Abstract
Excessive lipid accumulation in an obese state is linked with activation and release of detrimental cytokines and chemokines that promote metabolic dysregulation. In fact, emerging experimental evidence shows that abnormal modulation of T-cells in an obese state correlates with the development and progression of insulin resistance. Importantly, the evolving concept linking insulin resistance with impaired immunological mechanisms such as T-cell responses provides new prospects for understanding the role of inflammation in moderating metabolic complications.
Keywords: Obesity, Inflammation, Insulin resistance, T-cells, Type 2 diabetes mellitus
Graphical abstract
Highlights
-
•
Dysregulated immune responses are linked with metabolic complications.
-
•
Impaired T-cell modulation obesity correlate with insulin resistance.
-
•
Modulation of T-cell responses is essesntial for understanding the impact of inflammation on metabolic complications.
Abbreviations
- AT
adipose tissue
- DIO
diet induced obese
- IFN-γ:
interferon gamma
- IL:
interleukin
- IR
insulin resistance
- MHC
histocompatibility complex
- PRISMA-P:
Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols
- PROSPERO
prospective register of a systematic review
- Stat3
signal transducer and activator of transcription 3
- T2D
type 2 diabetes
- Th1/Th2
T helper 1/2
1. Introduction
Obesity is an independent risk factor of metabolic complications such as insulin resistance (IR) and inflammation during the pathogenesis of type 2 diabetes mellitus (T2D) [1]. Increased adipose tissue (AT) mass is a hallmark of chronic low grade inflammation that is characterised by progressive infiltration of T-cells [2,3]. T-cells play an important role in orchestrating the adaptive immune response and are the second largest cell population in AT followed by macrophages [4]. Briefly, findings have shown that CD4+ and CD8+ T-cells can infiltrate both visceral and subcutaneous AT, with pro-inflammatory T helper (Th)-1, Th17, and CD8+ T-cells, concomitant to the development of IR in healthy overweight or obese human subjects [5]. Hence, T-cells are considered to play an important role in obesity-induced inflammation and IR.
Although previous studies have described T-cell involvement in AT inflammation [6,7], the exact mechanisms and sequence of events in obesity-induced inflammation and the development of IR is unknown. Moreover, conflicting reports on T-cell activation and function in obese AT have been reported. For instance, contrary to their well-known co-stimulatory effects, B7, CD28 and CD40L molecules have in fact been shown to maintain immune homeostasis by regulating the development of IR and ameliorating AT inflammation in diet-induced obese (DIO) mice [[8], [9], [10]]. On the other hand, recent findings show that OX40, a secondary co-stimulatory molecule could exacerbate AT inflammation and IR by promoting T-cell activation in a DIO mouse model [11]. This is in agreement with other studies showing an increased AT infiltration of both pro-inflammatory and anti-inflammatory T-cell subsets in an obese state [12,13]. Surprisingly, contradictory data presented by others have described decreased anti-inflammatory T-cells, particularly the regulatory T-cells (Treg) subset in various experimental models of obesity, including human studies [3,14,15]. Therefore, it remains essential to establish the precise involvement of T-cells in AT inflammation and IR in obesity and T2D. To explore such consequence, the current study synthesised and critically assessed available literature reporting on the role of T-cells in modulating AT inflammation and IR in obesity and T2D.
2. Methods
This mini-review was prepared in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 guidelines [16]. Moreover, it forms part of a big project assessing published studies on T-cell function in T2D which was registered with the international prospective register of a systematic review (PROSPERO), registration number: CRD42018099745 and has been published [17].
2.1. Search strategy
A comprehensive search was conducted on the Cochrane Library, Embase and PubMed electronic databases from inception up to 28 March 2019 by two investigators (TMN and PVD). Unpublished and ongoing studies as well as review articles were screened for primary findings. In cases of disagreements, the third reviewer (BBN) was consulted for arbitration. The search strategy was adapted to each database using keywords and medical subjects heading (MeSH) terms such as “T-cells”, “adipose tissue”, “obesity”, “insulin resistance”, “type 2 diabetes mellitus” and their respective synonyms and associated words or phrases. No language restrictions were applied to the search strategy.
2.2. Study selection
This review included both animal and human studies reporting on the role or effect of T-cells in obesity-induced AT inflammation and IR. However, reviews, editorials, books, and letters were excluded. Two investigators (TMN and PVD) independently reviewed all relevant articles and identified eligible studies. Any disagreements were resolved by consulting BBN.
2.3. Data extraction
The main outcome of this study was to determine the role of T-cells in obesity-induced inflammation and IR. Briefly, the extracted data items included; names of the authors, year of publication, experimental model used, interventions used and main findings of each study. The Mendeley reference manager version 1.19.4-dev2 software (Elsevier, Amsterdam, Netherlands) was used to manage extracted information including identifying and removing study duplicates.
2.4. Quality assessment
Two investigators (TMN and VM) with the assistance of a third reviewer (PVD), assessed the quality of individual studies included in this review by following Animal Research: Reporting In Vivo Experiments (ARRIVE) guidelines [18]. The modified Downs and Black checklist [19] was used to assess quality of included human studies.
3. Results
3.1. Characteristic features of included studies
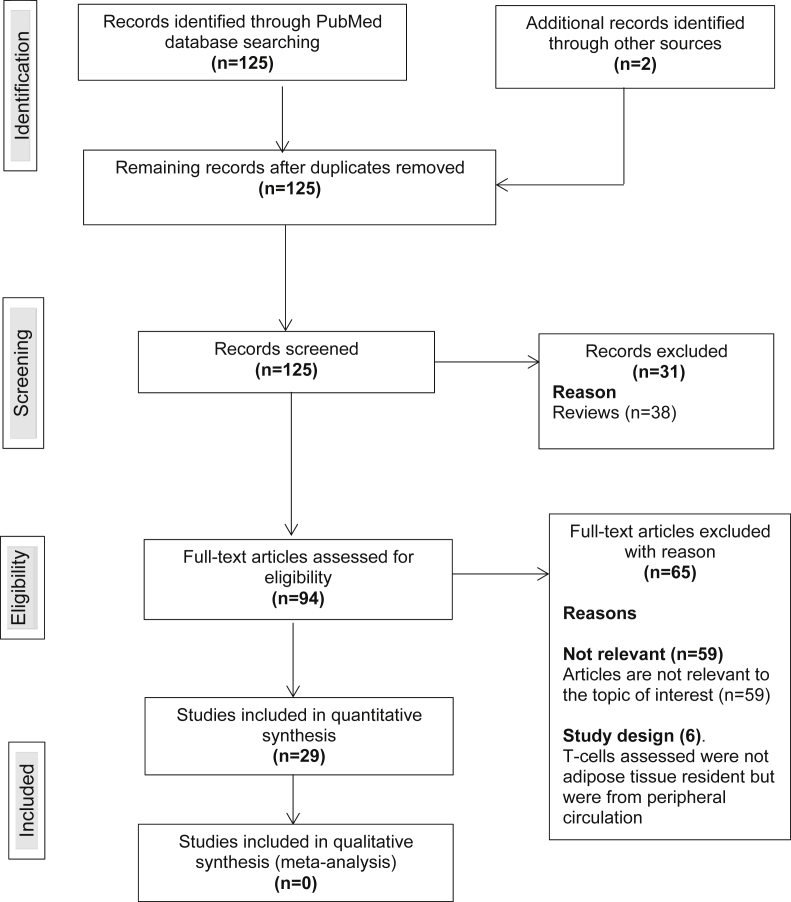
An overall number of 125 studies were identified and screened for eligibility and a total of 29 articles met the inclusion criteria. All included studies were published between 2008 and 2017. A total of 31 articles were excluded because they were review articles and 59 were irrelevant. Few studies (n = 6) were excluded due to study design, that is, the T-cells assessed in these studies were not AT resident but from peripheral circulation [20,21] (Fig. 1). Of the included studies, 24 were animal studies, 11 were human studies and 6 reported on both animals and humans (Table 1, Table 2). All human studies were observational studies.
Fig. 1.
Flow chart of study selection procedures.
Table 1.
An overview on included animal studies (n = 24).
| Author and year | Experimental model | Intervention used | Role of T-cells/Findings |
|---|---|---|---|
| Kintscher et al., 2008 [22] | Male C57BL/6 J mice | None | Infiltration of pro-inflammatory T-cells in visceral adipose tissue (AT) preceded that of macrophages. Furthermore, the T-cells were identified in the initiation of AT inflammation and the development of IR. |
| Winer et al., 2009 [12] | RAG-null and diet induced obese (DIO) C57BL/6 J mice | CD4+ T cell transfer | Increased infiltration of pathogenic interferon gamma (IFN-γ) secreting Th1 cells, Th2 and Tregs was identified in an obese state. Moreover, RAG-null mice showed exacerbated obesity and insulin resistance (IR). However, the transfer of CD4+ T-cells into RAG-null DIO mice reversed weight gain and IR. |
| Rocha et al., 2009 [6] | Male DIO C57BL/6 mice | None | Visceral AT of DIO C57BL/6 mice had higher numbers of both CD4+ and CD8+ T-cells than lean controls. In vitro T-cells from obese AT released IFN-γ than in controls |
| Nishimura et al., 2009 [23] | Male DIO C57BL/6 J and CD8null mice | CD8+ T cell transfer | There was increased infiltration of CD8+ T-cells that preceded the accumulation of macrophages in AT of DIO mice. However, genetic depletion of CD8+ T-cells lowered macrophage infiltration and reversed IR. Conversely, the adoptive transfer of CD8+ T-cells to CD8-null mice aggravated AT inflammation |
| Feuerer et al., 2009 [14] Zúñiga et al., 2010 [30] |
DIO C57BL/6 mice Male DIO C57BL/6 J and IL17-null mice |
Anti-IL-2 None |
AT resident Tregs were decreased in obese mice and had no suppressive activity but a normal proliferative response in obesity. Stimulation of Tregs by exogenous anti-interleukin (IL)-2 ameliorated obesity-induced inflammation and IR mediated by increased levels of IL-10 Increased infiltration of IL-17 producing T-cells in obese AT inhibited adipogenesis, moderated infiltration of immune cells in AT and regulated glucose metabolism. Moreover, IL-17 deficient mice developed severe obesity and display altered glucose metabolism compared to the wild type. |
| Yang et al., 2010 [7] | Male DIO C57BL/6 mice were | None | AT T-cells from DIO mice released increased levels of pro-inflammatory cytokines such as IFN-γ upon T-cell receptor (TCR) ligation. Moreover, compared to splenic T-cells, AT T-cells exhibited markedly restricted TCR diversity. Interestingly, removal of T-cells in epidydimal fat enhanced insulin sensitivity in early stage of obesity |
| Strissel et al., 2010 [24] | Male DIO C57BL/6 mice | None | Enhanced priming for IFN-γ production suggested the contribution of CD4+ and/or CD8+ T-cells to cell-mediated immune responses promoting AT inflammation and IR in obesity. T-cell enrichment and IFN-γ gene induction occurred subsequent to AT macrophage recruitment and the development of IR |
| Miller et al., 2010 [33] | Genetically obese diabetic (ob/ob) and ST2-null mice | Recombinant IL-33 | Treatment of AT cultures in vitro with IL-33 induced production of Th2 cytokines, and reduced expression of adipogenic and metabolic genes. Moreover, administration of recombinant IL-33 to ob/ob mice led to reduced adiposity and fasting glucose as well as improved glucose and insulin tolerance. HFD fed mice lacking endogenous ST2 (a receptor for IL-33) had increased body weight, impaired insulin secretion and glucose regulation compared to WT controls on HFD |
| Deiuliis et al., 2011 [3] | Male Foxp3-GFP ‘‘knockin’’ mice | None | DIO resulted in increased CD4+and CD8+ T-cells, with a significantly decreased Treg in visceral AT. Moreover, the number of Tregs inversely correlated with macrophages in the AT. |
| Priceman et al., 2013 [26] | DIO Stat3-null C57BL/6 mice | None | Regulation of AT T cell subsets by transcriptional factor, signal transducer and activator of transcription 3 (Stat3) is crucial for DIO and IR. The activity of Stat3 is elevated in both obese visceral AT and its resident T-cells. Stat3 in T-cells of DIO mice promoted the release of IFN-γ and blunts Tregs in visceral AT. Moreover, mice Stat3 null T-cells showed reduced DIO and improved IR and glucose tolerance, and suppressed visceral AT inflammation. |
| Morris et al., 2013 [27] | Male DIO C57BL/6 J mice | None | High fat diet (HFD)-induced obesity promoted conventional CD4+ T-cell proliferation in mice visceral (AT). Dietary obesity was shown to activate the proliferation of IFN-γ producing CD4+ T cells in adipose tissue |
| Montes et al., 2013 [36] | Male DIO C57BL/6 mice | AntiCTLA-4 Ig and AntiCD40L | CD4+, CD8+ and Tregs were increased in AT of DIO compared to lean controls. However, the administration of co-stimulatory inhibitors in DIO mice reduced inflammation but did not improve glucose tolerance |
| Jiang et al., 2013 [28] | Male DIO CD11-null C57BL/6 J mice | None | CD8+ T-cells in AT of obese mice showed activated phenotypes with increased proliferation and IFN-γ expression. CD11a-null DIO mice displayed markedly reduced T-cell accumulation and activation in AT. Furthermore, CD8+ T-cells from wild type mice, but not from CD11adeficient mice, infiltrated into AT of recipient obese wild type mice |
| Deng et al., 2013 [25] | Male DIO Major histocompatibility complex class II (MHC II)-null C57BL/6 mice | None | Expression MHC II in adipocyte was increased in obesity, which was parallel to increased pro-inflammatory and reduced anti-inflammatory AT T-cells. This exacerbated AT macrophage accumulation and M1 polarisation. Alternatively, MHC II-null mice developed less AT inflammation and IR than wild type mice, despite developing similar adiposity. |
| Zhong et al., 2014 [8] | Male B7-null DIO mice C57BL/6 | Adoptive transfer of Tregs | Reduced B7 expressions in obesity directly impaired Treg proliferation and function in obese mice and led to exacerbated AT inflammation and IR. B7-null mice had enhanced AT inflammation and IR in both obese and lean mice. However, adoptive transfer of Tregs reversed IR and AT inflammation in B7 KO mice. |
| Yi et al., 2014 [9] | DIO CD40-null C57BL/6 mice | None | CD40 deficiency mice exhibited exacerbated AT inflammation and IR with CD8+ T-cells being the major contributor. Contrary to its costimulatory effects, CD40 in fact regulated the development of IR DIO mice by ameliorating AT inflammation. |
| Wolf et al., 2014 [37] | Male DIO CD40-null and Rag1-null C57BL/6 mice | Anti-CD40 antibody Adoptive transfer of CD40-null T-cells |
CD40 deficient mice exhibited increased weight gain, accumulation of inflammatory cells, impaired insulin secretion and enhanced pro-inflammatory gene expression in AT. Conversely, therapeutic activation of CD40 signalling blocked further weight gain, lowered glucose levels, improved insulin sensitivity and suppressed AT inflammation. Furthermore, repopulation of Rag1-null mice with CD40-null T-cells provoked AT inflammation and IR. |
| Fabrizi et al., 2014 [31] | IL-21-null DIO C57BL/6 mice | None | IL-21 and IL-21R mRNA expression was upregulated in DIO and wild type mice in parallel to macrophage and inflammatory markers. Furthermore, DIO IL-21-null mice, showed reduced AT inflammation and improved IR due to increased infiltration of Tregs in AT. |
| Chatzigeorgiou et al., 2014 [10] | Male DIO CD40-null C57BL/6 mice | None | DIO CD40-null mice displayed worsened AT inflammation and IR when compared to wild-type mice. The worsened IR was associated with excessive AT inflammation mediated by increased accumulation of CD8+ T-cells and M1 macrophages. However, CD40L mice ameliorated IR and AT inflammation. |
| Poggi et al., 2015 [38] | Male DIO CD28-null C57Bl/6 mice | Anti-CTLA4 | CD28 deficiency decreased pathogenic T-cells and Treg content within AT without changing macrophages number. CTLA4-Ig injections reduced the number T-cells in AT but not inflammatory cytokines levels |
| Han et al., 2015 [32] | DIO C57BL/6 FOXP3 mice | IL-33 injections | DIO mice exhibited reduced AT-resident ST2+ Tregs thereby promoting inflammation and IR. However, this effect was completely reversed by treatment with IL-33. Furthermore, IL-33 administration also increased the proportion of ST2 expressing Tregs in the AT by 3-fold in DIO mice. |
| Liu et al., 2017 [11] | Male DIO C57BL/6, OX40-KO and B6.Rag2/Il2rg double knock mice | None | Increased expression of OX40 (CD134) on CD4+ T cells, infiltration and expression of pro-inflammatory cells and genes respectively, was observed in the AT of DIO mice. Furthermore, DIO OX40-null mice exhibited significantly reduced weight gain and lower fasting glucose levels than the OX40 knocked in mice. |
| Chen et al., 2017 [15] | Male C57BL/6 J | VAT antigens | Oral treatment of visceral AT mixture antigens effectively inhibited weight gain, and improved IR in HFD mice by increasing the numbers of CD4+Foxp3+ Tregs that were depleted in obesity |
Table 2.
An overview of included human studies (n = 11).
| Author and year | Experimental model | Intervention used | Role of T-cells/Findings |
|---|---|---|---|
| Kintscher et al., 2008 [22] | Individuals with T2D | None | Adipose tissue (AT) T-cell infiltration correlated with increased waist circumference in patients with type 2 diabetes mellitus (T2D). |
| Zeyda et al., 2011 [39] | Overweight and obese humans | None | Th1 and CD8+ T-cells were significantly upregulated in obese AT and correlated with AT inflammation. Surprisingly, Th2 and Tregs were also increased in visceral AT of individuals with obesity compared to lean counterparts |
| Deiuliis et al., 2011 [3] | Obese humans | None | Humans with obesity showed increased CD4+and CD8+ T-cells with a decreased Tregs in visceral AT. |
| Yang et al., 2010 [7] | Obese humans | None | There was increased infiltration of CD4+ and CD8+ T-cells in visceral AT of obese individuals compared to lean |
| Fabbrini et al., 2013 [68] | Obese humans with metabolically abnormal IR | None | The number of AT resident CD4+T-cells that produce interleukin (IL)-22 and IL-17 were 3–10 fold higher in obesity compared to lean subjects. |
| Deng et al., 2013 [25] | Obese women | None | Obesity enhanced major histocompatibility complex class II (MHC II) expression in adipocytes. Briefly, adipocytes activated AT resident CD4+ T-cells via MHC class II and leptin to induce AT inflammation |
| Zhong et al., 2014 [8] | Obese humans | None | Reduced B7 expression in obesity impaired regulatory T-cells (Treg) proliferation and function and led to exacerbated AT inflammation and IR |
| McLaughlin et al., 2014 [5] | Overweight and obese humans | None | CD4+ and CD8+ T-cells infiltrated AT with pro-inflammatory T-helper (Th)1, Th17 and CD8+ T-cells being significantly more frequent. Levels of Th2 in AT were inversely associated with systemic IR. |
| Fabrizi et al., 2014 [31] | Obese humans | None | IL-21 and IL-21R messenger RNA expression was upregulated in stromal vascular fraction from human obese subjects in parallel to macrophage and inflammatory markers. |
| Dalmas et al., 2014 [40] | Obese individuals with and without T2D | None | There was increased infiltration of IL-17 and IL-22-producing CD4+ T-cells in individuals with T2D. Moreover, CD4+ T-cell derived IL-22 amplified IL-1β driven inflammation in visceral AT and this was correlated with deterioration of glucose homeostasis. |
| Travers et al., 2015 [13] | Overweight and obese humans | None | Expression of CD4+ T-cells, macrophages and FOXP3 RNA transcripts were elevated in obesity. Furthermore, AT CD4+ and CD8+ T-cells expressed increased expression of CD69 and CD25 which was associated with increasing degree of obesity. In addition, increased T-cell activation correlated with increased expression and secretion of both pro and anti-inflammatory cytokines in AT. |
NB: All studies were observational studies.
3.2. Quality assessment and risk of bias
All included articles were published in peer-reviewed journals. For the animal studies, the ARRIVE guidelines were used to assess the quality of the included studies since it provides a precise method for scoring in vivo models. The median score and range of the 24 included studies was 16 (13–18) out of a possible score of 20, thus all studies met the minimum requirements for publication. Overall, all studies scored high in the introduction domain with a median of 4 (3–4) out of a possible score of 4 (overall agreement 92.97%, kappa = 0.96). Furthermore, the studies also scored high in the method and discussion domains with a median of 7 (5–9) out of the possible score of 9 (overall agreement 76.39%, kappa = 0.58) and 3 (2–3) out of a possible score of 3 (overall agreement 94.44%, kappa = 0.89), respectively. However, the studies scored low in the results section due to the study design, for example no baselines results and adverse events reported, resulting in a median of 2 (0–2) out of the possible score of 4 (overall agreement 69.79%, kappa = 0.40) (Table 1S).
For human studies, the Blacks and downs checklist was used to appraise the included studies and they all scored poorly (<13 points). The median score range of the 11 included studies was 10 (8–13). Overall, the included studies had a lower risk of reporting bias with a median of 5 (4–7) out of the possible score of 10 (overall agreement 90.91%, kappa = 0.82). In addition, the studies also had a relatively low risk of internal validity bias with a median of 3 (3–4) out of the possible score of 7 (overall agreement 88.31%, kappa = 0.95). However, all studies performed poorly on the external validity and selection bias domains (except one study) with a median of 0 (0–2) out of the possible score of 3 (overall agreement 87.88%, kappa = 0.76) and 1 (1–3) out of the possible score of 6 (overall agreement 81.82%, kappa = 0.64), respectively (Table 2S).
3.3. Overview of included animal studies on the role of T-cells
The search retrieved 24 studies that reported on the role of T-cells in AT inflammation and IR in various experimental models of obesity, published between 2008 and 2017. The sections below briefly discuss the different types of T-cells and their role in modulating obesity associated complications.
3.3.1. Infiltration of Th1, CD4+ and CD8+ T-cells in AT of obese animals promotes inflammation and IR
The overall evidence presented in this review clearly shows that DIO mice are among the well-recognized animal models used to investigate the role of T-cells in obesity (Table 1). Generally, these animals are fed a high caloric diet, usually rich in fat, which results in the development of obesity, mimicking that which is observed in humans. After just five weeks of high fat diet-feeding, Kintscher and colleagues were the first to show that infiltration of pro-inflammatory T-cells in the AT may occur before macrophages as a primary event in AT inflammation, concomitant to the development of obesity-induced IR [22]. These findings were further supported by subsequent studies that reported on an increased infiltration of interferon gamma (IFN-γ) producing Th1, CD8+ T-cells in AT of DIO mice when compared to controls [6,12,[23], [24], [25]]. Thus, suggesting that T-cells in the AT are likely to play a major role in mediating inflammation and IR in DIO mice, including humans. In RAG-null (which are mice lacking CD3 or T-cell receptor) and CD8-null mouse models, it was further demonstrated that high fat diet feeding exacerbated AT inflammation [12,23]. However, the transfer of CD4+ and CD8+ T-cells in these respective models alleviated IR and aggravated AT inflammation, respectively.
In addition to increased CD4+ and CD8+ AT infiltration in DIO mice, the T-cells were reported to release increased pro-inflammatory IFN-γ cytokine which significantly contributed to AT inflammation [7,[26], [27], [28]]. Remarkably, the removal of T-cells from DIO mice improved IR in early stages of obesity [7]. Like IFN-γ, the signal transducer and activator of transcription 3 (Stat3) is known to be central in modulating cytokine-dependent inflammation and immunity within an obese state [29]. Indeed results summarised in this review showed that Stat3 transcriptional factor levels were elevated in both AT and AT-resident T-cells, this consequence promoted the release of IFN-γ in DIO mice [26]. This study further demonstrated that Stat3-null mice showed improved IR and reduced AT inflammation. However, it is clear that other important components such as T-cell receptors remain important in regulating an inflammatory response within diverse specific disease conditions. For example, a sub-analysis of mice lacking T-cell α-chain (CD11a-null) showed markedly reduced accumulation and activation of T-cells in AT [28]. On the other hand, increased infiltration of interleukin (IL)-17 producing T-cells in AT was reported in DIO mice [30]. However, contrary to its well-established pro-inflammatory effects, IL-17 in fact regulated IR and reduced obesity, as well as AT inflammation in these mice. Nonetheless, a sub-analysis of IL-17-null DIO revealed increased obesity and IR [30], suggesting the diverse regulatory effects of Il-17 cytokine on AT inflammation in an obese state.
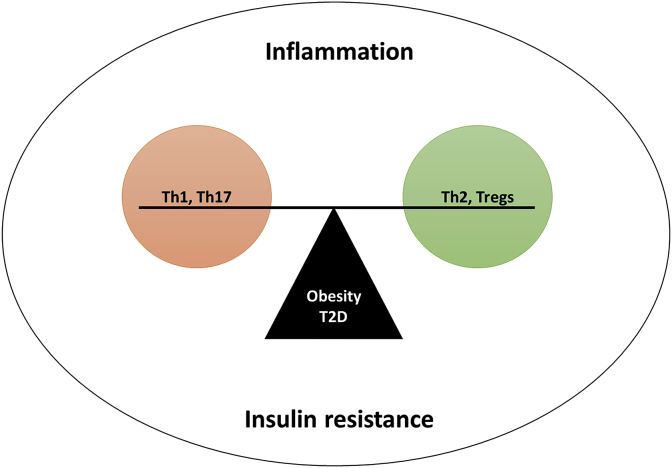
3.3.2. The levels of Th2 and tregs are reduced in AT of obese animals
It is well-established that T-cell anti-inflammatory subsets (Th2 and Tregs) decrease whilst the pro-inflammatory subsets increase as obesity progresses. Moreover, an imbalance between the modulation of Th1/Th17 and Th2/and Tregs has been associated with an exacerbated inflammatory response and the development of IR (Fig. 2). Evidence presented in this review shows that in addition to increased expression of major histocompatibility complex (MHC) class II, frequency of pro-inflammatory Th subsets and cytokines in AT were inversely proportional to the levels of Th2 subset and IL-13 cytokine in DIO mice [12,25]. Interestingly, MHC-null mice developed less AT inflammation and IR when compared to wild types, despite developing similar obesity associated abnormalities [25].
Fig. 2.
Effective modulation of Th1/Th17 and Th2/and Tregs remains important in the regulation and amelioration of insulin resistance.
Winer and associates were the first to report on decreased Tregs in AT of DIO mice, which was linked with the progression of obesity linked complications [12]. These findings have also been supported by subsequent mice studies presented by others [3,14,15,25,31,32]. Briefly, it has been shown that stimulation of Tregs with IL-2 improves AT inflammation and IR mediated by increased IL-10 [14]. Furthermore, administration of visceral AT antigens could effectively increase the number of Tregs resulting in the inhibition of weight gain and IR in mice on high fat diet [15]. Moreover, this study showed that the number of Tregs inversely correlated with macrophages in the AT. Alternatively, the expression of pro-inflammatory IL-21 and its receptor's (IL-21R) mRNA were upregulated in the AT of DIO mice [31]. Interestingly in DIO IL-22-null mice, amelioration of AT inflammation and reversal of IR was linked to elevated number of Tregs. The overall findings are consistent that an obese state, illustrating that high fat feeding is responsible for reduced AT-resident ST2+ (IL-33R) Tregs promote AT inflammation and IR, as demonstrated in DIO or ST2-null mice [32,33]. Thus, suggesting that some interventions, as seen with administration of IL-33 in DIO mice [32,33], can be further developed to directly or indirectly induce the release of Th2 cytokines leading to increased number of ST2+ Tregs.
3.3.3. Double-edged sword effect of T-cell co-stimulatory molecules in obese animals
For the successful activation of T-cells, both T-cell receptor and co-stimulatory molecule signals are required. Thus in the absence of a co-stimulatory signals, a hypo-responsive state of T-cells termed anergy is induced despite active TCR signalling and IL-2 expression [34,35]. Therefore, co-stimulatory signals are essential for T-cell activation and function. Inhibition of CD40 signalling pathway by administration of anti-CD40L in DIO mice reduced accumulation of pro-inflammatory macrophages (M1) and AT inflammation and but did not improve IR [36]. However contrary to this, CD40-null mice showed exacerbated IR and AT inflammation mediated by increased accumulation of CD8+ T-cells and M1 macrophages [9,10,37]. Conversely, the activation of CD40 signalling improved IR and suppressed AT inflammation and the repopulation of RAG-null mice with CD40-null T-cells triggered AT inflammation and IR [37]. In agreement with findings by Montes and colleagues, DIO CD28-null mice showed a decrease of both pro-inflammatory T-cells and Tregs in AT without changing macrophages number [38]. However contrary to this, B7-null mice exhibited enhanced AT inflammation and IR in both DIO and lean mice [8]. In addition, it was shown that adoptive transfer of Tregs into B7-null mice could reverse AT inflammation and IR. Moreover, the same study reported on the decreased expression of B7 expression in an obese state [8]. The inhibition of another co-stimulatory molecule, CTLA-4, in DIO mice could reduce the number of T-cells in AT but not the levels of pro-inflammatory cytokines [36,38]. On the other hand, the expression of another T-cell co-stimulatory marker, OX40, was reported to be increased on CD4+ T-cells in the AT of DIO mice [11]. Conversely, a sub-analysis of OX40-null mice showed significantly less weight gain and improved IR compared to the OX40 knocked in mice.
3.4. The impaired modulation of T-cells in obese human subjects promotes inflammation and IR
The search retrieved eleven human studies that reported on the role of T-cells in AT inflammation and IR, published between 2008 and 2015. The specific focus here was to establish whether the modulatory effect of T-cells on obesity associated complications compares to that observed in animal models.
Like the evidence observed in DIO mice (Table 1), increased infiltration of T-cells in obese AT of human subjects was consistent with exacerbated inflammation and it correlated with increased waist circumference [22]. An overwhelming number of studies presented in Table 2 reported on increased infiltration of Th1, CD4+ and CD8+ T-cells in AT of individuals with obesity when compared to lean counterparts [3,5,7,13,39]. Here, AT infiltrating T-cells were triggered in individuals with obesity, and this was demonstrated by elevation of activation markers such as CD69 and CD25, which are known to indicate immune activation and indirectly the degree of obesity in this case [13]. This was consistent with enhancement of pro-inflammatory cytokines like IL-17, IL-21 and IL-22 [31,40]. Elevated CD4+ T-cells in AT of individuals with obesity was also attributed to enhanced the expression of MHC class II [25], which strongly highlighted the consistent modulatory effects of T-cells in obesity induced inflammation.
Furthermore, obese individuals have been shown to present with reduced expression of B7 co-stimulatory molecule, which directly impairs both the proliferation and function of Tregs in AT [8]. In accordance with this, individuals with obesity display reduced levels of Tregs in AT, inversely correlating with IR [3,5]. However, contrary to this, increased AT infiltration by Th2 and Tregs was in fact reported in individuals with obesity [13,39].
4. Discussion
Obesity and its associated complications is persistently linked with impaired immune response and an aggravated inflammatory response [41]. However, the pathological mechanisms involved in these processes are not clearly understood. Therefore, this review aimed to synthesise and critically assess available literature on the role of T-cell function in AT inflammation in obesity or T2D. Most of the included studies showed a strong correlation between increased infiltration of Th1, CD4+ and CD8+ T-cells with an exacerbated pro-inflammatory state, leading to the development of IR. Experimental models of obesity and T2D persistently showed an enhanced infiltration of IFN-γ secreting Th1 cells concomitant to reduced levels of Tregs [3,6,12,40]. Evidence presented in this study clearly demonstrated that nutrition plays a major role in the development of metabolic complications, since it was apparent that high fat feeding promoted spontaneous development of obesity that was accompanied by impaired T-cell function in both animals and human subjects [5,12,13,23]. Although a detailed molecular signature that better describes the complex relationship between diet and metabolic dysregulation is not completely understood, AT function within an obese state remains a major focus of ongoing studies [42,43].
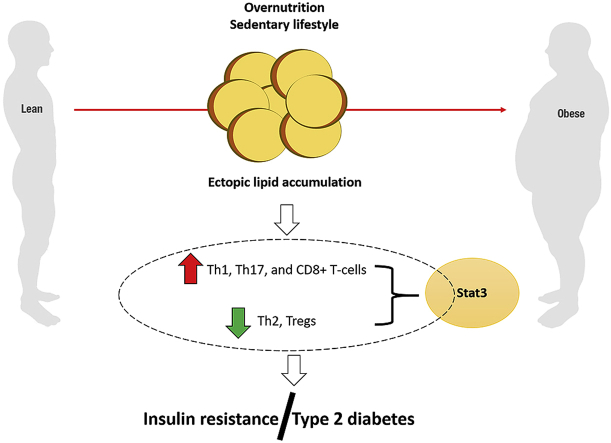
Nevertheless, as an endocrine organ, the AT can greatly modulate an inflammatory response by promoting secretion of cytokines and chemokines such as IL-6, IL-8, and MCP-1 that are implicated in promoting ectopic lipid accumulation [41]. In fact, accumulative data summarised in this review showed a strong association between an abnormal inflammatory response and impaired glucose homeostasis that is characterised by an IR state [5,8,9,22]. Anyhow, a vicious circle has been acknowledged between IR and ectopic lipid accumulation, together increasing the risk for the development of metabolic inflammation [1,44,45]. The current study shows that adaptive immunity, especially regulation of T-cells is central in the development of metabolic inflammation and IR [46]. For instance, one study showed that the regulation of AT T-cell subsets by Stat3 is crucial in the pathogenesis of IR [26]. The activity of Stat3 appears to be elevated in both obese visceral AT and its resident T-cells. Evidence presented in Table 1 indicates that activation of Stat3 promotes the release of IFN-γ and hinders that of Tregs in visceral AT of obese mice. Similarly, Stat3 null mice showed improved glucose tolerance and suppressed visceral AT inflammation. These findings suggest that besides vast involvement in other physiological processes [47], the STAT pathway plays a major role in modulating inflammatory response in obesity.
Evidence synthesised in this review also highlights the impact co-stimulatory molecules could have in modulating inflammatory responses within an obese state by inducing T-cell activation [10]. In fact, overwhelming evidence presented here suggests that their signalling pathways may in actual fact have a protective role in obesity and in the pathogenesis of T2D [9,37]. This evidence suggests the potential exploration of co-stimulatory molecules in understanding the role of T-cells in regulating pro-inflammatory responses and most importantly to determine ways to alleviate obesity-induced metabolic complications. In the context of obesity, CD40L is of particular interest since it has been shown that its administration could alleviate AT inflammation and IR in an obese state [36]. However, further studies are needed to confirm this aspect.
Furthermore, it is well-established that metabolites produced in the AT or other metabolic tissues may play an important role in immune response regulation [48–51. In fact, it is now well-established that AT is an active secretory organ that releases metabolites which have the ability to modulate body weight, insulin sensitivity and inflammation [48]. In the context of the latter, AT releases both pro- and anti-inflammatory adipokines which when imbalanced, contribute to the pathogenesis of obesity-linked complications [49]. One of the most studied AT derived adipokines is leptin, a pro-inflammatory metabolite that is significantly increased in obesity and has the ability to initiate and propagate a pro-inflammatory response [[49], [50], [51]]. Briefly, the binding of leptin to its specific receptor (Lep-R) expressed on T-cells is associated with activation of the Janus tyrosine kinase (JAK) pathway, which may results in the phosphorylation and activation of Stat3 [52,53]. Activation of Stat3 is positively correlated with elevated levels of detrimental cytokines such as IL-6 in obese individuals [53]. Interestingly, like leptin, IL-6 has the ability to activate the JAK-Stat3 signalling pathway [29]. Therefore, consistent with data summarised in this review [26], enhanced leptin secretion as a result of excess AT storage in an obese state may significantly contribute to the activation of the JAK-Stat3 signalling pathway in T-cells, thus contribute to aggravation of obesity-associated pro-inflammation.
On the other hand, AT is also known to secrete adipokines that oppose the actions of leptin and inhibit the pro-inflammatory stimuli. One of these adipokines is adiponectin, an anti-inflammatory metabolite that has been shown to increase insulin sensitivity and block lipid oxidation by activating the energy sensing, AMP-activated protein kinase (AMPK) [54,55]. Notably, adiponectin levels are significantly decreased in conditions of obesity, including individuals with T2D [56,57]. Concomitant to this, systematic and vector infusion of adiponectin in DIO mice has been shown to significantly inhibit the secretion and actions of IL-6 and TNF-α [58,59]. The latter has the ability to further activate and proliferate T-cells [60]. In addition, adiponectin inhibited cytotoxic activities of natural killer cells, the secretion of TNF-α and IFN-γ as well as the signalling of pro-inflammatory nuclear factor kappa-light-chain-enhancer of activated B-cells (NF-kB) through the activation of AMPK [61,62]. Moreover, adiponectin can also prevent atherogenesis by inhibiting the expression of the chemokine receptor 3 (CXCR3) on activated macrophages and thus reduce the infiltration of T-cells into the atheroma [63]. These findings are consistent with its effect in blocking the differentiation of Th1 and Th17 cells in rodents [64]. Interestingly, the inhibitory effect of adiponectin on T-cell differentiation has been attributed to its ability to block the CD40-dependent co-stimulatory signalling [64]. Although studies included in the review did not particularly describe their role in T-cell regulation, in the context of obesity, AT derived metabolites such as leptin and adiponectin are skewed towards the pro-inflammatory subset, which could induce and worsen the activation of pro-inflammatory T-cells.
In summary, and to our knowledge, this is the first systematic review to comprehensively describe the role of T-cells in obesity, linking an exacerbated inflammatory state and IR. In addition, this review highlights the potential protective effects that could be established by effective regulation of T-cells, leading to the amelioration of obesity associated complications such as T2D. Therefore, this study paves the way for future studies to explore novel avenues in developing new drugs that alleviate AT inflammation and IR linked with an obese state. Also of note, are the limitaions of the current review. Firstly, the included number of studies is low especially human studies. Furthermore, all human studies were observational studies whose evidence is of low quality. Lastly, due to unavailability of human participants’ characteristics, we were unable to correlate any biochemical and immune markers with degree of AT inflammation and IR. However, further studies are required to address this aspect.
5. Concluding remarks
Lifestyle modification, including over nutrition coupled with physical inactivity significantly contribute to the development of metabolic complications, including obesity and T2D. Diverse molecular pathways and biological interactions have been explored to understand the impact of these complications to human health. In fact, much attention has been focused on the role of inflammation and immune response in the development of metabolic abnormalities. Data summarised in this review demonstrates that increased infiltration of Th1, CD4+ and CD8+ T-cells in an obese state coupled with decreased levels of Th2 and Tregs greatly impacts human health by exacerbating inflammation and IR. Furthermore, despite the double-edged sword effect of T-cell co-stimulatory molecules, therapeutic interventions targeting CD40L signalling may have the potential to alleviate inflammation and IR linked with obesity. Further studies assessing therapeutic interventions aimed at modulating these pathways in metabolic disease are needed.
Authorship
TMN, PVD and BBN conceptualised, designed and drafted the review. All authors, including VM wrote and approved the final manuscript.
Conflict of interest disclosure
Authors don't have any competing interests to disclose.
Acknowledgements
The current study is partially funded by the National Research Foundation (NRF) of South Africa (Grant Number: 107519 to BB Nkambule). BB Nkambule is also a University of KwaZulu-Natal Developing Research Innovation, Localisation and Leadership in South Africa (DRILL) fellow. DRILL, is a NIH D43 grant (D43TW010131) awarded to UKZN in 2015 to support a research training and induction programme for early career academics. PV Dludla was partially supported as a Post-Doctoral Fellow by funding from Research Capacity Division of the South African Medical Research Council (SAMRC). The grant holder acknowledges that opinions, findings, and conclusions or recommendations expressed in any publication generated by the NRF or SAMRC supported research are those of the authors, and that the NRF accepts no liability whatsoever in this regard.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.metop.2019.100015.
Contributor Information
Tawanda Maurice Nyambuya, Email: mnyambuya@nust.na.
Phiwayinkosi Vusi Dludla, Email: pdludla@mrc.ac.za.
Vuyolwethu Mxinwa, Email: vuyomxinwa@gmail.com.
Bongani Brian Nkambule, Email: nkambuleb@ukzn.ac.za.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Shoelson S.E., Lee J., Goldfine A.B., Shoelson S.E., Lee J., Goldfine A.B. Inflammation and insulin resistance. J Clin Investig. 2006;116:1793–1801. doi: 10.1172/JCI29069. [and] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dandona P., Aljada A., Bandyopadhyay A. Inflammation : the link between insulin resistance , obesity and diabetes. Trends Immunol. 2004;25:4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 3.Deiuliis J., Shah Z., Shah N., Needleman B., Mikami D., Narula V. Visceral adipose inflammation in obesity is associated with critical alterations in tregulatory cell numbers. PLoS One. 2011;6:1–11. doi: 10.1371/journal.pone.0016376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mraz M., Haluzik M. The role of adipose tissue immune cells in obesity and low-grade inflammation. J Endocrinol. 2014;222:113–127. doi: 10.1530/JOE-14-0283. [DOI] [PubMed] [Google Scholar]
- 5.McLaughlin T., Liu L., Lamendola C., Shen L., Morton J., Rivas H. T-cell profile in adipose tissue is associated with insulin resistance and systemic inflammation in humans. Arterioscler Thromb Vasc Biol. 2014;34:2637–2643. doi: 10.1161/ATVBAHA.114.304636.T-Cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rocha V.Z., Folco E.J., Sukhova G., Gotsman I., Vernon A.H., Libby P. Interferon-gamma, a Th1 cytokine, regulates fat inflammation a role for adaptive immunity in obesity. Circ Res. 2009;103:467–476. doi: 10.1161/CIRCRESAHA.108.177105.Interferon-gamma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang H., Youm Y., Vandanmagsar B., Ravussin A., Gimble J.M., Greenway F. Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: implications for systemic inflammation and insulin resistance. J Immunol. 2010;185:1836–1845. doi: 10.4049/jimmunol.1000021.Obesity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhong J., Rao X., Braunstein Z., Taylor A., Narula V., Hazey J. T-cell costimulation protects obesity-induced adipose inflammation and insulin resistance. Diabetes. 2014;63:1289–1302. doi: 10.2337/db13-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yi Z., Stunz L.L., Bishop G.A. CD40-mediated maintenance of immune homeostasis in the adipose tissue microenvironment. Diabetes. 2014;63:2751–2760. doi: 10.2337/db13-1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chatzigeorgiou A., Seijkens T., Zarzycka B., Engel D., Poggi M. Blocking CD40-TRAF6 signaling is a therapeutic target in obesity-associated insulin resistance. Proc Natl Acad Sci U S A. 2014;111:2686–2691. doi: 10.1073/pnas.1400419111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu B., Yu H., Sun G., Sun X., Jin H., Zhang C. OX40 promotes obesity-induced adipose inflammation and insulin resistance. Cell Mol Life Sci. 2017;74:3827–3840. doi: 10.1007/s00018-017-2552-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winer S., Chan Y., Paltser G., Truong D., Tsui H., Bahrami J. Normalization of obesity-associated insulin resistance through immunotherapy: CD4+ T cells control glucose homeostasis. Nat Med. 2009;15:921–929. doi: 10.1038/nm.2001. [Normalization] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Travers R.L., Motta A.C., Betts J.A., Bouloumié A., Thompson D. The impact of adiposity on adipose tissue-resident lymphocyte activation in humans. Int J Obes. 2015;39:762–769. doi: 10.1038/ijo.2014.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feuerer M., Herrero L., Cipolletta D., Naaz A., Wong J., Nayer A. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X., Zhang D., Chen X., Meng G., Zheng Q., Mai W. Oral administration of visceral adipose tissue antigens ameliorates metabolic disorders in mice and elevates visceral adipose tissue-resident CD4+CD25+Foxp3+ regulatory T cells. Vaccine. 2017;35:4612–4620. doi: 10.1016/j.vaccine.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 16.Shamseer L., Moher D., Ghersi D., Liberati A., Petticrew M., Shekelle P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. Br Med J. 2015;7647:1–25. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 17.Nyambuya T.M., Dludla P.V., Nkambule B.B. T cell activation and cardiovascular risk in type 2 diabetes mellitus: a protocol for a systematic review and meta-analysis. BMC Syst Rev. 2018;7:1–6. doi: 10.1186/s13643-018-0835-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kilkenny C., Browne W.J., Cuthill I.C., Emerson M., Altman D.G. Improving bioscience research reporting: the arrive guidelines for reporting animal research. PLoS Biol. 2010;8:1–5. doi: 10.3390/ani4010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Connor S.R., Tully M.A., Ryan B., Bradley J.M., Baxter G.D., McDonough S.M. Failure of a numerical quality assessment scale to identify potential risk of bias in a systematic review: a comparison study. BMC Res Notes. 2015;8:1–7. doi: 10.1186/s13104-015-1181-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Viardot A., Heilbronn L., Samocha-Bonet D., Mackay F., Campbell L., Samaras K. Obesity is associated with activated and insulin resistant immune cells. Diabetes Metab Res Rev. 2012;28:1–18. doi: 10.1002/dmrr. [DOI] [PubMed] [Google Scholar]
- 21.Ahmad R., Al-Roub A., Koshy M., Sindhu S., Behbehani K. Relationship of Il-5 with Th1 and Th2 cytokines in individuals with or without type-2 diabetes. J Glycomics Lipidomics. 2015;5:1–4. doi: 10.4172/2153-0637.1000134. [DOI] [Google Scholar]
- 22.Kintscher U., Hartge M., Hess K., Foryst-ludwig A., Clemenz M., Wabitsch M. T-lymphocyte infiltration in visceral adipose tissue a primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance. Arterioscler Thromb Vasc Biol. 2008;28:1304–1310. doi: 10.1161/ATVBAHA.108.165100. [DOI] [PubMed] [Google Scholar]
- 23.Nishimura S., Manabe I., Nagasaki M., Eto K., Yamashita H., Ohsugi M. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 24.Strissel K.J., DeFuria J., Shaul M.E., Bennett G., Greenberg A.S., Obin M.S. T-cell recruitment and Th1 polarization in adipose tissue during diet-induced obesity in C57BL/6 mice. Obesity. 2010;18:1918–1925. doi: 10.1038/oby.2010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deng T., Lyon C.J., Minze L.J., Jianxin L., Zou J., Liu J.Z. Class II major histocompatibility complex plays an essential role in obesity-induced adipose inflammation. Cell Metabol. 2013;17:411–422. doi: 10.1016/j.cmet.2013.02.009.Class. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Priceman S.J., Kujawski M., Shen S., Cherryholmes G.A., Lee H., Zhang C. Regulation of adipose tissue T cell subsets by Stat3 is crucial for diet-induced obesity and insulin resistance. Proc Natl Acad Sci U S A. 2013;110:13079–13084. doi: 10.1073/pnas.1311557110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morris D.L., Cho K.W., Delproposto J.L., Oatmen K.E., Geletka L.M., Martinez-santibanez G. Adipose tissue macrophages function as antigen-presenting cells and regulate adipose tissue CD4 + T cells in Mmce. Diabetes. 2013;62 doi: 10.2337/db12-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang E., Perrard X.D., Yang D., Khan I.M., Perrard J.L., Wayne C. Essential role of CD11a in CD8+ T-cell accumulation and activation in adipose tissue. Arterioscler Thromb Vasc Biol. 2014;34:34–43. doi: 10.1161/ATVBAHA.113.302077.Essential. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wunderlich C.M., Hövelmeyer N., Wunderlich F.T. Mechanisms of chronic JAK-STAT3-SOCS3 signaling in obesity. JAK-STAT. 2013;2:e238781–e2387817. doi: 10.4161/jkst.23878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zúñiga L a, Shen W., Joyce-shaikh B., Pyatnova E a, Richards A.G., Thom C. IL-17 regulates adipogenesis, glucose homeostasis, and obesity. J Immunol. 2010;185:6947–6959. doi: 10.4049/jimmunol.1001269.IL-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fabrizi M., Marchetti V., Mavilio M., Marino A., Casagrande V., Cavalera M. IL-21 is a major negative regulator of IRF4-dependent lipolysis affecting tregs in adipose tissue and systemic insulin sensitivity. Diabetes. 2014;63:2086–2096. doi: 10.2337/db13-0939. [DOI] [PubMed] [Google Scholar]
- 32.Han J.M., Wu D., Denroche H.C., Yao Y., Verchere C.B., Levings M.K. IL-33 Reverses an obesity-induced deficit in visceral adipose tissue ST2+ T regulatory cells and ameliorates adipose tissue inflammation and insulin resistance. J Immunol. 2015;194:4777–4783. doi: 10.4049/jimmunol.1500020. [DOI] [PubMed] [Google Scholar]
- 33.Miller A.M., Asquith D.L., Hueber A.J., Anderson L.A., Holmes W.M., Mckenzie A.N. IL-33 induces protective effects in adipose tissue inflammation during obesity in mice. Circ J. 2010;107:650–658. doi: 10.1161/CIRCRESAHA.110.218867.IL-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harber M., Sundstedt A., Wraith D. Cambridge Univ Press; 2000. The role of signals 1 and 2 in T-cell activation; p. 3994.http://journals.cambridge.org/fulltext_content/ERM/ERM2_09/S1462399400002143sup004.htm [Google Scholar]
- 35.Xing Y., Hogquist K.A. T-cell tolerance : central and peripheral. Cold Spring Harb Perspect Biol. 2012;4:1–16. doi: 10.1101/cshperspect.a006957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Montes V.N., Turner M.S., Subramanian S., Ding Y., Hayden-ledbetter M., Slater S. T cell activation inhibitors reduce CD8 + T cell and pro- inflammatory macrophage accumulation in adipose tissue of obese mice. PLoS One. 2013;8:1–13. doi: 10.1371/journal.pone.0067709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wolf D., Jehle F., Michel N.A., Bukosza E.N., Rivera J., Chen Y.C. Coinhibitory suppression of t cell activation by CD40 protects against obesity and adipose tissue inflammation in mice. Circulation. 2014;129:2414–2425. doi: 10.1161/CIRCULATIONAHA.113.008055. [DOI] [PubMed] [Google Scholar]
- 38.Poggi M., Morin S.O., Bastelica D., Govers R., Canault M., Bernot D. CD28 deletion improves obesity-induced liver steatosis but increases adiposity in mice. Int J Obes. 2015;39:977–985. doi: 10.1038/ijo.2015.26. [DOI] [PubMed] [Google Scholar]
- 39.Zeyda M., Huber J., Prager G., Stulnig T.M. Inflammation correlates with markers of T-cell subsets including regulatory T cells in adipose tissue from obese patients. Obesity. 2011;19:743–748. doi: 10.1038/oby.2010.123. [DOI] [PubMed] [Google Scholar]
- 40.Dalmas E., Venteclef N., Caer C., Poitou C., Cremer I., Aron-Wisnewsky J. T cell-derived IL-22 amplifies IL-1β-driven inflammation in human adipose tissue: relevance to obesity and type 2 diabetes. Diabetes. 2014;63 doi: 10.2337/db13-1511. 1966–77. [DOI] [PubMed] [Google Scholar]
- 41.Gustafson B., Hammarstedt A., Andersson C.X., Smith U. Inflammed adipose tissue. Arterioscler Thromb Vasc Biol. 2007;27:2276–2283. doi: 10.1161/ATVBAHA.107.147835. [DOI] [PubMed] [Google Scholar]
- 42.Gómez-Hernández A., Beneit N., Díaz-Castroverde S., Escribano Ó. Differential role of adipose tissues in obesity and related metabolic and vascular complications. Internet J Endocrinol. 2016;2016:1–15. doi: 10.1155/2016/1216783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Russo L., Lumeng C.N. Properties and functions of adipose tissue macrophages in obesity. Immunology. 2018;155:407–417. doi: 10.1111/imm.13002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singer K., Lumeng C.N. The initiation of metabolic inflammation in childhood obesity. J Clin Investig. 2017;127:65–73. doi: 10.1172/JCI88882.Inflammation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dludla P.V., Nkambule B.B., Jack B., Mkandla Z., Mutize T., Silvestri S. Inflammation and oxidative stress in an obese state and the protective effects of gallic acid. Nutrients. 2019;11:1–29. doi: 10.3390/nu11010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xia C., Rao X., Zhong J. Role of T lymphocytes in type 2 diabetes and diabetes-associated inflammation. J Diabetes Res. 2017:1–6. doi: 10.1155/2017/6494795. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kiu H., Nicholson S.E. Biology and significance of the JAK/STAT signalling pathways. Growth Factors. 2012;30:88–106. doi: 10.3109/08977194.2012.660936.Biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Piya M.K., Mcternan P.G., Kumar S. Adipokine inflammation and insulin resistance: the role of glucose , lipids and endotoxin. J Endocrinol. 2013;216:T1–T15. doi: 10.1530/JOE-12-0498. [DOI] [PubMed] [Google Scholar]
- 49.Ouchi N., Parker J.L., Lugus J.J., Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921.Adipokines. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Y., Proence R., Maffei M., Barone M., Leopold L., Friedman J.M. Positional cloning of the mouse obese gene and human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 51.Francisco V., Pino J., Campos-cabaleiro V., Ruiz-fernández C., Mera A., Gonzalez-gay M.A. Obesity, fat mass and immune system: role for leptin. Front Physiol. 2018;9:1–20. doi: 10.3389/fphys.2018.00640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Procaccini C., De Rosa V., Galgani M., Carbone F., Rocca C La, Formisano L. Role of adipokines signaling in the modulation of T cells function. Front Immunol. 2013;4:1–12. doi: 10.3389/fimmu.2013.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Papathanassoglou E., El-Haschimi K., Li X.C., Matarese G., Strom T., Mantzoros C. Leptin receptor expression and signaling in lymphocytes: kinetics during lymphocyte activation, role in lymphocyte survival, and response to high fat diet in mice. J Immunol. 2006;176:7745–7752. doi: 10.4049/jimmunol.176.12.7745. [DOI] [PubMed] [Google Scholar]
- 54.Stelzer I., Zelzer S., Raggam R.B., Uller F.P.R., Truschnig-wilders M., Meinitzer A. Link between leptin and interleukin-6 levels in the initial phase of obesity related inflammation. Transl Res. 2012;159:118–124. doi: 10.1016/j.trsl.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 55.Yamauchi T., Kamon J., Minokoshi Y., Ito Y., Waki H., Uchida S. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 56.Ouchi N., Walsh K. Adiponectin as an anti-inflammatory factor. Int J Chem. 2007;380:24–30. doi: 10.1016/j.cca.2007.01.026.Adiponectin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bjornstad P., Truong U., Dorosz J.L., Cree-green M., Baumgartner A., Coe G. Cardiopulmonary dysfunction and adiponectin in adolescents with type 2 diabetes. J Am Heart Assoc. 2016;5:1–14. doi: 10.1161/JAHA.115.002804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abdella N.A., Mojiminiyi O.A. Clinical applications of adiponectin measurements in type 2 diabetes mellitus: screening, diagnosis, and marker of diabetes control. Diabetes Mark. 2018:1–6. doi: 10.1155/2018/5187940. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maeda N., Shimomura I., Ken K., Nishizawa H., Matsuda M., Nagaretani H. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8:731–737. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- 60.Zhang L., Meng S., Tu Q., Yu L., Tang Y., Dard M.M. Adiponectin ameliorates experimental periodontitis in diet-induced obesity mice. PLoS One. 2014;9 doi: 10.1371/journal.pone.0097824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mehta A.K., Gracias D.T., Croft M., Jolla L., States U. TNF activity and T-cells. Cytokine. 2018;101:14–18. doi: 10.1016/j.cyto.2016.08.003. [TNF] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim K., Kim J.K., Han S.H., Lim J., Kim K Il, Cho D.H. Adiponectin as a negative regulator of NK cell cytotoxicity. J Immunol. 2006;176:5958–5964. doi: 10.4049/jimmunol.176.10.5958. [DOI] [PubMed] [Google Scholar]
- 63.Pallmer K., Oxenius A., Gross C.C. Recognition and regulation of T cells by NK cells. Front Immunol. 2016;7:1–13. doi: 10.3389/fimmu.2016.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Okamoto Y., Folco E.J., Minami M., Wara A.K., Feinberg M.W., Sukhova G.K. Adiponectin inhibits the production of CXC receptor 3 chemokine ligands in macrophages and reduces T-lymphocyte recruitment in atherogenesis. Circ Res. 2008;102:218–225. doi: 10.1161/CIRCRESAHA.107.164988. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.