Abstract
Introduction
Since the influenza A/H1N1 pandemic of 2009 to 2010, numerous studies have described the clinical course and outcome of the different subtypes of influenza (A/H1N1, A/H3N2, and B). A recent systematic literature review concluded that there were no appreciable differences in either clinical presentation or disease severity among these subtypes, but study parameters limit the applicability of these results to military populations. We sought to evaluate differences in disease severity among influenza subtypes in a cohort of healthy, primarily outpatient adult U.S. Department of Defense beneficiaries.
Materials and Methods
From 2009 to 2014, we enrolled otherwise healthy adults age 18 to 65 years with influenza-like illness in an observational cohort study based in 5 U.S. military medical centers. Serial nasopharyngeal swabs were collected for determination of etiology and viral shedding by polymerase chain reaction. The presence and severity of symptoms was assessed by interview and patient diary.
Results
Over a 5-year period, a total of 157 adults with laboratory-confirmed influenza and influenza subtype were enrolled. Of these, 69 (44%) were positive for influenza A(H1N1), 69 (44%) for influenza A(H3N2), and 19 (12%) for influenza B. About 61% were male, 64% were active duty military personnel, and 72% had received influenza vaccine in the past 8 months. Almost 10% were hospitalized with influenza. Seasonal influenza virus distribution among enrollees mirrored that of nationwide trends each year of study. Individuals with A/H1N1 had upper respiratory composite scores that were lower than those with A/H3N2. Multivariate models indicated that individuals with A(H1N1) and B had increased lower respiratory symptom scores when compared to influenza A(H3N2) (A[H1N1]: 1.51 [95% CI 0.47, 2.55]; B: 1.46 [95% CI 0.09, 2.83]), whereas no other differences in symptom severity scores among influenza A(H1N1), influenza A(H3N2), and influenza B infection were observed. Overall, influenza season (maximum in 2012–2013 season) and female sex of the participant were found to be associated with increased influenza symptom severity.
Conclusions
Our study of influenza in a cohort of otherwise healthy, outpatient adult Department of Defense beneficiaries over 5 influenza seasons revealed few differences between influenza A(H1N1), influenza A(H3N2), and influenza B infection with respect to self-reported disease severity or clinical outcomes. This study highlights the importance of routine, active, and laboratory-based surveillance to monitor ongoing trends and severity of influenza in various populations to inform prevention measures.
INTRODUCTION
Since its emergence and ascent to pandemic status,1,2 influenza A(H1N1)pdm09 has continued to circulate. Although seasonal influenza A virus (ie, influenza A[H3N2])) was the predominant strain from 2010 to 2013,3–5 a resurgence of influenza A(H1N1)pdm09 occurred in 2013 to 2014, again becoming the leading cause of influenza-associated illness, hospitalization, and mortality in the United States.6
Clinical descriptions of influenza A(H1N1)pdm09 are numerous.7–13 When compared to influenza A(H3N2), the most notable difference of influenza A(H1N1)pdm09 infection as it emerged was younger host age.7–9 Otherwise, with respect to risk groups, clinical course, clinical outcome, and hospitalization rates, the differences were few.7–10 Some even associated influenza A(H1N1)pdm09 infection with a milder course.11–13 This is also true of comparisons of symptom severity and clinical outcomes with influenza B.14,15
Previous comparative studies of influenza A(H1N1)pdm09, influenza A(H3N2), and influenza B utilized either historical controls or contemporaneous controls during, or immediately after, the pandemic. Additionally, many of these comparisons were among hospitalized populations.14–20 As such, these characterizations of influenza A(H1N1)pdm09 may have been shaped by trends in health care seeking behavior, access to care, antiviral use, and/or vaccination uptake around the time of the pandemic. Studies comparing disease severity and clinical outcomes among the different influenza subtypes in various populations are warranted. In June 2009, we initiated an observational cohort study of influenza-like illness (ILI) at 5 geographically diverse U.S. military medical centers.21–22 This infrastructure has proven invaluable for the study of influenza, where major shifts in predominant types/subtypes can occur from year to year. Herein, using data from 2009 to 2014, we compared characteristics of influenza A(H1N1)pdm09, influenza A(H3N2) infection, and influenza B, using prospectively collected virologic, clinical, and symptom severity data in a geographically diverse, healthy, and predominantly outpatient population.
METHODS
Study Overview
The Acute Respiratory Infection Consortium Natural History Study21 is an observational, longitudinal cohort study of ILI among otherwise healthy Department of Defense (DoD) members and beneficiaries. Participating centers include: (1) Naval Medical Center Portsmouth, Virginia, (2) Naval Medical Center San Diego, California, (3) Madigan Army Medical Center, Tacoma, Washington, DC, (4) San Antonio Military Medical Center, Texas, and (5) Walter Reed National Military Medical Center, Bethesda, Maryland.
Study Population and Procedures
From October 2009 to May 2014, patients 18 to 65 years and presenting within 72 hours after ILI onset (temperature ≥ 100.4° F and sore throat or one of the following: cough, sputum production, shortness of breath, or chest pain) were recruited. Both inpatient and outpatient subjects were eligible. Patients with type 1 or 2 diabetes, immunodeficiency besides human immunodeficiency virus, chronic obstructive pulmonary disease, cystic fibrosis, severe asthma, chronic neuromuscular disease, chronic heart disease, or chronic kidney disease were excluded. Women with a current high risk or complicated pregnancy and patients with a poorly controlled psychiatric disorder were also excluded.
After obtaining informed consent, patient data were recorded through a standard questionnaire, and a nasopharyngeal swab (Nylon-flocked, Copan Diagnostics, Corona, California) was collected. After enrollment, participants returned at 3 subsequent time points (days 3 ± 1, 7 ± 2, and 28 ± 7) and symptom data and an nasopharyngeal swab were again collected. Participants were also asked to complete a daily symptom diary for 7 days, beginning at illness onset23 Patients were instructed by study personnel on appropriate completion of their symptom diary. Recording of symptom severity on the days before enrollment was by participant recall.
Clinical Characteristics and Severity Measures
Presence and severity of symptoms were recorded on a 4-point scale (0: none; 1: mild; 2: moderate; and 3: severe) similar to that previously described.20 Participants were trained by study personnel on the definitions. Symptom severity was quantified for each day of symptom data using the following 6 measures: (1) individual symptom score for 20 symptoms, (2) upper respiratory infection (URI) symptom score, calculated as the sum of severity scores for earache, runny nose, sore throat, and sneezing, (3) lower respiratory infection (LRI) symptom score, calculated as the sum of severity scores for cough, difficulty breathing, hoarseness, and chest discomfort, (4) gastrointestinal (GI) score, calculated as the sum of severity scores for nausea, vomiting, diarrhea, abdominal pain, and appetite loss), (5) systemic symptom score, calculated as the sum of severity scores for chills, muscle ache, headache, and fatigue, and (6) total composite score, calculated as the sum of severity scores for upper respiratory, lower respiratory, GI, and systemic composite scores. Participants only contributed 1 ILI episode to the analysis (the following episodes were dropped). Overall episode scores (upper respiratory, lower respiratory, GI, ILI, GI, systemic symptom score, and total) were calculated based on the maximum symptom scores reported during the episode.
Influenza Testing and Subtyping
Swabs were placed immediately into viral transport media, stored at −70° or − 80°F, and shipped on dry ice to the Naval Health Research Center (San Diego, California). All specimens were tested for influenza by real-time reverse transcription polymerase chain reaction (PCR).24 Determination of influenza species and subtype was performed on all influenza-positive specimens. Influenza viral load was determined by comparison of influenza-specific quantitative PCR assays against 2 housekeeping gene quantitative PCR assays among cases for whom specimens were available. Viral load was normalized by comparison to standards of known concentration, and then to measured amounts of housekeeping gene signals (manuscript in preparation). We assessed viral co-detection (ie, human rhinovirus, adenovirus, respiratory syncytial virus, coronavirus, parainfluenza virus, human metapneumovirus, and bocavirus) with 1 of 3 multiplex assays (xTAG Respiratory Viral Panel, Luminex, Austin, Texas; PLEX-ID Viral IC Spectrum, Abbott, Chicago, Illinois; or Target-enriched multiplex PCR, Diatherix Laboratories, Inc., Huntsville, Alabama).25–27
Statistical Analysis
We compared categorical variables (subject and clinical characteristics, symptom severity) by influenza subtype using chi-square tests, or Fisher’s exact tests if appropriate. For continuous outcomes, we performed ANOVA and t-tests. P-values < 0.05 were considered statistically significant. Linear mixed effects models with random effects for participant were run to evaluate differences in symptom scores by day of episode and influenza subtype, controlling for season, sex, and age. Maximum symptom scores during the episode were compared among the influenza subtypes using multivariate linear models, controlling for factors such as season, sex, age, and race/ethnicity. Analyses were performed using R 3.5.28,29
RESULTS
Between October 2009 and May 2014, 930 adult enrollees with demographic information had specimens available, among whom 159 cases of influenza were identified (69 influenza A(H1N1)pdm09, 69 influenza A(H3N2), 19 influenza B, and 2 untyped influenza, Supplemental Figure 1). In 2009 to 2010, all (n = 8) had influenza A(H1N1)pdm09 (Figure 1). In the 3 seasons that followed, A/H3N2 predominated (48% in 2010–2011; 47% in 2011–2012; 74% in 2012–2013). In 2013 to 2014, a resurgence of influenza A(H1N1)pdm09 occurred in the United States, and the pandemic strain was again the predominant cause (75%). Rates of influenza B remained relatively low between 2010 and 2014 (0% in 2009/10; 10% in 2010/11; 16% in 2011/12; 14% in 2012/13; and 14% in 2013/14). These annual trends were generally reflective of the nationwide trends as reported by the Centers For Disease Control and Prevention.30–34
Figure 1.
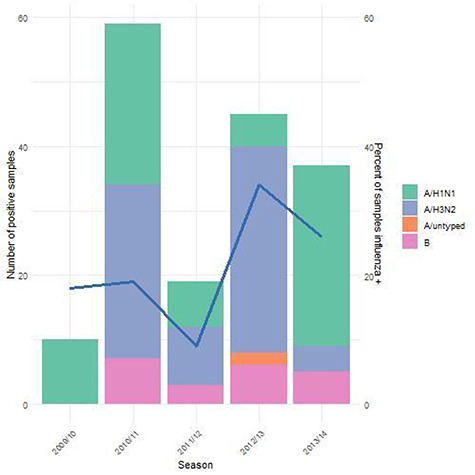
Influenza subtypes by season, 2009/10 to 2013/14 (bar chart, first y-axis), and percent of samples that were tested that were influenza positive (blue line, second y-axis).
Among the 159 participants with influenza, 157 had subtype and symptom severity data. The median age of this group was 32 years, 61% were male, 50% were White (non-Hispanic), and 64% were active duty military personnel. The baseline demographic characteristics were similar among all 3 influenza subtypes, with the exception of military status (Table I). Similar proportions in each group had been vaccinated during the 8 months before enrollment (71% (A/H1N1), 75% (A/H3N2), 63% (B); P = 0.56), and among the active military participants, vaccination rates were 88% to 98%. Among the 113 participants who had received an influenza vaccine within the past 8 months (median months since vaccination = 4), 63% received injectable and 37% received mist. Mist vaccines were used primarily in the November 2010 season (44% of vaccinations during that season were mist).
Table I.
Demographic and Risk Factor Characteristics of Study Participants With Influenza Infection, by Subtype
| Variable | Description | A/H1N1 | A/H3N2 | B |
|---|---|---|---|---|
| Age | 18–24 y | 17 (25) | 9 (13) | 4 (21) |
| 25–34 y | 28 (41) | 34 (49) | 4 (21) | |
| 35+ y | 24 (35) | 26 (38) | 11 (58) | |
| Sex | Male | 40 (58) | 41 (59) | 14 (74) |
| Site | MAMC | 8 (12) | 7 (10) | 3 (16) |
| NMCP | 11 (16) | 11 (16) | 4 (21) | |
| NMCSD | 28 (41) | 30 (43) | 11 (58) | |
| SAMMC | 20 (29) | 18 (26) | 1 (5) | |
| WRNMMC | 2 (3) | 3 (4) | 0 (0) | |
| Race | White | 41 (59) | 26 (38) | 11 (58) |
| Black | 11 (16) | 14 (20) | 2 (11) | |
| Hispanic | 12 (17) | 21 (30) | 5 (26) | |
| Unknown/other | 5 (7) | 8 (12) | 1 (5) | |
| >High school education | 39 (57) | 43 (62) | 9 (47) | |
| Military status*,+ | Active duty | 42 (61) | 50 (72) | 8 (42) |
| Dependent | 17 (25) | 16 (23) | 5 (26) | |
| Retired | 10 (14) | 3 (4) | 6 (32) | |
| Season*,^ | 2009/10 | 8 (12) | 0 (0) | 0 (0) |
| 2010/11 | 22 (32) | 25 (36) | 5 (26) | |
| 2011/12 | 7 (10) | 9 (13) | 3 (16) | |
| 2012/13 | 5 (7) | 31 (45) | 6 (32) | |
| 2013/14 | 27 (39) | 4 (6) | 5 (26) | |
| Vaccinated in past 8 m | 49 (71) | 52 (75) | 12 (63) | |
| Vaccinated in past 8 m – active military | 40 (98) | 45 (88) | 7 (88) |
* P < 0.05 across all 3 types.
^ P < 0.05 for H1 vs. H3.
+ P < 0.05 for A vs. B.
MAMC, Madigan Army Medical Center; NMCP, Naval Medical Center Portsmouth; NMCSD, Naval Medical Center San Diego; SAMMC, San Antonio Military Medical Center; WRNMMC, Walter Reed National Military Medical Center.
Symptom severity and clinical outcomes univariate analysis is presented in Table II. Cough, fatigue, chills, and muscle aches were most frequently reported as moderate or severe symptoms among those with ILIs (Supplemental Figure 2). Lower respiratory, GI, systemic, and total severity scores revealed no differences among the 3 influenza subtypes, although there was a statistically significant difference between A/H1N1 and A/H3N2 for the upper respiratory score (5.6 vs. 6.6, P = 0.037). Duration of both severe and moderate to severe symptoms was similar among the 3 influenza subtypes with a median of 3 days for severe symptoms and 6 days for moderate to severe symptoms. There were no differences among the influenza subtypes with respect to use of antipyretics, antivirals, antibiotics, or hospitalization.
Table II.
Mean (range) of the Maximum Symptom Severity Scores and N (%) With Certain Clinical Characteristics, Testing for Differences by Influenza Subtype
| A/H1N1 | A/H3N2 | B | |
|---|---|---|---|
| N = 157 | 69 (44%) | 69 (44%) | 19 (12%) |
| Lower respiratory scorea | 6.9 (1,12) | 6.1 (2,12) | 7.4 (4,11) |
| Upper respiratory scorea,* | 5.6 (0,12) | 6.6 (0,12) | 6.3 (1,11) |
| Gastrointestinal scorea | 4.8 (0,15) | 4.8 (0,15) | 5.6 (0,13) |
| Systemic scorea | 8.7 (4,12) | 9.0 (3,12) | 9.8 (5,12) |
| Total severity scorea | 25.9 (10,44) | 26.6 (11,50) | 29.1 (16,46) |
| Duration of limited activitya | 5.0 (1,9) | 4.7 (0,9) | 5.1 (0,9) |
| Duration of severe symptomsa | 3.9 (0,9) | 3.2 (0,9) | 3.7 (0,9) |
| Duration of moderate-severe symptomsa | 6.1 (1,9) | 6.2 (2,9) | 6.5 (2,9) |
| Antivirals takenb | 22 (32%) | 15 (22%) | 4 (21%) |
| Antibiotics takenb | 7 (10%) | 10 (14%) | 6 (32%) |
| Antipyretics takenb | 36 (52%) | 44 (64%) | 12 (63%) |
| Hospitalizedb | 8 (12%) | 4 (6%) | 3 (16%) |
aANOVA (for three-way comparison A/H1N1 vs. A/H3N2 vs. B) and t-tests (H1N1 vs. A/H3N2, and A vs. B).
bChi-square and Fisher’s exact tests.
* P-value < 0.05 comparing A/H1N1 and A/H3N2.
Participants recorded data on symptom presence and severity daily over 9 days, starting from illness onset (Figure 2). For all 3 subtypes, symptom scores peaked 1–3 days after onset of illness, and decreased thereafter. There were no detectable differences with respect to any of the composite symptom severity scores by day and influenza subtype, controlling for season, sex, and age. When considering the maximum symptom scores reported during the influenza episode (Supplemental Table), women had significantly higher upper respiratory, lower respiratory, GI, systemic, and total symptom scores (P < 0.01). Influenza season appeared to impact influenza severity, particularly regarding LRI and total symptom scores, independent of other risk factors. In addition, individuals with A(H1N1) and B had higher LRI scores than did individuals with influenza A(H3N2) (A[H1N1]: 1.51, 95% confidence interval [CI] 0.47, 2.55; B: 1.46, 95% CI 0.09, 2.83), controlling for age, sex, educational history, and influenza season. These results remained similar when time since vaccination was accounted for in the model and time since vaccination was not statistically significantly related to any of the severity scores; however, the estimated effect was positive for each of the scores (Supplemental Table II).
Figure 2.
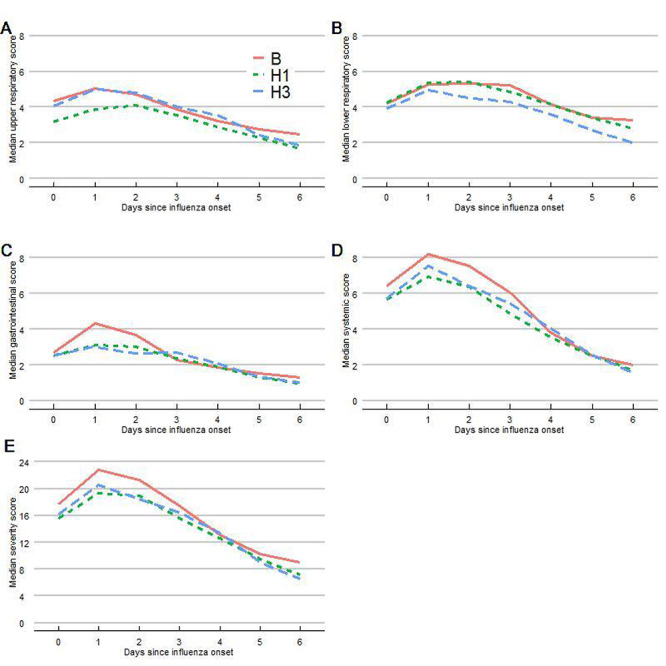
Influenza disease severity scores by day of influenza-like illness.
DISCUSSION
As the cause of the most recent influenza pandemic in human history, influenza A(H1N1)pdm09 virus ascended, receded, and resurged between 2009 and 2014, contributing along with influenza A(H3N2) and influenza B to the seasonal burden of influenza in the United States and elsewhere. Although initially characterized as a relatively mild, self-limiting illness like that of seasonal influenza, continuous evaluation of comparative disease severity is necessary.
We found few differences in the epidemiologic, virologic, and clinical characteristics of influenza A(H1N1)pdm09 as compared to influenza A(H3N2) infection and influenza B in our population of generally healthy adults. To our knowledge, no other prospective, comparative study of influenza A(H1N1)pdm09, influenza A(H3N2), and influenza B infection beyond the immediate postpandemic period (2010–2011) in a primarily healthy, outpatient U.S. population has been done. In the 2013 to 2014 influenza season, influenza A(H1N1)pdm09 was again the predominant cause of influenza in the United States,6 but published clinical descriptions from that season are limited. A systematic literature review that encompassed our studied time period showed similar results of comparable clinical presentation and disease severity regardless of influenza subtypes.14 The review included a heterogeneous international collection of studies comprised of subjects of all ages in various settings, and incorporated studies predating the 2009 pandemic. Additionally, per the authors’ report, few of the included studies adjusted for potential confounders, including age and vaccination status. Thus, our study focusing on a highly vaccinated and generally healthy adult cohort adds to the literature while having direct military relevance.
Most previous comparative studies were conducted immediately following the pandemic (ie, 2010–2011).14–20 Several suggested that influenza A(H1N1)pdm09 infections in the postpandemic era were increasingly severe, and that older age groups were affected,16,17,19,20 whereas 1 study showed no such differences.18 These studies were conducted in hospitalized populations, and many included those with comorbidities,18–20 which may account for the observed differences. A study of a population similar to ours, otherwise healthy adults with ILI presenting to military treatment facilities in San Antonio, Texas, between 2005 and 2011, showed no significant difference in the severity of infection caused by different influenza subtypes.35 By contrast, a study comparing the clinical presentation of different influenza strains among service members presenting with ILI to camp clinics performed by the Singapore military from May 2009 to June 2010, did suggest differences.13 A variety of factors may account for why an apparent difference in clinical presentation by influenza strain was detected in the Singapore military study but not in our study of U.S. military service members and beneficiaries, including the different locales (tropical vs. temperate), the timing (peripandemic vs. late postpandemic) and the collection of a prospective symptom diary. This observed variation in clinical presentation supports the need for continued epidemiologic surveillance and the importance of ongoing investigation into the pathogenic properties and evolution of different influenza strains.
The impact of routine influenza vaccination and the timing of that vaccination on subsequent disease risk and severity is still incompletely understood. It is well recognized that current influenza vaccines are only moderately effective.36 The H1N1 antigen has consistently been a component of monovalent and seasonal influenza vaccines since the pandemic. It is not currently known whether repeated vaccination with the same antigen influences host immunity. This is of concern for military personnel for whom annual vaccination is mandatory and vaccination rates are >90%. We previously reported that, among those with breakthrough disease (ie, vaccine failures), vaccination was associated with a reduction in influenza A(H3N2) disease severity.37 In this study, there was no statistically significant difference in symptom severity according to timing of vaccination when the analysis was restricted to individuals who had received vaccination during the influenza season of interest. Whether that is because of a true lack of difference or reflects a power limitation is unknown and merits further study.
Our study had numerous strengths. First, our prospective evaluation was conducted over 5 successive seasons. Therefore, we were able to minimize potential biases stemming from increased health care seeking behavior, case detection, or study participation during postpandemic periods. Second, our data in a healthy, outpatient adult population complement the data previously published on subtype analyses, which have predominantly been in hospitalized populations with significant comorbidities. Third, the seasonal distributions of influenza types/subtypes were reflective of nationwide surveillance data. Lastly, enrollment was restricted to individuals 18 to 65 years, a militarily relevant group that has been previously shown to be at higher risk for influenza A(H1N1)pdm09 infection.
There are limitations. Only a few hospitalized patients were enrolled. It is possible that severe illness and hospitalization for influenza A(H1N1)pdm09 does occur among otherwise healthy individuals. However, the likelihood is low. Because individuals with comorbidities were excluded, these findings are not applicable to individuals at increased risk for complications.
Influenza A(H1N1)pdm09 bears few, if any, epidemiologic, virologic, and clinical differences from influenza A(H3N2) and influenza B. These conclusions were drawn through ongoing, active surveillance for influenza at multiple military medical centers in the United States. Nevertheless, continued surveillance for disease trends and novel influenza variants remains warranted to help inform future prevention policies.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful for the contributions of the Acute Respiratory Infection Consortium team of clinical research coordinators, clinical site managers, data managers, and administrative support personnel to the success of this project.
The contents of this publication are the sole responsibility of the author(s) and do not necessarily reflect the views, opinions, or policies of Uniformed Services University of the Health Sciences (USUHS), the Department of Defense (DoD), the Departments of the Army, Navy, or Air Force. Mention of trade names, commercial products, or organizations does not imply endorsement by the U.S. Government. One or more authors are military service members or employees of the U.S. Government. This work was prepared as part of their official duties. Title 17 U.S.C. §105 provides that “Copyright protection under this title is not available for any work of the United States Government.” Title 17 U.S.C. §101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person’s official duties. The views expressed are those of the authors and do not reflect the official views of the Uniformed Services University of the Health Sciences, The Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., the National Institutes of Health or the Department of Health and Human Services, Brooke Army Medical Center, the U.S. Army Medical Department, the U.S. Army Office of the Surgeon General, the Department of Defense, or the Departments of the Army, Navy or Air Force. The investigators have adhered to the policies for protection of human subjects as prescribed in 45CFR46.The authors have no conflicts of interest to disclose.
FUNDING
This work (IDCRP-045) was conducted by the Infectious Disease Clinical Research Program (IDCRP), a Department of Defense (DoD) program executed by the Uniformed Services University of the Health Sciences (USUHS) through a cooperative agreement with The Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. (HJF). This project has been funded in whole, or in part, with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH), under Inter-Agency Agreement Y1-AI-5072, and the Armed Forces Health Surveillance Center, Global Emerging Infections Surveillance and Response System.
The Infectious Disease Institutional Review Board of the Uniformed Services University approved the study (IDCRP-045).
References
- 1. Centers for Disease Control and Prevention : Swine influenza A (H1N1) infection in two children--Southern California, March–April 2009. MMWR Morb Mortal Wkly Rep 2009; 58(15): 400–2. [PubMed] [Google Scholar]
- 2. World Health Organization : New influenza A (H1N1) virus: global epidemiological situation, June 2009. Wkly Epidemiol Rec 2009; 84(25): 249–57. [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention : Update: influenza activity--United States, 2010–11 season, and composition of the 2011–12 influenza vaccine. MMWR Morb Mortal Wkly Rep 2011; 60(21): 705–12. [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention : Update: influenza activity - United States, 2011–12 season and composition of the 2012–13 influenza vaccine. MMWR Morb Mortal Wkly Rep 2012; 61(22): 414–20. [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention : Influenza activity--United States, 2012–13 season and composition of the 2013–14 influenza vaccine. MMWR Morb Mortal Wkly Rep 2013; 62(23): 473–9. [PMC free article] [PubMed] [Google Scholar]
- 6. Centers for Disease Control and Prevention, Epperson S, Blanton L, et al. : Influenza activity - United States, 2013–14 season and composition of the 2014–15 influenza vaccines. MMWR Morb Mortal Wkly Rep 2014; 63(22): 483–90. [PMC free article] [PubMed] [Google Scholar]
- 7. Belongia EA, Irving SA, Waring SC, et al. : Clinical characteristics and 30-day outcomes for influenza A 2009 (H1N1), 2008-2009 (H1N1), and 2007-2008 (H3N2) infections. JAMA 2010; 304(10): 1091–8. [DOI] [PubMed] [Google Scholar]
- 8. Cowling BJ, Chan KH, Fang VJ, et al. : Comparative epidemiology of pandemic and seasonal influenza A in households. N Engl J Med 2010; 362(23): 2175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carcione D, Giele C, Dowse GK, et al. : Comparison of pandemic (H1N1) 2009 and seasonal influenza, Western Australia, 2009. Emerg Infect Dis 2010; 16(9): 1388–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shiley KT, Nadolski G, Mickus T, Fishman NO, Lautenbach E: Differences in the epidemiological characteristics and clinical outcomes of pandemic (H1N1) 2009 influenza, compared with seasonal influenza. Infect Control Hosp Epidemiol 2010; 31(7): 676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tang JW, Tambyah PA, Lai FY, et al. : Differing symptom patterns in early pandemic vs seasonal influenza infections. Arch Intern Med 2010; 170(10): 861–7. [DOI] [PubMed] [Google Scholar]
- 12. To KK, Wong SS, Li IW, et al. : Concurrent comparison of epidemiology, clinical presentation and outcome between adult patients suffering from the pandemic influenza A (H1N1) 2009 virus and the seasonal influenza A virus infection. Postgrad Med J 2010; 86(1019): 515–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yap J, Tan CH, Cook AR, et al. : Differing clinical characteristics between influenza strains among young healthy adults in the tropics. BMC Infect Dis 2012; 12: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Caini S, Kroneman M, Wiegers T, El Guerche-Seblan C, Paget J: Clinical characteristics and severity of influenza infections by virus type, subtype and lineage: a systematic literature review. Influenza Other Respi Viruses 2018; 12: 780–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cohn R, Babushkin F, Gellder K, Finn T: Characteristics of hosptialized adult patients with laboratory documented influenza A, B and respiratory syncytial virus - a single center retrospective observational study. PLoS One 14(3): e0214517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bolotin S, Pebody R, White PJ, et al. : A new sentinel surveillance system for severe influenza in England shows a shift in age distribution of hospitalised cases in the post-pandemic period. PLoS One 2012; 7(1): e30279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lehners N, Geis S, Eisenbach C, Neben K, Schnitzler P: Changes in severity of influenza A (H1N1)pdm09 infection from pandemic to first postpandemic season, Germany. Emerg Infect Dis 2013; 19(5): 748–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rahamat-Langendoen JC, Tutuhatunewa ED, Scholvinck EH, et al. : Influenza in the immediate post-pandemic era: a comparison with seasonal and pandemic influenza in hospitalized patients. J Clin Virol 2012; 54(2): 135–40. [DOI] [PubMed] [Google Scholar]
- 19. Viasus D, Cordero E, Rodriguez-Bano J, et al. : Changes in epidemiology, clinical features and severity of influenza A (H1N1) 2009 pneumonia in the first post-pandemic influenza season. Clin Microbiol Infect 2012; 18(3): E55–62. [DOI] [PubMed] [Google Scholar]
- 20. Yang SQ, Qu JX, Wang C, Yu XM, Liu YM, Cao B: Influenza pneumonia among adolescents and adults: a concurrent comparison between influenza A (H1N1) pdm09 and a (H3N2) in the post-pandemic period. Clin Respir J 2014; 8(2): 185–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Christian Coles PD, Millar EV, PhD TB, MC USN, Ottolini MG, USAF MC: The acute respiratory infection consortium: a multi-site, multi-disciplinary clinical research network in the Department of Defense. Mil Med 2019; 184(Issue Supplement_2): 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen WJ, Arnold JC, Fairchok MP, et al. : Epidemiologic, clinical, and virologic characteristics of human rhinovirus infection among otherwise healthy children and adults: rhinovirus among adults and children. J Clin Virol 2015; 64: 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Powers JH 3rd, Bacci ED, Leidy NK, et al. : Performance of the inFLUenza patient-reported outcome (FLU-PRO) diary in patients with influenza-like illness (ILI). PLoS One 2018 22; 13(3): e0194180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shu B, Wu KH, Emery S, et al. : Design and performance of the CDC real-time reverse transcriptase PCR swine flu panel for detection of 2009 a (H1N1) pandemic influenza virus. J Clin Microbiol 2011; 49(7): 2614–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee CK, Lee HK, Ng CW, et al. : Comparison of Luminex NxTAG respiratory pathogen panel and xTAG respiratory viral panel FAST version 2 for the detection of respiratory viruses. Ann Lab Med 2017; 37(3): 267–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen KF, Rothman RE, Ramachandran P, et al. : Rapid identification viruses from nasal pharyngeal aspirates in acute viral respiratory infections by RT-PCR and electrospray ionization mass spectrometry. J Virol Methods 2011; 173(1): 60–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hassoun A, Huff MD, Asis E, Chahal K, Azarbal A, Lu S: Effect of target-enriched multiplex polymerase chain reaction on patient outcomes and costs during the 2013-14 influenza season. J Hosp Infect 2017; 96(4): 366–70. [DOI] [PubMed] [Google Scholar]
- 28. R Core Team (2018). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria: URL https://www.R-project.org/). [Google Scholar]
- 29. Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team (2018). _nlme: Linear and Nonlinear Mixed Effects Models_. R package version 3.1–137, <URL:https://CRAN.Rproject.org/package=nlme >.).
- 30. Centers for Disease Control and Prevention : Influenza activity --- United States and worldwide, June 13–September 25, 2010. MMWR Morb Mortal Wkly Rep 2010; 59(39): 1270–3. [PubMed] [Google Scholar]
- 31. Centers for Disease Control and Prevention : Influenza activity -- United States, October 3, 2010-February 5, 2011. MMWR Morb Mortal Wkly Rep 2011; 60(6): 175–81. [PubMed] [Google Scholar]
- 32. Centers for Disease Control and Prevention : Influenza activity -- United States, September 30–November 24, 2012. MMWR Morb Mortal Wkly Rep 2012; 61(48): 990–3. [PubMed] [Google Scholar]
- 33. Centers for Disease Control and Prevention : Influenza activity -- United States, September 29–December 7, 2013. MMWR Morb Mortal Wkly Rep 2013; 62(50): 1032–6. [PMC free article] [PubMed] [Google Scholar]
- 34. Centers for Disease Control and Prevention : Influenza activity -- United States, September 28–December 6, 2014. MMWR Morb Mortal Wkly Rep 2014; 63(50): 1189–94. [PMC free article] [PubMed] [Google Scholar]
- 35. Scheuller HS, Park J, Lott L, Tavish M, Danaher P: Comparison of clinical features in a population of basic military trainees versus the general Department of Defense Beneficiary Population Presenting with influenza. Mil Med 2017; 182: e1917–192. [DOI] [PubMed] [Google Scholar]
- 36. Gaglani M, Pruszynski J, Murthy K, et al. : Influenza vaccine effectiveness against 2009 pandemic influenza A(H1N1) virus differed by vaccine type during 2013-2014 in the United States. J Infect Dis 2016; 213(10): 1546–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Deiss RG, Arnold JC, Chen WJ, et al. : Vaccine-associated reduction in symptom severity among patients with influenza A/H3N2 disease. Vaccine 2015; 33(51): 7160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


