., Dequanter D., Blecic S., El Afia F., Distinguin L., Chekkoury-Idrissi Y., Hans S., Delgado I.L., Calvo-Henriquez C., Lavigne P., Falanga C., Barillari M.R., Cammaroto G., Khalife M., Leich P., Souchay C., Rossi C., Journe F., Hsieh J., Edjlali M., Carlier R., Ris L., Lovato A., De Filippis C., Coppee F., Fakhry N., Ayad T., Saussez S. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur. Arch. Oto-Rhino-Laryngol. 2020 doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledur P.F., Karmirian K., Pedrosa C., Souza L.R.Q., Assis-de-Lemos G., Martins T.M., Ferreira J., de Azevedo Reis G.F., Silva E.S., Silva D., Salerno J.A., Ornelas I.M., Devalle S., Madeiro da Costa R.F., Goto-Silva L., Higa L.M., Melo A., Tanuri A., Chimelli L., Murata M.M., Garcez P.P., Filippi-Chiela E.C., Galina A., Borges H.L., Rehen S.K. Zika virus infection leads to mitochondrial failure, oxidative stress and DNA damage in human iPSC-derived astrocytes. Sci. Rep. 2020;10(1):1218. doi: 10.1038/s41598-020-57914-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Fu L., Gonzales D.M., Lavi E. Coronavirus neurovirulence correlates with the ability of the virus to induce proinflammatory cytokine signals from astrocytes and microglia. J. Virol. 2004;78(7):3398–3406. doi: 10.1128/jvi.78.7.3398-3406.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K., Wohlford-Lenane C., Perlman S., Zhao J., Jewell A.K., Reznikov L.R., Gibson-Corley K.N., Meyerholz D.K., McCray P.B., Jr. Middle East respiratory syndrome coronavirus causes multiple organ damage and lethal disease in mice transgenic for human dipeptidyl peptidase 4. J. Infect. Dis. 2016;213(5):712–722. doi: 10.1093/infdis/jiv499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Xue Q., Xu X. Involvement of the nervous system in SARS-CoV-2 infection. Neurotox. Res. 2020;38(1):1–7. doi: 10.1007/s12640-020-00219-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Zai J., Wang X., Li Y. Potential of large "first generation" human-to-human transmission of 2019-nCoV. J. Med. Virol. 2020;92(4):448–454. doi: 10.1002/jmv.25693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippi A., Domingues R., Setz C., Outeiro T.F., Krisko A. SARS-CoV-2: at the crossroad between aging and neurodegeneration. Mov. Disord. 2020;35(5):716–720. doi: 10.1002/mds.28084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magro G. SARS-CoV-2 and COVID-19: is interleukin-6 (IL-6) the ’culprit lesion’ of ARDS onset? What is there besides Tocilizumab? SGP130Fc. Cytokine X: 100029. 2020 doi: 10.1016/j.cytox.2020.100029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone K.E., Ramakrishna C., Gonzalez J.M., Stohlman S.A., Bergmann C.C. Glia expression of MHC during CNS infection by neurotropic coronavirus. Adv. Exp. Med. Biol. 2006;581:543–546. doi: 10.1007/978-0-387-33012-9_99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangale V., Syage A.R., Ekiz H.A., Skinner D.D., Cheng Y., Stone C.L., Brown R.M., O’Connell R.M., Green K.N., Lane T.E. Microglia influence host defense, disease, and repair following murine coronavirus infection of the central nervous system. Glia. 2020 doi: 10.1002/glia.23844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., Chang J., Hong C., Zhou Y., Wang D., Miao X., Li Y., Hu B. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in wuhan, China. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda T., Sankowski R., Staszewski O., Prinz M. Microglia heterogeneity in the single-cell era. Cell Rep. 2020;30(5):1271–1281. doi: 10.1016/j.celrep.2020.01.010. [DOI] [PubMed] [Google Scholar]
- Matias I., Buosi A.S., Gomes F.C. Functions of flavonoids in the central nervous system: astrocytes as targets for natural compounds. Neurochem. Int. 2016;95:85–91. doi: 10.1016/j.neuint.2016.01.009. [DOI] [PubMed] [Google Scholar]
- Matias I., Morgado J., Gomes F.C.A. Astrocyte heterogeneity: impact to brain aging and disease. Front. Aging Neurosci. 2019;11:59. doi: 10.3389/fnagi.2019.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauvais-Jarvis F. Aging, male sex, obesity, and metabolic inflammation create the perfect storm for COVID-19. Diabetes. 2020 doi: 10.2337/dbi19-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCray P.B., Jr., Pewe L., Wohlford-Lenane C., Hickey M., Manzel L., Shi L., Netland J., Jia H.P., Halabi C., Sigmund C.D., Meyerholz D.K., Kirby P., Look D.C., Perlman S. Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus. J. Virol. 2007;81(2):813–821. doi: 10.1128/JVI.02012-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGonagle D., Sharif K., O’Regan A., Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun. Rev. 2020;19(6):102537. doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J., Hlh U.K., Across Speciality Collaboration COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesci I., Macia A., Saleh A., Martin-Sancho L., Yin X., Snethlage C., Avansini S., Chanda S.K., Muotri A. 2020. Sofosbuvir protects human brain organoids against SARS-CoV-2. bioRXiv THE PREPRINTS SERVER FOR BIOLOGY. [DOI] [Google Scholar]
- Mlakar J., Korva M., Tul N., Popovic M., Poljsak-Prijatelj M., Mraz J., Kolenc M., Resman Rus K., Vesnaver Vipotnik T., Fabjan Vodusek V., Vizjak A., Pizem J., Petrovec M., Avsic Zupanc T. Zika virus associated with microcephaly. N. Engl. J. Med. 2016;374(10):951–958. doi: 10.1056/NEJMoa1600651. [DOI] [PubMed] [Google Scholar]
- Montalvan V., Lee J., Bueso T., De Toledo J., Rivas K. Neurological manifestations of COVID-19 and other coronavirus infections: a systematic review. Clin. Neurol. Neurosurg. 2020;194:105921. doi: 10.1016/j.clineuro.2020.105921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes C.A., Santos G., de Sampaio e Spohr T.C., D’Avila J.C., Lima F.R., Benjamim C.F., Bozza F.A., Gomes F.C. Activated microglia-induced deficits in excitatory synapses through IL-1beta: implications for cognitive impairment in sepsis. Mol. Neurobiol. 2015;52(1):653–663. doi: 10.1007/s12035-014-8868-5. [DOI] [PubMed] [Google Scholar]
- Mukherjee S., Akbar I., Kumari B., Vrati S., Basu A., Banerjee A. Japanese Encephalitis Virus-induced let-7a/b interacted with the NOTCH-TLR7 pathway in microglia and facilitated neuronal death via caspase activation. J. Neurochem. 2019;149(4):518–534. doi: 10.1111/jnc.14645. [DOI] [PubMed] [Google Scholar]
- Nakagaki K., Nakagaki K., Taguchi F. Receptor-independent spread of a highly neurotropic murine coronavirus JHMV strain from initially infected microglial cells in mixed neural cultures. J. Virol. 2005;79(10):6102–6110. doi: 10.1128/JVI.79.10.6102-6110.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netland J., Meyerholz D.K., Moore S., Cassell M., Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J. Virol. 2008;82(15):7264–7275. doi: 10.1128/JVI.00737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden D.M., Godbout J.P. Review: microglia of the aged brain: primed to be activated and resistant to regulation. Neuropathol. Appl. Neurobiol. 2013;39(1):19–34. doi: 10.1111/j.1365-2990.2012.01306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes P., Nogueira R., Coelho J., Rodrigues F., Salomao N., Jose C., de Carvalho J., Rabelo K., de Azeredo E., Basilio-de-Oliveira R., Basilio-de-Oliveira C., Dos Santos F., Paes M. A stillborn multiple organs’ investigation from a maternal DENV-4 infection: histopathological and inflammatory mediators characterization. Viruses. 2019;11(4) doi: 10.3390/v11040319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (OPAS), P.-A. O. o. H Folha informativa – COVID-19 (doença causada pelo novo coronavírus) 2020. https://www.paho.org/bra/index.php?option=com_content&view=article&id=6101:covid19&Itemid=875 Retrieved August 3, 2020, from.
- Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., Guo L., Guo R., Chen T., Hu J., Xiang Z., Mu Z., Chen X., Chen J., Hu K., Jin Q., Wang J., Qian Z. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020;11(1):1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson J., Mims C.A. Differential susceptibility of cultured neural cells to the human coronavirus OC43. J. Virol. 1985;53(3):1016–1019. doi: 10.1128/jvi.53.3.1016-1019.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plog B.A., Nedergaard M. The glymphatic system in central nervous system health and disease: past, present, and future. Annu. Rev. Pathol. 2018;13:379–394. doi: 10.1146/annurev-pathol-051217-111018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabelo K., Souza L.J., Salomao N.G., Oliveira E.R.A., Sentinelli L.P., Lacerda M.S., Saraquino P.B., Rosman F.C., Basilio-de-Oliveira R., Carvalho J.J., Paes M.V. Placental inflammation and fetal injury in a rare zika case associated with guillain-barre syndrome and abortion. Front. Microbiol. 2018;9:1018. doi: 10.3389/fmicb.2018.01018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos C., Sanchez G., Pando R.H., Baquera J., Hernandez D., Mota J., Ramos J., Flores A., Llausas E. Dengue virus in the brain of a fatal case of hemorrhagic dengue fever. J. Neurovirol. 1998;4(4):465–468. doi: 10.3109/13550289809114548. [DOI] [PubMed] [Google Scholar]
- Ray N.B., Power C., Lynch W.P., Ewalt L.C., Lodmell D.L. Rabies viruses infect primary cultures of murine, feline, and human microglia and astrocytes. Arch. Virol. 1997;142(5):1011–1019. doi: 10.1007/s007050050136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J.P., Chesney E., Oliver D., Pollak T.A., McGuire P., Fusar-Poli P., Zandi M.S., Lewis G., David A.S. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatr. 2020;7(7):611–627. doi: 10.1016/S2215-0366(20)30203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomão N., Rabelo K., Basilio-de-Oliveira C., Basilio-de-Oliveira R., Geraldo L., Lima F., Dos Santos F., Nuovo G., Oliveira E.R.A., Paes M. Fatal dengue cases reveal brain injury and viral replication in brain-resident cells associated with the local production of pro-inflammatory mediators. Viruses. 2020;12(6) doi: 10.3390/v12060603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankowski R., Mader S., Valdes-Ferrer S.I. Systemic inflammation and the brain: novel roles of genetic, molecular, and environmental cues as drivers of neurodegeneration. Front. Cell. Neurosci. 2015;9:28. doi: 10.3389/fncel.2015.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savarin C., Bergmann C.C. Fine tuning the cytokine storm by IFN and IL-10 following neurotropic coronavirus encephalomyelitis. Front. Immunol. 2018;9:3022. doi: 10.3389/fimmu.2018.03022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew M.V. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32(12):638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soreq L., Consortium U.K.B.E., North American Brain Expression C., Rose J., Soreq E., Hardy J., Trabzuni D., Cookson M.R., Smith C., Ryten M., Patani R., Ule J. Major shifts in glial regional identity are a transcriptional hallmark of human brain aging. Cell Rep. 2017;18(2):557–570. doi: 10.1016/j.celrep.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel M., Schneider K., Weber F., Weidmann M., Hufert F.T. Interaction of severe acute respiratory syndrome-associated coronavirus with dendritic cells. J. Gen. Virol. 2006;87(Pt 7):1953–1960. doi: 10.1099/vir.0.81624-0. [DOI] [PubMed] [Google Scholar]
- Steardo L., Steardo L., Jr., Zorec R., Verkhratsky A. Neuroinfection may contribute to pathophysiology and clinical manifestations of COVID-19. Acta Physiol. (Oxf) 2020;229(3) doi: 10.1111/apha.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanik M., Formanova P., Bily T., Vancova M., Eyer L., Palus M., Salat J., Braconi C.T., Zanotto P.M.A., Gould E.A., Ruzek D. Characterisation of Zika virus infection in primary human astrocytes. BMC Neurosci. 2018;19(1):5. doi: 10.1186/s12868-018-0407-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su S., Wong G., Shi W., Liu J., Lai A.C.K., Zhou J., Liu W., Bi Y., Gao G.F. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24(6):490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun N., Perlman S. Spread of a neurotropic coronavirus to spinal cord white matter via neurons and astrocytes. J. Virol. 1995;69(2):633–641. doi: 10.1128/jvi.69.2.633-641.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun N., Grzybicki D., Castro R.F., Murphy S., Perlman S. Activation of astrocytes in the spinal cord of mice chronically infected with a neurotropic coronavirus. Virology. 1995;213(2):482–493. doi: 10.1006/viro.1995.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzumura A., Lavi E., Bhat S., Murasko D., Weiss S.R., Silberberg D.H. Induction of glial cell MHC antigen expression in neurotropic coronavirus infections. Characterization of the H-2-inducing soluble factor elaborated by infected brain cells. J. Immunol. 1988;140(6):2068–2072. [PubMed] [Google Scholar]
- Templeton S.P., Kim T.S., O’Malley K., Perlman S. Maturation and localization of macrophages and microglia during infection with a neurotropic murine coronavirus. Brain Pathol. 2008;18(1):40–51. doi: 10.1111/j.1750-3639.2007.00098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisoncik J.R., Korth M.J., Simmons C.P., Farrar J., Martin T.R., Katze M.G. Into the eye of the cytokine storm. Microbiol. Mol. Biol. Rev. 2012;76(1):16–32. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay M.E., Stevens B., Sierra A., Wake H., Bessis A., Nimmerjahn A. The role of microglia in the healthy brain. J. Neurosci. 2011;31(45):16064–16069. doi: 10.1523/JNEUROSCI.4158-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trzeciak A., Lerman Y.V., Kim T.H., Kim M.R., Mai N., Halterman M.W., Kim M. Long-term microgliosis driven by acute systemic inflammation. J. Immunol. 2019;203(11):2979–2989. doi: 10.4049/jimmunol.1900317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai T.T., Chen C.L., Lin Y.S., Chang C.P., Tsai C.C., Cheng Y.L., Huang C.C., Ho C.J., Lee Y.C., Lin L.T., Jhan M.K., Lin C.F. Microglia retard dengue virus-induced acute viral encephalitis. Sci. Rep. 2016;6:27670. doi: 10.1038/srep27670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruta R., Oda Y. A clinical perspective of sepsis-associated delirium. J. Intensive Care. 2016;4:18. doi: 10.1186/s40560-016-0145-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaira L.A., Salzano G., Deiana G., De Riu G. Anosmia and ageusia: common findings in COVID-19 patients. Laryngoscope. 2020;130(7):1787. doi: 10.1002/lary.28692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gool W.A., van de Beek D., Eikelenboom P. Systemic infection and delirium: when cytokines and acetylcholine collide. Lancet. 2010;375(9716):773–775. doi: 10.1016/S0140-6736(09)61158-2. [DOI] [PubMed] [Google Scholar]
- van Marle G., Antony J., Ostermann H., Dunham C., Hunt T., Halliday W., Maingat F., Urbanowski M.D., Hobman T., Peeling J., Power C. West Nile virus-induced neuroinflammation: glial infection and capsid protein-mediated neurovirulence. J. Virol. 2007;81(20):10933–10949. doi: 10.1128/JVI.02422-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., Mehra M.R., Schuepbach R.A., Ruschitzka F., Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velandia-Romero M.L., Calderon-Pelaez M.A., Castellanos J.E. In vitro infection with dengue virus induces changes in the structure and function of the mouse brain endothelium. PloS One. 2016;11(6) doi: 10.1371/journal.pone.0157786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A., Nedergaard M. Physiology of astroglia. Physiol. Rev. 2018;98(1):239–389. doi: 10.1152/physrev.00042.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viel T., Chinta S., Rane A., Chamoli M., Buck H., Andersen J. Microdose lithium reduces cellular senescence in human astrocytes - a potential pharmacotherapy for COVID-19? Aging (Albany NY) 2020;12(11):10035–10040. doi: 10.18632/aging.103449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bernhardi R., Eugenin-von Bernhardi L., Eugenin J. Microglial cell dysregulation in brain aging and neurodegeneration. Front. Aging Neurosci. 2015;7:124. doi: 10.3389/fnagi.2015.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Xu W., Hu G., Xia S., Sun Z., Liu Z., Xie Y., Zhang R., Jiang S., Lu L. SARS-CoV-2 infects T lymphocytes through its spike protein-mediated membrane fusion. Cell. Mol. Immunol. 2020 doi: 10.1038/s41423-020-0424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S.R., Leibowitz J.L. Coronavirus pathogenesis. Adv. Virus Res. 2011;81:85–164. doi: 10.1016/B978-0-12-385885-6.00009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler D.L., Sariol A., Meyerholz D.K., Perlman S. Microglia are required for protection against lethal coronavirus encephalitis in mice. J. Clin. Invest. 2018;128(3):931–943. doi: 10.1172/JCI97229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Xu X., Chen Z., Duan J., Hashimoto K., Yang L., Liu C., Yang C. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav. Immun. 2020 doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Zhong S., Liu J., Li L., Li Y., Wu X., Li Z., Deng P., Zhang J., Zhong N., Ding Y., Jiang Y. Detection of severe acute respiratory syndrome coronavirus in the brain: potential role of the chemokine mig in pathogenesis. Clin. Infect. Dis. 2005;41(8):1089–1096. doi: 10.1086/444461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Han M., Li T., Sun W., Wang D., Fu B., Zhou Y., Zheng X., Yang Y., Li X., Zhang X., Pan A., Wei H. Effective treatment of severe COVID-19 patients with tocilizumab. Proc. Natl. Acad. Sci. U. S. A. 2020;117(20):10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y., Yang Y., Wang F., Ren H., Zhang S., Shi X., Yu X., Dong K. Clinical characteristics and outcomes of patients with severe covid-19 with diabetes. BMJ Open Diabetes Res. Care. 2020;8(1) doi: 10.1136/bmjdrc-2020-001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanuck S.F. Microglial phagocytosis of neurons: diminishing neuronal loss in traumatic, infectious, inflammatory, and autoimmune CNS disorders. Front. Psychiatr. 2019;10:712. doi: 10.3389/fpsyt.2019.00712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R.D., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann J., Hafezi W., Dockhorn A., Lorentzen E.U., Krauthausen M., Getts D.R., Muller M., Kuhn J.E., King N.J.C. Enhanced viral clearance and reduced leukocyte infiltration in experimental herpes encephalitis after intranasal infection of CXCR3-deficient mice. J. Neurovirol. 2017;23(3):394–403. doi: 10.1007/s13365-016-0508-6. [DOI] [PubMed] [Google Scholar]