Abstract
Objectives
This study aimed to investigate the predictive value of cognitive/functional measures in combination with hippocampal volume (HCV) on the probability of conversion from mild cognitive impairment (MCI) to Alzheimer’s disease (AD).
Methods
The Rey Auditory Verbal Learning Test for immediate memory, Mini-Mental State Examination, a functional assessment for independent daily activities and Alzheimer’s Disease Assessment Scale were used as cognitive/functional measures and HCV as neuroimaging measure. Logistic regression and Cox proportional hazard analyses were used to explore the measures’ predictive values for AD conversion and time to conversion.
Results
The probability of conversion from MCI to AD was associated with cognitive function, but this was moderated by HCV: higher at lower HCV and lower at higher HCV. General cognitive/functional measures were less predictive than immediate memory in predicting time to conversion to AD at small HCVs.
Conclusion
Effectiveness of cognitive measures and subtle functional abnormality in predicting conversion from MCI to AD is dependent on HCV, thus combined evaluation should be considered. A combination of HCV and immediate memory appear to perform best in predicting time to conversion.
Keywords: Brain/cognitive reserve, Hippocampus, Mild cognitive impairment, MRI, Neuropsychological tests
Alzheimer’s disease (AD) is a progressive degenerative disorder that involves cognitive decline severe enough to substantially impair daily activities. Cognitive decline accompanied by preserved daily activities has been specified as mild cognitive impairment (MCI), and is commonly known to be the prodromal phase of AD (Petersen et al., 1999). Approximately half of those with MCI progress to AD within 5 years (Pandya, Clem, Silva, & Woon, 2016). Identifying those who will progress to AD and predicting time to conversion remains an important clinical challenge.
Cognitive and functional performance is the central component of AD/MCI diagnostic. Thus, it is to be expected that cognitive performance is a sensitive predictor of conversion from MCI to AD (Belleville et al., 2017). A combination of measures from a range of domains typically provides a better predictor of disease progression (Belleville et al., 2017). Additionally, although intact daily function is the main clinical differentiator of MCI and AD diagnosis, subtle decline in daily function, while it remains in the normal range, is still predictive of conversion from MCI to AD (Gomar et al., 2011; Li et al., 2017). Furthermore, a combination of cognitive/functional measures with neuroimaging measures has been reported to produce significantly higher predictive accuracy (Devanand et al., 2008; Falahati, Westman, & Simmons, 2014; Moradi et al., 2015). Our recent study showed that the combination of a new hippocampal index—hippocampus to cerebellum volume ratio, HCCR—and Mini-Mental State Examination (MMSE) could reliably identify those who progress from MCI to AD within 5 years with an area under receiver operating characteristic curve of 0.9 (Tabatabaei-Jafari, Walsh, Shaw, & Cherbuin, 2018).
Cognitive/functional impairment is positively associated with neurodegeneration, but this association is not straightforward and there is a mismatch between the extent of neural pathology and the severity of cognitive/functional impairment (Steffener & Stern, 2012). Although a combination of cognitive performance and neuroimaging measures has been previously shown to have a higher predictive value compared with either measure alone, the relative contribution of these measures to each other across their range is not well understood. To answer these important questions, this study aimed to investigate the predictive value of cognitive/functional measures across the range of hippocampal volumes (HCVs), in those who have a diagnosis of MCI and convert to AD within 5 years. HCV and cognitive/functional measures were selected on the basis of established associations with MCI and AD (Jack et al., 2005; Li et al., 2017; Tabatabaei-Jafari, Shaw, & Cherbuin, 2015; Tabatabaei-Jafari et al., 2018). We hypothesized that HCV would moderate the predictive value of cognitive/functional performance. Additionally, we aimed to investigate how well a combination of these measures would predict time to conversion from MCI to AD.
Method
Study Participants
Data used in the preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). The ADNI was launched in 2003 as a public–private partnership, led by Principal Investigator Michael W. Weiner, MD. The primary goal of ADNI has been to test whether serial magnetic resonance imaging (MRI), positron emission tomography (PET), other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of MCI and early AD.
All participants of ADNI 1/GO/2 who were diagnosed with MCI at baseline were considered for inclusion. Those who were stable for at least 6 months after baseline diagnosis were included if they converted to AD within 5 years (MCIc; n = 183) or remained stable for more than 5 years (MCIs; n = 112).
Details of the diagnostic criteria can be found at the ADNI web site (http://www.adni-info.org/Scientists/AboutADNI.aspx). Briefly, participants were classified as MCI if they had an MMSE greater than 24, a CDR of 0.5, a subjective report of memory concern, an objective memory loss, preserved daily living activity and did not meet diagnostic criteria for dementia. Participants were classified as having AD if they had MMSE scores less than 26, CDR 0.5 or 1.0 and fulfill criteria for clinically probable AD according to the Institute of Neurological and Communicative Diseases and Stroke/Alzheimer’s Disease and Related Disorders Association.
Neuroimaging Acquisition and Processing
Participants underwent high-resolution MRI brain scans on 1.5 (N = 165) or 3 T (N = 130) scanners from General Electric, Siemens, or Philips (Milwaukee, WI; Germany; the Netherlands, respectively) using a standardized ADNI acquisition protocol for 3D MP-RAGE sequence (Jack et al., 2008). Images which had undergone specific ADNI preprocessing correction steps to standardize images from different sites and platforms, were obtained for this study: (a) Grad wrap; a specific correction of image geometry distortion due to nonlinearity, (b) B1 nonuniformity; B1 calibration to correct the image intensity nonuniformity that results when RF transmission is performed with a more uniform body coil while reception is performed with a less uniform head coil, (c) N3 correction; a histogram peak sharpening algorithm applied after grad wrap and B1 correction.
FreeSurfer version 5.3 (http://surfer.nmr.mgh.harvard.edu/) was used for automatic volumetric segmentation. The output images were visually checked for accurate segmentation.
Measures
One neuroimaging, three cognitive and one functional measure that have been extensively used for diagnostic purposes and cognitive and functional evaluation in clinical trials (Estévez-Gonzalez, Kulisevsky, Boltes, Otermin, & Garcia-Sanchez, 2003; Ito, Hutmacher, & Corrigan, 2012; Petersen et al., 2005) and with established associations with AD and predictive of MCI conversion (Ito et al., 2012; Li et al., 2017) were considered.
Hippocampal Volume
The hippocampus is one of the first brain areas to be impacted by AD pathology, and one of the areas with greatest shrinkage over the course of the disease (Tabatabaei-Jafari et al., 2015). It is also the most sensitive structural predictor of AD conversion in MCI individuals (Eckerstrom et al., 2008). Therefore, HCV, the total volume of the left and right hippocampi adjusted for age, field strength, and ICV using the residual regression method described elsewhere (Pintzka, Hansen, Evensmoen, & Haberg, 2015) was investigated as neuroimaging predictor.
Mini-Mental State Examination
The MMSE (Folstein, Folstein, & McHugh, 1975) is the most widely used screening instrument for AD/dementia (Arevalo-Rodriguez et al., 2015). It consists of 11 items with total scores ranging between 0 and 30, which lower scores reflecting more severe cognitive impairment. The items evaluate orientation in time and space (10 points), immediate recall (3 points), attention and calculation (5 points), delayed recall (3 points), language naming (2 points), following command (3 points), repetition (1 point), reading (1 point), writing (1 point), and visuospatial (1 point).
The Alzheimer’s Disease Assessment Scale
The modified 13-items Alzheimer’s Disease Assessment Scale (ADAS)-cog version (Petersen et al., 2005) was used here to assess general cognitive function. The modified ADAS consists of word recall (10 items), commends (5 items), construction (5 items), naming (5 items), ideational praxis (5 items), orientation (8 items), word recognition (12 items), recall instruction (5 items), spoken language (5 items), word finding (5 items), comprehension (5 items), delayed word recall (10 items), and number cancelation (5 items) in total 85 scores, which the higher score the severest the cognitive impairment.
Rey Auditory Verbal Learning Test
The Rey Auditory Verbal Learning Test (RAVLT) was used to evaluate episodic memory (Rey, 1941, 1964). It involves free recall of a list of 15 words in any order over five sequential trials. It is followed by recall of a second list of 15 words. Finally, the participant is asked to remember as many words as possible from the first list immediately following the second list recall and after 30 min. The scoring system of the RAVLT based on the correct number of words in each trial (5 in total) and evaluates a wide diversity of learning and memory functions including immediate memory, learning, and forgetting. The immediate recall score, RAVLT immediate, was considered for this study based on our introductory analyses that showed better predictive value for immediate memory compared with RAVLT learning and percentage of forgetting. The RAVLT immediate was computed as the total scores of trials 1–5.
The Functional Assessment Questionnaire
The Functional Assessment Questionnaire (FAQ) assesses abilities of daily living with total scores ranging from 0 to 30. A score of 0 indicates “no impairment” and 30 “severely impaired” (Ito et al., 2012; Pfeffer, Kurosaki, Harrah, Chance, & Filos, 1982). The total FAQ score is the sum of 10 daily activities, with each activity being rated from 0 to 3 (0 = normal, 1 = has difficulty but does by self, 2 = requires assistance, 3 = dependent). Evaluated activities are (a) writing checks, paying bills, or balancing a checkbook, (b) assembling tax records, business affairs, or other papers, (c) shopping alone for clothes, household necessities, or groceries, (d) playing a game of skill such as bridge or chess or working on a hobby, (e) heating water, making a cup of coffee, turning off the stove, (6) preparing a balanced meal, (f) keeping track of current events, (g) paying attention to and understanding a TV program, book, or magazine, (h) remembering appointments, family occasions, holidays, medications, and (i) traveling out of the neighborhood, driving, or arranging to take public transportation.
Statistical Analysis
Statistical analyses were performed using the R statistical software (version 3.3.2). No missing values were present in the measures of interest. Mahalanobis distance was used for detection of univariate and multivariate outliers. No influential outlier was detected. Group differences in demographic variables were assessed by t-test for continuous variables and chi square tests for categorical variables. Univariate and bivariate models were used to investigate prediction of conversion from MCI to AD within 5 years as well as prediction of the time to conversion. Each bivariate model consisted of standardized values of HCV and one of four cognitive/functional measures as well as their interaction. The alpha level was set at <0.05.
Prediction of AD Conversion
Logistic regression analysis (package Stats; version 3.3.2 and package Caret; version 6.3–73) was used to quantify the magnitude of predictive values of the measures for predicting MCI conversion to AD. Univariate and bivariate models were applied. The odds ratios were used to quantify the magnitude of the main and interaction effects of the predictors. To graphically illustrate the effect of HCV, the probability of conversion for the cognitive/functional measures at different categories of HCV was investigated. Participants were categorized into three groups; small HCV for those with HCV less than 5,500 mm3 (smaller than 1 SD), medium HCV for the volume between 5500 and 7500 mm3 (within 1 SD), and large HCV for those with larger than 7,500 mm3 (larger than 1 SD).
Prediction of Time to AD Conversion
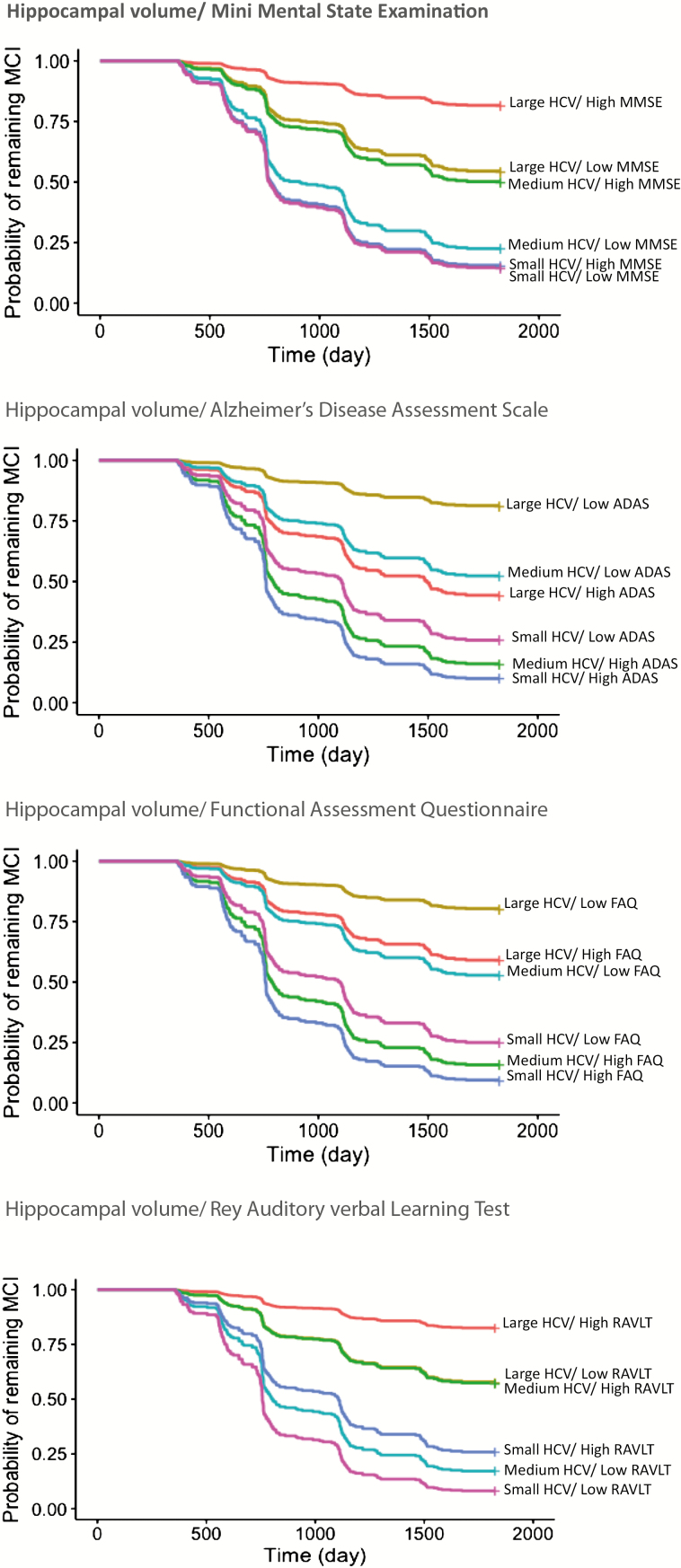
Cox proportional hazard analysis (package survival; version 2.40-1) was used to predict the time to AD conversion using the univariate and bivariate models. The hazard ratio for 1 SD change in the measures was used to quantify the magnitude of the main and interactive effects of the measures. In the case of the presence of interactive effect, to better interpret the effect the analyses were repeated with HCV as a categorical variable (small, medium, and large) in the model. To graphically illustrate the contribution of cognitive/functional measures and HCV on probability of remaining MCI over time, separate Kaplan–Meier curves were plotted for different combinations of categorical levels of HCV (small, medium, and large as defined above) and cognitive/functional measures (low and high). Cognitive/functional measures were categorized into low and high based on the median: 27 for MMSE, 13 for ADAS, 2 for FAQ, and 31 for RAVLT. Participants were categorized into six combinations for each cognitive/functional measure (Supplementary Figure 1). For example, for ADAS, they were categorized into small HCV/low ADAS, small HCV/high ADAS, medium HCV/low ADAS, medium HCV/high ADAS, large HCV/low ADAS, and large HCV/high ADAS.
Results
Participants’ Characteristics
Two hundred ninety-five MCI participants were categorized as MCI who subsequently converted to AD within 5 years (MCIc; n = 183), and MCI who were stable for more than 5 years (MCIs; n = 112). MCIs participants were about 2 years younger than MCIc but there were no significant differences in sex ratio or education between the two groups. The proportion of APOE e4 carriers was significantly higher in MCIc than MCIs. All the measures of interest (HCV and cognitive/functional measures) were significantly different between the groups (Table 1).
Table 1.
Participants Characteristics and Measurements
| Characteristics/Measures | MCIs | MCIc | Group difference |
|---|---|---|---|
| Sample size | 112 | 183 | |
| Age; year, mean (SD) | 71.95 (7.65) | 74.31 (6.90) | Yes |
| Age range, year | 57–88 | 55–89 | — |
| Male sex; N (%) | 72 (64.29) | 112 (61.20) | No |
| Education; year, mean (SD) | 15.75 (3.03) | 16.03 (2.73) | No |
| APOE e4; N (%) | 40 (35.71) | 124 (67.76) | Yes |
| One allele | 32 (28.57) | 93 (49.21) | Yes |
| Two alleles | 8 (7.14) | 31 (17.32) | Yes |
| Age at diagnosis change; year, mean (SD) | — | 76.83 (7.05) | — |
| Time to diagnosis change; year, mean (SD) | — | 2.40 (0.89) | — |
| MMSE, mean (SD) | 28.11 (1.49) | 26.93(1.73) | Yes |
| ADAS, mean (SD) | 13.45 (5.45) | 20.19 (5.49) | Yes |
| RAVLT immediate, mean (SD) | 38.40 (10.34) | 28.85 (7.11) | Yes |
| FAQ, mean (SD) | 1.75 (3.00) | 4.96 (4.62) | Yes |
| HCV,a mm3, mean (SD) | 7052.82 (909.03) | 6223.92 (875.56) | Yes |
Note. MCIc = mild cognitive impairment converted to Alzheimer’s disease within 5 years; MCIs = mild cognitive impairment stable for 5 or more years; APOE e4 = apolipoprotein E allele 4; MMSE = mini-mental state examination; ADAS = Alzheimer Disease Assessment Scale (cognitive subscale); RAVLT = Rey Auditory Verbal Learning Test; FAQ = functional assessment questionnaire.
aAdjusted by age, field strength, and intracranial volume.
Prediction of AD Conversion
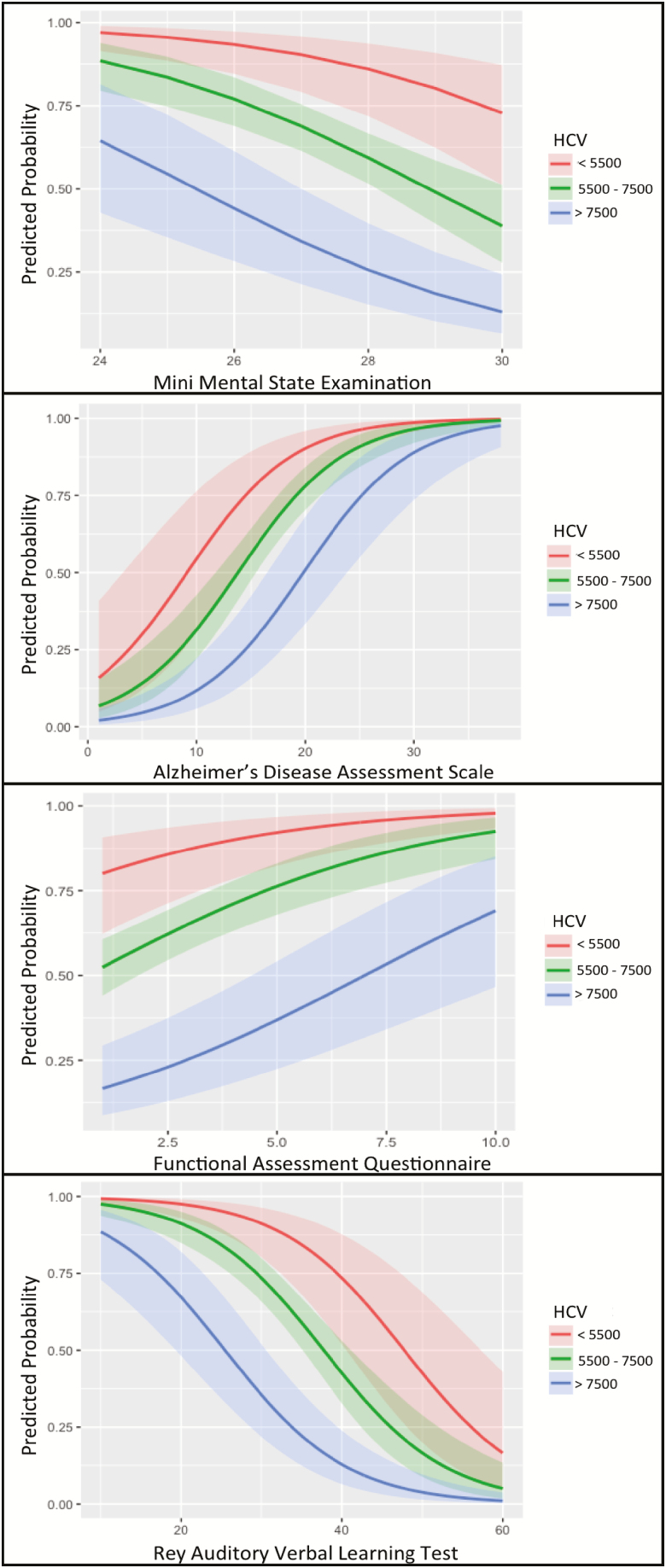
HCV, MMSE, ADAS, RAVLT, and FAQ were evaluated separately (univariate model) and all were significant predictors of AD conversion. Each cognitive/functional predictor remained a significant predictor of conversion from MCI to AD when HCV was added to the model, and HCV also remained a significant predictor. Additionally, HCV had additive effects with ADAS, RAVLT, and FAQ, whereas HCV and MMSE had interactive effects (Table 2). A graphical illustration (Figure 1) of the probability of conversion for the measures at three different categories of HCV (small, medium, and large) suggests that having a medium to large HCV had a protective effect against conversion in MMSE from 24 to 30. However that protective effect was smaller at lower MMSE scores. The same pattern was demonstrated in the normal range of FAQ, that is, having a medium to large HCV had a protective effect against conversion but the protection was lower when FAQ scores were closer to upper limit of the normal range. The pattern was relatively different for ADAS and RAVLT, where larger HCV was protective in medium ADAS or RAVLT.
Table 2.
Logistic Regression and Cox Proportional Hazard Results: Bivariate Models
| Prediction of conversion | Prediction of time to conversion | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Coef. | SE | OR (95% CI) | Z, p value | Coef. | SE | HR (95% CI) | Z, p value |
| HCV and MMSE | ||||||||
| HCV | −0.92 | 0.16 | 0.40 (0.29–0.54) | −5.67, p < .0001 | −0.53 | 0.08 | 0.59 (0.51–0.68) | −6.89, p < .00001 |
| MMSE | −0.63 | 0.15 | 0.53 (0.39–0.71) | −4.17, p < .0001 | −0.40 | 0.08 | 0.66 (0.57–0.78) | −5.01, p < .0001 |
| HCV: MMSE | −0.35 | 0.17 | 0.71 (0.50–0.98) | −2.05, p < .05 | −0.25 | 0.08 | 0.78 (0.66–0.91) | −3.18, p = .002 |
| HCV and ADAS | ||||||||
| HCV | −0.66 | 0.17 | 0.52 (0.37–0.72) | −3.86, p = .0001 | −0.41 | 0.08 | 0.67 (0.57–0.78) | −5.05, p < .0001 |
| ADAS | 1.18 | 0.19 | 3.26 (2.27–4.83) | 6.16, p < .0001 | 0.64 | 0.08 | 1.91 (1.62–2.25) | 7.79, p < .0001 |
| HCV: ADAS | 0.34 | 0.22 | 1.41 (0.92–2.14) | 1.59, p = .11 | 0.23 | 0.09 | 1.26 (1.07–1.49) | 2.70, p < .01 |
| HCV and FAQ | ||||||||
| HCV | −0.95 | 0.17 | 0.39 (0.27–0.54) | −5.56, p < .0001 | −0.50 | 0.07 | 0.61 (0.53–0.70) | −6.99, p < .0001 |
| FAQ | 1.05 | 0.22 | 2.84 (1.90–4.55) | 4.72, p < .0001 | 0.38 | 0.06 | 1.46 (1.30–1.65) | 6.28, p < .0001 |
| HCV: FAQ | 0.15 | 0.23 | 1.16 (0.73–1.77) | 0.65, p = 0.52 | 0.06 | 0.06 | 1.06 (0.95–1.19) | 1.09, p = 0.28 |
| HCV and RAVLT | ||||||||
| HCV | −0.92 | 0.17 | 0.40 (0.28–0.55) | −5.44, p < .0001 | −0.49 | 0.08 | 0.61 (0.53–0.71) | −6.46, p < .0001 |
| RAVLT | −0.18 | 0.20 | 0.31 (0.21–0.44) | −6.04, p < .0001 | −0.75 | 0.10 | 0.47 (0.39–0.58) | −7.45, p < .0001 |
| HCV: RAVLT | −0.09 | 0.22 | 0.91 (0.59–1.40) | −0.41, p = .68 | −0.17 | 0.84 | 0.84 (0.70–1.01) | −1.84, p = .07 |
Note. MMSE = mini-mental state examination (standardized); ADAS = Alzheimer Disease Assessment Scale (standardized); RAVLT = Rey Auditory Verbal Learning Test (immediate; standardized); FAQ = Functional Assessment Questionnaire (standardized); HCV = hippocampal volume adjusted by age, field strength, and intracranial volume (standardized).
Figure 1.
Predicted probabilities of conversion to Alzheimer’s: Predicted probabilities of cognitive measures at different hippocampal volumes. HCV has a reciprocal impact on predicted probability of the cognitive measures for conversion to Alzheimer’s. HCV = hippocampal volume adjusted by age, field strength, and intracranial volume; MMSE = mini-mental state examination; ADAS = Alzheimer Disease Assessment Scale (cognitive subscale); RAVLT = Rey Auditory Verbal Learning Test (immediate memory subscale); FAQ = Functional Assessment Questionnaire.
Prediction of Time to Conversion
All the measures significantly predicted time to AD conversion in separate univariate analyses (likelihood ratio test between 33 and 90, df = 1, p < .0001). Each cognitive/functional predictor remained a significant predictor when HCV was added to the model, and HCV also remained a significant predictor. Additionally, HCV had additive effects with RAVLT, and FAQ, whereas HCV and MMSE, and HCV and ADAS had interactive effects (Table 2).
The analyses were repeated using categories of HCV (small, medium, and large) in the models instead of HCV as a continuous variable (Table 3). The results revealed that MMSE was not a predictor of conversion in small HCV, and that having a medium to large HCV, respectively, associated with 45% and 81% lower risk of conversion from MCI to AD over time compared with small HCV. An additional 32% decrease in the risk of conversion was demonstrated for every 1 SD higher MMSE score in medium HCV but not in large HCV in comparison with small HCV. Similarly, ADAS was not predictive in small HCV and having a medium to large HCV was associated with 90% and 99.5% lower risk of conversion over time compared with small HCV. An additional 10% increase in the risk of conversion was demonstrated for every 1 SD higher ADAS score in medium HCV but not in large HCV in comparison with small HCV. In contrast, RAVLT was predictive in all HCV categories including small HCV, although an additional 4% decrease in the risk of conversion was detected for every 1 SD higher RAVLT in medium HCV.
Table 3.
Risk of Conversion from MCI to AD Over Time (Cox Proportional Hazard) by Hippocampal Volume Categories
| Variables | Coef. | SE | HR (95% CI) | Z, p Value |
|---|---|---|---|---|
| MMSE and HCV | ||||
| Medium HCV category | −0.59 | 0.18 | 0.55 (0.39–0.79) | −3.29, p = .001 |
| Large HCV category | −1.68 | 0.31 | 0.19 (0.10–0.35) | −5.35, p < .0001 |
| MMSE | −0.07 | 0.14 | 0.93 (0.70–1.23) | −0.5, p = .61 |
| Medium HCV category: MMSE | −0.38 | 0.17 | 0.68 (0.49–0.96) | −2.21, p = .03 |
| Large HCV category: MMSE | −0.60 | 0.31 | 0.55 (0.30–1.00) | −1.95, p = .05 |
| ADAS and HCV | ||||
| Medium HCV category | −2.28 | 0.69 | 0.10 (0.03–0.39) | −3.33, p = .0008 |
| Large HCV category | −3.11 | 1.05 | 0.04 (0.01–0.35) | −2.96, p = .003 |
| ADAS | 0.03 | 0.03 | 1.03 (0.97–1.08) | 0.94, p = .35 |
| Medium HCV category: ADAS | 0.10 | 0.03 | 1.10 (1.04–1.17) | 3.14, p = .003 |
| Large HCV category: ADAS | 0.10 | 0.06 | 1.10 (0.99–1.23) | 1.71, p = .09 |
| FAQ and HCV | ||||
| Medium HCV category | −0.63 | 0.25 | 0.53 (0.33–0.87) | −2.53, p = .01 |
| Large HCV category | −2.08 | 0.44 | 0.13 (0.05–0.30) | −4.74, p < .0001 |
| FAQ | 0.06 | 0.03 | 1.06(1.00–1.13) | 1.89, p = .06 |
| Medium HCV category: FAQ | 0.03 | 0.04 | 1.03 (0.96–1.10) | 0.81, p = .42 |
| Large HCV category: FAQ | 0.08 | 0.06 | 1.09 (0.97–1.21) | 1.47, p = .14 |
| RAVLT and HCV | ||||
| Medium HCV category | 0.82 | 0.66 | 2.27 (0.62–8.28) | 1.25, p = .21 |
| Large HCV category | 1.11 | 1.42 | 3.02 (0.19–49.31) | 0.78, p = .44 |
| RAVLT | −0.04 | 0.02 | 0.96 (0.93–0.99) | −2.05, p = .04 |
| Medium HCV category: RAVLT | −0.04 | 0.02 | 0.96 (0.92–0.99) | −2.01, p = .045 |
| Large HCV category: RAVLT | −0.09 | 0.05 | 0.92 (0.83–1.01) | −1.86, p = .06 |
Note. MMSE = mini-mental state examination (standardized); ADAS = Alzheimer Disease Assessment Scale (standardized); RAVLT = Rey Auditory Verbal Learning Test (immediate; standardized); FAQ = Functional Assessment Questionnaire (standardized); HCV = hippocampal volume adjusted by age, field strength, and intracranial volume (standardized).
Kaplan–Meier curves (Figure 2) revealed that the contribution of cognitive/functional measures in predicting the probability of remaining MCI over time was not constant at all HCV categories. For example, MMSE was not a determinant factor at small HCV, while it was at medium to large HCV.
Figure 2.
Kaplan–Meier plots for remaining stable over time: Illustrating the contribution of cognitive/functional measure and hippocampal volume on probability of remaining stable over time in MCI. Participants were categorized into six combinations based on three levels of HCV (small, medium, and large) and two levels of cognitive/functional measures (low and high). HCV = hippocampal volume adjusted by age, field strength, and intracranial volume; MMSE = mini-mental state examination; ADAS = Alzheimer Disease Assessment Scale (cognitive subscale); RAVLT = Rey Auditory Verbal Learning Test (immediate memory subscale); FAQ = Functional Assessment Questionnaire.
Discussion
This study aimed to investigate the predictive value of cognitive/functional measures in combination with HCV to predict conversion from MCI to AD within 5 years, as well as their capacity to predict time to conversion. The results demonstrated that the predictive value of cognitive/functional measures is dependent on HCV. The findings revealed that (a) in predicting the conversion from MCI to AD, the predictive value of cognitive/functional measures was higher at lower HCV, while it was lower at higher HCV, and (b) in predicting the time to AD conversion, the cognitive/functional measures were somewhat more predictive when HCV was in the medium range (5,500 to 7,500 mm3) than at smaller or larger volumes, except for the immediate memory test that remained predictive across all HCV. The effect of HCV in predicting time to conversion was interactive with general cognitive measures (MMSE and ADAS) but additive with the functional assessment (FAQ) and immediate memory test (RAVLT).
These findings are important because they demonstrate that severity of cognitive impairment or subtle functional impairment and severity of neural pathology are both important in predicting probability of AD conversion. Although cognitive/functional performance is closely linked with neuropathology, the association is not straightforward. There is an imperfect overlap between cognitive deficit and pathology severity (Neuropathology Group, Medical Research Council Cognitive & Aging, 2001). Individual variability in brain/cognitive reserve is the most likely explanation for this effect (Medaglia, Pasqualetti, Hamilton, Thompson-Schill, & Bassett, 2017; Steffener & Stern, 2012; Stern, 2009). Taking the severity of the pathology into account when evaluating cognitive/functional performance is a practical way to take into account the moderating effect of brain/cognitive reserve.
There is accumulating evidence showing that individuals with larger brain/cognitive reserve may cope better with neural damage, that is, at a given level of observed pathology, cognitive impairment is lower in those with larger brain/cognitive reserve (Stern, 2009). Diversity in efficacy and capacity of neural networks as well as compensatory neural mechanisms such as using alternative neural networks may underlie this coping mechanism such that cognitive function may be maintained for some time in the context of increasing neurodegeneration. When brain/cognitive reserve is exhausted, further neurodegeneration cannot be compensated for and failure in cognitive processes clinically manifest as conversion from CN to MCI or MCI to AD (Steffener & Stern, 2012). Therefore, since individuals vary in their levels of brain/cognitive reserve, cognitive and functional performance alone is not a perfect predictor of decline. Cognitive reserve has been indirectly estimated in the literature by proxy variables including education, IQ, literacy, occupational complexity, participation in leisure activities and even personality variables (Steffener & Stern, 2012). However, the accurate measurement of brain/cognitive reserve is still the subject of ongoing research and much controversy (Steffener, Brickman, Rakitin, Gazes, & Stern, 2009; Steffener, Reuben, Rakitin, & Stern, 2011; Stern et al., 2008; Zarahn, Rakitin, Abela, Flynn, & Stern, 2007). Altogether, a practical way to deal with the concealing effect of cognitive reserve is to take into account the severity of neuropathology when evaluating cognitive/functional performance.
In addition to predicting the likelihood of converting from MCI to AD, the prediction of time to conversion is also of clinical significance but has proven difficult to achieve. Our results suggest that combining HCV and cognitive/functional measures is more effective in predicting time to conversion. However, the effect of HCV differs for different cognitive/functional measures. It has an interactive effect with MMSE and ADAS but an additive effect with FAQ or RAVLT immediate. That is, the increase in the risk of AD conversion for each one-point decrease in the MMSE (or increase in ADAS) is not constant at different HCV values and is smaller at larger HCV. In contrast, the increase in the risk is constant for every one-point decrease on the RAVLT immediate (or higher FAQ) at any HCV values. This may be because HCV is more reflective of AD related pathology than MMSE and ADAS. As a consequence, at HCV less than 5,500 mm3, one unit difference in MMSE (or ADAS) is less influential than at larger HCV. This may explain the fact that MMSE and ADAS are not predictive of time to conversion at HCV less than 5,500 mm3 and at more than 7,500 mm3, but predictive in the mid-range of HCV (5,500–7,500 mm3).
In contrast, the combined evaluation of performance in a specific domain (such as immediate memory) and the brain structure underpinning that performance (HCV) may provide a more precise evaluation of the degree of neurodegeneration and the level of brain/cognitive reserve exhaustion. This may explain our findings that RAVLT immediate and HCV are more sensitive predictors of time to conversion. It may also explain the lack of interactive effect between these two measures.
It is important to note that because MMSE, ADAS, and FAQ evaluate performance across a larger number of neural networks, they may reflect the development of AD pathology across any of those networks and thus also predict the risk of AD conversion. However, because only part of their variability is related to hippocampal function, they do not appear to be as predictive of time to conversion than RAVLT immediate.
Many studies conducted to date have focused on combining MRI and cognitive/functional measures for improved diagnosis or prediction of AD conversion. Our study, in contrast, investigated the nature of the interaction between MRI and cognitive/functional measures in predicting AD conversion and time to conversion. Understanding the relationship between structural and cognitive/functional measures not only emphasizes the benefit of combining these measures for diagnostic/ prognostic purpose, it may also help better conceptualize the impact of brain/cognitive reserve on clinical/MRI measures.
In conclusion, AD is pathologically characterized by degenerative processes, the severity of which can be measured with neuroimaging techniques. The functional consequence of the degeneration can be concurrently assessed with cognitive/functional tools. A combination of both neuroimaging and cognitive/functional indexes are superior in predicting disease progression than either alone. However, the present findings indicate that the relative contribution of neuroimaging and cognitive/functional measures is not constant in predicting progression from MCI to AD. Cognitive/functional measures are predictors of conversion but their predictive values are not constant at all levels of HCVs. Additionally, the most effective combination of measures to predict time to conversion is likely to involve those that assess hippocampal volume in conjunction with one of its main functions, immediate memory.
Funding
Data collection and sharing for this work was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI; National Institutes of Health Grant U01 AG024904). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Alzheimer’s Association; Alzheimer Drug Discovery Foundation; BioClinica, Inc.; Biogen Idec, Inc.; Bristol- Myers Squibb Company; Eisai, Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; GE Healthcare; Innogenetics, N.V.; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Medpace, Inc.; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; Neu-roRx Research; Novartis Pharmaceuticals Corporation; Pfizer, Inc.; Piramal Imaging; Servier; Synarc, Inc.; and Takeda Pharmaceutical Company. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California. This research was partly undertaken on the National Computational Infrastructure (NCI) facility in Canberra, Australia, which is supported by the Australian Commonwealth Government and was supported by an Australian Government Research Training Program (RTP) Scholarship.
Supplementary Material
Acknowledgments
Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf.
Conflict of Interest
None reported.
References:
- Arevalo-Rodriguez I., Smailagic N., Roque I. F. M., Ciapponi A., Sanchez-Perez E., Giannakou A.,…Cullum S (2015). Mini-Mental State Examination (MMSE) for the detection of Alzheimer’s disease and other dementias in people with mild cognitive impairment (MCI). Cochrane Database Systematic Reviews, ( 3), CD010783. doi:10.1002/14651858.CD010783.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belleville S., Fouquet C., Hudon C., Zomahoun H. T. V., & Croteau J.; Consortium for the Early Identification of Alzheimer’s Disease-Quebec (2017). Neuropsychological measures that predict progression from mild cognitive impairment to Alzheimer’s type dementia in older adults: A systematic review and meta-analysis. Neuropsychology Review, 27, 328–353. doi:10.1007/s11065-017-9361-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devanand D. P., Liu X., Tabert M. H., Pradhaban G., Cuasay K., Bell K.,…Pelton G. H (2008). Combining early markers strongly predicts conversion from mild cognitive impairment to Alzheimer’s disease. Biological Psychiatry, 64, 871–879. doi:10.1016/j.biopsych.2008.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckerstrom C., Olsson E., Borga M., Ekholm S., Ribbelin S., Rolstad S.,…Malmgren H (2008). Small baseline volume of left hippocampus is associated with subsequent conversion of MCI into dementia: The Goteborg MCI study. Journal of the Neurological Sciences, 272(1–2), 48–59. doi:10.1016/j.jns.2008.04.024 [DOI] [PubMed] [Google Scholar]
- Estévez-González A., Kulisevsky J., Boltes A., Otermín P., & García-Sánchez C (2003). Rey verbal learning test is a useful tool for differential diagnosis in the preclinical phase of Alzheimer’s disease: Comparison with mild cognitive impairment and normal aging. International Journal of Geriatric Psychiatry, 18, 1021–1028. doi:10.1002/gps.1010 [DOI] [PubMed] [Google Scholar]
- Falahati F., Westman E., & Simmons A (2014). Multivariate data analysis and machine learning in Alzheimer’s disease with a focus on structural magnetic resonance imaging. Journal of Alzheimer’s Disease, 41, 685–708. doi:10.3233/JAD-131928 [DOI] [PubMed] [Google Scholar]
- Folstein M. F., Folstein S. E., & McHugh P. R (1975). “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12, 189–198. doi:10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Gomar J. J., Bobes-Bascaran M. T., Conejero-Goldberg C., Davies P., & Goldberg T. E.; Alzheimer’s Disease Neuroimaging Initiative (2011). Utility of combinations of biomarkers, cognitive markers, and risk factors to predict conversion from mild cognitive impairment to Alzheimer disease in patients in the Alzheimer’s disease neuroimaging initiative. Archives of General Psychiatry, 68, 961–969. doi:10.1001/archgenpsychiatry.2011.96 [DOI] [PubMed] [Google Scholar]
- Ito K., Hutmacher M. M., & Corrigan B. W (2012). Modeling of Functional Assessment Questionnaire (FAQ) as continuous bounded data from the ADNI database. Journal of Pharmacokinetics and Pharmacodynamics, 39, 601–618. doi:10.1007/s10928-012-9271-3 [DOI] [PubMed] [Google Scholar]
- Jack C. R. Jr., Bernstein M. A., Fox N. C., Thompson P., Alexander G., Harvey D.,…Weiner M. W (2008). The Alzheimer’s Disease Neuroimaging Initiative (ADNI): MRI methods. Journal of Magnetic Resonance Imaging, 27, 685–691. doi:10.1002/jmri.21049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack C. R., Jr., Shiung M. M., Weigand S. D., O’Brien P. C., Gunter J. L., Boeve B. F.,…Petersen R. C (2005). Brain atrophy rates predict subsequent clinical conversion in normal elderly and amnestic MCI. Neurology, 65, 1227–1231. doi:10.1212/01.wnl.0000180958.22678.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K., Chan W., Doody R. S., Quinn J., & Luo S.; Alzheimer’s Disease Neuroimaging Initiative (2017). Prediction of conversion to Alzheimer’s disease with longitudinal measures and time-to-event data. Journal of Alzheimer’s Disease, 58, 361–371. doi:10.3233/JAD-161201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medaglia J. D., Pasqualetti F., Hamilton R. H., Thompson-Schill S. L., & Bassett D. S (2017). Brain and cognitive reserve: Translation via network control theory. Neuroscience and Biobehavioral Reviews, 75, 53–64. doi:10.1016/j.neubiorev.2017.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradi E., Pepe A., Gaser C., Huttunen H., & Tohka J.; Alzheimer’s Disease Neuroimaging Initiative (2015). Machine learning framework for early MRI-based Alzheimer’s conversion prediction in MCI subjects. Neuroimage, 104, 398–412. doi:10.1016/j.neuroimage.2014.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuropathology Group, Medical Research Council Cognitive Function and Ageing Study (2001). Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS). Lancet, 357(9251), 169–175. doi:10.1016/s0140-6736(00)03589-3 [DOI] [PubMed] [Google Scholar]
- Pandya S. Y., Clem M. A., Silva L. M., & Woon F. L (2016). Does mild cognitive impairment always lead to dementia? A review. Journal of the Neurological Sciences, 369, 57–62. doi:10.1016/j.jns.2016.07.055 [DOI] [PubMed] [Google Scholar]
- Petersen R. C., Smith G. E., Waring S. C., Ivnik R. J., Tangalos E. G., & Kokmen E (1999). Mild cognitive impairment: Clinical characterization and outcome. Archives of Neurology, 56, 303–308. doi:10.1001/archneur.56.3.303 [DOI] [PubMed] [Google Scholar]
- Petersen R. C., Thomas R. G., Grundman M., Bennett D., Doody R., Ferris S.,…Thal L. J.; Alzheimer’s Disease Cooperative Study Group (2005). Vitamin E and donepezil for the treatment of mild cognitive impairment. The New England Journal of Medicine, 352, 2379–2388. doi:10.1056/NEJMoa050151 [DOI] [PubMed] [Google Scholar]
- Pfeffer R. I., Kurosaki T. T., Harrah C. H., Jr., Chance J. M., & Filos S (1982). Measurement of functional activities in older adults in the community. Journal of Gerontology, 37, 323–329. doi:10.1093/geronj/37.3.323 [DOI] [PubMed] [Google Scholar]
- Pintzka C. W., Hansen T. I., Evensmoen H. R., & Haberg A. K (2015). Marked effects of intracranial volume correction methods on sex differences in neuroanatomical structures: A HUNT MRI study. Frontiers in Neuroscience, 9, 238. doi:10.3389/fnins.2015.00238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey A. (1941). L’examen psychologique dans les cas d’encéphalopathie traumatique. (Les problems.) [The psychological examination in cases of traumatic encepholopathy. Problems]. Archives de Psychologie, 28, 215–285. [Google Scholar]
- Rey A. (1964). L’examen Clinique en Psychologie. Paris: Presses Universitaires de France. [Google Scholar]
- Steffener J., Brickman A. M., Rakitin B. C., Gazes Y., & Stern Y (2009). The impact of age-related changes on working memory functional activity. Brain Imaging and Behavior, 3, 142–153. doi:10.1007/s11682-008-9056-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffener J., Reuben A., Rakitin B. C., & Stern Y (2011). Supporting performance in the face of age-related neural changes: Testing mechanistic roles of cognitive reserve. Brain Imaging and Behavior, 5, 212–221. doi:10.1007/s11682-011-9125-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffener J., & Stern Y (2012). Exploring the neural basis of cognitive reserve in aging. Biochimica et Biophysica Acta, 1822, 467–473. doi:10.1016/j.bbadis.2011.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. (2009). Cognitive reserve. Neuropsychologia, 47, 2015–2028. doi:10.1016/j.neuropsychologia.2009.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y., Zarahn E., Habeck C., Holtzer R., Rakitin B. C., Kumar A.,…Brown T (2008). A common neural network for cognitive reserve in verbal and object working memory in young but not old. Cerebral Cortex (New York, N.Y.: 1991), 18, 959–967. doi:10.1093/cercor/bhm134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabatabaei-Jafari H., Shaw M. E., & Cherbuin N (2015). Cerebral atrophy in mild cognitive impairment: A systematic review with meta-analysis. Alzheimer’s & Dementia (Amsterdam, Netherlands), 1, 487–504. doi:10.1016/j.dadm.2015.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabatabaei-Jafari H., Walsh E., Shaw M. E., & Cherbuin N.; Alzheimer’s Disease Neuroimaging Initiative (ADNI) (2018). A simple and clinically relevant combination of neuroimaging and functional indexes for the identification of those at highest risk of Alzheimer’s disease. Neurobiology of Aging, 69, 102–110. doi:10.1016/j.neurobiolaging.2018.05.006 [DOI] [PubMed] [Google Scholar]
- Zarahn E., Rakitin B., Abela D., Flynn J., & Stern Y (2007). Age-related changes in brain activation during a delayed item recognition task. Neurobiology of Aging, 28, 784–798. doi:10.1016/j.neurobiolaging.2006.03.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.