Abstract
Methamphetamine‐associated cardiomyopathy (MACM) is an increasingly recognized disease entity in the context of a rapidly spreading methamphetamine epidemic. MACM may afflict individuals with a wide range of ages and socioeconomic backgrounds. Presentations can vary greatly and may involve several complications unique to the disease. Given the public health significance of this disease, there is a relative dearth of consensus material to guide clinicians in understanding, diagnosing, and managing MACM. This review therefore aims to: (1) describe pathologic mechanisms of methamphetamine as they pertain to the development, progression, and prognosis of MACM, and the potential to recover cardiac function; (2) summarize existing data from epidemiologic studies and case series in an effort to improve recognition and diagnosis of the disease; (3) guide short‐ and long‐term management of MACM with special attention to expected or potential sequelae of the disease; and (4) highlight pivotal unanswered questions in need of urgent investigation from a public health perspective.
Keywords: cardiomyopathy, diagnosis, heart failure, management, methamphetamine
Subject Categories: Cardiomyopathy, Heart Failure
Nonstandard Abbreviations and Acronyms
- BP
blood pressure
- CMR
cardiac magnetic resonance imaging
- EF
ejection fraction
- HR
heart rate
- LV
left ventricular
- MACM
methamphetamine‐associated cardiomyopathy
- PAH
pulmonary arterial hypertension
Amphetamines were first synthesized in the late 1920s as a mimetic to the popular drug ephedrine.1 The drugs were used mostly for nasal decongestion, asthma, narcolepsy, depression, and weight loss, and less commonly for heart block, myasthenia gravis, dysmenorrhea, and persistent hiccups.2 A patent on amphetamines in 1920s forced competitors to synthesize methamphetamines,3 which were applied to many of the same indications as amphetamines. However, recognition of their severe addiction potential led to manufacturing restrictions, but the drugs found their way to the black market, thus beginning the epidemic.
A consistent uptrend in the use of methamphetamines has been reported. In the United States, methamphetamine use between 2015 and 2016 increased dramatically from 0.5% to 0.8% of the total population (1.6 versus 2.6 million users), possibly because of greater availability, increasing potency, and reports of the “dark net” using cryptocurrencies like bitcoin to facilitate transactions.4, 5, 6
A 2016 Drug Enforcement Administration report identified methamphetamines as the greatest drug threat, second only to opioids. In 2005, the Rand Corporation estimated annual costs of methamphetamine treatment, the excess healthcare use associated with use and dependence, productivity losses, cost of methamphetamine‐associated crime, production of methamphetamines, the intangible burden borne by those addicted, and child endangerment at ≈$23.4 billion.7 Globally, premature death or disability attributable to amphetamine use has significantly increased over the past decade.8
Physiologic Effects of Methamphetamines
Methamphetamine, a member of the phenethylamine family, is a potent and highly addictive drug that often entails frequent and long‐term use. An addition of a methyl group to its sister drug amphetamine renders methamphetamine more lipophilic, resulting in increased blood‐brain barrier penetration, rapid onset of action, and prolonged activity.9, 10, 11 Crystal meth is a highly volatile form that can be easily smoked or injected, resulting in an onset within minutes and rapid peak concentrations, while remaining detectable in plasma for >24 hours (half‐life is ≈9 to 12 hours, independent of the route of administration).12
Methamphetamine increases postsynaptic catecholamine concentrations via several mechanisms, including: increasing cytosolic vesicular release of dopamine and norepinephrine at neuron terminals, decreasing the breakdown of catecholamines by blocking monoamine oxidase inhibitor, preventing presynaptic reuptake of catecholamines, and increasing diffusion of catecholamines into the postsynaptic space.13 Methamphetamine may also bind to other receptors expressed in the cardiovascular system, including the trace amino acid receptor, α‐2 adrenergic receptor, and ς‐1 receptor.13
Although methamphetamine may affect several organ systems, the most clinically relevant effects are neurologic and cardiovascular. In the central nervous system, methamphetamine elicits euphoria and increased alertness. Increased concentration of dopamine at the nucleus accumbens may promote the use‐reward relationship responsible for addiction.14 Contrarily, long‐term use leads to reduced dopamine synthesis and receptor downregulation, causing deficits in memory, attention, and decision‐making functions.15 Cardiovascular effects reflect a heightened catecholaminergic state (ie, elevated heart rate [HR], blood pressure [BP], and myocardial contractility), direct vasoconstriction or vasospasm, and possibly modulation of reactive oxygen species, inflammation, and reduced NO‐mediated vasodilation.13
Patterns of methamphetamine use may influence the degree of cardiovascular effect. Although the euphoric effect often begins to recede by 4 hours, increases in HR and BP may persist for >24 hours.11, 12 Therefore, repetitive dosing can lead to “stacking” of the cardiovascular effects. “Binge” use, defined as use for several days after which a period of abstinence ensues, has been associated with sensitization to the HR and BP effects in animals, resulting in cardiac inflammation and necrosis.16, 17
The effects of methamphetamine on the cardiovascular system may be enhanced by the concomitant use of other drugs, mainly alcohol, cocaine, and opioids.18, 19, 20 The combination of methamphetamine and alcohol increases rate‐pressure product compared with methamphetamine alone, possibly because of increased methamphetamine absorption and distribution, coupled with decreased metabolism.21, 22, 23, 24 Concomitant methamphetamine and cocaine is also associated with additive effects on vasoconstriction and myocardial oxygen consumption, causing greater cardiomyocyte damage in a rat model.25 Opioids, which reduce oxygenation via their respiratory depressant effects, may dysregulate the myocardial oxygen supply and demand balance when used in conjunction with methamphetamines.18
Pathophysiologic Mechanisms of Methamphetamine‐Associated Cardiomyopathy
The pathophysiologic mechanisms of methamphetamine‐associated cardiomyopathy (MACM) are thought to be multifactorial, involving processes linked to both direct and indirect myocardial damage (Figure 1). The drug may cause direct myocardial injury through several pathways, including: augmented free radical production promoting oxidative stress, apoptosis via increased p53 activity, mitochondrial dysfunction, altered gene expression, and defects in intracellular calcium hemostasis.26 These processes contribute to contractile dysfunction via detrimental effects on electrical‐mechanical coupling and calcium signaling and by increasing inflammation. Chronically, inflammation and loss of myocytes with replacement fibrosis lead to dilated cardiac chambers with impaired systolic function (clinically denoted as dilated cardiomyopathy and heart failure with reduced ejection fraction [EF]).18, 27, 28, 29 These direct effects of methamphetamines on the myocardium are likely in addition to and compounded by the indirect pathologic effects of short‐term and prolonged states of heightened sympathetic activity. However, as a testament to the degree of effect from direct myocardial toxicity, He30 demonstrated that methamphetamine‐induced histopathological changes in rat myocardium still occurred despite controlling for sympathetic‐related elevations in HR and BP by use of a β blocker.
Figure 1. Proposed pathophysiologic mechanisms for the development of methamphetamine‐associated cardiomyopathy.
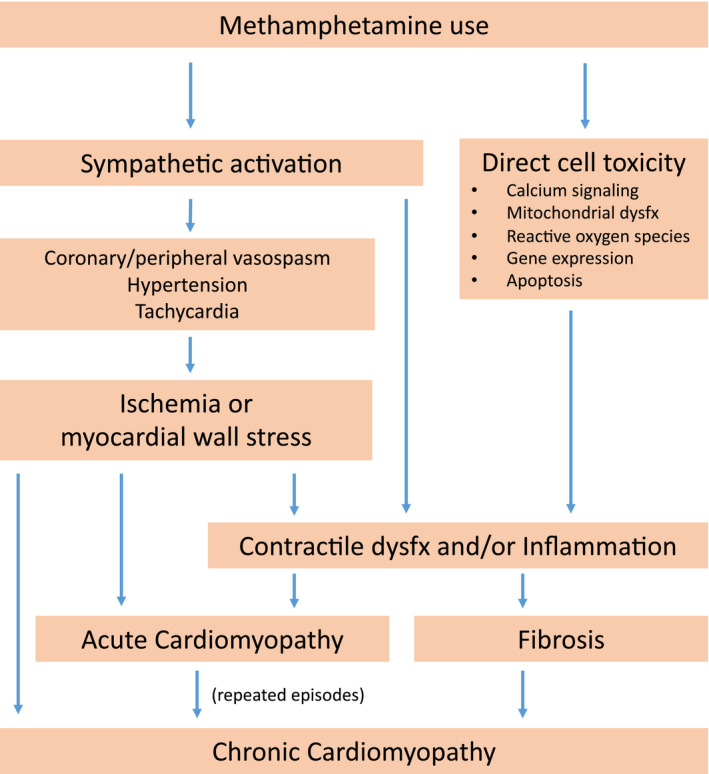
Dysfx indicates dysfunction.
Methamphetamines may cause acute myocardial ischemia through vasospasm of epicardial coronary arteries and the microvasculature.13, 31, 32, 33 In the long‐term setting, methamphetamines may cause ischemic disease by accelerating the development of atherosclerosis, as seen in both animal and human studies.18, 19, 34, 35 However, most case reports of MACM documented relatively normal coronary arteries.36, 37 Repeated or prolonged episodes of hypertension, tachycardia, or both may also summate to a chronic dilated cardiomyopathy with reduced EF. Catecholaminergic surges may also be responsible for the Takotsubo or “reverse Takotsubo” pattern previously reported.38
Histologically, methamphetamine use is associated with myocytolysis and vacuolization, spotty fibrosis, contraction band necrosis, atrophied and hypertrophied myocytes, edema, and disrupted mitochondria in rat models.27, 31, 39, 40 These findings are corroborated by a human autopsy study, demonstrating extensive myocardial remodeling with perivascular and interstitial fibrosis, cellular vacuolization, and ongoing myocyte destruction with proliferation of fibromyocytes in the interstitium.28 In a series of 100 cases of methamphetamine‐related deaths, cardiovascular pathological characteristics were found in 68% of the cases, including microscopic hemorrhages, fiber hypertrophy, focal necrosis, and perivascular fibrosis.34
Characteristics of MACM
Epidemiological Characteristics and Risk Factors
MACM is an increasingly recognized disease entity in the midst of a growing methamphetamine epidemic.41, 42, 43 The percentage of MACM among new cardiomyopathy cases in southern California has steadily increased from 1.8% in 2009 to 5.6% in 2014.43 An Australian autopsy study of all methamphetamine‐related deaths found cardiomyopathy, mostly dilated, in 5.5% of all cases.18
Patients with MACM tend to be of younger age compared with patients with cardiomyopathy attributable to other causes.38, 41, 43 A preponderance of cases among men (60%–93%)29, 36, 37, 38, 42, 43 as well as ethnic vulnerability to developing MACM have been described.37, 41, 43 However, these trends may represent the respective populations being studied. Genetic predisposition to development of cardiomyopathy in methamphetamine users may exist via the CYP2D6 enzyme, the initial and rate‐limiting step during metabolism of methamphetamine.44 A single study involving 56 patients showed a trend toward increased dilated cardiomyopathy among extensive metabolizers after adjusting for age and sex.45
Patterns of methamphetamine use may play an important role in the development of MACM as the pathologic effects of the drug have been shown to be dose dependent and amplified by repetitive use, binge pattern use, and concomitant use of other substances (see Physiologic Effects of Methamphetamines). However, in an autopsy study of methamphetamine‐related deaths, route of administration did not influence rates of cardiomegaly, left ventricular (LV) hypertrophy, coronary artery disease, cardiomyopathy, or replacement fibrosis.18 Reported duration of use before onset of symptoms appears highly variable, ranging from <7 months up to 15 years.29, 32, 46, 47, 48 Use of multiple illicit drugs has been shown to be common among patients with MACM,18, 29, 43 and a higher rate of alcohol abuse has been reported in patients with MACM compared with methamphetamine users without cardiomyopathy.49 Comorbid conditions commonly described in HF populations, including diabetes mellitus, hypertension, and obstructive coronary disease, are less frequent among MACM patients, perhaps because of young age at disease onset.29, 36, 41, 50
Presentation
Patients with MACM generally present with severe heart failure with reduced EF (New York Heart Association class III to IV symptoms),29, 36 while cardiogenic shock has also been cited.29, 48, 51 Chest pain is also a common complaint in both short‐ and long‐term settings.29, 52, 53 Although nonspecific, significant tachycardia has been reported, which may reflect compensatory response to low cardiac output, hypotension, hypoxia, and hyperthermia, but may also be an independent effect from catecholamine excess attributable to methamphetamines.12, 32, 48, 54 BP may be high but can be low once cases progress to cardiogenic shock. MACM patients are at significantly greater odds of multiple readmissions (≥3 admissions in <6 months) compared with patients with cardiomyopathy attributable to other causes.41 Patients may show evidence of other methamphetamine‐related, noncardiac findings, including cachexia, diaphoresis, track marks or excoriations, excessive talking, dry mouth with tooth decay (“meth mouth”), clenched jaw, hyperthermia, and mydriasis.55
Electrocardiogram
One study comparing the ECG findings of patients with history of methamphetamine use with age‐ and sex‐matched controls found that tachyarrhythmia, right axis deviation, lateral T‐wave inversion, P pulmonale, LV hypertrophy, QT prolongation, and inferior Q waves were all significantly more prevalent.56
Biomarkers
The utility of biomarkers in diagnosis of MACM is likely similar to that of the general HF population. In a recent retrospective study of 714 emergency department patients who tested positive for methamphetamine and were screened for HF, 63% had elevated brain natriuretic peptide (>100 pg/mL; mean value, 1256±1209 pg/mL), of whom 72% had LV dysfunction (mean EF, 34±18%) and 50% had severely reduced LV function. Among methamphetamine‐positive patients with elevated brain natriuretic peptide, creatinine was significantly higher but not troponin I.57
Echocardiography
Typical findings reported have included severe LV dilatation and reduced systolic function, left atrial enlargement, right ventricular dilatation, and dysfunction. Significant mitral and tricuspid valvular regurgitation were common as well as pulmonary hypertension, pericardial and pleural effusions, and ascites.36, 37, 38, 42 Global LV hypokinesis has been most commonly cited, while Takotsubo or “reverse” Takotsubo (hyperkinetic apex with hypokinetic base) appearance has also been described.46, 47, 58 In comparison to echocardiographic findings of young adults with other dilated cardiomyopathies, patients with MACM had significantly larger left atrial and left and right ventricular size, lower LVEF, and higher rate of mitral regurgitation.38 Patients with MACM may be especially prone to developing intracardiac thrombi, with a reported prevalence as high as 33% for LV thrombus and 3.3% for right ventricular thrombus.29, 59 This is likely a reflection of both severe cardiac dysfunctions and the heightened neurohormonal activation and prothrombotic state seen in MACM.29
Other Cardiovascular Manifestations
In addition to cardiomyopathy, methamphetamine use is associated with other acute and chronic cardiac complications. Acute myocardial infarction has been documented in several case reports, often being the result of vasospasm or coronary thrombosis in the absence of underlying coronary artery disease.32, 33, 60, 61, 62 Although symptomatic coronary artery disease is not common among patients presenting with MACM,36 accelerated atherosclerosis has been demonstrated in rat models and described at autopsy in methamphetamine‐related deaths with plaque burden greater than expected for age.35, 63 An epidemiological cross‐sectional study found a significant association between methamphetamine use and acute myocardial infarction while controlling for other risk factors, including cocaine, alcohol, and tobacco use.64
Acute aortic dissection, induced by hypertensive crisis secondary to methamphetamines, has been reported, perhaps suggesting that routine screening for methamphetamines is useful among patients presenting with aortic dissection.65, 66
Arrhythmias are not uncommon among patients using methamphetamines. One study of amphetamine and methamphetamine users found that among 230 cases, 3.5% presented with cardiac arrhythmias, including ventricular tachycardia, premature atrial beats, paroxysmal supraventricular tachycardia, and premature ventricular beats, and 34.3% had sinus tachycardia with long QT.67 Sudden cardiac death has also been associated with methamphetamine use, as might be expected in the setting of increased rates of fibrosis and heightened catecholaminergic state.68, 69
Pulmonary arterial hypertension (PAH) has been associated with methamphetamine use and may be found in conjunction with cardiomyopathy, although concomitance has not been clearly established. In a retrospective study of patients with PAH, those with idiopathic PAH were 10‐fold more likely to have used methamphetamines compared with patients with secondary PAH.70 A recent study also described patients with methamphetamine‐associated PAH as having more severe clinical presentations and a more rapidly progressive form of the disease compared with patients with idiopathic PAH.71
Outcomes and Recovery of Cardiac Function
Given the general severity of presentation, prognosis is often poor for patients with MACM. However, an important feature of MACM is the potential for recovery of cardiac function with abstinence from methamphetamines. In rat models, cardiac lesions induced by long‐term methamphetamine exposure have been shown to be reversible after withdrawal of the drug.39, 72 In a case series of 19 patients with MACM presenting with severe LV dysfunction (EF, 20±11%) and hemodynamic instability, 6 demonstrated recovery of LV function at 6 weeks. Smaller LV and left atrial size, shorter duration of methamphetamine use, reverse Takotsubo pattern, and no evidence of myocardial fibrosis appeared to predict early recovery.46 Schürer et al, using endomyocardial biopsy, showed that in patients with MACM who became abstinent, fibrotic burden was an independent predictor of cardiac recovery (Figure 2).29 Several case reports using cardiac magnetic resonance imaging (CMR) to identify fibrosis also describe recovery of cardiac function in patients with MACM without evidence of late gadolinium enhancement.73
Figure 2. Histological examples of patients with discontinued and continued methamphetamine (MA) abuse over different time periods.
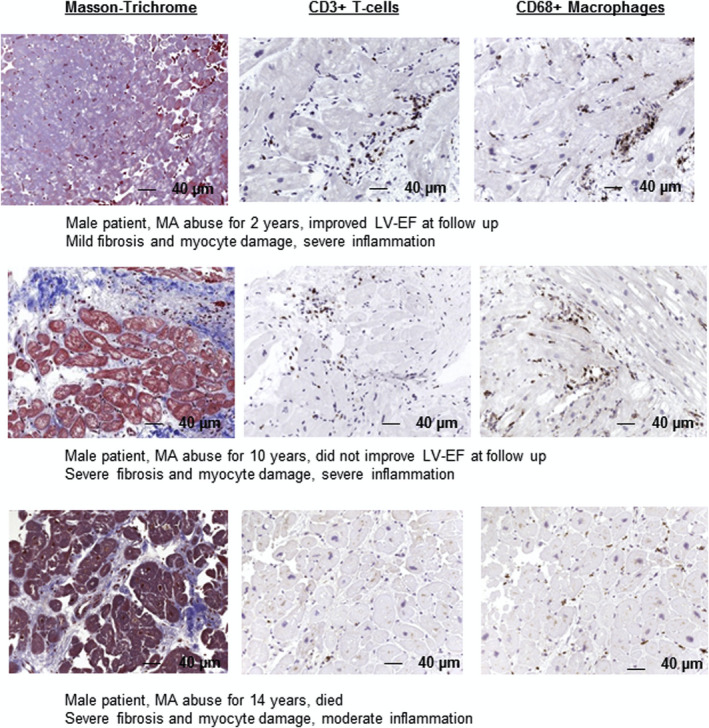
Longer and continued MA abuse (>5 years) is associated with a high degree of fibrosis and myocyte damage (magnification >200). Reprinted from Schürer et al29 with permission. Copyright ©2017, Elsevier. LV‐EF indicates left ventricular ejection fraction.
Diagnostic Approach
No consensus on diagnostic criteria for MACM currently exists. In the literature, the presumptive diagnosis of MACM has been based on recent methamphetamine use or positive urine toxicological findings, and exclusion of other common causes of HF. We recommend obtaining a detailed history on drug use, including duration and frequency (Table). Physical examination may uncover signs of methamphetamine use. Urine toxicological findings are helpful to detect recent use, as methamphetamine may be present in the urine for up to 2 and 4 days with short‐ and long‐term use, respectively. Caution is advised as high false‐positive rates are possible with medications, such as pseudoephedrine, ephedrine, phenylephrine, bupropion, trazadone, promethazine, and ranitidine.74 An echocardiogram is useful in detecting findings of severe LV dysfunction or biventricular failure with global hypokinesis and Takotsubo or reversed Takotsubo‐like appearance. Contrast echocardiography may be needed to confirm the presence of intracardiac thrombus.75 Ischemic evaluation is often warranted, especially in patients presenting with chest pain, troponin elevation, and wall motion abnormalities. CMR may be useful for assessment of the presence and degree of myocardial fibrosis, a strong predictor of reversibility of cardiac function, and the presence of intracardiac thrombus.76, 77
Table 1.
Diagnostic Evaluation of Patients With Suspected MACM
| Element of Presentation | Description |
|---|---|
| History of present illness | |
| Methamphetamine use patterns (dose and frequency, duration of use, binge use pattern, concomitant other drug or alcohol abuse) | Helps determine if methamphetamine is a factor in developing cardiomyopathy as well as suggest potential for cardiac recovery |
| Symptoms | |
| Chest pain | May be attributable to pulmonary congestion, acute coronary syndrome, or aortic dissection |
| Shortness of breath, orthopnea, PND | Volume overload, informs need for diuresis |
| Physical examination | |
| Vital signs: tachycardia, hypertension, and hyperthermia | Typical with active methamphetamine use |
| Poor dentition, cachexia | Provides evidence of methamphetamine use in patient not forthcoming; also corroborates urine toxicological results |
| Displaced PMI, parasternal heave, S3, systolic murmur | Consistent with dilated cardiomyopathy |
| Elevated JVD, crackles, leg edema, ascites | Volume overload, informs need for diuresis |
| Cold extremities, low BP, decreased urine output, altered mental status | Poor perfusion attributable to cardiogenic shock |
| Laboratory findings | |
| Urine toxicological findings | Positive for methamphetamine only if recent use; false positives occur because of cross‐reactivity with commonly used drugs/substances |
| Troponin | Mostly attributable to heart failure but may also suggest acute coronary syndrome |
| Brain natriuretic peptide | Typically elevated in patients with MACM |
| Lactate | Elevated levels indicate hypoperfusion and possible cardiogenic shock |
| BUN/creatinine | Acute kidney injury may be secondary to cardiorenal syndrome, but could also be attributable to rhabdomyolysis |
| Creatine kinase | Should be checked in all patients to assess presence of rhabdomyolysis |
| Imaging | |
|
Echocardiography: dilated atria and ventricles, Takotsubo or reverse Takotsubo, mitral and tricuspid regurgitation, pulmonary hypertension, pleural effusion, intracardiac thrombus |
Smaller ventricles and Takotsubo appearance may be more likely to recovery of cardiac function; special attention should be given to identifying intracardiac thrombus (contrast echocardiography and MRI) |
| Right heart catherization: elevated right and left ventricular filling pressures, pulmonary hypertension | Methamphetamine often causes biventricular dysfunction with or without PH |
| Coronary angiogram: nonobstructive CAD and vasospasm | Methamphetamine accelerates atherosclerosis; however, most patients with myocardial infarction demonstrate nonobstructive CAD; if suspicion is high for vasospasm but it is not seen, consider provocative measures |
| Prognostication | |
| Cardiac MRI/endomyocardial biopsy | Fibrotic burden may predict cardiac recovery if abstinence is achieved |
BP indicates blood pressure; BUN, blood urea nitrogen; CAD, coronary artery disease; JVD, jugular venous distention; MACM, methamphetamine‐associated cardiomyopathy; MRI, magnetic resonance imaging; PH, pulmonary hypertension; PMI, point of maximal impulse; and PND, paroxysmal nocturnal dyspnea.
Management
Short‐Term Management
Guidelines for the treatment of MACM have not been established, and management should be inferred from other forms of acute HF. In cases of cardiogenic shock, choice of intravenous vasoactive therapies, such as inotropes and vasopressors, should take into account potential sequelae or complications of methamphetamine (Figure 3). Tachycardia is often present because of methamphetamine‐mediated sympathetic hyperactivity. Benzodiazepines, such as midazolam, may be used to attenuate agitation and sympathetic stimulation.48, 78 Despite evidence of β‐blocker safety in the setting of amphetamine‐induced tachycardia and hypertension, initiation of lower doses and careful dose titration are advised in patients with significant cardiac dysfunction.79, 80 Drugs with a mixed α‐1 and β‐adrenergic receptor antagonist, like labetalol and carvedilol, provide more complete adrenergic blockade.81 The use of intravenous esmolol may be a prudent choice in patients with methamphetamine‐induced tachycardia and hemodynamic instability. Ivabradine may also reduce HR without lowering BP significantly.82 Inotropic or vasopressor support may be required to maintain peripheral perfusion in patients with severe HF and cardiogenic shock but may be limited by worsening tachycardia and vasoconstriction.83 To overcome the limitations of these drugs, mechanical circulatory support may be useful for hemodynamic stabilization and as a bridge to recovery.29, 48, 51
Figure 3. Management of acute and chronic methamphetamine (MA)–associated cardiomyopathy presentations.
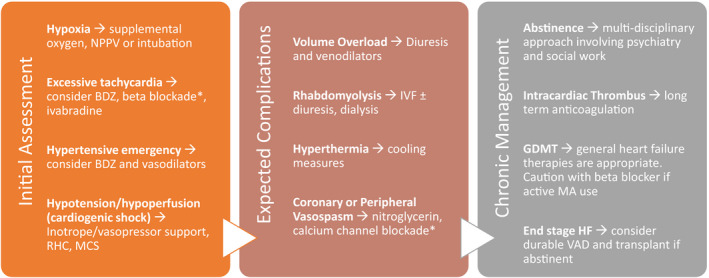
*Caution is advised if patient is actively using MAs. BDZ indicates benzodiazepines; GDMT, guideline‐directed medical therapy; HF, heart failure; IVF, intravenous fluid; MCS, mechanical circulatory support; NPPV, noninvasive positive pressure ventilation; RHC, right heart catheterization; and VAD, ventricular assist device.
Hyperthermia can be seen in patients using methamphetamines, and cooling measures may be considered to prevent neurologic damage, improve tachycardia, and decrease the risk of arrhythmias.84, 85 Hyperthermia is peripherally mediated, involving excessive muscle activity. Because the hypothalamic set point is not altered, antipyretics may not be effective. In refractory cases, benzodiazepines or paralysis with nondepolarizing neuromuscular blocking agents (ie, rocuronium or vecuronium) can be considered.
Proactive screening for intracardiac thrombi in patients presenting with MACM is advised given the elevated risk.29 Contrast echocardiography and CMR will provide a higher sensitivity for the detection of intracardiac thrombi.75, 77, 86 Prophylactic anticoagulation is indicated in the hospitalized patient, and long‐term anticoagulation should be considered in those with severe LV dysfunction.87
In patients presenting with MACM complicated by acute coronary syndrome, coronary vasospasm must be considered as a cause.32, 33 Diagnostic coronary angiography should be used according to guidelines and, if vasospasm is suspected, vasodilatory medications should be considered. If β blockade is initiated, a β blocker with α‐1 receptor antagonist activity (ie, carvedilol and labetalol) may be better tolerated.
Rhabdomyolysis is a common and challenging complication of methamphetamine use and often leads to acute kidney injury.88 Electrolyte disturbances and acidosis may contribute to proarrhythmic risk and negative inotropy. Usual management of rhabdomyolysis requires rapid administration of isotonic fluid that may be prohibitive in a patient presenting in acute HF. Active surveillance for rhabdomyolysis by checking creatinine kinase and urine studies early on may be prudent. If rhabdomyolysis is suspected, hemodialysis with ultrafiltration should be considered early to prevent imminent, severe renal damage.
Long‐Term Management
Currently, no studies have evaluated medical therapies specifically in patients with MACM. However, until further data are available, patients with MACM should be treated with guideline‐directed medical therapy for heart failure with reduced EF (Figure 3).29 Abstinence from methamphetamines may allow significant recovery of cardiac function; therefore, resources should be dedicated to that end. Because of the high risk for intracardiac thrombi, long‐term anticoagulation should be considered.
Consideration for primary prevention implantable cardioverter‐defibrillator implantation should follow the usual criteria for heart failure with reduced EF. As some patients with MACM may recover cardiac function, patients should be reassessed after a period of abstinence from methamphetamines. CMR can help to predict cardiac recovery in MACM patients.73, 76 A wearable cardioverter‐defibrillator may be appropriate as a bridge to recovery or decision. More important, high defibrillation thresholds have been reported in patients with MACM, making defibrillation threshold testing and empiric consideration of high‐output devices mandatory.89 Given the tendency to have severely reduced EF, many patients with MACM will be indicated for an implantable cardioverter‐defibrillator. However, one recent study found that only 14% of these patients actually received a device.49 This is presumably because of social factors that make providers apprehensive about offering implantation of an intracardiac device. Because of the intense addiction nature of the drug and perhaps other concomitant psychiatric illness, methamphetamine users often lack insight into the severity of their own disease, therefore limiting their ability to follow up after device implantation. Homelessness, which is common among methamphetamine users, can also make attending clinic appointments difficult. Without proper device surveillance, complications, such as pocket infection, lead fracture, or battery drain, may go unaddressed for extended periods. Increased prevalence of intravenous drug use also predisposes patients with MACM to developing infectious endocarditis with a possible complication being lead infection requiring device explant.
Patients with severely impaired cardiac function who are refractory to medical therapy should be considered for mechanical circulatory support as destination therapy or as a bridge to transplantation; however, similar social limitations to those mentioned above apply here as well. Furthermore, even with established abstinence from methamphetamines, patients with a previous history of substance abuse may demonstrate poor compliance after receiving mechanical circulatory support or transplantation, thus leading to poor outcomes.90, 91, 92
Promotion of Abstinence From Methamphetamines
The importance of abstinence from methamphetamines cannot be overstated from a public health perspective. Although the specifics of cessation efforts are beyond the scope of this review, we advise that clinicians seek a multipronged approach with the help of primary care providers, psychiatry, social work, and substance abuse counselors. Psychosocial interventions, including securing housing, establishing a support network, and receiving cognitive therapy, are important factors. Medical interventions should be considered with the aid of an addiction medicine specialist. Patients may also benefit from admission to dedicated rehabilitation centers.
Future Considerations
MACM is increasingly recognized as a severe form of cardiomyopathy, poised to become more prevalent in the midst of a growing methamphetamine epidemic worldwide. There should be a sense of urgency and commitment from the medical community to further characterize this disease so that we may be better equipped to care for this population. Risk factors for developing MACM should be identified through careful epidemiologic studies in conjunction with basic science investigation. Prospective studies are needed to determine the natural history of the disease, determine expected complications, and evaluate how therapies may improve longitudinal outcomes. Prognostic factors must be explored further, especially those related to predicting cardiac recovery, as this determination will most significantly impact management. CMR in this regard appears particularly promising. Finally, medical and governmental resources should be appropriately dedicated to methamphetamine cessation and rehabilitation, not only from a public health perspective but to reduce overall healthcare cost burden as well.
Disclosures
None.
(J Am Heart Assoc. 2020;9:e016704 DOI: 10.1161/JAHA.120.016704.)
For Disclosures, see page 9.
References
- 1. Rasmussen N. Making the first anti‐depressant: amphetamine in American medicine, 1929–1950. J Hist Med Allied Sci. 2006;61:288–323. [DOI] [PubMed] [Google Scholar]
- 2. Vearrier D, Greenberg MI, Miller SN, Okaneku JT, Haggerty DA. Methamphetamine: history, pathophysiology, adverse health effects, current trends, and hazards associated with the clandestine manufacture of methamphetamine. Dis Mon. 2012;58:38–89. [DOI] [PubMed] [Google Scholar]
- 3. Rasmussen N. Amphetamine‐type stimulants: the early history of their medical and non‐medical uses. Int Rev Neurobiol. 2015;120:9–25. [DOI] [PubMed] [Google Scholar]
- 4. Romanelli F, Smith KM. Clinical effects and management of methamphetamine abuse. Pharmacotherapy. 2006;26:1148–1156. [DOI] [PubMed] [Google Scholar]
- 5. National Institute on Drug Abuse (NIDA) Epidemiologic Trends in Drug Abuse: Proceedings of the Community Epidemiology Work Group, Highlights and Executive Summary. Bethesda, MD: U.S. Department of Health and Human Services, National Institutes of Health, 2012. [Google Scholar]
- 6. United Nations Office on Drugs and Crime, World Drug Report 2017. ISBN: 978‐92‐1‐148291‐1, eISBN: 978‐92‐1‐060623‐3, United Nations publication, Sales No. E.17.XI.6.
- 7. Nicosia N, Pacula RL, Kilmer B, Lundberg R, Chiesa J. The economic cost of methamphetamine use in the United States, 2005. Santa Monica, CA: Rand Corporation, 2009. [Google Scholar]
- 8. Global, regional, and national disability‐adjusted life‐years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1603–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goodwin JS, Larson GA, Swant J, Sen N, Javitch JA, Zahniser NR, De Felice LJ, Khoshbouei H. Amphetamine and methamphetamine differentially affect dopamine transporters in vitro and in vivo. J Biol Chem. 2009;284:2978–2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nichols DE. Medicinal chemistry and structure–activity relationships In: Cho AK, Segal DS, eds. Amphetamine and Its Analogs. San Diego, CA: Academic Press; 1994:3–41. [Google Scholar]
- 11. Kirkpatrick MG, Gunderson EW, Johanson CE, Levin FR, Foltin RW, Hart CL. Comparison of intranasal methamphetamine and d‐amphetamine self‐administration by humans. Addiction. 2012;107:783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cruickshank CC, Dyer KR. A review of the clinical pharmacology of methamphetamine. Addiction. 2009;104:1085–1099. [DOI] [PubMed] [Google Scholar]
- 13. Kevil CG, Goeders NE, Woolard MD, Bhuiyan MS, Dominic P, Kolluru GK, Arnold CL, Traylor JG, Orr AW. Methamphetamine use and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2019;39:1739–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kish SJ. Pharmacologic mechanisms of crystal meth. CMAJ. 2008;178:1679–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annu Rev Pharmacol Toxicol. 2007;47:681–698. [DOI] [PubMed] [Google Scholar]
- 16. Varner KJ, Ogden BA, Delcarpio J, Meleg‐Smith S. Cardiovascular responses elicited by the “binge” administration of methamphetamine. J Pharmacol Exp Ther. 2002;301:152–159. [DOI] [PubMed] [Google Scholar]
- 17. Yoshida K, Morimoto A, Makisumi T, Murakami N. Cardiovascular, thermal and behavioral sensitization to methamphetamine in freely moving rats. J Pharmacol Exp Ther. 1993;267:1538–1543. [PubMed] [Google Scholar]
- 18. Darke S, Duflou J, Kaye S. Prevalence and nature of cardiovascular disease in methamphetamine‐related death: a national study. Drug Alcohol Depend. 2017;179:174–179. [DOI] [PubMed] [Google Scholar]
- 19. Kaye S, Darke S, Duflou J, McKetin R. Methamphetamine‐related fatalities in Australia: demographics, circumstances, toxicology and major organ pathology. Addiction. 2008;103:1353–1360. [DOI] [PubMed] [Google Scholar]
- 20. Bujarski S, Roche DJ, Lunny K, Moallem NR, Courtney KE, Allen V, Hartwell E, Leventhal A, Rohrbaugh T, Ray LA. The relationship between methamphetamine and alcohol use in a community sample of methamphetamine users. Drug Alcohol Depend. 2014;142:127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mendelson J, Jones RT, Upton R, Jacob P III. Methamphetamine and ethanol interactions in humans. Clin Pharmacol Ther. 1995;57:559–568. [DOI] [PubMed] [Google Scholar]
- 22. Kirkpatrick MG, Gunderson EW, Levin FR, Foltin RW, Hart CL. Acute and residual interactive effects of repeated administrations of oral methamphetamine and alcohol in humans. Psychopharmacology. 2012;219:191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liang M, Liu Y, Zheng N, Ananda S, Liu L. Distribution of methamphetamine and its metabolite amphetamine in acute and subacute ethanol‐methamphetamine combination abuse model rats. J Anal Toxicol. 2012;36:30–35. [DOI] [PubMed] [Google Scholar]
- 24. Singh AK. Alcohol interaction with cocaine, methamphetamine, opioids, nicotine, cannabis, and gamma‐hydroxybutyric acid. Biomedicines. 2019;7:E16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Welder AA. A primary culture system of postnatal rat heart cells for the study of cocaine and methamphetamine toxicity. Toxicol Lett. 1992;60:183–196. [DOI] [PubMed] [Google Scholar]
- 26. Jafari Giv M. Exposure to amphetamines leads to development of amphetamine type stimulants associated cardiomyopathy (ATSAC). Cardiovasc Toxicol. 2017;17:13–24. [DOI] [PubMed] [Google Scholar]
- 27. Lord KC, Shenouda SK, McIlwain E, Charalampidis D, Lucchesi PA, Varner KJ. Oxidative stress contributes to methamphetamine‐induced left ventricular dysfunction. Cardiovasc Res. 2010;87:111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Karch SB. The unique histology of methamphetamine cardiomyopathy: a case report. Forensic Sci Int. 2011;212:e1–e4. [DOI] [PubMed] [Google Scholar]
- 29. Schurer S, Klingel K, Sandri M, Majunke N, Besler C, Kandolf R, Lurz P, Luck M, Hertel P, Schuler G, et al. Clinical characteristics, histopathological features, and clinical outcome of methamphetamine‐associated cardiomyopathy. JACC Heart Fail. 2017;5:435–445. [DOI] [PubMed] [Google Scholar]
- 30. He SY. Methamphetamine‐induced toxicity in cultured adult rat cardiomyocytes. Nihon Hoigaku Zasshi. 1995;49:175–186. [PubMed] [Google Scholar]
- 31. He SY, Matoba R, Fujitani N, Sodesaki K, Onishi S. Cardiac muscle lesions associated with chronic administration of methamphetamine in rats. Am J Forensic Med Pathol. 1996;17:155–162. [DOI] [PubMed] [Google Scholar]
- 32. Hong R, Matsuyama E, Nur K. Cardiomyopathy associated with the smoking of crystal methamphetamine. JAMA. 1991;265:1152–1154. [PubMed] [Google Scholar]
- 33. Chen JP. Methamphetamine‐associated acute myocardial infarction and cardiogenic shock with normal coronary arteries: refractory global coronary microvascular spasm. J Invasive Cardiol. 2007;19:E89–E92. [PubMed] [Google Scholar]
- 34. Akhgari M, Mobaraki H, Etemadi‐Aleagha A. Histopathological study of cardiac lesions in methamphetamine poisoning‐related deaths. Daru. 2017;25:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Karch SB, Stephens BG, Ho CH. Methamphetamine‐related deaths in San Francisco: demographic, pathologic, and toxicologic profiles. J Forensic Sci. 1999;44:359–368. [PubMed] [Google Scholar]
- 36. Wijetunga M, Seto T, Lindsay J, Schatz I. Crystal methamphetamine‐associated cardiomyopathy: tip of the iceberg? J Toxicol Clin Toxicol. 2003;41:981–986. [DOI] [PubMed] [Google Scholar]
- 37. Yeo KK, Wijetunga M, Ito H, Efird JT, Tay K, Seto TB, Alimineti K, Kimata C, Schatz IJ. The association of methamphetamine use and cardiomyopathy in young patients. Am J Med. 2007;120:165–171. [DOI] [PubMed] [Google Scholar]
- 38. Ito H, Yeo KK, Wijetunga M, Seto TB, Tay K, Schatz IJ. A comparison of echocardiographic findings in young adults with cardiomyopathy: with and without a history of methamphetamine abuse. Clin Cardiol. 2009;32:E18–E22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Islam MN, Jesmine K, Kong Sn Molh A, Hasnan J. Histopathological studies of cardiac lesions after long term administration of methamphetamine in high dosage–part II. Leg Med (Tokyo). 2009;11(suppl 1):S147–S150. [DOI] [PubMed] [Google Scholar]
- 40. Yi SH, Ren L, Yang TT, Liu L, Wang H, Liu Q. Myocardial lesions after long‐term administration of methamphetamine in rats. Chin Med Sci J. 2008;23:239–243. [DOI] [PubMed] [Google Scholar]
- 41. Diercks DB, Fonarow GC, Kirk JD, Jois‐Bilowich P, Hollander JE, Weber JE, Wynne J, Mills RM, Yancy C, Peacock WF IV. Illicit stimulant use in a United States heart failure population presenting to the emergency department (from the Acute Decompensated Heart Failure National Registry Emergency Module). Am J Cardiol. 2008;102:1216–1219. [DOI] [PubMed] [Google Scholar]
- 42. Neeki MM, Kulczycki M, Toy J, Dong F, Lee C, Borger R, Adigopula S. Frequency of methamphetamine use as a major contributor toward the severity of cardiomyopathy in adults </=50 years. Am J Cardiol. 2016;118:585–589. [DOI] [PubMed] [Google Scholar]
- 43. Sliman S, Waalen J, Shaw D. Methamphetamine‐associated congestive heart failure: increasing prevalence and relationship of clinical outcomes to continued use or abstinence. Cardiovasc Toxicol. 2016;16:381–389. [DOI] [PubMed] [Google Scholar]
- 44. Lin LY, Di Stefano EW, Schmitz DA, Hsu L, Ellis SW, Lennard MS, Tucker GT, Cho AK. Oxidation of methamphetamine and methylenedioxymethamphetamine by CYP2D6. Drug Metab Dispos. 1997;25:1059–1064. [PubMed] [Google Scholar]
- 45. Sutter ME, Gaedigk A, Albertson TE, Southard J, Owen KP, Mills LD, Diercks DB. Polymorphisms in CYP2D6 may predict methamphetamine related heart failure. Clin Toxicol (Phila). 2013;51:540–544. [DOI] [PubMed] [Google Scholar]
- 46. Voskoboinik A, Ihle JF, Bloom JE, Kaye DM. Methamphetamine‐associated cardiomyopathy: patterns and predictors of recovery. Intern Med J. 2016;46:723–727. [DOI] [PubMed] [Google Scholar]
- 47. Srikanth S, Barua R, Ambrose J. Methamphetamine‐associated acute left ventricular dysfunction: a variant of stress‐induced cardiomyopathy. Cardiology. 2008;109:188–192. [DOI] [PubMed] [Google Scholar]
- 48. Morrison LK, Kromm J, Gaudet J, Zuege D, Button B, Warshawski F, Lucyk SN. Rescue extracorporeal membrane oxygenation therapy in methamphetamine toxicity. CJEM. 2018;20(S2):S14–S19. [DOI] [PubMed] [Google Scholar]
- 49. Zhao SX, Kwong C, Swaminathan A, Gohil A, Crawford MH. Clinical characteristics and outcome of methamphetamine‐associated pulmonary arterial hypertension and dilated cardiomyopathy. JACC Heart Fail. 2018;6:209–218. [DOI] [PubMed] [Google Scholar]
- 50. Dadpour B, Dabbagh Kakhki VR, Afshari R, Dorri‐Giv M, Mohajeri SA, Ghahremani S. Myocardial perfusion and left ventricular function indices assessed by gated myocardial perfusion SPECT in methamphetamine abusers. Nucl Med Commun. 2016;37:1302–1305. [DOI] [PubMed] [Google Scholar]
- 51. Stokes MB, Fernando H, Taylor AJ. Cardiogenic shock secondary to methamphetamine induced cardiomyopathy requiring veno‐arterial extra‐corporeal membrane oxygenation. Int J Cardiol. 2016;207:134–135. [DOI] [PubMed] [Google Scholar]
- 52. Qutrio Baloch Z, Hussain M, Agha Abbas S, Perez JL, Ayyaz M. Methamphetamine‐induced cardiomyopathy (MACM) in a middle‐aged man: a case report. Emerg (Tehran). 2018;6:e9. [PMC free article] [PubMed] [Google Scholar]
- 53. Sadeghi R, Agin K, Taherkhani M, Najm‐Afshar L, Nelson LS, Abdollahi M, Shadnia S. Report of methamphetamine use and cardiomyopathy in three patients. Daru. 2012;20:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Won S, Hong RA, Shohet RV, Seto TB, Parikh NI. Methamphetamine‐associated cardiomyopathy. Clin Cardiol. 2013;36:737–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hart CL, Gunderson EW, Perez A, Kirkpatrick MG, Thurmond A, Comer SD, Foltin RW. Acute physiological and behavioral effects of intranasal methamphetamine in humans. Neuropsychopharmacology. 2008;33:1847–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Paratz ED, Zhao J, Sherwen AK, Scarlato RM, MacIsaac AI. Is an abnormal ECG just the tip of the ICE‐berg? Examining the utility of electrocardiography in detecting methamphetamine‐induced cardiac pathology. Heart Lung Circ. 2017;26:684–689. [DOI] [PubMed] [Google Scholar]
- 57. Richards JR, Harms BN, Kelly A, Turnipseed SD. Methamphetamine use and heart failure: prevalence, risk factors, and predictors. Am J Emerg Med. 2018;36:1423–1428. [DOI] [PubMed] [Google Scholar]
- 58. Chehab O, Ioannou A, Sawhney A, Rice A, Dubrey S. Reverse Takotsubo cardiomyopathy and cardiogenic shock associated with methamphetamine consumption. J Emerg Med. 2017;53:e81–e83. [DOI] [PubMed] [Google Scholar]
- 59. Janardhanan R, Kannan A. Methamphetamine cardiotoxicity: unique presentation with multiple bi‐ventricular thrombi. Am J Med. 2016;129:e3–e4. [DOI] [PubMed] [Google Scholar]
- 60. Khaheshi I, Mahjoob MP, Esmaeeli S, Eslami V, Haybar H. Simultaneous thrombosis of the left anterior descending artery and the right coronary artery in a 34‐year‐old crystal methamphetamine abuser. Korean Circ J. 2015;45:158–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Turnipseed SD, Richards JR, Kirk JD, Diercks DB, Amsterdam EA. Frequency of acute coronary syndrome in patients presenting to the emergency department with chest pain after methamphetamine use. J Emerg Med. 2003;24:369–373. [DOI] [PubMed] [Google Scholar]
- 62. Al Shehri MA, Youssef AA. Acute myocardial infarction with multiple coronary thromboses in a young addict of amphetamines and benzodiazepines. J Saudi Heart Assoc. 2016;28:180–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gao B, Li L, Zhu P, Zhang M, Hou L, Sun Y, Liu X, Peng X, Gu Y. Chronic administration of methamphetamine promotes atherosclerosis formation in ApoE‐/‐ knockout mice fed normal diet. Atherosclerosis. 2015;243:268–277. [DOI] [PubMed] [Google Scholar]
- 64. Westover AN, Nakonezny PA, Haley RW. Acute myocardial infarction in young adults who abuse amphetamines. Drug Alcohol Depend. 2008;96:49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Swalwell CI, Davis GG. Methamphetamine as a risk factor for acute aortic dissection. J Forensic Sci. 1999;44:23–26. [PubMed] [Google Scholar]
- 66. Wako E, LeDoux D, Mitsumori L, Aldea GS. The emerging epidemic of methamphetamine‐induced aortic dissections. J Card Surg. 2007;22:390–393. [DOI] [PubMed] [Google Scholar]
- 67. Bazmi E, Mousavi F, Giahchin L, Mokhtari T, Behnoush B. Cardiovascular complications of acute amphetamine abuse: cross‐sectional study. Sultan Qaboos Univ Med J. 2017;17:e31–e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kaye SM, Mcketin R. Cardiotoxicity associated with methamphetamine use and signs of cardiovascular pathology among methamphetamine users. NDARC Technical Report 2005;No. 238.
- 69. Karch SB. Karch's Pathology of Drug Abuse. 3rd ed Boca Raton, FL: CRC Press; 2002. [Google Scholar]
- 70. Chin KM, Channick RN, Rubin LJ. Is methamphetamine use associated with idiopathic pulmonary arterial hypertension? Chest. 2006;130:1657–1663. [DOI] [PubMed] [Google Scholar]
- 71. Zamanian RT, Hedlin H, Greuenwald P, Wilson DM, Segal JI, Jorden M, Kudelko K, Liu J, Hsi A, Rupp A, et al. Features and outcomes of methamphetamine associated pulmonary arterial hypertension. Am J Respir Crit Care Med. 2018;197:788–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Islam MN, Kuroki H, Hongcheng B, Ogura Y, Kawaguchi N, Onishi S, Wakasugi C. Cardiac lesions and their reversibility after long term administration of methamphetamine. Forensic Sci Int. 1995;75:29–43. [DOI] [PubMed] [Google Scholar]
- 73. Pujol‐Lopez M, Ortega‐Paz L, Flores‐Umanzor EJ, Perea RJ, Bosch X. Cardiac magnetic resonance as an alternative to endomyocardial biopsy to predict recoverability of left ventricular function in methamphetamine‐associated cardiomyopathy. JACC Heart Fail. 2017;5:853–854. [DOI] [PubMed] [Google Scholar]
- 74. Brahm NC, Yeager LL, Fox MD, Farmer KC, Palmer TA. Commonly prescribed medications and potential false‐positive urine drug screens. Am J Health Syst Pharm. 2010;67:1344–1350. [DOI] [PubMed] [Google Scholar]
- 75. Abdelmoneim SS, Pellikka PA, Mulvagh SL. Contrast echocardiography for assessment of left ventricular thrombi. J Ultrasound Med. 2014;33:1337–1344. [DOI] [PubMed] [Google Scholar]
- 76. Lopez JE, Yeo K, Caputo G, Buonocore M, Schaefer S. Recovery of methamphetamine associated cardiomyopathy predicted by late gadolinium enhanced cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2009;11:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Srichai MB, Junor C, Rodriguez LL, Stillman AE, Grimm RA, Lieber ML, Weaver JA, Smedira NG, White RD. Clinical, imaging, and pathological characteristics of left ventricular thrombus: a comparison of contrast‐enhanced magnetic resonance imaging, transthoracic echocardiography, and transesophageal echocardiography with surgical or pathological validation. Am Heart J. 2006;152:75–84. [DOI] [PubMed] [Google Scholar]
- 78. Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr, Ganiats TG, Holmes DR Jr, Jaffe AS, Jneid H, Kelly RF, Kontos MC, et al. 2014 AHA/ACC guideline for the management of patients with non‐ST‐elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:e344–e426. [DOI] [PubMed] [Google Scholar]
- 79. Richards JR, Albertson TE, Derlet RW, Lange RA, Olson KR, Horowitz BZ. Treatment of toxicity from amphetamines, related derivatives, and analogues: a systematic clinical review. Drug Alcohol Depend. 2015;150:1–13. [DOI] [PubMed] [Google Scholar]
- 80. Richards JR. Beta blockers and the cardiac complications of methamphetamine. Heart Lung Circ. 2017;26:416–417. [DOI] [PubMed] [Google Scholar]
- 81. Richards JR, Lange RA, Arnold TC, Horowitz BZ. Dual cocaine and methamphetamine cardiovascular toxicity: rapid resolution with labetalol. Am J Emerg Med. 2017;35:519.e1–519.e4. [DOI] [PubMed] [Google Scholar]
- 82. Dillinger JG, Maher V, Vitale C, Henry P, Logeart D, Manzo Silberman S, Allee G, Levy BI. Impact of ivabradine on central aortic blood pressure and myocardial perfusion in patients with stable coronary artery disease. Hypertension. 2015;66:1138–1144. [DOI] [PubMed] [Google Scholar]
- 83. Thiele H, Ohman EM, de Waha‐Thiele S, Zeymer U, Desch S. Management of cardiogenic shock complicating myocardial infarction: an update 2019. Eur Heart J. 2019;40:2671–2683. [DOI] [PubMed] [Google Scholar]
- 84. Amin AS, Meregalli PG, Bardai A, Wilde AA, Tan HL. Fever increases the risk for cardiac arrest in the Brugada syndrome. Ann Intern Med. 2008;149:216–218. [DOI] [PubMed] [Google Scholar]
- 85. Akhtar MJ, al‐Nozha M, al‐Harthi S, Nouh MS. Electrocardiographic abnormalities in patients with heat stroke. Chest. 1993;104:411–414. [DOI] [PubMed] [Google Scholar]
- 86. Weinsaft JW, Kim HW, Shah DJ, Klem I, Crowley AL, Brosnan R, James OG, Patel MR, Heitner J, Parker M, et al. Detection of left ventricular thrombus by delayed‐enhancement cardiovascular magnetic resonance prevalence and markers in patients with systolic dysfunction. J Am Coll Cardiol. 2008;52:148–157. [DOI] [PubMed] [Google Scholar]
- 87. Yew KL, Go CS, Razali F, Rajendran P, Ooi PS, Anum A. Methamphetamine‐associated reversible cardiomyopathy and stroke risk. Eur Rev Med Pharmacol Sci. 2014;18:2403–2404. [PubMed] [Google Scholar]
- 88. Richards JR, Johnson EB, Stark RW, Derlet RW. Methamphetamine abuse and rhabdomyolysis in the ED: a 5‐year study. Am J Emerg Med. 1999;17:681–685. [DOI] [PubMed] [Google Scholar]
- 89. Malhotra R, Patel S, Ramchand T, Al Nimri O. Higher defibrillation threshold in methamphetamine cardiomyopathy patients with implantable cardioverter‐defibrillator. Indian Pacing Electrophysiol J. 2017;17:167–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Hanrahan JS, Eberly C, Mohanty PK. Substance abuse in heart transplant recipients: a 10‐year follow‐up study. Prog Transplant. 2001;11:285–290. [DOI] [PubMed] [Google Scholar]
- 91. Shapiro PA, Williams DL, Foray AT, Gelman IS, Wukich N, Sciacca R. Psychosocial evaluation and prediction of compliance problems and morbidity after heart transplantation. Transplantation. 1995;60:1462–1466. [DOI] [PubMed] [Google Scholar]
- 92. Cogswell R, Smith E, Hamel A, Bauman L, Herr A, Duval S, John R, Roman D, Adatya S, Colvin‐Adams M, et al. Substance abuse at the time of left ventricular assist device implantation is associated with increased mortality. J Heart Lung Transplant. 2014;33:1048–1055. [DOI] [PubMed] [Google Scholar]


