Abstract
Objective:
Recognizing the adverse outcomes that occur to obese adults over the age of 65 with loss of muscle mass or strength, or sarcopenia is important. We will review the definitions of Sarcopenic Obesity, and attempt to link the epidemiological data with the molecular pathways. Upon understanding the model of sarcopenic obesity, we will discuss targeted interventions and further challenges to address this geriatrics syndrome.
Key findings:
As our understanding of this syndrome is growing, more data is emerging to help define Sarcopenic Obesity across different populations. We now have a better understanding of biological pathways in aging such as changes in body composition, sex-specific hormones, pro-inflammatory markers, and myocellular mechanisms. We will review a comprehensive model that shows the interactions between the different pathways leading to sarcopenic obesity. Such a model will explain the promising interventions in place and invite future ones.
Conclusion:
Sarcopenic Obesity is an important geriatric syndrome with significant clinical and healthcare implications. Further research is needed to harmonize definitions, clarifying mechanisms contributing to syndrome and use evidence-based interventions to target biological mechanisms in both research and clinical settings.
Keywords: body composition, obesity, older adults, sarcopenia
INTRODUCTION
Perhaps the two most currently emerging and challenging epidemiological trends in developing countries are the aging of the population and the obesity epidemic.(1) Over the past 10 years, the population age 65 and over in the United States increased from 37.2 million in 2006 to 49.2 million in 2016 (a 33% increase) and is projected to almost double to 98 million in 2060. Obesity trends are also rising, and the increasing prevalence of over the next 20–30 years will place a substantial strain on the finances and resources of the United States healthcare system.(2) These trends have also been observed worldwide.(3) The prevalence of obesity in combination with sarcopenia, the age-related loss of muscle mass and strength, increases in adults aged 65 years and older.(4) A major subset of adults over the age of 65 is now classified as having sarcopenic obesity, a high-risk geriatric syndrome predominantly observed in an ageing population that is at risk of synergistic complications from both sarcopenia and obesity.(5) In this paper, we will discuss the definition of sarcopenic obesity, as well as the epidemiological and molecular pathways that link to adverse events in clinical populations. Understanding of these pathways will ultimately permit the development of interventions that can meaningfully address this syndrome and its profound implications in a high-risk population of older adults.
Age-related changes in body composition.
The aging process leads to considerable changes in body composition that affect one’s physical and biological processes, specifically on the distribution of lean muscle and fat mass. Aging promotes an adipogenic state from the third decade until the seventh decade due to multiple factors. These factors include a decrease in basal metabolic rate,(6) reduced physical activity, and other proposed hormonal and inflammatory etiologies.(7–9) The combination of these factors leads to increased fat mass deposition and reduction in fat-free mass. While fat mass peaks in the seventh decade, it drops significantly after this time. Such changes also affect the distribution of fat mass in older adults. Aging leads to a loss of subcutaneous fat (peripherally first and then centrally), an accumulation of visceral fat, and ectopic fat deposition (e.g., muscle, liver, bone marrow).(10) This is partly explained by the reduced capacity for lipid accumulation with age among other unknown etiologies.(11)
Lean mass peaks in the fourth decade of life and its decline accelerates after the seventh decade.(12) Grip strength follows such a pattern as well. In a large cohort study combining 12 population-based studies of 49,964 participants, males were stronger than females from adolescence onwards, with a peak median grip strength of 51kg between ages 29–39, compared to 31kg in females between ages 26–42.(13) By age 80, weak grip strength, defined as less than 2.5 standard deviations below the gender-specific mean, reached a prevalence of 23% in males and 27% in females by age 80. In a separate study using data from the Baltimore Longitudinal Study on Aging, muscle quality declined in both arms and legs with age in cross-sectional analyses, using cross-sectional area and fat-free mass.(14) Importantly, the relationship between muscle quality and age was dependent on how muscle mass was estimated and whether subjects are studied in a cross-sectional or longitudinal study design. This age-related reduction in lean muscle mass and strength(15) accounts, in part, for the reduced resting metabolic rates.(16) Skeletal muscle metabolism is a major determinant of resting energy expenditure. Other etiological factors that cause a decline in resting metabolic rates include reduced physical activity,(17) reduced mitochondrial volume and reduced oxidative capacity.(18, 19) Age-related decreases in the components of total energy expenditure (e.g., resting metabolic rates, thermic effect of food and physical activity) contribute largely to the gradual increase in body fat. The reduction in energy expenditure in aging is typically not proportionally associated with a reduced drive to eat. The small yearly positive changes in energy balance might lead to weight gain.(20)
Age-specific Physiological decline
The relationship between age and physiological function remains poorly defined and there are no unique physiological markers that reliably predict physiological aging. This could be due to a variety of confounding genetic and lifestyle factors. The relationship between age and reduced physical activity has been well-established. For instance, the overwhelming majority of older people in the United Kingdom do not meet the minimum physical activity levels needed to maintain health.(8) The sedentary lifestyles that predominate in older age results in premature onset of ill health, disease and frailty.(8) The evidence demonstrates that regular physical activity is safe not only for healthy but even for frail older adults.(21) Furthermore, the risks of developing major cardiovascular and metabolic diseases, obesity, falls, cognitive impairments, osteoporosis and muscular weakness are mitigated by regular exercise. For example, even short-term exercises (e.g., aerobic, resistance, balance, flexibility) have been shown to reduce the risk of falls.(22) Regular exercise has also been one of the few interventions to reduce the risk of osteoporotic fractures among older adults.{Giangregorio, 2014 #121;Thornley, 2020 #122} What is defined as regular exercise includes completing activities ranging from low intensity walking through to more vigorous sports and resistance exercises. Among multiple physiological functions, oxygen uptake (VO2) max was most closely associated with age. However, a high level of variance between the age and VO2max has also been observed.(23) In conjunction with the reduction in Type II muscle fibers, their loss leads may promote sarcopenia due to changes in oxidation and glycolytic energy expenditures.(24) This is significant as Type II muscle fibers are larger in size when compared to Type I muscle fibers and thus are able to produce greater and quicker power, although for a shorter period of time, which may be an important consideration for activities of daily living.
Inflammatory and Hormonal Changes with Aging
The role of inflammation in the aging process has been extensively studied and multiple pathways have been implicated. Higher plasma concentrations of cytokines such as interleukin (IL)-6 and tumor necrosis factor (TNF)-α are associated with lower muscle mass and lower muscle strength in well-functioning older men and women.(7) It is also hypothesized that the rise in the above cytokines as well as in insulin growth factor (IGF)-1, as often observed in healthy older persons, may contribute to the loss of muscle mass, strength, and ultimately to progressive disability and death.(9) Batsis outlined the interrelated pathways that link inflammatory and hormonal changes that may promote sarcopenic obesity (Figure 1). (25) Diet and exercise interventions may alter these inflammatory cytokines and alter the relative ratio of adipokines. Such complex pathways are thought to lead to intramuscular fat deposition that may contribute to the impairment in functional status observed in this population. Importantly, Figure 2 demonstrates magnetic resonance imaging that reflects fat infiltration of one’s quadriceps muscle. This deposition is the hallmark of the causative pathways that may lead to sarcopenic obesity.
Figure #1: A proposed model of mechanisms leading to sarcopenic obesity.
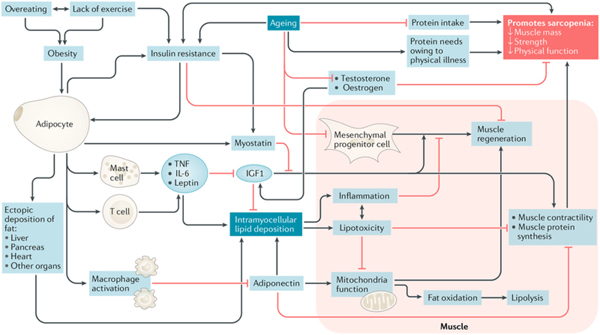
The proposed interplay between adipose and muscle tissue, which is believed to contribute to the development of sarcopenic obesity, is shown. The black lines are stimulatory, while red lines with flat ends indicate inhibition. IGF1, insulin- like growth factor 1; TNF, tumor necrosis factor. (25)
Figure 2: Magnetic Resonance Imaging of individuals with and without obesity.
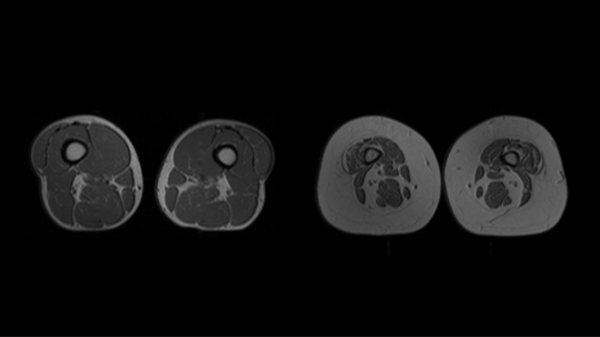
Cross- sectional magnetic resonance imaging of the quadriceps area of an individual without obesity with normal muscle characteristics (part a) and an individual with obesity with small muscles and infiltration by adipose tissue (part b) is shown. More muscle tissue is visible in part a than in part b.
Metabolic impairments observed in the Invecchiare in Chianti, Aging in the Chianti Area (InCHIANTI) and National Health and Nutrition Examination Survey (NHANES) datasets have consistently demonstrated the association with high BMI and low muscle strength or mass, and increased levels of the cytokine IL-6 and inflammatory marker, Creactive protein.(26–28) Changes in estrogen, testosterone and growth hormone are also implicated in inflammatory changes that promote phenotypic changes in fat and muscle mass. These hormones decline sharply with age.(28) Andropause and menopause are well defined with wide implications on aging. For instance, the effect of estrogen decreases during menopause whose effect extends past bone. Bone mass decreases with age after menopause in addition to increases in fat mass and decreases in lean mass.(29) Total and bioavailable testosterone correlate with age and show the greatest decline after the age of 70, and appears to be central in the development of sarcopenia as it increases both muscle mass and activates satellite cells leading to increased muscle function.(30) Finally, growth hormone deficiency has been implicated in the loss of muscle mass; however, it is unclear if growth hormone deficiency leads to muscle strength loss. A variety of other hormones as outlined in Figure 1, including adipoines (adiponectin) and myokines (myostatin) appear to play minor roles in age-related alterations in muscle mass and function.
Aging and Sarcopenia
As our understanding of sarcopenia improves, there is increasing evidence that muscle mass and muscle strength may not be synonymous or causally related. Recent longitudinal and intervention-based studies have clearly demonstrated that muscle atrophy is a relatively small contributor to the loss of muscle strength.(31) More attention has been focused on the subclinical deficits in the structure and function of the nervous system and/or impairments in of skeletal muscle to produce effective power as potential antecedents to reduced muscle strength.(32) Therefore, aging affects the muscular strength in more complex ways than previously perceived. More research is underway to further understand the neuromuscular complexity of reduced muscle strength.
Assessment of Body Composition
Many techniques are available to assess body composition, ranging from simple indirect measures to more sophisticated direct volumetric methods. Some of the more commonly used methods include anthropometry, tracer dilution, dual-energy X-ray absorptiometry (DEXA), air displacement plethysmography and bioelectrical impedance analysis. Gold standard methods of assessing muscle and fat include computer tomography and magnetic resonance imaging. Their precision and accuracy varies, and have been reviewed elsewhere.(33) In older adults, DEXA scans offers the dual benefit of bone density measurement as well as measurement of visceral fat which can be of high clinical utility. Findings from epidemiological studies over the past 30 years have demonstrated that visceral adipose tissue, is an independent risk marker of cardiovascular and metabolic morbidity and mortality.(34) This joint position statement from the International Atherosclerosis Society and the International Chair on Cardiometabolic Risk Working Group on Visceral Obesity summarize the evidence for visceral adiposity and ectopic fat as emerging risk factors for type 2 diabetes, atherosclerosis, and cardiovascular disease. (34)
Commonly used anthropometric measures are often using in the definitions of sarcopenic obesity. However, body mass index (BMI), while helpful from a population-based standpoint, has many limitations and exhibits poor sensitivity.(35) For instance, as individuals age, vertebral compression result in a reduction in height,(36) and may affect the BMI ratio. The redistribution of body mass and loss of lean mass described above also interferes with the ability of BMI to accurately capture the extent of adiposity.
Assessment of Sarcopenia
Muscle mass can be assessed using standard measures of body composition described earlier. Multiple methods can be used to assess muscular strength including grip strength,(37) sit-to-stand testing,(38) and short performance physical battery of lower extremity strength.(39) Grip strength has the advantage of ease of use in the clinical setting and its validation in prognostication.(40) Leg isometric strength has also been studied and is strongly correlated to fat-free mass.(41) The relationship between muscle quality and age is dependent on how muscle mass is estimated and on whether subjects are studied cross-sectionally or longitudinally. In addition, creatine may measure a muscle property not accounted by other measures.(14) It has been suggested that creatine (methyl-d3) dilution (D3-C), may more accurately assess muscle mass.(42) At a cellular level, estimates of total body muscle can be obtained from endogenous metabolites of skeletal muscle, such as creatinine, 3-methylhistidine, urinary creatinine excretion, and D3-creatine. D3-creatine is still in early clinical phase of development, and the issue of normative data is lacking. A critical need to develop such standards to reliably identify homogeneous populations with sarcopenia is needed prior to its routine use in clinical practice and intervention studies.(43)
Diagnosis of Sarcopenic Obesity
The diagnosis of sarcopenic obesity is highly debated and complex. In our opinion, the definition consists of separately defining sarcopenia and obesity. While we recognize that these have been highly variable within the literature, we present information that permit classification of participants using a given definition.
The Foundation for the National Institutes of Health Biomarkers Consortium Sarcopenia Project used an evidence-based approach to develop these criteria.(44) The final recommended cutpoints for weakness established in 2014 are grip strength <26kg for men and <16kg for women, and for low lean mass, appendicular lean mass adjusted for body mass index <0.789 for men and <0.512 for women.(44) In an updated consensus by the same group, the Sarcopenia Definitions and Outcomes Consortium was formed to develop evidence-based diagnostic cut-points for lean mass and/or muscle strength that identify people at increased risk of mobility disability. The conclusion was that grip strength - absolute or adjusted for BMI - is an important discriminator of mobility disability and other endpoints.(45)
An additional effort to include gait speed in conjunction with grip strength in a clinical algorithm was developed by the European Working Group on Sarcopenia in Older People (EWGSOP). This group developed a consensus diagnostic criteria for age-related sarcopenia.(46) In 2019, the EWGSOP revised the algorithm in which the grip strength or the chair test was used to assess the probability of sarcopenia. The diagnosis was then further confirmed by muscle quality or quantity imaging tests such DEXA, magnetic resonance imaging, bioelectrical impedance analysis, or computer tomography scan. The severity of sarcopenia was then determined by physical performance testing such as timed up and go test, 400 m walk test, short physical performance battery or gait speed.(47)
The differences in defining sarcopenia and sarcopenic obesity has led to significant differences in estimating the prevalence of this condition in older adults and markedly hampers the scientific advancement of the field. Prevalence of sarcopenic obesity in older adults varies up to 19–26-fold depending on current research definitions when applied to National Health and Nutrition Examination 1999–2004 data.(4) Despite the large variation in the prevalence of sarcopenic obesity across different obesity definitions,(48–51) recent evidence suggests that waist circumference as a surrogate for central obesity, is best associated with poorer muscle function.(51) Grip strength—absolute or adjusted for body mass index—is an important discriminator of mobility disability and other endpoints. Additional research is needed to develop a predictive risk model that takes into account sarcopenia components as well as age, sex, race, and comorbidities.(45) Unfortunately, the few studies that have evaluated the concordance of definitions demonstrate minimal overlap. Specifically, the concordance rates are 2.5% between the EWGSOP and the International Working Group on Sarcopenia (IWGS), 1.8% between the EWGSOP and the FNIH, and 1.0% between the IWGS and FNIH among community-dwelling older adults in Bavaria, Germany.(52) An international consensus on an evidence-based definition of sarcopenia is needed and will facilitate more effective research and interventions. Ethnic/racial and country-specific definitions may be necessary that reflect a study population. Alternatively, worldwide consensus aggregating large-scale, longitudinal, epidemiological cohorts to increase sample sizes to ascertain its impact on long-term functional impairment are critically needed.
Epidemiologic of Sarcopenic Obesity and Adverse Events
Despite such challenges, epidemiological evidence using well-established datasets has demonstrated a relationship between sarcopenic obesity and impaired long-term outcomes. Both sarcopenia and sarcopenic obesity are related to incident functional decline and disability in cross-sectional and longitudinal studies.(53) Cross-sectional data using NHANES 1999–2004 demonstrated significantly higher rates of physical limitations in both males and females.(54) Low muscle strength, defined as the lowest sex-specific tertile of knee extensor strength, and a BMI≥30kg/m2 had significantly lower walking speeds and steeper declines and higher risk of developing new mobility disability over a six-year follow-up.(55) There may be sex-specific differences in mortality risk in those with sarcopenia and sarcopenic obesity, as older women with sarcopenia have an increased all-cause mortality risk independent of obesity.(56) When different body composition measures and muscle strength measures are used to determine the functional decline in older men and women, low muscle mass was not significantly associated with such functional decline.(57) Low muscle strength co-existent with obesity, but not muscle mass, was predictive of increased falls risk score in middle-aged and older adults. In clinical settings, muscle function assessments may be useful for predicting falls risk in participants with obesity.
Falls are an important cause of mortality and morbidity in older adults. (58) In a study of falls risk in middle-aged and older adults, sarcopenic obesity defined used muscle mass had no significant relationship with falls among any categories irrespective of obesity type (global vs. central).(59) In contrast, in the same study, the multivariable linear regression analyses revealed mild but significantly increased falls risk scores for low muscle strength with obesity.(59) In a multiethnic cohort of postmenopausal women, sarcopenic obesity-related fall risk was high in women younger than 65 and those age 65 and older when defined using body fat percentage greater than 42%.(60)
Increasingly, epidemiological studies using the Korean NHANES, the British Regional Heart Study, the English Longitudinal Study of Aging, and a Japanese cohort have demonstrated marked associations between sarcopenic obesity and risk of medical comorbidity. Using the Korean NHANES, sarcopenic obesity defined using appendicular skeletal mass normalized for body weight below 2 standard deviations and with a BMI ≥27kg/m2 demonstrated an odds ratio (OR) 3.51 (2.15,5.75) in development of radiographic knee osteoarthritis.(61) Depression was strongly associated in a cross-sectional study using appendicular skeletal mass, grip strength and gait speed for sarcopenia, and percent body fat for obesity.(62) This was also observed longitudinally with low grip strength and obesity defined using a BMI≥30kg/m2, with a OR 1.79 (1.10,2.89) over six year follow-up.(63) Psychological stress was also highest in individuals with sarcopenic obesity, with a higher degree of psychological health (OR 1.79 [1.10,2.89]) and higher stress (OR 6.05 [1.89,19.38]).(64) Lastly, the risk of type 2 diabetes was markedly higher (HR 3.57 [2.04,6.24]) in the English Longitudinal Study of Aging in individuals with sarcopenic obesity defined using grip strength and BMI.(65)
Sarcopenia and visceral obesity have been suggested to aggravate each other, resulting in a vicious, bi-directional cycle. In a study of 379 Korean men and women (mean age 51.9±14.6 years) from the Korean Sarcopenic Obesity Study, visceral fat area was an independent negative predictor of the changes in appendicular lean soft tissue after adjusting for confounders.(66) Participants with visceral obesity and low muscle strength had significantly higher risk of mortality, worsening disability and hospitalization.(67)
Both obesity and low handgrip strength, independent of each other, predict the risk of death in adult men and women with additive pattern. The predictive value of obesity varies by age, whereas low muscle strength predicts mortality in all age groups aged>50 years and across all BMI categories. Data from adults between ages 50–91 years in a Finnish cohort demonstrated that the highest mortality was among those with low handgrip strength and obesity (HR 1.23 [1.04–1.46].(68) The NHANES III data demonstrated sex-specific differences in mortality using body fat and muscle mass cutoffs.(69) (70) A meta-analysis of all sarcopenic obesity definitions demonstrated differential mortality estimates for muscle mass (HR 1.06 [0.91,1.22]) as compared to muscle strength (HR 1.23 [1.09,1.38]).(71) When promoting health among older adults, more attention should be paid to physical fitness in addition to body weight and adiposity.(68) The LIFE study is a key study that reduced mobility disability in older adults through a walking and resistance program.{Pahor, 2014 #78} Other health promotion studies have demonstrated the importance of physical fitness as a surrogate for better health, yet future agreement among experts is needed before implementation into clinical practice.{Kitzman, 2016 #123;Nicklas, 2019 #124;Racette, 2017 #125;Sui, 2007 #126}. These are important effects that go beyond cardiometabolic factors and changes in cognition.{Edwards, 2017 #127;Lee, 1999 #128;Rheaume, 2011 #129}
What did the Epidemiological data show us?
Long-term associations observed in the epidemiological literature are strongly related to definitions of sarcopenia and obesity. Despite the lack of consensus on the definition of sarcopenic obesity, the detrimental clinical implications of this syndrome are unequivocal. Muscle strength is likely a stronger predictor of decline than muscle mass. This correlation can put more emphasis on the clinical screening for sarcopenic obesity with simple tests such as grip strength. The synergy of low muscle mass/strength and obesity (body fat or elevated BMI) is associated with significantly higher morbidity/mortality than either one alone. Therefore, treatments should address both obesity and reduced muscle mass/strength.
Caloric Restriction
Energy or caloric restriction is the hallmark of any intentional weight loss program. Calorie restriction triggers a complex series of intricate events, including activation of cellular stress response elements, improved autophagy, modification of apoptosis, and alteration in hormonal balance.(72) Caloric restriction aids with healthy aging through weight loss. In the CALERIE (Comprehensive Assessment of Long term Effects of Reducing Intake of Energy) study, weight-loss was strongly associated with improvements in VO2 and knee strength at 2-years.(73) On the other hand, caloric restriction on its own, which in most cases can entail a 20–40% reduction of food consumption relative to normal intake, is a significant intervention that can also result in detrimental effects.(74) Caution should be exercised with caloric restriction to avoid loss of lean muscle mass and strength. Generally, loss of weight is ¼ muscle and ¾ fat.(75) Combining caloric restriction with aerobic and resistance exercises can mitigate this potential detrimental effect and can provide greater improvement in physical function.(76) Also of concern during weight-loss efforts is loss of bone density which, without a resistance exercise component, can result in a decrease in hip bone density; resistance exercises can potential lead to corresponding increased hip bone mineral density.(77)
Aerobic and Resistance Exercise
Multiple modalities have investigated the effects of resistance training, aerobic training, or combination training on sarcopenic obesity. Older adults with sarcopenic obesity who engaged in the resistance training, aerobic training, and combination training demonstrated increased muscle mass and reduced total fat mass and visceral fat area compared with those without training.(78) The muscle strength performance and serum IGF-1 level improvements were most pronounced in a resistance/aerobic training group over an 8-week period. Compared with resistance training alone, protein supplementation combined with resistance training may have a stronger effect in preventing aging-related muscle mass attenuation and leg strength loss in older people.(79)
The improvement in VO2max and other marker has been shown to be possible in older adults using exercise programs to improve measures of physical function and preclinical disability with impairments in physical performance.(80) Binder et al randomized 150 older adults (mean age 83±4 years) with mild to moderate frailty who participated in a 9-month low-intensity home exercise vs. an exercise-training program. The latter program had improvements of 1.0 vs. 5.2 points for the modified physical performance score, 0.9 to 3.6 ml/kg/min for VO2 peak, and 1.6 to 4.9 points for the functional status questionnaire. The above findings and an increasingly emerging evidence argue to the continued need to advocate for more physical activity in the elderly.
Aerobic and resistance exercise, but not weight-loss, though, has a marked effect on the molecular and cellular level of the type II muscle fibers. Significant reductions in markers of muscle inflammation and anabolism, including Toll-like Receptor 4, insulin growth factors and type II fiber sizes were observed with exercise in frail older adults with obesity.(24, 81) Yet, a combined aerobic/resistance program was found to be more effective than either alone in improving muscle protein synthesis and myocellular quality during weight-loss interventions.(82)
Caloric Restriction with Exercise
Few studies specifically evaluate individuals with sarcopenic obesity. A randomized controlled trial in older female participants (aged 70 years and over) with sarcopenic obesity were enrolled in a three-month study of a 60-minute exercise class twice weekly, nutrition (consisting of essential amino acid supplementation and tea fortified with catechins), both, or health education classes. The combined intervention led to significant declines of total body fat (OR 4.42 [1.21,16.19]) and improved walking speed (OR 3.05 [1.01,9.19]).(83) A pivotal trial by Villareal et al randomized four groups (aerobic, resistance, combined, control) and found that the combined aerobic/resistance group had a mean 9% weight-loss, but marked improvements of 21% vs 14% in the aerobic or resistance groups in physical performance status.(84) Changes were also observed in undercarboxylated osteocalcin, sclerostin, and improvements in pancreatic insulin secretion.(85) Although, weight loss from lifestyle interventions result in significant decreases in total and free estradiol levels in frail, older men with obesity, this did not result in a clinically important increase in total testosterone nor a significant increase in free testosterone. The combination of diet and exercise in frail, older adults with obesity leads to increases in testosterone and reduced estradiol.(86) The implications of these findings are currently unclear and require further investigation.
Conclusions
Sarcopenic obesity is prevalent and increases the risk of decline in the older adults. We propose a shift to translate research-based findings to clinical environments from muscle mass to muscle strength. Sarcopenic obesity has detrimental effects on quality of life, independence, and mortality. We advocate a focus on physical function and quality of life. Many evidence-based interventions involve dietary and changes in physical activity. Gaining an understanding of the underlying behavioral and biological mechanisms is critical in targeting interventions. More research is needed to harmonize the definitions and clarify mechanisms contributing to this geriatric syndrome which could then lead to personalized targeting of interventions to the individual participant.
Acknowledgments
FINANCIAL SUPPORT:
Dr. Batsis receives funding from the National Institute on Aging of the National Institutes of Health under Award Number K23AG051681. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- BMI
Body mass index
- CALERIE
Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy
- DEXA
Dual-energy X-ray absorptiometry
- EWGSOP
European Working Group on Sarcopenia in Older People
- FNIH
Foundation for the National Institutes of Health
- HR
Hazard ratio
- IGF
Insulin-like growth factor
- IL-6
Interleukin-6
- InCHIANTI
Invecchiare in Chianti, aging in the Chianti area
- IWGS
International Working Group on Sarcopenia
- NHANES
National Health and Nutrition Examination Survey
- OR
Odds ratio
- VO2
Oxygen uptake
Footnotes
CONFLICT OF INTERESTS: None
REFERENCES
- 1.Mokdad AH, Bowman BA, Ford ES et al. (2001) The continuing epidemics of obesity and diabetes in the United States. Jama 286, 1195–1200. [DOI] [PubMed] [Google Scholar]
- 2.Ard J (2015) Obesity in the US: what is the best role for primary care? BMJ 350, g7846. [DOI] [PubMed] [Google Scholar]
- 3.Malenfant JH & Batsis JA (2019) Obesity in the geriatric population–a global health perspective. Journal of Global Health Reports 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Batsis JA, Barre LK, Mackenzie TA et al. (2013) Variation in the prevalence of sarcopenia and sarcopenic obesity in older adults associated with different research definitions: dual-energy X-ray absorptiometry data from the National Health and Nutrition Examination Survey 1999–2004. J Am Geriatr Soc 61, 974–980. [DOI] [PubMed] [Google Scholar]
- 5.Batsis JA & Villareal DT (2018) Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nature Reviews Endocrinology 14, 513–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruggiero C, Metter EJ, Melenovsky V et al. (2008) High basal metabolic rate is a risk factor for mortality: the Baltimore Longitudinal Study of Aging. J Gerontol A Biol Sci Med Sci 63, 698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Visser M, Pahor M, Taaffe DR et al. (2002) Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci 57, M326–332. [DOI] [PubMed] [Google Scholar]
- 8.McPhee JS, French DP, Jackson D et al. (2016) Physical activity in older age: perspectives for healthy ageing and frailty. Biogerontology 17, 567–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cappola AR, Xue QL, Ferrucci L et al. (2003) Insulin-like growth factor I and interleukin-6 contribute synergistically to disability and mortality in older women. The Journal of clinical endocrinology and metabolism 88, 2019–2025. [DOI] [PubMed] [Google Scholar]
- 10.Cartwright MJ, Tchkonia T Kirkland JL (2007) Aging in adipocytes: potential impact of inherent, depot-specific mechanisms. Exp Gerontol 42, 463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sepe A, Tchkonia T, Thomou T et al. (2011) Aging and regional differences in fat cell progenitors - a mini-review. Gerontology 57, 66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sayer AA, Syddall H, Martin H et al. (2008) The developmental origins of sarcopenia. J Nutr Health Aging 12, 427–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dodds RM, Syddall HE, Cooper R et al. (2014) Grip strength across the life course: normative data from twelve British studies. PLoS One 9, e113637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Metter EJ, Lynch N, Conwit R et al. (1999) Muscle quality and age: cross-sectional and longitudinal comparisons. J Gerontol A Biol Sci Med Sci 54, B207–218. [DOI] [PubMed] [Google Scholar]
- 15.Abizanda P, Romero L, Sanchez-Jurado PM et al. (2016) Energetics of Aging and Frailty: The FRADEA Study. J Gerontol A Biol Sci Med Sci 71, 787–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallagher D, Belmonte D, Deurenberg P et al. (1998) Organ-tissue mass measurement allows modeling of REE and metabolically active tissue mass. Am J Physiol 275, E249–258. [DOI] [PubMed] [Google Scholar]
- 17.Wilson MM & Morley JE (2003) Invited review: Aging and energy balance. J Appl Physiol (1985) 95, 1728–1736. [DOI] [PubMed] [Google Scholar]
- 18.Conley KE, Esselman PC, Jubrias SA et al. (2000) Ageing, muscle properties and maximal O(2) uptake rate in humans. J Physiol 526 Pt 1, 211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conley KE, Jubrias SA Esselman PC (2000) Oxidative capacity and ageing in human muscle. J Physiol 526 Pt 1, 203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doucet E, Imbeault P, St-Pierre S et al. (2000) Appetite after weight loss by energy restriction and a low-fat diet-exercise follow-up. Int J Obes Relat Metab Disord 24, 906–914. [DOI] [PubMed] [Google Scholar]
- 21.Pahor M, Guralnik JM, Ambrosius WT et al. (2014) Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA 311, 2387–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sherrington C, Tiedemann A, Fairhall N et al. (2011) Exercise to prevent falls in older adults: an updated meta-analysis and best practice recommendations. New South Wales public health bulletin 22, 78–83. [DOI] [PubMed] [Google Scholar]
- 23.Pollock RD, Carter S, Velloso CP et al. (2015) An investigation into the relationship between age and physiological function in highly active older adults. J Physiol 593, 657–680; discussion 680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nilwik R, Snijders T, Leenders M et al. (2013) The decline in skeletal muscle mass with aging is mainly attributed to a reduction in type II muscle fiber size. Exp Gerontol 48, 492–498. [DOI] [PubMed] [Google Scholar]
- 25.Batsis JA & Villareal DT (2018) Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol 14, 513–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Batsis JA, Mackenzie TA, Jones JD et al. (2016) Sarcopenia, sarcopenic obesity and inflammation: Results from the 1999–2004 National Health and Nutrition Examination Survey. Clin Nutr 35, 1472–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Batsis JA, Germain CM, Vasquez E et al. (2016) Prevalence of weakness and its relationship with limitations based on the Foundations for the National Institutes for Health project: data from the Health and Retirement Study. European journal of clinical nutrition 70, 1168–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schrager MA, Metter EJ, Simonsick E et al. (2007) Sarcopenic obesity and inflammation in the InCHIANTI study. J Appl Physiol (1985) 102, 919–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tremollieres FA, Pouilles JM Ribot CA (1996) Relative influence of age and menopause on total and regional body composition changes in postmenopausal women. Am J Obstet Gynecol 175, 1594–1600. [DOI] [PubMed] [Google Scholar]
- 30.Morley JE (2017) Hormones and Sarcopenia. Curr Pharm Des 23, 4484–4492. [DOI] [PubMed] [Google Scholar]
- 31.Clark BC & Manini TM (2012) What is dynapenia? Nutrition 28, 495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carson RG (2018) Get a grip: individual variations in grip strength are a marker of brain health. Neurobiol Aging 71, 189–222. [DOI] [PubMed] [Google Scholar]
- 33.Kuriyan R (2018) Body composition techniques. Indian J Med Res 148, 648–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neeland IJ, Ross R, Despres JP et al. (2019) Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol 7, 715–725. [DOI] [PubMed] [Google Scholar]
- 35.Batsis JA, Mackenzie TA, Bartels SJ et al. (2016) Diagnostic accuracy of body mass index to identify obesity in older adults: NHANES 1999–2004. Int J Obes (Lond) 40, 761–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu W, Perera S, Medich D et al. (2011) Height loss, vertebral fractures, and the misclassification of osteoporosis. Bone 48, 307–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bohannon RW (2019) Minimal clinically important difference for grip strength: a systematic review. J Phys Ther Sci 31, 75–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bohannon RW (2006) Reference values for the five-repetition sit-to-stand test: a descriptive meta-analysis of data from elders. Percept Mot Skills 103, 215–222. [DOI] [PubMed] [Google Scholar]
- 39.Guralnik JM, Ferrucci L, Simonsick EM et al. (1995) Lower-Extremity Function in Persons over the Age of 70 Years as a Predictor of Subsequent Disability. New England Journal of Medicine 332, 556–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rantanen T, Masaki K, He Q et al. (2012) Midlife muscle strength and human longevity up to age 100 years: a 44-year prospective study among a decedent cohort. Age (Dordr) 34, 563–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hulens M, Vansant G, Lysens R et al. (2001) Study of differences in peripheral muscle strength of lean versus obese women: an allometric approach. International Journal of Obesity 25, 676–681. [DOI] [PubMed] [Google Scholar]
- 42.Buehring B, Siglinsky E, Krueger D et al. (2018) Comparison of muscle/lean mass measurement methods: correlation with functional and biochemical testing. Osteoporos Int 29, 675–683. [DOI] [PubMed] [Google Scholar]
- 43.Rubbieri G, Mossello E Di Bari M (2014) Techniques for the diagnosis of sarcopenia. Clin Cases Miner Bone Metab 11, 181–184. [PMC free article] [PubMed] [Google Scholar]
- 44.Studenski SA, Peters KW, Alley DE et al. (2014) The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci 69, 547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cawthon PM, Travison TG, Manini TM et al. (2019) Establishing the Link Between Lean Mass and Grip Strength Cut-points With Mobility Disability and Other Health Outcomes: Proceedings of the Sarcopenia Definition and Outcomes Consortium Conference. J Gerontol A Biol Sci Med Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cruz-Jentoft AJ, Baeyens JP, Bauer JM et al. (2010) Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 39, 412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cruz-Jentoft AJ, Bahat G, Bauer J et al. (2019) Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 48, 601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim TN, Yang SJ, Yoo HJ et al. (2009) Prevalence of sarcopenia and sarcopenic obesity in Korean adults: the Korean sarcopenic obesity study. Int J Obes (Lond) 33, 885–892. [DOI] [PubMed] [Google Scholar]
- 49.Johnson Stoklossa CA, Sharma AM, Forhan M et al. (2017) Prevalence of Sarcopenic Obesity in Adults with Class II/III Obesity Using Different Diagnostic Criteria. Journal of nutrition and metabolism 2017, 7307618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Du K, Goates S, Arensberg MB et al. (2018) Prevalence of sarcopenia and sarcopenic obesity vary with race/ethnicity and advancing age. Divers Equal Health Care 15, 175–183. [Google Scholar]
- 51.Khor EQ- E, Lim JP, Tay L et al. (2019) Obesity Definitions in Sarcopenic Obesity: Differences in Prevalence, Agreement and Association with Muscle Function. The Journal of Frailty & Aging. [DOI] [PubMed] [Google Scholar]
- 52.Kemmler W, Teschler M, Weissenfels A et al. (2017) Prevalence of sarcopenia and sarcopenic obesity in older German men using recognized definitions: high accordance but low overlap! Osteoporos Int 28, 1881–1891. [DOI] [PubMed] [Google Scholar]
- 53.Baumgartner RN, Wayne SJ, Waters DL et al. (2004) Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes Res 12, 1995–2004. [DOI] [PubMed] [Google Scholar]
- 54.Batsis JA, Mackenzie TA, Lopez-Jimenez F et al. (2015) Sarcopenia, sarcopenic obesity, and functional impairments in older adults: National Health and Nutrition Examination Surveys 1999–2004. Nutr Res 35, 1031–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stenholm S, Alley D, Bandinelli S et al. (2009) The effect of obesity combined with low muscle strength on decline in mobility in older persons: results from the InCHIANTI study. Int J Obes (Lond) 33, 635–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Batsis JA, Mackenzie TA, Barre LK et al. (2014) Sarcopenia, sarcopenic obesity and mortality in older adults: results from the National Health and Nutrition Examination Survey III. European Journal of Clinical Nutrition 68, 1001–1007. [DOI] [PubMed] [Google Scholar]
- 57.Schaap LA, Koster A Visser M (2013) Adiposity, muscle mass, and muscle strength in relation to functional decline in older persons. Epidemiol Rev 35, 51–65. [DOI] [PubMed] [Google Scholar]
- 58.Tinetti ME (2003) Clinical practice. Preventing falls in elderly persons. N Engl J Med 348, 42–49. [DOI] [PubMed] [Google Scholar]
- 59.Scott D, Sanders KM, Aitken D et al. (2014) Sarcopenic obesity and dynapenic obesity: 5-year associations with falls risk in middle-aged and older adults. Obesity (Silver Spring) 22, 1568–1574. [DOI] [PubMed] [Google Scholar]
- 60.Follis S, Cook A, Bea JW et al. (2018) Association Between Sarcopenic Obesity and Falls in a Multiethnic Cohort of Postmenopausal Women. J Am Geriatr Soc 66, 2314–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee S, Kim TN Kim SH (2012) Sarcopenic obesity is more closely associated with knee osteoarthritis than is nonsarcopenic obesity: a cross-sectional study. Arthritis Rheum 64, 3947–3954. [DOI] [PubMed] [Google Scholar]
- 62.Ishii S, Chang C, Tanaka T et al. (2016) The Association between Sarcopenic Obesity and Depressive Symptoms in Older Japanese Adults. PLoS One 11, e0162898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hamer M, Batty GD Kivimaki M (2015) Sarcopenic obesity and risk of new onset depressive symptoms in older adults: English Longitudinal Study of Ageing. Int J Obes (Lond) 39, 1717–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cho Y, Shin SY Shin MJ (2015) Sarcopenic obesity is associated with lower indicators of psychological health and quality of life in Koreans. Nutr Res 35, 384–392. [DOI] [PubMed] [Google Scholar]
- 65.Cuthbertson DJ, Bell JA, Ng SY et al. (2016) Dynapenic obesity and the risk of incident Type 2 diabetes: the English Longitudinal Study of Ageing. Diabet Med 33, 1052–1059. [DOI] [PubMed] [Google Scholar]
- 66.Kim TN, Park MS, Ryu JY et al. (2014) Impact of visceral fat on skeletal muscle mass and vice versa in a prospective cohort study: the Korean Sarcopenic Obesity Study (KSOS). PLoS One 9, e115407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rossi AP, Bianchi L, Volpato S et al. (2017) Dynapenic Abdominal Obesity as a Predictor of Worsening Disability, Hospitalization, and Mortality in Older Adults: Results From the InCHIANTI Study. The Journals of Gerontology: Series A 72, 1098–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stenholm S, Mehta NK, Elo IT et al. (2014) Obesity and muscle strength as long-term determinants of all-cause mortality--a 33-year follow-up of the Mini-Finland Health Examination Survey. Int J Obes (Lond) 38, 1126–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Batsis JA, Mackenzie TA, Emeny RT et al. (2017) Low Lean Mass With and Without Obesity, and Mortality: Results From the 1999–2004 National Health and Nutrition Examination Survey. J Gerontol A Biol Sci Med Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Batsis JA, Mackenzie TA, Barre LK et al. (2014) Sarcopenia, sarcopenic obesity and mortality in older adults: results from the National Health and Nutrition Examination Survey III. Eur J Clin Nutr 68, 1001–1007. [DOI] [PubMed] [Google Scholar]
- 71.Tian S & Xu Y (2016) Association of sarcopenic obesity with the risk of all-cause mortality: A meta-analysis of prospective cohort studies. Geriatr Gerontol Int 16, 155–166. [DOI] [PubMed] [Google Scholar]
- 72.Golbidi S, Daiber A, Korac B et al. (2017) Health Benefits of Fasting and Caloric Restriction. Curr Diab Rep 17, 123. [DOI] [PubMed] [Google Scholar]
- 73.Racette SB, Rochon J, Uhrich ML et al. (2017) Effects of Two Years of Calorie Restriction on Aerobic Capacity and Muscle Strength. Med Sci Sports Exerc 49, 2240–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee C & Longo V (2016) Dietary restriction with and without caloric restriction for healthy aging. F1000Res 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Heymsfield SB, Gonzalez MC, Shen W et al. (2014) Weight loss composition is one-fourth fat-free mass: a critical review and critique of this widely cited rule. Obes Rev 15, 310–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Villareal DT, Chode S, Parimi N et al. (2011) Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med 364, 1218–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Soltani S, Hunter GR, Kazemi A et al. (2016) The effects of weight loss approaches on bone mineral density in adults: a systematic review and meta-analysis of randomized controlled trials. Osteoporosis International 27, 2655–2671. [DOI] [PubMed] [Google Scholar]
- 78.Chen HT, Chung YC, Chen YJ et al. (2017) Effects of Different Types of Exercise on Body Composition, Muscle Strength, and IGF-1 in the Elderly with Sarcopenic Obesity. J Am Geriatr Soc 65, 827–832. [DOI] [PubMed] [Google Scholar]
- 79.Liao CD, Tsauo JY, Wu YT et al. (2017) Effects of protein supplementation combined with resistance exercise on body composition and physical function in older adults: a systematic review and meta-analysis. Am J Clin Nutr 106, 1078–1091. [DOI] [PubMed] [Google Scholar]
- 80.Binder EF, Schechtman KB, Ehsani AA et al. (2002) Effects of exercise training on frailty in community-dwelling older adults: results of a randomized, controlled trial. J Am Geriatr Soc 50, 1921–1928. [DOI] [PubMed] [Google Scholar]
- 81.Lambert CP, Wright NR, Finck BN et al. (2008) Exercise but not diet-induced weight loss decreases skeletal muscle inflammatory gene expression in frail obese elderly persons. J Appl Physiol (1985) 105, 473–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Colleluori G, Aguirre L, Phadnis U et al. (2019) Aerobic Plus Resistance Exercise in Obese Older Adults Improves Muscle Protein Synthesis and Preserves Myocellular Quality Despite Weight Loss. Cell Metab 30, 261–273.e266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim H, Kim M, Kojima N et al. (2016) Exercise and Nutritional Supplementation on Community-Dwelling Elderly Japanese Women With Sarcopenic Obesity: A Randomized Controlled Trial. J Am Med Dir Assoc 17, 1011–1019. [DOI] [PubMed] [Google Scholar]
- 84.Villareal DT, Aguirre L, Gurney AB et al. (2017) Aerobic or Resistance Exercise, or Both in Dieting Obese Older Adults. N Engl J Med 376, 1943–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Colleluori G, Napoli N, Phadnis U et al. (2017) Effect of Weight Loss, Exercise, or Both on Undercarboxylated Osteocalcin and Insulin Secretion in Frail, Obese Older Adults. Oxid Med Cell Longev 2017, 4807046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Armamento-Villareal R, Aguirre LE, Qualls C et al. (2016) Effect of lifestyle intervention on the hormonal profile of frail, obese older men. The journal of nutrition, health & aging 20, 334–340. [DOI] [PMC free article] [PubMed] [Google Scholar]


