Abstract
ADHD is among the many syndromes in the psychiatric nosology for which etiological signal and clinical prediction are weak. Reducing phenotypic and mechanistic heterogeneity should be useful to arrive at stronger etiological and clinical prediction signals. We highlight key conceptual and methodological issues, highlighting the role of dimensional features aligned with RDoC and cognitive, personality, and temperament theory as well as neurobiology. We describe several avenues of work in this area, utilizing different statistical, computational, and machine learning approaches to resolve heterogeneity in ADHD. We offer methodological and conceptual recommendations. Methodologically, we propose that an integrated approach utilizing theory and advanced computational logic to address targeted questions, with consideration of developmental context, can render the heterogeneity problem tractable for ADHD. Conceptually, we conclude that the field is on the cusp of justifying an emotionally dysregulated sub-profile in ADHD that may be useful for clinical prediction and treatment testing. Cognitive profiles, while more nascent, may be useful for clinical prediction and treatment assignment in different ways depending on developmental stage. Targeting these psychological profiles for neurobiological and etiological study to capture different pathophysiological routes remains a near-term opportunity. Subtypes are likely to be multifactorial, cut across multiple dimensions, and be dependent for their ultimate selection on the research or clinical outcomes of interest. In this context parallel profiles based on cognition, emotion, and specific neural signatures appear to be on the horizon, each with somewhat different utilities. Efforts to integrate such cross-cutting profiles within a conceptual dysregulation framework are well underway.
Keywords: ADHD, heterogeneity, subtypes, Emotion, cognition, machine learning
Introduction
ADHD exhibits complex yet clinically relevant and unresolved heterogeneity (1, 2). It ranges widely in severity with the mild end almost indistinguishable from typical childhood behavior. Comorbidity is common, outcomes and treatment response are hard to predict, and etiological studies suggest multiple routes to the disorder. ADHD has no universal biological correlate. Because outcomes are unpredictable and often severe, understanding different profiles and pathways to account for outcomes and mechanisms is urgently important. ADHD thus exemplifies many of the issues about within-syndrome heterogeneity that challenge etiological research in the DSM at large (3). Heterogeneity requires better resolution for identifying stronger mechanistic signals (4). We recap critical conceptual contexts then selectively review avenues of potential progress in ADHD.
Conceptual background
The DSM is reflects a deep tradition, but is not the only psychopathology framework available. A long-standing empirical tradition in child psychopathology seeks data-driven, empirical solutions to organize psychopathology via consideration of behavioral or problem dimensions. This body of work, over hundreds of studies, began to be developed well before the DSM-III was published in 1980 (5). That tradition identifies two correlated domains of psychopathology (externalizing and internalizing). Structured hierarchically under these domains are less than a dozen non-orthogonal problem dimensions (6). These can be utilized to form an array of profile solutions corresponding to clinical phenomenon of interest (e.g., dysregulation (7)), although the feature set will be primarily descriptive and likely insufficient for questions related to processes or mechanisms. Whereas it is encouraging that the empirical dimensions map to a certain extent onto the DSM, results are not isomorphic with regard to heterogeneity. For example, ADHD is a single dimension in some dimensional psychopathology models, but is bi-dimensional (based on factor analytic studies) in DSM. Hybrid dimensional and categorical models of the nosology, such as the HiTop proposal, (8) are now gaining traction. These typically classified ADHD, or at least hyperactivity-impulsivity, under behavior or impulse disorders due to its strong correlation with oppositional, conduct, and aggressive behaviors (inattention sometimes “loads” equally with mood or anxiety conditions). Yet often overlooked in large multi-disorder correlational studies are ADHD’s equally compelling correlations with neurodevelopmental problems such as learning disabilities, intellectual development, motor development, and autism spectrum disorder. These are influential in developmental theory of ADHD (9). Thus, consideration both of this empirical tradition and of a developmental perspective are advisable for capturing ADHD heterogeneity (10).
Second, other approaches beyond the “clinical problem” domain are important to recall. For over a century, psychologists have sought to link personality and temperament traits “inward” to biology and “outward” to psychopathology. Online Supplement part E provides background on that tradition and an explanation of personality and temperament traits (for review see (11)). Higher order traits are similar in children and adults. For example, the classic three-to-five higher order traits in adult personality include extraversion/surgency, neuroticism, and conscientiousness/constraint (12). One child temperament model (13) highlights (a) surgency with sub-features of activity level and positive-affect, similar to extraversion; (b) negative affectivity, meaning prone to fearfulness, worry, and anger, similar to neuroticism; and (c) effortful control, the ability to over-ride an immediate impulse, similar to conscientiousness or constraint. Personality traits in adults are, of course, more elaborated and differentiated than in children. Progression of traits and their integration from infant-to-toddler-to-child-to-adolescent-to-adult remains a critical area for developmental science (13) and for ADHD. However, this developmental understanding is increasingly promising, bolstered by updated theories about neurobiology (14) (for more background, see (11, 13, 15, 16) and Online Supplement Part E). Psychopathology is hypothesized to relate to particular trait configurations (11, 13).
A modern incarnation of this logic is the RDoC proposal from NIMH (17, 18). It does not directly address heterogeneity or the idea of multiple mechanisms in an existing disorder. However, its logic can facilitate work in this area just as the personality-temperament traditions have done. RDoC proposes to link dimensions of psychological functioning such as negative emotional valence, working memory, and other transdiagnostic features, with neurobiological systems, and then to psychopathology, to advance knowledge about psychopathology. It thus equally elevates psychological and neural phenomena, and highlights dimensional measures.
RDoC, trait theory, and dimensional measures of psychopathology all offer a critically useful perspective that warrants integration to solve the heterogeneity problem in ADHD. Yet the challenges cannot be overlooked: (a) Dimensions require conversion into decision algorithms or cut points for clinical decision making; this is tractable if we retain clarity about goals (e.g., clinical versus biological discovery). (b) The structure of relations among the dimensions is missed in studying a single dimension (as RDoC can lead to), sacrificing potentially essential information about trait combinations in clinical syndromes. Configural trait models can address this. (c) A one-to-one correspondence of neurobiological or psychological function to psychopathology is unlikely (15, 19, 20).
Third, it is crucial to recognize the importance of integrating data driven and conceptual efforts and assumptions. Alone, mathematical methods cannot “reveal” nature’s true categorical structure in a multidimensional space (21, 22). Nearly unlimited ways are available to divide a multivariate sample. Assumptions, beliefs, theory, and goals are necessary in order to choose how to do so and to interpret results. See Online Supplement Part A and Part B and Figures S1 and S2 for more explanation, including the role of sample size. In short, if we fail to recognize the critical role of our beliefs, assumptions, and goals, we may be misled by a given data “solution” or may misapply our conclusions to clinical situations or populations for which they are inappropriate. Therefore, we highlight the value of an integrated approach to data-driven studies in which conceptual models, assumptions, and goals are explicitly (rather than implicitly) combined with data-driven approaches. This logic is summarized in Figure 1 (also see Online Supplement Part B).
Figure 1: Recommended Workflow for examining heterogeneity in ADHD and similar conditions.
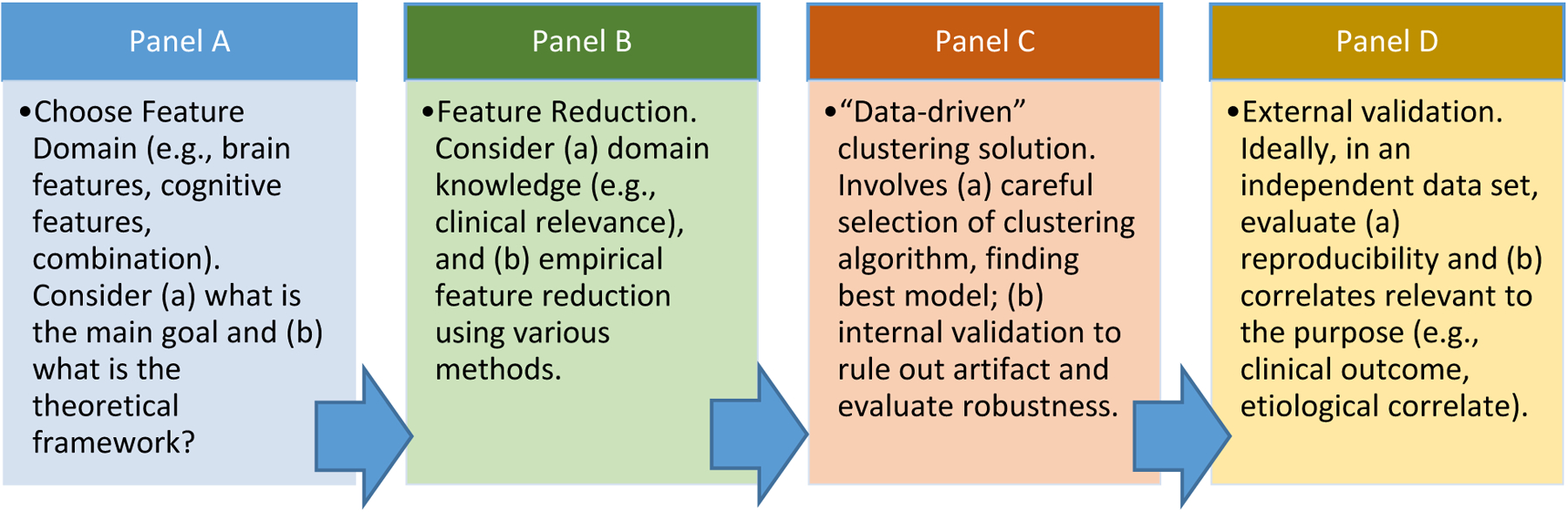
Schematic or conceptual workflow guiding our view of how data-driven and machine learning approaches are best utilized in psychopathology research. The problem of heterogeneity appears tractable using approaches that include (a) careful feature selection based on both theoretical and empirical considerations, (b) exploration and novel discovery that targets specific questions of interest, followed by (c) internal or statistical validation (various robustness indexes, simulation studies), and (d) external validation in independent datasets. For example, external validation might include further exploration of clinical utility (e.g., predictive accuracy differential) or enhanced etiological signal. Because the research space is multi-level (one can study physiology, psychology, behavior) and multi-dimensional, and because the intended purpose can vary (clinical prediction, discovery of etiology, etc.), different clustering solutions will be valid and correct in different research contexts. The role of conceptual purpose, theory, and assumptions about the nature of the phenomenon should be explicit. Then, feature domain can be chosen. Feature reduction can then proceed with the end goal in mind, as in the functional random forest (128) described in the Online Supplement (part B). Then, the clustering solution can be explored using unsupervised (discovery-based) approaches. Internal and external validation then follow. Internal validation evaluates the likelihood of artifact in the data, and external validation evaluates the usefulness of the solution. Different solutions can be pitted against one another competitively to determine which is most useful for a goal. The approach can assume a non-zero number of groups within statistical and machine-learning contexts, with a specific, hypothesis driven, targeted set of features (21). Doing so strengthens feature selection, goal identification, and interpretation of solutions to answer targeted questions.
This review highlights approaches using measures of the child’s psychology and neurobiology (e.g., cognition, emotion, brain imaging). Space does not permit consideration of equally important literatures on environmental/contextual variation, genetic variation, pharmacogenomics, and G × E interaction (23–27) in ADHD (for additional review see (28, 29)).
ADHD heterogeneity using DSM ADHD symptoms
The DSM provides three well known subtypes/presentations. An older conceptual or clinical idea is that there is a sub-population associated with ADHD that is inattentive (and maybe impulsive) but hypoactive. Introduced in DSM-III (APA, 1980), and formalized in DSM-IV, the concept has now shifted more heavily to the concept of sluggish-cognitive tempo (30)—which may represent a group only partially overlapping with ADHD.
A substantial literature has sought to improve on the DSM presentations via revised clustering of the 18 DSM symptoms—typically using latent class/profile analysis (a type of maximum likelihood analysis). This approach identifies classes of ADHD roughly corresponding to DSM inattentive and combined presentations, but further divided by the severity within those domains (e.g., mild inattentive, severe inattentive), and these results tend to replicate reasonably well (31, 32). However, individual profiles change over development in a manner that exceeds expectations from normative behavioral change, at least when rating scales are relied on for assessment (10, 32). For example, hyperactivity normatively declines during late childhood and adolescence, suggesting that we could expect a child’s ADHD presentation to develop from combined to inattentive. Yet the opposite frequently occurs as well. Overall, it is not clear that these solutions improve on existing utilization of symptom severity in regard to clinical evaluation or for mapping neurobiology (33, 34).
A potentially promising approach uses item response theory (35) or versions of a bifactor model to account for shared and distinct underlying dimensions within ADHD symptoms. The bifactor model is beginning to replicate, albeit with developmental variation. This model appears to improve the account of ADHD heterogeneity in relation to comorbidity and clinical prediction (36–38). (For a criticism of the bifactor approach, see (39)).
Another approach applies network modeling (40, 41) to interrogate symptom structure (see online Supplement Part C for explanation and contrast with bifactor model). Rather than assume a latent disorder or trait causes observed symptoms of psychopathology, it assumes that the confluence of symptoms is the disorder (40). In other words, symptoms lead to one another and accumulate until the syndrome is in place. A network is made up of nodes and edges. Nodes are the features of interest (e.g., symptoms of ADHD). Edges are the relations among them (e.g., correlations) (40, 41). In the case of ADHD, the network approach appears to enable further traction related to developmental change and population specificity of syndrome structure and heterogeneity. Martel et al (42) reported that symptoms differentiated with age, but a small core set of symptoms drove the others. Somewhat similar findings were reported by Silk et al (43). This approach may help identify unique pathways to syndrome emergence via one symptom leading to another set of symptoms (44), perhaps in different ways for different subgroups. Such insights if confirmed could open avenues for more targeted intervention.
Overall, contemporary approaches to refining symptom structure are poised to bring useful improvements in characterizing ADHD heterogeneity that are relevant to clinical assessment within the DSM context. They have not been much used to relate symptoms to cognitive, affective, or neural mechanisms, but this work is on the horizon.
ADHD heterogeneity via broader dimensional feature set: cognition and temperament
Even more promising may be approaches using neurobiologically and psychometrically informed dimensional measures of psychological or biological functioning. This approach relates to RDoC, goes outside of the DSM symptom lists, and can emphasize cognitive or emotional domains as well as neurobiological measures.
Cognitive heterogeneity in ADHD.
A key set of conceptual proposals for ADHD mechanism has involved neuropsychological or cognitive differentiation within ADHD, via different kinds of attentional breakdowns. This approach dates back half a century. It has included a long-standing interest in frontal lobe-related executive functions, reward or reinforcement, and arousal systems (45, 46). Barkley (46–48) sought to integrate multiple neuropsychological and cognitive domains related to ADHD, emphasizing response inhibition and temporal information processing. In the 1980s and 1990s, Sergeant, van der Meere, and colleagues advocated an energetic state perspective in which arousal or else activation is a key moderator of performance (49–51). Long standing ideas about reinforcement or reward-based mechanisms in ADHD have remained of interest (52–56), motivated by sophisticated neurobiological theories (57–60) and treatment utility (61). Sonuga Barke (62) introduced the idea of a dual-pathway to ADHD that might integrate different accounts. Multi-pathway approaches have been further articulated to attempt to integrate the picture, building on the idea that only some children have a problem with a given function (such as executive functioning) while others have a different dysfunction. Table 1 summarizes many of these ideas.
Table 1: A selection of proposals for addressing ADHD mechanistic heterogeneity.
The table includes illustrative conceptual and empirical citations for reference. As it shows, the proposals though many, fall into a few basic themes related to clinical symptom profiles, cognition/executive function, motivation/reward function, emotional regulation/temperament, and neurobiological proposals involving clues from MRI, EEG, and other methods. Cognitive proposals are the most numerous, emphasizing functions such as working memory, processing speed, inhibition, timing, reward discounting, and delay aversion. Etiological proposals are numerous; one is included one here as an exemplar.
| Perspective | General proposal for heterogeneity | Example papers |
|---|---|---|
| Clinical | Sluggish cognitive tempo | (30, 140–142) |
| Clinical | Non-hyperactive/hypoactive | DSM-III |
| Clinical | Predominantly hyp, inat, comb | DSM-IV |
| Neurobiological-cognition | Executive dysfunction subtype | (65) |
| Neurobiological-cognition | inhibition, working memory, time processing | (69, 143) |
| Neurobiological-cognition | EF, time processing | (69, 70, 143) |
| Neurobiological-motivation | EF vs delay aversion | (62, 71) |
| Neurobiological-motivation | EF, Reward response/discounting | (59, 60, 66, 144) |
| Emotion-regulation/temperament | Callous-unemotional | (145) |
| Emotion-regulation/temperament | Emotional dysregulation | (82, 146) |
| Emotion-regulation/temperament | Irritability | (81, 94) |
| Emotion-regulation/temperament | Surgent, Negative affect, cog control | (83) |
| Neurobiology | Differential cortical-subcortical engagement | (69, 143) |
| Neurobiology | Differential rates of neurodevelopment | (131) |
| Etiological | Perinatal exposure vs genetic or GxE | (147) |
We attempted to evaluate these ideas by integrating theory and method (Figure 1) (63). We administered a broad range of measures of executive function, arousal, reaction time, and other functions selected to reflect commonly studied domains in ADHD. After this conceptual feature selection, we turned to a data-driven approach. We introduced the use of a clustering method from graph theory called community detection (64) (see Online Supplement, Part D for explanation) to identify profiles in ADHD (63). Internal validation provided strong evidence of >0 clusters or profiles. The resulting profiles suggested a subgroup with top-down or executive function problems (65), as well as an additional subgroup with bottom up (e.g., signal detection response) weakness (66). Notably, similar profiles were seen in both ADHD and typically developing populations (63). Such nested context may be necessary to isolate deficits specific to ADHD. This work partially reproduced in an independent sample (67). That effort yielded a result consistent with the same principles: more than 0 but fewer than 5 profiles justified by internal validation, and distinctions along conceptually top-down versus bottom-up functions. However, the specific profiles differed.
Vaidya and colleagues (68) examined executive functions as assessed via rating scales in children with ADHD, autism, and typically developing controls. First, they used community detection to propose profiles and applied a support-vector-machine algorithm to predict group membership in a confirmation sample. Then, they sought biological validation with task-based functional MRI in a subset of the youth. Although the measurement method was quite different (ratings versus laboratory tests), results provided again support multiple cognitive profiles in ADHD (also see (62, 66, 69–71)). That study illustrates the potential and importance in combining discovery and validation and the potential for clinical prediction.
Longitudinal studies evaluating whether cognitive development modulates ADHD outcome are emerging as another view of heterogeneity. For example, a cohort of preschoolers with hyperactivity followed by Halperin and colleagues yielded several contributions. In one finding (72) of 214 preschoolers followed over time, ADHD childhood status varied in relation to whether hyperactive preschoolers were impaired focally in neuropsychological measures of attention/executive function or globally in a range of neuropsychological abilities. In a related finding (73), cognitive control was related to inattention symptoms, but stimulus-driven processes related to hyperactivity over time. In an older sample from childhood into early adolescence followed by our group (74), working memory change was associated with symptom recovery over time, while response inhibition and reward processing changes were not. (But see (75) for a discrepant set of findings).
Overall, these various findings illustrate that adding neurobiologically informed measures of cognition can generate novel insights into heterogeneity. Findings are broadly but not specifically reproducible, in that studies consistently find useful cognitive sub-profiles in ADHD but not necessarily the same sub-profiles across studies. They also confirm clinical observation for an initial argument of at least one executive dysfunction subgroup in ADHD (65). Several interpretive challenges remain, however, regarding generalizability, reproducibility, and specific feature selection and external validation relevant to a given goal (for more discussion see (76).
Further progress may be possible using computational decompositions of cognitive performance. For example, we have used signal detection theory and diffusion models to isolate cognitive processes related to ascending noradrenergic systems (77, 78). The results, combined with use of latent variable modeling to increase measurement reliability, have helped clarify and amplify the locus of genetic effects in ADHD via cognition— i.e., polygenic effects on ADHD proceed via working memory and arousal, but not other cognitive functions (79). In another study, these computational phenotypes helped isolate shared and unique cognitive features for the disorders that inform neurobiological study (80).
Emotional and trait heterogeneity in ADHD.
Another long-standing conceptual focus concerns emotional and/or anger dysregulation as an important feature and source of heterogeneity in ADHD (81). This relates to a proposal by Barkley and colleagues (82) and others, and a related proposal using a different conceptual framework by Nigg and colleagues (11, 83) and others that ADHD reflects variation in etiological pathways across temperament or emotion regulation systems (see below). Several groups have worked on ADHD in relation to temperament. For example, Halperin and colleagues (84) have effectively pursued the hypothesis that negative emotionality and anger dysregulation influences development of ADHD via disruption of executive functioning, from preschool into the early school years. In a 9 year prospective sample, Miller and colleagues reported links between infant temperament and childhood ADHD that were moderated by sex and parenting style, suggesting subtyping via contextual moderation and temperament (85, 86). Auerbach and colleagues have also linked early temperament to subsequent ADHD with potential contextual and genetic moderation (87, 88). Sullivan and colleagues also noted potential early markers of ADHD risk (89).
Formal mathematical efforts to discover emotion-related sub-profiles were slower to emerge, however. Therefore, drawing upon related developmental theory about ADHD (83, 87) we evaluated trait ratings (online Supplement Part E). We stress that using trait ratings is only one approach to the much broader domain of emotion regulation (see (90)). In initial, relatively constrained linear models, traits were strongly correlated with ADHD but not isomorphic with it, suggesting traits brought new, useful information (91, 92).
Subsequently, we applied the Rothbart et al. perspective (13) (Online Supplement Part E) to feature selection. We identified three reproducible profiles in ADHD samples (which did not appear in a non-ADHD sample) (a) normative temperament ratings (and by implication, normative emotional regulation), (b) high surgency, extraversion, or sensation seeking, (c) high anger and other negative affect (93–98). (See Online Supplement Part #E for details of the measure and profiles). These profiles converge with the developmental proposal by Nigg and colleagues (83).The identification of a group with high negative affect converges with growing evidence of the importance of irritability and emotional lability in ADHD (99–101). Figure 2 illustrates that these profiles were quite stable over a 1 year period and moderately stable over two years; they were somewhat more stable than DSM profiles that rely on ratings data. Figure 3 illustrates these groups’ proportional breakdown in relation to corresponding DSM-5 profiles, showing that these are not isomorphic but that traits introduce new information.
Figure 2: Alluvial plot illustrating stability of temperament heterogeneity in ADHD.

This plot represents just under 400 children with ADHD at 3 times points (see (93, 94)). Time point 1 ages are 7–11, time point 2 are 8–12, and time point 3 are 9–13. The plot illustrates the proportion of children in each initial profile (by different colors), and then where they go at the next time point (by shading of the colors). Tracing the flows, the chart shows several points. (a) The Irritable and Surgent profile are highly stable over a 1 year follow (note the dark and light blue and the dark and light red from year 1 to year 2). However, (b) over a 2 year period, many of the Surgent profile convert to a mild profile, and a subgroup of the irritable, while remaining dysregulated, convert to a Surgent profile. The mild profile can become Surgent (usually, only temporarily, as show in the pale yellow path from mild to Surgent to mild) or Irritable (and then remain there, note steep pale yellow path up to and remaining at irritable). To summarize, only a small fraction of Irritable children transition to the emotionally-regulated group. In addition, once a child transitions to the Irritable group from Mild or Surgent, they often remain there at least in this age range. In contrast, the Surgent and mild groups are less stable, with significant transitions between these two groups across years, as well as some transitions into the Irritable group. We hypothesize that anger dysregulation may inform impairment in both dysregulated profiles.
Figure 3: DSM-5 presentations and proposed emotion regulation profiles are not the same.

Sunburst plot showing the relation among DSM baseline presentations and temperament-based baseline profiles. The inner (blue) ring shows the proportion of children in the ADHD sample in each temperament profile at baseline (ages 7–11). The outer (yellow) ring shows for the same children their DSM-5 assigned clinical profile at the same point in time. The mapping thus shows that the ADHD-combined type (pale yellow) is evenly divided between Surgent and Irritable profiles, but that all are in a dysregulated group. The Inattentive DSM profile (gold) is divided between irritable, Surgent, and mild profiles, exemplifying heterogeneity. The small number of hyperactive profile children are exclusively in the mild profile of temperament, underscoring the likelihood that this group may less severe of a group than the combined type with which it is often associated. Data from (93).
External validation with regard to both biological signal and clinical outcome was supportive. The emotionally dysregulated groups had higher genetic loadings for ADHD liability (102), distinct EEG profiles in (103), and in preliminary evidence distinct functional connectivity on MRI. Most important for our purposes, reasonably consistent evidence indicates that these profiles predict clinical outcomes over a 1–3 year period better than symptom severity, baseline comorbidity, impairment, ADHD subtypes, or ADHD symptom profiles and that they predict over and above other clinical information (93–95, 98). Children in the irritable profile were more than twice as likely as other groups to have onset of additional psychiatric disorder one year later.
Thus, approaches using emotion and temperament appear poised to converge on a useful reframing of heterogeneity that may relate to developmental outcome as well as particular aspects of neurobiology.
Starting with neurobiological dimensions instead of behavior to address ADHD
Until this point, we highlighted use of an expanded feature of either cognition or ratings of temperament to characterize heterogeneity in ADHD, in low-dimensional data sets. A further set of RDoC-compatible dimensions arise from direct measures of neurobiology that have strong empirical or theoretical links to psychological, mental, or behavioral constructs (but see (20) for cautions).
EEG subtypes within ADHD.
Within ADHD, electroencephalogram (EEG) features have received longstanding attention, mostly on frequency patterns in the resting state EEG. Early studies converged on two ADHD profiles characterized by relatively greater frontal activity in (a) slow (particularly theta) versus fast (particularly beta) frequency bands, interpreted as reflecting cortical under-arousal (104), and (b) a small but consistent group with relatively high beta activity initially interpreted as a cortical hyper-arousal profile (105). Although intriguing, this proposal lacked a theoretical interpretation, after the arousal-based interpretation of these frequency patterns was challenged, and clinical or concurrent validation was lacking (106).
In the largest and most carefully done EEG heterogeneity study of ADHD to date, Loo et al. (107) addressed clinical validation empirically in children with ADHD (n=620) and without ADHD (n=121). They identified five distinct EEG sub-profiles each with an elevation in one of the traditionally measured EEG frequency bands (e.g., high delta, high theta, etc.). Children with and without ADHD were distributed across all five profiles, raising again the possibility of nested variation similar to some cognitive profiling studies, including ours (108–112). The EEG-based groups differed in age, mandating more examination of developmental trends and potential population admixture. Profiles appeared psychologically meaningful: a group with elevations at lower frequency bands exhibited cognitive impairment, whereas those with elevations in higher frequencies (alpha, beta) exhibited emotional dysregulation. Loo et al.’s findings may converge with our own recent work suggesting EEG-measured alpha power distinguishes subgroups with ADHD with varying degree of negative emotionality (103).
Overall, EEG-based profiles hold promise, but the literature to date underscores both (a) the interpretive challenges associated with lack of strong theory, and (b) the utility of identifying the goal, such as clinical validation. Feature selection has also been limited; whether use of more high-dimensional EEG input features will be productive remains untested.
MRI subtypes within ADHD.
In a major review in 2012, Willcutt et al (10) noted that at that time there were minimal contemporary MRI studies of the ADHD DSM profiles. Now, several studies have attacked this classification problem, productively using machine learning methods (109, 113–116). More work in this area will be useful evaluating alternative sub-profiling schemes (see recent discussion in (29)). We focus here, however, on the clustering problem--on efforts to discover new and more informative neurobiological ‘types’ of ADHD. Recent reviews have highlighted the diversity of brain imaging findings associated with ADHD (117), underscoring the need for clarification of heterogeneity.
Efforts at an integrated conception have considered variation in the involvement of parallel frontal-subcortical-thalamic loops (118, 119), or the relative importance of top-down or bottom-up signaling (120). While many of these ideas are focused on function, heterogeneity can be adjudicated via studies of brain structure as well. For example, variation in rate of development of cortical thickness may be important to ADHD outcome (15, 121–123). Consideration of widespread intrinsic functional networks allows for hypotheses related to differential disruption or cross talk among these networks (124). Although it is early days in this area for ADHD, several groups have worked in this direction in recent years with initial encouraging results (125). However, outcomes depend on levels of analysis and feature set.
For example, we (126) identified ADHD sub-populations based on a targeted analysis of variation in nucleus accumbens activity (NA). NA resting state functional connectivity corresponded to reward processing deficits--but only in a discrete subset of participants with ADHD. This finding helps amplifying the search for mechanisms in that subgroup. However, how these participants clustered using this feature set is very specific and does not take into account the rest of the brain’s organization. An overall picture of heterogeneity requires pairing this solution with alternatives using a different classification logic.
Therefore, we identified and validated a different set of sub-groups based on specified individual network phenomenon utilizing a novel clustering method called GIMME (127). Here, we classified individuals with ADHD into groups with distinct network phenomenon based on higher order brain systems (e.g., fronto-parietal, cingulo-opercular). We validated these findings via simulation. We expect this finding to be useful in searches for mechanistic linkage to neurodevelopmental, genetic, or environmental influences.
These latter two findings taken together underscore that there exists more than one valid solution depending on the approach and features used. There is simply more than one “right” answer. The importance of a given valid division depends on the question or outcome of interest (e.g., diagnosis, prognosis, treatment outcome, biological signal, etiology, etc.). (See online supplement Part B and Figures S1, S2 for details of possible methodological solutions such as the functional random forest (128)).
In short, the literature specific to characterizing heterogeneity in ADHD based on MRI is maturing at a rapid pace. What is clear is that unique patterns in brain physiology exist in individuals with ADHD. The task now is determine their best use, identify the best formulation for a given goal, and to maximize this information to improve long term outcomes.
Conceptual Contexts Revisited
Dysregulation as an organizing framework for many findings.
The various psychological mechanisms involved in ADHD and the theories put forward about them can be schematized within a model of self-regulation (15). One widely used conceptual framework in this vein is the triarchic model, depicted in the Online Supplement (Part F, Figure S5). (Such models require partially collapsing the artificial distinction between cognition and emotion). If ADHD broadly captures a population of individuals with varying kinds of dysregulatory problems (and risk for serious dysregulation-related complications), such an integrated conception helps organize where the most tractable paths for differentiation may lie for a given goal. Considering this model and the findings highlighted herein, we can postulate:
The clinical features that comprises the ADHD syndrome for a given individual manifest through a subset of particular breakdowns within self-regulation;
The specific breakdowns vary between groups individuals, and
Differentiating these may enable identification of neurobiological correlates and prediction of different clinical pathways within the ADHD population.
While different solutions will be useful in different ways, we highlight two exemplar applications of the general self-regulation model based on this review.
Cognitive profiles as useful in different ways at different developmental stages.
It appears that appropriate feature selection and validation may differentiate ADHD sub-groups characterized by (a) disruption of relatively late-stage “top down” cognitive operations (working memory) versus (b) relatively early-stage “bottom up” operations (reward temporal discounting, arousal state). Such cognitive profiles need more work, and may not map on to the temperament profiles. With regard to utility, the identification of a subgroup of youth with ADHD characterized by executive dysfunction may suggest interventions related to that weakness—adapted to developmental stage. For example, a body of work has argued that preschool intervention to help children with weak executive functioning may enhance academic outcome (129, 130). Such intervention may also secondarily support emotion regulation. In adolescence, variation in trajectory of working memory development may inform clinical persistence or desistence (131). Additionally, a subgroup with a low arousal profile may require a different approach (or may be particularly responsive to a particular medication class). These hypotheses could be tested.
Temperament profiles as useful for clinical prediction in childhood.
Support is quite promising for evaluating emotionally dysregulated ADHD sub-profiles, at least in pre-adolescent children. These profiles, varying in positive or negative valence dysregulation but possibly anchored by anger-dysregulation are promising with regard to (a) accessing developmental science of temperament, (b) detecting a stronger biological signal, and (c) enhancing clinical prediction. Such an approach thus warrants consideration in the nosology. However, this perspective also has limitations. It may not detect distinct etiological signals, or it may only be useful for predicting some clinical outcomes and not others. Developmental stage may be particularly important. The temperament profiles may be most useful in outlining early developmental sequences and childhood functioning and outcome. Figure 4 illustrates a set of hypotheses for follow up study.
Figure 4:

Potential ADHD heterogeneity related to clinical outcome and treatment
A developmental story may be possible.
We can speculatively propose the following in regard to possible developmental and clinical heterogeneity. In early life, ADHD can emerge through breakdowns in the regulation of approach signaling (Surgent/exuberant) or negative emotion regulation (irritability, anger, negative affectivity). These profiles may be modulated by a recursive process in early life by which extreme negative emotionality disrupts top down control and this in turn leads to further emotional dysregulation. When compensatory mechanisms are insufficient, such processes emanate in emergence of ADHD in an emotionally dysregulated profile or profiles (14, 74, 78, 83, 131–133). In adolescence, distinct cognitive profiles re-emerge that both reflect continuation or potential recovery of ADHD with or without an emotional dysregulation profile (74, 78, 131). Further empirical integration remains a priority.
Next Steps and Conclusion
Next Steps.
Key next steps are several. In the context of the current review, we suggest five of the most central questions, taking into account the purpose of a given heterogeneity proposal or clustering solution.
In neurobiological work (e.g., EEG, MRI, physiology), what is the most useful level of analysis for identifying clinically-actionable predictors or mechanisms that differentiate children for particular clinical or other goals? Here network cross-talk, functional versus structural features, degree of data reduction, and other considerations require specification in regard to utility or other goal.
In psychological work, do working memory (in the control domain) and arousal (in the energetic state domain) capture distinct features of heterogeneity related to clinical prediction or are they overlapping?
Relatedly, do these psychological profiles serve as useful targets for neurobiological study, enhancing understanding of pathways mechanistically?
Are temperament, cognitive, or neural profiles useful in detecting differential etiological sources, particularly those that may be modifiable?
Are temperament, cognitive, or neural subtypes useful in determining specific differential treatment response? We proposed hypotheses for both temperament and cognitive profiles. EEG or functional MRI profiles could be targetable by refined neurofeedback approaches or transcranial neural stimulation technologies.
Conclusion.
Resolving heterogeneity in ADHD requires at least three considerations. First, identifying the goals and using theory to explicitly guide analytic decision making is essential to the many valid solutions in complex multifactorial space. Second, ADHD-related cognitive and emotional features should be considered in both (a) incentive context (positive and negative valence) and (b) developmental context. Third, and crucially, work in this area still has relatively rarely considered psychosocial variations that may modulate subtypes, such as social adversity (134, 135).
These considerations, in conjunction with theory and dimensional feature sets outside of the DSM symptom list, can make the problem of heterogeneity tractable. It can lead to improvements in clinical characterization and detection of neurobiology. It therefore begins to be possible to foresee integrating an RDoC framework with developmental theory and with DSM syndromes to work toward a functional or mechanistic nosology.
We offer a cautionary yet optimistic concluding note. Most ADHD subtyping work to date has addressed relatively small data sets varying in their depth of clinical characterization. It has not engaged large data sets very often. Sample size is an important concern with regard to appropriate model selection, reproducibility, and sufficient representation of full population variation to isolate heterogeneity. Larger samples are now increasingly available—for example, the Adolescent Brain Cognitive Development (ABCD) study of over 11,000 children over a 10 year period (136–139). While ABCD and other data sets like it cannot avoid some lack of depth in ADHD sub-phenotyping, they can be very helpful in identifying heterogeneity in clinical change and in brain and other neurobiological profile. Findings from such large data sets then can be ‘yoked’ with smaller, but more deeply phenotyped samples to address various issues in discovery and validation.
Overall, the future is bright. The problem of creating a neurobiologically and clinically superior nosology to reduce ADHD heterogeneity is becoming tractable. We are hopeful that a better clinical characterization and the corresponding neurophysiology of ADHD is coming on to the horizon and will facilitate clinical prediction and etiological mapping efforts. We anticipate parallel efforts across the nosology.
Supplementary Material
Acknowledgements
Support was provided by NIH grants R37-MH-59105 (PI: Nigg), R01-MH099064 (PI: Nigg), R01 MH115357 (MPIs: Fair, Nigg); R01 MH096773 (PI: Fair). Dr. Karalunas was supported by NIH grant K23-MH108656.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
Damien Fair is a founder of Nous Imaging, Inc. but its activities are unrelated to the current study and any potential conflict of interest has been reviewed and managed by OHSU. The remaining authors have declared that they have no biomedical financial interests or potential conflicts of interest.
Contributor Information
Joel T Nigg, Department of Psychiatry, Oregon Health & Science University.
Sarah L. Karalunas, Department of Psychiatry, Oregon Health & Science University
Eric Feczko, Department of Behavioral Neuroscience, Oregon Health & Science University.
Damien A. Fair, Department of Behavioral Neuroscience, Oregon Health & Science University
References
- 1.American Psychiatric Association; (2013): Diagnostic and Statistical Manual of Mental Disorders. 5th Edition. [Google Scholar]
- 2.Luo Y, Weibman D, Halperin JM, Li X (2019): A Review of Heterogeneity in Attention Deficit/Hyperactivity Disorder (ADHD). Front Hum Neurosci. 13:42–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poland J, Tekin S (2017): Extraordinary science and psychiatry. Cambridge: MIT Press. [Google Scholar]
- 4.First MB (2016): Current State of Psychiatric Nosology. Computational Psychiatry: The MIT Press. [Google Scholar]
- 5.Achenbach TM (1966): The classification of children’s psychiatric symptoms: a factor-analytic study. Psychol Monogr. 80:1–37. [DOI] [PubMed] [Google Scholar]
- 6.Rescorla LA, Althoff RR, Ivanova MY, Achenbach TM (2019): Effects of society and culture on parents’ ratings of children’s mental health problems in 45 societies. Eur Child Adolesc Psychiatry. 28:1107–1115. [DOI] [PubMed] [Google Scholar]
- 7.Aitken M, Battaglia M, Marino C, Mahendran N, Andrade BF (2019): Clinical utility of the CBCL Dysregulation Profile in children with disruptive behavior. J Affect Disord. 253:87–95. [DOI] [PubMed] [Google Scholar]
- 8.Conway CC, Forbes MK, Forbush KT, Fried EI, Hallquist MN, Kotov R, et al. (2019): A Hierarchical Taxonomy of Psychopathology Can Transform Mental Health Research. Perspect Psychol Sci. 14:419–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frick PJ, Nigg JT (2012): Current issues in the diagnosis of attention deficit hyperactivity disorder, oppositional defiant disorder, and conduct disorder. Annu Rev Clin Psychol. 8:77–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willcutt EG, Nigg JT, Pennington BF, Solanto MV, Rohde LA, Tannock R, et al. (2012): Validity of DSM-IV attention deficit/hyperactivity disorder symptom dimensions and subtypes. J Abnorm Psychol. 121:991–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nigg JT (2006): Temperament and developmental psychopathology. J Child Psychol Psychiatry. 47:395–422. [DOI] [PubMed] [Google Scholar]
- 12.Trull TJ, Widiger TA (2013): Dimensional models of personality: the five-factor model and the DSM-5. Dialogues Clin Neurosci. 15:135–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rothbart MK (2011): Becoming who we are : temperament and personality in development. New York: Guilford Press. [Google Scholar]
- 14.Posner MI, Rothbart MK (2000): Developing mechanisms of self-regulation. Dev Psychopathol. 12:427–441. [DOI] [PubMed] [Google Scholar]
- 15.Nigg JT (2017): Annual Research Review: On the relations among self-regulation, self-control, executive functioning, effortful control, cognitive control, impulsivity, risk-taking, and inhibition for developmental psychopathology. J Child Psychol Psychiatry. 58:361–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kagan J, Snidman N (2004): The long shadow of temperament. Cambridge, Massachusetts: Harvard University Press. . [Google Scholar]
- 17.Cuthbert BN, Insel TR (2013): Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 11:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, et al. (2010): Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 167:748–751. [DOI] [PubMed] [Google Scholar]
- 19.Varoquaux G, Poldrack RA (2019): Predictive models avoid excessive reductionism in cognitive neuroimaging. Curr Opin Neurobiol. 55:1–6. [DOI] [PubMed] [Google Scholar]
- 20.Poldrack RA, Yarkoni T (2016): From Brain Maps to Cognitive Ontologies: Informatics and the Search for Mental Structure. Annu Rev Psychol. 67:587–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Everitt BS, Landau S, Leese M, Stahl D (2011): Cluster Analysis, 5th Ed New York: Wiley Publishers. [Google Scholar]
- 22.Sterba SK, Bauer DJ (2010): Matching method with theory in person-oriented developmental psychopathology research. Dev Psychopathol. 22:239–254. [DOI] [PubMed] [Google Scholar]
- 23.Nigg J, Nikolas M, Burt SA (2010): Measured gene-by-environment interaction in relation to attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 49:863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Assary E, Vincent JP, Keers R, Pluess M (2018): Gene-environment interaction and psychiatric disorders: Review and future directions. Semin Cell Dev Biol. 77:133–143. [DOI] [PubMed] [Google Scholar]
- 25.Palladino VS, McNeill R, Reif A, Kittel-Schneider S (2019): Genetic risk factors and gene-environment interactions in adult and childhood attention-deficit/hyperactivity disorder. Psychiatr Genet. 29:63–78. [DOI] [PubMed] [Google Scholar]
- 26.Nikolas MA, Klump KL, Burt SA (2015): Parental involvement moderates etiological influences on attention deficit hyperactivity disorder behaviors in child twins. Child Dev. 86:224–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chronis-Tuscano A, Raggi VL, Clarke TL, Rooney ME, Diaz Y, Pian J (2008): Associations between maternal attention-deficit/hyperactivity disorder symptoms and parenting. J Abnorm Child Psychol. 36:1237–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo Y, Weibman D, Halperin JM, Li X (2019): A Review of Heterogeneity in Attention Deficit/Hyperactivity Disorder (ADHD). Front Hum Neurosci. 13:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pruim RHR, Beckmann CF, Oldehinkel M, Oosterlaan J, Heslenfeld D, Hartman CA, et al. (2019): An Integrated Analysis of Neural Network Correlates of Categorical and Dimensional Models of Attention-Deficit/Hyperactivity Disorder. Biol Psychiatry Cogn Neurosci Neuroimaging. 4:472–483. [DOI] [PubMed] [Google Scholar]
- 30.Becker SP, Leopold DR, Burns GL, Jarrett MA, Langberg JM, Marshall SA, et al. (2016): The Internal, External, and Diagnostic Validity of Sluggish Cognitive Tempo: A Meta-Analysis and Critical Review. J Am Acad Child Adolesc Psychiatry. 55:163–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neuman RJ, Todd RD, Heath AC, Reich W, Hudziak JJ, Bucholz KK, et al. (1999): Evaluation of ADHD typology in three contrasting samples: a latent class approach. J Am Acad Child Adolesc Psychiatry. 38:25–33. [DOI] [PubMed] [Google Scholar]
- 32.Todd RD, Huang H, Todorov AA, Neuman RJ, Reiersen AM, Henderson CA, et al. (2008): Predictors of stability of attention-deficit/hyperactivity disorder subtypes from childhood to young adulthood. J Am Acad Child Adolesc Psychiatry. 47:76–85. [DOI] [PubMed] [Google Scholar]
- 33.Chhabildas N, Pennington BF, Willcutt EG (2001): A Comparison of the Neuropsychological Profiles of the DSV-IV Subtypes of ADHD. J Abnorm Child Psychol. 29:529–540. [DOI] [PubMed] [Google Scholar]
- 34.Khan SA, Faraone SV (2006): The genetics of ADHD: a literature review of 2005. Current Psychiatry Reports. 8:393. [DOI] [PubMed] [Google Scholar]
- 35.Li JJ, Reise SP, Chronis-Tuscano A, Mikami AY, Lee SS (2016): Item Response Theory Analysis of ADHD Symptoms in Children With and Without ADHD. Assessment. 23:655–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crisan DR, Tendeiro JN, Wanders RBK, van Ravenzwaaij D, Meijer RR, Hartman CA (2019): Practical consequences of model misfit when using rating scales to assess the severity of attention problems in children. Int J Methods Psychiatr Res.e1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sturm A, McCracken JT, Cai L (2019): Evaluating the Hierarchical Structure of ADHD Symptoms and Invariance Across Age and Gender. Assessment. 26:508–523. [DOI] [PubMed] [Google Scholar]
- 38.Martel MM, Roberts B, Gremillion M, von Eye A, Nigg JT (2011): External validation of bifactor model of ADHD: explaining heterogeneity in psychiatric comorbidity, cognitive control, and personality trait profiles within DSM-IV ADHD. J Abnorm Child Psychol. 39:1111–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greene AL, Eaton NR, Li K, Forbes MK, Krueger RF, Markon KE, et al. (2019): Are fit indices used to test psychopathology structure biased? A simulation study. J Abnorm Psychol. 128:740–764. [DOI] [PubMed] [Google Scholar]
- 40.McNally RJ (2016): Can network analysis transform psychopathology? Behav Res Ther. 86:95–104. [DOI] [PubMed] [Google Scholar]
- 41.Borsboom D, Cramer AO (2013): Network analysis: an integrative approach to the structure of psychopathology. Annu Rev Clin Psychol. 9:91–121. [DOI] [PubMed] [Google Scholar]
- 42.Martel MM, Levinson CA, Langer JK, Nigg JT (2016): A network analysis of developmental change in ADHD symptom structure from preschool to adulthood. Clin Psychol Sci. 4:988–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silk TJ, Malpas CB, Beare R, Efron D, Anderson V, Hazell P, et al. (2019): A network analysis approach to ADHD symptoms: More than the sum of its parts. PLoS One. 14:e0211053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McElroy E, Fearon P, Belsky J, Fonagy P, Patalay P (2018): Networks of Depression and Anxiety Symptoms Across Development. J Am Acad Child Adolesc Psychiatry. 57:964–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shue KL, Douglas VI (1992): Attention deficit hyperactivity disorder and the frontal lobe syndrome. Brain Cogn. 20:104–124. [DOI] [PubMed] [Google Scholar]
- 46.Barkley RA, Grodzinsky G, DuPaul GJ (1992): Frontal lobe functions in attention deficit disorder with and without hyperactivity: a review and research report. J Abnorm Child Psychol. 20:163–188. [DOI] [PubMed] [Google Scholar]
- 47.Barkley RA (1997): Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull. 121:65–94. [DOI] [PubMed] [Google Scholar]
- 48.Parry PA, Douglas VI (1983): Effects of reinforcement on concept identification in hyperactive children. J Abnorm Child Psychol. 11:327–340. [DOI] [PubMed] [Google Scholar]
- 49.Metin B, Roeyers H, Wiersema JR, van der Meere J, Sonuga-Barke E (2012): A meta-analytic study of event rate effects on Go/No-Go performance in attention-deficit/hyperactivity disorder. Biol Psychiatry. 72:990–996. [DOI] [PubMed] [Google Scholar]
- 50.Wender PH (1973): Some speculations concerning a possible biochemical basis of minimal brain dysfunction. Ann N Y Acad Sci. 205:18–28. [DOI] [PubMed] [Google Scholar]
- 51.Zentall S (1975): Optimal stimulation as theoretical basis of hyperactivity. Am J Orthopsychiatry. 45:549–563. [DOI] [PubMed] [Google Scholar]
- 52.de Castro Paiva GC, de Souza Costa D, Malloy-Diniz LF, Marques de Miranda D, Jardim de Paula J (2019): Temporal Reward Discounting in Children with Attention Deficit/Hyperactivity Disorder (ADHD), and Children with Autism Spectrum Disorder (ASD): A Systematic Review. Dev Neuropsychol. 44:468–480. [DOI] [PubMed] [Google Scholar]
- 53.Yang DY, Chi MH, Chu CL, Lin CY, Hsu SE, Chen KC, et al. (2019): Orbitofrontal dysfunction during the reward process in adults with ADHD: An fMRI study. Clin Neurophysiol. 130:627–633. [DOI] [PubMed] [Google Scholar]
- 54.Mies GW, de Water E, Wiersema JR, Scheres A (2019): Delay discounting of monetary gains and losses in adolescents with ADHD: Contribution of delay aversion to choice. Child Neuropsychol. 25:528–547. [DOI] [PubMed] [Google Scholar]
- 55.Mies GW, Ma I, de Water E, Buitelaar JK, Scheres A (2018): Waiting and working for rewards: Attention-Deficit/Hyperactivity Disorder is associated with steeper delay discounting linked to amygdala activation, but not with steeper effort discounting. Cortex. 106:164–173. [DOI] [PubMed] [Google Scholar]
- 56.van Hulst BM, de Zeeuw P, Bos DJ, Rijks Y, Neggers SF, Durston S (2017): Children with ADHD symptoms show decreased activity in ventral striatum during the anticipation of reward, irrespective of ADHD diagnosis. J Child Psychol Psychiatry. 58:206–214. [DOI] [PubMed] [Google Scholar]
- 57.Tripp G, Wickens JR (2008): Research review: dopamine transfer deficit: a neurobiological theory of altered reinforcement mechanisms in ADHD. J Child Psychol Psychiatry. 49:691–704. [DOI] [PubMed] [Google Scholar]
- 58.Johansen EB, Killeen PR, Russell VA, Tripp G, Wickens JR, Tannock R, et al. (2009): Origins of altered reinforcement effects in ADHD. Behav Brain Funct. 5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sagvolden T, Sergeant JA (1998): Attention deficit/hyperactivity disorder--from brain dysfunctions to behaviour. Behav Brain Res. 94:1–10. [PubMed] [Google Scholar]
- 60.Sagvolden T, Johansen EB, Aase H, Russell VA (2005): A dynamic developmental theory of attention-deficit/hyperactivity disorder (ADHD) predominantly hyperactive/impulsive and combined subtypes. Behav Brain Sci. 28:397–419; discussion 419–368. [DOI] [PubMed] [Google Scholar]
- 61.De Meyer H, Beckers T, Tripp G, van der Oord S (2019): Reinforcement Contingency Learning in Children with ADHD: Back to the Basics of Behavior Therapy. J Abnorm Child Psychol. 47:1889–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sonuga-Barke EJ (2002): Psychological heterogeneity in AD/HD--a dual pathway model of behaviour and cognition. Behav Brain Res. 130:29–36. [DOI] [PubMed] [Google Scholar]
- 63.Fair DA, Bathula D, Nikolas MA, Nigg JT (2012): Distinct neuropsychological subgroups in typically developing youth inform heterogeneity in children with ADHD. Proc Natl Acad Sci U S A. 109:6769–6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Newman ME (2006): Modularity and community structure in networks. Proc Natl Acad Sci U S A. 103:8577–8582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nigg JT, Willcutt EG, Doyle AE, Sonuga-Barke EJ (2005): Causal heterogeneity in attention-deficit/hyperactivity disorder: do we need neuropsychologically impaired subtypes? Biol Psychiatry. 57:1224–1230. [DOI] [PubMed] [Google Scholar]
- 66.Sonuga-Barke EJ (2005): Causal models of attention-deficit/hyperactivity disorder: from common simple deficits to multiple developmental pathways. Biol Psychiatry. 57:1231–1238. [DOI] [PubMed] [Google Scholar]
- 67.Roberts BA, Martel MM, Nigg JT (2017): Are There Executive Dysfunction Subtypes Within ADHD? J Atten Disord. 21:284–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vaidya CJ, You X, Mostofsky S, Pereira F, Berl MM, Kenworthy L (2019): Data-driven identification of subtypes of executive function across typical development, attention deficit hyperactivity disorder, and autism spectrum disorders. J Child Psychol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Castellanos FX, Tannock R (2002): Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nat Rev Neurosci. 3:617–628. [DOI] [PubMed] [Google Scholar]
- 70.Sonuga-Barke E, Bitsakou P, Thompson M (2010): Beyond the dual pathway model: evidence for the dissociation of timing, inhibitory, and delay-related impairments in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 49:345–355. [DOI] [PubMed] [Google Scholar]
- 71.Sonuga-Barke EJ, Sergeant JA, Nigg J, Willcutt E (2008): Executive dysfunction and delay aversion in attention deficit hyperactivity disorder: nosologic and diagnostic implications. Child Adolesc Psychiatr Clin N Am. 17:367–384, ix. [DOI] [PubMed] [Google Scholar]
- 72.Rajendran K, O’Neill S, Marks DJ, Halperin JM (2015): Latent profile analysis of neuropsychological measures to determine preschoolers’ risk for ADHD. J Child Psychol Psychiatry. 56:958–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Miller CJ, Miller SR, Healey DM, Marshall K, Halperin JM (2013): Are cognitive control and stimulus-driven processes differentially linked to inattention and hyperactivity in preschoolers? J Clin Child Adolesc Psychol. 42:187–196. [DOI] [PubMed] [Google Scholar]
- 74.Karalunas SL, Gustafsson HC, Dieckmann NF, Tipsord J, Mitchell SH, Nigg JT (2017): Heterogeneity in development of aspects of working memory predicts longitudinal attention deficit hyperactivity disorder symptom change. J Abnorm Psychol. 126:774–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Coghill DR, Hayward D, Rhodes SM, Grimmer C, Matthews K (2014): A longitudinal examination of neuropsychological and clinical functioning in boys with attention deficit hyperactivity disorder (ADHD): improvements in executive functioning do not explain clinical improvement. Psychol Med. 44:1087–1099. [DOI] [PubMed] [Google Scholar]
- 76.Karalunas SL, Nigg JT (2019): Heterogeneity and subtyping in attention-deficit/hyperactivity disorder— Considerations for emerging research using person-centered computational approaches. Biol Psychiatry. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Karalunas SL, Huang-Pollock CL, Nigg JT (2012): Decomposing attention-deficit/hyperactivity disorder (ADHD)-related effects in response speed and variability. Neuropsychology. 26:684–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Karalunas SL, Geurts HM, Konrad K, Bender S, Nigg JT (2014): Annual research review: Reaction time variability in ADHD and autism spectrum disorders: measurement and mechanisms of a proposed trans-diagnostic phenotype. J Child Psychol Psychiatry. 55:685–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nigg JT, Gustafsson HC, Karalunas SL, Ryabinin P, McWeeney SK, Faraone SV, et al. (2018): Working Memory and Vigilance as Multivariate Endophenotypes Related to Common Genetic Risk for Attention-Deficit/Hyperactivity Disorder. J Am Acad Child Adolesc Psychiatry. 57:175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Karalunas SL, Hawkey E, Gustafsson H, Miller M, Langhorst M, Cordova M, et al. (2018): Overlapping and distinct cognitive impairments in attention-deficit/hyperactivity and autism spectrum disorder without intellectual disability. Journal of abnormal child psychology. 46:1705–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shaw P, Stringaris A, Nigg J, Leibenluft E (2014): Emotion dysregulation in attention deficit hyperactivity disorder. Am J Psychiatry. 171:276–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Barkley RA, Fischer M (2010): The unique contribution of emotional impulsiveness to impairment in major life activities in hyperactive children as adults. J Am Acad Child Adolesc Psychiatry. 49:503–513. [DOI] [PubMed] [Google Scholar]
- 83.Nigg JT, Goldsmith HH, Sachek J (2004): Temperament and attention deficit hyperactivity disorder: the development of a multiple pathway model. Journal of Clinical Child & Adolescent sychology. 33:42–53. [DOI] [PubMed] [Google Scholar]
- 84.Rabinovitz BB, O’Neill S, Rajendran K, Halperin JM (2016): Temperament, executive control, and attention-deficit/hyperactivity disorder across early development. J Abnorm Psychol. 125:196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Miller NV, Degnan KA, Hane AA, Fox NA, Chronis-Tuscano A (2019): Infant temperament reactivity and early maternal caregiving: independent and interactive links to later childhood attention-deficit/hyperactivity disorder symptoms. J Child Psychol Psychiatry. 60:43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Miller NV, Hane AA, Degnan KA, Fox NA, Chronis-Tuscano A (2019): Investigation of a developmental pathway from infant anger reactivity to childhood inhibitory control and ADHD symptoms: interactive effects of early maternal caregiving. J Child Psychol Psychiatry. 60:762–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Auerbach JG, Atzaba-Poria N, Berger A, Landau R (2004): Emerging developmental pathways to ADHD: possible path markers in early infancy. Neural Plast. 11:29–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Einziger T, Levi L, Zilberman-Hayun Y, Auerbach JG, Atzaba-Poria N, Arbelle S, et al. (2018): Predicting ADHD Symptoms in Adolescence from Early Childhood Temperament Traits. J Abnorm Child Psychol. 46:265–276. [DOI] [PubMed] [Google Scholar]
- 89.Sullivan EL, Holton KF, Nousen EK, Barling AN, Sullivan CA, Propper CB, et al. (2015): Early identification of ADHD risk via infant temperament and emotion regulation: a pilot study. J Child Psychol Psychiatry. 56:949–957. [DOI] [PubMed] [Google Scholar]
- 90.(2014): Handbook of emotion regulation, 2nd ed New York, NY, US: Guilford Press. [Google Scholar]
- 91.Martel MM, Nigg JT (2006): Child ADHD and personality/temperament traits of reactive and effortful control, resiliency, and emotionality. J Child Psychol Psychiatry. 47:1175–1183. [DOI] [PubMed] [Google Scholar]
- 92.Martel MM, Nigg JT, von Eye A (2009): How do trait dimensions map onto ADHD symptom domains? J Abnorm Child Psychol. 37:337–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Karalunas SL, Fair D, Musser ED, Aykes K, Iyer SP, Nigg JT (2014): Subtyping attention-deficit/hyperactivity disorder using temperament dimensions: toward biologically based nosologic criteria. JAMA psychiatry. 71:1015–1024. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 94.Karalunas SL, Gustafsson HC, Fair D, Musser ED, Nigg JT (2019): Do we need an irritable subtype of ADHD? Replication and extension of a promising temperament profile approach to ADHD subtyping. Psychol Assess. 31:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Martel MM (2016): Dispositional trait types of ADHD in young children. Journal of attention disorders. 20:43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Martel MM, Nigg JT, Lucas RE (2008): Trait mechanisms in youth with and without attention-deficit/hyperactivity disorder. Journal of Research in Personality. 42:895–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Martel MM, Goth-Owens T, Martinez-Torteya C, Nigg JT (2010): A person-centered personality approach to heterogeneity in attention-deficit/hyperactivity disorder (ADHD). J Abnorm Psychol. 119:186–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Smith TE, Martel MM (2019): Trait-Based Profiles of ADHD in Adolescents and Young Adults. J Clin Child Adolesc Psychol. 48:440–454. [DOI] [PubMed] [Google Scholar]
- 99.Fernandez de la Cruz L, Simonoff E, McGough JJ, Halperin JM, Arnold LE, Stringaris A (2015): Treatment of children with attention-deficit/hyperactivity disorder (ADHD) and irritability: results from the multimodal treatment study of children with ADHD (MTA). J Am Acad Child Adolesc Psychiatry. 54:62–70.e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Leibenluft E, Cohen P, Gorrindo T, Brook JS, Pine DS (2006): Chronic Versus Episodic Irritability in Youth: ACommunity-Based, Longitudinal Study of Clinical and Diagnostic Associations. J Child Adolesc Psychopharmacol. 16:456–466. [DOI] [PubMed] [Google Scholar]
- 101.Stringaris A (2011): Irritability in children and adolescents: a challenge for DSM-5. Eur Child Adolesc Psychiatry. 20:61–66. [DOI] [PubMed] [Google Scholar]
- 102.Nigg JT, Karalunas S, Gustafsson H, Bhatt P, Ryabinin P, Mooney MA, et al. (2019): Evaluating chronic emotional dysregulation and irritability in relation to ADHD and depression genetic risk in children with ADHD. Journal of Child Psychology and Psychiatry. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Alperin BR, Smith CJ, Gustafsson HC, Figuracion MT, Karalunas SL (2019): The relationship between alpha asymmetry and ADHD depends on negative affect level and parenting practices. J Psychiatr Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Clarke AR, Barry RJ, McCarthy R, Selikowitz M (2001): EEG-defined subtypes of children with attention-deficit/hyperactivity disorder. Clin Neurophysiol. 112:2098–2105. [DOI] [PubMed] [Google Scholar]
- 105.Clarke AR, Barry RJ, Dupuy FE, Heckel LD, McCarthy R, Selikowitz M, et al. (2011): Behavioural differences between EEG-defined subgroups of children with attention-deficit/hyperactivity disorder. Clin Neurophysiol. 122:1333–1341. [DOI] [PubMed] [Google Scholar]
- 106.Clarke AR, Barry RJ, Dupuy FE, McCarthy R, Selikowitz M, Johnstone SJ (2013): Excess beta activity in the EEG of children with Attention-Deficit/Hyperactivity Disorder: A disorder of arousal? Int J Psychophysiol. 89:314–319. [DOI] [PubMed] [Google Scholar]
- 107.Loo SK, McGough JJ, McCracken JT, Smalley SL (2018): Parsing heterogeneity in attention-deficit hyperactivity disorder using EEG-based subgroups. Journal of Child Psychology and Psychiatry. 59:223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bergwerff CE, Luman M, Weeda WD, Oosterlaan J (2017): Neurocognitive profiles in children with ADHD and their predictive value for functional outcomes. Journal of attention disorders. 1087054716688533. [DOI] [PubMed] [Google Scholar]
- 109.Fair DA, Nigg JT, Iyer S, Bathula D, Mills KL, Dosenbach NUF, et al. (2013): Distinct neural signatures detected for ADHD subtypes after controlling for micro-movements in resting state functional connectivity MRI data. Front Syst Neurosci. 6:80–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lambek R, Sonuga-Barke E, Tannock R, Sørensen AV, Damm D, Thomsen PH (2018): Are there distinct cognitive and motivational sub-groups of children with ADHD? Psychol Med. 48:1722–1730. [DOI] [PubMed] [Google Scholar]
- 111.Mostert JC, Hoogman M, Onnink AMH, van Rooij D, von Rhein D, van Hulzen KJ, et al. (2018): Similar subgroups based on cognitive performance parse heterogeneity in adults with ADHD and healthy controls. Journal of attention disorders. 22:281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.van Hulst BM, De Zeeuw P, Durston S (2015): Distinct neuropsychological profiles within ADHD: a latent class analysis of cognitive control, reward sensitivity and timing. Psychol Med. 45:735–745. [DOI] [PubMed] [Google Scholar]
- 113.Shao L, Xu Y, Fu D (2018): Classification of ADHD with bi-objective optimization. J Biomed Inform. 84:164–170. [DOI] [PubMed] [Google Scholar]
- 114.Qureshi MN, Min B, Jo HJ, Lee B (2016): Multiclass Classification for the Differential Diagnosis on the ADHD Subtypes Using Recursive Feature Elimination and Hierarchical Extreme Learning Machine: Structural MRI Study. PLoS One. 11:e0160697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lecei A, van Hulst BM, de Zeeuw P, van der Pluijm M, Rijks Y, Durston S (2019): Can we use neuroimaging data to differentiate between subgroups of children with ADHD symptoms: A proof of concept study using latent class analysis of brain activity. Neuroimage Clin. 21:101601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Qian X, Castellanos FX, Uddin LQ, Loo BRY, Liu S, Koh HL, et al. (2019): Large-scale brain functional network topology disruptions underlie symptom heterogeneity in children with attention-deficit/hyperactivity disorder. Neuroimage Clin. 21:101600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rubia K (2018): Cognitive Neuroscience of Attention Deficit Hyperactivity Disorder (ADHD) and Its Clinical Translation. Front Hum Neurosci. 12:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Arnsten AF, Rubia K (2012): Neurobiological circuits regulating attention, cognitive control, motivation, and emotion: disruptions in neurodevelopmental psychiatric disorders. J Am Acad Child Adolesc Psychiatry. 51:356–367. [DOI] [PubMed] [Google Scholar]
- 119.Rubia K (2011): “Cool” inferior frontostriatal dysfunction in attention-deficit/hyperactivity disorder versus “hot” ventromedial orbitofrontal-limbic dysfunction in conduct disorder: a review. Biol Psychiatry. 69:e69–87. [DOI] [PubMed] [Google Scholar]
- 120.Petrovic P, Castellanos FX (2016): Top-Down Dysregulation-From ADHD to Emotional Instability. Front Behav Neurosci. 10:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shaw P, Malek M, Watson B, Greenstein D, de Rossi P, Sharp W (2013): Trajectories of cerebral cortical development in childhood and adolescence and adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 74:599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ernst M (2014): The triadic model perspective for the study of adolescent motivated behavior. Brain Cogn. 89:104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ernst M, Gowin JL, Gaillard C, Philips RT, Grillon C (2019): Sketching the Power of Machine Learning to Decrypt a Neural Systems Model of Behavior. Brain Sci. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Castellanos FX, Proal E (2012): Large-scale brain systems in ADHD: beyond the prefrontal-striatal model. Trends Cogn Sci. 16:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dajani DR, Burrows CA, Nebel MB, Mostofsky SH, Gates KM, Uddin LQ (2019): Parsing Heterogeneity in Autism Spectrum Disorder and Attention-Deficit/Hyperactivity Disorder with Individual Connectome Mapping. Brain Connect. 9:673–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Costa Dias TG, Iyer SP, Carpenter SD, Cary RP, Wilson VB, Mitchell SH, et al. (2015): Characterizing heterogeneity in children with and without ADHD based on reward system connectivity. Dev Cogn Neurosci. 11:155–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gates KM, Molenaar PC, Iyer SP, Nigg JT, Fair DA (2014): Organizing heterogeneous samples using community detection of GIMME-derived resting state functional networks. PLoS One. 9:e91322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Feczko E, Balba NM, Miranda-Dominguez O, Cordova M, Karalunas SL, Irwin L, et al. (2018): Subtyping cognitive profiles in Autism Spectrum Disorder using a Functional Random Forest algorithm. Neuroimage. 172:674–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Diamond A, Lee K (2011): Interventions shown to aid executive function development in children 4 to 12 years old. Science. 333:959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sasser TR, Bierman KL, Heinrichs B, Nix RL (2017): Preschool Intervention Can Promote Sustained Growth in the Executive-Function Skills of Children Exhibiting Early Deficits. Psychol Sci. 28:1719–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Halperin JM, Schulz KP (2006): Revisiting the role of the prefrontal cortex in the pathophysiology of attention-deficit/hyperactivity disorder. Psychol Bull.560–581. [DOI] [PubMed] [Google Scholar]
- 132.:Posner MI, Rothbart MK (2018): Temperament and brain networks of attention. Philos Trans R Soc Lond B Biol Sci. 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hawkey EJ, Tillman R, Luby JL, Barch DM (2018): Preschool Executive Function Predicts Childhood Resting-State Functional Connectivity and Attention-Deficit/Hyperactivity Disorder and Depression. Biol Psychiatry Cogn Neurosci Neuroimaging. 3:927–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Miller LL, Gustafsson HC, Tipsord J, Song M, Nousen E, Dieckmann N, et al. (2018): Is the Association of ADHD with Socio-Economic Disadvantage Explained by Child Comorbid Externalizing Problems or Parent ADHD? J Abnorm Child Psychol. 46:951–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Russell G, Ford T, Rosenberg R, Kelly S (2014): The association of attention deficit hyperactivity disorder with socioeconomic disadvantage: alternative explanations and evidence. J Child Psychol Psychiatry. 55:436–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Casey BJ, Cannonier T, Conley MI, Cohen AO, Barch DM, Heitzeg MM, et al. (2018): The Adolescent Brain Cognitive Development (ABCD) study: Imaging acquisition across 21 sites. Dev Cogn Neurosci. 32:43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Garavan H, Bartsch H, Conway K, Decastro A, Goldstein RZ, Heeringa S, et al. (2018): Recruiting the ABCD sample: Design considerations and procedures. Dev Cogn Neurosci. 32:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jernigan TL, Brown SA, Dowling GJ (2018): The Adolescent Brain Cognitive Development Study. J Res Adolesc. 28:154–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Volkow ND, Koob GF, Croyle RT, Bianchi DW, Gordon JA, Koroshetz WJ, et al. (2018): The conception of the ABCD study: From substance use to a broad NIH collaboration. Dev Cogn Neurosci. 32:4–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.McBurnett K, Pfiffner LJ, Frick PJ (2001): Symptom properties as a function of ADHD type: an argument for continued study of sluggish cognitive tempo. J Abnorm Child Psychol. 29:207–213. [DOI] [PubMed] [Google Scholar]
- 141.Carlson CL, Mann M (2000): Attention-deficit/hyperactivity disorder, predominantly inattentive subtype. Child Adolesc Psychiatr Clin N Am. 9:499–510, vi. [PubMed] [Google Scholar]
- 142.Barkley RA (2014): Sluggish cognitive tempo (concentration deficit disorder?): current status, future directions, and a plea to change the name. J Abnorm Child Psychol. 42:117–125. [DOI] [PubMed] [Google Scholar]
- 143.Nigg JT, Casey BJ (2005): An integrative theory of attention-deficit/ hyperactivity disorder based on the cognitive and affective neurosciences. Dev Psychopathol. 17:785–806. [DOI] [PubMed] [Google Scholar]
- 144.Sonuga-Barke EJ, Dalen L, Remington B (2003): Do executive deficits and delay aversion make independent contributions to preschool attention-deficit/hyperactivity disorder symptoms? J Am Acad Child Adolesc Psychiatry. 42:1335–1342. [DOI] [PubMed] [Google Scholar]
- 145.Musser ED, Galloway-Long HS, Frick PJ, Nigg JT (2013): Emotion regulation and heterogeneity in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 52:163–171.e162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Barkley R (2010): Deficient emotional self-regulation: a core component of attention-deficit/hyperactivity disorder. J ADHD and Related Disorders. 1:5–37. [Google Scholar]
- 147.Swanson JM, Kinsbourne M, Nigg J, Lanphear B, Stefanatos GA, Volkow N, et al. (2007): Etiologic subtypes of attention-deficit/hyperactivity disorder: brain imaging, molecular genetic and environmental factors and the dopamine hypothesis. Neuropsychol Rev. 17:39–59. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


