Transbronchial biopsies to obtain lung tissue specimens remain the gold standard to identify acute lung allograft rejection. However, bronchoscopic biopsy practices, including biopsy schedule, frequency, follow-up after abnormal results or results suggesting rejection, and the role and composition of a biopsy review panel, differ between transplant centers (1). Because of this heterogeneity, there have been few studies for at least a decade analyzing posttransplant biopsy data collected prospectively from multiple centers. A study by Todd and colleagues (pp. 576–585) published in this issue of the Journal is a welcome exception (2).
In this multicenter study, Todd and colleagues present a careful and extensive analysis of 2,026 lung biopsies obtained during surveillance (83.4%) and for-cause (16.6%) transbronchial biopsies from 400 lung transplant recipients to determine the incidence and severity of acute rejection within the first year after transplant with a focus on identifying potential risk factors for acute rejection. Results were obtained from five high-volume transplant centers in North America that used nonidentical but congruous biopsy schedules, which increases the priority of this study. Todd and colleagues report an incidence of acute rejection of 53.3%, with the majority of patients experiencing mild A1 rejection. High-level HLA mismatch between donor and recipient was associated with an increased risk for acute rejection. Double lung transplantation and the use of induction immunosuppression were associated with a decreased risk for acute rejection during the first year after transplantation. When Todd and colleagues normalized for number of biopsies performed during the first year after transplant and analyzed time-independent variables associated with acute rejection, they found that patients with double lung transplantation and patients with fewer than four HLA mismatches continued to have a decreased risk for acute rejection (2).
These results are consistent with previous findings, highly reproducible, and clinically useful based on the solid study design with prospective data collection from multiple centers. However, surveillance transbronchial biopsy has inherent limitations. It is invasive and costly, is subject to sampling errors, and is not capable of anticipating alloimmune events (3). Therefore, new diagnostic venues that can be combined with available pathological data should be explored.
An evolving body of recent evidence consistently supports that antibody-mediated rejection is an important contributor to acute and chronic lung allograft rejection after lung transplantation and that Foxp3+ regulatory CD4+ (cluster of differentiation 4–positive) T lymphocytes play a central role in recovery from acute injuries in lung allografts regardless of the cause of the injuries (4, 5). Indeed, since their discovery in 1995, regulatory T cells have been characterized as master regulatory cells with simultaneous, multidirectional functions in immune tolerance that are involved in both innate and adaptive immunity (6–8). These findings should be duly translated into clinical practice in a “bench-to-bedside manner” for assessment of regulatory T-cell function along with the routine tests currently utilized throughout the lung transplant process, including transbronchial biopsies.
Our increased understanding of the underlying immunology along with evolving analytic technologies provide the basis for new surveillance approaches with the aim of better predicting immune-mediated allograft damage that will determine whether the patient will suffer chronic lung allograft dysfunction (CLAD) or be free of CLAD. For instance, noninvasive biomarkers, including regulatory T cells circulating in the blood (9) and immune-cell–based assays that replicate antidonor alloimmune responses ex vivo (10), have recently been described and are associated with short-term and long-term transplant outcomes. The evaluation of key cellular events and signaling pathways underlying detectable posttransplant immunologic processes will help to more accurately quantify lung injuries associated with acute rejection in lung allografts. This includes evaluation of acute rejection with biomarkers identified with the evolving “-omics” technologies, including direct genome sequencing, genomics, transcriptomics, proteomics, and metabolomic analyses. Most notably, molecular measurement of gene expression using machine-learning–based microarray analysis has been developed over the last 3 years to overcome the limitations of conventional diagnostics used after abdominal organ transplantation (11, 12). The scientific community should be able to use this evolving artificial intelligence technology in an integrated manner for complex analyses not only of gene transcript data but also combining -omics data with clinical variables or risk factors that may impact transplant outcomes.
In the lungs, immune regulation is more complex than in other solid organs, and the lungs possess their own secondary lymphoid tissue, bronchus-associated lymphoid tissue. Foxp3+ regulatory CD4+ T lymphocytes have been very recently found to regulate immune tolerance in lung allografts (4). Diagnostic approaches need to be sophisticated enough to predict lung injuries in transplanted allografts and eventually the incidence of CLAD. By keeping abreast of recent findings detailing the basic immunology in lung allografts after transplantation with a special focus on newer key players, including regulatory T cells, next-generation pulmonary diagnostics should be able to transform the surveillance paradigm from “Detect” to “Detect, Quantify, and Predict” by synchronously analyzing all the translatable data with the assistance of artificial intelligence technology (Figure 1).
Figure 1.
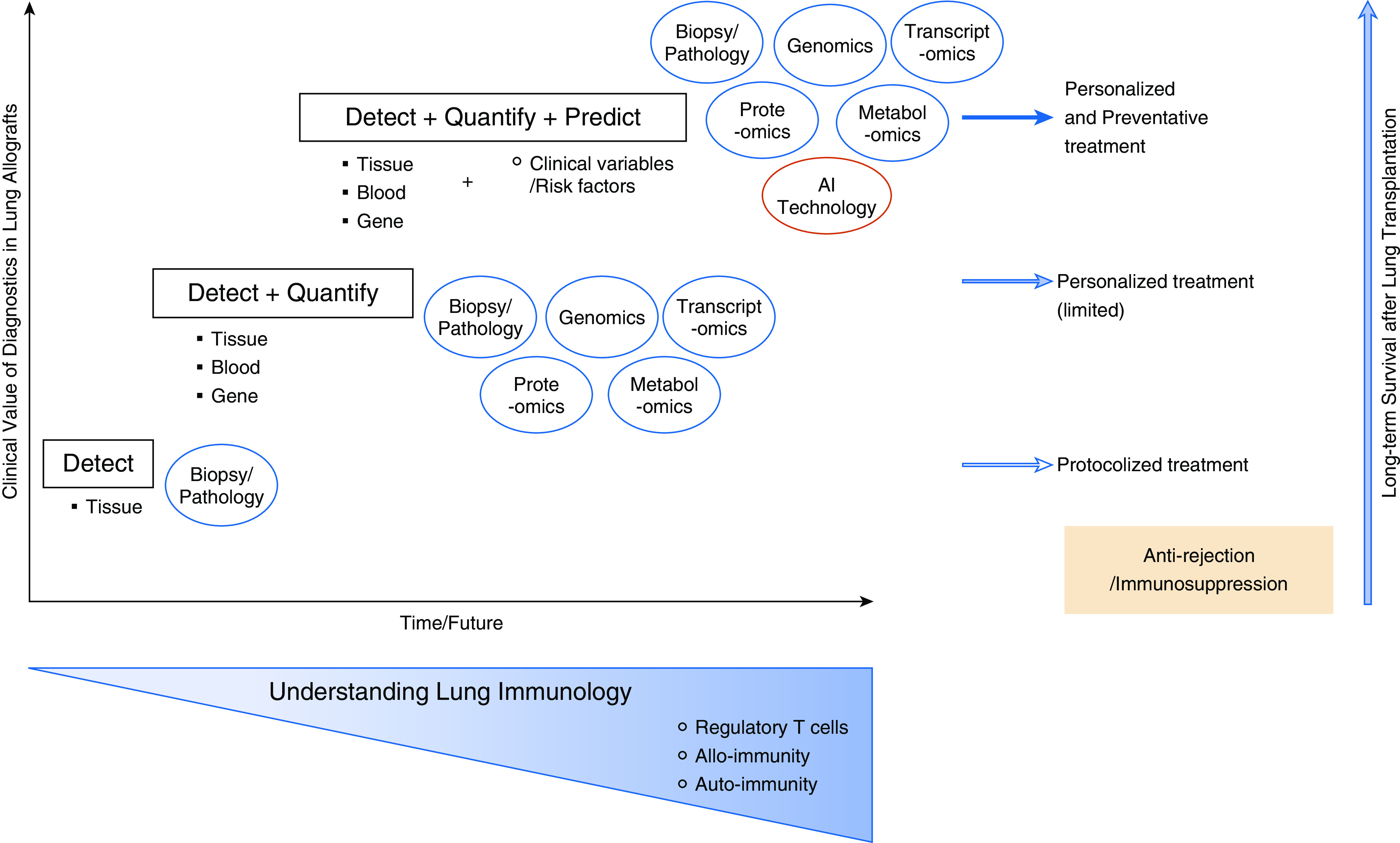
Clinical value of diagnostics in lung transplant recipients and implications for care. AI = artificial intelligence.
We urgently need a strategic approach to validate an accurate predictive model for graft rejection in lung transplant recipients that duly incorporates the crosstalk between immune cells and lung allografts, similar to a model tested for liver transplant recipients (13). Biopsy data remains an integral part of such a model; however, partnering bronchoscopy with evolving technologies should yield diagnostic data that facilitates personalized and preventative treatments, including immunosuppression regimens, that mitigate CLAD and optimize long-term outcomes after lung transplantation (14). Todd and colleagues should be congratulated for their thorough and important study utilizing a multicenter database with prospective collection of transbronchial biopsy data. Their results and clinical interpretation highlight the significance of acute rejection events in determining outcomes after lung transplantation. This study is one step of many that need to be taken toward overcoming the challenge of suboptimal long-term outcomes after lung transplantation.
Supplementary Material
Footnotes
Originally Published in Press as DOI: 10.1164/rccm.202005-1821ED on June 30, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Arcasoy SM, Berry G, Marboe CC, Tazelaar HD, Zamora MR, Wolters HJ, et al. Pathologic interpretation of transbronchial biopsy for acute rejection of lung allograft is highly variable. Am J Transplant. 2011;11:320–328. doi: 10.1111/j.1600-6143.2010.03382.x. [DOI] [PubMed] [Google Scholar]
- 2. Todd JL, Neely ML, Kopetskie H, Sever ML, Kirchner J, Frankel CW, et al. Risk factors for acute rejection in the first year after lung transplant: a multicenter study. Am J Respir Crit Care Med. 2020;202:576–585. doi: 10.1164/rccm.201910-1915OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bhorade SM, Husain AN, Liao C, Li LC, Ahya VN, Baz MA, et al. Interobserver variability in grading transbronchial lung biopsy specimens after lung transplantation. Chest. 2013;143:1717–1724. doi: 10.1378/chest.12-2107. [DOI] [PubMed] [Google Scholar]
- 4. Li W, Gauthier JM, Higashikubo R, Hsiao HM, Tanaka S, Vuong L, et al. Bronchus-associated lymphoid tissue-resident Foxp3+ T lymphocytes prevent antibody-mediated lung rejection. J Clin Invest. 2019;129:556–568. doi: 10.1172/JCI122083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guo Y, Wang Q, Li D, Onyema OO, Mei Z, Manafi A, et al. Vendor-specific microbiome controls both acute and chronic murine lung allograft rejection by altering CD4+ Foxp3+ regulatory T cell levels. Am J Transplant. 2019;19:2705–2718. doi: 10.1111/ajt.15523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wing JB, Tanaka A, Sakaguchi S. Human FOXP3+ regulatory T cell heterogeneity and function in autoimmunity and cancer. Immunity. 2019;50:302–316. doi: 10.1016/j.immuni.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 7. Gauthier JM, Harrison MS, Krupnick AS, Gelman AE, Kreisel D. The emerging role of regulatory T cells following lung transplantation. Immunol Rev. 2019;292:194–208. doi: 10.1111/imr.12801. [DOI] [PubMed] [Google Scholar]
- 8. Harper IG, Gjorgjimajkoska O, Siu JHY, Parmar J, Mulder A, Claas FHJ, et al. Prolongation of allograft survival by passenger donor regulatory T cells. Am J Transplant. 2019;19:1371–1379. doi: 10.1111/ajt.15212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ius F, Salman J, Knoefel AK, Sommer W, Nakagiri T, Verboom M, et al. Increased frequency of CD4+ CD25high CD127low T cells early after lung transplant is associated with improved graft survival: a retrospective study. Transpl Int. 2020;33:503–516. doi: 10.1111/tri.13568. [DOI] [PubMed] [Google Scholar]
- 10. Juvet SC, Sanderson S, Hester J, Wood KJ, Bushell A. Quantification of CD4(+) T cell alloreactivity and its control by regulatory T cells using time-lapse microscopy and immune synapse detection. Am J Transplant. 2016;16:1394–1407. doi: 10.1111/ajt.13607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Crespo E, Roedder S, Sigdel T, Hsieh SC, Luque S, Cruzado JM, et al. Molecular and functional noninvasive immune monitoring in the ESCAPE study for prediction of subclinical renal allograft rejection. Transplantation. 2017;101:1400–1409. doi: 10.1097/TP.0000000000001287. [DOI] [PubMed] [Google Scholar]
- 12. Stites E, Kumar D, Olaitan O, John Swanson S, Leca N, Weir M, et al. High levels of dd-cfDNA identify patients with TCMR 1A and borderline allograft rejection at elevated risk of graft injury. Am J Transplant. doi: 10.1111/ajt.15822. [online ahead of print] 13 Feb 2020; DOI: 10.1111/ajt.15822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Han JW, Joo DJ, Kim JH, Rha MS, Koh JY, Park HJ, et al. Early reduction of regulatory T cells is associated with acute rejection in liver transplantation under tacrolimus-based immunosuppression with basiliximab induction. Am J Transplant. doi: 10.1111/ajt.15789. [online ahead of print] 22 Jan 2020; DOI: 10.1111/ajt.15789. [DOI] [PubMed] [Google Scholar]
- 14. Shigemura N, Toyoda Y. Elderly patients with multiple comorbidities: insights from the bedside to the bench and programmatic directions for this new challenge in lung transplantation. Transpl Int. 2020;33:347–355. doi: 10.1111/tri.13533. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


