Abstract
Background
Atrial fibrillation (AF) is associated with a 5‐fold increased stroke risk. While most patients with AF warrant anticoagulation, optimal treatment remains uncertain for patients with AF without cardiovascular comorbidities because the risk of stroke in this population has not been well‐characterized.
Methods and Results
Participants (N=28 253; 55% women, mean age 64.6±9.4 years), from the REGARDS (Reasons for Geographic and Racial Differences in Stroke) study (2003–present) were classified into 1 of 4 groups based on the presence or absence of AF and the presence or absence of cardiovascular comorbidities. Cox proportional hazards analysis was used to compare the risk of stroke between groups. During 244 560 person‐years of follow‐up (median 8.7 years), 1206 strokes occurred. Compared with patients with neither AF nor cardiovascular comorbidities, we did not find an increased stroke risk (hazard ratio [HR], 1.23; 95% CI, 0.62–2.18 [P=0.511]) among participants with AF alone. Participants without AF but with cardiovascular comorbidities had both an elevated stroke risk (HR, 1.77; 95% CI, 1.48–2.18 [P<0.0001]) and an increased risk of cardioembolic stroke (HR, 2.34; 95% CI, 1.48–3.90 [P=0.0002]).
Conclusions
In this large cohort of participants with AF without cardiovascular comorbidities, we found that AF itself, without cardiovascular comorbidities, did not confer increased risk of stroke. Cardiovascular comorbidities, however, were associated with an increased risk of both stroke of any type and cardioembolic stroke, even in the absence of AF.
Keywords: arrhythmia, atrial fibrillation, comorbidities, risk, stroke
Subject Categories: Atrial Fibrillation, Cerebrovascular Disease/Stroke, Risk Factors
Nonstandard Abbreviations and Acronyms
- AF
atrial fibrillation
- ACC/AHA/HRS
American College of Cardiology/American Heart Association/Heart Rhythm Society
- BMI
body mass index
- HR
hazard ratio
- REGARDS
Reasons for Geographic and Racial Differences in Stroke
- TOAST
Trial of Org 10172 in Acute Stroke Treatment (ischemic stroke specification schema)
Clinical Perspective
What Is New?
In the absence of comorbidities, atrial fibrillation may not confer an increased risk of all‐cause stroke, although there may be an increased risk of cardioembolic stroke.
Use of stroke risk stratification methods, such as the CHA2DS2VASc score, should be used to identify patients at low risk of thromboembolism.
What Are the Clinical Implications?
Further studies that aim to tease out the risk of stroke caused by arrhythmia itself and the risk caused by associated comorbidities may help refine risk stratification, prescribing practices, and patient outcomes.
Introduction
Atrial fibrillation (AF) confers a 2‐fold increased risk of all‐cause mortality.1 This is chiefly mediated by the 5‐fold increased risk of stroke among patients with AF2, 3 and the fact that strokes are markedly more debilitating in those with AF.4, 5 For the majority of patients with nonvalvular AF with a CHA2DS2VASc score of ≥2,6 anticoagulant therapy is indicated.7, 8 However, among patients with “lone AF,” or AF without cardiovascular comorbidities, the risk/benefit profile of anticoagulation remains unclear.
Lone AF has been variably defined over the preceding decades, leading to ambiguity about its prognosis and treatment.9, 10 Even after accounting for variable definitions, the risk of stroke among patients with AF without cardiovascular comorbidities is unknown—there is no consensus in the literature. While some studies report a risk of stroke indistinguishable from the general population,11, 12 others have found a markedly elevated stroke risk.13, 14, 15 These inconsistencies may be attributable to differences in study design, such as the use of actuarial estimates for the reference group11 or by failing to adjust for confounders13 (such as race,16, 17, 18 income,19 educational attainment,20 and geographical region21) that are known to be associated with both lone AF and stroke but are not on the causal pathway. Because of this ambiguity, the American College of Cardiology/American Heart Association/Heart Rhythm Society (ACC/AHA/HRS) guidelines7, 8 offer only class IIa and IIb recommendations for thromboprophylaxis management in these patients. In addition, the European Society of Cardiology guideline22 recommendations differ from those of the ACC/AHA/HRS guidelines for patients with a CHA2DS2VASc score of 1.23 This variability in practice is a consequence of the paucity of conclusive evidence regarding risks and benefits in this population.
We hypothesized that participants in the REGARDS (Reasons for Geographic and Racial Differences in Stroke) study with AF alone would not have an increased risk of stroke when compared with the reference group of those with neither AF nor cardiovascular comorbidities. Secondary aims included comparing the risk of stroke in patients with cardiovascular comorbidities without AF with the risk of stroke in those with neither AF nor cardiovascular comorbidities, comparison of the incidence and proportion of the TOAST (Trial of Org 10172 in Acute Stroke Treatment [ischemic stroke specification schema])24 subtypes of ischemic stroke in each group, and examining the consistency of these associations in prespecified subgroups.
Methods
Study Design and Participants
Qualified researchers trained in human subject confidentiality protocols may request access to the data that support the findings of this study by contacting the REGARDS Operations Center at 888‐734‐2738.
We divided REGARDS study participants into 4 groups based on the presence or absence of AF and the presence or absence of cardiovascular comorbidities. Group 1 (reference group) included participants with no AF and no cardiovascular comorbidities. Group 2 included participants without AF, but with cardiovascular comorbidities. Group 3 included participants with AF, but without cardiovascular comorbidities. Group 4 included participants with both AF and cardiovascular comorbidities.
The design of the REGARDS study has been previously described.25 Briefly, the REGARDS study is a longitudinal population‐based cohort study of 30 239 black and white participants 45 years and older recruited between 2003 and 2007 designed to understand regional and racial disparities in stroke risk. Blacks (42%) and residents of the stroke belt (56%; Alabama, Arkansas, Georgia, Louisiana, Mississippi, North Carolina, South Carolina, and Tennessee) were systematically oversampled. Study methods were approved by institutional review boards at participating institutions. All participants provided written informed consent. The authors had full access to the data and take responsibility for its integrity and analysis.
Exposure Variables
AF was defined by either evidence of AF on study ECG or self‐reported prior physician diagnosis of AF. The ECGs were read by electrocardiographers blinded to clinical data at Wake Forest School of Medicine (Winston Salem, NC). AF by ECG and self‐report have been shown to be similarly predictive of stroke.26 Self‐reported history of any of the following was considered to represent cardiovascular comorbidity: physician diagnosis of diabetes mellitus, myocardial infarction, coronary angioplasty or stenting, coronary artery bypass surgery, surgery for peripheral artery disease, amputation for peripheral artery disease, heart failure (defined as the presence of orthopnea or paroxysmal nocturnal dyspnea), transient ischemic attack, or use of oral hypoglycemics, insulin, or antihypertensives.27 In addition, any of the following were considered evidence of cardiovascular comorbidities: ECG evidence of prior myocardial infarction, systolic blood pressure >140 mm Hg, diastolic blood pressure >90 mm Hg, fasting blood glucose >126 mg/dL, or nonfasting blood glucose >200 mg/dL. Echocardiographic data and comprehensive medical histories regarding prior mechanical or bioprosthetic valve implantation were not available, so possible valvular heart disease cannot be excluded.
Outcome Variables
Details of stroke adjudication have been previously reported.28 Briefly, report of a possible stroke29 triggered a request for medical records that were centrally adjudicated by a panel of blinded stroke expert physicians.30 Incident stroke cases adjudicated by October of 2016 were included. Stroke cause was ascertained from medical record review and assigned TOAST categories (large‐artery atherosclerosis, cardioembolism, small‐vessel occlusion, stroke of other determined cause, and stroke of undetermined cause).24
Statistical Analysis
Among 30 239 REGARDS study participants, 1930 were excluded for prior stroke and 56 withdrew consent, leaving 28 253 eligible patients. Baseline characteristics are reported as mean±SD for continuous variables and frequency (percentage) for categorical variables. Unadjusted analyses used ANOVA for continuous variables and chi‐square tests for categorical variables. Two‐sided P values <0.05 were considered statistically significant.
Cox proportional hazards modeling was used to compare stroke risk between groups, with group 1 being the reference. Hazard ratios (HRs) are reported with 95% CIs. Time‐independent proportionality assumptions were initially assessed by examining the Martingale residual plot.31 With inclusion of the natural logarithm of follow‐up time as a time‐dependent covariate, there was no evidence of substantial departures from the assumption of proportionality.32
Multivariable analysis was conducted with 4 models. Initial analysis was unadjusted, with subsequent analyses iteratively adjusting for covariates believed to be clinically important, including demographics (model 1 adjusted for age, sex, race, education, income, and geographic region), then modifiable risk factors (model 2 added high‐density lipoprotein cholesterol, total cholesterol, body mass index [BMI], and smoking), then medication use (model 2 added aspirin use and warfarin use).
Sensitivity analysis examined the proportion, incidence rates, and adjusted HR for the various TOAST subtypes.24 We also explored the consistency of the associations in prespecified subgroups by testing for interaction between the exposure variable and the subgroups, comparing the risks in subgroups separated by age, sex, race, smoking status, and BMI. Kaplan–Meier plots and log‐rank test were used to compare the stroke‐free survival between groups. Analyses were conducted using SAS version 9.4 (SAS Institute Inc.).
Results
Among 28 253 eligible participants, 7837 (27.7%) had neither AF nor cardiovascular comorbidities, 18 103 (64.1%) had no AF but did have cardiovascular comorbidities, 386 (1.4%) had AF but no cardiovascular comorbidities, and 1927 (6.8%) had both AF and cardiovascular comorbidities. Baseline characteristics of the study population are provided in Table 1. There were significant between‐group differences in all covariates.
Table 1.
Baseline Characteristics of the REGARDS Study Participants
| Variable | Group 1 No AF, No Comorbidities n=7837 (27.7%) | Group 2 No AF, Has Comorbidities n=18 103 (64.1%) | Group 3 Has AF, No Comorbidities n=386 (1.4%) | Group 4 Has AF, Has Comorbidities n=1927 (6.8%) | P Valuea |
|---|---|---|---|---|---|
| Age, y | 62.0±9.2 | 65.4±9.2 | 65.6±9.9 | 67.7±9.6 | <0.0001 |
| Men, % | 43.3 | 44.8 | 45.3 | 46.6 | 0.034 |
| CHA2DS2VAScb | 1.03±0.82 | 2.83±1.16 | 1.29±0.89 | 3.37±1.24 | <0.0001 |
| White race, % | 71.7 | 53.2 | 82.1 | 60.1 | <0.0001 |
| Education, % | <0.0001 | ||||
| <High school | 6.2 | 14.1 | 6.2 | 14.8 | |
| High school graduate | 22.2 | 27.1 | 26.2 | 27.4 | |
| Some college | 27.2 | 26.8 | 26.9 | 26.5 | |
| College graduate | 44.4 | 32.0 | 40.7 | 31.4 | |
| Income, % | <0.0001 | ||||
| <$20 000 | 10.7 | 19.6 | 13.0 | 23.4 | |
| $21 000 to $34 000 | 19.8 | 25.6 | 24.9 | 25.7 | |
| $35 000 to $74 000 | 33.7 | 29.0 | 28.2 | 26.9 | |
| ≥$75 000 | 24.0 | 13.6 | 20.0 | 10.8 | |
| Region, % | <0.0001 | ||||
| Stroke buckle | 20.3 | 21.1 | 22.0 | 23.0 | |
| Stroke belt | 33.3 | 35.2 | 31.6 | 35.5 | |
| Elsewhere | 46.4 | 43.8 | 46.4 | 41.5 | |
| Smoking status, % | <0.0001 | ||||
| Never | 50.4 | 44.4 | 43.6 | 40.9 | |
| Former | 35.9 | 40.9 | 44.2 | 45.6 | |
| Current | 13.7 | 14.7 | 12.2 | 13.5 | |
| Systolic BP, mm Hg | 118.5±11.2 | 131.1±16.9 | 118.3±10.8 | 129.7±17.5 | <0.0001 |
| Diastolic BP, mm Hg | 73.6±7.7 | 77.9±10.1 | 72.7±7.9 | 76.2±10.2 | <0.0001 |
| BMI, kg/m2 | 27.1±5.0 | 30.3±6.4 | 26.9±4.9 | 30.1±6.6 | <0.0001 |
| Total cholesterol, mg/dL | 200.3±37.5 | 190.2±40.2 | 195.3±39.7 | 183.2±41.3 | <0.0001 |
| HDL cholesterol, mg/dL | 54.9±16.4 | 51.0±16.0 | 53.6±17.3 | 49.6±16.3 | <0.0001 |
| LDL cholesterol, mg/dL | 121.6±33.2 | 112.0±34.9 | 116.6±32.6 | 105.6±34.0 | <0.0001 |
| Triglycerides, mg/dL | 118.9±77.0 | 136.7±88.4 | 127.1±107.7 | 139.3±89.8 | <0.0001 |
| eGFR | 88.4±19.2 | 85.2±24.9 | 85.7±19.3 | 80.5±26.2 | <0.0001 |
| Aspirin use, % | 28.3 | 47.1 | 36.0 | 51.9 | <0.0001 |
| Warfarin use, % | 0.6 | 1.9 | 18.7 | 21.7 | <0.0001 |
Continuous variables are listed as mean±SD. Categorical variables are listed as proportion (percentage). Baseline characteristics of the 28 253 eligible participants from the REGARDS (Reasons for Geographic and Racial Differences in Stroke) study at baseline are provided. There were significant between‐group differences at baseline in all covariates assessed. AF indicates atrial fibrillation; BMI, body mass index; BP, blood pressure; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; and LDL, low‐density lipoprotein.
P value as calculated by ANOVA for continuous and chi‐square for categorical variables.
Stroke risk score (see reference 6).
After 244 560 person‐years of follow‐up (median 8.7; interquartile range, 5.8–11.6), 1206 strokes occurred. The observed incidence rates per 1000 person‐years of follow‐up and multivariable‐adjusted HRs are provided in Table 2. The HR for stroke was not significantly elevated in participants with AF without cardiovascular comorbidities (HR, 1.23; CI, 0.62–2.18), after adjusting for covariates.
Table 2.
HRs for Stroke
| Group 1 No AF, No Comorbidities n=7837 (27.7%) | Group 2 No AF, Has Comorbidities n=18 103 (64.1%) | Group 3 Has AF, No Comorbidities n=386 (1.4%) | Group 4 Has AF, Has Comorbidities n=1927 (6.8%) | |
|---|---|---|---|---|
| Strokes | 175 | 867 | 18 | 146 |
| Total follow‐up, person‐y | 72 900 | 153 578 | 3330 | 14 752 |
| Stroke incidence rate (per 1000 person‐y) | 2.4 | 5.6 | 5.4 | 9.9 |
| Unadjusted HR | 1.0 (reference) | 2.34 (1.99–2.76) | 2.24 (1.33–3.54) | 4.08 (3.27–5.08) |
| Model 1 | 1.0 (reference) | 1.79 (1.50–2.14) | 1.55 (0.84–2.62) | 2.79 (2.20–3.54) |
| Model 2 | 1.0 (reference) | 1.83 (1.53–2.20) | 1.38 (0.70–2.42) | 2.91 (2.27–3.73) |
| Model 3 | 1.0 (reference) | 1.77 (1.48–2.14) | 1.23 (0.62–2.18) | 2.52 (1.93–3.28) |
Model 1 adjusts for age, sex, race, education, income, and geographic region. Model 2 adjusts for the covariates in model 1, with the addition of high‐density lipoprotein cholesterol, total cholesterol, body mass index, and smoking. Model 3 adjusts for the covariates in model 2, with the addition of regular aspirin use and warfarin use. Raw incidence rates and multivariable‐adjusted hazard ratios (HRs) for stroke in groups based on the presence or absence of atrial fibrillation (AF) and the presence or absence of cardiovascular comorbidities are provided. After adjustment for covariates, the hazard for stroke was not significantly elevated in group 3 (patinets with AF without comorbidities).
As a sensitivity analysis, we examined the proportion of strokes in each group by TOAST subtype. Participants with AF, whether with or without cardiovascular comorbidities, had a much higher fraction of cardioembolic strokes (69.2% and 54.8% for participants with AF versus 12.9% and 16.7% for participants without AF, respectively) (Table 3). Despite the similarity in total stroke risk in groups 1 and 3, the risk of cardioembolic stroke differed substantially, with covariate‐adjusted HRs of 2.34 (95% CI, 1.48–3.90) for group 2 and 3.12 (95% CI, 1.15–8.46) for group 3, compared with group 1 (Table 4). Stroke‐free survival and cardioembolic stroke‐free survival by group are depicted in Figure 1.
Table 3.
TOAST Stroke Subtype Incidence Rates
| Group 1 No AF, No Comorbidities n=7837 (27.7%) | Group 2 No AF, Has Comorbidities n=18 103 (64.1%) | Group 3 Has AF, No Comorbidities n=386 (1.4%) | Group 4 Has AF, Has Comorbidities n=1927 (6.8%) | |
|---|---|---|---|---|
| Total strokes | 175 | 867 | 18 | 146 |
| Total stroke incidence rate | 2.4 | 5.6 | 5.4 | 9.9 |
| Ischemic stroke incidence rate | 2.1 | 5.1 | 3.9 | 9.2 |
| Cardioembolic | 0.27 (12.9%) | 0.85 (16.7%) | 2.70 (69.2%) | 5.04 (54.8%) |
| Large‐vessel | 0.24 (11.6%) | 0.65 (12.7%) | ··· | 1.30 (14.1%) |
| Small‐vessel | 0.33 (15.5%) | 0.81 (15.9%) | ··· | 0.40 (4.4%) |
| Other | 0.11 (5.1%) | 0.25 (4.9%) | ··· | 0.34 (3.7%) |
| Unknown | 1.15 (54.8%) | 2.54 (49.9%) | 1.20 (30.8%) | 2.12 (23.0%) |
| Hemorrhagic stroke incidence rate | 0.3 | 0.6 | 1.5 | 0.7 |
Incidence rates are per 1000 person‐years of follow‐up. Percentages refer to the fraction of all ischemic strokes in a given group assigned to each TOAST (Trial of Org 10172 in Acute Stroke Treatment [ischemic stroke classification stroke subtype]). Raw incidence rates by stroke subtype in each group, as well as proportions of all strokes attributed to each cause. AF indicates atrial fibrillation.
Table 4.
HRs for Cardioembolic Stroke
| Group 1 No AF, No Comorbidities n=7837 (27.7%) | Group 2 No AF, Has Comorbidities n=18 103 (64.1%) | Group 3 Has AF, No Comorbidities n=386 (1.4%) | Group 4 Has AF, Has Comorbidities n=1927 (6.8%) | |
|---|---|---|---|---|
| Cardioembolic strokes | 23 | 165 | 9 | 86 |
| Cardioembolic stroke incidence rate (per 1000 person‐y) | 0.31 | 0.94 | 3.74 | 5.43 |
| Unadjusted HR | 1.0 (reference) | 3.40 (2.24–5.39) | 8.54 (3.75–17.85) | 18.38 (11.81–29.79) |
| Model 1 | 1.0 (reference) | 2.57 (1.64–4.25) | 4.77 (1.74–11.23) | 11.95 (7.26–19.68) |
| Model 2 | 1.0 (reference) | 2.38 (1.51–3.96) | 4.01 (1.33–9.94) | 11.02 (6.62–18.33) |
| Model 3 | 1.0 (reference) | 2.34 (1.48–3.90) | 3.12 (1.15–8.46) | 8.25 (4.79–14.21) |
Model 1 adjusts for age, sex, race, education, income, and geographic region. Model 2 adjusts for the covariates in model 1, with the addition of high‐density lipoprotein cholesterol, total cholesterol, body mass index, and smoking. Model 3 adjusts for the covariates in model 2, with the addition of regular aspirin use and warfarin use. Incidence rates and hazard ratios (HRs) specific to cardioembolic stroke for each group. The hazard for cardioembolic stroke remained elevated in group 3 after adjustment. AF indicates atrial fibrillation.
Figure 1. Stroke‐free survival and cardioembolic stroke–free survival.
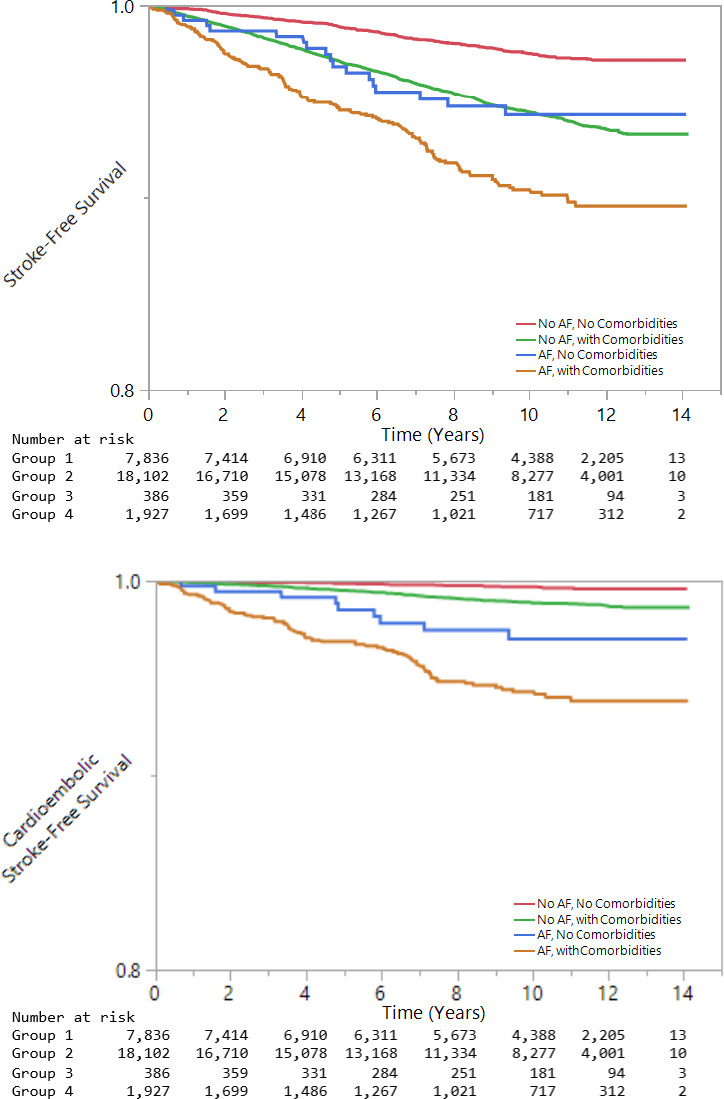
Kaplan–Meier curves depict stroke‐free survival and cardioembolic stroke–free survival, by groups.
Subgroup analyses (Figure 2) demonstrated consistency in these relationships when stratified by sex, race, BMI, and smoking, although an interaction between age (<median age of 64 versus ≥64 years) and group emerged as an effect modifier (P=0.02), with younger participants having a higher magnitude of risk explained by group than older participants.
Figure 2. Hazard ratios (HRs) for stroke in subgroups.
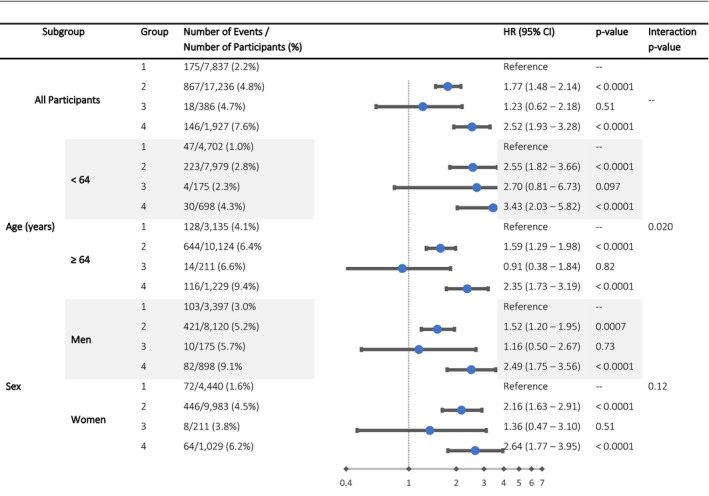
Interaction analysis in prespecified subgroups demonstrates consistency in the reported relationships overall, although group assignment (reflecting the presence or absence of atrial fibrillation and the presence or absence of comorbidities) appears to explain more of the risk of stroke among participants younger than the median. Model is adjusted for age, sex, race, education, income, geographic region, high‐density lipoprotein cholesterol, total cholesterol, body mass index (BMI), smoking, regular aspirin use, and warfarin use.
Discussion
In this analysis of the REGARDS study cohort, we found no evidence of an increased risk of stroke among participants with AF without cardiovascular comorbidities, compared with the reference group of those with neither AF nor cardiovascular comorbidities. This finding was consistent in subgroup analyses. However, there was evidence of effect‐modification between age (<64 versus ≥64 years) and group. For those with cardiovascular comorbidities without AF, we found an increased risk of cardioembolic stroke, even in the absence of AF.
Our finding that AF without cardiovascular comorbidities does not convey an increased overall stroke risk is consistent with much of the published literature,11, 12 although there has been recent research from the Framingham Heart Study13 suggesting that patients with AF without cardiovascular comorbidities may have an increased risk of major adverse cardiovascular events. The current guidelines do not recommend anticoagulation for men with a CHA2DS2VASc score of 0 or women with a CHA2DS2VASc score of 1,7, 8, 22 as this subgroup of patients has a risk of stroke comparable to the population at large.33 Given this, it is reasonable to conclude that anticoagulants are not indicated, as the risks of therapy likely outweigh the benefits in this low‐risk population.34
Atrial Cardiopathy and Stroke
There is ongoing debate about the relative contribution to stroke risk attributable to clinical AF per se and the often‐associated cardiovascular comorbidities. The observation that AF confers a relatively small additive risk of stroke in the absence of cardiovascular comorbidities may be explained by the theory of cardiopathy‐dependent risk of atrioembolic stroke,35, 36, 37, 38 as opposed to the traditionally held arrhythmia‐dependent risk of stroke, in which the fibrillating atrium or the postconversion atrium itself causes stasis and thrombogenesis.39 If AF is in the causal pathway for atrioembolic stroke, then patients with AF without cardiovascular comorbidities (group 3) should have a markedly increased risk of stroke. Our findings of no increased risk of stroke in this group adds support to the hypothesis that arrhythmia alone may be insufficient for increasing stroke risk.
In addition to AF appearing insufficient to increase stroke risk in the absence of cardiovascular comorbidities, there is a growing body of literature suggesting that some patients have a heightened risk of atrioembolic stroke, even without AF. For instance, patients without AF can still have left atrial appendiceal thrombi40 or greatly impaired left atrial function,41 which can lead to stroke in some cases.42 In addition, the lack of temporal relationship between arrhythmia and stroke has been noted,43 to the extent that many people with presumed AF‐related stroke had been in sinus rhythm continuously for over 1 year at the time of stroke.44 In light of these findings being discrepant with clinical arrhythmia being required for atrioembolic stroke, the theory of fibrotic atrial cardiopathy has been advanced,35 in which a chronically diseased atrium develops both electrophysiologic and mechanical dysfunction.36 Our findings of a 2‐fold increased risk of cardioembolic stroke in patients without AF but with cardiovascular comorbidities may support this theory.
Stroke Subtyping by TOAST Criteria
As the REGARDS study collected data on ischemic stroke cause, we explored the proportion of strokes in each group by TOAST subtype. We found that: (1) the majority of strokes that occurred in patients with AF without cardiovascular comorbidities were cardioembolic; and (2) the relative risk of cardioembolic stroke was comparatively high in this group, with a 10‐fold increased incidence rate, compared with those with neither AF nor cardiovascular comorbidities. Although the absolute risk, reflected in the observed incidence rate of 2.70 cardioembolic strokes per 1000 person‐years of follow‐up in patients with AF without cardiovascular comorbidities, is low, the markedly higher morbidity and mortality associated with cardioembolic stroke45, 46 (as compared with other stroke subtypes) could suggest a benefit for therapeutic anticoagulation, even in this group that does not currently receive anticoagulation because of a low overall stroke risk. Even after accounting for the contribution of covariates, a 3‐fold increased risk of cardioembolic stroke remains.
We note that this finding may be explained by the fact that attribution of stroke cause is dependent on known cardiovascular comorbidities—if someone with AF without cardiovascular comorbidities experiences a stroke, it may be classified as cardioembolic, when the same stroke might be classified as of unknown cause if AF were not present. In light of this probable confounding in TOAST assignment by the presence of AF, the observed findings may not be meaningfully interpretable. In contrast, the finding of increased risk of cardioembolic stroke among patients with cardiovascular comorbidities in the absence of AF (group 2) is not confounded in this manner.
Interactions
In subgroup analyses, there was no significant interaction by sex, race, BMI, or smoking status, but the age×group interaction term was significant. Specifically, among patients with AF without cardiovascular comorbidities, participants older than the median (64 years) had no increased risk of stroke, while those younger than the median had an adjusted HR for stroke of 2.70 (95% CI, 0.81–6.73). Although the 95% CI crosses 1.0, the point estimate suggests that, in the absence of cardiovascular comorbidities, AF may convey more risk of stroke among younger participants than among older participants. This finding should be considered hypothesis‐generating, particularly in light of the fact that the P value for interaction is not statistically significant when correcting for multiple comparisons. Further studies focusing on the AF‐attributable risk of stroke in younger adults could prove valuable.
Comparison to Prior Literature
To the best of our knowledge, our subcohort of participants with AF without cardiovascular comorbidities is larger than any previously reported in the literature. Despite this, we found no increased risk of total stroke in patients with AF without cardiovascular comorbidities, but did find evidence of both effect modification by age and that social and demographic covariates (eg, smoking and income) accounted for some of the stroke risk. This may explain some of the discrepant findings previously reported in the literature. For example, in Swedish registries, patients with AF without cardiovascular comorbidities had HRs for stroke of 3.1 (95% CI, 2.6–3.7) in women and 2.2 (95% CI, 1.8–2.5) in men, but this analysis only matched on age and sex,14 as did a Scottish cohort study that also found an increased stroke risk.15 Other studies did not differentiate stroke from other cardiovascular outcomes, making interpretation of stroke risk difficult.13, 47, 48
The most plausible explanation for these discrepant conclusions about stroke risk hinges on the interplay between socioeconomic status, AF, and risk of stroke. In our study, we adjusted for age, sex, race, education, income, geographic region, high‐density lipoprotein cholesterol, total cholesterol, BMI, smoking, aspirin use, and warfarin use. To the best of our knowledge, this is the most complete adjustment performed in analyses of patients with AF without cardiovascular comorbidities. In contrast, prior analyses that have found significant differences in risk of stroke or cardiovascular outcomes only matched for sex and age.13, 14, 15, 47, 48 Given that prior literature has demonstrated that race, educational achievement, and income are each correlated with risk of stroke,16, 17, 18, 19, 20 we suspected that lack of adjustment for these confounders could explain their results. To determine how our results would compare, we repeated our analyses, this time only adjusting for age and sex, finding that participants with AF without cardiovascular comorbidities would have an increased risk of stroke of any type (HR, 1.86; CI, 1.21–2.85). This illustrates that, with regard to stroke risk in patients with AF, there is evidence of effect modification (by age) and confounding, with smoking, high‐density lipoprotein, and income having the largest impact (proportional hazards P values of <0.0001). Future studies should capture these categories of data to allow for adjustment to more accurately quantify the stroke risk attributable to AF.
Limitations and Strengths
Our study should be interpreted in the context of its limitations. The inclusion of only 2 races limits generalizability. Echocardiographic measures of left atrial size and function were not available. Similarly, echocardiographic data and history of prior mechanical or bioprosthetic valve implantation was not available to separate participants by valvular AF versus nonvalvular AF.49 Participants were categorized into groups as of study enrollment, with incident AF not being considered in our analysis. In addition, the comparatively small number of events observed in the AF without cardiovascular comorbidities group limited power, although we did demonstrate a statistically significant difference in risk of cardioembolic stroke. Classification of a TOAST stroke cause is somewhat reviewer‐dependent, although prior studies have demonstrated high inter‐rater reliability, particularly for cardioembolic strokes.50 In particular, a history of AF may influence TOAST classification, as discussed above. The exclusion of participants with prebaseline stroke may have limited our power to detect between‐group differences, as participants with prior stroke are those with the highest risk of future stroke. However, the clinical difficulty in differentiating recrudescence of prior ischemic symptoms from incident stroke may have confounded our outcome measure. AF may have been present but clinically undetected in the groups with no known AF, although this is a limitation of any study in the absence of continuous cardiac monitoring. Similarly, we did not have data to subtype AF into paroxysmal, persistent, and long‐standing persistent, each of which may have a differential attributable risk of stroke. Finally, our study was observational, so any hypotheses regarding the utility of anticoagulant therapy for prevention of stroke in people without AF must be tested in subsequent clinical studies.
Despite these limitations, we feel that our study adds substantively to the existing literature owing to the size of the subcohort of participants with AF without cardiovascular comorbidities. In addition, our study is the first analysis of a large cohort of patients with AF without cardiovascular comorbidities that was able to adjust for race, education, and income by utilizing a biracial nationwide prospective cohort in which there was formal adjudication of events from the medical record and long‐term follow‐up was available.
Conclusions
We found that the risk of stroke in patients with AF without cardiovascular comorbidities is not significantly elevated in comparison to patients with neither AF nor cardiovascular comorbidities, after adequately adjusting for covariates. However, patients with cardiovascular comorbidities, even in the absence of AF, had a heightened risk of cardioembolic stroke.
Sources of Funding
This research project is supported by cooperative agreement U01 NS041588, cofunded by the National Institute of Neurological Disorders and Stroke (NINDS) and the National Institute on Aging (NIA), National Institutes of Health, and Department of Health and Human Service. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NINDS or the NIA. Representatives of the NINDS were involved in the review of the article but were not directly involved in the collection, management, analysis, or interpretation of the data.
Disclosures
None.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS study investigators and institutions can be found at: https://www.uab.edu/soph/regardsstudy/.
(J Am Heart Assoc. 2020;9:e016380 DOI: 10.1161/JAHA.120.016380.)
For Sources of Funding and Disclosures, see page 9.
References
- 1. Ruddox V, Sandven I, Munkhaugen J, Skattebu J, Edvardsen T, Otterstad JE. Atrial fibrillation and the risk for myocardial infarction, all‐cause mortality and heart failure: a systematic review and meta‐analysis. Eur J Prev Cardiol. 2017;24:1555–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population‐based estimates. Am J Cardiol. 1998;82:2N–9N. [DOI] [PubMed] [Google Scholar]
- 3. Wolf PA, Dawber TR, Thomas HE, Kannel WB. Epidemiologic assessment of chronic atrial‐fibrillation and risk of stroke—Framingham Study. Neurology. 1978;28:973–977. [DOI] [PubMed] [Google Scholar]
- 4. Steger C, Pratter A, Martinek‐Bregel M, Avanzini M, Valentin A, Slany J, Stollberger C. Stroke patients with atrial fibrillation have a worse prognosis than patients without: data from the Austrian Stroke registry. Eur Heart J. 2004;25:1734–1740. [DOI] [PubMed] [Google Scholar]
- 5. Lin HJ, Wolf PA, Kelly‐Hayes M, Beiser AS, Kase CS, Benjamin EJ, D'Agostino RB. Stroke severity in atrial fibrillation. The Framingham Study. Stroke. 1996;27:1760–1764. [DOI] [PubMed] [Google Scholar]
- 6. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 7. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2014;130:E199–E267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr, Ellinor PT, Ezekowitz MD, Field ME, Furie KL, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. Circulation. 2019;140:e125–e151. [DOI] [PubMed] [Google Scholar]
- 9. Evans W, Swann P. Lone auricular fibrillation. Br Heart J. 1954;16:189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wyse DG, Van Gelder IC, Ellinor PT, Go AS, Kalman JM, Narayan SM, Nattel S, Schotten U, Rienstra M. Lone atrial fibrillation does it exist? J Am Coll Cardiol. 2014;63:1715–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kopecky SL, Gersh BJ, McGoon MD, Whisnant JP, Holmes DR Jr, Ilstrup DM, Frye RL. The natural history of lone atrial fibrillation. A population‐based study over three decades. N Engl J Med. 1987;317:669–674. [DOI] [PubMed] [Google Scholar]
- 12. Jahangir A, Lee V, Friedman PA, Trusty JM, Hodge DO, Kopecky SL, Packer DL, Hammill SC, Shen WK, Gersh BJ. Long‐term progression and outcomes with aging in patients with lone atrial fibrillation: a 30‐year follow‐up study. Circulation. 2007;115:3050–3056. [DOI] [PubMed] [Google Scholar]
- 13. Kim EJ, Yin X, Fontes JD, Magnani JW, Lubitz SA, McManus DD, Seshadri S, Vasan RS, Ellinor PT, Larson MG, et al. Atrial fibrillation without comorbidities: prevalence, incidence and prognosis (from the Framingham Heart Study). Am Heart J. 2016;177:138–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Andersson T, Magnuson A, Bryngelsson IL, Frobert O, Henriksson KM, Edvardsson N, Poci D. Gender‐related differences in risk of cardiovascular morbidity and all‐cause mortality in patients hospitalized with incident atrial fibrillation without concomitant diseases: a nationwide cohort study of 9519 patients. Int J Cardiol. 2014;177:91–99. [DOI] [PubMed] [Google Scholar]
- 15. Stewart S, Hart CL, Hole DJ, McMurray JJ. A population‐based study of the long‐term risks associated with atrial fibrillation: 20‐year follow‐up of the Renfrew/Paisley study. Am J Med. 2002;113:359–364. [DOI] [PubMed] [Google Scholar]
- 16. Thomas KL, Piccini JP, Liang L, Fonarow GC, Yancy CW, Peterson ED, Hernandez AF; Get With the Guidelines Steering C and Hospitals . Racial differences in the prevalence and outcomes of atrial fibrillation among patients hospitalized with heart failure. J Am Heart Assoc. 2013;2:e000200 DOI: 10.1161/JAHA.113.000200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rodriguez CJ, Soliman EZ, Alonso A, Swett K, Okin PM, Goff DC Jr, Heckbert SR. Atrial fibrillation incidence and risk factors in relation to race‐ethnicity and the population attributable fraction of atrial fibrillation risk factors: the Multi‐Ethnic Study of Atherosclerosis. Ann Epidemiol. 2015;25:71–76, 76.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kabra R, Cram P, Girotra S, Vaughan Sarrazin M. Effect of race on outcomes (stroke and death) in patients >65 years with atrial fibrillation. Am J Cardiol. 2015;116:230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Misialek JR, Rose KM, Everson‐Rose SA, Soliman EZ, Clark CJ, Lopez FL, Alonso A. Socioeconomic status and the incidence of atrial fibrillation in whites and blacks: the Atherosclerosis Risk in Communities (ARIC) study. J Am Heart Assoc. 2014;3:e001159 DOI: 10.1161/JAHA.114.001159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Soliman EZ, Zhang ZM, Judd S, Howard VJ, Howard G. Comparison of risk of atrial fibrillation among employed versus unemployed (from the REasons for Geographic and Racial Differences in Stroke Study). Am J Cardiol. 2017;120:1298–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cushman M, Cantrell RA, McClure LA, Howard G, Prineas RJ, Moy CS, Temple EM, Howard VJ. Estimated 10‐year stroke risk by region and race in the United States: geographic and racial differences in stroke risk. Ann Neurol. 2008;64:507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016;18:1609–1678. [DOI] [PubMed] [Google Scholar]
- 23. Andrade JG, Macle L, Nattel S, Verma A, Cairns J. Contemporary atrial fibrillation management: a comparison of the current AHA/ACC/HRS, CCS, and ESC guidelines. Can J Cardiol. 2017;33:965–976. [DOI] [PubMed] [Google Scholar]
- 24. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE III. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 25. Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS, Howard G. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25:135–143. [DOI] [PubMed] [Google Scholar]
- 26. Soliman EZ, Howard G, Meschia JF, Cushman M, Muntner P, Pullicino PM, McClure LA, Judd S, Howard VJ. Self‐reported atrial fibrillation and risk of stroke in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. Stroke. 2011;42:2950–2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pullicino PM, McClure LA, Wadley VG, Ahmed A, Howard VJ, Howard G, Safford MM. Blood pressure and stroke in heart failure in the REasons for Geographic And Racial Differences in Stroke (REGARDS) study. Stroke. 2009;40:3706–3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Howard VJ, Kleindorfer DO, Judd SE, McClure LA, Safford MM, Rhodes JD, Cushman M, Moy CS, Soliman EZ, Kissela BM, et al. Disparities in stroke incidence contributing to disparities in stroke mortality. Ann Neurol. 2011;69:619–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Meschia JF, Brott TG, Chukwudelunzu FE, Hardy J, Brown RD Jr, Meissner I, Hall LJ, Atkinson EJ, O'Brien PC. Verifying the stroke‐free phenotype by structured telephone interview. Stroke. 2000;31:1076–1080. [DOI] [PubMed] [Google Scholar]
- 30. Hatano S. Experience from a multicentre stroke register: a preliminary report. Bull World Health Organ. 1976;54:541–553. [PMC free article] [PubMed] [Google Scholar]
- 31. Lin DY, Wei LJ, Ying Z. Checking the Cox model with cumulative sums of martingale‐based residuals. Biometrika. 1993;80:557–572. [Google Scholar]
- 32. Ng'Andu NH. An empirical comparison of statistical tests for assessing the proportional hazards assumption of Cox's model. Stat Med. 1997;16:611–626. [DOI] [PubMed] [Google Scholar]
- 33. Olesen JB, Torp‐Pedersen C, Hansen ML, Lip GY. The value of the CHA2DS2‐VASc score for refining stroke risk stratification in patients with atrial fibrillation with a CHADS2 score 0‐1: a nationwide cohort study. Thromb Haemost. 2012;107:1172–1179. [DOI] [PubMed] [Google Scholar]
- 34. Friberg L, Skeppholm M, Terent A. Benefit of anticoagulation unlikely in patients with atrial fibrillation and a CHA2DS2‐VASc score of 1. J Am Coll Cardiol. 2015;65:225–232. [DOI] [PubMed] [Google Scholar]
- 35. Hirsh BJ, Copeland‐Halperin RS, Halperin JL. Fibrotic atrial cardiomyopathy, atrial fibrillation, and thromboembolism mechanistic links and clinical inferences. J Am Coll Cardiol. 2015;65:2239–2251. [DOI] [PubMed] [Google Scholar]
- 36. Guichard JB, Nattel S. Atrial cardiomyopathy a useful notion in cardiac disease management or a passing fad? J Am Coll Cardiol. 2017;70:756–765. [DOI] [PubMed] [Google Scholar]
- 37. Kamel H, Okin PM, Elkind MSV, Iadecola C. Atrial fibrillation and mechanisms of stroke time for a new model. Stroke. 2016;47:895–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kamel H, Okin PM, Longstreth WT Jr, Elkind MS, Soliman EZ. Atrial cardiopathy: a broadened concept of left atrial thromboembolism beyond atrial fibrillation. Future Cardiol. 2015;11:323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lip GY, Lowe GD. ABC of atrial fibrillation. Antithrombotic treatment for atrial fibrillation. BMJ. 1996;312:45–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Omran H, Rang B, Schmidt H, Illien S, Schimpf R, Maccarter D, Kubini R, Von Der Recke G, Tiemann K, Becher H, et al. Incidence of left atrial thrombi in patients in sinus rhythm and with a recent neurologic deficit. Am Heart J. 2000;140:658–662. [DOI] [PubMed] [Google Scholar]
- 41. Ozer N, Tokgozoglu L, Ovunc K, Kabakci G, Aksoyek S, Aytemir K, Kes S. Left atrial appendage function in patients with cardioembolic stroke in sinus rhythm and atrial fibrillation. J Am Soc Echocardiogr. 2000;13:661–665. [DOI] [PubMed] [Google Scholar]
- 42. Patel SM, Ackerman MJ, Asirvatham SJ. Left atrial appendage dysfunction in a patient with premature ventricular contractions—a risk factor for stroke? Indian Pacing Electrophysiol J. 2013;13:136–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Martin DT, Bersohn MM, Waldo AL, Wathen MS, Choucair WK, Lip GYH, Ip J, Holcomb R, Akar JG, Halperin JL; Investigators I . Randomized trial of atrial arrhythmia monitoring to guide anticoagulation in patients with implanted defibrillator and cardiac resynchronization devices. Eur Heart J. 2015;36:1660–1668. [DOI] [PubMed] [Google Scholar]
- 44. Brambatti M, Connolly SJ, Gold MR, Morillo CA, Capucci A, Muto C, Lau CP, Van Gelder IC, Hohnloser SH, Carlson M, et al. Temporal relationship between subclinical atrial fibrillation and embolic events. Circulation. 2014;129:2094–2099. [DOI] [PubMed] [Google Scholar]
- 45. Feigin VL, Lawes CM, Bennett DA, Anderson CS. Stroke epidemiology: a review of population‐based studies of incidence, prevalence, and case‐fatality in the late 20th century. Lancet Neurol. 2003;2:43–53. [DOI] [PubMed] [Google Scholar]
- 46. Yip PK, Jeng JS, Lee TK, Chang YC, Huang ZS, Ng SK, Chen RC. Subtypes of ischemic stroke. A hospital‐based stroke registry in Taiwan (SCAN‐IV). Stroke. 1997;28:2507–2512. [DOI] [PubMed] [Google Scholar]
- 47. Kopecky SL, Gersh BJ, McGoon MD, Chu CP, Ilstrup DM, Chesebro JH, Whisnant JP. Lone atrial fibrillation in elderly persons: a marker for cardiovascular risk. Arch Intern Med. 1999;159:1118–1122. [DOI] [PubMed] [Google Scholar]
- 48. Weijs B, de Vos CB, Tieleman RG, Peeters FE, Limantoro I, Kroon AA, Cheriex EC, Pisters R, Crijns HJ. The occurrence of cardiovascular disease during 5‐year follow‐up in patients with idiopathic atrial fibrillation. Europace. 2013;15:18–23. [DOI] [PubMed] [Google Scholar]
- 49. Martins RP, Galand V, Colette E, Behar N, Pavin D, Leclercq C, Daubert JC, Mabo P. Defining nonvalvular atrial fibrillation: a quest for clarification. Am Heart J. 2016;178:161–167. [DOI] [PubMed] [Google Scholar]
- 50. Meschia JF, Barrett KM, Chukwudelunzu F, Brown WM, Case LD, Kissela BM, Brown RD Jr, Brott TG, Olson TS, Rich SS, et al. Interobserver agreement in the Trial of Org 10172 in acute stroke treatment classification of stroke based on retrospective medical record review. J Stroke Cerebrovasc Dis. 2006;15:266–272. [DOI] [PMC free article] [PubMed] [Google Scholar]


