Background
Gastric cancer remains the 3rd leading cause of cancer-related mortality and the 5th most common cancer worldwide.1 There is marked global variation of disease with areas of high versus low incidence. While the United States (US) is considered a low incidence country overall, incidence rates differ markedly among certain populations including some racial and ethnic minorities and immigrant populations, where rates might even approach that of endemic countries.2, 3 Of particular concern, non-cardia gastric adenocarcinoma (NCGA) is increasing among some populations in the US, including women below age 50 and Hispanic men.3 In the US, gastric cancer is the 15th most common cancer, with estimated 26,240 new cases and 10,800 deaths in 2018.2
NCGA is classified according to two histologic subtypes based on the Lauren classification: intestinal-type and diffuse-type.4 Intestinal-type GA is the result of complex interactions between genetic, environmental, and microbial-level determinants and represents the malignant transformation of a series of discrete histopathologic premalignant stages; this is in contrast to the diffuse-type GA, where the pathogenesis is less understood and no distinct precursor lesions have been identified. Gastric intestinal metaplasia (GIM) is one premalignant lesion for intestinal-type GA and, histologically, is typified by the replacement of the native gastric foveolar and/or glandular epithelium by intestinal-type epithelium.5 A diagnosis of GIM is strongly associated with risk of developing dysplasia and intestinal-type gastric cancer. Decades ago, Correa et al described a stepwise process whereby normal gastric mucosa progresses through discrete histopathologic stages to non-atrophic chronic gastritis, atrophic gastritis, intestinal metaplasia, and dysplasia, prior to malignant transformation to invasive intestinal-type adenocarcinoma.6 While chronic infection with Helicobacter pylori (H. pylori) is thought to be the primary trigger to the cascade, histologic progression is multifactorial and necessitates contributions from H. pylori virulence factors and environmental exposures amidst a background of genetic susceptibility and aberrant host responses.7–9 The so-called Correa cascade is most applicable to intestinal-type gastric cancer as compared to diffuse-type gastric adenocarcinoma.
Globally, the estimated annual incidence rates of gastric cancer among patients with GIM are highly variable in the literature, with ranges anywhere from 72 to 1950 per 100,000 people.10–15 Previously reported incidence rates are higher among patients with concomitant low-grade or high-grade dysplasia, and limit strong conclusions as to the malignant risk attributable to GIM alone.11, 13 The variability in estimated incidence among the studies, though, allows opportunity to identify prognostic factors which could be used for risk stratification to identify which patients with GIM are more likely to have neoplastic transformation and thus might benefit most from surveillance for early disease detection.16, 17 Potential prognostic factors include the extent of GIM12, 18, 19, the histopathologic subtype20–22, family history of gastric cancer12, 20, 23, 24, H. pylori virulence factors19, 25–27 or other noninvasive biomarkers (e.g. pepsinogen (PG) I and II)28, alcohol consumption29, tobacco use30, dietary habits31–33, and racial or ethnic background12, 13.
The reported prevalence of GIM from large international databases of gastric biopsies varies widely, ranging from 3.4% to 29.6%.10, 34–36 GIM can be diagnosed incidentally on random biopsies of normal appearing mucosa or targeted biopsies of subtle mucosal abnormalities. Despite the known increased risk of gastric cancer among patients with GIM, there are no randomized controlled trials that have evaluated the benefits or harms of surveillance endoscopy among patients with GIM.37 This has led to consensus-based recommendations for surveillance endoscopy in limited subgroups of patients with suspected higher risk of developing gastric cancer.16, 17 Vance et al surveyed 227 academic and private practice gastroenterologists in the US and found wide variability in the knowledge and practices related to endoscopic surveillance in patients with GIM. The survey highlighted the need for societal guidelines for clear guidance in clinical practice and future research.38 Therefore, the American Gastroenterological Association (AGA) prioritized this topic for the generation of clinical guidelines for gastric intestinal metaplasia.39
The technical review was divided into two reports. The first focused generating evidence profiles that directly informed four distinct PICO questions.40 The primary objective of this technical review is to summarize and analyze the indirect evidence informing the guideline, with the secondary objective to serve as a comprehensive resource for GIM epidemiology based on a systematic review.
Methods
Overview
The technical reviews and their accompanying guideline were conducted using the GRADE (Grading of Recommendation, Assessment, Development, and Evaluation) framework.41 The AGA Clinical Guidelines Committee selected the members of the guideline and technical review panels based on their clinical content and methodological expertise after undergoing a vetting process to exclude any conflict of interest. The guideline panel defined the scope of the guideline and developed focused clinical questions that were deemed relevant for clinical practice. The clinical questions aimed to 1) define risk factors for progression from GIM to gastric cancer, 2) quantify the risk of neoplastic progression and the impact of risk factor modification, and 3) define the risk versus benefit profile of endoscopic surveillance of GIM among patients deemed high or low-risk for gastric cancer according to predefined risk factors based on the literature. The technical review panel then formulated the clinical questions, identified the patient-important outcomes, and systematically reviewed the literature to summarize the available body of evidence for each question. Additionally, the technical review panel reviewed the literature systematically for indirect evidence that could assist the guideline panel in making informed decisions for the questions. Such evidence included 1) the prevalence of GIM in the US overall, in different racial and ethnic subgroups in the US, and in different regions worldwide; 2) identification of risk factors for GIM and quantification of the respective associated risk; and 3) the risk of incident neoplasia (i.e. dysplasia or gastric cancer) among patients diagnosed with GIM in the US, worldwide, and among those with risk factors for gastric cancer.
Formulating the Clinical Questions
The questions identified by the guideline panel as clinically relevant were formulated by the technical review panel using the PICO format. The PICO format frames clinical questions by defining a specific population (P), intervention (I), comparator (C), and outcome (O). The panel finalized four questions, which are detailed in Table 1. The first part of the technical review was dedicated to summarizing the evidence that directly informed the PICO questions.40
Table 1:
PICO questions, outcomes, and evidence needed to inform PICO questions
| PICO Question | Patient-Important Outcomes | Evidence needed to inform PICO questions |
|---|---|---|
| 1. Among patients with GIM, does testing for H. pylori and treating if positive vs no testing affect outcomes? | Early cancer detection Reduced gastric cancer morbidity/mortality Endoscopy complications Costs Psychological harms |
1. Incidence and prevalence of GIM in the US population 2. Incidence of stomach cancer in the general population 3. Prevalence of concurrent stomach cancer in patients with GIM 4. Incidence of stomach cancer in patients with GIM after GIM diagnosis 5. Risk of progression to gastric cancer in patients with GIM Subgroups: Family history of gastric cancer, Race/Ethnicity, smoking status, histologic features, extent of GIM, biomarkers. 6. Potential adverse consequences of performing surveillance upper endoscopy for patients with GIM 7. Benefits of performing surveillance upper endoscopy for patients with GIM |
| 2. Among patients with GIM who are identified as low risk, does subsequent upper endoscopic surveillance vs no follow up affect outcomes? | ||
| 3. Among patients with GIM who are identified as high risk, does subsequent upper endoscopic surveillance vs no follow up affect outcomes? | ||
| 4. Among patients with GIM without dysplasia does short term upper endoscopic follow up (< 1 year) to determine the extent (using biopsies) of GIM vs no short term follow up affect outcomes? |
The technical review panel, in conjunction with the guideline panel, also formulated questions that could inform the PICO questions indirectly. We aimed to define the burden of GIM in the US by assessing its prevalence in the US and comparing it with other countries and regions globally, as well as in the context of gastric cancer incidence rates. We aimed to define the rate of neoplastic progression from GIM to incident dysplasia or gastric cancer. We additionally aimed to define each of the above in the context of established risk factors for intestinal-type NCGA. Potential risk determinants identified by clinical content experts included: extensive GIM (defined as GIM involving the corpus) versus limited GIM (defined as GIM involving only the antrum, based on sufficient histologic evaluation of both antrum and corpus), GIM histopathologic subtype (incomplete versus complete), the geographical region based on the United Nations Standard Country Codes (M49)42, racial or ethnic groups, the presence of H. pylori or its virulence factors, noninvasive biomarkers (e.g. PG), family history of gastric cancer in a first-degree family member, smoking history, alcohol use history, pernicious anemia and autoimmune gastritis.
To limit disagreements regarding certain concepts, the panel agreed on specific definitions for histologic progression and regression, extensive and limited GIM, and complete and incomplete GIM prior to the systematic review, as detailed in the first part of the technical review.40
The operative link for gastric intestinal metaplasia (OLGIM) is a histopathologic classification system used to stage intestinal metaplasia by based on severity and extent GIM. It has less interobserver agreement compared to the operative link for gastric atrophy (OLGA).43 It ranges from Stage 0 to IV and a recent meta-analysis of case-control studies showed an association of advanced stages (III/IV) with higher risk of gastric cancer.44
H. pylori and its virulence factors and serum PG I and PG I/II ratio have been considered as biomarkers for to identify patients at high risk of developing gastric cancer. 26–28 Both PG I and PG II are secreted by the chief and foveolar cells in the gastric corpus and fundus, however, PG II is also secreted by pyloric glands in the antrum and Brunner’s glands in the duodenum. Alterations in the levels of PG I and PG I/II ratio have been identified as indicators of chronic atrophic gastritis, the step preceding intestinal metaplasia in the Correa cascade.45
The Systematic Review Process
The systematic review is reported in concordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement and the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) proposal.46, 47 As detailed above, a protocol was developed a priori by the technical review panel in conjunction with the guideline panel, to guide the systematic review.
Literature Search Strategy
Guided by the technical review panel, an experienced medical librarian, conducted a comprehensive search of the following databases from its earliest inception to July 2017 with a complete updated search performed in September 2018: MEDLINE Epub Ahead of Print, MEDLINE In-Process & Other Non-Indexed Citations, MEDLINE Daily, MEDLINE, EMBASE Classic, EMBASE, and Wiley’s Cochrane Library. The search was limited to English language and human adults. Controlled vocabulary supplemented with keywords was used to search for studies of prevalence of GIM, surveillance in GIM, testing and treating for H. pylori in patients with GIM, and the incidence of gastric cancer among patients with GIM. The final strategy is available in Appendix Document 1. The reference lists of previously published systematic reviews, prior guidelines, and the included references were also searched to identify relevant studies that might have been missed by our search strategy.
Eligibility Criteria
The inclusion and exclusion criteria were based on the above formulated clinical questions. Randomized controlled trials, nonrandomized comparative studies, and single arm noncomparative studies were eligible for inclusion. We excluded studies without data on GIM diagnosed histologically, or if we were not able to separate the results by GIM status.
We initially aimed to abstract prevalence data only from studies which included 100 or more patients; however, we modified the threshold to 250 patients or more after our search identified a large number of studies that reported GIM prevalence data. We additionally performed a sensitivity analysis for those studies that included prevalence data for 100–250 patients and confirmed the low impact of these smaller studies. We included studies that reported risk factors of interest regardless of the number of patients with GIM, so long as the full study population was 250 or larger. For studies that reported the incidence of gastric cancer among patients with GIM, we included studies with at least 20 patients diagnosed with GIM based on histology. This threshold was chosen due to the fewer number of studies that reported incidence rates.
We excluded studies of GIM of the cardia due to the different biology of cancers in this anatomic subsite compared to GA of the noncardia.7, 48 We also excluded studies of patients with prior gastric cancer and pediatric patients. Unless they reported outcomes of interest, we did not include studies restricted to: 1) atrophic gastritis, gastric dysplasia, or H. pylori infection (without GIM); 2) studies that compared different biopsy protocols; 3) studies that only used the operative link of gastric atrophy (OLGA) stage; and 4) studies that did not report data which could be abstracted specifically for patients with GIM. We also excluded case reports and narrative reviews. Authors of abstracts published after 2015 were contacted to obtain full-text reports or data, which were otherwise excluded if non-response.
Study Selection
The references identified using the above search strategy were reviewed according to the standard systematic review methods. The title and abstract of each identified reference were reviewed by two blinded independent investigators for eligibility and full-text retrieval. When disagreement was encountered at this stage, the reference was included for full-text retrieval. Each full-text manuscript was then evaluated by two independent blinded investigators. Disagreement was solved by consensus between the two investigators, and if it was not resolved, a third investigator from the team was consulted. The above process was performed using piloted standardized Research Electronic Data Capture (REDCap) forms designed by the technical review team.49
To identify studies that reported the risk or rate of progression from GIM to gastric cancer based on the preidentified risk factors, we queried our extraction REDCap forms for the references that reported any information about the risk factors. Then, we manually crossed those references with the references that reported risk of progression data. A similar process was used to identify studies that assessed the prevalence of the risk factors in patients with GIM or the prevalence of GIM in certain suspected high-risk groups.
Data Extraction and Outcome Measures
Data was extracted from each eligible reference by two independent blinded reviewers and disagreements were solved by consensus. A standardized electronic extraction form was designed using REDCap.49 The form was designed to be adaptive to the study design and the questions that are answered in each study. We collected the study-level data from each eligible study. The baseline characteristics included the country where the study was performed, age and gender of the patients, and the number of patients for each risk factor. For randomized controlled trials that did not report our outcomes of interest, we contacted the corresponding, first and/or senior authors to attempt to obtain the missing data. When a study had multiple publications, we harmonized the information from all the publications and used the most recent data available.
The outcomes of interest for each PICO question are outlined in Table 1. We used the relative risk and/or incidence rate ratios when comparative studies were available and provided enough data. For studies of incidence, we calculated both the cumulative incidence (the probability of developing the outcome over a specified period) and the incidence rate (the number of patients who developed the outcome per unit of time), when available. To calculate the incidence, we extracted the number of patients with GIM without dysplasia and/or the number of person-years as the denominator, and the number of patients who developed gastric cancer or dysplasia, regardless of the dysplasia grade due to the variability in reporting, as the numerator. For prevalence studies, we calculated the number of patients who underwent gastric biopsies, regardless of the indication, and were found to have GIM as the highest histopathologic lesion (i.e. no concomitant neoplasia). The prevalence and incidence were extracted in a similar fashion for the different risk factors, when available, for the purpose of subgroup analysis.
The subgroups of interest are: the topographic extent of GIM (limited versus extensive), OLGIM (the operative link for gastric intestinal metaplasia) stage, histologic subtypes (complete versus incomplete), H. pylori status and H. pylori virulence factors (e.g. CagA, VacA), serum PG (I, II, and I/II ratio), race and ethnicity, geographical region (M49 code), smoking status, alcohol use, dietary habits, first-degree relative with history of gastric cancer, pernicious anemia and autoimmune gastritis. For H. pylori status, we used 2 different thresholds to divide the studies to represent low-prevalence (15%) and high-prevalence areas (75%) based on agreement among the technical review panel.
Data Analysis
We used the DerSimonian-Liard random-effects model to pool the relative risk and/or incidence rate ratios when comparative studies were available.50 To pool prevalence, cumulative incidence and incidence rates, we used the inverse-variance fixed-effects model to calculate the pooled estimate using the Freeman-Tukey double arcsine transformation.51, 52 We elected to use the fixed-effects model to pool the prevalence and incidence studies as we presumed that larger studies were more likely to be more inclusive and representative of the general population. The fixed-effects model will give such studies, appropriately, higher weights in the pooled estimates. When evaluating the risk factors, if the risk factor was a binary variable, we used relative risks to assess the effect of the risk factor. If the risk factor was categorical with more than 2 categories, we used subgroup analysis and the interaction test to estimate and compare the effects between the different categories.53 We also conducted meta-regression to assess the correlation between the prevalence of GIM and the prevalence of H. pylori in each study. We used the I2 statistic to measure statistical heterogeneity and we used an I2 of 50% as threshold to investigate significant heterogeneity.54 If there was a sufficient number of studies without significant statistical heterogeneity, we used the asymmetry tests to assess publication bias.55 As sensitivity analyses, we still conducted the random effects model for the pooled prevalence and incidence estimates. Additionally, to account for the possible limitations related to the use of the inverse-variance method when pooling prevalence and incidence data when studies were sparse, we repeated all the analyses using the generalized linear mixed model.56 The statistical analysis was conducted using R version 3.4.4 and the package meta.57, 58
Risk of Bias and Quality of Evidence Assessment
Risk of Bias Assessment in Individual Studies
To assess the risk of bias in studies of prevalence and/or incidence, we used the Joanna Briggs Institute tool for critical appraisal of prevalence studies.59 For randomized controlled trials and nonrandomized comparative studies included in the first report of the technical review, we used the Cochrane Collaboration’s tool for assessing risk of bias and a modified version of the Newcastle-Ottawa scale.60, 61 The risk of bias assessment tools were built into our adaptive REDCap data extraction form and each study was assessed by two independent investigators.49
Risk of Bias Assessment Across Studies
We used the GRADE framework to assess the quality (certainty) of evidence derived from the systematic review and meta-analysis.41 In this approach, the evidence is graded for each outcome as high, moderate, low, or very low. Evidence derived from randomized controlled trials start as high quality, while evidence derived from observational studies start as low quality. Subsequently, the evidence can be downgraded for risk of bias, inconsistency, indirectness, imprecision, publication bias, and/or other factors. The evidence can be upgraded when there is a large magnitude of effect or dose-response relationship. For evidence on prevalence and incidence, the quality of evidence starts as high and is downgraded as described here.
Evidence-to-Decision Framework
Because this technical review was used to inform the development of clinical guidelines alongside a comprehensive risk-benefit analysis and the accompanying quality of evidence, information about additional factors such as patients’ preferences and values, resource utilization, and cost-effectiveness were considered and noted when available.
Results
Search Strategy and Study Selection
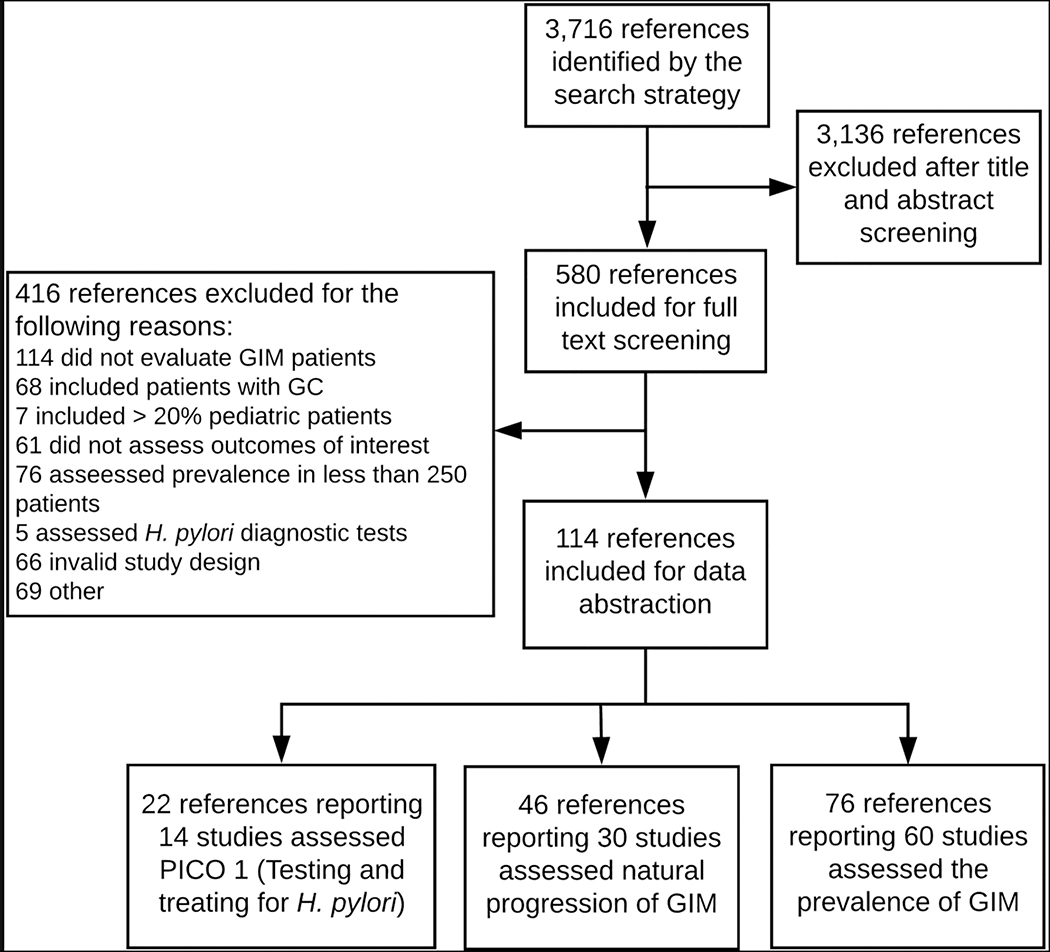
The search strategy identified 3716 potential references. After removing duplicates and reviewing the titles and abstracts, 580 articles were eligible for full-text review. After application of inclusion and exclusion criteria, 121 studies were ultimately included for data abstraction. The flow of the selection process and the reasons for exclusion are outlined in Figure 1. As detailed above, this review is limited to studies reporting GIM prevalence. Using the threshold of 250 people for prevalence studies, we identified 53 studies that reported the prevalence of GIM and provided population specifics. Additionally, we identified 6 studies the reported the prevalence of GIM among patients infected with H. pylori and 1 study that reported the prevalence of GIM among patients with first-degree relatives with a history of gastric cancer.
Figure 1.
PRISMA flow diagram of study selection.
Baseline Characteristics
The baseline characteristics of the studies are summarized in Table 2. The study designs were as follows: 45 cross-sectional, 7 retrospective cohort, 6 prospective cohort, and two randomized controlled trials (RCTs). The studies reported the prevalence of GIM in 12 different geographical regions and 29 countries.
Table 2:
Summary of studies and patients included
| Study First author’s last name, Publication year (Country), and Study design |
Demographics: Mean Age (SD) or Range in years*; Gender; Ethnicities/Races; |
Settings (pathology database, population based) Biopsy protocol**: | Total subjects (N) Time period |
Subgroups |
|---|---|---|---|---|
| Abangah, 201692 (Iran) Cross-sectional |
Age: 48, Range 21–84 Male: 64.8% Ethnicities: Southern Asia |
Population undergoing endoscopy Biopsy protocol: Two biopsies from antrum, 2 from body and 1 from incisura |
1000 48 mos. |
- H. pylori positive 80.8% - Smoking history 40.3% - Alcohol consumption 27.9% - Histological predictors: complete GIM 67%, Incomplete GIM 33% - Atrophic Gastritis 7.9% - Dysplasia 1.2% |
| Ajdarkosh, 201478 (Iran) Cross-sectional |
Age: 58 Male: 47.5% Ethnicities: Southern Asia |
Population undergoing endoscopy Biopsy protocol: Biopsies from antrum and body (did not report number of biopsies) |
688 36 mos. |
- H. pylori positive 64.5% |
| Al-Knawy, 199993 (Saudi Arabia) Cross-sectional |
Age: 43, SD 17.6 Male: 53.3% Ethnicities: Western Asia |
Population undergoing endoscopy Biopsy protocol: Biopsies from antrum (did not report number of biopsies) |
778 Not reported |
- H. pylori positive 75.4% - Histological predictors: 1. Complete GIM 85.6 % 2. Incomplete GIM 14.3 |
| Almouradi, 201362 (USA) Retrospective cohort |
Age: Not reported for total population Male: 46.5% Ethnicities/Races: North America/Black, Hispanic, White, and Asian |
Population undergoing endoscopy | 677 7 mos. |
- H. pylori positive 43% - Only 437 patients had gastric biopsies. |
| Aydin, 2017106 (Turkey) Retrospective cohort |
Age: 38.22, SD 14.64, Range 18–88 Male: 44.6% Ethnicities: Western Asia |
Population undergoing endoscopy Biopsy protocol: Biopsies from antrum (did not report number of biopsies) |
682 14 mos. |
- H. pylori positive 69.6% - Atrophic Gastritis 69.6% |
| Brkic, 201787 (Croatia) Cross-sectional |
Age: more than 2/3 were > 50 Male: 47% Ethnicities: Southern Europe |
Population undergoing endoscopy Biopsy protocol: Two biopsies from antrum and 2 from body |
871 24 mos. |
- H. pylori positive 41% - Atrophic Gastritis 3.5–5% |
| Chang, 200282 (Taiwan) Cross-sectional |
Age: 48.4, Range 30–82 Male: 57.3% Ethnicities: Eastern Asia |
Population undergoing endoscopy Biopsy protocol: Two biopsies from antrum and 2 from body |
274 2 mos. |
- H. pylori positive 62.4 % |
| Correa, 1990107 (Colombia) Prospective cohort |
Age: Not reported Male: 50% Ethnicities/Races: South America/ Hispanic |
Population undergoing endoscopy Biopsy protocol: Two biopsies from antrum and 1 from body |
1788 61 mos. |
No data for subgroups reported |
| Craanen, 199194 (Netherlands) Cross-sectional |
Age: 57.8, SD 16.8 Male: Not reported Ethnicities: Western Europe |
Population undergoing endoscopy Biopsy protocol: Biopsies from antrum (did not report number of biopsies) |
533 19 mos. |
- Histological subtypes: 1. Complete GIM (type I) 98.5% 2. Incomplete GIM: type II 77.8% and type III 15.5% |
| Cu, 2000 (Vietnam)108 Cross-sectional |
Age: Range 16–78 Male: 68.9% Ethnicities: South-Eastern Asia |
Population undergoing endoscopy Biopsy protocol: Two biopsies taken from antrum and body |
347 48 mos. |
- H. pylori positive 40.6% |
| Den Hoed, 2011109 (Netherlands) Cross-sectional |
Age: 53.1, Range 17–86 Male: 49.8% Ethnicities: Western Europe |
Population undergoing endoscopy Biopsy protocol: Two biopsies taken from antrum and body |
383 Not reported |
- H. pylori positive 22% - Smoking history: 25% current, 24% ex-smoker - Alcohol consumption: 61% - Atrophic gastritis 9.4% |
| El-Serag, 199963 (USA) Cross-sectional |
Age: 57–58, SD 14–15 Male: Not reported for the total population Ethnicities/Races: North America/White and Hispanic |
Population undergoing endoscopy Biopsy protocol: Two gastric biopsies were obtained from the antrum, corpus, and cardia |
302 Not reported |
- H. pylori positive 43.4% - Atrophic gastritis 9.9% |
| Eriksson, 200889 (Finland) Cross-sectional |
Age: 54, SD 16 Male: 39.9% Ethnicities: Northern Europe |
Population undergoing endoscopy Biopsy protocol: Two biopsies from antrum and 2 from body |
505 6 mos. |
- H. pylori positive 12.4 % - Histological subtype: 1. Complete GIM 16% 2. Incomplete GIM 8.3% |
| Fennerty, 199264 (USA) Cross-sectional |
Age: 63 Range 24–94 Male: 99.5% Ethnicities/Races: North America/ White 81.6%, Hispanic 13.2%, Native American 2.5%, Black 2.3%, Asian 0.5% |
Population undergoing endoscopy Biopsy protocol: Minimum 2 biopsies from incisura |
440 24 mos. |
- H. pylori positive 45 % |
| Gomez, 201365 (USA) Cross-sectional |
Age: Median 53 Male: 55.7% Ethnicities: North America |
Population undergoing endoscopy | 300 3 mos. |
- H. pylori positive 2% - Family history of stomach cancer 3% - Smoking history: 42% - Alcohol consumption 35% |
| Haroon, 2013110 (Pakistan) Cross-sectional |
Age: 41.3, SD 13.3 Range 16–75 Male: 50% Ethnicities: Southern Asia |
Population undergoing endoscopy Biopsy protocol: Two biopsies taken from antrum and body |
375 6 mos. |
- H. pylori positive 47% - Atrophic gastritis 9.6% - Dysplasia1.3% |
| Haziri, 2017111 (Kosovo) Cross-sectional |
Age: most subjects 40–49 Male: 60.2% Ethnicities: Southern Europe |
Population undergoing endoscopy Biopsy protocol: Multiple biopsies from antrum and body |
802 Not reported |
- H. pylori positive 59.7% - Atrophic Gastritis 17.6% |
| Hong, 201484 (China) Cross-sectional |
Age: 45.8, SD 12.1 Male: 72.9% Ethnicities: Eastern Asia |
Population undergoing endoscopy Biopsy protocol: Two biopsies from antrum, 2 from body and 1 from incisura |
9297 116 mos. |
- Dysplasia 2.3% |
| Hsu, 2007112 (Taiwan) Prospective cohort |
Age: 53.6 Male: 66.2% Ethnicities: Eastern Asia |
Population undergoing endoscopy Biopsy protocol: Two biopsies from antrum and 2 from body |
1225 96 mos. |
- H. pylori positive 50.4% - Smoking history 27.8% - Alcohol consumption 13.1% |
| Huang, 2012113 (China) Cross-sectional |
Age: 44.7, SD 13.5 Male: 60% Ethnicities: Eastern Asia |
Population undergoing endoscopy Biopsy protocol: Two biopsies taken from antrum |
406 56 mos. |
- H. pylori positive 24.1% |
| Ibrisim, 2008114 (Turkey) Cross-sectional |
Age: 46.36, SD 11.4 Male; 46.7% Ethnicities: Western Asia |
Population undergoing endoscopy Biopsy protocol: Two biopsies from antrum, 2 from body and 1 from incisura |
289 35 mos. |
- H. pylori positive 39.1% - Histological predictors: 1. Complete GIM 6.9% 2. Incomplete GIM 19.4 - Atrophic gastritis 25.6% - Dysplasia 3.8% |
| Imai, 2013115 (Japan) Cross-sectional |
Age: Not reported Male: 50% Ethnicities/Races: Eastern Asia |
Autopsy study Biopsy protocol: Stomachs from autopsies |
937 84 mos. |
No subgroups |
| Isajevs, 201485 (Lithuania) Cross-sectional |
Age: Not reported Male: Not reported Ethnicities: Northern Europe |
Population undergoing endoscopy Biopsy protocol: Two biopsies from antrum, 2 from body and 1 from incisura |
835 Not reported |
- H. pylori positive 57.8% - Atrophic Gastritis 33.4% - OLGA stages: stage 0 = 556, stage I =184, stage II = 70, stage III = 20, stage IV = 5 - OLGIM stage: stage 0 = 591, stage I =155, stage II = 66, stage III = 19, stage IV = 4 |
| Jedrychowski, 201081 (Poland) Cross-sectional |
Age: Not reported Male: 58.5% Ethnicities: Eastern Europe |
Population undergoing endoscopy Biopsy protocol: Two biopsies from antrum |
1,290 Not reported |
No data for subgroups reported |
| Jiang, 201734 (China) Cross-sectional |
Age: 50.98, SD 13.3 Male: 50% Ethnicities: Eastern Asia |
Population undergoing endoscopy Biopsy protocol: One biopsy from antrum (3 cm from the pylorus) |
28,745 Not reported |
- Atrophic gastritis 8.54% - Dysplasia 5.81% |
| Joo, 201379 (Korea) Cross-sectional |
Age: 48.7, SD 11.3, Range 15–98 Male: 58.6% Ethnicities: Eastern Asia |
Population undergoing endoscopy | 4,023 12 mos. |
- H. pylori positive 59.8% - Family history of stomach cancer 11.4% - Smoking history 21.9 % - Alcohol consumption 61.8% - Atrophic gastritis 40.7% |
| Kang, 201535 (Korea) Cross-sectional |
Age: 56.23, SD 9.76, Range 40–93 Male: 43.62% Ethnicities: Eastern Asia |
Medical records of patients who underwent endoscopy | 40,821 78 mos. |
- Atrophic gastritis 27.97% |
| Kim, 200814 (Korea) Prospective cohort |
Age: 46.7 Male: 79.2% Ethnicities: Eastern Asia |
Population undergoing endoscopy Biopsy protocol: Two biopsies from antrum and 2 from body |
1,790 72 mos. |
- H. pylori positive 81.2% |
| Lahner, 2014116 (Italy) Cross-sectional |
Age: 58, Range 50–65 Male: 36.6% Ethnicities: Southern Europe |
Population undergoing endoscopy Biopsy protocol: Two biopsies from antrum, 2 from body and 1 from incisura |
979 Not reported |
- H. pylori positive 34% - Family history of stomach cancer 11.6% - Smoking history 35.7% - Alcohol consumption 32.5% - Atrophic Gastritis 10% |
| Mansour-Ghanaei, 201276 (Iran) Cross-sectional |
Age: 43.45, SD 10.6 Gender: 49.4% Ethnicities: Southern Asia |
Population undergoing endoscopy Biopsy protocol: Two biopsies from antrum, 2 from body and 1 from incisura |
1095 36 mos. |
- H. pylori positive 76.6% - Family history of stomach cancer 46% - Atrophic gastritis 4.3% - Dysplasia 2% |
| Micu, 2010117 (Romania) Cross-sectional |
Age: 57, Range 16–89 Male: 48.1% Ethnicities: Eastern Europe |
Population undergoing endoscopy Biopsy protocol: Biopsies were taken from antrum and body (did not report number of biopsies) |
3096 49 mos. |
- H. pylori positive 46.1% |
| Nam, 201497 (Korea) Cross-sectional |
Age: 48.2, SD 10.8, Range 21–68 Male: 49.2% Ethnicities: Eastern Asia |
Population undergoing endoscopy Biopsy protocol: Two biopsies from antrum and 2 from body |
632 20 mos |
- H. pylori positive 59% - Family history of stomach cancer 10.6% - Smoking history 23.4 % - Alcohol consumption 60.3% |
| Nasser, 2015118 (Lebanon) Cross-sectional |
Age: Median 50, Range 20–87 Male: 50% Ethnicities: Western Asia |
Population undergoing endoscopy | 300 27 mos. |
- H. pylori positive 52% - Smoking history: 42% - Alcohol consumption 20.7% |
| Niknam, 201596 (Iran) Cross-sectional |
Age: 39, SD 15 Male: 30% Ethnicities: Southern Asia |
Population undergoing endoscopy Biopsy protocol: Two biopsies from antrum and 2 from body |
1,376 Not reported |
- H. pylori positive 76.5% - Histological subtypes: 1. Complete GIM 2.6% 2. Incomplete GIM 0.4% |
| Olmez, 2015119 (Turkey) Retrospective cohort |
Age: 57, SD 15, Range 17–98 Male: 59.5% Ethnicities: Western Asia |
Population undergoing endoscopy | 4,050 52 mos. |
- Histological subtypes: 1. Complete GIM (type I) 8.2% 2. Incomplete GIM: type II 32% and type III 38% |
| Ozdil, 2010120 (Turkey) Retrospective cohort |
Age: 45.97, SD 15.15, Range 18–94 Male: 40% Ethnicities: Western Asia |
Population undergoing endoscopy Biopsy protocol: Biopsies from antrum and body (did not report number of biopsies) |
3,301 23mos |
- H. pylori positive 71.3% |
| Petersson, 200274 (Sweden) Cross-sectional |
Age: 60, Range 35–85 Male: 54.5% Ethnicities: Northern Europe |
Population undergoing endoscopy Biopsy protocol: Three biopsies from antrum and 3 from body |
502 Not reported |
- H. pylori positive 40% - Histological subtypes: 1. Complete GIM (type I) 15% 2. Incomplete GIM: type II 64% and type III 21% - Atrophic gastritis 48$ - Pernicious anemia 5.4 % |
| Plummer, 200772, 75 (Venezuela) RCT |
Age: Not reported for the total population Male: 47% Ethnicities/Races: South America (Hispanic) |
Population undergoing endoscopy Biopsy protocol: Three biopsies from antrum and 1 from body |
2,200/2131 36 mos. |
- H. pylori/cagA status: cagA(+) 40%, cagA(-) 44%, uninfected 16%. - Histological subtype: 1. Complete GIM (type I) 70.4% 2. Incomplete GIM: type II 15.2% and type III 14.3% - Atrophic gastritis15% - Dysplasia 6% |
| Saragih, 2007121 (Indonesia) Retrospective cohort |
Age: Not reported Male: Not reported Ethnicities: South-Eastern Asia |
Population undergoing endoscopy Biopsy protocol: Biopsies were taken from antrum, body and incisura (did not report number of biopsies) |
2903 96 mos. |
- H. pylori positive 9% |
| Sobala, 199386 (UK) Cross-sectional |
Age: median 49, Range 18–88 Male: 60.6% Ethnicities: Northern Europe |
Population undergoing endoscopy Biopsy protocol: Two biopsies taken from antrum |
350 Not reported |
- H. pylori positive 63.1% - Histologic subtype: 1. Complete GIM (type I) 61% 2. Incomplete GIM: type II 64%, type III 6%, and multiple 29% |
| Song, 201510 (Sweden) Cross-sectional |
Age: 56 Male: 44.5% Ethnicities: Northern Europe |
Patients in a national database who underwent endoscopy with biopsies | 342,297 264 mos. |
- Atrophic gastritis 4.2% - Dysplasia 0.6% |
| Song, 201780 (Korea) Cross-sectional |
Age: 52.76, SD 10.74 Male: 48.5% Ethnicities: Eastern Asia |
Population undergoing endoscopy | 662 91 mos. |
- H. pylori positive 61.5% - Family history of stomach cancer 10.3% - Smoking history 39.2 % - Alcohol consumption 71.4% - Atrophic gastritis 29% |
| Sonnenberg, 201536 (USA) Cross-sectional |
Age: Not reported Male: 38% Ethnicities: North America |
Patients in a national pathology database who underwent endoscopy | 895,323 72 mos. |
- H. pylori positive 10.6% - Atrophic gastritis 12.8% |
| Tsukanov, 2011122 (Siberia) Cross-sectional |
Age: 36.1(male), 42.3(female) Male: 47.5% Ethnicities: Eastern Europe 32.4% Eastern Asia 67.6% |
Population undergoing endoscopy Biopsy protocol: One biopsy from antrum, 1 from body and 1 from incisura |
2129 Not reported |
- H. pylori positive 94.1% |
| Ucuncu, 2016123 (Turkey) Cross-sectional |
Age: 43.70, Range 18–65 Male: 39.5% Ethnicities: Western Asia |
Population undergoing endoscopy Biopsy protocol: Biopsies were taken from antrum and body (did not report number of biopsies) |
3096 24 mos. |
- H. pylori positive 28.4% - Smoking history 18.8% - Alcohol history 1.3% - Atrophic gastritis 5.8% |
| Wang, 1998124 (Taiwan) Cross-sectional |
Age: 44.3, SD 11.1 Male: 55.2% Ethnicities: Eastern Asia |
Population undergoing endoscopy Biopsy protocol: Four biopsies from antrum and 4 from body |
302 Not reported |
- H. pylori positive 61.3% |
| Wang, 201783 (China) Cross-sectional |
Age: 53.1, SD 11.8 Male: 42.3% Ethnicities: Eastern Asia |
Population undergoing endoscopy Biopsy protocol: Two biopsies from antrum, 2 from body and 1 from incisura |
331 14 mos. |
- H. pylori positive 43.2% - Family history of stomach cancer 6.34% - Smoking History: 13.6% current, 26.6% ex-smoker - Alcohol consumption: 33.5% current, 42.9% ex-drinker - Atrophic gastritis 46.5% - Pepsinogen positive (PG I level ≤70 ng/mL and PG I/II ≤7) 5.4% |
| Whiting, 2002125 (UK) Prospective cohort |
Age: Not reported Male: Not reported Ethnicities: Northern Europe |
Population undergoing endoscopy | 1753 120 mos. |
- Atrophic gastritis 1.6% - Dysplasia 0.9% |
| Xia, 200088 (Australia) Cross-sectional |
Age: 52.1, Range 17–85 Male: 48.5% Ethnicities/Races: Australia |
Population undergoing endoscopy Biopsy protocol: Two biopsies from antrum, 2 from body and 1 from incisura |
268 Not reported |
- H. pylori positive 42% - Atrophic gastritis 19% |
| Yee, 2009126 (Hong Kong) Retrospective cohort |
Age: 45.4 Range 14–89 Male: 39% Ethnicities: Eastern Asia |
Population undergoing endoscopy Biopsy protocol: Biopsies were taken from antrum and body (did not report number of biopsies) |
1751 72 mos. |
- H. pylori positive 45% |
| You, 199390 (China) Cross-sectional |
Age: Range 35–64 Male: 52.7% Ethnicities: Eastern Asia |
Population undergoing endoscopy Biopsy protocol: Seven to eight biopsies divided by antrum, body and incisura |
3400 Not reported |
- Atrophic gastritis 44.8% - Dysplasia 20.1% |
| You, 2006127 (China) RCT |
Age: 46.8 Male: 50% Ethnicities: Eastern Asia |
Population undergoing endoscopy Biopsy protocol: Four biopsies from antrum, 2 from body and 1 from incisura |
3411/3344 12 mos. |
- H. pylori positive 67.1% - Atrophic gastritis 45.2% - Dysplasia 13.6% |
| Zabaleta, 201166 (USA) Cross-sectional |
Age: 48–56, SD 11–12 Male: 20–36% Ethnicities/Races: North America/Black 63.4%, White 36.6% |
Population undergoing endoscopy Biopsy protocol: One biopsy from antrum, body and incisura |
569 Not reported |
- H. pylori positive 32% - Smoking history 34.4%% - Dysplasia 0.4% |
| Summary of studies that only included H. pylori infected patients only or studies that only included patients with first degree family history of gastric cancer | ||||
| Chen, 200167 (China/Netherlands) Cross-sectional |
Age: Chinese 40.9; Dutch 49.7 Male: Chinese 57%; Dutch 58.2% Ethnicities: Eastern Asia (Chinese) 57.0%; Western Europe (Dutch) 58.2% |
H. pylori positive patients that had endoscopy Biopsy protocol: Four biopsies from antrum, and 4 from body |
526 Not reported |
- Atrophic gastritis: Chines 52%; Dutch 42% |
| Eidt, 199468 (Germany) Cross-sectional |
Age: 51.4 Male: 60% Ethnicities: Western Europe |
H. pylori positive patients that had endoscopy Biopsy protocol: Two biopsies from antrum |
2692 24 mos |
No data for subgroups reported |
| Guarner, 200169 (Mexico) Cross-sectional |
Age: 51.8; SD 9.5 Male: Not reported Ethnicities: Central America |
H pylori positive patients that had endoscopy. Biopsy protocol: 3 from the antrum, 1 from the incisura angularis, and 3 from the corpus. |
368 Not reported |
- Atrophic gastritis 62% |
| Lee, 201370 (Taiwan) Prospective cohort |
Age: 49.2, SD 12.8 Male: 45.8% Ethnicities: Eastern Asia |
H pylori positive patients that had endoscopy. Biopsy protocol: Biopsies from antrum and body (did not report number of biopsies) |
4121/1762 48 mos. |
- Atrophic gastritis 59.9% - Dysplasia 0.3% |
| Leodolter, 201371 (Germany) Retrospective cohort |
Age: 54.8 Male: 53.2% Ethnicities: Western Europe |
H. pylori positive patients that had endoscopy Biopsy protocol: Two biopsies from antrum, and 2 from body |
845 48 mos. |
- Atrophic gastritis 3.2% |
| Leung, 200577 (China) Cross-sectional |
Age: Median 24; Range 8–66 Male: 47% Ethnicities: Eastern Asia |
Patients with first degree family history of gastric cancer that had endoscopy Biopsy protocol: Four biopsies from antrum, and 4 from body |
270 31 mos. |
- H. pylori positive 59.6% - Smoking history 19.6% - Alcohol consumption 33% |
| Sadjadi, 201415 (Iran) Prospective cohort |
Age: 53.1, SD 9.9 Male: 49.1% Ethnicities: Southern Asia |
H. pylori positive patients that had endoscopy Biopsy protocol: Two biopsies from antrum, 2 from body and 1 from incisura |
928 121.8 mos. |
- Family history of stomach cancer 20.7% - Smoking history 39.1% - Alcohol consumption 4.7% - Dietary intake: high salt (> 6 grams) 81.1% |
Ages are means with standard deviation unless otherwise specified.
Risk of Bias Assessment
The overall risk of bias in the individual studies is summarized in Appendix Document 2. Most of the individual studies were at moderate to high risk of bias. The most common limitation was the sampling frame, with 15% of the studies using a sample frame that we considered relevant to our target population, i.e. patients undergoing endoscopic evaluation in the United States. Approximately 65% of the studies reported the prevalence of GIM in patients who had biopsies obtained from both the antrum and corpus, while the remaining either obtained biopsies from the antrum only or did not specify. The risk of bais across the studies and the certainty of evidence for each outcome are summarized in Table 3.
Table 3:
Certainty of Evidence
| Outcome | N/n | Estimate (95% CI) | Certainty of Evidence | Risk of Bias | Inconsistency | Imprecision | Indirectness | |
|---|---|---|---|---|---|---|---|---|
| Prevalence of GIM in the US | 6/897,731 | 4.8% (4.8 – 4.9) | ⊕⊕⊕○ Moderate |
Serious | Not serious1 | Not serious | Not serious | |
| Prevalence of GIM in the different regions | South Asia | 5/4,534 | 9.5% (8.7 – 10.4) | ⊕⊕○○ Low |
Serious | Serious | Not serious | Not serious |
| West Asia | 7/12,496 | 14.1% (13.5 – 14.7) |
⊕⊕○○ Low |
Serious | Serious | Not serious | Not serious | |
| North America | 6/897,731 | 4.8% (4.8 – 4.9) |
⊕⊕⊕○ Moderate |
Serious | Not serious1 | Not serious | Not serious | |
| South Europe | 3/2,652 | 17.5% (16.0 – 18.9) |
⊕⊕○○ Low |
Serious | Serious | Not serious | Not serious | |
| East Asia | 16/97,940 | 21.0% (20.7 – 21.2) |
⊕⊕○○ Low |
Serious | Serious2 | Not serious | Not serious | |
| South America | 2/3,919 | 23.9% (22.6 – 25.3) |
⊕⊕⊕○ Moderate |
Serious | Not serious | Not serious | Not serious | |
| West Europe | 2/916 | 16.6% (14.2 – 19.1) |
⊕○○○ Very low |
Serious3 | Serious | Serious4 | Not serious | |
| South-East Asia | 2/3,250 | 6.5% (5.7 – 7.4) |
⊕⊕○○ Low |
Serious | Serious | Not serious | Not serious | |
| North Europe | 6/346,215 | 3.4% (3.3 – 3.5) |
⊕⊕○○ Low |
Serious | Serious | Not serious | Not serious | |
| East Europe | 3/4,732 | 18.7% (17.6 – 19.8) |
⊕⊕○○ Low |
Serious | Serious | Not serious | Not serious | |
| Australia | 1/268 | 16.0% (11.9 – 20.7) |
⊕○○○ Very low |
Serious | Not serious | Very serious5 | Not serious | |
| Prevalence of GIM based on cagA status | cagA positive | 3/1,347 | 36.4% (33.8 – 39.0) |
⊕○○○ Very low |
Very serious6,7 | Serious | Serious | Serious |
| cagA negative | 2/1,002 | 21.3% (18.8 – 24.0) |
||||||
| Uninfected | 2/719 | 17.8% (15.1 – 20.7) |
||||||
| Prevalence of GIM in the US based on race/ethnicity7 | Non-Hispanic Black | 2/566 | 15.9% (13.0 – 19.0) |
⊕⊕○○ Low |
Serious8 | Not serious | Serious4 | Not serious |
| Hispanic | 2/220 | 23.3% (17.8 – 29.1) |
⊕○○○ Very low |
Serious8 | Serious | Very serious5 | Not serious | |
| Non-Hispanic White | 3/610 | 12.2% (9.7 – 15.0) |
⊕⊕○○ Low |
Serious8 | Not serious | Serious4 | Not serious | |
| Asian | 1/27 | 14.8% (3.5 – 31.1) |
⊕○○○ Very low |
Serious8 | Not serious | Very serious5 | Not serious | |
| Native American | 1/11 | 18.2% (0.5 – 47.4) |
⊕○○○ Very low |
Serious8 | Not serious | Very serious5 | Not serious | |
| Relative risk of finding GIM on gastric biopsies in patients on high vs. low salt diet | 2/4,890 | 1.18 (0.99 – 1.40) |
⊕○○○ Very low |
Very serious9 | Not serious | Not serious | Serious | |
| Relative risk of finding GIM on gastric biopsies in patients on low vs. high fruits/vegetables diet | 2/2,174 | 1.42 (1.13 – 1.79) |
⊕○○○ Very low |
Very serious9 | Not serious | Not serious | Serious | |
| Relative risk of finding GIM on gastric biopsies in patients with high vs. low dairy products intake | 1/4,931 | 1.72 (1.43 – 2.05) |
⊕○○○ Very low |
Very serious9 | Not serious | Not serious | Serious | |
| Prevalence of GIM in patients with pernicious anemia | 1/27 | 88.9% (70.8 – 97.6) |
⊕○○○ Very low |
Serious10 | Not serious | Very serious5 | Not serious | |
| Relative risk of finding GIM on gastric biopsies in patients with first-degree family history of gastric cancer vs. patients with no family history | 5/4,791 | 1.46 (0.97 – 2.21) |
⊕○○○ Very low |
Serious | Not serious | Serious | Serious | |
| Relative risk of finding GIM on gastric biopsies in patients who smoke tobacco versus non-smokers | 7/7,971 | 1.57 (1.24 – 1.98) |
⊕○○○ Very low |
Very serious9,11 | Not serious | Not serious | Serious | |
| Relative risk of finding GIM on gastric biopsies in patients who drink alcohol versus patients who did not drink alcohol | 6/6,775 | 1.29 (1.12 – 1.50) |
⊕○○○ Very low |
Very serious9,11 | Not serious | Not serious | Serious | |
| Prevalence of incomplete GIM in patients with GIM found during gastric biopsies | 13/2,742 | 47.7% (45.8 – 49.6) |
⊕○○○ Very low |
Serious | Very serious12 | Not serious | Serious | |
| Prevalence of extensive GIM in patients with GIM found during gastric biopsies | 9/3,558 | 30.3% (28.8 – 31.8) |
⊕○○○ Very low |
Serious | Serious13 | Not serious | Serious | |
the observed inconsistency was explained by the differences in the risk of bias between the individual studies.
the observed inconsistency was partially explained by the differences in the risk of bias between the individual studies.
the study by den Hod et al was well conducted with low risk of bias and we have higher certainty in its estimate (7%, 95% CI 5 – 10%) compared to the pooled estimate.
due to small total number of included patients.
due to very small total number of included patients.
the method of diagnosing H. pylori and cagA status differed between the studies.
the comparison between the subgroups is based on a Chi squared test which has inherent methodological limitations.
the studies did not report obtaining biopsies routinely from both antrum and corpus.
the relative risk estimate were not adjusted for other possible risk factors, that may contribute to risk of bias as in Appendix 2.
it was not clear how the subgroup of patients with pernicious anemia was identified.
the definition of exposure to smoking or alcohol was not clear in most of the studies (current use vs. prior use vs. never).
the observed inconsistency was not explained by differences in risk of bias or geographical region
the observed inconsistency was partially explained by the prevalence of H. pylori infection
Acronyms: N, number of studies; n, total number of patients; CI, confidence interval; GIM, gastric intestinal metaplasia; H. pylori, helicobacter pylori
The Prevalence of GIM
The United States
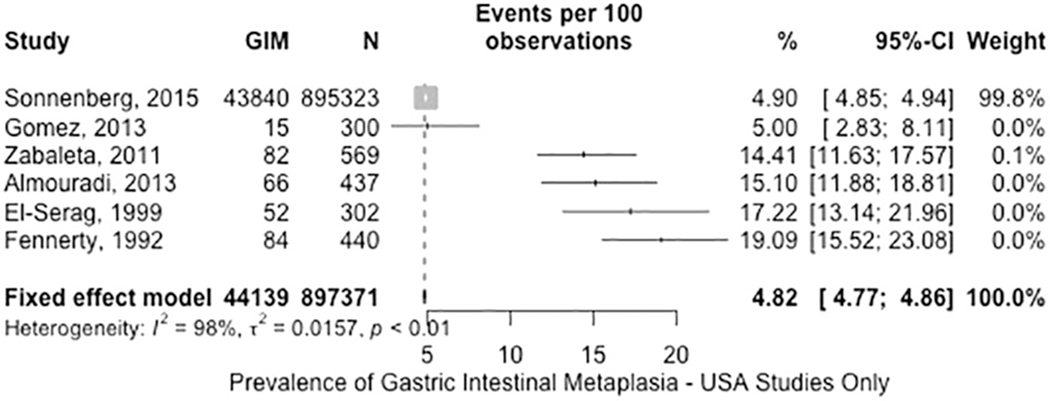
Of the 53 studies, 6 reported GIM prevalence in the US (n= 897,371). The fixed-effects pooled prevalence of GIM was 4.8% (95% CI: 4.8–4.9%) in patients who underwent gastric biopsies regardless of the indication (Figure 2, moderate certainty in evidence). Although the point estimates of the studies ranged between 4.9% and 19.1%, the observed inconsistency was explained by risk of bias related to patient selection. The study by Sonnenberg et al. dominated the other studies due to its large sample size and had the highest influence on the estimate.36 The study was well-designed and based on a large national pathology database with coverage of 46 states. Relevant major limitations of this study were that 1) it could not be confirmed whether all included individuals had biopsies obtained from both the antrum and corpus; 2) the indication for upper endoscopy with biopsies; and 3) individual-level details above basic demographics. The assessment for publication bias was not appropriate due to the substantial statistical heterogeneity.36, 62–66
Figure 2.
Prevalence of GIM in US patients who underwent gastric biopsies.
Worldwide GIM Prevalence (including US)
There were significant differences across geographic regions based on subgroup analysis (p < 0.01, Appendix Figure 1), hence we presented the pooled estimates separately. The fixed-effects pooled prevalence of GIM was lowest in studies from Northern Europe 3.4% (low certainty in evidence), followed by Northern America 4.8% (moderate certainty), South-East Asia 6.5% (low certainty), Southern Asia 9.5% (low certainty), Western Asia 14.1% (low certainty), Australia 16.0% (very low certainty), Western Europe 16.6% (very low certainty), Southern Europe 17.5% (low certainty), Eastern Europe 18.7% (low certainty), Eastern Asia 21.0% (low certainty), and highest in South America 23.9% (moderate certainty). We did not assess for publication bias due to the substantial statistical heterogeneity. The certainty in evidence was rated down for risk of bias when the studies with the highest influence on the pooled estimate did not report that biopsies were routinely obtained from both the antrum and corpus. We rated down for inconsistency when it was not possible to explain it (e.g. based on risk of bias) and for imprecision when the total number of patients was less than 1,000.
The Prevalence of GIM in Proposed High-Risk Groups
Helicobacter pylori exposed patients
Forty-four of the 53 studies (83%) reported on H. pylori prevalence among the study population. We conducted exploratory analyses by categorizing the studies based on the prevalence of H. pylori. When we stratified studies based on prevalence of H. pylori exposure (above vs below 15%), the studies with over 15% H pylori exposure among included individuals reported higher GIM prevalence (Appendix Figure 2). Raising the the threshold H pylori prevalence to 75% yielded similar findings (Appendix Figure 3). However, the studies within each subgroup were inconsistent (e.g. the point estimate for GIM prevalence ranged from 3 to 48% in the subgroup of studies with H. pylori prevalence greater than 15%) and were limited by moderate to high risk of bias in general.
To further investigate the correlation between H. pylori prevalence and GIM prevalence, we also performed univariate meta-regression to assess whether the variability of H pylori prevalence between studies could explain the variability of GIM prevalence between the studies. However, we found no correlation between these two variables (p= 0.85, Appendix Figure 4). This observation could relate to differences in the methods used to diagnose H. pylori and definitions of H. pylori ‘positivity’ (i.e. prior exposure versus active infection).
We identified 6 studies (n= 7,121) that reported the prevalence of GIM in H. pylori -exposed patients with fixed-effects pooled GIM prevalence of 25% (95%CI: 24.0 – 26.0%). Based on the subgroup interaction test, there was a statistically significant difference in GIM prevalence based on the geographical regions (p < 0.01; Appendix Figure 5).15, 67–71
H. pylori associated virulence factors
Three studies (n= 3,068) reported GIM prevalence among H. pylori exposed patients according to the presence or expression of cytotoxin-associated gene A (cagA) status, but otherwise no other H. pylori associated virulence factors. cagA presence or expression status was assessed by variable methods, such as polymerase chain reaction and serologic testing for antibodies, respectively. For the purpose of this review, we considered either the presence of cagA gene or its expression as “CagA positive” although we acknowledge that not all individuals infected with cagA gene positive H pylori strains will mount a serologic response to CagA. The prevalence of GIM was highest in CagA-positive H. pylori exposed patients (36.4%), followed by CagA-negative H. pylori exposed patients (21.3%), and lowest in patients without H. pylori exposure (17.8%) (Appendix Figure 6, very low certainty in evidence).66, 69, 72 It is important to note that the high pooled prevalence in the patients without H. pylori infection was limited by inconsistency (studies from the U.S. and Mexico) and imprecision.
Ethnic and Racial Subgroups
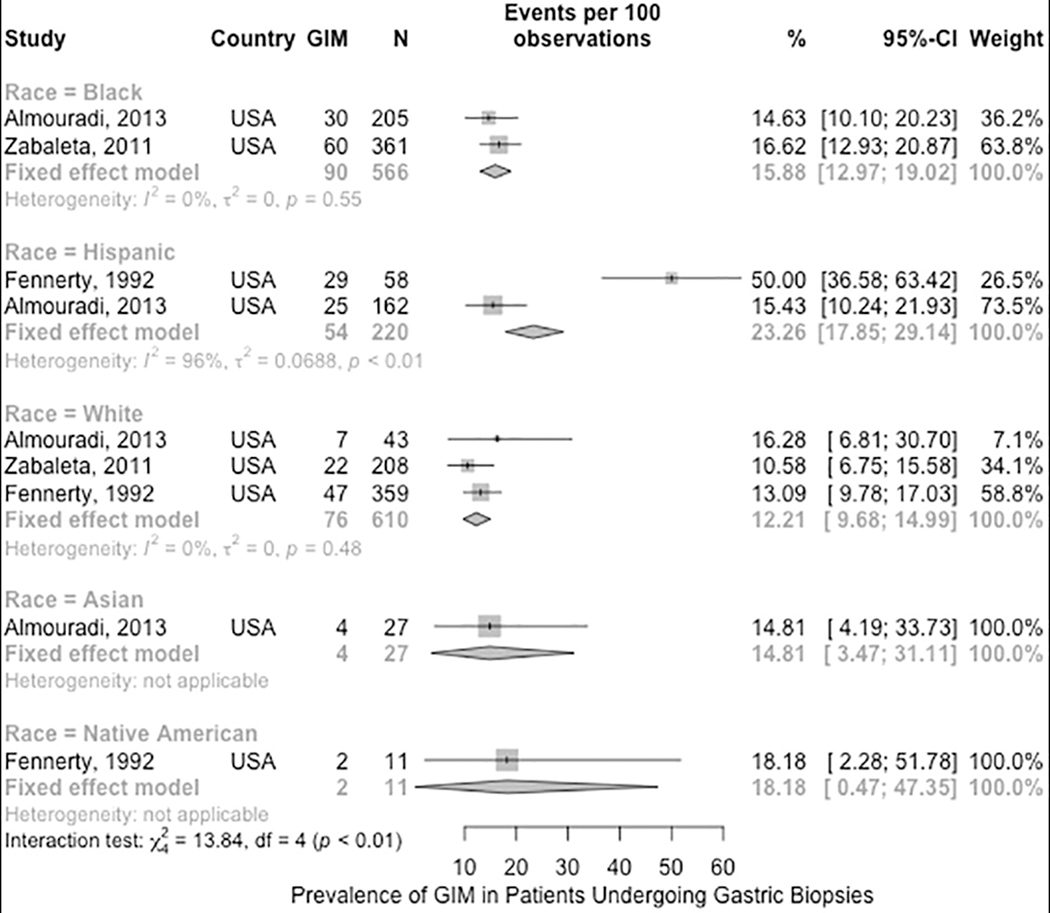
We performed subgroup analysis to compare the prevalence of GIM between different racial and ethnic groups in the US including non-Hispanic Blacks, Hispanics, non-Hispanic Whites, Asians, and Native Americans (3 studies, n = 1,434). Based on the subgroup interaction test, there was a statistically significant difference between the groups, with a higher prevalence of GIM among Hispanics compared to the other groups (p < 0.01, low to very low certainty in evidence). The higher prevalence among Hispanics was driven mostly by the small subgroup of patients (n= 58) from the study by Fennerty et al 64, which reported a GIM prevalence of 50%, compared to the larger subgroup (n= 162) from the study by Almouradi et al 62, which reported a prevalence of 15.4%; the latter point estimate is comparable to the non-Hispanic subgroups. All of the pooled estimates were limited by serious to very serious imprecision, as the number of patients within the subgroups ranged from 11 to 610 (Figure 3).62, 64, 66 A cross-sectional study by Choi et al. used a large national pathology database and showed that patients of Hispanic and certain Asian ethnicities (Korean, Chinese, Vietnamese, and Japanese) have higher GIM prevalence, 12.7% to 39.9%, compared to other races and ethnicities grouped together.73 The study did not report the prevalence of GIM in the other races and ethnicities separately which limited our ability to pool it with the other studies.
Figure 3.
Prevalence of GIM in US patients who underwent gastric biopsies by race/ethnicity.
Pernicious Anemia
One study reported the prevalence of GIM in 27 patients with known pernicious anemia as 88.9% (95%CI: 70.8 – 96.6%).74 Details regarding the methods used to identify and select the patients who had pernicious anemia were not clear in the study, and raise concern for selection bias; this is in addition to the very serious imprecision due to the very small sample size. (Appendix Figure 7, very low certainty in evidence).
First Degree Family History of Gastric Cancer
Five studies (n= 4,791 patients) reported the prevalence of GIM in patients with a first-degree relative with gastric cancer.15, 65, 75–77 The random-effects pooled relative risk of diagnosing GIM in patients with vs. without a family history of gastric cancer was 1.46 (95%CI 0.97 – 2.21). The relative risks from the individual studies were not adjusted for confounding factors and the studies were observational cross-sectional studies (Appendix Figure 8, very low certainty in evidence). Of note, while one of the included studies was limited to H. pylori exposed patients, our results did not change when we performed a sensitivity analysis excluding this study (data not shown).15 A study by Leung et al reported a 30% prevalence of GIM among patients with a first-degree family history of gastric cancer; however, because this was a single-arm noncomparative study that was limited to patients with a positive family history, it was not eligible for pooled analysis of risk estimates.77
Smoking and Alcohol Use History
Seven studies (n= 7,971) reported the prevalence of GIM among patients with former or current tobacco use. The random-effects pooled unadjusted relative risk of diagnosing GIM in patients with history of current vs. former smoking or never smoking was 1.57 (95% CI: 1.24 – 1.98).15, 65, 77–81 Six of the seven studies (n=6,775) also reported the prevalence of GIM based on the history of alcohol use. The random-effects pooled unadjusted relative risk of having GIM in current vs former or never alcohol users was 1.29 (95%CI: 1.12 – 1.50).15, 65, 77–80
All studies were observational cross-sectional studies conducted mostly in Asian countries and the relative risks were not adjusted for confounding factors. Additionally, the studies inconsistently differentiated between current, prior and never smokers or alcohol users (Appendix Figures 9 and 10, very low certainty in evidence).
In both analyses, the results were unchanged when we excluded studies limited to H. pylori-exposed patients15 or to patients with first-degree family history of gastric cancer.77
Dietary Habits
Three studies (n=6,136) reported the prevalence of GIM in patients according to dietary habits. The unadjusted relative risks of having GIM in patients consuming high vs low salt diets, low versus high fruit/vegetable intakes, and high vs low dairy product intakes were 1.18 (95%CI: 0.99 – 1.40), 1.42 (95%CI: 1.13 – 1.79), and 1.72 (95%CI: 1.43 – 2.05). None of the studies were conducted in the U.S. nor were any of the populations comparable to the U.S. population. The relative risks were not adjusted for confounders and the studies were limited by moderate to high risk of bias (Appendix Figure 11, very low certainty in evidence).15, 79, 81 The results were unchanged when we excluded the study which was limited to H. pylori-exposed patients15
Pepsinogen (PG) Level
Our comprehensive search did not identify any studies that reported data specifically related to GIM prevalence in patients based on pepsinogen levels. Because the vast majority of the studies which used pepsinogen as a biomarker were focused on gastric atrophy28, distinction of GIM in the absence of gastric atrophy was not possible. Chang et al showed that patients with GIM had low PG I levels and PG I/PG II ratio compared to patients without GIM.82 In this study, a PG I/PG II ratio < 7.5 was also associated with GIM in a multivariable regression that adjusted for H. pylori seropositivity, age and the presence of duodenal ulcers. Wang et al showed an inverse correlation between OLGIM stages and PG I/PG II ratio, regardless of the H. pylori status.83
The Prevalence of GIM Subcategories
GIM Extent
There is some heterogeneity in the literature regarding the definition of extensive versus limited GIM based on topographic extent. Consistent with the first part of this technical review, we defined extensive GIM as GIM involving at least the corpus (i.e. corpus and antrum/incisura, or corpus alone), while limited GIM was defined as GIM involving only the antrum/incisura.40 This distinction necessitates formal histologic assessment of both locations. Thus, only those studies where at least one biopsy was taken from the antrum and corpus separately were eligible for this subgroup analysis. We acknowledge that the yield is expectedly higher with multiple biopsies from antrum and corpus, but the limited number of studies precluded our exclusion of studies based on the number of biopsies taken. In the first part of our review, we found that the relative risk of incident gastric cancer in patients with extensive GIM vs limited GIM was 2.07 (95% CI 0.97 to 4.42; 2 studies with n = 222).40
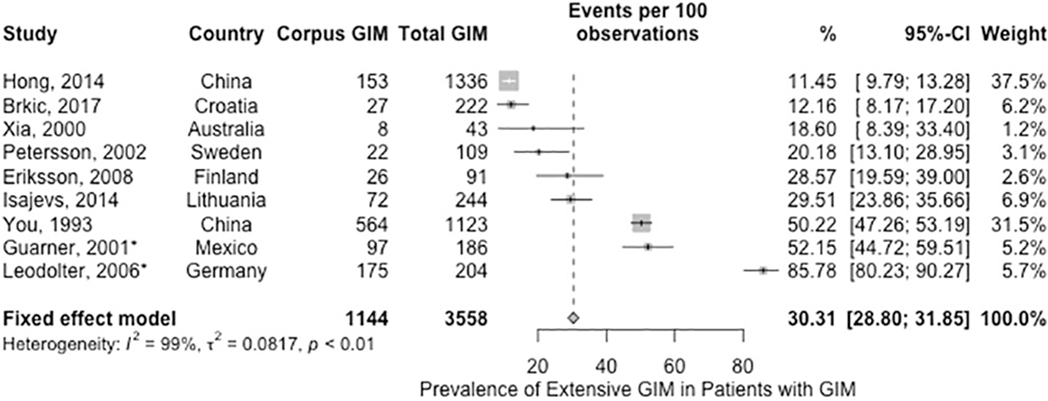
Based on 9 studies (n= 3,558) which included data on GIM prevalence, among patients with GIM who had biopsies obtained from both antrum and corpus the fixed-effects estimated pooled prevalence of extensive GIM was 30.3% (95%CI: 28.8% to 31.8%). None of the studies were from the U.S. and the point estimates of the individual studies ranged between 11–85%; the observed inconsistency was not completely explained by the prevalence of H. pylori, geographical region or risk of bias (Figure 4, very low certainty in evidence).69, 71, 74, 84–90 When we performed a sensivity analysis excluding two studies limited to H. pylori-exposed patients 69, 71 (n= 3168), the estimated pooled prevalence of extensive GIM was slightly lower at 25.6% (95% CI: 24.1 – 27.2). We identified one study by Lahner et al, which was published after our updated search date (September 2018) that otherwise met inclusion criteria.91 The study included 201 patients with GIM and reported 25.9% prevalence of extensive GIM, which is consistent with our estimated pooled prevalence.
Figure 4.
Prevalence of extensive GIM among patients found to have GIM on gastric biopsies (*Guarner 2001 and Leodolter 2006 were studies of H. pylori-infected patients).
Histopathological Subtype
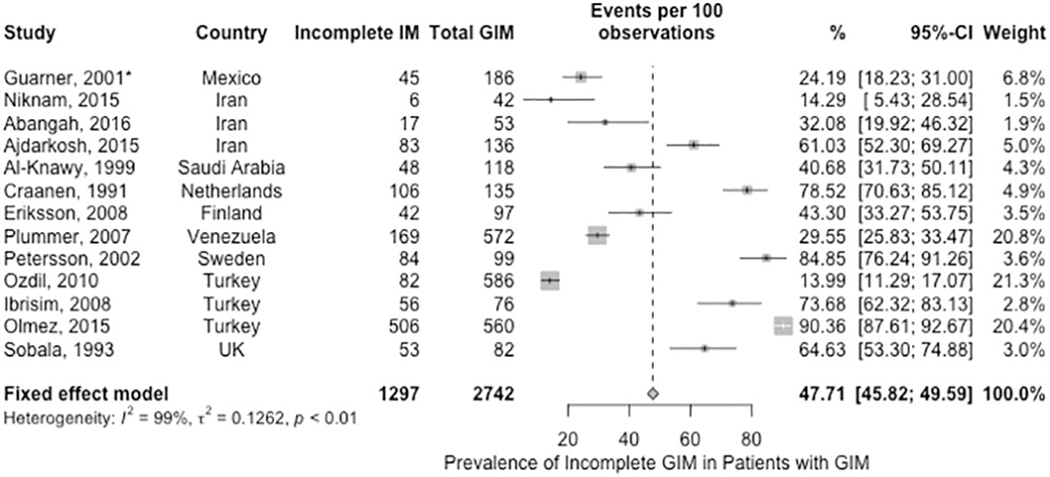
As reported in the first part of this technical review, incomplete GIM is associated with a higher relative risk of incident gastric cancer compared to complete GIM (RR 3.33, 95% CI 1.96 – 5.64; 7 studies with n= 2031).40 Based on 13 studies (n= 2,742), the fixed-effects estimated pooled prevalence of incomplete GIM among patients with GIM was 47.7% (95%CI: 45.8% to 49.6%). The point estimates of the individual studies ranged from 14–90%; the observed inconsistency was not completely explained by H. pylori prevalence, geographical region, or risk of bias (Figure 5, very low certainty of evidence) 72, 74, 78, 89, 92–96 The pooled prevalence did not change significantly when we excluded the one study that included H. pylori exposed patients only.69
Figure 5.
Prevalence of incomplete GIM among pateints found to have GIM on gastric biopsies (*Guarner 2001 was a study of of H. pylori-infected patients).
OLGIM Stages
Although our search criteria did not identify any study that showed an association of OLGIM stages with increased risk of neoplasia, we conducted an exploratory analysis to assess the prevalence of the different OLGIM stages among patients with GIM. Based on three non-US studies (n= 620), the prevalence of the different OLGIM stages decreased as the the stage became more advanced. The fixed-effects estimated pooled prevalence of OLGIM stage I, II, III and IV were 55.5% (95% CI: 51.6 – 59.4%), 26.1% (95%CI: 22.7 – 29.6%, I2 = 20%), 10.8% (95%CI: 8.5 – 13.4%), and 6.4% (95%CI: 4.6 – 8.5%), respectively (Appendix Figure 12).83, 85, 97
Sensitivity Analyses
We repeated all the analyses of prevalence using the generalized linear mixed model and inverse variance random-effects model to assess the robustness of our findings and their sensitivity to the statistical method we used. The pooled estimates did not differ whether we used the inverse-variance method or the generalized linear mixed model.
Publication Bias
We could not assess for publication bias in any of the meta-analyses due to the small number of studies and/or the substantial statistical heterogeneity.
Summary and Conclusions
Here we synthesized the findings of the first comprehensive review of the prevalence of GIM and associated risk factors for subsequent diagnosis of gastric neoplasia using standard methodology for systematic reviews and meta-analyses in order to inform the AGA Guidelines on Cancer Surveillance in Patients with GIM.
As we report in the first part of this technical review, patients with a diagnosis of GIM have a higher risk of incident gastric neoplasia compared to patients without GIM and, importantly, there are distinct subgroups of patients with GIM who have a 2- to 4.5-fold higher risk of developing incident gastric cancer above that of GIM alone. These groups include those with extensive GIM, incomplete GIM, or a first-degree family history of gastric cancer. We quantified this risk in the first part of the technical review, while here we report their prevalence based on systematic review. The data presented here are relevant across stakeholders including patients, healthcare providers, and policy makers. These evidence profiles are intended not only to guide clinical decision making for GIM such as risk stratification for endoscopic surveillance, but also to direct the research agenda given the breadth of knowledge gaps we have highlighted here and in the first part of the technical review.40
One area in need of immediate comparative studies, particularly in the US, is with respect to best-practice protocols for GIM identification and risk stratification. Prior studies have established that the likelihood of detecting GIM on gastric biopsies correlates with the number of gastric biopsies obtained and that sampling error undermines the optimal detection of GIM.85, 95, 98–102 Professional societies have attempted to standardize the number and methods used to obtain random gastric biopsies; however, there is remarkable variability in practice.38, 103, 104
This technical review has multiple strengths. It is based on a systematic comprehensive search of the available literature with adherence to all high quality measures of the standard systematic review methodology. We involved both clinical and methodologic expertise and utilized the GRADE framework to assess the quality of the available evidence. We were able to identify gaps in the literature to direct future work and efforts. Although such gaps limited our ability to directly inform our PICO questions, we identified indirect evidence to assist the guidelines panel in making evidence-based decisions regarding the PICO questions.
Our work is not without inherent limitations, however. Most of our findings are based on observational studies and should be interpreted with caution. As noted previously, despite the large number of the studies that we identified, the pooled prevalence estimates were influenced by large studies from pathological databases; Hence, it is arguable that our pooled prevalence estimates could have underestimated or overestimated the true prevalence of GIM. They could have underestimated the prevalence due to the variability in practice when it comes to obtaining gastric biopsies in terms of the location and the number of biospies obtained leading to missed GIM cases. A systematic review of optimal endoscopic and histologic protocols for the identification of GIM was outside of the scope of this review as determined by the AGA. On the other hand, the pathology databases receive samples from clinicians performing endoscopic procedures for certain indications. Individuals who have risk factors for GIM, such as H. pylori infection and smoking, tend to have symptoms, such as dyspepsia, that necessitate endoscopic evaluation. Enrichment of the study population with symptomatic individuals who more often have risk factors for GIM and who are also more likely to have biopsies obtained for diagnostic evaluation potentially overestimates GIM prevalence in the general population. Indeed, in this review we provided estimates of the prevalence of the risk factors that could be associated with higher risk of gastric neoplasia among patients with GIM, such as GIM histologic subtypes. Analyzing clinical predictors of having these risk factors, however, was outside our scope.
As noted in our statistical analysis section, we elected to use the fixed-effects model as we presumed that differences between the studies were related to sampling error rather than differences between the included patients. While we acknowledge that this approach has limitations in the setting of high heterogeneity, we accepted this tradeoff as the fixed-effects model ensured studies with larger sample sizes, which are less affected by sampling error, were allocated higher weights compared to smaller studies when pooled. To ensure statistical rigor was maintained, we additionally used the generalized linear mixed model and random-effects model to assess the sensitivity of our pooled estimates to the change in the statistical method; importantly, our overall conclusions were preserved.56
Based on the U.S. Census Bureau data from July 2017, there are around 252 million adults in the US.105 Based on the pooled prevalence estimate form our technical reviews, we can estimate that there are approximately 12.1 million adults with GIM in the US. We also estimated the incidence of gastric cancer in patients with GIM to be 82 cases per 100,000 person-years, which equates to approximately 10,000 new cases of gastric cancer annually in association with GIM. Based on publically available population-based data in the United States2, there are an estimated 26,240 new cases annually with an overall incidence rate estimated to be 7.2 cases per 100,000 persons, although the incidence ranges from 4.7 to 13.7 depending on gender and racial/ethnic subgroup. Hence, potentially up to 40% of the newly diagnosed (noncardia) gastric cancers in the US may be in the context of associated GIM. Notably, because gastric cancer screening and GIM surveillance do not occur routinely in the US, the majority of incident cases are diagnosed in an advanced stage when treatment options are noncurative.
In conclusion, we have summarized the available evidence and provided estimates of the prevalence of GIM, the incidence rate of gastric cancer in patients with GIM overall and also based on proposed risk factors including clinicodemographic and individual lifestyle factors, extent of GIM, and histologic subclassification of GIM. Our comprehensive evidence profiles are an important comprehensive resource for clinicians, researchers, and patients to assist informed clinical decision making regarding endoscopic surveillance for GIM, as well as to guide the research agenda moving forward. Indeed, we have identified gaps in the literature for future research and most importantly the need to standardize endoscopic and histologic assessment practices and to better define risk factors for developing gastric cancer that could be used to stratify patients with GIM and guide the need for and frequency of endoscopic surveillance.
Supplementary Material
Appendix Doxument 1. Detailed search strategy.
Appendix Document 2. Risk of bias table and summary (Petersson*, 2002 is limited to the subgroup of patients with pernicious anemia).
Appendix Figure 1. Prevalence of GIM in patients who underwent gastric biopsies worldwide by geographical regions.
Appendix Figure 2. Prevalence of GIM in patients who underwent gastric biopsies by low prevalence of H. pylori (≤15%).
Appendix Figure 3. Prevalence of GIM in patients who underwent gastric biopsies by high prevalence of H. pylori (>75%).
Appendix Figure 4. Meta-regression of proportion of patients with GIM on proportion of patients with H. pylori exposure.
Appendix Figure 5. Prevalence of GIM among H. pylori-exposed patients who underwent gastric biopsies by geographical regions.
Appendix Figure 6. Prevalence of GIM among patients who underwent gastric biopsies by H. pylori and cagA status.
Appendix Figure 7. Prevalence of GIM among patients with pernicious anemia who underwent gastric biopsies.
Appendix Figure 8. Relative risk of finding GIM on gastric biopsies in patients with versus without first-degree family history of gastric cancer (*Sadjadi 2014 was a study of H. pylori-infected patients).
Appendix Figure 9. Relative risk of finding GIM on gastric biopsies in patients who smoke tobacco versus nonsmokers and previous smokers (*Sadjadi 2014 was a study of H. pylori-infected patients; **Leung 2005 was a study of patients with first-degree family history of gastric cancer).
Appendix Figure 10. Relative risk of finding GIM on gastric biopsies in patients who drink alcohol versus patients who do not drink alcohol (*Sadjadi 2014 was a study of H. pylori-infected patients; **Leung 2005 was a study of patients with first-degree family history of gastric cancer).
Appendix Figure 11. Relative risk of finding GIM on gastric biopsies in patients with high versus low salt intake, low versus high frutis and vegetable intake, and high versus low dairy products intake (*Sadjadi 2014 was a study of H. pylori-infected patients).
Appendix Figure 12. Prevalence of OLGIM stages among patients found to have GIM on gastric biopsies.
Acronyms
- NCGA
non-cardia gastric adenocarcinoma
- GA
gastric adenocarcinoma
- GIM
gastric intestinal metaplasia
- H. pylori
Helicobacter pylori
- US
United States
- AGA
American Gastroenterological Association
- GRADE
Grading of Recommendation, Assessment, Development, and Evaluation
- OLGIM
the operative link for gastric intestinal metaplasia
- OLGA
the operative link for gastric atrophy
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- MOOSE
Meta-analysis Of Observational Studies in Epidemiology
- CI
confidence interval
- cagA
cytotoxin-associated gene A
- vacA
vacuolating cytotoxin A
- PG
pepsinogen
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2.Cronin KA, Lake AJ, Scott S, et al. Annual Report to the Nation on the Status of Cancer, part I: National cancer statistics. Cancer 2018;124:2785–2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson WF, Rabkin CS, Turner N, et al. The Changing Face of Noncardia Gastric Cancer Incidence Among US Non-Hispanic Whites. J Natl Cancer Inst 2018;110:608–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lauren P The Two Histological Main Types of Gastric Carcinoma: Diffuse and So-Called Intestinal-Type Carcinoma. An Attempt at a Histo-Clinical Classification. Acta Pathol Microbiol Scand 1965;64:31–49. [DOI] [PubMed] [Google Scholar]
- 5.Dixon MF, Genta RM, Yardley JH, et al. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol 1996;20:1161–81. [DOI] [PubMed] [Google Scholar]
- 6.Correa P, Piazuelo MB. The gastric precancerous cascade. J Dig Dis 2012;13:2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Correa P, Piazuelo MB, Wilson KT. Pathology of gastric intestinal metaplasia: clinical implications. Am J Gastroenterol 2010;105:493–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Correa P, Houghton J. Carcinogenesis of Helicobacter pylori. Gastroenterology 2007;133:659–72. [DOI] [PubMed] [Google Scholar]
- 9.Wroblewski LE, Peek RM Jr., Wilson KT. Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev 2010;23:713–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song H, Ekheden IG, Zheng Z, et al. Incidence of gastric cancer among patients with gastric precancerous lesions: observational cohort study in a low risk Western population. BMJ 2015;351:h3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee TY, Wang RC, Lee YC, et al. The Incidence of Gastric Adenocarcinoma Among Patients With Gastric Intestinal Metaplasia: A Long-term Cohort Study. J Clin Gastroenterol 2016;50:532–7. [DOI] [PubMed] [Google Scholar]
- 12.Reddy KM, Chang JI, Shi JM, et al. Risk of Gastric Cancer Among Patients With Intestinal Metaplasia of the Stomach in a US Integrated Health Care System. Clin Gastroenterol Hepatol 2016;14:1420–5. [DOI] [PubMed] [Google Scholar]
- 13.Li D, Bautista MC, Jiang SF, et al. Risks and Predictors of Gastric Adenocarcinoma in Patients with Gastric Intestinal Metaplasia and Dysplasia: A Population-Based Study. Am J Gastroenterol 2016;111:1104–13. [DOI] [PubMed] [Google Scholar]
- 14.Kim N, Park RY, Cho SI, et al. Helicobacter pylori infection and development of gastric cancer in Korea: long-term follow-up. J Clin Gastroenterol 2008;42:448–54. [DOI] [PubMed] [Google Scholar]
- 15.Sadjadi A, Derakhshan MH, Yazdanbod A, et al. Neglected role of hookah and opium in gastric carcinogenesis: a cohort study on risk factors and attributable fractions. Int J Cancer 2014;134:181–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dinis-Ribeiro M, Areia M, de Vries AC, et al. Management of precancerous conditions and lesions in the stomach (MAPS): guideline from the European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter Study Group (EHSG), European Society of Pathology (ESP), and the Sociedade Portuguesa de Endoscopia Digestiva (SPED). Endoscopy 2012;44:74–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Committee ASoP, Evans JA, Chandrasekhara V, et al. The role of endoscopy in the management of premalignant and malignant conditions of the stomach. Gastrointest Endosc 2015;82:1–8. [DOI] [PubMed] [Google Scholar]
- 18.Shichijo S, Hirata Y, Niikura R, et al. Histologic intestinal metaplasia and endoscopic atrophy are predictors of gastric cancer development after Helicobacter pylori eradication. Gastrointest Endosc 2016;84:618–24. [DOI] [PubMed] [Google Scholar]
- 19.den Hollander WJ, Holster IL, den Hoed CM, et al. Surveillance of premalignant gastric lesions: a multicentre prospective cohort study from low incidence regions. Gut 2018. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez CA, Pardo ML, Liso JM, et al. Gastric cancer occurrence in preneoplastic lesions: a long-term follow-up in a high-risk area in Spain. Int J Cancer 2010;127:2654–60. [DOI] [PubMed] [Google Scholar]
- 21.Filipe MI, Munoz N, Matko I, et al. Intestinal metaplasia types and the risk of gastric cancer: a cohort study in Slovenia. Int J Cancer 1994;57:324–9. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez CA, Sanz-Anquela JM, Companioni O, et al. Incomplete type of intestinal metaplasia has the highest risk to progress to gastric cancer: results of the Spanish follow-up multicenter study. J Gastroenterol Hepatol 2016;31:953–8. [DOI] [PubMed] [Google Scholar]
- 23.den Hoed CM, Holster IL, Capelle LG, et al. Follow-up of premalignant lesions in patients at risk for progression to gastric cancer. Endoscopy 2013;45:249–56. [DOI] [PubMed] [Google Scholar]
- 24.Yaghoobi M, McNabb-Baltar J, Bijarchi R, et al. What is the quantitative risk of gastric cancer in the first-degree relatives of patients? A meta-analysis. World J Gastroenterol 2017;23:2435–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang JQ, Zheng GF, Sumanac K, et al. Meta-analysis of the relationship between cagA seropositivity and gastric cancer. Gastroenterology 2003;125:1636–44. [DOI] [PubMed] [Google Scholar]
- 26.Dinis-Ribeiro M, Yamaki G, Miki K, et al. Meta-analysis on the validity of pepsinogen test for gastric carcinoma, dysplasia or chronic atrophic gastritis screening. J Med Screen 2004;11:141–7. [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez CA, Figueiredo C, Lic CB, et al. Helicobacter pylori cagA and vacA genotypes as predictors of progression of gastric preneoplastic lesions: a long-term follow-up in a high-risk area in Spain. Am J Gastroenterol 2011;106:867–74. [DOI] [PubMed] [Google Scholar]
- 28.Huang YK, Yu JC, Kang WM, et al. Significance of Serum Pepsinogens as a Biomarker for Gastric Cancer and Atrophic Gastritis Screening: A Systematic Review and Meta-Analysis. PLoS One 2015;10:e0142080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han X, Xiao L, Yu Y, et al. Alcohol consumption and gastric cancer risk: a meta-analysis of prospective cohort studies. Oncotarget 2017;8:83237–83245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Praud D, Rota M, Pelucchi C, et al. Cigarette smoking and gastric cancer in the Stomach Cancer Pooling (StoP) Project. Eur J Cancer Prev 2018;27:124–133. [DOI] [PubMed] [Google Scholar]
- 31.D’Elia L, Rossi G, Ippolito R, et al. Habitual salt intake and risk of gastric cancer: a meta-analysis of prospective studies. Clin Nutr 2012;31:489–98. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Z, Xu G, Ma M, et al. Dietary fiber intake reduces risk for gastric cancer: a meta-analysis. Gastroenterology 2013;145:113–120 e3. [DOI] [PubMed] [Google Scholar]
- 33.Fang X, Wei J, He X, et al. Landscape of dietary factors associated with risk of gastric cancer: A systematic review and dose-response meta-analysis of prospective cohort studies. Eur J Cancer 2015;51:2820–32. [DOI] [PubMed] [Google Scholar]
- 34.Jiang JX, Liu Q, Zhao B, et al. Risk factors for intestinal metaplasia in a southeastern Chinese population: an analysis of 28,745 cases. J Cancer Res Clin Oncol 2017;143:409–418. [DOI] [PubMed] [Google Scholar]
- 35.Kang JH, Lim YJ, Kang JH, et al. Prevalence of Precancerous Conditions and Gastric Cancer Based upon the National Cancer Screening Program in Korea for 7 Years, Single Center Experience. Gastroenterol Res Pract 2015;2015:571965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sonnenberg A, Genta RM. Changes in the Gastric Mucosa With Aging. Clin Gastroenterol Hepatol 2015;13:2276–81. [DOI] [PubMed] [Google Scholar]
- 37.O’Connor A, McNamara D, O’Morain CA. Surveillance of gastric intestinal metaplasia for the prevention of gastric cancer. Cochrane Database Syst Rev 2013:CD009322. [DOI] [PubMed] [Google Scholar]
- 38.Vance RB, Jr., Kubiliun N, Dunbar KB. How Do We Manage Gastric Intestinal Metaplasia? A Survey of Clinical Practice Trends for Gastrointestinal Endoscopists in the United States. Dig Dis Sci 2016;61:1870–8. [DOI] [PubMed] [Google Scholar]
- 39.Gupta S, Li D, El Serag H, et al. American Gastroenterological Institute Guidelines for Management of Gastric Intestinal Metaplasia. Gastroenterology 2019;In Press. [Google Scholar]
- 40.Gawron A, Shah S, Altayar O, et al. AGA Institute Technical Review on Gastric Intestinal Metaplasia – Part 1. Gastroenterology 2019;In Press. [Google Scholar]
- 41.Guyatt GH, Oxman AD, Schunemann HJ, et al. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol 2011;64:380–2. [DOI] [PubMed] [Google Scholar]
- 42.United Nations. Statistical Division. Standard country or area codes for statistical use (M49). New York: United Nations, 1999. [Google Scholar]
- 43.Capelle LG, de Vries AC, Haringsma J, et al. The staging of gastritis with the OLGA system by using intestinal metaplasia as an accurate alternative for atrophic gastritis. Gastrointest Endosc 2010;71:1150–8. [DOI] [PubMed] [Google Scholar]
- 44.Yue H, Shan L, Bin L. The significance of OLGA and OLGIM staging systems in the risk assessment of gastric cancer: a systematic review and meta-analysis. Gastric Cancer 2018;21:579–587. [DOI] [PubMed] [Google Scholar]
- 45.Agreus L, Kuipers EJ, Kupcinskas L, et al. Rationale in diagnosis and screening of atrophic gastritis with stomach-specific plasma biomarkers. Scand J Gastroenterol 2012;47:136–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006–12. [DOI] [PubMed] [Google Scholar]
- 47.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- 48.Cancer Genome Atlas Research N, Analysis Working Group: Asan U, Agency BCC, et al. Integrated genomic characterization of oesophageal carcinoma. Nature 2017;541:169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- 51.Freeman MF, Tukey JW. Transformations Related to the Angular and the Square Root. Ann. Math. Statist. 1950;21:607–611. [Google Scholar]
- 52.Barendregt JJ, Doi SA, Lee YY, et al. Meta-analysis of prevalence. J Epidemiol Community Health 2013;67:974–8. [DOI] [PubMed] [Google Scholar]
- 53.Deeks JJ, Altman DG, Bradburn MJ. Statistical Methods for Examining Heterogeneity and Combining Results from Several Studies in Meta-Analysis. Systematic Reviews in Health Care 2008. [Google Scholar]
- 54.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ioannidis JP, Trikalinos TA. The appropriateness of asymmetry tests for publication bias in meta-analyses: a large survey. CMAJ 2007;176:1091–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stijnen T, Hamza TH, Ozdemir P. Random effects meta-analysis of event outcome in the framework of the generalized linear mixed model with applications in sparse data. Stat Med 2010;29:3046–67. [DOI] [PubMed] [Google Scholar]
- 57.R Core Team. R: A Language and Environment for Statistical Computing: R Foundation for Statistical Computing, 2018. [Google Scholar]
- 58.Schwarzer G meta: {A}n {R} package for meta-analysis. R News 2007;7:40–45. [Google Scholar]
- 59.Munn Z, Moola S, Lisy K, et al. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc 2015;13:147–53. [DOI] [PubMed] [Google Scholar]
- 60.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Volume 2018 Ottawa, Canda: Department of Epidemiology and Commuunity Medicine, University of Ottawa, 2011. [Google Scholar]
- 62.Almouradi T, Hiatt T, Attar B. Gastric Intestinal Metaplasia in an Underserved Population in the USA: Prevalence, Epidemiologic and Clinical Features. Gastroenterol Res Pract 2013;2013:856256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.El-Serag HB, Sonnenberg A, Jamal MM, et al. Characteristics of intestinal metaplasia in the gastric cardia. Am J Gastroenterol 1999;94:622–7. [DOI] [PubMed] [Google Scholar]
- 64.Fennerty MB, Emerson JC, Sampliner RE, et al. Gastric intestinal metaplasia in ethnic groups in the southwestern United States. Cancer Epidemiol Biomarkers Prev 1992;1:293–6. [PubMed] [Google Scholar]
- 65.Gomez JM, Frye JW, Patrie JT, et al. The Presence of Gastric Intestinal Metaplasia in Patients Undergoing EGD with Biopsy is Associated with a Family History of Gastric Cancer in the United States. Journal of Gastroenterology and Hepatology Research 2013;2:726–9. [Google Scholar]
- 66.Zabaleta J, Camargo MC, Ritchie MD, et al. Association of haplotypes of inflammation-related genes with gastric preneoplastic lesions in African Americans and Caucasians. Int J Cancer 2011;128:668–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen XY, van Der Hulst RW, Shi Y, et al. Comparison of precancerous conditions: atrophy and intestinal metaplasia in Helicobacter pylori gastritis among Chinese and Dutch patients. J Clin Pathol 2001;54:367–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Eidt S, Stolte M. Antral intestinal metaplasia in Helicobacter pylori gastritis. Digestion 1994;55:13–8. [DOI] [PubMed] [Google Scholar]
- 69.Guarner J, Herrera-Goepfert R, Mohar A, et al. Gastric atrophy and extent of intestinal metaplasia in a cohort of Helicobacter pylori-infected patients. Hum Pathol 2001;32:31–5. [DOI] [PubMed] [Google Scholar]
- 70.Lee YC, Chen TH, Chiu HM, et al. The benefit of mass eradication of Helicobacter pylori infection: a community-based study of gastric cancer prevention. Gut 2013;62:676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Leodolter A, Ebert MP, Peitz U, et al. Prevalence of H pylori associated “high risk gastritis” for development of gastric cancer in patients with normal endoscopic findings. World J Gastroenterol 2006;12:5509–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Plummer M, van Doorn LJ, Franceschi S, et al. Helicobacter pylori cytotoxin-associated genotype and gastric precancerous lesions. J Natl Cancer Inst 2007;99:1328–34. [DOI] [PubMed] [Google Scholar]
- 73.Choi CE, Sonnenberg A, Turner K, et al. High Prevalence of Gastric Preneoplastic Lesions in East Asians and Hispanics in the USA. Dig Dis Sci 2015;60:2070–6. [DOI] [PubMed] [Google Scholar]
- 74.Petersson F, Borch K, Franzen LE. Prevalence of subtypes of intestinal metaplasia in the general population and in patients with autoimmune chronic atrophic gastritis. Scand J Gastroenterol 2002;37:262–6. [DOI] [PubMed] [Google Scholar]
- 75.Kato I, Vivas J, Plummer M, et al. Environmental factors in Helicobacter pylori-related gastric precancerous lesions in Venezuela. Cancer Epidemiol Biomarkers Prev 2004;13:468–76. [PubMed] [Google Scholar]
- 76.Mansour-Ghanaei F, Joukar F, Baghaei SM, et al. Gastric precancerous lesions in first degree relatives of patients with known gastric cancer: a cross-sectional prospective study in Guilan Province, north of Iran. Asian Pac J Cancer Prev 2012;13:1779–82. [DOI] [PubMed] [Google Scholar]
- 77.Leung WK, Ng EK, Chan WY, et al. Risk factors associated with the development of intestinal metaplasia in first-degree relatives of gastric cancer patients. Cancer Epidemiol Biomarkers Prev 2005;14:2982–6. [DOI] [PubMed] [Google Scholar]
- 78.Ajdarkosh H, Sohrabi M, Moradniani M, et al. Prevalence of gastric precancerous lesions among chronic dyspeptic patients and related common risk factors. Eur J Cancer Prev 2015;24:400–6. [DOI] [PubMed] [Google Scholar]
- 79.Joo YE, Park HK, Myung DS, et al. Prevalence and risk factors of atrophic gastritis and intestinal metaplasia: a nationwide multicenter prospective study in Korea. Gut Liver 2013;7:303–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Song JH, Kim YS, Heo NJ, et al. High Salt Intake Is Associated with Atrophic Gastritis with Intestinal Metaplasia. Cancer Epidemiol Biomarkers Prev 2017;26:1133–1138. [DOI] [PubMed] [Google Scholar]
- 81.Jedrychowski W, Popiela T, Drews M, et al. Effect of Helicobacter pylori infection, smoking and dietary habits on the occurrence of antrum intestinal metaplasia. Clinico-epidemiological study in Poland. Pol J Pathol 1999;50:289–95. [PubMed] [Google Scholar]
- 82.Chang CH, Wu MS, Chang YT, et al. Risk factors for intestinal metaplasia in adult residents of Matzu: a cross-sectional study. J Formos Med Assoc 2002;101:835–40. [PubMed] [Google Scholar]
- 83.Wang X, Lu B, Meng L, et al. The correlation between histological gastritis staging- ‘OLGA/OLGIM’ and serum pepsinogen test in assessment of gastric atrophy/intestinal metaplasia in China. Scand J Gastroenterol 2017;52:822–827. [DOI] [PubMed] [Google Scholar]
- 84.Hong JB, Zuo W, Wang AJ, et al. Gastric ulcer patients are more susceptible to developing gastric cancer compared with concomitant gastric and duodenal ulcer patients. Oncol Lett 2014;8:2790–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Isajevs S, Liepniece-Karele I, Janciauskas D, et al. The effect of incisura angularis biopsy sampling on the assessment of gastritis stage. Eur J Gastroenterol Hepatol 2014;26:510–3. [DOI] [PubMed] [Google Scholar]
- 86.Sobala GM, O’Connor HJ, Dewar EP, et al. Bile reflux and intestinal metaplasia in gastric mucosa. J Clin Pathol 1993;46:235–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brkić N, Terzić V, Švagelj M, et al. The prevalence and characteristics of Helicobacter pylori-associated gastritis in dyspeptic patients in Eastern Croatia, determined by immunohistochemistry. Periodicum Biologorum 2017;119:75–80. [Google Scholar]
- 88.Xia HH, Kalantar JS, Talley NJ, et al. Antral-type mucosa in the gastric incisura, body, and fundus (antralization): a link between Helicobacter pylori infection and intestinal metaplasia? Am J Gastroenterol 2000;95:114–21. [DOI] [PubMed] [Google Scholar]
- 89.Eriksson NK, Karkkainen PA, Farkkila MA, et al. Prevalence and distribution of gastric intestinal metaplasia and its subtypes. Dig Liver Dis 2008;40:355–60. [DOI] [PubMed] [Google Scholar]
- 90.You WC, Blot WJ, Li JY, et al. Precancerous gastric lesions in a population at high risk of stomach cancer. Cancer Res 1993;53:1317–21. [PubMed] [Google Scholar]
- 91.Lahner E, Carabotti M, Esposito G, et al. Occurrence and predictors of metaplastic atrophic gastritis in a nation-wide consecutive endoscopic population presenting with upper gastrointestinal symptoms. Eur J Gastroenterol Hepatol 2018. [DOI] [PubMed] [Google Scholar]
- 92.Abangah G, Rahmani A, Hafezi-Ahmadi MR, et al. Precancerous histopathologic lesions of upper gastrointestinal tract among dyspeptic patients upon endoscopic evaluations. J Gastrointest Cancer 2016;47:1–7. [DOI] [PubMed] [Google Scholar]
- 93.Al-Knawy B, Morad N, Jamal A, et al. Helicobacter pylori and intestinal metaplasia with its subtypes in the gastric antrum in a Saudi population. Scand J Gastroenterol 1999;34:562–5. [DOI] [PubMed] [Google Scholar]
- 94.Craanen ME, Blok P, Dekker W, et al. Prevalence of subtypes of intestinal metaplasia in gastric antral mucosa. Dig Dis Sci 1991;36:1529–36. [DOI] [PubMed] [Google Scholar]
- 95.Guarner J, Herrera-Goepfert R, Mohar A, et al. Diagnostic yield of gastric biopsy specimens when screening for preneoplastic lesions. Hum Pathol 2003;34:28–31. [DOI] [PubMed] [Google Scholar]
- 96.Niknam R, Manafi A, Maghbool M, et al. Is endoscopic nodular gastritis associated with premalignant lesions? Neth J Med 2015;73:236–41. [PubMed] [Google Scholar]
- 97.Nam JH, Choi IJ, Kook MC, et al. OLGA and OLGIM stage distribution according to age and Helicobacter pylori status in the Korean population. Helicobacter 2014;19:81–9. [DOI] [PubMed] [Google Scholar]
- 98.Varbanova M, Wex T, Jechorek D, et al. Impact of the angulus biopsy for the detection of gastric preneoplastic conditions and gastric cancer risk assessment. J Clin Pathol 2016;69:19–25. [DOI] [PubMed] [Google Scholar]
- 99.de Vries AC, Haringsma J, de Vries RA, et al. Biopsy strategies for endoscopic surveillance of pre-malignant gastric lesions. Helicobacter 2010;15:259–64. [DOI] [PubMed] [Google Scholar]
- 100.Stolte M, Muller H, Talley NJ, et al. In patients with Helicobacter pylori gastritis and functional dyspepsia, a biopsy from the incisura angularis provides useful diagnostic information. Pathol Res Pract 2006;202:405–13. [DOI] [PubMed] [Google Scholar]
- 101.Eriksson NK, Farkkila MA, Voutilainen ME, et al. The clinical value of taking routine biopsies from the incisura angularis during gastroscopy. Endoscopy 2005;37:532–6. [DOI] [PubMed] [Google Scholar]
- 102.El-Zimaity HM, Graham DY. Evaluation of gastric mucosal biopsy site and number for identification of Helicobacter pylori or intestinal metaplasia: role of the Sydney System. Hum Pathol 1999;30:72–7. [DOI] [PubMed] [Google Scholar]
- 103.Bisschops R, Areia M, Coron E, et al. Performance measures for upper gastrointestinal endoscopy: A European Society of Gastrointestinal Endoscopy quality improvement initiative. United European Gastroenterol J 2016;4:629–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Committee ASoP, Sharaf RN, Shergill AK, et al. Endoscopic mucosal tissue sampling. Gastrointest Endosc 2013;78:216–24. [DOI] [PubMed] [Google Scholar]
- 105.U.S. Census Bureau Population Division. Estimates of the Total Resident Population and Resident Population Age 18 Years and Older for the United States, States, and Puerto Rico: July 1, 2017. (SCPRC-EST2017–18+POP-RES).
- 106.Aydın H, Kartal A, Oduncu M. Co-Occurrence of Helicobacter Pylori and Intestinal Metaplasia in Patients with Dyspepsia Journal of Clinical and Analytical Medicine 2017;8:223–5. [Google Scholar]
- 107.Correa P, Haenszel W, Cuello C, et al. Gastric precancerous process in a high risk population: cohort follow-up. Cancer Res 1990;50:4737–40. [PubMed] [Google Scholar]
- 108.Cu PQ, Huyen NX, Luan TT, et al. Helicobacter Pylori and Precancerous Gastric Lesions. Digestive Endoscopy 2001;12:221–224. [Google Scholar]
- 109.den Hoed CM, van Eijck BC, Capelle LG, et al. The prevalence of premalignant gastric lesions in asymptomatic patients: predicting the future incidence of gastric cancer. Eur J Cancer 2011;47:1211–8. [DOI] [PubMed] [Google Scholar]
- 110.Haroon S, Faridi N, Lodhi FR, et al. Frequency of precancerous lesions in endoscopic gastric biopsies in chronic gastritis. J Coll Physicians Surg Pak 2013;23:247–50. [PubMed] [Google Scholar]
- 111.Haziri A, Juniku-Shkololli A, Gashi Z, et al. Helicobacter pylori infection and precancerous lesions of the stomach. Med Arh 2010;64:248–9. [PubMed] [Google Scholar]
- 112.Hsu PI, Lai KH, Hsu PN, et al. Helicobacter pylori infection and the risk of gastric malignancy. Am J Gastroenterol 2007;102:725–30. [DOI] [PubMed] [Google Scholar]
- 113.Huang C, Chen Q, Jiang J, et al. Gastric metaplasia and Helicobacter pylori infection in hemodialysis patients. Ren Fail 2012;34:420–4. [DOI] [PubMed] [Google Scholar]
- 114.Ibrisim D, Cevikbas U, Akyuz F, et al. Intestinal metaplasia in portal hypertensive gastropathy: a frequent pathology. Eur J Gastroenterol Hepatol 2008;20:874–80. [DOI] [PubMed] [Google Scholar]
- 115.Imai T, Murayama H. Time trend in the prevalence of intestinal metaplasia in Japan. Cancer 1983;52:353–61. [DOI] [PubMed] [Google Scholar]
- 116.Lahner E, Zullo A, Hassan C, et al. Detection of gastric precancerous conditions in daily clinical practice: a nationwide survey. Helicobacter 2014;19:417–24. [DOI] [PubMed] [Google Scholar]
- 117.Micu G, Staniceanu F, Zurac S, et al. Regression of precancerous epithelial alteration in patients with Helicobacter pylori chronic gastritis. Rom J Intern Med 2010;48:89–99. [PubMed] [Google Scholar]
- 118.Nasser SC, Slim M, Nassif JG, et al. Influence of proton pump inhibitors on gastritis diagnosis and pathologic gastric changes. World J Gastroenterol 2015;21:4599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Olmez S, Aslan M, Erten R, et al. The Prevalence of Gastric Intestinal Metaplasia and Distribution of Helicobacter pylori Infection, Atrophy, Dysplasia, and Cancer in Its Subtypes. Gastroenterol Res Pract 2015;2015:434039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ozdil K, Sahin A, Kahraman R, et al. Current prevalence of intestinal metaplasia and Helicobacter pylori infection in dyspeptic adult patients from Turkey. Hepatogastroenterology 2010;57:1563–6. [PubMed] [Google Scholar]
- 121.Saragih JB, Akbar N, Syam AF, et al. Incidence of helicobacter pylori infection and gastric cancer : an 8-year hospital based study. Acta Med Indones 2007;39:79–81. [PubMed] [Google Scholar]
- 122.Tsukanov VV, Butorin NN, Maady AS, et al. Helicobacter pylori Infection, Intestinal Metaplasia, and Gastric Cancer Risk in Eastern Siberia. Helicobacter 2011;16:107–12. [DOI] [PubMed] [Google Scholar]
- 123.Üçüncü MZ, Kabul Gürbulak E, Gürbulak B, et al. Relationship between atrophic gastritis, gastric intestinal metaplasia and Helicobacter pylori on endoscopic screening of upper gastrointestinal tract and a brief review of the literature. European Surgery 2016;48:99–104. [Google Scholar]
- 124.Wang CC, Wu MS, Wang HH, et al. Helicobacter pylori infection and age on the development of intestinal metaplasia--a multiple logistic regression analysis. Hepatogastroenterology 1998;45:2234–7. [PubMed] [Google Scholar]
- 125.Whiting JL, Sigurdsson A, Rowlands DC, et al. The long term results of endoscopic surveillance of premalignant gastric lesions. Gut 2002;50:378–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yee YK, Wong KW, Hui CK, et al. Prevalence and time trend of intestinal metaplasia in Hong Kong. J Gastroenterol Hepatol 2009;24:896–9. [DOI] [PubMed] [Google Scholar]
- 127.You WC, Brown LM, Zhang L, et al. Randomized double-blind factorial trial of three treatments to reduce the prevalence of precancerous gastric lesions. J Natl Cancer Inst 2006;98:974–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix Doxument 1. Detailed search strategy.
Appendix Document 2. Risk of bias table and summary (Petersson*, 2002 is limited to the subgroup of patients with pernicious anemia).
Appendix Figure 1. Prevalence of GIM in patients who underwent gastric biopsies worldwide by geographical regions.
Appendix Figure 2. Prevalence of GIM in patients who underwent gastric biopsies by low prevalence of H. pylori (≤15%).
Appendix Figure 3. Prevalence of GIM in patients who underwent gastric biopsies by high prevalence of H. pylori (>75%).
Appendix Figure 4. Meta-regression of proportion of patients with GIM on proportion of patients with H. pylori exposure.
Appendix Figure 5. Prevalence of GIM among H. pylori-exposed patients who underwent gastric biopsies by geographical regions.
Appendix Figure 6. Prevalence of GIM among patients who underwent gastric biopsies by H. pylori and cagA status.
Appendix Figure 7. Prevalence of GIM among patients with pernicious anemia who underwent gastric biopsies.
Appendix Figure 8. Relative risk of finding GIM on gastric biopsies in patients with versus without first-degree family history of gastric cancer (*Sadjadi 2014 was a study of H. pylori-infected patients).
Appendix Figure 9. Relative risk of finding GIM on gastric biopsies in patients who smoke tobacco versus nonsmokers and previous smokers (*Sadjadi 2014 was a study of H. pylori-infected patients; **Leung 2005 was a study of patients with first-degree family history of gastric cancer).
Appendix Figure 10. Relative risk of finding GIM on gastric biopsies in patients who drink alcohol versus patients who do not drink alcohol (*Sadjadi 2014 was a study of H. pylori-infected patients; **Leung 2005 was a study of patients with first-degree family history of gastric cancer).
Appendix Figure 11. Relative risk of finding GIM on gastric biopsies in patients with high versus low salt intake, low versus high frutis and vegetable intake, and high versus low dairy products intake (*Sadjadi 2014 was a study of H. pylori-infected patients).
Appendix Figure 12. Prevalence of OLGIM stages among patients found to have GIM on gastric biopsies.