Abstract
Background
Rapid influx of energy caused by fasting/refeeding repeatedly enhances fatty acid synthesis leading to triacylglycerol accumulation and production of reactive oxygen species (ROS), increasing the risk of non-alcoholic steatohepatitis (NASH). Previous studies have reported that the ingestion of butyrate is effective at preventing hepatic disorders, which are accompanied by fat accumulation and inflammation. The aim of this study is to reveal the mechanism of action of butyrate, and thus we investigated the effects of dietary butyrate on the expressions of antioxidant enzymes in the livers of rats during refeeding following fasting.
Methods
Thirty-seven male rats were divided into six groups (6–7 animals per group): non-fasting, fasting, refeeding with a high sucrose diet as control for 12 or 24 h, and refeeding with a high sucrose diet containing 5% sodium butyrate (NaB) for 12 or 24 h. All groups except the non-fasting group were fasted for 72 h before refeeding. Statistical analysis was conducted among 4 refeeding groups (refeeding with the control diet for 12 or 24 h, and refeeding with a diet containing NaB for 12 or 24 h).
Results
Supplementation with NaB significantly reduced (p < 0.05) fatty acid synthase (Fas) gene expression and increased the expression of the carnitine palmitoyltransferase 1α (Cpt1a) gene, resulting in reduced triacylglycerol content in the livers of rats refed the NaB diet compared with controls at 24 h after the start of refeeding. The mRNA levels of the genes related to glutathione synthesis were significantly higher (p < 0.05) in the livers of the butyrate group than the control group. In addition, the mRNA level of Foxo3a, a transcription factor that regulates the expressions of antioxidant enzymes, was higher in the butyrate group than controls. The acetylation levels of histone H4 around the Foxo3a gene tended to be increased (p = 0.055) by refeeding with the NaB diet.
Conclusion
NaB supplementation in the diet for refeeding reduced the rate of lipid synthesis and stimulated fatty acid oxidation in the liver, which inhibited fat accumulation and the risk of NASH. The transcriptional regulation of Foxo3a involves histone acetylation around the gene.
Keywords: Sodium butyrate, Liver, Non-alcoholic steatohepatitis (NASH)
Abbreviations: HDAC, histone deacetylase; NaB, sodium butyrate; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; ROS, reactive oxygen species; SCFAs, short-chain fatty acids
1. Introduction
The liver coordinates a series of metabolic adaptations during feeding and fasting to enable the continuous production and delivery of energy to critical organs. During starvation, the liver releases glycogen-derived glucose into the circulation, followed by the production of de novo glucose from amino acids. Then, fatty acid oxidation is enhanced to provide ketones as an alternative energy source [1]. Rapid carbohydrate influx, such as refeeding after starvation, enhances fatty acid synthesis, leading to triacylglycerol accumulation in the liver. In addition, disturbances in energy metabolism related to repeated dietary restrictions and rebound effects to overeating are considered as risk factors for non-alcoholic fatty liver disease (NAFLD), the incidence of which has increased in recent years [2]. Although NAFLD is a benign and simple steatosis, a recent study reported that 1%–3% of Japanese adults have nonalcoholic steatohepatitis (NASH) [3]. When fatty acids are rapidly oxidized in mitochondria and peroxisomes in the liver, high levels of reactive oxygen species ROS are produced that function as apoptotic signals [4]. Therefore, excessive mitochondrial function might induce excessive oxidative stress, which leads to the increased expression of inflammatory cytokines and further progression to NASH [5].
Short-chain fatty acids (SCFAs) such as acetate, propionate, and butyrate are generated by the bacterial fermentation of dietary fiber in the colon. Inulin and guar gum have protective effects for high-fat diet-induced obesity and hepatic steatosis, and it was suggested that these effects of dietary fibers are related to SCFAs [6,7]. Previous studies reported that butyrate reduced liver damage in several animal models [8,9]. In a rat model of type 2 diabetes induced by combination of a high-fat diet and a low-dose streptozotocin injection, the daily intraperitoneal injection of sodium butyrate (NaB) suppressed fat accumulation and gluconeogenesis in the liver as effectively as metformin [8], a drug for diabetes. C57BL/6J mice fed a Western diet fortified with fructose, fat, and cholesterol for 6 weeks developed NASH, while supplementation with NaB led to reduced liver steatosis and hepatic inflammation without any effects on body weight gain [9].
Butyrate has multiple effects on mammalian cells including inhibition of proliferation, induction of differentiation, and induction or repression of gene expression. It was suggested that these effects are derived in part by the inhibition of histone deacetylase (HDAC) activity. Butyrate inhibits most HDAC except for class III HDAC and class II HDAC6 and HDAC10 [10]. Acetylation of histones H3 and H4 is a pivotal post-translational modification related to chromatin structure alterations and transcriptional regulation around the genes [11]. Indeed, among SCFAs, butyrate was shown to prevent high fat-diet induced hepatic insulin resistance [12]. However, whether butyrate acts as an HDAC inhibitor to affect lipid metabolism and antioxidant systems in the liver is poorly understood.
Refeeding after fasting markedly changes energy metabolism, especially in the liver, where large amounts of carbohydrates and lipids flow from the portal vein, and enhanced mitochondrial functions and antioxidant mechanisms are required to effectively process them. Feeding with a high sucrose diet after fasting is thought to be a risk for NASH that is associated with fat accumulation and oxidative stress in the liver. A previous study showed that drinking a sucrose solution for 9 weeks induced insulin resistance and steatosis in rats [13]. The aim of this study is to reveal the mechanism of action of butyrate, and we investigated the effect of the administration of NaB with a high sucrose diet after fasting on the expressions of genes related to energy metabolism and antioxidant systems in the liver.
2. Material and methods
2.1. Animals
Six-week-old Sprague-Dawley male rats (SLC, Hamamatsu, Japan) were maintained under a stable temperature (23 ± 2 °C) and humidity (55 ± 5%) with a light-dark cycle (7:00–19:00) according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Rats with free access to a diet shown in Table 1 and tap water for 8–9 days. Thirty-seven rats were divided into six groups: non-fasting (n = 6), fasting (n = 7), refeeding with a high sucrose diet as a control for 12 h (n = 6) or 24 h (n = 6), and refeeding with a high sucrose diet containing NaB for 12 h (n = 6) or 24 h (n = 6). All groups except the non-fasting group were fasted for 72 h and then refed the control diet or the diet containing 5% NaB (Table 1) for 12 or 24 h. Each group was sacrificed under isoflurane inhalation anesthesia between 9:00 and 11:00 and the serum and livers were collected. The experimental procedures conformed to the guidelines of the Animal Usage Committee of the University of Shizuoka (No. 165139).
Table 1.
Diet composition (g/kg).
| Diet before starvation | Diet for refeeding |
||
|---|---|---|---|
| Control | NaB | ||
| Α-Corn starch | 629 | 229 | 179 |
| Sucrose | 200 | 400 | 400 |
| Casein | 200 | 200 | 200 |
| Corn Oil | 70 | 70 | 70 |
| Sodium butyrate | – | – | 50 |
| Fiber (Cellulose) | 50 | 50 | 50 |
| AIN93G-Mineral mixture | 35 | 35 | 35 |
| AIN93G-Vitamin mixture | 10 | 10 | 10 |
| L-Cystein | 3 | 3 | 3 |
| Choline bitartrate | 2.5 | 2.5 | 2.5 |
| Tert-Butylhydroquinone |
0.014 |
0.014 |
0.014 |
| Total | 1000 | 1000 | 1000 |
2.2. Blood biochemical parameters and triacylglycerol content in the liver
Serum triacylglycerol (Fujifilm Wako Shibayagi, Gunma, Japan), glucose (Fujifilm Wako Shibayagi), insulin (Fujifilm Wako Shibayagi) and β-hydroxybutyrate (BioVision, Milpitas, CA) concentration were determined using commercial kits.
2.3. SOD activity and GSSG/GSH ratio in the liver
Around 100 mg liver tissue was weighed and homogenized in 0.25 M sucrose buffer. The supernatants were used to determine manganese superoxide dismutase (SOD) activity using a SOD assay kit WST (Dojinnkagaku, Kumamoto, Japan).
Another 100 mg liver tissue was homogenized in 5% sulfosalicylic acid buffer. The supernatants were diluted 10 times with pure water and used to determine total glutathione (GSH) and oxidized glutathione (GSSG), using a GSSG/GSH Quantification Kit (Dojinnkagaku).
2.4. Quantitative reverse transcription (RT)-PCR
Total RNA was extracted by acid guanidinium thiocyanate-phenol-chloroform extraction as described by Chomczynski and Sacchi [14]. One microgram of total RNA samples was converted into cDNA by reverse transcription using Superscript III reverse transcriptase (Invitrogen, Waltham, MA) according to the manufacturer’s instructions. PCR amplification was performed on a Light Cycler 480 instrument system (Roche Diagnostics, Basel, Switzerland) and SYBR Green I Master (Roche Diagnostics), according to the manufacturer’s instructions. The cycle threshold (CT) values of test genes and Tf2b detected by qRT-PCR were converted to signal intensities by the delta-delta method, which calculates the difference of 1 CT-value as a 2-fold difference between samples. We analyzed sterol regulatory element binding-protein (SREBP) 1, fatty acid synthase (FAS), peroxisome proliferator-activated receptor (PPAR) α, carnitine palmitoyltransferase (CPT) 1α and acyl CoA oxidase (ACO) 1 to investigate the regulation of lipid metabolism. SOD2 and catalase were analyzed to investigate the possibility in removal of ROS. γ-Glutamylcyclotransferase (Ggct), 5-oxoprolinase (Oplah), glutamic-oxaloacetic transaminase 1 (Got1), glutamate cysteine ligase, modifier subunit (Gclm), glutamate-cysteine ligase, catalytic subunit (Gclc) and glutathione synthetase (Gss) were analyzed to investigate the GSH synthesis pathway. Forkhead box O3a (FoxO3a) is a transcriptional factor which regulates cellular homeostasis and stress response. The sequences of the PCR primer pairs and fragment sizes are shown in Supplemental table 1.
2.5. Chromatin immunoprecipitation assay (ChIP assay)
Livers were homogenized and incubated in a fixation solution (1% formaldehyde, 4.5 mM HEPES [pH 8.0], 9 mM NaCl, 0.09 mM EDTA, 0.04 mM EGTA) in 10% phosphate-buffered saline (PBS) for 30 min at 37 °C. Reactions were terminated by adding glycine to a final concentration of 150 mM. After washing in FACS solution (PBS, 2% bovine serum, 0.05% NaN3), the samples were sonicated in SDS lysis buffer (50 mM Tris-HCl [pH 8.0], 10 mM EDTA [pH 8.0], 1% SDS, 0.5 mM phenylmethanesulfonylfluoride) to generate DNA fragments of 200–500 bp. The immunoprecipitation was performed using anti-acetyl-histone H3 (#06–559, Sigma-Aldrich, MO, USA) and anti-acetyl-histone H4 (#06–598, Sigma-Aldrich) antibodies. The precipitated DNA was subjected to real-time PCR using primers corresponding to the indicated sites in the promoter/enhancer and transcribed regions. The CT values of the ChIP signals detected by real-time PCR were converted to percentages of the signal for input DNA using the delta-delta method, with formula 100 × [2(CTinput−CTIPsample)]. The primer sequences used in ChIP assays are listed in Supplemental Table 2.
2.6. Statistical analysis
The results were expressed as the means ± standard error of the mean (SEM). The data were analyzed using the Kruskal-Wallis test, followed by a post hoc Steel-Dwass an multiple comparison among 4 refeeding groups (refeeding with the control diet for 12 or 24 h, and refeeding with a diet containing NaB for 12 or 24 h). P < 0.05 was considered statistically significant.
3. Results
3.1. Effects of refeeding with a control diet or a diet containing sodium butyrate after starvation on liver triacylglycerol and blood biochemical parameters
Food intake from the start of refeeding was significantly higher at 24 h than 12 h after the start of refeeding, and it was not different between the control diet and the NaB diet for 12 h and 24 h period, respectively (Table 2). Liver weight of rats refed the control diet tended to increase (p = 0.051) from 12 h to 24 h after start of refeeding. The concentrations of serum triacylglycerol, glucose, and insulin were decreased after 3 days of fasting, and they were not significantly different between rats refed control or NaB diet (Table 2). We determined the concentration of serum β-hydroxybutyrate, a ketone body utilized as an energy source during starvation, which inhibits HDAC by a mechanism similar to that of butyrate. The serum β-hydroxybutyrate concentration was higher in rats refed the NaB diet for 12 h and lower in rats refed the control diet for 24 h (Table 2).
Table 2.
Effects of refeeding a control diet or a diet containing sodium butyrate after starvation on liver triacylglycerol content and biochemical parametaers in serum.
| non-Fasting (n = 6) | Fasting (n = 7) | Refeeding 12 h |
Refeeding 24 h |
|||
|---|---|---|---|---|---|---|
| Control |
NaB |
Control |
NaB |
|||
| (n = 6) | (n = 6) | (n = 6) | (n = 6) | |||
| Food consumption (g) | – | – | 15.7 ± 0.4 a | 14.1 ± 0.4 a | 23.2 ± 0.7 b | 21.5 ± 1.4 b |
| Liver weight (g/100g BW) | 4.39 ± 0.10 | 2.71 ± 0.05 | 4.33 ± 0.12 | 4.14 ± 0.25 | 4.87 ± 0.10 | 4.74 ± 0.21 |
| Liver triacylglycerol (mg/g liver) | 7.83 ± 0.14 | 6.30 ± 0.45 | 8.46 ± 0.46 | 7.86 ± 0.59 | 10.3 ± 0.9 | 7.59 ± 0.77 |
| Serum triacylglycerol (mg/dL) | 152 ± 15 | 20.4 ± 2.7 | 157 ± 10 | 147 ± 13 | 138 ± 16 | 188 ± 25 |
| Serum glucose (mg/dL) | 160 ± 6 | 134 ± 9 | 180 ± 11 | 205 ± 14 | 188 ± 22 | 163 ± 7 |
| Serum insulin (ng/mL) | 1.04 ± 0.13 | 0.07 ± 0.00 | 1.59 ± 0.24 | 1.65 ± 0.28 | 1.59 ± 0.20 | 1.31 ± 0.26 |
| Serum β-hydroxybutyrate (nmol/μL) | 0.06 ± 0.02 | 1.32 ± 0.32 | 0.14 ± 0.03 ab | 0.25 ± 0.06 b | 0.06 ± 0.02 a | 0.19 ± 0.02 ab |
Data are expressed as means ± SEM for 6–7 rats.
The data were analyzed using the Kruskal-Wallis test, followed by a post hoc Steel-Dwass an multiple comparison among 4 refeeding groups.
a-b: Different letters indicate significant differences (p < 0.05).
3.2. Effects of starvation and refeeding with a control diet or a diet containing sodium butyrate on the mRNA levels of genes related to lipid metabolism in the liver
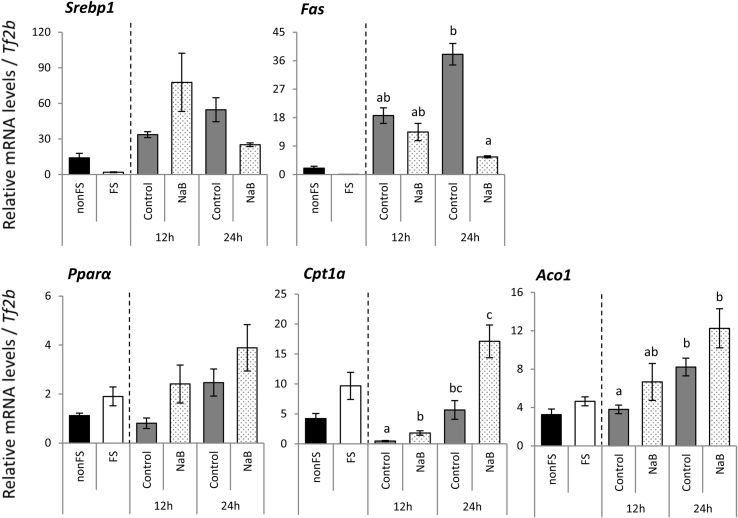
While refeeding with a high sucrose diet induced the expression of genes related to fatty acid synthesis, supplementation of a diet with NaB for refeeding significantly suppressed the mRNA levels of Fas at 24 h after the start of refeeding (Fig. 1). Conversely, the mRNA levels of Cpt1α, which are related to fatty acid oxidization, was significantly higher in rats refed the NaB diet than rats refed the control diet at 12 h after the start of refeeding. Similarly, the mRNA levels of Aco1 was higher in rats refed the NaB diet for 24 h and lower in rats refed the control diet for 12 h (Fig. 1).
Fig. 1.
Effects of starvation and refeeding with a control diet or a diet containing NaB on the mRNA levels of genes related to lipid metabolism in the liver
The mRNA levels were quantified by real-time RT-PCR and normalized to Tf2b mRNA abundance. Data are expressed as the means ± SEM for 6–7 animals.
The data were analyzed using the Kruskal-Wallis test, followed by a post hoc Steel-Dwass an multiple comparison among 4 refeeding groups.
a∼c: Different letters indicate significant differences (p < 0.05).
3.3. Effects of starvation and refeeding with a control diet or a diet containing sodium butyrate on SOD activity and GSSG/GSH ratio
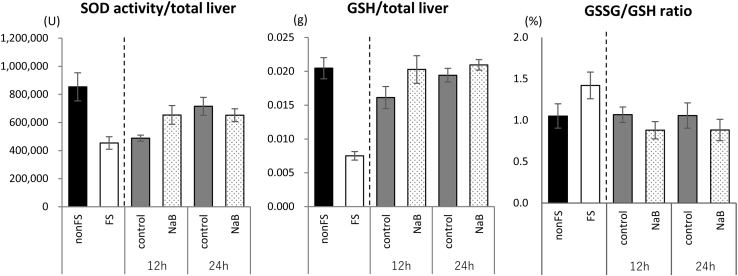
To examine whether NaB supplementation exerted a protective effect against oxidative stress, we determined the SOD activity and GSH level in the liver. SOD activity and the amount of GSH were decreased after 3 days of fasting, and those were not significantly different between rats refed control or NaB diet (Fig. 2). The GSSG/GSH ratio was not significantly different between rats refed control or NaB diet (Fig. 2).
Fig. 2.
Effects of starvation and refeeding with a control diet or a diet containing NaB on SOD activity and GSSG/GSH ratio
Data are the means ± SEM for 6–7 animals.
The data were analyzed using the Kruskal-Wallis test, followed by a post hoc Steel-Dwass an multiple comparison among 4 refeeding groups.
3.4. Effects of starvation and refeeding with a control diet or a diet containing sodium butyrate on the mRNA levels of genes related to antioxidant enzymes in the liver
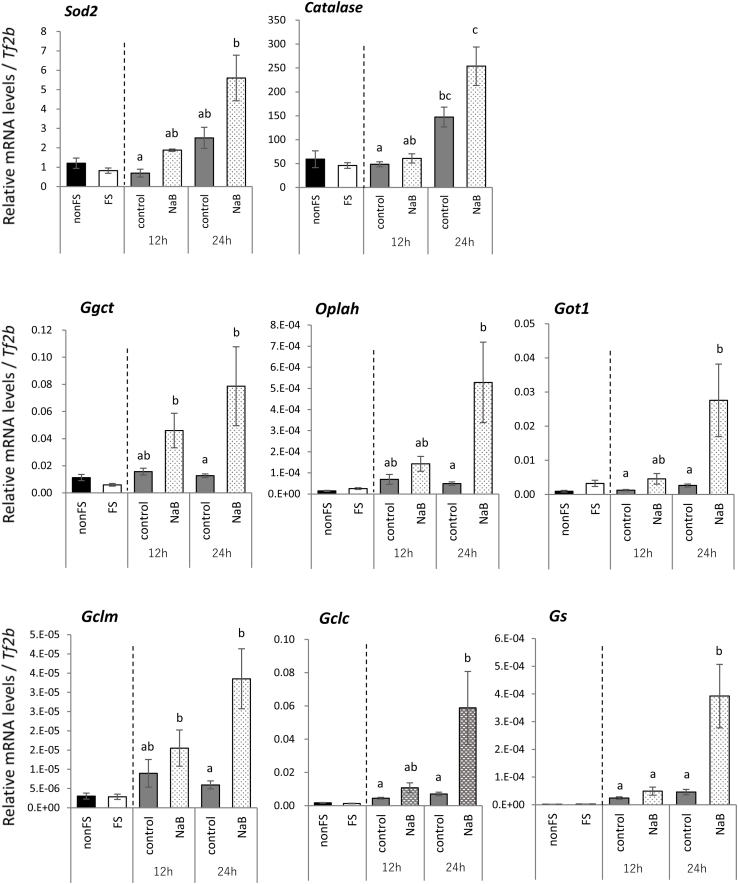
SOD2 (Mn-SOD) present in mitochondria is induced by oxidative stress during inflammation and has cytoprotective functions. In rats refed the NaB diet, the mRNA level of Sod2 tended to be higher (p = 0.051) than in rats refed the control diet at 12 h after the start of refeeding (Fig. 3). The mRNA level of Catalase, which is responsible for the elimination of H2O2, was elevated from 12 h to 24 h after the start of refeeding, and higher in rats refed the NaB diet for 24 h than in rats refed the control diet for 12 h (Fig. 3). In addition, the mRNA levels of genes involved in GSH synthesis (Ggct, Oplah, Got1, Gclm, Gclc, and Gs) were significantly higher in rats refed the NaB diet than in rats refed the control diet at 24 h after the start of refeeding (Fig. 3).
Fig. 3.
Effects of starvation and refeeding with a control diet or a diet containing NaB on the mRNA levels of antioxidant enzymes in the liver of rats
The mRNA levels were quantified by real-time RT-PCR and normalized to Tf2b mRNA abundance. Data are expressed as the means ± SEM for 6–7 rats.
The data were analyzed using the Kruskal-Wallis test, followed by a post hoc Steel-Dwass an multiple comparison among 4 refeeding groups.
a∼c: Different letters indicate significant differences (p < 0.05).
3.5. Effects of starvation and refeeding with a control diet or a diet containing sodium butyrate on Foxo3a expression and histone H3/H4 acetylation around the gene
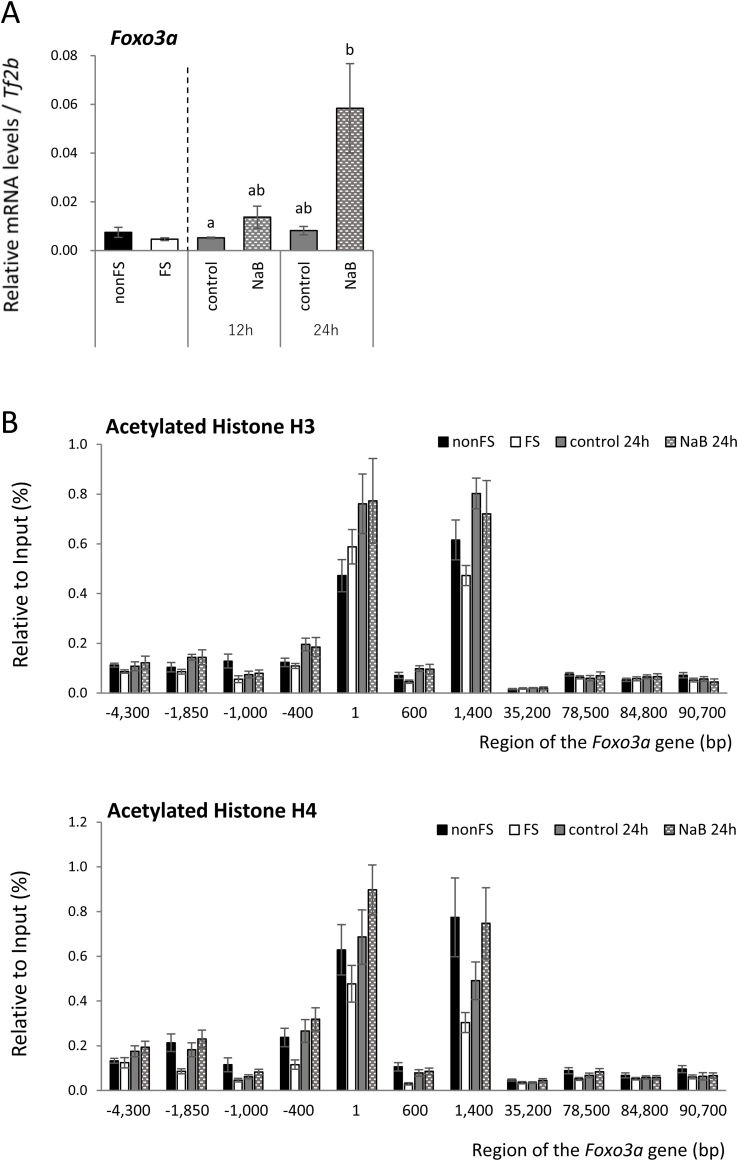
Forkhead box O3a (FoxO3a) regulates the transcription of genes involved in apoptosis, oxidative stress, glucose metabolism, and DNA damage repair including SOD2 and catalase, two scavenger proteins that have essential roles in oxidative detoxification in mammals [15]. The mRNA expression of Foxo3a in this study was higher in rats refed the NaB diet for 24 h than in rats refed the control diet for12 h (Fig. 4).
Fig. 4.
Effects of starvation and refeeding with a control diet or a diet containing NaB on Foxo3a mRNA levels and histone H3 and H4 acetylation levels around the Foxo3a gene.
A) Foxo3a mRNA levels were normalized to Tf2b mRNA abundance.
The data were analyzed using the Kruskal-Wallis test, followed by a post hoc Steel-Dwass an multiple comparison among 4 refeeding groups.
a∼b: Different letters indicate significant differences (p < 0.05).
B) The abscissa denotes the region on the Foxo3a gene relative to the transcription initiation site.
The data were analyzed using the Kruskal-Wallis test, followed by a post hoc Steel-Dwass an multiple comparison between control and NaB group.
To investigate the role of histone acetylation in the transcriptional regulation of Foxo3a and the effect of butyrate, we performed ChIP assays using anti-acetylated histone antibodies. The ChIP signals for normal rabbit IgG around the Foxo3a gene were <0.05% of the input. In rats fasted for 3 days, the signals of acetylated histone H4 were lower than those of non-fasted rats in the enhancer/promotor and transcription regions (from −1,850 bp to 1,400 bp). In addition, the signals of acetylated histone H4 at 1,400 bp downstream from the transcription start site tended to be higher (p = 0.055) in rats re-fed the NaB diet than in rats refed the control diet (Fig. 4).
4. Discussion
Excessive energy intake plays an important role in the development of fatty liver, and the weight loss by means of food restriction is the effective way to reduce hepatic fat [16]. While obesity increases a risk of NAFLD, some patients are reported to develop to fatty liver in spite of low BMI, especially in those who have been refed following rapid weight loss [17]. One of the reasons why fatty liver occurs after rapid weight loss/malnutrition is considered to be attributable to insufficient secretion of triglyceride from the liver due to lack of apolipoprotein B [18,19]. Therefore, it is very likely that the cycles of refeeding high sucrose and high fat diet after strict starvation are a putative risk factor for NAFLD. In this study, we showed that refeeding with a sucrose-fortified control diet increased the mRNA expression of Fas and its regulator, Srebp1. NaB supplementation of the diet for refeeding led to an suppression in the mRNA expressions of genes related to fatty acid synthesis at 24 h after the start of refeeding. By contrast, the mRNA expressions of genes related to β-oxidation were enhanced by refeeding with the NaB diet. A previous study reported that the oral administration of butyrate as well as acetate and propionate suppressed high-fat induced obesity by the activation of AMPK [20]. In addition, the administration of butyrate prevented high-fat diet-induced insulin resistance, which promoted energy expenditure and induced mitochondria functions [21]. Thus, in this study it was likely that AMPK was activated by butyrate resulting in the reduced accumulation of fat.
We showed that supplementation with NaB increased the mRNA levels of antioxidant enzymes. The mRNA expression of Sod2 was higher in rats fed the NaB containing diet for 24 h than in rats fed the control diet for 12 h. SOD activity per total liver tissue was decreased by starvation and tended to recover in the NaB group compared with the control group at 12 h after the start of refeeding. These results indicate that the enzyme activity of SOD and the expression of Sod2 was enhanced in response to refeeding after fasting, and that the antioxidant capacity was enhanced by supplementation with NaB. The increased expressions of genes related to β-oxidation such as Cpt1a were observed in the livers of animals refed the diet containing NaB, and therefore it is likely that increased Sod2 expression in the NaB group was related to the increased level of ROS, which is produced in mitochondria during increased β-oxidation.
GSH is a tripeptide consisting of glutamate, cysteine, and glycine, and its biosynthesis cycle requires γ-glutamylcyclotransferase, 5-oxoprolinase, glutamate cysteine ligase, and glutathione synthase [22]. Because mitochondrial GSH has a central role in ROS regulation, the depletion of GSH may be relevant to the development of steatohepatitis and NASH [23]. In this study, the expressions of genes related to GSH biosynthesis were upregulated by refeeding with NaB at 24 h after the start of refeeding. No significant difference was observed in the GSSG/GSH ratio, a classical marker of oxidative stress level, between the NaB and control groups, suggesting that refeeding with NaB did not induce excessive oxidative stress. These results indicate that refeeding with NaB after starvation likely to increase amount of GSH in the liver, which protects against oxidative damage.
Butyrate has multiple effects on mammalian cells including the inhibition of proliferation, induction of differentiation, and transcriptional regulation of gene expression, some of which are related to HDAC inhibition [10]. A previous study demonstrated that β-hydroxybutyrate, which has a structure similar to butyrate, suppressed oxidative stress by increasing histone acetylation levels by the inhibition of HDAC1 and HDAC2 [24]. Indeed, the intravenous administration of β-hydroxybutyrate enhanced histone H3 acetylation levels around Foxo3a, a transcription factor that has a key role in oxidative stress responses, and increased the mRNA expression of the Foxo3a gene [24]. In this study, we demonstrated that oral supplementation with NaB markedly enhanced the expression of Foxo3a. ChIP assays showed that the histone H3 and H4 acetylation levels at the Foxo3a promoter region were reduced by starvation although no differences in the histone H3 acetylation levels were observed between the NaB and control groups. However, the histone H4 acetylation levels in the Foxo3a transcription region tended to be higher in the NaB group than in the control group.
Few studies have investigated epigenetic regulatory mechanisms including histone modifications, whereby butyrate modulates gene expressions to prevent fatty liver. When rats were orally administrated a NaB-containing high-fat diet, the acetylation of histone H3K9 on the PPARα promoter and the expression of the PPARα gene were reported to be enhanced and to have a protective role against NAFLD [25]. Although the expression of HDAC1 was reversely correlated with the acetylation level of histone H3K9 on the PPARα promoter, it is unclear how HDAC1 identified target genes [25]. However, it is likely that the expression levels of HDACs and/or the binding levels of HDACs to the Foxo3a gene are modulated by NaB supplementation. Future studies are needed to explain whether the binding of HDAC1 around the Foxo3a gene is reduced by NaB administration, and how HDAC activity is involved in the fluctuation of histone acetylation levels on the Foxo3a gene. Therefore, a limitation of this study is that it is unclear whether the changes caused by NaB administration were really due to inhibition of HDACs. Future investigations with not only NaB but also a positive control of HDAC inhibitor, such as trichostatin A, are needed to clarify the detailed mechanism.
There is a possible limitation in applying this study to the treatment and prevention of NAFLD and NASH, which are public health issues. Although butyric acid is contained in the daily product such as cheese and butter, it is not practical to ingest it in large amounts. Clostridium butyricum probiotics is a possible tool, since it was reported that supplementation of Clostridium butyricum decreased accumulation of lipid droplets in the liver in high-fat diet induced fatty liver in rats [26].
Taken together, the results of the present study suggest that NaB supplementation in the diet for refeeding may reduce the rate of lipid synthesis and stimulate fatty acid oxidation in the liver, which inhibited fat accumulation and the risk of NASH. Furthermore, NaB increased the expressions of antioxidant enzymes including Sod2, and the putative transcription factor Foxo3, which was related to the reduced risk of NASH by butyrate through a decrease in oxidative damage and inflammation.
CRediT authorship contribution statement
Kazue Honma: Conceptualization, Methodology, Formal analysis, Writing - original draft, Funding acquisition. Kaho Oshima: Investigation, Visualization, Writing - original draft. Saeko Takami: Investigation, Visualization, Writing - original draft. Toshinao Goda: Supervision, Resources, Writing - review & editing.
Declaration of competing interest
None.
Acknowledgments
We thank Sarah Williams, PhD, and J. Ludovic Croxford, PhD, from Edanz Group (www.edanzediting.com) for editing a draft of this manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.metop.2020.100043.
Contributor Information
Kazue Honma, Email: honmak@u-shizuoka-ken.ac.jp.
Kaho Oshima, Email: f15107@u-shizuoka-ken.ac.jp.
Saeko Takami, Email: s16217@u-shizuoka-ken.ac.jp.
Toshinao Goda, Email: gouda@u-shizuoka-ken.ac.jp.
Funding
This work was supported by a grant from Sapporo Bioscience Foundation, Japan and Japan Society for the Promotion of ScienceKAKENHI Grant Number 18K17927.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Lee J., Choi J., Scafidi S., Wolfgang M.J. Hepatic fatty acid oxidation restrains systemic catabolism during starvation. Cell Rep. 2016;16:201–212. doi: 10.1016/j.celrep.2016.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology (Baltimore, Md) 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 3.Hashimoto E., Tokushige K. Prevalence, gender, ethnic variations, and prognosis of NASH. J Gastroenterol. 2011;46(Suppl 1):63–69. doi: 10.1007/s00535-010-0311-8. [DOI] [PubMed] [Google Scholar]
- 4.Fernandez-Sanchez A., Madrigal-Santillan E., Bautista M., Esquivel-Soto J., Morales-Gonzalez A., Esquivel-Chirino C. Inflammation, oxidative stress, and obesity. Int J Mol Sci. 2011;12:3117–3132. doi: 10.3390/ijms12053117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tariq Z., Green C.J., Hodson L. Are oxidative stress mechanisms the common denominator in the progression from hepatic steatosis towards non-alcoholic steatohepatitis (NASH)? Liver Int : Off J Int Assoc Study Liver. 2014;34:e180–e190. doi: 10.1111/liv.12523. [DOI] [PubMed] [Google Scholar]
- 6.den Besten G., Gerding A., van Dijk T.H., Ciapaite J., Bleeker A., van Eunen K. Protection against the metabolic syndrome by guar gum-derived short-chain fatty acids depends on peroxisome proliferator-activated receptor gamma and glucagon-like peptide-1. PloS One. 2015;10 doi: 10.1371/journal.pone.0136364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weitkunat K., Schumann S., Petzke K.J., Blaut M., Loh G., Klaus S. Effects of dietary inulin on bacterial growth, short-chain fatty acid production and hepatic lipid metabolism in gnotobiotic mice. J Nutr Biochem. 2015;26:929–937. doi: 10.1016/j.jnutbio.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Khan S., Jena G. Sodium butyrate reduces insulin-resistance, fat accumulation and dyslipidemia in type-2 diabetic rat: a comparative study with metformin. Chem Biol Interact. 2016;254:124–134. doi: 10.1016/j.cbi.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Jin C.J., Sellmann C., Engstler A.J., Ziegenhardt D., Bergheim I. Supplementation of sodium butyrate protects mice from the development of non-alcoholic steatohepatitis (NASH) Br J Nutr. 2015;114:1745–1755. doi: 10.1017/S0007114515003621. [DOI] [PubMed] [Google Scholar]
- 10.Davie J.R. Inhibition of histone deacetylase activity by butyrate. J Nutr. 2003;133 doi: 10.1093/jn/133.7.2485S. 2485s-93s. [DOI] [PubMed] [Google Scholar]
- 11.Grunstein M. Histone acetylation in chromatin structure and transcription. Nature. 1997;389:349–352. doi: 10.1038/38664. [DOI] [PubMed] [Google Scholar]
- 12.McNabney S.M., Henagan T.M. Short chain fatty acids in the colon and peripheral tissues: a focus on butyrate, colon cancer, obesity and insulin resistance. Nutrients. 2017;9 doi: 10.3390/nu9121348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burgeiro A., Cerqueira M.G., Varela-Rodriguez B.M., Nunes S., Neto P., Pereira F.C. Glucose and lipid dysmetabolism in a rat model of prediabetes induced by a high-sucrose diet. Nutrients. 2017;9 doi: 10.3390/nu9060638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 15.Huang H., Tindall D.J. Dynamic FoxO transcription factors. J Cell Sci. 2007;120:2479–2487. doi: 10.1242/jcs.001222. [DOI] [PubMed] [Google Scholar]
- 16.Marchesini G., Petta S., Dalle Grave R. Diet, weight loss, and liver health in nonalcoholic fatty liver disease: pathophysiology, evidence, and practice. Hepatology (Baltimore, Md) 2016;63:2032–2043. doi: 10.1002/hep.28392. [DOI] [PubMed] [Google Scholar]
- 17.Tsai J.H., Ferrell L.D., Tan V., Yeh M.M., Sarkar M., Gill R.M. Aggressive non-alcoholic steatohepatitis following rapid weight loss and/or malnutrition. Mod Pathol : Off J Un States Can Acad Pathol Inc. 2017;30:834–842. doi: 10.1038/modpathol.2017.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia A.E., Kasim N., Tamboli R.A., Gonzalez R.S., Antoun J., Eckert E.A. Lipoprotein profiles in class III obese caucasian and african American women with nonalcoholic fatty liver disease. PloS One. 2015;10 doi: 10.1371/journal.pone.0142676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagayoshi A., Matsuki N., Saito H., Tsukamoto K., Kaneko K., Wakashima M. Defect in assembly process of very-low-density lipoprotein in suncus liver: an animal model of fatty liver. J Biochem. 1995;117:787–793. doi: 10.1093/oxfordjournals.jbchem.a124777. [DOI] [PubMed] [Google Scholar]
- 20.den Besten G., Bleeker A., Gerding A., van Eunen K., Havinga R., van Dijk T.H. Short-chain fatty acids protect against high-fat diet-induced obesity via a PPARgamma-dependent switch from lipogenesis to fat oxidation. Diabetes. 2015;64:2398–2408. doi: 10.2337/db14-1213. [DOI] [PubMed] [Google Scholar]
- 21.Gao Z., Yin J., Zhang J., Ward R.E., Martin R.J., Lefevre M. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58:1509–1517. doi: 10.2337/db08-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kageyama S., Ii H., Taniguchi K., Kubota S., Yoshida T., Isono T. Mechanisms of tumor growth inhibition by depletion of gamma-glutamylcyclotransferase (GGCT): a novel molecular target for anticancer therapy. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19072054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mantena S.K., King A.L., Andringa K.K., Eccleston H.B., Bailey S.M. Mitochondrial dysfunction and oxidative stress in the pathogenesis of alcohol- and obesity-induced fatty liver diseases. Free Radic Biol Med. 2008;44:1259–1272. doi: 10.1016/j.freeradbiomed.2007.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimazu T., Hirschey M.D., Newman J., He W., Shirakawa K., Le Moan N. Suppression of oxidative stress by beta-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science (New York, NY) 2013;339:211–214. doi: 10.1126/science.1227166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun B., Jia Y., Hong J., Sun Q., Gao S., Hu Y. Sodium butyrate ameliorates high-fat-diet-induced non-alcoholic fatty liver disease through peroxisome proliferator-activated receptor alpha-mediated activation of beta oxidation and suppression of inflammation. J Agric Food Chem. 2018;66:7633–7642. doi: 10.1021/acs.jafc.8b01189. [DOI] [PubMed] [Google Scholar]
- 26.Seo M., Inoue I., Tanaka M., Matsuda N., Nakano T., Awata T. Clostridium butyricum MIYAIRI 588 improves high-fat diet-induced non-alcoholic fatty liver disease in rats. Dig Dis Sci. 2013;58:3534–3544. doi: 10.1007/s10620-013-2879-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.