Abstract
Sexual dimorphism in body size, aggression, and dispersal patterns may affect the degree to which males and females perceive aggression from either sex as stressful. Whereas male macaques typically disperse to new groups at maturity, thus encountering many unfamiliar individuals of both sexes, females are philopatric, usually only encountering unfamiliar males who transfer into their natal groups. In rare circumstances, however, group fusions can expose both males and females to many novel individuals, which often increases aggression. Here, we use a captive new group formation of rhesus macaques (Macaca mulatta) as a model of social instability during fusions and examine differences in male and female chronic stress responses to male-pattern and female-pattern trauma (i.e., trauma inflicted by males or by females, respectively). We found that male- but not female-pattern traumas predicted hair cortisol concentrations during the first nine months after new group formation, but in opposite ways for males and females. A greater number of male-pattern traumas was linked to elevated hair cortisol concentrations in females but slightly lower hair cortisol concentrations in males. We suggest that the apparent importance of male-pattern trauma, but not female-pattern-trauma, in predicting higher hair cortisol concentrations in females can be attributed to the more acutely intense but less persistent nature of male aggression toward females.
Keywords: group fusion, wounding, trauma, social instability, cercopithecine primates
Introduction
Most cercopithecine primates live in stable multi-female groups in which the females typically remain in their natal groups throughout their lives whereas males disperse at sexual maturity (Moore 1984; Pusey and Packer 1987). As a result of their different life histories, males, but not females, are guaranteed to experience a change in their social environment at least once in their lives. However, social change can occur for females, too, such as when social relationships change (Ehardt and Bernstein 1986, Engh et al. 2006), large numbers of females mature at once (Samuels and Henrickson 1983), or a group fuses with another (Jaffe and Isbell 2010). Under such conditions, aggression and physiological stress may increase. For example, in wild vervet monkeys (Chlorocebus pygerythrus) undergoing group fusion, agonistic interactions between adult females increased immediately and then declined as individuals sorted out their positions in the dominance hierarchy (Jaffe and Isbell 2010), and in captive rhesus macaques (Macaca mulatta), trauma and diarrhea were associated with uncertainty in dominance relationships (Beisner et al. 2014). Aggression and physiological stress may also be exacerbated in captive environments because animals cannot easily escape aggressors, and management decisions to remove individuals, introduce unfamiliar individuals, or combine groups affect the social environment (Brent et al. 1997; Clarke et al. 1996). Importantly, these captive situations differ from social changes in wild groups in that captive individuals do not make their own decisions about whether to join or leave groups. However, if we are interested in the evolved responses of individuals to changes in their social milieu, then removing individuals, introducing individuals, or combining groups in captivity may elicit similar responses to those that evolved as responses to emigration or death, immigration, or group fusions, respectively.
Social instability is presumably stressful. One reason for this is that aggressive social interactions become more common and may lead to physical trauma (Wooddell et al. 2016). How stressful this situation is for an individual might depend on both the severity of trauma they are likely to experience from an aggressive encounter and whether aggressive interactions are chronic or transient. In cercopithecines, female-female aggression can take the form of ongoing harassment of particular individuals or matrilines in both socially stable and unstable conditions (Aureli et al 1992, Gygax et al. 1997). Rates of aggression between unrelated females may further increase under the higher density conditions of captivity (Judge and de Waal 1997) and during periods of social instability (Bernstein and Gordon 1974). Thus, when females experience aggression from other females, particularly in captivity and during periods of social instability, this may be a chronic source of stress. For males, aggression from females may be an acute stressor, though likely not as severe as male aggression toward females because, with their heavier bodies and larger canines, males pose a threat to female aggressors.
Whereas females might experience chronic stress due to aggression from other females, more severe acute stress might result from male aggression. Cercopithecines are typically sexually dimorphic in both body size and canine size (e.g., rhesus macaques: Feeroz et al. 2010). Because males are larger, have longer canines, and can therefore cause more serious trauma than females, even a transient bout of aggression from a male toward a female might cause more physiological stress than repeated aggression from a female would. In contrast, male aggression toward males may be a chronic stressor, as males direct most of their aggression toward each other (Kulik et al. 2015) and ascendance in the male dominance hierarchy can be achieved and maintained through repeated aggression (Georgiev et al. 2016, Higham and Maestripieri 2010). Thus, males might be expected to experience aggression from other males as more stressful than aggression from females, because the former is both more severe and more frequent.
Rhesus macaques are typical cercopithecines in that they live in large nepotistic social groups characterized by obvious dominance hierarchies in both males and females (Thierry 2004). Dominance relationships are established and reinforced through frequent agonism (Bernstein and Gordon 1974, Bernstein and Ehardt 1985). Although most such interactions are merely threats, contact aggression is still common, particularly in captivity (Southwick 1969), and in groups undergoing periods of social instability (Ehardt and Bernstein 1986, Gygax et al. 1997). The sexes are dimorphic in body size, with adult males weighing 1.1-1.6 times that of adult females (Feeroz et al. 2010). Physical trauma differs depending on the sex of the aggressor, with male-inflicted injuries often involving tooth punctures and severe lacerations (male-pattern traumas) and female-inflicted injuries often involving crushing trauma to extremities, bruising, and superficial lacerations (female-pattern traumas) (Bohm and Gilbert 2012).
Here, we examine physical trauma and its effects on hair cortisol concentrations in captive, group-housed rhesus macaques for nine months before and after a new group was formed at the California National Primate Research Center (CNPRC). New group formations simulate the social instability created by group fusions in the wild. We examine whether the severity and persistence of social stressors alter the effects of those stressors on hair cortisol. We focus on physical trauma because it reflects an extreme end of social aggression that would be expected to elicit a stress response in the recipient. Since high-ranking individuals have more to lose in periods of social instability and severe aggression may be associated with uncertainty in dominance relationships, we also examine whether sex-specific traumas are correlated with lower ranks and lower dominance certainty at nine months after group formation.
First, we predict that male-pattern trauma will lead to higher hair cortisol concentrations in both males and females, with females experiencing a greater effect because they are at a disadvantage in agonistic encounters with males. Second, we predict that female-pattern trauma will also lead to higher hair cortisol concentrations in both males and females, with females experiencing a greater effect because female-female aggression can be persistent and female aggression poses less of a threat to males than to other females. Third, we predict that higher-ranking individuals will have higher hair cortisol concentrations than lower-ranking individuals, as previous studies have found in some cercopithecines during periods of social instability (Higham et al. 2013, Sapolsky 1990). Finally, we predict that those with less certainty in their dominance relationships will have higher hair cortisol than those with greater dominance certainty, because uncertainty about one’s dominance relationships is presumably a stressor.
Methods
Study site and subjects
The study focused on a new social group (NC21-B) formed at the CNPRC in their outdoor breeding colony on 7 May, 2012. The group was housed in a 0.2 ha field cage that contained multiple perches, swings, and A-frames for protection from rain and wind. The new group initially comprised 111 individuals. Behavioral management staff monitored the new group, and sometimes temporarily or permanently removed individuals from the field cage for medical treatment or management purposes. Data on individuals’ ages and housing histories were obtained from CNPRC records. Individuals placed in the new group came from matrilines from an established large outdoor social group, several small outdoor-housed peer groups, and several indoor-housed animals. Individuals included in the study (n = 49 individuals, 39 females) ranged in age from 0.75 to 4.9 years, with a mean of 2.2 years. Our sample includes all individuals present at hair sample collections at baseline and at nine months after group formation, excluding one very old female because she was an outlier for age.
We collected hair samples from the study subjects on 15 May 2012, one week after the new group was formed, and again on 12 February 2013, approximately nine months after the initial group formation, during routine health checks conducted by CNPRC staff. Management staff sedated animals using weight-specific doses of ketamine and shaved hair from the inner thigh of each individual present in the enclosure. We placed each sample in an aluminum foil pouch and labeled the samples with the individual’s identification number and the date of collection. Foil pouches were sealed and stored at room temperature until laboratory processing for assay.
Hair cortisol processing
Hair samples were processed for cortisol following a protocol modified from Davenport et al. (2006). Briefly, hair samples were weighed, washed twice in isopropanol, dried, and ground in a Retsch Ball Mill. We extracted hormones from the powdered hair using methanol, dried the samples under a stream of air, and reconstituted the extracts in 0.4 ml of diluent using buffer solution from commercially available assay kits (Salimetrics, State College, PA). We stored samples in a freezer at -80 degrees Celsius until assay for cortisol concentrations. For more details, see Linden et al. (in press) and Vandeleest et al. (2019). Hair cortisol concentrations from the assay were converted to pg cortisol/mg hair for statistical analyses.
Trauma and health data collection
We extracted trauma and health data for 9-month periods before individuals were moved for group formation (8 August 2011-6 May 2012) and after group formation (7 May 2012-12 February 2013) from health records maintained by management and veterinary staff at CNPRC. We scaled severity of wounding from 0 to 5, with 0 indicating no wounding and 5 representing the most severe injuries, for both Male-Pattern Traumas and Female-Pattern Traumas (R. Samak and D. Hannibal, unpub. data; Table S1). We included all hospitalizations during the 9-month period and any injuries treated during the health check at which hair samples were collected.
Statistical analyses
We compared rates of hospitalization with female- and male-pattern traumas before and after new group formation with paired Wilcoxon signed-ranks tests, examining males and females separately (Table S2, Supplementary Materials). Since we were interested in socially inflicted trauma, we included only socially housed individuals in the sample before group formation (n = 45 individuals, 37 females).
We used network analysis to calculate rank and dominance certainty. First, we constructed a win-loss matrix based on dyadic interactions involving aggressive and submissive behaviors from data collected routinely by a single behavioral management staff member between 6 July 2012 and 5 February 2013 using event sampling. For each interaction, the observer recorded the individual identification number of the initiator and the recipient and noted all behaviors from Table S3 (Supplementary Materials) that were exhibited during the interaction. Then, with the Perc package for R (Fujii et al., 2015), we used indirect pathways to calculate imputed dominance probabilities for relationship between each pair in the network, ranging from 0 (complete certainty that the row individual is subordinate to the column individual) to 1 (complete certainty that the row individual is dominant to the column individual), with 0.5 representing complete uncertainty about the dominance relationship. The Perc package also identified the best rank order based on the imputed dominance probability matrix, using simulated annealing runs that compare costs of various rank orders. These methods are described in greater detail by Fushing et al. (2011). For analyses, Rank is the proportion of individuals in the group that the focal individual outranks, such that the highest-ranked individual has a value of 1 and the lowest-ranked has a value of 0. We calculated each individual’s average dominance probability to obtain its Dominance Certainty score (see Vandeleest et al. 2016). This score, ranging from 0.5 (maximally uncertain relationships) to 1.0 (maximally certain relationships), provides a metric for each individual’s general certainty in dominance relationships, regardless of whether it is dominant or subordinate.
We analyzed hair cortisol at baseline and hair cortisol nine months after group formation using models fitted in R (R Core Team 2016). We fitted multi-level models to hair cortisol data with varying intercepts for the subgroup in which the individual had previously been housed using the Bayesian map2stan function from the rethinking package (McElreath 2016a), which estimates parameters in the model using Markov Chain Monte Carlo algorithms implemented in RStan (Stan Development Team 2017). We used regularizing (“weakly informative”) priors, which reduce overfitting (McElreath 2016b). This approach allows better estimation of varying effects via pooling of information across clusters and is more robust for small sample sizes than traditional frequentist multi-level models (Gelman and Hill 2007; McElreath 2016b). Models were fitted to examine several possible influences, including age, sex, sex-specific traumas, and hospitalizations (Table 1). No males experienced Female-Pattern Trauma during the baseline period, so we could not include an interaction with Sex in models of Female-Pattern Trauma. We used an information theoretic approach to compare models, calculating WAIC scores and weights (McElreath 2016b, Gelman et al. 2014). By comparing several models to a null model including only varying effects for Subgroup (Q0; Table 1), we were able to ask several questions. We first asked whether hair cortisol varies by age and sex classes (Q1; Table 1). The other models asked whether hospitalization (Q2a; Table 1), male-pattern trauma (Q3a; Table 1), or female-pattern trauma (Q4a; Table 1) influence hair cortisol beyond the effects of age and sex, and if so, whether those effects are different for males and females (Q2b, Q3b, and Q4b; Table 1). By examining these questions under baseline conditions as well as the period following the new group formation, we were able to determine whether our results apply generally or are specific to periods of social instability.
Table 1.
Questions and models tested for Baseline and 9-month Hair Cortisol.
| Model | Question | Variables |
|---|---|---|
| Q0 | Null model | [Varying effects for Subgroup, also included in all models below] |
| Q1 | Does hair cortisol differ by age and sex class? | Y = Age + Sex |
| Q2a | Does Hospitalization influence hair cortisol beyond the effects of age and sex? | Y = Age + Sex + Hosp |
| Q2b | Does the impact of Hospitalization differ for males and females? | Y = Age + Sex + Hosp + Sex*Hosp |
| Q3a | Does Male-Pattern Trauma influence hair cortisol beyond the effects of age and sex? | Y = Age + Sex + MPT |
| Q3b | Does the impact of Male-Pattern Trauma differ for males and females? | Y = Age + Sex + MPT + Sex*MPT |
| Q4a | Does Female-Pattern Trauma influence hair cortisol beyond the effects of age and sex? | Y = Age + Sex + FPT |
| Q4b | Does the impact of Female-Pattern Trauma differ for males and females? | Y = Age + Sex + FPT + Sex*FPT |
Using the subset of individuals for which we had data on dominance interactions (n=41) we fitted linear regression models with varying effects for Subgroup to the 9-month Hair Cortisol using Age, Sex, Rank, and Dominance Certainty as predictors, and allowing interactions. We compared models containing subsets of these predictors, allowing up to three predictors, using WAIC. We included a model with just Age and Sex as predictors to assess whether effects of Rank or Dominance Certainty improved prediction over this model. We generated figures with estimated means and 95% credible intervals also using the rethinking package in R.
Results
Rates of hospitalizations and traumas
Rates of hospitalization, male-pattern trauma, and female-pattern trauma increased for both males and females after the new group formation. These rates did not increase equally for both sexes, however. Hospitalizations of females increased (0.89 traumas/individual before to 1.0 after) to a lesser degree than hospitalizations of males (0.5 hospitalizations/individual before to 1.5 after). Among females, female-pattern traumas increased only slightly (0.35 traumas/female before to 0.38 after), whereas male-pattern traumas showed a more marked increase (0.30 traumas/female before to 0.49 after). Males experienced no female-pattern traumas before the new group formation, but increased to a rate of 0.5 traumas/male after new group formation. Male-pattern traumas experienced by males increased from 0.13 traumas/male before to 0.40 after the new group formation. There were no significant differences between counts before and after for either sex, possibly due to the low numbers of events per individual (hospitalization count for females: p = 0.69; hospitalization count for males: p = 0.10; male-pattern trauma count for females: p = 0.36; male-pattern trauma count for males: p = 0.37; female-pattern trauma count for females: p = 0.92; female-pattern trauma count for males: p = 0.37).
Effects of age and sex on hair cortisol prior to group formation (baseline)
The model that best balanced fit and parsimony in predicting hair cortisol concentrations before the group formation included only Age and Sex (Q1; Table 1). This model had the lowest WAIC score. In this model, older individuals had significantly lower hair cortisol concentrations than did younger individuals, and females had significantly lower hair cortisol concentrations than did males (Table S4).
Effects of age, sex, and male-pattern trauma on hair cortisol following group formation
The model that best balanced fit and parsimony in predicting hair cortisol concentrations after the group formation included Age, Sex, Male-Pattern Trauma, and an interaction between Sex and Male-Pattern Trauma (model Q3b; Table 1). We note that our sample included only 10 males. Baseline patterns remained in that older individuals had lower hair cortisol concentrations than did younger individuals, and females had lower hair cortisol concentrations than did males. In addition, an interaction between Sex and Male-Pattern Trauma appeared. Experiencing more male-pattern traumas during the period after group formation led to higher hair cortisol concentrations in females but lower concentrations in males (Fig. 1). We identified three potential outliers (1 female, 2 males) with high cortisol concentrations which could have driven this relationship, and refitted the models excluding them (Table 2) but model Q3b remained the highest-ranked model with Age, Sex, and Male-Pattern Trauma continuing their influences.
Figure 1.
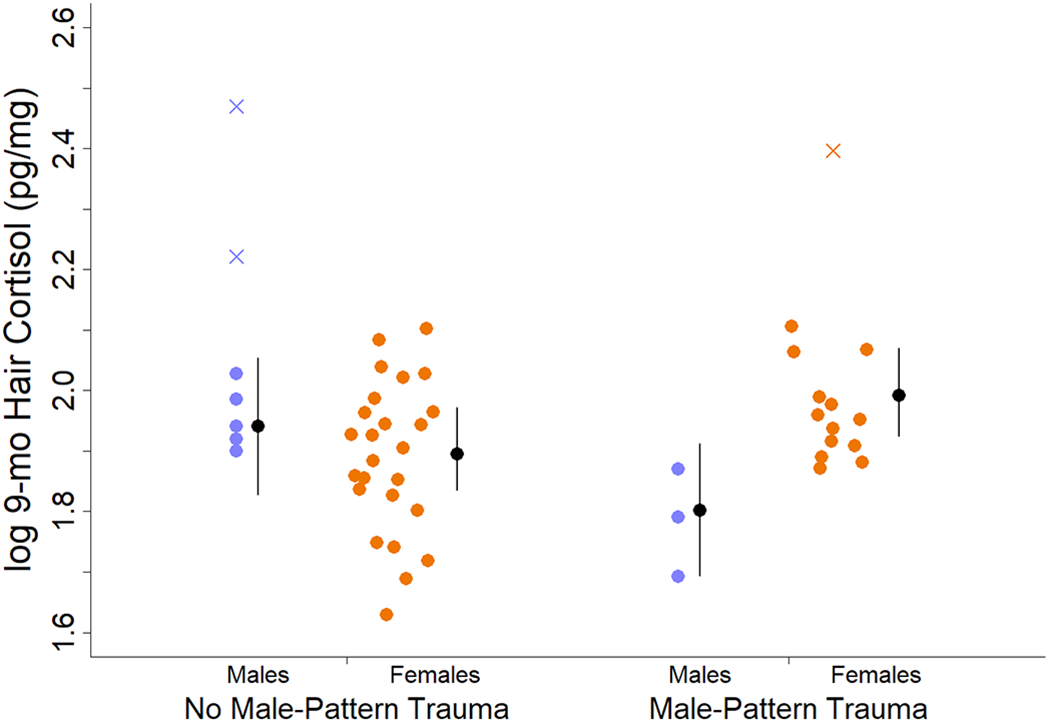
Log 9-month Hair Cortisol concentrations relative to Male-Pattern Trauma. Blue circles indicate data for males, orange circles indicate data for females, jittered on the x-axis to avoid overlapping points. X marks indicate data points for outliers excluded from analysis. Black circles indicate estimated mean Hair Cortisol concentrations. Black lines indicate 95% credible interval for mean.
Table 2.
Model coefficients and standard errors for 9-month Hair Cortisol, excluding outliers.
| Q3b coeff (SE) | Q0 coeff (SE) | Q4a coeff (SE) | Q3a coeff (SE) | Q1 coeff (SE) | Q4b coeff (SE) | Q2b coeff (SE) | Q2a coeff (SE) | |
|---|---|---|---|---|---|---|---|---|
| WAIC | −70.8 | −66.8 | −65.1 | −64.1 | −63.9 | −63.9 | −62.8 | −61.7 |
| dWAIC | 0 | 4.0 | 5.7 | 6.7 | 6.9 | 6.9 | 8.0 | 9.1 |
| Weight | 0.76 | 0.10 | 0.04 | 0.03 | 0.02 | 0.02 | 0.01 | 0.01 |
| Intercept | 2.01 (0.06) | 1.91 (0.02) | 1.93 (0.06) | 1.94 (0.06) | 1.94 (0.06) | 2.08 (0.07) | 2.02 (0.07) | 1.94 (0.06) |
| Age | −0.01 (0.003) | −0.07 (0.04) | −0.01 (0.01) | −0.01 (0.01) | −0.01 (0.01) | −0.01 (0.01) | −0.01 (0.01) | |
| Sex | −0.05 (0.05) | −0.03 (0.05) | −0.04 (0.05) | −0.03 (0.05) | −0.05 (0.07) | −0.07 (0.07) | 0.03 (0.05) | |
| Hospitalization | −0.14 (0.09) | 0.002 (0.04) | ||||||
| Male-Pattern Trauma | −0.15 (0.09) | 0.06 (0.05) | ||||||
| Female-Pattern Trauma | −0.07 (0.04) | −0.19 (0.12) | ||||||
| Hospitalization * Sex | 0.16 (0.09) | |||||||
| Male-Pattern Trauma*Sex | 0.24 (0.09) | |||||||
| Female-Pattern Trauma*Sex | 0.17 (0.13) |
To examine whether this result was driven by greater severity of traumas in females than males, we fitted an ordered categorical model with a cumulative probability link to the maximum severity scores of individuals who experienced male-pattern trauma (n=18), using the rethinking package in R (McElreath 2016a). This method estimated the probability of receiving a maximum severity score of k or lower, for k=1 to 3 (the highest maximum severity experienced by individuals in our sample during this period). We found, however, that females who suffered male-pattern traumas had significantly lower severity trauma scores than did males (ɑcutoff1 = −7.19±3.40; ɑcutoff2 = −0.62±1.28; ßsex = −7.94±3.40, p=0.03).
Our finding that females suffered lower-severity male-pattern traumas comes despite the systematic removal of males who suffered severe trauma. Fitting a logistic regression model to data on individuals who were removed temporarily or permanently from the group (n=158 removal events of 96 unique individuals), we found that males removed for treatment because they experienced trauma were more likely to be permanently removed from the group than were females, who were more likely to be returned to the group (ɑ = -2.33±0.37; ßsex = 1.06±0.50, p=0.035). This reveals that females still suffered less severe male-pattern traumas than the males remaining in the group.
Effects of age and dominance status on hair cortisol nine months after group formation
Both Rank and Dominance Certainty were important predictors of hair cortisol in our models. Of the six highest weighted models (accounting for 81% of model weight), five included Rank and two included Dominance Certainty (Table 3). However, the highest weighted model included Age as well as Rank and Dominance Certainty. Younger animals, those with lower rank, and those with greater dominance certainty had higher hair cortisol concentrations (Fig. 2).
Table 3.
Model coefficients and standard errors for 9-month Hair Cortisol by age, sex, rank and dominance probability.
| Model | WAIC | dWAIC | Weight | Intercept | Age (SE) | Sex (SE) | Percent Outranked (SE) | Dominance Certainty (SE) | interaction |
|---|---|---|---|---|---|---|---|---|---|
| Age + Rank + Dominance Certainty | −33.5 | 0 | 0.42 | 1.75 (0.20) | −0.019 (0.008) | −0.21 (0.10) | 0.66 (0.33) | ||
| Age + Rank | −31.0 | 2.5 | 0.12 | 2.11 (0.08) | −0.012 (0.007) | −0.12 (0.10) | |||
| Age + Rank + Age*Rank | −30.5 | 3.0 | 0.09 | 2.23 (0.12) | −0.027 (0.015) | −0.33 (0.21) | 0.025 (0.022) | ||
| Age + Dominance Certainty | −30.2 | 3.2 | 0.08 | 1.91 (0.20) | −0.02 (0.008) | 0.30 (0.32) | |||
| Rank | −29.4 | 4.1 | 0.05 | 2.04 (0.06) | −0.18 (0.09) | ||||
| Age + Sex + Rank | −29.0 | 4.5 | 0.04 | 2.12 (0.08) | −0.012 (0.008) | −0.009 (0.062) | −0.12 (0.10) | ||
| Age + Sex | −28.6 | 4.9 | 0.04 | 2.09 (0.08) | −0.015 (0.007) | −0.017 (0.062) | |||
| Rank + Dominance Certainty | −28.5 | 5.0 | 0.03 | 1.88 (0.21) | −0.23 (0.11) | 0.26 (0.33) | |||
| Age + Sex + Dominance Certainty | −28.4 | 5.1 | 0.03 | 1.90 (0.19) | −0.02 (0.009) | −0.018 (0.063) | 0.34 (0.30) | ||
| Sex + Rank | −27.4 | 6.1 | 0.02 | 2.05 (0.07) | −0.026 (0.062) | −0.16 (0.10) | |||
| Rank + Dominance Certainty + Rank*Dominance Certainty | −27.2 | 6.3 | 0.02 | 1.51 (0.53) | 0.43 (0.83) | 0.80 (0.79) | −0.93 (1.17) | ||
| Age + Dominance Certainty + Age*Dominance Certainty | −27.1 | 6.4 | 0.02 | 1.87 (0.37) | −0.030 (0.038) | 0.38 (0.56) | 0.011 (0.050) | ||
| Sex + Rank + Sex*Rank | −27.1 | 6.4 | 0.02 | 2.17 (0.11) | −0.16 (0.12) | −0.52 (0.26) | 0.40 (0.28) | ||
| Sex + Rank + Dominance Certainty | −26.3 | 7.2 | 0.01 | 1.89 (0.21) | −0.032 (0.062) | −0.21 (0.11) | 0.26 (0.33) | ||
| Dominance Certainty | −25.4 | 8.1 | 0.01 | 2.03 (0.21) | −0.11 (0.29) | ||||
| Sex + Dominance Certainty | −23.6 | 9.9 | 0 | 2.04 (0.22) | −0.045 (0.065) | −0.07 (0.31) | |||
| Sex + Dominance Certainty + Sex*Dominance Certainty | −21.3 | 12.2 | 0 | 2.11 (0.75) | −0.14 (0.78) | −0.19 (1.13) | 0.14 (1.17) |
N=41
Figure 2.
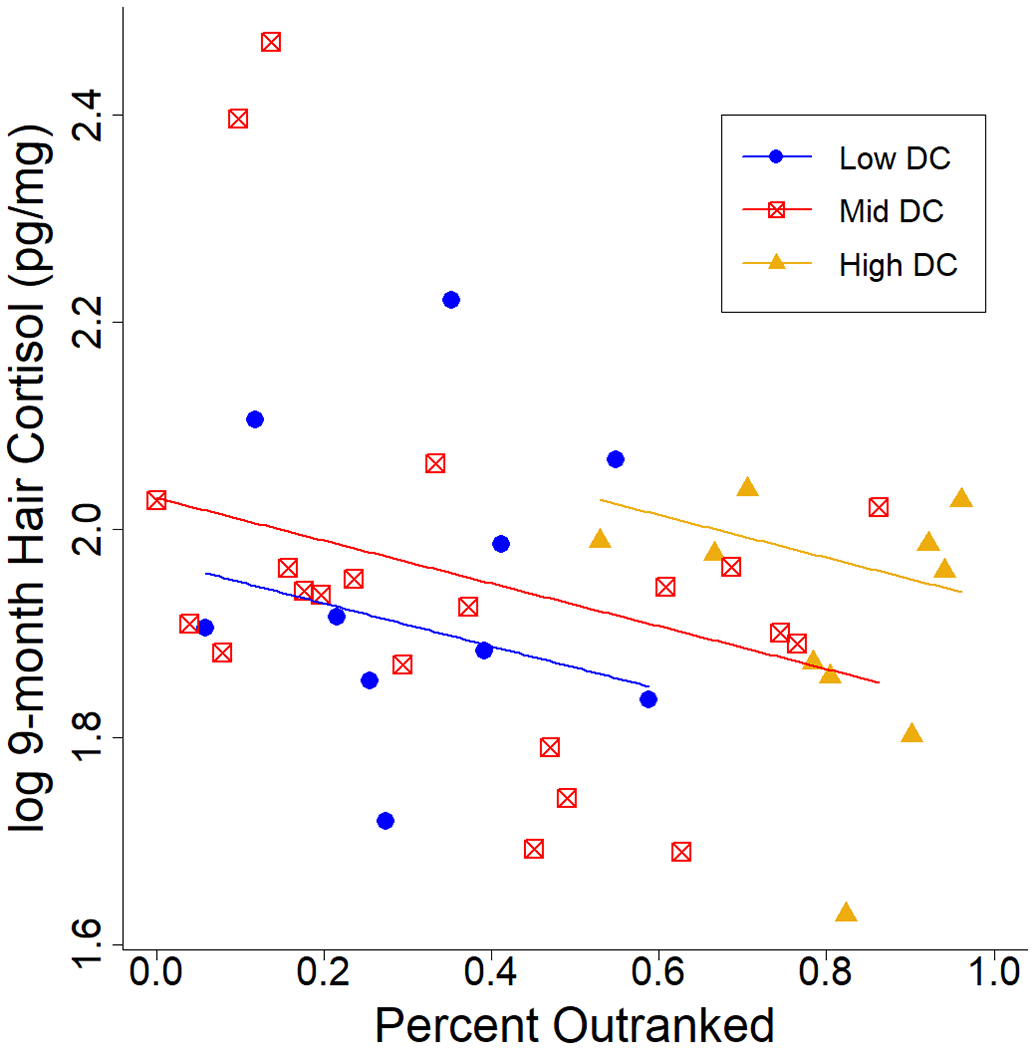
Log 9-month hair cortisol by rank for individuals of different dominance certainties, assuming mean age. Rank is shown here as Percent Outranked, a number between 0 and 1 such that the highest-ranking individual is 1.0 and the lowest-ranking is 0. Colors indicate dominance certainty for highest quartile (yellow), middle two quartiles (red), and lowest quartile (blue). Lines represent model-estimated mean hair cortisol concentrations for the mean dominance certainty in each dominance certainty group. Note that ages vary within each dominance certainty group and dominance certainty is shown here in categories for visualization purposes only.
Discussion
Our first prediction was that both males and females who experienced male-pattern trauma following the new group formation would have higher hair cortisol concentrations than those who did not, with this effect being stronger in females than in males. The results supported this prediction for females, but were opposite from expected for males. Second, we predicted that both males and females who experienced female-pattern trauma following new group formation would have higher hair cortisol concentrations than those who did not, with this effect being stronger in females than in males. Contrary to our predictions, female-pattern trauma was not important in predicting hair cortisol concentrations for either sex. Our third and fourth predictions were that we would see higher hair cortisol concentrations in higher-ranking individuals and those with less dominance certainty, respectively. Contrary to both predictions, more highly ranked individuals had lower hair cortisol concentrations, and those with greater dominance certainty had higher hair cortisol concentrations. We consider explanations for these results below.
Male-pattern trauma and hair cortisol concentrations
Our finding that females who experienced male-pattern trauma had higher hair cortisol concentrations than those who did not during the period of social instability suggests that the severity of injuries caused by male aggression makes that aggression particularly stressful for females. Because males are larger than females and can cause severe trauma, it is perhaps not surprising that females experiencing male-pattern trauma would exhibit the most extreme response in Hypothalamic-Pituitary-Adrenal (HPA) activity, although we note that our post-hoc analysis of trauma severity showed that females suffered less severe male-pattern traumas than males did. We did not find a relationship between male-pattern trauma and hair cortisol at baseline, suggesting that male aggression becomes particularly stressful for females during periods of social instability. Perhaps this is because under more stable conditions, females are better able to draw on social support to cope with stressors, for example, through grooming or proximity to social partners (Brent et al. 2011).
Males remaining in the group at nine months who experienced male-pattern traumas, including large lacerations that partially transected muscle tissue, had lower hair cortisol concentrations compared to those males not experiencing male-pattern traumas. Our sample size for males was limited, and this result should therefore be interpreted with caution. If our pattern of low hair cortisol concentrations for males experiencing male-pattern trauma does reflect a biological reality, we can think of three potential explanations: evolved mechanisms for dealing with social stressors, confounding effects of metabolically costly physical activity, or HPA axis down-regulation.
Evolved mechanisms for dealing with social stressors
Because male-male aggression (or the threat of aggression) is comparatively common in rhesus macaques, particularly during periods of social instability, male rhesus macaques may have evolved strategies that minimize stress experienced during these interactions (male takeovers: Neville 1968, Georgiev et al. 2016; intra-group aggression: Higham and Maestripieri 2010, Kulik et al. 2015, Southwick 1969). In contrast, females in the wild typically remain in their natal groups with female kin (Moore 1984; Pusey and Packer 1987), and, although they interact with unfamiliar males who occasionally emigrate into their natal group, they rarely interact with many unfamiliar males at once under such extreme social instability as occurs when a new group is formed in captivity. Therefore, females may lack coping mechanisms for experiencing frequent aggression from many unfamiliar males. While such evolved differences between males and females could explain why males do not react as strongly to male-pattern trauma as females do, the question remains why males who experienced male-pattern trauma actually have slightly lower hair cortisol concentrations than males who did not.
Confounding effects of costly metabolic activity
Males who avoid male-pattern trauma may do so through other metabolically costly behaviors, which are known to increase cortisol production (Sapolsky et al. 2000). For example, they might avoid trauma by running away, fighting back, or maintaining heightened vigilance. It could even be that the males who do not suffer male-pattern trauma are the ones causing the trauma in others, which would involve physical activity for the aggressor.
HPA axis down-regulation
Finally, because rhesus macaque males direct more aggression toward other males than toward females (Kulik et al. 2015), males who experience chronic stress due to aggression from other males may have suffered HPA axis dysregulation, leading to a reduction in cortisol production despite continued experience of stress. If male aggression toward females is more transient, then females would not experience this dysregulation, consistent with our observations. Although stress is associated with increased cortisol production as part of an adaptive fight-or-flight response, chronic stress can eventually lead to down-regulation of the HPA axis. Such HPA axis dysregulation has been demonstrated in humans with post-traumatic stress disorder, major depression, and other stress-related disorders (Heim et al. 2000; Pochigaeva et al. 2017; Steudte et al. 2013). In nonhuman primates, similar down-regulation of the HPA axis has been found in captive rhesus macaques under chronic stress (Capitanio et al. 1998), in socially subordinate female marmosets following a new group formation (Saltzman et al. 2006), and in individually-housed captive rhesus macaques with a history of self-inflicted bite wounds (Novak 2003). Another study reported that rhesus macaques who experienced early adversity via maternal separation had lower hair cortisol concentrations 1.5 years later (Feng et al. 2011). Thus, low cortisol concentrations do not necessarily indicate that the individual is not under intense stress; rather, they may indicate that the individual has been unable to cope with chronic stress, eventually resulting in dysregulation of the HPA axis (Linden et al. in press).
One argument against this explanation is that HPA axis down-regulation would only result after an initial period of chronic stress, during which individuals would still produce high concentrations of cortisol. This makes it unclear whether we should expect high or low cortisol concentrations in a long-term measure of HPA activity such as hair, even if chronic stress has led to down-regulation of cortisol production. Future studies could examine both short-term and long-term measures of cortisol, as well as tests of HPA axis regulation such as Dexamethasone Suppression tests, over a period of social instability to test whether some individuals are experiencing HPA axis down-regulation. It is also possible that HPA axis dysregulation preceded the group formation, and individuals with dysregulated HPA axes were disproportionately recipients of trauma. For example, certain personality traits might lead to HPA axis dysregulation in periods of chronic stress (Linden et al. in press), while also predisposing individuals to socially inflicted trauma. Only some of the individuals in our group had been assessed for temperament, which precluded examining both personality and trauma in a single model. Studies could address this in the future by thoroughly assessing temperament of all individuals prior to a new group formation.
Unfortunately, we do not have behavioral data about physical activity or hormonal data about HPA axis regulation for this group during the time period of the study. Future research could include such data to account for the effects of physical activity on HPA axis activity and examine alterations in HPA axis regulation before and after a new group formation. As a final point, our sample consisted primarily of females, and perhaps our sample size for males was too small to detect broader patterns in male responses to male-pattern trauma. Although it is typical for both wild and naturalistic captive groups to consist of more females than males, future studies could examine multiple group formations to increase the sample size of males.
Female-pattern trauma and hair cortisol concentrations in both sexes
We found no relationship between female-pattern trauma and hair cortisol concentrations for either sex. This was surprising, as such trauma could be severe. The majority of individuals who suffered female-pattern trauma (9 of 15) had such severe injuries to extremities that fingertips or tail tips had to be amputated. We offer several explanations similar to those offered above for our male-pattern trauma results. Female-female aggression is common in rhesus macaques, particularly during periods of social instability or when unfamiliar females interact (Bernstein and Gordon 1974, Hausfater 1972, Payne et al. 2003, Wooddell et al. 2016). Perhaps evolved mechanisms for dealing with similarly high rates of female aggression under naturalistic conditions allowed individuals in our sample to cope with the trauma they experienced without adverse physiological effects. Alternatively, perhaps individuals who did not suffer female-pattern trauma did so through metabolically costly behaviors (e.g., running, vigilance) that led to increased cortisol concentrations equivalent to those seen in injured individuals, or female aggression did greatly intensify, even if actual rates of trauma did not, leading to HPA axis dysregulation in some individuals. Studies incorporating behavioral data on individual activity levels and measures of HPA axis regulation could test these three potential explanations.
Rank and dominance certainty
We also found that age and rank, and, to a lesser extent, dominance certainty, affect 9-month hair cortisol. Our results are consistent with the known decline of cortisol with age (Gust et al. 2000). Interestingly, rank and dominance certainty are positively correlated (Pearson’s r = 0.59, p<0.01) in our sample, but when considering the two together, they had opposing effects: more highly ranked individuals had lower cortisol concentrations whereas those with greater dominance certainty had higher cortisol concentrations.
Previous work has suggested rank and social stability interact in their effects on cortisol production. Under socially stable conditions, lower-ranked cercopithecines tend to have higher cortisol concentrations than those of higher rank, but during periods of social instability this pattern can reverse (Gust et al. 1993, Sapolsky 1990). The interpretation for this pattern is that under stable conditions, high rank confers benefits in terms of reduced stress, but high-ranking individuals have more to lose during periods of social instability and therefore perceive the situation as more stressful. However, other studies have found more complex relationships between rank and cortisol. For example, one study of male rhesus macaques found no relationship between rank and cortisol except during periods of social instability, when more highly ranked males had higher cortisol concentrations (Higham et al. 2013). One complication is that even within the same social group, the degree of social instability can vary at different positions in the dominance hierarchy, influencing individuals’ cortisol production. Sapolsky (1992) found that male olive baboons’ (Papio anubis) basal cortisol concentrations depended on uncertainty in dominance relationships with males close to their own rank, and these effects differed depending on whether the uncertainty resulted from the individual rising or falling in rank. However, in chacma baboons (P. ursinus), females’ glucocorticoid concentrations increased during periods of instability but, unlike male olive baboons, their positions in the dominance hierarchy had no effect (Engh et al. 2006). Other studies of rank and cortisol have failed to find a clear relationship, though low-ranking individuals may be more variable in their cortisol concentrations (Bercovitch and Clarke 1995).
Our results did not match our predictions based on the most common patterns in other studies of cercopithecines. Our study took place during a period of social instability, following a new group formation, when high-ranking individuals would be expected to have higher cortisol concentrations. We found the opposite. However, we have only one timepoint for hair cortisol concentrations after the group formation, nine months later, and perhaps by then social instability had decreased to the point where high-ranking individuals were no longer at risk of losing their status. If the group was comparatively stable for the majority of the nine-month period following the group formation, our results may then be attributed to differences in the outcomes of aggressive interactions for high- and low-ranking individuals under stable social conditions. Under these conditions, individuals of high rank tend to benefit more from the formalized dominance hierarchy than those of low rank do (Maestripieri and Georgiev 2015). High-ranking individuals might be injured during aggressive interactions, but they “win” more of these interactions, which is why they attain high rank. Low-ranking individuals who are injured during aggressive encounters are also likely “losing” many of these interactions, which might mean they continue to receive harassment from higher-ranking individuals or have reduced access to resources such as food, space, or social partners. Future research could measure HPA activity repeatedly to document whether high-ranking individuals had higher hair cortisol concentrations when social instability was most intense, immediately following the new group formation. Alternatively, previous work has shown higher cortisol in low-ranking individuals during periods of social instability in species with high rates of displacement of aggression (Abbott et al. 2003). Future studies could examine this with data on rates of displacement of aggression in a newly formed group. We also found that individuals with greater dominance certainty had higher hair cortisol concentrations. As with our results for trauma, tests of HPA axis regulation might also shed light on these findings. If intense uncertainty in dominance relationships led to HPA axis down-regulation, for example, that would also explain the patterns we see here. Behavioral data on social interactions would also be useful. Perhaps individuals with lower dominance certainty avoid social interactions while those with greater dominance certainty engage more. That engagement could be physiologically stressful, leading to higher cortisol concentrations.
Future research should test the potential explanations we offer above by examining behavioral data on physical activity and HPA axis responsivity to suppression and activation before and throughout periods of social instability. If males have evolved coping mechanisms for male aggression that females lack or females perceive male aggression as more stressful than males do, both males and females should exhibit typical cortisol responses to dexamethasone suppression tests, regardless of their experience of male-pattern trauma. In this case, behavioral data coupled with repeated measures of cortisol concentrations could provide a clearer picture of how individuals of different age-sex classes respond to threats of aggression, in addition to actual injuries as examined here. If our findings here were confounded by metabolically costly behaviors, then behavioral data on physical activity should predict hair cortisol concentrations better than the experience of trauma does in future studies. If our findings reflect down-regulation of the HPA axis due to chronic stress for males experiencing male-pattern trauma, males but not females should exhibit an altered cortisol response to a dexamethasone suppression test, as in Capitanio et al. (1998). If down-regulation is due to previous experience of chronic stress, which both alters HPA activity and increases likelihood of experiencing male-pattern traumas, it should be evident prior to the period of instability as well. If the stress of the new group formation causes HPA axis down-regulation, the altered cortisol response should emerge during the period of social instability.
Conclusions
We found that male-pattern trauma led to greater hair cortisol concentrations in females, but not males, following a new group formation. We suggest that for females, male aggression is acutely severe. Our other results were unexpected and more difficult to explain. Throughout the discussion we have offered suggestions for how future research can disentangle the different hypotheses we offer using data on behavior, personality, and HPA axis regulation.
Supplementary Material
Acknowledgments:
This work was supported by two research grants to JBL from the Department of Anthropology at UC Davis and by a UC Davis Faculty Research Grant to LAI. The lab in which hair cortisol analysis was performed is supported by NIH R01HD068335 (McCowan).We thank the CNPRC staff who assisted in hair sample collection, those who collected and maintained colony records used in this study, E. Van Cleave, M. Tran, R. Montez, E. Rivera, L. Milgrom, H. Martyn, J. Hook, and M. Gelatos for help with hair processing, B. Beisner, J. Vandeleest, and D. Hannibal for helpful discussion, and H. Martin for assistance with statistical analyses. The CNPRC is funded by the base grant P51OD011157.
Footnotes
Compliance with Ethical Standards
The authors declare they have no conflict of interest. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution at which the studies were conducted. All procedures were approved by the Animal Care and Use Committee of the University of California, Davis. Hair sample collections were conducted under IACUC protocol #18090.
Literature Cited
- Abbott DH, Keverne EB, Bercovitch FB, Shively CA, Mendoza SP, Saltzman W, Snowdon CT, Ziegler TE, Banjevic M, Garland T, Sapolsky RM (2003) Are subordinates always stressed? A comparative analysis of rank differences in cortisol levels among primates. Horm Behav 43: 67–82. 10.1016/S0018-506X(02)00037-5 [DOI] [PubMed] [Google Scholar]
- Aureli F, Cozzolino R, Cordischi C, Scucchi S (1992) Kin-oriented redirection among Japanese macaques: an expression of a revenge system? Anim Behav 44: 283–291. 10.1016/0003-3472(92)90034-7 [DOI] [Google Scholar]
- Beisner BA, Vandeleest JJ, Hsieh F, Fujii K, McCowan B (2014) Mean dominance relationship certainty is better than rank at predicting diarrhea incidence and wounding in captive rhesus macaques (Macaca mulatta). Amer J Primatol 76(S1): 72 10.1002/ajp.22382 [DOI] [PubMed] [Google Scholar]
- Bercovitch FB, Clarke AS (1995) Dominance rank, cortisol concentrations, and reproductive maturation in male rhesus macaques. Physiol Behav 58: 215–221. 10.1016/0031-9384(95)00055-N [DOI] [PubMed] [Google Scholar]
- Bernstein IS, Ehardt CL (1985) Agonistic aiding: kinship, rank, age, and sex influences. Amer J Primatol 8: 37–52. 10.1002/ajp.1350080105 [DOI] [PubMed] [Google Scholar]
- Bernstein IS, Gordon TP (1974) The function of aggression in primate societies: uncontrolled aggression may threaten human survival, but aggression may be vital to the establishment and regulation of primate societies and sociality. Amer Sci 62: 304–311. [PubMed] [Google Scholar]
- Bohm RP, Gilbert MH (2012) Emergency medicine and critical care for nonhuman primates, in: Abee CR, Mansfield K, Tardif S, and Morris T (eds.) Nonhuman Primates in Biomedical Research, Volume 1: Biology and Management, second edition Elsevier, Waltham, MA, pp. 359–389. 10.1016/B978-0-12-381365-7.00015-7 [DOI] [Google Scholar]
- Brent L, Kessel AL, Barrera H (1997) Evaluation of introduction procedures in captive chimpanzees. Zoo Biol 16: 335–342. [DOI] [Google Scholar]
- Brent LJN, Semple S, Dubuc C, Heistermann M, MacLarnon A (2011) Social capital and physiological stress levels in free-ranging adult female rhesus macaques. Physiol Behav 102: 76–83. 10.1016/j.physbeh.2010.09.022 [DOI] [PubMed] [Google Scholar]
- Capitanio JP, Mendoza SP, Lerche NW, Mason WA (1998) Social stress results in altered glucocorticoid regulation and shorter survival in simian acquired immune deficiency syndrome. Proc Natl Acad Sci, USA 95: 4714–4719. 10.1073/pnas.95.8.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke MR, Harrison RM, Didier ES (1996) Behavioral, immunological, and hormonal response associated with social change in rhesus monkeys (Macaca mulatta). Amer J Primatol 39: 223–233. [DOI] [PubMed] [Google Scholar]
- Davenport MD, Tiefenbacher S, Lutz CK, Novak MA, Meyer JS (2006) Analysis of endogenous cortisol concentrations in the hair of rhesus macaques. Gen Comp Endocrinol 147: 255–261. 10.1016/j.ygcen.2006.01.005 [DOI] [PubMed] [Google Scholar]
- Ehardt CL, Bernstein IS (1986) Matrilineal overthrows in rhesus monkey groups. Int J Primatol 7: 157–181. 10.1007/BF02692316 [DOI] [Google Scholar]
- Engh AL, Beehner JC, Bergman TJ, Whitten PL, Hoffmeier RR, Seyfarth RM, Cheney DL (2006) Female hierarchy instability, male immigration and infanticide increase glucocorticoid levels in female chacma baboons. Anim Behav 71: 1227–1237. 10.1016/j.anbehav.2005.11.009 [DOI] [Google Scholar]
- Feeroz MM, Schillaci MA, Begum S, Hasan MK, Aziz MA, Rabiul Alam SM, Rahman SM, Akhtar F, Engel GA, Jones-Engel L (2010) Morphometric assessment of rhesus macaques (Macaca mulatta) from Bangladesh. Primate Conserv 25: 119–125. 10.1896/052.025.0105 [DOI] [Google Scholar]
- Feng X, Wang L, Yang S, Qin D, Wang J, Li C, Lv L, Ma Y, Hu X (2011) Maternal separation produces lasting changes in cortisol and behavior in rhesus monkeys. Proc Natl Acad Sci, USA 108: 14312–14317. 10.1073/pnas.1010943108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii K, Jin J, Shev A, Beisner B, McCowan B, Fushing H (2015) Perc: using percolation and conductance to find information flow certainty in a direct network R Package Version 0.1.0 ed. Available at http://cran.r-project.org/package=Perc.
- Fushing H, McAssey MP, McCowan B (2011) Computing a ranking network with confidence bounds from a graph-based beta random field. Proc Roy Soc A 467: 3590–3612. 10.1098/rspa.2011.0268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman A, Hill J (2007) Data analysis using regression and multilevel/hierarchical models. Cambridge University Press, New York. [Google Scholar]
- Gelman A, Hwang J, Vehtari A (2014) Understanding predictive information criteria for Bayesian models. Stat Comput 24: 997–1016. 10.1007/s11222-013-9416-2 [DOI] [Google Scholar]
- Georgiev AV, Christie D, Rosenfield KA, Ruiz-Lambides AV, Maldonado E, Thompson ME, Maestripieri D (2016) Breaking the succession rule: the costs and benefits of an alpha-status take-over by an immigrant rhesus macaque on Cayo Santiago. Behaviour 153: 323–351. 10.1163/1568539X-00003344 [DOI] [Google Scholar]
- Gust DA, Gordon TP, Hambright MK, Wilson ME (1993) Relationship between social factors and pituitary-adrenocortical activity in female rhesus monkeys (Macaca mulatta). Horm Behav 27: 318–331. 10.1006/hbeh.1993.1024 [DOI] [PubMed] [Google Scholar]
- Gust DA, Wilson ME, Stocker T, Conrad S, Plots PM, Gordon TP (2000) Activity of the Hypothalamic-Pituitary-Adrenal Axis is altered by aging and exposure to social stress in female rhesus monkeys. J Clin Endocrinol Metab 85: 2556–2563. 10.1210/jcem.85.7.6696 [DOI] [PubMed] [Google Scholar]
- Gygax L, Harley N, Kummer H (1997) A matrilineal overthrow with destructive aggression in Macaca fascicularis. Primates 38: 149–158. 10.1007/BF02382005 [DOI] [Google Scholar]
- Hausfater G (1972) Intergroup behavior of free-ranging rhesus monkeys (Macaca mulatta). Folia Primatol 18: 78–107. 10.1159/000155471 [DOI] [PubMed] [Google Scholar]
- Heim C, Ehlert U, Hellhammer DH (2000) The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology 25: 1–35. 10.1016/S0306-4530(99)00035-9 [DOI] [PubMed] [Google Scholar]
- Higham JP, Heistermann M, Maestripieri D (2013) The endocrinology of male rhesus macaque social and reproductive status: a test of the challenge and social stress hypotheses. Behav Ecol Sociobiol 67: 19–30. 10.1007/s00265-012-1420-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higham JP, Maestripieri D (2010) Revolutionary coalitions in male rhesus macaques. Behaviour 147: 1889–1908. 10.1163/000579510X539709 [DOI] [Google Scholar]
- Jaffe KE, Isbell LA (2010) Changes in ranging and agonistic behavior of vervet monkeys (Cercopithecus aethiops) after predator-induced group fusion. Amer J Primatol 72: 634–644. 10.1002/ajp.20821 [DOI] [PubMed] [Google Scholar]
- Judge PG, de Waal FB (1997) Rhesus monkey behavior under diverse population densities: coping with long-term crowding. Anim Behav 54: 643–662. 10.1006/anbe.1997.0469 [DOI] [PubMed] [Google Scholar]
- Kulik L, Amici F, Langos D, Widdig A (2015) Sex differences in the development of aggressive behavior in rhesus macaques (Macaca mulatta). Int J Primatol 36: 764–789. 10.1007/s10764-015-9853-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden JB, Capitanio JP, McCowan B, Isbell LA (in press) Coping style and cortisol levels in infancy predict hair cortisol following new group formation in captive rhesus macaques (Macaca mulatta). Amer J Primatol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestripieri D, Georgiev AV (2015) What cortisol can tell us about the costs of sociality and reproduction among free-ranging rhesus macaque females on Cayo Santiago. Amer J Primatol 78: 92–105. 10.1002/ajp.22368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElreath R (2016a) rethinking: Statistical Rethinking book package R package version 1.59. [Google Scholar]
- McElreath R (2016b) Statistical rethinking: a Bayesian course with examples in R and STAN. CRC Press, Boca Raton, FL. [Google Scholar]
- Moore J (1984) Female transfer in primates. Int J Primatol 5: 537–589. 10.1007/BF02692285 [DOI] [Google Scholar]
- Novak MA (2003) Self-injurious behavior in rhesus monkeys: new insights into its etiology, physiology, and treatment. Amer J Primatol 59: 3–19. 10.1002/ajp.10063 [DOI] [PubMed] [Google Scholar]
- Payne HFP, Lawes MJ, Henzi SP (2003) Fatal attack on an adult female Cercopithecus mitis erythrarchus: implications for female dispersal in female-bonded societies. Int J Primatol 24: 1245–1250. 10.1023/B:IJOP.0000005990.39403.96 [DOI] [Google Scholar]
- Pochigaeva K, Druzhkova T, Yakovlev A, Onufriev M, Grishkina M, Chepelev A, Guekht A, Gulyaeva N (2017) Hair cortisol as a marker of hypothalamic-pituitary-adrenal axis activity in female patients with major depressive disorder. Metab Brain Dis 32: 577–583. 10.1007/s11011-017-9952-0 [DOI] [PubMed] [Google Scholar]
- Pusey AE and Packer C (1987) Dispersal and philopatry, in: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, and Struhsaker TT (eds), Primate Societies. University of Chicago Press, Chicago, IL, pp. 250–266. [Google Scholar]
- R Core Team (2016) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: https://www.R-project.org/. [Google Scholar]
- Saltzman W, Hogan BK, Abbott DH (2006) Diminished cortisol levels in subordinate female marmosets are associated with altered central drive to the hypothalamic-pituitary-adrenal axis. Biol Psychiatry 60: 843–849. 10.1016/j.biopsych.2005.12.006 [DOI] [PubMed] [Google Scholar]
- Samuels A, Henrickson RV (1983) Outbreak of severe aggression in captive Macaca mulatta. Amer J Primatol 5: 277–281. 10.1002/ajp.1350050314 [DOI] [PubMed] [Google Scholar]
- Sapolsky RM (1990) Adrenocortical function, social rank, and personality among wild baboons. Biol Psychiatry 28: 862–878. 10.1016/0006-3223(90)90568-M [DOI] [PubMed] [Google Scholar]
- Sapolsky RM (1992) Cortisol concentrations and the social significance of rank instability among wild baboons. Psychoneuroendocrinology 17: 701–709. 10.1016/0306-4530(92)90029-7 [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU (2000) How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 21: 55–89. 10.1210/edrv.21.1.0389 [DOI] [PubMed] [Google Scholar]
- Southwick CH (1969) Aggressive behaviour of rhesus monkeys in natural and captive groups, in: Garattini S, Sigg EB (eds), Aggressive Behaviour: Proceedings of the International Symposium on the Biology of Aggressive Behavior. Amsterdam: Excerpta Medica Foundation; pp. 32–43. [Google Scholar]
- Stan Development Team (2017) RStan: the R interface to Stan R package version 2.15.1. http://mc-stan.org
- Steudte S, Kirschbaum C, Gao W, Alexander N, Schönfeld S, Hoyer J, Stalder T (2013) Hair cortisol as a biomarker of traumatization in healthy individuals and posttraumatic stress disorder patients. Biol Psychiatry 74: 639–646. 10.1016/j.biopsych.2013.03.011 [DOI] [PubMed] [Google Scholar]
- Thierry B (2004) Social epigenesis, in: Thierry B, Singh M, and Kaumanns W (eds), Macaque Societies: A Model for the Study of Social Organization. Cambridge University Press, Cambridge, UK: pp. 267–290. [Google Scholar]
- Vandeleest JJ, Beisner BA, Hannibal DL, Nathman AC, Capitanio JP, Hsieh F, Atwill ER, McCowan B (2016) Decoupling social status and status certainty effects on health in macaques: a network approach. PeerJ 4: e2394 10.7717/peerj.2394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandeleest JJ, Capitanio JP, Hamel A, Meyer J, Novak M, Mendoza SP, McCowan B (2019) Social stability influences the association between adrenal responsiveness and hair cortisol concentrations in rhesus macaques. Psychoneuroendocrinology 100, 164–171. 10.1016/j.psyneuen.2018.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooddell LJ, Kaburu SSK, Rosenberg KL, Meyer JS, Suomi SJ, Dettmer AM (2016) Matrilineal behavioral and physiological changes following the removal of a non-alpha matriarch in rhesus macaques (Macaca mulatta). PLoS ONE 11: e0157108 10.1371/journal.pone.0157108 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


