Abstract
Dietary protein may help prevent age-related declines in strength and functional capacity. This study examines the independent relationship between dietary protein and longitudinal changes in physical functioning among adults participating in the Framingham Offspring Study from examination 5 (1991–1995) to examination 8 (2005–2008). Protein intakes were derived from 3-day diet records during examinations 3 and 5; functional status was determined over 12 years using 7 items selected from standardized questionnaires. Multivariable models adjusted for age, sex, education, physical activity, smoking, height, and energy intake. Functional tasks that benefitted most from a higher-protein diet (≥1.2 g/kg/day vs. <0.8 g/kg/day) were doing heavy work at home, walking 1/2 mile (0.8 km), going up and down stairs, stooping/kneeling/crouching, and lifting heavy items. Those with higher protein intakes were 41% less likely (95% CI: 0.43, 0.82) to become dependent in 1 or more of the functional tasks over follow-up. Higher physical activity and lower body mass index were both independently associated with less functional decline. The greatest risk reductions were found among those with higher protein intakes combined with either higher physical activity, more skeletal muscle mass, or lower body mass index. This study demonstrates that dietary protein intakes above the current US Recommended Daily Allowance may slow functional decline in older adults.
Keywords: activities of daily living, aging, body composition, body mass index, cohort studies, dietary proteins, exercise
Aging is associated with progressive decline in muscle mass, strength, and physical functioning (1–3). Lean muscle mass declines from 50% of total body weight in young adults to 25% in adults aged 75–80 years (4). Age-related muscle loss potentially influences physical functioning adversely, particularly in the lower extremities (5), leading to increased risk of falling, restricted mobility, functional decline, and reduced life expectancy (6). At all ages, maintenance of lean mass requires a balance of muscle protein synthesis and breakdown.
Dietary protein stimulates muscle protein synthesis by activating the mammalian target of rapamycin (mTOR) signaling cascade (7). The current US Recommended Dietary Allowance for protein intake is 0.8 g/kg/day for adults (8). Recent studies suggest that for older people to maintain muscle mass and optimal physical functioning, greater amounts of dietary protein may be required (9,10) to overcome age-related anabolic resistance (11,12). One international panel of experts recommended protein intakes of 1.0–1.2 g/kg/day for adults ages 65 years or older, with even higher intakes recommended for older adults who are more physically active (13).
Some evidence provides support for beneficial effects of higher intakes of dietary protein during aging. For example, adults in the highest (vs. lowest) quintile of protein intake in the Health, Aging, and Body Composition (Health ABC) Study had 40% less decline in lean mass and appendicular lean mass over 3 years (14). In a prospective study, elderly subjects with higher intakes of protein (1.20–1.76 g/kg/day vs. <0.8 g/kg/day) had fewer health problems over 10 years of follow-up (15), and in the Women’s Health Initiative, women aged 65–79 years who had higher protein intakes had lower risks of frailty over 3 years of follow-up (16).
Several studies suggest that the greatest anabolic stimulus results from the combination of high intakes of dietary protein and resistance exercise (17,18). Questions remain about whether higher protein intake alone is sufficient to maintain muscle mass and functional performance among older adults.
The objective of this study was to examine the independent association of dietary protein intakes on long-term changes in physical functioning over more than a decade among initially middle-aged and older adults in the Framingham Offspring Study (FOS) and to determine whether any such protein-related association on functional status was modified by physical activity, level of skeletal muscle mass (SMM), or body mass index (BMI).
METHODS
Study population
The FOS began in 1971 in Framingham, Massachusetts, with the recruitment of 5,124 offspring (and their spouses) of participants in the original Framingham Study. After a second examination visit in 1980, 8 years after enrollment, FOS subjects were examined every 4 years.
For the current analyses, we excluded subjects who: 1) failed to attend examination visits 3, 4, or 5 and provide dietary record data (n = 1,840); 2) reported extreme intakes (for men, <1,200 or >4,000 calories per day; for women, <1,000 or >3,500 calories per day) or extreme intakes of protein-source foods (n = 322); 3) were less than 50 years old at examinations 3–5 (n = 868); 4) failed to attend or provide functional status data at examination 5, 6, 7, and 8 (n = 129); 5) failed to have more than 1 functional status measurement (n = 115); or 6) had prevalent cancer (except nonmelanoma skin cancer) at time of baseline functional assessment (n = 71). This left a total of 1,779 subjects for these analyses. However, we further excluded subjects from task-specific analyses who were dependent in those tasks at baseline. For example, subjects who were unable to walk 1/2 mile at baseline were excluded from analyses related to becoming dependent in that task at the end of follow-up. In addition, for later analyses designed to examine, for example, the risk of developing 1 or more dependencies, we included only those subjects who were independent in all tasks at baseline. The current analyses were conducted under the approval of the Boston University Institutional Review Board.
Dietary assessment
Dietary data were collected using 3-day diet records following standardized procedures and supervised by a trained nutritionist during examination cycles 3 (1983–1988) and 5 (1991–1995). Subjects used 2-dimensional food models to aid in estimating portion sizes. The dietary records were analyzed for nutrient content using the Nutrition Data System of the University of Minnesota (19).
Each subject’s dietary protein intake was expressed in 2 ways: 1) as grams per kilogram (g/kg) of total body weight per day, to allow for direct comparison with US Department of Agriculture recommended dietary intakes; and 2) as sex-specific weight-adjusted protein intakes (g/day) estimated using residuals from the linear regression models. The latter was used to account for intake differences attributable to body weight. The protein residual from the regression model, which is uncorrelated with body weight, was added to the sex-specific group mean weight to express intake on a more readily interpretable scale. Each subject’s usual protein intake was estimated as the mean intake level from all 6 dietary records (2 sets of 3-day records). Earlier studies have shown that dietary protein intake may be accurately estimated with 5–8 days of dietary records (20,21).
Functional status outcome assessment
Data on the functional status of FOS subjects were collected at each examination starting with examination 5 (1991–1995) and ending with examination 8 (2005–2008). Two well-validated scales were employed for this purpose: the Nagi scale (22) (a self-reported functional status scale of 11 items) and the Rosow-Breslau scale (23) (a self-reported 6-item scale measuring functional status on gross-mobility tasks).
For this study, we selected 7 tasks from the Rosow-Breslau and Nagi scales that were most related to the need for strength or endurance. The 3 Rosow-Breslau tasks included heavy work at home (e.g., shoveling or washing windows, walls, or floors), walking 1/2 mile (0.8 km, or about 4–6 blocks), and going up and down a flight of stairs. Subjects were asked to report their ability to do these tasks independently; those using a cane or other assistive device were considered independent as long as they did not require assistance from another person. The 4 tasks selected from Nagi Scale included pushing/pulling heavy objects (e.g., heavy living room chair); stooping, kneeling, or crouching; lifting >10 lb (>4.54 kg), for example, a very heavy bag of groceries); and lifting <10 lb (e.g., a bag of potatoes). Response categories included no difficulty, a little difficulty, some difficulty, a lot of difficulty, and unable to do. These responses were then dichotomized by collapsing the first 3 response categories (able) and the last 2 (unable).
Assessment of potential confounding and effect modification
Each subject’s educational level was categorized as having at least some college education versus less. Weight and height were measured to the nearest 0.25 pounds and 0.25 inches, respectively, using a standard counterbalance scale with a measuring bar. BMI at each examination was calculated as the subject’s examination-specific weight (in kg) divided by mean adult height (ages 18–60 for women and ages 21–60 for men) in meters squared. The use of mean height helps to reduce random measurement error and the effect of height loss after the age of 60.
Cigarette smoking information was collected routinely at every examination visit. Subjects who smoked at least 1 cigarette per day were considered current smokers, and amount smoked was estimated as the mean of cigarettes smoked per day during the exposure period (examinations 3–5). Alcohol intake was similarly estimated as mean intake per day (grams of alcohol) from examinations 3–5. Physical activity was assessed at examinations 2 and 4 by asking each subject to report number of hours/day spent in sleep and in sedentary, light, moderate, and vigorous activities in a 24-hour period. A physical activity index was derived by summing the product of the hours spent in moderate and vigorous activities multiplied times a weight based on the estimated energy expenditure (oxygen consumption) required for that activity as previously described (24).
Each subject’s SMM was determined using data from bioelectrical impedance analysis (BIA) and an equation derived by Janssen et al. (25):
where height was measured in centimeters, BIA resistance in ohms, sex was coded as men = 1 and women = 0, and age was in years. Estimated SMM (kilograms) was then converted to percentage SMM (%SMM) as follows:
Based on sensitivity analyses and statistical power considerations, %SMM was classified as low (<36% for men and <26% for women) or high (≥36% for men and ≥26% for women). These sex-specific cutoff values resulted in about 40% of subjects (both men and women) being classified as having low %SMM (reference group).
Statistical analysis
There were 2 analytical aims for this study: 1) to estimate the independent association of dietary protein intake on functional outcomes in adults, ages 50 or older; and 2) to determine whether physical activity, SMM, or BMI modifies the association of dietary protein with functional status over time. For these analyses, dietary protein was categorized in 2 ways, as g/kg/day (<0.8, 0.8–1.1, and ≥1.2) and as weight-adjusted protein derived from the residuals of a linear regression model, classified as sex-specific quintiles of intake as follows: quintile 1 (mean intakes: for men, 56.4 g/day; for women, 50.2 g/day); quintiles 2–4 (mean intakes: for men, 83.3 g/day; for women, 71.4 g/day); quintile 5 (mean intakes: for men, 115.6 g/day; for women, 97.8 g/day).
At each of examination visits 5 through 8, subjects were classified according to their ability to carry out each of the 7 selected functional tasks. In each category of protein intake, subjects who were independent in a given task at examination 5 (1991–1995) were followed prospectively through examination 8 (2005–2008), approximately 12 years, for the development of task-specific functional dependence by the end of the follow-up period. Additional analyses calculated the risk of becoming dependent in 1 or more functional disabilities and 2 or more functional disabilities. Person-years of follow-up was estimated as time from the end of the protein exposure period (at examination 5) to the first of the following events: 1) date of development of disability in any of the specific tasks of interest; 2) loss to follow-up; 3) death; or 4) end of the data collection period for this study (examination 8). The median follow-up time for all subjects was 13 years. The group with the lowest protein intake served as the reference category for all analyses. Cox proportional hazards models were used to estimate the risk of becoming dependent in each of the functional task outcomes over 12 years of follow-up.
To explore effect modification of protein intake on functional outcomes according to physical activity level, activity was dichotomized as lower or higher (i.e., lowest 2 sex-specific quintiles of activity vs. upper 3 quintiles of activity) using a sensitivity analysis. Possible effect modification by %SMM (<36% SMM vs. ≥36% for men and <26% SMM vs. ≥26% for women) or BMI (<28 versus ≥28) was also evaluated. Our evaluation of effect modification involved examining the stratum-specific hazard ratio estimates to determine whether the sum of the estimates from individual strata was consistent with an additive effect.
To assess overall functional decline, a weighted composite score using the 7 selected tasks from the Rosow-Breslau and Nagi scales was created with input from experts in the field of disability assessment. Each task was given a weight based on the level of strength and/or endurance needed to complete the task. The Rosow-Breslau tasks were scored as follows: 1) heavy work around the house (washing windows walls or floors, shoveling): 0 = unable, 4 = able; 2) walking up and down stairs to second floor: 0 = unable, 3 = able; and 3) walking 1/2 mile (about 4–6 blocks): 0 = unable, 4 = able. The tasks from the Nagi scale were scored as follows: 1) pulling or pushing large objects (e.g., living room chair): 0 = unable, 1 = a lot of difficulty, 2 = some difficulty, 3 = a little difficulty, 4 = no difficulty; 2) stooping, crouching, or kneeling: 0 = unable, 1 = a lot of difficulty, 2 = some difficulty, 3 = a little difficulty, 4 = no difficulty; 3) lifting or carrying weights over 10 lb (e.g., very heavy bag of groceries): 0 = unable, 1 = a lot of difficulty, 2 = some difficulty, 3 = a little difficulty, 4 = no difficulty; and 4) lifting or carrying weights under 10 lb (e.g., bag of potatoes): 0 = unable, 0.5 = a lot of difficulty, 1 = some difficulty, 1.5 = a little difficulty, 2 = no difficulty. Each subject’s functional score was the sum of all points for the 7 specified tasks (with a maximum total score of 25 points), calculated separately for each year from examinations 5 through 8. To explore the change in the functional status score over time according to usual protein intake group, longitudinal mixed models for repeated measures data were used. Subject-specific random intercepts were used to account for the correlation between the functional status scores measured over time. An unstructured covariance matrix assumption was used. All statistical analyses were carried out using SAS, version 9.3 (SAS Institute Inc., Cary, North Carolina).
RESULTS
Table1 shows the subject characteristics according to daily protein intake. Adults consuming 1.2 g/kg/day or more of protein were leaner, more physically active, drank more alcohol, and consumed a lower proportion of carbohydrates per day.
Table 1.
Mean Values and Standard Deviations of Baseline Characteristics According to Category of Protein Intake Among Subjects 50 Years of Age or Older, Framingham Offspring Study, Massachusetts, 1991–2008
| Characteristic | Category of Daily Protein Intake | P Valuea | ||
|---|---|---|---|---|
| <0.8 g/kg (n = 352) | 0.8–1.1 g/kg (n = 952) | ≥1.2 g/kg (n = 475) | ||
| Age, years | 56.4 (6.1) | 56.5 (5.7) | 56.0 (5.6) | 0.3692 |
| Height, cm | 167.6 (9.4) | 167.6 (9.4) | 166.8 (9.3) | 0.2757 |
| Weight, kg | 85.9 (17.9) | 75.5 (14.4) | 69.7 (12.7) | <0.0001 |
| BMIb | 30.5 (5.5) | 26.8 (3.8) | 24.9 (3.3) | <0.0001 |
| Physical activity index | 12.8 (8.9) | 12.5 (8.4) | 13.8 (8.9) | 0.0223 |
| No. of cigarettes/day | 3.8 (9.6) | 3.5 (9.2) | 4.0 (10.3) | 0.6903 |
| Alcohol intake, g/day | 9.2 (18.5) | 10.6 (14.7) | 12.8 (18.1) | 0.0063 |
| Nutrient intakes | ||||
| Energy intake, kcals | 1,494 (345.8) | 1,808 (434.6) | 2,227 (525.9) | <0.0001 |
| Fat, % kcals | 34.0 (6.2) | 34.5 (6.5) | 34.6 (6.6) | 0.3075 |
| Carbohydrates, % kcals | 48.8 (8.0) | 46.8 (7.9) | 45.6 (7.6) | <0.0001 |
| Protein, % kcals | 15.9 (3.3) | 17.1 (3.1) | 18.1 (3.0) | <0.0001 |
| Protein, g/kg/day | 0.7 (0.1) | 1.0 (0.1) | 1.4 (0.2) | <0.0001 |
| Protein (weight-adjusted)c | 54.0 (8.2) | 75.6 (10.7) | 101.3 (15.8) | <0.0001 |
| College educationd | 28.8 | 29.7 | 28.9 | 0.9913 |
| Male sexd | 43.2 | 46.6 | 48.6 | 0.1263 |
Abbreviations: BMI, body mass index; SD, standard deviation.
a P values comparing means (analysis of variance) and proportions (Mantel-Haenszel χ2).
b BMI was calculated as weight (kg)/height (m)2.
c Protein intake in grams per day adjusted for body weight, using residual method.
d Values are expressed as column percentages.
Table2 shows the unadjusted cumulative incidence of functional disability in each of the 7 observed tasks. There was a tendency for subjects with the lowest protein intakes (whether expressed as g/kg/day or g/day of weight-adjusted protein intake) to have a higher prevalence of disabilities at the end of 12 years of follow-up. For example, 18.0% of those in the lowest category of weight-adjusted protein residuals were unable to walk 1/2 mile by examination 8 compared with only 10.1% of those in the highest quintile of intake. The tasks for which there were the highest levels of disability were heavy work at home and walking 1/2 mile.
Table 2.
Prevalence of Functional Dependence in Selected Tasks at Examination 8 (2005–2008) Among Subjects Who Were Independent in Those Tasks at Examination 5 (1991–1995), Framingham Offspring Study, Massachusetts
| Scale and Functional Task | Daily Protein Intake | Daily Intake as Weight-Adjusted Protein Residualsa | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <0.8 g/kg | 0.8–1.1 g/kg | ≥1.2 g/kg | P for Trendb | Low | Moderate | High | P for Trendb | |||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |||
| Rosow-Breslau | ||||||||||||||
| Heavy work | 51 | 18.2 | 126 | 15.4 | 61 | 14.9 | 0.2775 | 52 | 18.6 | 138 | 15.0 | 48 | 15.4 | 0.3157 |
| Walk 1/2 milec | 56 | 18.5 | 106 | 12.4 | 45 | 10.6 | 0.0030 | 54 | 18.0 | 121 | 12.5 | 32 | 10.1 | 0.0037 |
| Flight of stairs | 26 | 8.6 | 42 | 4.9 | 28 | 6.5 | 0.3815 | 25 | 8.3 | 53 | 5.5 | 18 | 5.6 | 0.1696 |
| Nagi | ||||||||||||||
| Push/pull heavy objects | 23 | 7.6 | 46 | 5.4 | 23 | 5.4 | 0.2446 | 25 | 8.5 | 51 | 5.3 | 16 | 5.0 | 0.0741 |
| Stoop, kneel, crouch | 42 | 14.6 | 83 | 9.9 | 31 | 7.5 | 0.0025 | 37 | 12.9 | 94 | 10.0 | 25 | 8.0 | 0.0488 |
| Lift >10 lbc | 23 | 7.7 | 45 | 5.3 | 24 | 5.6 | 0.3010 | 21 | 7.1 | 53 | 5.5 | 18 | 5.6 | 0.4498 |
| Lift <10 lb | 13 | 4.2 | 16 | 1.8 | 8 | 1.8 | 0.0526 | 13 | 4.3 | 16 | 1.6 | 8 | 2.5 | 0.1452 |
a Sex-specific quintiles of weight-adjusted protein residuals. Low intake: quintile 1; moderate intake: quintiles 2–4; high intake: quintile 5.
b P value from Mantel-Haenszel χ2 test.
c One half mile is approximately 0.8 km, and 10 lb is approximately 4.54 kg.
To adjust for potential confounding, we used Cox proportional hazards models (Table3) to estimate hazard ratios for becoming dependent in each of the 7 functional tasks over 12 years. The confounding factors retained in the final models included age, sex, education, physical activity, cigarettes smoked per day, height, and total energy intake. For nearly all tasks, there was an inverse linear association between protein intake and risk of disability. Those with the highest protein intakes had the lowest risk of becoming dependent over 12 years. The strongest and most consistent findings were the beneficial associations of protein intake with walking 1/2 mile; climbing stairs; stooping, kneeling or crouching down; and lifting objects weighing <10 pounds.
Table 3.
Risk of Becoming Dependent in Selected Functional Tasks Over 12 Years, According to Protein Intake, Framingham Offspring Study, Massachusetts, 1991–2008
| Functional Task | No. | Daily Protein Intake | Daily Intake as Weight-Adjusted Protein Residualsa | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.8–1.1 g/kgb | ≥1.2 g/kgb | Moderateb | Highb | ||||||
| HRc | 95% CI | HRc | 95% CI | HRc | 95% CI | HRc | 95% CI | ||
| Heavy work at home | 1,633 | 0.84 | 0.62, 1.12 | 0.68 | 0.46, 1.01 | 0.77 | 0.58, 1.04 | 0.74 | 0.49, 1.13 |
| Walk 1/2 miled | 1,734 | 0.52 | 0.39, 0.71 | 0.39 | 0.26, 0.60 | 0.60 | 0.44, 0.82 | 0.42 | 0.26, 0.68 |
| Flight of stairs | 1,748 | 0.46 | 0.30, 0.71 | 0.43 | 0.24, 0.75 | 0.69 | 0.44, 1.09 | 0.47 | 0.24, 0.93 |
| Push or pull heavy objects | 1,724 | 0.95 | 0.62, 1.48 | 1.01 | 0.57, 1.78 | 0.91 | 0.59, 1.40 | 0.67 | 0.35, 1.29 |
| Stoop, kneel, crouch | 1,679 | 0.54 | 0.39, 0.74 | 0.32 | 0.20, 0.51 | 0.70 | 0.40, 0.98 | 0.39 | 0.23, 0.66 |
| Lift >10 lbd | 1,736 | 0.74 | 0.48, 1.15 | 0.54 | 0.30, 0.99 | 0.72 | 0.46, 1.12 | 0.55 | 0.28, 1.07 |
| Lift <10 lb | 1,776 | 0.42 | 0.21, 0.81 | 0.18 | 0.07, 0.51 | 0.37 | 0.19, 0.74 | 0.26 | 0.09, 0.78 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
a Sex-specific quintiles of weight-adjusted protein residuals. Low intake: quintile 1; moderate intake: quintiles 2–4; high intake: quintile 5.
b The reference category for protein intake (g/kg/day) was an intake <0.8 g/kg. The reference category for protein intake (residuals) was low protein intake (quintile 1).
c Adjusted for age, sex, education, physical activity, cigarettes per day, height, and total energy intake.
d One half mile is approximately 0.8 km, and 10 lb is approximately 4.54 kg.
Table4 examines the association of protein intake with the risk of becoming dependent in ≥1 and ≥2 of the 7 selected functional tasks over 12 years. For all subjects combined, those with the highest protein intakes were approximately 40% less likely to become dependent in 1 or more functional tasks and 50% less likely to become dependent in 2 or more functional tasks over 12 years. The hazard ratios were slightly stronger for women than for men.
Table 4.
Risk of Becoming Dependent in Multiple Functional Tasks According to Protein Intake, Framingham Offspring Study, Massachusetts, 1991–2008
| Task Dependence | Daily Protein Intake | Daily Intake as Weight-Adjusted Protein Residualsa | ||||||
|---|---|---|---|---|---|---|---|---|
| 0.8–1.1 g/kgb | ≥1.2 g/kgb | Moderateb | Highb | |||||
| HRc | 95% CI | HRc | 95% CI | HRc | 95% CI | HRc | 95% CI | |
| Dependent in ≥1 task | ||||||||
| All | 0.82 | 0.64, 1.04 | 0.59 | 0.43, 0.82 | 0.79 | 0.62, 1.01 | 0.61 | 0.42, 0.86 |
| Men | 0.71 | 0.49, 1.05 | 0.60 | 0.36, 1.00 | 0.88 | 0.60, 1.29 | 0.70 | 0.39, 1.25 |
| Women | 0.86 | 0.62, 1.18 | 0.55 | 0.36, 0.86 | 0.71 | 0.52, 0.98 | 0.53 | 0.33, 0.84 |
| Dependent in ≥2 tasks | ||||||||
| All | 0.67 | 0.49, 0.90 | 0.49 | 0.33, 0.74 | 0.79 | 0.58, 1.07 | 0.51 | 0.32, 0.81 |
| Men | 0.74 | 0.42, 1.30 | 0.90 | 0.45, 1.82 | 0.81 | 0.47, 1.39 | 0.65 | 0.29, 1.48 |
| Women | 0.64 | 0.45, 0.92 | 0.35 | 0.21, 0.59 | 0.78 | 0.53, 1.13 | 0.44 | 0.24, 0.79 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
a Sex-specific quintiles of weight-adjusted protein residuals. Low intake: quintile 1; moderate intake: quintiles 2–4; high intake: quintile 5.
b The reference category for protein intake (g/kg/day) was an intake of <0.8 g/kg. The reference category for protein intake (residuals) was low protein intake (quintile 1).
c Adjusted for age, sex, education, physical activity, cigarettes per day, height, and total energy intake.
Table5 shows results with %SMM added to the multivariable model as a potential causal intermediate. Nearly all of the effect estimates were attenuated, leading us to conclude that %SMM is likely to be a causal intermediate in the association between dietary protein and functional outcomes.
Table 5.
Evaluating Percentage of Skeletal Muscle Mass as a Possible Causal Intermediate in the Association Between Protein Intake and Functional Dependence at End of Follow-Up, Framingham Offspring Study, Massachusetts, 1991–2008
| Task Dependence | Daily Protein Intake | Daily Intake as Weight-Adjusted Protein Residualsa | ||||||
|---|---|---|---|---|---|---|---|---|
| 0.8–1.1 g/kgb | ≥1.2 g/kgb | Moderate | High | |||||
| HRc | 95% CI | HRc | 95% CI | HRc | 95% CI | HRc | 95% CI | |
| Dependent in ≥1 task | ||||||||
| All | 1.05 | 0.80, 1.37 | 0.85 | 0.59, 1.23 | 0.88 | 0.68, 1.14 | 0.68 | 0.46, 0.99 |
| Men | 1.00 | 0.63, 1.60 | 0.98 | 0.53, 1.83 | 0.98 | 0.62, 1.54 | 0.81 | 0.40, 1.62 |
| Women | 1.02 | 0.73, 1.43 | 0.74 | 0.47, 1.17 | 0.79 | 0.57, 1.09 | 0.59 | 0.37, 0.94 |
| Dependent in ≥2 tasks | ||||||||
| All | 0.83 | 0.59, 1.17 | 0.67 | 0.42, 1.06 | 0.88 | 0.62, 1.24 | 0.57 | 0.34, 0.95 |
| Men | 1.31 | 0.61, 2.81 | 1.66 | 0.66, 4.15 | 1.01 | 0.51, 2.02 | 0.86 | 0.31, 2.35 |
| Women | 0.77 | 0.52, 1.15 | 0.50 | 0.28, 0.88 | 0.87 | 0.58, 1.30 | 0.53 | 0.29, 0.97 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
a Sex-specific quintiles of weight-adjusted protein residuals. Low intake: quintile 1; moderate intake: quintiles 2–4; high intake: quintile 5.
b The reference category for protein intake (g/kg/day) was an intake of <0.8 g/kg. The reference category for protein intake (residuals) was low protein intake (quintile 1).
c Adjusted for age, sex, education, physical activity, cigarettes per day, height, total energy intake, and percentage of skeletal muscle mass.
Finally, Table6 presents the independent and combined associations of dietary protein and physical activity, SMM, and BMI. Subjects who were more active and consumed more protein were 61% less likely (95% CI: 0.24, 0.64) to become dependent in 1 or more functional tasks over 12 years. Higher physical activity alone led to a 43% reduction in risk, while being in the highest protein category alone was associated with a 51% reduction in risk of dependence in 1 or more tasks. In these stratified analyses, the association of dietary protein appeared to be modified by the amount of skeletal muscle mass. The effect estimates for higher %SMM combined with higher dietary protein intakes on functional dependence were stronger for women than for men (66% and 33% reduced risks, respectively). Finally, both men and women with a BMI <28 were less likely to experience functional decline. In particular, women with a lower BMI who consumed more protein had an even greater reduction in risk of functional decline. In general, the associations of dietary protein with % SMM and BMI were approximately additive.
Table 6.
Modification of the Association Between Dietary Protein and Functional Dependence According to Physical Activity, Percentage of Skeletal Muscle Mass, and Body Mass Index, Framingham Offspring Study, Massachusetts, 1991–2008
| Protein Intakea | Low Physical Activityb | High Physical Activityb | Low % SMMc | High %SMMc | BMI ≥28d | BMI <28d | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HRe | 95% CI | HRe | 95% CI | HRe | 95% CI | HRe | 95% CI | HRe | 95% CI | HRe | 95% CI | |
| All subjects | ||||||||||||
| Low | 1.00 | Referent | 0.57 | 0.37, 0.89 | 1.00 | Referent | 0.71 | 0.46, 1.10 | 1.00 | Referent | 0.67 | 0.45, 1.01 |
| Moderate | 0.68 | 0.48, 0.97 | 0.50 | 0.34, 0.74 | 0.96 | 0.68, 1.34 | 0.54 | 0.39, 0.76 | 1.00 | 0.72, 1.38 | 0.57 | 0.42, 0.77 |
| High | 0.49 | 0.29, 0.82 | 0.39 | 0.24, 0.64 | 0.65 | 0.39, 1.08 | 0.45 | 0.29, 0.71 | 0.78 | 0.46, 1.33 | 0.48 | 0.32, 0.71 |
| Men | ||||||||||||
| Low | 1.00 | Referent | 0.63 | 0.31, 1.30 | 1.00 | Referent | 1.05 | 0.49, 2.25 | 1.00 | Referent | 0.65 | 0.32, 1.34 |
| Moderate | 0.80 | 0.44, 1.43 | 0.57 | 0.30, 1.10 | 1.23 | 0.68, 2.22 | 0.73 | 0.40, 1.34 | 1.14 | 0.71, 1.83 | 0.62 | 0.39, 0.97 |
| High | 0.76 | 0.32, 1.79 | 0.42 | 0.19, 0.97 | 0.88 | 0.35, 2.21 | 0.67 | 0.30, 1.54 | 0.75 | 0.35, 1.62 | 0.61 | 0.32, 1.17 |
| Women | ||||||||||||
| Low | 1.00 | Referent | 0.56 | 0.31, 0.99 | 1.00 | Referent | 0.57 | 0.34, 0.98 | 1.00 | Referent | 0.67 | 0.40, 1.11 |
| Moderate | 0.59 | 0.38, 0.93 | 0.46 | 0.28, 0.75 | 0.79 | 0.52, 1.19 | 0.43 | 0.29, 0.65 | 0.88 | 0.56, 1.38 | 0.51 | 0.34, 0.77 |
| High | 0.36 | 0.19, 0.70 | 0.38 | 0.20, 0.70 | 0.55 | 0.29, 1.02 | 0.34 | 0.20, 0.59 | 0.85 | 0.40, 1.81 | 0.40 | 0.23, 0.67 |
Abbreviations: BMI, body mass index; CI, confidence interval; HR, hazard ratio; %SMM, percentage skeletal muscle mass.
a Protein intake from sex-specific residuals. Low intake: quintile 1; moderate intake: quintiles 2–4; high intake: quintile 5.
b Low physical activity: quintiles 1–2; high physical activity: quintiles 3–5 (using sex-specific quintiles).
c Low %SMM: <36% for men and <26% for women; high %SMM: ≥36% for men and ≥26% for women.
d BMI was calculated as weight (kg)/height (m)2.
e Adjusted for age, sex, education, physical activity, cigarettes per day, height, and total energy intake.
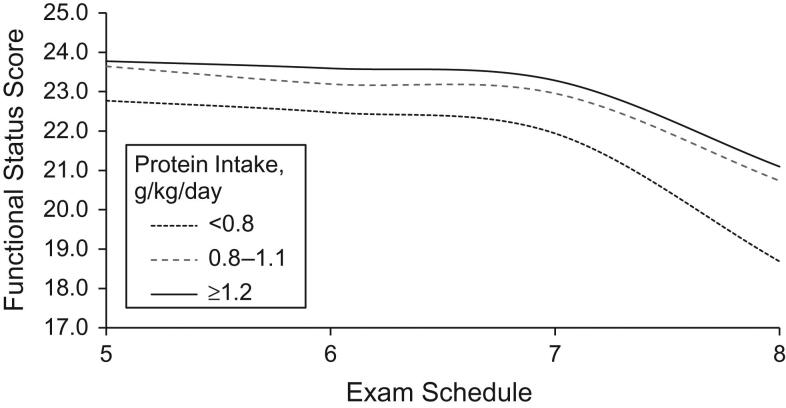
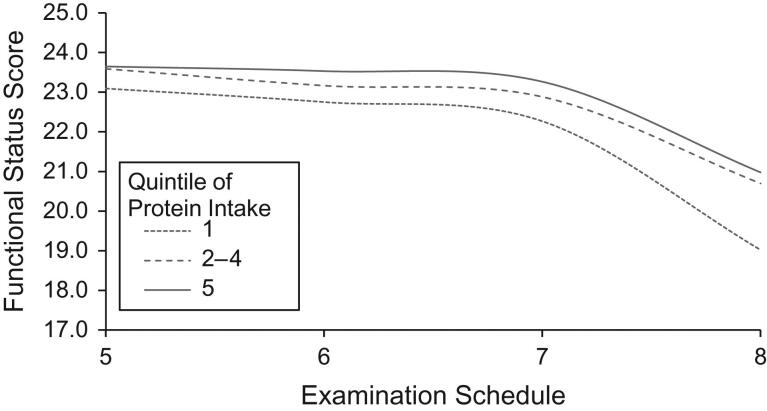
The associations between dietary protein intake and functional status scores at examinations 5 through 8 are shown in Figures1 and2. Figure1 examines protein intake as g/kg/day while Figure2 shows results associated with weight-adjusted protein residuals. The results are similar. All groups of subjects experienced declining functional scores over time, particularly starting at examination 7. Those in the higher 2 categories of protein intake had less functional decline throughout the follow-up period than those in the lowest category of intake.
Figure 1.
The association between categories of usual protein intake per kilogram of body weight and functional status scores among subjects 50 years of age or older, Framingham Offspring Study, Massachusetts, 1991–2008.
Figure 2.
The association between categories of usual weight-adjusted protein intake estimated using the residual method and functional status scores among subjects 50 years of age or older, Framingham Offspring Study, Massachusetts, 1991–2008.
DISCUSSION
In this study, higher dietary protein intake was associated with a lower risk of becoming dependent in functional tasks requiring strength and endurance. The types of tasks most affected by protein consumption included doing heavy work at home; walking 1/2 mile; going up and down a flight of stairs; stooping, kneeling, or crouching down; and lifting heavy items like bags of groceries. In these analyses, the estimated hazard ratios for higher dietary protein intakes were somewhat stronger for women than for men. It is possible that the weaker estimates in men could be due to some degree of bias in the reporting of disability. Over 12 years of follow-up, subjects consuming more dietary protein were also less likely to become dependent in multiple functional tasks. In addition, higher levels of moderate and vigorous activity, in particular, as well as higher baseline levels of SMM and lower BMI were independently associated with greater preservation of functional performance over time and strengthened the beneficial association between dietary protein and functional performance.
This study adds to the evidence that dietary protein plays an important role in the maintenance of functional independence during the later stages of life. It also supports previous findings that suggest that older adults may benefit from protein intakes that are above the current US dietary recommendations. Previous studies of protein requirements have been based on nitrogen-balance studies, which have been conducted mainly among young adults (26). It is increasingly recognized that such short-term nitrogen-balance studies provide only limited information on protein requirements for older adults. The current analysis supports previous suggestions that a more meaningful approach would be to more directly evaluate the association between dietary protein and health and functional outcomes (27–29).
During the middle and older adult years, there are changes in body composition and lifestyle that promote the loss of lean body mass and the acquisition of greater fat mass. This transition is likely to be associated with a higher risk of functional decline and increased morbidity and mortality (30,31). Reviews of the evidence in recent years have shown that dietary protein and/or amino acid intake combined with resistance exercise can stimulate muscle protein synthesis and slow breakdown (resulting in positive net protein balance) despite advancing age (32). A review by Volpi et al. (10) concluded that to retain muscle mass, strength, and optimal physical functioning among older adults, the current recommendation for dietary protein intakes for older adults of 0.8 g/kg/day should be raised.
Loss of muscle strength, often a surrogate marker for physical functioning, is associated with reduced functional capacity, decreased skeletal muscle mass, impaired muscle quality, neurological dysfunction, and other comorbid conditions (33). In the longitudinal InCHIANTI Study among community-dwelling adults aged ≥65 years, obese subjects with less muscle strength were more likely to experience declines in walking speed and development of new mobility disabilities compared with normal-weight individuals without such strength loss (34). Some prior studies have shown that adequate protein intake combined with strength training can result in substantial improvements in muscle mass, strength, and physical performance in older adults (35,36). These results are consistent with our findings that those with a higher physical activity level, higher %SMM, and lower BMI at baseline were less likely to develop functional decline with age.
A clinical trial of frail elderly demonstrated that 30 g of supplemental protein (vs. placebo) led to greater improvements in leg extension strength and physical performance on the Short Physical Performance Battery (35). When combined with resistance exercise training, protein supplementation among frail elderly subjects led to increased muscle mass, strength, and physical performance (37). This study is consistent with the present findings that higher physical activity level and higher protein intake at baseline reduced functional decline over time.
There are several important strengths of this study, starting with the long follow-up period from baseline protein intake to final measurement of functional status. Physical functioning outcomes were measured by well-validated Rosow-Breslau and Nagi scales at 4 sequential exams. In addition, baseline protein intake was derived from detailed 3-day dietary records. Another important strength of this study was the use of 2 different approaches for expressing protein intake. This approach allowed for the direct comparison of protein intake with the current dietary recommendations and for accounting for possible confounding by the subject’s body size that may be a concern when expressing protein intake as grams per kilogram per day. The similarity in the results of these 2 different approaches provides greater confidence in the overall findings.
There are several limitations of this study. One is the lack of performance-based measures of functional status. Limited power for sex-specific analyses is another such limitation. In this study, subjects’ SMMs were measured by BIA, and while this is not a gold standard approach for measuring skeletal muscle mass, a previous cross-validation study found that BIA more accurately and precisely measured SMM than did magnetic resonance imaging (25). Dietary intakes as well as physical activity levels, alcohol intake, and cigarette smoking were all self-reported, which is a limitation of the analyses. In addition, these analyses do not provide data on the type or source of dietary protein. Further, it is possible that functional impairments may be underreported in this study although it seems unlikely that such underreporting would be biased with respect to the exposure. Nonetheless, residual confounding is possible. Finally, incomplete information on comorbidities that may influence functional status change is a limitation of this study. However, most of these comorbidities are unlikely to be associated with dietary protein and are therefore unlikely to confound the results.
In summary, this study supports the premise that raising the protein guidelines to above the current US dietary recommendations in older adults may help to maintain functional performance into later adult years. It also supports the importance of physical activity and maintenance of a healthy body weight in the prevention of age-related functional decline.
ACKNOWLEDGMENTS
Author affiliations: Preventive Medicine and Epidemiology, Department of Medicine, Boston University School of Medicine, Boston, Massachusetts (Jabed Mustafa, R. Curtis Ellison, Martha R. Singer, M. Loring Bradlee, Bindu Kalesan, Lynn L. Moore); and Endocrinology, Diabetes, Nutrition, and Weight Management, Department of Medicine, Boston University School of Medicine, Boston, Massachusetts (Michael F. Holick).
Conflict of interest: none declared.
Abbreviations
- BIA
bioelectrical impedance analysis
- BMI
body mass index
- FOS
Framingham Offspring Study
- SMM
skeletal muscle mass
REFERENCES
- 1. Candow DG,Chilibeck PD. Differences in size, strength, and power of upper and lower body muscle groups in young and older men.J Gerontol A Biol Sci Med Sci.2005;60(2):148–156. [DOI] [PubMed] [Google Scholar]
- 2. Lauretani F,Russo CR,Bandinelli S,et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia.J Appl Physiol (1985).2003;95(5):1851–1860. [DOI] [PubMed] [Google Scholar]
- 3. Baumgartner RN,Waters DL,Gallagher D,et al. Predictors of skeletal muscle mass in elderly men and women.Mech Ageing Dev.1999;107(2):123–136. [DOI] [PubMed] [Google Scholar]
- 4. Short KR,Vittone JL,Bigelow ML,et al. Age and aerobic exercise training effects on whole body and muscle protein metabolism.Am J Physiol Endocrinol Metab.2004;286(1):E92–E101. [DOI] [PubMed] [Google Scholar]
- 5. Montero-Fernández N,Serra-Rexach JA. Role of exercise on sarcopenia in the elderly.Eur J Phys Rehabil Med.2013;49(1):131–143. [PubMed] [Google Scholar]
- 6. Cederholm T,Cruz-Jentoft AJ,Maggi S. Sarcopenia and fragility fractures.Eur J Phys Rehabil Med.2013;49(1):111–117. [PubMed] [Google Scholar]
- 7. Wullschleger S,Loewith R,Hall MN. TOR signaling in growth and metabolism.Cell.2006;124(3):471–484. [DOI] [PubMed] [Google Scholar]
- 8. Institute of Medicine Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients).Washington, DC:The National Academies Press;2005. [Google Scholar]
- 9. Houston DK,Tooze JA,Garcia K,et al. Protein intake and mobility limitation in community-dwelling older adults: the Health ABC study.J Am Geriatr Soc.2017;65(8):1705–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Volpi E,Campbell WW,Dwyer JT,et al. Is the optimal level of protein intake for older adults greater than the recommended dietary allowance? J Gerontol A Biol Sci Med Sci.2013;68(6):677–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kumar V,Selby A,Rankin D,et al. Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men.J Physiol.2009;587(1):211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cuthbertson D,Smith K,Babraj J,et al. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle.FASEB J.2005;19(3):422–424. [DOI] [PubMed] [Google Scholar]
- 13. Bauer J,Biolo G,Cederholm T,et al. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group.J Am Med Dir Assoc.2013;14(8):542–559. [DOI] [PubMed] [Google Scholar]
- 14. Houston DK,Nicklas BJ,Ding J,et al. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: the Health, Aging, and Body Composition (Health ABC) Study.Am J Clin Nutr.2008;87(1):150–155. [DOI] [PubMed] [Google Scholar]
- 15. Vellas BJ,Hunt WC,Romero LJ,et al. Changes in nutritional status and patterns of morbidity among free-living elderly persons: a 10-year longitudinal study.Nutrition.1997;13(6):515–519. [DOI] [PubMed] [Google Scholar]
- 16. Beasley JM,LaCroix AZ,Neuhouser ML,et al. Protein intake and incident frailty in the Women’s Health Initiative observational study.J Am Geriatr Soc.2010;58(6):1063–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Symons TB,Sheffield-Moore M,Mamerow MM,et al. The anabolic response to resistance exercise and a protein-rich meal is not diminished by age.J Nutr Health Aging.2011;15(5):376–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Drummond MJ,Dreyer HC,Pennings B,et al. Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging.J Appl Physiol (1985).2008;104(5):1452–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schakel SF,Sievert YA,Buzzard IM. Sources of data for developing and maintaining a nutrient database.J Am Diet Assoc.1988;88(10):1268–1271. [PubMed] [Google Scholar]
- 20. Nelson M,Black AE,Morris JA,et al. Between- and within-subject variation in nutrient intake from infancy to old age: estimating the number of days required to rank dietary intakes with desired precision.Am J Clin Nutr.1989;50(1):155–167. [DOI] [PubMed] [Google Scholar]
- 21. Prentice RL,Mossavar-Rahmani Y,Huang Y,et al. Evaluation and comparison of food records, recalls, and frequencies for energy and protein assessment by using recovery biomarkers.Am J Epidemiol.2011;174(5):591–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nagi SZ.An epidemiology of disability among adults in the United States.Milbank Mem Fund Q Health Soc.1976;54(4):439–467. [PubMed] [Google Scholar]
- 23. Rosow I,Breslau N. A Guttman health scale for the aged.J Gerontol.1966;21(4):556–559. [DOI] [PubMed] [Google Scholar]
- 24. Kannel WB,Sorlie P. Some health benefits of physical activity. The Framingham Study.Arch Intern Med.1979;139(8):857–861. [PubMed] [Google Scholar]
- 25. Janssen I,Heymsfield SB,Baumgartner RN,et al. Estimation of skeletal muscle mass by bioelectrical impedance analysis.J Appl Physiol (1985).2000;89(2):465–471. [DOI] [PubMed] [Google Scholar]
- 26. Rand WM,Pellett PL,Young VR. Meta-analysis of nitrogen balance studies for estimating protein requirements in healthy adults.Am J Clin Nutr.2003;77(1):109–127. [DOI] [PubMed] [Google Scholar]
- 27. Fukagawa NK.Protein requirements: methodologic controversy amid a call for change.Am J Clin Nutr.2014;99(4):761–762. [DOI] [PubMed] [Google Scholar]
- 28. De Buyser SL,Petrovic M,Taes YE,et al. Physical function measurements predict mortality in ambulatory older men.Eur J Clin Invest.2013;43(4):379–386. [DOI] [PubMed] [Google Scholar]
- 29. Studenski S,Perera S,Patel K,et al. Gait speed and survival in older adults.JAMA.2011;305(1):50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bales CW,Ritchie CS. Sarcopenia, weight loss, and nutritional frailty in the elderly.Annu Rev Nutr.2002;22:309–323. [DOI] [PubMed] [Google Scholar]
- 31. Janssen I,Heymsfield SB,Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability.J Am Geriatr Soc.2002;50(5):889–896. [DOI] [PubMed] [Google Scholar]
- 32. Koopman R,van Loon LJ. Aging, exercise, and muscle protein metabolism.J Appl Physiol (1985).2009;106(6):2040–2048. [DOI] [PubMed] [Google Scholar]
- 33. Cruz-Jentoft AJ,Baeyens JP,Bauer JM,et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People.Age Ageing.2010;39(4):412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stenholm S,Alley D,Bandinelli S,et al. The effect of obesity combined with low muscle strength on decline in mobility in older persons: results from the InCHIANTI study.Int J Obes (Lond).2009;33(6):635–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tieland M,van de Rest O,Dirks ML,et al. Protein supplementation improves physical performance in frail elderly people: a randomized, double-blind, placebo-controlled trial.J Am Med Dir Assoc.2012;13(8):720–726. [DOI] [PubMed] [Google Scholar]
- 36. Daly RM,O’Connell SL,Mundell NL,et al. Protein-enriched diet, with the use of lean red meat, combined with progressive resistance training enhances lean tissue mass and muscle strength and reduces circulating IL-6 concentrations in elderly women: a cluster randomized controlled trial.Am J Clin Nutr.2014;99(4):899–910. [DOI] [PubMed] [Google Scholar]
- 37. Tieland M,Dirks ML,van der Zwaluw N,et al. Protein supplementation increases muscle mass gain during prolonged resistance-type exercise training in frail elderly people: a randomized, double-blind, placebo-controlled trial.J Am Med Dir Assoc.2012;13(8):713–719. [DOI] [PubMed] [Google Scholar]