Abstract
Purpose
To evaluate the relationship between refractive error, circadian phase, and melatonin with consideration of prior light exposure, physical activity, and sleep.
Methods
Healthy young myopic (spherical equivalent refraction [SER] ≤−0.50DS) and emmetropic adults underwent noncycloplegic autorefraction and axial length (AL) measures. Objective measurements of light exposure, physical activity, and sleep were captured across 7 days by wrist-worn Actiwatch-2 devices. Questionnaires assessed sleep quality and chronotype. Hourly evening saliva sampling during a dim-light melatonin onset (DLMO) protocol evaluated circadian phase, and both morning serum and saliva samples were collected. Liquid chromatography/mass spectrometry quantified melatonin.
Results
Subjects (n = 51) were aged 21.4 (interquartile range, 20.1−24.0) years. Melatonin was significantly higher in the myopic group at every evening time point and with both morning serum and saliva sampling (P ≤ 0.001 for all). DLMO-derived circadian phase did not differ between groups (P = 0.98). Multiple linear regression analysis demonstrated significant associations between serum melatonin and SER (B = –.34, β = –.42, P = 0.001), moderate activity (B = .009, β = .32, P = 0.01), and mesopic illumination (B = –.007, β = –.29, P = 0.02), F(3, 46) = 7.23, P < 0.001, R2 = 0.32, R2adjusted = .28. Myopes spent significantly more time exposed to “indoor” photopic illumination (3 to ≤1000 lux; P = 0.05), but “indoor” photopic illumination was not associated with SER, AL, or melatonin, and neither sleep, physical activity, nor any other light exposure metric differed significantly between groups (P > 0.05 for all).
Conclusions
While circadian phase is aligned in adult myopes and emmetropes, myopia is associated with both elevated serum and salivary melatonin levels. Prospective studies are required to ascertain whether elevated melatonin levels occur before, during, or after myopia development.
Keywords: circadian rhythm, circadian phase, DLMO (dim-light melatonin onset), melatonin, myopia, refractive error, light exposure, physical activity
During childhood, visual experience guides homeostatic eye growth. This complex regulatory process aims to achieve a fine balance between the eye's axial length and optical power so that light is focused directly on the retina. Failure of this process leads to refractive error, including myopia, a progressive condition typically caused by excessive eye growth and characterized by blurred distance vision.1 In addition to causing blurred distance vision, increasing levels of myopia are associated with an increased lifetime risk of sight-threatening ocular pathology.2 It is therefore of concern that myopia is predicted to affect almost 50% of the global population by the year 2050.3
Both genetic and environmental factors contribute to myopia development; however, the exact pathophysiology is unclear. It is thought that exposure to modern urban environments, prioritizing education and providing less opportunity for spending time outdoors, plays a major role in the recent surge of myopia.4,5 Observational studies and randomized controlled trials have established that spending more time outdoors protects against myopia onset.6–14 This finding has been used to develop antimyopia public health policies in countries with high myopia prevalence.15–17 However, the precise protective elements by which the outdoor environment shields against myopia are unclear.18 This is problematic, especially in countries where ultraviolet (UV) radiation is high. Advising children to spend more time outdoors may be counterintuitive to decades of public health campaigning aimed at preventing UV-related skin damage and pathology. Therefore, defining the beneficial aspects of the outdoor environment and exploring the potential biological pathways by which time outdoors protects against myopia are urgently required to refine global public health guidelines.
Spending more time outdoors reduces opportunity for near work and a sedentary lifestyle, both observed risk factors for myopia.19,20 However, as time spent outdoors has been found to independently predict myopia incidence7 and remains protective against myopia development after controlling for near work and physical activity,21 it is unlikely that these factors are crucial protective aspects of time outdoors. Potential protective features of the outdoor environment include a greater illuminance and broader spectrum of light, a higher spatial frequency content of visual input, and reduced opportunity for the peripheral retina to receive growth-promoting defocus signals.2,22 The mechanisms by which these features may act, alone or in combination, to regulate homeostatic eye growth also remain unclear.
Increasing evidence implicates circadian rhythms in refractive error development (see Chakraborty et al.23 for a comprehensive review). Supportive of the hypotheses presented by Chakraborty et al.,23 recent work demonstrates that knockout of the circadian gene Bmal1 in the mouse retina or the circadian genes cycle and period in Drosophila melanogaster both result in myopic phenotypes,24 and findings from a recent meta-analysis of genome-wide association studies suggest that genetic factors that control circadian rhythms are involved in human myopia development.25
Circadian rhythms are biological processes that display an endogenous, entrainable oscillation of approximately 24 hours. In humans, these rhythms form a hierarchy of oscillators, orchestrated by a central pacemaker located in the suprachiasmatic nuclei (SCN), to synchronize our physiology and behaviors to the changing demands of the day-night cycle.26 In addition to internal regulation by the central pacemaker, temporal information can be elicited from various external cues, including light exposure, physical activity, mealtimes, and social interactions. Rhythmic changes in the light-dark cycle and alterations thereof provide the most potent zeitgeber to our circadian system.27,28
When light stimulates the retina, photoreceptor signals are integrated through short-wavelength-sensitive intrinsically photosensitive retinal ganglion cells to initiate the release of dopamine from amacrine cells and the inhibition of melatonin synthesis in the pineal gland. Dopamine and melatonin are mutually inhibitory hormones that oscillate in antiphase with circadian rhythmicity, helping to coordinate the circadian system.29 Typically, dopamine synthesis peaks during daylight hours and melatonin secretion peaks at night. The secretion of these hormones can be influenced by altering environmental light exposure patterns, encouraging or suppressing secretion earlier or later in the 24-hour cycle. For example, greater levels of light exposure in the morning advance melatonin secretion, and conversely, if more light is delivered in the evening, a common phenomenon in modern life, melatonin secretion is delayed.30,31
A role for dopamine in eye growth signaling has been strongly implicated by previous research,23,32,33 and dopamine is proposed to mediate the protective effects of increased time outdoors against myopia by regulating circadian physiology.23,34 Circadian rhythms are known to exist within the eye,35,36 and various ocular parameters have been seen to demonstrate diurnal oscillation.37–39 Briefly, greater levels of dopamine appear protective against experimental myopia. In the chick eye, daytime levels of retinal dopamine and its principal metabolite, 3,4-dihydroxyphenylacetic acid, have been shown to reduce in response to form deprivation40,41 or lens-induced myopia42 and to recover to original levels 1 week following the removal of form deprivation.41 Moreover, application of a dopamine agonist, apomorphine, by eye drop or subconjunctival injection increases retinal dopamine and mitigates against form deprivation myopia40,43,44 and lens-induced myopia in various animal models.45,46 Dopamine is known to contribute to the regulation of ocular rhythms35,36 and is upregulated in both retinal and neural tissue when light is detected by the retina.47,48 Furthermore, intravitreal injection of a dopamine antagonist, spiperone, abolishes the protective effect of bright light against form deprivation myopia.49
Due to the challenges associated with quantifying retinal dopamine in humans, research exploring dopaminergic pathways and myopia has been mostly restricted to animal models. One refractive error study evaluated circulating dopamine levels in young adults and reported significantly lower serum levels in adult myopes compared with nonmyopes at the end of winter but not summer.50 However, due to dopamine's complex synthesis by both the central nervous system and peripheral organs, as well as its inability to cross the blood-brain barrier, it is unclear whether measuring circulating dopamine in isolation of its metabolites is appropriate and whether results can be extrapolated to usefully indicate retinal dopamine levels in the context of refractive error studies.51
For these reasons, studies evaluating the relationship between human myopia and circadian rhythms have assessed the circadian hormone melatonin, a well-documented robust biomarker of internal circadian phase.52 Due to melatonin's short half-life, the 24-hour oscillation of circulating melatonin measured under constant routine conditions accurately reflects the oscillation of the endogenous pacemaker. However, frequent sampling of circulating melatonin is invasive, time-consuming, and expensive. Consequently, alternative biomarkers that require less sampling, such as the dim-light melatonin onset (DLMO), have been developed to assess circadian phase.53,54
In 2017, Kearney et al.50 reported a significant association between human refractive error, axial length, and morning serum melatonin; circulating melatonin was almost three times higher in myopes compared with nonmyopes. Melatonin has a dynamic circadian secretion profile, and Kearney et al. sampled melatonin from a single point in this cycle. As such, the authors were not able to ascertain whether the difference in melatonin between the myopic and nonmyopic group was reflective of a phase shift in melatonin's secretion and/or a higher concentration in circulating melatonin sustained across all or part of the 24-hour cycle.
The present cross-sectional study builds on Kearney et al.50 by further exploring differences between melatonin concentrations in young adults with and without myopia. In particular, investigating whether circadian phase (identified by DLMO) differs between myopes and emmetropes and whether saliva samples obtained through noninvasive methods elicit interrefractive group differences in melatonin concentration comparable to those seen in serum. Furthermore, participants’ prior light exposure, physical activity, and sleep were considered in the present evaluation of melatonin and refractive error.
Methods
The study was approved by the Ulster University Research Ethics Committee (REC/18/0089) and adhered to the tenets of the Declaration of Helsinki, and subjects provided written informed consent prior to participation. Recruitment and data collection occurred over 8 weeks at the end of winter (February to March 2019) prior to the onset of daylight saving time (DST, March 31, 2019). Daylength for each subject's participation period was recorded from an online database (www.timeanddate.com), and these data were considered during data analysis. Consented subjects were requested to attend three study appointments at Ulster University's Coleraine campus (55°N).
Subjects were aged 18 to 30 years and had not previously taken part in studies of refractive error and melatonin in our laboratory (Kearney et al.). Participant age was limited as melatonin concentration is known to reduce with age.55 Individuals were excluded if they were taking prescribed medication (e.g., melatonin supplements) or diagnosed with a medical condition known to affect systemic melatonin concentration, were a regular smoker (including the use of a nicotine vaporizer), were pregnant, were breastfeeding, had irregular menstrual cycles, currently were undergoing myopia intervention, were diagnosed with ocular disease, had experienced previous ocular surgery, had completed regular nightshift work within the past year, had traveled across more than one time zone in the past month, had a physical disability that would prevent them from completing the protocol, or demonstrated a noncycloplegic hyperopic autorefraction greater than +2.00 DS.
Visit 1
A brief ocular and clinical history was taken from each subject to confirm eligibility. Parental history of myopia was established through a validated questionnaire,56 and any prescribed or nonprescribed medication and supplements were noted. The impact of menstrual cycle on melatonin status is unclear.57 For this reason, female subjects were asked to provide the date of the most recent menstruation to estimate menstrual phase during sample collection (menstrual phase [from days 1 to 5]; follicular phase [from days 1 to 13]; ovulation phase [day 14]; luteal phase [from days 15 to 28]). In line with Kearney et al.,50 open-field noncycloplegic autorefraction (SRW-5000; Shin‐Nippon, Tokyo, Japan) was performed bilaterally while subjects viewed a distance target. Myopia was defined as spherical equivalent refraction (SER) ≤−0.50DS in either eye, and subjects were assigned to the “myopic” or “emmetropic” group. Axial length (IOLMaster; ZEISS, Carl Zeiss Meditec AG Jena, Germany) was then measured in the right eye. To elicit information on sleep quality and chronotype (i.e., whether individuals are morning type or evening type), subjects completed the Pittsburgh Sleep Quality Index (PSQI)58 and the Horne Ostberg Morningness-Eveningness Questionnaire (MEQ),59 respectively. Subjects were fitted with an Actiwatch-2 device (Actiwatch-2; Philips Respironics, Murrysville, Pennsylvania, United States) on their nondominant wrist. The Actiwatch-2 is a validated objective method of measuring sleep/wake patterns and has been used in numerous myopia-related investigations to quantify light exposure and physical activity.60–62 The device contains a solid-state piezoelectric accelerometer with a sampling rate of 32 Hz to measure physical activity in counts per minute (CPM) and a silicone photodiode light sensor to measure photopic illuminance between 5 and 100,000 lux (wavelength range 400–900 nm; peak sensitivity 570 nm). Subjects were requested to refrain from removing the device during the sampling period, except when in water for more than 30 minutes, and were told to keep the light sensor uncovered by clothing. Devices were programmed to record at 30-second epochs and dispensed for 7 days.
Visit 2
One week later, subjects returned to the laboratory at 18:45:00 for the dim-light melatonin phase assessment. Wrist-worn devices were returned to the researcher and data downloaded. Subjects were requested to eat their evening meal prior to 18:00:00 and to refrain from alcohol, cigarettes, nicotine, aspirin, anti-inflammatory-type medications, and certain foods rich in melatonin (bananas, grapes, cereals, olives, nuts, etc.) 12 hours prior to the laboratory visit. Room lights were switched off and residual environmental lighting was below 30 lux. Screen use was permitted but only at the lowest brightness intensity and 100% red saturation. Subjects provided hourly saliva samples from 19:00:00 until habitual bedtime. Habitual bedtime was defined for each subject as their average bedtime recorded by the Actiwatch device over the previous 7 nights. A small pastry was provided after the 20:00:00 sample. Eating was prohibited within 30 minutes of sampling. Where habitual bedtime had not been reached after the 23:00:00 sample, subjects were allowed to return home but were requested to continue hourly sampling at home in a similar dim environment until habitual bedtime. Previous research has found that samples can be reliably collected in the home environment.63,64 Samples collected at home were prelabeled, immediately stored in the individual's fridge, and returned to the primary researcher (SCF) the following morning.
Visit 3
On waking the next day, subjects provided a saliva sample prior to getting out of bed and turning room lights on. Collection time was recorded. Subjects attended the laboratory at 08:20:00 to return all saliva samples, and between 08:30:00 and 10:00:00, serum and final saliva samples were collected in a seated position under uniform indoor photopic illumination.
Sample Collection and Melatonin Analysis
Blood samples were collected from the antecubital vein. Samples were centrifuged at 1800 g for 15 minutes at 4°C within 1 hour of collection, and 1000 µL of serum was isolated from the centrifuged sample. Saliva samples were collected via the “passive drool” method with subjects in a seated position. Subjects were asked to rinse their mouth with water 10 minutes prior to saliva collection and to refrain from using lip makeup during the sampling period. Subjects were then instructed to tilt their head forward and let saliva pool on the floor of their mouths before drooling the sample through a SalivaBio Collection Aid (Salimetrics LLC, State College, PA, USA) into a polypropylene vial. All samples were stored at −80°C prior to analysis.
Melatonin quantitation in all biomatrices was conducted by online solid-phase extraction (SPE) coupled with high-performance liquid chromatography tandem mass spectrometry (HPLC-MS/MS). HPLC was a Shimadzu Nexera XR (Kyoto, Japan), and separation was performed in gradient mode. Mass spectrometer was an API 4000 triple quadrupole (QQQ) (AB Sciex, Warrington, UK) equipped with a turbo ion spray source. Melatonin powder, ≥98%, was purchased from Sigma-Aldrich (Dorset, UK). Melatonin-d4 (internal standard, ISTD), was purchased from Cambridge Bioscience (Cambridge, UK). All other reagents were from Sigma-Aldrich unless otherwise stated. Methods for all biomatrices were developed and validated in accordance to The International Council for Harmonisation (ICH) of Technical Requirements for Pharmaceuticals for Human Use guidelines for bioanalytical method validation (ICH M10, 2019) by the Mass Spectrometry Centre at Ulster University (Appendix).
DLMO
The DLMO was calculated dynamically using the “3k” threshold method.52,65 Individual thresholds were calculated for each subject as the mean plus two standard deviations of three low daytime melatonin concentrations. A dynamic threshold was chosen in light of previously identified refractive error–related variances in melatonin concentration.50 Published research reports that this dynamic method is acceptable when using an hourly sampling protocol and provides a better estimate of the point at which melatonin levels begin to rise, compared with alternative methods such as the low static threshold approach.66 DLMO was defined for each subject as the time at which melatonin rose above the “3k” threshold, determined by linear interpolation between the points immediately above and below the threshold.
Actiwatch Data Analysis
Actiwatch data were downloaded and analyzed using the Philips Respironics software (Actiware 6.0.9). An automatic software algorithm determined major rest intervals on each actogram. Actograms were visually analyzed, and periods where the device had been removed for 15 minutes or more were excluded. This process removed 2.6% of data in total across all participants. Actiware software summary sleep statistics were generated and mean values extracted and used in analysis.60,67–70 Raw light exposure and physical activity data were exported to an Excel file. To align with previous myopia research,61,62 time spent “outdoors” was defined as the time spent exposed to illuminations greater than 1000 lux. Light exposure data were subsequently categorized to determine the daily time (in minutes) subjects spent exposed to scotopic (≤0.01 lux), mesopic (0.01 to ≤3 lux), indoor photopic (3 to ≤1000 lux), and outdoor photopic (>1000 lux) illumination. Furthermore, both the mean time at which exposure to illuminations greater than 1000 lux (MLiT1000) occurred and the standard deviation of MLiT1000 were calculated as described by Reid et al.71 This allowed examination of relations between refractive error, circadian phase or melatonin, and the timing and spread of “outdoor” light exposure. Briefly, MLiT1000 was defined as the average clock time of all light exposure data points above 1000 lux. Physical activity data were categorized to determine the time spent in sedentary (≤59 CPM), light (59 to ≤399 CPM), moderate (399 to ≤1404 CPM), and vigorous (>1404 CPM) activity during active periods. Cutoff points for physical activity categories were derived from a validation study evaluating the Actiwatch-2 device in a similar young adult cohort.72
Statistical Analysis
Statistical analyses were performed in SPSS (IBM SPSS Statistics for Windows Version 25.0; SPSS, Chicago, IL). Data were analyzed for normality using the Shapiro-Wilk test. Parametric data were analyzed using independent sample t-tests. Nonparametric data were analyzed using the Mann-Whitney U test for independent samples. Spearman's correlation assessed the correlation between melatonin, SER, axial length, objective light exposure, physical activity, and sleep metrics. Pearson's chi-square test assessed whether PSQI or MEQ scores were associated with sex or the presence/absence of myopia. Multiple regression analysis evaluated the strength of association between significant variables and melatonin concentration. Results are presented as median (interquartile range). In all instances, a P value of ≤0.05 was considered statistically significant. Holm-Bonferroni correction was applied to control for multiple statistical testing of salivary melatonin across the sampling period.73
Results
Fifty-one subjects completed the protocol across 8 weeks. Noncycloplegic spherical equivalent refraction did not differ significantly between right and left eyes (P = 0.94). Only right eye data are considered further. Table 1 summarizes sex, age, and refractive and axial length data, and Table 2 summarizes subjective and objective measures of chronotype, sleep, activity, and light exposure data for all subjects and by refractive group. Briefly, chronotype, sleep quality and duration, and physical activity metrics did not differ significantly between refractive groups. Neither refractive group nor gender were significantly associated with higher proportions of subjects categorized as having “good” or “poor” PSQI sleep quality (χ2 = .35, P = 0.56 and χ2 = .13, P = 0.72, respectively), and chronotype classification, as determined by the MEQ, was not significantly associated with refractive group (χ2 = 2.74, P = 0.26) or sex (χ2 = 3.12, P = 0.21). Neither date nor daylength at time of assessment differed significantly between refractive groups (P = 0.73 for both). As a group, myopes spent statistically more time exposed to “indoor” photopic conditions compared with their nonmyopic peers (P = 0.05, Table 3). However, the amount of time individuals spent “indoors” was not significantly correlated to SER or axial length (Spearman's rank, ρ = –.17, P = 0.24 and ρ = .20, P = 0.16, respectively), and no other light exposure parameter differed significantly between groups (Tables 2 and 3). Of note, MLiT1000 tended to be earlier for myopes compared with emmetropes, but the difference between refractive error groups did not reach statistical significance (P = 0.07), and MLiT1000 was not significantly correlated to either SER or axial length (AL) (Spearman's rank, ρ = –.17, P = 0.23 and ρ = –.08, P = 0.58, respectively).
Table 1.
Demographics for All Subjects, Myopes, and Emmetropes [median (IQR)]
| Characteristic | All Subjects (n = 51) | Myopes (n = 28) | Emmetropes (n = 23) | P Value |
|---|---|---|---|---|
| Sex, female/male, n | 29/22 | 17/11 | 12/11 | |
| Age (y) | 21.4 (20.1 to 24.0) | 21.7 (20.2 to 23.4) | 21.2 (20.0 to 24.4) | 0.86 |
| SER (DS)* | −0.94 (−3.28 to +0.28) | −3.18 (−4.26 to −1.78) | 0.44 (−0.03 to +0.58) | <0.0001† |
| Axial length (mm)* | 24.24 (23.57 to 25.40) | 25.32 (24.72 to 25.84) | 23.57 (23.04 to 23.86) | <0.0001† |
Values are presented as median (interquartile range) unless otherwise indicated.
P value for two-tailed Mann-Whitney U test compares myopes to emmetropes.
*Indicates independent samples t-test comparing myopes to emmetropes.
Indicates significance at P ≤ 0.05.
Table 2.
Subjective PSQI and MEQ Outputs and Objective Light Exposure, Physical Activity, and Sleep Metrics Derived From Actiwatch Devices for All Subjects, Myopes, and Emmetropes
| Characteristic | All Subjects (n = 51) | Myopes (n = 28) | Emmetropes (n = 23) | P Value |
|---|---|---|---|---|
| MEQ | ||||
| MEQ score | 48 (43–55) | 47 (42–54) | 48 (44–56.50) | 0.38 |
| MEQ chronotype | 23 intermediate; 4 moderate evening; 1 moderate morning | 16 intermediate; 3 moderate evening; 4 moderate morning | ||
| PSQI | ||||
| PSQI score | 5 (4–7) | 5 (4–7.25) | 5 (4–6) | 0.82 |
| Bedtime (hh:mm:ss)* | 23:45:00 (23:00:00–00:00:00) | 00:00:00 (23:11:15–00:30:00) | 23:30:00 (23:30:00–00:00:00) | 0.06 |
| Waketime (hh:mm:ss) | 8:00:00 (7:30:00–8:02:30) |
8:00:00 (7:45:00–8:18:45) |
8:00:00 (7:30:00–8:00:00) |
0.27 |
| Sleep onset (min) | 20 (15–30) | 20 (15–26.25) | 20 (15–30) | 0.43 |
| Time asleep (h) | 7 (6.5–8) | 7 (6.38–7.63) | 7 (7–8) | 0.08 |
| Sleep quality | 12 good; 16 poor | 8 good; 15 poor | ||
| Light exposure | ||||
| Daylength (hh:mm:ss) | 11:06:35 (10:26:25–12:09:41) | 11:20:06 (10:26:14–12:10:48) | 11:06:35 (10:15:29–11:49:23) | 0.73 |
| Total daily light exposure (lux) | 128,387 (81,145–208,614) | 137,715 (93,134–201,631) | 124,000 (70,491–200,492) | 0.52 |
| Mean daily light exposure (lux) | 119 (80–177) | 127 (93–174) | 105 (66–208) | 0.65 |
| Maximum light exposure (lux)* | 12,873 (8033–16,706) | 15,145 (8523–19,529) | 12,036 (7950–14,512) | 0.26 |
| MLiT1000 (hh:mm:ss) | 13:22:29 (13:05:30–13:49:34) | 13:19:08 (12:39:28–13:46:41) | 13:28:51 (13:17:25–13:57:50) | 0.07 |
| SD MLiT1000 (hh:mm:ss) | 01:50:47 (01:34:14–02:06:44) | 01:55:21 (01:37:07–02:14:09) | 01:40:04 (01:33:26–01:59:00) | 0.18 |
| Physical activity | ||||
| Total daily physical activity (counts) | 255,214 (227,523–310,234) | 246,192 (230,252–287,957) | 278,343 (226,483–334,183) | 0.36 |
| Mean daily physical activity (CPM) | 221 (190–268) | 216 (191–262) | 224 (190–285) | 0.38 |
| Sleep metrics | ||||
| Bedtime* | 00:16:17 (23:42:38–00:50:30) | 00:12:47 (23:39:38–1:02:32) | 00:20:00 (23:59:36–00:40:33) | 0.79 |
| Get-up time* | 8:45:40 (8:08:12–9:05:29) | 8:47:51 (8:10:34–9:04:33) | 8:38:47 (8:04:26–9:03:03) | 0.85 |
| Mid-sleep time* | 4:31:47 (3:59:31–4:55:08) | 4:30:43 (3:54:53–5:02:45) | 4:32:10 (4:06:12–4:49:32) | 0.72 |
| Total sleep* | 6:58:04 (6:25:49–7:24:00) | 7:02:22 (6:29:15–7:32:06) | 6:55:47 (6:25:49–7:19:40) | 0.98 |
| Sleep onset (min) | 22.64 (16.79–33.43) | 23.32 (18.27–33.47) | 22.57 (16.21–32.04) | 0.61 |
| Sleep efficiency (%) | 82.74 (79.76–85.75) | 83.29 (81.11–86.08) | 81.51 (78.82–84.88) | 0.34 |
Values are presented as median (interquartile range) unless otherwise indicated. P value for two-tailed Mann-Whitney U test compares myopes to emmetropes.
Subjects were labeled as having good sleep quality if PSQI score was <5 and poor sleep quality if PSQI score was ≥5.58
Indicates independent samples t-test comparing myopes to emmetropes.
Table 3.
Daily Time (Minutes) Spent in Different Intensities of Light Exposure (Scotopic, Mesopic, Indoor Photopic, and Outdoor Photopic) and Physical Activity (Sedentary, Light, Moderate, and Vigorous Activity During Active Periods) for All Subjects, Myopes, and Emmetropes
| Characteristic | All Subjects (n = 51) | Myopes (n = 28) | Nonmyopes (n = 23) | P Value |
|---|---|---|---|---|
| Light exposure | ||||
| Scotopic (<0.01 lux) |
556 (495–616) | 564 (476-615) | 547 (517–614) | 0.65 |
| Mesopic (0.01 to <3 lux) |
200 (161–258) | 185 (152–247) | 242 (173–264) | 0.23 |
| Indoor photopic (3 to <1000 lux)* |
633 (543–711) | 649 (584–742) | 606 (511–687) | 0.05† |
| Outdoor photopic (≥1000 lux) |
26 (16–43) | 27 (19–44) | 26 (14–41) | 0.58 |
| Physical activity | ||||
| Sedentary activity (<59 CPM)* |
315 (244–360) | 321 (241–368) | 317 (274–347) | 0.85 |
| Light activity (59 to <399 CPM)* |
399 (342–430) | 401 (344–429) | 383 (347–439) | 0.84 |
| Moderate activity (399 to <1404 CPM) |
247 (215–300) | 248 (223–299) | 228 (212–330) | 0.91 |
| Vigorous activity (≥1404 CPM) |
16 (10–27) | 16 (10–23) | 15 (10–35) | 0.33 |
Values are presented as median (interquartile range) unless otherwise indicated. P value for two-tailed Mann-Whitney U test compares myopes to emmetropes.
Indicates independent samples t-test comparing myopes to emmetropes.
Indicates significance at P ≤ 0.05.
Melatonin Characteristics
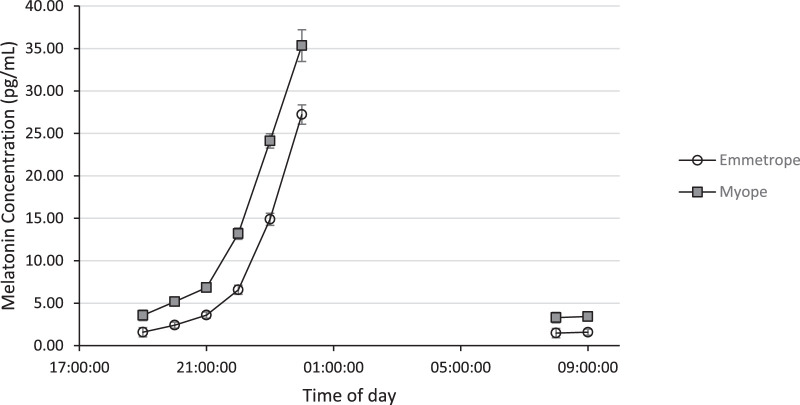
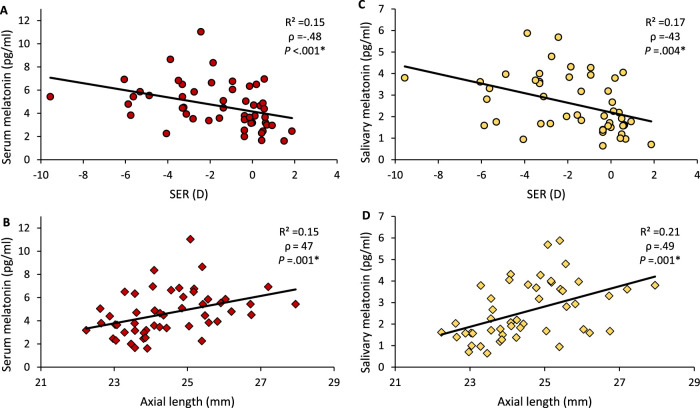
At every time point sampled, serum and salivary melatonin levels were significantly higher in the myopic group (Fig. 1 and Table 4, P ≤ 0.001 for all) and correlated to both SER and axial length (Fig. 2 and Table 5). As indicated in Table 4, eight saliva samples displayed a melatonin concentration below the assay's limit of detection (LOD) and were not included in analysis. Sample collection time did not vary significantly between refractive groups for any time point (Mann-Whitney, P > 0.05 for all). Furthermore, daylength at time of sampling was not significantly associated with morning serum melatonin (Spearman's rank, ρ = –.015, P = 0.92).
Figure 1.
Median salivary melatonin concentration (pg/mL) at each collection time point for myopes (gray filled square) and emmetropes (open circle). Error bars denote 95% confidence intervals.
Table 4.
Melatonin Concentration in Serum (pg/mL) and Saliva (pg/mL) Samples Collected at Different Time Points for All Subjects, Myopes, and Emmetropes
| Melatonin Concentration | ||||
|---|---|---|---|---|
| Time Point | All Subjects | Myopes | Emmetropes | P Value |
| Serum | ||||
| 09:10:00 | n = 51 | n = 28 | n = 23 | |
| (08:57:30–09:25:00) | 4.46 (3.28–5.85) | 5.43 (4.32–6.53) | 3.45 (2.75–4.52) | <0.001*,† |
| Saliva | ||||
| 08:00:00 | n = 47 | n = 28 | n = 19 (+4 <LOD) | |
| (07:27:30–08:06:15) | 2.62 (1.46–3.62) | 3.30 (2.37–4.34) | 1.48 (0.86–2.53) | 0.001*,† |
| 09:10:00 | n = 49 | n = 28 | n = 21 (+2 <LOD) | |
| (09:00:00–09:20:00) | 2.07 (1.59–3.69) | 3.43 (1.96–3.94) | 1.59 (1.37–2.03) | 0.001* |
| 19:00:00 | n = 49 | n = 28 | n = 21 (+2 <LOD) | |
| 2.25 (1.56–3.80) | 3.56 (2.37–4.09) | 1.58 (1.23–1.95) | <0.0001* | |
| 20:00:00 | n = 51 | n = 27 | n = 23 | |
| 2.97 (2.18–5.67) | 5.18 (2.86–5.86) | 2.41 (1.75–3.08) | <0.0001*,† | |
| 21:00:00 | n = 51 | n = 28 | n = 23 | |
| 4.39 (3.53–7.33) | 6.83 (4.24–7.98) | 3.59 (2.5–4.3) | <0.001* | |
| 22:00:00 | n = 51 | n = 28 | n = 23 | |
| 8.38 (6.51–15.01) | 13.19 (7.73–17.00) | 6.57 (5.65–8.16) | <0.0001* | |
| 23:00:00 | n = 51 | n = 28 | n = 23 | |
| 19.11 (14.14–24.93) | 24.11 (17.2–28.48) | 14.88 (13.32–18.77) | <0.0001*,† | |
| n = 41 | n = 23 | n = 18 | ||
| 00:00:00 (00:00:00–00:00:00) | 30.18 (27.13–35.54) | 35.35 (30.71–37.44) | 27.22 (24.16–29.13) | <0.0001* |
| DLMO | n = 48 | n = 27 | n = 21 | |
| 20:22:46 (20:16:22–20:31:04) | 20:22:49 (20:16:00–20:32:59) | 20:22:43 (20:18:18–20:28:22) | 0.98 | |
Values are presented as median (interquartile range) unless otherwise indicated. P value for two-tailed Mann-Whitney U test compares myopes to emmetropes. Holm-Bonferroni correction was applied to control for multiple statistical tests.
Indicates independent samples t-test comparing myopes to emmetropes.
Indicates significance at P ≤ 0.05.
Figure 2.
Scatter graphs showing the correlation between morning serum (A, B) and salivary (C, D) melatonin, SER (top row), and axial length (bottom row). ρ = Spearman's correlation coefficient. *Significant at P ≤ 0.05.
Table 5.
Relationship Between Salivary Melatonin Concentration at Different Sampling Times and Both SER and AL
| SER | AL | |||||
|---|---|---|---|---|---|---|
| Sampling Time | ρ | R 2 | P | ρ | R 2 | P |
| 08:00:00 | −.41 | .14 | 0.004* | .39 | .16 | 0.007* |
| 09:10:00 | −.43 | .17 | 0.004* | .49 | .21 | 0.001* |
| 19:00:00 | −.51 | .20 | 0.001* | .57 | .25 | <0.001* |
| 20:00:00 | −.49 | .19 | 0.002* | .50 | .22 | 0.001* |
| 21:00:00 | −.47 | .18 | 0.002* | .51 | .23 | 0.001* |
| 22:00:00 | −.52 | .18 | 0.001* | .54 | .22 | <0.001* |
| 23:00:00 | −.48 | .20 | 0.002* | .47 | .22 | 0.001* |
| 00:00:00 | −.54 | .21 | 0.001* | .53 | .23 | 0.001* |
ρ = Spearman's correlation coefficient.
Holm-Bonferroni correction was applied to control for multiple statistical tests.
Indicates significance at P ≤ 0.05.
Serum melatonin levels exhibited a weak positive correlation with time spent in moderate activity (ρ = .29, R² = .07, P = 0.04) (Table 6). No other sleep, physical activity, or light exposure metric was significantly associated with serum melatonin concentration (Spearman's rank, P > 0.05 for all). Less mesopic light exposure tended to be correlated with higher levels of serum melatonin (ρ = –.27, R² = .08, P = 0.06), but this relationship did not reach statistical significance. Multiple linear regression analysis evaluated the relative strength of association between morning serum melatonin and refractive error (B = –.34, β = –.42, P = 0.001), moderate activity (B = .009, β = .32, P = 0.01), and mesopic light exposure (B = –.007, β = –.29, P = 0.02). All variables contributed significantly to explaining the variance in serum melatonin measures, F(3, 46) = 7.23, P < 0.001, R2 = 0.32, R2adjusted = .28, and based on standardized coefficients (β), refractive error was the strongest contributor.
Table 6.
Relationship Between Daily Time (Minutes) Spent in Different Intensities of Both Light Exposure and Physical Activity, and Morning Serum Melatonin Concentration (pg/mL)
| Serum Melatonin | |||
|---|---|---|---|
| Characteristic | ρ | R 2 | P |
| Light exposure | |||
| Scotopic (<0.01 lux) |
.07 | .02 | 0.61 |
| Mesopic (0.01 to <3 lux) |
−.27 | .08 | 0.06 |
| Indoor photopic (3 to <1000 lux) |
.04 | .003 | 0.80 |
| Outdoor photopic (≥1000 lux) |
.10 | .01 | 0.51 |
| Physical activity | |||
| Sedentary activity (<59 CPM) |
−.22 | .06 | 0.12 |
| Light activity (59 to <399 CPM) |
−.20 | .03 | 0.15 |
| Moderate activity (399 to <1404 CPM) |
.29 | .07 | 0.04* |
| Vigorous activity (≥1404 CPM) |
.26 | .05 | 0.07 |
ρ = Spearman's correlation coefficient.
Indicates significance at P ≤ 0.05.
Collection time for corresponding morning serum and saliva samples did not vary significantly (Student's t-test, P > 0.05), and morning salivary melatonin levels were significantly correlated to corresponding serum concentrations of melatonin (P < 0.001) (Fig. 3).
Figure 3.
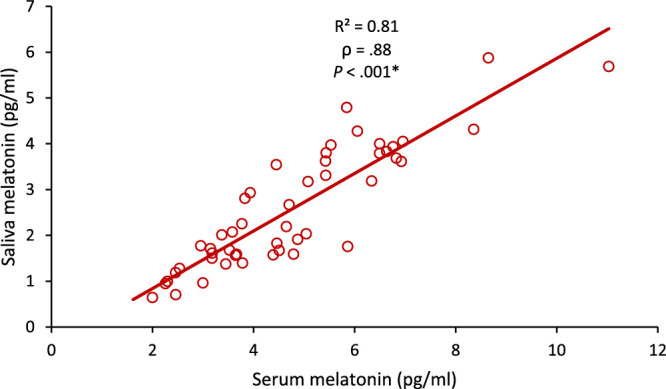
Scatter graph showing the correlation of salivary melatonin with serum melatonin levels. ρ = Spearman's correlation coefficient. *Significant at P ≤ 0.05.
DLMO-Derived Circadian Phase
DLMO was successfully determined for 48 subjects (27 myopes and 21 emmetropes); two emmetropes had daytime melatonin levels below the LOD, and one myopic subject provided only two “daytime” samples, which prevented the determination of DLMO. DLMO did not differ significantly between refractive error groups (Table 4, P = 0.98) and was not associated with SER or axial length (Spearman's rank, ρ = . 06, P = 0.71 and ρ = –.11, P = 0.45, respectively). Lower levels of PSQI-reported sleep duration were significantly associated with a later DLMO (Spearman's rank, ρ = –.34, P =.02). Otherwise, DLMO was not significantly associated with any other self-reported questionnaire data or any objective sleep, light exposure, or physical activity metric (Spearman's rank, P > 0.05 for all).
Age was not significantly correlated with serum melatonin or DLMO (Spearman's correlation, ρ = .028, P = 0.85 and ρ = –.106, P = 0.47, respectively), and neither serum melatonin nor DLMO were significantly associated with sex (Mann-Whitney, P = 0.68 and P = 0.67, respectively). Twenty-three women provided information regarding their menstrual phase. At the time of sampling, women reported being in either the luteal (n = 15) or follicular phase (n = 8) of their menstrual cycle. In this small group of women, menstrual phase was not associated with serum melatonin (independent samples t-test, t(21) = −1.31, P = 0.21) or DLMO (Mann-Whitney, P = 0.73).
Discussion
In light of accumulating evidence implicating circadian dysregulation as a factor in myopia development,23 the present study explored circadian phase and melatonin concentration in young adult myopes and emmetropes, with consideration of prior light exposure, physical activity, and sleep. The outcomes confirm the report of Kearney et al.50 that serum melatonin is significantly elevated in adult myopes and, furthermore, illustrate that this association and the correlation between melatonin, axial length, and magnitude of myopia is demonstrable through the less invasive method of saliva sampling. The elevation of melatonin in samples obtained from myopic subjects was consistent throughout the sampling period, and there was no difference in circadian phase between refractive groups as assessed by DLMO. The present cross-sectional investigation cannot identify whether the association between higher melatonin in myopes is a cause or consequence of myopia but indicates that refractive error explains some of the individual variability seen in melatonin profiles.74,75 Carefully designed prospective studies are now required to better understand the relationship between melatonin, circadian rhythms, and refractive error.
The data presented here demonstrate that elevated systemic melatonin levels are associated with increasing levels of myopia, a greater amount of time spent undertaking moderate physical activity, and less time exposed to mesopic illumination (0.01 to ≤3 lux). Together, these variables explain 32% of the individual variability in morning serum melatonin, with refractive error the most potent contributor to the model. Both physical activity and light exposure provide temporal cues to our circadian system and are known to influence melatonin secretion.76 The outcomes of the present study offer further support for a contributing role of circadian physiology to ocular growth control mechanisms, but the exact nature of this role cannot be determined from the present investigation.
Our results are in contrast with those reported by two studies from the Ostrin laboratory evaluating refractive error and melatonin.37,68 In neither study was a significant difference in melatonin concentration found between refractive groups. Abbott et al.68 collected a single morning saliva sample between 09:00:00 and 11:00:00 and found no significant difference in melatonin concentration between myopes and emmetropes. Burfield et al.37 collected saliva samples every 4 hours for 24 hours to assess the diurnal variation in melatonin. Burfield et al.37 reported no significant difference in the diurnal amplitude, acrophase (peak), or mean concentration of melatonin between myopes and emmetropes. The disparity in outcomes between the present study and those reported by Ostrin's group may reflect differences in melatonin quantification methodology, participant demographics, and/or geographical location. Of particular note, both studies from the Ostrin laboratory analyzed saliva samples for melatonin concentration using a commercial enzyme-linked immunosorbent assay (ELISA) kit. Compared with the highly specific liquid chromatography/mass spectrometry (LC/MS) methods used in the present study, the ELISA technique is typically limited by lower specificity for the target compound. As such, cross-reactivity of compounds with a similar molecular structure to melatonin during ELISA analysis may have masked interrefractive group differences in previous work.
Patterns of light exposure identified within the refractive groups in the present study are consistent with previous reports evaluating light exposure profiles and refractive error in adulthood, which fail to identify a relationship between recent light exposure history and SER or AL.77 While at the group level, myopes spent on average significantly more time exposed to “indoor” levels of illumination compared with emmetropes, recent light exposure history in relation to scotopic, mesopic, or “outdoor” light levels did not differ by group in these adult subjects. This finding contrasts with published data from children. Myopic children have been shown to spend significantly less time outdoors (>1000 lux) and more time in mesopic illumination (1–30 lux) compared with their nonmyopic peers.61,62 A significant association has also been reported between greater average daily light exposure and less axial elongation in both children and young adults.78,79 Childhood data gathered during a period of dynamic eye growth may better inform our understanding of the environmental factors that promote myopia onset and progression, compared with adult data taken when ocular growth and myopia progression are less active.
Other factors that may have restricted the present study's ability to expose relationships between previous light exposure history, melatonin, and refractive measures are the time of year and geographical location at which data were collected. Chronobiology research has shown that brighter daytime light exposure, which is typically accompanied by longer photoperiods and a shortening of the melatonin secretion phase, is associated with acute elevation in the amplitude of nocturnal melatonin secretion.80–82 Such findings have been observed experimentally and seasonally.80–83 By contrast, in the present study, there were no significant differences in total, average, or maximum light exposure between myopes and emmetropes that could explain the differences seen in melatonin concentration. Neither scotopic, “indoor,” or “outdoor” light exposure metrics (at wrist level) nor the timing or spread of “outdoor” light exposure (MLiT1000) were significantly associated with melatonin levels. Light exposure profiles were evaluated at the end of winter in Northern Ireland (latitude of 55°N). Irrespective of refractive error, subjects spent an average of only 26 (interquartile range, 16–43) minutes per day exposed to illuminations >1000 lux. The low frequency of outdoor light exposure may have also limited opportunity for exploring associations between both the timing and spread of outdoor light exposure (MLiT1000) and refractive error, circadian phase, and melatonin. Differences in light exposure profiles between refractive groups may be better evaluated during summer months when individuals are less constrained by weather and daylength. Light exposure profiles may also be better represented by measuring light exposure at eye level, rather than at the wrist, now possible with advances in technology.84
Reduced subjective sleep quality and quantity, both outputs of the circadian system, have been reported in myopic children.85,86 These associations have not been consistently demonstrated in studies evaluating sleep and refractive error in adults, either using subjective (questionnaire) or objective (actigraphy) methods of profiling sleep.68,86 The present study found no association between objective or subjective sleep metrics and refractive error. This is not surprising. In addition to questionnaire-associated recall bias, an inherent limitation of both questionnaire and actigraph data is that they primarily reflect sleep-wake cycles, which are heavily influenced by social factors, rather than internal circadian time.
The present study benefits from adherence to a strict sample collection protocol with comprehensive exclusion criteria and a highly specific LC/MS analysis technique. There were no significant differences in sample collection times between myopes and emmetropes or between corresponding morning saliva and serum samples. Melatonin concentration is known to increase when moving from a supine to a standing position and vice versa; therefore, all human tissue samples were collected in a seated position. A dim uniform lighting environment was maintained during the DLMO protocol. While subjects were permitted to use screens, these were all set to the lowest screen intensity and 100% red saturation. The amount of time each individual subject spent using screens was not recorded during the DLMO protocol. Recently published research demonstrates that the circadian system is highly sensitive to low evening light exposures and that there is significant interindividual variability in relation to this stimulus.75 As such, future research should consider the use of short-wavelength blocking goggles and prohibiting screen use to further control environmental lighting impacts during DLMO phase assessments.
In conclusion, these findings demonstrate for the first time that adult myopia is associated with significantly elevated levels of circulating melatonin in both morning and evening samples but that circadian phase, as indicated by DLMO, does not differ between myopes and emmetropes. This study also establishes that associations between melatonin and refractive error are detectable through noninvasive saliva sampling. Accumulating research from animal models implies that disrupted circadian rhythms contribute to myopia development, but there are few data from human subjects to corroborate this proposition. The present study presents robust evidence that circulating levels of melatonin, a key circadian hormone, are significantly different in adult myopes compared with their emmetropic peers. The significant association found between refractive error and melatonin cannot be explained by differences in circadian phase, recent light exposure history, physical activity, or sleep. Well-designed prospective studies are now required to ascertain the relevance of these findings to the onset and development of myopia, particularly whether elevated melatonin levels are evident before and during myopia development. Accruing such knowledge may provide insight into the factors promoting childhood myopia and how increased time spent outdoors helps to regulate homeostatic eye growth.
Acknowledgments
The authors thank Anna Hordiyenko for her assistance in data collection, and Sara Dobbin for her assistance in sample analysis.
Supported by a Department for the Economy (Northern Ireland) PhD studentship.
Disclosure: S.C. Flanagan, None; D. Cobice, None; P. Richardson, None; J.J. Sittlington, None; K.J. Saunders, None
Appendix
Chromatographic Conditions
StrataX online SPE (25 µm 20, 20 mm) column was purchased from Phenomenex (Macclesfield, Cheshire, UK). SPE mobile phase (A) was 20 mM ammonium acetate/methanol (95:5, v/v). Analytical column Kinetex reverse phase C18 3.0 × 150 mm; 3 µm was purchased from Phenomenex (Macclesfield, UK). Flow rate was set at 0.3 mL/min (constant), column temperature was set at 35°C, and injection volume was 10 µL. Sample diluent was 0.1% ascorbic acid with 0.001% ETDA in 90:10 (methanol/water, v/v).Mobile phases were (B) 90:10 (v/v) (10 mM ammonium formate adjusted to pH 3.0 with formic acid/methanol) and (C) methanol.
Gradient Profile
.
| Time (min) | B (%) | C (%) |
|---|---|---|
| 0.00 | 80 | 20 |
| 3.00 | 80 | 20 |
| 15.00 | 0 | 100 |
| 15.10 | 80 | 20 |
| 20.10 | 80 | 20 |
Mass Spectrometer Conditions
Analysis was carried out in positive ion mode using multiple reaction monitoring (MRM) mode. Online tuning was performed to maximize parent isolation and daughter generation. Ionization and fragmentation conditions were set as follows: CAD (Arb): 6, CUR (Arb): 40, GSI (Arb): 35; GSII (Arb) 40; IS: (V) 5500; temp (°C): 500.
MRM Transitions
.
| Parent | Quantifier | Qualifier | CE | DP | CXP | EP | |
|---|---|---|---|---|---|---|---|
| Compound | (m/z) | (m/z) | (m/z) | (V) | (V) | (V) | (V) |
| Melatonin | 233.1 | 174.2 | 159.0 | 23/49 | 96 | 10/12 | 10 |
| ISTD | 237.1 | 178.1 | NA | 23 | 96 | 10 | 10 |
Sample Preparation and HPLC/MS/MS Analysis
Saliva
Accurately pipette 200 µL of saliva into a 1.5-mL Eppendorf tube, add 200 µL of water and 25 µL of the internal standard working solution (1 ng/mL), and mix well. Then, add 450 µL precipitation reagent (cold acetonitrile), mix with vortex (1 minute, full speed), and centrifuge for 10 minutes at 13,000 rpm at room temperature. Then transfer 250 µL of the supernatant to an HPLC insert and then into a 1.5-mL HPLC vial and store at −20°C until analysis. The limit of quantitation (LOQ) was 1.0 pg mL−1, the LOD was 0.2 pg mL−1, the dynamic range was 1.0–100 pg mL−1 (R2 of nominal versus calculated was 0.997 with no intercept bias). The accuracy at low Quality control (QC) (5 pg mL−1) and high QC (25 pg mL−1) were within 85% to 115% precision (low QC [n = 6, coefficient of variation (CV) = 4.1%], high QC [n = 6, CV = 2.9%]). Results are an average of n = 2 preparation.
Serum
Serum melatonin was quantified using liquid chromatography followed by online solid-phase extraction and tandem mass spectrometry analysis (LC‐online SPE‐MS/MS) as previously described by Kearney et al.50 In brief, accurately pipette 400 µL of serum into a 1.5-mL Eppendorf tube; add 25 µL of the internal standard working solution (1 ng/mL), 50 µL of serum media solution, and 700 µL of precipitation reagent (cold acetonitrile) mix with vortex (1 minute, full speed); and centrifuge for 10 minutes at 13,000 rpm. Then transfer 800 µL of the supernatant to a 1.5-mL amber HPLC vial and store at −20°C until analysis. The LOQ was 2.5 pg mL−1, the LOD was 1.0 pg mL−1, and the dynamic range was 2.5–200 pg mL−1 (R2 of nominal versus calculated was 0.992 with no intercept bias). The accuracy at low QC (25 pg mL−1) and high QC (100 pg mL−1) were within 85% to 115% precision (low QC [n = 6, CV = 3.7%], high QC [n = 6, CV = 4.2%]). Results are average of n = 2 preparation.
References
- 1. Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004; 43: 447–468. [DOI] [PubMed] [Google Scholar]
- 2. Flitcroft DI. The complex interactions of retinal, optical and environmental factors in myopia aetiology. Prog Retin Eye Res. 2012; 31: 622–660. [DOI] [PubMed] [Google Scholar]
- 3. Holden BA, Fricke TR, Wilson DA, et al.. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016; 123: 1036–1042. [DOI] [PubMed] [Google Scholar]
- 4. Morgan IG, Rose KA. Myopia: is the nature-nurture debate finally over? Clin Exp Optom. 2019; 102: 3–17. [DOI] [PubMed] [Google Scholar]
- 5. Morgan I, Rose K. How genetic is school myopia? Prog Retin Eye Res. 2005; 24: 1–38. [DOI] [PubMed] [Google Scholar]
- 6. Guo Y, Liu LJ, Tang P, et al.. Outdoor activity and myopia progression in 4-year follow-up of Chinese primary school children: the Beijing Children Eye Study. PLoS One. 2017; 12: e0175921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guggenheim JA, Northstone K, McMahon G, et al.. Time outdoors and physical activity as predictors of incident myopia in childhood: a prospective cohort study. Invest Ophthalmol Vis Sci. 2012; 53: 2856–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xiong S, Sankaridurg P, Naduvilath T, et al.. Time spent in outdoor activities in relation to myopia prevention and control: a meta-analysis and systematic review. Acta Ophthalmol. 2017; 95: 551–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wu PC, Tsai CL, Wu HL, Yang YH, Kuo HK. Outdoor activity during class recess reduces myopia onset and progression in school children. Ophthalmology. 2013; 120: 1080–1085. [DOI] [PubMed] [Google Scholar]
- 10. Hua WJ, Jin JX, Wu XY, et al.. Elevated light levels in schools have a protective effect on myopia. Ophthalmic Physiol Opt. 2015; 35: 252–262. [DOI] [PubMed] [Google Scholar]
- 11. Dirani M, Tong L, Gazzard G, et al.. Outdoor activity and myopia in Singapore teenage children. Br J Ophthalmol. 2009; 93: 997–1000. [DOI] [PubMed] [Google Scholar]
- 12. Jin JX, Hua WJ, Jiang X, et al.. Effect of outdoor activity on myopia onset and progression in school-aged children in northeast China: the Sujiatun Eye Care Study. BMC Ophthalmol. 2015; 15: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cao K, Wan Y, Yusufu M, Wang N. Significance of outdoor time for myopia prevention: a systematic review and meta-analysis based on randomized controlled trials. Ophthalmic Res. 2020; 63: 97–105. [DOI] [PubMed] [Google Scholar]
- 14. He M, Xiang F, Zeng Y, et al.. Effect of time spent outdoors at school on the development of myopia among children in China: a randomized clinical trial. JAMA. 2015; 314: 1142–1148. [DOI] [PubMed] [Google Scholar]
- 15. Hobday R. Myopia and daylight in schools: a neglected aspect of public health? Perspect Public Health. 2016; 136: 50–55. [DOI] [PubMed] [Google Scholar]
- 16. Seet B, Wong TY, Tan DT, et al.. Myopia in Singapore: taking a public health approach. Br J Ophthalmol. 2001; 85: 521–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Resnikoff S, Jonas JB, Friedman D, et al.. Myopia-a 21st century public health issue. Invest Ophthalmol Vis Sci. 2019; 60: Mi–Mii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lingham G, Mackey DA, Lucas R, Yazar S. How does spending time outdoors protect against myopia? A review. Br J Ophthalmol. 2020;104:593–599. [DOI] [PubMed] [Google Scholar]
- 19. O'Donoghue L, Kapetanankis VV, McClelland JF, et al.. Risk factors for childhood myopia: findings from the NICER study childhood myopia risk factors: NICER study. Invest Ophthalmol Vis Sci. 2015; 56: 1524–1530. [DOI] [PubMed] [Google Scholar]
- 20. Harrington SC, Stack J, O'Dwyer V. Risk factors associated with myopia in schoolchildren in Ireland. Br J Ophthalmol. 2019; 103: 1803–1809. [DOI] [PubMed] [Google Scholar]
- 21. Rose KA, Morgan IG, Ip J, et al.. Outdoor activity reduces the prevalence of myopia in children. Ophthalmology. 2008; 115: 1279–1285. [DOI] [PubMed] [Google Scholar]
- 22. French AN, Ashby RS, Morgan IG, Rose KA. Time outdoors and the prevention of myopia. Exp Eye Res. 2013; 114: 58–68. [DOI] [PubMed] [Google Scholar]
- 23. Chakraborty R, Ostrin LA, Nickla DL, et al.. Circadian rhythms, refractive development, and myopia. Ophthalmic Physiol Opt. 2018; 38: 217–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stone RA, McGlinn AM, Chakraborty R, et al.. Altered ocular parameters from circadian clock gene disruptions. PLoS One. 2019; 14: e0217111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hysi PG, Choquet H, Khawaja AP, et al.. Meta-analysis of 542,934 subjects of European ancestry identifies new genes and mechanisms predisposing to refractive error and myopia. Nature Genetics. 2020;52: 401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bell-Pedersen D, Cassone VM, Earnest DJ, et al.. Circadian rhythms from multiple oscillators: lessons from diverse organisms. Nat Rev Genet. 2005; 6: 544–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guido ME, Garbarino-Pico E, Contin MA, et al.. Inner retinal circadian clocks and non-visual photoreceptors: novel players in the circadian system. Prog Neurobiol. 2010; 92: 484–504. [DOI] [PubMed] [Google Scholar]
- 28. Baver SB, Pickard GE, Sollars PJ, Pickard GE. Two types of melanopsin retinal ganglion cell differentially innervate the hypothalamic suprachiasmatic nucleus and the olivary pretectal nucleus. Eur J Neurosci. 2008; 27: 1763–1770. [DOI] [PubMed] [Google Scholar]
- 29. Tosini G, Dirden JC. Dopamine inhibits melatonin release in the mammalian retina: in vitro evidence. Neurosci Lett. 2000; 286: 119–122. [DOI] [PubMed] [Google Scholar]
- 30. Lewy AJ, Bauer VK, Cutler NL, et al.. Morning vs evening light treatment of patients with winter depression. Arch Gen Psychiatry. 1998; 55: 890–896. [DOI] [PubMed] [Google Scholar]
- 31. Duffy JF, Kronauer RE, Czeisler CA. Phase-shifting human circadian rhythms: influence of sleep timing, social contact and light exposure. J Physiol. 1996; 495: 289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Feldkaemper M, Schaeffel F. An updated view on the role of dopamine in myopia. Exp Eye Res. 2013; 114: 106–119. [DOI] [PubMed] [Google Scholar]
- 33. Zhou X, Pardue MT, Iuvone PM, Qu J. Dopamine signaling and myopia development: what are the key challenges. Prog Retin Eye Res. 2017; 61: 60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stone RA, Pardue MT, Iuvone PM, Khurana TS. Pharmacology of myopia and potential role for intrinsic retinal circadian rhythms. Exp Eye Res. 2013; 114: 35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Besharse JC, McMahon DG. The retina and other light-sensitive ocular clocks. J Biol Rhythm. 2016; 31: 223–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Green CB, Besharse JC. Retinal circadian clocks and control of retinal physiology. J Biol Rhythm. 2004; 19: 91–102. [DOI] [PubMed] [Google Scholar]
- 37. Burfield HJ, Carkeet A, Ostrin LA. Ocular and systemic diurnal rhythms in emmetropic and myopic adults. Invest Ophthalmol Vis Sci. 2019; 60: 2237–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Burfield HJ, Patel NB, Ostrin LA. Ocular biometric diurnal rhythms in emmetropic and myopic adults. Invest Ophthalmol Vis Sci. 2018; 59: 5176–5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nickla DL. Ocular diurnal rhythms and eye growth regulation: where we are 50 years after Lauber. Exp Eye Res. 2013; 114: 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stone RA, Lin T, Laties AM, Iuvone PM. Retinal dopamine and form-deprivation myopia. Proc Natl Acad Sci USA. 1989; 86: 704–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pendrak K, Nguyen T, Lin T, et al.. Retinal dopamine in the recovery from experimental myopia. Curr Eye Res. 1997; 16: 152–157. [DOI] [PubMed] [Google Scholar]
- 42. Guo S, Sivak J, Callender M, Diehl-Jones B. Retinal dopamine and lens-induced refractive errors in chicks. Curr Eye Res. 1995; 14: 385–389. [DOI] [PubMed] [Google Scholar]
- 43. Dong F, Zhi Z, Pan M, et al.. Inhibition of experimental myopia by a dopamine agonist: different effectiveness between form deprivation and hyperopic defocus in guinea pigs. Mol Vis. 2011; 17: 2824. [PMC free article] [PubMed] [Google Scholar]
- 44. Iuvone PM, Tigges M, Stone RA, Lambert S, Laties AM. Effects of apomorphine, a dopamine receptor agonist, on ocular refraction and axial elongation in a primate model of myopia. Invest Ophthalmol Vis Sci. 1991; 32: 1674–1677. [PubMed] [Google Scholar]
- 45. Schmid KL, Wildsoet CF.. Inhibitory effects of apomorphine and atropine and their combination on myopia in chicks. Optom Vis Sci. 2004; 81: 137–147. [DOI] [PubMed] [Google Scholar]
- 46. Nickla DL, Totonelly K, Dhillon B. Dopaminergic agonists that result in ocular growth inhibition also elicit transient increases in choroidal thickness in chicks. Exp Eye Res. 2010; 91: 715–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Iuvone PM, Galli C, Garrison-Gund C, Neff NH. Light stimulates tyrosine hydroxylase activity and dopamine synthesis in retinal amacrine neurons. Science. 1978; 202: 901–902. [DOI] [PubMed] [Google Scholar]
- 48. Cohen Y, Peleg E, Belkin M, Polat U, Solomon AS. Ambient illuminance, retinal dopamine release and refractive development in chicks. Exp Eye Res. 2012; 103: 33–40. [DOI] [PubMed] [Google Scholar]
- 49. Ashby RS, Schaeffel F. The effect of bright light on lens compensation in chicks. Invest Ophthalmol Vis Sci. 2010; 51: 5247–5253. [DOI] [PubMed] [Google Scholar]
- 50. Kearney S, O'Donoghue L, Pourshahidi LK, Cobice D, Saunders KJ. Myopes have significantly higher serum melatonin concentrations than non-myopes. Ophthal Physiol Opt. 2017; 37: 557–567. [DOI] [PubMed] [Google Scholar]
- 51. Meiser J, Weindl D, Hiller K. Complexity of dopamine metabolism. Cell Commun Signal. 2013; 11: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Benloucif S, Burgess HJ, Klerman EB, et al.. Measuring melatonin in humans. J Clin Sleep Med. 2008; 4: 66–69. [PMC free article] [PubMed] [Google Scholar]
- 53. Lewy AJ, Cutler NL, Sack RL. The endogenous melatonin profile as a marker for circadian phase position. J Biol Rhythm. 1999; 14: 227–236. [DOI] [PubMed] [Google Scholar]
- 54. Pandi-Perumal SR, Smits M, Spence W, et al.. Dim light melatonin onset (DLMO): a tool for the analysis of circadian phase in human sleep and chronobiological disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2007; 31: 1–11. [DOI] [PubMed] [Google Scholar]
- 55. Iguchi H, Kato K-I, Ibayashi H. Age-dependent reduction in serum melatonin concentrations in healthy human subjects. J Clin Endocrinol Metab. 1982; 55: 27–29. [DOI] [PubMed] [Google Scholar]
- 56. Breslin KMM, O'Donoghue L, Saunders KJ. A prospective study of spherical refractive error and ocular components among Northern Irish schoolchildren (the NICER study). Invest Ophthalmol Vis Sci. 2013; 54: 4843–4850. [DOI] [PubMed] [Google Scholar]
- 57. Baker FC, Driver HS. Circadian rhythms, sleep, and the menstrual cycle. Sleep Med. 2007; 8: 613–622. [DOI] [PubMed] [Google Scholar]
- 58. Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989; 28: 193–213. [DOI] [PubMed] [Google Scholar]
- 59. Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976; 4: 97–110. [PubMed] [Google Scholar]
- 60. Ostrin LA, Sajjadi A, Benoit JS. Objectively measured light exposure during school and summer in children. Optom Vision Sci. 2018; 95: 332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Landis EG, Yang V, Brown DM, Pardue MT, Read SA. Dim light exposure and myopia in children. Invest Ophthalmol Vis Sci. 2018; 59: 4804–4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Read SA, Collins MJ, Vincent SJ. Light exposure and physical activity in myopic and emmetropic children. Optom Vis Sci. 2014; 91: 330–341. [DOI] [PubMed] [Google Scholar]
- 63. Burgess HJ, Wyatt JK, Park M, Fogg LF. Home circadian phase assessments with measures of compliance yield accurate dim light melatonin onsets. Sleep. 2015; 38: 889–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Pullman RE, Roepke SE, Duffy JF. Laboratory validation of an in-home method for assessing circadian phase using dim light melatonin onset (DLMO). Sleep Med. 2012; 13: 703–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Voultsios A, Kennaway DJ, Dawson D. Salivary melatonin as a circadian phase marker: validation and comparison to plasma melatonin. J Biological Rhythm. 1997; 12: 457–466. [DOI] [PubMed] [Google Scholar]
- 66. Molina TA, Burgess HJ. Calculating the dim light melatonin onset: the impact of threshold and sampling rate. Chronobiol Int. 2011; 28: 714–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ostrin LA, Abbott KS, Queener HM. Attenuation of short wavelengths alters sleep and the ipRGC pupil response. Ophthal Physiol Opt. 2017; 37: 440–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Abbott KS, Queener HM, Ostrin LA. The ipRGC-driven pupil response with light exposure, refractive error, and sleep. Optom Vis Sci. 2018; 95: 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ostrin LA, Jnawali A, Carkeet A, Patel NB. Twenty-four hour ocular and systemic diurnal rhythms in children. Ophthal Physiol Opt. 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Akacem LD, Wright KP Jr, LeBourgeois MK. Sensitivity of the circadian system to evening bright light in preschool-age children. Physiol Rep. 2018; 6: e13617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Reid KJ, Santostasi G, Baron KG, Wilson J, Kang J, Zee PC. Timing and intensity of light correlate with body weight in adults. PLoS One. 2014; 9:e92251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lee P, Tse C. Calibration of wrist-worn ActiWatch 2 and ActiGraph wGT3X for assessment of physical activity in young adults. Gait Posture. 2019; 68: 141–149. [DOI] [PubMed] [Google Scholar]
- 73. Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6: 65–70. [Google Scholar]
- 74. Burgess HJ, Fogg LF. Individual differences in the amount and timing of salivary melatonin secretion. PLoS One. 2008; 3: e3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Phillips AJ, Vidafar P, Burns AC, et al.. High sensitivity and interindividual variability in the response of the human circadian system to evening light. PNAS. 2019; 116: 12019–12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hébert M, Martin SK, Lee C, Eastman CI. The effects of prior light history on the suppression of melatonin by light in humans. J Pineal Res. 2002; 33: 198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ostrin LA. Objectively measured light exposure in emmetropic and myopic adults. Optom Vis Sci. 2017; 94: 229–238. [DOI] [PubMed] [Google Scholar]
- 78. Read SA, Collins MJ, Vincent SJ. Light exposure and eye growth in childhood. Invest Ophthalmol Vis Sci. 2015; 56: 6779–6787. [DOI] [PubMed] [Google Scholar]
- 79. Ulaganathan S, Read SA, Collins MJ, Vincent SJ. Influence of seasons upon personal light exposure and longitudinal axial length changes in young adults. Acta Ophthalmol. 2019; 97: e256–e265. [DOI] [PubMed] [Google Scholar]
- 80. Takasu NN, Hashimoto S, Yamanaka Y, et al.. Repeated exposures to daytime bright light increase nocturnal melatonin rise and maintain circadian phase in young subjects under fixed sleep schedule. Am J Physiol Regul Integr Comp Physiol. 2006; 291: R1799–R1807. [DOI] [PubMed] [Google Scholar]
- 81. Wehr TA. The durations of human melatonin secretion and sleep respond to changes in daylength (photoperiod). J Clin Endocrinol Metab. 1991; 73: 1276–1280. [DOI] [PubMed] [Google Scholar]
- 82. Vondravsová D, Hájek I, Illnerová H. Exposure to long summer days affects the human melatonin and cortisol rhythms. Brain Res. 1997; 759: 166–170. [DOI] [PubMed] [Google Scholar]
- 83. Adamsson M, Laike T, Morita T. Annual variation in daily light exposure and circadian change of melatonin and cortisol concentrations at a northern latitude with large seasonal differences in photoperiod length. J Physiol Anthropol BioMed Central. 2017; 36: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Wen L, Cheng Q, Lan W, et al.. An objective comparison of light intensity and near-visual tasks between rural and urban school children in China by a wearable device Clouclip. Transl Vis Sci Techn. 2019; 8: 15–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Jee D, Morgan IG, Kim EC. Inverse relationship between sleep duration and myopia. Acta Ophthalmol. 2016; 94: e204–e210. [DOI] [PubMed] [Google Scholar]
- 86. Ayaki M, Torii H, Tsubota K, Negishi K. Decreased sleep quality in high myopia children. Sci Rep. 2016; 6: 33902. [DOI] [PMC free article] [PubMed] [Google Scholar]