Abstract
Background and aims
Vitamin D exists as an inactive 25-hydroxyvitamin D (25(OH)D) in the bloodstream, which is converted to active 1,25-dihydroxyvitaminD (1,25(OH)2D) in target tissues. Cohort studies reporting cardiovascular disease among individuals with low vitamin D are inconsistent and solely measure 25(OH)D. Psoriasis, a chronic inflammatory disease, is a vitamin D deficient state and is associated with increased cardiovascular disease risk. While serum 25(OH)D is routinely measured, we hypothesized that measurement of 1,25(OH)2D in psoriasis may perform better than 25(OH)D in capturing cardiovascular risk.
Methods
Consecutive psoriasis patients (N=122) at baseline underwent FDG PET/CT and CCTA scans to measure visceral adipose volume, aortic vascular uptake of FDG, and coronary plaque burden respectively. Blood levels of both 1,25(OH)2D and 25(OH)D were measured by chemiluminescence (LIASON XL DIaSorin, Stillwater, MN).
Results
The psoriasis cohort was middle-aged (mean ± SD: 49.6 ± 13.0), predominantly male (n=71, 58%), in majority Caucasians (n=98, 80%), and had moderate-to-severe skin disease [psoriasis area severity index score, PASI score, med. (IQR): 5.5 (3.2–10.7)], with almost one-fourth of the cohort on biologic psoriasis therapy for skin disease management (n=32, 27%) at baseline. Interestingly, serum levels of 1,25(OH)2D but not 25 (OH) D were found to be inversely associated with visceral adipose, a marker of cardiometabolic risk in fully adjusted models (β=−0.43, p=0.026 and β=−0.26 p=0.13). Similarly, we found an inverse relationship between 1,25(OH)2D, but not 25(OH)D, and aortic vascular uptake of FDG independent of traditional risk factors (β=−0.19, p=0.01). Finally, we found that serum 1,25(OH)2D, but not 25(OH)D, was inversely associated with non-calcified coronary plaque burden, as measured by CCTA independent of traditional risk factors (β=−0.18, p=0.03).
Conclusions
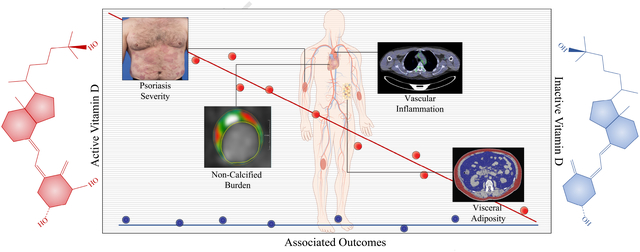
In conclusion, we demonstrate that low 1,25(OH)2D levels were associated with visceral adipose volume, vascular uptake of FDG and coronary plaque burden independent of traditional risk factors, suggesting that 1,25(OH)2D may better capture the cardiometabolic risk associated with vitamin D deficient states.
Graphical Abstract
Introduction
Vitamin D is a fat-soluble steroid pro-hormone derived from two environmental sources. Vitamin D2 (Ergocalciferol) is obtained from the diet, while the majority of Vitamin D3 (Cholecalciferol) is synthesized in the skin upon exposure to UVB radiation from sunlight1, 2. When these pro-hormones reach the circulation they first undergo hydroxylation in the liver to produce biologically inactive 25-hydroxy vitamin D 25(OH)D, followed by further hydroxylation in the kidney to biologically active 1,25 dihydroxy vitamin D (1,25(OH)2D) when needed1, 2. The highly potent 1,25(OH)2D functions at circulating concentrations of pg/ml to tightly regulate serum calcium and phosphate concentration3. Furthermore, the observation that 1,25(OH)2D may regulate expression of over 1000 genes in the human genome, particularly those involved in cell proliferation, inflammation and innate immunity, suggests that this hormone may be beneficial in the treatment of a plethora of human diseases, especially in states of chronic inflammation2.
Vitamin D deficiency has been shown to be associated with multiple sclerosis, type 1 diabetes, inflammatory bowel disease and rheumatoid arthritis4–7, 8. Vitamin D is also used routinely as a topical agent to alleviate the symptoms of psoriasis9. Psoriasis is a chronic inflammatory skin disease that affects an estimated 3% of the adult population. In addition to the skin manifestations, psoriasis patients, particularly those with severe disease, have increased risk of myocardial infarction, stroke and overall cardiovascular mortality10–12.
Furthermore, psoriasis is associated with higher sub-clinical aortic vascular uptake of 18F-fluorodeoxyglucose (FDG), as well as elevated lipid-rich non-calcified coronary plaque burden when compared to healthy controls13, 14. Finally, psoriasis disease symptoms are improved with biologic agents that target TNF-α, IL-17 or IL-12/23 signaling pathways and hence this disease state may be used as a human model to study the effects of different therapeutic strategies on inflammatory atherogenesis15, 16.
Given that psoriasis is associated with increased cardiovascular risk and that numerous studies, including large-scale randomized clinical trials, have investigated the link between circulating vitamin D levels and risk of cardiovascular disease but remain inconclusive17, 18, we sought to explore associations between inactive (25(OH)D) or active (1,25(OH)2D) vitamin D levels and sub-clinical cardiovascular disease in psoriasis. While low circulating vitamin D (<20ng/ml) have higher cardiovascular risk, vitamin D supplementation has no clear beneficial effects17–19. However, a reason for the discrepancy in these studies may be the inability to report levels of biologically active 1,25(OH)2D vitamin D. To this end, we used a fully-automated, chemi-luminescent assay to measure the serum levels of both 1,25(OH)2D and 25(OH)D in a cross-sectional cohort of psoriasis patients who have undergone measurements of aortic vascular uptake of FDG and coronary artery disease20,21, 22. The objective was to investigate relationships of serum 1,25(OH)2D and 25(OH)D with psoriasis severity, aortic vascular uptake of FDG and non-calcified coronary plaque burden. Such a study would provide useful information regarding the utility of measuring inactive and active forms of vitamin D when making predictions of disease prognosis.
Materials and methods
Study design and selection of study groups
In a prospective, observational design, 290 participants were recruited in an ongoing cohort study to understand the association between psoriasis and cardiometabolic disease under the Psoriasis, Atherosclerosis and Cardiometabolic Initiative from 1 January, 2013 through 31 October, 2018, with 238 completing one-year follow-up. Out of 290 participants, 122 consecutive psoriasis patients were included in our analyses based on whom active and inactive vitamin D levels have been analyzed till date. Approval for this research was granted by the Institutional Review Board of the National Heart Lung and Blood Institute prior to patient recruitment, in accordance with the Declaration of Helsinki. All patients provided informed consent before study participation. Please refer to Supplementary materials for detailed inclusion and exclusion criteria of psoriasis participants.
Clinical assessment
All patients were seen at the NIH Clinical Center for visits. During these visits, patients received a detailed history and physical exam. All patients underwent 18-FDG PET/CT (18F-fluorodeoxyglucose Positron Emission Tomography) imaging and laboratory testing. Coronary Computed Tomography Angiography (CCTA) scans were acquired on all patients who provided consent and lacked contraindications. A study provider confirmed the onset and duration of psoriasis and assessed psoriasis severity using the Psoriasis Area and Severity Index (PASI) score, which combines the severity of lesions and the area affected into a single score, considering erythema, induration, and desquamation within each lesion.
FDG PET/CT analysis
After fasting overnight, patients were given a 10 mCi dose of 18-FDG. Approximately 60 min after administration of 18-FDG load, PET/CT images were acquired using a Siemens Biograph mCT PET/CT 64-slice scanner (Siemens Medical Solutions USA, Malvern, PA, USA). Axial 1.5 mm thick slices of the aorta were analyzed by placing regions of interest (ROIs) around the vessel walls at the level of key anatomical landmarks, with careful avoidance of adjacent structures. Mean and maximum standard uptake values (SUVs) were calculated using Extended Brilliance Workspace (Phillips Electronics, NV, USA). To standardize the values, target-to-background ratios (TBR) were calculated by dividing the maximum SUVs of each slice by the 18-FDG uptake measured in the lumen of the vena cava, and we report the average TBR in the aorta for each patient16.
Coronary CT angiography analysis
Eligible patients who consented to receive CCTA were scanned on a 320 detector row unit (Aquilion ONE ViSION Edition; Toshiba Medical Systems, Otawara, Japan) using standard techniques during their baseline visits on the same day that fasting blood samples were drawn. We evaluated CCTA data in all patients who had analyzable scans and evaluated all three epicardial coronary arteries individually. Plaque morphology and composition were then analyzed using the dedicated software program, QAngio CT (Medis, The Netherlands). Total, non-calcified, and dense-calcified plaque burden were quantified on the basis of pre-defined Hounsfield unit ranges. Indices for total, non-calcified and dense-calcified plaque burden were then calculated by dividing the plaque volume of each vessel by its total length, attenuated for luminal intensity. We report total burden, non-calcified and dense-calcified burden for each patient13, 23.
1,25(OH)2D and 25(OH)D measurements
Levels of 1,25(OH)2D were determined from human serum at baseline with a fully automated and sensitive immunoassay that uses a recombinant fusion construct of the vitamin D receptor ligand binding domain for specific capture of 1,25(OH)2D (DiaSorin, Saluggia, Italy). The limit of quantitation for this 1,25(OH)2D assay is 5 ng/mL and the reference interval determined in healthy controls ranged between 25.0 and 86.5 ng/mL with a median of 48.1 ng/mL22.
Statistical analysis
Data were reported as mean with standard deviation for parametric variables, median with interquartile range (IQR) for non-parametric variables and percentages for categorical variables. In baseline analyses, parametric and non-parametric variables were compared between the two groups using student t-test and Mann-Whitney U test, respectively. In longitudinal analyses, parametric variables were analyzed using paired-t test and non-parametric variables using Wilcoxon signed-rank test. Dichotomous variables were analyzed using Pearson’s chi-square test at baseline and McNemar’s test in longitudinal analysis. In multivariable linear and logistic regression analyses, the potential confounding variables were determined and added to the base model by purposeful selection. Standardized beta values from these analyses were reported, which indicate number of standard deviations change in the outcome variable per standard deviation change in the predicting variable. P-value<0.05 was deemed significant. All statistical analyses were performed using STATA 12 (Stata Corp., College Station, TX, USA) by National Institutes of Health staff, blinded to clinical demographics and imaging characteristics.
Results
Using a fully automated immunoassays for the specific detection of vitamin D metabolites, we measured the serum levels of cohort of psoriasis patients and healthy controls. The psoriasis cohort (N=122) was middle-aged (mean ± SD: 49.6 ± 13.0), predominantly male (n=71, 58%) in majority Caucasians (n=98, 80%) and had moderate-to-severe skin disease [psoriasis area severity index score, PASI score, med. (IQR): 5.5 (3.2–10.7)], with almost one-fourth of the cohort on biologic psoriasis therapy for skin disease management (n=32, 27%) at baseline. Patients had a low cardiovascular risk by Framingham 10-year risk [3.0 (1.0–6.0)], despite being obese (30.0 ± 6.4), and almost half having a history of dyslipidemia (n = 60, 49%), with one-fourth being on statin use (n = 39, 32%) (Table 1). The psoriasis cohort had serum levels of 1,25(OH)2D and 25(OH)D at 52.4 ± 1.4 ng/ml and 28.7 ± 1.3 ng/ml respectively (Table 1). A value of 25(OH)D greater than 20ng/ml is considered adequate according to the Institute of Medicine guidelines24.
Table 1: Psoriasis patients at baseline.
Characteristics of psoriasis patients at baseline.
| Variable | Baseline (N=122) |
|---|---|
| Demographic and clinical characteristics | |
| Age, years | 49.6 ± 13.0 |
| Males | 71 (58) |
| Caucasian | 98 (80) |
| Hypertension | 35 (29) |
| Hyperlipidemia | 60 (49) |
| Type-2 diabetes | 13(11) |
| Body Mass Index | 30.0 ± 6.4 |
| Waist-to-hip ratio | 0.95 (0.89–1.00) |
| Current smoker | 11 (9) |
| Statin use | 39 (32) |
| Clinical and lab values | |
| Total cholesterol, mg/dl | 183.8 ± 35.0 |
| HDL cholesterol, mg/dl | 55.3 ± 17.2 |
| LDL cholesterol, mg/dl | 104.2 ± 29.6 |
| Triglycerides, mg/dl | 125.2 ± 83.9 |
| Framingham Risk Score | 3 (1–6) |
| High-sensitivity C-reactive protein, mg/L | 2.1 (0.87–4.5) |
| Psoriasis characterization | |
| Psoriasis Area Severity Index Score | 5.5 (3.2–10.7) |
| Systemic or biologic treatment | 32 (27) |
| Vitamin D parameters | |
| 1,25 (OH) 2D (ng/ml) | 52.4 ± 15.2 |
| 25 (OH) D (ng/ml) | 28.7 ± 14.0 |
| Vascular characterization | |
| Aortic target-to-background ratio (TBR) | 1.74 ± 0.27 |
| Non-calcified coronary burden, mm2 (×100) | 1.13 ± 0.59 |
| Adiposity | |
| Visceral adiposity (cm3) | 16346±9489 |
| Subcutaneous adiposity (cm3) | 21375±12239 |
Values reported in the table as mean ± SD or median (IQR) for continuous data and N (%) for categorical data.
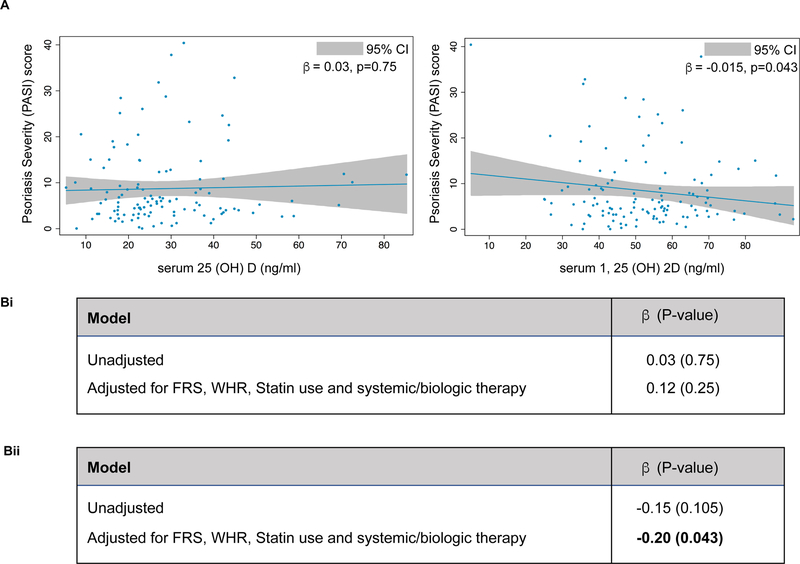
On comparing these vitamin D levels with PASI score, a measure of PSO disease severity, there was an inverse relationship between serum 1,25(OH)2D and PASI score (β=−0.15, p=0.11), which reached significance although borderline when adjusted for Framingham risk score, waist-to-hip ratio, statin use and systemic/biological therapy (β=−0.20, p=0.043) (Figure 1). In contrast, we did not observe an association between serum 25(OH)D and PASI score in our cohort (β=0.03, p=0.75), even upon adjustment for Framingham risk score, waist-to-hip ratio, statin use and systemic/biological therapy (β=0.12, p=0.25) (Figure 1).
Figure 1: Psoriasis severity and vitamin D levels.
(A) Relationship of vitamin D (inactive and active) with a measurement of psoriasis severity. (B) Multivariable regression analysis between PASI score and serum 25(OH)D (i) and 1, 25(OH)2D (ii) in psoriasis patients.
Obesity is a cardiovascular risk factor, which is increased with psoriasis severity25. In addition to traditional assessments of obesity such as body mass index and waist-to-hip ratio, our psoriasis cohort has undertaken direct measurements of abdominal adiposity using 18F-FDG-PET/CT26. This technique enables the distinction between metabolic activity in visceral versus subcutaneous adipose regions26. Given that accumulation of visceral but not subcutaneous fat causes cardiovascular disease, we hypothesized an inverse relationship between serum 1,25(OH) D and visceral adiposity (Table 2)27. Serum 1,25(OH)2D and 25(OH)D both inversely associated with visceral adipose, only 1,25(OH)2D reached significance (1,25(OH)2D: β=−0.26, p=0.039 and 25(OH)D: β=−0.08, p=0.55), which persisted even after adjustment for Framingham risk score, waist-to-hip ratio, statin use and treatment with systemic or biologics (1,25(OH)2D: β=−0.43, p=0.026 and 25(OH)D: β=−0.26, p=0.13) (Table 2A). Consistent with our hypothesis, while 1,25(OH)2D and 25(OH)D showed an inverse relationship with subcutaneous adipose, neither association reached statistical significance (1,25(OH)2D: β=−0.12, p=0.35 and 25(OH)D: β=−0.23, p=0.06) (Table 2B).
Table 2: Adiposity and vitamin D levels.
(A) Relationship of vitamin D (inactive and active) with visceral adiposity. Multivariable regression analysis between visceral adiposity and serum 25(OH)D (i) or 1,25 (OH)2D (ii) in psoriasis patients. (B) Relationship of vitamin D (inactive and active) with subcutaneous adiposity. Multivariable regression analysis between subcutaneous adiposity and serum 25(OH)D (i) or 1,25 (OH)2D (ii) in psoriasis patients.
| Model | β (p-value) |
|---|---|
| Unadjusted | −0.08 (0.55) |
| Adjusted for FRS, WHR, statin use and systemic/biologic therapy | −0.26 (0.13) |
| Model | β (p -value) |
| Unadjusted | −0.26 (0.039) |
| Adjusted for FRS, WHR, statin use and systemic/biologic therapy | −0.43 (0.026) |
| Model | |
| Unadjusted | −0.23 (0.06) |
| Adjusted for FRS, WHR, statin use and systemic/biologic therapy | −0.22 (0.09) |
| Model | |
| Unadjusted | −0.12 (0.35) |
| Adjusted for FRS, WHR, statin use and systemic/biologic therapy | −0.03 (0.84) |
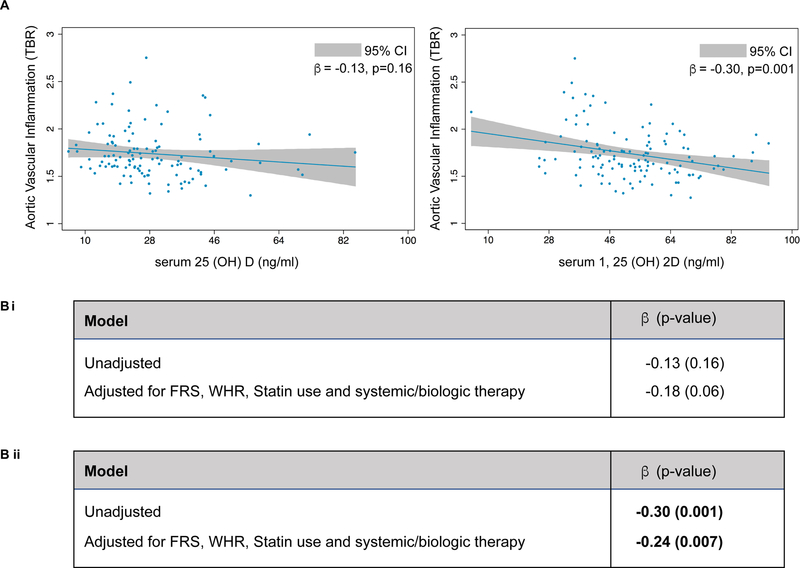
Our previous reported associations have shown that psoriasis patients have greater aortic vascular uptake of FDG when compared to healthy controls20, 28. This led to the hypothesis that serum vitamin D levels in our cohort may also be related to vascular uptake of FDG. Vascular uptake of FDG (as measured by TBR) was significantly lower in psoriasis subjects with high levels of both serum 25(OH)D and 1,25(OH)2D but only the association with 1,25(OH)2D, was significant (β=−0.30, p=0.001) (Figure 2). This relationship was maintained even when adjusted for Framingham risk score, waist-to-hip ratio, statin use and systemic/biological therapy (β=−0.24, p=0.007) (Figure 2).
Figure 2: Aortic vascular uptake of 18F-fluorodeoxyglucose and vitamin D level.
(A) Relationship of vitamin D (inactive and active) with aortic vascular uptake of 18F-fluorodeoxyglucose (TBR-target-to-background ratio). (B) Multivariable regression analysis between aortic vascular inflammation and serum 25(OH)D (i) and 1, 25(OH)2D (ii) in psoriasis patients.
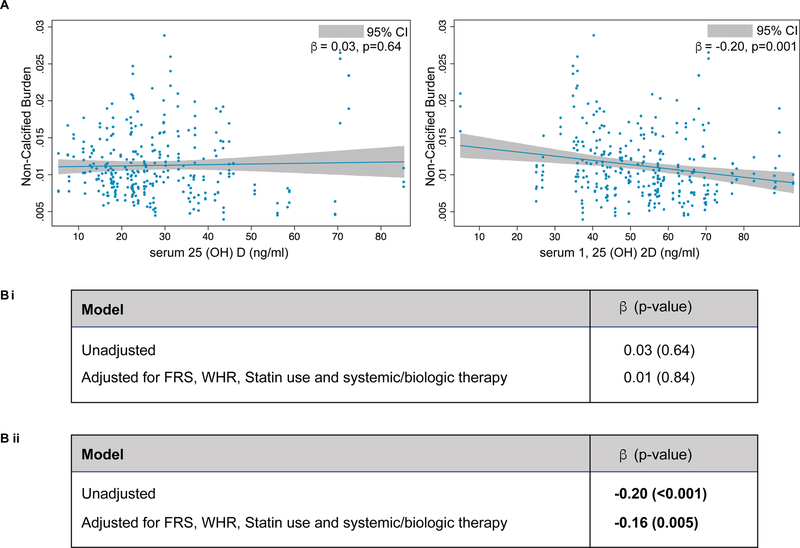
We have also shown that patients with psoriasis have increased lipid-rich non-calcified coronary plaque burden13. Hence, we investigated if serum vitamin D levels were related to non-calcified coronary plaque burden in our cohort of psoriasis patients (Figure 3). Similarly, we found that serum 1,25(OH)2D, but not 25(OH)D, was inversely associated with non-calcified calcified burden (NCB), as measured by coronary computed tomography angiography (CCTA) (β= −0.20, p<0.001 vs. β= 0.03, p=0.64) (Figure 3) which was consistent even after adjustment for Framingham risk score, waist-to-hip ratio, statin use and systemic/biological therapy (β=−0.16, p=0.005) (Figure 3). Significant associations between serum levels of either form of vitamin D and dense calcified burden in this disease cohort were not observed (data not shown)
Figure 3: Coronary plaque burden and vitamin D levels.
(A) Relationship of vitamin D (inactive and active) with lipid-rich coronary plaque burden. (B) Multivariable regression analysis between lipid-rich coronary plaque burden and serum 25(OH)D (i) and 1,25(OH)2D (ii) in psoriasis patients.
Given the association between 1,25 (OH) 2D and psoriasis severity, we hypothesized that patients who had a clinical improvement in their skin disease severity would also have improvement in 1,25(OH)2D levels. Demographic/clinical characteristics of psoriasis patients who had improvement in skin disease severity are shown in Supplementary Table 1. At one-year, 78/122 (64%) patients had an improvement in psoriasis skin disease severity [7.1 (4.5–14.7) vs 3.2 (1.9–5.5), p<0.001] following (n=51) 67% of the patients being on systemic or biologic treatment at 1-year. Concurrently, high-sensitivity c-reactive protein levels decreased significantly at one-year [2.4 (0.9–7.1) vs 1.2 (0.6–3.6), p<0.001]. Interestingly, 1,25(OH)2D levels increased at one-year follow-up [50.8 ± 16.1 to 52.6 ± 14.7, p=0.03] whereas serum 25(OH)D decreased at one-year (29.3 ± 14.84 vs 27.4 ± 11.8, p=0.03) following improvement in psoriasis.
Discussion
Using a longitudinal cohort study design in a well phenotyped sample of psoriasis patients with 1-year follow-up, we demonstrate that psoriasis severity has an inverse dose-response relationship with levels of the active form of vitamin D 1,25(OH)2D, but a similar relationship with inactive 25(OH)D was not observed. Similarly, active vitamin D levels were inversely associated with PASI score, visceral adiposity, vascular uptake of FDG and coronary plaque burden. Finally, treating psoriasis with biological therapies was associated with an increase in 1,25(OH)2D vitamin D levels at one-year and a concurrent decrease in 25(OH)D vitamin D levels at follow-up. Our study highlights the need that any future epidemiological studies should consider measurement of both circulating 25(OH)D and 1,25(OH)2D (active and inactive forms of vitamin D) when investigating the effects of vitamin D supplementation on disease states.
It is well known that systemic inflammation is causal in atherosclerosis development29, 30. Therefore, targeting inflammatory pathways to reduce residual risk for coronary artery disease beyond traditional risk factors is an emerging area of therapeutic interest29, 30. Chronic systemic inflammatory disorders such as psoriasis are associated with increased myocardial infarction and cardiovascular mortality independent of traditional risk factors and quelling systemic inflammation using anti-inflammatory treatments has been shown to have cardiovascular benefits over time13, 16, 31. Vitamin D is a potent anti-inflammatory molecule used clinically for numerous inflammatory diseases, and hence also been proposed to have beneficial effects on cardiovascular disease. Notably, individuals deficient in circulating vitamin D 25(OH)D (less than 20ng/ml) have increased risk of cardiovascular events32, yet randomized clinical trials have failed to demonstrate any significant beneficial effect of vitamin D supplementation on heart disease17, 32, 33. Here we suggest a possible reason for these unexpected findings.
Given that 1,25(OH)2D is the biologically active form of vitamin D, while 25(OH)D is in an inactive state until further hydroxylated in the kidney by 1β-hydroyxylase, it is perhaps not surprising that our findings suggest robust inverse associations between 1,25(OH)2D, but not 25(OH)D, and sub-clinical cardiovascular disease1, 2. Indeed, previous reports have indicated roles for 1,25(OH)2D through vitamin D receptor (VDR) activation in several stages of atherosclerosis32, 34. Briefly, 1,25(OH)2D -VDR signaling suppresses endothelial cell activation by transcriptionally suppressing inflammatory cytokines NFκB, IL-6, IL-8, IL-1β or adhesion molecule expression35–37. Vitamin D may also have a direct effect on lipid metabolism by reducing triglyceride levels or ApoA1 expression or indirectly by suppressing lipolysis via lowering parathyroid hormone (PTH) expression32. On the vasculature itself, 1,25(OH)2D has been shown to both inhibit foam cell formation and enhance cholesterol efflux38, 39. A damaged endothelial monolayer may also be repaired by 1,25(OH)2D via increase vascular endothelial growth factor (VEGF) while a stiffening artery may be relaxed by nitric oxide release mediated by 1,25(OH)2D induction endothelin (ET-1) signaling40, 41.
Given the data presented, it is intriguing to consider 1β-hydroyxylase as playing a role in cardiometabolic risk. This enzyme is essential for the conversion of 25(OH)D to 1,25(OH)2D in the kidney, which prompts the question whether this enzyme is defective under inflammatory conditions or activated in response to anti-inflammatory therapeutics used to treat psoriasis. Measurements of 1β-hydroyxylase activity in psoriasis patients and pre-clinical mouse models are yet to be determined42. It is interesting to note, however, that mice lacking the 1β-hydroyxylase gene (Cy27B1) exhibit the expected rickets phenotype along with myocardial hypertrophy and increased progression of atherosclerosis43.
Potential flaws in this observational study is its small sample size and open label use of biologic agent. Additionally, psoriasis is a typically season/weather dependent disease, where improvement in psoriasis severity in the longer summer months is observed44. Given that the majority of the active vitamin D precursors are synthesized in the skin, one would predict that the associations with cardiovascular risk may also be seasonally dependent. The fact that our patients at baseline were chosen consecutively, and those followed to one-year revisited the clinic within one-month of the one year anniversary, overcomes any concerns that we had over seasonal variations of vitamin D levels. To our knowledge, large-scale seasonal variations of cardiovascular events linked to serum vitamin D levels have not been determined. Finally, we use surrogates of cardiovascular disease rather than hard cardiovascular events, however, FDG PET/CT and CCTA surrogates have been extensively validated and prospectively predict cardiovascular events.
In conclusion, we demonstrate that psoriasis severity has an inverse dose-response relationship with levels of the active form of vitamin D 1,25(OH)2D, but a similar relationship with inactive 25(OH)D was not observed. Similarly, circulating 1,25(OH)2D levels are inversely associated with markers of visceral adiposity, vascular uptake of FDG and coronary plaque burden independent of traditional risk factors. Finally, psoriasis treatment was associated with an increase in active vitamin D levels at one-year and a concurrent decrease in inactive vitamin D levels at follow-up. Our study highlights that any future epidemiological studies should consider measurement of both circulating 25(OH)D and 1,25(OH)2D when investigating the effects of vitamin D supplementation on disease states.
Supplementary Material
Highlights.
Serum 1,25(OH)2D but not 25(OH)D levels inversely associated with psoriasis disease severity.
Serum 1,25(OH)2D but not 25(OH)D levels inversely associated with surrogate markers of cardiovascular risk in psoriasis.
Serum 1,25(OH)2D but not 25(OH)D levels inversely associated with visceral adiposity in psoriasis.
Serum 1,25(OH)2D but not 25(OH)D levels increase with psoriasis disease improvement.
Acknowledgements:
We would like to thank the NIH Clinical Center outpatient clinic-7 clinical care team for the excellent care they provide our participants and to Joel Gelfand for guidance throughout this project. We would also like to acknowledge Jeremy Seeman for technical expertise in measuring active and inactive forms of vitamin D.
Financial support
This study was supported by the National Heart, Lung and Blood Institute (NHLBI) Intramural Research Program (HL006193- 05). This research was also made possible through the NIH Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from the Doris Duke Charitable Foundation (DDCF Grant #2014194), the American Association for Dental Research, the Colgate-Palmolive Company, Genentech, Elsevier, and other private donors.
The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
Dr. Mehta is a full-time US government employee and has served as a consultant for Amgen, Eli Lilly, and Leo Pharma receiving grants/other payments; as a principal investigator and/or investigator for AbbVie, Celgene, Janssen Pharmaceuticals, Inc, and Novartis receiving grants and/or research funding; and as a principal investigator for the National Institute of Health receiving grants and/or research funding.
All other authors declare no conflicts of interests in relation to the work presented in this manuscript.
References:
- [1].Jones G, The discovery and synthesis of the nutritional factor vitamin D, International journal of paleopathology, 2018;23:96–99. [DOI] [PubMed] [Google Scholar]
- [2].Pike JW and Christakos S, Biology and Mechanisms of Action of the Vitamin D Hormone, Endocrinology and metabolism clinics of North America, 2017;46:815–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Renkema KY, Alexander RT, Bindels RJ, et al. , Calcium and phosphate homeostasis: concerted interplay of new regulators, Annals of medicine, 2008;40:82–91. [DOI] [PubMed] [Google Scholar]
- [4].Dadaei T, Safapoor MH, Asadzadeh Aghdaei H, et al. , Effect of vitamin D3 supplementation on TNF-alpha serum level and disease activity index in Iranian IBD patients, Gastroenterology and hepatology from bed to bench, 2015;8:49–55. [PMC free article] [PubMed] [Google Scholar]
- [5].Hong Q, Xu J, Xu S, et al. , Associations between serum 25-hydroxyvitamin D and disease activity, inflammatory cytokines and bone loss in patients with rheumatoid arthritis, Rheumatology (Oxford, England), 2014;53:1994–2001. [DOI] [PubMed] [Google Scholar]
- [6].Sotirchos ES, Bhargava P, Eckstein C, et al. , Safety and immunologic effects of high- vs low-dose cholecalciferol in multiple sclerosis, Neurology, 2016;86:382–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Nardin M, Verdoia M, Schaffer A, et al. , Vitamin D status, diabetes mellitus and coronary artery disease in patients undergoing coronary angiography, Atherosclerosis, 2016;250:114–121. [DOI] [PubMed] [Google Scholar]
- [8].Deluca HF, History of the discovery of vitamin D and its active metabolites, BoneKEy reports, 2014;3:479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kuehl B and Shear NH, The Evolution of Topical Formulations in Psoriasis, Skin therapy letter, 2018;23:5–9. [PubMed] [Google Scholar]
- [10].Gelfand JM, Dommasch ED, Shin DB, et al. , The risk of stroke in patients with psoriasis, The Journal of investigative dermatology, 2009;129:2411–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gelfand JM, Neimann AL, Shin DB, et al. , Risk of myocardial infarction in patients with psoriasis, Jama, 2006;296:1735–1741. [DOI] [PubMed] [Google Scholar]
- [12].Ahlehoff O, Gislason GH, Lindhardsen J, et al. , Prognosis following first-time myocardial infarction in patients with psoriasis: a Danish nationwide cohort study, Journal of internal medicine, 2011;270:237–244. [DOI] [PubMed] [Google Scholar]
- [13].Lerman JB, Joshi AA, Chaturvedi A, et al. , Coronary Plaque Characterization in Psoriasis Reveals High-Risk Features That Improve After Treatment in a Prospective Observational Study, Circulation, 2017;136:263–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Naik HB, Natarajan B, Stansky E, et al. , Severity of Psoriasis Associates With Aortic Vascular Inflammation Detected by FDG PET/CT and Neutrophil Activation in a Prospective Observational Study, Arteriosclerosis, thrombosis, and vascular biology, 2015;35:2667–2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Harrington CL, Dey AK, Yunus R, et al. , Psoriasis as a human model of disease to study inflammatory atherogenesis, American journal of physiology. Heart and circulatory physiology, 2017;312:H867–h873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Dey AK, Joshi AA, Chaturvedi A, et al. , Association Between Skin and Aortic Vascular Inflammation in Patients With Psoriasis: A Case-Cohort Study Using Positron Emission Tomography/Computed Tomography, JAMA cardiology, 2017;2:1013–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Manson JE, Cook NR, Lee IM, et al. , Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease, The New England journal of medicine, 2019;380:33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhang R, Li B, Gao X, et al. , Serum 25-hydroxyvitamin D and the risk of cardiovascular disease: dose-response meta-analysis of prospective studies, The American journal of clinical nutrition, 2017;105:810–819. [DOI] [PubMed] [Google Scholar]
- [19].Tunon J, Cristobal C, Tarin N, et al. , Coexistence of low vitamin D and high fibroblast growth factor-23 plasma levels predicts an adverse outcome in patients with coronary artery disease, PloS one, 2014;9:e95402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Joshi AA, Lerman JB, Dey AK, et al. , Association Between Aortic Vascular Inflammation and Coronary Artery Plaque Characteristics in Psoriasis, JAMA cardiology, 2018;3:949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gruson D, Ferracin B, Ahn SA, et al. , 1,25-Dihydroxyvitamin D to PTH(1–84) Ratios Strongly Predict Cardiovascular Death in Heart Failure, PloS one, 2015;10:e0135427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Valcour A, Zierold C, Podgorski AL, et al. , A novel, fully-automated, chemiluminescent assay for the detection of 1,25-dihydroxyvitamin D in biological samples, The Journal of steroid biochemistry and molecular biology, 2016;164:120–126. [DOI] [PubMed] [Google Scholar]
- [23].Salahuddin T, Natarajan B, Playford MP, et al. , Cholesterol efflux capacity in humans with psoriasis is inversely related to non-calcified burden of coronary atherosclerosis, European heart journal, 2015;36:2662–2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Pludowski P, Holick MF, Grant WB, et al. , Vitamin D supplementation guidelines, The Journal of steroid biochemistry and molecular biology, 2018;175:125–135. [DOI] [PubMed] [Google Scholar]
- [25].Fleming P, Kraft J, Gulliver WP, et al. , The Relationship of Obesity With the Severity of Psoriasis: A Systematic Review, Journal of cutaneous medicine and surgery, 2015;19:450–456. [DOI] [PubMed] [Google Scholar]
- [26].Rivers JP, Powell-Wiley TM, Dey AK, et al. , Visceral Adiposity in Psoriasis is Associated With Vascular Inflammation by (18)F-Fluorodeoxyglucose Positron-Emission Tomography/Computed Tomography Beyond Cardiometabolic Disease Risk Factors in an Observational Cohort Study, JACC. Cardiovascular imaging, 2018;11:349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Matsuzawa Y, The role of fat topology in the risk of disease, International journal of obesity (2005), 2008;32 Suppl 7:S83–92. [DOI] [PubMed] [Google Scholar]
- [28].Naik HB, Natarajan B, Stansky E, et al. , Severity of Psoriasis Associates With Aortic Vascular Inflammation Detected by FDG PET/CT and Neutrophil Activation in a Prospective Observational Study, Arteriosclerosis, thrombosis, and vascular biology, 2015;35:2667–2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Santos-Gallego CG, Picatoste B and Badimon JJ, Pathophysiology of acute coronary syndrome, Current atherosclerosis reports, 2014;16:401. [DOI] [PubMed] [Google Scholar]
- [30].Raggi P, Genest J, Giles JT, et al. , Role of inflammation in the pathogenesis of atherosclerosis and therapeutic interventions, Atherosclerosis, 2018;276:98–108. [DOI] [PubMed] [Google Scholar]
- [31].Mehta NN, Shin DB, Joshi AA, et al. , Effect of 2 Psoriasis Treatments on Vascular Inflammation and Novel Inflammatory Cardiovascular Biomarkers: A Randomized Placebo-Controlled Trial, Circulation. Cardiovascular imaging, 2018;11:e007394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Pilz S, Verheyen N, Grubler MR, et al. , Vitamin D and cardiovascular disease prevention, Nature reviews. Cardiology, 2016;13:404–417. [DOI] [PubMed] [Google Scholar]
- [33].Scragg R, Stewart AW, Waayer D, et al. , Effect of Monthly High-Dose Vitamin D Supplementation on Cardiovascular Disease in the Vitamin D Assessment Study : A Randomized Clinical Trial, JAMA cardiology, 2017;2:608–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Norman PE and Powell JT, Vitamin D and cardiovascular disease, Circulation research, 2014;114:379–393. [DOI] [PubMed] [Google Scholar]
- [35].Kudo K, Hasegawa S, Suzuki Y, et al. , 1alpha,25-Dihydroxyvitamin D(3) inhibits vascular cellular adhesion molecule-1 expression and interleukin-8 production in human coronary arterial endothelial cells, The Journal of steroid biochemistry and molecular biology, 2012;132:290–294. [DOI] [PubMed] [Google Scholar]
- [36].Stach K, Kalsch AI, Nguyen XD, et al. , 1alpha,25-dihydroxyvitamin D3 attenuates platelet activation and the expression of VCAM-1 and MT1-MMP in human endothelial cells, Cardiology, 2011;118:107–115. [DOI] [PubMed] [Google Scholar]
- [37].Won S, Sayeed I, Peterson BL, et al. , Vitamin D prevents hypoxia/reoxygenation-induced blood-brain barrier disruption via vitamin D receptor-mediated NF-kB signaling pathways, PloS one, 2015;10:e0122821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Oh J, Weng S, Felton SK, et al. , 1,25(OH)2 vitamin d inhibits foam cell formation and suppresses macrophage cholesterol uptake in patients with type 2 diabetes mellitus, Circulation, 2009;120:687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Yin K, You Y, Swier V, et al. , Vitamin D Protects Against Atherosclerosis via Regulation of Cholesterol Efflux and Macrophage Polarization in Hypercholesterolemic Swine, Arteriosclerosis, thrombosis, and vascular biology, 2015;35:2432–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zhong W, Gu B, Gu Y, et al. , Activation of vitamin D receptor promotes VEGF and CuZn-SOD expression in endothelial cells, The Journal of steroid biochemistry and molecular biology, 2014;140:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Absi M and Ward DT, Increased endothelin-1 responsiveness in human coronary artery smooth muscle cells exposed to 1,25-dihydroxyvitamin D(3), American journal of physiology. Cell physiology, 2013;304:C666–672. [DOI] [PubMed] [Google Scholar]
- [42].Baumer Y, Ng Q, Sanda GE, et al. , Chronic skin inflammation accelerates macrophage cholesterol crystal formation and atherosclerosis, JCI insight, 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Christakos S, Dhawan P, Verstuyf A, et al. , Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects, Physiological reviews, 2016;96:365–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Pascoe VL and Kimball AB, Seasonal variation of acne and psoriasis: A 3-year study using the Physician Global Assessment severity scale, Journal of the American Academy of Dermatology, 2015;73:523–525. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.