Abstract
Background
The Lake Victoria basin is one of the most persistent hotspots of schistosomiasis in Africa, the intestinal form of the disease being studied more often than the urogenital form. Most schistosomiasis studies have been directed to Schistosoma mansoni and their corresponding intermediate snail hosts of the genus Biomphalaria, while neglecting S. haematobium and their intermediate snail hosts of the genus Bulinus. In the present study, we used DNA sequences from part of the cytochrome c oxidase subunit 1 (cox1) gene and the internal transcribed spacer 2 (ITS2) region to investigate Bulinus populations obtained from a longitudinal survey in Lake Victoria and neighbouring systems during 2010–2019.
Methods
Sequences were obtained to (i) determine specimen identities, diversity and phylogenetic positions, (ii) reconstruct phylogeographical affinities, and (iii) determine the population structure to discuss the results and their implications for the transmission and epidemiology of urogenital schistosomiasis in Lake Victoria.
Results
Phylogenies, species delimitation methods (SDMs) and statistical parsimony networks revealed the presence of two main groups of Bulinus species occurring in Lake Victoria; B. truncatus/B. tropicus complex with three species (B. truncatus, B. tropicus and Bulinus sp. 1), dominating the lake proper, and a B. africanus group, prevalent in banks and marshes. Although a total of 47 cox1 haplotypes, were detected within and outside Lake Victoria, there was limited haplotype sharing (only Haplotype 6 was shared between populations from Lake Victoria open waters and neighbouring aquatic systems) – an indication that haplotypes are specific to habitats.
Conclusions
The Bulinus fauna of Lake Victoria consists of at least B. truncatus, B. tropicus, Bulinus sp. 1 (B. trigonus?) and B. ugandae. The occurrence and wide distribution of Bulinus species in Lake Victoria potentially implies the occurrence of urogenital schistosomiasis in communities living along the shores and on islands of the lake who depend solely on the lake for their livelihood. More in-depth studies are needed to obtain a better picture of the extent of the disease in the Lake Victoria basin. 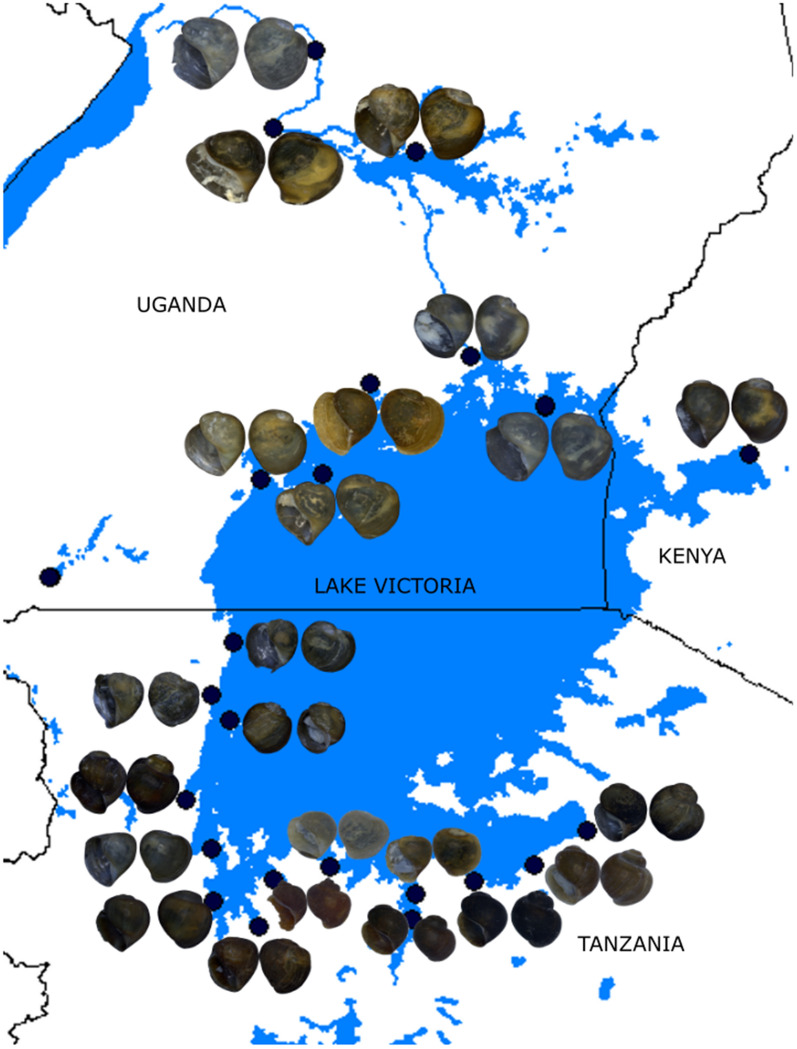
Keywords: African lakes, Epidemiology, Phylogeography, Neglected tropical diseases, Schistosoma haematobium
Background
Schistosomiasis is a parasitic disease caused by digenean trematodes of the genus Schistosoma and is a socio-notable disease in tropical and subtropical regions. It is prevalent in more than 78 countries and territories infecting more than 250 million people worldwide, most of whom inhabit sub-Saharan Africa [1, 2]. Although more than 20 Schistosoma species are recognised, only Schistosoma mansoni and S. haematobium are ubiquitously known in sub-Saharan Africa due to their capability to cause intestinal and urogenital schistosomiasis, respectively [1, 3, 4]. The highest infections and disease burdens are frequently found in school-aged children, particularly in settings with poor hygiene and sanitary facilities [5]. Human hosts infected with these Schistosoma species experience acute hyperaemia, abnormal growth, internal haemorrhaging, fibrosis and tissue thickening [6]. As a result, infection with S. mansoni culminates with liver fibrosis, portal hypertension and ascites, while bladder cancer is the final stage of a S. haematobium infection [7]. Furthermore, genital schistosomiasis complications associated with S. haematobium infections include hypertrophic and ulcerative lesions of the female genital tract [8]. Schistosoma species, like other digenean trematodes, utilise pulmonate snails to complete their two-host life-cycles; i.e. Biomphalaria spp. for S. mansoni and Bulinus spp. for S. haematobium [1, 3, 4].
The Lake Victoria ecoregion of the East African Rift System, is characterized by a wealth of extraordinary freshwater biodiversity that has accumulated throughout the Quaternary, including almost 700 species of cichlid fishes [9, 10]. Major geological and climatological changes occurred in this region during this period. These changes are linked to the development of the East African Rift. More recently, anthropogenic pressures in the Lake Victoria ecoregion have grown exponentially due to multifactorial stressors such as habitat degradation, pollution, exploitation, the introduction of invasive species, ecosystem modifications and climate change [11, 12]. Insights into the consequences of recent and historic environmental changes in the region are crucial to understanding the diversification dynamics of freshwater biota. Effects of ecosystem changes on the community composition and demography of benthic organisms remain poorly assessed since few studies have been conducted apart from cichlid fishes and the schistosome intermediate host snail genus Biomphalaria [13].
Lake Victoria is endowed with a remarkable mollusc fauna, although it is less diverse than in lakes Malawi and Tanganyika, perhaps due to its younger age and relative shallowness [10, 14]. Despite its young age of about 400,000 years, Lake Victoria has experienced three major desiccation events within the last 100,000 years [15, 16]. The current water body arose about 14,600 years ago [15, 16], which is relatively shorter for snail species radiation [10, 17]. Nevertheless, Brown et al. [17] listed 28 gastropod species in Lake Victoria, of which six are medically significant species within genera Bulinus (4 species) and Biomphalaria (2 species). Lake Victoria, which is shared between Tanzania, Uganda and Kenya, therefore plays a significant role in the persistence of schistosomiasis in these surrounding countries [18–20]. Despite the increasing efforts to control schistosomiasis with praziquantel through mass drug administration (MDA) programmes, East African countries are still among the hotspots for this parasitic disease. Herein, the majority of schistosomiasis cases are reported from fishing communities and particularly in school-aged children surrounding Lake Victoria [21–24]. The vast majority of studies focusing on Schistosoma and their intermediate hosts in Lake Victoria and neighbouring aquatic systems have mainly focused on S. mansoni and Biomphalaria spp. [13, 18, 25] while overlooking Bulinus spp. and their potential role in the urogenital schistosomiasis transmission (i.e. S. haematobium). However, identifying these potential Bulinus hosts is an initial step in estimating the extent and relevance of urogenital schistosomiasis in the given area [26, 27].
The genus Bulinus consists of 37 species occurring mainly in Africa, the Middle East and in the Mediterranean Area [17]. The recognized Bulinus species fall into four groups, namely the Bulinus africanus, B. reticulatus, and B. forskalii species groups, and the B. truncatus/B. tropicus species complex. Many species within these groups except, for example, B. tropicus and B. ugandae are involved in the transmission of S. haematobium [28, 29]. Moreover, B. africanus group species play an important role in the transmission of S. haematobium and S. bovis in Central East Africa [17]. However, precise species identification of snails of the genus Bulinus is often difficult because of strong morphological similarities and overlap among species, the coexistence of different forms and groups in a narrow area and the lack of well-defined criteria by which to distinguish species [17, 29]. Additionally, some studies have also reported the existence of cryptic species in some localities [30, 31], which further exacerbates the taxonomic uncertainties within genus Bulinus.
The knowledge of the number of Bulinus species occurring within or nearby Lake Victoria is obscure. For instance, Mandahl-Barth [29] recognised B. trigonus and B. transversalis as independent species, but Brown [17] viewed them as lacustrine morphs of B. tropicus and B. truncatus or synonyms of unnamed Bulinus species (Bulinus sp.). Moreover, there is a scarcity of information on the geographical distribution patterns of Bulinus species in the lake. Bulinus trigonus and B. transversalis have their type-localities in the Tanzanian side of Lake Victoria, while B. ugandae was first found in Jinja Bay, Uganda [17]. Surveys by Mwambungu [32] reported the occurrence of B. ugandae in the Speke Gulf of the lake in Tanzania, Ngupula & Kayanda [33] found B. ugandae and B. transversalis in Uganda and Opisa et al. [34] and Nyakaana et al. [35] reported the existence of B. globosus in the lake shores in Kenya and Uganda. Although the separation of B. ugandae from B. globosus is dubious and the overall taxonomy of Bulinus spp. in Lake Victoria is uncertain [17], it is not clear if all the four Bulinus groups are represented in the lake. Moreover, knowledge of how the four groups may be spatially distributed remains questionable. Moreover, Bulinus species such as B. ugandae and B. trigonus, whose type-materials come from Lake Victoria, are not endemic to the lake, similar morphs have been recorded in lakes Mutanda and Edward as well [17].
In many biological cases where conventional analyses have failed to identify species, molecular techniques, particularly phylogenetic approaches using DNA sequence data, have proven successful. For example, the application of markers, such as cytochrome c oxidase subunit 1 (cox1) and nuclear genes such as the internal transcribed spacer (ITS) regions, 28S and 18S, have facilitated species identification of Bulinus spp. [31, 36, 37]. In the present study we, therefore, used two more variable genetic markers, cox1 and ITS2, to investigate the phylogeography of Bulinus species occurring in Lake Victoria. This information is invaluable in improving our understanding of Bulinus species identities and phylogenetic relationships, as well as the epidemiology of the potential urogenital schistosomiasis.
Therefore, we combine mitochondrial DNA (mtDNA) and nuclear DNA (nDNA) markers to investigate Bulinus populations obtained from a longitudinal survey in Lake Victoria and neighbouring aquatic systems to (i) determine the identity, diversity and phylogenetic position of the species, (ii) reconstruct phylogeographical affinities and (iii) determine the population structures of the species. We discuss the results and their implications for the potential transmission and epidemiology of urogenital schistosomiasis in Lake Victoria.
Methods
Source of material for genomic DNA
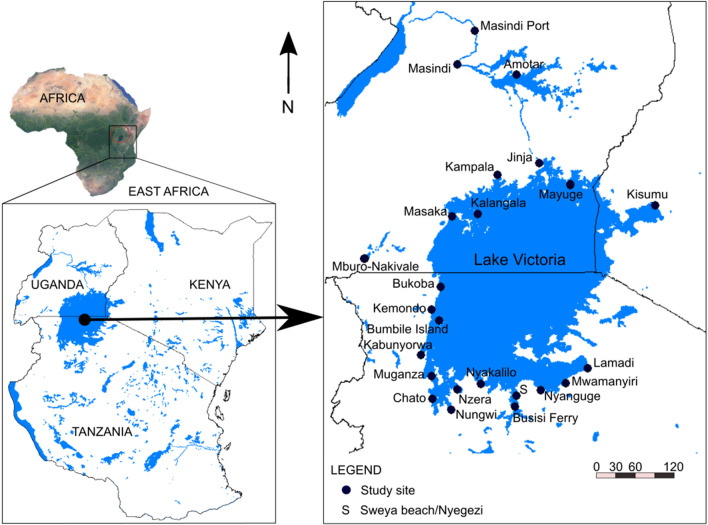
Pulmonate snails of the genus Bulinus were collected from 20 locations around Lake Victoria and (for comparative purposes) from an additional four locations in the neighbouring aquatic systems of the River Nile and Lake Mburo-Nakivale (Fig. 1, Table 1). Sampling was carried out in open waters, on shoreline banks, around islands and in bordering marsh habitats where water was either stagnant or relatively calm. Specimens were hand-picked off water plants, rocks, stones or the floor bottom where they were more easily accessible or collected with strainers, long handheld scoops and dredges in more challenging situations (e.g. deeper waters). Dredging was carried out repeatedly per site in depths from 2 m down to approximately 25 m in the Kenyan and Ugandan part of the lake. In most of the sites, sampling was carried out close to active anthropogenic activities (e.g. fish landing sites or ferry docks) and for at least 30–60 min. All specimens were collected during various field trips from 2010–2019 and snails identified as Bulinus spp. were preserved in 80% ethanol.
Fig. 1.
Study area map of East African countries that share Lake Victoria and the sampling sites for this study are shown
Table 1.
Locality, voucher, sequence and haplotype information for the Bulinus spp. from Lake Victoria studied
| Species | Locality | Country | Latitude | Longitude | Voucher No. | Sequence ID | Habitat | Haplotype ID | GenBank ID | |
|---|---|---|---|---|---|---|---|---|---|---|
| cox1 | ITS2 | |||||||||
| B. truncatus | Igabiro | Tanzania | − 1.17769 | 31.87792 | UGSB 22907 | Bkt26885 | Stone and rocks | BKT1 | MT707360 | MT707212 |
| B. truncatus | Igabiro | Tanzania | − 1.17769 | 31.87792 | UGSB 22908 | BKt26886 | Stone and rocks | BKT2 | MT707361 | |
| B. truncatus | Kemondo | Tanzania | − 1.47796 | 31.7498 | UGSB 22909 | Ket26887 | Stone and rocks | KET1 | MT707362 | MT707222 |
| B. truncatus | Kemondo | Tanzania | − 1.47796 | 31.7498 | UGSB 22910 | Ket26888 | Stone and rocks | KET2 | MT707363 | |
| Bulinus sp. 2 | Bumbire Island | Tanzania | − 1.61476 | 31.85625 | UGSB 22911 | Bit26889 | Island | BIT1 | MT707364 | |
| Bulinus sp. 2 | Bumbire Island | Tanzania | − 1.61476 | 31.85625 | UGSB 22912 | Bit26890 | Island | BIT2 | MT707365 | MT707234 |
| Bulinus sp. 2 | Bumbire Island | Tanzania | − 1.61476 | 31.85625 | UGSB 22946 | Bit26924 | Island | BIT3 | MT707366 | |
| Bulinus sp. 2 | Bumbire Island | Tanzania | − 1.61476 | 31.85625 | UGSB 23464 | Bit27073 | Island | BIT4 | MT707367 | |
| Bulinus sp. 2 | Bumbire Island | Tanzania | − 1.61476 | 31.85625 | UGSB 23465 | Bit27074 | Island | BIT5 | MT707368 | |
| Bulinus sp. 2 | Kabunyorwa | Tanzania | − 2.06018 | 31.61382 | UGSB 22913 | Kbt26891 | Papyrus | KBT1 | MT707369 | |
| Bulinus sp. 2 | Kabunyorwa | Tanzania | − 2.06018 | 31.61382 | UGSB 22914 | Kbt26892 | Papyrus | KBT2 | MT707370 | MT707235 |
| B. truncatus | Muganza | Tanzania | − 2.33702 | 31.75166 | UGSB 22915 | Mut26893 | Open water | MUT1 | MT707371 | |
| B. truncatus | Muganza | Tanzania | − 2.33702 | 31.75166 | UGSB 22916 | Mut26894 | Open water | MUT2 | MT707372 | MT707220 |
| Bulinus sp. 2 | Muganza | Tanzania | − 2.33702 | 31.75166 | UGSB 22940 | Mut26918 | Water Hyasin | MUT3 | MT707373 | MT707236 |
| Bulinus sp. 2 | Muganza | Tanzania | − 2.33702 | 31.75166 | UGSB 22941 | Mut26919 | Water Hyasin | MUT4 | MT707374 | |
| B. truncatus | Muganza | Tanzania | -2.33702 | 31.75166 | UGSB 22945 | Mut26923 | Open water | MUT5 | MT707375 | |
| Bulinus sp. 2 | Chato | Tanzania | − 2.63292 | 31.76368 | UGSB 23463 | Cht27072 | Papyrus | CHT1 | MT707376 | |
| Bulinus sp. 2 | Nungwe | Tanzania | − 2.77446 | 32.0136 | UGSB 22919 | Nut26897 | Papyrus | NUT1 | MT707377 | |
| Bulinus sp. 2 | Nungwe | Tanzania | − 2.77446 | 32.0136 | UGSB 22920 | Nut26898 | Papyrus | NUT2 | MT707378 | MT707233 |
| Bulinus sp. 2 | Nungwi | Tanzania | − 2.77446 | 32.0136 | UGSB 23466 | Nut27075 | Papyrus | NUT3 | MT707379 | |
| B. tropicus | Nzera | Tanzania | − 2.51209 | 32.09845 | UGSB 22921 | Nzt26899 | Sand beach | NZT1 | MT707380 | |
| B. tropicus | Nzera | Tanzania | − 2.51209 | 32.09845 | UGSB 22922 | Nzt26900 | Sand beach | NZT2 | MT707381 | MT707229 |
| B. truncatus | Nyakalilo | Tanzania | − 2.43669 | 32.41158 | UGSB 22923 | Nyt26901 | Stone beach | NYT1 | MT707382 | MT707221 |
| Bulinus sp. 2 | Nyakalilo | Tanzania | − 2.43669 | 32.41158 | UGSB 22924 | Nyt26902 | Papyrus | NYT2 | MT707383 | MT707237 |
| Bulinus sp. 2 | Busisi | Tanzania | − 2.72626 | 32.87034 | UGSB 22925 | But26903 | Water Hyasin | BUT1 | MT707384 | MT707230 |
| Bulinus sp. 2 | Busisi | Tanzania | − 2.72626 | 32.87034 | UGSB 22926 | But26904 | Water Hyasin | BUT2 | MT707385 | |
| Bulinus sp. 2 | Nyegezi A | Tanzania | − 2.585 | 32.88541 | UGSB 22927 | Sat26905 | Water Hyasin | SAT1 | MT707386 | MT707238 |
| B. truncatus | Nyegezi A | Tanzania | − 2.585 | 32.88541 | UGSB 22928 | Sat26906 | Stone and rocks | SAT2 | MT707387 | MT707223 |
| B. truncatus | Nyegezi B | Tanzania | − 2.58434 | 32.88331 | UGSB 22929 | Sbt26907 | Stone and rocks | SBT1 | MT707387 | MT707216 |
| B. truncatus | Nyegezi B | Tanzania | − 2.58434 | 32.88331 | UGSB 22930 | Sbt26908 | Stone and rocks | SBT2 | MT707387 | |
| B. tropicus | Nyegezi C | Tanzania | − 2.58388 | 33.51714 | UGSB 22942 | Sct26920 | Sand beach | SCT1 | MT707390 | MT707228 |
| Bulinus sp. 1 | Nyegezi C | Tanzania | − 2.58388 | 33.51714 | UGSB 22943 | Sct26921 | Sand beach | SCT2 | MT707391 | |
| Bulinus sp. 1 | Nyegezi C | Tanzania | − 2.58388 | 33.51714 | UGSB 22944 | Sct26922 | Sand beach | SCT3 | MT707392 | MT707226 |
| Bulinus sp. 2 | Nyanguge | Tanzania | − 2.51911 | 33.20884 | UGSB 22933 | Ngt26911 | Marshes/papyrus | NGT1 | MT707393 | |
| Bulinus sp. 2 | Nyanguge | Tanzania | − 2.51911 | 33.20884 | UGSB 22934 | Ngt26912 | Marshes/papyrus | NGT2 | MT707394 | MT707232 |
| Bulinus sp. 2 | Nyanguge | Tanzania | − 2.51911 | 33.20884 | UGSB 23467 | Ngt27076 | Marshes/papyrus | NGT3 | MT707395 | |
| Bulinus sp. 2 | Mwamanyiri | Tanzania | − 2.43022 | 33.5424 | UGSB 22935 | Mwt26913 | Marshes/papyrus | MWT1 | MT707396 | MT707231 |
| Bulinus sp. 2 | Mwamanyiri | Tanzania | − 2.43022 | 33.5424 | UGSB 22936 | Mwt26914 | Marshes/papyrus | MWT2 | MT707397 | |
| Bulinus sp. 2 | Mwamanyiri | Tanzania | − 2.43022 | 33.5424 | UGSB 23468 | Mwt27077 | Marshes/papyrus | MWT3 | MT707398 | |
| Bulinus sp. 2 | Lamadi | Tanzania | − 2.23738 | 33.84236 | UGSB 22937 | Lat26915 | Marshes/papyrus | LAT1 | MT707399 | |
| Bulinus sp. 2 | Lamadi | Tanzania | − 2.23738 | 33.84236 | UGSB 22938 | Lat26916 | Marshes/papyrus | LAT2 | MT707400 | MT707240 |
| Bulinus sp. 2 | Lamadi | Tanzania | − 2.23738 | 33.84236 | UGSB 23469 | Lat27078 | Marshes/papyrus | LAT4 | MT707401 | |
| Bulinus sp. 2 | Kisumu | Kenya | − 0.12739 | 34.74232 | UGSB 23446 | Kik27055 | Water Hyasin | KIK1 | MT707402 | |
| Bulinus sp. 2 | Kisumu | Kenya | − 0.12739 | 34.74232 | UGSB 23447 | Kik27056 | Water Hyasin | KIK2 | MT707403 | MT707239 |
| Bulinus sp. 2 | Kisumu | Kenya | − 0.12739 | 34.74232 | UGSB 23448 | kik27057 | Water Hyasin | KIK3 | MT707406 | |
| B. truncatus | Nile | Uganda | 0.42084 | 33.19639 | UGSB 23452 | Niu27061 | Open water | JIU1 | MT707407 | MT707214 |
| B. truncatus | Mayuge | Uganda | 0.14067 | 33.60258 | UGSB 16758 | Myu22513 | Open water | MYU1 | MT707404 | |
| B. truncatus | Mayuge | Uganda | 0.14067 | 33.60258 | UGSB 16757 | Myu22514 | Open water | MYU2 | MT707405 | |
| B. truncatus | Mayuge | Uganda | 0.14067 | 33.60258 | UGSB 23453 | Myu27062 | Open water | MYU3 | MT707408 | |
| B. truncatus | Mayuge | Uganda | 0.14067 | 33.60258 | UGSB 23454 | Myu27063 | Open water | MYU4 | MT707409 | |
| B. truncatus | Mayuge | Uganda | 0.14067 | 33.60258 | UGSB 23603 | Myu27108 | Open water | MYU5 | MT707411 | |
| B. truncatus | Mayuge | Uganda | 0.14067 | 33.60258 | UGSB 23604 | Myu27109 | Open water | MYU6 | MT707410 | MT707218 |
| B. truncatus | Masindi | Uganda | 2.12852 | 32.32919 | UGSB 23457 | Msu27066 | Nile river | MSU1 | MT707412 | |
| B. truncatus | Masindi | Uganda | 2.12852 | 32.32919 | UGSB 23458 | Msu27067 | Nile river | MSU2 | MT707413 | |
| B. truncatus | Masindi | Uganda | 2.12852 | 32.32919 | UGSB 23459 | Msu27068 | Nile river | MSU3 | MT707414 | |
| B. truncatus | Masindi | Uganda | 2.12852 | 32.32919 | UGSB 23460 | Msu27069 | Nile river | MSU4 | MT707415 | MT707217 |
| B. truncatus | Masindi | Uganda | 2.12852 | 32.32919 | UGSB 23607 | Msu27112 | Nile river | MSU5 | MT707416 | |
| B. truncatus | Masindi Port | Uganda | 1.69249 | 32.09664 | UGSB 16759 | Mpu22515 | Nile river | MPU1 | MT707417 | |
| B. truncatus | Masindi Port | Uganda | 1.69249 | 32.09664 | UGSB 16760 | Mpu22516 | Nile river | MPU2 | MT707418 | |
| B. truncatus | Masindi Port | Uganda | 1.69249 | 32.09664 | UGSB 23610 | Mpu27115 | Nile river | MPU3 | MT707419 | MT707219 |
| Bulinus sp. 2 | Masaka | Uganda | − 0.27263 | 32.02691 | UGSB 23461 | Mku27070 | Water Hyasin | MKU1 | MT707420 | |
| Bulinus sp. 2 | Kampala | Uganda | − 0.27263 | 32.02691 | UGSB 23596 | Kau27101 | Water Hyasin | KAU1 | MT707421 | MT707241 |
| B. truncatus | Kampala | Uganda | − 0.27263 | 32.02691 | UGSB 23598 | Kau27103 | Open water | KAU2 | MT707422 | MT707215 |
| Bulinus sp. 2 | Kampala | Uganda | − 0.27263 | 32.02691 | UGSB 23599 | Kau27104 | Water Hyasin | KAU3 | MT707423 | |
| Bulinus sp. 2 | Amotar | Uganda | 1.55822 | 32.88828 | UGSB 23613 | Amu27118 | Marshes/papyrus | AMU1 | MT707424 | |
| B. truncatus | Kalangala | Uganda | 0.30371 | 32.28927 | UGSB 16774 | Klu22530 | Open water | KLU1 | MT707425 | |
| B. truncatus | Kalangala | Uganda | 0.30371 | 32.28927 | UGSB 23616 | Klu27121 | Open water | KLU2 | MT707426 | MT707213 |
| B. truncatus | Lake Mburo | Uganda | − 0.638 | 30.9528 | UG3 | BspJDUG3 | MNU1 | MT707427 | ||
| B. truncatus | River Rwizi | Uganda | − 0.6863 | 30.8856 | UG19 | BspJUG19 | MNU2 | MT707428 | ||
| B. truncatus | River Rwizi | Uganda | − 0.6863 | 30.8856 | UG22 | BspJUG22 | MNU3 | MT707429 | ||
| B. truncatus | Lake Mburo | Uganda | − 0.6951 | 30.8514 | UG27 | BspJUG27 | MNU4 | MT707430 | ||
| B. truncatus | Lake Nakivale | Uganda | − 0.8205 | 30.8559 | UG7 | BspJDUG7 | MNU5 | MT707431 | ||
| Bulinus sp. 2 | Lake Nakivale | Uganda | − 0.8205 | 30.8559 | UG98 | BspJUG98 | MNU6 | MT707432 | ||
| B. forskalii | Lake Nakivale | Uganda | − 0.8205 | 30.8559 | UG76 | BspJUG76 | MNU7 | MT707403 | ||
| B. truncatus | Lake Albert | Uganda | A1 | HQ121558 | LAU1 | HQ121558 | ||||
| B. truncatus | Lake Albert | Uganda | A2 | HQ121559 | LAU2 | HQ121559 | ||||
| B. truncatus | Lake Albert | Uganda | A3 | HQ121560 | LAU3 | HQ121560 | ||||
| B. truncatus | Katosho swamp | Tanzania | T1 | HQ121562 | KST1 | HQ121562 | ||||
| B. truncatus | Lake Albert | Uganda | BO (Booma) | GU176747 | LAU4 | GU176747 | ||||
| B. truncatus | Lake Albert | Uganda | 1PD (Piida) | GU176748 | LAU5 | GU176748 | ||||
| B. truncatus | Lake Albert | Uganda | TO (Toonya) | GU176749 | LAU6 | GU176749 | ||||
| B. truncatus | Nyanguge | Tanzania | Nyanguge | AM286313 | NGT | AM286313 | ||||
| Bulinus sp. 2 | Kisumu | Kenya | ADC farm | AM286297 | AFK1 | AM286297 | ||||
| B. truncatus | Lake Sagara | Tanzania | T04em43A | AM286298 | LST | AM286298 | ||||
Abbreviation: UGSB, University of Giessen Systematics and Biodiversity collection
DNA extraction, amplification and sequencing
Genomic DNA was extracted using the CTAB method [38] from 2–5 specimens per locality for a total of 74 specimens. A 655-bp target fragment of the mtDNA cox1 gene was amplified using primers and PCR conditions given by Folmer et al. [39]. In a few cases, the region was amplified using the primers LCO1490 [39] and COR722B [40] and PCR conditions as detailed by Kane et al. [37]. Primers LT1 and ITS2-RIXO and PRC conditions stated by Almeyda-Artigas et al. [41] and Bargues et al. [42] were used to amplify the rDNA ITS2 region. Sanger sequencing was performed by LGC Genomics GmbH (Berlin, Germany).
Phylogenetic analyses
Chromatograms were assembled and inspected using Geneious version 8.0.6 (Biomatters, Auckland, New Zealand; Kearse et al. [43]). Multiple alignments were generated for each marker, with the ClustalW tool [44] implemented in BioEdit version 7.0.5.3 [45]. Newly generated sequences from 74 specimens were combined with 57 additional available sequence data from GenBank to expand our datasets (Additional file 1: Table S1). The online program MAFFT [46], was used to align the ITS2 partition. The phylogenetic trees of the concatenated datasets of 620 bp cox1 and ITS2 were estimated using Maximum Likelihood (ML) and Bayesian Inference (BI) analyses. The cox1 and ITS2 partitions were concatenated using Sequences Matrix version 1.2.8 [47]. In both cases, Indoplanorbis exustus was used as the outgroup. The best sequence evolutionary model to each partition was evaluated with jModelTest version 2.1.4 [48]. Based on the Akaikeʼs information criterion (AIC), HYK + G and GTR+G were selected as the best evolutionary models for cox1 and ITS2 datasets, respectively. ML analysis was conducted using Randomized Accelerated Maximum Likelihood (RAxML version 7.0.4; [49]) with a bootstrap of 1000 replicates. Bayesian inference analysis, to obtain an ultrametric tree for the General Mixed Yule Coalescent (GMYC) model of species delimitation [50], was carried out using BEAST version 1.8.4 [51]. Runs consisted of 5,000,000 MCMC generations, sampling every 500th tree. Validation of convergence and mixing was assessed in Tracer 1.5 [52] to ensure that all effective sample size (ESS) values were > 200. We used TreeAnnotator 1.8.4 (BEAST package) to identify the maximum clade credibility (MCC) tree by discarding 50% of the trees as ‘burn-in’.
We applied two DNA-based species delimitation methods (SDMs) with single and multiple delimiting thresholds to resolve the species boundaries in Bulinus specimens incorporated. These were the Poisson Tree Process (PTP [53]) and the GMYC method as mentioned above. Both mPTP (maximum likelihood, PTP and Bayesian, bPTP) and GMYC analyses were carried out with the web-based service at https://species.h-its.org/.
Phylogeographical and population analyses
Phylogeographical analyses were performed for the novel cox1 sequences of the Bulinus specimens from Lake Victoria and the neighbouring systems (i.e. Lake Mburo-Nakivale and the River Nile). The dataset consisted of the 74 sequences generated herein. The relationships between haplotypes were identified through a statistical parsimony network constructed in TCS version 1.21 [54] with 95% confidence.
For genetic diversity, differentiation and population expansion or shrinkage cox1 sequences belonging to the Bulinus specimens from Lake Victoria basin were split into two groups representing B. truncatus and Bulinus sp. 2. Bulinus truncatus sequences were divided into three subpopulations based on habitat, namely, lentic sand substrate, lentic stones and rock substrates and lotic habitats. The sequences forming the Bulinus sp. 2 group were also divided into three subpopulations based on lentic habitats; islands, papyrus swamps and marshes (water hyacinth). We estimated haplotype diversity (h) and nucleotide diversity (π) [55] using DnaSP version 6.12.03 [56]. Moreover, we performed analyses of molecular variance (AMOVA), to examine the amount of genetic variability within and between populations, using Arlequin version 3.5.2.2 [57].
The mitochondrial DNA sequence data were also tested for deviation from neutral expectations (e.g. population expansion events). Genetic equilibrium was assessed using Arlequin version 3.5.2.2 [57] by calculating Tajima’s D [58] and Fu’s Fs [59]. Under the assumption of selective neutrality, Arlequin version 3.5.2.2 was also used for mismatch distribution analysis of pairwise differences within and between populations. The relative population sizes (θ0 and θ1) and relative time since population expansion (τ) were estimated also using Arlequin version 3.5.2.2. The estimated τ value was used to estimate time since expansion using the formula τ= 2 µt, where µ is the mutation rate per site per generation and τ is the time since population expansion [60]. In the present study, the substitution rate of 1.22 ± 0.27% per million years was applied for the mtDNA (cox1) region [61]. Additionally, a Mantel test for matrix correspondence between genetic and geographical distances was performed using GenAlEx version 6.5. [62] to test the isolation by distance (IBD). The input matrices for genetic distance were constructed in Mega X [63].
Results
Species identification and phylogenetic relationships
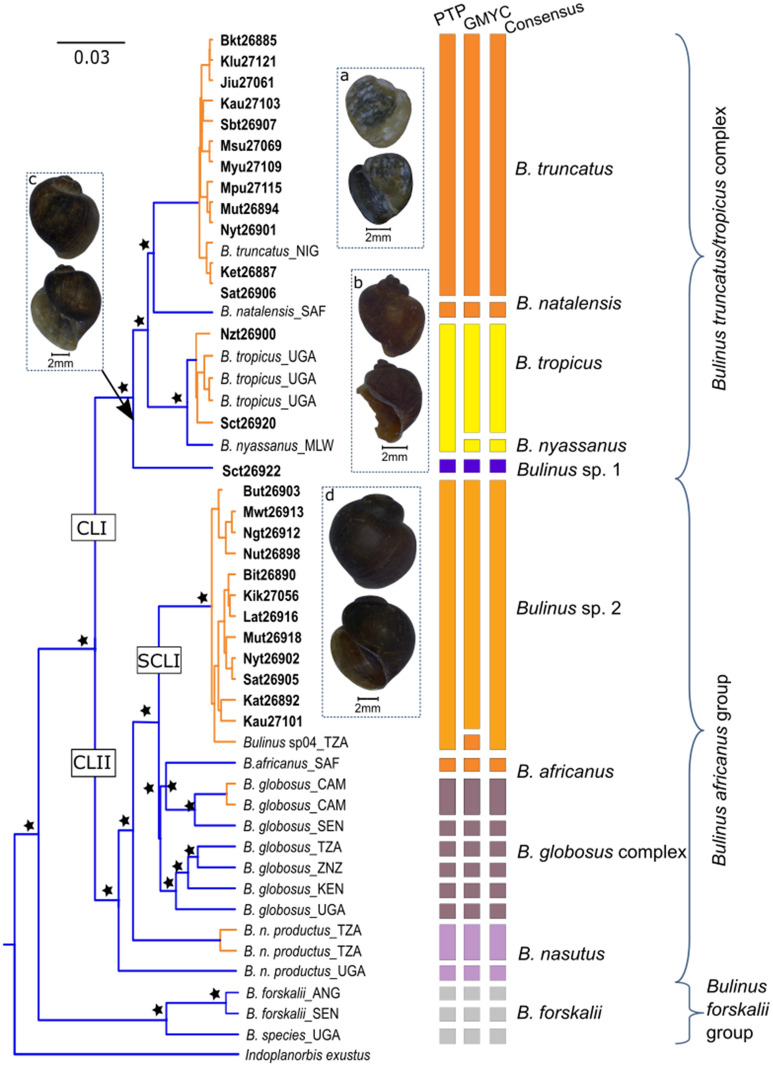
Both Maximum Likelihood (ML) and Bayesian Inference (BI) analyses of concatenated genes (cox1 and ITS2) generated strongly supported phylogenies that revealed the presence of two main Bulinus groups in Lake Victoria (Fig. 2). Clade I comprised of B. truncatus/tropicus complex and Clade II contained the B. africanus group Moreover, Clade I exhibited a complex structure that corresponded to Bulinus specimens that inhabited open waters and sandy beaches of Lake Victoria. For instance, specimen labelled Sct26922, collected from Nyegezi on the Tanzanian side of the lake, was found in shallow waters near sandy beaches coexisting with a physid species. Both species delimitation methods (SDMs), PTP and GMYC, categorised the specimen as a unique molecular operational taxonomic unit (MOTU; Bulinus sp. 1.). Clade I also contained Bulinus samples collected outside the lake albeit within the lake basin i.e. the Lake Mburo-Nakivale and Nile River ecosystems, denoting that these species are not endemic to Lake Victoria. Moreover, combined phylogenetic and SDMs analyses revealed the presence of B. truncatus and B. tropicus in Lake Victoria, although B. truncatus are more widely distributed than B. tropicus.
Fig. 2.
The BI phylogenetic tree of Bulinus species with bars, on the right, denoting different species delimitation results, based on the dataset of concatenated cox1 and ITS2 sequences. Within the phylogeny, nodes supported and shared between BI and ML methods are marked with stars where support equates to 90–100% (ML) and 0.95–1 (BI). Names in bold are for specimens collected in the present study and the rest have been retrieved from GenBank: B. truncatus (a); B. tropicus (b); Bulinus sp. 1 (c); Bulinus sp. 2 (d). Locality details are provided in Table 1. Abbreviations: CLI, Clade I; CLII, Clade II; SCI, Subclade I; SCII, Subclade II. The three-letter abbreviation for countries is also given. Notes: the blue colour represents different species, while green stands for the same species according to species delimitation methods. The three-letter abbreviations represent countries: NIG, Nigeria; SAF, South Africa; UGA, Uganda; MLW, Malawi; TZA, Tanzania; CAM, Cameroon; SEN, Senegal; ZNZ, Zanzibar; KEN, Kenya; ANG, Angola. The information for sequences retrieved from the GenBank is presented in Additional file 1: Table S1
Novel sequences forming subclade I (SCI, Fig. 2) were isolated from Bulinus specimens collected from the banks and marshes surrounding the lake and small islands, particularly Bumbire in Tanzania and Mayuge in Uganda. Although these Bulinus specimens formed a well-defined and supported clade in both analyses (ML = 100% and BI = 1.0), they did not intermingle with other species within the clade; they formed a definite group of their own (Bulinus sp. 2). As shown in Fig. 2, despite the complexity or the presence of cryptic species in GenBank sequences designated as B. globosus, SDMs treated Bulinus specimens from the banks and surrounding marshes, regardless of the location they were collected, as one species (MOTU). The specimens from the banks matched only with Bulinus sp. T04em43A (GenBank: AM286298) from Lake Sagara in Tanzania, and accordingly, SDMs placed them under the same MOTU. The phylogeny and SDMs from cox1 also identified Bulinus nasutus productus and B. forskalii collected from Lake Mburo-Nakivale system within the Lake Victoria basin. Similar results are shown for cox1 analyses (Additional file 2: Figure S1)
Phylogeographical and population analyses
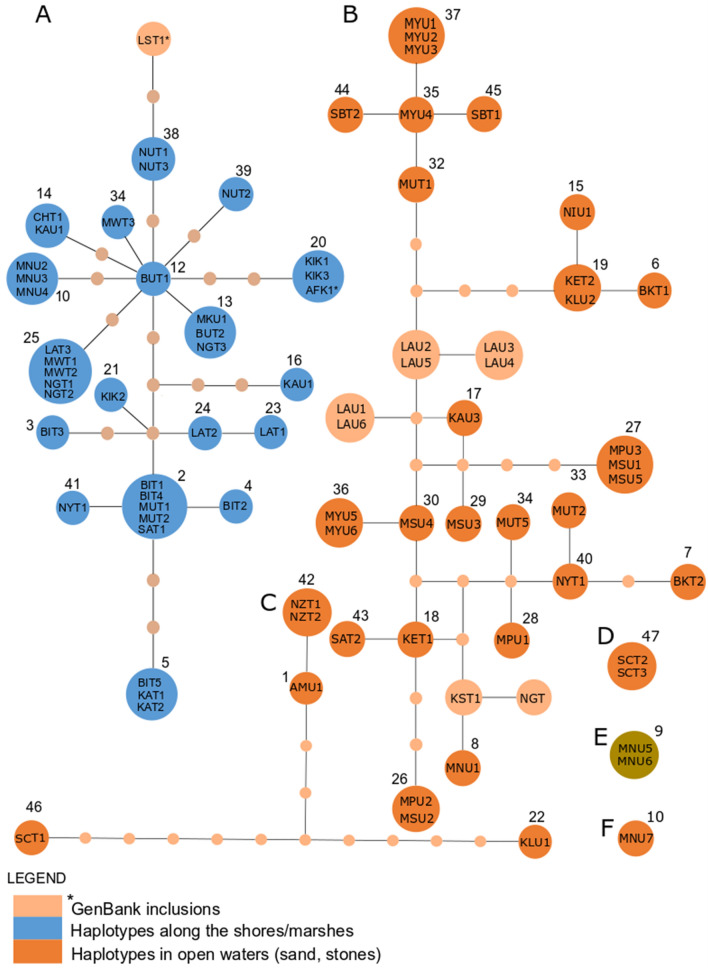
Although the phylogeographical analysis of the present study did not acquire sufficient samples from the Kenyan side and small islands, in particular, TCS networks supported the phylogenies (Fig. 3) that Lake Victoria is dominated by two distinct clades of Bulinus species; species occurring in the lake proper and those inhabiting the banks and surrounding marshes. However, at the confidence limit of 0.95, the dataset comprising specimens from the banks and marshes represented B. africanus group species (A in Fig. 3) while those from the open water (lake proper) revealed three separate networks: B. truncatus/B. tropicus complex, i.e. B. truncatus (B in Fig. 3), B. tropicus (C in Fig. 3) and an undefined species Bulinus sp. 1 (D in Fig. 3). Bulinus nasutus productus and B. forskalii from Lakes Mburo-Nakivale systems formed separate networks (E and F in Fig. 3, respectively). Similar to phylogeny and SDMs, TCS analysis revealed a Bulinus specimen collected from Nyegezi in Tanzania (Haplotype 47) as a distinct species (D in Fig. 3). Generally, the TCS analysis showed that Bulinus species had shared haplotypes distributed throughout Lake Victoria, indicating that these species are not localised in the lake (Fig. 4). Moreover, the TCS analysis corroborated phylogenies and SDMs that specimens sampled from the banks and surrounding marshes of Lake Victoria relate to potentially undescribed bulinid species, Bulinus sp. T04em43A (GenBank: AM286298) from Lake Sagara, Tanzania and Bulinus sp. K3.03 (GenBank: AM286297) from Lake Victoria in Kisumu, Kenya.
Fig. 3.
Statistical parsimony network of cox1 sequences (connecting limit: 95%) of Bulinus species from Lake Victoria; Bulinus sp. 2 (a), B. truncatus (b), B. tropicus (c), Bulinus sp. 1 (d), B. nasutus (e) and B. forskalii (f). The size of the circles corresponds to the number of individuals belonging to the respective haplotype. Mutational steps for the missing haplotypes are presented as small circles, and numbers correspond to the number of individuals with a given haplotype. Green stands for GenBank material
Fig. 4.
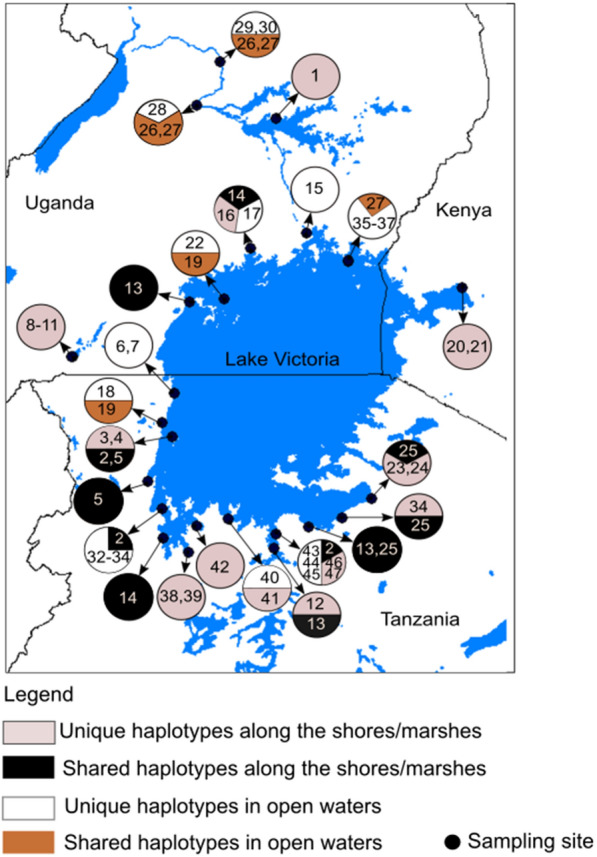
A map of Lake Victoria showing Bulinus species haplotypes distribution. Specimen details are provided in Table 1
The mtDNA loci showed high overall haplotype (h) and nucleotide (π) diversity among populations (0.984 and 0.071). The population analysis of B. truncatus revealed 22 haplotypes, out of which 2 haplotypes were shared between sand beaches and river systems (Table 2). On the other hand, Bulinus sp. 2 (Clade II) population consisted of 17 haplotypes and a least one haplotype was shared between two habitats i.e. islands, papyrus and water hyacinths. Nevertheless, no haplotype was shared among the three habitats; an indication that haplotypes are specific to habitats. Nucleotide and haplotype diversities were also high within each habitat (Table 2).
Table 2.
Results of genetic diversities, AMOVAs and mismatch distribution for populations of Bulinus spp. in Lake Victoria
| Bulinus truncatus | Bulinus sp. 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Mean (n = 29) | Lentic sand substrate (n = 14) | Lentic stone & rocks substrate (n = 8) | River systems (n = 7) | Mean (n = 31) | Islands (n = 5) | Swamp papyrus (n = 15) | Marshes water hyacinth (n = 11) | |
| Haplotype (h) | 22 | 11 | 8 | 5 | 17 | 4 | 10 | 7 |
| Haplotype diversity (h) | 0.978 ± 0.005 | 0.956 ± 0.0156 | 1.000 ± 0.022 | 0.905 ± 0.040 | 0.940 ± 0.005 | 0.900 ± 0.016 | 0.895 ± 0.022 | 0.909 ± 0.041 |
| Nucleotide diversity (π) | 0.01067 | 0.01134 | 0.01014 | 0.08387 | 0.00753 | 0.005 | 0.010 | 0.084 |
| FST (P-value) | 0.034 (0.045) | 0.014 (0.297) | 0.048 (0.072) | 0.047 (0.027) | 0.064 (0.020) | 0.077 (0.081) | − 0.016 (0.432) | 0.080 (0.000) |
| Tajima’s D (P-value) | − 0.126 (0.484) | − 1.092 (0.144) | 0.096 (0.575) | 0.618 (0.733) | − 1.158 (0.112) | − 1.162 (0.058) | − 0.606 (0.279) | − 0.317 (0.414) |
| Fu’s FS (P-value) | − 1.735 (0.237) | − 2.549 (0.097) | − 3.273 (0.022) | 0.617 (0.585) | − 5.439 (0.014) | − 0.445 (0.277) | − 2.088 (0.149) | 0.644 (0.312) |
Note: The ordering of specimens was based on the habitats they were found
The inbreeding coefficients (FST), defined from the AMOVA, for B. truncatus and Bulinus sp. 2 populations were 0.034 (P = 0.045) and 0.064 (P = 0.020) respectively. These FST values demonstrate an apparently low genetic differentiation between habitats. Table 2 summarises the genetic variations of the Bulinus in these groups occurring in Lake Victoria. Generally, FST values (0.021–0.023) between and within habitats groups were low (Table 2) indicating that the gene flow among Bulinus species populations and subpopulations within the Lake Victoria is high. The AMOVA concurs with the haplotype network, in which there was no clear demarcation between the localities where a given specimen was collected and its genetic affiliation with other haplotypes (Figs. 3, 4).
The estimates of Tajima’s D and Fu’s Fs test of Bulinus populations from Lake Victoria (i.e. within the lake, banks and surrounding marshes) were negative and statistically significant (Table 2), which denotes that the Bulinus species in the lake have undergone a recent population expansion. With a 95% confidence interval (CI), estimates of θ0 and θ1 for Bulinus species indicated that populations expanded, both demographically and spatially, from a compact to a considerable size (Table 3). Using the tau values (τ) of 3.787 and 4 for the B. truncatus in the open water and Bulinus sp. 2 occurring in the banks and marshes of Lake Victoria, we roughly estimate the starting time for Bulinus rapid population expansion to be between 207,694 (± 107,823) and 464,678 (± 278,312) years ago (Table 3).
Table 3.
Results of the mismatch distribution analyses for the demographic and spatial expansions of the Bulinus species from Lake Victoria populations and time since expansion
| Bulinus truncatus | Bulinus sp. 2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Demographic expansion | Spatial expansion | Demographic expansion | Spatial expansion | |||||||||
| Lentic sand substrate | Lentic stones & rock substrates | Lotic habitats | Lentic sand substrate | Lentic stones & rock substrates | Lotic habitats | Islands | Swamp papyrus | Marshesa | Islands | Swamp papyrus | Marshesa | |
| SSD (P-value) | 0.095 (0.280) | 0.079 (< 0.0001) | 0.024 (0.160) | 0.095 (0.430) | 0.069 (0.040) | 0.020 (0.600) | 0.023 (0.130) | 0.087 (0.010) | 0.069 (0.170) | 0.021 (0.370) | 0.059 (0.100) | 0.063 (0.410) |
| RI (P-value) | 0.310 (0.280) | 0.219 (< 0.0001) | 0.073 (0.280) | 0.310 (0.510) | 0.219 (0.110) | 0.073 (0.640) | 0.032 (0.450) | 0.092 (0.580) | 0.213 (0.200) | 0.032 (0.680) | 0.092 (0.430) | 0.213 (0.390) |
| Theta0/Theta | 0.000 | 1.900 | 0.000 | 0.05589 | 0.001 | 0.001 | 0.000 | 5.500 | 1.622 | 0.010 | 0.010 | 1.180 |
| Theta1 | 16.211 | 3414.978 | 34.961 | 46.951 | 3414.978 | 49.882 | ||||||
| τ (Cl) | 3.469 (2.258-6.211) | 4.000 (2.822–7.725) | 5.556 (2.994–7.803) | 3.233 (1.555-6.146) | 5.811 (2.309–7.509) | 5.363 (2.788–7.639) | 6.277 (3.646–8.352) | 5.000 (3.555–13.682) | 5.438 (3.344–10.984) | 6.121 (3.805–7.578) | 7.234 (2.901–8.282) | 5.453 (2.619–10.173) |
| T in years | 222,824 | 256,951 | 356,946 | 207,694 | 373,288 | 344,497 | 403,243 | 321,188 | 349,293 | 393,184 | 464,678 | 350,287 |
| ΔT in years | ± 77,788 | ± 75,655 | ± 164,610 | ± 107,823 | ± 224,936 | ± 165,400 | ± 169,000 | ± 92,843 | ± 134,498 | ± 148,784 | ± 278,312 | ± 182,033 |
Abbreviations: SSD, sum of squared deviations; RI, raggedness index; CI, 95% confidence interval; τ, population parameter Tau; T, time since expansion
aWater hyacinth
There was no significant correlation between genetic and geographical distances within the Bulinus population (B. truncatus) inhabiting the proper lake (r2 = 0.018, P > 0.05) or those (Bulinus sp. 2) from the banks, islands and marshes (r2 = 0.0038, P > 0.05). Overall all Bulinus samples from Lake Victoria did not exhibit any correlation between genetic variations and distance (r2 = 0.0175, P > 0.05), indicating the variation in genetic distance is mainly due to taxonomic differences as already shown by both phylogeny and parsimony networks.
Discussion
Identity of Bulinus in Lake Victoria and their phylogenetic affinities
The present study, to our knowledge, is the first to apply molecular techniques on the longitudinally surveyed Bulinus species occurring in Lake Victoria. A majority of studies on molluscs in Lake Victoria have been conducted on Biomphalaria species for their role in the spread of intestinal schistosomiasis [13, 18, 25]. The present study provides molecular-based evidence on the presence of two Bulinus groups in the lake; B. truncatus/B. tropicus occupying the open waters, covering sand beaches, stones and submerged rocks, while B. africanus group dominates the banks, small islands and surrounding marshes. Although the number of species determined by PTP and GMYC was slightly indecisive, the present study supports previous findings [27, 31, 35–37] that molecular methods could delineate the monophyletic subclade comprising of B. truncatus and its sibling B. tropicus (Fig. 2), which are morphologically difficult to distinguish [17].
From Mandahl-Barth [64] to present, the taxonomy of Bulinus species in Lake Victoria is in scrutiny. According to Brown [17], four species of Bulinus occur in Lake Victoria and the most common are the coexisting diploid and tetraploid populations forming the B. truncatus/B. tropicus complex that lack an apparent taxonomic boundary. Other Bulinus material was classified as B. trigonus and B. transversalis [64], though Brown [17] suggested that they might be lacustrine morphs of B. tropicus and B. truncatus. However, the present molecular analysis of material from Bumbire Island, the type-locality for B. transversalis [17], grouped the material with Bulinus sp. 2, which is regarded by the present study as B. ugandae. Nonetheless, the specimens from the island were smaller than those collected from the banks and marshes elsewhere. Although potentially topotypic material was collected and a single species only occurred there, we cannot conclude that Bulinus sp. 2 is, in fact, B. transversalis. Morphological characteristics of the snails studied here suggest that nowadays the waters around the island are rather inhabited by B. ugandae.
Phylogenetic analysis accompanied by SDMs also revealed a unique MOTU of Bulinus, Bulinus sp. 2, in Lake Victoria (Fig. 2, Clade II/Subclade I), which was strongly supported as sister to B. globosus in the B. africanus group. Although our phylogenetic analyses did not find sequences of Bulinus from Lake Victoria in the GenBank database to compare with, our sampling is reasonable to relate the Bulinus sp. 2 to B. ugandae. In our perusal of the literature regarding genus Bulinus in Lake Victoria, only B. ugandae shares similar features to the present material. Both Mandahl-Barth [29] and Brown [17] while scrutinising the morphological characters of B. ugandae, they questioned its taxonomic position in relation to B. globosus. Loker et al. [65] also acknowledged the challenging task of separating accurately B. globosus and B. ugandae from the Lake Victoria region. Moreover, Mwambungu [32] encountered B. ugandae in the Speke Gulf of the lake on the Tanzanian side and Ngupula & Kayanda [33] found B. ugandae and B. transversalis in Uganda. A study Opisa et al. [34] also found B. globosus distributed along the shores of Lake Victoria in Kisumu, Kenya. The close relatedness of the present specimen and Bulinus sp. (GenBank: AM286297) from Kisumu in Kenya [26] further shows a wide distribution of B. ugandae in Lake Victoria. While the separation of B. ugandae from B. globosus morphologically is paradoxical [17, 29], most workers used the names interchangeably.
In this analysis, we also found a unique MOTU of Bulinus (Bulinus sp. 1) which were collected in the southern part of Lake Victoria at Sweya beach in Nyegezi, Mwanza. The strong phylogenetic support for Bulinus sp. 1 (BS = 100%, PP = 1.00; Fig. 2) within the B. truncatus/B. tropicus clade and the separation of B. truncatus and B. tropicus haplotypic networks (Fig. 3), is a clear indication that Bulinus sp. 1 is a different species. The closest match to the cox1 sequences of Bulinus sp. 1 was 97.22% with B. tropicus (GenBank: KJ157492) from Cameroon [36]. Morphologically, Bulinus sp. 1 were similar to other members of the B. truncatus/B. tropicus species complex except that they were found co-existing with B. tropicus and physids in much shallower water on the mud-covered sand beach. Given that this species is neither B. truncatus nor B. tropicus nor B. transversalis (see above), we remain with B. trigonus as the sole known member of the B. truncatus/B. tropicus complex for Lake Victoria. More research is, however, needed to decide whether Bulinus sp. 1 indeed represents B. trigonus.
It is noteworthy that the shallow lake systems west of Lake Victoria harbour at least two different Bulinus species (i.e. B. nasutus productus and B. forskalii; see Figs. 2, 3, 4). Summarizing the current Bulinus diversity (Table 4), the Lake Victoria fauna consists of at least four species: B. truncatus; B. tropicus; Bulinus sp. 1 (B. trigonus?); and B. ugandae (Bulinus sp. 2).
Table 4.
Species diversity of the genus Bulinus in the Lake Victoria basin
| Species | Occurrence | Role as host | Reference | Present study |
|---|---|---|---|---|
| B. africanus | Near LV in Kenya, Mwanza, Tanzania | Main host in South Africa, NW Tanzania | Brown [17] | Not found |
| B. globosus | Mwanza, LV, Kisumu | Southern Africa, Main host in NW Tanzania | Loker et al [65]; Opisa et al [34] | Not found |
| B. forskalii | LV | not confirmed | Brown [17] | B. forskalii (not found in lake proper) |
| B. nasutus productus | Eastern shore LV | Main host in NW Tanzania | Brown [17] Mandahl-Barth [14] | B. nasutus productus (not found in lake proper) |
| B. tropicus | Not mentioned before | Not known | B. tropicus | |
| B. reticulatus | Near Kisumu and Mwanza | Not known | Brown [17]; Loker et al [65] | Not found |
| B. trigonus | LV and Lake Edward | B. truncatus: main host in NE, W and N Africa | Brown [17] | B. trigonus? |
| B. transversalis | LV and Victoria Nile | Not known | Brown [17]; Mandahl-Barth [14] | Not found |
| B. ugandae | LV, NW Tanzania | Not known | Brown [17]; Mandahl-Barth [14] | B. ugandae |
Notes: Taxa mentioned in the literature, their distribution, assumed or proven roles as intermediate hosts for S. haematobium are provided. Where possible, findings from the recent study are compared to the previous information
Abbreviation: LV, Lake Victoria
Genetic population analysis
The genetic variation, analysis of molecular variance (AMOVA), and isolation by distance showed Bulinus species populations in Lake Victoria to be panmictic. The overall FST value (0.034) in cox1 was significantly low, which may be explained by high gene flow rates among Bulinus populations in Lake Victoria to favour the evolution of phenotypic plasticity within species [66]. Also, AMOVA produced FST values within populations ranging from 0.00–0.080, meaning Bulinus species in Lake Victoria consist of overlapping populations. However, the ranges of genetic differentiation between populations (0.00–0.08) are comparable to previous studies on Bulinus species [67–69], who attributed the variations to self-fertilization within the populations. Given the size of the lake and high gene flow observed, it can be hypothesized that Bulinus species in Lake Victoria could be both cross and self-fertilizers. The cross-fertilization and pathogenesis in the banks and surrounding marshes may be increased due to intrusion of water weeds water hyacinth (Eichhornia crassipes), which are implicated in creating new habitats for snails [70, 71]. Moreover, our findings corroborate Standley et al. [13] who argued about the impossibility of sudden demographical events that would influence the genetic diversity and population structure of snail populations in Lake Victoria.
Studies in Lake Victoria have shown that, despite its large size, it is one of the youngest large lakes in the African Rift and has existed only 400,000 years ago with three complete desiccations in between, and the current water body was refilled about 14,600 years ago [15]. In contrast, our findings showed the Bulinus populations in Lake Victoria began spatial and demographic expansion about 99,700–743,000 years before the present. The explanation may be twofold, (i) the snails colonized the lake from neighbouring aquatic systems during the last refilling and (ii) the lake did not completely dry to reflect the 100,000 years of Milankovitch climate forcing cycles [10, 15]. Both scenarios could be associated with the low levels of genetic variation and population structure indices at the intrapopulation level within the Bulinus species in Lake Victoria [29]. Our results, however, support the scenario that the current biota in Lake Victoria recolonized the refilling lake from refugia as argued by Nalugwa et al. [72] given that about 100,000 years ago Lake Victoria probably collected its waters from regions near Lake Tanganyika [10]. The occurrence of Bulinus species in Lake Sagara in the Ugalla-Malagarasi drainage system in western Tanzania [37] and B. truncatus in Lakes Kivu and Tanganyika (Katosho swamp) [73], respectively, similar to those found in Lake Victoria, further supports the invasion theory.
Ecological aspects
Lake Victoria experienced tremendous ecological perturbations in the Anthropocene, and human activities nowadays might contribute significantly to the mixing of populations across the lake and adjacent aquatic ecosystems [74]. Even though we found no indication of such human effects for the Bulinus populations studied, future studies employing more sensitive markers should focus on these potentially confounding factors affecting population structures across the lake. Differential impacts of human disturbances on snail existence and abundances have been demonstrated in the Kenyan part of Lake Victoria [75]. Whereas some species might disappear, others, including intermediate host snails, i.e. pulmonates generally, might be even favoured by eutrophication processes and as such might increase the risks of transmission [75, 76]. The general abundance of pulmonate snails is high throughout the lake and marsh systems (FC and CA, personal observations). This in concert with reduced predator pressure from molluscivorous fishes might account for the comparatively high biomasses of certain gastropod species including some of the Bulinus spp. There is evidence for the roles of habitats in shaping (eco-)morphotypes in the less diverse Biomphalaria in Lake Victoria [77]. Such effects remain to be studied in detail for Bulinus, although our results so far indicated a link between habitat types and genetic diversity.
Parasitological implications of Bulinus species in Lake Victoria
Lake Victoria is one of the most well-known hotspots of schistosomiasis worldwide with fishing communities and school-aged children reported to be the most infected demographic groups in the surrounding countries of Kenya, Tanzania and Uganda [18–20, 23, 24]. However, a vast majority of reports on schistosomiasis in the lake and banks have focused on Biomphalaria species and their consequential S. mansoni [13, 18, 25]. There are two specific or subspecific forms of Biomphalaria species that preserve transmission of schistosomiasis in the lake: (i) B. sudanica, mainly found along the shores and surrounding marshes and swamps; and (ii) B. choanomphala, a more in-depth water inhabitant of Lake Victoria (Stanley et al. [18], but see Zhang et al. [25] for a discussion on species identified). The present findings showed that two dominant taxa of Bulinus occur in the lake: (i) B. ugandae (Bulinus sp. 2), mainly found along the banks and surrounding marshes and swamps in the mainland and islands; and (ii) members of B. truncatus/B. tropicus complex, which are found in open water habitats.
Although the present study did not test the collected snails for patent and prepatent infections with Schistosoma spp. or other digenean trematodes, the presence of certain Bulinus species in Lake Victoria potentially implies the presence of S. haematobium. Both B. truncatus/B. tropicus complex, B. africanus and B. forskalii group members have already been implicated in the transmission of S. haematobium elsewhere in Africa [17, 31, 78]. Bulinus nasutus productus has been known to occur around the eastern shore of the lake [33] and was now also found in the west. This species has been shown to be involved in S. haematobium transmission [12]. Even if B. tropicus is not known to be an intermediate host for Schistosoma species [17], the present findings are particularly important because hitherto the morphological distinction within B. truncatus/B. tropicus complex is challenging [17]. Bulinus truncatus is not yet known to be a host in equatorial Africa; however, there is potential [17] since it is the main host in the regions up the Nile river (Nile Province of South Sudan) where high prevalences of S. haematobium infections have been reported [79]. Bulinus ugandae is apparently not known to host S. haematobium but screening for B. globosus should continue in and around Lake Victoria. Given that B. africanus group members are found close by (B. nasustus and B. forskalii in satellite lakes that are hydrologically connected to Lake Victoria), there is a hidden risk for the prevalence of S. haematobium. Therefore, the occurrence and wide distribution of Bulinus species in Lake Victoria potentially threaten the health of communities living along the shores and on islands of the lake who depend on the lake for their livelihood. This situation is even triggered by the increasing pollution of the lake, which has recently been demonstrated to worsen the infection risks [80], this is yet another factor complicating the combat of schistosomiasis in this hotspot [24]. Future studies should undertake more experimental approaches to snail transmission. Another promising tool in predicting and identifying transmission potential (contamination and exposure) is the environmental DNA approach [81]. This has very recently been successfully used for environmental surveillance of schistosomiasis [82].
Previous studies on the prevalence of S. mansoni and S. haematobium showed the species were partitioned according to distance from the lake, i.e. S. mansoni occurred close to the lake and S. haematobium further on the hinterland [83]. Additionally, the spatial distribution of S. haematobium was in line with the presence of streams and ponds [79]. These observations imply that intermediate host species of Biomphalaria and Bulinus, the respective intermediate hosts for S. mansoni and S. haematobium, likely occur inside and outside the lake, respectively [18]. Our results, on the other hand, corroborate the previous observations that arrange of Bulinus species are present in the lake and are confirmed here to be widespread, but their role in S. haematobium transmission remains uncertain. A widely neglected aspect relates to schistosomiasis as a disease of veterinary concern [27]. Bulinus tropicus and B. ugandae are a well-known host for S. bovis, a parasite extensively infecting livestock [81]. Zoonotic schistosomiasis is currently largely underestimated [84] but could be studied in the setting of Lake Victoria in the future. Zoonotic schistosomiasis could be of high concern for both livestock and also wildlife existing in the adjacent world-famous national parks.
Conclusions
This study has reported two major Bulinus groups and at least four species occurring in Lake Victoria, B. truncatus/B. tropicus complex and B. africanus inhabiting vegetation-free sand and stone beaches, and banks and surrounding marshes/papyrus beds on the mainland and islands. These findings reflect previous findings on Biomphalaria species. Since in this study, we did not trace how far deep B. truncatus/B. tropicus complex can occur, we recommend a depth abundance relationship analysis for Bulinus species be carried out. Our findings also conclude that the assumed B. ugandae dominates the banks and surrounding marshes. Bulinus trigonus might indeed be a separate species whereas the B. transversalis remains to be studied genetically. Following our findings, a parasitological examination of Bulinus species around the lake is paramount to understanding their role in the epidemiology of urogenital schistosomiasis and its subsequential control. It is also recommended to study in parallel patterns in co-occurring Biomphalaria spp. throughout seasonal cycles and along environmental gradients.
Supplementary information
Additional file 1: Table S1. Summary of additional sequence data from the crater lakes and other regions retrieved from GenBank with the localities and haplotypes noted. Additionally, the locality, voucher, sequence and haplotype information for the Bulinus species from Lake Victoria studied for the first time herein are also given. Abbreviation: UGSB, University of Giessen Systematics and Biodiversity.
Additional file 2: Figure S1. The BI phylogenetic tree of Bulinus species with bars, on the right, denoting different species delimitation results, based on the dataset of concatenated cox1 sequences. Within the phylogeny, nodes supported and shared between BI and ML methods are marked with stars where support equates to 90–100% (ML) and 0.95–1 (BI). Names in bold denote specimens collected in the present study and the rest have been retrieved from the GenBank. Locality details are provided in Table 1. Blue colour represents different species, while green represents the same species as resolved by species delimitation methods. The information for sequences retrieved from the GenBank is presented in Additional file 1: Table S1. Abbreviations: SC1, Subclade 1; SC2, Subclade 2; SC3, Subclade 3; SC4, Subclade 4. The three-letter abbreviations represent countries: NIG, Nigeria; SAF, South Africa; UGA, Uganda; MLW, Malawi; TZA, Tanzania; CAM, Cameroon; SEN, Senegal; ZNZ, Zanzibar; KEN, Kenya; ANG, Angola; EGY, EGYPT; DRC, Democratic Republic of Congo.
Acknowledgments
We are grateful to Joseph Jude Agaba for sharing samples from western Uganda. We thank Silvia Nachtigall for technical assistance in the laboratory. We are also grateful to Richard Massinde of the University of Dar es Salaam for his technical assistance in the field. National Councils for Science and Technology (Uganda, Kenya) and Tanzania Commission for Science and Technology (COSTECH) are also acknowledged for providing the relevant collection permits.
Abbreviations
- SDMs
Species delimitation methods
- PTP
Poisson tree processes
- GMYC
Generalized mixed Yule coalescent
- AMOVA
Analysis of molecular variance
- ML
Maximum likelihood
- BI
Bayesian inference
- NCBI
The National Center for Biotechnology Information
Authors’ contributions
FC and CA conceived the study. FC carried out the sampling in the Tanzanian side of Lake Victoria. FC also produced the sequences and performed data analyses, with the help of AM, IT and AFS. IT and CA collected part of the material from Kenyan and Ugandan sides, and all authors were involved in data interpretation. Figures were produced by FC. All authors critically reviewed and approved the final manuscript.
Funding
FC was supported by the Alexander von Humboldt Research Fellowship for postdoctoral researchers. CA gratefully acknowledges support from the German Research Foundation (DFG).
Availability of data and materials
All data generated or analysed in the course of this study are included in the article, its additional files or have been deposited in the University of Giessen Systematics and Biodiversity (UGSB) repository, which are available upon request. Additionally, newly generated sequences were deposited in the GenBank database under the accession numbers MT707360-MT707433 (cox1) and MT707212-MT707241 (ITS2).
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Fred D. Chibwana, Email: fredchibwana@udsm.ac.tz, Email: fredchibwana@yahoo.com
Immaculate Tumwebaze, Email: tumwebazeimmaculate@gmail.com.
Anna Mahulu, Email: a_mahulu@yahoo.com.
Arthur F. Sands, Email: francis.sands@yahoo.com
Christian Albrecht, Email: christian.albrecht@allzool.bio.uni-giessen.de.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13071-020-04281-1.
References
- 1.Colley DG, Bustinduy AL, Secor WE, King CH. Human schistosomiasis. Lancet. 2014;383:2253–2264. doi: 10.1016/S0140-6736(13)61949-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. Schistosomiasis and soil transmitted helminthiases: numbers of people treated in 2017. Wkly Epidemiol Rec. 2018;93:681–92. [PubMed]
- 3.Chitsulo L, Engels D, Montresor A, Savioli L. The global status of schistosomiasis and its control. Acta Trop. 2000;77:41–51. doi: 10.1016/s0001-706x(00)00122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hotez PJ, Kamath A. Neglected tropical diseases in sub-Saharan Africa: review of their prevalence, distribution, and disease burden. PLoS Negl Trop Dis. 2009;3:e412. doi: 10.1371/journal.pntd.0000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burke ML, Jones MK, Gobert GN, Li YS, Ellis MK, McManus DP. Immunopathogenesis of human schistosomiasis. Parasite Immunol. 2009;31:163–176. doi: 10.1111/j.1365-3024.2009.01098.x. [DOI] [PubMed] [Google Scholar]
- 7.WHO . Progress report 2001–2011 and strategic plan 2012–2020. Geneva: World Health Organization; 2013. pp. 1–80. [Google Scholar]
- 8.O’Brien DP, Ford N, Djirmay AG, Calmy A, Vitoria M, Jensen TO, Christinet V. Female genital schistosomiasis and HIV: research urgently needed to improve understanding of the health impacts of this important coinfection. J Acquir Immune Defic Syndr. 2019;80:489–493. doi: 10.1097/QAI.0000000000001957. [DOI] [PubMed] [Google Scholar]
- 9.Danley PD, Husemann M, Ding B, DiPietro LM, Beverly EJ, Peppe DJ. The impact of the geological history and paleoclimate on the diversification of East African cichlids. Int J Evol Biol. 2012;2012:e574851. doi: 10.1155/2012/574851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salzburger W, Van Bocxlaer B, Cohen AS. Ecology and evolution of the African Great Lakes and their faunas. Annu Rev Ecol Evol Syst. 2014;45:519–545. [Google Scholar]
- 11.Verschuren D, Johnson TC, Kling HJ, Edgington DN, Leavitt PR, Brown ET, et al. History and timing of human impact on Lake Victoria, East Africa. Proc R Soc. B. 2002;269:289–294. doi: 10.1098/rspb.2001.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sayer CA, Máiz-Tomé L, Darwall WRT. Freshwater biodiversity in the Lake Victoria Basin: guidance for species conservation, site protection, climate resilience and sustainable livelihoods. Cambridge: IUCN; 2018. [Google Scholar]
- 13.Standley CJ, Goodacre SL, Wade CM, Stothard JR. The population genetic structure of Biomphalaria choanomphala in Lake Victoria, East Africa: implications for schistosomiasis transmission. Parasit Vectors. 2014;7:524. doi: 10.1186/s13071-014-0524-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mandahl-Barth G. The species of the genus Bulinus, intermediate hosts of Schistosoma. Bull World Health Organ. 1965;33:33–44. [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson TC, Kelts K, Odada E. The Holocene history of Lake Victoria. Ambio. 2000;29:2–11. [Google Scholar]
- 16.Stager JC, Johnson ATC. The late Pleistocene desiccation of Lake Victoria and the origin of its endemic biota. Hydrobiologia. 2008;596:5–16. [Google Scholar]
- 17.Brown DS. Freshwater snails of Africa and their medical importance. 2. London: Taylor & Francis; 1994. [Google Scholar]
- 18.Gouvras AN, Allan F, Kinung’hi S, Rabone M, Emery A, Angelo T, et al. Longitudinal survey on the distribution of Biomphalaria sudanica and B. choanomophala in Mwanza region, on the shores of Lake Victoria, Tanzania: implications for schistosomiasis transmission and control. Parasit Vectors. 2017;10:316. doi: 10.1186/s13071-017-2252-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiegand RE, Mwinzi PNM, Montgomery SP, Chan YYL, Andiego K, Omedo M, et al. A persistent hotspot of Schistosoma mansoni infection in a five-year randomized trial of praziquantel preventative chemotherapy strategies. J Infect Dis. 2017;216:1425–1433. doi: 10.1093/infdis/jix496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Exum NG, Kibira SPS, Ssenyonga R, Nobili J, Shannon AK, Ssempebwa JC, et al. The prevalence of schistosomiasis in Uganda: a nationally representative population estimate to inform control programs and water and sanitation interventions. PLoS Negl Trop Dis. 2019;13:e0007617. doi: 10.1371/journal.pntd.0007617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sang HC, Muchiri G, Ombok M, Odiere MR, Mwinzi PNM. Schistosoma haematobium hotspots in south Nyanza, western Kenya: prevalence, distribution and co-endemicity with Schistosoma mansoni and soil-transmitted helminths. Parasit Vectors. 2014;7:125. doi: 10.1186/1756-3305-7-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rowel C, Fred B, Betson M, Sousa-Figueiredo JC, Kabatereine NB, Stothard JR. Environmental epidemiology of intestinal schistosomiasis in Uganda: population dynamics of Biomphalaria (Gastropoda: Planorbidae) in Lake Albert and Lake Victoria with observations on natural infections with digenetic trematodes. Biomed Res Int. 2015;2015:717261. doi: 10.1155/2015/717261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kittur N, King CH, Campbell CH, Kinung’hi S, Mwinzi PNM, Karanja DMS, et al. Persistent hotspots in schistosomiasis consortium for operational research and evaluation studies for gaining and sustaining control of schistosomiasis after four years of mass drug administration of praziquantel. Am J Trop Med Hyg. 2019;101:617–627. doi: 10.4269/ajtmh.19-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mutuku MW, Laidemitt MR, Beechler BR, Mwangi IN, Otiato FO, Agola EL, et al. A search for snail-related answers to explain differences in response of Schistosoma mansoni to praziquantel treatment among responding and persistent hotspot villages along the Kenyan Shore of Lake Victoria. Am J Trop Med Hyg. 2019;101:65–77. doi: 10.4269/ajtmh.19-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang SM, Bu L, Laidemitt MR, Lu L, Mutuku MW, Mkoji GM, et al. Complete mitochondrial and rDNA complex sequences of important vector species of Biomphalaria, obligatory hosts of the human-infecting blood fluke, Schistosoma mansoni. Sci Rep. 2018;8:7341. doi: 10.1038/s41598-018-25463-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kane RA, Southgate VR, Rollinson D, Littlewood DTJ, Lockyer AE, Pags JR, et al. A phylogeny based on three mitochondrial genes supports the division of Schistosoma intercalatum into two separate species. Parasitology. 2008;127:131–137. doi: 10.1017/s0031182003003421. [DOI] [PubMed] [Google Scholar]
- 27.Tumwebaze I, Clewing C, Dusabe MC, Tumusiime J, Kagoro-Rugunda G, Hammoud C, et al. Molecular identification of Bulinus spp. intermediate host snails of Schistosoma spp. in crater lakes of western Uganda with implications for the transmission of the Schistosoma haematobium group parasites. Parasit Vectors. 2019;12:565. doi: 10.1186/s13071-019-3811-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madsen H. Schistosoma intermediate host snails. In: Jamieson BGM, editor. Schistosoma Biology, Pathology and Control. 1. Boca Raton: CRC Press; 2017. [Google Scholar]
- 29.Mandahl-Barth G. Intermediate hosts of Schistosoma: African Biomphalaria and Bulinus. II. Bull World Health Organ. 1957;17:1–65. [PMC free article] [PubMed] [Google Scholar]
- 30.Jørgensen A, Stothard JR, Madsen H, Nalugwa A, Nyakaana S, Rollinson D. The ITS2 of the genus Bulinus: novel secondary structure among freshwater snails and potential new taxonomic markers. Acta Trop. 2013;128:218–225. doi: 10.1016/j.actatropica.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 31.Abe EM, Guo YH, Shen H, Mutsaka-Makuvaza MJ, Habib MR, Xue JB, et al. Phylogeography of Bulinus truncatus (Audouin, 1827) (Gastropoda: Planorbidae) in selected African countries. Trop Med Infect Dis. 2018;3:127. doi: 10.3390/tropicalmed3040127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mwambungu J. The diversity of benthic mollusks of Lake Victoria and Lake Burigi. Tanzania J Sci. 2004;30:21–32. [Google Scholar]
- 33.Ngupula GW, Kayanda R. Benthic macrofauna community composition, abundance and distribution in the Tanzanian and Ugandan inshore and offshore waters of Lake Victoria. African J Aquat Sci. 2010;35:185–192. [Google Scholar]
- 34.Opisa S, Odiere MR, Jura WGZO, Karanja DMS, Mwinzi PNM. Malacological survey and geographical distribution of vector snails for schistosomiasis within informal settlements of Kisumu City, western Kenya. Parasit Vectors. 2011;4:226. doi: 10.1186/1756-3305-4-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nyakaana S, Stothard JR, Nalugwa A, Webster BL, Lange CN, Jørgensen A, et al. Bulinus globosus (Planorbidae; Gastropoda) populations in the Lake Victoria basin and coastal Kenya show extreme nuclear genetic differentiation. Acta Trop. 2013;128:226–233. doi: 10.1016/j.actatropica.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 36.Zein-Eddine R, Djuikwo-Teukeng FF, Al-Jawhari M, Senghor B, Huyse T, Dreyfuss G. Phylogeny of seven Bulinus species originating from endemic areas in three African countries, in relation to the human blood fluke Schistosoma haematobium. BMC Evol Biol. 2014;14:271. doi: 10.1186/s12862-014-0271-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kane RA, Stothard JR, Emery AM, Rollinson D. Molecular characterization of freshwater snails in the genus Bulinus: a role for barcodes? Parasit Vectors. 2008;1:15. doi: 10.1186/1756-3305-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilke T, Davis G, Qiu D, Spear R. Extreme mitochondrial sequence diversity in the intermediate schistosomiasis host Oncomelania hupensis robertsoni: another case of ancestral polymorphism? Malacologia. 2006;48:143–157. [Google Scholar]
- 39.Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol. 1994;3:294–299. [PubMed] [Google Scholar]
- 40.Wilke T, Davis GM. Infraspecific mitochondrial sequence diversity in Hydrobia ulvae and Hydrobia ventrosa (Hydrobiidae: Rissooidea: Gastropoda): do their different life histories affect biogeographic patterns and gene flow? Biol J Linn Soc. 2000;70:89–105. [Google Scholar]
- 41.Almeyda-Artigas RJ, Bargues MD, Mas-Coma S. ITS-2 rDNA sequencing of Gnathostoma species (Nematoda) and elucidation of the species causing human gnathostomiasis in the Americas. J Parasitol. 2000;86:537. doi: 10.1645/0022-3395(2000)086[0537:IRSOGS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 42.Bargues MD, Vigo M, Horak P, Dvorak J, Patzner RA, Pointier JP, et al. European Lymnaeidae (Mollusca: Gastropoda), intermediate hosts of trematodiases, based on nuclear ribosomal DNA ITS-2 sequences. Infect Genet Evol. 2001;1:85–107. doi: 10.1016/s1567-1348(01)00019-3. [DOI] [PubMed] [Google Scholar]
- 43.Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, et al. Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinforma Appl Note. 2012;28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- 46.Katoh K, Standley DM. MAFFT Multiple Sequence Alignment Software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vaidya G, Lohman DJ, Meier R. SequenceMatrix: concatenation software for the fast assembly of multi-gene datasets with character set and codon information. Cladistics. 2011;27:171–180. doi: 10.1111/j.1096-0031.2010.00329.x. [DOI] [PubMed] [Google Scholar]
- 48.Posada D. jModelTest: phylogenetic model averaging. Mol Biol Evol. 2008;25:1253–1256. doi: 10.1093/molbev/msn083. [DOI] [PubMed] [Google Scholar]
- 49.Stamatakis A. Phylogenetics RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinforma Appl Note. 2006;22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- 50.Fujisawa T, Barraclough TG. Delimiting species using single-locus data and the generalized mixed Yule coalescent approach: a revised method and evaluation on simulated data sets. Syst Biol. 2013;62:707–724. doi: 10.1093/sysbio/syt033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Drummond AJ, Suchard MA, Dong X, Andrew R. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol Biochem Parasitol. 2012;29:1969–1973. doi: 10.1093/molbev/mss075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rambaut A, Drummond AJ. Tracer 1.5, 2009. MCMC trace file analyser. 2009. http://tree.bio.ed.ac.uk/software/tracer/. Accessed 30 April 2020
- 53.Zhang J, Kapli P, Pavlidis P, Stamatakis A. Phylogenetics a general species delimitation method with applications to phylogenetic placements. Bioinformatics. 2013;29:2869–2876. doi: 10.1093/bioinformatics/btt499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clement M, Posada D, Crandall KA. TCS: a computer program to estimate gene genealogies. Mol Ecol. 2000;9:1657–1659. doi: 10.1046/j.1365-294x.2000.01020.x. [DOI] [PubMed] [Google Scholar]
- 55.Nei M. Molecular evolutionary genetics. New York: Columbia University Press; 1987. [Google Scholar]
- 56.Rozas J, Ferrer-Mata A, Carlos anchez-DelBarrio JS, Guirao-Rico S, Librado P, an Ramos-Onsins SE, et al. DnaSP 6 DNA sequence polymorphism analysis of large data sets. Mol Biol Evol. 2017;34:3299–3302. doi: 10.1093/molbev/msx248. [DOI] [PubMed] [Google Scholar]
- 57.Excoffier L, Lischer HEL. Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Mol Ecol Resour. 2010;10:564–567. doi: 10.1111/j.1755-0998.2010.02847.x. [DOI] [PubMed] [Google Scholar]
- 58.Tajima F. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics. 1989;123:585–595. doi: 10.1093/genetics/123.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fu YX. Statistical tests of neutrality of mutations against population growth, hitchhiking and background selection. Genetics. 1997;147:915–925. doi: 10.1093/genetics/147.2.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Slatkin M, Hudsont RR. Pairwise comparisons of mitochondrial DNA sequences in stable and exponentially growing populations. Genetics. 1991;129:555–562. doi: 10.1093/genetics/129.2.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wilke T, Schultheiß R, Albrecht C. As time goes by: a simple fool’s guide to molecular clock approaches in invertebrates. Am Malacol Bull. 2009;27:25–45. [Google Scholar]
- 62.Peakall R, Smouse PE. GenAlEx 65: genetic analysis in Excel. Population genetic software for teaching and research an update. Bioinformatics. 2012;28:2537–2539. doi: 10.1093/bioinformatics/bts460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mandahl-Barth G. The freshwater mollusks of Uganda and adjacent territories. Ann Mus Congo Tervuren, Zool. 1954;32:1–206. [Google Scholar]
- 65.Loker ES, Moyo HG, Gardner SL. Trematode-gastropod associations in nine non-lacustrine habitats in the Mwanza region of Tanzania. Parasitol. 1981;83:381–399. [Google Scholar]
- 66.Hollander J, Ahlgren J, Brönmark C. Rates of gene flow in a freshwater snail and the evolution of phenotypic plasticity. Biol J Linn Soc. 2017;121:764–770. [Google Scholar]
- 67.Charbonnel N, Angers B, Rasatavonjizay R, Bremond P, Debain C, Jarne P. The influence of mating system, demography, parasites and colonization on the population structure of Biomphalaria pfeifferi in Madagascar. Mol Ecol. 2002;11:2213–2228. doi: 10.1046/j.1365-294x.2002.01586.x. [DOI] [PubMed] [Google Scholar]
- 68.Mavarez J, Amarista M, Pointier JP, Jarne P. Fine-scale population structure and dispersal in Biomphalaria glabrata, the intermediate snail host of Schistosoma mansoni, in Venezuela. Mol Ecol. 2002;11:879–889. doi: 10.1046/j.1365-294x.2002.01486.x. [DOI] [PubMed] [Google Scholar]
- 69.Nalugwa A, Kristensen TK, Nyakaana S, Jørgensen A. Mitochondrial DNA variations in sibling species of the Bulinus truncatus/tropicus complex in Lake Albert, Western Uganda. Zool Stud. 2010;49:515–522. [Google Scholar]
- 70.Carolus H, Muzarabani KC, Hammoud C, Schols R, Volckaert FAM, Barson M, et al. A cascade of biological invasions and parasite spillback in man-made Lake Kariba. Sci Total Environ. 2019;659:1283–1292. doi: 10.1016/j.scitotenv.2018.12.307. [DOI] [PubMed] [Google Scholar]
- 71.Plummer ML. Impact of invasive water hyacinth (Eichhornia crassipes) on snail hosts of schistosomiasis in Lake Victoria, East Africa. Ecohealth. 2005;2:81–86. [Google Scholar]
- 72.Nalugwa A, Jørgensen A, Nyakaana S, Kristensen TK, Jørgensen A. Molecular phylogeny of Bulinus (Gastropoda: Planorbidae) reveals the presence of three species complexes in the Albertine Rift freshwater bodies. Int J Genet Mol Biol. 2010;2:130–139. [Google Scholar]
- 73.Rollinson D, Stothard JR, Southgate VR. Interactions between intermediate snail hosts of the genus Bulinus and schistosomes of the Schistosoma haematobium group. Parasitology. 2001;123:245–260. doi: 10.1017/s0031182001008046. [DOI] [PubMed] [Google Scholar]
- 74.Van Damme D, Seddon M, Carr JA. The status and distribution of freshwater molluscs in the Lake Victoria Basin. In: Sayer CA, Máiz-Tomé L, Darwall WRT, editors. Freshwater biodiversity in the Lake Victoria Basin guidance for species conservation, site protection, climate resilience and sustainable livelihoods. Cambridge: IUCN; 2018. pp. 65–81. [Google Scholar]
- 75.Lange CN, Kristensen TK, Madsen H. Gastropod diversity, distribution and abundance in habitats with and without anthropogenic disturbances in Lake Victoria, Kenya. Afr J Aquat Sci. 2013;38:295–304. [Google Scholar]
- 76.Standley CJ, Vounatsou P, Gosoniu L, Jørgensen A, Adriko M, Lwambo NJ, et al. The distribution of Biomphalaria (Gastropoda: Planorbidae) in Lake Victoria with ecological and spatial predictions using Bayesian modeling. Hydrobiologia. 2012;683:249–264. [Google Scholar]
- 77.Standley CJ, Wade CM, Stothard JR. A fresh insight into transmission of schistosomiasis: a misleading tale of Biomphalaria in Lake Victoria. PLoS ONE. 2011;6:10. doi: 10.1371/journal.pone.0026563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Deganello R, Cruciani M, Beltramello C, Duncan O, Oyugi V, Montresor A. Schistosoma hematobium and S. mansoni among children, southern Sudan. Emerg Infect Dis. 2007;13:1504–1506. doi: 10.3201/eid1310.070356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Siza JE, Kaatano GM, Chai JY, Eom KS, Rim HJ, Yong TS, et al. Prevalence of schistosomes and soil-transmitted helminths among school children in Lake Victoria basin, Tanzania. Korean J Parasitol. 2015;53:515–524. doi: 10.3347/kjp.2015.53.5.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Becker JM, Ganatra AA, Kandie F, Mühlbauer L, Ahlheim J, Brack W, et al. Pesticide pollution in freshwater paves the way for schistosomiasis transmission. Sci Rep. 2020;10:3650. doi: 10.1038/s41598-020-60654-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stothard JR, Campbell SJ, Osei-Atweneboana MY, Durant T, Stanton MC, Biritwum NK, et al. Towards interruption of schistosomiasis transmission in sub-Saharan Africa: developing an appropriate environmental surveillance framework to guide and to support ‘end game’ interventions. Infect Dis Poverty. 2017;6:10. doi: 10.1186/s40249-016-0215-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sengupta ME, Hellström M, Kariuki HC, Olsen A, Thomsen PF, Mejer H, et al. Environmental DNA for improved detection and environmental surveillance of schistosomiasis. Proc Natl Acad Sci. 2019;116:8931–8940. doi: 10.1073/pnas.1815046116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Angelo T, Buza J, Kinung’hi SM, et al. Geographical and behavioral risks associated with Schistosoma haematobium infection in an area of complex transmission. Parasit Vectors. 2018;11:481. doi: 10.1186/s13071-018-3064-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Standley CJ, Mugisha L, Dobson AP, Stothard JR. Zoonotic schistosomiasis in non-human primates: past, present and future activities at the human-wildlife interface in Africa. J Helminthol. 2012;86:131–140. doi: 10.1017/S0022149X12000028. [DOI] [PubMed] [Google Scholar]
- 85.Akinwale OP, Kane RA, Rollinson D, Stothard JR, Ajayi MB, Akande DO, et al. Molecular approaches to the identification of Bulinus species in south-west Nigeria and observations on natural snail infections with schistosomes. J Helminthol. 2011;85:283–293. doi: 10.1017/S0022149X10000568. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Summary of additional sequence data from the crater lakes and other regions retrieved from GenBank with the localities and haplotypes noted. Additionally, the locality, voucher, sequence and haplotype information for the Bulinus species from Lake Victoria studied for the first time herein are also given. Abbreviation: UGSB, University of Giessen Systematics and Biodiversity.
Additional file 2: Figure S1. The BI phylogenetic tree of Bulinus species with bars, on the right, denoting different species delimitation results, based on the dataset of concatenated cox1 sequences. Within the phylogeny, nodes supported and shared between BI and ML methods are marked with stars where support equates to 90–100% (ML) and 0.95–1 (BI). Names in bold denote specimens collected in the present study and the rest have been retrieved from the GenBank. Locality details are provided in Table 1. Blue colour represents different species, while green represents the same species as resolved by species delimitation methods. The information for sequences retrieved from the GenBank is presented in Additional file 1: Table S1. Abbreviations: SC1, Subclade 1; SC2, Subclade 2; SC3, Subclade 3; SC4, Subclade 4. The three-letter abbreviations represent countries: NIG, Nigeria; SAF, South Africa; UGA, Uganda; MLW, Malawi; TZA, Tanzania; CAM, Cameroon; SEN, Senegal; ZNZ, Zanzibar; KEN, Kenya; ANG, Angola; EGY, EGYPT; DRC, Democratic Republic of Congo.
Data Availability Statement
All data generated or analysed in the course of this study are included in the article, its additional files or have been deposited in the University of Giessen Systematics and Biodiversity (UGSB) repository, which are available upon request. Additionally, newly generated sequences were deposited in the GenBank database under the accession numbers MT707360-MT707433 (cox1) and MT707212-MT707241 (ITS2).