Abstract
Background & Aims
The effects of vitamin D on risk of colorectal cancer precursors are not clear. We examined the influence of vitamin D supplementation on risk of colorectal adenomas and serrated polyps in a prespecified ancillary study of a large-scale prevention trial (the vitamin D and omegA-3 trial, VITAL) of individuals who were free of cancer and cardiovascular disease at enrollment.
Methods
In VITAL trial, 25,871 adults with no history of cancer or cardiovascular disease (12,786 men 50 y or older and 13,085 women 55 y or older) were randomly assigned to groups given daily dietary supplements (2000 IU vitamin D3 and 1 g marine n-3 fatty acid) or placebo. Patients were assigned to groups from November 2011 through March 2014 and the study ended on December 31, 2017. We confirmed conventional adenomas and serrated polyps by reviewing histopathology reports from participants who had reported a diagnosis of polyps and were asked by their doctors to return for a repeated endoscopy in 5 years or less. We calculated the odds ratios (ORs) and 95% CIs by logistic regression, after adjusting for age, sex, n-3 treatment assignment, and history of endoscopy at time of randomization.
Results
During a median follow-up of 5.3 years, we documented 308 cases of conventional adenomas in 12,927 participants in the vitamin D group and 287 cases in 12,944 participants in the placebo group (OR for the association of vitamin D supplementation with adenoma, 1.08; 95% CI, 0.92–1.27). There were 172 cases of serrated polyps in the vitamin D group and 169 cases in the placebo group (OR for the association of vitamin D supplementation with serrated polyp, 1.02; 95% CI, 0.82–1.26). Supplementation was not associated with polyp size, location, multiplicity, or histologic features. We found evidence for an interaction between vitamin D supplementation and serum level of 25-hydroxyvitamin D, measured in 15,787 participants. Among individuals with serum levels of 25-hydroxyvitamin D below 30 ng/mL, the OR associated with supplementation for conventional adenoma was 0.82 (95% CI, 0.60–1.13), whereas among individuals with serum levels of 25-hydroxyvitamin D above 30 ng/mL, the OR for conventional adenoma was 1.20 (95% CI, 0.92–1.55) (P for interaction=.07). There was a significant interaction between vitamin D supplementation and serum level of 25-hydroxyvitamin D in their association with advanced adenoma (P for interaction=.04).
Conclusions
Based on an ancillary study of data from the VITAL trial, daily vitamin D supplementation (2000 IU) was not associated with risk of colorectal cancer precursors in average-risk adults not selected for vitamin D insufficiency. A potential benefit for individuals with low baseline level of vitamin D requires further investigation.
ClinicalTrials.gov number
Keywords: Chemoprevention, primary prevention, nutrition, colon cancer
Introduction
Colorectal cancer is the third most common cancer and cause of cancer death in each sex in the United States.1 It can develop from two distinct groups of precursor lesions, including conventional adenomas and serrated polyps.2 Several dietary factors have been implicated in colorectal carcinogenesis, including vitamin D, which can be synthesized in the skin upon exposure to ultraviolet B radiation and consumed from foods and supplements. Once released into circulation, vitamin D is metabolized in the liver to 25-hydroxyvitamin [25(OH)D] and then in the kidney and other organs including the colon to the bioactive form, 1,25-dihydroxyvitamin D [1,25(OH)2D]. Through binding to the vitamin D receptor expressed in most tissues, 1,25(OH)2D may exert a wide spectrum of anticancer properties, including suppression of inflammation, regulation of cellular proliferation, and induction of apoptosis and differentiation.3, 4
Numerous prospective studies have reported a beneficial association with colorectal neoplasia of a range of markers for vitamin D status, including circulating 25(OH)D, dietary and supplement intake, and predicted 25(OH)D based on major determinants of vitamin D status.5–8 However, the evidence from randomized controlled trials (RCTs) remains inconclusive.9–13 A recent meta-analysis of 13 RCTs with a median follow-up ranging from 1 to 6.2 years showed no evidence of an effect of vitamin D supplementation for cancer incidence (relative risk, 1.03; 95% confidence interval [CI], 0.91–1.15).14 These discrepant findings between observational studies and RCTs may reflect no appreciable biological association or could be attributed to some limitations of RCTs, including poor adherence, inadequate vitamin D dose and duration, low prevalence of vitamin D deficiency of the study population, and a combination of these factors.15–17
Despite these data, however, the role of vitamin D in the early stage of colorectal carcinogenesis remains unclear. No RCT has yet assessed the effect of vitamin D supplementation on incident colorectal polyps among average-risk individuals. Therefore, we examined the influence of vitamin D supplementation on the risk of colorectal adenomas and serrated polyps in a prespecified ancillary study of a large-scale prevention trial, the VITamin D and OmegA-3 TriaL (VITAL) among individuals free of cancer and cardiovascular disease at enrollment.18 The initial findings of VITAL showed no effect of vitamin D on incidence of all types of cancer, including colorectal cancer (n=98), after a median follow-up of 5.3 years.19
Materials and methods
Study population
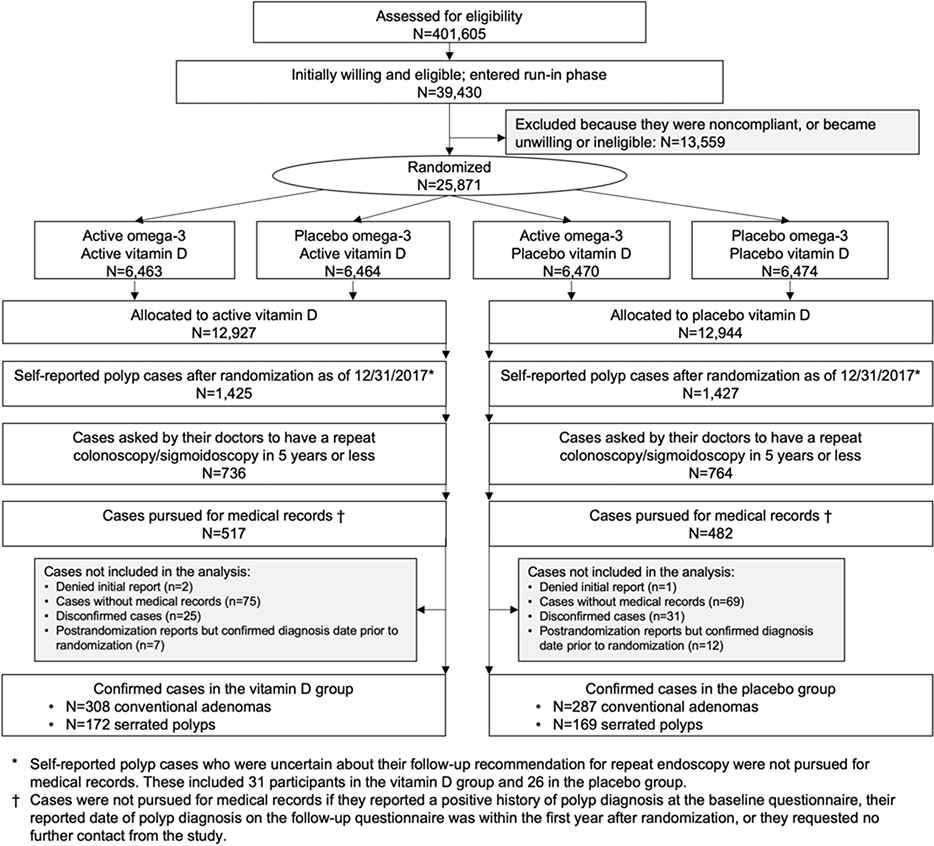
Details of the VITAL design and follow-up have been described previously.18–20 Briefly, VITAL is a completed randomized, double-blind, placebo-controlled trial, with a two-by-two factorial design, of vitamin D3 (2000 IU per day) and marine n-3 fatty acid (1 g per day) in the primary prevention of cardiovascular disease and cancer among 12,786 men aged ≥50 and 13,085 women aged ≥55 in the United States (Figure 1). Details of statistical power calculation for the primary and secondary endpoints have been described previously.18 The vitamin D dose was chosen based on the totality of prior evidence to reach the postulated optimal value of ≥90 nmol/L in the active vitamin D group and a difference in achieved 25(OH)D levels of approximately 30–50 nmol/L between the active treatment and placebo groups.18 Details about the inclusion and exclusion criteria and compliance of the trial are provided in the Supplementary Methods. All participants provided written informed consent. The trial was approved by the institutional review board of Partners Healthcare-Brigham and Women’s Hospital, Boston. All authors had access to the study data and reviewed and approved the final manuscript.
Figure 1.
Flow diagram of the VITAL polyp ancillary study
Outcome ascertainment
Annual questionnaires were administered to assess compliance, side effects, diagnoses of major illnesses, risk-factor updates, and endoscopic use. Approximately 59% of participants reported that they had received colonoscopy or sigmoidoscopy during the study period. On the 4-year questionnaire, participants were asked if they had been diagnosed with any colorectal polyp in the past 4 years, and if yes, whether they had been asked by the doctors to return for a repeated colonoscopy or sigmoidoscopy in 5 years or less. To confirm polyp cases and identify high-risk cases in a cost-efficient manner, we only acquired medical records from participants who answered yes to both questions, who are more likely to have polyps than those based on self-reported polyps alone. Participants who answered “not sure” to the question regarding repeated colonoscopy or sigmoidoscopy were not pursued for medical records (N=31 in the vitamin D group and 26 in the placebo group). Similar questions and follow-up procedures were used in the 5-year questionnaire. We performed medical record review in 36% (517/1,425) of the self-reported polyp cases in the vitamin D group and 34% (482/1,427) in the placebo group. Details on polyp ascertainment are provided in Figure 1.
Investigators blinded to randomization status reviewed the collected endoscopic and pathologic records and extracted data on polyp size, number, and histologic subtype at each anatomic sublocation, including proximal colon that encompasses cecum, ascending colon, hepatic flexure, transverse colon, and splenic flexure; distal colon that encompasses descending and sigmoid colon; and rectum that encompasses rectum and rectosigmoid junction. We defined two case groups – conventional adenomas and serrated polyps. Conventional adenomas included tubular adenoma, tubulovillous adenoma, and villous adenoma; those adenomas might or might not have high-grade dysplasia. We further defined patients with advanced conventional adenomas as those having at least one conventional adenoma with endoscopic size of 10 mm or greater or with advanced histology (tubulovillous or villous histology or high-grade dysplasia). Serrated polyps encompassed hyperplastic polyp, traditional serrated adenoma, and sessile serrated polyp with or without cytological dysplasia. If a participant had both conventional adenoma and serrated polyp on an endoscopy, he or she was counted in both case groups.
Assessment of covariates and plasma 25(OH)D
Participants completed a baseline questionnaire regarding their diet, clinical and lifestyle risk factors, including family history of colorectal cancer, history of colonoscopy or sigmoidoscopy in the past 10 years, smoking, body weight, height, alcohol consumption, physical activity, medication use, and use of dietary supplements. Blood samples were obtained at baseline from all willing participants and assayed for serum 25(OH)D (n=15,787) at Quest Diagnostics using liquid chromatography–tandem mass spectrometry. We participated in the 25(OH)D standardization program of the Centers for Disease Control and Prevention.
Statistical analysis
Descriptive statistics were calculated separately for the vitamin D and placebo groups. We performed intention-to-treat analysis to examine the effect of vitamin D supplementation. Logistic regression was used to calculate the odds ratio (OR) and 95% CI for the risk of conventional adenomas and serrated polyps comparing the vitamin D to placebo groups. Consistent with our prior study,19 we adjusted for age, sex, and randomization group in the n-3 portion of the trial (n-3 or placebo group). We further adjusted for use of colonoscopy and sigmoidoscopy in the past 10 years prior to randomization.
In a prespecified analysis, we examined the treatment effect within strata defined by baseline serum 25(OH)D levels at <30 and ≥30 ng/mL, the cutoff for vitamin D sufficiency recommended by the Endocrine Society.21 We calculated the P for interaction using the Wald test for the product term between the binary serum 25(OH)D variable and vitamin D treatment assignment. We also performed subgroup analyses according to polyp features, including size (<10 mm, ≥10 mm), sublocation (proximal colon, distal colon, rectum), multiplicity (single, multiple >=2), histology (for conventional adenoma only: tubular, tubulovillous, villous, or high-grade dysplasia), and malignant potential (for conventional adenoma only: advanced and nonadvanced). We assessed the difference in the treatment effects across different polyp groups and calculated the P for heterogeneity among cases only, with the case group classification as the dependent variable and treatment assignment as the independent variable. Finally, we performed exploratory stratified analysis and assessed interaction by Wald test according to several factors at randomization, including age, sex, race/ethnicity, family history of colorectal cancer, body mass index (BMI), physical activity, smoking, alcohol, regular aspirin use, history of colonoscopy or sigmoidoscopy in the past 10 years, history of colorectal polyps, and group assignment for the n-3 treatment.
Results
Table 1 shows the basic characteristics of participants at randomization, which were generally well balanced between the treatment groups. The mean age was 67 years, 51% were females, and 20% were African Americans. Among the total of 25,871 participants, 2,852 reported a diagnosis of colorectal polyps on the questionnaires, of which 1,500 (53%) reported that they had been asked by their doctors to return for a repeat colonoscopy or sigmoidoscopy in 5 years. Among those, we pursued medical records from 999 individuals (67%) and confirmed the diagnosis of conventional adenomas in 308 individuals from the vitamin D group and 287 from the placebo groups; and 172 cases of serrated polyps from the vitamin D group (including 56 with mixed/serrated adenomas only and 116 with at least one hyperplastic polyp) and 169 cases of serrated polyps from the placebo group (including 44 with mixed/serrated adenomas only and 125 with at least one hyperplastic polyp) (Figure 1). The mean interval (standard deviation) between randomization and polyp diagnosis was 3.2 (1.2) years in the vitamin D group and 3.1 (1.2) years in the placebo group (Supplementary Figure 1).
Table 1.
Baseline characteristics of participants according to vitamin D supplementationa
| Variable | Placebo group (n=12,944) | Vitamin D group (n=12,927) | P value |
|---|---|---|---|
| Age, year | 67.1 (7.1) | 67.1 (7.1) | 0.84 |
| Women, % | 6538 (51) | 6547 (51) | 0.83 |
| Race/ethnicity, % | 0.97 | ||
| Non-Hispanic white | 9033 (71) | 9013 (71) | |
| African American | 2553 (20) | 2553 (20) | |
| Others | 1071 (9) | 1081 (9) | |
| Family history of colorectal cancer, % | 1574 (13) | 1609 (14) | 0.51 |
| Colonoscopy or sigmoidoscopy in the past 10 years, % | 9654(75) | 9671(75) | 0.67 |
| Colonoscopy in the past 10 years, % | 9467 (73) | 9448 (73) | 0.93 |
| Sigmoidoscopy in the past 10 years, % | 1659 (14) | 1540 (13) | 0.04 |
| History of colorectal polyps, % | 3211 (25) | 3224 (25) | 0.80 |
| Smoking status, % | 0.88 | ||
| Never | 6620 (52) | 6565 (52) | |
| Past | 5221 (41) | 5243 (41) | |
| Current | 915 (7) | 921 (7) | |
| Body mass index, kg/m2 | 28.1 (5.8) | 28.1 (5.7) | 0.50 |
| Physical activity, MET-hours/week | 22.9 (25.9) | 22.5 (25.8) | 0.15 |
| Use of aspirin, % | 5814 (46) | 5756 (45) | 0.53 |
| Use of vitamin D supplements, %b | 5533 (43) | 5497 (43) | 0.72 |
| Use of calcium supplements, % | 2539 (20) | 2627 (20) | 0.16 |
| Use of multivitamin supplements, % | 5656 (44) | 5750 (45) | 0.20 |
| Alcohol use, % | 0.31 | ||
| Never | 4017 (32) | 3977 (31) | |
| Rarely to less than once per week | 968 (8) | 941 (7) | |
| 1–6/week | 4385 (34) | 4511 (36) | |
| Daily | 3364 (26) | 3274 (26) | |
| Serum 25(OH)D, ng/mLc | 30.8 (10.0) | 30.9 (10.0) | 0.35 |
Abbreviations: DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; MET, metabolic equivalent.
Mean (standard deviation) and percentage are presented for continuous and categorical variables, respectively.
To be eligible for the trial, participants are required to limit consumption of supplemental vitamin D to no more than 800 IU/day from all supplemental sources combined.
Serum data were available in 7,891 participants in the control group (61%) and 7,896 in the intervention group (61%) who provided a blood sample at enrollment.
Vitamin D treatment was not associated with risk of either conventional adenomas (multivariable OR=1.08, 95% CI, 0.92–1.27) or serrated polyps (OR=1.02, 95% CI, 0.82–1.26) (Table 2). A statistically nonsignificant interaction with baseline serum 25(OH)D levels was observed (P=0.07); vitamin D treatment showed a suggestively inverse association with conventional adenomas among individuals with baseline serum 25(OH)D levels of <30 ng/mL (OR=0.82, 95% CI, 0.60–1.13), but not among those with ≥30 ng/mL (OR=1.20, 95% CI, 0.92–1.55). A similar interaction was found for advanced adenomas (P=0.04) and the OR associated with vitamin D treatment was 0.60 (95% CI, 0.30–1.20) for baseline 25(OH)D of <30 ng/mL and 1.50 (95% CI, 0.87–2.61) for ≥30 ng/mL (Supplementary Table 1).
Table 2.
Association of vitamin D supplementation with risk of conventional adenomas and serrated polypsa
| Conventional adenomas |
Serrated polyps |
|||
|---|---|---|---|---|
| Placebo group | Vitamin D group | Placebo group | Vitamin D group | |
| Overall | ||||
| No. of cases | 287 | 308 | 169 | 172 |
| OR (95% CI)a | 1 (ref) | 1.08 (0.92–1.28) | 1 (ref) | 1.02 (0.82–1.26) |
| OR (95% CI)b | 1 (ref) | 1.08 (0.92–1.27) | 1 (ref) | 1.02 (0.82–1.26) |
| By serum 25(OH)D level at randomization | ||||
| <30 ng/mL | ||||
| No. of cases | 88 | 71 | 53 | 49 |
| OR (95% CI)b | 1 (ref) | 0.82 (0.60–1.13) | 1 (ref) | 0.94 (0.64–1.39) |
| ≥30 ng/mL | ||||
| No. of cases | 109 | 132 | 73 | 73 |
| OR (95% CI)b | 1 (ref) | 1.20 (0.92–1.55) | 1 (ref) | 0.98 (0.71–1.37) |
| P for interactionc | 0.07 | 0.87 | ||
Abbreviations: CI, confidence interval; OR, odds ratio.
Logistic regression was adjusted for age, sex, and fish oil treatment assignment.
Logistic regression was further adjusted for use of colonoscopy or sigmoidoscopy in the past 10 years prior to randomization.
P for interaction was calculated by Wald test for the product term between vitamin D randomization assignment and baseline serum 25(OH)D level (binary).
Because our participants were not screened uniformly for colorectal polyps before random assignment, some polyps diagnosed during the intervention period may have been prevalent at baseline. To address this, we conducted a sensitivity analysis excluding participants with colorectal polyps that occurred within the first 2 years after the start of the trial. Similar null results were found (for conventional adenomas [n=481]: OR=1.07, 95% CI, 0.89–1.28; for serrated polyps [n=273]: OR=1.09, 95% CI, 0.86–1.38). Moreover, because regular screening endoscopy was not protocol-mandated, we performed another sensitivity analysis by restricting to individuals who reported use of colonoscopy or sigmoidoscopy during the study period. The results were essentially unchanged (for conventional adenomas [n=560]: OR=1.00, 95% CI, 0.85–1.19; for serrated polyps [n=322]: OR=0.95, 95% CI, 0.76–1.19).
No statistically significant heterogeneity in the treatment effect was found according to polyp size, location, multiplicity, or histology (Supplementary Table 2). No statistically significant interactions were detected by demographic and lifestyle factors, endoscopic history, and n-3 fatty acid treatment (Supplementary Table 3).
Discussion
In this large-scale primary prevention trial in a population not selected for vitamin D insufficiency, supplementation with vitamin D at a dose of 2000 IU per day for a median period of 5.3 years did not reduce the risk of conventional adenomas or serrated polyps compared to the placebo. Stratified analysis indicated a potential benefit for conventional adenomas among individuals with low serum 25(OH)D at randomization. These findings provide novel data on the effect of vitamin D supplementation on early stage of colorectal carcinogenesis and have implications for future studies.
In contrast to the inconsistent data for incidence of colorectal neoplasia, both observational studies22–24 and RCTs25–27 have associated high levels of vitamin D with lower cancer mortality and favorable survival among patients with established colorectal cancer, indicating a potential benefit of vitamin D for inhibiting cancer progression. Indeed, we recently reported in VITAL a 25% reduction in cancer mortality associated with vitamin D supplementation after excluding the first two years of follow-up, whereas no benefit was found for incidence of any cancer, including colorectal cancer.19 Despite these data, the impact of vitamin D on the very early stage of colorectal carcinogenesis remains largely unknown. This is an important question to address because of the lengthy and multistep process of colorectal carcinogenesis from normal mucosa to premalignant lesions and ultimately to invasive carcinoma.
Two groups of precursor lesions have been identified for colorectal cancer, including conventional adenomas and serrated polyps.2 In contrast to conventional adenomas that develop and progress to CRC through a series of mutations in oncogenes and tumor suppressor genes, serrated polyps are characterized by hypermethylation of CpG islands and BRAF mutation, and primarily contribute to the development of microsatellite instable CRC.28 Increasing evidence indicates a difference in the environmental and genetic risk factors for conventional adenomas and serrated polyps.29–32
Several observational studies have linked higher intake of vitamin D and levels of circulating 25(OH)D to lower risk of conventional adenomas,33, 34 although residual confounding cannot be ruled out. In contrast, the only RCT that specifically examined colorectal neoplasia as the primary endpoint did not find any benefit of vitamin D supplementation at a dose of 1000 IU per day on recurrence of conventional adenomas.13 However, because that study was conducted among participants who already had a history of conventional adenoma, it is still unclear whether vitamin D can protect against polyp occurrence in average-risk individuals. The null findings in the current study suggest that daily supplementation of vitamin D at a dose of 2000 IU does not affect the overall risk of conventional adenoma among individuals not selected for vitamin D insufficiency. Nevertheless, we observed a potential interaction with baseline serum 25(OH)D levels. Vitamin D supplementation showed a suggestively beneficial association with risk of conventional adenomas, particularly advanced conventional adenomas, in individuals with 25(OH)D below 30 ng/mL, while no association was found for those with 25(OH)D of at least 30 ng/mL. Similar findings have been reported for colorectal cancer in an observational analysis of the Women’s Health Initiative.9 These data are also consistent with the recent findings of a large pooled analysis of 17 cohorts that the optimal 25(OH)D concentrations for colorectal cancer risk reduction ranged between 30 and 40 ng/mL, and that no further risk reduction was observed for 25(OH)D at 40 ng/mL or higher.7 Therefore, given the limited number of participants with low baseline vitamin D in our study, further studies are needed to test the effect of vitamin D supplementation for adenoma prevention among individuals with or at risk of vitamin D deficiency (e.g., below 20 ng/mL).
On the other hand, for serrated polyps, because their clinical significance was not realized until recently, very limited data exist regarding their relationship with vitamin D. We recently reported in a large prospective cohort study an association between higher vitamin D intake and lower risk of serrated polyps, regardless of the size of polyps.32 In contrast, in a secondary analysis of the RCT of vitamin D and calcium supplementation among individuals with a history of conventional adenomas, no effect was observed for either intervention on incidence of serrated polyps in the treatment phase, although an elevated risk of serrated polyps was found in the observational phase for calcium treatment.35 In the current study, we did not observe any benefit of vitamin D on risk of serrated polyps. Therefore, these data indicate that vitamin D supplementation may not have a substantial influence on the serrated pathway of colorectal cancer.
The strengths of our study include the RCT design, high adherence to the intervention regimen, detailed assessment of covariates that allows for subgroup analysis, and measurement of baseline serum 25(OH)D levels in >60% of participants that enabled examination of the influence of baseline vitamin D levels on the treatment effects. Moreover, our study enrolled 20% of African Americans, for whom there are data suggesting that vitamin D may be more beneficial.36–38 However, we did not observe any racial/ethnic difference in the current study.
Our study also has several limitations. First, because regular screening endoscopy was not protocol-mandated, it is likely that not all polyps were diagnosed. Also, due to resource constraints, we were only able to perform medical record review for a subset of polyp cases that were recommended by their doctors to undergo surveillance colonoscopy within 5 years. However, given the randomization design and large sample size, no difference between the vitamin D treatment and placebo groups was found in the proportion of medical record review among the self-reported polyp cases (36% vs. 34%) or the proportion of endoscopic examination of participants during the study period (59% for both groups). Second, because of the evolving nature and lack of consensus regarding the diagnostic criteria of specific subtypes of serrated polyps, we were unable to distinguish sessile serrated polyps and traditional serrated adenomas from hyperplastic polyps. Third, because a single-dose of vitamin D supplementation was used in the trial, we were unable to assess the dose-response relationship. Fourth, given multiple testing conducted in the study and the limited number of cases, the findings of subgroup and stratified analyses should be interpreted cautiously. Finally, data of genetic variants that may influence metabolism and biological activity of vitamin D are not available. There is evidence that the effect of vitamin D on colorectal neoplasia may vary by the genetic variants in the vitamin D receptor or vitamin D-binding protein.11, 39
In conclusion, we found that vitamin D supplementation at a dose of 2000 IU per day was not associated with risk of colorectal premalignant lesions. A potential benefit for individuals with low baseline vitamin D status requires further investigation.
Supplementary Material
Need to Know.
Background
The effects of vitamin D on risk of colorectal cancer precursors are not clear.
Findings
Based on an ancillary study of data from a large randomized trial of average-risk adults, daily vitamin D supplementation (2000 IU) was not associated with risk of conventional adenomas or serrated polyps.
Implications for patient care
The potential benefit of vitamin D supplementation for individuals with low baseline level of vitamin D requires further investigation.
Acknowledgements
VITAL Investigators, Staff, and Study Participants
The authors thank the VITAL investigators, staff, and the trial participants for their outstanding dedication and commitment.
Members of the VITAL Research Group
VITAL Steering Committee:
JoAnn E. Manson (Chair), Julie E. Buring (Chair), Nancy R. Cook, I-Min Lee, William Christen, Shari S. Bassuk, Samia Mora, Heike Gibson, David Gordon, Trisha Copeland, Denise D’Agostino, Georgina Friedenberg, Claire Ridge, Vadim Bubes, Edward L. Giovannucci, Walter C. Willett (all at Brigham and Women’s Hospital, Harvard Medical School, Boston; Drs. Manson, Buring, Cook, Lee, Giovannucci and Willett are also at the Harvard T.H. Chan School of Public Health).
Scientific consultants:
John Baron (University of North Carolina, Chapel Hill), Michael Holick (Boston Medical Center), Bruce Hollis (University of South Carolina).
Other Members of the VITAL Research Group:
(Brigham and Women’s Hospital): Christine M. Albert, Diane Gold, Meryl LeBoff, Olivia Okereke, Aruna Pradhan, Howard Sesso, Wendy Chen, Paulette Chandler, J. Michael Gaziano, Olga Demler, Kathryn Rexrode, Karen Costenbader, John Forman, Erik Alexander, Sonia Friedman, Jeffrey Katz, Shumin Zhang, Jennifer Lin, Joseph Walter, Julie Duszlak, Kate Kalan, Jean MacFadyen, Natalya Gomelskaya, David Bates, Ara Sarkissian, Mary Breen, Yeulolani Andrade, Manickavasagar Vinayagamoorthy, Chunying Li, Eunjung Kim, Franco Giulianini, Gregory Kotler, Marty Van Denburgh, Rimma Dushkes, Yanyan Liu, Eduardo Pereira, Lisa Fields-Johnson, George Menjin, Lucy Liu, Lauren Girard, Scott Zeller, Naomi Riches, Katelyn Hasson, Ellen Bhang, Maria Revilla, Elena McCarthy, Alex Moran, Kristen Haise, Leah Arsenault, Philomena Quinn, Sancia Grimes, Ivan Fitchorov, Kurt Schwerin, Shamikhah Curry, Annie Murray, Angela Zhang, Diana Walrond-Williams, Alison Weinberg, Chris Pfeffer, Margarette Haubourg, Viviane Nguyen, Henry Ouellette, Rolando Rodriguez, Tony Montgomery, Keith Morse, Vincent Guzman, Megan Perry, Sandra Weekes, Doug Smith, Allison Clar, Sara Curran, Yaneve Fonge, David Hibbert, Louisa Paine, Kelly Royce, Courtney Splaine, Jennifer McMahon, David Eldridge, Laura Hand, Kay Inandan, Meghan Rieu Werden, Harriet Samuelson, Andrea Hrbek, Megan Mele, Eileen Bowes, Mary Anne Ryan
(Massachusetts General Hospital, Boston): Carlos Camargo, Jacqueline Danik, Ravi Thadhani
(Vanderbilt University, Nashville): Thomas Wang
(Rush University Medical Center, Chicago): Raj C. Shah
(University of California, San Francisco): Michelle A. Albert
(Emory University): Carlos Kase
(Centers for Disease Control and Prevention, Vitamin D Standardization Program): Hubert Vesper and Julianne Botelho.
Data and Safety Monitoring Board
(Voting Members): Lawrence S. Cohen, MD; Theodore Colton, ScD; Mark A. Espeland, PhD; Craig Henderson, MD; Alice H. Lichtenstein, ScD; Rebecca A. Silliman, MD, PhD; and Nanette Wenger, MD (Chair). Ex-officio members include Josephine Boyington, PhD, MPH; Rebecca Costello, PhD; Cindy Davis, PhD; Peter Greenwald, MD; Gabriela Riscuta, MD; and Harold Seifried, PhD.
Funding support:
The work is supported by grants (U01 CA138962, R01 CA138962, P01 CA87969, R01 CA137178, R35 CA197735, K99 CA215314, and R00 CA215314) from the National Cancer Institute, the National Heart, Lung, and Blood Institute, the Office of Dietary Supplements, the National Institute of Neurological Disorders and Stroke, and the National Center for Complementary and Integrative Health. M. Song was supported by a Mentored Research Scholar Grant in Applied and Clinical Research (MRSG-17-220-01 - NEC) from the American Cancer Society. A. Chan is a Stuart and Suzanne Steele MGH Research Scholar.
Pharmavite LLC of Northridge, California (vitamin D) and Pronova BioPharma of Norway and BASF (Omacor fish oil) donated the study agents, matching placebos, and packaging in the form of calendar packs. Quest Diagnostics (San Juan Capistrano, CA) measured serum 25-hydroxyvitamin D at no cost to the study.
VITAL was approved by the Institutional Review Board of Partners Healthcare/Brigham and Women’s Hospital, and the study agents have received Investigational New Drug Approval from the U.S. Food and Drug Administration.
VITAL is registered at clinicaltrials.gov (NCT01169259). The VITAL website is www.vitalstudy.org.
Role of funder/sponsor statement
The funding organization or sponsor had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Abbreviations
- 1,25(OH)2D
1,25-dihydroxyvitamin D
- 25(OH)D
25-hydroxyvitamin D
- BMI
body mass index
- CI
confidence interval
- OR
odds ratio
- RCT
randomized controlled trial
- VITAL
VITamin D and OmegA-3 TriaL
Footnotes
Conflict of interest:
Dr. Fuchs reports consulting role for Agios, Bain Capital, Bayer, Celgene, Dicerna, Five Prime Therapeutics, Gilead Sciences, Eli Lilly, Entrinsic Health, Genentech, KEW, Merck, Merrimack Pharmaceuticals, Pfizer, Sanofi, Taiho, and Unum Therapeutics. He also serves as a Director for CytomX Therapeutics and owns unexercised stock options for CytomX and Entrinsic Health. Dr. Meyerhardt is a consultant for Array pharmaceutical, Taiho, Ignyta, and COTA.
Data sharing statement:
The complete de-identified patient data set collected for the VITAL intervention phase will be made available to others upon request after November 10, 2020 (jmanson@rics.bwh.harvard.edu).
Access to data and data analysis
Drs. JoAnn E. Manson and Julie E. Buring, the principal investigators of VITAL, and Dr. Edward L Giovannucci, the principal investigator of the VITAL polyp ancillary study, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019. Epub 2019/01/09. [DOI] [PubMed] [Google Scholar]
- 2.Leggett B, Whitehall V. Role of the serrated pathway in colorectal cancer pathogenesis. Gastroenterology. 2010;138(6):2088–100. Epub 2010/04/28. [DOI] [PubMed] [Google Scholar]
- 3.Song M, Garrett WS, Chan AT. Nutrients, foods, and colorectal cancer prevention. Gastroenterology. 2015;148(6):1244–60 e16 Epub 2015/01/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feldman D, Krishnan AV, Swami S, et al. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer. 2014;14(5):342–57. Epub 2014/04/08. [DOI] [PubMed] [Google Scholar]
- 5.Giovannucci E, Liu Y, Rimm EB, et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst. 2006;98(7):451–9. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs ET, Hibler EA, Lance P, et al. Association between circulating concentrations of 25(OH)D and colorectal adenoma: a pooled analysis. Int J Cancer. 2013;133(12):2980–8. Epub 2013/06/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCullough ML, Zoltick ES, Weinstein SJ, et al. Circulating Vitamin D and Colorectal Cancer Risk: An International Pooling Project of 17 Cohorts. J Natl Cancer Inst. 2019;111(2):158–69. Epub 2018/06/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Touvier M, Chan DS, Lau R, et al. Meta-analyses of vitamin D intake, 25-hydroxyvitamin D status, vitamin D receptor polymorphisms, and colorectal cancer risk. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2011;20(5):1003–16. Epub 2011/03/08. [DOI] [PubMed] [Google Scholar]
- 9.Wactawski-Wende J, Kotchen JM, Anderson GL, et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. The New England journal of medicine. 2006;354(7):684–96. [DOI] [PubMed] [Google Scholar]
- 10.Scragg R, Khaw KT, Toop L, et al. Monthly High-Dose Vitamin D Supplementation and Cancer Risk: A Post Hoc Analysis of the Vitamin D Assessment Randomized Clinical Trial. JAMA Oncol. 2018;4(11):e182178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barry EL, Peacock JL, Rees JR, et al. Vitamin D Receptor Genotype, Vitamin D3 Supplementation, and Risk of Colorectal Adenomas: A Randomized Clinical Trial. JAMA Oncol. 2017;3(5):628–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lappe J, Watson P, Travers-Gustafson D, et al. Effect of Vitamin D and Calcium Supplementation on Cancer Incidence in Older Women: A Randomized Clinical Trial. JAMA. 2017;317(12):1234–43. [DOI] [PubMed] [Google Scholar]
- 13.Baron JA, Barry EL, Mott LA, et al. A Trial of Calcium and Vitamin D for the Prevention of Colorectal Adenomas. The New England journal of medicine. 2015;373(16):1519–30. Epub 2015/10/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goulao B, Stewart F, Ford JA, et al. Cancer and vitamin D supplementation: a systematic review and meta-analysis. Am J Clin Nutr. 2018;107(4):652–63. Epub 2018/04/11. [DOI] [PubMed] [Google Scholar]
- 15.Holick MF. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med. 2006;354(21):2287–8. [DOI] [PubMed] [Google Scholar]
- 16.Giovannucci E Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med. 2006;354(21):2287–8. [PubMed] [Google Scholar]
- 17.Forman MR, Levin B. Calcium plus vitamin D3 supplementation and colorectal cancer in women. N Engl J Med. 2006;354(7):752–4. Epub 2006/02/17. [DOI] [PubMed] [Google Scholar]
- 18.Manson JE, Bassuk SS, Lee IM, et al. The VITamin D and OmegA-3 TriaL (VITAL): rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp Clin Trials. 2012;33(1):159–71. Epub 2011/10/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manson JE, Cook NR, Lee IM, et al. Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. The New England journal of medicine. 2019;380(1):33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bassuk SS, Manson JE, Lee IM, et al. Baseline characteristics of participants in the VITamin D and OmegA-3 TriaL (VITAL). Contemp Clin Trials. 2016;47:235–43. Epub 2016/01/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. The Journal of clinical endocrinology and metabolism. 2011;96(7):1911–30. Epub 2011/06/08. [DOI] [PubMed] [Google Scholar]
- 22.Zgaga L, Theodoratou E, Farrington SM, et al. Plasma vitamin D concentration influences survival outcome after a diagnosis of colorectal cancer. J Clin Oncol. 2014;32(23):2430–9. Epub 2014/07/09. [DOI] [PubMed] [Google Scholar]
- 23.Vaughan-Shaw PG, Zgaga L, Ooi LY, et al. Low plasma vitamin D is associated with adverse colorectal cancer survival after surgical resection, independent of systemic inflammatory response. Gut. 2019. Epub 2019/04/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ng K, Meyerhardt JA, Wu K, et al. Circulating 25-hydroxyvitamin d levels and survival in patients with colorectal cancer. J Clin Oncol. 2008;26(18):2984–91. Epub 2008/06/21. [DOI] [PubMed] [Google Scholar]
- 25.Ng K, Nimeiri HS, McCleary NJ, et al. Effect of High-Dose vs Standard-Dose Vitamin D3 Supplementation on Progression-Free Survival Among Patients With Advanced or Metastatic Colorectal Cancer: The SUNSHINE Randomized Clinical Trial. JAMA. 2019;321(14):1370–9. Epub 2019/04/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Urashima M, Ohdaira H, Akutsu T, et al. Effect of Vitamin D Supplementation on Relapse-Free Survival Among Patients With Digestive Tract Cancers: The AMATERASU Randomized Clinical Trial. JAMA. 2019;321(14):1361–9. Epub 2019/04/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Fang F, Tang J, et al. Association between vitamin D supplementation and mortality: systematic review and meta-analysis. BMJ. 2019;366:l4673 Epub 2019/08/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rashtak S, Rego R, Sweetser SR, et al. Sessile Serrated Polyps and Colon Cancer Prevention. Cancer Prev Res (Phila). 2017;10(5):270–8. Epub 2017/03/23. [DOI] [PubMed] [Google Scholar]
- 29.Bailie L, Loughrey MB, Coleman HG. Lifestyle Risk Factors for Serrated Colorectal Polyps: A Systematic Review and Meta-analysis. Gastroenterology. 2017;152(1):92–104. Epub 2016/09/19. [DOI] [PubMed] [Google Scholar]
- 30.Burnett-Hartman AN, Passarelli MN, Adams SV, et al. Differences in epidemiologic risk factors for colorectal adenomas and serrated polyps by lesion severity and anatomical site. Am J Epidemiol. 2013;177(7):625–37. Epub 2013/03/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davenport JR, Su T, Zhao Z, et al. Modifiable lifestyle factors associated with risk of sessile serrated polyps, conventional adenomas and hyperplastic polyps. Gut. 2018;67(3):456–65. Epub 2016/11/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He X, Wu K, Ogino S, et al. Association Between Risk Factors for Colorectal Cancer and Risk of Serrated Polyps and Conventional Adenomas. Gastroenterology. 2018;155(2):355–73 e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi YJ, Kim YH, Cho CH, et al. Circulating levels of vitamin D and colorectal adenoma: A case-control study and a meta-analysis. World J Gastroenterol. 2015;21(29):8868–77. Epub 2015/08/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei MY, Garland CF, Gorham ED, et al. Vitamin D and prevention of colorectal adenoma: a meta-analysis. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2008;17(11):2958–69. Epub 2008/11/08. [DOI] [PubMed] [Google Scholar]
- 35.Crockett SD, Barry EL, Mott LA, et al. Calcium and vitamin D supplementation and increased risk of serrated polyps: results from a randomised clinical trial. Gut. 2018. Epub 2018/03/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fiscella K, Winters P, Tancredi D, et al. Racial disparity in death from colorectal cancer: does vitamin D deficiency contribute? Cancer. 2011;117(5):1061–9. Epub 2010/10/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pibiri F, Kittles RA, Sandler RS, et al. Genetic variation in vitamin D-related genes and risk of colorectal cancer in African Americans. Cancer causes & control : CCC. 2014;25(5):561–70. Epub 2014/02/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andersen SW, Shu XO, Cai Q, et al. Total and Free Circulating Vitamin D and Vitamin D-Binding Protein in Relation to Colorectal Cancer Risk in a Prospective Study of African Americans. Cancer Epidemiol Biomarkers Prev. 2017;26(8):1242–7. Epub 2017/05/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gibbs DC, Fedirko V, Um C, et al. Associations of Circulating 25-Hydroxyvitamin D3 Concentrations With Incident, Sporadic Colorectal Adenoma Risk According to Common Vitamin D-Binding Protein Isoforms. Am J Epidemiol. 2018;187(9):1923–30. Epub 2018/05/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.