Abstract
Background
Palliative care supports quality of life, symptom control, and goal setting in heart failure (HF) patients. Unlike hospice, palliative care does not restrict life‐prolonging therapy. This study examined the association between palliative care during hospitalization for HF on the subsequent transitions and procedures.
Methods and Results
Veterans admitted to hospitals with HF from 2010 to 2015 were randomly selected for the Veterans Administration External Peer Review Program. Variables pertaining to demographic, clinical, laboratory, and usage were captured from Veterans Administration electronic records. Patients receiving hospice services before admission were excluded. Patients who received palliative care were propensity matched to those who did not. The primary outcomes were whether the patient experienced transitions or procedures in the 6 months after admission. Transitions included multiple readmissions (≥2) or intensive care admissions and procedures included mechanical ventilation, pacemaker implantation, or defibrillator implantation. Among 57 182 hospitalized HF patients, 1431 received palliative care, and were well matched to 1431 without (standardized mean differences ≤ ±0.05 on all matched variables). Palliative care was associated with significantly fewer multiple rehospitalizations (30.9% versus 40.3%, P<0.001), mechanical ventilation (2.8% versus 5.4%, P=0.004), and defibrillator implantation (2.1% versus 3.6%, P=0.01). After adjustment for facility fixed effects, palliative care consultation was associated with a significantly reduced hazard of multiple readmissions (adjusted hazard ratio=0.73, 95% CI, 0.64–0.84) and mechanical ventilation (adjusted hazard ratio=0.76, 95% CI, 0.67–0.87).
Conclusions
Palliative care during HF admissions was associated with fewer readmissions and less mechanical ventilation. When available, engagement of HF patients and caregivers in palliative care for symptom control, quality of life, and goals of care discussions may be associated with reduced rehospitalizations and mechanical ventilation.
Keywords: hospice, palliative care, readmission
Subject Categories: Heart Failure, Quality and Outcomes
Clinical Perspective
What Is New?
The multidisciplinary approach of palliative care includes interventions aimed at minimizing suffering and maximizing quality of life in heart failure patients without the need to forgo life‐prolonging care as in hospice.
This analysis found that palliative care consultation during heart failure hospitalization reduced transitions, which included ≥2 rehospitalizations, and procedures such as mechanical ventilation.
What Are the Clinical Implications?
The availability of palliative care for heart failure patients during hospitalization may limit these outcomes.
As health systems develop population health approaches to delivery of care, palliative care for heart failure patients could be considered as an adjunct to improve patient quality of life, symptom management, and goal setting.
Nonstandard Abbreviations and Acronyms.
ACCF/AHA American College of Cardiology Foundation/American Heart Association
AICD automated implantable cardiac defibrillator
BNP B‐type natriuretic peptide
BUN blood urea nitrogen
HF heart failure
HR hazard ratio
ICD‐9 International Classification of Diseases, Ninth Revision
ICU intensive care unit
LOS length of stay
SMD standard mean difference
VA EPRP Veterans Administration External Peer Review Program
VAMC Veterans Affairs Medical Center
VA Veterans Administration
As heart failure (HF) progresses, patients experience high symptom burden that negatively impacts function, creates suffering, and increases mortality.1 By 2030, the prevalence of HF will grow by 46%, resulting in >8 million adults living with the condition, and an estimated $69.7 billion in total costs.2 Palliative care has become recognized as a beneficial component of HF management, particularly for symptom management and quality of life.3, 4, 5 The 2013 American College of Cardiology Foundation/American Heart Association Guideline for the Management of Heart Failure recommended palliative care for some hospitalized patients and care coordination for chronic HF.6 However, palliative care remains underutilized among the HF population.3, 7, 8
Critical and holistic thinking is essential to ensure the well‐being of patients with HF and their families, as well as the minimization of unnecessary healthcare usage. Palliative care focuses on improving a patient's quality of life by managing pain and other distressing symptoms of a serious illness. Palliative care can be provided concurrent with other medical treatments.9 The multidisciplinary approach of palliative care includes interventions aimed at minimizing suffering and maximizing quality of life.
Understanding the association of palliative care with transitions and procedures for patients living with HF can provide important insights into the use of palliative care. In other function‐limiting and life‐limiting conditions, such as terminal cancer and advanced cognitive impairment, outcomes have centered on repeat transitions in care settings or procedures such as intensive care unit admission, mechanical ventilation, or feeding tube insertion.10, 11, 12 In the HF population, inclusion of additional procedures, such as cardiac surgery or placement of a pacemaker or defibrillator, may be important markers.13
This retrospective, propensity‐score matched cohort study examined the association of palliative care engagement during HF hospitalization with transitions and procedures. The study's hypothesis was that among patients admitted to the hospital with HF, palliative care services would be associated with fewer transitions and procedures in the 6 months after hospitalization than a matched HF cohort who did not receive palliative care.
Methods
Data Availability
Based on restrictions in the Data Use Agreements used in this study, the authors are unable to make a data set available. Methodology questions may be directed to the corresponding author.
Study Design
This retrospective propensity‐matched analysis identified HF admissions from the Veterans Administration External Peer Review Program, which randomly selected medical records for review related to quality and performance during hospitalization. Trained nurses reviewed the selected records for data on performance and exclusionary conditions.14 The External Peer Review Program cohort used for this analysis included patients admitted with heart failure from October 2009 to September 2015. In the case of multiple readmissions, the analysis focused on the first readmission (index admission). Patients on hospice before admission as defined by Veterans Administration (VA) or Medicare records were excluded. A total of 124 Veterans Affairs Medical Center acute care hospitals were included.
Ethics
Before data collection, Institutional Review Board approval was obtained from the Providence Veterans Affairs Medical Center. This retrospective study was performed on clinically collected data and informed consent was waived.
Main Exposure: Palliative Care Encounter
Palliative care was operationalized as at least 1 hospital medical encounter with a palliative care professional occurring between the admission date and 3 days after the discharge. Within the VA, hospital encounters can be completed after discharge because care coordination is non‐billable and attached to the initial encounter. Encounters were included for up to 3 days after the discharge date. The VA uses this method of encounter measurement for workload capture.
To exclude patients who had hospice before admission, usage was examined in the year before the admission date from VA encounters, VA billing records for hospice services, and Medicare records for hospice care.
Outcomes
The primary outcome of interest was transitions and procedures 6 months after discharge. Using VA and Medicare records, we identified the number of hospitalizations after discharge, intensive care unit admissions, and hospice enrollments. Procedures were captured with International Classification of Diseases, Ninth Revision (ICD‐9) procedure codes after the initial palliative care encounter and included mechanical ventilation, pacemaker placement, automated cardiac defibrillator placement, cardiac surgery including coronary bypass or valve replacement, and insertion of a feeding tube.
Covariates for Propensity Matching
From the VA data infrastructure, demographics such as age, sex, and race were identified for each patient. In addition, comorbidity data were gathered to complete the Elixhauser comorbidity index from the year before admission based on VA encounter coding. Vital signs, laboratory, and ejection fraction data were included from the admission of interest. Laboratory variables used for the analysis were prioritized on the nearest proximity to the time of admission. Not all patients had an echocardiogram during the admission of interest. If the ejection fraction was not available from the admission of interest, data were extrapolated 6 months before or after the admission. Prior‐year VA costs and hospital days were included as matching variables. Prior work found that prior year VA costs helped to account for unobserved variance.15 To address potential mismatch in exposure to the composite outcome, days alive after hospitalization was included in the propensity matching.
Facility Palliative Care Availability
To address variation among VA hospitals in the availability of palliative care that could be correlated with other practice patterns, we also controlled for facility effects in the percentage of HF admissions with palliative care encounters (Figure S1).
Missing Data
Missing data were rare with respect to demographic information. Comorbidities were scored as present if the code was used within the prior year. For laboratory and clinical data, multiple imputations were used if the information was not present in the VA medical record. A listing of matching variables is included in Table S1.
Statistical Analysis
We estimated propensity scores using a logistic model of palliative care on patient covariates. Covariates were maintained as continuous during the matching. Patients in the palliative care cohort were matched on a 1:1 ratio to those in the non‐palliative‐care cohort using greedy, nearest‐neighbor matching with a radius of 0.01 and without replacement. The standardized mean difference was examined to assess covariate balance. Past literature found a mean standardized difference of <0.20 appropriate for suitable matching on variables.16
We analyzed transitions and procedures in the matched sample in 3 ways. In the first analysis, the outcome was operationalized as the proportion of patients having a transition or procedure within 6 months after admission. Proportions were compared using Chi‐square statistics. The second analysis examined time until the first transition or procedure. A Cox proportional hazard model was used to adjust residual mortality risk and to censor for death.17 To confirm that death is a competing risk in the model, the cause‐specific hazard model, as well as, the sub‐distribution hazard model were developed and yielded similar results.18 A third model included adjustment for facility availability of palliative care for HF as a fixed effect. Kaplan–Meier curves were created from the time of discharge to the outcome and compared with a log‐rank test.
Results
Baseline Characteristics
Table 1 describes the admitted (n=58 712), the palliative care (n=1431), and the matched (n=1431) cohorts. There were significant measurable differences between the overall cohort and the palliative cohort with respect to age, comorbidity, clinical data, prior usage, cost, and mortality. The palliative cohort was older with more comorbidity, lower ejection fraction, more days in the hospital, more costs, and higher post‐hospitalization mortality rates. Propensity analysis was used to obtain a comparison cohort and the propensity matching reduced the standard mean difference (SMD) to <0.20 in all matched variables. Both the palliative and matched cohorts were older (75.8 versus 75.7 years, SMD 0.01), mostly men (98.7% versus 98.6%, SMD 0.01), and had multiple comorbid conditions as determined by the mean Elixhauser comorbidity index (6.3 versus 6.3, SMD 0.01). The palliative and matched cohorts accrued significant medical costs in the year before index admission ($43 363 versus $42 076, SMD 0.02), respectively. Overall, 39.9% of the palliative care cohort had died within 6 months of discharge compared with 37.9% of the matched cohort (SMD 0.04).
Table 1.
Comparison of the Matched Cohorts
| Admitted Cohort n=58 712 | Palliative Cohort n=1431 | Matched Cohort n=1431 | Standardized Differencea | ||
|---|---|---|---|---|---|
| Mean (SD) or % (n) | Mean (SD) or % (n) | Mean (SD) or % (n) | Palliative vs Matched | Palliative vs Admitted | |
| Demographics | |||||
| Age, y | 70.85 (11.39) | 75.84 (11.14) | 75.70 (11.02) | 0.01 | 0.44 |
| Men | 98.15 (57 626) | 98.67 (1412) | 98.60 (1411) | 0.01 | 0.04 |
| Race | 0.00 | 0.15 | |||
| White | 74.77 (43 897) | 80.57 (1153) | 80.92 (1158) | ||
| Black | 23.39 (13 730) | 18.03 (258) | 17.96 (257) | ||
| Other | 1.85 (1085) | 1.40 (20) | 1.12 (16) | ||
| Comorbidities | |||||
| MI | 22.08 (12 965) | 29.91 (428) | 30.89 (442) | −0.02 | 0.18 |
| Diabetes mellitus | 54.06 (31 741) | 50.73 (726) | 49.34 (706) | 0.03 | −0.07 |
| Lymphoma | 1.73 (1017) | 2.24 (32) | 1.75 (25) | 0.04 | 0.04 |
| Solid tumor | 13.70 (8042) | 18.94 (271) | 18.52 (265) | 0.01 | 0.14 |
| Metastatic disease | 1.38 (812) | 4.05 (58) | 3.42 (49) | 0.03 | 0.16 |
| Elixhauser | 5.43 (2.86) | 6.26 (2.98) | 6.25 (2.88) | 0.01 | 0.29 |
| Laboratory data | |||||
| Renal function | |||||
| Blood urea nitrogen | 26.63 (14.13) | 32.49 (15.58) | 32.75 (16.85) | −0.02 | 0.39 |
| Creatinine | 1.54 (0.87) | 1.71 (0.90) | 1.73 (0.99) | −0.02 | 0.19 |
| Sodium | 138.50 (3.97) | 138.06 (4.67) | 138.11 (4.20) | −0.01 | −0.10 |
| Brain natriuretic peptide | 1415 (1292) | 1753 (1479) | 1750 (1601) | 0.00 | 0.24 |
| Potassium | 4.17 (0.54) | 4.25 (0.59) | 4.24 (0.56) | 0.01 | 0.13 |
| Hematocrit | 37.09 (6.09) | 36.22 (6.32) | 35.89 (6.03) | 0.05 | −0.14 |
| Clinical data | |||||
| Ejection fraction | 40.19 (16.28) | 36.96 (17.40) | 36.81 (15.90) | 0.01 | −0.19 |
| Blood pressure (mean arterial) | 97.78 (15.32) | 92.06 (14.51) | 91.64 (15.09) | 0.03 | −0.38 |
| Pulse | 80.80 (16.79) | 81.92 (17.18) | 81.77 (16.49) | 0.01 | 0.07 |
| Body mass index | 31.45 (7.52) | 29.34 (6.96) | 29.20 (6.71) | 0.02 | −0.29 |
| Usage data | |||||
| Hospitalizations in prior 12 mo (mean, n) | 1.06 (1.50) | 1.62 (2.02) | 1.39 (1.69) | 0.12 | 0.31 |
| Mean hospitalization length in prior 12 mo, d | 7.70 (22.99) | 10.43 (22.37) | 9.88 (25.25) | 0.02 | 0.12 |
| Days alive after index admission | 1183 (865) | 553 (629) | 553 (608) | 0.00 | −0.83 |
| Death in 6 mo after index admission | 14.54 (8535) | 39.90 (571) | 37.88 (542) | 0.04 | 0.59 |
| Baseline cost | |||||
| Total cost in prior 12 mo | 32 729 (47 040) | 43 363 (53 359) | 42 076 (57 756) | 0.02 | 0.21 |
MI indicates myocardial infarction.
The standardized difference is the difference of the group means divided by the standard deviation of the cohort.
Outcomes
Transitions and procedures within 6 months of discharge are displayed in Table 2. The palliative care cohort was associated with fewer multiple readmissions (2+ readmissions) compared with the matched cohort (30.9% versus 40.3%, P<0.001). There was no statistically significant difference in intensive care unit admission (15.9% versus 17.8%, P=0.162). Compared with the matched cohort, the palliative cohort had less mechanical ventilation (2.8% versus 5.4%, P=0.004). Palliative care was associated with lower automated implantable cardiac defibrillator placement (2.1% versus 3.6%, P=0.01) but no difference in pacemaker placement (0.4% versus 0.4%, P=1.0), cardiac surgery (0.5% versus 0.8%, P=0.34), or hemodialysis (3.4% versus 4.5%, P=0.15). Our secondary outcome of hospice use in the 6 months after discharge was significantly higher in the palliative cohort (34.8% versus 18.3%, P<0.001).
Table 2.
Transitions and Procedures in the Matched Cohorts
| Palliative Care Cohort n=1431 | Matched Cohort n=1431 | P Value | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Transitions | |||||
| Intensive care unit admission | 227 | 15.9 | 255 | 17.8 | 0.1619 |
| Readmission (n≥2) | 442 | 30.9 | 577 | 40.3 | <0.0001 |
| Hospice admission | 498 | 34.8 | 262 | 18.3 | <0.0001 |
| Procedures | |||||
| Mechanical ventilation | 40 | 2.8 | 78 | 5.4 | 0.0004 |
| Pacemaker | 6 | 0.4 | 6 | 0.4 | 1.0000 |
| Defibrillator implantation | 30 | 2.1 | 52 | 3.6 | 0.0137 |
| Cardiac surgery | 7 | 0.5 | 11 | 0.8 | 0.3443 |
| Hemodialysis | 49 | 3.42 | 64 | 4.47 | 0.1499 |
| Feeding tube | 6 | 0.4 | 7 | 0.5 | 0.7810 |
Table 3 describes the results of the proportional hazard model which found a significant 25% reduction in the hazard of the multiple readmissions (≥2) outcome in the palliative care cohort (hazard ratio [HR] 0.76, 95% CI, 0.68–0.85) with censoring for death during the 6‐month follow‐up. Inclusion of the facility availability of palliative care as a fixed effect in the analysis did not significantly alter the results (adjusted HR 0.73, 95% CI, 0.64–0.84). Palliative care was similarly associated with a decline in the hazard of mechanical ventilation after adjusting for facility fixed effects (adjusted HR 0.76, 95% CI, 0.67–0.87). Figure illustrates the association of palliative care and the comparison group for the multiple readmissions (Figure A) and mechanical ventilation (Figure B) outcomes over the follow‐up period.
Table 3.
Hazard Ratios of Palliative vs Control for Transitions and Procedures Within 180 Days of Discharge
| Transition | Unadjusted HR (95% CI) | Adjusted HR (95% CI)a | Adjusted HR With Facility Fixed Effects (95% CI)a |
|---|---|---|---|
| Readmissions (n≥2) | 0.76 (0.68–0.85) | 0.70 (0.62–0.79) | 0.73 (0.64–0.84) |
| Mechanical ventilation | 0.79 (0.71–0.88) | 0.77 (0.69–0.86) | 0.76 (0.67–0.87) |
HR indicates hazard ratio.
Adjusted for mortality risk and censored for death.
Figure 1. Kaplan–Meier curve for multiple readmissions and mechanical ventilation.
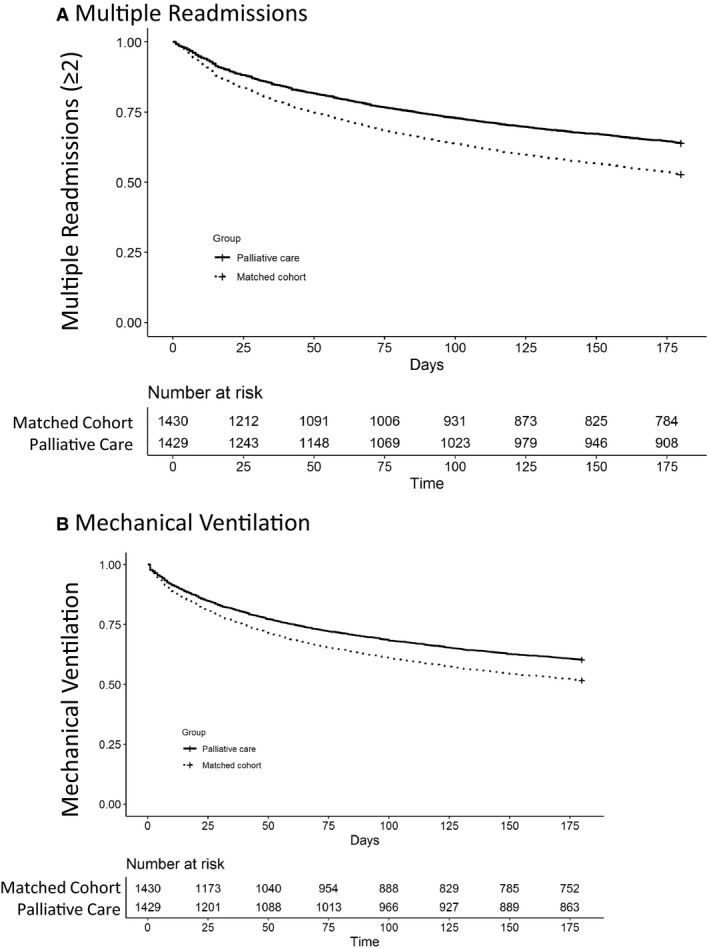
For the 180‐day follow‐up period, cohorts were tracked for first occurrence of multiple readmissions (A) and mechanical ventilation (B). The population at risk after censoring for death and outcome is described in the tables below the curves. In both panels, palliative care was significantly different than the matched cohort (P<0.001).
Patients who received palliative care were more likely to receive palliative care on a subsequent hospitalization (14.4% versus 8.3%, P<0.001) or during an outpatient encounter (20.6% versus 3.4%, P<0.001).
Discussion
Past evidence supports palliative care as an effective intervention for improving quality of life in HF.19, 20, 21 The cohort with palliative care was older with more comorbidity, usage, and cost in the prior year, necessitating the use of a propensity‐score matched comparison cohort. Using this propensity‐score matched cohort, we found that palliative care consultation during admission for HF was associated with fewer rehospitalizations and increased enrollment in hospice in the 6 months after hospitalization. These findings add to an increasing number of analyses that found associations between palliative care and positive outcomes for patients experiencing HF.
Palliative care is one additional service cardiologists can use in their comprehensive management of patients with HF. The observed association with reduced rehospitalization within 6 months among patients who received palliative care provides additional support that a palliative approach may be used to help guide goals of care conversations with patients living with HF. Also, engagement of palliative care during hospitalization may build an ongoing relationship that increases palliative care and facilitates discussion during subsequent hospitalizations or outpatient visits. Prior evidence suggests that palliative care might reduce healthcare costs.22, 23, 24, 25 When allowed, palliative care may be used concurrently with curative, life‐preserving treatment. For this analysis, patients in the palliative care cohort still opted for procedures such as hemodialysis and defibrillator implantation.
Prior outcomes of palliative care studies, developed in dementia and oncology research11, 12, 26 focused on similar outcomes (Table S2). In the HF population, other care procedures, such as implantation of an automated cardiac defibrillator, pacemaker, or cardiac surgery, have strong evidence in advanced HF. However, HF is a progressive disease with increasing symptom severity and functional decline. This decline in function, while on a different trajectory than dementia and oncology, is challenging for HF patients. Palliative care brings an opportunity to individualize care management toward the patient's goals through goals of care conversations, symptom management, or continuity across settings.
Importantly, palliative care is not universally available. In this study, we found a wide variability in the palliative care encounters for patients hospitalized with HF among medical centers (Figure S2). We postulate that palliative care is underutilized in part because of the misconception that it is synonymous with hospice.7, 27, 28 While both hospice and palliative care focus on symptom control, hospice enrolls patients who meet reimbursement eligibility criteria (a life expectancy of <6 months) and cannot be provided concurrently with curative treatment for the terminal condition. Palliative care and hospice can be provided wherever a patient resides (eg, home, hospice center, hospital, long‐term care facility, etc).9 In an integrated system such as the VA, palliative care can be delivered concurrently with more aggressive HF therapy.29 Palliative care uses a shared decision‐making strategy, which is influenced by a complex interplay among patient, family, provider, and systemic factors.30, 31, 32, 33 Prior work found that this approach is associated with a decrease in HF symptoms, increased satisfaction, reduced cost, and reduced transitions.24, 25, 34 With similar mortality between the groups, the stark difference in hospice enrollment between the palliative and matched cohorts demonstrates that the palliative approach may increase hospice referral and suggests that there is an HF population with an unmet need who may benefit from the additional layers of support that concurrent palliative care offers.
Strengths
This is a large study representing >58 000 patients. The study population represents patients from Veterans Affairs Medical Centers across the United States, allowing for geographic and racial diversity. The palliative cohort was successfully matched on a 1:1 ratio and the non‐palliative care cohort and multiple data sources were combined to provide comprehensive measurement of transitions and procedures in the cohort. These data sources were also used to exclude patients on hospice before HF admission and to measure the transition to hospice after admission which is critical to examine the impact of concurrent palliative care and HF care.
Limitations
Propensity matching is challenging for patients with palliative care, as there is a degree of unmeasured confounding in the selection of patients who receive palliative care. As with all matched cohort analyses, we were unable to demonstrate a causal relationship with this analysis. Other limitations of this study are related to the available sample. The population demographics and the availability of the data in the electronic medical record limit generalizability to the VA healthcare system, given the patients were predominantly male Veterans and there was no external validation. Additionally, the availability of concurrent palliative care and HF care is limited in health systems that are not integrated. Our data did not comment on critical components of end‐of‐life care such as functional status, quality of life, and caregiver support, as these variables were not available in the electronic medical record.
Conclusions
This study demonstrated an association between palliative care services in admitted HF patients and reduced multiple readmissions and mechanical ventilation. As HF progression is characterized by progressive functional decline, there is a growing understanding that concurrent palliative care can play an important role in attenuating the impact of HF, controlling symptoms, and providing continuity of care to meet the patients’ goals.
Translational Outlook
The symptoms and progression of HF lead to a decline in functional abilities. Palliative care has become increasingly recognized as a beneficial component of HF management, particularly in improving symptoms and quality of life. In patients admitted to the hospital with HF, this study found that palliative care was associated with less multiple rehospitalizations and mechanical ventilation. However, palliative care was not available at all medical centers. Targeted collaborations and increasing the palliative care workforce will be critical to meeting the growing HF demand.
Sources of Funding
Dr Rudolph is supported by the Department of Veterans Affairs Health Services Research and Development Center of Innovation in Long Term Services and Supports (CIN 13‐419) and the Department of Veterans Affairs Health Services Research Partnered Evaluation (SDR 15‐465). Mr Bowen and Dr Diop are supported by the Primary Care Population Medicine Program at the Warren Alpert School of Medicine at Brown University. Ms Jiang and Drs Wu, Gozalo, Cornell, and Rudolph are employees of the United States Department of Veterans Affairs. Sponsors Role: The authors retained full independence in the conduct and communication of this research.
Disclosures
None.
Supporting information
Tables S1 and S2 Figures S1 and S2
Acknowledgments
The statements and opinions expressed are those of the authors and do not represent the official policy or procedures of the United States Government or the Department of Veterans Affairs. We are thankful to Robert McConeghy and Elizabeth Archambault for editorial comments on the article.
Author contributions: Ms Jiang and Drs Wu and Rudolph had full access to the data and take responsibility for the analysis. Study Conception and Design: Jiang, Wu, Rudolph; Acquisition and interpretation of data: Bowen, Diop, Jiang, Gozalo, Wu, and Rudolph; Manuscript preparation and critical revision: Bowen, Diop, Jiang, Cornell, Gozalo, Wu, and Rudolph. All authors provided final manuscript approval.
(J Am Heart Assoc. 2020;9:e013989 DOI: 10.1161/JAHA.119.013989.)
For Sources of Funding and Disclosures, see page 7.
References
- 1. Shah AB, Morrissey RP, Baraghoush A, Bharadwaj P, Phan A, Hamilton M, Kobashigawa J, Schwarz ER. Failing the failing heart: a review of palliative care in heart failure. Rev Cardiovasc Med. 2013;14:41–48. [DOI] [PubMed] [Google Scholar]
- 2. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–e322. [DOI] [PubMed] [Google Scholar]
- 3. Robinson MR, Al‐Kindi SG, Oliveira GH. Trends in palliative care use in elderly men and women with severe heart failure in the United States. JAMA Cardiol. 2017;2:344. [DOI] [PubMed] [Google Scholar]
- 4. Brannstrom M, Boman K. Effects of person‐centred and integrated chronic heart failure and palliative home care. PREFER: a randomized controlled study. Eur J Heart Fail. 2014;16:1142–1151. [DOI] [PubMed] [Google Scholar]
- 5. Wiskar K, Toma M, Rush B. Palliative care in heart failure: review. Trends Cardiovasc Med. 2018;28:445–450. [DOI] [PubMed] [Google Scholar]
- 6. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:1810–1852. [DOI] [PubMed] [Google Scholar]
- 7. Khan RF, Feder S, Goldstein NE, Chaudhry SI. Symptom burden among patients who were hospitalized for heart failure. JAMA Intern Med. 2015;175:1713–1715. [DOI] [PubMed] [Google Scholar]
- 8. Diop MS, Rudolph JL, Zimmerman KM, Richter MA, Skarf LM. Palliative care interventions for patients with heart failure: a systematic review and meta‐analysis. J Palliat Med. 2017;20:84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. American Academy of Hospice and Palliative Medicine . How to define hospice and palliative care. 2017;2019.
- 10. Aaltonen M, Raitanen J, Forma L, Pulkki J, Rissanen P, Jylha M. Burdensome transitions at the end of life among long‐term care residents with dementia. J Am Med Dir Assoc. 2014;15:643–648. [DOI] [PubMed] [Google Scholar]
- 11. Gozalo P, Teno JM, Mitchell SL, Skinner J, Bynum J, Tyler D, Mor V. End‐of‐life transitions among nursing home residents with cognitive issues. N Engl J Med. 2011;365:1212–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Miller SC, Lima JC, Intrator O, Martin E, Bull J, Hanson LC. Palliative care consultations in nursing homes and reductions in acute care use and potentially burdensome end‐of‐life transitions. J Am Geriatr Soc. 2016;64:2280–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Farmakis D, Parissis J, Lekakis J, Filippatos G. Acute heart failure: epidemiology, risk factors, and prevention. Rev Esp Cardiol (Engl Ed). 2015;68:245–248. [DOI] [PubMed] [Google Scholar]
- 14. Goulet JL, Erdos J, Kancir S, Levin FL, Wright SM, Daniels SM, Nilan L, Justice AC. Measuring performance directly using the veterans health administration electronic medical record: a comparison with external peer review. Med Care. 2007;45:73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu WC, Taveira TH, Jeffery S, Jiang L, Tokuda L, Musial J, Cohen LB, Uhrle F. Costs and effectiveness of pharmacist‐led group medical visits for type‐2 diabetes: a multi‐center randomized controlled trial. PLoS One. 2018;13:e0195898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity‐score matched samples. Stat Med. 2009;28:3083–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bowen GS, Diop MS, Jiang L, Wu WC, Rudolph JL. A multivariable prediction model for mortality in individuals admitted for heart failure. J Am Geriatr Soc. 2018;66:902–908. [DOI] [PubMed] [Google Scholar]
- 18. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 19. Lewin WH, Cheung W, Horvath AN, Haberman S, Patel A, Sullivan D. Supportive cardiology: moving palliative care upstream for patients living with advanced heart failure. J Palliat Med. 2017;20:1112–1119. [DOI] [PubMed] [Google Scholar]
- 20. Akyar I, Dionne‐Odom JN, Bakitas MA. Using patients and their caregivers feedback to develop ENABLE CHF‐PC: an early palliative care intervention for advanced heart failure. J Palliat Care. 2019;34:103–110. [DOI] [PubMed] [Google Scholar]
- 21. Campbell RT, Petrie MC, Jackson CE, Jhund PS, Wright A, Gardner RS, Sonecki P, Pozzi A, McSkimming P, McConnachie A, et al. Which patients with heart failure should receive specialist palliative care? Eur J Heart Fail. 2018;20:1338–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pattenden JF, Mason AR, Lewin RJ. Collaborative palliative care for advanced heart failure: outcomes and costs from the ‘Better Together’ pilot study. BMJ Support Palliat Care. 2013;3:69–76. [DOI] [PubMed] [Google Scholar]
- 23. Twaddle ML, Maxwell TL, Cassel JB, Liao S, Coyne PJ, Usher BM, Amin A, Cuny J. Palliative care benchmarks from academic medical centers. J Palliat Med. 2007;10:86–98. [DOI] [PubMed] [Google Scholar]
- 24. Brumley R, Enguidanos S, Jamison P, Seitz R, Morgenstern N, Saito S, McIlwane J, Hillary K, Gonzalez J. Increased satisfaction with care and lower costs: results of a randomized trial of in‐home palliative care. J Am Geriatr Soc. 2007;55:993–1000. [DOI] [PubMed] [Google Scholar]
- 25. Wong FK, Ng AY, Lee PH, Lam PT, Ng JS, Ng NH, Sham MM. Effects of a transitional palliative care model on patients with end‐stage heart failure: a randomised controlled trial. Heart. 2016;102:1100–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mitchell SL, Teno JM, Kiely DK, Shaffer ML, Jones RN, Prigerson HG, Volicer L, Givens JL, Hamel MB. The clinical course of advanced dementia. N Engl J Med. 2009;361:1529–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fendler TJ, Swetz KM, Allen LA. Team‐based palliative and end‐of‐life care for heart failure. Heart Fail Clin. 2015;11:479–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hui D, De La Cruz M, Mori M, Parsons HA, Kwon JH, Torres‐Vigil I, Kim SH, Dev R, Hutchins R, Liem C, et al. Concepts and definitions for “supportive care”, “best supportive care”, “palliative care”, and “hospice care” in the published literature, dictionaries, and textbooks. Support Care Cancer. 2013;21:659–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Way D, Ersek M, Montagnini M, Nathan S, Perry SA, Dale H, Savage JL, Luhrs CA, Shreve ST, Jones CA. Top ten tips palliative care providers should know about caring for veterans. J Palliat Med. 2019;22:708–713. [DOI] [PubMed] [Google Scholar]
- 30. McDermott E, Selman LE. Cultural factors influencing advance care planning in progressive, incurable disease: a systematic review with narrative synthesis. J Pain Symptom Manage. 2018;56:613–636. [DOI] [PubMed] [Google Scholar]
- 31. Ornstein KA, Aldridge MD, Mair CA, Gorges R, Siu AL, Kelley AS. Spousal characteristics and older adults’ hospice use: understanding disparities in end‐of‐life care. J Palliat Med. 2016;19:509–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Carr D, Moorman SM, Boerner K. End‐of‐life planning in a family context: does relationship quality affect whether (and with whom) older adults plan?. J Gerontol B Psychol Sci Soc Sci. 2013;68:586–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Johnson KS, Kuchibhatla M, Payne R, Tulsky JA. Race and residence: intercounty variation in black‐white differences in hospice use. J Pain Symptom Manage. 2013;46:681–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sidebottom AC, Jorgenson A, Richards H, Kirven J, Sillah A. Inpatient palliative care for patients with acute heart failure: outcomes from a randomized trial. J Palliat Med. 2015;18:134–142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1 and S2 Figures S1 and S2
Data Availability Statement
Based on restrictions in the Data Use Agreements used in this study, the authors are unable to make a data set available. Methodology questions may be directed to the corresponding author.


