Abstract
Background
A dramatic increase in aging populations and low birth rates rapidly drive aging societies and increase aging-associated neurodegenerative diseases. However, functional food or medicinal formulations to prevent geriatric brain disorders are not readily available. Panax ginseng is a candidate, since ginseng has long-been consumed as a rejuvenating agent. However, the underlying molecular mechanisms and the components of ginseng that are responsible for brain rejuvenation and human longevity are unknown. Accumulating evidence shows that gintonin is a candidate for the anti-aging ingredient of ginseng, especially in brain senescence.
Methods
Gintonin, a glycolipoprotein complex, contains three lipid-derived G protein-coupled receptor ligands: lysophosphatidic acids (LPAs), lysophosphatidylinositols (LPIs), and linoleic acid (LA). LPA, LPI, and LA act on six LPA receptor subtypes, GPR55, and GPR40, respectively. These G protein-coupled receptors are distributed within the nervous and non-nervous systems of the human body.
Results
Gintonin-enriched fraction (GEF) exhibits anti-brain senescence and effects against disorders such as Alzheimer's disease (AD), Huntington's disease (HD), and Parkinson's disease (PD). Oral administration of gintonin in animal models of d-galactose-induced brain aging, AD, HD, and PD restored cognitive and motor functions. The underlying molecular mechanisms of gintonin-mediated anti-brain aging and anti-neurodegenerative diseases include neurogenesis, autophagy stimulation, anti-apoptosis, anti-oxidative stress, and anti-inflammatory activities. This review describes the characteristics of gintonin and GEF, and how gintonin exerts its effects on brain aging and brain associated-neurodegenerative diseases.
Conclusion
Finally, we describe how GEF can be applied to improve the quality of life of senior citizens in aging societies.
Keywords: Panax ginseng, Gintonin, Brain aging, Neurodegenerative diseases, Rejuvenation
1. Introduction
Aging is an inevitable biological process and results in complex and physiological changes to the body.1 Although there are many theories on aging, they fall into two generally accepted categories: programmed factors vs. damage-related factors.2 The theory of programmed factors is that organisms have a biological timetable overseeing growth, development, aging, and death. During these periods, gene expression is altered to control maintenance, repair, and defense mechanisms. On the other hand, the theory of damage-related factors holds that internal, external, or environmental insults damage cells and organs, and accumulate in organisms.2 Both theories agree that aging organisms are unable to maintain their normal functions, resulting in death. Despite the enormous molecular complexity of aging, there are several molecular mechanisms in which the aging process can considered in a generic sense, for example, disruption of hormonal axes, accumulated oxidative stress, chronic inflammation, increasing nucleic acid instability, and a reduction in metabolic efficiency. Among these common aging factors, inflammation and oxidative stress appear to be detrimental in all of the physiological aspects of aging.3
An aging society is a society whose median age rises due to rising life expectancy and/or declining birthrates.4 The United Nations standards define an aging society as a country in which the share of population aged over 65 years exceeds 7% of the whole population.5 Currently, both developed countries and many developing countries are rapidly becoming aging societies, with increasing life expectancies.5 Aging societies usually accompany decreases in the economically active population.6, 7 Therefore, such countries make financial investments to boost birthrates.6, 7 In addition, senior citizens in aging societies show greater cognitive decline and higher occurrences of chronic diseases and age-related neurodegenerative diseases such as AD, PD and HD than younger populations, although some cases of these diseases are due to family heritage or genetic factors.8, 9, 10 Thus, humans are now living longer but not always living healthier. Currently, although many countries around the world are rapidly become aging societies, there are no functional foods or medicines to delay brain aging and further prevent aged-related neurodegenerative diseases.11 At a cellular level, brain aging is characterized by increasing inflammation, oxidative stress, increased genomic instability, altered metabolism and the destruction of protein homeostasis, and decline of autophagy activity, which causes the accumulation of cellular toxic peptides or proteins.2 Recent evidence shows that ginseng gintonin, a herbal medicine, provides a promise in protecting the brain from aging and aging-related neurodegenerative diseases.12
This review provides a brief history of gintonin isolation from ginseng, chemical compositions of gintonin, and gintonin synthesis. We further focus on the underlying molecular mechanisms of gintonin-mediated effects against brain aging and neurodegenerative diseases, both in vitro and in vivo, and the possible applications of gintonin in an aging population that is accompanied by brain aging neurodegenerative disease.
2. Ginseng, gintonin, and gintonin-enriched fraction (GEF)
2.1. Ginseng
Panax ginseng is one of the most widely consumed herbal nutritional products in the world. Ginseng has been used to promote rejuvenation and longevity; against stress, weakness and fatigue; and for both mental and physical states.13 Although the root of Panax ginseng is used in medicinal food recipes, it is also added to confectionary, food products, and drinks.14, 15, 16 Recent studies have shown that medicinal formulations of the extract are consumed for their medicinal purposes in several countries. However, relatively little is known about the underlying molecular mechanisms and which components of ginseng are responsible for its efficacy. The active components, ginsenosides (also called ginseng saponins), which are a kind of plant glycoside, were first found in ginseng.17 and gintonin was recently isolated from a crude ginseng total saponin fraction.18 Since ginsenosides have been well characterized in previous reports,17 we will focus on gintonin.
2.2. Gintonin: a non-saponin bioactive component of ginseng
Before technical methods to purify individual ginsenosides from ginseng root were developed, a simple butanol fraction of ginseng, which is also known as a crude ginseng total saponin fraction, was usually used for in vitro and in vivo studies.19 As a dried powder, the color of crude ginseng total saponin fraction ranges from weak to thick brown depending on the content of other ginseng components besides ginsenosides, since the color of pure ginsenosides is white.20 Thus, the percentage of pure ginsenosides is low in crude ginseng total saponin fractions, although active components that are brown in crude ginseng total saponin fraction have been unidentified. In early studies before novel components were isolated from crude ginseng total saponin fraction, they were shown to work in a different manner from pure ginsenosides, in terms of elicit of [Ca2+]i transients and Ca2+-activated Cl− channel regulation in Xenopus oocytes, since they activate Ca2+-activated chloride channels through unknown membrane protein signaling pathways through phospholipase C (PLC)-IP3-transient cytosolic Ca2+ release.21 However, individual pure ginsenoside are unable to do this, indicating the possibility that crude ginseng total saponin fraction contains unknown active ingredients besides ginsenosides.21, 22, 23, 24 Interestingly, the degree of Ca2+-activated Cl− channel activation by crude ginseng total saponin fraction is dependent on its color, since thick brown crude ginseng total saponin fraction is more active in activation of Ca2+-activated Cl− channels than that of a weak brown color.
Later, Pyo et al.25 isolated the active ingredient that induced Ca2+-activated Cl− channel activation from crude ginseng total saponin fraction through multiple steps using organic solvents (i.e., methanol and butanol), fractionation, and diethylaminoethyl (DEAE) sepharose anion exchange chromatography. The active ingredient was named gintonin: a [Ca2+]i transient induction agent of ginseng in mammalian cells. However, individual pure ginsenosides did not activate Ca2+-activated Cl− channels at all.25 Chemical characteristics of gintonin include carbohydrates, lipids, and ginseng proteins complex. The yield of gintonin is about 0.2% in 4-year-old white and 6-year-old red ginseng, although the amount gintonin is still higher than individual ginsenosides of ginseng.25 Other ginseng species, such as American ginseng, also contain an amount of gintonin comparable with Panax ginseng.25 It appears that gintonin can be further divided into six different subtypes through size-exclusion gel filtration. The native molecular weight of gintonin is about 67 kDa, but its apparent molecular weight is about 13 kDa according to SDS-PAGE analysis, indicating that gintonin might be a kind of pentamer.25 In qualitative assays, gintonin consists of ginseng proteins with rich hydrophobic amino acids, and carbohydrates rich with glucose ≫ glucosamine, and lipid components of linoleic ≫ palmitic > oleic = stearic acids.25
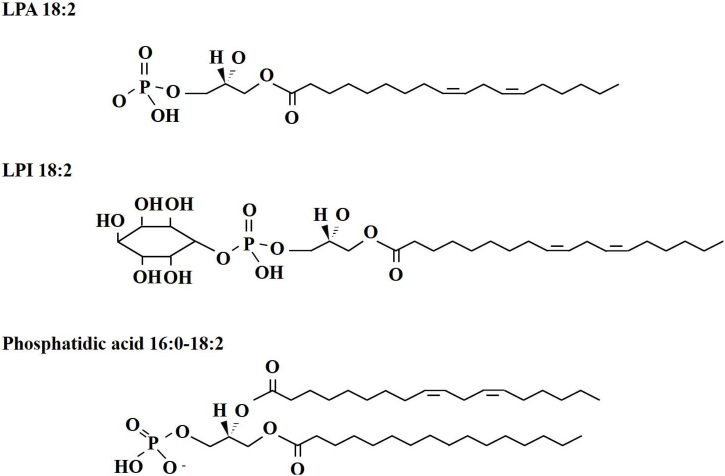
Hwang et al.26 for the first time identified that the active ingredient of gintonin is lysophosphatidic acids (LPAs). which are known as endogenous ligands of plasma membrane G protein-coupled LPA receptors (Fig. 1). Before their identification in animal systems, LPAs were first known as serum factors in trace amounts, since serum factors induce mitogenic effects in cultured fibroblasts and retraction in neuronal cells.27, 28, 29 Interestingly, the fatty acid composition of LPAs in gintonin is mostly linoleic acid (C18:2), whereas the fatty acid components of LPAs in animals and other plants are oleic acid (C18:1), palmitic acid (C16:0), or stearyl acid (C18:0).30 In addition, LPAs, which are hydrophobic lipid components of gintonin, tightly bind to ginseng protein components, since LPAs are partially separated from ginseng protein components only after long-term methanol extraction of gintonin, as LPAs are tightly bound to serum albumin.30 Proteomic analysis of protein components of gintonin shows that the main protein components of gintonin are ginseng latex-like protein 151 and ginseng major protein.31, 32
Fig. 1.
The chemical structures of four main bioactive components of gintonin. Gintonin was prepared from Panax ginseng root. LPAs was proved as the main functional ingredients of gintonin. Gintonin LPAs act as exogenous ligand for G protein-coupled LPA receptors. Recently, gintonin LPI and linoleic acid was also proved as a ligand of GPR40 and GPR55.49
Gintonin binds and activates six subtypes of LPA receptor in a differential manner to elicit [Ca2+]i transients in mammalian cells.26 Thus, gintonin exhibits different affinities for six LPA receptor subtypes for Ca2+ mobilization, in increasing order of affinity from LPA2, LPA5, LPA1, LPA3, LPA4, to LPA6, but does not induce changes to intracellular cAMP concentration.26 Gintonin has also been isolated from ginseng leaf and stem, in addition to ginseng root.33 Interestingly, although ginseng leaf contains a lot of gintonin, it was not easy to remove lead-derived chlorophyll from gintonin without using hexane.33 Gintonin has also been isolated using only ethanol instead of methanol and butanol.34 In vitro studies using neuronal and non-neuronal cells show that gintonin, but not pure ginsenosides, induces [Ca2+]i transients via LPA receptor activation and acts as an exogenous LPA receptor ligand, which is key to gintonin's in vitro and in vivo biological effects, as described below.
2.3. Gintonin-enriched fraction (GEF)
Instead of using multiple steps for gintonin preparation using organic chemicals such as methanol and butanol, a brief and cost-effective method was developed to obtain a large amount of gintonin using only fermented ethanol and water, after much trial and error.35 After extraction of ginseng with ethanol, the extract is fractioned with water to obtain water-soluble and water-insoluble fractions. The water-insoluble precipitate, rather than the water-soluble supernatant, still induced a large [Ca2+]i transient in neuronal cells, as does gintonin.35 The water-insoluble precipitate fraction was designated as the gintonin-enriched fraction (GEF). The yield of GEF was approximately 1.3%, which is 6-fold higher than that obtained previously. GEF utilizes the same signal transduction pathway as gintonin does, and induces [Ca2+]i transients in cultured mouse cortical astrocytes. Most of the water-soluble supernatant fractionation does not induce [Ca2+]i transients and mainly contains ginsenosides and carbohydrates.35 The apparent molecular weight of GEF is about 13 kDa by SDS-PAGE, which is equivalent to that obtained by the previous gintonin preparation method.35 Interestingly, the main difference between gintonin and GEF is that more ginseng proteins exist in GEF preparations, since GEF staining by Coomassie brilliant blue after SDS-PAGE is stronger than that for gintonin.35 Because GEF can be prepared through water precipitation of ginseng ethanol extract and is easily reproducible with high yields, it could be used for preclinical animal studies and commercially utilized for the development of gintonin-derived medicinal food and natural medicine for application in humans.36
3. Characteristics of the lipid components of gintonin and GEF
Gas chromatography–mass spectrometry for fatty acid analysis and liquid chromatography–tandem mass spectrometry for GEF phospholipids analysis has revealed that gintonin contains many kinds of bioactive lipids.37 Further quantifying analysis for fatty acids, lysophospholipids (LPLs), and phospholipids (PLs) revealed that GEF contains about 7.5% linoleic (C18:2), 2.8% palmitic (C16:0), and 1.5% oleic acids (C18:1). GEF contains about 0.2% LPA C18:2, 0.06% LPA C16:0, and 0.02% LPA C18:1. GEF contains 0.08% lysophosphatidylcholine (LPC), 0.03% lysophosphatidylethanolamine (LPE), and 0.13% lysophosphatidylinositols (LPIs), which act as an endocannabinoid GPR55 ligand.38, 39, 40, 41 The order of lysophospholipids in GEF is LPA > LPI > LPC > LPE. GEF also contains about 1% phosphatidic acid (PA) C16:0–18:2, 0.5% PA C18:2–18:2, and 0.2% PA C16:0–18:1, which are now considered second messengers.42 The majority of lipid components of gintonin or GEF have negative rather than positive or neutral charges (Fig. 1). This might be the reason that gintonin can be separated from other ginseng components through anion exchange chromatography.25 Polyunsaturated linoleic acid, which is an endogenous GPR40 ligand.43, 44, 45, 46 is the most abundant component of gintonin and GEF. Linoleic acid (C18:2) is the main fatty acid component of LPA, other LPLs, and PAs in GEF, raising the possibility that linoleic acid (C18:2) is the primary fatty acid compound of LPA, LPL, PA and other lipid synthesis components in ginseng. Thus, gintonin contains three ligands of G protein-coupled receptors such as LPA receptors, GPR40, GPR55, and PA, a second messenger, which can also be also converted into LPAs if they are attacked by phospholipases in the digestive system. Linoleic acid (C18:2) is also an essential fatty acid in animals. Linoleic acid (C18:2) derived from GEF could also be further transformed into other physiologically active lipid-derived agents as a precursor of arachidonic acid and prostaglandins in animals.47
3.1. Bioactive lipid syntheses of gintonin and formation of bioactive complexes with ginseng proteins
Gintonin is a kind of glycolipoprotein in ginseng.25 Although LPA and other bioactive phospholipid syntheses in ginseng have not been directly demonstrated, they can be inferred from plant LPA and lipid synthetic processes. Plant LPAs and other lysophospholipids can originate from water soluble glycerol 3-phosphate (G3P) by transfer of an acyl group, of which might mostly be linoleic acid (C18:2) in ginseng as described above, by glycerol 3-phosphate acyltransferase. Interestingly, most animal LPAs have oleic acid (C18:1) or palmitic acid rather than linoleic acid (C18:2), as mentioned above. Choline, ethanolamine, or an inositol group can be also added to the phosphate group of LPA to produce LPC, LPE or LPI.48 Interestingly, in gintonin and GEF, the major group added to LPA to produce LPI is inositol. LPAs can be further acylated to form PAs by LPA acyltransferase,48 of which the second acyl group could also be palmitic (C16:0), linoleic (C18:2), or oleic acid (C18:1) in order of prevalence in GEF (Fig. 1). Thus, in LPA and PA syntheses, the main acyl group will be in the order of linoleic (C18:2) ≫ palmitic (C16:0) > oleic acid (C18:1). PAs could be further processed to form other PLs or glycerolipids.48 Thus, LPAs, and other lysophospholipids like LPIs and PAs can be intermediate metabolites for the synthesis of diverse glycerolipids and PLs in ginseng. Interestingly, these bioactive lipids are isolated as a complex with ginseng proteins such as GLP151 and ginseng major protein, which produces a unique gintonin.25, 30 In addition, it was revealed that the amounts of LPAs in gintonin were much higher than those in other foodstuffs or herbal medicines with an abundance of linoleic (C18:2).25, 30 Bioactive lipid components in gintonin and GEF might be derived from hydrolysis of ginseng plasma membrane lipids during organic extraction with high temperature and bind to ginseng proteins. Further study is needed to elucidate the origins of these bioactive lipids in gintonin and why gintonin exists as a complex with ginseng proteins.
3.2. Mode of action of gintonin: involvement of gintonin- or GEF-mediated regulations of adrenergic, cholinergic, glutamatergic, serotonergic receptor regulations, and autophagic stimulation via LPA receptors
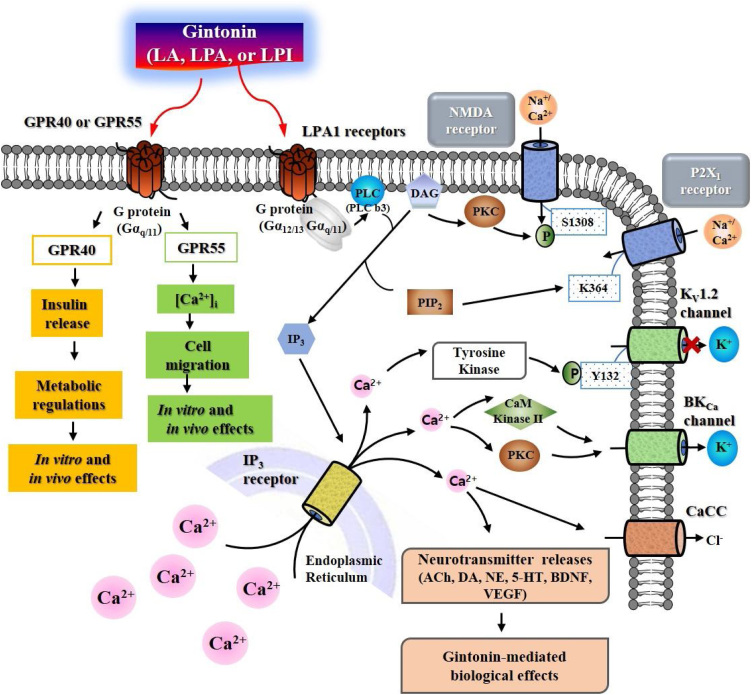
It appears that the primary targets on the plasma membrane of gintonin are LPA receptors,26 since LPAs were first found in gintonin and LPAs are more abundant than other bioactive ligands such as LPIs, LPCs, and LPEs.37 Recently, it was demonstrated that gintonin linoleic acid and LPI interact with GPR40 and GPR55 to exhibit cellular effects such as insulin secretion and Ca2+-mediated cell migration, respectively.49 Thus, gintonin contains at least three different ligand complexes for G protein-coupled receptor regulation such as LPA receptors, GPR40, and GPR55 (Fig. 2).49 Interestingly, although the signaling pathways of GPR40 are different from that of LPA receptors,49 LPA receptors and GPR55 share common signaling pathways, in that activation of both receptors elicits [Ca2+]i transients and induces Ca2+-dependent cell migration.49 Gintonin-mediated activation of GPR55 and LPA receptors is coupled to [Ca2+]i transients through Gαq/11 activation-phospholipase C activation-IP3 formation-[Ca2+]i transient induction. Thus, gintonin produces second messenger Ca2+ via LPA receptors and GPR55. Ca2+, an intracellular mediator of gintonin, initiates a cascade of amplifications inducing further intracellular effects and/or intercellular communications by activation of Ca2+-dependent kinases, receptors, and gliotransmitters, and neurotransmitter release.18 Recent studies have shown that gintonin-mediated [Ca2+]i transients in mammalian cells are further directly and indirectly coupled to the regulation of adrenergic, cholinergic, glutamatergic, and serotonergic systems through the release of respective neurotransmitters in the central and peripheral nervous systems (Fig. 2).50, 51, 52, 53 Gintonin enhances brain neurotrophic BDNF and VEGF release and expression.54, 55 Gintonin also stimulates autophagic flux in primary astrocytes.56 Thus, gintonin-mediated regulations of various receptors, neurotrophic and autophagic systems are further coupled to diverse in vitro and in vivo biological effects such as synaptic transmission, cell migration, cell proliferation, glycogenolysis, anti-depression, and neurogenesis in neuronal cells (Fig. 2). Furthermore, oral gintonin or GEF treatment is associated with improvement of learning and memory,51, 52 and enhancement of physical stamina via catecholamine-glycogenolysis axis.50 Gintonin also shows anti-metastatic effects via autotaxin inhibition.57 The detailed in vivo effects of gintonin and/or GEF on brain aging, brain aging-related neurodegenerative diseases and autophagic regulations will be described below section. However, other ginseng components, such as ginsenosides and acidic polysaccharides, do not show the same effects as gintonin.18
Fig. 2.
Signal transduction of ginseng gintonin on the mammalian cell plasma membrane via LPA receptors, GPR40, and GPR55. Gintonin activates LPA GPCRs, GPR40 and GPR55, respectively, which can lead to intracellular responses through the regulations of ion channels and receptors.18 In nervous system, gintonin-mediated signaling transduction pathway can be also coupled to neurotransmitter (i.e., acetylcholine, dopamine, norepinephrine, and serotonin) release for intercellular communications and to stimulations of BDNF and VEGF releases.18 The released neurotransmitters and neurotrophic factors further regulate their respective receptors to exhibit ginseng gintonin-mediated in vivo biological effects. Thus, in vivo biological effects of ginseng gintonin might be achieved via LPA receptors, GPR40, and/or GPR55 and indirectly via activations of respective receptors by ligands released by gintonin treatment. ACh, acetylcholine; DA, dopamine; NE, norepinephrine; 5-HT, serotonin.
3.3. Anti-brain aging effects of gintonin
Aging-related studies in animals can be tedious, since researchers have to wait for a long time for the animals to grow old enough. Recently, several methods have been developed to induce rapid brain, as well as other organ, aging through treatment with biochemical agents. One of these methods uses d-galactose. d-Galactose has been widely used to induce rapid aging of body organs, including the brain. Because d-galactose-induced aging resembles that in humans, this method is widely used for anti-aging pharmacological agent development in aging model systems.58, 59, 60, 61 The basic molecular mechanisms of d-galactose-induced brain aging are due to the excessive production of reactive oxygen species (ROS) and subsequent ROS-induced mitochondrial dysfunctions in nervous and non-nervous systems. Thus, the d-galactose-induced aging model is more similar to the damaged-related factors of aging theory than the programmed factor theory.62, 63
The hippocampus is part of brain's limbic system. It plays important roles in the consolidation of diverse cognitive information from short-term memory to long-term memory. Brain aging affects hippocampal functions and induces memory dysfunctions.64 However, gintonin administration enhances hippocampal-dependent cognitive functions.54 Gintonin co-administration with d-galactose reversed d-galactose alone-induced hippocampal senescence.65 Thus, long-term treatment of d-galactose induces hippocampal aging by reducing the hippocampal cell proliferation, differentiation, and maturation observed in human brain aging. Interestingly, hippocampal LPA1 receptor expression is also diminished under d-galactose insult.65 Co-administration of gintonin with d-galactose reverses d-galactose-induced reduction of cell proliferation, increases differentiation of immature neurons into mature neurons, and increases LPA1 receptor expression in the mouse hippocampus. Gintonin also increases the expression of phosphorylated cyclic adenosine monophosphate response element binding protein (pCREB), which plays an important role in cognitive functions. In addition, in an electrophysiological study, d-galactose decreased the long-term potentiation (LTP) amplitude, which is a basic cellular mechanism that underlies learning and memory.65 Co-administration of gintonin in d-galactose mice enhanced LTP and restored cognitive functions compared with those in mice treated with d-galactose alone. The LPA and LPA1 receptors play a crucial role in early brain development.65 Relatively little is known about the role of the LPA1 receptor in the adult brain, as opposed to the developmental brain. In the d-galactose-induced brain aging model, GEF administration recovers a decrease in adult LPA1 receptor expression, which raises the possibility that brain senescence might accompany a decrease in adult hippocampal LPA1 receptor expression levels and that LPA1 receptor restoration by gintonin administration might contribute to the maintenance of hippocampal homeostasis related with cognitive functions under oxidative stress. This concept might be supported by LPA1 receptor null-mice that show cognitive and attention deficits.12
3.4. Anti-Alzheimer's disease effects of gintonin
AD represents a severe geriatric brain disease, since it usually occurs in those aged over 65 years. AD occurs in about 10% of people over 65 years and the occurrence dramatically increases in those aged over 80 years.66 AD severity or AD-related social problems in aging societies are due to the fact that therapeutic drugs have not yet been developed, and as such, AD is considered an incurable disease. The number of AD patients is growing every year, and increasing the economic and psychological burdens in many households. The worldwide prevalence of AD is estimated to double and 115 million within the next 20 years.67 AD is clinically characterized by dysfunction in learning and memory, as well as deterioration of other cognitive and noncognitive mental and motor functions, and whole brain atrophy, which makes social activities impossible.66, 67 The main causes of AD are the long-term accumulations of amyloid-β (Aβ) and tau proteins in the brain. Aβ and tau protein are neurotoxic; it accumulates into aggregates, and induces inflammation and oxidative stresses in the brain. It also induces cell death, destroys synaptic connections among neurons, and finally results in the clinical signs and symptoms of AD.66, 67, 68 A series of events occur in Aβ aggregate formation, from the accumulation of amyloid-β protein precursors (AβPPs) in neurons and their subsequent accumulation, resulting in the death of brain cells and eventually AD. This is the “amyloid cascade hypothesis”,69 although this hypothesis has recently been challenged by the observations that many young adults and older persons without dementia show substantial Aβ plaque accumulations in the brain and that inhibitors of Aβ plaque formation failed to show a therapeutic effect on AD in clinical trials.70, 71
Gintonin decreases Aβ1–42 release and attenuates Aβ1–42-induced cytotoxicity in SH-SY5Y neuroblastoma cells.72 In studies of the molecular basis of gintonin-induced anti-AD effects using SH-SY5Y neuroblastoma cells, gintonin stimulated non-amyloidogenic rather than amyloidogenic pathways to produce soluble AβPPα (sAβPPα), which is has neuroprotective and neurotrophic effects, so is beneficial to neurons.72 Gintonin promotes non-amyloidogenic sAβPPα release via Ca2+-dependent metalloprotease α-secretase activation, and protein-trafficking processes.72 In vivo studies have shown that gintonin has anti-AD effects in short- and long-term Aβ-induced cognitive dysfunction in wild-type animals. As a short-term treatment for 2 weeks, gintonin rescued acute Aβ1–42-induced cognitive dysfunction in mice.72
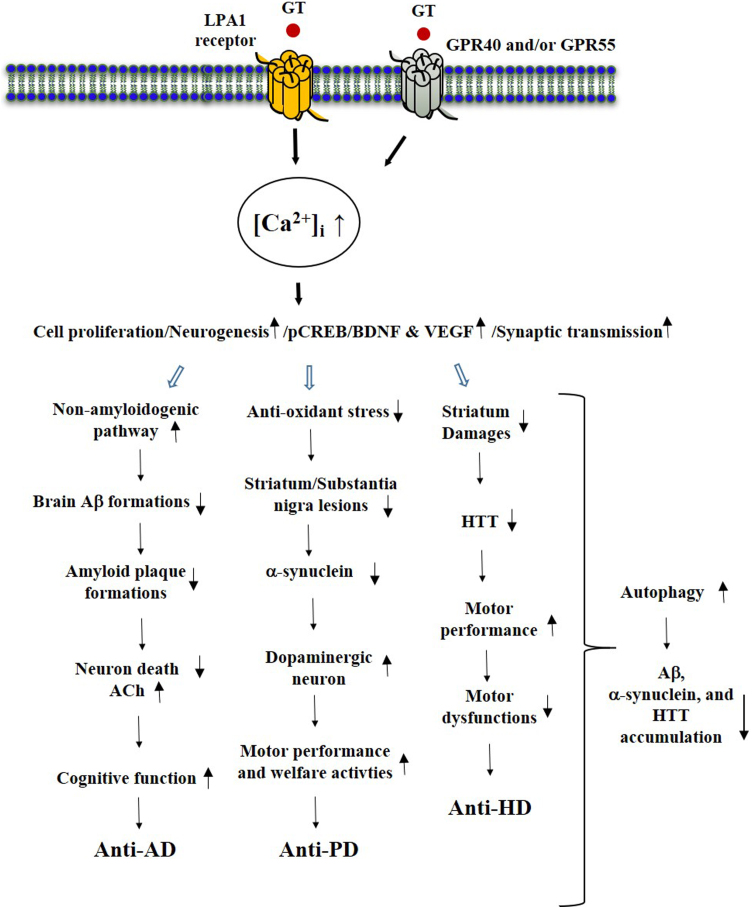
In transgenic AD animal models, which are widely used to model brain senescence-related AD induction, it usually takes 9 months to observe memory dysfunctions and amyloid plaque accumulation in the cortex and hippocampus. In the brain aging AD model, long-term oral administration of gintonin for 3 months significantly reduced amyloid plaque deposition in the cortex and hippocampus, and rescued short- and long-term memory and spatial working memory impairments.72 Long-term oral administration of gintonin also significantly showed anti-neuroinflammatory effects by reducing microglial Iba-1 expression in the cortex and hippocampus.72 Thus, the gintonin-mediated anti-AD effects inhibit Aβ formation, decrease microglial activation, and increase beneficial sAβPPα via LPA1 receptor signaling pathways. This results in decreased amyloid plaque formation/accumulation, as well as decreased neuroinflammation in the brain, and recovery of brain function, resulting in enhancement of cognitive functions. This was the first indication that ginseng gintonin intake can prevent Aβ formation and amyloid plaque accumulation in the brains of aged AD model animals (Fig. 3).
Fig. 3.
Effects of gintonin and GEF on geriatric brain diseases. Long-term oral administration of ginseng gintonin attenuates geriatric brain diseases. The common mode of action of gintonin against neurodegenerative diseases is LPA receptors activation and anti-apoptosis, anti-inflammation, anti-oxidative stress, and autophagic stimulations. GPR40 and GPR55 might be involved in geriatric brain diseases but not yet clearly demonstrated as much as LPA and LPA1 receptor. HTT, huntingtin.
3.5. Gintonin effects on hippocampal cholinergic systems in AD animal models
In addition to the amyloid-induced AD hypothesis, another hypothesis has been circulating for four decades. This is that AD is due to a loss of cholinergic innervations in the cerebral cortex in aged populations. In fact, brain acetylcholine concentration also declines with brain aging and in AD patients.73 Brain cholinergic dysfunctions including muscarinic and/or nicotinic acetylcholine receptor-related synaptic malfunctions and decreases in brain acetylcholine concentrations are correlated with cognitive impairment. This leads to the “cholinergic hypothesis of AD”, since Aβ accumulation induces death of cholinergic neurons, resulting in decreases in brain acetylcholine concentrations.74, 75 Even in normal animals, administration of cholinergic antagonists, such as scopolamine, induces transient cognitive deficits,76 indicating that the brain's cholinergic system is important in cognitive functions in animals and humans. This hypothesis is supported by the development and clinical application of a cholinesterase inhibitor. Acetylcholinesterase inhibitors, such as donepezil, are also clinically approved, although these drugs only partially alleviate AD symptoms without additional AD therapy.77
In an in vitro study, gintonin stimulated acetylcholine release from cultured mouse hippocampal neural progenitor cells (NPCs). Gintonin-mediated stimulation of acetylcholine release achieved via [Ca2+]i transients through LPA receptor signaling pathways and gintonin treatment also increased the expression of choline acetyltransferase (ChAT), an enzyme involved with acetylcholine synthesis.52 Thus, gintonin increases acetylcholine release from neurons after enhancement by acetylcholine synthesis. In in vivo studies, oral administration of GEF for 3 weeks significantly attenuated scopolamine-induced memory impairments. In addition, oral administration of gintonin also significantly attenuated Aβ-induced cholinergic dysfunctions by increasing ChAT activity and decreasing acetylcholine esterase (AChE) activity, and finally an increase in brain acetylcholine concentration. In the transgenic AD mouse model described above,52 oral administration of gintonin for 3 months also attenuated AD-related hippocampal cholinergic impairment by increasing cholinergic neurons. Thus, gintonin recovers the brain hippocampal cholinergic system damaged by amyloid plaque accumulations in an AD animal model by enhancing acetylcholine release, increasing brain ChAT expression, and decreasing brain AChE expression. Gintonin-mediated cholinergic regulation contributes to the amelioration of aging-related AD.52 This was the second indication that gintonin has anti-AD effects (Fig. 3).
3.6. Gintonin effects on hippocampal neurogenesis in an AD animal model
It was believed for a long time that adult brain neurogenesis does not occur and that recovery of brain neurons is irreversible after brain damage induced by stroke-induced hypoxia or neurodegenerative disease like AD. However, recent studies have demonstrated that adult neurogenesis including neuronal differentiation, migration and maturations can be observed in limited areas of the brain such as the hippocampus and third ventricle.77 Now it is believed that adult brain neurogenesis can occur indefinitely.78 Currently, adult brain neurogenesis has received much attention for its clinical applications, since many animal studies have shown that adult animal brain neurogenesis can be applied to restore cognitive function, and treat stroke, depression, and neurodegenerative diseases.79 The LPA and LPA1 receptors are mainly involved in embryonic brain development with diverse functions in cell proliferation, morphological changes, and migration to target areas of the brain.80, 81 Thus, the LPA1 receptor is abundantly expressed during prenatal stages and gradually decreases postnatally.81, 82 Recent studies have shown that the LPA1 receptor is also involved in adult brain functions such as cognition, depression, emotion, neurogenesis, mental diseases, and social activity.83 In addition to gintonin's ability to inhibit β-amyloid plaque formation in the cortex and hippocampus, restore β-amyloid-induced cognitive dysfunction, and restoration the cholinergic system in an AD animal model, Kim et al.12, 84 showed that gintonin stimulates neuronal cell proliferation, in vitro and in vivo, in the mouse hippocampus. In an in vitro study, gintonin treatment increased 5-bromo-2′-deoxyuridine (BrdU) incorporation into hippocampal NPCs. Gintonin increased NeuN-positive neurons, which are biomarkers of mature neurons. However, the actions of gintonin were blocked by an LPA1/3 receptor antagonist and a Ca2+ chelator, showing that gintonin-mediated hippocampal neuronal cell proliferation and maturation was achieved via the Ca2+-dependent signaling pathway, probably with LPA1/3 receptor regulation. An in vivo study using adult wild-type animals and aging-related AD model animals, 3-month oral administration of GEF also increased hippocampal BrdU incorporation, which indicates an increase in neuronal cell proliferation, in both adult wild-type and transgenic AD mice.84 This evidence supports that long-term oral gintonin administration induces adult brain neurogenesis in normal animals and even AD model animal.84, 65 This was the third indication that gintonin has anti-AD effects; this time via brain neurogenesis (Fig. 3). Collectively, in AD animal models, the gintonin-mediated anti-AD effects include (1) activation of non-amyloidogenic pathways, resulting in the formation of beneficial sAβPPα rather than neurotoxic Aβ; (2) restoration of the brain cholinergic system by increasing ChAT and decreasing AChE activity; and (3) stimulation of hippocampal neurogenesis in both adult wild-type and AD model animals.52, 72, 84
3.7. Effects of gintonin on motor performance
Impaired motor functions or movement disorders in elderly people in aging societies are another important manifestation of aging-related brain diseases. Motor functions of aged individuals become slow and unnatural; walking becomes more difficult and posture becomes slouched.85, 86 Gintonin has been proven preclinically to enhance motor functions through peripheral LPA receptor regulation. Lee et al.50 compared rota-rod motor performance in fasting mice between a control (saline) vehicle group and an oral gintonin-treated group, and found that gintonin increased blood glucose via liver glycogenolysis and enhanced motor activity, compared with the saline vehicle group. The molecular mechanisms of gintonin-mediated enhancement of motor performance with increased blood glucose include that gintonin treatment first increases catecholamines such as epinephrine and norepinephrine from the adrenal gland of the kidney.50 The released catecholamines interact with the liver β-adrenergic receptor to initiate liver glycogenolysis and increase blood glucose. Thus, increased blood glucose through liver β-adrenergic receptor activation might act as an energy source for motor performance even in the fasting state, indicating the possibility that gintonin can act as an invigorator for physical stamina in elderly people.
3.8. GEF protects brain dopaminergic neurons in a Parkinson's disease (PD) animal model
PD is a disease that affects motor function and is the second-most prevalent and age-associated neurodegenerative disease after AD.87 Symptoms of PD include tremors, muscular rigidity, akinesia, bradykinesia, postural instability, and abnormal gait.88 These symptoms are caused by insufficient dopamine production in the substantia nigra and striatum due to the selective death of dopaminergic neurons.88, 89, 90 In an animal study using 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), which impairs dopaminergic neurons in the substantia nigra and striatum through ROS formation and induces symptoms similar to human PD such as motor dysfunctions,91 oral administration of GEF increased the survival rate of these mice.92, 93 Choi et al.92 and Jo et al.93 showed that oral GEF administration in a PD mouse model significantly ameliorated the effects of neurological deficits such as motor performance, as measured with pole and rotarod tests, and social welfare activities in nest building tests. GEF administration also increased spontaneous locomotive activity that was measured by travel distance, compared to MPTP alone.93 Amelioration of neurological deficits by GEF in PD animal models was supported by immuno-histochemical assays showing a reduction in the loss of tyrosine hydroxylase-positive neurons, microglial activation, and activation of inflammatory mediators (interleukin-6, tumor necrosis factor, and cyclooxygenase-2). GEF administration also inhibits phosphorylation of mitogen-activated protein kinases and nuclear factor-kappa B signaling pathways.
In apoptosis-related evaluation regarding the reduced number of intact substantia nigra and striatum cells after MPTP exposure, the expression of Bax, the main regulatory factor in the process of proapoptotic cell death by inducing mitochondrial pore formation in the developing cerebellum, was enhanced, After GEF co-administration with MPTP, fewer Bax-immunoreactive cells were observed, suggesting that the MPTP-mediated Bax protein induction was repressed by GEF co-treatment. Meanwhile, anti-apoptotic Bcl2 was reduced by MPTP, while GEF co-treatment increased Bcl2 expression92 These results demonstrate the neuroprotective effects of GEF in the MPTP-exposed mouse substantia nigra and striatum in a PD animal model.4 Activation of nuclear factor erythroid 2-related factor 2/heme oxygenase-1 (Nrf2/HO-1) is a potential therapeutic target for brain neurodegeneration, since they are activated to protect cells under ROS insults. GEF administration enhances Nrf2/HO-1 expression in the mouse substantia nigra and striatum.92, 93
In addition to damage to dopaminergic neurons in the substantia nigra and striatum, another characteristic of PD is the accumulation of α-synuclein-containing inclusion bodies within dopaminergic neurons. These inclusions are known as Lewy bodies.94 Accumulations of α-synuclein in Lewy bodies are caused by impaired axonal transport.95 GEF treatment to neurons reduces the accumulation of α-synuclein.93 The molecular mechanisms in GEF-mediated amelioration in the MPTP-induced PD animal model are achieved via multiple pathways such as LPA receptor regulation, anti-apoptosis, anti-inflammation, and anti-oxidant stress.92, 93 Gintonin administration also increased LPA1/3 receptor gene expression in the substantia nigra and striatum in MPTP-treated mice. However, co-administration of the LPA1/3 receptor antagonist blocked GEF-mediated increase of the LPA1/3 receptor gene expression and attenuated increases in tyrosine hydroxylase and Nrf2 protein expression in the substantia nigra and striatum,92 showing the involvement of LPA receptors in GEF-mediated anti-PD (Fig. 3).
3.9. GEF protects brain striatal neurons in a Huntington's disease (HD) animal model
Huntington's disease (HD), also known as Huntington's chorea, is an inherited brain disorder. The genetic mutation responsible for HD is an abnormal expansion of a CAG trinucleotide repeat in the HTT gene that encodes for huntingtin (HTT).96, 97, 98 Symptoms of HD usually begin between 30 and 50 years of age.96 The damaged brain area includes the basal ganglia.97 The earliest symptoms are a general lack of coordination of movement and an unsteady gait, often followed by abnormal mood and/or mental abilities.98, 99 As the disease advances, uncoordinated, jerky body movements become more apparent and worsen, until coordinated movement becomes difficult and the person is unable to talk.99 The general symptoms become similar to PD when HD worsens,99 and the person's mental abilities generally decline, becoming dementia-like.100 Patients usually die 20 years after onset and death results from fatal aspiration of pneumonia.99
Jang et al.101 demonstrated that GEF mitigates the neurological symptoms and immuno-histochemical impairments of the striatum in an animal model of HD. They used two in vitro and in vivo methods to demonstrate the anti-HD effects of GEF. One used 3-nitropropionic acid (3-NPA), which is produced in fungus-contaminated corn and induces an irreversible inhibition of complex II in the mitochondria, impairing cellular respiration and energy metabolism by inhibiting succinate dehydrogenase (SDH) in specific brain sites including the striatum and cortex, resulting in similar symptoms to human HD.101 In a 3-NPA model of HD, GEF attenuated mitochondrial dysfunction. i.e., succinate dehydrogenase and MitoSOX activities, apoptosis, microglial activation, and mRNA expression of inflammatory mediators (i.e., IL-1β, IL-6, TNF-α, COX-2, and iNOS) in the striatum after 3-NPA-intoxication.101 However, administration of GEF increases the Nrf2 signaling pathway and inhibits mitogen-activated protein kinases (MAPKs) and the nuclear factor-κB (NF-κB) signaling pathway.101 The other models used STHdh cells, which produce aggregated huntingtin protein when exposed to H2O2 and damage neurons,102 and an in vivo adeno-associated viral (AAV) vector-infected mouse model of HD, which also overproduces aggregated huntingtin protein. In both these in vitro and in vivo models, GEF reduced mutant HTT aggregates, protected STHdh cells, reduced cell death, and reduced aggregates N171-82Q-mutant HTT in the striatum. GEF has beneficial effects in a chemical-induced HD model with antioxidant and anti-inflammatory activities through brain LPA1 receptor regulation. In addition, gintonin also exerts neuroprotective effects in STHdh cells and an AAV vector-infected model of HD by reducing mutant HTT.101 Thus, gintonin exhibits anti-HD activities in a chemical model using 3-NPA, an STHdh cell model and AAV vector-infected mutant model of HD. Taken together, gintonin or GEF shows anti-neurodegenerative activities in animal models of AD, PD, and HD. The common molecular mechanisms by which gintonin exerts anti-brain aging and anti-neurodegenerative effects in diseases such as AD, PD, and HD include anti-apoptotic, anti-inflammatory, and anti-oxidant effects via LPA receptor regulations12, 52, 65, 84, 92, 93, 101 (Fig. 3).
3.10. A role of gintonin-mediated autophagy stimulation in neurodegenerative diseases
Autophagy is a type of intracellular recycling program that removes damaged or old cellular organelles, and protein aggregates or mis-folded protein components.103 In animal brain experiments, autophagy-related proteasome activity decreases in an age-dependent manner more predominantly in neurons than in glial cells.104 The autophagy-related gene Atg is involved in normal autophagy, and deletion or mutation of this gene induces disturbances in cellular homeostasis, leading to neurodegenerative diseases.105 Thus, if autophagy is dysregulated in the aging brain, defective proteins and damaged organelles cannot be clearly removed from neurons.106, 107, 108 Accumulation of waste proteins mainly in neurons leads to neuropathological conditions, since they are more vulnerable to the toxicity exerted by misfolded proteins103 and have lower capacity to combat inflammation and/or oxidative stress.109 Thus, autophagy dysfunction is a common feature of most neurodegenerative diseases in the aged population with AD.110, 111 PD,112 and HD113, 114 Formation of abnormal protein aggregates composed of Aβ amyloid115 and tau proteins result in AD.116 Lewy body α-synuclein aggregates result in PD,117 and HTT protein aggregates result in HD.118 Thus, depending on the brain disease, the aggregated proteins in the brain differ from each other. The aggregated proteins in AD, PD, and HD can cause synaptic impairment, damage to organelles, neuronal cell death, and finally, clinical symptoms.109
It was reported recently that stimulation of astrocytic autophagy might be beneficial for neuroprotection in age-related neurodegenerative diseases.119 Astrocytes can mediate the clearance of Aβ through its uptake and degradation.120 In addition, astrocytes are involved in Aβ uptake and degradation121, 122 Regarding PD, it has been observed that α-synuclein, the main component of Lewy body inclusions, accumulates in astrocytes, and correlates with the extent of neuronal loss.123, 124 Furthermore, α-synuclein released from neurons is taken up by astrocytes.124, 125, 126 In the HD brain, disease progression correlates with an increase in reactive astrocytes, and HTT, which contains polyglutamine, also accumulates in astrocytes.127, 128 Loss of the glutamate transporter GLT1, which is involved in astrocytic autophagy, is a common characteristic found in human HD brain and animal models129, 130 Thus, uptake or removal of neurodegenerative disease-related proteins through astrocytic autophagy point to the contribution of astrocytes in neurodegenerative diseases.
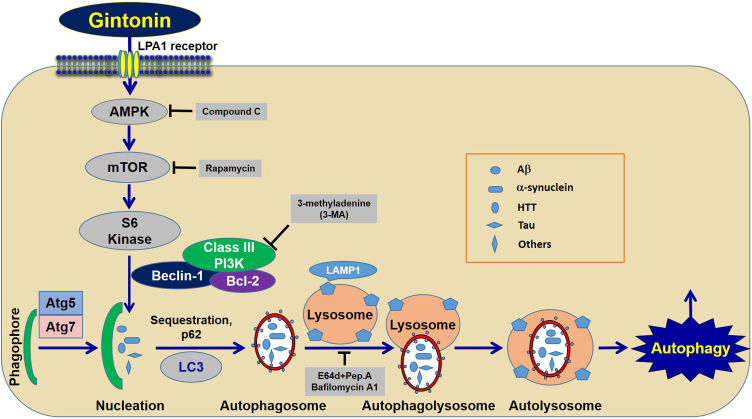
In a study of gintonin's role in brain autophagy, Rahman et al.56 demonstrated that treatment of gintonin to cortical astrocytes upregulated the autophagy marker proteins LC1-I and LC3-II. Gintonin-mediated marker protein increases were blocked by LPA1/3 receptor antagonists, showing that gintonin-mediated enhancement of autophagy marker protein was achieved via G protein-coupled LPA receptor regulation. Gintonin-mediated autophagy also increased the formation of LC3 puncta and the signaling pathway for gintonin-mediated enhancement of autophagy, including AMPK-mTOR pathways, since pretreatment with an AMPK inhibitor, compound C, inhibited LC3-II and LC3 puncta expression, whereas treatment with a mammalian target of rapamycin (mTOR) inhibitor, rapamycin, further enhanced LC3-II and LC3 puncta expression.131 However, gintonin-mediated autophagy enhancements were significantly blocked by an autophagy inhibitor, such as 3-methyladenine, and knockdown of beclin-1, Atg5, and Atg7 gene expression, which are necessary and key elements for vesicle initiation, nucleation, and elongation through phagophore formation.132 Deletion of the essential autophagic genes ATG7105 and ATG5106 can sufficiently induce the formation of cytoplasmic inclusions and neurodegeneration in neurons, indicating that normal autophagy is necessary for the homeostasis of neurons. When astrocytes were pretreated with a lysosomotropic agent, E-64d/peps A or bafilomycin A1, gintonin-mediated autophagy significantly increased the levels of LC3-II along with the formation of LC3 puncta, also indicating a role for astrocytes in autophagy. Gintonin-mediated enhancement of autophagic flux led to an increase in lysosome-associated membrane protein 1 and degradation of ubiquitinated p62/SQSTM1. Thus, gintonin could be a candidate as an astrocytic autophagy enhancer via the LPA1 receptor-AMPK-mTOR-mediated signaling pathway (Fig. 4).
Fig. 4.
Signaling pathway for gintonin-mediated autophagic influx via LPA1 receptor. Gintonin stimulates astrocytic autophagy via down-streams of autophagic processes.56 Stimulations of autophagy flux by gintonin might play a role for housekeeper by cleaning brain toxic wastes such as Aβ, α-synuclein, HTT and others, although it requires more studies to show that gintonin-mediated astrocytic autophagy is coupled to in vivo anti-neurodegenerative diseases.
Astrocytes are a type of brain glial cell and constitute about 20% of all glial cells. They play a housekeeping role in the brain and have diverse functions such as glycogen storage for energy supply to neurons, BBB formation, uptake of excitatory neurotransmitter released from neurons, and synaptic transmission regulation through the formation of tripartite synapses with neurons.120, 133 In addition to the in vitro and in vivo neuroprotective effects of gintonin, we showed in previous reports that gintonin stimulates gliotransmitter release, astrocytic glycogenolysis, and formation of vascular endothelial growth factor (VEGF) in hypoxic astrocytes.134, 135, 136 Gintonin also stimulates cortical astrocytic autophagy.56 Although there is currently no direct evidence regarding whether gintonin-mediated stimulation of autophagy in the brain is linked to the amelioration of neurodegeneration (Fig. 4), it seems that gintonin-mediated stimulation of brain astrocytic autophagy could be an attractive therapeutic target for aging-related brain diseases.
3.11. Development of medicinal food or natural medicines using ginseng gintonin is necessary for elderly people in aging societies
Most well-known traditional medicines contain ligands that act on the GPCR or ligand-gated ion channels in the plasma membrane of animal cells. Many of these medicines treat various types of diseases. In fact, GPCR-related drugs account for more than half of those in pharmaceutical markets. For example, morphine and morphine derivatives, which are derived from the herbal poppy, are widely used as anti-nociceptive agent through the regulations of endogenous opioid receptors.137 Another recent example, ω-conotoxins, which are peptides isolated from Cone snails and inhibit voltage-gated Ca2+ channels with high affinity, are commercialized as specific painkillers for chronic pain.138 Ginseng usually does not show many side effects, even when taken in the long-term, compared to other traditional medicines. Ginseng saponins (or ginsenosides) were first isolated from ginseng but they did not show a role as a receptor ligand of GPCR or ligand-gated ion channels, since they did not show selectivity in receptors and ligand-gated ion channels, but rather interactions with plasma membrane receptors or ligand-gated ion channels in a non-specific and ambiguous manner and with very low affinity.17
Recently, gintonin was isolated from ginseng and gintonin acts as an exogenous ligand for the LPA receptors, GPR40 and GPR55.49 Gintonin attenuates aging related-neurodegenerative diseases such as AD, PD, and HD,12, 91, 92, 100 and stimulates autophagy in neuronal cells56 and is a good candidate for ginseng-derived functional foods or natural medicines against aging-related brain diseases. This is important, since AD, PD, and HD are becoming increasingly more prevalent in our aging society, and dramatically increasing compared with other chronic diseases. These diseases impose huge psychological burdens on families and economic burdens on society, especially in countries with aging societies. Gintonin (or GEF) will be first the ginseng product to target GPCRs such as the LPA receptors GPR40 or GPR55, for brain rejuvenation in senior citizens. Now, further trials are necessary to shift the paradigm of ginseng-related product production from old-fashioned products using dried ginseng root itself or simple ginseng extract to more refined- and disease targeted-oriented ginseng product production.
4. Conclusion
Recent preclinical studies have shown that gintonin and/or GEF ameliorates brain aging-related neurodegenerative disease such as AD, PD, and HD. The mode of actions underlying anti-neurodegenerative diseases by gintonin and/or GEF treatment might be derived from (1) increases in brain BDNF and VEGF expression and increases of acetylcholine and dopamine synthesis enzymes; (2) stimulations of hippocampal and striatal neurogenesis; (3) attenuations of brain apoptosis due to oxidative stress by increasing Nrf2/HO-1 and neuro-inflammation by inhibition of ROS formation and inflammatory cytokine production in brain; (4) restoration of the BBB damaged by oxidative stress and neuro-inflammation; and (5) gintonin-mediated stimulation of cortical astrocytic autophagy. These beneficial effects gintonin might provide a molecular bases to inhibit accumulation or facilitation of clean-up of Aβ, α-synuclein or HTT. In the future, further clinical investigations might be required to examine the efficacy, safety or side effects, and tolerability for the prevention and attenuation of brain aging and neurodegenerative diseases.
Author contributions
Conceptualization: SHC, RL, SMN, DGK and SYN. Methodology: SHC, RL, SMN and DGK. Formal analysis: SHC, RL, SMN and DGK. Writing-original draft: SHC, SYN. Writing-review & editing: SHC, IHC, HCK, YC, HR, and SYN. Supervision: SHC, IHC, HCK, YC, HR, and SYN.
Conflicts of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be SHR construed as potential conflicts of interest.
Funding
This work was supported by the Ministry of Science, ICT, and Future Planning for the Basic Science Research Program (NFR 2020RIFIA1058460) and the Brain Research Program (NRF2016M3C7A1913894) to S.Y. Nah. This paper was supported by Konkuk University Researcher Fund in 2020.
Ethical statement
Not applicable.
Data availability
The data for this review will be made available upon request.
References
- 1.Medvedev Z.A. An attempt at a rational classification of theories of ageing. Biol Rev Camb Philos Soc. 1990;65:375–398. doi: 10.1111/j.1469-185x.1990.tb01428.x. [DOI] [PubMed] [Google Scholar]
- 2.Jin K. Modern biological theories of aging. Aging Dis. 2010;1:72–74. [PMC free article] [PubMed] [Google Scholar]
- 3.Bondy S.C., Campbell A. Humana Press; 2016. Inflammation, aging, and oxidative stress; p. 405. [Google Scholar]
- 4.Lutz W., Sanderson W., Scherbov S. The coming acceleration of global population ageing. Nature. 2008;451:716–719. doi: 10.1038/nature06516. [DOI] [PubMed] [Google Scholar]
- 5.United Nations Development Programme . September 2005. UN Human Development Report 2005, International Cooperation at a Crossroads-Aid, Trade and Security in an Unequal World (PDF). United Nations Development Programme. [Google Scholar]
- 6.Weil D.N. vol. 1. 1997. The economics of population aging; pp. 967–1014. (Handbook of population and family economics). [Google Scholar]
- 7.Fries J.F. Aging, natural death, and the compression of morbidity. N Engl J Med. 1980;303:130–135. doi: 10.1056/NEJM198007173030304. [DOI] [PubMed] [Google Scholar]
- 8.Wyss-Coray T. Ageing, neurodegeneration and brain rejuvenation. Nature. 2016;539:180–186. doi: 10.1038/nature20411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hindle J.V. Ageing, neurodegeneration and Parkinson's disease. Age Ageing. 2010;39:156–161. doi: 10.1093/ageing/afp223. [DOI] [PubMed] [Google Scholar]
- 10.Bano D., Zanetti F., Mende Y., Nicotera P. Neurodegenerative processes in Huntington's disease. Cell Death Dis. 2011;2:e228. doi: 10.1038/cddis.2011.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watson R. Academic Press; 2017. Nutrition and functional foods for healthy aging. [Google Scholar]
- 12.Kim H.J., Jung S.W., Kim S.Y., Cho I.H., Kim H.C., Rhim H. Panax ginseng as an adjuvant treatment for Alzheimer's disease. J Ginseng Res. 2018;42:401–411. doi: 10.1016/j.jgr.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gawa-Ochiai K., Kawasaki K. Panax ginseng for frailty-related disorders: a review. Front Nutr. 2018;5:140. doi: 10.3389/fnut.2018.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Triyannanto E., Lee J.H., Lee K.T. Effects of sucrose stearate addition on the quality improvement of ready-to-eat Samgyetang during storage at 25 °C. Korean J Food Sci Anim Resour. 2014;34:683–691. doi: 10.5851/kosfa.2014.34.5.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Majori S., Pilati D., Gazzani D., Paiano J., Ferrari S., Sannino A. Energy drink and ginseng consumption by Italian university students: a cross-sectional study. J Prev Med Hyg. 2018;59:63–74. doi: 10.15167/2421-4248/jpmh2018.59.1.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Sanctis V., Sanctis N., Soliman A.T., Elsedfy H., Di Maio S., El Kholy M. Caffeinated energy drink consumption among adolescents and potential health consequences associated with their use: a significant public health hazard. Acta Biomed. 2017;88:222–231. doi: 10.23750/abm.v88i2.6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nah S.Y. Ginseng ginsenoside pharmacology in the nervous system: involvement in the regulation of ion channels and receptors. Front Physiol. 2014;5:98. doi: 10.3389/fphys.2014.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi S.H., Jung S.W., Lee B.H., Kim H.J., Hwang S.H., Kim H.K. Ginseng harmacology: a new paradigm based on gintonin-lysophosphatidic acid receptor interactions. Front Pharmacol. 2015;6:245. doi: 10.3389/fphar.2015.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nah S.Y., Kim D.H., Rhim H. Ginsenosides: are any of them candidates for drugs acting on the central nervous system? CNS Drug Rev. 2007;13:381–404. doi: 10.1111/j.1527-3458.2007.00023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim Y.S., Cho I.H., Jeong M.J., Jeong S.J., Nah S.Y., Cho Y.S. Therapeutic effect of total ginseng saponin on skin wound healing. J Ginseng Res. 2011;35:360–367. doi: 10.5142/jgr.2011.35.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi S., Rho S.H., Jung S.Y., Kim S.C., Park C.S., Nah S.Y. A novel activation of Ca(2+)-activated Cl(−) channel in Xenopus oocytes by Ginseng saponins: evidence for the involvement of phospholipase C and intracellular Ca(2+) mobilization. Br J Pharmacol. 2001;132:641–648. doi: 10.1038/sj.bjp.0703856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi S., Kim H.J., Ko Y.S., Jeong S.W., Kim Y.I., Simonds W.F. G alpha(q/11) coupled to mammalian phospholipase C beta 3-like enzyme mediates the ginsenoside effect on Ca(2+)-activated Cl(−) current in the Xenopus oocyte. J Biol Chem. 2001;276:48797–48802. doi: 10.1074/jbc.M104346200. [DOI] [PubMed] [Google Scholar]
- 23.Jeong S.M., Lee J.H., Kim S., Rhim H., Lee B.H., Kim J.H. Ginseng saponins induce store-operated calcium entry in Xenopus oocytes. Br J Pharmacol. 2004;142:585–593. doi: 10.1038/sj.bjp.0705797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee J.H., Jeong S.M., Lee B.H., Kim J.H., Ko S.R., Kim S.H. Effect of calmodulin on ginseng saponin-induced Ca2+-activated Cl− channel activation in Xenopus laevis oocytes. Arch Pharm Res. 2005;28:413–420. doi: 10.1007/BF02977670. [DOI] [PubMed] [Google Scholar]
- 25.Pyo M.K., Choi S.H., Hwang S.H., Shin T.J., Lee B.H., Lee S.M. Novel glycolipoproteins from Ginseng. J Ginseng Res. 2008;35:92–103. [Google Scholar]
- 26.Hwang S.H., Shin T.J., Choi S.H., Cho H.J., Lee B.H., Pyo M.K. Gintonin, newly identified compounds from ginseng, is novel lysophosphatidic acids-protein complexes and activates G protein-coupled lysophosphatidic acid receptors with high affinity. Mol Cells. 2012;33:151–162. doi: 10.1007/s10059-012-2216-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tigyi G., Dyer D.L., Miledi R. Lysophosphatidic acid possesses dual action in cell proliferation. Proc Natl Acad Sci U S A. 1994;91:1908–1912. doi: 10.1073/pnas.91.5.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dyer D., Tigyi G., Miledi R. The effect of active serum albumin on PC12 cells: I. Neurite retraction and activation of the phosphoinositide second messenger system. Brain Res Mol Brain Res. 1992;14:293–301. doi: 10.1016/0169-328x(92)90096-t. [DOI] [PubMed] [Google Scholar]
- 29.Tigyi G., Henschen A., Miledi R. A factor that activates oscillatory chloride currents in Xenopus oocytes copurifies with a subfraction of serum albumin. J Biol Chem. 1991;266:20602–20609. [PubMed] [Google Scholar]
- 30.Tigyi G., Miledi R. Lysophosphatidates bound to serum albumin activate membrane currents in Xenopus oocytes and neurite retraction in PC12 pheochromocytoma cells. J Biol Chem. 1992;267:21360–21367. [PubMed] [Google Scholar]
- 31.Nam M.H., Heo E.J., Kim J.Y., Kim S.I., Kwon K.H., Seo J.B. Proteome analysis of the responses of Panax ginseng C. A. Meyer leaves to high light: use of electrospray ionization quadrupole-time of flight mass spectrometry and expressed sequence tag data. Proteomics. 2003;3:2351–2367. doi: 10.1002/pmic.200300509. [DOI] [PubMed] [Google Scholar]
- 32.Sun H., Kim M.K., Pulla R.K., Kim Y.J., Yang D.C. Isolation and expression analysis of a novel major latex-like protein (MLP151) gene from Panax ginseng. Mol Biol Rep. 2010;37:2215–2222. doi: 10.1007/s11033-009-9707-z. [DOI] [PubMed] [Google Scholar]
- 33.Pyo M.K., Choi S.H., Shin T.J., Hwang S.H., Lee B.H., Kang J. A simple method for the preparation of crude gintonin from ginseng root, stem, and leaf. J Ginseng Res. 2011;35:209–218. doi: 10.5142/jgr.2011.35.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi S.H., Shin T.J., Lee B.H., Hwang S.H., Kang J., Kim H.J. An edible gintonin preparation from ginseng. J Ginseng Res. 2011;35:471–478. doi: 10.5142/jgr.2011.35.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi S.H., Jung S.W., Kim H.S., Kim H.J., Lee B.H., Kim J.Y. A brief method for preparation of gintonin-enriched fraction from ginseng. J Ginseng Res. 2015;39:398–405. doi: 10.1016/j.jgr.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moon J., Choi S.H., Shim J.Y., Park H.J., Oh M.J., Kim M. Gintonin administration is safe and potentially beneficial in cognitively impaired elderly. Alzheimer Dis Assoc Disord. 2018;32:85–87. doi: 10.1097/WAD.0000000000000213. [DOI] [PubMed] [Google Scholar]
- 37.Cho H.J., Choi S.H., Kim H.J., Lee B.H., Rhim H., Kim H.C. Bioactive lipids in gintonin-enriched fraction from ginseng. J Ginseng Res. 2019;43:209–217. doi: 10.1016/j.jgr.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lauckner J.E., Jensen J.B., Chen H.Y., Lu H.C., Hille B., Mackie K. GPR55 is a cannabinoid receptor that increases intracellular calcium and inhibits M current. Proc Natl Acad Sci U S A. 2008;105:2699–2704. doi: 10.1073/pnas.0711278105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Piñeiro R., Maffucci T., Falasca M. The putative cannabinoid receptor GPR55 defines a novel autocrine loop in cancer cell proliferation. Oncogene. 2011;30:142–152. doi: 10.1038/onc.2010.417. [DOI] [PubMed] [Google Scholar]
- 40.Oka S., Kimura S., Toshida T., Ota R., Yamashita A., Sugiura T. Lysophosphatidylinositol induces rapid phosphorylation of p38 mitogen-activated protein kinase and activating transcription factor 2 in HEK293 cells expressing GPR55 and IM-9 lymphoblastoid cells. J Biochem. 2010;147:671–678. doi: 10.1093/jb/mvp208. [DOI] [PubMed] [Google Scholar]
- 41.Piñeiro R., Falasca M. Lysophosphatidylinositol signalling: new wine from an old bottle. Biochim Biophys Acta. 2012;1821:694–705. doi: 10.1016/j.bbalip.2012.01.009. [Google Scholar] [CrossRef] [DOI] [PubMed] [Google Scholar]
- 42.Agwu D.E., McPhail L.C., Sozzani S., Bass D.A., McCall C.E. Phosphatidic acid as a second messenger in human polymorphonuclear leukocytes. Effects on activation of NADPH oxidase. J Clin Invest. 1991;88:531–539. doi: 10.1172/JCI115336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tomita T., Hosoda K., Fujikura J., Inagaki N., Nakao K. The G-protein-coupled long-chain fatty acid receptor GPR40 and glucose metabolism. Front Endocrinol (Lausanne) 2014;5:152. doi: 10.3389/fendo.2014.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feng X.T., Leng J., Xie Z., Li S.L., Zhao W., Tang Q.L. GPR40: a therapeutic target for mediating insulin secretion (review) Int J Mol Med. 2012;30:1261–1266. doi: 10.3892/ijmm.2012.1142. [DOI] [PubMed] [Google Scholar]
- 45.Burant C.F., Viswanathan P., Marcinak J., Cao C., Vakilynejad M., Xie B. TAK-875 versus placebo or glimepiride in type 2 diabetes mellitus: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2012;379:1403–1411. doi: 10.1016/S0140-6736(11)61879-5. [DOI] [PubMed] [Google Scholar]
- 46.Yamada H., Yoshida M., Ito K., Dezaki K., Yada T., Ishikawa S.E. Potentiation of glucose-stimulated insulin secretion by the GPR40-PLC-TRPC pathway in pancreatic β-cells. Sci Rep. 2016;6:25912. doi: 10.1038/srep25912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salem N., Jr., Pawlosky R., Hibbeln W.J. In vivo conversion of linoleic acid to arachidonic acid in human adults. Prostagl Laukm Essent Fatty Acids. 1999;60:407–410. doi: 10.1016/s0952-3278(99)80021-0. [DOI] [PubMed] [Google Scholar]
- 48.Lee B.H., Choi S.H., Kim H.J., Jung S.W., Kim H.K., Nah S.Y. Plant lysophosphatidic acids: a rich source for bioactive lysophosphatidic acids and their pharmacological applications. Biol Pharm Bull. 2016;39:156–162. doi: 10.1248/bpb.b15-00575. [DOI] [PubMed] [Google Scholar]
- 49.Cho Y.J., Choi S.H., Lee R., Hwang H., Rhim H., Cho I.H. Ginseng gintonin contains ligands for GPR40 and GPR55. Molecules. 2020;25:E1102. doi: 10.3390/molecules25051102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee B.H., Kim J., Lee R.M., Choi S.H., Kim H.J., Hwang S.H. Gintonin enhances performance of mice in rotarod test: Involvement of lysophosphatidic acid receptors and catecholamine release. Neurosci Lett. 2016;612:256–260. doi: 10.1016/j.neulet.2015.12.026. [DOI] [PubMed] [Google Scholar]
- 51.Park H., Kim S., Rhee J., Kim H.J., Han J.S., Nah S.Y. Synaptic enhancement induced by gintonin via lysophosphatidic acid receptor activation in central synapses. J Neurophysiol. 2015;113:1493–1500. doi: 10.1152/jn.00667.2014. [DOI] [PubMed] [Google Scholar]
- 52.Kim H.J., Shin E.J., Lee B.H., Choi S.H., Jung S.W., Cho I.H. Oral administration of gintonin attenuates cholinergic impairments by scopolamine, amyloid-β protein, and mouse model of Alzheimer's disease. Mol Cells. 2015;38:796–805. doi: 10.14348/molcells.2015.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim H.J., Park S.D., Lee R.M., Lee B.H., Choi S.H., Hwang S.H. Gintonin attenuates depressive-like behaviors associated with alcohol withdrawal in mice. J Affect Disord. 2017;215:23–29. doi: 10.1016/j.jad.2017.03.026. [DOI] [PubMed] [Google Scholar]
- 54.Kim S., Kim M.S., Park K., Kim H.J., Jung S.W., Nah S.Y. Hippocampus-dependent cognitive enhancement induced by systemic gintonin administration. J Ginseng Res. 2016;40:55–61. doi: 10.1016/j.jgr.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hwang S.H., Lee B.H., Choi S.H., Kim H.J., Won K.J., Lee H.M. Effects of gintonin on the proliferation, migration, and tube formation of human umbilical-vein endothelial cells: involvement of lysophosphatidic-acid receptors and vascular-endothelial-growth-factor signaling. J Ginseng Res. 2016;40:325–333. doi: 10.1016/j.jgr.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rahman M.A., Hwang H., Nah S.Y., Rhim H. Gintonin stimulates autophagic flux in primary cortical astrocytes. J Ginseng Res. 2020;44:67–78. doi: 10.1016/j.jgr.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hwang S.H., Lee B.H., Kim H.J., Cho H.J., Shin H.C., Im K.S. Suppression of metastasis of intravenously-inoculated B16/F10 melanoma cells by the novel ginseng-derived ingredient, gintonin: involvement of autotaxin inhibition. Int J Oncol. 2013;42:317–326. doi: 10.3892/ijo.2012.1709. [DOI] [PubMed] [Google Scholar]
- 58.Lu J., Wu D.M., Zheng Y.L., Hu B., Zhang Z.F. Purple sweet potato color alleviates d-galactose-induced brain aging in old mice by promoting survival of neurons via PI3K pathway and inhibiting cytochrome C-mediated apoptosis. Brain Pathol. 2010;20:598–612. doi: 10.1111/j.1750-3639.2009.00339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nam S.M., Choi J.H., Yoo D.Y., Kim W., Jung H.Y., Kim J.W. Valeriana officinalis extract and its main component, valerenic acid, ameliorate d-galactose-induced reductions in memory, cell proliferation, and neuroblast differentiation by reducing corticosterone levels and lipid peroxidation. Exp Gerontol. 2013;4 8:1369–1377. doi: 10.1016/j.exger.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 60.Nam S.M., Kim J.W., Yoo D.Y., Yim H.S., Kim D.W., Choi J.H. Physical exercise ameliorates the reduction of neural stem cell, cell proliferation and neuroblast differentiation in senescent mice induced by d-galactose. BMC Neurosci. 2014;15:116. doi: 10.1186/s12868-014-0116-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rehman S.U., Shah S.A., Ali T., Chung J.I., Kim M.O. Anthocyanins reversed d-galactose-induced oxidative stress and neuroinflammation mediated cognitive impairment in adult rats. Mol Neurobiol. 2017;54:255–271. doi: 10.1007/s12035-015-9604-5. [DOI] [PubMed] [Google Scholar]
- 62.Du Z., Yang Q., Zhou T., Liu L., Li S., Chen S. d-Galactose-induced mitochondrial DNA oxidative damage in the auditory cortex of rats. Mol Med Rep. 2014;10:2861–2867. doi: 10.3892/mmr.2014.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cui X., Zuo P., Zhang Q., Li X., Hu Y., Long J. Chronic systemic d-galactose exposure induces memory loss, neurodegeneration, and oxidative damage in mice: protective effects of R-α-lipoic acid. J Neurosci Res. 2006;83:1584–1590. doi: 10.1002/jnr.20845. [DOI] [PubMed] [Google Scholar]
- 64.Baptista P., Andrade J.P. Adult hippocampal neurogenesis: regulation and possible functional and clinical correlates. Front Neuroanat. 2018;12:44. doi: 10.3389/fnana.2018.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nam S.M., Hwang H., Seo M., Chang B.J., Kim H.J., Choi S.H. Gintonin attenuates d-galactose-induced hippocampal senescence by improving long-term hippocampal potentiation, neurogenesis, and cognitive functions. Gerontology. 2018;64:562–575. doi: 10.1159/000491113. [DOI] [PubMed] [Google Scholar]
- 66.Querfurth H.W., LaFerla F.M. Alzheimer's disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 67.GBD 2016 Dementia Collaborators Global, regional, and national burden of Alzheimer's disease and other dementias, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:88–106. doi: 10.1016/S1474-4422(18)30403-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hardy J., Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer's disease. Trends Pharmacol Sci. 1991;12:383–388. doi: 10.1016/0165-6147(91)90609-v. [DOI] [PubMed] [Google Scholar]
- 69.Hardy J.A., Higgins G.A. Alzheimer's disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 70.Hughes T.M., Kuller L.H., Barinas-Mitchell E.J., McDade E.M., Klunk W.E., Cohen A.D. Arterial stiffness and beta-amyloid progression in nondemented elderly adults. JAMA Neurol. 2014;71:562–568. doi: 10.1001/jamaneurol.2014.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jagust W.J., Mormino E.C. Lifespan brain activity, β-amyloid, and Alzheimer's disease. Trends Cogn Sci. 2011;15:520–526. doi: 10.1016/j.tics.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hwang S.H., Shin E.J., Shin T.J., Lee B.H., Choi S.H., Kang J. Gintonin, a ginseng-derived lysophosphatidic acid receptor ligand, attenuates Alzheimer's disease-related neuropathies: involvement of non-amyloidogenic processing. J Alzheimers Dis. 2012;31:207–223. doi: 10.3233/JAD-2012-120439. [DOI] [PubMed] [Google Scholar]
- 73.Francis P., Palmer A., Snape M., Wilcock G. The cholinergic hypothesis of Alzheimer's disease:a review of progress. J Neurol Neurosurg Psychiatry. 1999;66:137–147. doi: 10.1136/jnnp.66.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Drachman D.A., Leavitt J. Human memory and the cholinergic system. Arch Neurol. 1974;30:113–121. doi: 10.1001/archneur.1974.00490320001001. [DOI] [PubMed] [Google Scholar]
- 75.Bartus R.T., Dean R.L., Beer B., Lippa A.S. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–417. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- 76.Balmus I.M., Ciobica A. Main plant extracts’ active properties effective on scopolamine-induced memory loss. Am J Alzheimers Dis Other Demen. 2017;32:418–428. doi: 10.1177/1533317517715906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Birks J.S., Harvey R.J. Donepezil for dementia due to Alzheimer's disease. Cochrane Database Syst Rev. 2018;6:CD001190. doi: 10.1002/14651858.CD001190. [DOI] [PubMed] [Google Scholar]
- 78.Rashid M.H., Zahid M.F., Zain S., Kabir A., Hassan S.U. The neuroprotective effects of exercise on cognitive decline: a preventive approach to Alzheimer disease. Cureus. 2020;12:e6958. doi: 10.7759/cureus.6958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Akers K.G., Chérasse Y., Fujita Y., Srinivasan S., Sakurai T., Sakaguchi M. Concise review: regulatory influence of sleep and epigenetics on adult hippocampal neurogenesis and cognitive and emotional function. Stem Cells. 2018;36:969–976. doi: 10.1002/stem.2815. [DOI] [PubMed] [Google Scholar]
- 80.Choi J.W., Herr D.R., Noguchi K., Yung Y.C., Lee C.W., Mutoh T. LPA receptors: subtypes and biological actions. Annu Rev Pharmacol Toxicol. 2010;50:157–186. doi: 10.1146/annurev.pharmtox.010909.105753. [DOI] [PubMed] [Google Scholar]
- 81.Yung Y.C., Stoddard N.C., Chun J. LPA receptor signaling: pharmacology, physiology, and pathophysiology. J Lipid Res. 2014;55:1192–1214. doi: 10.1194/jlr.R046458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Suckau O., Gross I., Schrötter S., Yang F., Luo J., Wree A. LPA1, LPA2, LPA4, and LPA6 receptor expression during mouse brain development. Dev Dyn. 2019;248:375–395. doi: 10.1002/dvdy.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Moreno-Fernandez R.D., Tabbai S., Castilla-Ortega E., Perez-Martin M., Estivill-Torrus G., Rodriguez de Fonseca F. Stress, depression, resilience and ageing: a role for the LPA–LPA1 pathway. Curr Neuropharmacol. 2018;16:271–283. doi: 10.2174/1570159X15666170710200352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim H.J., Kim D.J., Shin E.J., Lee B.H., Choi S.H., Hwang S.H. Effects of gintonin-enriched fraction on hippocampal cell proliferation in wild-type mice and an APPswe/PSEN-1 double Tg mouse model of Alzheimer's disease. Neurochem Int. 2016;101:56–65. doi: 10.1016/j.neuint.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 85.Doherty T.J., Vandervoort A.A., Taylor A.W., Brown W.F. Effects of motor unit losses on strength in older men and women. J Appl Physiol. 1993;74:868–874. doi: 10.1152/jappl.1993.74.2.868. [DOI] [PubMed] [Google Scholar]
- 86.Lanza I.R., Towse T.F., Caldwell G.E., Wigmore D.M., Kent-Braun J.A. Effects of age on human muscle torque, velocity, and power in two muscle groups. J Appl Physiol. 2003;95:2361–2369. doi: 10.1152/japplphysiol.00724.2002. [DOI] [PubMed] [Google Scholar]
- 87.Elbaz A., Carcaillon L., Kab S., Moisan F. Epidemiology of Parkinson's disease. Rev Neurol. 2016;172:14–26. doi: 10.1016/j.neurol.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 88.Jankovic J. Parkinson's disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79:368–376. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- 89.Brichta L., Greengard P., Flajolet M. Advances in the pharmacological treatment of Parkinson's disease: targeting neurotransmitter systems. Trends Neurosci. 2013;36:543–554. doi: 10.1016/j.tins.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 90.Kalia L.V., Lang A.E. Parkinson's disease. Lancet. 2015;386:896–912. doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- 91.Kin K., Yasuhara T., Kameda M., Date I. Animal models for Parkinson's disease research: trends in the 2000s. Int J Mol Sci. 2019;20:E5402. doi: 10.3390/ijms20215402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Choi J.H., Jang M., Oh S., Nah S.Y., Cho I.H. Multi-target protective effects of gintonin in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-mediated model of Parkinson's disease via lysophosphatidic acid receptors. Front Pharmacol. 2018;9:515. doi: 10.3389/fphar.2018.00515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jo M.G., Ikram M., Jo M.H., Yoo L., Chung K.C., Nah S.Y. Gintonin mitigates MPTP-induced loss of nigrostriatal dopaminergic neurons and accumulation of α-synuclein via the Nrf2/HO-1 pathway. Mol Neurobiol. 2019;56:39–55. doi: 10.1007/s12035-018-1020-1. [DOI] [PubMed] [Google Scholar]
- 94.Vijitruth R., Liu M., Choi D.Y., Nguyen X.V., Hunter R.L., Bing G. Cyclooxygenase-2 mediates microglial activation and secondary dopaminergic cell death in the mouse MPTP model of Parkinson's disease. J Neuroinflamm. 2006;27:6. doi: 10.1186/1742-2094-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.De Vos K.J., Grierson A.J., Ackerley S., Miller C.C. Role of axonal transport in neurodegenerative diseases. Annu Rev Neurosci. 2008;31:151–173. doi: 10.1146/annurev.neuro.31.061307.090711. [DOI] [PubMed] [Google Scholar]
- 96.Ross C.A., Tabrizi S.J. Huntington's disease: from molecular pathogenesis to clinical treatment. Lancet Neurol. 2011;10:83–98. doi: 10.1016/S1474-4422(10)70245-3. [DOI] [PubMed] [Google Scholar]
- 97.Dayalu P., Albin R.L. Huntington disease: pathogenesis and treatment. Neurol Clin. 2015;33:101–114. doi: 10.1016/j.ncl.2014.09.003. [Google Scholar] [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 98.The Huntington's Disease Collaborative Research Group A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- 99.Fukumoto T., Miyamoto R. Hung-up knee Jerk in Huntington's disease. N Engl J Med. 2020;382:e10. doi: 10.1056/NEJMicm1910309. [DOI] [PubMed] [Google Scholar]
- 100.Vogel A., Jørgensen K., Larsen I.U. Normative data for Emotion Hexagon test and frequency of impairment in behavioral variant frontotemporal dementia, Alzheimer's disease and Huntington's disease. Appl Neuropsychol Adult. 2020;14:1–6. doi: 10.1080/23279095.2020.1720686. [DOI] [PubMed] [Google Scholar]
- 101.Jang M., Choi J.H., Chang Y., Lee S.J., Nah S.Y., Cho I.H. Gintonin, a ginseng-derived ingredient, as a novel therapeutic strategy for Huntington's disease: activation of the Nrf2 pathway through lysophosphatidic acid receptors. Brain Behav Immun. 2019;80:146–162. doi: 10.1016/j.bbi.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 102.Túnez I., Tasset I., Pérez-De La Cruz V., Santamaría A. 3-Nitropropionic acid as a tool to study the mechanisms involved in Huntington's disease: past, present and future. Molecules. 2010;15:878–916. doi: 10.3390/molecules15020878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Singer E., Walter C., Weber J.J., Krahl A.C., Mau-Holzmann U.A., Rischert N. Reduced cell size, chromosomal aberration and altered proliferation rates are characteristics and confounding factors in the STHdh cell model of Huntington disease. Sci Rep. 2017;7:16880. doi: 10.1038/s41598-017-17275-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mizushima N., Levine B., Cuervo A.M., Klionsky D.J. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rubinsztein D.C., Marino G., Kroemer G. Autophagy and aging. Cell. 2011;146:682–695. doi: 10.1016/j.cell.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 106.Komatsu M., Waguri S., Chiba T., Murata S., Iwata J., Tanida I. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- 107.Toth M.L., Sigmond T., Borsos E., Barna J., Erdelyi P., Takacs-Vellai K. Longevity pathways converge on autophagy genes to regulate life span in Caenorhabditis elegans. Autophagy. 2008;4:330–338. doi: 10.4161/auto.5618. [DOI] [PubMed] [Google Scholar]
- 108.Simonsen A., Cumming R.C., Brech A., Isakson P., Schubert D.R., Finley K.D. Promoting basal levels of autophagy in the nervous system enhances longevity and oxidant resistance in adult Drosophila. Autophagy. 2008;4:176–184. doi: 10.4161/auto.5269. [PubMed] [Google Scholar] [DOI] [PubMed] [Google Scholar]
- 109.Meng Q., Cai D. Defective hypothalamic autophagy directs the central pathogenesis of obesity via the IkappaB kinase beta (IKKbeta)/NF-kappaB pathway. J Biol Chem. 2011;286:32324–32332. doi: 10.1074/jbc.M111.254417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cuervo A.M., Macian F. Autophagy and the immune function in aging. Curr Opin Immunol. 2014;29:97–104. doi: 10.1016/j.coi.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nixon R.A., Wegiel J., Kumar A., Yu W.H., Peterhoff C., Cataldo A. Extensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy study. J Neuropathol Exp Neurol. 2005;64:113–122. doi: 10.1093/jnen/64.2.113. [DOI] [PubMed] [Google Scholar]
- 112.Yu W.H., Cuervo A.M., Kumar A., Peterhoff C.M., Schmidt S.D., Lee J.H. Macroautophagy – a novel beta-amyloid peptide-generating pathway activated in Alzheimer's disease. J Cell Biol. 2005;171:87–98. doi: 10.1083/jcb.200505082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Anglade P., Vyas S., Javoy-Agid F., Herrero M.T., Michel P.P., Marquez J. Apoptosis and autophagy in nigral neurons of patients with parkinson's disease. Histol Histopathol. 1997;12:25–31. [PubMed] [Google Scholar]
- 114.Rui Y.N., Xu Z., Patel B., Chen Z., Chen D., Tito A. Huntingtin functions as a scaffold for selective macroautophagy. Nat Cell Biol. 2015;17:262–275. doi: 10.1038/ncb3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ochaba J., Lukacsovich T., Csikos G., Zheng S., Margulis J., Salazar L. Potential function for the huntingtin protein as a scaffold for selective autophagy. Proc Natl Acad Sci U S A. 2014;111:9–894. doi: 10.1073/pnas.1420103111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Spilman P., Podlutskaya N., Hart M.J., Debnath J., Gorostiza O., Bredesen D. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer's disease. PLoS ONE. 2010;5:e9979. doi: 10.1371/journal.pone.0009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rodriguez-Navarro J.A., Rodriguez L., Casarejos M.J., Solano R.M., Gomez A., Perucho J. Trehalose ameliorates dopaminergic and tau pathology in parkin deleted/tau overexpressing mice through autophagy activation. Neurobiol Dis. 2010;39:423–438. doi: 10.1016/j.nbd.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 118.Pan T., Kondo S., Le W., Jankovic J. The role of autophagy-lysosome pathway in neurodegeneration associated with Parkinson's disease. Brain. 2008;131:1969–1978. doi: 10.1093/brain/awm318. [DOI] [PubMed] [Google Scholar]
- 119.Ravikumar B., Vacher C., Berger Z., Davies J.E., Luo S., Oroz L.G. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet. 2004;36:585–595. doi: 10.1038/ng1362. [DOI] [PubMed] [Google Scholar]
- 120.Metaxakis A., Ploumi C., Tavernarakis N. Autophagy in age-associated neurodegeneration. Cells. 2018;7:E37. doi: 10.3390/cells7050037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sung K., Jimenez-Sanchez M. Autophagy in astrocytes and its implications in neurodegeneration. J Mol Biol. 2020 doi: 10.1016/j.jmb.2019.12.041. S0022-2836:30023-1. [DOI] [PubMed] [Google Scholar]
- 122.Koistinaho M., Lin S., Wu X., Esterman M., Koger D., Hanson J. Apolipoprotein E promotes astrocyte colocalization and degradation of deposited amyloid-beta peptides. Nat Med. 2004;10:719–726. doi: 10.1038/nm1058. [DOI] [PubMed] [Google Scholar]
- 123.Lin Y.T., Seo J., Gao F., Feldman H.M., Wen H.L., Penney J. APOE4 causes widespread molecular and cellular alterations associated with Alzheimer's disease phenotypes in human iPSC-derived brain cell types. Neuron. 2018;98:1141–1154. doi: 10.1016/j.neuron.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wakabayashi K., Hayashi S., Yoshimoto M., Kudo H., Takahashi H. NACP/alpha-synuclein-positive filamentous inclusions in astrocytes and oligodendrocytes of Parkinson's disease brains. Acta Neuropathol. 2000;99:14–20. doi: 10.1007/pl00007400. [DOI] [PubMed] [Google Scholar]
- 125.Lee H.J., Suk J.E., Patrick C., Bae E.J., Cho J.H., Rho S. Direct transfer of alpha-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J Biol Chem. 2010;285:9262–9272. doi: 10.1074/jbc.M109.081125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Braidy N., Gai W.P., Xu Y.H., Sachdev P., Guillemin G.J., Jiang X.M. Uptake and mitochondrial dysfunction of alpha-synuclein in human astrocytes, cortical neurons and fibroblasts. Transl Neurodegener. 2013;2:20. doi: 10.1186/2047-9158-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lindström V., Gustafsson G., Sanders L.H., Howlett E.H., Sigvardson J., Kasrayan A. Extensive uptake of α-synuclein oligomers in astrocytes results in sustained intracellular deposits and mitochondrial damage. Mol Cell Neurosci. 2017;82:143–156. doi: 10.1016/j.mcn.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 128.Shin J.Y., Fang Z.H., Yu Z.X., Wang C.E., Li S.H., Li X.J. Expression of mutant huntingtin in glial cells contributes to neuronal excitotoxicity. J Cell Bio. 2005;171:1001–1012. doi: 10.1083/jcb.200508072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Singhrao S.K., Thomas P., Wood J.D., MacMillan J.C., Neal J.W., Harper P.S. Huntingtin protein colocalizes with lesions of neurodegenerative diseases: an investigation in Huntington's, Alzheimer's, and Pick's diseases. Exp Neurol. 1998;150:213–222. doi: 10.1006/exnr.1998.6778. [DOI] [PubMed] [Google Scholar]
- 130.Liévens J.C., Woodman B., Mahal A., Spasic-Boscovic O., Samuel D., Kerkerian-Le Goff L. Impaired glutamate uptake in the R6 Huntington's disease transgenic mice. Neurobiol Dis. 2001;8:807–821. doi: 10.1006/nbdi.2001.0430. [DOI] [PubMed] [Google Scholar]
- 131.Faideau M., Kim J., Cormier K., Gilmore R., Welch M., Auregan G. In vivo expression of polyglutamine-expanded huntingtin by mouse striatal astrocytes impairs glutamate transport: a correlation with Huntington's disease subjects. Hum Mol Genet. 2010;19:3053–3067. doi: 10.1093/hmg/ddq212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lipinski M.M., Zheng B., Lu T., Yan Z., Py B.F., Ng A. Genome-wide analysis reveals mechanisms modulating autophagy in normal brain aging and in Alzheimer's disease. Proc Natl Acad Sci U S A. 2010;107:4–69. doi: 10.1073/pnas.1009485107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Farhy-Tselnicker I., Allen N.J. Astrocytes, neurons, synapses: a tripartite view on cortical circuit development. Neural Dev. 2018;13:7. doi: 10.1186/s13064-018-0104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kim H., Lee B.H., Choi S.H., Kim H.J., Jung S.W., Hwang S.H. Gintonin stimulates gliotransmitter release in cortical primary astrocytes. Neurosci Lett. 2015;603:19–24. doi: 10.1016/j.neulet.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 135.Choi S.H., Kim H.J., Cho H.J., Park S.D., Lee N.E., Hwang S.H. Gintonin-mediated release of astrocytic vascular endothelial growth factor protects cortical astrocytes from hypoxia-induced cell damages. J Ginseng Res. 2019;43:305–311. doi: 10.1016/j.jgr.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Choi S.H., Kim H.J., Cho H.J., Park S.D., Lee N.E., Hwang S.H. Gintonin, a ginseng-derived exogenous lysophosphatidic acid receptor ligand, protects astrocytes from hypoxic and re-oxygenation stresses through stimulation of astrocytic glycogenolysis. Mol Neurobiol. 2019;56:3280–3294. doi: 10.1007/s12035-018-1308-1. [DOI] [PubMed] [Google Scholar]
- 137.Trang T., Al-Hasani R., Salvemini D., Salter M.W., Gutstein H., Cahill C.M. Pain and poppies: the good, the bad, and the ugly of opioid analgesics. J Neurosci. 2015;35:13879–13888. doi: 10.1523/JNEUROSCI.2711-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Deer T.R., Pope J.E., Hanes M.C., McDowell G.C. Intrathecal therapy for chronic pain: a review of morphine and ziconotide as firstline options. Pain Med. 2019;20:784–798. doi: 10.1093/pm/pny132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data for this review will be made available upon request.