Abstract
Addictions are characterized by choices made to satisfy the addiction despite the risk it could produce an adverse consequence. Here, we developed a murine version of a ‘risky decision-making’ task (RDT), in which mice could respond on a touchscreen panel to obtain either a large milkshake reward associated with varying probability of footshock, or a smaller amount of the same reward that was never punished. Results showed that mice shifted choice from the large to small reward stimulus as shock probability increased. Immunohistochemical analysis revealed more Fos-positive cells in prelimbic cortex (PL) and basal amygdala (BA) after RDT testing, and a strong anti-correlation between infralimbic cortex (IL) activity and choice of the large reward stimulus under likely (75–100% probability) punishment. These findings establish an assay for risky choice in mice and provide preliminary insight into the underlying neural substrates.
Introduction
A defining feature of addictions is that users continue to seek out and engage with the addicted substance/activity despite the threat of danger, illness, or other adverse consequence. As a basis for studying the underlying neurobiology of such compulsive behavior in addictions, various methods in rodents have been devised to assess the persistence of reward seeking behavior in the face of potential punishment [1–5]. Many of these paradigms draw on a long literature describing the suppressive effects of electric shock on an instrumental response for reward [6–9].
In a recent example of this approach, Setlow and colleagues developed an assay for assessing the willingness of rats to make an operant response (lever press or nose poke) for a preferred reward (large versus small quantity) despite an increasing probability of concurrent footshock [10, 11]. These authors have employed the rat ‘risky decision-making’ task (RDT) to examine the effects of exposure to abused drugs, reveal individual differences in tolerance of risk, and examine neural substrates of a risky choice [12–18].
The main aim of the current study was to develop a murine version of the RDT in order to exploit the powerful genetic tools that can be employed in the mouse to study neural substrates of risky choice behavior. With that goal in mind, immediate-early gene (Fos) mapping was conducted as a preliminary step towards identifying neural correlates of RDT testing in the mouse.
To accomplish these aims, 8 male and 7 female 8 week old C57BL/6J mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA) and housed (2–4 same-sex mice per cage in a temperature- and humidity-controlled vivarium under a 12 h light/dark cycle (lights on 0630h, testing during light on phase). Mice were 9 weeks old at the start of testing and 13 weeks old at its completion. Experimental procedures were approved by the NIAAA Animal Care and Use Committee and followed the NIH guidelines outlined in Using Animals in Intramural Research and the local Animal Care and Use Committees. While the sample constituted both sexes, the study was not explicitly designed or powered to test for potential sex differences [19]. C57BL/6J was chosen as the representative genetic strain for most murine addiction work [20]; however, there are likely marked strain differences in RDT performance, as there are for other measures of punished reward seeking behavior [21].
The RDT was modified from a version of the task developed for rats [18], adapted for use in the Bussey-Saksida Touch Screen System (model 80614, Lafayette Instruments, Lafayette, IN, USA) [22, 23] (Figure 1A–D). Body weight was maintained at 85% free feeding weight throughout testing to motivate responding. Mice were first familiarized with 2 mL of the liquid reward (Yazoo strawberry milkshake, FrieslandCampina, Amersfoort, Netherlands) presented in plastic dish in the home cage for 30 minutes. The next day, mice had as single session in the test apparatus with reward available in the reward receptacle.
Figure 1: Touchscreen-based risky decision-making task (RDT) for mice.
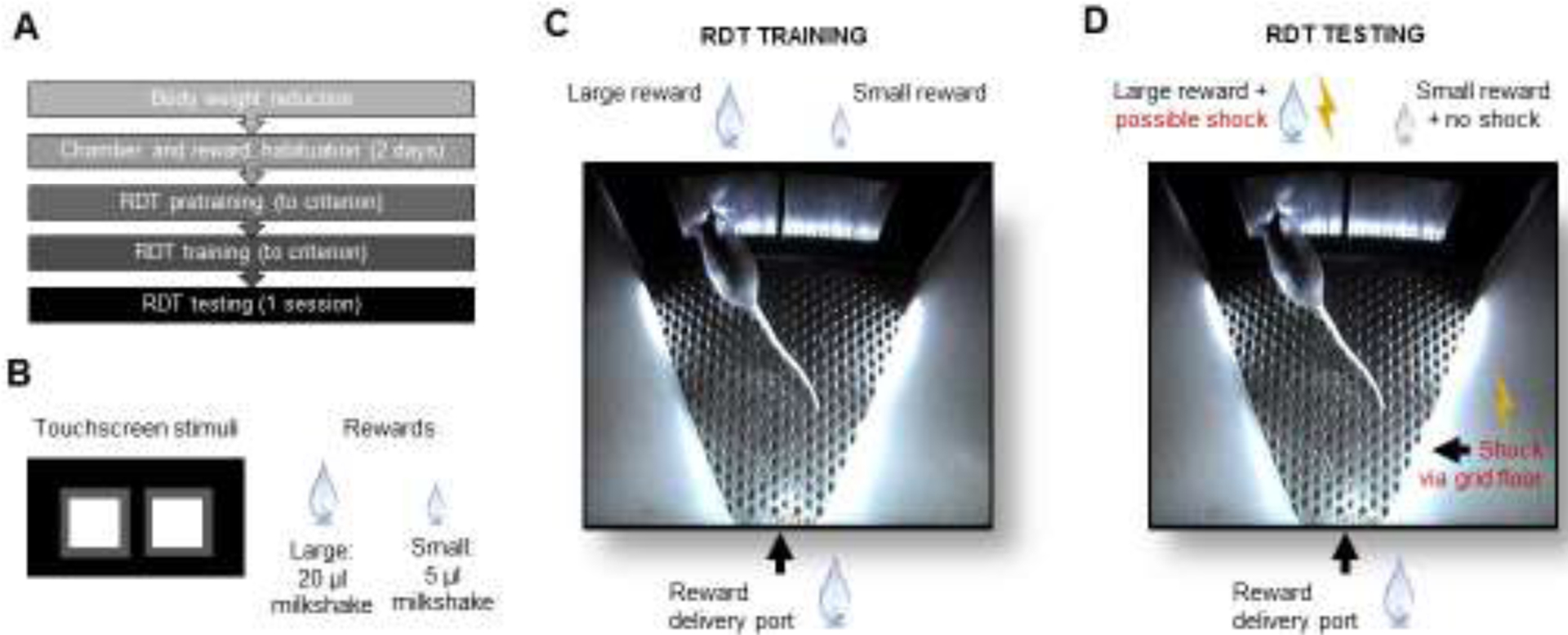
(A) Sequence of experimental stages. (B) Mouse-eye view of the choice-stimuli displayed on the touchscreen. Large and small reward options. (C) During RDT training, response on the left versus right stimulus produces the large or small reward, respectively. (D) During RDT testing, response on the left versus right stimulus produces the large reward and possible shock or small reward and no shock, respectively.
Pre-training began on the following session. Mice were trained to associate the randomly timed dispensing of 40 × 5 μL rewards with the presentation of a 2-second, 65-dB tone and illumination of the magazine light. Mice consuming all 40 rewards in a 30 minute session proceeded to instrumental pre-training, which entailed 3 successive phases, as in other touchscreen tasks [24]. In phase 1, the mouse initiated a trial by placing its head into the food magazine (as detected by an infrared beam), which extinguished the magazine light and resulted in the appearance of a 6.5 cm2 2-dimensional patterned stimulus (randomly selected from a stimulus-set) in 1 of the 2 touchscreen windows for 20 seconds. When the stimulus disappeared, reward was delivered in conjunction with the tone and magazine light cues.
Mice obtaining 30 or more rewards in the 30 minute session moved to phase 2. In phase 2, the mouse was required to touch the window in which a stimulus was presented to receive a reward. Mice obtaining 30 or more rewards in the 30 minute session moved to phase 3. Phase 3 was the same as phase 2, with the exception that touches of the window not containing the timulus extinguished the house light and produced a 20 second ‘timeout’ period during which a new trial could not be initiated. If no response was made, the same stimulus was presented in the same window until a response was made. Mice touching the stimulus-containing screen on >75% of 30 trials (excluding non-responses) within a 30 minute session moved to training proper. Mice completed pre-training in 6.8 ± 0.6 sessions.
Reward discrimination training began with forced trials (Figure 2A–B), which entailed presentation of a 6.5 cm2 white square stimulus for 20 seconds in 1 of the touchscreen windows. Touching the stimulus extinguished the stimulus and resulted in either a 5 μL (small) or 20 μL (large) reward. Touching the blank window had no programmed consequences. No touch produced a 20 second timeout (house light extinguished, no trial initiation possible); this trial was recorded as an omission and repeated later in the session. An 8 second period was imposed after each trial before the next trial could be initiated (with a magazine head entry). The left/right location of the small and large reward stimulus was fixed for each mouse and counterbalanced across mice. Presentation of small and large reward trials was randomized across trials. After completing 20x small and 20x large of these forced trials (excluding omission trials), the session continued with choice trials.
Figure 2: Schematics of task-flow during RDT training and testing.
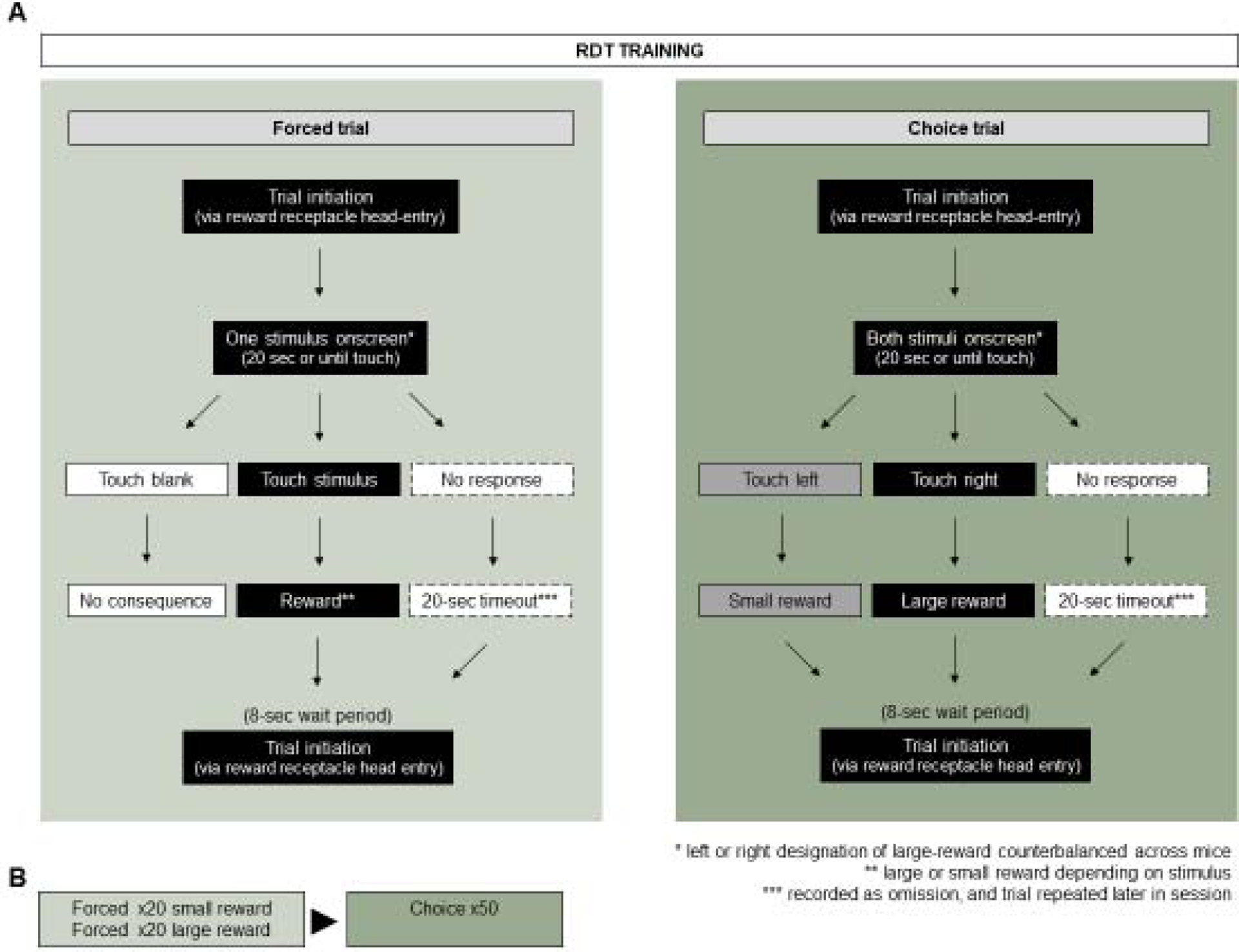
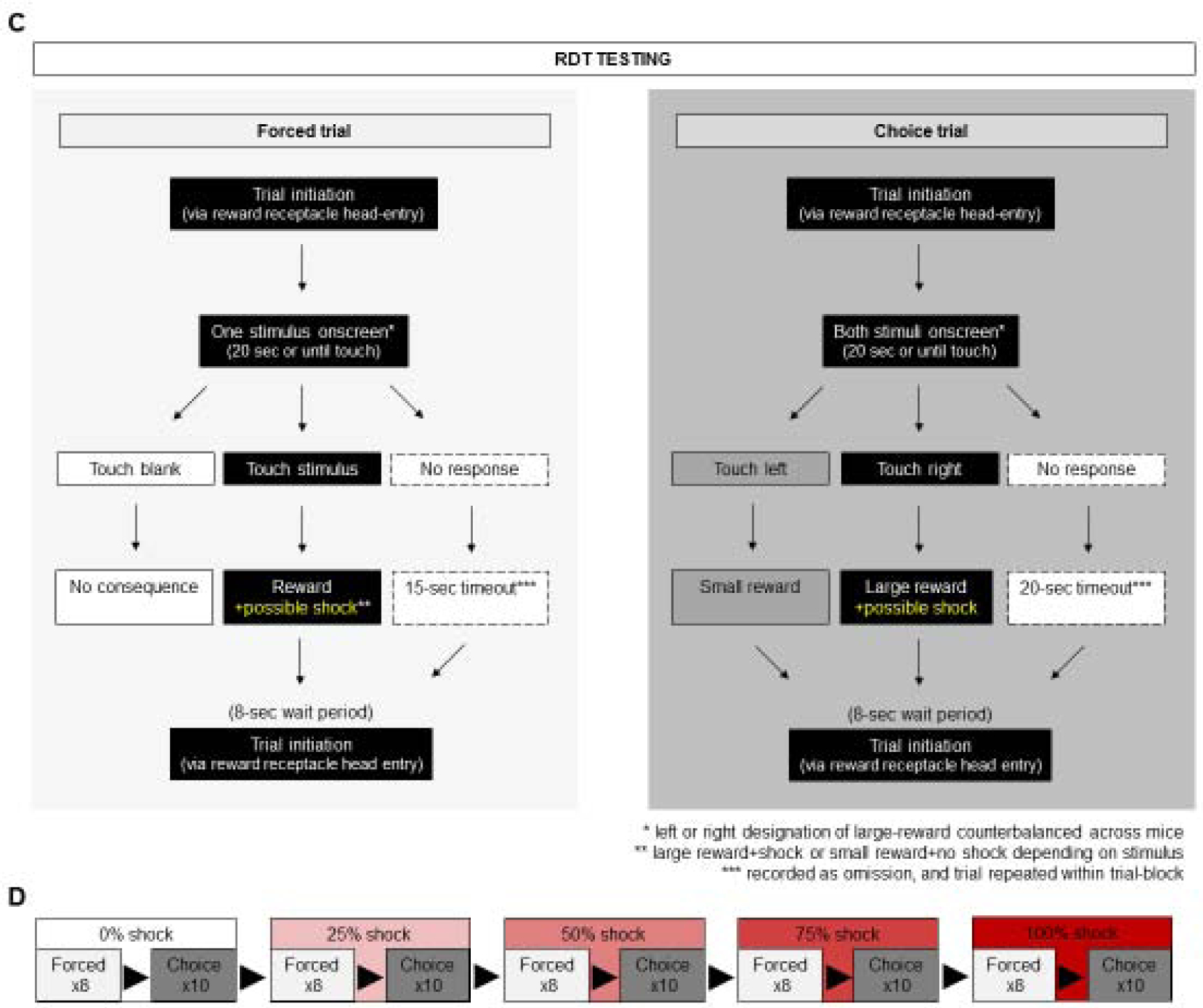
(A) Task-flow on forced and choice trials during RDT training. (B) RDT training sessions comprise 40-forced trials followed by 50 choice-trials. (C) Task-flow on forced and choice trials during RDT testing. (D) RDT training sessions comprise 5 successive blocks of 8-forced trials followed by 10 choice-trials, in which shock-probability following a large reward choice increases in 25% increments.
Choice trials entailed 50x simultaneous presentations of both stimuli for 20 seconds. The mouse was free to touch either the small or large reward stimulus to obtain the corresponding reward and extinguish the house stimulus. No touch within 20 seconds produced a 20 second timeout and the trial was recorded as an omission. An 8 second period was imposed after each trial before the next trial could be initiated (with a magazine head entry). Training continued over daily sessions until all 90 trials were completed (40x forced, 50x free) within 90 minutes, and the large reward stimulus was selected on >80% of the choice trials on each of 2 consecutive sessions. Mice completed RDT training in 4.2 ± 0.2 sessions.
RDT testing (Figure 2C,D) was organized in 5× 18-trial blocks. Each block began with 8x forced (4x small, 4x large) trials, entailing the same procedure as RDT training except for the possibility that touching the large reward stimulus would result in concomitant delivery of a 0.1 mA, 0.5-second, scrambled footshock through the grid floor. Within each block, forced trials were followed by 10x choice trials, entailing the same procedure as RDT training except for continued possibility of footshock. The probability of shock for forced and choice trials increased across the 5 blocks: 0%, 25%, 50%, 75%, and 100%. A no-touch choice on forced or choice trials produced a timeout of 20 seconds and the trial was recorded as an omission. Each trial was initiated by a head entry, with a minimum wait time of 8 seconds (no maximum) imposed after reward collection/timeout. The session ended after all 90 trials were completed or 90 minutes had elapsed. The maximum session duration mice were given to complete training and testing was 90 minutes (average time taken to complete testing = 53.05 ± 23.33 minutes). Data were excluded for 1 mouse not exhibiting >80% large reward stimulus preference on the 0% probability of shock during a forced trial block.
For RDT choice trials, reward preference (number of large and small reward stimulus choices made in each choice block of the 10 total trials presented), omission rate (number of omissions per 10 trial choice block), and their choice latency were calculated and analyzed with a repeated measures (for trial block) analysis of variance (ANOVA), followed by Bonferroni corrected paired t-tests (significance threshold: P<.05).
Results showed that preference for the large reward stimulus decreased (F(4,44)=22.04, P<.01), and preference for the small reward stimulus increased (F(4,44)=4.03, P=.007). This decrease was evident across the choice trial blocks, such that the large reward stimulus was significantly preferred for on the 0%, 25%, or 50% blocks, but large versus small stimulus choice was equivalent on the 75% and 100% blocks (Figure 3A). The rate of omission (F(4,44)=7.03, P<.001) and the latency to choose large (F(4,45)=2.66, P=.05), but not small (P>.05), reward stimulus also increased across the choice trial blocks though, interestingly, the increase in both measures reached significance on the 75% and not 100%, relative to the 0% probability block (Figure 3B,C). The 75% probability block may be a critical stage in the transition from large to small stimulus preference, such that mice retained some willingness to choose the large reward the despite the risk but did so hesitantly, unlike at the 100% probability, where choice had shifted almost entirely to the small reward option.
Figure 3: Performance during RDT testing.
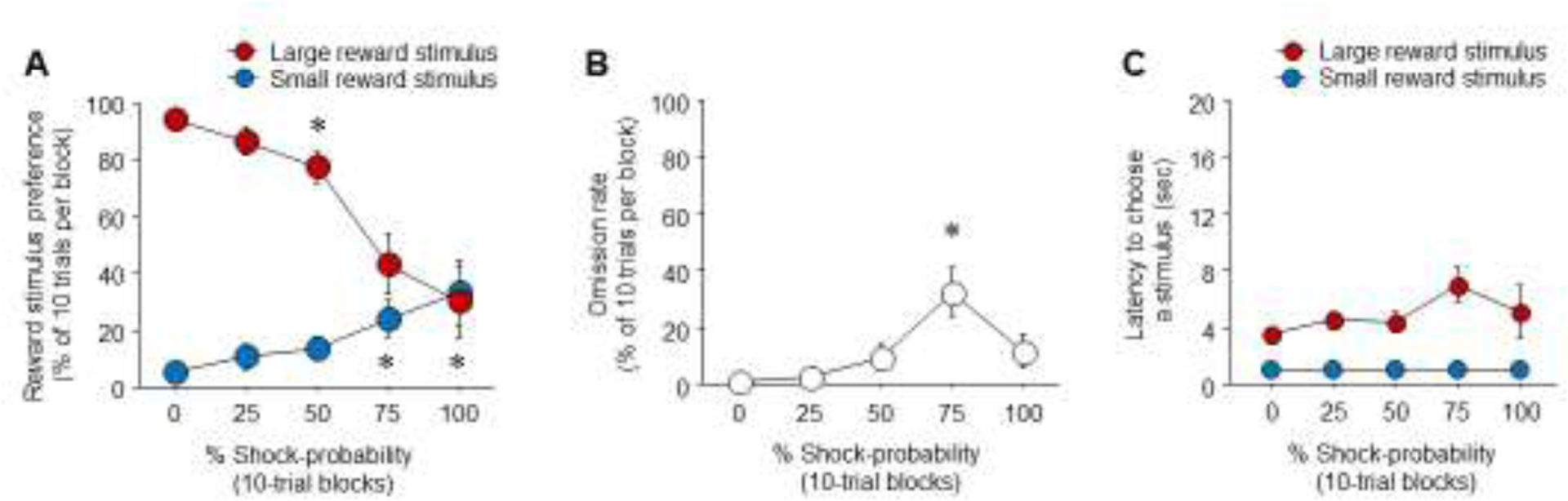
(A) Reward preference shifts from the large to small reward option with increasing probability the large reward option will result in shock. (B) Increased omission rate at the 75% shock-probability block. (C) Increased latency to choose the large reward stimulus at the 75% shock-probability block. #versus small reward on same shock-probability block. n=11. Data are means ± SEM.
Overall, these patterns of RDT in C57BL/6J mice are largely in line with those previously reported by Setlow and colleagues in rats [12–18], despite some noteworthy methodological differences including current use of a fixed (versus individually calibrated) intensity of shock across subjects and a single (versus repeated) RDT testing session. This latter difference is notable in view of the demands being placed on the subject in a single session – i.e., online learning about the changing task contingencies and the resultant modification of behavior accordingly – as opposed to the expression of relatively stable performance after repeated sessions [15]. Therefore, the effects of a manipulation that alters single-session performance, or the observation of neural correlates of such performance (see Fos data below), must be interpreted with an awareness that multiple factors are likely at play.
At the completion of testing, mice were sacrificed for RDT-related Fos analysis in a set of brain regions implicated in punishment and conflict [25]. Another group underwent the same procedures except that no shock (NS) was delivered during RDT testing, and as expected, showed no change in behavior changed across ‘shock’ trial blocks (Figure S1). In both groups, 30 minutes after the session (120 minutes post start), mice were deeply anaesthetized with a ketamine/xylazine cocktail and transcardially perfused with ice cold phosphate buffered saline (PBS, pH 7.4) followed by ice cold 4% paraformaldehyde (PFA). Coronal sections (50 μm thick) were cut on a vibratome (Leica VT1000 S, Leica Biosystems Inc, Buffalo Grove, IL, USA) and stored free floating in phosphate buffer (PB) 0.1M at 4° C for >1 week.
Sections were rinsed 3X for 10 minutes in PBS, blocked in 10% normal goat serum, and 1% bovine serum albumin in PBS-TritonX (0.3%) for 2 hours and incubated over 2 nights in rabbit anti-c-Fos (9F6) (catalogue# 2250S, 1:1000, Cell Signaling Technology, Danvers, MA, USA) in a dilution of 1% normal goat serum and 0.1% bovine serum albumin in PBS-TritonX (0.3%) at 4°C on a platform rocker. Sections were then rinsed 3X for 10 minutes in PBS and incubated in anti-rabbit Alexa 488 secondary antibody (catalogue# A-11034, 1:500, Invitrogen, Eugene, OR, USA) in a dilution of 1% normal goat serum and 0.1% bovine serum albumin in PBS-TritonX (0.3%) at room temperature on a platform rocker for 2 hours. Sections were rinsed in PBS 2X for 10 minutes and then counterstained with Hoechst 33342 (5 μg/mL, Life Technologies, H1399) in PBS and rinsed 1X 0.1M PB for 10 minutes. Serial sections were mounted on slides, air dried, and coverslipped with Fluoromount Aqueous Mounting Medium (product#: F4680, Sigma Aldrich) and sealed with clear nail polish.
Images for all the sections were acquired using a slide scanner (Olympus, BX61, VS120 with VS_ASW software) with a 20x objective (U Plan S Apo; 20x NA 0.75). For image analysis, the VSI reader plugin (BIOP, Zurich, Switzerland) for Fiji software (https://imagej.net/Fiji) [26] was used and a contour of each brain region of interest manually drawn with reference to a brain atlas to obtain high resolution images. Fos-positive cells were quantified in a semi-automated manner using a custom written macro. Parameters for nuclei size and circularity were manually adjusted for each region. Cells were counted (blind to group) in 3 sections from each hemisphere, for 6 datapoints per region/mouse. Correction for double counting was unnecessary because sections were nonconsecutive.
Counts were made in a total of eleven brain regions: prelimbic cortex (PL) (from AP= +1.42, ML= ±0.05, DV= −1.5 to AP= +2.0, ML= ±0.25, DV= −2.25), infralimbic cortex (IL) (from AP= +2.0, ML= ±0.05, DV= −2.5 to AP= +1.54, ML= ±0.25, DV= −3.5), basolateral amygdala (BA) (from AP= −1.15, ML= ±2.75, DV= −5.0 to AP= −2.56, ML= ±3.65, DV= −6.0), medial habenula (MHb) (from AP= −0.94, ML= ±0.23, DV= −2.25 to AP= −2.05, ML= ±0.45, DV= −2.75), lateral habenula (LHb) (from AP= −1.05, ML= ±0.35, DV= −2.4 to AP= −2.15, ML= ±0.95, DV= −3.15), dorsal hippocampal CA3 (from AP= −1.54, ML= ±1.45, DV= −1.95 to AP= −2.48, ML= ±2.65, DV= −3.0), and dorsal hippocampal dentate gyrus (from AP= −1.54, ML= ±0.45, DV= −2.05 to AP = −2.48, ML= ±2.25, DV= −2.35).
Groups were compared for Fos counts using the Games-Howell test to accommodate for unequal sample size in the groups. This indicated significant but selective differences across the 7 regions examined. The RDT group had more Fos-positive cells in PL and BA, but fewer cells in mHb, relative to the NS controls (all P<0.05), and similar counts in IL, lHb, CA3, or DG (Figure 3B–F, S2). An important caveat to these data is that although the NS and RDT groups had similar food restriction and training histories, and equivalent exposure to the test chamber and to handling during the test session (as opposed to, e.g., a simple home cage control), their test experience differed in various ways in addition to whether they or not they were asked to make ‘risky decisions,’ including shock-receipt, rewards earned and general activity level. These variables could be controlled to some extent by, for example, using a yoked group in which the task parameters matched those the risky group with the exception of the shock being presented at random and therefore uncoupled from the response for the large reward [1].
Nonetheless, PL and BA activation after RDT testing generally agree with the engagement of these regions in tasks that involve learning about associations between discrete environmental stimuli and shock, or conflict between approach and avoidance responses [27]. Human functional neuroimaging and rodent behavioral studies have implicated the lHB habenula in various measures of punishment [28], whereas the mHb has been less well studied. Intriguingly, however, the mHb is proposed to have a role in motivation and drug withdrawal negative states [29, 30], encouraging further investigation of the lesser mHb activation seen after RDT testing.
Next, we performed Pearson’s correlations to examine whether regional Fos activity was related to differences in RDT performance across individual mice. Interestingly, only one brain region, IL, showed a relationship between activity and reward preference that varied with shock probability. Fos counts in IL were significantly anti-correlated with %large reward stimulus choice, specifically at the 75% (r= −0.78, Fisher z-transformation P<.001) and 100% (r= −0.71, Fisher z-transformation P<.001), but not lower, shock probability blocks (Figure 3G). By contrast, IL Fos counts did not significantly correlate with %small reward stimulus at any shock probability (all P>.05, highest value, 75% shock r= 0.55).
Thus, despite the absence of an overall group difference in IL Fos counts between the RDT tested mice and controls, within the RDT group higher IL IEG activity strongly associated with greater avoidance of the large reward stimulus when it was likely to result in shock. This finding is consistent with a role for IL in inhibiting and flexibly adapting reward seeking under punishment in other behavioral tasks [31–33]. Indeed, pharmacological inactivation of IL and PL has recently been shown to increase punished large reward stimulus choice in the rat RDT task when shock probabilities increase over trial blocks, as in the current study [16]. These data provide a foundation from which to interrogate the role of these mPFC regions and their circuit connections using the mouse version of the RDT task.
In sum, the current findings provide an initial characterization of a murine version of an RDT task originally developed for rats [10, 11] and preliminary evidence of corticolimbic regions mediating this measure of risky choice decisions.
Supplementary Material
Figure S1: Performance of non-shocked IEG controls during RDT testing. (A) Reward preference did not shift across trial-blocks when the large reward option will not result in shock. (B) Omission rate remained unchanged across trial-blocks. (C) Choice-latency did not change across trial-blocks. n=4. Data are means ± SEM.
Figure S2: IEG activity associated with RDT testing. Examples of Fos immunohistochemical labeling in RDT tested mice (A,B) and controls (C,D). (E) No difference in Fos counts in CA3. (F) No difference in Fos counts in DG. n=4–11. Scale bars=1 mm. Data are means ± SEM.
Figure 4: IEG activity associated with RDT testing.
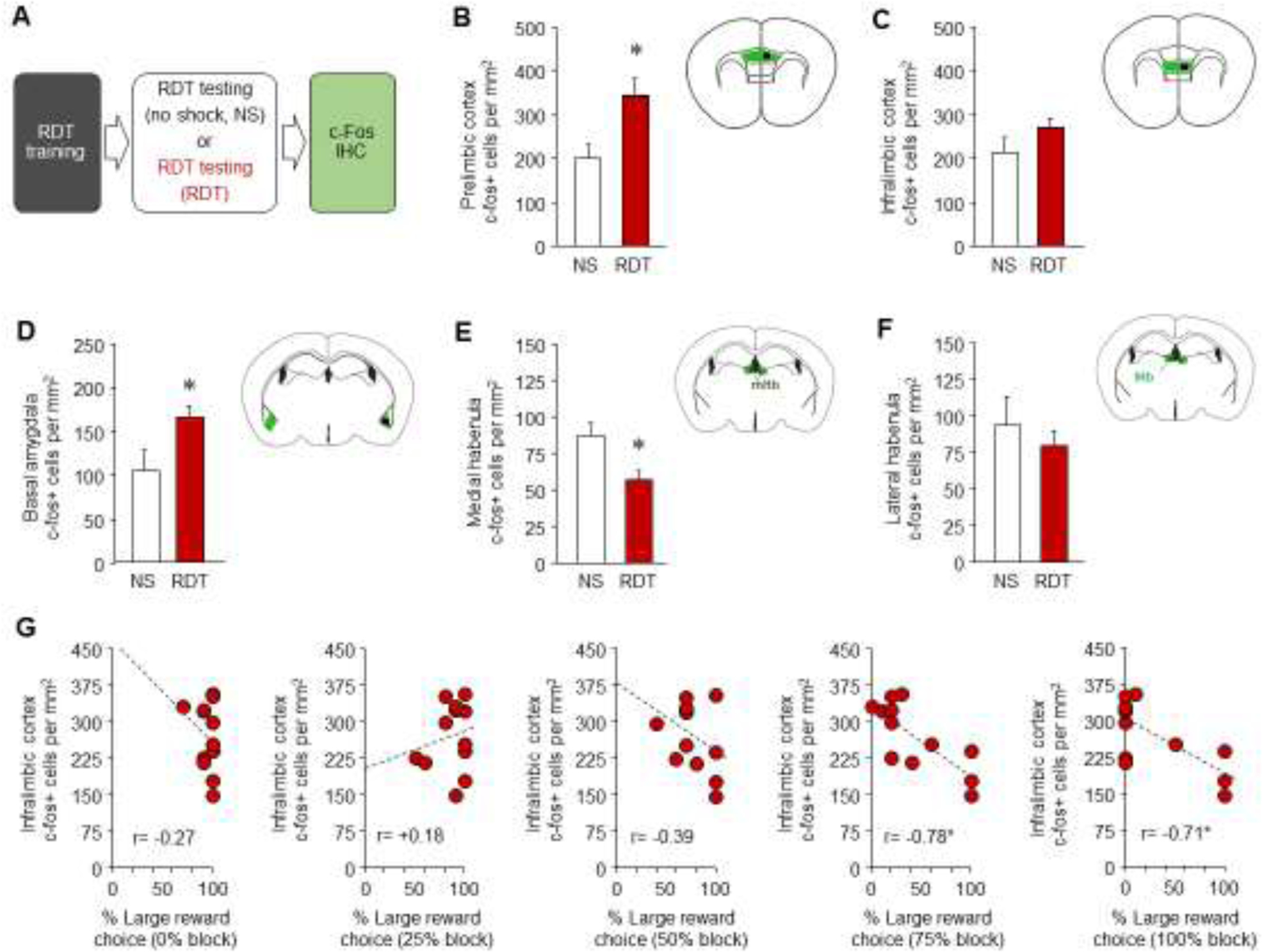
(A) Immunohistochemical labeling and quantification of Fos-positive cells was conducted after RDT testing or a non-shocked (NS) RDT testing equivalent session. (B) Higher Fos counts in PL after RDT. (C) No difference in Fos counts in IL. (D) Higher Fos counts in BA after RDT. (E) Lower Fos counts in mHb after RDT. (F) No difference in Fos counts in lHb. (G) Fos counts in IL anti-correlate with large reward choice during the 75% and 100% shock-probability blocks. *versus NS. n=11 RDT, n=4 NS. Data are means ± SEM.
Acknowledgements
Research supported by the NIAAA Intramural Research Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Jean-Richard-Dit-Bressel P, Killcross S, McNally GP, Behavioral and neurobiological mechanisms of punishment: implications for psychiatric disorders, Neuropsychopharmacology 43(8) (2018) 1639–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Orsini CA, Blaes SL, Setlow B, Simon NW, Recent Updates in Modeling Risky Decision Making in Rodents, Methods Mol Biol 2011 (2019) 79–92. [DOI] [PubMed] [Google Scholar]
- [3].Hopf FW, Lesscher HM, Rodent models for compulsive alcohol intake, Alcohol 48(3) (2014) 253–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Marchant NJ, Campbell EJ, Pelloux Y, Bossert JM, Shaham Y, Context-induced relapse after extinction versus punishment: similarities and differences, Psychopharmacology (Berl) 236(1) (2019) 439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Everitt BJ, Giuliano C, Belin D, Addictive behaviour in experimental animals: prospects for translation, Philos Trans R Soc Lond B Biol Sci 373(1742) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Vogel JR, Beer B, Clody DE, A simple and reliable conflict procedure for testing anti-anxiety agents, Psychopharmacologia 21(1) (1971) 1–7. [DOI] [PubMed] [Google Scholar]
- [7].Evenden JL, Varieties of impulsivity, Psychopharmacology (Berl) 146(4) (1999) 348–61. [DOI] [PubMed] [Google Scholar]
- [8].Pollard GT, Howard JL, The Geller-Seifter conflict paradigm with incremental shock, Psychopharmacology (Berl) 62(2) (1979) 117–21. [DOI] [PubMed] [Google Scholar]
- [9].Hunt HF, Brady JV, Some Effects of Punishment and Intercurrent Anxiety on a Simple Operant, Journal of Comparative and Physiological Psychology 48(4) (1955) 305–310. [DOI] [PubMed] [Google Scholar]
- [10].Orsini CA, Moorman DE, Young JW, Setlow B, Floresco SB, Neural mechanisms regulating different forms of risk-related decision-making: Insights from animal models, Neurosci Biobehav Rev 58 (2015) 147–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Simon NW, Gilbert RJ, Mayse JD, Bizon JL, Setlow B, Balancing risk and reward: a rat model of risky decision making, Neuropsychopharmacology 34(10) (2009) 2208–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Orsini CA, Blaes SL, Dragone RJ, Betzhold SM, Finner AM, Bizon JL, Setlow B, Distinct relationships between risky decision making and cocaine self-administration under short-and long-access conditions, Prog Neuropsychopharmacol Biol Psychiatry 98 (2020) 109791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Blaes SL, Orsini CA, Mitchell MR, Spurrell MS, Betzhold SM, Vera K, Bizon JL, Setlow B, Monoaminergic modulation of decision-making under risk of punishment in a rat model, Behav Pharmacol 29(8) (2018) 745–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mitchell MR, Weiss VG, Beas BS, Morgan D, Bizon JL, Setlow B, Adolescent risk taking, cocaine self-administration, and striatal dopamine signaling, Neuropsychopharmacology 39(4) (2014) 955–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Deng JV, Orsini CA, Shimp KG, Setlow B, MeCP2 Expression in a Rat Model of Risky Decision Making, Neuroscience 369 (2018) 212–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Orsini CA, Heshmati SC, Garman TS, Wall SC, Bizon JL, Setlow B, Contributions of medial prefrontal cortex to decision making involving risk of punishment, Neuropharmacology 139 (2018) 205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Orsini CA, Hernandez CM, Singhal S, Kelly KB, Frazier CJ, Bizon JL, Setlow B, Optogenetic Inhibition Reveals Distinct Roles for Basolateral Amygdala Activity at Discrete Time Points during Risky Decision Making, J Neurosci 37(48) (2017) 11537–11548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Orsini CA, Trotta RT, Bizon JL, Setlow B, Dissociable roles for the basolateral amygdala and orbitofrontal cortex in decision-making under risk of punishment, J Neurosci 35(4) (2015) 1368–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Orsini CA, Willis ML, Gilbert RJ, Bizon JL, Setlow B, Sex differences in a rat model of risky decision making, Behav Neurosci 130(1) (2016) 50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Radke AK, Jury NJ, Kocharian A, Marcinkiewcz CA, Lowery-Gionta EG, Pleil KE, McElligott ZA, McKlveen JM, Kash TL, Holmes A, Chronic EtOH effects on putative measures of compulsive behavior in mice, Addict Biol 22(2) (2017) 423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Halladay LR, Kocharian A, Holmes A, Mouse strain differences in punished ethanol self-administration, Alcohol 58 (2017) 83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bergstrom HC, Lipkin AM, Lieberman AG, Pinard CR, Gunduz-Cinar O, Brockway ET, Taylor WW, Nonaka M, Bukalo O, Wills TA, Rubio FJ, Li X, Pickens CL, Winder DG, Holmes A, Dorsolateral Striatum Engagement Interferes with Early Discrimination Learning, Cell Rep 23(8) (2018) 2264–2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Radke AK, Kocharian A, Covey DP, Lovinger DM, Cheer JF, Mateo Y, Holmes A, Contributions of nucleus accumbens dopamine to cognitive flexibility, The European journal of neuroscience (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Brigman JL, Ihne J, Saksida LM, Bussey TJ, Holmes A, Effects of Subchronic Phencyclidine (PCP) Treatment on Social Behaviors, and Operant Discrimination and Reversal Learning in C57BL/6J Mice, Front Behav Neurosci 3 (2009) 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Whittle N, Hauschild M, Lubec G, Holmes A, Singewald N, Rescue of impaired fear extinction and normalization of cortico-amygdala circuit dysfunction in a genetic mouse model by dietary zinc restriction, J Neurosci 30(41) (2010) 13586–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A, Fiji: an open-source platform for biological-image analysis, Nat Methods 9(7) (2012) 676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Diehl MM, Bravo-Rivera C, Rodriguez-Romaguera J, Pagan-Rivera PA, Burgos-Robles A, Roman-Ortiz C, Quirk GJ, Active avoidance requires inhibitory signaling in the rodent prelimbic prefrontal cortex, Elife 7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lawson RP, Seymour B, Loh E, Lutti A, Dolan RJ, Dayan P, Weiskopf N, Roiser JP, The habenula encodes negative motivational value associated with primary punishment in humans, Proc Natl Acad Sci U S A 111(32) (2014) 11858–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Batalla A, Homberg JR, Lipina TV, Sescousse G, Luijten M, Ivanova SA, Schellekens AFA, Loonen AJM, The role of the habenula in the transition from reward to misery in substance use and mood disorders, Neurosci Biobehav Rev 80 (2017) 276–285. [DOI] [PubMed] [Google Scholar]
- [30].Molas S, DeGroot SR, Zhao-Shea R, Tapper AR, Anxiety and Nicotine Dependence: Emerging Role of the Habenulo-Interpeduncular Axis, Trends Pharmacol Sci 38(2) (2017) 169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Halladay LR, Kocharian A, Piantadosi PT, Authement ME, Lieberman AG, Spitz NA, Coden K, Glover LR, Costa VD, Alvarez VA, Holmes A, Prefrontal Regulation of Punished Ethanol Self-administration, Biol Psychiatry (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Cameron CM, Murugan M, Choi JY, Engel EA, Witten IB, Increased Cocaine Motivation Is Associated with Degraded Spatial and Temporal Representations in IL-NAc Neurons, Neuron 103(1) (2019) 80–91 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].St Onge JR, Floresco SB, Prefrontal cortical contribution to risk-based decision making, Cereb Cortex 20(8) (2010) 1816–28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Performance of non-shocked IEG controls during RDT testing. (A) Reward preference did not shift across trial-blocks when the large reward option will not result in shock. (B) Omission rate remained unchanged across trial-blocks. (C) Choice-latency did not change across trial-blocks. n=4. Data are means ± SEM.
Figure S2: IEG activity associated with RDT testing. Examples of Fos immunohistochemical labeling in RDT tested mice (A,B) and controls (C,D). (E) No difference in Fos counts in CA3. (F) No difference in Fos counts in DG. n=4–11. Scale bars=1 mm. Data are means ± SEM.


