Abstract
Background
In 1993, the US Food and Drug Administration established guidelines to increase diversity by sex and race/ethnicity of participants in clinical trials supporting novel drug approvals. In this study we investigated the 10‐year trends of participation of women and minorities in pivotal trials supporting approval of new molecular entities in cardiometabolic drugs from January 2008 to December 2017.
Methods and Results
A list of new molecular entities was abstracted from publicly available data at Drugs@Fda. Sex and race/ethnicity data were collected from trial publications. Linear regression analysis was performed to assess the relation between drug approval year and proportion of women and minorities enrolled. Thirty‐five novel cardiovascular (n=24) and diabetes mellitus (n=11) drugs were approved by the US Food and Drug Administration during the study period. The median number of participants supporting each drug was 5930 (interquartile range, 3175–10 942). Women represented 36% (n=108 052) of trial participants (n=296 163). Women were underrepresented compared with their proportion of the disease population in trials of coronary heart disease (participation‐to‐prevalence ratio, 0.52), heart failure (participation‐to‐prevalence ratio, 0.58), and acute coronary syndrome (participation‐to‐prevalence ratio, 0.68). Among trial participants, 81% were white, 4% black, 12% Asian, and 11% Hispanic/Latino. There was no significant association between enrollment of women (P=0.29) or underrepresented minorities (P=0.45) with the drug approval year.
Conclusions
Over the past decade (2008–2017), women and minorities, particularly blacks, have continued to be inadequately represented in pivotal cardiometabolic clinical trials that support US Food and Drug Administration approval of new molecular entities. This may have major implications in determining efficacy of such therapies in these groups, and may impair generalizability of trial results to routine clinical practice.
Keywords: cardiometabolic drugs, clinical trials, minorities, women
Subject Categories: Race and Ethnicity, Women, Pharmacology, Clinical Studies
Nonstandard Abbreviations and Acronyms
- DM
diabetes mellitus
- FDA
US Food and Drug Administration
- NDA
new drug application
- NME
new molecular entity
- PPR
participation‐to‐prevalence ratio
Clinical Perspective
What Is New?
Women and racial minorities are underrepresented in pivotal efficacy trials for novel cardiometabolic drugs approved by the US Food and Drug Administration, with no clear evidence of improvement in the recent decade.
Women accounted for 36% of the study trial populations, whereas blacks constituted only 4%.
What Are the Clinical Implications?
Inadequate representation of women and racial minorities in clinical trials can have major implications in determining the effects of therapy in these groups, and may impair the generalizability of the utility of the drug when distributed broadly in clinical practice.
Further efforts are needed to enhance participant inclusion to generate more complete information about any variation in drug therapies between demographic subgroups.
Cardiovascular and cardiometabolic diseases are the leading cause of mortality worldwide,1 with diabetes mellitus (DM) increasing the risk of cardiovascular disease by about four times in women.2 Despite the growing burden, there is a concerning lack of diversity by race and sex in clinical trials evaluating the safety and efficacy of drugs for these diseases.3, 4 Adequate involvement of both men and women in drug trials is vital to discern any sex‐based difference in drug effects.5 Moreover, demographic characteristics, such as race, may also have a contrasting effect on drug response, which may inadvertently lead to variation in treatment outcomes and survival.6
To counter this disparity, since 1993, the US Food and Drug Administration (FDA) has implemented guidelines encouraging greater participation of women7 and the need for diverse demographic enrollment.8 Although their policies may have gradually increased participation, women and racial minorities continue to be underrepresented in cardiometabolic trials.9, 10, 11 Sex disparity in clinical trial enrollment was also highlighted in a recent study,11 which addressed participation of women relative to their disease population in core cardiovascular trials supporting new drug applications (NDAs). However, no study has addressed whether involvement of women and racial minorities has changed over time for trials evaluating cardiometabolic drugs. Therefore, we sought to investigate sex and racial disparity in pivotal efficacy trials of novel cardiometabolic drugs approved in the past decade. We also analyzed the temporal trends of participation among these demographic groups.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request. As publicly available data were used, approval from the institutional review board was not required for this study.
Data Sources and Extraction
Novel cardiometabolic drug approvals from January 2008 to December 2017 were abstracted from the FDA website (Drugs@FDA) under NDA and biological license applications. Only new molecular entities (NMEs) approved under submission classification “Type 1: New Molecular Entity” were included in our study. Drugs approved for hyperlipidemia were also extracted under cardiovascular indication. Data including (1) drug name, (2) year of approval, (3) drug indication, and (4) approval pathway were obtained from the drug approval label, which was also used to identify all pivotal clinical trials listed in Section 14 under “Clinical Studies.” If a trial did not reach the analysis phase, it was excluded from our study.
To minimize the chances of missing data, the pivotal trials were subsequently searched on http://www.clinicaltrials.gov and PubMed. Data for (5) total population, (6) participation by sex, (7) race, (8) ethnicity of participants, (9) location, and (10) funding source of every pivotal trial associated with an NME were extracted from the study publication for total number of participants for whom corresponding demographic information was available. When a corresponding publication of the trial could not be found, data were obtained directly from the approval label. The Clinical Trials website was also reviewed for any additional information not available from the trial publication.
Race was captured in three categories, including (1) white, (2) black, and (3) Asian. If ethnicity was reported, it was recorded as Hispanic/Latino. Drug approval pathway was classified as (1) expedited pathway or (2) standard approval. We divided location/region of trial enrollment into (1) exclusively North America, including United States, Canada, and Mexico; (2) Europe; (3) the rest of the world—regions excluding North America and Europe; and (4) multiregional. Funding source was categorized as (1) government or (2) industry funding. Because biopharmaceutical companies are largely responsible for conducting clinical research required to advance and commercialize an NME,12 we further divided industry funding into (1) US‐based industry, (2) non–US‐based industry, or (3) collaborative (sponsored by both US‐ and non–US‐based company). This was defined by the location of the company headquarters from the industry sponsor's website.
Subgroup analysis by sex was conducted by examining the approval label and the FDA clinical and statistical reviews available at https://www.accessdata.fda.gov/scripts/cder/daf/ for each NME to identify and describe sex‐specific differences in the efficacy of the drug on the basis of analysis of pivotal trials. Any statement describing variable treatment effect of the drug between the two sexes for both binary end points (event yes/no) as well as continuous end points (eg, changes from baseline in glycated hemoglobin) was recorded separately for each therapeutic area. Trials reporting sex‐stratified hazard ratio (HR) with 95% CI for binary end points in approval label or FDA clinical and statistical review were also recorded for indexing of any sex‐based difference in the efficacy of drug effect. If no efficacy analysis by sex was found in the FDA clinical and statistical review for an NME, it was recorded as “no sex analysis conducted.” Moreover, we also examined approval label Section 6, “Adverse Reactions,” to identify any sex‐based difference in drug‐related adverse events. If no statement about difference in adverse events was found in the approval label, it was recorded as “not reported.”
To maximize quality and accuracy of data, the corresponding publications were searched and data were extracted by two independent investigators (I.S., T.J.S.). In instances where subsequent trial publication could not be found and data were not available in approval label, or if there was a discrepancy in data abstracted, a third reviewer (M.S.K.) was consulted. Details of data extraction for each drug, along with any discrepancy observed between data reported in trial publication and approval label, are displayed in Table S1.
Statistical Analysis
Trials were grouped according to the year the drug was approved. Continuous variables are reported as mean (SD) or median (interquartile range), and categorical variables are expressed as frequency and percent. Participation by sex, race, and ethnicity of every pivotal trial of each NME was calculated as a percentage of total participants in the study population. This percentage participation was further evaluated by drug approval year and by therapeutic group to assess any noticeable trends over the previous decade. An independent‐sample t test was used to assess for differences between two groups. For more than two groups, one‐way analysis of variance was used to test the significance of means and a post‐hoc analysis was done to identify groups that were significantly different at P<0.05. Missing data for race were obtained by adding the total population of trials that did not report the particular race and calculating it as a percentage of total participants in the overall study population.
To examine representation of women in our trials relative to the overall proportion of women in the disease population, we used the metric of “participation‐to‐prevalence ratio” (PPR), as suggested by Poon et al.13 We searched the Global Burden of Disease database14 to identify recent global prevalence of disease among both men and women for our disease populations. Global Burden of Disease is considered one of the most comprehensive epidemiologic data sets available globally, with the World Health Organization now regularly developing Global Burden of Disease estimates at global, national, and regional levels for >100 diseases and injuries by age, sex, and region.15 If estimated prevalence for our disease indication was not available in the Global Burden of Disease database, a comprehensive literature search was conducted using PubMed to obtain peer‐reviewed journal articles that estimated global prevalence of disease by sex. If global prevalence information was unavailable after searching both sources, then estimated prevalence data from studies conducted in North America were preferred. The most recent published data were used whenever possible.
PPR is calculated by dividing the percentage of women among total trial participants by the percentage of women among the disease population as follows:
The estimated proportion of women in the disease population was calculated by dividing the estimated prevalence of a particular disease area among women by overall prevalence (men and women) of that disease (Table S2). We divided disease population among eight key areas, namely acute coronary syndrome, stable coronary heart disease, heart failure, atrial fibrillation, hypertension, pulmonary arterial hypertension, DM, and hypercholesterolemia. Any drug that did not fall into either of the categories just indicated was listed as “other.” As suggested by Poon et al13 and Eshera et al,9 a PPR ratio between 0.8 and 1.2 would indicate adequate representation of women in trials relative to disease population, whereas a PPR <0.8 or >1.2 would represent underrepresentation or overrepresentation of women in trials, respectively. The PPR was only calculated for the female participation, and not race or ethnicity, in view of the limited number of trials within each disease group that reported participation of these demographic groups.
A simple linear regression analysis was performed to assess the trend in demographic characteristics of the patient samples from the years 2008 through 2017, using year of drug approval as the independent variable. The dependent variables were percentage of women and percentage of underrepresented minorities (black, Asian, and Hispanic/Latino). We applied simple linear regression after ensuring that the data met all the assumptions necessary to apply the test, which included ruling out autocorrelation between the two variables. P<0.05 was considered significant. SPSS version 23 (IBM Corp, Armonk, NY) and Microsoft Excel (Microsoft Corp, Redmond, WA) were used for analysis.
Results
General Characteristics
The characteristics of approved NMEs in the previous decade are presented in Table 1. There were 35 novel cardiometabolic drugs approved by FDA from January 2008 through December 2017. Data were analyzed from a total of 143 pivotal trials (57 cardiovascular and 86 DM) supporting approval of these drugs. Corresponding trial publications were found for all drugs except four (ie, azilsartan, lomitapide mesylate, insulin degludec, and lixisenatide), whereas a minor discrepancy between approval label and trial publication data was noted for rivaroxaban and vorapaxar sulfate (Table S1). The median number of trials per drug was 3 (interquartile range, 1–6). All trials (296 163 participants) enrolled both male and female participants. The median number of participants supporting each drug was 5930 (interquartile range, 3175–10 942). The majority of participants were enrolled in atrial fibrillation drug trials (13 trials; 94 624 participants), followed by DM (86 trials; 64 282 participants) and heart failure (4 trials; 44 923 participants) (Table S3).
Table 1.
Characteristics of Novel Cardiovascular and Diabetes Mellitus Drugs Approved in the Past Decade
| Drug | Approval Year | Approval Pathway | Therapeutic Area | Disease Indication | No. of Trials | Total Population, N | Women, n (%) |
|---|---|---|---|---|---|---|---|
| Regadenoson | 2008 | Standard | Cardiovascular | Other | 2 | 1871 | 577 (30.8) |
| Clevidipine | 2008 | Standard | Cardiovascular | Hypertension | 6 | 1847 | 522 (28.3) |
| Dronedarone | 2009 | Expedited | Cardiovascular | AF | 4 | 6492 | 2703 (41.6) |
| Prasugel | 2009 | Expedited | Cardiovascular | ACS | 1 | 13 608 | 3539 (26.0) |
| Saxagliptin | 2009 | Standard | Diabetes mellitus | DM | 6 | 4148 | 2130 (51.4) |
| Pitvastatin | 2009 | Standard | Cardiovascular | Hypercholesterolemia | 5 | 3375 | 1775 (52.6) |
| Liraglutide | 2010 | Standard | Diabetes mellitus | DM | 5 | 3978 | 1830 (46.0) |
| Dabigatran | 2010 | Expedited | Cardiovascular | AF | 1 | 18 113 | 6599 (36.4) |
| Azilsartan | 2011 | Standard | Cardiovascular | Hypertension | 7 | 5941 | 2911 (49.0) |
| Linagliptin | 2011 | Standard | Diabetes mellitus | DM | 8 | 3800 | 1824 (48.0) |
| Rivaroxaban | 2011 | Standard | Cardiovascular | AF | 3 | 9359 | 5458 (58.3) |
| Ticagrelor | 2011 | Standard | Cardiovascular | ACS | 1 | 18 624 | 5288 (28.4) |
| Lomitapide mesylate | 2012 | Standard | Cardiovascular | Hypercholesterolemia | 1 | 29 | 13 (44.8) |
| Apixaban | 2012 | Expedited | Cardiovascular | AF | 2 | 23 800 | 8738 (36.7) |
| Alogliptin benzoate | 2013 | Standard | Diabetes mellitus | DM | 9 | 6035 | 3081 (51.1) |
| Mipomersen | 2013 | Standard | Cardiovascular | Hypercholesterolemia | 1 | 51 | 29 (56.9) |
| Canagliflozin | 2013 | Standard | Diabetes mellitus | DM | 8 | 6729 | 3027 (50.0) |
| Riociguat | 2013 | Standard | Cardiovascular | PAH | 2 | 704 | 522 (74.1) |
| Macitentan | 2013 | Standard | Cardiovascular | PAH | 1 | 742 | 571 (77.0) |
| Dapagliflozin | 2014 | Standard | Diabetes mellitus | DM | 11 | 5930 | 2952 (49.8) |
| Vorapaxar sulfate | 2014 | Standard | Cardiovascular | CHD | 1 | 26 449 | 6326 (23.9) |
| Empagliflozin | 2014 | Standard | Diabetes mellitus | DM | 6 | 4826 | 2141 (44.4) |
| Edoxaban | 2015 | Standard | Cardiovascular | AF | 2 | 29 347 | 11 566 (39.4) |
| Ivabradine hydrochloride | 2015 | Expedited | Cardiovascular | HF | 3 | 36 524 | 8668 (23.7) |
| Cangrelor | 2015 | Standard | Cardiovascular | ACS | 1 | 10 942 | 3051 (27.9) |
| Sacubitril/valsartan | 2015 | Expedited | Cardiovascular | HF | 1 | 8399 | 1832 (21.8) |
| Insulin degludec | 2015 | Standard | Diabetes mellitus | DM | 9 | 5625 | 2454 (43.6) |
| Selexipag | 2015 | Standard | Cardiovascular | PAH | 1 | 1156 | 923 (79.8) |
| Alirocumab | 2015 | Standard | Cardiovascular | Hypercholesterolemia | 5 | 3499 | 1372 (39.2) |
| Evolocumab | 2015 | Standard | Cardiovascular | Hypercholesterolemia | 4 | 3175 | 1502 (47.3) |
| Lixisenatide | 2016 | Standard | Diabetes mellitus | DM | 11 | 11 147 | 4451 (39.9) |
| Betrixaban | 2017 | Expedited | Cardiovascular | AF | 1 | 7513 | 4088 (54.4) |
| Semaglutide | 2017 | Standard | Diabete mellitus | DM | 6 | 7215 | 3129 (43.4) |
| Ertugliflozin | 2017 | Standard | Diabete mellitus | DM | 7 | 4849 | 2333 (48.1) |
| Angiotensin II acetate | 2017 | Expedited | Cardiovascular | Other | 1 | 321 | 126 (39.3) |
ACS indicates acute coronary syndrome; AF, atrial fibrillation; CHD, coronary heart disease; DM, diabetes mellitus; HF, heart failure; and PAH, pulmonary arterial hypertension.
Data for region of clinical trial enrollment were available for 117 (82%) pivotal trials (Table 2). Of these trials, 93 (209 427 participants) were multiregional and 16 (32 273 participants) were conducted in North America and 8 (39 306 participants) were based in Europe, whereas no trials were conducted exclusively elsewhere in the world. All drug trials were sponsored by pharmaceutical companies, with 15 drugs (52 trials; 115 830 participants) sponsored by US‐based companies, 17 drugs (68 trials; 165 429 participants) sponsored by non–US‐based companies, and 3 drugs (23 trials; 14 904 participants) sponsored collaboratively by a US‐ and non–US‐based company. Eight drugs (14 trials; 114 770 participants) were approved via the expedited pathway.
Table 2.
Representation of Women in Pivotal Drug Trials
| No. of Trials | Overall Population, N | Women, n (%) | P Value | |
|---|---|---|---|---|
| Overall | 143 | 296 163 | 108 052 (36.4) | |
| Year of drug approval | ||||
| 2008 | 8 | 3718 | 1100 (29.6) | 0.29a |
| 2009 | 16 | 27 623 | 10 147 (36.7) | |
| 2010 | 6 | 22 091 | 8429 (38.2) | |
| 2011 | 19 | 37 724 | 15 481 (41.0) | |
| 2012 | 3 | 23 829 | 8751 (36.7) | |
| 2013 | 21 | 14 261 | 7230 (50.7) | |
| 2014 | 18 | 37 205 | 11 419 (30.7) | |
| 2015 | 26 | 98 667 | 31 368 (31.8) | |
| 2016 | 11 | 11 147 | 4451 (39.9) | |
| 2017 | 15 | 19 898 | 9676 (48.6) | |
| Location | ||||
| North America | 16 | 32 273 | 9595 (29.7) | <0.01 |
| Western/Central Europe | 8 | 39 306 | 9765 (24.8) | |
| Multiregional | 93 | 209 427 | 81 643 (39.0) | |
| Funding | ||||
| US pharmaceutical | 52 | 115 830 | 39 695 (34.3) | 0.19 |
| Non–US pharmaceutical | 68 | 165 429 | 61 134 (37.0) | |
| Collaboration | 23 | 14 904 | 7223 (48.5) | |
| Approval pathway | ||||
| Expedited pathway | 14 | 114 770 | 36 293 (31.6) | 0.03 |
| Standard pathway | 129 | 181.393 | 71 759 (39.6) | |
Simple linear regression used.
Trends in Participation of Women
The trend for participation of women across pivotal drug trials is highlighted in Table 2. The total number of women enrolled in these trials was 108 052, accounting for 36% of the 296 163 total participants. Throughout the previous decade, the year 2008 had the lowest participation of women (30%), whereas 2013 had the highest (51%) (Figure 1). The enrollment of women as a percentage of overall enrollment did not increase significantly over time (r=0.38, P=0.285).
Figure 1. Percentage of men and women participating overall in cardiovascular and diabetes mellitus pivotal drug trials according to year of drug approval.
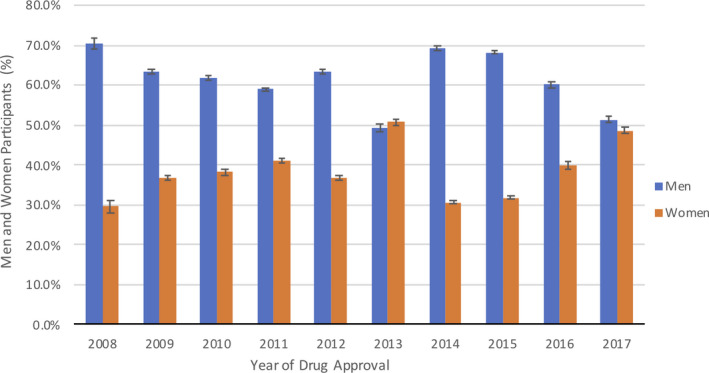
Representation of women was highest (39%) in multiregional trials and lowest (25%) in Western and Central European trials, which represented a statistically significant difference (P<0.01). Drugs sponsored collaboratively by US‐ and non–US‐based pharmaceutical companies had the highest participation of women (48%), followed by non‐US (37%) and US (34%) companies. There was a statistically significant difference (P=0.03) in enrollment of women between drugs approved via the expedited pathway (31.6% participants women) and drugs approved via standard protocol (39.6% participants women).
The highest percentage of women enrolled was seen in pulmonary arterial hypertension trials (77%), followed by hypercholesterolemia (46%) and DM (46%) trials, whereas the lowest was in heart failure (23%) trials (Table S3). Women were proportionally underrepresented in trials of coronary heart disease (PPR, 0.52), heart failure (PPR, 0.58), and acute coronary syndrome (PPR, 0.68). However, women were overrepresented in pulmonary arterial hypertension trials (PPR, 1.35) (Figure 2). Because 2 of the 3 pulmonary arterial hypertension drugs were approved in 2013, we conducted a secondary analysis by excluding those studies and adjusting the regression analysis for indication. No significant increase in enrollment of women as a percentage of overall enrollment was observed (r=0.39, P=0.257).
Figure 2. Participation of women in pivotal drug trials: prevalence‐corrected estimate.
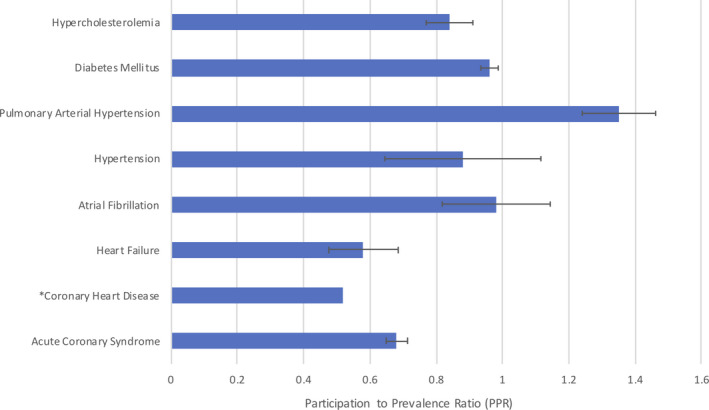
*Coronary heart disease participation‐to‐prevalence ratio was dependent upon 1 trial, so the 95% CI could not be calculated.
All 35 novel cardiometabolic drugs reported a sex‐based analysis of efficacy, whereas 11 cardiometabolic drugs reported a conclusive statement on sex‐based analysis of safety of drugs in pivotal trials to determine any variable effect of drug‐related adverse events and efficacy between sexes. Of the 11 novel DM drugs, 8 showed no indications of a sex‐based difference in efficacy. Variation in efficacy was recorded with saxagliptin, empagliflozin, and semaglutide, where saxagliptin and empagliflozin had a better treatment effect in men than women, and semaglutide had a better effect in women compared with men at the 0.5‐mg dose (Table S4). Variations in drug‐related adverse events were reported in 4 novel DM drugs, with genital mycotic infection being the most frequently reported adverse event for both men and women. Use of dapagliflozin, empagliflozin, and ertugliflozin showed genital mycotic infections occurring more frequently in women compared with men, whereas canagliflozin use led uncircumcised men to be more likely to develop genital mycotic infections (Table S4).
Of the 24 novel cardiovascular drugs, 21 demonstrated similar treatment effects for men and women. Table S5 lists 13 cardiovascular drugs with a binary efficacy end point and HR with 95% CI, according to sex. All 13 of these drugs showed overlapping 95% CIs for men and women, indicating similar drug effects for both sexes. Three hypercholesterolemia drugs, pitavastatin, mipomersen, and alirocumab, showed dissimilarities in drug efficacy. Pitavastatin and mipomersen showed a greater low‐density liprotein cholesterol–lowering effect in women, whereas alirocumab showed a higher percentage change in lowering of low‐density liprotein cholesterol in men compared with women (Table S5). No variation in drug‐related adverse events by sex was reported for any novel cardiovascular drug (Table S6). Results were not adjusted for other factors such as age or weight.
Trends in Participation of Racial/Ethnic Minorities
Race was reported for 34 of the 35 drug programs with data analyzed for 125 trials that reported inclusion by race (Table 3). Three of these trials enrolled only Asian participants, so they were excluded from the final analysis. Of the remaining trials across both therapeutic areas, whites were reported in a total of 122 trials, blacks in 104 trials, and Asians in 76 trials. Of the overall enrolled population among trials reporting data, whites represented 81% (218 054 of 269 176), blacks 4% (6325 of 175 487), and Asians 12% (22 076 of 178 004) of the total study population. Data for ethnicity were reported in 51 trials, with 11% of patients being Hispanic/Latino (Table S7).
Table 3.
Representation of Ethnic/Racial Minorities in Pivotal Drug Trials
| No. of Trials | Overall Population, N | White, n (%) | Black, n (%) | Asian, n (%) | Hispanic/Latino, n (%) | |
|---|---|---|---|---|---|---|
| Overall | 143 | 296 163 | 218 054 (73.6) | 6325 (2.1) | 22 076 (7.5) | 6333 (2.1) |
| Year of drug approval | ||||||
| 2008 | 8 | 3718 | 1427 (38.4) | 97 (2.6) | 1 (0.0) | 8 (0.2) |
| 2009 | 16 | 27 623 | 23 285 (84.3) | 561 (2.0) | 802 (2.9) | 533 (1.9) |
| 2010 | 6 | 22 091 | 15 742 (71.3) | 420 (1.9) | 2898 (13.1) | NR |
| 2011 | 19 | 37 724 | 31 211 (82.7) | 1565 (4.1) | 3005 (8.0) | 961 (2.5) |
| 2012 | 3 | 23 829 | 15 131 (63.5) | 182 (0.8) | 2548 (10.7) | NR |
| 2013 | 21 | 14 261 | 8585 (60.2) | 622 (4.4) | 1772 (12.4) | 1334 (9.4) |
| 2014 | 18 | 37 205 | 27 900 (75.0) | 251 (0.7) | 2355 (6.3) | 312 (0.8) |
| 2015 | 26 | 98 667 | 70 091 (71.0) | 1007 (1.0) | 4674 (4.7) | 719 (0.7) |
| 2016 | 11 | 11 147 | 8028 (72.0) | 372 (3.3) | 771 (6.9) | 888 (8.0) |
| 2017 | 15 | 19 898 | 16 654 (83.7) | 918 (4.6) | 1519 (7.6) | 1578 (7.9) |
| Location | ||||||
| North America | 16 | 32 273 | 26 156 (81.0) | 824 (2.6) | 317 (1.0) | 975 (3.0) |
| Europe | 8 | 39 306 | 23 444 (59.6) | 2 (0.0) | 3081 (7.8) | 1 (0.0) |
| Multiregional | 93 | 209 427 | 158 462 (75.7) | 3848 (1.8) | 16 850 (8.0) | 4028 (1.9) |
| P value | NA | NA | 0.01 | <0.01 | 0.11 | 0.36 |
| Funding | ||||||
| US pharmaceutical | 52 | 115 830 | 83 084 (71.7) | 1782 (1.5) | 3494 (3.0) | 2001 (1.7) |
| Non–US pharmaceutical | 68 | 165 429 | 103 814 (62.8) | 3571 (2.2) | 9850 (6.0) | 4020 (2.4) |
| Collaboration | 23 | 14 904 | 7770 (52.1) | 402 (2.7) | 3026 (20.3) | 312 (2.1) |
| P value | NA | NA | 0.55 | 0.84 | 0.15 | 0.20 |
| Approval pathway | ||||||
| Expedited pathway | 14 | 114 770 | 60 097 (52.4) | 1377 (1.2) | 7631 (6.6) | 531 (0.5) |
| Standard pathway | 129 | 181 393 | 130 452 (71.9) | 4378 (2.4) | 8739 (4.8) | 5802 (3.2) |
| P value | NA | NA | 0.81 | 0.54 | 0.29 | NA |
NA indicates not applicable; and NR, not reported.
The white population was predominant in all 3 locations. Eighty‐one percent (26 156 of 32 273) whites were reported in North American trials, followed by 76% (158 462 of 209 427) in multiregional trials and 60% (23 444 of 39 306) in European trials. The black population made up 3% (824 of 32 273) of the North American trials and 2% (3848 of 209 427) of the multiregional trials. Asians made up 1% (317 of 32 273) of the North American trials, 8% (3081 of 39 306) of the European trials, and 8% (16 850 of 209 427) of the multiregional trials. Hispanic/Latinos were underrepresented, with only 3% (975 of 32 273) reported in North American trials and 2% (4028 of 20 947) in multiregional trials. A statistically significant relation was seen between proportion of white population (P=0.01) and black population (P<0.01) and location of trial (Table 3).
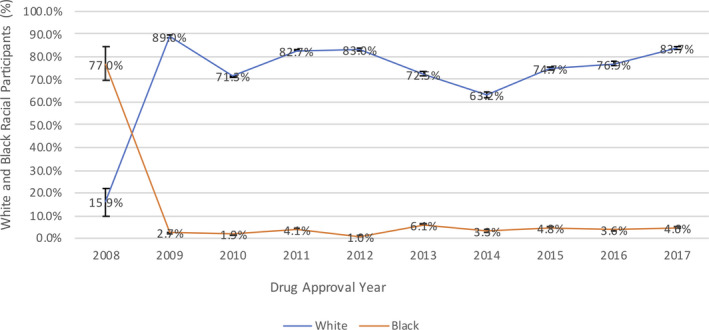
In trials reporting results for both white and black populations, inclusion of the black minority group remained <10% each year, except for 2008, owing to the predominantly black population in the 1 trial that reported data on racial minorities. Figure 3 demonstrates the percentage of whites enrolled in the same drug trials that reported results for the black racial minority group as well over the past decade. No significant association was noted between underrepresented minorities and year of drug approval (r=0.27, P=0.45).
Figure 3. Comparison of overall percentage of whites and blacks enrolled in pivotal cardiovascular and diabetes mellitus drug trials according to year of drug approval.
Discussion
Our analysis has highlighted that the proportion of women and minorities enrolled in pivotal trials for novel cardiometabolic drugs during the past decade remains disproportionately low, with no clear evidence of consistent improvement between 2008 and 2017 by either by sex or race/ethnicity.
Another recent report showed a similar sex disparity in cardiovascular trials supporting NDAs.11 Our study has expanded on the previous study by showing similar patterns of sex disparity in pivotal trials supporting novel DM and hyperlipidemia drugs. In addition, we have shown that representation of racial and ethnic minorities remains low in cardiometabolic drug trials. Furthermore, temporal trends have shown little progress in representation of women and racial minorities in cardiometabolic drug trials during the past decade.
The sex differences observed in clinical trials were previously justified by voicing safety concerns for women who may volunteer, especially those of reproductive age, which may also serve as an exclusion criterion for various studies. Previous studies have demonstrated that women perceiving increased risk of harm or being less aware of cardiovascular risk factors were less willing to participate in clinical trials.16, 17, 18, 19 Beyond participant factors, there may have been subconscious biases that led physicians and other investigators into screening more men than women.20
Besides these barriers, however, sex representation in clinical trials also varies with indication and is strongly attributed to disease prevalence in the population.11 Our results concur with a previous study11 highlighting heart failure as having particularly poor representation of women, where participation of women ranged from 22% to 24% across trials of 2 novel drugs, despite women making up 40% of those with heart failure with reduced ejection fraction disease.21 Comparatively, we found a 46% representation of women in DM drug trials, which more closely reflects their global disease burden of 55%.1 Despite the FDA's efforts to curb sex disparity over the years,7, 8 our results show no significant increase in overall participation of women over time.
Our analysis has also shown that FDA medical and statistical reviews of all novel cardiometabolic drugs included a sex‐based efficacy analysis. This is in contrast to previous studies,9, 10, 13 where much lower rates of sex‐based safety and efficacy analysis were observed. The trend of reporting sex‐based analysis has gradually increased over the years. For example, Poon et al13 reported a sex‐based analysis of safety and efficacy in 72% of NDAs approved by the FDA between 2007 and 2009, whereas Eshera et al9 reported the number of sex‐based analyses to have increased to include up to 92% NDAs approved between 2010 and 2012, to highlight any variation in efficacy or safety of drug between the 2 groups. This upward trend may be attributed to continued efforts by the FDA toward implementing guidelines22 and better compliance of trial sponsors in following these guidelines and conducting sex‐based analyses during the clinical trial.
Moreover, we observed few clinically meaningful differences in the efficacy and safety of drugs when analyzed by sex. For trials reporting binary efficacy end points, HR and 95% CI showed little sex‐based difference in drug efficacy. However, interpreting these results requires caution, as they did not consider multiple comparisons or adjust for other factors that may have affected outcomes. Differences in drug efficacy were noted in 3 hypercholesterolemia and 3 DM drugs, where women demonstrated better low‐density liprotein cholesterol lowering from baseline than men with 2 hypercholesterolemia drugs, and men had better change from baseline glycated hemoglobin with 2 DM drugs. Only 1 drug, empagliflozin, showed differences in both efficacy and adverse‐event rate by sex, where a greater treatment effect was noted in men; however, genital mycotic infections occurred more frequently in women. These differences in efficacy and the frequency of adverse events may be due to sex‐specific pharmacokinetic or pharmacodynamic factors23, 24 that could increase sensitivity to some drugs.
Furthermore, the majority of the clinical trials we analyzed had predominantly white participants with low enrollment rates of minority racial groups across both therapeutic areas. The FDA recommends Hispanic/Latino to be reported in the category of ethnicity and not race.25 However, some studies reported Hispanic participants as being in the race category. This means the Hispanic ethnicity may be applicable to people of different races in some studies. Studies that did not individually report ethnicity may have included Hispanic participants within the different race categories, which makes it difficult to assess and draw conclusions on overall inclusion of these racial groups, but it may explain why ethnicity was only reported in 51 of the 143 pivotal trials. A marked racial and ethnic disparity was observed in our analysis. Blacks comprise of 13% of the US population,26 but only represent 4% of participants in the trial populations analyzed. This finding concurs with recent studies,9, 27 emphasizing the need to establish more stringent guidelines to encourage inclusion of minority groups. Racial/ethnic heterogeneity in cardiovascular disease risk has been widely documented, with a higher prevalence of hypertension in blacks28 and a higher prevalence of DM in Hispanic/Latinos29 when compared with other racial groups. In addition, there is heterogeneity of genetic ancestry among self‐reported racial groups, and therefore assessment of individual genomic information would likely be more informative in predicting treatment outcomes than self‐reported race. This heterogeneity may lead to contrasting drug response, thereby making it crucial to assess safety and efficacy of drugs in groups with differing genetic composition.
There are some limitations to our study. First, PPR does not calculate prevalence of disease for the same age as the trial participants and the prevalence of disease may not be inclusive of all patients in the disease population. Although we tried our best to extract prevalence of disease from global data, the 3 disease populations that used prevalence data of North America to calculate percentage of disease of women in the population may not be reflective of expected prevalence across countries included in global trials. Second, although our study included all pivotal trials present in the approval label, it did not evaluate representation of women in the early‐phase studies. Third, a large number of participants had missing data for race and ethnicity. Missing data were recorded for 8% of whites, 40% of blacks, and 40% of Asians of the overall study population. This makes it difficult to generalize and draw conclusions on overall inclusion of various racial groups.
The high missing data rate among minorities may be attributed to the racial barrier between the minority participants and trial investigators, whereby participants of a different sociocultural background may not feel comfortable trusting the investigator or trial sponsors.30 This may lead to a low retention rate where minority group participants may only feel comfortable continuing with follow‐up if adequate trust has been established with the interviewers or field staff over time.31 An effective way to maximize participation may be done by increasing diversity among data collectors and the trial investigation team.31 Racial matching between the trial investigating team and participants may lead to an increased level of trust, thus contributing to increased enrollment in trials and fewer missing data. Furthermore, most of these clinical trials were multinational and thus not exclusively conducted in the United States. This could have had an impact when recruiting racial groups in which disease prevalence may also significantly differ according to demographic subgroup.
Conclusions
Our study has demonstrated the current trends in demographic data for clinical trials of cardiovascular and DM drugs that were submitted to the FDA for approval between 2008 and 2017. Persistent sex disparities remain, with women being inadequately represented in these trials. This participation disparity can limit information about the effects of therapy in women and impair generalizability of the drug's utility when released broadly in clinical practice. Furthermore, racial minorities, particularly blacks, have continued to be underrepresented in study trial populations, with participation rates remaining unchanged over the time frame studied. Therefore, to generate more complete information about the effects of new therapies, and to ensure clinical trials meet the needs of the populations seen in routine clinical practice, further efforts are needed to enhance the representativeness of clinical trials according to race and sex. Likewise, future studies are encouraged to identify factors contributing to such gaps in representativeness and to develop strategies and improve participant inclusivity and representation within clinical trials for cardiometabolic drugs.
Sources of Funding
Dr Michos is supported by the Amato Fund for Women's Cardiovascular Health Research at Johns Hopkins University.
Disclosures
S.J.G. has received a Young Investigator Award from the Heart Failure Society of America/Emergency Medicine Foundation Acute Heart Failure, funded by Novartis; has received research support from Amgen, AstraZeneca Bristol‐Myers Squibb, and Novartis; serves on an advisory board for Amgen Cytokinetics; and serves as a consultant for Amgen and Merck. J.B. is a consultant for Abbott, Amgen, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, CVRx, Janssen, LivaNova, Luitpold, Medtronic, Merck, Novartis, Relypsa, and Vifor. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S7
(J Am Heart Assoc. 2020;9:e015594 DOI: 10.1161/JAHA.119.015594.)
For Sources of Funding and Disclosures, see page 11.
References
- 1. Virani S, Alonso A, Benjamin E, Bittencourt M, Callaway C, Carson A, Chamberlain A, Chang A, Cheng S, Delling F, et al. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–e596. [DOI] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention . Diabetes and women. 2018. Available at: https://www.cdc.gov/features/diabetes-women/index.html. Accessed April 15, 2020.
- 3. US General Accounting Office . Women's Health: FDA needs to ensure more study of gender differences in prescription drug testing. 1992. Available at: http://archive.gao.gov/d35t11/147861.pdf. Accessed April 15, 2020.
- 4. Meinert CL, Gilpin AK, Unalp A, Dawson C. Gender representation in trials. Control Clin Trials. 2000;21:462–475. [DOI] [PubMed] [Google Scholar]
- 5. Fadiran EO, Zhang L. Effects of Sex Differences in the Pharmacokinetics of Drugs and Their Impact on the Safety of Medicines in Women. Medicines for Women. Switzerland: Springer International Publishing; 2015:41–68. [Google Scholar]
- 6. Temple R, Stockbridge NL. BiDil for heart failure in black patients: the U.S. Food and Drug Administration perspective. Ann Intern Med. 2007;146:57–62. [DOI] [PubMed] [Google Scholar]
- 7. U.S. Food and Drug Administration . Guideline for the study and evaluation of gender differences in the clinical evaluation of drugs. 1993;58:39406–39416. [PubMed] [Google Scholar]
- 8. U.S. Food and Drug Administration . Guidance for industry and Food and Drug Administration staff: evaluation of sex‐specific data in medical device clinical studies. 2014. Available at: www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM283707.pdf. Accessed April 15, 2020.
- 9. Eshera N, Itana H, Zhang L, Soon G, Fadiran E. Demographics of clinical trials participants in pivotal clinical trials for new molecular entity drugs and biologics approved by FDA from 2010 to 2012. Am J Ther. 2015;22:435–455. [DOI] [PubMed] [Google Scholar]
- 10. Yang Y, Carlin A, Faustino P, Motta M, Hamad M, He R, Watanuki Y, Pinnow E, Khan M. Participation of women in clinical trials for new drugs approved by the food and drug administration in 2000–2002. J Womens Health. 2009;18:303–310. [DOI] [PubMed] [Google Scholar]
- 11. Scott P, Unger E, Jenkins M, Southworth M, McDowell T, Geller R, Elahi M, Temple R, Woodcock J. Participation of women in clinical trials supporting FDA approval of cardiovascular drugs. J Am Coll Cardiol. 2018;71:1960–1969. [DOI] [PubMed] [Google Scholar]
- 12. Bodenheimer T. Uneasy alliance: clinical investigators and the pharmaceutical industry. N Engl J Med. 2000;342:1539–1544. [DOI] [PubMed] [Google Scholar]
- 13. Poon R, Khanijow K, Umarjee S, Fadiran E, Yu M, Zhang L, Parekh A. Participation of women and sex analyses in late‐phase clinical trials of new molecular entity drugs and biologics approved by the FDA in 2007–2009. J Womens Health. 2013;22:604–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. GBD Compare, IHME Viz Hub. Vizhub.healthdata.org. Available at: https://vizhub.healthdata.org/gbd-compare/. Accessed January 9, 2020.
- 15. The Global Burden of Disease concept. Who.int. Available at: https://www.who.int/quantifying_ehimpacts/publications/en/9241546204chap3.pdf. Accessed March 14, 2020.
- 16. Whyte J, Woodcock J, Wang J. Review of the drug trials snapshots program of the US food and drug administration, women in cardiovascular drug trials. JAMA Intern Med. 2017;177:724–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ding EL, Powe NR, Manson JE, Sherber NS, Braunstein JB. Sex differences in perceived risks, distrust, and willingness to participate in clinical trials: a randomized study of cardiovascular prevention trials. Arch Intern Med. 2007;167:905–912. [DOI] [PubMed] [Google Scholar]
- 18. Doyal L. Sex, gender, and health: the need for a new approach. BMJ. 2001;323:1061–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Peterson ED, Lytle BL, Biswas MS, Coombs L. Willingness to participate in cardiac trials. Am J Geriatr Cardiol. 2004;13:11–15. [DOI] [PubMed] [Google Scholar]
- 20. Melloni C, Berger J, Wang T, Gunes F, Stebbins A, Pieper K, Dolor R, Douglas P, Mark D, Newby L. Representation of women in randomized clinical trials of cardiovascular disease prevention. Circ Cardiovasc Qual Outcomes. 2010;3:135–142. [DOI] [PubMed] [Google Scholar]
- 21. Lee D, Gona P, Vasan R, Larson M, Benjamin E, Wang T, Tu J, Levy D. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction. Circulation. 2009;119:3070–3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. U.S. Food and Drug Administration . FDA Action Plan to Enhance the Collection and Availability of Demographic Subgroup Data. 2014. Available at: https://www.fda.gov/downloads/regulatoryinformation/legislation/significantamendmentstothefdcact/fdasia/ucm410474.pdf. Accessed March 16, 2020.
- 23. Soldin O, Chung S, Mattison D. Sex differences in drug disposition. J Biomed Biotechnol. 2011;2011:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Coker SJ. Drugs for men and women—how important is gender as a risk factor for TdP? Pharmacol Ther. 2008;119:186–194. [DOI] [PubMed] [Google Scholar]
- 25. Guidance for industry and FDA Staff, Collection Of Race And Ethnicity Data In Clinical Trials. 2017. Available at: https://www.fda.gov/media/102838/download. Accessed April 15, 2020.
- 26. U.S. Census Bureau . QuickFacts: United States, 2016. Available at: www.census.gov/quickfacts/table/PST045215/. Accessed April 15, 2020.
- 27. Chen A, Wright H, Itana H, Elahi M, Igun A, Soon G, Pariser A, Fadiran E. Representation of women and minorities in clinical trials for new molecular entities and original therapeutic biologics approved by FDA CDER from 2013 to 2015. J Womens Health. 2018;27:418–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kramer H, Han C, Post W, Goff D, Diezroux A, Cooper R, Jinagouda S, Shea S. Racial/Ethnic differences in hypertension and hypertension treatment and control in the multi‐ethnic study of atherosclerosis (MESA). Am J Hypertens. 2004;17:963–970. [DOI] [PubMed] [Google Scholar]
- 29. McBean A, Li S, Gilbertson D, Collins A. Differences in diabetes prevalence, incidence, and mortality among the elderly of four racial/ethnic groups: whites, blacks, hispanics, and asians. Diabetes Care. 2004;27:2317–2324. [DOI] [PubMed] [Google Scholar]
- 30. Weisfeld V, English R, Claiborne A. A Public Engagement And Clinical Trials. Washington, DC: National Academies Press; Recruitment Challenges in Clinical Trials for Different Diseases and Conditions. 2012. Available at: https://www.ncbi.nlm.nih.gov/books/NBK92105. Accessed May 5, 2020. [Google Scholar]
- 31. Yancey AK, Ortega AN, Kumanyika SK. Effective recruitment and retention of minority research participants. Annu Rev Public Health. 2006;27:1–28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S7


