Abstract
Childhood glaucoma is an important cause of blindness world-wide. Eleven genes are currently known to cause inherited forms of glaucoma with onset before age 20. While all the early-onset glaucoma genes cause severe disease, considerable phenotypic variability is observed among mutations carriers. In particular, FOXC1 genetic variants are associated with a broad range of phenotypes including multiple forms of glaucoma and also systemic abnormalities, especially hearing loss. FOXC1 is a member of the forkhead family of transcription factors and is involved in neural crest development necessary for formation of anterior eye structures and also pharyngeal arches that form the middle ear bones. In this study we review the clinical phenotypes reported for known FOXC1 mutations and show that mutations in patients with reported ocular anterior segment abnormalities and hearing loss primarily disrupt the critically important forkhead domain. These results suggest that optimal care for patients affected with anterior segment dysgenesis should include screening for FOXC1 mutations and also testing for hearing loss.
Keywords: Glaucoma, genes, childhood, variable phenotype, hearing loss, anterior segment dysgenesis, FOXC1
Introduction
Glaucoma is a significant cause of blindness in children world-wide (Beck, 2011a; Haddad et al., 2007). Childhood forms of glaucoma are frequently characterized by high intraocular pressure (IOP) resulting from abnormalities of the eye fluid drainage structures (trabecular meshwork), however familial forms of normal-tension glaucoma are also known. High IOP causes irreversible damage to the optic nerve and in elastic pediatric eyes can cause ocular enlargement (buphthalmos) with the associated complications of high myopia, retinal detachment and corneal decompensation related to fracture of the corneal basement membranes. As curative therapies for glaucoma do not currently exist, affected children are subject to a lifetime of medical and surgery treatments directed toward lowering elevated intraocular pressure (Zagora et al., 2015; Ben-Zion et al., 2011; Beck et al., 2011b).
The discovery of genes responsible for pediatric glaucoma is an important step toward the development of clinically useful gene-based screening tests and novel and potentially curative genetic therapies. Eleven genes responsible for childhood forms of glaucoma have been identified so far (Table 1). Four genes are now known to cause congenital glaucoma: CYP1B1 and LTBP2 causing autosomal recessive disease (Ali et al., 2009; Bejjani et al., 1998; Stoilov et al., 1997) and TIE2 (TEK) and ANGPT1 cause dominantly inherited congenital glaucoma with variable expressivity related to development of Schlemm’s canal (Souma et al., 2016; Thomson et al., 2017). Mutations in three genes coding for transcription factors involved in ocular development can cause early-onset glaucoma and anterior segment dysgenesis: FOXC1 (Axenfeld-Rieger syndrome) (Nishimura et al., 1998), PITX2 (Rieger Syndrome) (Semina et al., 1996), PAX6 (Aniridia and Peter’s anomaly) (Jordan et al., 1992; Prosser et al., 1998). Recently CPAMD8 mutations have been identified as a cause of a unique form of autosomal recessive anterior segment dysgenesis that can include congenital cataracts (Cheong et al., 2016; Hollmann et al., 2017). Dominant MYOC (myocilin) missense alleles cause juvenile (onset after age 3) glaucoma (Fingert et al., 2002; Wiggs et al., 1998; Stone et al., 1997). Myocilin is an extracellular matrix protein and disease-causing missense alleles induce ER stress from the misfolded protein response (Donegan et al., 2015). Loss of function MYOC mutations in mice and humans do not cause glaucoma (Kim et al., 2001; Wiggs et al., 2001). FOXC1, PITX2 and PAX6 are regulatory genes that influence development of the ocular anterior segment including structures involved in glaucoma (Fan and Wiggs, 2010). Loss of function dominant alleles cause clinically evident developmental defects that can include glaucoma (Allen et al., 2015). OPTN (optineurin) and TBK1 (tank binding protein 1) cause dominantly inherited early-onset normal tension glaucoma, characterized by profound optic atrophy in the setting of normal IOP (Fingert et al., 2011; Hauser et al., 2006; Rezaie et al., 2002).
Table 1.
Childhood glaucoma genes and phenotypes
| Gene | Protein | Inheritance | Phenotypes |
|---|---|---|---|
| CYP1B1 | Cytochrome P450 1B1 | AR | Congenital and juvenile glaucoma |
| LTBP2 | Latent transforming growth factor binding protein 2 | AR | Congenital glaucoma |
| CPAMD8 | C3 and PZP like alpha-2-macroglobulin domain containing 8 | AR | Anterior segment dysgenesis |
| PITX2 | Paired like homeodomain 2 | AD | Anterior segment dysgenesis and classic Reiger syndrome |
| FOXC1 | Forkhead box C1 | AD | Congenital glaucoma, anterior segment dysgenesis, Axenfeld-Rieger syndrome, juvenile open angle glaucoma |
| PAX6 | Paired box 6 | AD | Aniridia, corneal keratitis, Peter’s anomaly |
| MYOC | Myocilin | AD | Juvenile open angle glaucoma, adult-onset open angle glaucoma |
| TIE2 (TEK) | TEK receptor tyrosine kinase | AD | Congenital glaucoma with variable expressivity |
| ANGPT1 | Angiopoietin 1 | AD | Congenital glaucoma |
| OPTN | Optineurin | AD | Normal tension glaucoma |
| TBK1 | TANK binding kinase 1 | AD | Normal tension glaucoma |
Abbreviations: AD, Autosomal dominant; AR, Autosomal recessive.
Variable phenotypes
Phenotypic variation has been observed in patients with disease caused by childhood glaucoma genes, especially for patients with mutations in CYP1B1, MYOC, PAX6 and FOXC1. Many patients with CYP1B1 mutations are diagnosed with congenital glaucoma during infancy, however some patients do not show evidence of the disease until later in childhood or even teenage years (López-Garrido et al., 2013; Khan et al., 2011; Suri et al., 2009). Similarly, while many MYOC mutations cause disease before age 20, several mutations, including the well-studied Q368X, are known to be responsible for disease in individuals who are not diagnosed with glaucoma until later in life (Nag et al., 2018; Allingham et al., 1998). PAX6 mutations are classically known to cause aniridia (Prosser et al., 1998) but can also cause autosomal dominant keratitis due to limbal stem cell deficiency (Mirzayans et al., 1995; Li et al., 2015). FOXC1 mutations can be responsible for disease with onset ranging from birth (Siggs et al., 2019) to adult (Bailey et al., 2016). Additionally our NEIGHBORHOOD consortium has recently identified SNPs in the FOXC1 5’ UTR that are significantly associated with adult-onset POAG, suggesting that variable expression of FOXC1 may contribute to POAG more commonly (Cooke Bailey et al., 2016).
FOXC1 ocular phenotypes
Forkhead transcription factors are a family of proteins that share a highly conserved forkhead DNA-binding domain and are required for regulation of embryogenesis, cell migration, differentiation and fate determination (Golson and Kaestner, 2016). FOXC1 codes for a member of the forkhead transcription factor family that is required for the migration and specification of the periocular mesenchyme neural-crest derived mesenchymal cells that give rise to important ocular structures related to glaucoma including the stroma of the ciliary body and iris and the trabecular meshwork (Akula et al., 2019).
Both deletions and duplications involving FOXC1 have been implicated in ocular disease (Lehmann et al., 2000; Nishimura et al., 2001) indicating gene dosage as a critical factor in disease development. FOXC1 null mice exhibit clinical features of anterior segment dysgenesis including iris hypoplasia, corectopia, and embryotoxon in mice (Kume et al., 1998; Gould et al., 2004).
FOXC1 mutations can cause a broad range of ocular phenotypes: Axenfeld-Rieger syndrome (Nishimura et al., 1998), Peters Anomaly (Honkanen et al., 2003), congenital glaucoma (Siggs et al., 2019), and more recently adult-onset primary open angle glaucoma (Bailey et al., 2016). Frequently FOXC1 mutations are associated with Axenfeld-Rieger anomaly defined by anterior segment dysgenesis with characteristic posterior embryotoxon, iris hypoplasia, and corectopia (Seifi and Walter, 2018). Axenfeld-Rieger syndrome describes patients with Axenfeld-Rieger anomaly and additional systemic features that may include a flat mid-face due to maxillary hypoplasia and a flat broad nose, teeth abnormalities, redundant umbilical skin and congenital heart defects (Lewis et al., 2017). Many patients with Axenfeld-Rieger anomaly or syndrome will also develop glaucoma, however the severity of the anterior segment dysgenesis does not predict glaucoma risk. Recent studies suggest that patients with truncating FOXC1 mutations are more likely to be diagnosed with congenital glaucoma (Siggs et al., 2019).
FOXC1 systemic phenotypes
FOXC1 mutation carriers may also exhibit a range of systemic abnormalities. Patients with large-scale deletions or duplications of the 6pter-6p24 region that includes FOXC1, FOXFQ and FOXF2 can present with a syndromic phenotype defined by hearing loss, cardiac abnormalities, short stature, dental abnormalities, facial dysmorphism and hypertelorism (Gould et al., 2004). De Hauwere syndrome describes a subset of the 6pter-6p24 deletion patients that are characterized by Axenfeld-Rieger syndrome, hydrocephalus and hearing loss (Lowry et al., 2007). Dandy-Walker malformation involving the cerebellum has also been described in patients with FOXC1 mutations (Aldinger et al., 2009). FOXC1 is an important component of the signaling pathways necessary for cardiac development and mutations can cause congenital heart disease and abnormal valve formation (Zhu, 2016). Involvement of FOXC1 in the neural crest migration forming the pharyngeal arches and cardiac neural crest likely underlie these systemic findings in FOXC1 mutation carriers (Kume et al., 2001).
Patients with FOXC1 point mutations and indels (nonsense, frameshift or missense alleles) also can present with a range of ocular and systemic phenotypes (Table 2). Systemic phenotypes associated with FOXC1 mutations are similar in range and scope to those identified in patients with large-scale deletions and duplications suggesting that genetic abnormalities involving FOXC1 are important drivers of the 6pter-6p25 syndromic clinical features.
Table 2.
FOXC1 mutations and ocular and hearing phenotypes
| Protein variant | Protein domain | cDNA variant | Hearing Phenotype | Ocular Phenotype | Reference |
|---|---|---|---|---|---|
| Q2X | Active 1 | c.4C>T | NR | ARA | Komatireddy et al., 2003 |
| R4fs | Active 1 | c.12delC | NR | Glaucoma | Chakrabarti et al., 2009 |
| S9fsX89 | Active 1 | c.26–47ins | Deafness | Iris hypoplasia, corectopia | Kawase et al., 2001 |
| Q23X | Active 1 | c.67C>T | Hearing loss | ARA, glaucoma | Mirzayans et al., 2001 |
| R28_30del | Active 1 | c.81_89del9 | NR | Glaucoma | Chakrabarti et al., 2009 |
| A31_33del | Active 1 | c.92_100del9 | NR | Glaucoma | Kaur et al., 2009 |
| A31fsX41 | Active 1 | c.93_102del10 | No systemic findings | ARA, glaucoma | Michael et al., 2016 |
| A31fsX41 | Active 1 | c.93_102del10 | No systemic findings | ARA, glaucoma | Mears et al., 1998 |
| G33fsX41 | Active 1 | c.99_108del10 | NR | ARA | Nishimura et al., 2001 |
| G34fsX8 | Active 1 | c.100_109del10 | NR | PE, glaucoma | Souzeau et al., 2017 |
| A39fsX42 | Active 1 | c.116_123del8 | NR | ARA | Nishimura et al., 2001 |
| Y47X | Active 1 | c.141C>G | NR | Glaucoma | Medina-Trillo et al., 2015 |
| S48X | Active 1 | c.143C>A | No systemic findings | PE, corectopia | Weisschuh et al., 2006 |
| A51fsX73 | Active 1 | c.153_163del11 | NR | ARA, glaucoma | Nishimura et al., 1998 |
| Y64X | After Active 1 |
c.192C>G | NR | ARA, glaucoma | Carmona et al., 2017 |
| Q70fsX73 | Forkhead | c.210delG | Hearing loss | ARA, glaucoma | Swiderski RE et al. 1999 |
| P79T | Forkhead | c.235C>A | Hearing loss | ARA, glaucoma | Suzuki T et al., 2001 |
| P79R | Forkhead | c.236C>G | NR | Iris hypoplasia, glaucoma | Weisschuh et al., 2006 |
| P79L | Forkhead | c.236C>T | NR | ARA | Saleem et al., 2003 |
| P79L | Forkhead | c.236C>T | NR | ARA | Nishimura et al., 1998 |
| S82T | Forkhead | c.245G>C | Hearing loss | ARA, glaucoma | Mears et al., 1998 |
| A85P | Forkhead | c.253G>C | NR | ARA, glaucoma | Fuse et al., 2007 |
| L86F | Forkhead | c.256C>T | NR | ARA, glaucoma | Saleem et al., 2003 |
| I87M | Forkhead | c.261C>G | No systemic findings | ARA, glaucoma | Mears et al., 1998 |
| T88fsX100 | Forkhead | c.262_265insC | NR | ARA | Nishimura et al., 2001 |
| A90T | Forkhead | c.268G>A | No systemic findings | PE, glaucoma | Souzeau et al., 2017 |
| A90D | Forkhead | c.269C>A | NR | Glaucoma | Siggs et al., 2019 |
| I91S | Forkhead | c.272T>G | NR | ARA, glaucoma | Kawase et al., 2001 |
| I91T | Forkhead | c.272T>C | NR | ARA | Mortemousque et al.,2004 |
| D96GfsX210 | Forkhead | c.286dupG | NR | PE, glaucoma | D’Haene et al., 2011 |
| D96fsX305 | Forkhead | c.286insG | NR | ARA, glaucoma | Kawase et al., 2001 |
| Q106X | Forkhead | c.316C>T | NR | ARA, glaucoma | D’Haene et al., 2011 |
| Q106X | Forkhead | c.316C>T | NR | ARA, glaucoma | Souzeau et al., 2017 |
| Q106RfsX75 | Forkhead | c.317delA | Normal hearing | ARA, glaucoma | Kim et al., 2013 |
| M109V | Forkhead | c.325A>G | Hearing loss | Corectopia | D’Haene et al., 2011 |
| F112SfsX69 | Forkhead | c.335del | Hearing Loss | ARA, glaucoma | D’Haene et al., 2011 |
| F112S | Forkhead | c.335T>C | NR | ARA, glaucoma | Nishimura et al., 1998 |
| F112S | Forkhead | c.335T>C | NR | ARA, glaucoma | Honkanen et al., 2003 |
| Y115S | Forkhead | c.339T>C | Middle-ear deafness | ARA, glaucoma | Weisschuh et al., 2006 |
| D117TfsX64 | Forkhead | c.349delG | NR | ARA, glaucoma | Siggs et al., 2019 |
| Q120X | Forkhead | c.358C>T | NR | ARA, glaucoma | Weisschuh et al., 2008 |
| Q123X | Forkhead | c.367C>T | NR | ARA, glaucoma | Komatireddy et al., 2003 |
| I126M | Forkhead | c.378C>G | NR | ARA, glaucoma | Nishimura et al., 1998 |
| H128R | Forkhead | c.378A>G | NR | Glaucoma | Chakrabarti et al., 2009 |
| R127H | Forkhead | c.380G>A | NR | ARA, glaucoma | Kawase et al., 2001 |
| R127L | Forkhead | c.380T>G | NR | ARA, glaucoma | Du et al., 2016 |
| L130F | Forkhead | c.388C>T | NR | ARA, glaucoma | Ito et al., 2007 |
| S131L | Forkhead | c.392C>T | NR | ARA, glaucoma | Nishimura et al., 1998 |
| S131X | Forkhead | c.392C>A | NR | Glaucoma | D’Haene et al., 2011 |
| S131W | Forkhead | c.392C>G | NR | ARA | D’Haene et al., 2011 |
| C135Y | Forkhead | c.402G>A | NR | Glaucoma | Chakrabarti et al., 2009 |
| V137del | Forkhead | c.409_411del | NR | PE, glaucoma | Siggs et al., 2019 |
| K138E | Forkhead | c.412A>G | NR | PE, glaucoma | D’Haene et al., 2011 |
| P146fs | Forkhead | c.437_453del17 | Hearing loss | ARA, glaucoma | Fuse et al., 2007 |
| G149D | Forkhead | c.446G>A | NR | ARA, glaucoma | Weisschuh et al., 2006 |
| W152R | Forkhead | c.454T>C | Mild deafness | ARA, glaucoma | Michael et al., 2016 |
| W152G | Forkhead | c.454T>G | NR | Glaucoma | Ito et al., 2009 |
| W152X | Forkhead | c.456G>A | NR | ARA, glaucoma | Cella et al., 2006 |
| T153P | Forkhead | c.457A>C | Hearing loss | PE, glaucoma | Siggs et al., 2019 |
| M161V | Forkhead | c.481A>G | Middle-ear deafness | ARA, glaucoma | Weisschuh et al., 2006 |
| M161K | Forkhead | c.482T>A | NR | ARA, glaucoma | Panicker et al., 2002 |
| M161K | Forkhead | c.482T>A | NR | ARA, glaucoma | Komatireddy et al., 2003 |
| E163X | Forkhead | c.487G>T | Hearing Loss | Glaucoma | Siggs et al., 2019 |
| G165R | Forkhead | c.494G>C | NR | ARA, glaucoma | Murphy et al., 2004 |
| R169P | Forkhead | c.506G>C | Hearing loss | ARA | Murphy et al., 2004 |
| R170W | Forkhead | c.508C>T | Hearing Loss | ARA, glaucoma | Gripp et al., 2013 |
| Q200fsX109 | After Forkhead |
c.599_617del19 | NR | ARA | Souzeau et al., 2017 |
| P202RfsX113 | After Forkhead |
c.605delC | NR | ARA, glaucoma | D’Haene et al., 2011 |
| A204RfsX111 | After Forkhead |
c.609delC | NR | ARA, glaucoma | Kelberman et al., 2011 |
| I223PfsX87 | Inhibitory | c.666_681del16 | NR | PE glaucoma | Souzeau et al., 2017 |
| G231VfsX73 | Inhibitory | c.692_696del5 | NR | ARA, glaucoma | D’Haene et al., 2011 |
| L240VfsX65 | Inhibitory | c.718_719delCT | No systemic findings | ARA, glaucoma | Cella et al., 2006 |
| L240VfsX65 | Inhibitory | c.718_719delCT | NR | Glaucoma | Siggs et al., 2019 |
| L240RfsX75 | Inhibitory | c.719delT | + | Glaucoma | Hariri et al.,2018 |
| L246fsX68 | Inhibitory | c.738delG | NR | Iris atrophy, glaucoma | Weisschuh et al., 2006 |
| D261RfsX45 | Inhibitory | c.780dup | NR | NR | D’Haene et al., 2011 |
| S272RfsX43 | Inhibitory | c.816_817delinsG | NR | NR | D’Haene et al., 2011 |
| A291fs | Inhibitory | c.853dup25 | NR | Glaucoma | Chakrabarti et al., 2009 |
| P297S | Inhibitory | c.889C>T | NR | Glaucoma | Fetterman et al., 2009 |
| P297S | Inhibitory | c.889C>T | NR | Glaucoma | Medina-Trillo et al., 2016 |
| S309CfsX84 | Inhibitory | c.925_949del25 | NR | Glaucoma | Souzeau et al., 2017 |
| E327AfsX200 | Inhibitory | c.980_981del | NR | NR | D’Haene et al., 2011 |
| G379Gins | After Inhibitory |
c.1142_1144insGGC | No systemic findings | Iris atrophy, glaucoma | Yang et al., 2015 |
| M400SfsX129 | After inhibitory |
c.1193_1196dup | Congenital deafness | Iris atrophy, glaucoma | Reis et al., 2016 |
| S422X | After Inhibitory |
c.1265C>A | NR | ARA, glaucoma | Souzeau et al., 2017 |
| G452insR | After Inhibitory |
c.1362_1364insCGG | No systemic findings | Iris atrophy, glaucoma | Yang et al., 2015 |
| Y497X | Active 2 | c.1491C>G | NR | Glaucoma | D’Haene et al., 2011 |
| Y497X | Active 2 | c.1491C>G | Hearing Loss | PE, glaucoma | Souzeau et al., 2017 |
| N503fsX15 | Active 2 | c.1511delT | NR | ARA, glaucoma | Weisschuh et al., 2006 |
| F504fsX518 | Active 2 | c.1512delG | NR | ARA | Nishimura et al., 2001 |
Abbreviations: ARA, Axenfeld-Rieger anomaly; PE, Posterior embryotoxon; NR = Not reported.
FOXC1 and Hearing Loss
Patients with large-scale deletions and other copy number variations (CNVs) involving chromosome 6p25 and FOXC1 frequently are affected with hearing loss in addition to anterior ocular dysgenesis (D’haene et al., 2011; Gould et al., 2004). Although a precise role for FOXC1 in hearing or ear development is not well understood, during development, neural crest cells migrate from the dorsal hindbrain to specific locations in pharyngeal arch (PA) 1 and 2, to form the middle ear bones (malleus, incus and stapes) (Ritter and Martin, 2019). As FOXC1 contributes to neural crest migration in the pharyngeal arches, its possible that FOXC1 mutations can interfere with this process. Defective FOXC1 can lead to abnormal development and ossification of facial bones (Xu et al., 2018) and Foxc1−/− mice have abnormal cranial facial bone development, and failed ossification of the middle ear bones (Inman et al., 2013).
To gain a better understanding of the role of FOXC1 in hearing and deafness we reviewed published reports of FOXC1 variants and recorded information on hearing and ocular findings (Tables 2 and 3 and Figure 1) by searching PubMed with terms “FOXC1” and “Mutation” or “6p25” or “Ring chromosome 6”. We excluded publications that were not in English, did not describe human genetic variants or were not accessible online.
Table 3.
Chromosome 6p25 abnormalities and ocular and hearing phenotypes
| 6p25 Variant | Hearing Phenotype | Ocular Phenotype | Reference |
|---|---|---|---|
| .084 Mb deletion | NR | PE, iris atrophy, glaucoma | D’Haene et al, 2011 |
| 0.98 Mb deletion | Hearing loss | ARA, glaucoma | Reis et al, 2012 |
| 1.10 Mb deletion | Hearing loss | ARA, glaucoma | Reis et al, 2012 |
| 1.3 Mb deletion | Hearing loss | ARA | Reis et al, 2012 |
| 1.3 Mb deletion | Hearing Loss | Congenital glaucoma | Siggs et al., 2019 |
| 1.4 Mb deletion | NR | Normal Ophthalmic exam | Ovaert et al., 2017 |
| 1.5 Mb deletion | Normal hearing | Axenfeld-Rieger syndrome, congenital glaucoma | Reis et al, 2012 |
| 1.5 Mb deletion | NR | PE, iris atrophy, glaucoma | Sadagopan et al., 2015 |
| 2.1 Mb deletion | Conductive hearing defect | Anterior segment dysgenesis | Anderlid et al, 2003 |
| 2.1 Mb deletion | Abnormal auditory brainstem response | Congenital glaucoma | Nakane et al., 2013 |
| 2.21 MB deletion | Hearing loss | Myopia | Bedoyan et al, 2011 |
| 2.54 Mb deletion | Hearing Loss | ARA | Vernon et al., 2013 |
| 2.6 Mb deletion | Middle ear malformations and hearing loss | Iris hypoplasia, glaucoma | D’Haene et al, 2011 |
| 2.6 Mb duplication | NR | PE, iris atrophy, glaucoma | Sadagopan et al., 2015 |
| 2.7 Mb deletion | Sensorineural deafness | PE, iris atrophy, glaucoma | Martinez-Glez et al., 2007 |
| 3.4 Mb deletion | Hearing loss | Glaucoma | Weegerink et al, 2016 |
| 3.4 Mb deletion | Hearing loss | Glaucoma | Weegerink et al, 2016 |
| 3.4 Mb deletion | Hearing loss | PE | Weegerink et al, 2016 |
| 3.4 Mb deletion | Middle ear hearing loss | PE, glaucoma | D’Haene et al, 2011 |
| 3.9 Mb deletion | Normal hearing | Strabismus | Cellini et al, 2012 |
| 34 kb deletion | Hearing loss | PE, glaucoma | D’Haene et al, 2011 |
| 4.7 Mb deletion | Hearing loss | ARA | D’Haene et al, 2011 |
| 4.8 Mb deletion | Conductive hearing loss | PE, iris atrophy | Le Caignec et al., 2005 |
| 5.06 Mb deletion and 1 Mb duplication | Hearing loss | Corectopia | Linhares et al, 2015 |
| 5.4 kb deletion | NR | PE | D’Haene et al, 2011 |
| 5.5 Mb deletion | Hearing loss | PE, corneal opacity | Le Caignec et al., 2005 |
| 6 Mb deletion | Normal auditory brainstem response, but no speech | Normal Ophthalmic exam | Piccione et al., 2012 |
| 6.6 Mb deletion | Normal hearing | ARA, glaucoma | Tonoki et al, 2011 |
| 6p25 microdeletion | Sensorineural deafness | ARA | Kapoor et al, 2011 |
| 6p25-6p22 deletion | NR | ARA | Suzuki et al., 2006 |
| 6p25-6pter deletion | Normal hearing | ARA | Maclean et al, 2005 |
| 6p25-6pter deletion | Normal hearing | PE, glaucoma | Tonoki et al, 2011 |
| 6p25-6pter deletion | Normal hearing | Axenfeld-Rieger syndrome, congenital glaucoma | Reis et al, 2012 |
| 6p25-6pter deletion | Hearing loss | ARA, glaucoma | Gould et al, 2004 |
| 6p25-6pter deletion | Normal hearing | ARA | Gould et al, 2004 |
| 6pter deletion | Normal hearing | PE | Lin et al, 2005 |
| 6p25 to 6pter deletion | Hearing loss | ARA | Lin et al, 2005 |
| 6pter microdeletion | Normal hearing | Anterior segment dysgenesis | Guillen-Navarro et al, 1997 |
| Ring chromosome 6, 6 Mb deletion on 6p | Hearing loss | Peter’s anomaly, glaucoma | Zhang et al, 2004 |
| Ring chromosome 6, 1.8 Mb distal 6p deletion | Hearing loss | Ocular features not recorded | Pace et al., 2017 |
| Ring chromosome 6, 6p deletion | Normal auditory brainstem response, but no speech | PE, iris atrophy, glaucoma | Corona-Rivera et al., 2018 |
| Ring chromosome 6, 6p25.2 deletion 1.78 Mb | NR | Anterior segment dysgenesis, microphthalmia | Zhang et al., 2016 |
Abbreviations: ARA, Axenfeld-Rieger anomaly; PE, Posterior embryotoxon; NR, Not reported.
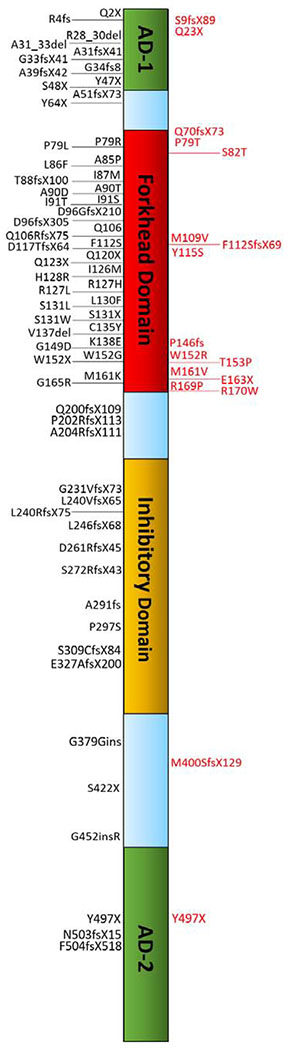
Figure 1. Gene location of FOXC1 mutations.
The location of the mutations listed in Table 2 are shown. Variants shown in red font are reported in patients with hearing loss. Abbreviations: AD1, Active Domain 1; AD2, Active Domain 2.
Our review identified 82 different FOXC1 human mutations (Table 2) and 42 6p25 deletions, duplications or ring chromosomes that include the FOXC1 genomic region (Table 3). Of the 82 FOXC1 mutations reported in patients with ocular disease, 17 reported abnormal hearing (Table 2; Figure 1). Fifteen of the 17 mutations found in patients reporting hearing loss either caused a frameshift or premature stop codon in Active Domain 1, leading to the loss of the forkhead domain, or a framshrift, nonsense or missense change located in the forkhead domain itself (Figure 1). Only one mutation within the forkhead domain (Q106RfsX75) reported normal hearing (Kim et al., 2013). Unfortunately, in many cases, there is no mention of the hearing phenotype or the case is reported with “no systemic findings,” making it difficult to determine whether or not hearing tests were conducted (Table 2).
Of the 38 reported cases of 6p25 deletions or duplications 20 (53%) have described hearing defects as part of the clinical presentation and 2 of 4 patients with ring chromosome 6 involving the FOXC1 genomic region also reported hearing loss (Table 3). Nine patients with 6p deletions reported have normal hearing and two patients were reported to have normal auditory brainstem response but no speech (Table 3) suggesting variable expressivity of the hearing phenotype. Similar to the reports for the FOXC1 mutations (Table 2) 7 of the 6p25 deletion, duplication or ring chromosome reports did not comment on hearing.
The results of this literature review show that FOXC1 mutations that cause both anterior segment dysgenesis and hearing loss most likely disrupt the critical forkhead domain. The forkhead domain is necessary for proper FOXC1 nuclear localization and DNA binding, and disruptions to this part of the gene are the most deleterious to protein function (Saleem et al., 2004). There are however, many patients with FOXC1 mutations involving the forkhead domain that do not report hearing problems. This observation may be due to variable expressivity of the hearing phenotype, or could implicate a second gene or other factors that impact hearing pathogenesis. Alternatively, hearing tests may not have been done or may not have been noted in the report.
Summary
Currently 11 genes are known to cause early-onset glaucoma and variable phenotypes in mutation carriers is frequently observed. In this review we focused on the spectrum of phenotypes found in patients with FOXC1 mutations with an emphasis on hearing loss. We determined that of the majority of FOXC1 mutations reported in the literature in patients with anterior segment dysgenesis and hearing loss disrupt the critically important forkhead domain necessary for DNA binding and transcriptional regulation. We also find that approximately 50% of patients reported with 6p25 deletions, duplications or ring chromosomes also report hearing abnormalities. These results overall suggest that FOXC1 mutations are capable of causing hearing defects and that patients with FOXC1 mutations should undergo hearing testing.
Highlights.
Eleven genes responsible for childhood forms of glaucoma are currently known.
Variable clinical features can be observed in patients with mutations in childhood glaucoma genes.
FOXC1 mutations can cause ocular and systemic disease.
FOXC1 mutations causing ocular disease and hearing loss are primarily located in the forkhead domain.
Acknowledgements
This work was supported in part by the March of Dimes Foundation, NIH/NEI P30 EY014104 and a student fellowship grant from Yale University Medical School (ACG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akula M, Park JW, West-Mays JA, 2019. Relationship between neural crest cell specification and rare ocular diseases. J Neurosci Res. 97, 7–15. doi: 10.1002/jnr.24245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldinger KA, Lehmann OJ, Hudgins L, Chizhikov VV, Bassuk AG, Ades LC, Krantz ID, Dobyns WB, Millen KJ, 2009. FOXC1 is required for normal cerebellar development and is a major contributor to chromosome 6p25.3 Dandy-Walker malformation. Nat Genet. 41, 1037–1042. doi: 10.1038/ng.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M, McKibbin M, Booth A, Parry DA, Jain P, Riazuddin SA, Hejtmancik JF, Khan SN, Firasat S, Shires M, Gilmour DF, Towns K, Murphy AL, Azmanov D, Tournev I, Cherninkova S, Jafri H, Raashid Y, Toomes C, Craig J, Mackey DA, Kalaydjieva L, Riazuddin S, Inglehearn CF, 2009. Null mutations in LTBP2 cause primary congenital glaucoma. Am J Hum Genet. 84, 664–671. doi: 10.1016/j.ajhg.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen KF, Gaier ED, Wiggs JL, 2015. Genetics of Primary Inherited Disorders of the Optic Nerve: Clinical Applications. Cold Spring Harb Perspect Med. 5, a017277. doi: 10.1101/cshperspect.a017277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allingham RR, Wiggs JL, De La Paz MA, Vollrath D, Tallett DA, Broomer B, Jones KH, Del Bono EA, Kern J, Patterson K, Haines JL, Pericak-Vance MA, 1998. Gln368STOP myocilin mutation in families with late-onset primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 39, 2288–2295. [PubMed] [Google Scholar]
- Anderlid BM, Schoumans J, Hallqvist A, Ståhl Y, Wallin A, Blennow E, Nordenskjöld M, 2003. Cryptic subtelomeric 6p deletion in a girl with congenital malformations and severe language impairment. Eur J Hum Genet. 11, 89–92. [DOI] [PubMed] [Google Scholar]
- Bailey JN, Loomis SJ, Kang JH, Allingham RR, Gharahkhani P, Khor CC, Burdon KP, Aschard H, Chasman DI, Igo RP Jr, Hysi PG, Glastonbury CA, Ashley-Koch A, Brilliant M, Brown AA, Budenz DL, Buil A, Cheng CY, Choi H, Christen WG, Curhan G, De Vivo I, Fingert JH, Foster PJ, Fuchs C, Gaasterland D, Gaasterland T, Hewitt AW, Hu F, Hunter DJ, Khawaja AP, Lee RK, Li Z, Lichter PR, Mackey DA, McGuffin P, Mitchell P, Moroi SE, Perera SA, Pepper KW, Qi Q, Realini T, Richards JE, Ridker PM, Rimm E, Ritch R, Ritchie M, Schuman JS, Scott WK, Singh K, Sit AJ, Song YE, Tamimi RM, Topouzis F, Viswanathan AC, Verma SS, Vollrath D, Wang JJ, Weisschuh N, Wissinger B, Wollstein G, Wong TY, Yaspan BL, Zack DJ, Zhang K, Study EN; ANZRAG Consortium, Weinreb RN, Pericak-Vance MA, Small K, Hammond CJ, Aung, Liu Y, Vithana EN, MacGregor S, Craig JE, Kraft P, Howell G, Hauser MA, Pasquale LR, Haines JL, Wiggs JL, 2016. Genome-wide association analysis identifies TXNRD2, ATXN2 and FOXC1 as susceptibility loci for primary open-angle glaucoma. Nat Genet. 48, 189–194. doi: 10.1038/ng.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AD, 2011a. Primary congenital glaucoma in the developing world. Ophthalmology. 118, 229–230. doi: 10.1016/j.ophtha.2010.06.015. [DOI] [PubMed] [Google Scholar]
- Beck AD, Lynn MJ, Crandall J, Mobin-Uddin O, 2011b. Surgical outcomes with 360-degree suture trabeculotomy in poor-prognosis primary congenital glaucoma and glaucoma associated with congenital anomalies or cataract surgery. J AAPOS. 15, 54–58. doi: 10.1016/j.jaapos.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedoyan JK, Lesperance MM, Ackley T, Iyer RK, Innis JW, Misra VK, 2011. A complex 6p25 rearrangement in a child with multiple epiphyseal dysplasia. Am J Med Genet A. 155A, 154–163. doi: 10.1002/ajmg.a.33751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejjani BA, Lewis RA, Tomey KF, Anderson KL, Dueker DK, Jabak M, Astle WF, Otterud B, Leppert M, Lupski JR, 1998. Mutations in CYP1B1, the gene for cytochrome P4501B1, are the predominant cause of primary congenital glaucoma in Saudi Arabia. Am J Hum Genet. 62, 325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Zion I, Tomkins O, Moore DB, Helveston EM, 2011. Surgical results in the management of advanced primary congenital glaucoma in a rural pediatric population. Ophthalmology. 118, 231–235.e1. doi: 10.1016/j.ophtha.2010.02.027. [DOI] [PubMed] [Google Scholar]
- Carmona S, da Luz Freitas M, Froufe H, Simões MJ, Sampaio MJ, Silva ED, Egas C, 2017. Novel de novo FOXC1 nonsense mutation in an Axenfeld-Rieger syndrome patient. Am J Med Genet A. 173, 1607–1610. doi: 10.1002/ajmg.a.38234. [DOI] [PubMed] [Google Scholar]
- Cella W, de Vasconcellos JP, de Melo MB, Kneipp B, Costa FF, Longui CA, Costa VP, 2006. Structural assessment of PITX2, FOXC1, CYP1B1, and GJA1 genes in patients with Axenfeld-Rieger syndrome with developmental glaucoma, 2006. Invest Ophthalmol Vis Sci. 47, 1803–1809. [DOI] [PubMed] [Google Scholar]
- Cellini E, Disciglio V, Novara F, Barkovich JA, Mencarelli MA, Hayek J, Renieri A, Zuffardi O, Guerrini R, 2012. Periventricular heterotopia with white matter abnormalities associated with 6p25 deletion. Am J Med Genet A. 158A, 1793–1797. doi: 10.1002/ajmg.a.35416. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Kaur K, Rao KN, Mandal AK, Kaur I, Parikh RS, Thomas R, 2009. The transcription factor gene FOXC1 exhibits a limited role in primary congenital glaucoma. Invest Ophthalmol Vis Sci. 50, 75–83. doi: 10.1167/iovs.08-2253. [DOI] [PubMed] [Google Scholar]
- Cheong SS, Hentschel L, Davidson AE, Gerrelli D, Davie R, Rizzo R, Pontikos N, Plagnol V, Moore AT, Sowden JC, Michaelides M, Snead M, Tuft SJ, Hardcastle AJ, 2016. Mutations in CPAMD8 Cause a Unique Form of Autosomal-Recessive Anterior Segment Dysgenesis. Am J Hum Genet. 99, 1338–1352. doi: 10.1016/j.ajhg.2016.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corona-Rivera JR, Corona-Rivera A, Zepeda-Romero LC, Rios-Flores IM, Rivera-Vargas J, Orozco-Vela M, Santana-Bejarano UF, Torres-Anguiano E, Pinto-Cardoso M, David D, Bobadilla-Morales L, 2018. Ring chromosome 6 in a child with anterior segment dysgenesis and review of its overlap with other FOXC1 deletion phenotypes. Congenit Anom (Kyoto). 2018 September 18. doi: 10.1111/cga.12309. [DOI] [PubMed] [Google Scholar]
- D’Haene B, Meire F, Claerhout I, Kroes HY, Plomp A, Arens YH, de Ravel T, Casteels I, De Jaegere S, Hooghe S, Wuyts W, van den Ende J, Roulez F, Veenstra-Knol HE, Oldenburg RA, Giltay J, Verheij JB, de Faber JT, Menten B, De Paepe A, Kestelyn P, Leroy BP, De Baere E, 2011. Expanding the spectrum of FOXC1 and PITX2 mutations and copy number changes in patients with anterior segment malformations. Invest Ophthalmol Vis Sci. 52, 324–33. doi: 10.1167/iovs.10-5309. [DOI] [PubMed] [Google Scholar]
- Donegan RK, Hill SE, Freeman DM, Nguyen E, Orwig SD, Turnage KC, Lieberman RL, 2015. Structural basis for misfolding in myocilin-associated glaucoma. Hum Mol Genet. 24, 2111–2124. doi: 10.1093/hmg/ddu730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du RF, Huang H, Fan LL, Li XP, Xia K, Xiang R, 2016. A Novel Mutation of FOXC1 (R127L) in an Axenfeld-Rieger Syndrome Family with Glaucoma and Multiple Congenital Heart Diseases. Ophthalmic Genet. 37, 111–115. doi: 10.3109/13816810.2014.924016. [DOI] [PubMed] [Google Scholar]
- Fan BJ, Wiggs JL, 2010. Glaucoma: genes, phenotypes, and new directions for therapy. J Clin Invest. 120, 3064–3072. doi: 10.1172/JCI43085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetterman CD, Mirzayans F, Walter MA, 2009. Characterization of a novel FOXC1 mutation, P297S, identified in two individuals with anterior segment dysgenesis. Clin Genet. 76, 296–299. doi: 10.1111/j.1399-0004.2009.01210.x. [DOI] [PubMed] [Google Scholar]
- Fingert JH, Robin AL, Stone JL, Roos BR, Davis LK, Scheetz TE, Bennett SR, Wassink TH, Kwon YH, Alward WL, Mullins RF, Sheffield VC, Stone EM, 2011. Copy number variations on chromosome 12q14 in patients with normal tension glaucoma. Hum Mol Genet. 20, 2482–2494. doi: 10.1093/hmg/ddr123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingert JH, Stone EM, Sheffield VC, Alward WL, 2002. Myocilin glaucoma. Surv Ophthalmol. 47, 547–561. [DOI] [PubMed] [Google Scholar]
- Fuse N, Takahashi K, Yokokura S, Nishida K, 2007. Novel mutations in the FOXC1 gene in Japanese patients with Axenfeld-Rieger syndrome. Mol Vis. 13, 1005–1009. [PMC free article] [PubMed] [Google Scholar]
- Golson ML, Kaestner KH, 2016. Fox transcription factors: from development to disease. Development. 143, 4558–4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould DB, Jaafar MS, Addison MK, Munier F, Ritch R, MacDonald IM, Walter MA, 2004. Phenotypic and molecular assessment of seven patients with 6p25 deletion syndrome: relevance to ocular dysgenesis and hearing impairment. BMC Med Genet. 5, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould DB, Smith RS, John SW, 2004. Anterior segment development relevant to glaucoma. Int J Dev Biol. 48, 1015–1029. [DOI] [PubMed] [Google Scholar]
- Gripp KW, Hopkins E, Jenny K, Thacker D, Salvin J, 2013. Cardiac anomalies in Axenfeld-Rieger syndrome due to a novel FOXC1 mutation. Am J Med Genet A. 161A, 114–119. doi: 10.1002/ajmg.a.35697. [DOI] [PubMed] [Google Scholar]
- Guillén-Navarro E, Wallerstein R, Reich E, Zajac L, Ostrer H, 1997. Robinow syndrome with developmental brain dysplasia. Am J Med Genet. 1997 November 28;73(1):98–9. [DOI] [PubMed] [Google Scholar]
- Haddad MA, Sei M, Sampaio MW, Kara-José N, 2007. Causes of visual impairment in children: a study of 3,210 cases. J Pediatr Ophthalmol Strabismus. 44, 232–240. [DOI] [PubMed] [Google Scholar]
- Hauser MA, Sena DF, Flor J, Walter J, Auguste J, Larocque-Abramson K, Graham F, Delbono E, Haines JL, Pericak-Vance MA, Rand Allingham R, Wiggs JL, 2006. Distribution of optineurin sequence variations in an ethnically diverse population of low-tension glaucoma patients from the United States. J Glaucoma. 15, 358–363. [DOI] [PubMed] [Google Scholar]
- Hollmann AK, Dammann I, Wemheuer WM, Wemheuer WE, Chilla A, Tipold A, Schulz-Schaeffer WJ, Beck J, Schütz E, Brenig B, 2017. Morgagnian cataract resulting from a naturally occurring nonsense mutation elucidates a role of CPAMD8 in mammalian lens development. PLoS One. 12, e0180665. doi: 10.1371/journal.pone.0180665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honkanen RA, Nishimura DY, Swiderski RE, Bennett SR, Hong S, Kwon YH, Stone EM, Sheffield VC, Alward WL, 2003. A family with Axenfeld-Rieger syndrome and Peters Anomaly caused by a point mutation (Phe112Ser) in the FOXC1 gene. Am J Ophthalmol. 135, 368–375. [DOI] [PubMed] [Google Scholar]
- Inman KE, Purcell P, Kume T, Trainor PA, 2013. Interaction between Foxc1 and Fgf8 during mammalian jaw patterning and in the pathogenesis of syngnathia. PLoS Genet. 9, e1003949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito YA, Footz TK, Berry FB, Mirzayans F, Yu M, Khan AO, Walter MA, 2009. Severe molecular defects of a novel FOXC1 W152G mutation result in aniridia. Invest Ophthalmol Vis Sci. 50, 3573–3579. doi: 10.1167/iovs.08-3032. [DOI] [PubMed] [Google Scholar]
- Ito YA, Footz TK, Murphy TC, Courtens W, Walter MA, 2007. Analyses of a novel L130F missense mutation in FOXC1. Arch Ophthalmol. 125, 128–135. [DOI] [PubMed] [Google Scholar]
- Jordan T, Hanson I, Zaletayev D, Hodgson S, Prosser J, Seawright A, Hastie N, van Heyningen V, 1992. The human PAX6 gene is mutated in two patients with aniridia. Nat Genet. 1, 328–332. [DOI] [PubMed] [Google Scholar]
- Kapoor S, Mukherjee SB, Shroff D, Arora R, 2011. Dysmyelination of the cerebral white matter with microdeletion at 6p25. Indian Pediatr. 48, 727–729. [DOI] [PubMed] [Google Scholar]
- Kaur K, Ragge NK, Ragoussis J, 2009. Molecular analysis of FOXC1 in subjects presenting with severe developmental eye anomalies. Mol Vis. 15, 1366–1373. [PMC free article] [PubMed] [Google Scholar]
- Kawase C, Kawase K, Taniguchi T, Sugiyama K, Yamamoto T, Kitazawa Y, Alward WL, Stone EM, Nishimura DY, Sheffield VC, 2001. Screening for mutations of Axenfeld-Rieger syndrome caused by FOXC1 gene in Japanese patients. J Glaucoma. 10, 477–482. [DOI] [PubMed] [Google Scholar]
- Kelberman D, Islam L, Holder SE, Jacques TS, Calvas P, Hennekam RC, Nischal KK, Sowden JC, 2011. Digenic inheritance of mutations in FOXC1 and PITX2 : correlating transcription factor function and Axenfeld-Rieger disease severity. Hum Mutat. 32, 1144–1152. doi: 10.1002/humu.21550. [DOI] [PubMed] [Google Scholar]
- Khan AO, Al-Abdi L, Mohamed JY, Aldahmesh MA, Alkuraya FS, 2011. Familial juvenile glaucoma with underlying homozygous p.G61E CYP1B1 mutations. J AAPOS. 15, 198–199. doi: 10.1016/j.jaapos.2011.01.156. [DOI] [PubMed] [Google Scholar]
- Kim BS, Savinova OV, Reedy MV, Martin J, Lun Y, Gan L, Smith RS, Tomarev SI, John SW, Johnson RL, 2001. Targeted Disruption of the Myocilin Gene (Myoc) Suggests that Human Glaucoma-Causing Mutations Are Gain of Function. Mol Cell Biol. 21, 7707–7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim GN, Ki CS, Seo SW, Yoo JM, Han YS, Chung IY, Park JM, Kim SJ. A novel forkhead box C1 gene mutation in a Korean family with Axenfeld-Rieger syndrome. Mol Vis. 2013. April 30;19:935–43. [PMC free article] [PubMed] [Google Scholar]
- Komatireddy S, Chakrabarti S, Mandal AK, Reddy AB, Sampath S, Panicker SG, Balasubramanian D, 2003. Mutation spectrum of FOXC1 and clinical genetic heterogeneity of Axenfeld-Rieger anomaly in India. Mol Vis. 9, 43–48. [PubMed] [Google Scholar]
- Kume T, Deng KY, Winfrey V, Gould DB, Walter MA, Hogan BL,1998. The forkhead/winged helix gene Mf1 is disrupted in the pleiotropic mouse mutation congenital hydrocephalus. Cell. 93, 985–996. [DOI] [PubMed] [Google Scholar]
- Kume T, Jiang H, Topczewska JM, Hogan BL, 2001. The murine winged helix transcription factors, Foxc1 and Foxc2, are both required for cardiovascular development and somitogenesis. Genes Dev. 15, 2470–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Caignec C, De Mas P, Vincent MC, Boceno M, Bourrouillou G, Rival JM, David A, 2005. Subtelomeric 6p deletion: clinical, FISH, and array CGH characterization of two cases. Am J Med Genet A. 132A, 175–180. [DOI] [PubMed] [Google Scholar]
- Lehmann OJ, Ebenezer ND, Jordan T, Fox M, Ocaka L, Payne A, Leroy BP, Clark BJ, Hitchings RA, Povey S, Khaw PT, Bhattacharya SS, 2000. Chromosomal duplication involving the forkhead transcription factor gene FOXC1 causes iris hypoplasia and glaucoma. Am J Hum Genet. 67, 1129–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CJ, Hedberg-Buenz A, DeLuca AP, Stone EM, Alward WLM, Fingert JH, 2017. Primary congenital and developmental glaucomas. Hum Mol Genet. 26, R28–R36. doi: 10.1093/hmg/ddx205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Xu F, Zhu J, Krawczyk M, Zhang Y, Yuan J, Patel S, Wang Y, Lin Y, Zhang M, Cai H, Chen D, Zhang M, Cao G, Yeh E, Lin D, Su Q, Li WW, Sen GL, Afshari N, Chen S, Maas RL, Fu XD, Zhang K, Liu Y, Ouyang H, 2015. Transcription Factor PAX6 (Paired Box 6) Controls Limbal Stem Cell Lineage in Development and Disease. J Biol Chem. 290, 20448–20454. doi: 10.1074/jbc.M115.662940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin RJ, Cherry AM, Chen KC, Lyons M, Hoyme HE, Hudgins L, 2005. Terminal deletion of 6p results in a recognizable phenotype. Am J Med Genet A. 136, 162–168. [DOI] [PubMed] [Google Scholar]
- Linhares ND, Svartman M, Rodrigues TC, Rosenberg C, Valadares ER, 2015. Subtelomeric 6p25 deletion/duplication: Report of a patient with new clinical findings and genotype-phenotype correlations. Eur J Med Genet. 58, 310–318. doi: 10.1016/j.ejmg.2015.02.011. [DOI] [PubMed] [Google Scholar]
- López-Garrido MP, Medina-Trillo C, Morales-Fernandez L, Garcia-Feijoo J, Martínez-de-la-Casa JM, García-Antón M, Escribano J, 2013. Null CYP1B1 genotypes in primary congenital and nondominant juvenile glaucoma. Ophthalmology. 120, 716–723. doi: 10.1016/j.ophtha.2012.09.016. [DOI] [PubMed] [Google Scholar]
- Lowry RB, Gould DB, Walter MA, Savage PR, 2007. Absence of PITX2, BARX1, and FOXC1 mutations in De Hauwere syndrome (Axenfeld-Rieger anomaly, hydrocephaly, hearing loss): a 25-year follow up. Am J Med Genet A. 143A, 1227–1230. [DOI] [PubMed] [Google Scholar]
- Maclean K, Smith J, St Heaps L, Chia N, Williams R, Peters GB, Onikul E, McCrossin T, Lehmann OJ, Adès LC, 2005. Axenfeld-Rieger malformation and distinctive facial features: Clues to a recognizable 6p25 microdeletion syndrome. Am J Med Genet A. 132A, 381–385. [DOI] [PubMed] [Google Scholar]
- Martinez-Glez V, Lorda-Sanchez I, Ramirez JM, Ruiz-Barnes P, Rodriguez de Alba M, Diego-Alvarez D, Ramos C, Searby CC, Nishimura DY, Ayuso C, 2007. Clinical presentation of a variant of Axenfeld-Rieger syndrome associated with subtelomeric 6p deletion. Eur J Med Genet. 50, 120–127. [DOI] [PubMed] [Google Scholar]
- Mears AJ, Jordan T, Mirzayans F, Dubois S, Kume T, Parlee M, Ritch R, Koop B, Kuo WL, Collins C, Marshall J, Gould DB, Pearce W, Carlsson P, Enerback S, Morissette J, Bhattacharya S, Hogan B, Raymond V, Walter MA, 1998. Mutations of the forkhead/winged-helix gene, FKHL7, in patients with Axenfeld-Rieger anomaly. Am J Hum Genet. 63, 1316–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina-Trillo C, Aroca-Aguilar JD, Méndez-Hernández CD, Morales L, García-Antón M, García-Feijoo J, Escribano J, 2016. Rare FOXC1 variants in congenital glaucoma: identification of translation regulatory sequences. Eur J Hum Genet. 24, 672–680. doi: 10.1038/ejhg.2015.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheal S, Siddiqui SN, Zafar SN, Villanueva-Mendoza C, Cortés-González V, Khan MI, den Hollander AI, 2016. A Novel Homozygous Mutation in FOXC1 Causes Axenfeld Rieger Syndrome with Congenital Glaucoma. PLoS One. 11, e0160016. doi: 10.1371/journal.pone.0160016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzayans F, Gould DB, Héon E, Billingsley GD, Cheung JC, Mears AJ, Walter MA, 2000. Axenfeld-Rieger syndrome resulting from mutation of the FKHL7 gene on chromosome 6p25. Eur J Hum Genet. 8, 71–74. [DOI] [PubMed] [Google Scholar]
- Mirzayans F, Pearce WG, MacDonald IM, Walter MA, 1995. Mutation of the PAX6 gene in patients with autosomal dominant keratitis. Am J Hum Genet. 57, 539–548. [PMC free article] [PubMed] [Google Scholar]
- Mortemousque B, Amati-Bonneau P, Couture F, Graffan R, Dubois S, Colin J, Bonneau D, Morissette J, Lacombe D, Raymond V, 2004. Axenfeld-Rieger anomaly: a novel mutation in the forkhead box C1 (FOXC1) gene in a 4-generation family. Arch Ophthalmol. 122, 1527–1533. [DOI] [PubMed] [Google Scholar]
- Murphy TC, Saleem RA, Footz T, Ritch R, McGillivray B, Walter MA, 2004. The wing 2 region of the FOXC1 forkhead domain is necessary for normal DNA-binding and transactivation functions. Invest Ophthalmol Vis Sci. 45, 2531–2538. [DOI] [PubMed] [Google Scholar]
- Nag A, Lu H, Arno M, Iglesias AI, Bonnemaijer P, Broer L, Uitterlinden AG, Klaver CC, van Duijn C, Hysi PG, Hammond CJ, 2017. Evaluation of the Myocilin Mutation Gln368Stop Demonstrates Reduced Penetrance for Glaucoma in European Populations. Ophthalmology. 124, 547–553. doi: 10.1016/j.ophtha.2016.11.018. [DOI] [PubMed] [Google Scholar]
- Nakane T, Kousuke N, Sonoko H, Yuko K, Sato H, Kubota T, Sugita K, 2013. 6p subtelomere deletion with congenital glaucoma, severe mental retardation, and growth impairment. Pediatr Int. 55, 376–381. doi: 10.1111/j.1442-200X.2012.03729.x. [DOI] [PubMed] [Google Scholar]
- Nishimura DY, Searby CC, Alward WL, Walton D, Craig JE, Mackey DA, Kawase K, Kanis AB, Patil SR, Stone EM, Sheffield VC, 2001. A spectrum of FOXC1 mutations suggests gene dosage as a mechanism for developmental defects of the anterior chamber of the eye. Am J Hum Genet. 68, 364–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura DY, Swiderski RE, Alward WL, Searby CC, Patil SR, Bennet SR, Kanis AB, Gastier JM, Stone EM, Sheffield VC, 1998. The forkhead transcription factor gene FKHL7 is responsible for glaucoma phenotypes which map to 6p25. Nat Genet. 19, 140–147. [DOI] [PubMed] [Google Scholar]
- Ovaert C, Busa T, Faure E, Missirian C, Philip N, Paoli F, Milh M, Mace L, Zaffran S, 2017. FOXC1 haploinsufficiency due to 6p25 deletion in a patient with rapidly progressing aortic valve disease. Am J Med Genet A. 173, 2489–2493. doi: 10.1002/ajmg.a.38331. [DOI] [PubMed] [Google Scholar]
- Pace NP, Maggouta F, Twigden M, Borg I, 2017. Molecular cytogenetic characterisation of a novel de novo ring chromosome 6 involving a terminal 6p deletion and terminal 6q duplication in the different arms of the same chromosome. Mol Cytogenet. 10, 9. doi: 10.1186/s13039-017-0311-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panicker SG, Sampath S, Mandal AK, Reddy AB, Ahmed N, Hasnain SE, 2002. Novel mutation in FOXC1 wing region causing Axenfeld-Rieger anomaly. Invest Ophthalmol Vis Sci. 43, 3613–3616. [PubMed] [Google Scholar]
- Piccione M, Antona R, Salzano E, Cavani S, Malacarne M, Morreale Bubella R, Pierluigi M, Viaggi CD, Corsello G, 2012. Array-CGH and clinical characterization in a patient with subtelomeric 6p deletion without ocular dysgenesis. Am J Med Genet A. 2012 January;158A(1):150–4. doi: 10.1002/ajmg.a.34308. [DOI] [PubMed] [Google Scholar]
- Prosser J, van Heyningen V, 1998. PAX6 mutations reviewed. Hum Mutat. 11, 93–108. [DOI] [PubMed] [Google Scholar]
- Reis LM, Tyler RC, Volkmann Kloss BA, Schilter KF, Levin AV, Lowry RB, Zwijnenburg PJ, Stroh E, Broeckel U, Murray JC, Semina EV, 2012. PITX2 and FOXC1 spectrum of mutations in ocular syndromes. Eur J Hum Genet. 20, 1224–1233. doi: 10.1038/ejhg.2012.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis LM, Tyler RC, Weh E, Hendee KE, Schilter KF, Phillips JA 3rd, Sequeira S, Schinzel A, Semina EV, 2016. Whole exome sequencing identifies multiple diagnoses in congenital glaucoma with systemic anomalies. Clin Genet. 90, 378–382. doi: 10.1111/cge.12816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaie T, Child A, Hitchings R, Brice G, Miller L, Coca-Prados M, Héon E, Krupin T, Ritch R, Kreutzer D, Crick RP, Sarfarazi M, 2002. Adult-onset primary open-angle glaucoma caused by mutations in optineurin. Science. 295, 1077–1079. [DOI] [PubMed] [Google Scholar]
- Ritter KE, Martin DM, 2019. Neural crest contributions to the ear: Implications for congenital hearing disorders. Hear Res. 376,22–32. doi: 10.1016/j.heares.2018.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadagopan KA, Liu GT, Capasso JE, Wuthisiri W, Keep RB, Levin AV, 2015. Anirdia-like phenotype caused by 6p25 dosage aberrations. Am J Med Genet A. 167A, 524–528. doi: 10.1002/ajmg.a.36890. [DOI] [PubMed] [Google Scholar]
- Saleem RA, Banerjee-Basu S, Murphy TC, Baxevanis A, Walter MA, 2004. Essential structural and functional determinants within the forkhead domain of FOXC1. Nucleic Acids Res. 32,4182–4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem RA, Murphy TC, Liebmann JM, Walter MA, 2003. Identification and analysis of a novel mutation in the FOXC1 forkhead domain. Invest Ophthalmol Vis Sci. 2003 November;44(11):4608–12. [DOI] [PubMed] [Google Scholar]
- Seifi M, Walter MA, 2018. Axenfeld-Rieger syndrome. Clin Genet. 93, 1123–1130. doi: 10.1111/cge.13148. Epub 2018 January 25. [DOI] [PubMed] [Google Scholar]
- Semina EV, Reiter R, Leysens NJ, Alward WL, Small KW, Datson NA, Siegel-Bartelt J, Bierke-Nelson D, Bitoun P, Zabel BU, Carey JC, Murray JC, 1996. Cloning and characterization of a novel bicoid-related homeobox transcription factor gene, RIEG, involved in Rieger syndrome. Nat Genet. 14, 392–399. [DOI] [PubMed] [Google Scholar]
- Siggs OM, Souzeau E, Pasutto F, Dubowsky A, Smith JEH, Taranath D, Pater J, Rait JL, Narita A, Mauri L, Del Longo A, Reis A, Chappell A, Kearns LS, Staffieri SE, Elder JE, Ruddle JB, Hewitt AW, Burdon KP, Mackey DA, Craig JE, 2019. Prevalence of FOXC1 Variants in Individuals With a Suspected Diagnosis of Primary Congenital Glaucoma. JAMA Ophthalmol. doi: 10.1001/jamaophthalmol.2018.5646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souma T, Tompson SW, Thomson BR, Siggs OM, Kizhatil K, Yamaguchi S, Feng L, Limviphuvadh V, Whisenhunt KN, Maurer-Stroh S, Yanovitch TL, Kalaydjieva L, Azmanov DN, Finzi S, Mauri L, Javadiyan S, Souzeau E, Zhou T, Hewitt AW, Kloss B, Burdon KP, Mackey DA, Allen KF, Ruddle JB, Lim SH, Rozen S, Tran-Viet KN, Liu X, John S, Wiggs JL, Pasutto F, Craig JE, Jin J, Quaggin SE, Young TL, 2016. Angiopoietin receptor TEK mutations underlie primary congenital glaucoma with variable expressivity. J Clin Invest. 126, 2575–2587. doi: 10.1172/JCI85830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souzeau E, Siggs OM, Zhou T, Galanopoulos A, Hodson T, Taranath D, Mills RA, Landers J, Pater J, Smith JE, Elder JE, Rait JL, Giles P, Phakey V, Staffieri SE, Kearns LS, Dubowsky A, Mackey DA, Hewitt AW, Ruddle JB, Burdon KP, Craig JE, 2017. Glaucoma spectrum and age-related prevalence of individuals with FOXC1 and PITX2 variants. Eur J Hum Genet. 25,1290. doi: 10.1038/ejhg.2017.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoilov I, Akarsu AN, Sarfarazi M, 1997. Identification of three different truncating mutations in cytochrome P4501B1 (CYP1B1) as the principal cause of primary congenital glaucoma (Buphthalmos) in families linked to the GLC3A locus on chromosome 2p21. Hum Mol Genet. 6, 641–647. [DOI] [PubMed] [Google Scholar]
- Stone EM, Fingert JH, Alward WL, Nguyen TD, Polansky JR, Sunden SL, Nishimura D, Clark AF, Nystuen A, Nichols BE, Mackey DA, Ritch R, Kalenak JW, Craven ER, Sheffield VC, 1997. Identification of a gene that causes primary open angle glaucoma. Science 275, 668–670. [DOI] [PubMed] [Google Scholar]
- Suri F, Yazdani S, Narooie-Nejhad M, Zargar SJ, Paylakhi SH, Zeinali S, Pakravan M, Elahi E, 2009. Variable expressivity and high penetrance of CYP1B1 mutations associated with primary congenital glaucoma. Ophthalmology. 116, 2101–2109. doi: 10.1016/j.ophtha.2009.04.045. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Nakamura M, Amano E, Mokuno K, Shirai S, Terasaki H, 2006. Case of chromosome 6p25 terminal deletion associated with Axenfeld-Rieger syndrome and persistent hyperplastic primary vitreous. Am J Med Genet A. 140, 503–508. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Takahashi K, Kuwahara S, Wada Y, Abe T, Tamai M, 2001. A novel (Pro79Thr) mutation in the FKHL7 gene in a Japanese family with Axenfeld-Rieger syndrome. Am J Ophthalmol. 32, 572–575. [DOI] [PubMed] [Google Scholar]
- Swiderski RE, Reiter RS, Nishimura DY, Alward WL, Kalenak JW, Searby CS, Stone EM, Sheffield VC, Lin JJ, 1999. Expression of the Mf1 gene in developing mouse hearts: implication in the development of human congenital heart defects. Dev Dyn. 216, 16–27. [DOI] [PubMed] [Google Scholar]
- Thomson BR, Souma T, Tompson SW, Onay T, Kizhatil K, Siggs OM, Feng L, Whisenhunt KN, Yanovitch TL, Kalaydjieva L, Azmanov DN, Finzi S, Tanna CE, Hewitt AW, Mackey DA, Bradfield YS, Souzeau E, Javadiyan S, Wiggs JL, Pasutto F, Liu X, John SW, Craig JE, Jin J, Young TL, Quaggin SE, 2017. Angiopoietin-1 is required for Schlemm’s canal development in mice and humans. J Clin Invest. 127, 4421–4436. doi: 10.1172/JCI95545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonoki H, Harada N, Shimokawa O, Yosozumi A, Monzaki K, Satoh K, Kosaki R, Sato A, Matsumoto N, Iizuka S, 2011. Axenfeld-Rieger anomaly and Axenfeld-Rieger syndrome: clinical, molecular-cytogenetic, and DNA array analyses of three patients with chromosomal defects at 6p25. Am J Med Genet A. 155A, 2925–2932. doi: 10.1002/ajmg.a.33858. [DOI] [PubMed] [Google Scholar]
- Vernon HJ, Bytyci Telegrafi A, Batista D, Owegi M, Leigh R, 2013. 6p25 microdeletion: white matter abnormalities in an adult patient. Am J Med Genet A. 161A, 1686–1689. doi: 10.1002/ajmg.a.35937. [DOI] [PubMed] [Google Scholar]
- Weegerink NJ, Swinnen FK, Vanakker OM, Casselman JW, Dhooge IJ, 2016. Phenotype of a Belgian Family With 6p25 Deletion Syndrome. Ann Otol Rhinol Laryngol. 2016 September;125(9):734–45. doi: 10.1177/0003489416650687. [DOI] [PubMed] [Google Scholar]
- Weisschuh N, Dressler P, Schuettauf F, Wolf C, Wissinger B, Gramer E, 2006. Novel mutations of FOXC1 and PITX2 in patients with Axenfeld-Rieger malformations. Invest Ophthalmol Vis Sci. 47, 3846–3852. [DOI] [PubMed] [Google Scholar]
- Weisschuh N, Wolf C, Wissinger B, Gramer E, 2008. A novel mutation in the FOXC1 gene in a family with Axenfeld-Rieger syndrome and Peters’ anomaly. Clin Genet. 74, 476–480. doi: 10.1111/j.1399-0004.2008.01025.x. [DOI] [PubMed] [Google Scholar]
- Wiggs JL, Allingham RR, Vollrath D, Jones KH, De La Paz M, Kern J, Patterson K, Babb VL, Del Bono EA, Broomer BW, Pericak-Vance MA, Haines JL, 1998. Prevalence of mutations in TIGR/Myocilin in patients with adult and juvenile primary open-angle glaucoma.Am J Hum Genet. 63, 1549–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggs JL, Vollrath D, 2001. Molecular and clinical evaluation of a patient hemizygous for TIGR/MYOC. Arch Ophthalmol. 119, 1674–1678. [DOI] [PubMed] [Google Scholar]
- Xu P, Balczerski B, Ciozda A, Louie K, Oralova V, Huysseune A, Crump JG, 2018. Fox proteins are modular competency factors for facial cartilage and tooth specification. Development. 26, 145(12). pii: dev165498. doi: 10.1242/dev.165498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HJ, Lee YK, Joo CK, Moon JI, Mok JW, Park MH, 2015. A Family with Axenfeld-Rieger Syndrome: Report of the Clinical and Genetic Findings. Korean J Ophthalmol. 29, 249–255. doi: 10.3341/kjo.2015.29.4.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagora SL, Funnell CL, Martin FJ, Smith JE, Hing S, Billson FA, Veillard AS, Jamieson RV, Grigg JR, 2015. Primary congenital glaucoma outcomes: lessons from 23 years of follow-up. Am J Ophthalmol. 2015 159, 788–796. doi: 10.1016/j.ajo.2015.01.019. [DOI] [PubMed] [Google Scholar]
- Zhang HZ, Li P, Wang D, Huff S, Nimmakayalu M, Qumsiyeh M, Pober BR, 2004. FOXC1 gene deletion is associated with eye anomalies in ring chromosome 6. Am J Med Genet A. 124A, 280–287. [DOI] [PubMed] [Google Scholar]
- Zhang R, Chen X, Li P, Lu X, Liu Y, Li Y, Zhang L, Xu M, Cram DS, 2016. Molecular characterization of a novel ring 6 chromosome using next generation sequencing. Mol Cytogenet. 9, 33. doi: 10.1186/s13039-016-0245-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, 2016. Forkhead box transcription factors in embryonic heart development and congenital heart disease. Life Sci. 144,194–201. doi: 10.1016/j.lfs.2015.12.001. [DOI] [PubMed] [Google Scholar]