Abstract
Aging is associated with changes in lower-body functioning. The extent to which lower-body function is associated with cognitive changes over time is unclear, especially among older Hispanics, a high-risk population for declines in physical and cognitive functioning. We sought to determine if the association between lower-body functioning and cognitive decline over 9-years differentially varied with respect to balance, gait speed, lower-body strength (chair stands), or a summary score of the three measures. This retrospective cohort study used clinical performance data from the Hispanic Established Populations for the Epidemiologic Study of the Elderly (H-EPESE). Cognitive function was measured using the Mini-Mental Status Exam. Linear mixed modeling was used to investigate the association between lower-body function and cognitive decline, controlling for patients’ demographic and health characteristics. We found that gait speed and timed chair stands but not balance were associated with accelerated cognitive decline in Mexican-Americans age 75 years and older. These parameters of lower-body function can be feasibly measured in any clinic. As limitations in lower-body functioning may be an early marker of cognitive decline, this suggests an opportunity for the development of interventions to slow cognitive and physical disablement and promote successful aging among persons older than 75 years.
Keywords: Cognitive aging, gait, balance, physical performance
Introduction
Advanced age is associated with increased risk of limitations in lower-body functioning, such as impaired balance and slow gait (Patel et al., 2014; Verghese et al., 2006). Such limitations have been linked to poor health outcomes, including increased morbidity, disability, and mortality (Boyd et al., 2005; Buchman et al., 2009; Newman et al., 2006). The Short Physical Performance Battery (SPPB) is a commonly used instrument in population-based and clinical studies that measures three aspects of lower body function: (1) repeated chair stands; (2) walking speed; and (3) balance tests. Although an SPPB summary score has been consistently associated with increased mortality (Pavasini et al., 2016), studies have shown that gait speed is the strongest of the three measures for predicting poor health outcomes (Veronese et al., 2018).
Reduced lower-body functioning is also a risk factor for cognitive decline (Buchman et al., 2007). In particular, slow gait has been consistently associated with increased cognitive decline (Buracchio et al., 2010; Camicioli et al., 1998; Mielke et al., 2013). Based on this evidence, the motoric cognitive risk (MCR) syndrome, characterized by slow gait and observable cognitive impairment, has been proposed as a low-cost method for identifying older adults who may be at an increased risk for cognitive impairment and dementia (Rosso et al., 2017; Verghese et al., 2013; 2015). Less research has considered other aspects of lower-body functioning that may be associated with cognitive decline, such as lower extremity muscle strength or balance (Veronese et al., 2016).
Gait speed, balance, and chair stands primarily measure functional mobility (Horak et al., 2016), postural stability (Mesbah et al., 2017), and lower body strength (Jones et al., 1999), respectively. While each clinical parameter may assess a specific aspect of lower-body functioning, achieving a high performance on any one task requires adequate performance on the other measures. For example, being able to quickly walk 8 ft requires a person to have sufficient lower body strength to move themselves forward and be able to maintain their balance during each phase of the gait cycle (Mantel et al., 2019; Shubert et al., 2006).
The interdependence between the three measures has likely contributed to prior studies having used the SPPB summary score when examining the association between lower-body functioning and cognitive function. However, individual measures could contribute proportionally more or less to this association (i.e., strong associative relationship between one or two measures and cognitive decline drives the association for the SPPB summary score, despite other measures having no actual relationship). Comparison of cognitive outcomes with respect to individual measures of lower-body function may identify more specific associations, which would be useful to guide clinical testing and assessment of older adults.
Our central hypothesis is the association between lower-body functioning and cognitive decline varies according to different measures of lower-body functioning. This hypothesis is based on evidence that pathological changes to particular brain regions may reduce performance in specific lower body tasks before changes in cognition are observed (Ezzati et al., 2015; Macfarlane et al., 2015; Rosso et al., 2017; Sorond et al., 2015). For instance, a study of 175 older adults (mean age 73 years) that assessed ten brain regions found that right hippocampal volume was the only brain region associated with both a decline in gait speed over a 14-year period and cognitive impairment (Rosso et al., 2017). Additionally, performance on the chair stands task was associated with white matter hyperintensities in the frontal and parietal lobes, whereas gait speed was associated with hyperintensities in the temporal lobe and anterior white matter.
Hispanics are the largest older adult minority group in the U.S. (U.S. Census Bureau, 2012; West et al., 2014) and the majority of Hispanics are Mexican American (Flores et al., 2017). Older Hispanics are a high-risk population to develop physical and cognitive impairment due to the high prevalence of chronic health conditions, increased longevity, and socioeconomic disadvantages (Angel et al., 2015; Kogan et al., 2012; Ottenbacher et al., 2009). Additionally, many older Hispanics to have worked in physically demanding jobs as young and middle-aged adults (Flippen & Tienda, 2000; Missikpode et al., 2016), which can contribute to physical limitations later in life (Missikpode et al., 2016). Finally, older Hispanics are less likely than non-Hispanic Whites to have completed a high school level of education (Ryan & Bauman, 2016). These education disparities can increase the risk for cognitive impairment among older Hispanics (Garcia et al., 2019; Meng & D’Arcy, 2012).
There is evidence for a bi-directional association between physical limitations and cognitive decline among older Mexican Americans. Physical frailty (Samper-Ternent et al., 2009), weak grip strength (Alfaro-Acha et al., 2006), and physical inactivity (Downer et al., 2016; Ottenbacher et al., 2014) have all been associated with worse cognitive outcomes among older Mexican Americans. Conversely, cognitive impairment is a risk factor for physical frailty (Raji et al., 2010), disability (Raji et al., 2004), and poor muscle strength (Raji et al., 2005). Additionally, cognitive impairment has been associated with greater decline in lower-body functioning for older Mexican American (Raji et al., 2002). However, to our knowledge, no studies have examined the association between lower-body functioning and rate of cognitive decline in this population.
Therefore, the objective of the present study is to examine the relationship between lower-body functioning and rate of cognitive decline among older Mexican-Americans. This analysis will test two hypotheses. First, greater severity of physical limitations as indicated by lower scores on the SPPB will be associated with greater cognitive decline. Second, among the measures of gait speed, balance, and chair stands included in the SPPB, slow gait will have the strongest association with cognitive decline.
Methods
Participants
This analysis used data from the Hispanic Established Populations for the Epidemiologic Study of the Elderly (H-EPESE). The H-EPESE is a longitudinal study of community dwelling Mexican-Americans aged 65 and older living in the Southwestern United States (Bassford, 1995; Markides et al., 1996). Details of the study design and participant recruitment have been described previously (Eschbach et al., 2004). Briefly, participants were identified by first selecting census tracts in counties where the Mexican American population comprised at least 6.6% of the total population. Next, census blocks were randomly selected to identify Mexican Americans aged 65 and older. A total of 3,050 participants were interviewed at the first observation wave in 1993–94. Participants have been interviewed approximately every 2 to 3 years and the eighth observation wave was completed in 2012–13.
The H-EPESE sample was replenished at Wave 5 (2004–05) with 902 new participants aged 75 and older. The new sample was selected using the same procedures as the original sample of 3050 participants. All 905 participants in the new sample were 75 or older because this was the minimum age of the surviving participants who were recruited when the study began in 1993–94.
The selection of the study cohort was as follows. A total of 2,069 participants were interviewed at Wave 5 (hereafter “baseline”). We excluded participants who required a proxy to complete the baseline interview because these participants were not asked to complete the tasks for lower-body functioning. We then excluded participants who were missing the measure of cognitive function at baseline or were not interviewed at one or more follow-up waves. The final analytic sample included 1,489 participants. The demographic characteristics, self-reported health conditions, and measures of lower-body functioning were derived from the baseline observation. Measures for cognitive functioning were derived from the 2004–05, 2006–07, 2010–11, and 2012–13 observation waves.
Measures
Cognition: Cognitive function was measured at each observation using the Mini-Mental State Examination (MMSE) (Folstein et al., 1975). The MMSE assesses orientation to time (5 points) and place (5 points), attention (5 points), registration (3 points), recall (3 points) and language and praxis (9 points). Higher scores on the MMSE indicate better cognitive functioning. The MMSE is frequently used to assess the cognitive function of older adults in clinical and community settings, including for racially and ethnically diverse populations and older adults with low educational attainment (Arévalo et al., 2020). We analyzed the MMSE as a continuous outcome as opposed to classifying participants’ cognitive status at each observation wave. This approach was used because the MMSE has known limitations for being able to accurately identify older adults with mild-to-moderate cognitive impairment (Mitchell, 2009), especially in populations with low educational attainment (Borson et al., 2005). H-EPESE participants can choose to receive an English or Spanish language version of the MMSE. The level of difficulty for individual MMSE items can vary depending on if the assessment is done in English or Spanish (Jones, 2006), but the total scores are comparable (Morales et al., 2006).
Lower-body functioning: Three performance-based measures of lower-body functioning were used in the analysis, including a timed 8 ft walk, timed repeated chair stands, and standing balance tasks (Markides et al., 2001; Panas et al., 2013). Consistent with prior research, performance on the 8 ft walk, chair stands, and balance tasks was categorized as unable, poor, moderate, good, and best (Panas et al., 2013). For the walking task, participants were instructed to walk as fast as they were able for 8 ft. The shortest time of two trials was recorded. The repeated chair stand task required participants to stand from a sitting position with arms crossed for five repetitions. The time to complete the 8 ft walk or chair stand task was used to group participants into quartiles and scored as 1 point (poor), 2 points (moderate), 3 points (good), or 4 points (best). The standing balance test included three tasks: maintaining side-by-side, semi-tandem, and tandem positions for 10 s. Participants were scored 1 point if they completed a side-by side stand but were unable to complete a semi-tandem stand (poor); 2 points if they completed a semi-tandem stand but were unable to complete a full tandem stand for >2 s (moderate); 3 points if they completed the full tandem stand for 2.1 to 9.9 s (good); and 4 points if they completed a full tandem stand for 10 s (best/highest). Participants unable to complete a task were assigned a score of 0 points for that task (unable to do). An overall lower-body functioning score was calculated by summing the scores from the three measures (range 0–12 points). Participants who were unable to do all three measures were given a value of 0. The remaining participants were categorized according to the distribution of the total score in the sample: 1 to 4 points (poor); 5 to 8 points (moderate); and 9 to 12 points (highest).
Covariates: Demographic characteristics included age, sex, years of education, marital status, and nativity (born in the U.S. or born in Mexico) were collected at baseline. Marital status was dichotomized according to being currently married (yes/no). Health characteristics included self-reported measures for diabetes, hypertension, arthritis, hip fracture, heart disease, stroke, Parkinson’s disease, and Alzheimer’s disease. A comorbidity index was calculated by summing the number of health conditions the participant reported (range 0–8).
Measures for hearing impairment, vision impairment, and high depressive symptoms were also controlled for in the analyses. Hearing was assessed by asking participants if he/she can usually hear (with or without a hearing aid) and understand what a person says without seeing their face if that person is in a quiet room and talks in a normal voice. Participants who responded no or yes with a hearing aid were classified as hearing impaired. Vision was assessed by asking participants if he/she can see well enough (with or without classes/contact lenses) to recognize a friend or a family member from across the street, across the room, or when at an arm’s length away. Participants who responded no to any of the three distances were classified as visually impaired. High depressive symptoms were defined as scoring 16 points or higher on the Center for Epidemiologic Studies Depression scale (Radloff, 1977).
Statistical analysis
The relationship between lower-body functioning at baseline and change in cognitive functioning was examined using linear mixed effects regression models. The 8 ft walk, chair stands, balance, and summary score were examined separately for a total of four regression models. The highest performing category for each task was treated as the reference category. Time was measured as the number of years since baseline. We assessed if poorer lower-body functioning was associated with greater cognitive decline over time by including an interaction term of lower-body functioning by time in each regression model. All tests of statistical significance were two sided with significance level, 0.05. Analyses were performed with SAS version 9.4 (SAS Institute, Inc., Cary, NC).
Results
Sample Characteristics
Descriptive characteristics are presented in Table 1. The mean age was 81.1 years and the mean years of education was 5.0. The majority of the sample was female, not married, and born in the U.S. The percentage of participants who were unable to perform the 8 ft walk, chair stands, and balance tasks were 17.9%, 24.3%, and 33.3%, respectively.
Table 1.
Baseline Characteristics of the Study Cohort (N = 1,489).
| Variables | n | Value | |
|---|---|---|---|
| Age, mean (SD) | 1,489 | 81.2 (4.7) | |
| Years of education, mean (SD) | 1,489 | 5.0 (4.0) | |
| Gender, n (%) | |||
| Male | 1,489 | 533 (37.1) | |
| Female | 936 (62.9) | ||
| Marital status, n (%) | |||
| Married | 1,486 | 651 (43.8) | |
| Not married | 835 (56.2) | ||
| Nativity, n (%) | |||
| Mexico born | 1,489 | 657 (44.1) | |
| US born | 832 (55.9) | ||
| Hearing impairment, n (%) | |||
| No | 1,485 | 1,127 (75.9) | |
| Yes | 358 (24.1) | ||
| Visual difficulty, n (%) | |||
| No | 1,489 | 1,302 (87.4) | |
| Yes | 187 (12.6) | ||
| Depression, n (%) | |||
| No | 1,467 | 1,224 (83.4) | |
| Yes | 243 (16.6) | ||
| Comorbidity index, mean (SD) | 1,489 | 1.9 (1.2) | |
| Physical functions, n (%) | |||
| Chair stands | Unable to do | 1,364 | 332 (24.3) |
| Poor | 259 (19.0) | ||
| Moderate | 208 (15.2) | ||
| Good | 209 (15.3) | ||
| Best | 356 (26.1) | ||
| Walking speed | Unable to do | 1,413 | 253 (17.9) |
| Poor | 161 (11.4) | ||
| Moderate | 342 (24.2) | ||
| Good | 453 (32.1) | ||
| Best | 204 (14.4) | ||
| Balancing | Unable to do | 1,432 | 477 (33.3) |
| Poor | 125 (8.7) | ||
| Moderate | 149 (10.4) | ||
| Good | 192 (13.4) | ||
| Best | 489 (34.1) | ||
| SPPB Score | Unable to do | 1,461 | 223 (15.3) |
| 1 to 4 | 275 (18.8) | ||
| 5 to 8 | 518 (35.5) | ||
| 9 to 12 | 445 (30.5) | ||
| Baseline MMSE score, mean (SD) | 1,489 | 21.9 (6.4) | |
Note. SPPB = Short Physical Performance Battery; MMSE = Mini-Mental State Examination; SD = standard deviation.
Lower-Body Function and Cognition
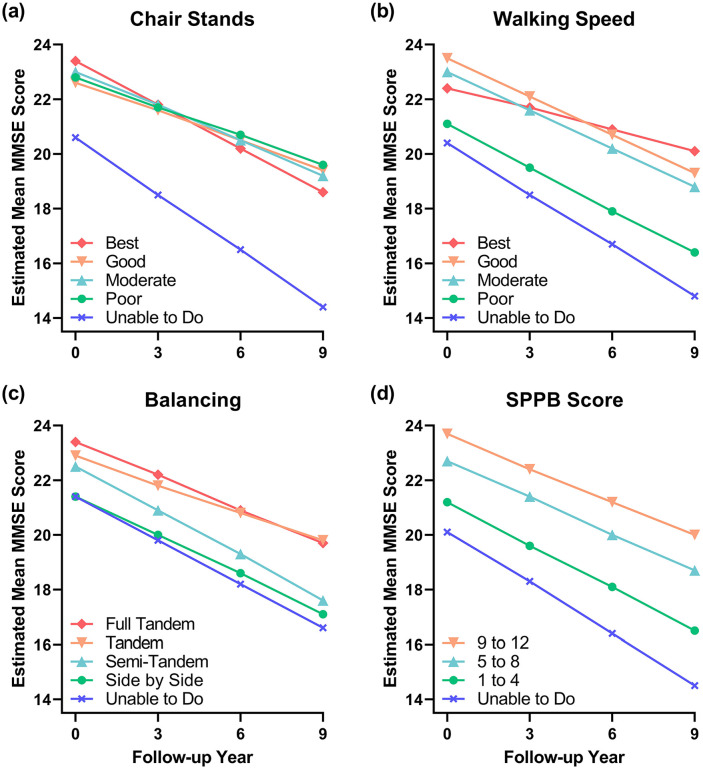
For easier interpretation, we present the results of the linear mixed effects regression model for each physical performance task as the average adjusted MMSE score at each observation wave (Table 2). We also provide a visual representation of the interaction between each measure of lower-body function and time in Figure 1. The parameter estimates from the linear mixed effects regression models are presented in Supplementary Table S1. Pairwise comparisons for the estimated mean MMSE scores and performance in each task at every observation wave are presented in Supplementary Table S2.
Table 2.
The Conditional Effect of Time on Cognitive Function for Performance in Each Lower-Body Function Tasks and the Estimated Mean of Mini-Mental State Examination (MMSE) Scores over 9 Years.
| Physical functions | Annual change (95% CI) | Estimated mean MMSE scoresa (95% CI) | |||
|---|---|---|---|---|---|
| Baseline | 3rd-year follow-up | 6th-year follow-up | 9th-year follow-up | ||
| Chair stands | |||||
| Unable to do | −0.69 (–0.82, –0.55) | 20.6 (19.9, 21.3) | 18.5 (17.9, 19.2) | 16.5 (15.7, 17.3) | 14.4 (13.3, 15.5) |
| Poor | −0.36 (–0.48, –0.24) | 22.8 (22.1, 23.6) | 21.7 (21.1, 22.4) | 20.7 (19.9, 21.4) | 19.6 (18.6, 20.5) |
| Moderate | −0.42 (–0.56, –0.28) | 23.0 (22.2, 23.9) | 21.8 (21.0, 22.5) | 20.5 (19.7, 21.3) | 19.2 (18.1, 20.3) |
| Good | −0.36 (–0.50, –0.23) | 22.6 (21.8, 23.5) | 21.6 (20.8, 22.3) | 20.5 (19.7, 21.3) | 19.4 (18.3, 20.4) |
| Best | −0.53 (–0.64, –0.42) | 23.4 (22.8, 24.0) | 21.8 (21.3, 22.3) | 20.2 (19.6, 20.8) | 18.6 (17.7, 19.4) |
| Walking speed | |||||
| Unable to do | −0.62 (–0.77, –0.47) | 20.4 (19.6, 21.2) | 18.5 (17.8, 19.2) | 16.7 (15.8, 17.5) | 14.8 (13.6, 16.0) |
| Poor | −0.52 (–0.70, –0.34) | 21.1 (20.1, 22.0) | 19.5 (18.7, 20.3) | 17.9 (16.9, 18.9) | 16.4 (15.0, 17.8) |
| Moderate | −0.47 (–0.58, –0.35) | 23.0 (22.4, 23.7) | 21.6 (21.0, 22.2) | 20.2 (19.5, 20.9) | 18.8 (17.9, 19.7) |
| Good | −0.46 (–0.56, –0.37) | 23.5 (22.9, 24.1) | 22.1 (21.6, 22.6) | 20.7 (20.2, 21.3) | 19.3 (18.6, 20.1) |
| Best | −0.26 (–0.39, –0.12) | 22.4 (21.6, 23.3) | 21.7 (20.9, 22.4) | 20.9 (20.1, 21.7) | 20.1 (19.0, 21.2) |
| Balancing | |||||
| Unable to do | −0.53 (–0.63, –0.42) | 21.4 (20.8, 22.0) | 19.8 (19.3, 20.3) | 18.2 (17.6, 18.8) | 16.6 (15.8, 17.5) |
| Poor | −0.48 (–0.68, –0.27) | 21.4 (20.3, 22.5) | 20.0 (19.0, 20.9) | 18.6 (17.4, 19.7) | 17.1 (15.5, 18.7) |
| Moderate | −0.54 (–0.71, –0.37) | 22.5 (21.6, 23.5) | 20.9 (20.1, 21.8) | 19.3 (18.3, 20.3) | 17.6 (16.3, 19.0) |
| Good | −0.35 (–0.49, –0.20) | 22.9 (22.0, 23.8) | 21.8 (21.1, 22.6) | 20.8 (19.9, 21.7) | 19.8 (18.6, 20.9) |
| Best | −0.41 (–0.50, –0.32) | 23.4 (22.8, 24.0) | 22.2 (21.7, 22.6) | 20.9 (20.4, 21.4) | 19.7 (18.9, 20.4) |
| SPPB score | |||||
| Unable to do | −0.62 (–0.79, –0.46) | 20.1 (19.2, 21.0) | 18.3 (17.5, 19.0) | 16.4 (15.4, 17.4) | 14.5 (13.2, 15.8) |
| 1 to 4 | −0.52 (–0.66, –0.39) | 21.2 (20.5, 22.0) | 19.6 (19.0, 20.3) | 18.1 (17.3, 18.9) | 16.5 (15.4, 17.6) |
| 5 to 8 | −0.44 (–0.53, –0.35) | 22.7 (22.2, 23.2) | 21.4 (20.9, 21.8) | 20.0 (19.5, 20.6) | 18.7 (18.0, 19.4) |
| 9 to 12 | −0.40 (–0.50, –0.31) | 23.7 (23.1, 24.2) | 22.4 (21.9, 22.9) | 21.2 (20.7, 21.8) | 20.0 (19.3, 20.8) |
Note. CI = confidence interval.
Estimated mean MMSE scores were adjusted for baseline demographic and health characteristics shown on Table 1.
Figure 1.
Cognitive functioning over time based on physical performance measurements. (a–d) Plots represent interactions between estimated mean Mini-Mental State Exam (MMSE) score and three performance-based measures of lower body function, as well as a composite physical function score, the Short Physical Performance Battery (SPPB).
Chair stands: Participants who were unable to perform the chair standing task had significantly lower cognitive functioning at baseline (mean MMSE score of 20.6, 95% confidence interval [CI]:19.9, 21.3) than participants in the highest performing quartile (mean MMSE score of 23.4, 95% CI:22.8, 24.0). These participants also exhibited significantly greater cognitively decline relative to those who scored 3 points (good). Being unable to do the chair stands was associated with an annual mean MMSE decline of 0.69 points, respectively, whereas performing in the good category was associated with a mean decline of 0.36 points. Participants who were classified as poor, moderate, or good performers did not have significantly different cognitive functioning at baseline and had similar rates of cognitive decline compared to the best performers.
Eight-foot walk: Participants who were unable to complete the 8 ft walk or were poor performers had significantly lower cognitive functioning at baseline (mean MMSE score 20.4 and 21.1, respectively) and showed faster cognitive decline compared to the highest performing (best) participants. Being unable to do or poorly performing the 8 ft walk was associated with an annual mean MMSE decline of 0.62 and 0.52 points, respectively, whereas performing in the best category was associated with a mean decline of 0.26 points. Moderate performers did not have significantly different cognitive functioning at baseline compared to the highest performers but did exhibit faster cognitive decline (moderate performers annually declined by 0.21 points more than the best performers, on average). Good and moderate performers showed more rapid cognitive decline than the highest performers (good performers annually declined by an average of 0.20 points more than the best performers).
Balance: Participants who were unable to do the balance task or could only complete the side-by-side task had significantly lower cognitive functioning at baseline compared to participants who could perform the full-tandem task. Participants who could complete the semi-tandem or tandem tasks had lower cognitive function than those who could perform the full-tandem, however these differences were not statistically significant. The differences in cognitive function between the four groups remained consistent over time and the balance by time interaction term was not statistically significant. However, as indicated by comparable mean MMSE scores (Table 2), ability to perform either the tandem or full-tandem task likely captures similar participants. Similarly, performing the side-by-side task was comparable to being unable to do the balance task.
Overall lower-body function: As expected, participants who scored 0, 1 to 4, and 5 to 8 points all had significantly lower cognitive functioning at baseline compared to participants who scored 9 to 12 points. Only participants who had a score 0 points had significantly greater cognitive decline compared to participants who scored between 9 and 12 points.
Discussion
Findings from this longitudinal study indicate that a relatively simple three-task battery of lower-body function was significantly associated with decline in cognitive functioning over a 9-year period among older Mexican-Americans.
Participants who were unable to do or were poor performers on any task had significantly lower cognitive functioning compared to the best performers, supporting our hypothesis. Participants with the highest SPPB scores (9–12) had significantly higher cognitive function compared to all other groups. While performance on balance testing was associated with lower cognitive function, we found no significant difference in rate of cognitive decline. Prior studies have similarly demonstrated the association between poor balance and low cognitive function (Gujord Tangen et al., 2014), although one study has reported that abnormal balance predicts cognitive decline over 2 years in older adults with Alzheimer’s (Rolland et al., 2009). However, it has been demonstrated that only the one-leg stand can significantly discriminate between functional levels (Curb et al., 2006).
Interestingly, results from our balance task indicate a high degree of similarity between the tandem and full-tandem performers, indicating that these two categories may be associated with similar patterns of cognition over 9 years. A similar pattern was observed for the side-by-side performers and those unable to do the balance task. While clinical measurement of performance-based tasks is one of the most reliable methods for ascertaining physical function, previous studies have found that a composite measure of survey data with objective clinical measures enables greater discrimination of physical function among the highest and poorest performers (Kasper et al., 2017). Application of survey data to our study may refine the associations observed here by further delineating individuals among the highest and lowest scoring groups of the balance task.
Importantly, faster rates of cognitive decline were observed for those unable to do the chair stands or gait speed task, as well as those who poorly performed in the gait speed task. These findings suggest that these two measures may be useful for identifying individuals with the greatest risk for cognitive decline. Gait speed may be particularly indicative of cognition over the next few years, as we observed a linear relationship between time to complete the 8ft walk and annual decline in MMSE score. Similar findings have been observed in younger older-adult (aged 60 years or older) populations (Alfaro-Acha et al., 2007) and non-Hispanic white older adults (Rosso et al., 2017; Verghese et al., 2013).
Our findings underscore the value of lower-body physical capacity, and declining gait speed in particular, as an early indicator of future cognitive decline. While our analysis was unable to identify causal factors, others have begun to shed light on potential mediators of the relationship between physical limitations and cognitive decline. For instance, decline in gait speed over 14 years predicted later cognitive impairment in initially healthy older adults, and this was partially attributable to decreased right hippocampal volume (Rosso et al., 2017). Similarly, striatal shape and volume abnormalities of the caudate nucleus, a brain region involved in motor and non-motor functions, have been associated with poor performance in the SPPB gait and balance tasks (Macfarlane et al., 2015). Others have hypothesized that the link between decline in both cognition and motor ability may be through age-associated increases in pro-inflammatory cytokines (“inflammaging”) causing adverse structural and functional changes in the central nervous system (CNS), namely reduced dopaminergic function (Rutherford et al., 2016). As dopamine is a neurotransmitter required for the execution of stable motor actions, as well as healthy cognitive processing, it may thus reasonably mediate the relationship between lower-body function and cognitive decline as observed in our study. Taken together, these findings support the theory that shared neuropathologic processes may manifest as the heterogeneous presentations seen in cognitive frailty or MCR syndrome.
Physical limitations may be an early marker of cognitive decline or dementia and can feasibly be measured in a clinical setting. Measuring these three parameters of lower-body function requires no special equipment, takes little time (<20 min), and can be conducted by any trained health professional, providing an ideal screening tool for any setting including low-income environments. High-risk individuals can be targeted for clinical interventions to improve physical function, which may be effective for slowing cognitive decline and delaying dementia onset. In particular, interventions focusing on lower-body strength may be most effective based on our finding that accelerated cognitive decline was observed in individuals with the lowest scores on the gait speed and chair stands tasks.
Our analyses revealed that, on average, even participants with high lower-body functioning experienced cognitive decline. However, person-level changes in cognitive functioning are highly variable (Wilson et al., 2002). Most older adults decline over a prolonged period of time, but this overall decline can include periods in which a person improves in cognitive functioning (Boyle et al., 2013). A prior study that used data from the H-EPESE identified three sub-trajectories of cognitive functioning over time (Downer et al., 2017). Approximately 31% of participants maintained high global cognition, wherein the average MMSE score changed from 27 points to just 26.2 points over the 8-year period. Future research using data from more extensive cognitive batteries should consider sub-trajectories of cognitive decline to determine if older Mexican-Americans with superior lower-body function show improvement in cognitive functioning or are able to maintain a high level of cognitive functioning over a long period of time.
Our study has several limitations. First, cognitive function was measured using only the MMSE. The MMSE can underestimate the cognitive functioning of Hispanic older adults and those with low education (Ramirez et al., 2006). The MMSE also has well-known limitations for detecting cognitive decline (Spencer et al., 2013) and mild-to-moderate cognitive impairment (Mitchell, 2009), especially for Hispanic older adults (Franco-Marina et al., 2010) and older adults with low education (Jones & Gallo, 2002) or impaired cognition (Tombaugh & McIntyre, 1992). Additionally, we were unable to examine specific cognitive domains that may be negatively affected by physical limitations, such as executive functioning (Daly et al., 2015). Measures of executive functioning in the MMSE include spelling world backward and item naming. However, individual MMSE items have been shown to be culturally biased (Ramirez et al., 2006) and to have varying levels of difficulty depending on if the assessment is done in English or Spanish (Jones, 2006). Thus, results based on individual items of the MMSE may be biased (Morales et al., 2006). In light of these limitations, it is important to replicate our findings using data from ethnically and racially diverse cohorts that include neuropsychological batteries that assess multiple cognitive domains.
A second limitation is that health conditions were based on self-report alone, which may have led to under-reporting of conditions and undiagnosed conditions may be missed. Third, participants who experience the most severe cognitive decline may have been observed only at baseline due to poor health preventing subsequent observation. Due to the requirement that participants be observed in at least one follow-up (2,069 interviewed at baseline, 1,489 in final analytic sample), the analytic sample may have been skewed toward a population with a healthier profile than the background population. Also, participants who required a proxy or who could not complete the physical functioning items had to be excluded, potentially limiting the generalizability of our findings to all Mexican-Americans aged 75 and older in the southwestern United States. Finally, we used measures of lower-body functioning that were collected at a single time point. Prior research indicates that while it is more common for older adults to experience increasing frailty and physical decline, many older adults can improve in physical functioning (Gill, 2014; Gill et al., 2006). Future research that investigates changes in lower-body functioning may reveal temporal relationships between limitations in lower-body functioning and cognitive impairment.
Conclusion
In summary, this study provided evidence for an association between simple clinical tests of lower-body function and cognitive decline over 9 years in older Mexican-Americans. While there is substantial literature demonstrating the relationship between gait speed and cognitive decline, we show reduced lower-body strength is associated with cognitive decline in Mexican-Americans. While our results do not provide evidence for a causal relationship between lower-body functioning and cognitive decline, this association suggests that those with impaired performance on gait speed or chair standing tasks may be at especially high-risk for cognitive decline. These physical limitations can feasibly be measured in any clinical setting, including low-resource or rural environments. Continued research is necessary to confirm these findings in other Hispanic populations, as well as to determine the efficacy of interventions that target physical limitations for preventing accelerated cognitive decline in high-risk individuals.
Supplemental Material
Supplemental material, Supplementary_Table_S1 for Mobility but Not Balance Limitations Are Associated With Cognitive Decline among Older Hispanics by Paul A. Wadsworth, Nai-Wei Chen, Mukaila Raji, Kyriakos S. Markides and Brian Downer in Gerontology and Geriatric Medicine
Supplemental material, Supplementary_Table_S2 for Mobility but Not Balance Limitations Are Associated With Cognitive Decline among Older Hispanics by Paul A. Wadsworth, Nai-Wei Chen, Mukaila Raji, Kyriakos S. Markides and Brian Downer in Gerontology and Geriatric Medicine
Footnotes
Author Contributions: Wadsworth, Markides, and Downer: Study conception and design; Chen: data analysis; Markides: Data acquisition; Wadsworth: visualization; Wadsworth, Raji, Chen, Downer: writing manuscript; All authors: interpretation of results, substantive edits, manuscript preparation. All authors have given final approval of this version of the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institute on Aging at the National Institutes of Health (Grant Numbers P30AG024832, P30AG059301, R01AG010939, T32AG051131, K01AG058789).
Supplemental Material: Supplemental material for this article is available online.
ORCID iD: Paul A. Wadsworth  https://orcid.org/0000-0002-4562-7830
https://orcid.org/0000-0002-4562-7830
References
- Alfaro-Acha A., Snih S. A., Raji M. A., Kuo Y.-F., Markides K. S., Ottenbacher K. J. (2006). Handgrip strength and cognitive decline in older Mexican Americans. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 61(8), 859–865. 10.1093/gerona/61.8.859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfaro-Acha A., Al Snih S., Raji M. A., Markides K. S., Ottenbacher K. J. (2007). Does 8-foot walk time predict cognitive decline in older Mexicans Americans? Journal of the American Geriatrics Society, 55(2), 245–251. 10.1111/j.1532-5415.2007.01039.x [DOI] [PubMed] [Google Scholar]
- Angel R. J., Angel J. L., Hill T. D. (2015). Longer lives, sicker lives? Increased longevity and extended disability among Mexican-origin elders. Journals of Gerontology - Series B Psychological Sciences and Social Sciences, 70(4), 639–649. 10.1093/geronb/gbu158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arévalo S. P., Kress J., Rodriguez F. S. (2020). Validity of cognitive assessment tools for older adult Hispanics: A systematic review. Journal of the American Geriatrics Society, 68(4), 882–888. 10.1111/jgs.16300 [DOI] [PubMed] [Google Scholar]
- Bassford T. L. (1995). Health status of Hispanic elders. In Espino D. V. (Ed.), Clinics in geriatric medicine (Vol. 11, pp. 25–38). National Academy Press. [PubMed] [Google Scholar]
- Borson S., Scanlan J. M., Watanabe J., Tu S.-P., Lessig M. (2005). Simplifying detection of cognitive impairment: Comparison of the Mini-Cog and Mini-Mental State Examination in a multiethnic sample. Journal of the American Geriatrics Society, 53(5), 871–874. 10.1111/j.1532-5415.2005.53269.x [DOI] [PubMed] [Google Scholar]
- Boyd C. M., Xue Q. L., Simpson C. F., Guralnik J. M., Fried L. P. (2005). Frailty, hospitalization, and progression of disability in a cohort of disabled older women. American Journal of Medicine, 118(11), 1225–1231. 10.1016/j.amjmed.2005.01.062 [DOI] [PubMed] [Google Scholar]
- Boyle P. A., Yu L., Wilson R. S., Schneider J. A., Bennett D. A. (2013). Relation of neuropathology with cognitive decline among older persons without dementia. Frontiers in Aging Neuroscience, 5(SEP), 50 10.3389/fnagi.2013.00050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman A. S., Boyle P. A., Wilson R. S., Tang Y., Bennett D. A. (2007). Frailty is associated with incident Alzheimer’s disease and cognitive decline in the elderly. Psychosomatic Medicine, 69(5), 483–489. 10.1097/psy.0b013e318068de1d [DOI] [PubMed] [Google Scholar]
- Buchman A. S., Wilson R. S., Bienias J. L., Bennett D. A. (2009). Change in frailty and risk of death in older persons. Experimental Aging Research, 35(1), 61–82. 10.1080/03610730802545051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buracchio T., Dodge H. H., Howieson D., Wasserman D., Kaye J. (2010). The trajectory of gait speed preceding mild cognitive impairment. Archives of Neurology, 67(8), 980–986. 10.1001/archneurol.2010.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camicioli R., Howieson D., Oken B., Sexton G., Kaye J. (1998). Motor slowing precedes cognitive impairment in the oldest old. Neurology, 50(5), 1496–1498. 10.1212/WNL.50.5.1496 [DOI] [PubMed] [Google Scholar]
- Curb J. D., Ceria-Ulep C. D., Rodriguez B. L., Grove J., Guralnik J., Willcox B. J., Donlon T., Masaki K. H., Chen R. (2006). Performance-based measures of physical function for high-function populations. Journal of the American Geriatrics Society, 54(5), 737–742. 10.1111/j.1532-5415.2006.00700.x [DOI] [PubMed] [Google Scholar]
- Daly M., McMinn D., Allan J. L. (2015). A bidirectional relationship between physical activity and executive function in older adults. Frontiers in Human Neuroscience, 8(JAN), 1044 10.3389/fnhum.2014.01044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downer B., Chen N. W., Raji M., Markides K. S. (2017). A longitudinal study of cognitive trajectories in Mexican Americans age 75 and older. International Journal of Geriatric Psychiatry, 32(10), 1122–1130. 10.1002/gps.4575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downer B., Kumar A., Veeranki S. P., Mehta H. B., Raji M., Markides K. S. (2016). Mexican-American Dementia Nomogram: Development of a Dementia Risk Index for Mexican-American Older Adults. Journal of the American Geriatrics Society, 64(12), e265–e269. 10.1111/jgs.14531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschbach K., Ostir G. V., Patel K. V., Markides K. S., Goodwin J. S. (2004). Neighborhood context and mortality among older Mexican Americans: Is there a barrio advantage? American Journal of Public Health, 94(10), 1807–1812. 10.2105/ajph.94.10.1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzati A., Katz M. J., Lipton M. L., Lipton R. B., Verghese J. (2015). The association of brain structure with gait velocity in older adults: A quantitative volumetric analysis of brain MRI. Neuroradiology, 57(8), 851–861. 10.1007/s00234-015-1536-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flippen C., Tienda M. (2000). Pathways to retirement: Patterns of labor force participation and labor market exit among the pre-retirement population by race, Hispanic origin, and sex. Journals of Gerontology - Series B Psychological Sciences and Social Sciences, 55(1), S14–S27. 10.1093/geronb/55.1.S14 [DOI] [PubMed] [Google Scholar]
- Flores A., López G., Radford J. (2017). 2015, Foreign-Born Population in the United States Statistical Portrait | Pew Research Center. https://www.pewresearch.org/hispanic/2017/05/03/2015-statistical-information-on-immigrants-in-united-states/
- Folstein M. F., Folstein S. E., McHugh P. R. (1975). “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–198. 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Franco-Marina F., García-González J. J., Wagner-Echeagaray F., Gallo J., Ugalde O., Sánchez-García S., Espinel C., Juárez-Cedillo T., Rodríguez M. A. V., García-Peña C. (2010). The Mini-mental state examination revisited: Ceiling and floor effects after score adjustment for educational level in an aging Mexican population. International Psychogeriatrics, 22(1), 72–81. 10.1017/S1041610209990822 [DOI] [PubMed] [Google Scholar]
- Garcia M. A., Downer B., Chiu C. T., Saenz J. L., Rote S., Wong R. (2019). Racial/Ethnic and nativity differences in cognitive life expectancies among older adults in the United States. Gerontologist, 59(2), 281–289. 10.1093/geront/gnx142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill T. M. (2014). Disentangling the disabling process: Insights from the precipitating events project. Gerontologist, 54(4), 533–549. 10.1093/geront/gnu067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill T. M., Gahbauer E. A., Allore H. G., Han L. (2006). Transitions between frailty states among community-living older persons. Archives of Internal Medicine, 166(4), 418–423. 10.1001/archinte.166.4.418 [DOI] [PubMed] [Google Scholar]
- Gujord Tangen G., Engedal K., Bergland A., Moger Anders T., Mengshoel Marit A. (2014). Relationships between balance and cognition in patients with subjective cognitive impairment, mild cognitive impairment, and Alzheimer disease. Physical Therapy, 94(8), 1123–1134. 10.2522/ptj.20l30298 [DOI] [PubMed] [Google Scholar]
- Horak F. B., Mancini M., Carlson-Kuhta P., Nutt J. G., Salarian A. (2016). Balance and gait represent independent domains of mobility in Parkinson disease. Physical Therapy, 96(9), 1364–1371. 10.2522/ptj.20150580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. J., Rikli R. E., Beam W. C. (1999). A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Research Quarterly for Exercise and Sport, 70(2), 113–119. 10.1080/02701367.1999.10608028 [DOI] [PubMed] [Google Scholar]
- Jones R. N. (2006). Identification of measurement differences between English and Spanish language versions of the Mini-Mental State Examination: Detecting differential item functioning using MIMIC modeling. Medical Care, 44(11 Suppl. 3), S124–S133. 10.1097/01.mlr.0000245250.50114.0f [DOI] [PubMed] [Google Scholar]
- Jones R. N., Gallo J. J. (2002). Education and sex differences in the mini-mental state examination: Effects of differential item functioning. Journals of Gerontology - Series B Psychological Sciences and Social Sciences, 57(6), 548–558. 10.1093/geronb/57.6.P548 [DOI] [PubMed] [Google Scholar]
- Kasper J. D., Chan K. S., Freedman V. A. (2017). Measuring physical capacity. Journal of Aging and Health, 29(2), 289–309. 10.1177/0898264316635566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogan M., Fischer-Smith T., Kaminsky R., Lehmicke G., Rappaport J. (2012). CSF-1R up-regulation is associated with response to pharmacotherapy targeting tyrosine kinase activity in AML cell lines. Anticancer Research, 32(3), 893–899. 10.1016/j.biotechadv.2011.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane M. D., Looi J. C. L., Walterfang M., Spulber G., Velakoulis D., Styner M., Crisby M., Örndahl E., Erkinjuntti T., Waldemar G., Garde E., Hennerici M., Bäzner H., Blahak C., Wallin A., Wahlund L.-O. (2015). Shape abnormalities of the caudate nucleus correlate with poorer gait and balance: Results from a subset of the ladis study. American Journal of Geriatric Psychiatry, 23(1), 59–71.e1. 10.1016/j.jagp.2013.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantel A., Trapuzzano A., Chizmar S., Haffke L., Dawson N. (2019). An investigation of the predictors of comfortable and fast gait speed in community-dwelling older adults. Journal of Geriatric Physical Therapy (2001), 42(4), E62–E68. 10.1519/JPT.0000000000000216 [DOI] [PubMed] [Google Scholar]
- Markides K. S., Black S. A., Ostir G. V., Angel R. J., Guralnik J. M., Lichtenstein M. (2001). Lower body function and mortality in Mexican American elderly people. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 56(4), M243–M247. 10.1093/gerona/56.4.M243 [DOI] [PubMed] [Google Scholar]
- Markides K. S., Stroup-Benham C. A., Goodwin J. S., Perkowski L. C., Lichtenstein M., Ray L. A. (1996). The effect of medical conditions on the functional limitations of Mexican-American elderly. Annals of Epidemiology, 6(5), 386–391. 10.1016/S1047-2797(96)00061-0 [DOI] [PubMed] [Google Scholar]
- Meng X., D’Arcy C. (2012). Education and dementia in the context of the cognitive reserve hypothesis: A systematic review with meta-analyses and qualitative analyses. PLoS One, 7(6), e38268 10.1371/journal.pone.0038268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesbah N., Perry M., Hill K. D., Kaur M., Hale L. (2017). Postural stability in older adults with Alzheimer disease. Physical Therapy, 97(3), 290–309. 10.2522/ptj.20160115 [DOI] [PubMed] [Google Scholar]
- Mielke M. M., Roberts R. O., Savica R., Cha R., Drubach D. I., Christianson T., Pankratz V. S., Geda Y. E., Machulda M. M., Ivnik R. J., Knopman D. S., Boeve B. F., Rocca W. A., Petersen R. C. (2013). Assessing the temporal relationship between cognition and gait: Slow gait predicts cognitive decline in the mayo clinic study of aging. Journals of Gerontology - Series A Biological Sciences and Medical Sciences, 68(8), 929–937. 10.1093/gerona/gls256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missikpode C., Michael Y. L., Wallace R. B. (2016). Midlife occupational physical activity and risk of disability later in life: National health and aging trends study. Journal of the American Geriatrics Society, 64(5), 1120–1127. 10.1111/jgs.14083 [DOI] [PubMed] [Google Scholar]
- Mitchell A. J. (2009). A meta-analysis of the accuracy of the mini-mental state examination in the detection of dementia and mild cognitive impairment. Journal of Psychiatric Research, 43(4), 411–431. 10.1016/j.jpsychires.2008.04.014 [DOI] [PubMed] [Google Scholar]
- Morales L. S., Flowers C., Gutierrez P., Kleinman M., Teresi J. A. (2006). Item and scale differential functioning of the Mini-Mental State Exam assessed using the Differential Item and Test Functioning (DFIT) framework. Medical Care, 44(11 Suppl. 3), 143–151. 10.1097/01.mlr.0000245141.70946.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman A. B., Simonsick E. M., Naydeck B. L., Boudreau R. M., Kritchevsky S. B., Nevitt M. C., Pahor M., Satterfield S., Brach J. S., Studenski S. A., Harris T. B. (2006). Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. Journal of the American Medical Association, 295(17), 2018–2026. 10.1001/jama.295.17.2018 [DOI] [PubMed] [Google Scholar]
- Ottenbacher A. J., Snih S., Al Bindawas S. M., Markides K. S., Graham J. E., Samper-Ternent R., Raji M. A., Ottenbacher K. J. (2014). Role of physical activity in reducing cognitive decline in older mexican-american adults. Journal of the American Geriatrics Society, 62(9), 1786–1791. 10.1111/jgs.12978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottenbacher K. J., Graham J. E., Al Snih S., Raji M., Samper-Ternent R., Ostir G. V., Markides K. S. (2009). Mexican Americans and frailty: Findings from the hispanic established populations epidemiologic studies of the elderly. American Journal of Public Health, 99(4), 673–679. 10.2105/AJPH.2008.143958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panas L. J., Siordia C., Angel R. J., Eschbach K., Markides K. S. (2013). Physical performance and short-term mortality in very old Mexican Americans. Experimental Aging Research, 39(5), 481–492. 10.1080/0361073X.2013.839021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel K. V., Phelan E. A., Leveille S. G., Lamb S. E., Missikpode C., Wallace R. B., Guralnik J. M., Turk D. C. (2014). High prevalence of falls, fear of falling, and impaired balance in older adults with pain in the United States: Findings from the 2011 National Health and Aging Trends Study. Journal of the American Geriatrics Society, 62(10), 1844–1852. 10.1111/jgs.13072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavasini R., Guralnik J., Brown J. C., di Bari M., Cesari M., Landi F., Vaes B., Legrand D., Verghese J., Wang C., Stenholm S., Ferrucci L., Lai J. C., Bartés A. A., Espaulella J., Ferrer M., Lim J.-Y., Ensrud K. E., Cawthon P. M., . . . Campo G. (2016). Short physical performance battery and all-cause mortality: Systematic review and meta-analysis. BMC Medicine, 14(1), 1–9. 10.1186/s12916-016-0763-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff L. S. (1977). The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1(3), 385–401. 10.1177/014662167700100306 [DOI] [Google Scholar]
- Raji M. A., Al Snih S., Ostir G. V., Markides K. S., Ottenbacher K. J. (2010). Cognitive status and future risk of frailty in older Mexican Americans. Journals of Gerontology - Series A Biological Sciences and Medical Sciences, 65 A(11), 1228–1234. 10.1093/gerona/glq121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raji M. A., Al Snih S., Ray L. A., Patel K. V., Markides K. S. (2004). Cognitive status and incident disability in older Mexican Americans: Findings from the Hispanic established population for the epidemiological study of the elderly. Ethnicity and Disease, 14(1), 26–31. [PubMed] [Google Scholar]
- Raji M. A., Kuo Y. F., Al Snih S., Markides K. S., Peek M. K., Ottenbacher K. J. (2005). Cognitive status, muscle strength, and subsequent disability in older Mexican Americans. Journal of the American Geriatrics Society, 53(9), 1462–1468. 10.1111/j.1532-5415.2005.53457.x [DOI] [PubMed] [Google Scholar]
- Raji M. A., Ostir G. V., Markides K. S., Goodwin J. S. (2002). The interaction of cognitive and emotional status on subsequent physical functioning in older Mexican Americans: Findings from the hispanic established population for the epidemiologic study of the elderly. Journals of Gerontology - Series A Biological Sciences and Medical Sciences, 57(10), M678–M682. 10.1093/gerona/57.10.M678 [DOI] [PubMed] [Google Scholar]
- Ramirez M., Teresi J. A., Holmes D., Gurland B., Lantigua R. (2006). Differential item functioning (DIF) and the Mini-Mental State Examination (MMSE): Overview, sample, and issues of translation. Medical Care, 44(11 Suppl. 3), S95–S106. 10.1097/01.mlr.0000245181.96133.db [DOI] [PubMed] [Google Scholar]
- Rolland Y., van Kan G. A., Nourhashemi F., Andrieu S., Cantet C., Guyonnet-Gillette S., Vellas B. (2009). An abnormal “One-leg Balance” test predicts cognitive decline during Alzheimer’s disease. Journal of Alzheimer’s Disease, 16(3), 525–531. 10.3233/JAD-2009-0987 [DOI] [PubMed] [Google Scholar]
- Rosso A. L., Verghese J., Metti A. L., Boudreau R. M., Aizenstein H. J., Kritchevsky S., Harris T., Yaffe K., Satterfield S., Studenski S., Rosano C. (2017). Slowing gait and risk for cognitive impairment. Neurology, 89(4), 336–342. 10.1212/WNL.0000000000004153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford B. R., Taylor W. D., Brown P. J., Sneed J. R., Roose S. P. (2016). Biological aging and the future of geriatric psychiatry. Journals of Gerontology - Series A Biological Sciences and Medical Sciences, 72(3), 343–352. 10.1093/gerona/glw241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan C., Bauman K. (2016). Educational attainment in the United States: 2015. Current Population Reports, 20, 1–11. [Google Scholar]
- Samper-Ternent R., Al Snih S., Raji M. A., Markides K. S., Ottenbacher K. J. (2009). Relationship between frailty and cognitive decline in older Mexican Americans. Journal of the American Geriatrics Society, 56(10), 1845–1852. 10.1111/j.1532-5415.2008.01947.x.Relationship [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shubert T. E., Schrodt L. A., Mercer V. S., Busby-Whitehead J., Giuliani C. A. (2006). Are scores on balance screening tests associated with mobility in older adults? Journal of Geriatric Physical Therapy (2001), 29(1), 35–39. [PubMed] [Google Scholar]
- Sorond F. A., Cruz-Almeida Y., Clark D. J., Viswanathan A., Scherzer C. R., De Jager P., Csiszar A., Laurienti P. J., Hausdorff J. M., Chen W. G., Ferrucci L., Rosano C., Studenski S. A., Black S. E., Lipsitz L. A. (2015). Aging, the central nervous system, and mobility in older adults: Neural mechanisms of mobility impairment. Journals of Gerontology - Series A Biological Sciences and Medical Sciences, 70(12), 1526–1532. 10.1093/gerona/glv130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer R. J., Wendell C. R., Giggey P. P., Katzel L. I., Lefkowitz D. M., Siegel E. L., Waldstein S. R. (2013). Psychometric limitations of the mini-mental state examination among nondemented older adults: An evaluation of neurocognitive and magnetic resonance imaging correlates. Experimental Aging Research, 39(4), 382–397. 10.1080/0361073X.2013.808109 [DOI] [PubMed] [Google Scholar]
- Tombaugh T. N., McIntyre N. J. (1992). The Mini-Mental State Examination: A comprehensive review. Journal of the American Geriatrics Society, 40(9), 922–935. 10.1111/j.1532-5415.1992.tb01992.x [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau. (2012). Resident Population by Race, Hispanic Origin, and Age: 2000 and 2009. https://www2.census.gov/library/publications/2010/compendia/statab/130ed/tables/11s0009.pdf
- Verghese J., Kowal P., Bennett D. A. (2015). Author response. Neurology, 85(4), 389 10.1212/01.wnl.0000470376.04336.ea [DOI] [PubMed] [Google Scholar]
- Verghese J., LeValley A., Hall C. B., Katz M. J., Ambrose A. F., Lipton R. B. (2006). Epidemiology of gait disorders in community-residing older adults. Journal of the American Geriatrics Society, 54(2), 255–261. 10.1111/j.1532-5415.2005.00580.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese J., Wang C., Lipton R. B., Holtzer R. (2013). Motoric cognitive risk syndrome and the risk of dementia. Journals of Gerontology - Series A Biological Sciences and Medical Sciences, 68(4), 412–418. 10.1093/gerona/gls191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veronese N., Stubbs B., Trevisan C., Bolzetta F., De Rui M., Solmi M., Sartori L., Musacchio E., Zambon S., Perissinotto E., Crepaldi G., Manzato E., Sergi G. (2016). What physical performance measures predict incident cognitive decline among intact older adults? A 4.4 year follow up study. Experimental Gerontology, 81, 110–118. 10.1016/j.exger.2016.05.008 [DOI] [PubMed] [Google Scholar]
- Veronese N., Stubbs B., Volpato S., Zuliani G., Maggi S., Cesari M., Lipnicki D. M., Smith L., Schofield P., Firth J., Vancampfort D., Koyanagi A., Pilotto A., Cereda E. (2018). Association between gait speed with mortality, cardiovascular disease and cancer: A systematic review and meta-analysis of prospective cohort studies. Journal of the American Medical Directors Association, 19(11), 981–988.e7. 10.1016/j.jamda.2018.06.007 [DOI] [PubMed] [Google Scholar]
- West L. A., Cole S., Goodkind D., He W. (2014). 65+ in the United States. Social Research. U.S. Census Bureau. [Google Scholar]
- Wilson R. S., Beckett L. A., Barnes L. L., Schneider J. A., Bach J., Evans D. A., Bennett D. A. (2002). Individual differences in rates of change in cognitive abilities of older persons. Psychology and Aging, 17(2), 179–193. 10.1037/0882-7974.17.2.179 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_Table_S1 for Mobility but Not Balance Limitations Are Associated With Cognitive Decline among Older Hispanics by Paul A. Wadsworth, Nai-Wei Chen, Mukaila Raji, Kyriakos S. Markides and Brian Downer in Gerontology and Geriatric Medicine
Supplemental material, Supplementary_Table_S2 for Mobility but Not Balance Limitations Are Associated With Cognitive Decline among Older Hispanics by Paul A. Wadsworth, Nai-Wei Chen, Mukaila Raji, Kyriakos S. Markides and Brian Downer in Gerontology and Geriatric Medicine