Abstract
Background & aims
The objective of the present study was to determine, for the first time, the prevalence and clinical features of food allergy in Portuguese adolescents.
Methods
Cross-sectional study performed in various secondary schools in central Portugal. Randomly selected adolescents replied to a validated food allergy questionnaire. Those who reported an adverse food reaction were seen at participating hospitals, where clinical history was taken, skin prick (SPT) and prick-prick skin (SPPT) tests were performed, and food allergen-specific IgE levels (sIgE) were determined. An open oral challenge was performed in selected cases. Cases of positive clinical history of immediate (up to 2 h after ingestion) reaction in association with positive food sIgE levels and/or SPT were classified as IgE-associated probable food allergy and as confirmed IgE-mediated food allergy if food challenges were positive. Cases of positive clinical history of delayed (more than 2 h after ingestion) and negative food sIgE levels independently of positive SPT or SPPT results, were classified as non-IgE associated probable food allergy.
Results
The prevalence of probable food allergy in Portuguese adolescents was 1.41% (95% CI: 0.90–2.03%), with fresh fruits, shellfish, nuts, and peanut as the most frequently implicated foods. IgE-mediated probable food allergy occurred in 1.23% (95% CI: 0.67–1.72%) of cases, with fresh fruits, shellfish, and nuts mainly involved. Cutaneous symptoms were most frequently reported.
Conclusions
The prevalence of probable food allergies in Portuguese adolescents is low, is mostly related to fresh fruits, shellfish, nuts, and peanut, and most frequently involves cutaneous symptoms.
Keywords: Adolescents, Adverse food reaction, Food allergy, Prevalence, Cutaneous tests, Open food challenge
Abbreviations: AFR, Adverse Food Reactions; CI, Confidence Interval; IgE, Immunoglobulin E; NPV, Negative Predictive Value; OAS, Oral Allergy Syndrome; OR, Odds ratio; PPV, Positive Predictive Value; SPT, Skin prick test; SPPT, Skin prick-prick test
Introduction
Food allergy is a relevant health problem which is associated with considerable morbidity, a non-negligible level of mortality (in cases of food-associated anaphylaxis), and lower quality of life.1,2 In fact, the impact of food allergies on dietary habits and social integration of food allergic patients is obvious,3,4 with many affected individuals having to undergo changes in their diet, including, in some cases, very restrictive diets, due to an adverse food reaction.5 In addition, food allergies are also a clear economic burden, namely regarding work or school absenteeism,6,7 with impact upon school performance8 and quality of life.9
However, not all adverse reactions to foods are regarded as being an immunologically mediated “food allergy”.1,10,11 Partly for this reason, the prevalence values of food allergies in the general adolescent population are not well known. Various meta-analyses have estimated the prevalence of food allergies to any food in schoolchildren to be between 7% and 40% when only self-reported values are analysed,9,12, 13, 14, 15 and between 1% and 3% when studies include diagnostic tests.14, 15, 16, 17, 18 As far as we know, besides a single study in children attending an allergy outpatient clinic,19 the only actual population-based studies on the prevalence of food allergies carried out in Portugal were performed by our group in children20 and adults,21 but no studies in adolescents have been published. Thus, the objective of our study was to determine the prevalence of both self-reported and probable food allergy, as well as to analyse the clinical features, involved foods, and associated factors in a general population of Portuguese adolescents.
Methods
Population and sample
For this study, we took into account the fact that 3168 adolescents aged between 1023 years old (mean age: 14.3 ± 1.1; 51.7% female) were registered in 7 secondary schools of the cities of Castelo Branco, and Covilhã, in central Portugal. Based on an estimated prevalence of 4%,13,22,23 and considering a 95% confidence interval and a margin of error of 2%, we calculated that we would need a representative sample of 399 adolescents (STATA Statistical Package®). Considering an expected reply rate of 40%, we reset the sample size to 779 adolescents.
Study design
This was a cross-sectional study performed in 2013–2015. A list of all students in each class of each school was obtained, and adolescents were selected by a simple randomisation process. A standardised screening questionnaire was given to each volunteer, and those who reported a previous adverse food reaction which was subsequently confirmed by telephone, were invited to an appointment at the outpatient allergy clinics of the participating hospitals, where a standardised food allergy-related clinical history was taken,24 skin prick tests (SPT) and, when food was available, prick-prick skin tests (SPPT) were performed with a standardised technique, and blood was collected for determination of food allergen-specific IgE levels. In selected cases, an open oral challenge was performed. In these cases, if the patients did not exclude the suspected food from the diet, an elimination diet was followed for a minimum of 7 days prior to the food challenge. Patients with a positive clinical history of immediate (up to 2 h after ingestion) reaction in association with positive food sIgE levels and/or skin prick tests (with or without performance of a positive open challenge) were classified as IgE-associated probable food allergy. Cases of positive clinical history or delayed (more than 2 h after ingestion) and negative food sIgE levels independently of positive SPT or SPPT results, were classified as non-IgE associated probable food allergy.
Questionnaire
A 17-item, previously validated questionnaire on adverse food reactions25 was given by hand to all volunteers. This questionnaire included demographic data, questions on the occurrence of previous episodes of adverse reactions to foods, types of foods involved, types of reactions, post-ingestion latency time until appearance of symptoms, date of latest reaction, need for medical assistance, and personal or family history of atopic diseases. Those adolescents who reported an adverse food reaction were subsequently contacted by phone by a trained allergist within the following 3 months (Fig. 1). Those who confirmed the previous self-report of an adverse reaction were invited to a full allergy screen at the participating hospitals.
Fig. 1.
Flowchart of the study design and investigations
Determination of allergen-specific IgE serum levels
In all individuals seen at the outpatient clinics, 5 ml of peripheral blood were taken for the determination of the levels of total serum IgE, aeroallergen-specific screening IgE (Phadiatop inhalant allergens®), and suspected food-specific IgE levels. No recombinant allergens or panallergens were used. A fluorometric (ImmunoCAP® 250 Phadia Diagnosis)-based technique was used (Phadia & Thermo Scientific, Uppsala, Sweden). Allergen-specific levels above 0.35 KUA/L were regarded as positive.
Skin prick tests
In vivo studies included SPT (LETI Laboratories, Spain; Bial-Aristegui, São Mamede do Coronado, Portugal; Stallergènes, Antony, France) for aeroallergens (house dust mites; cockroach; fungi; latex; cat and dog dander; weeds, tree, and grass pollens) and suspected foods and, when available, SPPT with native suspect foods, since the sensitivity of the latter test is higher when compared with SPT using commercial extracts.26 Tests were carried out in duplicate on the volar aspect of the forearms. A drop of each commercial extract was placed upon the skin, and each drop was pricked through using a metal lancet (Stallergènes, Antony, France). Histamine dihydrochloride as positive and saline solution as negative controls were used respectively. The mean weal diameter was recorded after 15 min. Weals with a mean diameter at least 3 mm greater than that of the negative control were regarded as positive. SPPT tests used the same methodology, but fresh foods were used.
Oral challenge
Open oral challenges were performed in cases with positive clinical history, SPT and/or SPPT, and sIgE levels to suspect foods, and also in those cases in which clinical history was unclear and SPT results, as well as specific IgE levels, were negative or discrepant. Open challenge tests were carried out with suspect food,22 in accordance with published guidelines.10,11,27 In those cases in which individuals did not avoid the suspect foods, in spite of having symptoms, an elimination diet for at least 7 days before the oral challenge was carried out and monitored.10,11,27, 28, 29 Oral challenges were performed at the hospitals, under direct clinical observation for 4 h after challenge. In all cases, volunteers were contacted by phone by the responsible allergist in the following 24 h. Volunteers who reported any symptoms were reassessed at hospital allergy services.
Statistical analysis
Data were analysed using the Software Package for Social Sciences (SPSS) version 20.0® (SPSS Inc., Chicago, IL, USA). Analysis of normality of distribution of variables was performed using the One Sample Kolmogorov-Smirnov test. Descriptive analysis was used for the characterization of the sample. Chi-Square test or Fischer's Exact Test were used in the case of nominal variables. Comparative analysis of quantitative variables was carried out using Student's t-test or Mann-Whitney U test depending on distribution of variables. Odds ratio values were calculated for analysis of possible risk factors for adverse for reactions. A p value of less than 0.05 was regarded as significant with all statistical tests.
Results
Determination of prevalence and clinical features of self-reported food allergy
Of the 3168 questionnaires that were handed out (Fig. 1), 1752 were returned correctly filled in and with the written informed consent (57.3% reply rate). The questionnaire was properly completed by 1702 individuals (97.2% of the total of returned questionnaires; mean age: 14.9 ± 2.1 years; median age: 14 years; 61.9% female). Of these, 183 adolescents reported previous adverse reactions (total of 239 episodes) upon ingestion of at least 1 food (11.01%). These reactions had most frequently taken place 4 months to 5 years before (42.0% of the cases). Most adolescents reported symptoms with more than 1 type of food (50.2%; 92/183). Regarding episodes of adverse food reaction, most commonly implicated foods were fresh fruits (59/239 episodes– 24.7%; 73/239 episodes – 30.5% if latex-related fruits were included as well), seafood (32/239 episodes – 13.4%), milk (30/239 episodes – 12.5%), and nuts (15/239 episodes, excluding peanut – 6.3%) (Fig. 2).
Fig. 2.
Most frequently implicated foods by self-report (Values in %); number of episodes = 239
Most frequently reported symptoms were cutaneous (urticaria/angioedema; 107 episodes – 44.7%), followed by abdominal (34 episodes – 14.2%), respiratory symptoms (18 episodes – 7.53%), or oral allergy syndrome (17 episodes – 7.1%). 49 episodes (20.5%), were difficult to define clinically (Fig. 3).
Fig. 3.
Distribution of self-reported symptom frequency by food type (Values in %); number of episodes = 239
In most of the reported episodes (43.5%), symptoms developed up to 30 min after ingestion, and in 30.95% of the cases had a delayed onset (2–24 h) (Fig. 4).
Fig. 4.
Self-reported time until development of symptoms upon food ingestion (Values in %); number of episodes = 239
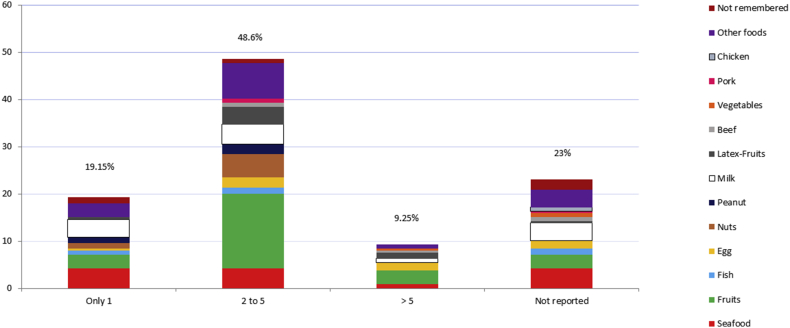
Most of the 183 adolescents who reported a total of 239 episodes of AFR mentioned 2 to 5 reactions with the same food (48.6%; 89/183 individuals, reporting 116 episodes), with fresh fruits being the most frequent food in this group (38 out of 116 episodes, and in 47 out of 116 episodes if latex related fruits were included). No individuals with latex sensitisation were found.
In addition, 35 out of 183 adolescents (19.15%) reported 46 episodes of an AFR, with seafood being the most frequently associated food in this group (10/46 episodes) (Fig. 5).
Fig. 5.
Self-reported number of episodes induced by the same food (Values in %); number of adolescents (cases) = 183
About 56% (102/183) of the adolescents needed medical treatment: 67% of them (68 cases) at a hospital emergency department, 12.5% (13 cases) by a general practitioner, 13.5% (14 cases) by self-medication, and 7% (7 cases) by an allergy specialist.
Most individuals who reported reactions (59%) had not been diagnosed an adverse food reaction, and only 30% had been given such a diagnosis by an allergist.
Having a personal (OR: 3.00; 95% CI: 1.80–5.00) or family history (OR: 2.60; 95% CI: 1.53–4.32) of atopy were factors significantly associated with an increased risk of having an adverse food reaction.
Determination of prevalence and clinical features of probable food allergy
Of the 183 individuals who reported an AFR, 44 (24%) declined to continue the study, and 2 adolescents (1.1%) did not complete the study (1 of them had been studied thoroughly already) (Fig. 1). The remaining 137 adolescents (74.9% of the total number of AFR cases) were subsequently seen at an allergy hospital appointment. Of these, 56 (40.9%) reported absence of symptoms upon subsequent ingestion of the suspect food in the period between completion of the questionnaire and the hospital appointment, and were therefore not further studied. Thus, the remaining 81 adolescents under study (59.1% of the 137 adolescents seen at the hospitals) completed the full allergy study (clinical history, SPT/SPPT, food-specific lgE levels, and open oral challenge tests, in some cases). We performed 32 open oral challenges in 27 volunteers, which were clearly positive in 17 of them: isolated OAS in 5 cases; OAS in association with diarrhoea and colicky abdominal pain in 2 cases; vomiting and diarrhoea in 4 cases; isolated generalised urticaria in 2 cases; generalised urticaria and angioedema of lips in 1 case; generalised urticaria and mild dyspnea in 1 case. All of these cases occurred 15–30 min after the onset of the tests; finally, there were 2 cases of delayed reaction: 1 involving colicky abdominal pain and diarrhoea starting 9–12 h after the challenge, and 1 consisting of mild urticarial rash and itchy skin which started 12 h after the test. No cases of delayed anaphylaxis were identified.
Upon completion of the study, 24 adolescents (1 had already been diagnosed IgE-mediated milk allergy in another hospital) were diagnosed an AFR with an immunological basis (24/1702; 1.41% of the total number of adolescents that filled in the questionnaire; 95% CI = 0.90–2.03%; mean age: 15.1 years, median age: 15 years, 54.1% female), and a probable IgE-mediated mechanism was detected in 21 of them (21/1702; l.23%; 95% CI: 0.67–1.72%.) (Table 1 shows the results for the newly diagnosed adolescents).
Table 1.
Characteristics of newly diagnosed Food Allergic patients
| Patient ID | Age | Sex | IgE levels (KUA) | Food Specific IgE | Personal History of atopy | Family History of atopy | SPT aero allergens | Sensitisation to more than one foodstuff | Foodstuff | Symptoms | Time to symptom development | Similar episodes with the same food | SPT with Commercial food extracts | Food Prick by Prick skin test | Open Food Challenge | Allergy mechanism |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| #1 | 12 | M | 269 | POS | Yes | No | POS | No | Other tree nuts | Anaphylaxis | <30 mins | 2 to 5 | POS | POS | Not performed | IgE mediated |
| #2 | 12 | M | 69 | Negative | Yes | No | Negative | No | Milk | Other symptoms | >24 h | >5 | Negative | Not performed | POS | Non IgE mediated |
| #3 | 12 | F | 258 | POS | Yes | Yes | POS | Yes | Peanut, Egg | UA/AE | <30 mins (peanut), | Only 1 | POS (peanut), Negative (egg) | POS (peanut), | Not performed | IgE mediated (only for peanut) |
| 2-24 h (Egg) | Negative (Egg) | |||||||||||||||
| #4 | 13 | M | 529 | POS | Yes | Yes | POS | No | Fruits | OAS | <30 mins | >5 | Negative | POS | Not performed | IgE mediated |
| #5 | 15 | F | 230 | POS | Yes | Yes | POS | No | Seafood | UA/AE | <30 mins | 2 to 5 | Negative | POS | POS | IgE mediated |
| #6 | 15 | F | 92 | POS | Yes | Yes | POS | No | Other tree nuts | Anaphylaxis | <30 mins | Only 1 | POS | POS | Not performed | IgE mediated |
| #7 | 15 | F | 86 | POS | Yes | Yes | POS | No | Fruits | OAS | <30 mins | >5 | POS | POS | Not performed | IgE mediated |
| #8 | 18 | F | 114 | POS | No | No | POS | No | Fruits | UA/AE | 30 mins to 2 h | >5 | POS | POS | POS | IgE mediated |
| #9 | 19 | M | 303 | POS | Yes | Yes | POS | No | Other tree nuts | UA/AE | 30 mins to 2 h | Only 1 | POS | Not performed | Not performed | IgE mediated |
| #10 | 16 | M | 164 | POS | Yes | Yes | POS | No | Fruits | OAS | <30 mins | 2 to 5 | POS | POS | POS | IgE mediated |
| #11 | 16 | M | 233 | POS | Yes | Yes | POS | No | Fruits | OAS | <30 mins | 2 to 5 | POS | POS | POS | IgE mediated |
| #12 | 16 | M | 238 | POS | Yes | No | POS | No | Fruits | OAS | <30 mins | >5 | POS | POS | POS | IgE mediated |
| #13 | 14 | F | 112 | POS | Yes | Yes | POS | No | Fruits | OAS | <30 mins | >5 | POS | POS | POS | IgE mediated |
| #14 | 17 | F | 279 | POS | Yes | Yes | POS | No | Seafood | Anaphylaxis | <30 mins | Only 1 | POS | POS | POS | IgE mediated |
| #15 | 16 | F | 1686 | POS | Yes | Yes | POS | No | Seafood | Anaphylaxis | <30 mins | Only 1 | POS | POS | POS | IgE mediated |
| #16 | 16 | M | 88,5 | POS | Yes | No | POS | No | Seafood | UA/AE | <30 mins | 2 to 5 | POS | POS | POS | IgE mediated |
| #17 | 15 | F | 112,7 | POS | Yes | Yes | POS | No | Seafood | UA/AE | <30 mins | 2 to 5 | POS | POS | POS | IgE mediated |
| #18 | 17 | M | 131,2 | POS | Yes | No | POS | Yes | Peanut, Seafood | Peanut: OAS Seafood: UA/AE | <30 mins | 2 to 5 | POS (peanut & seafood) | Not performed | POS (seafood) Negative (peanut) | IgE mediated (only for seafood) |
| #19 | 14 | F | 127,9 | POS | Yes | Yes | POS | No | Seafood | UA/AE, Abdominal | <30 mins | 2 to 5 | POS | POS | POS | IgE mediated |
| #20 | 15 | F | 34,9 | Negative | No | No | POS | No | Other tree nuts | Respiratory | 2–24 h | 2 to 5 | POS | Negative | POS | Non IgE mediated |
| #21 | 14 | F | 114,4 | POS | Yes | Yes | POS | No | Other tree nuts | UA/AE | <30 mins | 2 to 5 | POS | Not performed | POS | IgE mediated |
| #22 | 16 | M | 87,9 | Negative | No | No | Negative | No | Other tree nuts | UA/AE | 2–24 h | 2 to 5 | Negative | POS | POS | Non IgE mediated |
| #23 | 15 | M | 148 | POS | Yes | Yes | POS | No | Fruits | OAS | <30 mins | >5 | POS | Not performed | POS | IgE mediated |
Most frequently implicated foods were fresh fruits (30.8%). Most of these belonged to the Rosaceae family (80% of cases) — apple, pear, strawberry, and/or plum. Banana (Musaceae) and/or melon (Cucurbitaceae) were involved in 10% of cases, and orange and/or tangerines (Rutaceae) in 8% of cases; in the remaining cases, patients reported multiple sensitisations to these fruits. Other reported foods mostly included shellfish (26.9%, mainly crustaceans), nuts (23% walnut, cashew and hazelnut), peanut and milk (7.7% each), and egg (3.8% each). In the 20 cases in which an IgE-mediated association was newly found, specific lgE levels to implicated foods as well as Phadiatop were positive in all of them, and in addition, the mean total lgE serum levels were higher than compared with the group with non-IgE-mediated reactions (265.78 KUA/L versus 63.93 KUA/L, respectively; p < 0.001; Mann-Whitney U Test), Of the 3 adolescents in whom no IgE-associated mechanism was demonstrated, only 2 were atopic, 1 with a positive Phadiatop® test and 1 with positive SPT to aeroallergens. SPT performed with commercial food extracts were positive in 19 foods out of 22 in the group of adolescents with an lgE-associated mechanism and in 3 out of 9 foods tested in the non-IgE associated cases (general test sensitivity of 66.7%, specificity of 100%, PPV: 100%, NPV: 86.4%). No differences between commercial extracts were found. Fresh food SPPT were positive in 13 out of 15 cases in the group of adolescents with an IgE-associated mechanism and only 1 in the non-IgE associated cases (general test sensitivity of 87.5%; specificity: 100%, PPV: 100%, NPV: 91.7%).
The most prevalent symptoms in all studied cases were cutaneous (40% of cases), followed by OAS (32%) and anaphylaxis (16%), with the latter being associated with the ingestion of nuts and shellfish (2 cases each). Only in the 3 cases which were not IgE-associated were the symptoms delayed, appearing more than 2 h after ingestion, since in all cases with an IgE-association, symptoms appeared in less than 2 h upon ingestion.
Of all the adolescents who finished the study at the hospitals (81 individuals), 65 cases (80.2%) needed treatment for their symptoms, mostly at an emergency department. However, only 6 individuals with a diagnosis of probable food allergy (4 with anaphylaxis, 1 with respiratory symptoms, and 1 with cutaneous symptoms) sought medical attention. A high proportion of cases diagnosed with food allergy (either IgE- or non-IgE-associated) reported the presence of personal and/or family history of atopy.
No significant association factors were seen between sex, age, locality of origin (rural vs urban areas), type of food, and time elapsed since the latest reaction. In the same way, we found no significant association between severity of the food-induced reaction and total serum IgE levels.
Discussion
Our study determined, for the first time in Portugal, the prevalence of probable food allergy, the type of implicated foods, types of symptoms, and other associated factors in an adolescent population. We have shown that the prevalence of probable food allergies in this population is low, is mostly related to fresh fruits and nuts, and most frequently involves cutaneous symptoms.
The initial, written questionnaire showed that the values of self-reported food allergy (11.01%) were within the range described in other population-based studies (3–40%).9,12, 13, 14, 15, 16,22,30, 31, 32, 33 Similarly, the value obtained in our study for the prevalence of probable food allergy (1.41%) is similar to that reported for adolescents in the United States (2,5%)23,34 and Europe (0.5%–3.5%),15,33 although the latter values were obtained after performance of single or double-blind oral challenge tests.13,14,17,30,33,35
This discrepancy in prevalence results between self-reported and medically confirmed data (using in vitro and in vivo tests and/or oral challenge) has been described. Previous studies have shown that self-reports tend to overestimate food allergies.12, 13, 14, 15,17,22,30,33,36, 37, 38 This discrepancy may be partly due to an information bias based upon an enhanced self-perception of symptoms which are wrongly ascribed to food ingestion. Cultural factors, health literacy, or accessibility to a medical diagnosis may be involved15,33 (in our study, only 16% of the adolescents that reported food-associated symptoms had ever seen an allergist for that reason). Nevertheless, prevalence values across different studies are hardly comparable, given the heterogeneity of study designs and the types of population involved. In any case, the overestimation of self-reported food-related adverse food reactions may be worrying since it is frequently associated with inappropriate restriction diets with subsequent nutritional deficits.3
The implicated foods, both in self-reported allergies as well as in test-confirmed, probable food allergies, in our study are included in the so-called “big eight allergens”37 and are similar to those found in other population-based studies using similar methodology in Europe,9,13,14,16,17,22,30,39,40 Asia,38,41 and the United States.10,23,31,32,42,43 However, fresh fruits being the most frequently implicated foods, places our study in line with those performed in western and Mediterranean Europe,16,17,22 but not with those from northern Europe, North America9,10,13,14,23,30,31,43,44 or, surprisingly, the eastern Mediterranean Europe,33 probably due to differences in study methodology. In fact, these differences may be partly due to different food habits38 or concurrent pollen sensitisation, although we cannot exclude the possibility that the comparatively smaller size of our sample may have influenced our results. On the other hand, it is also fundamental to stress that, in contrast with our study, OAS is often not regarded as a symptom of food allergy, since it is frequently associated with pollen-induced respiratory symptoms in the same patient, as happened in our study, and is therefore regarded as a “secondary allergy” by various research groups.30,35,45
It is also important to highlight the discrepancy between the panels of implicated foods when we compared self-reported results with those obtained upon completion of the allergy study. Whereas the self-reported panel mainly included fresh fruits, milk, and shellfish, the confirmed (post-tests) panel essentially identified fresh fruit and nuts. Other studies have also identified similar situations in adolescents in Europe15,16,22,30,35 and this has been confirmed by meta-analyses,12,13,45 having such discrepancies partly ascribed to differences in the concept of adverse food reactions between patients and specialist doctors. This highlights the need for an adequate diagnostic approach to food-associated symptoms, so that subsequent detrimental situations may be averted or better controlled.46 These include inadequate diets,3 difficulties in the reintroduction of the “culprit” food in case allergy was not confirmed,47 stress and anxiety because of eventual accidental ingestion of suspected foods,2,4,48 or even bullying at school.49
Cutaneous symptoms were the most prevalent ones in our study, both in self-report and in those adolescents who completed the full allergy workup. This is in agreement with results from most other groups.10,11,13,16,22,23,30,35,37,43
An interesting aspect of our work was the analysis of data obtained from the self-reported symptoms. We found several possible associations between the ingestion of certain foods and the development of certain symptoms (fresh fruits, milk, and egg in relation to cutaneous manifestations, shellfish in relation to abdominal symptoms, fresh fruits in connection with OAS, and nuts and peanuts in anaphylaxis), associations which, except for the latter 2, had not been previously reported. On the other hand, bearing in mind the timeframe for the appearance of symptoms, we found 2 predominant response patterns, previously identified by Osterballe:30 an immediate type of reaction, arising in less than 30 min post-ingestion, and a more delayed, between 2 and 24 h post-ingestion, mainly associated with fresh fruits and milk, in both cases.
As far as adolescents with probable allergy are concerned, we also found an inverse relationship between symptom latency time and symptom severity. In addition, anaphylaxis cases were all associated with the ingestion of nuts and peanut. It should be stressed that the 3 non-IgE mediated cases had a latency time longer than 2 h and most IgE-mediated cases had developed within 30 min upon ingestion.
We also analysed eventual risk factors associated with food allergies. Multivariate analysis showed that a personal or a family history of atopy were significantly associated with a higher risk of having food allergies, as has been described in previous studies and metanalyses focusing on adolescents and children.1,10,13,14,50
One of the limitations of the present study was the fact that we could not perform double-blind, placebo-controlled food challenges, a test which is regarded as the “gold standard” for the final diagnosis of food allergy. In spite of this, the current report is the first population-based study in Portuguese adolescents. Furthermore, it yields information on probable food allergy in this population, based upon not only a positive clinical history/questionnaire, but also on diagnostic tests including SPT, food-specific IgE levels, and open oral food challenges (particularly in cases that were clinically less clear or in which there were diagnostic doubts), which makes it a very thorough study. In fact, a high proportion of population-based studies on food allergies performed in other countries only applied a questionnaire,16,30,35,37,43 and others only performed SPT or determination of food-specific serum IgE in suspect cases of food allergy.9,17,38
Another possible limitation of our study concerns the fact that 25.1% of the adolescents who reported adverse food reactions did not complete the study, which is partly explained by the clear national increase in the “Healthcare service usage” fees, during the implementation of the study. In addition, an increase in unstable employment during the period of the study limited absences from work by parents accompanying the adolescents in hospital visits. A relatively high drop-out rate and low participation are indeed limiting factors in population-based epidemiological studies, and this has been reported in multiple studies, with the reply rate being inversely associated with the magnitude of the study. The reply rate has varied between 40–50%14,22,30,35 and 61–86%9,15,16 when studies are only based upon questionnaires, and lower22,35 when a more thorough assessment (skin tests, blood tests, food challenges) is involved, with a Turkish study being the only exception we found.33 Nevertheless, our reply rate was quite acceptable for this type of study (57.3%). In spite of the relative limitations in our study, the size of our sample and the features of our work reached the predefined values in terms of statistical power, representativity, and proportionality for analysis. Relatively low reply rates may also lead to a selection bias, mainly with people who are more concerned about allergy problems and more prone to returning the questionnaire. However, if this were the case, we would expect to find high self-report prevalence rates, in comparison with other studies, and this was not the case. Finally, in order to more firmly extrapolate our results to other regions of Portugal, further studies carried out in other regions of the country, as well as a nationwide, multicentre study, are warranted.
Conclusions
The prevalence of probable food allergy in our sample of Portuguese adolescents was low. Fresh fruits, shellfish, nuts, and peanut were the main implicated foods, and the most frequently reported symptoms were cutaneous. There was a clear discrepancy between self-reported and probable food allergy, both in terms of prevalence and of the implicated foods.
This study is the first step towards a thorough study of food allergies and their repercussions in Portugal and may contribute towards a global characterization of food allergies in adolescents in Europe.
Consent for publication
All authors gave their written consent for publication.
Author’ contributions
CLI and LTB conceived and coordinated the study and participated in its design. CLI prepared the first draft and performed the clinical approach. LTB helped, reviewed and translated to draft the manuscript. SMN performed the statistical analysis. AR and LTB performed in vivo and food oral challenges tests. PF performed in vitro tests. OL and AMF applied the questionnaires. All authors read and approved the final version of this manuscript.
Availability of data and materials
Please contact corresponding author for primary data requests.
Funding
This project did not receive any specific funding from any agency, commercial or not not-for-profit sectors.
Ethical aspects
This study was performed in accordance with the Declaration of Helsinki and was approved by the Ethics Committees of the Amato Lusitano Hospital, the Administrative Sub-Region of Health of Castelo Branco and the University of Beira Interior. It was also approved by the Ministry of Education (DGIDC, Reg. Nº 0266300001 from January 2012). All volunteers and their legal guardians/parents gave their written informed consent.
Declaration of Competing Interests
The authors declare no conflicts of interest.
Acknowledgments
We would like to thank Nuno Dias for his support for the graphics design and Marisa Padilha, Ana Martins and Mónica Lopes (Faculty of Health Sciences of the University of Beira Interior) for their collaboration in the application of the questionnaire.
Footnotes
Full list of author information is available at the end of the article
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2020.100453.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Sicherer S.H. Epidemiology of food allergy. J Allergy Clin Immunol. 2011;127:594–602. doi: 10.1016/j.jaci.2010.11.044. [DOI] [PubMed] [Google Scholar]
- 2.Namork E., Fæste C.K., Stensby B.A., Egaas E., Løvik M. Severe allergic reactions to food in Norway: a ten year survey of cases reported to the food allergy register. Int J Environ Res Publ Health. 2011;8:3144–3155. doi: 10.3390/ijerph8083144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Silva D., Geromi M., Panesar S.S. On behalf of the EAACI Food Allergy and Anaphylaxis Guidelines Group. Acute and long-term management of food allergy: systematic review. Allergy. 2014;69:159–167. doi: 10.1111/all.12314. [DOI] [PubMed] [Google Scholar]
- 4.Lau G.Y., Patel N., Umasunthar T. Anxiety and stress in mothers of food-allergic children. Pediatr Allergy Immunol. 2014;25:236–242. doi: 10.1111/pai.12233. [DOI] [PubMed] [Google Scholar]
- 5.Sicherer S.H., Sampson H.A. Food allergy. J Allergy Clin Immunol. 2006;117(2 Suppl):S470–S475. doi: 10.1016/j.jaci.2005.05.048. [DOI] [PubMed] [Google Scholar]
- 6.Patel D.A., Holdford D.A., Edwards E., Carroll N.V. Estimating the economic burden of food-induced allergic reactions and anaphylaxis in the United States. J Allergy Clin Immunol. 2011;128:110–115. doi: 10.1016/j.jaci.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 7.Flabbee J., Petit N., Jay N. The economic costs of severe anaphylaxis in France: an inquiry carried out by the Allergy Vigilance Network. Allergy. 2008;63:360–365. doi: 10.1111/j.1398-9995.2007.01513.x. [DOI] [PubMed] [Google Scholar]
- 8.Calsbeek H., Rijken M., Bekkers M.J., Dekker J., van Berge Henegouwen G.P. School and leisure activities in adolescents and young adults with chronic digestive disorders: impact of burden of disease. Int J Behav Med. 2006;13:121–130. doi: 10.1207/s15327558ijbm1302_3. [DOI] [PubMed] [Google Scholar]
- 9.Marklund B., Ahlstedt S., Nordström G. Health-related quality of life in food hypersensitive schoolchildren and their families: parents' perceptions. Health Qual Life Outcome. 2006;4:48. doi: 10.1186/1477-7525-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.NIAID-Sponsored Expert Panel. Boyce J.A., Assa'ad A., Burks A.W. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126(6 Suppl):S1–S58. doi: 10.1016/j.jaci.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burks A.W., Tang M., Sicherer S. ICON: food allergy. J Allergy Clin Immunol. 2012;129:906–920. doi: 10.1016/j.jaci.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Rona R.J., Keil T., Summers C. The prevalence of food allergy: a meta-analysis. J Allergy Clin Immunol. 2007;120:638–646. doi: 10.1016/j.jaci.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 13.Nwaru B.I., Hickstein L., Panesar S.S. EAACI Food Allergy and Anaphylaxis Guidelines Group. The epidemiology of food allergy in Europe: a systematic review and meta-analysis. Allergy. 2014;69:62–75. doi: 10.1111/all.12305. [DOI] [PubMed] [Google Scholar]
- 14.Pereira B., Venter C., Grundy J., Clayton B., Arshad H., Dean T. Prevalence of sensitization to food allergens, reported adverse reaction to foods, food avoidance, and food hypersensitivity among teenagers. J Allergy Clin Immunol. 2005;116:884–892. doi: 10.1016/j.jaci.2005.05.047. [DOI] [PubMed] [Google Scholar]
- 15.Kavaliunas A., Surkiene G., Dubakiene R. Europrevall survey on prevalence and pattern of self-reported adverse reactions to food and food allergies among primary schoolchildren in Vilnius, Lithuania. Medicina. 2012;48:265–271. [PubMed] [Google Scholar]
- 16.Kanny G., Moneret-Vautrin D.A., Flabbee J., Beaudouin E., Morisset M., Thevenin F. Population study of food allergy in France. J Allergy Clin Immunol. 2001;108:133–140. doi: 10.1067/mai.2001.116427. [DOI] [PubMed] [Google Scholar]
- 17.Pénard-Morand C., Raherison C., Kopferschmitt C. Prevalence of food allergy and its relationship to asthma and allergic rhinitis in schoolchildren. Allergy. 2005;60:1165–1171. doi: 10.1111/j.1398-9995.2005.00860.x. [DOI] [PubMed] [Google Scholar]
- 18.Caffarelli C., Coscia A., Ridolo E. Parent's estimate of food allergy prevalence and management in Italian shool-aged children. Pediatr Int. 2011;53:505–510. doi: 10.1111/j.1442-200X.2010.03294.x. [DOI] [PubMed] [Google Scholar]
- 19.Bento M.L., Armando F., Cesar-Ramos J.M. Epidemiology of food allergy in Portugal. Pediatr Pulmonol. 2001;(Supplement 23):38–40. [PubMed] [Google Scholar]
- 20.Jorge A., Soares E., Sarinho E., Lorente F., Gama J., Taborda-Barata L. Prevalence and clinical features of adverse food reactions in Portuguese children. Allergy Asthma Clin Immunol. 2017;13:40. doi: 10.1186/s13223-017-0212-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lozoya-Ibáñez C., Morgado-Nunes S., Rodrigues A., Lobo C., Taborda-Barata L. Prevalence and clinical features of adverse food reactions in Portuguese adults. Allergy Asthma Clin Immunol. 2016;12:36. doi: 10.1186/s13223-016-0139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zuberbier T., Edenharter G., Worm M. Prevalence of adverse reactions to food in Germany-a population study. Allergy. 2004;59:338–345. doi: 10.1046/j.1398-9995.2003.00403.x. [DOI] [PubMed] [Google Scholar]
- 23.Liu A.H., Jaramillo R., Sicherer S.H. National prevalence and risk factors for food allergy and relationship to asthma: results from the National Health and Nutrition Examination Survey 2005-2006. J Allergy Clin Immunol. 2010;126:798–806. doi: 10.1016/j.jaci.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prates S. Colheita da História Clínica. Rev Port Imunoalergol. 2009;17(supl I):6–10. [Google Scholar]
- 25.Lozoya-Ibáñez C., Macedo A., Rodrigues A. Validation of a questionnaire for the study of food allergies in Portuguese adults. Allergy. 2011;66:S395. (Abstract) [Google Scholar]
- 26.Romano A., Di Fonso M., Giuffreda F. Food-dependent exercise-induced anaphylaxis: clinical and laboratory findings in 54 subjects. Int Arch Allergy Immunol. 2001;125:264–272. doi: 10.1159/000053825. [DOI] [PubMed] [Google Scholar]
- 27.Bindslev-Jensen C., Ballmer-Weber B.K., Bengtsson U. Standardization of food challenges in patients with immediate reactions to foods – position paper from the European Academy of Allergology and Clinical Immunology. Allergy. 2004;59:690–697. doi: 10.1111/j.1398-9995.2004.00466.x. [DOI] [PubMed] [Google Scholar]
- 28.Liebermann J.A., Sicherer S.H. Diagnosis of food allergy: epicutaneous skin tests, in vitro tests, and oral food challenge. Curr Allergy Asthma Rep. 2011;11:58–64. doi: 10.1007/s11882-010-0149-4. [DOI] [PubMed] [Google Scholar]
- 29.Fleischer D.M., Bock S.A., Spears G.C. Oral food challenges in children with a diagnosis of food allergy. J Pediatr. 2011;158:578–583. doi: 10.1016/j.jpeds.2010.09.027. [DOI] [PubMed] [Google Scholar]
- 30.Osterballe M., Hansen T.K., Mortz C.G., Høst A., Bindslev-Jensen C. The prevalence of food hypersensitivity in an unselected population of children and adults. Pediatr Allergy Immunol. 2005;16:567–573. doi: 10.1111/j.1399-3038.2005.00251.x. [DOI] [PubMed] [Google Scholar]
- 31.Soller L., Ben-Shosnan M., Harrington D.W. Overall prevalence of self-reported food allergy in Canada. J Allergy Clin Immunol. 2012;130:986–988. doi: 10.1016/j.jaci.2012.06.029. (letter) [DOI] [PubMed] [Google Scholar]
- 32.McGowan E.C., Keet C.A. Prevalence of self-reported food allergy in the national health and nutrition examination survey (NHANES) 2007-2010. J Allergy Clin Immunol. 2013;132:1216–1219. doi: 10.1016/j.jaci.2013.07.018. e5 (letter) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaya A., Erkoçoglu M., Civelek E., Çakir B., Kocabas C.N. Prevalence of confirmed IgE-mediated food allergy among adolescents in Turkey. Pediatr Allergy Immunol. 2013;24:456–462. doi: 10.1111/pai.12097. [DOI] [PubMed] [Google Scholar]
- 34.JJS Chafen, Newberry S.J., Riedl M.A. Diagnosing and managing common food allergies: a systematic review. J Am Med Assoc. 2010;303:1848–1856. doi: 10.1001/jama.2010.582. [DOI] [PubMed] [Google Scholar]
- 35.Roehr C.C., Edenharter G., Reimann S. Food allergy and non-allergic food hypersensitivity in children and adolescents. Clin Exp Allergy. 2004;34:1534–1541. doi: 10.1111/j.1365-2222.2004.02080.x. [DOI] [PubMed] [Google Scholar]
- 36.Osterballe M., Mortz C.G., Hansen T.K., Andersen K.E., Bindslev-Jensen C. The Prevalence of food hypersensitivity in young adults. Pediatr Allergy Immunol. 2009;20:686–692. doi: 10.1111/j.1399-3038.2008.00842.x. [DOI] [PubMed] [Google Scholar]
- 37.Vierk K.A., Khoeler K.M., Fein S.B., Street D.A. Prevalence of self-reported food allergy in American adults and use of food labels. J Allergy Clin Immunol. 2007;119:1504–1510. doi: 10.1016/j.jaci.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 38.Mahesh P.A., Wong G.W.K., Ogorodova L. Prevalence of food sensitization and probable food allergy among adults in India: the EuroPrevall INCO study. Allergy. 2016;71:1010–1019. doi: 10.1111/all.12868. [DOI] [PubMed] [Google Scholar]
- 39.Kummeling I., Mills E.N.C., Clausen M. The EuroPrevall surveys on the prevalence of food allergies in children and adults: background and study methodology. Allergy. 2009;64 doi: 10.1111/j.1398-9995.2009.02046.x. 1493-1467. [DOI] [PubMed] [Google Scholar]
- 40.Fernández-Rivas M., Barreales L., Mackie A.R. The EuroPrevall outpatient clinic study on food allergy: background and methodology. Allergy. 2015;70:576–584. doi: 10.1111/all.12585. [DOI] [PubMed] [Google Scholar]
- 41.Wong G.W.K., Mahesh P.A., Ogorodova L. The EuroPrevall-INCO surveys on the prevalence of food allergies in children from China, India and Russia: the study methodology. Allergy. 2010;65:385–390. doi: 10.1111/j.1398-9995.2009.02214.x. [DOI] [PubMed] [Google Scholar]
- 42.McClain S., Bowman C., Fernández-Rivas M., Ladics G.S., Van Ree R. Allergic sensitization: food- and protein-realted factors. Clin Transl Allergy. 2014;4:11. doi: 10.1186/2045-7022-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ben-Shoshan M., Harrington D.W., Soller L. A population-based study on peanut, tree nut, fish, shellfish, and sesame allergy prevalence in Canada. J Allergy Clin Immunol. 2010;125:1327–1335. doi: 10.1016/j.jaci.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 44.Gupta R.S., Springston E.E., Warrier M.R. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. 2011;128(1):e9–17. doi: 10.1542/peds.2011-0204. [DOI] [PubMed] [Google Scholar]
- 45.Zuidmeer L., Goldhahn K., Rona R.J. The prevalence of plant food allergies: a systematic review. J Allergy Clin Immunol. 2008;121:1210–1218. doi: 10.1016/j.jaci.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 46.Crevel R., Ronsmans S., Marsaux C., Bánáti D. ILSI Europe's food allergy task force: from defining the hazrd to assessing the risk from food allergens. J AOAC Int. 2018;101:91–95. doi: 10.5740/jaoacint.17-0397. [DOI] [PubMed] [Google Scholar]
- 47.Eigenmann P.A., Caubet J.-C., Zamora S.A. Continuing food-avoidance diets after negative food challenges. Pediatr Allergy Immunol. 2006;17:601–605. doi: 10.1111/j.1399-3038.2006.00455.x. [DOI] [PubMed] [Google Scholar]
- 48.Le T.M., Zijlstra W.T., van Opstal E.Y. Food avoidance in children with adverse food reactions: influence of anxiety and clinical parameters. Pediatr Allergy Immunol. 2013;24:650–655. doi: 10.1111/pai.12114. [DOI] [PubMed] [Google Scholar]
- 49.Shemesh E., Annunziato R.A., Ambrose M.A. Child and parental reports of bullying in a consecutive sample of children with food allergy. Pediatrics. 2013;131:e10. doi: 10.1542/peds.2012-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McBride D., Keil T., Grabenhenrich L. The EuroPrevall birth cohort study on food allergy: baseline characteristics of 12,000 newborns and their families from nine European countries. Pediatr Allergy Immunol. 2012;23:230–239. doi: 10.1111/j.1399-3038.2011.01254.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Please contact corresponding author for primary data requests.